Abstract
There has been a surge in COVID-19 vaccine–associated lymphadenopathy (LAD), including after the booster dose of vaccine. This can create diagnostic dilemmas in oncology patients as the relatively sudden LAD can mimic metastasis or cancer recurrence, at a risk of leading to additional but unnecessary anti-neoplastic therapy. Here we report the histopathologic features in a case of persistent LAD occurring in a patient with history of breast invasive ductal carcinoma which followed a COVID-19 vaccine booster. A needle core and then excisional biopsy showed atypical follicular hyperplasia with features that histologically and phenotypically could mimic follicular lymphoma, but the findings were ultimately interpreted to be reactive in nature and related temporally to COVID-19 vaccine. To our knowledge, this is the first case of an atypical lymphoproliferative lesion with features potentially mimicking lymphoma associated with COVID-19 vaccine.
Keywords: COVID-19, COVID-19 vaccine, COVID-19-related lymphadenopathy, Mimicker of lymphoma
Introduction
Coronavirus disease 2019 (COVID-19) is a global pandemic, representing one of the most severe coronavirus diseases in humans in the past two decades [1]. COVID-19 disease is caused by the recently emerged severe acute respiratory syndrome (SARS)–related coronavirus species 2 or SARS-CoV-2 [2, 3]. According to World Health Organization data, as of mid-May 2022, more than 525 million confirmed cases were documented worldwide, and more than 6.2 million deaths are attributed to COVID-19 [4]. Also as of mid-May 2022, over 11.5 billion vaccine doses have been administered worldwide and approximately 7 million doses are administered each day [5].
Although symptoms and severity vary widely among patients infected with COVID-19, the majority of symptomatic patients have respiratory-type symptoms with features typical of an infectious etiology including fever, chills, sore throat, nasal congestion, cough, shortness of breath, myalgia, loss of taste and smell, and diarrhea [6, 7]. Most patients with COVID-19 infection do not undergo tissue biopsy; thus, any characteristic histologic features of COVID-19 infection are only infrequently reported. To date, among the infrequently described histologic findings of COVID-19 infection, most reports have focused on the pulmonary pathology abnormalities including diffuse alveolar damage, organizing pneumonia, reactive type II pneumocyte hyperplasia, and chronic interstitial pneumonias [8–11].
COVID-19 vaccines are also associated with adverse events, which sometimes mimic COVID-19 infection itself and include expected vaccine side effects, such as headache, fatigue, muscle, and joint pain, fever, and chills [12–14]. Lymphadenopathy (LAD) arising as an adverse events following immunization (AEFI) is infrequent but increases with subsequent exposures, accounting for 0.51% of all AEFI following first dose of vaccine, 0.87% of AEFIs following 2nd dose, and 3.29% of AEFIs following the third booster dose [15]. The histologic features of COVID-19 vaccine–related LAD are not well established, given that the vast majority of vaccine-related LAD are not biopsied, and that the rarely reported biopsies are not described in detail. Here we report a case of LAD where an extensively worked-up axillary lymph node biopsy ipsilateral to a recent COVID-19 vaccination site showed atypical hyperplasia with features mimicking a follicular lymphoma with plasmacytic differentiation or other related neoplasm. This represents an important diagnostic pitfall for pathologists and clinicians to be aware of. To our knowledge, this is the first report of COVID-19 vaccine–related atypical lymphoid hyperplasia potentially mimicking lymphoma.
Report of case
A 70-year-old female presented to our hospital with persistent (5 months) and worsening right axillary lymphadenopathy. She had a history of invasive ductal carcinoma of left breast 5 years prior, T1cN0, stage la, Oncotype recurrence score 18, status post left lumpectomy, and left axillary sentinel lymph node biopsy, which was negative for malignancy. She was also treated with adjuvant radiation and anastrozole therapy. 18F-FDG PET/CT imaging studies showed at least 3 prominent right axillary lymph nodes, the largest measuring 12 mm with cortical thickening and SUV 7 and at least 5 prominent left axillary lymph nodes with cortical thickening, highest SUV 4.5 (Fig. 1). Core needle biopsy was done to rule out metastasis.
Fig. 1.
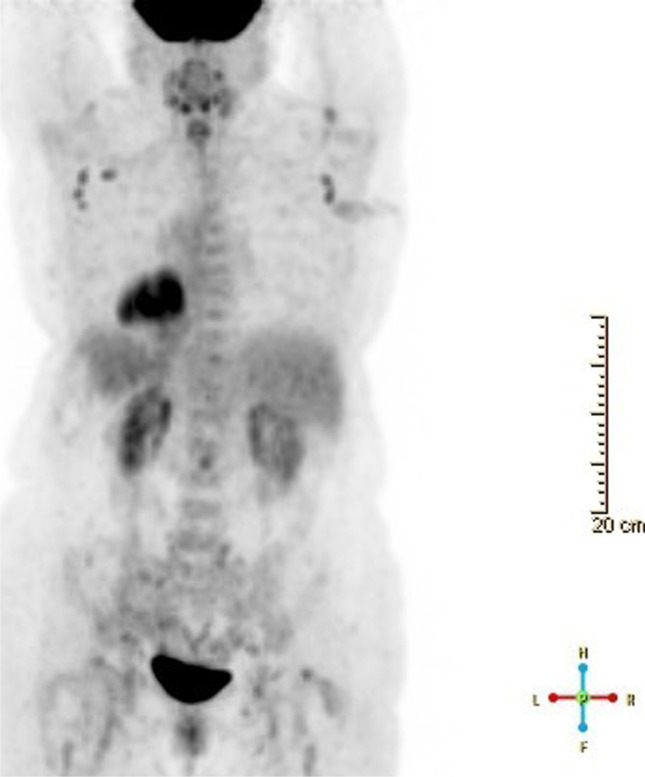
18F-FDG PET/CT. Imaging shows bilateral enlarged axillary lymph nodes, right external iliac lymph nodes and left inguinal lymph nodes
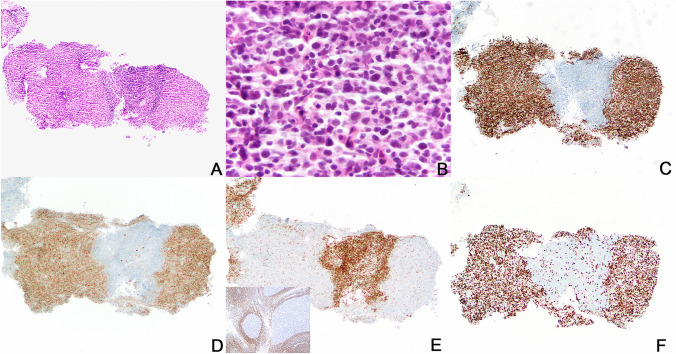
Histologic sections showed a small and fragmented biopsy of lymph node containing few prominent and abnormal-appearing secondary lymphoid follicles (Fig. 2). Histologic abnormalities included that the follicles had ill-defined borders with poorly defined to absent mantle zones, lacked normal polarization, and contained a relatively monotonous population of medium to large-sized centrocytic and centroblastic lymphoid cells. Compared to normal secondary follicles, there were infrequent apoptotic bodies and tingible body macrophages were absent. Follicular B cells expressed CD10 and BCL6 and were mostly negative for BCL2 by immunohistochemistry with a high Ki-67 proliferative rate of ~ 80%, but without typical polarization as would be seen in normal germinal centers (Fig. 2). Concurrent flow cytometry identified a monotypic CD10-positive kappa light chain–restricted B-cell population. Taken together, these findings were suspicious for a B-cell lymphoma of follicular center cell origin and an excisional biopsy was recommended for confirmation and grading. Cytogenetic FISH studies were negative for BCL6 and IRF4 rearrangements and negative for IGH::BCL2 fusion.
Fig. 2.
Core needle biopsy of axillary lymph node. Histologic sections show a small and fragmented biopsy of lymph node containing few prominent and atypical-appearing germinal centers (A, hematoxylin and eosin stain, 100 × magnification). The atypical follicles have ill-defined borders, lack mantle zones and germinal center polarization, and contain a relatively monotonous population of medium- to large-sized centrocytic and centroblastic lymphoid cells with decreased apoptotic bodies and no tingible body macrophages (B, hematoxylin and eosin stain, 500 × magnification). Follicular B cells express CD10 (C) and BCL6 (D) and are negative for BCL2 (E) by immunohistochemistry. For comparison, BCL2 immunostain in normal germinal centers of reactive tonsil is included in the inset of panel E. Compared to normal tonsil, the atypical follicles in the patient biopsy are irregular in shape and lack mantle zones. The Ki-67 proliferative rate is high, ~ 80%, without polarization although the entire germinal centers are not present in the biopsy and markedly hyperplastic germinal centers do not necessarily show polarization (F)
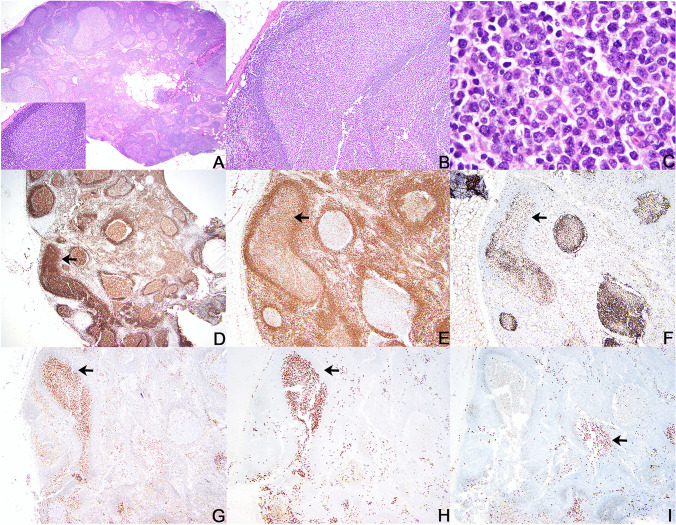
Excisional biopsy of the persistently enlarged 1.2-cm right axillary lymph node was performed 2 months later and showed mostly reactive follicular hyperplasia with few abnormal follicles. The abnormal follicles were characterized by germinal centers containing atypical populations of centroblastic, lymphoplasmacytic, and more plasmacytic-appearing cells (Fig. 3). The lymphoplasmacytic and plasmacytic cells were CD79a and IRF4/MUM1 positive with dim coexpression of BCL2, only rare expression of CD138, and decreased expression of CD20. The Ki67 proliferative rate was lower with unclear polarization in the abnormal follicles (Fig. 3). Kappa and lambda RNA in situ hybridization showed both kappa-restricted and lambda-restricted follicles (Fig. 3). The plasma cells outside the follicles were polytypic. CD30 was positive in reactive immunoblasts only and EBER in situ hybridization was negative. IgG4 + plasma cells were not increased. Clonality testing by polymerase chain reaction using BIOMED-2 primers in microdissected areas of abnormal follicles showed polyclonal immunoglobulin heavy chain and kappa light chain gene amplification products. Flow cytometric studies showed polytypic B-cells with a CD10-positive population that was lambda skewed. Although these were concerning features, the findings were considered to be atypical but not diagnostic of a lymphoma.
Fig. 3.
Excisional lymph node biopsy. Histologic section of right axillary lymph node shows intact nodal architecture with most secondary follicles showing normal shapes and distribution with well-defined mantle zones and polarized germinal centers containing tingible body macrophages (A, hematoxylin and eosin stain, 20 × magnification; inset hematoxylin and eosin stain, 200 × magnification). Occasional large atypical-appearing follicles have non-polarized germinal centers containing a relatively monotonous population of cells with variable plasmacytic morphologic features (B, hematoxylin and eosin stain, 100 × magnification; C, hematoxylin and eosin stain, 500 × magnification). The atypical follicles with increased plasmacytic cells show stronger expression of CD79a (D, arrow) and dim coexpression of BCL2 (E, arrow) and have an atypical low Ki67 proliferative rate (F, arrow) compared to adjacent normal follicles. IRF4/MUM1 is strongly expressed in the atypical follicles (G) and some show kappa restriction (H, arrow) while others show lambda restriction (I, arrow). Panels H and I depict kappa and lambda ultrasensitive RNA in situ hybridization assays
Subsequently, the patient’s hematologist indicated that the axillary lymphadenopathy developed within a month following a Pfizer BioNTech COVID-19 vaccine booster (3rd injection). The vaccine injection site was ipsilateral to the lymph node which was biopsied. Staging bone marrow biopsy was negative for lymphoma. Seven months after initial presentation, the patient’s LAD resolved and the patient remains asymptomatic.
Discussion
Most reports to date describing the histologic features of COVID-19 vaccine–related lesions have focused on vaccine-induced immune thrombotic thrombocytopenia, thrombosis, and vasculitis [16–20]. Development of LAD after COVID-19 vaccination is reported in several case reports and small series, with the abnormalities largely described in regard to clinical and radiology findings, including in the context of assessment of LAD developing post vaccine in cancer patients undergoing cancer surveillance [21–24]. In the vast majority of reports describing vaccine-related LAD, patients have not undergone tissue biopsy and histologic features are not described [21–24]. Rare reports of necrotizing LAD after COVID-19 vaccine are reported, where biopsy has shown histologic features similar to those of Kikuchi-Fujimoto disease (KFD) [25, 26].
COVID-19 vaccine–induced LAD has also been documented to involve atypical sites such as contralateral or bilateral axillary or supraclavicular regions, nuchal, hilar, and pectoral regions [27], and as generalized LAD [15]. Clinically and radiographically these LADs involving atypical sites mimicked metastasis or lymphomas; however, these previously documented reports were not also accompanied by biopsy and histopathologic studies, even though the initial radiologic interpretations raised concerns for metastasis or lymphoma [15, 27].
A review of literature has described 68 cases of COVID-19 vaccine–associated LAD [28]. These occurred predominantly in women (88.2%), and were identified after first or second dose of vaccines in individuals who were vaccinated with any of three different types of COVID-19 vaccines (Pfizer-BioNTech (n = 30, 44.1%), Moderna (n = 17, 25%), and Oxford-AstraZeneca (n = 1, 1.5%), not reported in n = 20 (29.4%)). Imaging abnormalities were identified from day 1 to 4 weeks after vaccination, and LAD persisted for up to 6 weeks. However, the histologic features of the LAD based on tissue biopsy were not described in this study [28]. Thus, at this time, the spectrum of histopathologic features of LAD occurring after COVID-19 vaccination is largely unknown, with a limited number of typical hyperplasias and rare necrotizing LAD reported.
In the case we present, COVID-19 vaccine–related LAD showed atypical lymphoid hyperplasia with features that could mimic lymphoma including abnormal lymphoid follicles containing monotonous centroblasts and increased lymphoplasmacytic and plasmacytic cells, monotypia (of B cells and plasma cells), and even some dim BCL2 expression in one of the two biopsies. These findings raised the question of grade 3 follicular lymphoma, pediatric-type follicular lymphoma, or partial involvement by a follicular lymphoma (or less likely marginal zone lymphoma) with plasmacytic differentiation. The large cells excluded in situ follicular neoplasia from the differential diagnosis. Features that precluded the diagnosis of a lymphoma included the lack of clear-cut even focal architectural effacement and knowledge that follicular hyperplasia with monotypic plasmacytoid cells exist and rarely may even show some BCL2 expression [29]. Also not supporting a diagnosis of lymphoma was that the follicles included some that were kappa monotypic and others lambda monotypic while many were polytypic. While unusual, the flow cytometric findings were also not clear in terms of diagnosing lymphoma in that the needle biopsy had CD10 + kappa-positive monotypic cells but the excision appeared polytypic with only a subset of CD10 + B-cells that were lambda skewed. Finally, by FISH studies, there was no evidence of BCL2 or BCL6 rearrangements and no evidence of B-cell monoclonality even within microdissected follicles. To our knowledge, this is the first reported case of atypical LAD that could mimic lymphoma associated with COVID-19 vaccine.
The case we reported highlights that post-vaccine LAD may show features mimicking lymphoma, an important diagnostic pitfall. In addition, this case illustrates the importance of awareness of the spectrum of reactive hyperplasias that can include monotypic plasmacytoid cells in germinal centers, and the importance of clinical findings and adequate tissue sampling in the overall interpretation of pathologic specimens. Interpretation in this case was initially complicated by lack of knowledge of recent COVID-19 vaccine booster, and the initial limited core needle biopsy.
Although similar histopathologic findings have not been reported in the context of COVID-19 vaccine or infection, they have been reported by Wang et al. in a series of 17 cases of unusual follicular hyperplasias with light chain–restricted germinal centers [29]. In this series, 13 of 17 cases had both kappa- and lambda-restricted germinal centers, similar to what we report herein. In our case, the unusual findings were associated with booster dose of COVID-19 vaccine, whereas in the report by Wang et al. the findings were variably associated with increased IgG4-positive plasma cells within germinal centers (7/17, 41%) and autoimmune disorders (7/17, 41%) [29]. There was no history of an autoimmune disorder in our patient and IgG4 + plasma cells were not increased.
Cases of vaccine-related LAD have been reported most commonly in association with DNA and RNA vaccines, which elicit both cell-mediated (T-cell) and humoral (B-cell) response [30, 31]. DNA and RNA vaccines have been demonstrated to elicit brisk B-cell proliferation in the germinal centers compared to their counterparts (peptide-based or inactivated viral particle-based) resulting in ensuing increased likelihood of patients developing lymphadenopathy [32, 33]. We postulate that the monotypic proliferations of B-cells and plasmacytic cells in the case we report occurring after a booster vaccine dose may be due to proliferation of memory B-cells which have already undergone clonal proliferation and affinity maturation as a result of the first and second dose of vaccination. More histopathologic studies of post-vaccine LADs, including after booster dose, would be necessary to confirm whether the phenomena of such pseudo-lymphomatous lesions are rare events or common entities post vaccine. Nonetheless, these findings in older adult populations may mimic lymphoma and be over diagnosed as nodal involvement by lymphoma of either follicular or marginal zone type, and therefore represent important diagnostic pitfalls for pathologists and clinicians to be aware of.
Author contribution
Ashish Patil, MD, PhD: collected data, reviewed literature, composed paper.
Steven H. Swerdlow, MD: reviewed data, composed and reviewed paper.
Izidore S. Lossos, MD: reviewed data, composed and reviewed paper.
Jennifer R Chapman, MD: collected data, reviewed data, reviewed literature, composed and reviewed paper.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sultana F et al (2022) A study of plithogenic graphs: applications in spreading coronavirus disease (COVID-19) globally. J Ambient Intell Humaniz Comput 1–21. 10.1007/s12652-022-03772-6 [DOI] [PMC free article] [PubMed]
- 2.Malik P, et al. COVID-19: a disease with a potpourri of histopathologic findings-a literature review and comparison to the closely related SARS and MERS. SN Compr Clin Med. 2021;3:2407–2434. doi: 10.1007/s42399-021-01029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muralidar S, Ambi SV, Sekaran S, Krishnan UM. The emergence of COVID-19 as a global pandemic: understanding the epidemiology, immune response and potential therapeutic targets of SARS-CoV-2. Biochimie. 2020;179:85–100. doi: 10.1016/j.biochi.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Coronavirus (2022) (COVID-19) Dashboard. https://covid19.who.int/. Accessed 25 July 2022
- 5.Our World in Data. https://ourworldindata.org. Accessed 25 July 2022
- 6.Baj J, et al. COVID-19: specific and non-specific clinical manifestations and symptoms: the current state of knowledge. J Clin Med. 2020;9:1753. doi: 10.3390/jcm9061753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dixon BE et al (2020) Symptoms and symptom clusters associated with SARS-CoV-2 infection in community-based populations: results from a statewide epidemiological study. medRxiv 2020.2010.2011.20210922. 10.1101/2020.10.11.20210922 [DOI] [PMC free article] [PubMed]
- 8.Zhang H, et al. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann Intern Med. 2020;172:629–632. doi: 10.7326/M20-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian S, et al. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pernazza A, et al. Early histologic findings of pulmonary SARS-CoV-2 infection detected in a surgical specimen. Virchows Arch. 2020;477:743–748. doi: 10.1007/s00428-020-02829-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calabrese F, et al. Pulmonary pathology and COVID-19: lessons from autopsy. The experience of European Pulmonary Pathologists. Virchows Arch. 2020;477:359–372. doi: 10.1007/s00428-020-02886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO (n. d) Statement for healthcare professionals: how COVID-19 vaccines are regulated for safety and effectiveness. https://www.who.int/news/item/17-05-2022-statement-for-healthcare-professionals-how-covid-19-vaccines-are-regulated-for-safety-and-effectiveness/. Accessed 25 July 2022
- 13.CDC (n. d) Possible side effects after getting a COVID-19 vaccine. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/expect/after.html
- 14.Shrestha S, et al. Adverse events related to COVID-19 vaccines: the need to strengthen pharmacovigilance monitoring systems. Drugs Ther Perspect. 2021;37:376–382. doi: 10.1007/s40267-021-00852-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centrumlareb B (2022) Overview of lymphadenopathy after Covid-19 booster vaccination. https://www.lareb.nl/media/o5rlkdvg/signal_2022_overview_lymphadenopathy_after_booster-covid-19.pdf. Accessed 25 July 2022
- 16.Sharifian-Dorche M, et al. Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination; a systematic review. J Neurol Sci. 2021;428:117607. doi: 10.1016/j.jns.2021.117607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oldenburg J, et al. Diagnosis and management of vaccine-related thrombosis following AstraZeneca COVID-19 vaccination: guidance statement from the GTH. Hamostaseologie. 2021;41:184–189. doi: 10.1055/a-1469-7481. [DOI] [PubMed] [Google Scholar]
- 18.Shakoor MT, Birkenbach MP, Lynch M. ANCA-associated vasculitis following Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021;78:611–613. doi: 10.1053/j.ajkd.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scharf RE, Alberio L. COVID-19: SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. Hamostaseologie. 2021;41:179–182. doi: 10.1055/a-1369-3488. [DOI] [PubMed] [Google Scholar]
- 20.Bostan E, Gulseren D, Gokoz O. New-onset leukocytoclastic vasculitis after COVID-19 vaccine. Int J Dermatol. 2021;60:1305–1306. doi: 10.1111/ijd.15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiller N, Goldberg SN, Cohen-Cymberknoh M, Vainstein V, Simanovsky N. Lymphadenopathy associated with the COVID-19 vaccine. Cureus. 2021;13:e13524. doi: 10.7759/cureus.13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Özütemiz C, et al. Lymphadenopathy in COVID-19 vaccine recipients: diagnostic dilemma in oncologic patients. Radiology. 2021;300:E296–e300. doi: 10.1148/radiol.2021210275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Co M, Wong PCP, Kwong A. COVID-19 vaccine associated axillary lymphadenopathy - a systematic review. Cancer Treat Res Commun. 2022;31:100546. doi: 10.1016/j.ctarc.2022.100546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim J, et al. COVID-19 vaccine-related axillary lymphadenopathy in breast cancer patients: case series with a review of literature. Semin Oncol. 2021;48:283–291. doi: 10.1053/j.seminoncol.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soub HA, et al. Kikuchi-Fujimoto disease following SARS CoV2 vaccination: case report. IDCases. 2021;25:e01253. doi: 10.1016/j.idcr.2021.e01253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan HM, Hue SS-S, Wee A, See KC. Kikuchi-Fujimoto disease post COVID-19 vaccination: case report and review of literature. Vaccines. 2021;9:1251. doi: 10.3390/vaccines9111251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cocco G, et al. Atypical Sites of lymphadenopathy after anti-COVID-19 vaccine: ultrasound features. Medicina. 2022;58:197. doi: 10.3390/medicina58020197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keshavarz P, Yazdanpanah F, Rafiee F, Mizandari M. Lymphadenopathy following COVID-19 vaccination: imaging findings review. Acad Radiol. 2021;28:1058–1071. doi: 10.1016/j.acra.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang XJ, Moore EM, Swerdlow SH, Aggarwal N. Light chain-restricted plasmacytoid cells in hyperplastic germinal centersA clinicopathologic investigation. Am J Clin Pathol. 2021;156:871–885. doi: 10.1093/ajcp/aqab043. [DOI] [PubMed] [Google Scholar]
- 30.Hiller N, Goldberg SN, Cohen-Cymberknoh M, Vainstein V, Simanovsky N. Lymphadenopathy associated with the COVID-19 vaccine. Cureus. 2021;13:e13524–e13524. doi: 10.7759/cureus.13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bshesh K, et al. Lymphadenopathy post-COVID-19 vaccination with increased FDG uptake may be falsely attributed to oncological disorders: a systematic review. J Med Virol. 2022;94:1833–1845. doi: 10.1002/jmv.27599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bettini E, Locci M. SARS-CoV-2 mRNA vaccines: immunological mechanism and beyond. Vaccines. 2021;9:147. doi: 10.3390/vaccines9020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lederer K, et al. SARS-CoV-2 mRNA vaccines foster potent antigen-specific germinal center responses associated with neutralizing antibody generation. Immunity. 2020;53:1281–1295.e1285. doi: 10.1016/j.immuni.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]