Abstract
Purpose
Obstructive sleep apnea (OSA) is underdiagnosed, partially from variable clinical presentations. Emphasis is often placed on Epworth Sleepiness Scale (ESS), a subjective measure of sleepiness, but variable in OSA. We hypothesized that daytime complaints measured with Language of Sleepiness Questionnaire (LOS) in OSA are not being captured by ESS.
Methods
Adults referred to a tertiary sleep clinic undergoing sleep studies completed ESS and LOS questionnaires (20 items with various patient-reported descriptors). LOS was examined in patients who had or did not have OSA without sleepiness based on ESS < 10. Cluster analysis was performed to assess whether or not groups of individuals differed based on classification with or without OSA and with or without ESS-based sleepiness.
Results
Approximately half the study population (n = 185 completed) had OSA. ESS score (mean ± SD) was 9.0 ± 5.4. There was no significant difference in ESS between patients with and without OSA (9.0 ± 5.1 vs 9.1 ± 5.7, p = 0.969). In patients with OSA, females, older patients and white patients were significantly less likely to have an ESS ≥ 10 when compared to patients with an ESS < 10. In patients with an ESS < 10, there were no significant differences in descriptors of sleepiness between patients with and without OSA with the most common descriptors selected being “I lack energy,” “I wake up sleepy,” “I keep waking up,” and “I don’t sleep enough.”
Conclusions
The ESS failed to discriminate patients with OSA from those without OSA. Despite an ESS < 10, both daytime and sleep complaints using the LOS questionnaire were present in patients with OSA. Asymptomatic OSA may be less common than previously reported.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11325-022-02703-1.
Keywords: Excessive daytime sleepiness, Obstructive sleep apnea, Epworth Sleepiness Scale, Hypersomnolence, Fatigue
Introduction
Obstructive sleep apnea (OSA) is amongst the most common respiratory disorders in the developed world, affecting one seventh of the word’s adult population [1]. OSA is associated with a wide range of adverse health sequelae yet this chronic disorder remains underdiagnosed and untreated [2]. Underdiagnosis may in part be due to the highly variable clinical presentation in OSA.
Excessive sleepiness is regarded as one of the cardinal manifestations of OSA, but subjective sleepiness is not universal [3–5]. Sleepiness is reported by 15–50% of people with OSA identified through general population screening [6]. Moreover, the definition and optimal measurement of daytime sleepiness remains inconsistent and an area of ongoing debate. The Epworth Sleepiness Scale (ESS) is a subjective measure of sleepiness that has been widely adopted in clinical practice to detect and gauge severity of excessive daytime sleepiness, yet the ESS has not been shown to be a reliable screening test for OSA [7]. Often times, a variety of words and phrases are used to describe excessive sleepiness, often interchangeably (e.g., fatigue, drowsiness, weariness, lethargy, and lack of energy) [8]. More so, emphasis is often placed on distinguishing differences between sleepiness and fatigue, but complaints of fatigue may be just as common as that of sleepiness in OSA. Chervin et al. examined preferred words used by patients in describing their complaints in almost 200 adults with moderate to severe OSA—fatigue, tiredness, and lack of energy were more frequently reported than sleepiness [9]. The proportion of patients who preferred the term sleepiness to describe their primary problem was only 22%, suggesting that patients with OSA and their providers may use a diverse set of words to describe their problem.
There is a need to understand better the ways in which sleepiness is described by patients with OSA. In this study, we explored various descriptors of sleepiness using the “Language of Sleepiness” questionnaire in patients referred to a sleep clinic who completed a sleep study. Screening for depression was included as a way to better characterize the patients. The main aims of our study were to (a) compare descriptors of sleepiness in patients diagnosed with OSA who report no daytime sleepiness as defined by the Epworth Sleepiness Scale < 10/24, and (b) to use cluster analysis to identify groups of individuals based on their responses and assess whether these groups differed by OSA status (AHI ≥ 5/h). We hypothesized that asymptomatic OSA is uncommon and that there are descriptors of sleepiness in OSA not being captured by the ESS that may ultimately facilitate diagnosis.
Methods
Participants
Adults aged 18–89 years being evaluated for the first time in the Sleep Clinic at the University of California San Diego between March 2020 and March 2021 were recruited. Patients previously seen for sleep consultation were excluded. Vulnerable patient groups including prisoners and those with cognitive impairment were excluded. The study was approved by the University of California San Diego Institutional Review Board and registered with ClinicalTrials.gov (NCT04323254). Participants electronically signed informed consent prior to initiating the study protocol.
Data collection
All patients completed consultation with a sleep specialist.
Questionnaires
All participants were given the clinical intake form and the following questionnaires using a secure, HIPAA-compliant electronic platform:
-
i)
Epworth Sleepiness Scale (ESS). The ESS is a self-administered questionnaire assessing an individual’s subjective propensity for daytime sleepiness. Subjects were asked how likely they are to doze off or fall asleep on a scale of 0–3, in 8 specific situations, with total scores ranging from 0 to 24. An ESS score of 10 or greater is considered indicative of subjective excessive daytime sleepiness [10].
-
ii)
“Language of Sleepiness” (LOS) questionnaire. The LOS is a 20-item questionnaire with various descriptors of sleepiness (Table 2). Patients were asked to rank the top 5 descriptors and rank the severity of each descriptor from 0 to 10 with 10 being the more severe. One descriptor, “I keep waking up” appears twice to assess consistency. The questionnaire was developed by our team based on clinical experience and refined based on expert input over the last 2 decades.
-
iii)
Functional Outcomes of Sleep Questionnaire (FOSQ-10). The FOSQ-10 is a 10-item questionnaire measuring the impact of daytime sleepiness on activities of daily living. The mean of the total subscales is multiplied by 5 for the final score, with total scores ranging from 5 to 20. A score of 17.8 is considered the cut-off for normal and abnormal functioning [11].
-
iv)
Beck Depression Inventory-II (BDI-II). The BDI-II is a 21-item, self-report rating inventory that measures characteristic attitudes and symptoms of depression.
Table 2.
OSA (AHI ≥ 5/h) patients without (ESS < 10/24) and with (ESS ≥ 10/24) excessive sleepiness
| ESS < 10, n = 48 | ESS ≥ 10, n = 42 | p value | |
|---|---|---|---|
| Age (years) | 57.3 ± 13.27 | 47.9 ± 14.0 | 0.002 |
| Male sex (%) | 26 (54%) | 32 (76%) | 0.029 |
| White race (%) | 36 (77%) | 16 (42%) | 0.024 |
| Current smoker (%) | 1 (2%) | 3 (7%) | 0.245 |
| Sleep aid use (%) | 9 (19%) | 9 (21%) | 0.789 |
| History of cardiovascular disease (%) | 7 (15%) | 3 (7%) | 0.262 |
| History of psychiatric disease (%) | 11 (23%) | 13 (31%) | 0.390 |
| Apnea–hypopnea index (AHI) | 20.6 ± 16.1 | 20.5 ± 20.4 | 0.984 |
| Oxyhemoglobin saturation nadir | 79.6 ± 9.8 | 80.2 ± 7.6 | 0.753 |
| FOSQ-10 | 15.3 ± 3.1 | 14.0 ± 2.9 | 0.045 |
| Beck Depression Inventory-II | 10.5 ± 8.0 | 14.31 ± 9.7 | 0.040 |
| “Language of Sleepiness” questionnaire | |||
| I can’t think clearly | 4.3 ± 2.9 | 3.9 ± 2.4 | 0.421 |
| I need to sleep in | 3.9 ± 2.7 | 5.1 ± 3.2 | 0.061 |
| I don’t dream | 3.3 ± 2.8 | 3.5 ± 2.8 | 0.701 |
| I get overtired | 5.0 ± 2.9 | 5.8 ± 2.7 | 0.178 |
| I lack energy | 5.8 ± 2.8 | 6.9 ± 2.5 | 0.046 |
| I need to nap a lot | 3.4 ± 2.6 | 5.1 ± 3.2 | 0.006 |
| I am exhausted | 5.0 ± 3.0 | 6.5 ± 2.7 | 0.018 |
| I can’t sleep enough | 5.0 ± 3.2 | 5.7 ± 2.9 | 0.280 |
| I don’t sleep enough | 5.5 ± 3.1 | 5.7 ± 3.3 | 0.724 |
| I am always tired | 5.7 ± 3.0 | 6.7 ± 2.8 | 0.116 |
| I keep waking up (1) | 6.4 ± 3.1 | 6.4 ± 3.0 | 0.989 |
| I am tired all of the time | 5.0 ± 2.9 | 6.6 ± 2.9 | 0.015 |
| I can’t stay awake | 2.5 ± 2.0 | 4.1 ± 2.6 | 0.001 |
| I need to drink coffee | 3.1 ± 2.8 | 4.7 ± 3.3 | 0.012 |
| I keep waking up (2) | 6.3 ± 3.2 | 6.5 ± 3.0 | 0.725 |
| I am fatigued | 5.4 ± 2.9 | 6.3 ± 2.7 | 0.136 |
| I wake up sleepy | 5.5 ± 3.0 | 6.1 ± 3.1 | 0.396 |
| It takes me a long time to wake up | 3.3 ± 2.5 | 4.2 ± 3.1 | 0.114 |
| I am often drowsy | 3.4 ± 2.2 | 5.3 ± 2.9 | 0.001 |
| I can’t concentrate | 4.6 ± 3.2 | 4.7 ± 3.11 | 0.897 |
Data are presented as mean ± standard deviation unless otherwise indicated. Bolded values indicate p < 0.05
The answers to the questionnaires were recorded to a secure electronic database, REDCap, along with the results of the overnight sleep study, when available, and medical history from the participant’s medical records. The clinical intake form and medical records included demographics, past medical history, medications, and smoking history. REDCap is a password-protected secure Web application and a HIPAA-compliant environment. Participants had a coded study identifier associated with their research documents and questionnaires.
Sleep studies
Sleep studies were performed at the University of California San Diego Sleep Center. The type of sleep study was determined by the evaluating physician at the time of the clinic visit, but was also influenced by insurance mandates. This study was completed during the coronavirus disease 2019 (COVID-19) pandemic. Due to safety concerns, the sleep laboratory was closed for a period of time and most sleep studies were completed as unattended home sleep studies. Attended, overnight polysomnography (PSG) was performed using the Alice 6 LDx Diagnostic sleep system including electroencephalography (F1-M1, C4-M1, O2-M1), bilateral electrooculography, continuous airflow with thermistor and nasal pressure transducer, thoracic, and abdominal respiratory inductance plethysmography, body position, submental and bilateral anterior tibial electromyography, and finger pulse oximetry. Unattended home sleep studies were performed using the Resmed ApneaLink Air™ system including continuous airflow, thoracic respiratory effort, body position, and finger pulse oximetry.
The UC San Diego Sleep Center is accredited by the American Academy of Sleep Medicine and all procedures followed AASM Version 2.6 Scoring Manual recommendations. Hypopneas were scored if the peak airflow signal excursions dropped by ≥ 30% of pre-event baseline for at least 10 s, and there was a > 4% desaturation from pre-event baseline (Acceptable AASM 1B criteria).
Statistical analysis
Data are presented as mean ± SD for continuous variables and n (%) for categorical variable. For comparison of demographic characteristics and sleep study measures between those with and without OSA, t-test was used for continuous variables and Pearson chi-square test for categorical variables.
For the initial analysis, we identified patients with OSA based on an AHI ≥ 5/h and determined which descriptors were most commonly used in association with OSA. In addition, we assessed to what extent the sleep disorder impacts the patient’s perception of his/her quality of life. These analyses included simple correlations for the linear scales and independent samples t-tests to compare different groups based on OSA severity and ESS scores.
We utilized agglomerative hierarchical clustering methods with an average linkage to identify groupings of patients who had similar responses to LOS questions [12]. Hierarchical cluster approaches assign all observations to their own cluster and sequentially merge clusters most similar until there is only one cluster. The optimal number of clusters is determined by visually inspecting a dendrogram (described in greater detail in the “Results” section). Further details about this method can be found elsewhere [12]. We included all LOS variables in the cluster analysis, excluding the second “I keep waking up” variable. After determining the optimal number of clusters and categorizing patients, we described clusters based on the distribution of values for LOS questions, and determined whether they distinguished subjects based on OSA status.
Descriptors of sleepiness were examined in patients with OSA of varying severity but an ESS < 10/24 and compared to patients without OSA. Statistical significance was defined as alpha = 0.05 (two-tailed) for all analyses. All statistical analyses were completed in SPSS (IBM Corp. Released 2021. IBM SPSS Statistics for Windows, Version 28.0, Armonk, NY: IBM Corp). Cluster analysis was completed using Stata SE version 15 (StataCorp, College Station, TX). Based on the exploratory nature of the analyses, no corrections were made for multiple comparisons.
Results
Participants
Of 249 participants, complete data were available in 185 participants. Compared to participants with incomplete data, participants with complete data were less likely to smoke, less likely to have a history of cardiovascular disease and had a lower apnea–hypopnea index (see supplement). All analyses reported focused on those with complete data.
Baseline characteristics of participants with complete data are shown in Table 1. The study sample was middle-aged, largely evenly divided between males and females and predominantly white. Approximately half the study population had OSA as defined by a total apnea–hypopnea index ≥ 5/h with a mean ± SD AHI of 20.5 ± 18.4/h. Patients with OSA were more likely to have a history of cardiovascular disease but had significantly lower BDI-II scores and were less likely to have a history of psychiatric disease or use sleep aids when compared to patients without OSA.
Table 1.
Baseline characteristics
| Total, n = 185 | AHI < 5/h, n = 95 | AHI ≥ 5/h, n = 90 | p value | |
|---|---|---|---|---|
| Age (years) | 46.6 ± 14.5 | 40.7 ± 12.0 | 52.9 ± 14.3 | < 0.001 |
| Male sex (%) | 103 (56%) | 45 (47%) | 58 (64%) | 0.019 |
| White race (%) | 112 (64%) | 60 (66%) | 52 (61%) | 0.512 |
| Current smoker (%) | 9 (5%) | 5 (5%) | 4 (4%) | 0.796 |
| Sleep aid use (%) | 51 (28%) | 33 (35%) | 18 (20%) | 0.028 |
| History of cardiovascular disease (%) | 11 (6%) | 1 (1%) | 10 (11%) | 0.004 |
| History of psychiatric disease (%) | 70 (38%) | 46 (48%) | 24 (27%) | 0.002 |
| Apnea–hypopnea index (AHI) | 10.9 ± 15.8 | 1.7 ± 1.3 | 20.5 ± 18.4 | < 0.001 |
| Oxyhemoglobin saturation nadir | 84.5 ± 8.1 | 88.9 ± 3.8 | 79.9 ± 8.8 | < 0.001 |
| ESS | 9.0 ± 5.4 | 9.1 ± 5.7 | 9.0 ± 5.1 | 0.969 |
| FOSQ-10 | 14.3 ± 3.3 | 14.0 ± 3.6 | 14.7 ± 3.1 | 0.117 |
| Beck Depression Inventory-II | 13.9 ± 9.7 | 15.5 ± 10.1 | 12.2 ± 9.0 | 0.021 |
Data are presented as mean ± standard deviation unless otherwise indicated. Bolded values indicate p < 0.05
Epworth Sleepiness Scale
In the study sample, ESS score (mean ± SD) was 9.0 ± 5.4/24. There was no significant difference in the ESS between patients with and without OSA defined by an apnea–hypopnea index ≥ 5/h (9.0 ± 5.1 vs 9.1 ± 5.7, p = 0.969). In patients with OSA, females, older patients, and white patients were significantly less likely to have an ESS ≥ 10/24 when compared to patients with an ESS < 10/24 (see Table 2).
“Language of Sleepiness” descriptors
Of the 20 “Language of Sleepiness” descriptors, significant differences were seen for “I lack energy,” “I can’t sleep enough,” “I am always tired,” “I am tired all of the time,” “I am fatigued,” and “I am often drowsy” between patients with and without OSA, with lower (less severe) scores reported in patients with OSA (Table 3).
Table 3.
Comparison of “Language of Sleepiness” descriptors between patients without (AHI < 5/h) and with OSA (AHI ≥ 5/h)
| Total, n = 185 | AHI < 5, n = 95 | AHI ≥ 5, n = 90 | p value | |
|---|---|---|---|---|
| I can’t think clearly | 4.4 ± 2.9 | 4.7 ± 3.0 | 4.1 ± 2.7 | 0.152 |
| I need to sleep in | 4.8 ± 3.1 | 5.2 ± 3.1 | 4.4 ± 3.0 | 0.080 |
| I don’t dream | 3.1 ± 2.7 | 2.8 ± 2.6 | 3.4 ± 2.8 | 0.168 |
| I get overtired | 5.6 ± 2.9 | 5.9 ± 3.0 | 5.4 ± 2.8 | 0.203 |
| I lack energy | 6.7 ± 2.7 | 7.1 ± 2.6 | 6.3 ± 2.7 | 0.042 |
| I need to nap a lot | 4.5 ± 3.1 | 4.8 ± 3.3 | 4.2 ± 3.0 | 0.202 |
| I am exhausted | 6.1 ± 3.0 | 6.5 ± 3.1 | 5.7 ± 2.9 | 0.092 |
| I can’t sleep enough | 5.8 ± 3.1 | 6.3 ± 3.1 | 5.3 ± 3.1 | 0.024 |
| I don’t sleep enough | 5.8 ± 3.2 | 6.1 ± 3.2 | 5.6 ± 3.1 | 0.292 |
| I am always tired | 6.7 ± 2.9 | 7.1 ± 2.9 | 6.2 ± 2.9 | 0.031 |
| I keep waking up (1) | 6.3 ± 3.2 | 6.3 ± 3.4 | 6.4 ± 3.0 | 0.703 |
| I am tired all of the time | 6.3 ± 3.0 | 6.8 ± 3.0 | 5.7 ± 3.0 | 0.018 |
| I can’t stay awake | 3.5 ± 2.6 | 3.7 ± 2.7 | 3.2 ± 2.4 | 0.193 |
| I need to drink coffee | 4.2 ± 3.3 | 4.5 ± 3.3 | 3.9 ± 3.2 | 0.212 |
| I keep waking up (2) | 6.3 ± 3.2 | 6.1 ± 3.4 | 6.4 ± 3.1 | 0.614 |
| I am fatigued | 6.4 ± 2.9 | 6.8 ± 2.8 | 5.9 ± 2.8 | 0.023 |
| I wake up sleepy | 6.1 ± 3.1 | 6.4 ± 3.1 | 5.8 ± 3.0 | 0.167 |
| It takes me a long time to wake up | 4.1 ± 3.0 | 4.5 ± 3.1 | 3.7 ± 2.8 | 0.060 |
| I am often drowsy | 4.7 ± 2.9 | 5.2 ± 3.0 | 4.3 ± 2.7 | 0.033 |
| I can’t concentrate | 4.9 ± 3.1 | 5.1 ± 3.1 | 4.6 ± 3.1 | 0.286 |
Data are presented as mean ± standard deviation. Bolded values indicate p < 0.05
In patients with an ESS < 10, there were no significant differences in descriptors of sleepiness between patients with and without OSA (Table 4) with the most common descriptors selected being “I lack energy,” “I wake up sleepy,” “I keep waking up,” and “I don’t sleep enough.” However, various complaints were common in those with ESS < 10 who are sometimes labeled “asymptomatic.”
Table 4.
Comparison of “Language of Sleepiness” descriptors for those with an ESS < 10 without and with OSA defined by an apnea–hypopnea index ≥ 5/h
| Epworth Sleepiness Scale < 10 | ||||
|---|---|---|---|---|
| Total, n = 102 | AHI < 5, n = 54 | AHI ≥ 5, n = 48 | p value | |
| Age (years) | 48.4 ± 14.9 | 40.4 ± 11.4 | 57.3 ± 13.3 | 0.000 |
| Male sex (%) | 48 (47%) | 22 (41%) | 26 (54%) | 0.175 |
| White race (%) | 68 (69%) | 32 (62%) | 36 (77%) | 0.386 |
| History of cardiovascular disease (%) | 7 (7%) | 0 (0%) | 7 (15%) | 0.004 |
| History of psychiatric disease (%) | 39 (38%) | 28 (52%) | 11 (23%) | 0.003 |
| Apnea–hypopnea index (AHI) | 10.6 ± 14.5 | 1.7 ± 1.4 | 20.6 ± 16.1 | 0.000 |
| Oxyhemoglobin saturation nadir | 84.5 ± 8.5 | 95.1 ± 1.8 | 79.6 ± 9.8 | 0.000 |
| FOSQ-10 | 15.2 ± 3.4 | 15.0 ± 3.6 | 15.3 ± 3.1 | 0.616 |
| Beck Depression Inventory-II | 12.3 ± 9.4 | 14 ± 10.2 | 10.4 ± 8.0 | 0.528 |
| “Language of Sleepiness” questionnaire | ||||
| I can’t think clearly | 4.4 ± 3.1 | 4.5 ± 3.3 | 4.3 ± 2.9 | 0.718 |
| I need to sleep in | 4.2 ± 2.8 | 4.5 ± 2.9 | 3.9 ± 2.7 | 0.253 |
| I don’t dream | 2,8 ± 2.6 | 2.5 ± 2.3 | 3.3 ± 2.8 | 0.123 |
| I get overtired | 5.2 ± 2.9 | 5.3 ± 3.0 | 5.0 ± 2.9 | 0.543 |
| I lack energy | 6.2 ± 2.9 | 6.5 ± 2.9 | 5.8 ± 2.8 | 0,240 |
| I need to nap a lot | 3.5 ± 2.8 | 3.7 ± 2.9 | 3.4 ± 2.6 | 0.624 |
| I am exhausted | 5.4 ± 3.0 | 5.7 ± 3.0 | 5.0 ± 3.0 | 0.244 |
| I can’t sleep enough | 5.4 ± 3.2 | 5.7 ± 3.1 | 5.0 ± 3.2 | 0.238 |
| I don’t sleep enough | 5.6 ± 3.2 | 5.7 ± 3.3 | 5.5 ± 3.1 | 0.700 |
| I am always tired | 6.0 ± 3.0 | 6.3 ± 2.9 | 5.7 ± 3.0 | 0.367 |
| I keep waking up (1) | 6.1 ± 3.4 | 5.9 ± 3.6 | 6.4 ± 3.1 | 0.384 |
| I am tired all of the time | 5.5 ± 2.9 | 5.9 ± 3.0 | 5.0 ± 2.9 | 0.164 |
| I can’t stay awake | 2.4 ± 2.1 | 2.4 ± 2.2 | 3.5 ± 2.0 | 0.801 |
| I need to drink coffee | 3.4 ± 2.9 | 2.7 ± 3.0 | 3.1 ± 2.8 | 0.330 |
| I keep waking up (2) | 6.1 ± 3.4 | 5.9 ± 3.6 | 6.3 ± 3.2 | 0.591 |
| I am fatigued | 5.9 ± 2.9 | 6.2 ± 2.8 | 5.4 ± 2.9 | 0.170 |
| I wake up sleepy | 5.5 ± 3.0 | 5.6 ± 3.1 | 5.5 ± 3.0 | 0.926 |
| It takes me a long time to wake up | 3.6 ± 2.8 | 3.9 ± 3.1 | 2.5 ± 1.2 | 0.216 |
| I am often drowsy | 3.7 ± 2.5 | 4.0 ± 2.7 | 2.2 ± 1.2 | 0.221 |
| I can’t concentrate | 4.7 ± 3.2 | 4.8 ± 3.3 | 3.2 ± 0.3 | 0.766 |
Data are presented as mean ± standard deviation unless otherwise indicated. Bolded values indicate p < 0.05
Cluster analysis
A dendrogram from the cluster analysis is shown in Fig. 1. Dendrograms are graphical depictions that map the hierarchical clustering of observations. Each cluster is represented at the bottom of the figure. The two closest clusters are merged until one cluster remains at the top of the figure. Vertical lines represent distance between clusters, and horizontal lines represent clusters that are merged. For example, cluster G1 and G2 merge, which is then subsequently merged with G3. The optimal number of clusters can be determined by identifying the number of vertical lines with the largest distance between cluster merges (e.g., horizontal lines). Based on the dendrogram, we decided that two clusters are appropriate. Please note that due to the large number of observations, we limited the dendrogram to the top 15 clusters, and thus the long lines at the bottom of the graph can be ignored.
Fig. 1.
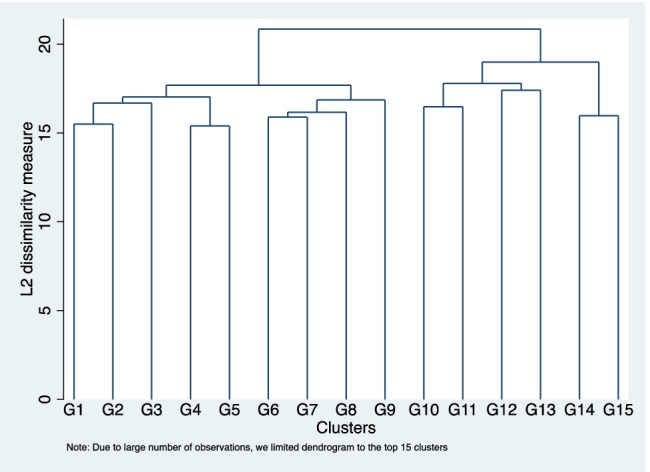
Dendrogram for Language of Sleepiness questions
Figure 2 depicts box plots of the severity ratings for each LOS item in cluster 1 (n = 127) and cluster 2 (n = 58). Cluster 1 is characterized by moderate to high severity for each LOS item except for “I don’t dream,” and “I can’t stay awake” (both of which for the most part had lower severity levels). Cluster 2 is characterized by low severity on most LOS items. OSA status did not differ across clustered groups (cluster 1 = 44.9% has AHI ≥ 5/h, cluster 2 = 56.9%, p = 0.129).
Fig. 2.
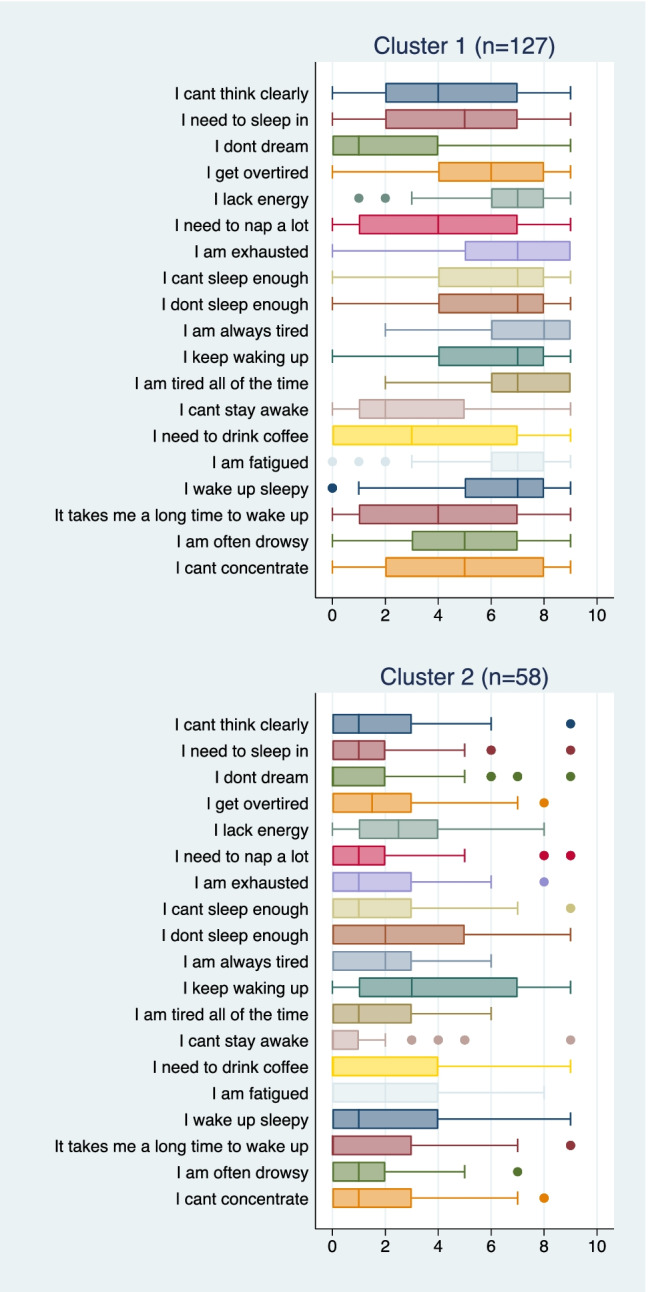
Distribution of severity levels for “Language of Sleepiness” items in cluster 1 and cluster 2
Discussion
In patients referred to our sleep clinic, excessive sleepiness using an Epworth Sleepiness Score ≥ 10 failed to discriminate patients with OSA from those without OSA. Based on the original observation of a mean ESS score of 12 in patients with moderate to severe OSA as compared to 6 in normal healthy controls, an ESS score of ≥ 10 has been widely adopted in clinical practice to define sleepiness [10]. However, few high quality studies have explored its test properties and when evaluating the performance of the ESS against polysomnography in identifying patients with OSA, the ESS had a large number of false negative results [7]. When considering an AHI of ≥ 5/h, the ESS has revealed a range of sensitivity of 0.27–0.72, specificity of 0.5–0.76, and accuracy of 51–59% [7]. No consistent association between severity of OSA and degree of excessive sleepiness has been shown [13–17]. In a large community-based cohort, the Sleep Heart Health Study, only 46% of 6440 participants with moderate to severe OSA based on an AHI of ≥ 15/h reported sleepiness defined using an ESS ≥ 10 or a report of at least frequently feeling unrested or sleepy [5]. Additionally, there are limited data on the ESS test–retest reliability. Yet, great weight is often placed on sleepiness and the ESS when diagnosing OSA and sleepiness is included as one diagnostic criteria for OSA by the International Classification of Sleep Disorders.
In Chervin’s observational study looking at preferred terms used to describe symptoms in OSA, fatigue, tiredness, and lack of energy were reported more frequently than sleepiness (57%, 61%, 62% vs 47%) and when asked to select the one most significant symptom, lack of energy was selected more than any other symptoms in patients with OSA [9]. Forty-four percent of patients considered lack of energy as their most limiting symptom compared to 16% for sleepiness. The authors also showed there was generally no association between each symptom and objective, “gold standard” polysomnographic measures of OSA severity or daytime sleepiness, arguing that the daytime subjective consequences of OSA are complicated. In a community sample of 837 adult men who completed a sleep study, sleepiness defined using ≥ 2 of 3 problems (feeling sleepy sitting quietly; feeling tired/fatigued/sleepy; trouble staying awake) but not the ESS was associated with OSA [18].
Our study confirms these findings and raises questions about whether sleepiness is a distinguishing characteristic of OSA. We found a range of descriptors of sleepiness used by patients with OSA. Additionally, there were relatively few differences in LOS item severity between those with and without an AHI of ≥ 5/h. Furthermore, clustering individuals based on profiles of sleepiness severity also did not fully distinguish individuals by OSA status. Paradoxically, patients with an AHI of ≤ 5/h had greater severity on some LOS items. The lack of significant differences in descriptors of sleepiness using the “Language of Sleepiness” between patients with and without OSA in our study is not surprising as sleepiness is multidimensional. Terri Young elegantly discusses the complexity of daytime sleepiness, with contributions from distinct drives of sleep and alertness, varying etiologies, state and trait considerations, and a wide spectrum of consequences, resulting in a need for a variety of definitions in order to address clinical questions [8]. Although relating to different underlying concepts, excessive sleepiness and fatigue can show overlapping features and patients can present with both complaints simultaneously [19]. Sleepiness and fatigue can also be independent manifestations of sleep disorders [20]. Thus, based on our findings, emphasis on sleepiness in OSA may be an impediment to accurate diagnosis.
Additionally, our study showed that females, older and white patients with OSA were significantly less likely to report sleepiness using an ESS ≥ 10. The potential gender differences in the expression of sleepiness is not new, with men reporting sleepy behavior and women reporting feelings of excessive sleepiness [8]. Similar to our study, in the Sleep Heart Health Study, Baldwin et al. found men and women answered questions on sleepiness differently [21]. Women reported feeling sleepy as often as men did, but women were less likely to have an ESS ≥ 10 and more likely to report feeling unrested than men. In men, the ESS was more strongly correlated with reports of feeling unrested or sleepy compared to women [21]. Chervin found the female gender was associated with a markedly increased frequency of all symptoms, although the differences for sleepiness was the smallest between females and males. In a study comparing clinical and polysomnographic features of OSA between 2052 males and 775 females with OSA, females more frequently reported fatigue and insomnia symptoms [22]. Some of these differences may be due to cultural factors. “Sleepiness” may be perceived as a sign of personal weakness while tiredness may be associated with working hard and thus be the less stigmatized term, but there may be also variation in sensitivity to the sensations of sleepiness [23]. Sforza et al. studied the presence of sleepiness in elderly patients with OSA and found the prevalence of EDS was low in healthy elderly patients with OSA highlighting the multiple potential influences on sleepiness [24].
Identifying and characterizing sleepiness may go beyond diagnosis and have prognostic and therapeutic implications in OSA. Using data from the Sleep Heart Health Study, the excessively sleepy symptom subtype was associated with more than threefold increased risk of prevalent heart failure and increased risk of cardiovascular disease compared with asymptomatic or moderately sleepy symptom subtypes [25]. In a 2012 meta-analysis of the effect of CPAP treatment on BP, reductions in DBP with PAP were predicted by ESS scores [26]. Much of the criticism for the negative randomized trials on CPAP in cardiovascular outcomes has been the exclusion of this sleepy subtype who may be at higher risk for cardiovascular disease. In aggregate, the data have found no cardiovascular benefit of CPAP in patients with OSA but subjects were not excessively sleepy [27–29].
Our study had limitations due to COVID-19 which was declared a pandemic by the WHO in March 2020 when patient enrollment began. The impact of COVID-19 on sleep is yet to be determined [30]. We had planned to examine the relationship between descriptors of sleepiness and objective polysomnographic measures of OSA pathology including various measures of OSA severity, but for safety reasons, most sleep studies were completed as unattended home sleep studies. Additionally, this study population ended up having mild OSA with only 29 patients with moderate and 15 with severe OSA, limiting analyses by OSA severity. Our study did not explore descriptors of sleepiness in sleep conditions other than OSA. The cohort was not selected for depressive symptoms and specific data on depressive course or sleep disturbances; thus, we were not able to closely examine the relationships of sleepiness and OSA with depression.
Our findings question the emphasis that is often placed on sleepiness and the ESS score in patients with OSA. Patients with OSA may use various daytime complaints and descriptors of sleepiness, and the clinical decision of whether or not to pursue diagnostic testing for OSA should not rely on a single ESS metric. Subjective measures of sleepiness likely only capture a limited aspect of a heterogeneous and multidimensional entity. Further studies exploring potential gender, age, and cultural influences on descriptors of sleepiness in various sleep disorders are required.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
All authors have seen and approved the manuscript.
Funding
Jazz Pharmaceuticals provided financial support in the form of a clinical investigator sponsored trial. The sponsor had no role in the design or conduct of this research. Dr. Kaufman was funded by the National Institute on Aging (NIH Grant #: K01AG061239). Dr. Malhotra is funded by the NIH.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (University of California San Diego Institutional Review Board – IRB project number 190489) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
Dr. Atul Malhotra reports income related to medical education from Livanova, Jazz, Equillium, and Corvus. ResMed provided a philanthropic donation to UC San Diego. All other authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–698. doi: 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young T, Evans L, Finn L, et al. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20(9):705–6. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 3.Davies RJ, Ali NJ, Stradling JR. Neck circumference and other clinical features in the diagnosis of the obstructive sleep apnoea syndrome. Thorax. 1992;47(2):101–5. doi: 10.1136/thx.47.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guilleminault C, Black JE, Palombini L, et al. A clinical investigation of obstructive sleep apnea syndrome (OSAS) and upper airway resistance syndrome (UARS) patients. Sleep Med. 2000;1(1):51–56. doi: 10.1016/S1389-9457(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 5.Kapur VK, Baldwin CM, Resnick HE, et al. Sleepiness in patients with moderate to severe sleep-disordered breathing. Sleep. 2005;28(4):472–7. doi: 10.1093/sleep/28.4.472. [DOI] [PubMed] [Google Scholar]
- 6.Gottlieb DJ, Punjabi NM. Diagnosis and management of obstructive sleep apnea: a review. JAMA. 2020;323(14):1389–1400. doi: 10.1001/jama.2020.3514. [DOI] [PubMed] [Google Scholar]
- 7.Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young TB. Epidemiology of daytime sleepiness: definitions, symptomatology, and prevalence. J Clin Psychiatry. 2004;65(Suppl 16):12–16. [PubMed] [Google Scholar]
- 9.Chervin RD. Sleepiness, fatigue, tiredness, and lack of energy in obstructive sleep apnea. Chest. 2000;118(2):372–9. doi: 10.1378/chest.118.2.372. [DOI] [PubMed] [Google Scholar]
- 10.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 11.Chasens ER, Ratcliffe SJ, Weaver TE. Development of the FOSQ-10: a short version of the Functional Outcomes of Sleep Questionnaire. Sleep. 2009;32(7):915–9. doi: 10.1093/sleep/32.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufman LRP. Finding groups in data: an introduction to cluster analysis. New York: Wiley; 1990. [Google Scholar]
- 13.Chervin RD, Aldrich MS. The Epworth Sleepiness Scale may not reflect objective measures of sleepiness or sleep apnea. Neurology. 1999;52(1):125–31. doi: 10.1212/WNL.52.1.125. [DOI] [PubMed] [Google Scholar]
- 14.Garbarino S, Scoditti E, Lanteri P, et al. Obstructive sleep apnea with or without excessive daytime sleepiness: clinical and experimental data-driven phenotyping. Front Neurol. 2018;9:505. doi: 10.3389/fneur.2018.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mediano O, Barcelo A, de la Pena M, et al. Daytime sleepiness and polysomnographic variables in sleep apnoea patients. Eur Respir J. 2007;30(1):110–13. doi: 10.1183/09031936.00009506. [DOI] [PubMed] [Google Scholar]
- 16.Olson LG, Cole MF, Ambrogetti A. Correlations among Epworth Sleepiness Scale scores, multiple sleep latency tests and psychological symptoms. J Sleep Res. 1998;7(4):248–53. doi: 10.1046/j.1365-2869.1998.00123.x. [DOI] [PubMed] [Google Scholar]
- 17.Walter TJ, Foldvary N, Mascha E, et al. Comparison of Epworth Sleepiness Scale scores by patients with obstructive sleep apnea and their bed partners. Sleep Med. 2002;3(1):29–32. doi: 10.1016/S1389-9457(01)00079-X. [DOI] [PubMed] [Google Scholar]
- 18.Adams RJ, Appleton SL, Vakulin A, et al. Association of daytime sleepiness with obstructive sleep apnoea and comorbidities varies by sleepiness definition in a population cohort of men. Respirology. 2016;21(7):1314–21. doi: 10.1111/resp.12829. [DOI] [PubMed] [Google Scholar]
- 19.Neu D, Linkowski P, le Bon O. Clinical complaints of daytime sleepiness and fatigue: how to distinguish and treat them, especially when they become 'excessive' or 'chronic'? Acta Neurol Belg. 2010;110(1):15–25. [PubMed] [Google Scholar]
- 20.Hossain JL, Ahmad P, Reinish LW, et al. Subjective fatigue and subjective sleepiness: two independent consequences of sleep disorders? J Sleep Res. 2005;14(3):245–53. doi: 10.1111/j.1365-2869.2005.00466.x. [DOI] [PubMed] [Google Scholar]
- 21.Baldwin CM, Kapur VK, Holberg CJ, et al. Associations between gender and measures of daytime somnolence in the Sleep Heart Health Study. Sleep. 2004;27(2):305–11. doi: 10.1093/sleep/27.2.305. [DOI] [PubMed] [Google Scholar]
- 22.Basoglu OK, Tasbakan MS. Gender differences in clinical and polysomnographic features of obstructive sleep apnea: a clinical study of 2827 patients. Sleep Breath. 2018;22(1):241–249. doi: 10.1007/s11325-017-1482-9. [DOI] [PubMed] [Google Scholar]
- 23.Dement WC, Hall J, Walsh JK. Tiredness versus sleepiness: semantics or a target for public education? Sleep. 2003;26(4):485–6. [PubMed] [Google Scholar]
- 24.Sforza E, Pichot V, Martin MS, et al. Prevalence and determinants of subjective sleepiness in healthy elderly with unrecognized obstructive sleep apnea. Sleep Med. 2015;16(8):981–6. doi: 10.1016/j.sleep.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Mazzotti DR, Keenan BT, Lim DC, et al. Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes. Am J Respir Crit Care Med. 2019;200(4):493–506. doi: 10.1164/rccm.201808-1509OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montesi SB, Edwards BA, Malhotra A, et al. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med. 2012;8(5):587–96. doi: 10.5664/jcsm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peker Y, Glantz H, Eulenburg C, et al. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA Randomized Controlled Trial. Am J Respir Crit Care Med. 2016;194(5):613–20. doi: 10.1164/rccm.201601-0088OC. [DOI] [PubMed] [Google Scholar]
- 28.Thunstrom E, Glantz H, Yucel-Lindberg T, et al (2017) CPAP does not reduce inflammatory biomarkers in patients with coronary artery disease and nonsleepy obstructive sleep apnea: a randomized controlled trial. Sleep. Nov 1;40(11). [DOI] [PubMed]
- 29.McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–31. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 30.Advani I, Gunge D, Banks S, et al. Is increased sleep responsible for reductions in myocardial infarction during the COVID-19 pandemic? Am J Cardiol. 2020;131:128–130. doi: 10.1016/j.amjcard.2020.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request.


