Abstract
Background:
Low protein diets have been linked to decreased bone strength in humans. Arginine and lysine can help improve the healing process and stimulate growth factors.
Aim:
To evaluate if dietary arginine and lysine combination aids in reducing the time frame for osseo-integration process and bone formation in animal models.
Materials and Methods:
Controls (Group I) and Experimental (Group II) consisted of twelve New Zealand rabbits. Animals in the experimental group were fed a conventional pellet food, water, and the amino acids L-Lysine and L-Arginine (Biovea, USA), whereas those in the control group were offered a standard diet. In both groups of animals, titanium implants measuring 2.5mm* 6mm were implanted in each tibial osteotomy. At the end of two weeks, four weeks, and eight weeks, the animals were euthanized. The tibial bone was removed and preserved in 1% formalin. The samples were analysed histologically for presence or absence of Necrosis, presence or absence of clot formation, Vascularization, Fibroblast, Osteoblasts and Osteoid Bone growth.
Results:
Histological outcomes on vascularization, fibroblasts, osteoblasts, osteoid bone growth inferred no significant variation between the control and experimental groups after 8 weeks (P>.05).
Conclusion:
Vascularity, clot organisation, osteoblasts, fibroblasts, and osteoid bone production in the protein fed experimental group animals were better in initial stages of healing when compared to control groups.
KEYWORDS: Amino acids, bone formation, healing, osseointegration, vascularization
INTRODUCTION
The amino acids are used as adjunct treatment therapy in conditions such as arthritis, muscle mass building, malnutrition, osteoporosis, HSV, cancer therapy, leukemia, skin cancer, and prostate cancer.[1,2] During the treatment procedures, the amino acids are administered through orally, intravenously, or intramuscularly.[3] While several amino acids have been linked to impacts on soft tissue, musculature, and neural conduction, amino acids such as L-Arginine and L-Lysine have been linked to osteogenesis and metabolism.[4,5]
Amino acids such as arginine and lysine have been shown to speed up the healing of fractures by increasing microcirculation, augmenting growth hormones, and increasing collagen synthesis. The nitric oxide synthase enzyme pathway is used to metabolize L-arginine in the body. The amino acid lysine is involved in collagen and osteopontin crosslinking.[6]
Inadequate lysine consumption causes a reduction in collagen production and an elevation in the risk of fractures. The arginine amino acid plays a role in serum insulin such as growth factor levels, wound healing, immune functions, and in enhancing growth hormone. The genetic studies by Jeong-Eun-Huh et al. indicate arginine can determine cell proliferation or differentiation. The oral administration and combination of lysine and arginine are indicated as one of the potential materials for accelerating or improving bone deformity. The prolonged oral administration of arginine and lysine are also indicated as nontoxic to other visceral organs functioning among humans and animals. This study was done to evaluate if dietary arginine and lysine combination aids in reducing the time frame for osseointegration process and bone formation.
MATERIALS AND METHODS
An animal ethical clearance pro forma was submitted to the institutional animal ethical committee and was approved. The experimental animals included were of New Zealand albino strain weighing equal to or about 2 kg and around 2 years of age. The E value (E = Total number of animals – number of groups) was used to determine the sample size. The study has two groups; hence, the E value was estimated as 10.
The groups were classified as:
Group I - Control where the animals were fed with standard diet
Group II - Experimental where the animals were fed with protein-rich diet.
Three animals were assigned to the control group, whereas nine animals were assigned to the experimental group (1:3). The control animals were provided a regular pellet diet, whereas the rabbits in experimental group were fed with regular diet supplemented amino acids L-lysine and L-arginine (Biovea, USA). The amino acid was administered intraorally using a 1 ml syringe once daily. Where in the control animals were fed with the standard pellet diet and water at room temperature and an implant armamentarium was arranged [Figure 1a-d].
Figure 1.
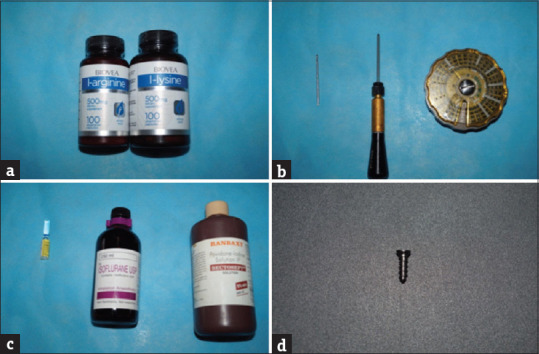
Amino acids administered and mini implant for placement (a) amino acid, (b) ketamine, isoflurane, and povidone iodine, (c) implant armamentarium (d) implant leforte screws
Titanium implants measuring 2.5 mm × 6 mm (Leforte screws) were embedded in each osteotomy done in the tibia [Figure 2a-i]. The experimental animals (2 weeks- 3 animals, 4 weeks- 3 animals, and 8 weeks- 3 animals) were fed with normal diet and arginine (biovea, USA) 50 mg/kg orally and lysine (biovea, USA) 50 mg/kg orally for 2 weeks, 4 weeks and 8 weeks respectively. The amino acids were orally administered by placing the nozzle of the syringe in the corner of the mouth as described by Flecknell [Figure 3a]. The control animals in group 1, group 2, and group 3 were maintained on a normal diet at the end of 2 weeks, 4 weeks, and 8 weeks, one animal from the control group and three animals from the experimental group were euthanized. The tibial bone was removed and preserved in 1% formalin [Figure 3b]. The samples were decalcified, processed, embedded, and sectioned. The slides were prepared for H and E staining.
Figure 2.
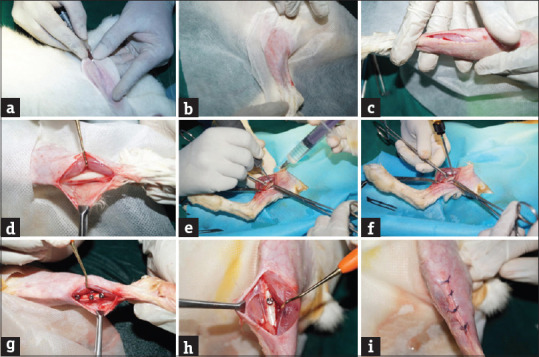
Surgical procedure for implant placement (a) incision, (b) surgical site (c) skin incision, (d) exposing bone, (e) osteotomy procedure with surgical drill and copious saline irrigation. (f) Implant placement, (g) implants in situ (experimental), (h) implants in situ (control), (i) closure of surgical site
Figure 3.
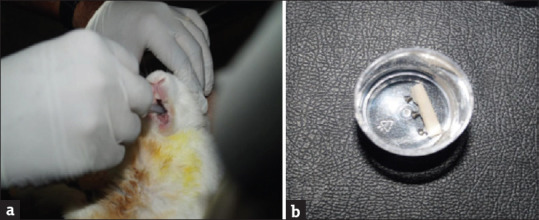
Harvested bone sample with implants in 10% formalin (a) feeding amino acids to the experimental animals, (b) harvested bone sample with implants in 10% formalin
Histological assessment
The specimen slides were examined under light microscope under ×4 and ×10 magnifications. Two separate observers recorded their interpretations of the specimens. The parameters observed for in the slide were presence or absence of necrosis [Figure 4], presence or absence of clot formation [Figure 4b], vascularization [Figure 4c], fibroblast [Figure 4d], osteoblasts [Figure 4e], and osteoid bone [Figure 4f]. The scoring for vascularization, fibroblast, osteoblasts, and osteoid bone was done over a scale of 0–4 (0 – Nil, 1– Mild, 2– Moderate, 3– Marked, and 4– Absolute) [Figure 4a-d].
Figure 4.
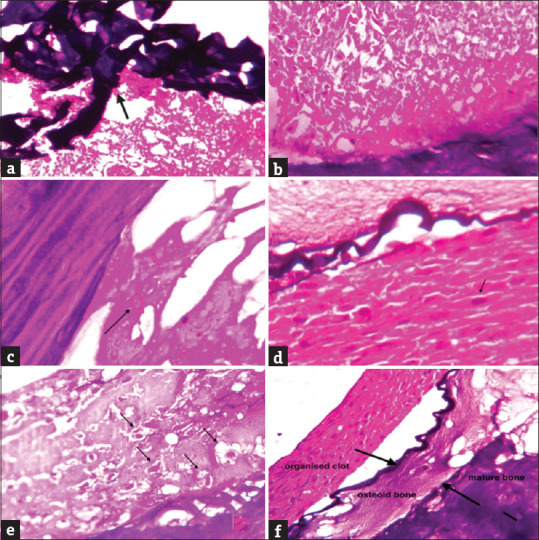
Histological outcomes assessed in the study (a) necrosis, (b) organized clot, (c) vascularization, (d) fibroblasts, (e) osteoblasts, (f) osteoid bone
Immunohistochemical analysis
Immunohistochemistry with SATB2 was performed to evaluate its expression in osteoblastic differentiation. SATB2 expression was evidenced in all test groups, osteoblastic differentiation at the implant tissue interface was noted. Osteoblasts were seen along the bony trabeculae clearly indicative of osteogenesis. Number of osteoblasts in the test groups was higher than the control group [Figure 5a and b].
Figure 5.
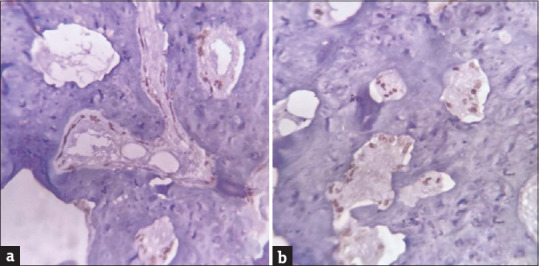
Immunohistochemical expression of SATB2 in osteoblasts (a) positive immunohistochemical expression of SATB2 in osteoblasts of control group, (b) positive immunohistochemical expression of SATB2 in osteoblasts of experimental group
RESULTS
Histological outcomes provided by the two independent observers were rated using kappa statistics. Independent samples t-test were performed for vascularization, fibroblasts, osteoblasts, and osteoid bone growth. However, there were no statistically significant differences observed with respect to the outcome measures between the control and the experimental groups after 8 weeks (P > 0.05) [Table 1].
Table 1.
Outcome measures evaluated in the study
| Outcome measure | Group I (control) | Group II (experimental) | P |
|---|---|---|---|
| Necrosis | Minimal | Minimal | >0.05 (NS) |
| Vascularization | Marked | Marked | >0.05 (NS) |
| Fibroblast growth | Marked | Marked | >0.05 (NS) |
| Osteoblast growth | Marked | Marked | >0.05 (NS) |
| Osteoid formation | Marked | Marked | >0.05 (NS) |
NS: Not significant
DISCUSSION
The osseointegration process depends on numerous factors such as the implant material itself, surface topography, or the texture, the type of bone, and host responses of the bone. The osseointegration process passes through different phases-osteophilic, osteoinductive, osteoconductive, and osteoadaptive. The first two phases of this osseointegration process require a better host bone bed and near-perfect intrinsic healing potential.[7] There are similarities between the bone fracture healing and the healing process of bone around implants. There are reports of use of lysine and arginine in accelerating the fracture healing, in osteomalacia, in osteoporosis, hence the current study was designed around the amino acid and the accelerated osseointegration process, modifying the healing potential.[8]
The vascularization process was graded at mild to moderate between 4 and 8 weeks in experimental group whereas during the same period in the control group it was graded as only mild. The formation of the immature fibroblasts is essential in terms of the presence of collagen, cell differentiation, and it is the most common type of connective tissue formed in the animals during the healing phase. The mesenchymal derived immature fibroblasts presence was mild to severe in the experimental group at 8 weeks indicating a better collagen formation.
There are numerous studies which are associated with amino acids and osteoblastic cells proliferation, in particular, the arginine and lysine amino acids are tested in osteopenic rats cell cultures for alkaline phosphatase activity and in modifying the mesenchymal stem cell signaling.[9,10,11] The present study seems to be having a correlation between the dietary amino acid animal group to nondietary amino acid animal group in terms of osteoblastic activity.
The new bone formation was also appreciated at the same 8-week period in the experimental group. Although there is a difference between the control group and experimental group, statistically not much significance is obtained. The study was based on oral administration of amino acids lysine and arginine. The absorption rate and bioavailability of the amino acids might be equal or lesser than the dosage administered orally. This could have reduced the effect at the implant site, a study indicated that there is a 20% of bioavailability of arginine for every 10 g administered orally.[12] The orally administered amino acid are nontoxic, tend to increase the calcium absorption at the gastrointestinal tract, and also reported to increase the inducible nitric oxide synthase activity which in turn increases the vascularity for better healing.[13]
The present study demonstrates a change with the use of orally administered amino acid around the implants, further studies can be directed toward the parenteral administration, increase in dosage, and effect targeted toward specific cells. Long-term studies on rate of bone maturation, human trials, and effect of amino acid on osseointegration of implants three dimensionally can be the future trends of this study.
CONCLUSION
This study observed vascularity, clot organization, osteoblasts, fibroblasts, and osteoid bone production in the protein-fed experimental group animals were better in the initial stages of healing when compared to control groups.
Financial support and sponsorship
Self-funded.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Flynn NE, Shaw MH, Becker JT. Amino acids in health and endocrine function. Adv Exp Med Biol. 2020;1265:97–109. doi: 10.1007/978-3-030-45328-2_6. [DOI] [PubMed] [Google Scholar]
- 2.Marcone GL, Rosini E, Crespi E, Pollegioni L. D-amino acids in foods. Appl Microbiol Biotechnol. 2020;104:555–74. doi: 10.1007/s00253-019-10264-9. [DOI] [PubMed] [Google Scholar]
- 3.Torricelli P, Fini M, Giavaresi G, Giardino R. Bone tissue cultures: An in vitro model for the evaluation of bone defect healing after L-arginine and L-lysine administration. Artif Cells Blood Substit Immobil Biotechnol. 2001;29:325–34. doi: 10.1081/bio-100104234. [DOI] [PubMed] [Google Scholar]
- 4.Fini M, Aldini NN, Canè V, Zaffe D, Giavaresi G, Rocca M, et al. Effects of essential amino acids and lactose on bony fractures and defects in rabbits: A preliminary histomorphometric study. Arch Orthop Trauma Surg. 1999;119:39–45. doi: 10.1007/s004020050352. [DOI] [PubMed] [Google Scholar]
- 5.Dallari D, Fini M, Stagni C, Torricelli P, Nicoli Aldini N, Giavaresi G, et al. In vivo study on the healing of bone defects treated with bone marrow stromal cells, platelet-rich plasma, and freeze-dried bone allografts, alone and in combination. J Orthop Res. 2006;24:877–88. doi: 10.1002/jor.20112. [DOI] [PubMed] [Google Scholar]
- 6.Munger RG, Cerhan JR, Chiu BC. Prospective study of dietary protein intake and risk of hip fracture in postmenopausal women. Am J Clin Nutr. 1999;69:147–52. doi: 10.1093/ajcn/69.1.147. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen JJ, Low SA, Ramseier NT, Hadap RV, Young NA, Wang M, et al. Analysis of the bone fracture targeting properties of osteotropic ligands. J Control Release. 2021;329:570–84. doi: 10.1016/j.jconrel.2020.09.047. [DOI] [PubMed] [Google Scholar]
- 8.Medvecky L, Giretova M, Stulajterova R, Danko J, Vdoviakova K, Kresakova L, et al. Characterization of properties, in vitro and in vivo evaluation of calcium phosphate/amino acid cements for treatment of osteochondral defects. Materials (Basel) 2021;14:436. doi: 10.3390/ma14020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meesters DM, Wijnands KA, Brink PR, Poeze M. Malnutrition and fracture healing: Are specific deficiencies in amino acids important in nonunion development? Nutrients. 2018;10:1597. doi: 10.3390/nu10111597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang B, Liang JM, Ding JN, Xu J, Xu JG, Chai YM. Dimethyloxaloylglycine-stimulated human bone marrow mesenchymal stem cell-derived exosomes enhance bone regeneration through angiogenesis by targeting the AKT/mTOR pathway. Stem Cell Res Ther. 2019;10:335. doi: 10.1186/s13287-019-1410-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alapure BV, Lu Y, He M, Chu CC, Peng H, Muhale F, et al. Accelerate healing of severe burn wounds by mouse bone marrow mesenchymal stem cell-seeded biodegradable hydrogel scaffold synthesized from arginine-based poly (ester amide) and chitosan. Stem Cells Dev. 2018;27:1605–20. doi: 10.1089/scd.2018.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tangphao O, Grossmann M, Chalon S, Hoffman BB, Blaschke TF. Pharmacokinetics of intravenous and oral L-arginine in normal volunteers. Br J Clin Pharmacol. 1999;47:261–6. doi: 10.1046/j.1365-2125.1999.00883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts A. The safety and regulatory process for amino acids in Europe and the United States. J Nutr. 2016;146:2635S–42S. doi: 10.3945/jn.116.234591. [DOI] [PubMed] [Google Scholar]


