Abstract
Objective:
The objective of the study was to use polymerase chain reaction (PCR) to examine and compare the genotype distribution of human papillomavirus (HPV) in oral lichen planus (OLP).
Materials and Methods:
Deoxyribonucleic acid (DNA) was extracted from 20 OLP biopsy specimens. Conventional PCR assay employing consensus HPV primers was used to identify HPV DNA. Positive PCR samples were then subjected to PCR assay with HPV type-specific primers.
Results:
Out of the total 20 OLP specimens evaluated, eight samples (40%) were positive for HPV. Females had a 41.7% higher HPV-positive rate than males. The most common type in the HPV type-specific PCR assay was HPV-18 (75%), which is a high-risk type of HPV linked to malignant diseases. The erosive kind of OLP had the greatest percentage of HPV positives (50%).
Conclusion:
The present study confirms the detection of HPV in OLP lesions, as determined by PCR-coupled HPV gene sequencing, as well as its likely mechanism of malignant transformation.
KEYWORDS: Human Papillomavirus 16, human papillomavirus 18, lichen planus, PCR, premalignant
INTRODUCTION
Oral lichen planus (OLP) is a chronic autoimmune condition that causes thickening or atrophy of the epithelium or ulceration in some cases.[1] The common clinical manifestations are reticular and erosive lesions. The reticular lesion, which affects the buccal mucosa, tongue, and gingiva, is generally asymptomatic and manifests as white keratotic lines with an erythematous margin. It affects people all around the globe, primarily in their fifth and sixth decades. Women are at two-fold times more risk than men.[2,3]
Although various molecular explanations have been proposed, the etiology and pathophysiology of OLP remain unexplained. OLP is caused by autoimmune T lymphocytes that cause atrophy of the epithelial cells, resulting in chronic inflammatory changes. As a result of the cell-mediated immunologic processes, the epithelial basal cell layer degenerates. The proinflammatory mediators such as nuclear factor kappa B may be activated in the chronic course of OLP, and suppression of the transforming growth factor control pathway promotes hyperproliferation of keratinocyte, resulting in white lesions. Stress, diabetes, hepatitis C, trauma, and hypersensitivity to medications and metals are the suspected causal factors associated with OLP.[3,4]
The World Health Organization referred OLP as an oral potentially malignant disorder (OPMD) with an undetermined risk of oncogenesis and recommended that OLP patients be closely monitored. It is a potentially precancerous stage of oral squamous cell carcinoma (OSCC).[5] OPMD comprises conditions such as leukoplakia, erythroplakia, lichen planus, discoid lupus erythematosus, and actinic cheilitis. Tobacco consumption and alcohol are the two major risk factors underlying OPMD. High-risk human papillomavirus (HPV), particularly genome of HPV-16 and 18, has recently been identified as an emerging risk of OPMD.[6]
HPV deoxyribonucleic acid (DNA) is twofold more commonly found in OLP lesions than in normal oral mucosa.[7] The highest prevalence of HPV DNA is found in the atrophic form of OLP.[4,8,9] The detection of HPV in OLP has been estimated to be around 0.5%–2.2%, depending on the regional variation.[1] The HPV viral protein E6 promotes p53 degradation, while the viral protein E7 inhibits the activity of the retinoblastoma protein. This causes disruption of the cell cycle, leading to oncogenic transformation.[4,6]
HPV are small double-stranded circular DNA infecting various epithelial tissues and have over 200 genotypes.[5] Depending on the clinical presentations, HPV is defined as high risk or low risk. Low-risk HPV manifests as benign proliferative lesions like warts, while high-risk types are frequently incorporated into host DNA. HPV-16 and 18 are referred as high-risk types. They are commonly found in oral dysplastic lesions and cervical, anal, and oropharyngeal cancers.[10,11]
The current study sought to investigate the prevalence of HPV DNA, HPV-16, and HPV-18 in tissue biopsies using conventional polymerase chain reaction (PCR) in subjects with OLP.
MATERIALS AND METHODS
Collection of biopsy specimens
A sample size of 20 was deemed optimum to evaluate and compare the genotype distribution of HPV in OLP with a precision of 10%. Twenty OLP biopsy specimens in DNA stabilizer were obtained from the Department of Oral Medicine and Radiology, from patients clinically diagnosed with OLP between 2020 and 2021. For DNA extraction, specimens that were proven to be OLP positive based on histological assessment were chosen. A consensus diagnosis was achieved by two competent oral and maxillofacial pathologists. The study was approved by the Institution Ethics Committee, and written informed consent was obtained from the study subjects. All the study subjects were between the age of 18 and 56 years, and there were eight males and 12 females. The inclusion criterion was OLP located in the oral cavity that was histopathologically diagnosed as OLP. The exclusion criteria were histopathologic detection of dysplasia in part of lesions and the specimens with poor tissue quality. Twenty tissue samples in a DNA stabilizer were used for HPV detection and typing HPV-16 and HPV-18 using a conventional single PCR assay. The reagents of the PCR assay are shown in Table 1. The quality and quantity of the DNA were estimated using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primer and ultraviolet–visible (UV–VIS) spectrophotometer, respectively. The quantity of DNA at 260/280 optical density was estimated to be 1.30–2.10 μg/mL, depicting a good detection range.
Table 1.
Reagents for PCR
| PCR components | Volume (µl) |
|---|---|
| Nuclease-free water | 10.70 |
| 10× reaction buffer with MgCl2 (1.5 mM) | 2.00 |
| dNTP mix (2.5 mM) | 2.00 |
| Primer FP (10 pmol/µl) | 2.00 |
| Primer RP (10 pmol/µl) | 2.00 |
| Taq DNA polymerase (5 U) | 0.30 |
| Template DNA (50 ng/µl) | 1.00 |
| Total volume | 20.0 |
PCR=polymerase chain reaction, dNTP=deoxyribose nucleotide triphosphate, FP=Forward Primer, RP=Reverse Primer
Genomic DNA extraction from the tissue sample using the cetyltrimethylammonium bromide method
The tissue samples stored at −20°C were transferred to a sterile mortar and pestle and grounded to a fine powder using liquid nitrogen. Then, 675 μl of extraction buffer was added and incubated at 37°C for 30 min. To the above, 75 μl of Sodium dodecyl sulfate (SDS) was added and incubated at 65°C for 2 h, followed by centrifugation at 10,000 rpm for 10 min at 4°C. The supernatant was collected in a sterile microcentrifuge tube. Equal volume of chloroform: isoamyl alcohol was added and centrifuged at 10,000 rpm for 10 min at 4°C. The aqueous phase was transferred to a sterile microcentrifuge tube. Then, 0.6 volumes of isopropanol was added and incubated at room temperature for 1 h. After incubation, it was centrifuged at 10,000 rpm for 10 min. The pellet was washed with 500 μl of 70% ethanol and centrifuged at 10,000 rpm for 10 min at room temperature. The pellet was air-dried and dissolved in 20 μl of sterile water.
DNA assessment based on PCR and gel electrophoresis
PCR was done with GAPDH primers to check the quality of DNA (product size: 496 bp; GAPDH F: TTCTGGGGACTGGCTTTCC; GAPDH R: AAAG TGGTCGTTGAGGGCAA). Bands were observed in all 20 samples. A no template control was included in the last lane to rule out contamination [Figure 1]. The DNA isolated was loaded onto 1.2% agarose gel electrophoresis to visualize the bands and determine their quality.
Figure 1.
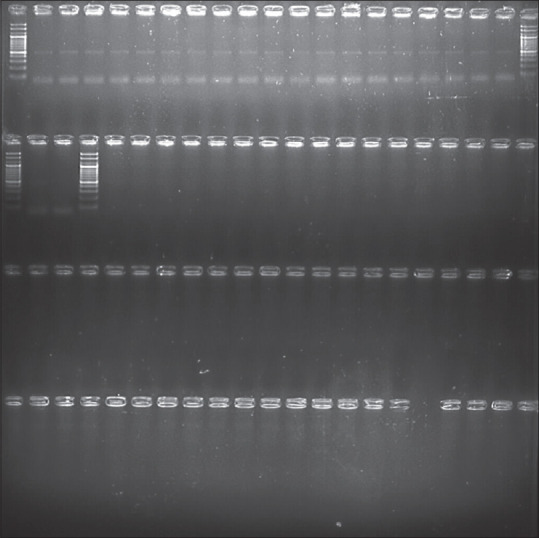
PCR by GAPDH primer. GAPDH = glyceraldehyde-3-phosphate dehydrogenase, PCR = polymerase chain reaction
Quantification of isolated DNA
The quantity of the isolated DNA was checked in UV–VIS spectrophotometer (Vivaspec Biophotometer, Germany). From the stock, 50 times dilution was obtained by mixing 1 μl of DNA with 49 μl of sterile distilled water. The A260/A280 ratio was recorded to check the purity of DNA preparation.
HPV analysis by PCR
For the detection of target HPV DNA and typing of HPV-16 and HPV-18, a single PCR assay was used. HPV detection was done by PGMY09/11 primers (200 bp HPV-18 and 500 bp HPV-16). PCR was carried out using the following primers for the E6 region: HPV forward primer 5′-CGTCCMARRGGAWACTGATC-3′ and HPV reverse primer 5′-GCMCAGGGWCATAAYAATGG-3′. The quantified DNA was used to detect the presence of HPV using the specific primers below [Figure 2]. On observation, eight samples turned positive for HPV. The PCR profile is shown in Table 2.
Figure 2.
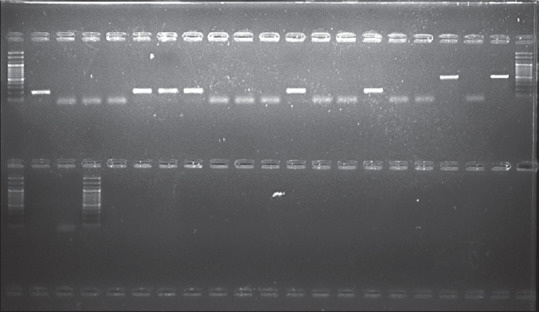
Agarose gel electrophoresis of HPV by single PCR assay. HPV = human papillomavirus, PCR = polymerase chain reaction
Table 2.
PCR profile
| Initial denaturation | 35 cycles | Final extension | ||
|---|---|---|---|---|
|
| ||||
| Denaturation | Annealing | Extension | ||
| 94°C for 3 min | 94°C for 50 s | 50°C for 40 s | 72°C for 1.30 min | 72°C for 6 min |
PCR=polymerase chain reaction
Statistical analysis
The data thus obtained was analyzed using the Statistical Packages for Social Sciences version 20.0 for Windows (IBM SPSS Statistics; SPSS South Asia Pvt Ltd, Bangalore, India). The Chi-square test was applied to evaluate the association between gender, site, and type of OLP. P ≤ 0.05 was considered statistically significant.
RESULTS
Eight samples (40%) out of a total of 20 specimens were positive for HPV in PCR analysis [Table 3]. In the HPV type-specific PCR assay, the frequency of positivity for each HPV-16 genome was 25% (2/20) and for each HPV-18 genome was 75% (6/20). The study sample included a male to female ratio of 8:12, with HPV-positive rates of 37.5% (3/8) in males and 41.7% (5/12) in females. Table 4 shows that there was a statistically insignificant difference in the percentage of HPV-positive males and females. The frequencies of HPV positivity in reticular and erosive OLP were 25% (2/8) and 50% (6/12), respectively. Twelve samples were obtained from the buccal mucosa and eight from the labial mucosa. According to the OLP site, HPV positivity rates were 50% (6/12) and 25% (2/8), respectively. However, an insignificant difference was found between the site and the type of lesion [Table 4].
Table 3.
Frequency distribution of gender, type, and site of OLP, HPV positivity, and HPV genome
| n (%) | |
|---|---|
| Gender | |
| Male | 8 (40) |
| Female | 12 (60) |
| Type of OLP | |
| Reticular | 8 (40) |
| Erosive | 12 (60) |
| Site of OLP | |
| Buccal | 12 (60) |
| Labial | 8 (40) |
| HPV | |
| Positive | 8 (40) |
| Negative | 12 (60) |
| HPV genome | |
| HPV-16 | 2 (25) |
| HPV-18 | 6 (75) |
HPV=human papillomavirus, OLP=oral lichen planus
Table 4.
Association of HPV in OLP based on gender, type, and site of OLP
| n (%) | Chi-square | P | ||
|---|---|---|---|---|
|
| ||||
| Negative | Positive | |||
| Gender | ||||
| Male | 5 (62.5) | 3 (37.5) | 0.03 | 0.852 |
| Female | 7 (58.3) | 5 (41.7) | ||
| Type of OLP | ||||
| Reticular | 6 (75) | 2 (25) | 1.25 | 0.264 |
| Erosive | 6 (50) | 6 (50) | ||
| Site of OLP | ||||
| Buccal | 6 (50) | 6 (50) | 1.25 | 0.264 |
| Labial | 6 (75) | 2 (25) | ||
HPV=human papillomavirus, OLP=oral lichen planus
DISCUSSION
OLP has regional variation in its prevalence, and its estimated global prevalence is 1.27%.[12] In India, OLP is reported to affect 2.6% of the population.[13] In a 10-year follow-up, the risk of oncogenesis of OLP ranges from 1.2% to 3.2%.[14] In contrast, Ma et al.[1] stated that the malignant transformation rate of OLP was 0–6.25%. HPV-16 has been established as the prime infectious genome in OSCC with a prevalence of 16%. Henceforth, the causal role of HPV-16 in OLP malignancy has to be investigated further with a long-term follow-up.
The findings of this study correlate with the earlier prevalence studies[2,3] of HPV in females. HPV-16 and HPV-18 disrupt normal cell cycle regulation by expressing E6 and E7 genes by binding similar oncoproteins to the neoplasm.[15] The strength of the relationship between HPV-16 and OLP was quite substantial with an odds ratio of 11.27 and has been involved in the pathogenesis of malignancy. The highest positivity rate for HPV-16 was reported by Kato et al.[14] However, the current study found a greater positivity rate of HPV-18 genome, which was in line with a similar previous study.[16]
HPV has a predilection for squamous cells, and the typical histopathologic characteristics of OLP are lymphocytic infiltration into the subepithelial layer in a band-like pattern, liquefactive basal layer degeneration, Civatte bodies with several eosinophilic colloid bodies, varying degrees of focal orthokeratosis or parakeratosis, and irregular acanthosis. The most frequent clinical manifestation is a reticulated pattern of white striae.[16] However, in our current investigation, we discovered a higher prevalence of erosive lesions. HPV infection rates in OLP have been reported at rates ranging from 15.4% to 42.6%.[14] Understanding the significance of HPV in oral malignancy is thus hampered by diverse frequencies, which may be attributable to changes in sample procedures such as biopsy methods, washings, brushings, patient demographics, and sensitivity of assays such as in situ hybridization and PCR.[11]
OLP has been documented to affect any site in the oral mucosa. However, the buccal mucosa, gingiva, and tongue are the most commonly affected regions,[16] which is consistent with our findings regarding the location of the lesion. Syrjänen et al.[7] and Gorsky et al.[17] suggested a possibly strong association between HPV, OSCC, and OLP in their systematic review and meta-analysis.
The HPV positivity rate of 40% reported in the study is comparable with the other PCR-based investigations that found the prevalence of HPV infection to range from 40.8%[18] to 41.5%.[14] Few other studies, on the other hand, have found a lower prevalence of HPV in OLP lesions, ranging from 17%,[19] 17.6%,[20] 25%,[21] 27.1%[11] to 29.6%.[22]
The variations are most likely attributable to the mean age of the study sample. Pattanshetty et al.[10] observed 65% of HPV-16/18–positive cases in the normal mucosa of tobacco users. Biopsy has been demonstrated to be a more reliable method for confirming HPV status than salivary analysis.[4] While PCR is frequently employed as a diagnostic method in HPV epidemiological studies, the economic and technical aspects are generally prohibitive for extensive screening programs. The innate advantage of the PCR assay is its ability to determine the very minuscule quantity of DNA of HPV. Simultaneously, stringent laboratory processes and controls are essential for decreasing contamination-related false-positive results.[23]
Limitations of the study
The smaller sample size is a significant limitation of the study. HPV-16 and HPV-18 are frequently reported in the normal oral mucosa. According to the current evidence, it may either serve as a source for new infection or a cause of recurrent lesions of HPV. To detect any changes in the normal oral mucosa, future case–control studies involving normal controls and prospective cohort studies with long-term patient follow-up are recommended to evaluate and incorporate preventive measures and targeted therapies against HPV infection.
CONCLUSION
The findings of our study confirm the presence of HPV in OLP lesions, as determined by PCR-coupled HPV gene sequencing, as well as its likely mechanism of malignant transformation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ma J, Zhang J, Zhang Y, Lv T, Liu J. The magnitude of the association between human papillomavirus and oral lichen planus: A meta-analysis. PLoS One. 2016;11:e0161339. doi: 10.1371/journal.pone.0161339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alrashdan MS, Cirillo N, Mccullough M. Oral lichen planus: A literature review and update. Arch Dermatol Res. 2016;308:539–51. doi: 10.1007/s00403-016-1667-2. [DOI] [PubMed] [Google Scholar]
- 3.Scully C, Carrozzo M. Oral mucosal disease: Lichen planus. Br J Oral Maxillofac Surg. 2008;46:15–21. doi: 10.1016/j.bjoms.2007.07.199. [DOI] [PubMed] [Google Scholar]
- 4.Sahebjamiee M, Sand L, Karimi S, Biettolahi J, Jabalameli F, Jalouli J. Prevalence of human papillomavirus in oral lichen planus in an Iranian cohort. J Oral Maxillofac Pathol. 2015;19:170–4. doi: 10.4103/0973-029X.164528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shang Q, Peng J, Zhou Y, Chen Q, Xu H. Association of human papillomacirus with oral lichen planus and oral leukoplakia: A meta-anaysis. J Evid Based Dent Pract. 2020;20:101485. doi: 10.1016/j.jebdp.2020.101485. [DOI] [PubMed] [Google Scholar]
- 6.Kaewmaneenuan N, Lekawanvijit S, Pongsiriwet S. High prevalence of human papillomavirus type 18 in oral potentially malignant disorders in Thailand. Asian Pacific J Cancer Prev. 2021;22:1875–81. doi: 10.31557/APJCP.2021.22.6.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Syrjänen S, Lodi G, Bultzingslowen I, Aliko A, Arduino P, Campisi G, et al. Human papillomaviruses in oral carcinoma and oral potentially malignant disorders : A systematic review. Oral Dis. 2011;17:58–72. doi: 10.1111/j.1601-0825.2011.01792.x. [DOI] [PubMed] [Google Scholar]
- 8.Poulopoulos A, Hempel M, Karakitsos G, Fakis A, Andreadis D. Assessment of HPV screening methods and sample storage in oral lichen planus lesions. Transl Res Oral Oncol. 2017;2:1–6. [Google Scholar]
- 9.Mattila R, Rautava J, Syrjänen S. Human papillomavirus in oral atrophic lichen planus lesions. Oral Oncol. 2012;48:980–4. doi: 10.1016/j.oraloncology.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Pattanshetty S, Kotrashetti VS, Nayak R, Bhat K, Somannavar P, Babji D. PCR based detection of HPV 16 and 18 genotypes in normal oral mucosa of tobacco users and non-users. Biotech Histochem. 2014;89:433–9. doi: 10.3109/10520295.2014.887143. [DOI] [PubMed] [Google Scholar]
- 11.Giovannelli L, Campisi G, Lama A, Giambalvo O, Osborn J, Margiotta V, et al. Human papillomavirus DNA in oral mucosal lesions. J Infect Dis. 2002;185:833–6. doi: 10.1086/339193. [DOI] [PubMed] [Google Scholar]
- 12.Arirachakaran P, Chansaengroj J, Lurchachaiwong W, Kanjanabud P, Thongprasom K, Poovorawan Y. Oral lichen planus in thai patients has a low prevalence of human papillomavirus. ISRN Dent. 2013;2013:362750. doi: 10.1155/2013/362750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vijayan A, Muthukrishnan A, Vidyadharan M, Nair A. Role of human papilloma virus in malignant transformation of oral lichen planus: A systematic review. J Pharm Bioallied Sci. 2021;13:S62–7. doi: 10.4103/jpbs.JPBS_836_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato S, Kawai R, Isomura M, Sato N, Yoshida W, Kamiya K. Human papillomavirus in oral lichen planus of japanese patients. J Hard Tissue Biol. 2015;24:181–8. [Google Scholar]
- 15.Subashini V, Ramani P, Anuja N, Sherlin HJ, Abhilasha R, Jayaraj G. Human papilloma virus -6, 16 in the pathogenesis of oral lichen planus -A systematic review. J Pharm Sci Res. 2017;9:2417–22. [Google Scholar]
- 16.Sameera A, Kotikalpudi R, Patel RK. Molecular detection of human papillomavirus DNA in oral lichen planus patients. J Clin Diagnostic Res. 2019;13:20–4. [Google Scholar]
- 17.Gorsky M, Epstein JB, Aviv T. Oral lichen planus: Malignant transformation and human papilloma virus : A review of potential clinical implications. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:461–4. doi: 10.1016/j.tripleo.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen H, Norrild JB, Vedtofte I, Praetorius F, Reibel J, Holmstrup I. Human papillomavirus in oral premalignant lesions. Eur J Cancer B Oral Oncol. 1996;32:264–70. doi: 10.1016/0964-1955(96)00011-5. [DOI] [PubMed] [Google Scholar]
- 19.Shroyer KR, Greer R., Jr Detection of human papillomavirus DNA by in situ DNA hybridization and polymerase chain reaction in premalignant and malignant oral lesiuns. Oral Surg Oral Med Oral Pathol. 1991;71:708–13. doi: 10.1016/0030-4220(91)90279-l. [DOI] [PubMed] [Google Scholar]
- 20.Campisi G, Giovannelli L, Arico P, Lama A, Di Liberto C, Ammatuna P, et al. HPV DNA in clinically different variants of oral leukoplakia and lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:705–11. doi: 10.1016/j.tripleo.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Farhadi S, Sadri D, Hashemi M. Archive of detection of human papillomavirus 33 in erosive oral lichen planus archive of SID. Int J Cancer Manag. 2020;13:e101488. [Google Scholar]
- 22.Sand L, Jalouli M, Larsson P, Hirsch J. Human papilloma viruses in oral lesions. Anticancer Res. 2000;20:1183–8. [PubMed] [Google Scholar]
- 23.Kumaraswamy K, Vidhya M. Human papilloma virus and oral infections : An update. J Cancer Res Ther. 2011;7:120–7. doi: 10.4103/0973-1482.82915. [DOI] [PubMed] [Google Scholar]


