Abstract
This study assesses whether associations of prenatal cannabis exposure and psychopathology persist into early adolescence.
Dramatic increases in cannabis use during pregnancy are alarming because of evidence that prenatal exposure may be associated with a host of adverse outcomes.1 We previously found that prenatal cannabis exposure (PCE) following maternal knowledge of pregnancy is associated with increased psychopathology during middle childhood using baseline data from the Adolescent Brain Cognitive Development (ABCD) study.2 Here, leveraging longitudinal ABCD study data (data release 4.0), we examined whether associations with psychopathology persist into early adolescence.
Methods
We estimated associations between retrospective report of maternal cannabis use during pregnancy (only before maternal knowledge of pregnancy [BK-PCE], before and after maternal knowledge of pregnancy [BAK-PCE], and no exposure [NE]) and longitudinal assessments (baseline [June 1, 2016, to October 15, 2018], 1-year follow-up, and 2-year follow-up) of psychopathology (Child Behavior Checklist3 subscales, n = 20; total reported psychoticlike experiences on the Prodromal Questionnaire–Brief Child Version4). PCE groups had greater attrition (χ2 = 34.2, P < .001). Participants provided assent and caregivers provided written informed consent to a protocol approved by the institutional review board of each data collection site. We followed the STROBE reporting guideline for cohort studies.
Associations between PCE groups (BK-PCE, BAK-PCE, and NE) and child psychopathology were estimated using mixed models. In addition to main associations of exposure and age, interactions (ie, [age + age2] × [BK-PCE + BAK-PCE]) modeled age-associated change. χ2 Tests of log likelihood compared models with and without predictors of interest (ie, PCE group, PCE group × age interaction) to determine significance.
Covariates included family and child variables (Table). False discovery rate (FDR) multiple comparison correction was used (n = 42; exposure main associations and interactions with age). Secondary analyses tested whether associations were robust to the additional inclusion of pregnancy-associated covariates with high levels of missingness in the entire sample and to polygenic risk scores for cannabis use disorder and proximal outcomes of interest (eg, polygenic risk for schizophrenia, depression) in the European ancestry subsample (n = 5110; genetic methodological details available elsewhere2).
Table. Prenatal Cannabis Exposure and Child Psychopathology as Children Enter Adolescence.
| Variable | Mean (SD)a | Mixed-model ANCOVA | P value for post hoc mixed-model, uncorrected | |||||
|---|---|---|---|---|---|---|---|---|
| Exposed pre- and postknowledge (n = 208) | Exposed preknowledge only (n = 391) | No exposure (n = 10 033) | χ2 | P value (FDR) | Pre- and postknowledge vs no | Preknowledge only vs no | Pre- and postknowledge vs preknowledge only | |
| CBCL | ||||||||
| Total problems | 31.47 (23.41) | 23.78 (19.66) | 16.7 (15.55) | 13.59 | .004 | <.001 | .29 | <.001 |
| Externalizing factor | 8.83 (7.8) | 6.05 (6.44) | 3.95 (4.93) | 16.29 | .002 | <.001 | .96 | <.001 |
| Rule-breaking behavior | 2.76 (2.66) | 1.8 (2.1) | 1.04 (1.52) | 33.08 | <.001 | <.001 | .37 | <.001 |
| Aggressive behavior | 6.07 (5.53) | 4.25 (4.66) | 2.91 (3.66) | 12.34 | .007 | <.001 | .78 | <.001 |
| Internalizing factor | 7.77 (7) | 6.36 (5.6) | 4.9 (4.9) | 5.06 | .14 | .04 | .23 | .08 |
| Withdrawn/depression | 1.96 (2.3) | 1.54 (1.92) | 1.06 (1.51) | 6.83 | .06 | .02 | .20 | .17 |
| Somatic complaints | 2.12 (2.1) | 1.87 (1.83) | 1.42 (1.65) | 1.23 | .58 | .51 | .34 | .67 |
| Anxious/depressed | 3.69 (3.49) | 2.95 (2.9) | 2.43 (2.69) | 7.01 | .06 | .01 | .26 | .04 |
| Social problems | 3.04 (2.86) | 2.13 (2.31) | 1.43 (1.91) | 18.09 | <.001 | <.001 | .46 | <.001 |
| Thought problems | 2.9 (2.89) | 2.19 (2.58) | 1.5 (1.86) | 17.85 | <.001 | <.001 | .06 | .02 |
| Attention problems | 4.99 (3.83) | 3.98 (3.69) | 2.71 (3.05) | 19.17 | <.001 | <.001 | .11 | .002 |
| Sluggish cognitive tempo | 1.1 (1.27) | 0.83 (1.13) | 0.48 (0.81) | 42.87 | <.001 | <.001 | <.001 | .05 |
| Stress problems | 5.06 (4.23) | 3.76 (3.49) | 2.74 (2.92) | 14.44 | .003 | <.001 | .44 | .005 |
| Obsessive-compulsive problems | 2.17 (2.13) | 1.72 (1.88) | 1.29 (1.56) | 13.16 | .005 | <.001 | .13 | .07 |
| ADHD problemsb | 4.24 (3.23) | 3.4 (2.98) | 2.35 (2.59) | 15.28 | .002 | <.001 | .10 | .005 |
| Anxiety problemsb | 3.13 (2.88) | 2.46 (2.37) | 1.96 (2.12) | 7.74 | .06 | .01 | .20 | .13 |
| Conduct problemsb | 3.14 (3.55) | 1.89 (2.59) | 1.1 (1.91) | 27.22 | <.001 | <.001 | .86 | <.001 |
| Depression problemsb | 2.23 (2.61) | 1.75 (2.12) | 1.31 (1.83) | 3.97 | .19 | .06 | .45 | .04 |
| Oppositional defiant problemsb | 2.82 (2.19) | 2.22 (2.13) | 1.61 (1.76) | 7.17 | .06 | .01 | .90 | .01 |
| Somatic problemsb | 1.51 (1.49) | 1.36 (1.39) | 1.03 (1.25) | 1.04 | .61 | .58 | .36 | .70 |
| PQ-BC | ||||||||
| Psychoticlike experiences | 3.14 (3.32) | 2.95 (2.97) | 2.01 (2.54) | 10.51 | .02 | .03 | .009 | .26 |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; ANCOVA, analysis of covariance; CBCL, Child Behavior Checklist; FDR, false discovery rate; PQ-BC, Prodromal Questionnaire–Brief Child Version.
Group means (SDs) of each raw CBCL3 subscale score and the total number of psychoticlike experiences reported, regardless of distress, on the PQ-BC.4 Twenty CBCL subscales including those based on factor analyses (n = 11; ie, total problems [includes externalizing, internalizing, social, thought, and attention problems subscales], externalizing problems [includes rule-breaking and aggressive behaviors], internalizing problems [includes withdrawn/depression, somatic complaints, and anxiety/depressed problems), other scales (n = 3; ie, sluggish cognitive tempo, stress problems, and obsessive-compulsive problems), and those judged by experts to be consistent with Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition) categories (n = 6; ie, depressive, anxiety, somatic, ADHD, oppositional defiant, and conduct problems). Mean values were calculated within-person before computing the group means (mean values were not calculated in analyses). ANCOVA χ2 and P values are from mixed-model comparing models with PCEs (ie, exposure pre- and postknowledge of pregnancy, exposure preknowledge of pregnancy only) to 1 without these terms, Benjamini-Hochberg FDR-corrected for multiple comparisons (n = 42 comparisons, df = 2; Figure). Linear mixed-model random intercepts included (1) family, (2) research sites, and (3) participant identification number. Fixed-effect covariates included (1) child age at each visit, (2) child sex (0 = male, 1 = female), (3-8) self-reported child race and ethnicity (African American, Asian, Hispanic, Native American, Pacific Islander, White), (9-12) current household income (<$50 000, <$75 000, <$100 000, >$100 000), (13) current maternal education, (14) pubertal status at baseline, (15-19) first-degree familial history of mental illness (depression, psychosis, anxiety, mania, antisocial behavior), (20-21) first-degree familial history of drug or alcohol problems, (22-24) prenatal exposure to alcohol, tobacco, or other drugs before maternal knowledge of pregnancy, (25-28) prenatal exposure to alcohol, tobacco, or other drugs after maternal knowledge of pregnancy, (29) child substance use (tobacco puff or alcohol sip), and (30) twin or triplet status. Follow-up analyses included pregnancy-associated variables, which were excluded from primary analyses because of high missingness (remaining n = 9367, k = 26 585 observations), including (1) length of time pregnant before maternal knowledge of pregnancy, (2) whether the pregnancy was unplanned (0 = planned, 1 = unplanned), (3) maternal age at birth, (4) prenatal vitamin usage, and (5) birth weight. Further follow-up analyses were restricted to participants of gnomically confirmed European ancestry and included covariates 1-10 (10 ancestry principal components) and 11-17 (polygenic risk for cannabis use disorder, cross-disorder risk, schizophrenia, major depressive disorder, ADHD, generalized anxiety disorder, and risk taking). For additional details regarding genetic analyses please refer to Paul et al.2
Using Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition) codes.
Results
A total of 391 individuals were in the BK-PCE group, 208 were in the BAK-PCE group, and 10 032 were in the NE group. Of those, 2379 (22%) self-reported as African American; 709 (7%), Asian/Asian American; 766 (7%), Hispanic; 378 (4%), Native American; 69 (0.6%), Pacific Islander; 8593 (81%), White; and 766 (7%), other. There were 10 631 individuals and 30 091 longitudinal assessments (baseline: n = 10 624; mean [IQR] age, 9.9 [8.9-11.1] years; 1-year follow-up: n = 10 094; mean [IQR] age, 10.9 [9.7-12.4] years; 2-year follow-up: n = 9373; mean [IQR] age, 12.0 [10.6-13.8] years). PCE was associated with persisting vulnerability to psychopathology throughout early adolescence (Figure and Table). These associations did not change with age (FDR-corrected P > .11). Significant findings were primarily driven by exposure following maternal knowledge of pregnancy (Figure and Table). Results remained FDR-significant when including covariates with high missingness (ie, pregnancy-associated covariates) with the exception of psychoticlike experiences (FDR-corrected P = .13; influential covariates were maternal age at birth and planned pregnancy). Associations remained directionally consistent and of similar magnitude in the BAK-PCE group after accounting for polygenic risk in the European ancestry subsample; 4 scales (ie, sluggish cognitive tempo, social problems, rule-breaking behavior, and Diagnostic and Statistical Manual of Mental Disorders [Fifth Edition] conduct problems) remained nominally significant.
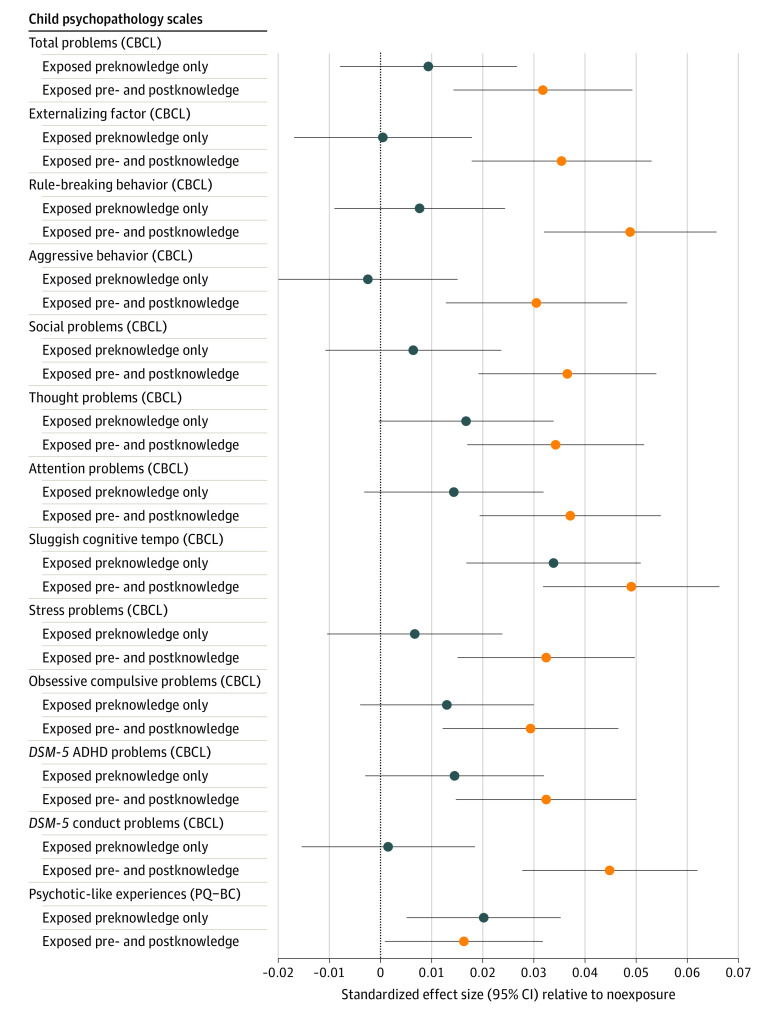
Figure. Prenatal Cannabis Exposure and Child Psychopathology as Children Enter Early Adolescence.
Standardized regression β effect sizes and 95% CIs from mixed-effect regressions assessing the association of prenatal cannabis exposure prior to maternal knowledge of pregnancy or prior to and postmaternal knowledge of pregnancy compared with no exposure. Nonsignificant outcomes (Table; somatic problems/complaints, internalizing factor, anxious/depressed, withdrawn/depressed, and Diagnostic and Statistical Manual of Mental Disorders [Fifth Edition] [DSM-5] depression, anxiety, and oppositional defiant) are not shown. As seen by the 95% CIs, prenatal cannabis exposure pre- and postmaternal knowledge of pregnancy was associated with increased risk for all significant measures. However, exposure only before maternal knowledge of pregnancy was only significantly associated with increased risk for sluggish cognitive tempo and psychoticlike experiences. ADHD indicates attention-deficit/hyperactivity disorder; CBCL, Child Behavior Checklist; PQ-BC, Prodromal Questionnaire–Brief Child Version.
Discussion
PCE is associated with persisting vulnerability to broad-spectrum psychopathology as children progress through early adolescence. Increased psychopathology may lead to greater risk for psychiatric disorders and problematic substance use as children enter peak periods of vulnerability in later adolescence.5 Larger associations in the BAK-PCE group may be attributable to the timing of cannabinoid receptor neural expression, which onsets in rodents at the equivalent of 5 to 6 weeks.6 Limitations include the small sample of prenatal cannabis-exposed offspring, potential underreporting of use during pregnancy, imprecise data on the timing/frequency/potency of cannabis exposure, and the lack of data on some potential confounders (eg, maternal stress during pregnancy). Evidence that the impact of PCE on psychopathology does not ameliorate as children enter adolescence further cautions against cannabis use during pregnancy.
References
- 1.Nashed MG, Hardy DB, Laviolette SR. Prenatal cannabinoid exposure: emerging evidence of physiological and neuropsychiatric abnormalities. Front Psychiatry. 2021;11:624275. doi: 10.3389/fpsyt.2020.624275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paul SE, Hatoum AS, Fine JD, et al. Associations between prenatal cannabis exposure and childhood outcomes: results from the ABCD study. JAMA Psychiatry. 2021;78(1):64-76. doi: 10.1001/jamapsychiatry.2020.2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles: An Integrated System of Multi-Informant Assessment. University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- 4.Karcher NR, Barch DM, Avenevoli S, et al. Assessment of the Prodromal Questionnaire-Brief Child version for measurement of self-reported psychoticlike experiences in childhood. JAMA Psychiatry. 2018;75(8):853-861. doi: 10.1001/jamapsychiatry.2018.1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solmi M, Radua J, Olivola M, et al. Age at onset of mental disorders worldwide: large-scale meta-analysis of 192 epidemiological studies. Mol Psychiatry. 2022;27(1):281-295. doi: 10.1038/s41380-021-01161-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu CS, Jew CP, Lu HC. Lasting impacts of prenatal cannabis exposure and the role of endogenous cannabinoids in the developing brain. Future Neurol. 2011;6(4):459-480. doi: 10.2217/fnl.11.27 [DOI] [PMC free article] [PubMed] [Google Scholar]