Abstract
The persistence of symptoms for a long time after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is now familiar as post-COVID syndrome (PCS). To the best of our knowledge, the risk of long-term clinical outcomes in children after SARS-CoV-2 infection is still unclear. Unlike in adults, current evidence suggests a lower prevalence of persistent symptoms in children. However, since several studies are characterized by great heterogeneity, it is difficult to accurately estimate the exact incidence of PCS in children. The presence and course of recovery depend on risk factors that are more common in adults than children. Proposed pathophysiological mechanisms in PCS in children include age-dependent immune responses, angiotensin-converting enzyme 2 expression, blood-brain barrier development or social issues affecting children behavior, such as school closure and social isolation. However, further longitudinal studies are required for unanswered issues to be clarified. The aim of the present review is to describe the long-term symptoms per biological system in children, potential risk factors and the role of the immune system in the presence of PCS.
Keywords: severe acute respiratory syndrome coronavirus 2, coronavirus disease 2019, children, post-COVID syndrome, clinical manifestations
1. Introduction
Τhe number of patients recovering from severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection is continuously increasing (1,2). Some patients of all ages require a clinical re-evaluation for persistent symptoms (3). This post-infection syndrome, which lasts ≥4 weeks and cannot be explained by an alternative diagnosis, was named as ‘Long COVID-19 syndrome’, ‘post-COVID syndrome (PCS)’, ‘post-acute COVID-19 syndrome’, ‘chronic COVID-19’ or ‘post-acute sequelae of SARS-CoV-2 infection (PASC)’ (4).
Similar postinfectious sequelae were also observed among patients recovering from other epidemic coronavirus diseases, severe acute respiratory syndrome Coronavirus (SARS-CoV) and Middle East respiratory syndrome (MERS), which included respiratory, mental and cognitive disorders (5). Post COVID-19 symptoms could be physical or psychological and typically include fatigue, cough, shortness of breath, chest pain, olfactory (dysosmia/anosmia) and gustatory (dysgeusia/ageusia) disturbances, depression, anxiety, post-traumatic stress disorder and concentration disturbances (6,7). Children appear to be less susceptible to PCS, but the reasons for this have not yet been fully understood.
The purpose of this review is to describe the reported long-term symptoms per biological system in children, the potential risk factors as well as the role of immune system in the presence of PCS.
2. Incidence of PCS in children
There are three definitions for PCS, according to US Centers for Disease Control and Prevention (CDC), the UK National Institute for Health and Care Excellence (NICE) and World Health Organization (WHO). CDC describes a wide range of ongoing, resolved or returning symptoms that occur in people at least four or more weeks after the onset of the infection, regardless of the presence, combination, duration, severity, or individuals' underlying conditions (8). NICE defines PCS as a cluster of signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks, are not explained by an alternative diagnosis and can fluctuate or change over time (9). According to NICE recommendations, PCS can also be considered before 12 weeks and clinical investigation for PCS is suggested simultaneously with the assessment of an alternative underlying disease (9).
In WHO definition, PCS occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months after the onset of COVID-19 with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis. Common symptoms include fatigue, shortness of breath, cognitive dysfunction, but also others, and generally have an impact on everyday functioning. Symptoms may be new onset after initial recovery from an acute episode of COVID-19 or persist from the initial illness. Symptoms may also fluctuate or relapse over time (10). According to the WHO, adults and children can experience PCS without knowing when they initially had their first infection. This phenomenon is suggestive of describing symptoms long after the recovery from acute disease and directly affects PCS surveillance especially in pediatric population, in which the exact disease onset is frequently unknown.
PCS in children has always been a diagnostic challenge, especially in distinguishing the symptoms related to SARS-CoV-2 infection from those related to the pandemic. The collection of data and analysis methods regarding PCS in children varies greatly among studies. Studies investigating PCS in children are characterized by a wide variety of COVID-19 diagnostic confirmation, since some are based on molecular testing (PCR) (11,12) or serological assays (13,14) or both (15,16), while others do not clearly define the diagnostic methods on which they relied to evaluate PCS in children (17,18). The collection of PCS information also significantly varies and some may be questioned as there is no clinical examination by a physician, but data were collected through questionnaires (19,20), telephone (21,22) or electronic applications (18,23).
In a systematic review including 14 studies on persistent symptoms in children and adolescents, the PCS prevalence in children had a surprisingly wide range between 4-66%, since few studies were settled in the absence of a control group (24). From these studies, only 3 included a control group of children and were able to justify a higher prevalence of persistent symptoms in children with COVID-19 than in healthy ones (24).
Recently, Molteni et al (23) recruited 1,334 children aged 5-17 years positive for SARS-CoV-2 and a matched group of children negative for SARS-CoV-2 in a prospective study based on a mobile application. Although the median duration of the disease varied significantly between the two groups (6 and 3 days, respectively), no significant differences in hospitalization or presentation in the emergency department were observed (approximately 2% of each group) (23). A limited but unignorable percentage of those children had persistent symptoms for at least 1 month and 2 months (4 and 2%, respectively) (23).
The prevalence of persistent symptoms for ≥3 months was initially shown to range from 0-27% (25,26). This variability is mainly attributed to the fluctuation in SARS-CoV-2 severity, the undetermined suspected or confirmed cases, the inconsistent methodological assessment and the transient follow-up times (25,26). Unlike PCS rates in adults (may surprisingly extend to one third of hospitalized patients), the estimated prevalence of PCS in children at least three months after the onset of the disease does not exceed 4% (13,14,27). Regarding age groups differences in PCS, children aged 6-11 years may even reach 60% of the pediatric population with PCS (14,28).
During the first two years of COVID-19 pandemic, it become evident that children represent only a minority of all confirmed COVID-19 cases worldwide which rarely exceed 20% according to Centers for Disease Control and Prevention (CDC) (29).
It is important to state that children <5 years are unable to describe symptoms, such as anosmia, ageusia or headache, that represent ‘classic’ acute phase symptoms, but also persistent COVID-19 manifestations. This leads to a significant restriction in COVID-19 diagnosis in children. Children exposed to SARS-CoV-2 who have not been diagnosed may require hospitalization in the future to relieve symptoms, such as cough or shortness of breath, that could constitute PCS manifestations (30). Hence, a major reason why PCS in children may be underdiagnosed can be justified as a result of the limited diagnostic tests performed on them during the acute phase of infection (31).
The incidence of PCS is difficult to estimate, as several studies indicate that the prevalence of commonly reported PCS symptoms, such as fatigue or headache, does not differ between children with positive and negative SARS-CoV-2 tests (31-33). However, Kikkenborg et al (34) showed that the incidence of persistent symptoms 2 months after COVID-19 was higher in convalescent adolescent patients than healthy controls.
Despite the subjective nature of the symptoms frequently described in children with PCS, Buonsenso et al (35) support that mental, muscoskeletal, and cardiovascular manifestations are considered the most PCS characteristic conditions of PCS in children. A recent meta-analysis showed that the incidence of PCS can approximately reach 30% of hospitalized COVID-19 children (36).
3. PCS symptoms in children
In contrast to the relatively early recognition of long-term impact of COVID-19 in older patients, there are limited data regarding the risk of persistent symptoms in children following COVID-19. The most common PCS symptoms in children and adults were depicted in Table I. Persistent symptoms commonly reported in children do not differ significantly compared to adults, including fatigue, respiratory distress, headache, myalgias, arthralgias, olfactory and gustatory disturbances, heart palpitations, concentration, and sleep disturbances (23,36,37). Therefore, 7% of children with COVID-19 and persistent symptoms had a reported poor quality of health (14). PCS can also be reported in asymptomatic children, while some manifestations can be misdiagnosed with Multisystem Inflammatory Syndrome in Children (MIS-C) (38).
Table I.
Comparison of post-COVID-19 syndrome symptoms reported from pediatric and adult studies (6,13,14,23,24,27,31,32,36,38,40-43,45,47,60,61,63,64).
| Symptom | Children, % | Adults, % |
|---|---|---|
| Fatigue | 3-87 | 28-87 |
| Myalgias/arthralgias | 1-61 | 3-25 |
| Rhinorrhea | 20-52 | 1-10 |
| Headache | 5-26 | 5-20 |
| Depression | 1-25 | 18-25 |
| Sore throat | 19-25 | 4-10 |
| Anosmia | 17-23 | 11-20 |
| Anxiety | 1-20 | 20-23 |
| Dyspnea | 6-15 | 12-30 |
| Chest pain | 5-12 | 5-22 |
| Concentration problem | 4-10 | 16-34 |
| Sleep disorders | 1-7 | 17-25 |
Symptoms of musculoskeletal system
Fatigue is one of the most common persistent physical symptoms regardless of the disease or hospitalization status and is commonly reported in adult females (6). The exact incidence of fatigue is difficult to be precisely estimated, given the large number of existing studies and the increased risk of recall bias from the participants (36).
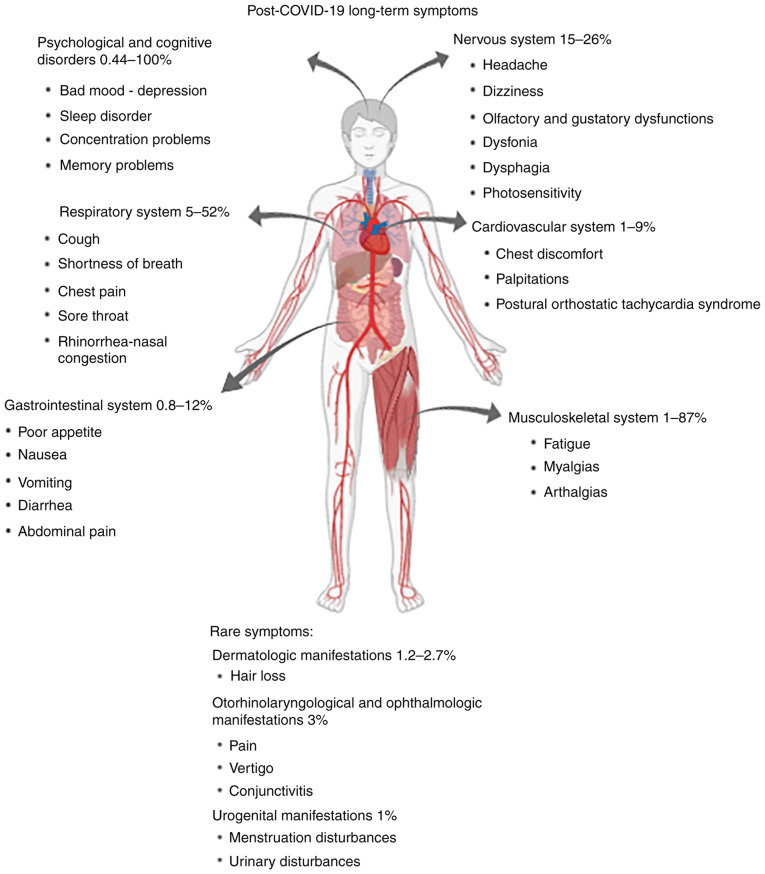
Although the incidence of fatigue in children can range between 3-87% (Fig. 1) (13,23,24), it is more common in children of older ages (23%). It is also regularly reported in 30% of adolescents before the pandemic (31,38,39). Although fatigue is well tolerable and resolves soon after the acute phase, it is possible to persist for 3 months or longer, especially in intensive care unit adult convalescent individuals (40). Notably, the prevalence of exercise intolerance has been estimated to reach 5% in children with PCS (36).
Figure 1.
Post-COVID-19 long-term symptoms per biological system in children and their reported frequencies.
Regarding myalgias and arthralgias, which may often accompany fatigue, the incidence can range between 1-61% (24). Aiyegbusi et al (41) support the idea that both are included in the most common PCS symptoms as well (25 and 20%, respectively) described by adult convalescent adult patients.
Symptoms of respiratory system
Approximately 30% of patients with severe COVID-19 hospitalized experience at least one persistent respiratory complication that often has a negative impact on individuals' daily activities (14,27,41). Many patients may experience persistent cough at least 2 weeks after the disease which resolved within 3 months, without excluding its persistence until 12 months (7,42-44). Chest pain due to respiratory complications, exertional or positional, is shown to not to be resolved soon after disease and can be persistent in up to 22% of adult patients for at least 3 months (45).
In children, sore throat and hoarseness are the most common PCS symptoms on the upper respiratory tract (23 and 5% of adolescents, respectively), while in another study rhinorrhea was included among the most common symptoms (52%) (Fig. 1) (23,31). Cough and shortness of breath are described in approximately 16 and 12% of adolescents, respectively, and are the most common respiratory symptoms of PCS (32). Persistent cough is characterized by a resolution potential of 4-8 weeks after disease onset and is prevalent in at least 1% of convalescent COVID-19 children, while shortness of breath is rare but not unignorable (13,45). The risk of persistent dyspnea was significantly higher in children exposed to SARS-CoV-2 than in healthy controls (46,47). In adults, a study including approximately 1700 hospitalized COVID-19 patients previously hospitalized with COVID-19 in Wuhan, China, showed that the prevalence of dyspnea remains consistent six and twelve months after the onset of the disease (26 and 30%, respectively) (43).
Symptoms of the gastrointestinal and cardiovascular systems
Gastrointestinal symptoms (GI), including abdominal pain, nausea, vomiting, poor appetite, and diarrhea, are common in children and adults with COVID-19 (48,49). Abdominal pain was the only persistent GI symptom that has been well described for more than 1 month in <5% of children and its incidence in adolescents reaches approximately 5% (13,31). In contrast diarrhea is considered a relatively common persistent symptom of COVID-19, encountered in approximately 6% of both convalescent adolescents and adult patients (31,40). Poor appetite, nausea, and vomiting are even rarely present within the first 2 weeks after acute phase, since they are reported in 2.3, 1.5, and 0.8%, respectively, but in adolescents the incidence of poor appetite, as reflected by the daily meal intake, can surprisingly reach 12% (31,48) (Fig. 1). In a 6-month surveillance study for persistent COVID-19 symptomatology, the prevalence of poor appetite, diarrhea and vomiting was higher (8, 5 and 5%, respectively) (7). In children, prolonged clinical manifestations from the GI tract due to PCS should not be confused with GI symptoms frequently present in MIS-C several weeks after exposure to SARS-CoV-2. According to a recent meta-analysis, the estimated prevalence of constipation, a symptom that presents in both physical and psychological disturbances, can even reach 2% of children with PCS (36).
Except for MIS-C, the cardiovascular risk for long-term clinical outcomes is generally lower in children compared to adults. Chest discomfort rarely exceeds 6 and 9% of convalescent COVID-19 children and adolescents, respectively, while palpitations and other heart rate abnormalities range between 1-2% (31,45) (Fig. 1).
In September 2020, a case of Postural Orthostatic Tachycardia Syndrome (POTS) three weeks after COVID-19 was described (50). Despite the resolution of acute phase symptomatology, POTS persisted for over 5 months and could not be explained by any other alternative medical condition (50). Autonomic impairment is a rare but notable immune-related condition often associated with many pathogens, including SARS, but with insufficient pathophysiological clarification (51). However, it still remains questionable whether there is a direct association of autonomic impairment with SARS-CoV-2 pediatric patients and continuous surveillance will assist in the elucidation of all possible underlying mechanisms.
Symptoms of central and peripheral nervous systems
Neurological complications of PCS in children are of major significance and might take years to present and evaluated. This has already been shown by other respiratory pathogens, such as pertussis, in which preschool children after natural infection were associated with an increased risk of epilepsy in adolescence (52). The most common prolonged neurological manifestations in adolescents are headache (26%) and dizziness with photosensitivity (15%) (32) (Fig. 1).
In a multicenter study based on neuroimaging and neurologic symptoms by Lindan et al (53), rare incidents of encephalomyelitis, myelitis, hypotonia, ataxia, ophthalmoplegia, and cranial nerve complications were observed in children with PCS. Guillain-Barre syndrome (GBS) with upper and lower extremities involvement is another rare but significant complication of the disease that often requires vigorous rehabilitation with motor physiotherapy in cases with sphincter disturbances or spastic quadriparesis (54). Continuous surveillance is essential for the evaluation of these complications.
Olfactory and gustatory dysfunctions are two of the most characteristic clinical manifestations of COVID-19 in both acute and convalescent phase and are significantly more common in children with COVID-19 than healthy controls (47). The majority of COVID-19 survivors describe a complete loss of smell within the first week of the convalescent phase (55). Therefore, more than 70% of adults describe a complete recovery from smell and taste disturbances within 10 days after their onset (56). Compared to women, male patients may have faster recovery rates during convalescence (57). The loss of smell has been described as one of the most common symptoms in children and adolescents (21%) with PCS and can remain unresolved for at least 5 months after the onset of the disease in 4.7% of them (31,45) (Fig. 1). Although anosmia can present as the only clinical manifestation in approximately 15% of children with acute COVID-19, it can be detected later in the clinical evaluation of the patient, since manifestations of the respiratory tract often proceed (23,45).
Dysphonia and dysphagia have been reported as a rare complication in 9 children after MIS-C that required 1-6 months to resolve after voice therapy or invasive procedures (58). According to a recent meta-analysis, the estimated prevalence of dysphonia and dysphagia has been evaluated in 0.5-2% of children with PCS (36).
Other physical clinical manifestations
A recent meta-analysis by Lopez-Leon et al (36) has evaluated the prevalence of some rare prolonged clinical manifestations of children with COVID-19. Dermatological manifestations, including hair loss, are prevalent in approximately 1.2-2.7% of children with PCS, while otorhinolaryngological symptoms (such as ear pain or vertigo) and ophthalmologic manifestations such as conjunctivitis and pain are estimated at approximately 3% of children after COVID-19(36). Urogenital symptoms, including menstruation disturbances or manifestations from the urinary tract, are notable but are rarely encountered (approximately 1%) (36).
Psychological and cognitive disorders
Psychological disorders are also common during recovery from COVID-19 and were described from the first months of the pandemic (45). In a longitudinal study of COVID-19 convalescent individuals from China, approximately 25% of participants reported at least one persistent psychological symptom 3 months after the onset of the disease (6). Among 142 ICU survivors, anxiety, depression and post-traumatic symptoms were reported in 23, 18, and 7%, respectively (59). Interestingly, COVID-19 patients with a free prior psychiatric history were associated with a higher risk of developing a 1st psychiatric episode at least 3 months following COVID-19 compared with those recovering from other medical illnesses, such as influenza and other respiratory infections (60). Depression was reported in approximately 15% of hospitalized patients with COVID-19, while sleep difficulties were encountered in approximately 25% of COVID-19 patients six months after discharge (9,60,61).
Cognitive disorders, such as concentration and memory problems, have also been reported in patients in the ICU and non-ICU. In a study from the United Kingdom that included 100 hospitalized and ICU-admitted COVID-19 patients after hospital discharge, approximately 16 and 34% of them reported a new-onset or worsened concentration problem, while a temporary memory impairment was reported in 18 and 19%, respectively (40). Logistic regression models in SARS-CoV-2 individuals without prior documented neurological symptoms have verified that even mild SARS-CoV-2 infection was associated with 18 times greater odds of cognitive decline, as defined by a 4-point decrease in Montreal Cognitive Assessment (MoCA) scores (62).
In addition to persistent physical symptoms, concerns have been raised about the long-term impact of SARS-CoV-2 on the psychological and mental health of convalescent children. A meta-analysis including 29 studies in children and adolescents from various countries underscores that 25% of children and adolescents recovered by COVID-19 express depressive symptoms, while increased anxiety disorders reached 20% (63). This study also showed that depression and anxiety rates were doubled during the pandemic compared to years before COVID-19(63). A recent meta-analysis showed that mood and sleep disturbances in children with COVID-19 can exceed 16 and 8% in the convalescent phase, while concentration and learning difficulties can be as common as in 6% of children with COVID-19(36). In contrast, speech disorders are rarely encountered (0.44%) in children after COVID-19(36).
The indirect effects of social isolation and school closures during the pandemic period had several consequences. The use of electronic media by children has increased worryingly (100%), they reduced physical activity (80%), increased their BMI (60%), increased learning difficulties (50%) and sleep disorders (45%) (64) (Fig. 1). The prevalence of weight disturbances has been estimated to reach 4% in children with PCS (36).
4. Risk factors associated with PCS in children
Gender plays a dominant role as a predisposing factor to persistent symptoms in children. Compared to men, women are characterized by an increased incidence of symptoms such as joint pain or psychological and cognitive disturbances that may even reach 79% (12,18). As in children, women are more prone to persistent COVID-19 compared to men (33% vs. 47%, respectively) (65). There are some of the studies in which male gender predominates in PCS in children and adolescents, however, these studies do not use a control group (26,65,66).
PCS is positively associated with the number of underlying medical conditions that consist risk factors for severe disease and prolonged hospitalization (65). Risk factors that are associated with PCS in children and adolescents include obesity, impaired medical history of mental health, allergies, prolonged hospitalization and MIS-C (31,45,65,67). Risk factors for PCS in adults include female gender, increased age, obesity, hypertension, asthma and immunosuppression with estimated odds ratios (ORs) ranging from 1.3 to 2.3(68). Although early published data were indicative of a 2-week recovery schedule in convalescent patients with mild disease and 3 months or longer for those with severe disease, the resolution is characterized by significant heterogeneity (69).
Role of adaptive immunity in PCS
According to the UK Office for National Statistics, the first dose of any COVID-19 vaccine results in a 13% decrease in self-reported PCS symptoms in individuals with confirmed COVID-19 before vaccination (70). Compared to the first, the second dose was associated with an additional 9% decrease in reported prolonged COVID-19 symptoms, and vaccinees developed long-term complications that significantly prevented daily activities less frequently than unvaccinated individuals (70). Antonelli et al (71) found that vaccinated individuals after the second dose have a reduced risk of persistent symptoms for at least 28 days by 50%. However, no significant correlations of prolonged symptoms with antibody titers elicited by vaccination or viral load in COVID-19 breakthrough vaccines have been established (72). Since the absolute frequencies of COVID-19 vaccination in children are lower compared to adults (73), this could be a reason why children could be more prone to PCS despite the low reported incidence of the syndrome.
According to current evidence, it is possible that certain immunological conditions could predispose to PCS. Increased levels of inflammatory cytokines during the acute phase of the disease in adults, such as interferon-α (IFN-α), granulocyte colony stimulating factor (G-CSF), TNF-α and interleukins IL-1β, IL-6, IL-13 and IL-17 or activation of lymphocyte subpopulations, such as Th9, CD8+ effector T cells or CD4+ effector memory T cells, could predispose to long-term complications of the disease (74). In a multicenter prospective study involving 175 patients who were followed for a year for long-term complications of the disease, a positive association of PCS and lower levels of IgM and IgG3 antibodies was observed (75). The decryption of the immune mechanisms elicited by SARS-CoV-2 infection will contribute to the detection of more prognostic immunobiomarkers related to PCS.
In a large-scale cohort study involving children and young adults with COVID-19, persistent symptoms were reported more frequently 3 months after the disease onset in the age group 11-17 years (32). Since the first trimester coincides with the peak of antibody levels after natural infection (our unpublished data), a possible association between antibody levels and PCS could be implemented. Most studies currently focus on PCS in adults and children aged <6 years (23,31). Since children aged 1-6 years have relatively higher antibody titers compared to other age groups of childhood (30), future studies should illuminate a possible underlying pathophysiological basis that associates immune responses with PCS in children <11 years old.
Based on these results and despite the limited data on the impact of vaccination in PCS in children, it is reasonable to support massive vaccination policies in children not only for critical prevention of COVID-19 and MIS-C, but also for long-term indirect effects.
5. Possible pathophysiological mechanisms for PCS in children
Children have fewer comorbidities and typically have milder clinical manifestations compared to adults who rarely require hospitalization. This mild clinical outcome of acute SARS-CoV-2 infection alongside the decreased hospitalization rates result in diminished follow-up assessment of children in healthcare facilities (46). In an observational study that investigated long-term complications of COVID-19 in children, approximately 40% of the initial population typically responded to follow-up evaluation (46). This is indicative of the underestimation of PCS in children, as it becomes difficult to record physical or psychological symptoms of patients lost to follow-up.
Why children are less likely to develop life-threatening physical complications of the disease is multifactorial and is attributed to the vital early mucosal immune response, the reduced cytokine release syndrome, and limited comorbidities (76). The expression of the angiotensin-converting enzyme 2 (ACE2) receptor and the transmembrane protease serine 2 (TMPRSS2) is quantitatively lower in children compared to adults (77).
The angiotropic nature of the disease has been well described in adults for acute and delayed manifestation and raised the awareness in children with the existence of post-inflammatory condition of MIS-C. Hyperinflammatory and hypercoagulable states alongside cytokine release syndrome can prove harmful to myocardial cells leading to a wide variety of cardiovascular complication including thromboembolic and ischemic events, myopericarditis, arrhythmias and heart failure in both adults and children (37,77,78). Furthermore, an increased expression of the ACE2 receptor has also been found in sustentacular cells of the olfactory epithelium (79). It is reasonable to hypothesize that decreased expression of the ACE2 receptor in the cardiovascular, respiratory, or GI system could be associated with lower proportion of long-term complications involving these systems.
In fatigue, possible pathophysiological mechanisms are multifactorial and range from central nervous system (CNS) implementation to psychological factors (68). Long-term fatigue is usually associated with prolonged hospitalization, CNS inflammation and sarcolemma damage of the skeletal muscles, but although all of these etiologies are relatively rare in children, they may only partially explain the decreased incidence of fatigue compared to adults (68). The impact of the pandemic in children should not be underestimated regarding only psychological, but also physical complications such as fatigue, although more evidence is required for this hypothesis to be enlightened.
Despite the underestimation of prolonged physical symptoms after COVID-19, the prevalence of psychological symptoms in children and adolescents is considerably higher compared to adults (80). Although school closure and lockdown measures are of great importance in preventing long-term manifestations of the disease, nevertheless they have a negative impact on the psychosocial balance of children (81). During the pandemic, children were forced to distance themselves from their friends, sports, and educational activities (81). The age-dependent vulnerability of the pediatric population to mental health disturbances, including depression and anxiety, requires a longitudinal assessment by specialists to evaluate long-term psychological aspects.
There is insufficient evidence regarding long-term neurologic and cognitive disorders of children. Immunologic response to CNS infections is modulated by astrocytes that act as antigen-presenting cells and by endothelial cells that mediate inflammatory response and cytokine release (81,82). However, the mechanisms by which SARS-CoV-2 traverses the blood-brain barrier and enters the CNS remains poorly understood. Given the neurotropic nature of SARS-CoV-2, chronic inflammation of the brain stem by leukocytes and the age-dependent blood-brain and blood-cerebrospinal fluid barrier of babies <4 months (68,82,83), long-term neurologic and cognitive impairment could not be excluded, but it is still difficult to accurately evaluate.
6. Postinfectious syndromes after other infectious diseases
The question on the existence of prolonged clinical manifestations attributed to different viral and non-viral pathogens has been set for decades (84). Some of the most common pathogens that are associated with post-infectious syndromes include respiratory tract infections (for example influenzae H1N1), EBV, measles, mumps, rubella, Ebola, Coxiella burnetti, Borrelia, Giardia lamblia, Dengue, West Nile virus, VZV, Coxsackie B, Chikungunya, Polio, Salmonella, Shigella, Yersinia, Campylobacter, and Streptococcus pyogenes (84,85). Post-Ebola syndrome is characterized by fatigue, myalgias, arthralgias, hearing loss, dysphagia, ophthalmologic manifestations, neurocognitive disorders, and sleep disturbances (86-89). Post-treatment Lyme disease syndrome is characterized by fatigue, myalgia, arthralgia, irritability, neurocognitive disorders, and sleep disturbances (90,91). Post-chikungunya syndrome is characterized by fatigue, myalgias, arthralgias, hearing loss, ophthalmologic manifestations, hair loss, irritability, neurocognitive disorders, sleep disturbances and depression (92-94). Q-fever fatigue syndrome is associated with fatigue, fever, myalgias, arthralgias, shortness of breath, headache, lymphadenopathy, ophthalmologic manifestations, teeth problems, fasciculation, irritability and neurocognitive disorders (95-98). However, most of these studies refer to adults, and there is not enough evidence in the pediatric population.
In contrast to the syndromes mentioned above, post-infectious syndrome after infectious mononucleosis has been well described in the pediatric population and especially in adolescents, with prolonged fatigue being the predominant and most characteristic manifestation that can last for over 6 months (99). Chronic fatigue syndrome has also been associated with other viral pathogens, including pandemic influenzae (H1N1), VZV, polio virus, Coxsackie virus B, West Nile and Dengue (100-105). Arthralgias, another common prolonged post-infectious syndrome, has been associated with mumps, rubella, and GI tract infections, such as Salmonella, Shigella, Yersinia, and Campylobacter, as reactive arthritis (84,106).
7. Conclusions
COVID-19 pandemic has resulted in a growing population of pediatric and elderly patients with a wide range of persistent symptoms several weeks after acute SARS-CoV-2 infection. Τhe prevalence of persistent symptoms in children is significantly limited compared to adults, but remains considerable. The differences between the three definitions of PCS (WHO, CDC and NICE) alongside the lack of uniformly data collection, analysis methods and control groups among the different studies raise questions about the incidence precision of PCS in children. In order the exact incidence of the syndrome in children to be approached and the underlying pathophysiological mechanisms to be enlightened, future longitudinal studies should include control groups, detailed record of physical and psychological clinical features and frequent follow-up schedules.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
FF, EBT and AM contributed to literature research, manuscript writing and review. FF and EBT designed the figure and tables. AM conceptualized and supervised the study. Data authentication is not applicable. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1. Center for Systems Science and Engineering: COVID-19 Map-Johns Hopkins coronavirus resource center. Johns Hopkins Coronavirus Resource Center, 2021. [Google Scholar]
- 2.Martins MM, Prata-Barbosa A, da Cunha AJLA. Update on SARS-CoV-2 infection in children. Paediatr Int Child Health. 2021;41:56–64. doi: 10.1080/20469047.2021.1888026. [DOI] [PubMed] [Google Scholar]
- 3.Greenhalgh T, Knight M, A'Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020;370(m3026) doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 4.Collins FS. NIH launches new initiative to study ‘Long COVID’. National Institutes of Health (NIH), Bethesda, MD, 2021. [Google Scholar]
- 5.Ahmed H, Patel K, Greenwood DC, Halpin S, Lewthwaite P, Salawu A, Eyre L, Breen A, O'Connor R, Jones A, Sivan M. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and middle east respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: A systematic review and meta-analysis. J Rehabil Med. 2020;52(jrm00063) doi: 10.2340/16501977-2694. [DOI] [PubMed] [Google Scholar]
- 6.Xiong Q, Xu M, Li J, Liu Y, Zhang J, Xu Y, Dong W. Clinical sequelae of COVID-19 survivors in Wuhan, China: A single-centre longitudinal study. Clin Microbiol Infect. 2021;27:89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, et al. 6-Month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention (CDC): Long COVID or Post-COVID Conditions. CDC, Atlanta, GA, 2022. [Google Scholar]
- 9. National Institute for Health and Care Excellence (NICE): COVID-19 rapid guideline: Managing the long-term effects of COVID-19. NICE, London, pp1-111, 2021. [PubMed] [Google Scholar]
- 10. World Health Organization (WHO): A clinical case definition of post COVID-19 condition by a Delphi consensus, 6 October 2021. WHO, Geneva, 2021. [Google Scholar]
- 11.Borch L, Holm M, Knudsen M, Ellermann-Eriksen S, Hagstroem S. Long COVID symptoms and duration in SARS-CoV-2 positive children-a nationwide cohort study. Eur J Pediatr. 2022;181:1597–1607. doi: 10.1007/s00431-021-04345-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matteudi T, Luciani L, Fabre A, Minodier P, Boucekine M, Bosdure E, Dubus JC, Colson P, Chabrol B, Morand A. Clinical characteristics of paediatric COVID-19 patients followed for up to 13 months. Acta Paediatr. 2021;110:3331–3333. doi: 10.1111/apa.16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blankenburg J, Wekenborg MK, Reichert J, Kirsten C, Kahre E, Haag L, Haag L, Schumm L, Czyborra P, Berner R, Armann JP. doi: 10.1101/2021.05.11.21257037. Mental health of adolescents in the pandemic: Long-COVID-19 or long-pandemic syndrome? medRxiv: doi: [DOI] [Google Scholar]
- 14.Radtke T, Ulyte A, Puhan MA, Kriemler S. Long-term symptoms after SARS-CoV-2 infection in children and adolescents. JAMA. 2021;326:869–871. doi: 10.1001/jama.2021.11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fink TT, Marques HHS, Gualano B, Lindoso L, Bain V, Astley C, Martins F, Matheus D, Matsuo OM, Suguita P, et al. Persistent symptoms and decreased health-related quality of life after symptomatic pediatric COVID-19: A prospective study in a Latin American tertiary hospital. Clinics (Sao Paulo) 2021;76(e3511) doi: 10.6061/clinics/2021/e3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roge I, Smane L, Kivite-Urtane A, Pucuka Z, Racko I, Klavina L, Pavare J. Comparison of persistent symptoms after COVID-19 and other non-SARS-CoV-2 infections in children. Front Pediatr. 2021;9(752385) doi: 10.3389/fped.2021.752385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erol N, Alpinar A, Erol C, Sari E, Alkan K. Intriguing new faces of Covid-19: Persisting clinical symptoms and cardiac effects in children. Cardiol Young. 2022;32:1085–1091. doi: 10.1017/S1047951121003693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller F, Nguyen V, Navaratnam A, Shrotri R, Kovar J, Hayward AC, Fragaszy E, Aldridge RW, Hardelid P. doi: 10.1097/INF.0000000000003715. Prevalence of persistent symptoms in children during the COVID-19 pandemic: Evidence from a household cohort study in England and Wales. medRxiv: 2021.05.28.21257602, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stephenson T, Shafran R, De Stavola B, Rojas N, Aiano F, Amin-Chowdhury Z, McOwat K, Simmons R, Zavala M, Consortium C, et al. Long COVID and the mental and physical health of children and young people: National matched cohort study protocol (the CLoCk study) BMJ Open. 2021;11(e052838) doi: 10.1136/bmjopen-2021-052838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zavala M, Ireland G, Amin-Chowdhury Z, Ramsay ME, Ladhani SN. doi: 10.1093/cid/ciab991. Acute and persistent symptoms in children with PCR-confirmed SARS-CoV-2 infection compared to test-negative children in England: Active, prospective, national surveillance. Clin Infect Dis: Nov 29, 2021 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osmanov IM, Spiridonova E, Bobkova P, Gamirova A, Shikhaleva A, Andreeva M, Blyuss O, El-Taravi Y, DunnGalvin A, Comberiati P, et al. Risk factors for long covid in previously hospitalised children using the ISARIC global follow-up protocol: A prospective cohort study. Eur Respir J. 2021;59(2101341) doi: 10.1183/13993003.01341-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rusetsky Y, Meytel I, Mokoyan Z, Fisenko A, Babayan A, Malyavina U. Smell status in children infected with SARS-CoV-2. Laryngoscope. 2021;131:E2475–E2480. doi: 10.1002/lary.29403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molteni E, Sudre CH, Canas LS, Bhopal SS, Hughes RC, Antonelli M, Murray B, Kläser K, Kerfoot E, Chen L, et al. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2. Lancet Child Adolesc Heal. 2021;5:708–718. doi: 10.1016/S2352-4642(21)00198-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmermann P, Pittet LF, Curtis N. How common is long COVID in children and adolescents? Pediatr Infect Dis J. 2021;40:e482–e487. doi: 10.1097/INF.0000000000003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buonsenso D, Munblit D, De Rose C, Sinatti D, Ricchiuto A, Carfi A, Valentini P. Preliminary evidence on long COVID in children. Acta Paediatr. 2021;110:2208–2211. doi: 10.1111/apa.15870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Say D, Crawford N, McNab S, Wurzel D, Steer A, Tosif S. Post-acute COVID-19 outcomes in children with mild and asymptomatic disease. Lancet Child Adolesc Heal. 2021;5:e22–e23. doi: 10.1016/S2352-4642(21)00124-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guler SA, Ebner L, Aubry-Beigelman C, Bridevaux PO, Brutsche M, Clarenbach C, Garzoni C, Geiser TK, Lenoir A, Mancinetti M, et al. Pulmonary function and radiological features 4 months after COVID-19: First results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J. 2021;57(2003690) doi: 10.1183/13993003.03690-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanichkachorn G, Newcomb R, Cowl CT, Murad MH, Breeher L, Miller S, Trenary M, Neveau D, Higgins S. Post-COVID-19 syndrome (long haul syndrome): Description of a multidisciplinary clinic at mayo clinic and characteristics of the initial patient cohort. Mayo Clin Proc. 2021;96:1782–1791. doi: 10.1016/j.mayocp.2021.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Centers for Disease Control and Prevention: COVID Data Tracker: Demographic Trends of COVID-19 cases and deaths in the US reported to CDC. US Department of Health and Human Services, CDC, Atlanta, GA, 2022. https://covid.cdc.gov/covid-data-tracker/#demographics. Accessed July 28, 2022. [Google Scholar]
- 30.Filippatos F, Tatsi EB, Dellis C, Efthymiou V, Margeli A, Papassotiriou I, Syriopoulou V, Michos A. Seroepidemiology of SARS-CoV-2 in pediatric population during a 16-month period prior to vaccination. J Med Virol. 2022;94:2174–2180. doi: 10.1002/jmv.27608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hyde Z. Difference in severe acute respiratory syndrome coronavirus 2 attack rate between children and adults may reflect bias. Clin Infect Dis. 2022;74:152–155. doi: 10.1093/cid/ciab183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stephenson T, Pinto Pereira SM, Shafran R, de Stavola BL, Rojas N, McOwat K, Simmons R, Zavala M, O'Mahoney L, Chalder T, et al. Physical and mental health 3 months after SARS-CoV-2 infection (long COVID) among adolescents in England (CLoCk): A national matched cohort study. Lancet Child Adolesc Heal. 2022;6:230–239. doi: 10.1016/S2352-4642(22)00022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chew-Graham CA, Briggs TA, Kane B. Long COVID in children and young people: uncertainty and contradictions. Br J Gen Pract. 2022;72:253–254. doi: 10.3399/bjgp22X719501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kikkenborg Berg S, Dam Nielsen S, Nygaard U, Bundgaard H, Palm P, Rotvig C, Vinggaard Christensen A. Long COVID symptoms in SARS-CoV-2-positive adolescents and matched controls (LongCOVIDKidsDK): A national, cross-sectional study. Lancet Child Adolesc Heal. 2022;6:240–248. doi: 10.1016/S2352-4642(22)00004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buonsenso D, Pujol FE, Munblit D, Pata D, McFarland S, Simpson FK. Clinical characteristics, activity levels and mental health problems in children with long coronavirus disease: A survey of 510 children. Future Microbiol. 2022;17:577–588. doi: 10.2217/fmb-2021-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez-Leon S, Wegman-Ostrosky T, del Valle CA, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, Villapol S. Long-COVID in children and adolescents: a systematic review and meta-analyses. Sci Rep. 2022;12(9950) doi: 10.1038/s41598-022-13495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ludvigsson JF. Case report and systematic review suggest that children may experience similar long-term effects to adults after clinical COVID-19. Acta Paediatr. 2021;110:914–921. doi: 10.1111/apa.15673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thallapureddy K, Thallapureddy K, Zerda E, Suresh N, Kamat D, Rajasekaran K, Moreira A. Long-term complications of COVID-19 infection in adolescents and children. Curr Pediatr Rep. 2022;10:11–17. doi: 10.1007/s40124-021-00260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rimes KA, Goodman R, Hotopf M, Wessely S, Meltzer H, Chalder T. Incidence, prognosis, and risk factors for fatigue and chronic fatigue syndrome in adolescents: A prospective community study. Pediatrics. 2007;119:e603–e609. doi: 10.1542/peds.2006-2231. [DOI] [PubMed] [Google Scholar]
- 40.Halpin SJ, McIvor C, Whyatt G, Adams A, Harvey O, McLean L, Walshaw C, Kemp S, Corrado J, Singh R, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J Med Virol. 2021;93:1013–1022. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- 41.Aiyegbusi OL, Hughes SE, Turner G, Rivera SC, McMullan C, Chandan JS, Haroon S, Price G, Davies EH, Nirantharakumar K, et al. Symptoms, complications and management of long COVID: A review. J R Soc Med. 2021;114:428–442. doi: 10.1177/01410768211032850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wanga V, Chevinsky JR, Dimitrov LV, Gerdes ME, Whitfield GP, Bonacci RA, Nji MAM, Hernandez-Romieu AC, Rogers-Brown JS, McLeod T, et al. Long-term symptoms among adults tested for SARS-CoV-2-United States, January 2020-April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1235–1241. doi: 10.15585/mmwr.mm7036a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang L, Yao Q, Gu X, Wang Q, Ren L, Wang Y, Hu P, Guo L, Liu M, Xu J, et al. 1-Year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet. 2021;398:747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tenforde MW, Kim SS, Lindsell CJ, Billig Rose E, Shapiro NI, Files DC, Gibbs KW, Erickson HL, Steingrub JS, Smithline HA, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network-United States, March-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. Gemelli Against COVID-19 Post-Acute Care Study Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Osmanov IM, Spiridonova E, Bobkova P, Gamirova A, Shikhaleva A, Andreeva M, Blyuss O, El-Taravi Y, DunnGalvin A, Comberiati P, et al. Risk factors for post-COVID-19 condition in previously hospitalised children using the ISARIC Global follow-up protocol: A prospective cohort study. Eur Respir J. 2022;59(2101341) doi: 10.1183/13993003.01341-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fainardi V, Meoli A, Chiopris G, Motta M, Skenderaj K, Grandinetti R, Bergomi A, Antodaro F, Zona S, Esposito S. Long COVID in children and adolescents. Life (Basel) 2022;12(285) doi: 10.3390/life12020285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tariq R, Saha S, Furqan F, Hassett L, Pardi D, Khanna S. Prevalence and mortality of COVID-19 patients with gastrointestinal symptoms: A systematic review and meta-analysis. Mayo Clin Proc. 2020;95:1632–1648. doi: 10.1016/j.mayocp.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang X, Xu H, Jiang H, Wang L, Lu C, Wei X, Liu J, Xu S. Clinical features and outcomes of discharged coronavirus disease 2019 patients: A prospective cohort study. QJM. 2020;113:657–665. doi: 10.1093/qjmed/hcaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miglis MG, Prieto T, Shaik R, Muppidi S, Sinn DI, Jaradeh S. A case report of postural tachycardia syndrome after COVID-19. Clin Auton Res. 2020;30:449–451. doi: 10.1007/s10286-020-00727-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carod-Artal FJ. Infectious diseases causing autonomic dysfunction. Clin Auton Res. 2018;28:67–81. doi: 10.1007/s10286-017-0452-4. [DOI] [PubMed] [Google Scholar]
- 52.Olsen M, Thygesen SK, Østergaard JR, Nielsen H, Henderson VW, Ehrenstein V, Nørgaard M, Sørensen HT. Hospital-diagnosed pertussis infection in children and long-term risk of epilepsy. JAMA. 2015;314:1844–1849. doi: 10.1001/jama.2015.13971. [DOI] [PubMed] [Google Scholar]
- 53.Lindan CE, Mankad K, Ram D, Kociolek LK, Silvera VM, Boddaert N, Stivaros SM, Palasis S. Neuroimaging manifestations in children with SARS-CoV-2 infection: A multinational, multicentre collaborative study. Lancet Child Adolesc Heal. 2021;5:167–177. doi: 10.1016/S2352-4642(20)30362-X. ASPNR PECOBIG Collaborator Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frank CHM, Almeida TVR, Marques EA, de Sousa Monteiro Q, Feitoza PVS, Borba MGS, Vasconcelos HL, de Souza Bastos M, Lacerda MVG. Guillain-Barré syndrome associated with SARS-CoV-2 infection in a pediatric patient. J Trop Pediatr. 2021;67(fmaa044) doi: 10.1093/tropej/fmaa044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hopkins C, Surda P, Whitehead E, Kumar BN. Early recovery following new onset anosmia during the COVID-19 pandemic-an observational cohort study. J Otolaryngol Head Neck Surg. 2020;49(26) doi: 10.1186/s40463-020-00423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cho RHW, To ZWH, Yeung ZWC, Tso EYK, Fung KSC, Chau SKY, Leung EYL, Hui TSC, Tsang SWC, Kung KN, et al. COVID-19 viral load in the severity of and recovery from olfactory and gustatory dysfunction. Laryngoscope. 2020;130:2680–2685. doi: 10.1002/lary.29056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meini S, Suardi LR, Busoni M, Roberts AT, Fortini A. Olfactory and gustatory dysfunctions in 100 patients hospitalized for COVID-19: Sex differences and recovery time in real-life. Eur Arch Otorhinolaryngol. 2020;277:3519–3523. doi: 10.1007/s00405-020-06102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Halfpenny R, Stewart A, Carter A, Wyatt M, Jephson C, O'Dwyer E, Cavalli L. Dysphonia and dysphagia consequences of paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) Int J Pediatr Otorhinolaryngol. 2021;148(110823) doi: 10.1016/j.ijporl.2021.110823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morin L, Savale L, Pham T, Colle R, Figueiredo S, Harrois A, Gasnier M, Lecoq AL, Meyrignac O, et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325:1525–1534. doi: 10.1001/jama.2021.3331. Writing Committee for the COMEBAC Study Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taquet M, Luciano S, Geddes JR, Harrison PJ. Bidirectional associations between COVID-19 and psychiatric disorder: Retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8:130–140. doi: 10.1016/S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mandal S, Barnett J, Brill SE, Brown JS, Denneny EK, Hare SS, Heightman M, Hillman TE, Jacob J, Jarvis HC, et al. ‘Long-COVID’: A cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2021;76:396–398. doi: 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Del Brutto OH, Wu S, Mera RM, Costa AF, Recalde BY, Issa NP. Cognitive decline among individuals with history of mild symptomatic SARS-CoV-2 infection: A longitudinal prospective study nested to a population cohort. Eur J Neurol. 2021;28:3245–3253. doi: 10.1111/ene.14775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Racine N, McArthur BA, Cooke JE, Eirich R, Zhu J, Madigan S. Global prevalence of depressive and anxiety symptoms in children and adolescents during COVID-19: A meta-analysis. JAMA Pediatr. 2021;175:1142–1150. doi: 10.1001/jamapediatrics.2021.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huss G, Magendie C, Pettoello-Mantovani M, Jaeger-Roman E. Implications of the COVID-19 pandemic for pediatric primary care practice in Europe. J Pediatr. 2021;233:290–291.e2. doi: 10.1016/j.jpeds.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stavem K, Ghanima W, Olsen MK, Gilboe HM, Einvik G. Persistent symptoms 1.5-6 months after COVID-19 in non-hospitalised subjects: A population-based cohort study. Thorax. 2021;76:405–407. doi: 10.1136/thoraxjnl-2020-216377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sterky E, Olsson-Åkefeldt S, Hertting O, Herlenius E, Alfven T, Ryd Rinder M, Rhedin S, Hildenwall H. Persistent symptoms in Swedish children after hospitalisation due to COVID-19. Acta Paediatr. 2021;110:2578–2580. doi: 10.1111/apa.15999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smane L, Roge I, Pucuka Z, Pavare J. Clinical features of pediatric post-acute COVID-19: A descriptive retrospective follow-up study. Ital J Pediatr. 2021;47(177) doi: 10.1186/s13052-021-01127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crook H, Raza S, Nowell J, Young M, Edison P. Long covid-mechanisms, risk factors, and management. BMJ. 2021;374(n1648) doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 69.McCue C, Cowan R, Quasim T, Puxty K, McPeake J. Long term outcomes of critically ill COVID-19 pneumonia patients: Early learning. Intensive Care Med. 2021;47:240–241. doi: 10.1007/s00134-020-06313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Office for National Statistics: Coronavirus (COVID-19) vaccination and self-reported long COVID in the UK. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19vaccinationandselfreportedlongcovidintheuk/25october2021. Accessed January 26, 2022. [Google Scholar]
- 71.Antonelli M, Penfold RS, Merino J, Sudre CH, Molteni E, Berry S, Canas LS, Graham MS, Klaser K, Modat M, et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID symptom study app: A prospective, community-based, nested, case-control study. Lancet Infect Dis. 2022;22:43–55. doi: 10.1016/S1473-3099(21)00460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pereira C, Harris BHL, Di Giovannantonio M, Rosadas C, Short CE, Quinlan R, Sureda-Vives M, Fernandez N, Day-Weber I, Khan M, et al. The Association between antibody response to severe acute respiratory syndrome coronavirus 2 infection and post-COVID-19 syndrome in healthcare workers. J Infect Dis. 2021;223:1671–1676. doi: 10.1093/infdis/jiab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Centers for Disease Control and Prevention: COVID Data Tracker: Trends in Demographic Characteristics of People Receiving COVID-19 Vaccinations in the United States. US Department of Health and Human Services, CDC, Atlanta, GA, 2022. https://covid.cdc.gov/covid-data-tracker/#vaccination-demographics-trends. Accessed July 28, 2022. [Google Scholar]
- 74.Acosta-Ampudia Y, Monsalve DM, Rojas M, Rodríguez Y, Zapata E, Ramírez-Santana C, Anaya JM. Persistent autoimmune activation and proinflammatory state in post-coronavirus disease 2019 syndrome. J Infect Dis. 2022;225:2155–2162. doi: 10.1093/infdis/jiac017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cervia C, Zurbuchen Y, Taeschler P, Ballouz T, Menges D, Hasler S, Adamo S, Raeber ME, Bächli E, Rudiger A, et al. Immunoglobulin signature predicts risk of post-acute COVID-19 syndrome. Nat Commun. 2022;13(446) doi: 10.1038/s41467-021-27797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Filippatos F, Tatsi EB, Michos A. Immune response to SARS-CoV-2 in children: A review of the current knowledge. Pediatr Investig. 2021;5:217–228. doi: 10.1002/ped4.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schuler BA, Habermann AC, Plosa EJ, Taylor CJ, Jetter C, Negretti NM, Kapp ME, Benjamin JT, Gulleman P, Nichols DS, et al. Age-determined expression of priming protease TMPRSS2 and localization of SARS-CoV-2 in lung epithelium. J Clin Invest. 2021;131(e140766) doi: 10.1172/JCI140766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Butowt R, von Bartheld CS. Anosmia in COVID-19: Underlying mechanisms and assessment of an olfactory route to brain infection. Neuroscientist. 2021;27:582–603. doi: 10.1177/1073858420956905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ravens-Sieberer U, Kaman A, Otto C, Adedeji A, Devine J, Erhart M, Napp AK, Becker M, Blanck-Stellmacher U, Löffler C, et al. Mental health and quality of life in children and adolescents during the COVID-19 pandemic-results of the copsy study. Dtsch Arztebl Int. 2020;117:828–829. doi: 10.3238/arztebl.2020.0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.de Figueiredo CS, Sandre PC, Portugal LCL, Mázala-de-Oliveira T, da Silva Chagas L, Raony Í, Ferreira ES, Giestal-de-Araujo E, Dos Santos AA, Bomfim PO. COVID-19 pandemic impact on children and adolescents' mental health: Biological, environmental, and social factors. Prog Neuropsychopharmacol Biol Psychiatry. 2021;106(110171) doi: 10.1016/j.pnpbp.2020.110171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim KS. Mechanisms of microbial traversal of the blood-brain barrier. Nat Rev Microbiol. 2008;6:625–634. doi: 10.1038/nrmicro1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Erickson MA, Banks WA. Age-associated changes in the immune system and blood-brain barrier functions. Int J Mol Sci. 2019;20(1632) doi: 10.3390/ijms20071632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bannister BA. Post-infectious disease syndrome. Postgrad Med J. 1988;64:559–567. doi: 10.1136/pgmj.64.753.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Choutka J, Jansari V, Hornig M, Iwasaki A. Unexplained post-acute infection syndromes. Nat Med. 2022;28:911–923. doi: 10.1038/s41591-022-01810-6. [DOI] [PubMed] [Google Scholar]
- 86.Wilson HW, Amo-Addae M, Kenu E, Ilesanmi OS, Ameme DK, Sackey SO. Post-ebola syndrome among ebola virus disease survivors in montserrado county, Liberia 2016. Biomed Res Int. 2018;2018(1909410) doi: 10.1155/2018/1909410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Clark DV, Kibuuka H, Millard M, Wakabi S, Lukwago L, Taylor A, Eller MA, Eller LA, Michael NL, Honko AN, et al. Long-term sequelae after Ebola virus disease in Bundibugyo, Uganda: A retrospective cohort study. Lancet Infect Dis. 2015;15:905–912. doi: 10.1016/S1473-3099(15)70152-0. [DOI] [PubMed] [Google Scholar]
- 88.Jagadesh S, Sevalie S, Fatoma R, Sesay F, Sahr F, Faragher B, Semple MG, Fletcher TE, Weigel R, Scott JT. Disability among ebola survivors and their close contacts in sierra leone: A retrospective case-controlled cohort study. Clin Infect Dis. 2018;66:131–133. doi: 10.1093/cid/cix705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sneller MC, Reilly C, Badio M, Bishop RJ, Eghrari AO, Moses SJ, Johnson KL, Gayedyu-Dennis D, Hensley LE, et al. A longitudinal study of ebola sequelae in Liberia. N Engl J Med. 2019;380:924–934. doi: 10.1056/NEJMoa1805435. PREVAIL III Study Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rebman AW, Bechtold KT, Yang T, Mihm EA, Soloski MJ, Novak CB, Aucott JN. The clinical, symptom, and quality-of-life characterization of a well-defined group of patients with posttreatment lyme disease syndrome. Front Med (Lausanne) 2017;4(224) doi: 10.3389/fmed.2017.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ursinus J, Vrijmoeth HD, Harms MG, Tulen AD, Knoop H, Gauw SA, Zomer TP, Wong A, Friesema IHM, Vermeeren YM, et al. Prevalence of persistent symptoms after treatment for lyme borreliosis: A prospective observational cohort study. Lancet Reg Health Eur. 2021;6(100142) doi: 10.1016/j.lanepe.2021.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paixão ES, Rodrigues LC, Costa MDCN, Itaparica M, Barreto F, Gérardin P, Teixeira MG. Chikungunya chronic disease: A systematic review and meta-analysis. Trans R Soc Trop Med Hyg. 2018;112:301–316. doi: 10.1093/trstmh/try063. [DOI] [PubMed] [Google Scholar]
- 93.Soumahoro MK, Gérardin P, Boëlle PY, Perrau J, Fianu A, Pouchot J, Malvy D, Flahault A, Favier F, Hanslik T. Impact of Chikungunya virus infection on health status and quality of life: A retrospective cohort study. PLoS One. 2009;4(e7800) doi: 10.1371/journal.pone.0007800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gérardin P, Fianu A, Malvy D, Mussard C, Boussaïd K, Rollot O, Michault A, Gaüzere BA, Bréart G, Favier F. Perceived morbidity and community burden after a Chikungunya outbreak: The TELECHIK survey, a population-based cohort study. BMC Med. 2011;9(5) doi: 10.1186/1741-7015-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hickie I, Davenport T, Wakefield D, Vollmer-Conna U, Cameron B, Vernon SD, Reeves WC, Lloyd A. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: Prospective cohort study. BMJ. 2006;333(575) doi: 10.1136/bmj.38933.585764.AE. Dubbo Infection Outcomes Study Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marmion BP, Shannon M, Maddocks I, Storm P, Penttila I. Protracted debility and fatigue after acute Q fever. Lancet. 1996;347:977–978. doi: 10.1016/s0140-6736(96)91469-5. [DOI] [PubMed] [Google Scholar]
- 97.Bronner MB, Haagsma JA, Dontje ML, Barmentloo L, Kouwenberg RMCEJ, Olde Loohuis AGM, de Groot A, Erasmus V, Polinder S. Long-term impact of a Q-fever outbreak: An evaluation of health symptoms, health-related quality of life, participation and health care satisfaction after ten years. J Psychosom Res. 2020;139(110258) doi: 10.1016/j.jpsychores.2020.110258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ayres JG, Flint N, Smith EG, Tunnicliffe WS, Fletcher TJ, Hammond K, Ward D, Marmion BP. Post-infection fatigue syndrome following Q fever. QJM. 1998;91:105–123. doi: 10.1093/qjmed/91.2.105. [DOI] [PubMed] [Google Scholar]
- 99.Katz BZ, Shiraishi Y, Mears CJ, Binns HJ, Taylor R. Chronic fatigue syndrome after infectious mononucleosis in adolescents. Pediatrics. 2009;124:189–193. doi: 10.1542/peds.2008-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ramblow J, Alexander M, LaPorte R, Kaufmann C, Kuller L. Epidemiology of the post-polio syndrome. Am J Epidemiol. 1992;136:769–786. doi: 10.1093/aje/136.7.769. [DOI] [PubMed] [Google Scholar]
- 101.Chia J, Chia A, Voeller M, Lee T, Chang R. Acute enterovirus infection followed by myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and viral persistence. J Clin Pathol. 2010;63:165–168. doi: 10.1136/jcp.2009.070466. [DOI] [PubMed] [Google Scholar]
- 102.Seet RC, Quek AM, Lim EC. Post-infectious fatigue syndrome in dengue infection. J Clin Virol. 2007;38:1–6. doi: 10.1016/j.jcv.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 103.Tsai SY, Yang TY, Chen HJ, Chen CS, Lin WM, Shen WC, Kuo CN, Kao CH. Increased risk of chronic fatigue syndrome following herpes zoster: A population-based study. Eur J Clin Microbiol Infect Dis. 2014;33:1653–1659. doi: 10.1007/s10096-014-2095-x. [DOI] [PubMed] [Google Scholar]
- 104.Garcia MN, Hause AM, Walker CM, Orange JS, Hasbun R, Murray KO. Evaluation of prolonged fatigue post-West Nile virus infection and association of fatigue with elevated antiviral and proinflammatory cytokines. Viral Immunol. 2014;27:327–333. doi: 10.1089/vim.2014.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Magnus P, Gunnes N, Tveito K, Bakken IJ, Ghaderi S, Stoltenberg C, Hornig M, Lipkin WI, Trogstad L, Håberg SE. Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) is associated with pandemic influenza infection, but not with an adjuvanted pandemic influenza vaccine. Vaccine. 2015;33:6173–6177. doi: 10.1016/j.vaccine.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 106.Pogreba-Brown K, Austhof E, Tang X, Trejo MJ, Owusu-Dommey A, Boyd K, Armstrong A, Schaefer K, Bazaco MC, Batz M, et al. Enteric pathogens and reactive arthritis: Systematic review and meta-analyses of pathogen-associated reactive arthritis. Foodborne Pathog Dis. 2021;18:627–639. doi: 10.1089/fpd.2020.2910. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.