Abstract
The fungal pathogen Candida albicans is naturally diploid, and current gene disruption strategies require two successive transformations. We describe here a genetic construct (UAU1) for which two copies may be selected. Insertion of UAU1 into one genomic site, after a single transformation, allows selection for segregants with two copies of the insertion. Major classes of segregants are those carrying homozygous insertion mutations and allelic triplications, which have two insertion alleles and a wild-type allele. Thus nonessential and essential genes may be distinguished rapidly through PCR tests for homozygosis and triplication. We find that homozygous mutations may be isolated at three nonessential loci (ADE2, RIM20, and YGR189), while only allelic triplications were found at two essential loci (SNF1 and CDC28). We have unexpectedly isolated homozygous mutants with mutations at CDC25; they are viable but defective in filamentation on serum-containing medium. The UAU1 cassette is thus useful to assess rapidly the essentiality of C. albicans genes.
Candida albicans is an opportunistic fungal pathogen. It has been of experimental interest for two main reasons. First, it is a significant pathogen that causes oral mucosal infections, vaginitis, nosocomial bloodstream infections, and a variety of deep tissue infections (26). Therefore, many experimental studies have focused on pathogenesis, drug resistance, and analysis of prospective drug targets. Second, it is a distant cousin of the most well-characterized unicellular eukaryote, Saccharomyces cerevisiae, so that the function of a C. albicans gene may be suggested by its role in S. cerevisiae. Yet, surprisingly, C. albicans may use gene products and regulatory pathways in novel ways (4, 16, 17, 28, 34, 37). The contrast between C. albicans and S. cerevisiae can provide unique insight into regulatory mechanisms, interpathway relationships, and general aspects of eukaryotic biology.
Molecular genetics has played an increasingly prominent role in studies of C. albicans, particularly with the partial genomic sequence as a tool for gene discovery. However, genetic methods are cumbersome for two reasons (30). First, C. albicans strains are diploid (or of higher ploidy) and there is no meiotic division, so that gene disruption mutants must be constructed through two successive transformations. Second, with current methods, disruption of one allele may be straightforward, but disruption of the second allele is infrequent if the second disruption construct is homologous to the first. These problems can make it difficult to construct a homozygous mutant and to determine whether a gene is essential for growth.
We describe here a genetic strategy that circumvents these difficulties. It yields homozygous insertion mutations after a single transformation. The strategy provides a rapid test for essential genes and should thus accelerate drug target validation. In addition, the strategy can provide a preliminary assessment of mutant phenotypes, and it lends itself to large-scale analysis of gene function.
MATERIALS AND METHODS
Plasmids.
The UAU1 (ura3-ARG4-ura3) (Fig. 1A) cassette is carried in plasmid pBME101, which was constructed as follows. Plasmid pRS424ARG4-URA3-BH1 (4) was digested with BsmI and SpeI, overhangs were blunted with Vent polymerase, and blunt ends were ligated to form plasmid pBME98. This plasmid was then digested with XhoI and SmaI, filled in, and religated to remove a polylinker ClaI site to yield plasmid pBME99. Five hundred base pairs of the 3′ end of URA3 was amplified from pRS424ARG4-URA3-BH1 with primers Kpn5′ and Xho3′-500 and ligated into the XhoI and KpnI sites of pBME99, generating plasmid pBME101. (Oligonucleotide sequences are listed in Table 1.)
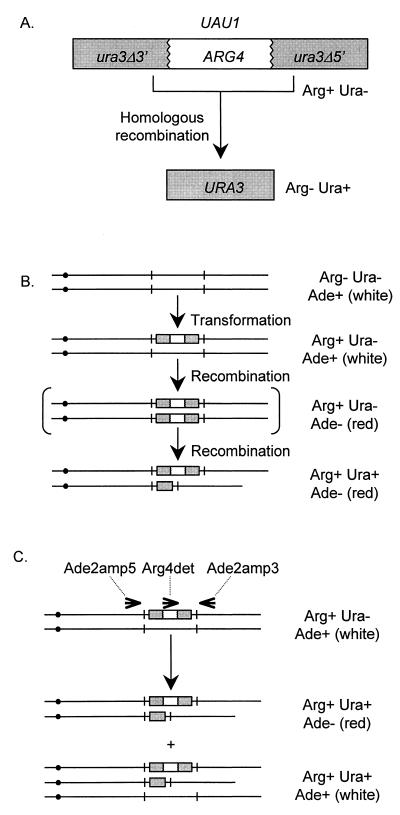
FIG. 1.
Genetic properties of UAU1. (A) Conversion of UAU1 to URA3. The UAU1 marker (top) comprises an intact ARG4 gene flanked by URA3 deletion derivatives ura3Δ3′ and ura3Δ5′. The URA3 segments are nonfunctional, so the UAU1 cassette confers an Arg+ Ura− phenotype. The URA3 segments share 530 bp of homology and can thus recombine to yield an intact URA3 gene. Recombination excises the ARG4 gene and results in an Arg− Ura+ phenotype. (B) Anticipated use of UAU1 to select for homozygous mutants (double-disruption selection). One allele of a gene (here ADE2) is disrupted with a UAU1 insertion through transformation with selection for an Arg+ phenotype. Growth of the transformant yields rare recombinant segregants in which the UAU1 insertion allele is homozygous. Such segregants may be selected after they undergo recombinational excision within one UAU1 cassette to yield a unique Arg+ Ura+ phenotype. (C) Outcome of double-disruption selection with an ade2::UAU1/ADE2 strain. Genotypes were determined with PCR primers depicted at the top. The ade2::UAU1/ADE2 strain yielded two classes of Arg+ Ura+ segregants. One class (homozygote) was Ade−, grew into red colonies, and yielded diagnostic ade2::UAU1 and ade2::URA3 PCR products (Fig. 2A, lanes 1, 2, 5, 7, and 9). The other class (allelic triplication) was Ade+, grew into white colonies, and yielded ade2::UAU1, ade2::URA3, and ADE2 PCR products (Fig. 2A, lanes 3, 4, 6, 8, and 10). The latter class is depicted as a trisome as an example, but it may arise through translocation, tandem duplication, or other genetic rearrangements.
TABLE 1.
Oligonucleotide sequences
| Oligo- nucleotide | Sequence (5′ to 3′) |
|---|---|
| 3-Detect | TGTGGAATTGTGAGCGGATAACAATTTCAC |
| 5-Detect | GTTTTCCCAGTCACGACGTTGTAAAACGAC |
| ade2-3DR | GGGTTGCCTTATCACCCAAGACATTCAACATAATAGCATTGGTGATGGAATGTGGAATTGTGAGCGGATA |
| ade2-5DR | GTCCATTATATGCTGAAAAATGGTGTCCTTTCACCAAAGAATTGGCTGTGGTTTTCCCAGTCACGACGTT |
| Ade2amp3 | CCATCTTTCGCTCTGGTCTAGTAG |
| Ade2amp5 | GTCGATGACTTGTTACACATTGGG |
| Arg4det | GGAATTGATCAATTATCTTTTGAAC |
| cdc25 3′DR | TAAATTTTACGTTTTTATTGATTCGCTCTACTGTACAAACACTATGCTTAGCAAATACTATGTGGAATTGTGAGCGGATA |
| cdc25 5′DR | AATATTATGAAACTTTTACTTGAAAAAAATTGGTCCATGTCGTATTACAATGAACCTGTAGTTTTCCCAGTCACGACGTT |
| Cdc25amp3 | CATTGTGTATTAGAAGTCTGTAGTTC |
| Cdc25amp5 | GGAATTGATATCTTTACTAATTGC |
| cdc28 3′DR | CAGACCACATATCTACCCCAGTGGAATATTGTTTCCCTCCTGTGGAATTGTGAGCGGATA |
| cdc28 5′DR | ATTCGAGGTATTAAACATTGTCATTCTCATCGAGTTTTACGTTTTCCCAGTCACGACGTT |
| cdc25del1 | TGAAAACGGTTTGAAACTCTTGA |
| cdc25del2 | CATGTGTGCCATTTTTTGGTGTG |
| cdc25del3 | TGTCAAATCAGATAAGTACACACCA |
| cdc25del4 | CCAATCAATTGACTAACTTTGTTGG |
| cdc25del5 | TATCAATGCTCTCACTGAGCCCTG |
| cdc25del6 | TTTGCTCACTCCGACCAAGTTC |
| Cdc28amp3 | CCCTGAATATCCTGAAAAGCAATCG |
| Cdc28amp5 | GAAGATGAAGGTGTACCTAGTACC |
| cdc28-C | CCCCTGAATATCCTGAAAAGCAATCG |
| cdc28-N | CCAACTATAGAACACACACATCCCAAGCC |
| Kpn5′ | GGGGGTACCTGATTTCTAGAAGGACCACC |
| Rim20amp3 | GATTCCATAAACCAGGTTTACTAG |
| Rim20amp5 | GAGTGTAATCATTTGTTGCAAGAG |
| SNF1 dr 3′ | TTGATTTCGATTGCTGAGACGATGAGTTTCCTGCAGCAACTGTGGAATTGTGAGCGGATA |
| SNF1 dr 5′ | GCAAGTTCCGATCGACCCCGCTGCAAATCCAGCAAATAGAGTTTTCCCAGTCACGACGTT |
| Snf1amp3 | GTCTTTCACCAATGCATGATTC |
| Snf1amp5 | CATAATGAAAATCAATCGCAAC |
| SNF1claI-5′ | CCATCGATGAATCAATATATAGAAGAAGG |
| SNF1ecoRI-3′ | GGGGAATTCGCTCATCTTTAATTAGTTTCG |
| Xho3′-500 | GGGCTCGAGCATCAATTTATGATTTTTGAAG |
| YGR189 3′DR | AGCTTGAAGAGGAGGAGGAAGATGATGATGATGATGATGAAGTGGTAGATGAAGGTGAGCGTGGAATTGTGAGCGGATA |
| YGR189 5′DR | CCATACTTATGTTATTGATTGGACCAAAGATGCAGTTACTTGGTCCGTTGACGGTAGTGTTTTCCCAGTCACGACGTT |
| Ygr189amp3 | GAACTGCATTGGATTTTCGC |
| Ygr189amp5 | CTACTACTTATGATCGTGGT |
Several of the gene disruption constructs described below were generated by in vivo recombination in S. cerevisiae, based on the method of Ma et al. (18). The key feature of this method is that linearization of a plasmid with CEN-ARS or 2 μm replicons causes a decrease in transformation efficiency in S. cerevisiae, but inclusion of a DNA fragment that bridges the linearization site restores efficient transformation. The retrieved plasmids largely contain the resealed plasmid carrying the bridging DNA fragment inserted through homologous recombination. We used this method to extend the homology that flanked gene disruption PCR products. We could thus use gene disruption primers with only 40 bases of homology to the targeted locus. The construction of plasmid pBME102, which carries the ade2::UAU1 gene disruption cassette, was typical. The UAU1 cassette was amplified by PCR from plasmid pBME101 with primers ade2-3DR and ade2-5DR (Table 1) to yield an ade2::UAU1 PCR product with 50 bp of ADE2 homology on each end. Approximately 1 μg of the 4.15-kbp PCR product, purified from an agarose gel, was cotransformed with 0.2 μg of EcoRI-linerized plasmid pRS314-ADE (44) into S. cerevisiae trp1 mutant strain AMP107 (36). EcoRI digestion linearized the plasmid by cleaving at a single site in the C. albicans ADE2 insert that lies between the regions of homology to primers ade2-3DR and ade2-5DR. Transformants were selected on SC-Trp plates, which selects for the plasmid TRP1 marker. A control transformation with no DNA yielded 0 colonies/plate, a control with linearized pRS314-ADE yielded 112 colonies/plate, and the cotransformation of linearized pRS314-ADE and the ade2::UAU1 PCR product yielded 773 colonies/plate. DNA prepared from Trp+ transformants (10) was transformed into Escherichia coli strain HB101 (Promega), with selection for ampicillin resistance. The structure of retrieved plasmid pBME102 was confirmed by restriction digestion.
The snf1::UAU1 gene disruption cassette was carried in plasmid pBME105. We first constructed plasmid pBME103 as follows: C. albicans genomic DNA was diluted 100-fold and PCR amplified with primers SNF1c1aI-5′ and SNF1ecoRI-3′, and the PCR product was ligated into plasmid pGEMT-Easy (Promega). We then moved the SNF1 insert as an EcoRI fragment into EcoRI-digested vector pRS424 (2) to create plasmid pBME104. Finally, plasmid pBME105 was generated by in vivo recombination of BsmI-linearized pBME104 and a snf1::UAU1 PCR product with 40 bp of SNF1 homology on each end, produced with template plasmid pBME101 and primers SNF1 dr 5′ and SNF1 dr 3′.
The cdc28::UAU1 cassette was carried in plasmid pBME108. We first constructed plasmid pBME100, which carries a 1.5-kbp clone of C. albicans CDC28, by PCR amplification of strain CAI4 DNA with primers cdc28-N and cdc28-C and ligation into vector pGEM-T (Promega). We also removed the XhoI site of vector pRS424 (2) by ligation of XhoI-digested and filled-in pRS424, yielding plasmid pBME106. We then constructed plasmid pBME107 by ligating the pBME100 insert, released with SacII and SpeI, into pBME106 digested with SacII and SpeI. Plasmid pBME108 was generated by in vivo recombination of XhoI-digested pRS424ΔXhoI-CDC28 and a cdc28::UAU1 PCR product produced from template plasmid pBME101 and primers cdc28 5′DR and cdc28 3′DR.
Strains.
The C. albicans strains (Table 2) are derivatives of strain BWP17 (ura3Δ:: λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG), which was derived from strain CAI4 (7) as described previously (44). The following UAU1 disruption heterozygotes were constructed through transformation of strain BWP17 with the restriction fragments indicated and selection for Arg+ transformants: strain BMY7, the NotI/EcoRV insert fragment from plasmid pBME 102; strain BMY18, the PvuII insert fragment from plasmid pBME105; and strain BMY22, the PvuII insert fragment from plasmid pBME108. The following UAU1 disruption heterozygotes were constructed through transformation of strain BWP17 with the PCR product indicated and selection for Arg+ transformants: strain BMY16, PCR with primers cdc25 5′dr and cdc25 3′dr on template pBME101; and strain DAY151, PCR with primers YGR189 5′DR and YGR 189 3′ DR on template pBME101. The UAU1 heterozygote BMY17 was constructed by homologous integration of the UAU1 cassette into URA3 sequences of a rim20::URA3 allele. Specifically, we transformed strain DAY18 (originally called Enx-het1 [44]), of genotype rim20::URA3/RIM20 ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG, with the PCR product of template pBME101 amplified with primers 5-Detect and 3-Detect, selected for Arg+ transformants, and screened for those that became Ura−. (This integration reaction is the reverse of the excision reaction diagrammed in Fig. 1A.) Integration of UAU1 cassettes in heterozygotes was confirmed through genomic DNA PCRs with the primer pairs listed in Table 2.
TABLE 2.
Double-disruption selection experiments
| Locus | Strain (genotypea) | Detection primers | Ura+ rate per divisionb | Arg+ Ura+ rate per divisionb | No. of homozygotesc |
|---|---|---|---|---|---|
| ADE2 | BMY7 (ade2::UAU1/ADE2) | Ade2amp5, Ade2amp3, Arg4det | 3.5 × 10−6 | 2.0 × 10−8 | 12 |
| YGR189 | DAY151 (ygr189::UAU1/YGR189) | Ygr189amp5, Ygr189amp3, Arg4det | 2.3 × 10−6 | 1.0 × 10−8 | 10 |
| RIM20 | BMY17 (rim20::UAU1/RIM20) | Rim20amp5, Rim20amp3, Arg4det | 1.5 × 10−5 | 6.8 × 10−8 | 11 |
| SNF1 | BMY18 (snf1::UAU1/SNF1) | Snflamp5, Snflamp3, Arg4det | 2.9 × 10−6 | 1.3 × 10−8 | 0 |
| CDC28 | BMY22 (cdc28::UAU1/CDC28) | Cdc28amp5, Cdc28amp3, Arg4det | 7.9 × 10−6 | 3.5 × 10−7 | 0 |
| CDC25 | BMY16 (cdc25::UAU1/CDC25) | Cdc25amp5, Cdc25amp3, Arg4det | 5.4 × 10−6 | 3.6 × 10−9 | 2 |
Strains carried the additional mutations ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG.
Recombination rates were calculated through the method of the median, as described in Materials and Methods.
The number of homozygous mutants found among 30 independent Arg+ Ura+ segregants from the heterozygote indicated. Genotypes were determined through PCR analysis, examples of which are shown in Fig. 2.
The extents of the deletion mutations are as follows. For ADE2, the deletion removes codons 195 to 310; the open reading frame (ORF) is 568 codons. For RIM20, the deletion removes codons 520 to 719; the ORF is 785 codons. For YGR189, the deletion removes codons 180 to 316; the ORF is 453 codons. For SNF1, the deletion removes codons 20 to 358; the ORF is 620 codons. For CDC28, the deletion removes codons 131 to 181; the ORF is 317 codons. For CDC25, the deletion removes codons 1000 to 1333; the ORF is 1333 codons. All of these deletions remove highly conserved regions of the respective proteins, including key residues required for SNF1 and CDC28 protein kinase activity. The deletion of CDC25 removes the GDP-GTP exchange factor domain (15), which is the most highly conserved region of the protein.
Media and growth conditions.
C. albicans and S. cerevisiae were routinely cultured in YPD plus uridine (2% Bacto Peptone, 1% yeast extract, 20% dextrose, and 80 μg uridine per ml). Selection was done on SD synthetic medium (6.7% yeast nitrogen base plus ammonium sulfate, without amino acids, and with 2% dextrose); auxotrophic supplements were added at standard concentrations (14) except that uridine was added at 80 μg per ml.
PCR detection.
Genotypes at each locus were determined through PCR assays with the primers indicated in Table 2. Flanking primers (illustrated by Ade2amp5 and Ade2amp3 in Fig. 1C) were used to detect wild-type alleles and URA3 insertion alleles. However, we found that detection of UAU1 insertion alleles was unreliable with flanking primers, presumably because of the more efficient synthesis of the smaller products from wild-type and URA3 insertion alleles. Therefore, we used a flanking primer and an internal ARG4 primer (illustrated by Ade2amp3 and Arg4det in Fig. 1C) in a second PCR to detect the presence of UAU1 insertion alleles. PCR was performed with total yeast genomic DNA as described previously (44). Reaction mixtures were typically heated to 94°C for 5 min, followed by 33 cycles of 94°C for 1 min, 50°C for 1 min, and 72°C for 3 min. After a final incubation at 72°C for 10 min, reaction mixtures were stored at 4°C.
Determination of recombination rates.
Recombination rates were determined by the method of Lea and Coulson, as described by Rattray and Symington (33). A UAU1 insertion heterozygote was streaked onto YPD and grown at 30°C for 2 to 3 days. Nine entire single colonies were used to inoculate separate 3-ml YPD broth cultures that were grown to saturation at 30°C. One milliliter of saturated culture was washed, suspended in 1 ml of sterile water, and diluted for plating. One hundred microliters of the undiluted suspension was plated on SC-Arg-Ura, 100 μl of a 1/100 dilution was plated on SC-Ura, and 20 μl (in a total volume of 200 μl) of a 1/10,000 dilution was plated on YPD. Colony counts of these plates were used to determine the median mitotic recombinant frequencies (Ura+/total or Ura+ Arg+/total) for each strain. Recombination rates (events per cell per generation) were calculated according to the formula rate = (0.4343 × median frequency)/(log N − log N0), where N is the total number of cells in the culture and N0 is the initial number of cells (one cell) that gave rise to the culture (33).
RESULTS
Selection for homozygous mutants.
We constructed a gene disruption cassette, UAU1 (ura3-ARG4-ura3), that can exist in two states (Fig. 1A). The cassette includes three segments: ura3Δ3′, a nonfunctional 3′ deletion copy of URA3; ARG4; and ura3Δ5′, a nonfunctional 5′ deletion copy of URA3 that shares 530 bp of sequence identity with the ura3Δ3′ segment. The cassette expresses ARG4, but not URA3, in this state (UAU1). The 530 bp of homology between the ura3 segments permits recombination to yield an intact URA3 gene and excision of ARG4. The resulting cassette expresses URA3 but not ARG4. We reasoned that the cassette would permit identification of homozygous mutants (Fig. 1B). A heterozygous UAU1 insertion, introduced by transformation, might occasionally become homozygous through gene conversion or a mitotic recombination event. The homozygous mutant would be uniquely capable of yielding segregants that express both ARG4 and URA3. Thus, homozygous mutants should be found among Arg+ Ura+ segregants in a population carrying a UAU1 insertion.
We used this rationale to construct homozygous ade2/ade2 mutants after one transformation of reference strain BWP17 (relevant genotype: arg4/arg4 ura3/ura3 ADE2/ADE2). The strain was transformed with an ade2::UAU1 DNA fragment. An Arg+ transformant (strain BMY7) had the genotype ade2::UAU1/ADE2, as verified by PCR and Southern blot analyses (Fig. 2A, lane H, and data not shown). Strain BMY7 yielded primarily white Ade+ colonies, and loss of ADE2 function creates red Ade− colonies. We grew 30 independent cultures of the heterozygote and plated aliquots to isolate Ura+ and Arg+ Ura+ segregants (Table 2). Ura+ segregants arose a rate of 3.5 × 10−6 per division as uniformly white Ade+ colonies. Arg+ Ura+ segregants arose at a rate of 2.0 × 10−8 per division. Among the Arg+ Ura+ colonies, 23% were red and Ade−. We used PCR analysis to determine the genotype of one randomly chosen Arg+ Ura+ segregant from each culture (Fig. 2B, lanes 1 to 10, and data not shown). We found 12 segregants of genotype ade2::UAU1/ade2::URA3, and these were phenotypically red and Ade−. The 18 remaining segregants were of genotype ade2::UAU1/ade2::URA3/ADE2 and were phenotypically white and Ade+. These latter Arg+ Ura+ Ade+ segregants thus have three copies of the ADE2 locus; the third copy may derive from trisomy or translocation (Fig. 1C). Our results indicate that homozygous mutants can be isolated after a single transformation.
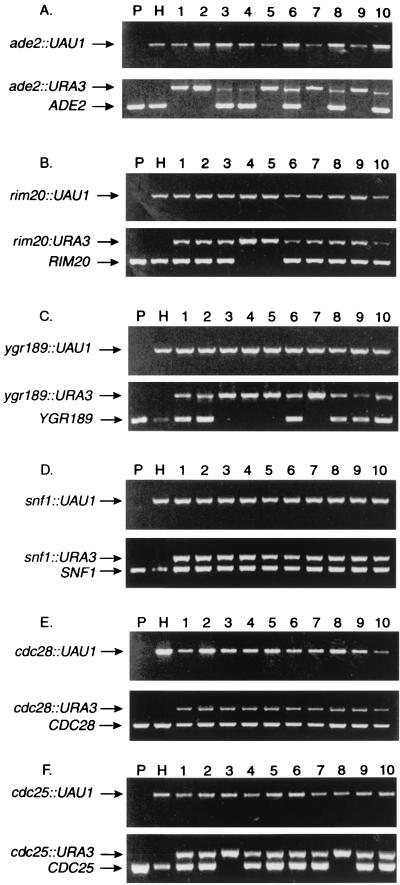
FIG. 2.
PCR analysis of double-disruption selection segregants. Each panel shows a composite of two agarose gels of PCRs conducted with genomic DNA templates. The upper-gel PCRs used amp3 and Arg4det primers; the lower-gel PCRs used amp3 and amp5 primers. (The amp3–amp5 PCRs are unreliable for detection of full-length UAU1 insertions, presumably because the smaller PCR products are amplified more efficiently.) Lanes: templates from parent strain BWP17 (lanes P), the respective UAU1 disruption heterozygote (lanes H), and 10 independent Arg+ Ura+ segregants from the disruption heterozygote (lanes 1 to 10). Panels show analyses for ADE2 (A), RIM20 (B), YGR189 (C), SNF1 (D), CDC28 (E), and CDC25 (F).
To determine whether this homozygosis strategy may be useful at other loci, we examined the genes RIM20 and YGR189. Previously constructed rim20/rim20 mutants are viable, so this gene is not essential (4). We constructed rim20::UAU1/RIM20 and ygr189::UAU1/YGR189 heterozygotes through one transformation (strains BMY17 and DAY151, respectively) and examined 30 independent cultures of each for production of Ura+ and Arg+ Ura+ segregants (Table 2). For both strains, these segregants arose at rates similar to those observed with the ade2::UAU1/ADE2 heterozygote. PCR analysis of genotype was again carried out on one Arg+ Ura+ segregant per culture (Figs. 2B and C and data not shown). For both genes, we found that one-third of the segregants were rim20::UAU1/rim20::URA3 or ygr189::UAU1/ygr189::URA3 homozygotes, respectively, and two-thirds were rim20::UAU1/rim20::URA3/RIM20 or ygr189::UAU1/ygr189::URA3/YGR189 triplication derivatives, respectively (Table 2). The rim20/rim20 homozygotes had a filamentation defect, as found for conventionally constructed homozygotes (4); the ygr189/ygr189 homozygotes had no obvious phenotype. Therefore, homozygous mutants have been isolated by selection from UAU1 insertion heterozygotes at three different C. albicans loci.
Homozygote-trisome (HT) test for essential genes.
Identification of essential genes in C. albicans is vital to assess prospective drug targets (30) and to consider more-refined functional tests (1). C. albicans genes have been considered essential if one allele can be disrupted but the second cannot. We reasoned that the UAU1 cassette might simplify and accelerate this assessment: a UAU1 insertion in nonessential genes yields both homozygous and triplication-bearing segregants, but a UAU1 insertion in an essential gene should yield only triplication-bearing segregants. Thus, we examined the consequences of UAU1 insertions in three likely essential genes: SNF1, CDC28, and CDC25.
Petter et al. have presented strong experimental evidence that SNF1 is essential for viability (29). We created a snf1::UAU1/SNF1 heterozygote and found that it produced Ura+ and Arg+ Ura+ segregants at rates similar to those for ADE2 and YGR189 insertions (Table 2). PCR genotyping of 30 independent Arg+ Ura+ segregants revealed that all were snf1::UAU1/snf1::URA3/SNF1 triplication derivatives (Table 2; Fig. 2D). Our failure to obtain snf1/snf1 homozygotes is consistent with the conclusion that SNF1 is an essential gene.
CDC28 specifies a cyclin-dependent protein kinase, and activity of such kinases is vital for cell cycle progression in all eukaryotes (21). The C. albicans CDC28 gene complements corresponding defects in other yeasts (3, 35), so we inferred that C. albicans CDC28 may be essential. A cdc28::UAU1/CDC28 heterozygote produced Ura+ and Arg+ Ura+ segregants at rates comparable to those for other insertion heterozygotes; PCR genotyping revealed that 30 independent Arg+ Ura+ segregants were cdc28::UAU1/cdc28::URA3/CDC28 triplication derivatives (Table 2; Fig. 2E). These results support the idea that C. albicans CDC28 is an essential gene.
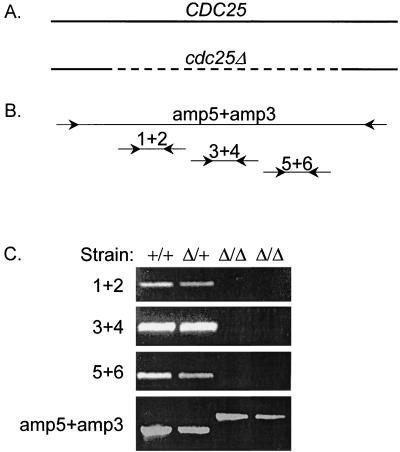
The S. cerevisiae CDC25 gene is essential for viability; Cdc25p is an activator of Ras proteins (39). C. albicans CDC25 complements an S. cerevisiae cdc25 mutant (8), so we inferred that CDC25 may be essential in C. albicans as well. A cdc25::UAU1/CDC25 heterozygote produced Ura+ segregants at rates comparable to those for other insertion heterozygotes and produced Arg+ Ura+ segregants at a slightly lower rate (Table 2). PCR genotyping revealed that 2 of 30 independent Arg+ Ura+ segregants were of genotype cdc25::UAU1/cdc25::URA3 (Fig. 2F, lanes 3 and 8); the remaining 28 segregants were cdc25::UAU1/cdc25::URA3/CDC25 triplication derivatives (Table 2; Fig. 2F). In principle, the DNA segment deleted from the CDC25 locus might have been translocated to a different region of the genome in the two segregants. Such a translocation would be undetected in flanking-primer PCRs but should be detected with PCRs of internal segments. However, three internal primer pairs yielded no PCR product from genomic templates prepared from the cdc25::UAU1/cdc25::URA3 segregants (Fig. 3). Therefore, CDC25 is not essential for viability in C. albicans. A recent study indicates that C. albicans Ras2p is a positive regulator of serum-induced filamentation (6). The two cdc25/cdc25 homozygotes grew at a slightly reduced rate and were partially defective in filamentation on agar containing serum. These findings support the idea that Cdc25p promotes Ras2p activity and filamentation but indicate that CDC25 is not an essential gene.
FIG. 3.
PCR analysis of cdc25Δ/cdc25Δ segregants. (A) Schematic diagram of wild-type and mutant CDC25 loci. The solid line represents the genomic CDC25 sequence. The dashed line represents the sequence deleted and replaced with UAU1 and URA3 in the cdc25::UAU1 and cdc25::URA3 alleles. (B) Locations of PCR primers. Each line represents a PCR amplification product that derives from the CDC25 sequences directly above (in panel A). The primer pair amp5 + amp3 (Cdc25amp5 and Cdc25amp3) can amplify wild-type and mutant CDC25 alleles. The primer pairs 1+2 (cdc25del1 and cdc25del2), 3+4 (cdc25del3 and cdc25del4), and 5+6 (cdc25del5 and cdc25del6) amplify regions that are deleted by the cdc25::UAU1 and cdc25::URA3 alleles. (C) PCR products from genomic templates. Genomic templates derived from strains BWP17 (CDC25/CDC25), BMY16 (cdc25::UAU1/CDC25), and the two cdc25::UAU1/cdc25::URA3 segregants were amplified by PCR with the primer pairs indicated, and PCR products were visualized after agarose gel electrophoresis.
DISCUSSION
Analysis of gene function in C. albicans is a critical step toward understanding its biology, pathogenesis, and vital processes. In the past, this analysis has required two successive transformations to create homozygous null mutations or conditionally expressed alleles (1, 7, 30, 43). These methods allow construction of precisely defined mutant strains that can be characterized via a battery of refined phenotypic tests. Here, we have described a double disruption selection strategy with a different purpose: to construct homozygous mutants rapidly for a preliminary assessment of gene function. Our strategy is not a replacement or a substitute for the careful crafting of homozygous mutants. Instead, it is a strategy that lends itself to large-scale analysis of gene function.
We have presented here an application of double-disruption selection that facilitates one important goal of C. albicans research: the identification of essential genes. We refer to this test as the HT test. The methodology fills an important need for basic research, because it is otherwise laborious to determine whether a gene is essential (29, 30), and current technology can yield conflicting results (5, 16, 22, 38). The methodology also fills an important need for genome-based pharmaceutical research, in which one must evaluate a large number of gene products as prospective drug targets (23). Finally, we expect that the technical simplicity of our method may encourage those who study a conserved gene in some other organism to test the homologous gene's function in C. albicans.
The results of HT tests were largely consistent with expectations based on prior studies: SNF1 and CDC28 are essential; ADE2 and RIM20 are not. However, the test also argued that CDC25 is not essential, because homozygous cdc25/cdc25 mutants were isolated. (The deletion that we constructed removes much of the domain that is necessary and sufficient for GDP-GTP exchange factor activity [15], so we can formally conclude only that Cdc25p exchange factor activity is not essential.) We note, though, that the cdc25/cdc25 homozygotes arose a lower rate than homozygous insertion mutations in other nonessential genes. This observation might be explained if viability of the cdc25/cdc25 mutant depended upon a genetic suppressor mutation, as is the case in S. cerevisiae (39). A second possibility is that CDC25 is completely dispensable, as is likely the case in Schizosaccharomyces pombe (19), but that the structure or position of the locus limits recombination. We note that the same ambiguities apply to traditional (two-transformation) gene disruption strategies: infrequent isolation of a targeted mutant may reflect either the inadvertent selection of the genetic suppressor or recombinational, rather than functional, properties of the locus. The ambiguities may be resolved by protein depletion experiments or use of dominant-negative mutants (1, 6). Thus, the advantage of the HT test is that it provides functional information rapidly that can justify more definitive but laborious experiments.
There is a circumstance—the case of preexisting triplicated alleles—in which the HT test might yield misleading results. There are three loci for which triplicated alleles have been reported, CHS2, FTR1, and FKS1 (GSC1) (9, 22, 32), although FKS1 (GSC1) is apparently not triplicated in all isolates of strain CAI4 (5). One can envision that insertion of the UAU1 cassette into CHS2, for example, would yield a genetic structure consistent with a genotype of chs2::UAU1/CHS2; the actual genotype would be chs2::UAU1/CHS2/CHS2. Subsequent Arg+ Ura+ selection would yield exclusively segregants of genotype chs2::UAU1/chs2::URA3/CHS2, not because CHS2 is essential but because the strain initially had triplicated alleles. Thus far, preexisting triplications have seldom been observed in C. albicans, so we expect that this limitation for the HT test will not outweigh its usefulness.
Our results with double-disruption selection raise an important question that we have not yet resolved: how large a DNA segment becomes homozygous in the Arg+ Ura+ homozygous disruption mutants? Given that natural C. albicans isolates are heterozygous for preexisting mutations (31, 40–42), it is possible that selection for homozygosity of a UAU1 insertion mutation may yield homozygosity of a linked mutation as well. In our small survey of known nonessential genes, we found only homozygotes that had phenotypes consistent with traditionally constructed mutants. However, the possibility that a linked mutation may become homozygous remains an important caveat for any conclusion derived solely from double-disruption selection.
Our estimates of mitotic recombination rates point to a surprising conclusion: that heterozygous mutations become homozygous at a rate of 2 × 10−3 per division. We calculate this rate from the rate of production of detectable homozygotes (∼1 × 10−8 per division) and the rate of recombination to generate URA3 from UAU1 (∼3 × 10−6 per division). This homozygosis rate is 102- to 103-fold higher than expected from studies of S. cerevisiae (24) and violates anecdotal common knowledge derived from use of Ura-blaster cassettes to create gene disruptions (7, 43). One simple explanation is that our estimate of the rate of recombination to generate URA3 from UAU1 may be artificially low because of a selective advantage of Arg+ cells during growth in broth culture. However, in coculture experiments, we have not detected such an advantage (B. Enloe and A. P. Mitchell, unpublished results). A second explanation is that our frequency estimates are in error because of differences in growth dynamics on the selective plates. Thus, for example, Ura+ colonies might arise only from preexisting recombinants, while Arg+ Ura+ colonies might arise during growth after plating. A third explanation is that the event that generates URA3 in a UAU1 insertion heterozygote is not equivalent to the event that generates URA3 in a homozygote or triplication derivative. For example, a single recombination event might generate two copies of the insertion mutation and simultaneously promote conversion of UAU1 to URA3 in one of the copies. Therefore, our rate estimates serve as an empirical guide, but we remain skeptical that interchromosomal recombination is so frequent in C. albicans.
Allelic triplications were detectable for all of the genes we examined, and we are uncertain of their genetic structure. One simple possibility is that they result from trisomy for the respective chromosome, perhaps in conjunction with an overall increase in ploidy. In support of this idea, we note that several different selections yield monosomic and trisomic C. albicans derivatives (12, 13, 27). A second possibility is that the triplications result from tandem duplication or translocation of a smaller genomic segment, a mechanism that also has experimental support (11, 20, 25). The UAU1 cassette may be a useful tool to define the genetic and environmental parameters that influence changes in gene dosage through either of these mechanisms.
ACKNOWLEDGMENTS
We are grateful to Dana Davis, Teresa Lamb, Vincent Bruno, and Wenjie Xu for many helpful discussions and for comments on the manuscript and to an anonymous referee for suggesting that CDC25 may have undergone a transposition.
We thank the Burroughs Wellcome Fund (Mycology Scholar Award) and the NIH (grant PO1 AI37194) for financial support.
REFERENCES
- 1.Care R S, Trevethick J, Binley K M, Sudbery P E. The MET3 promoter: a new tool for Candida albicans molecular genetics. Mol Microbiol. 1999;34:792–798. doi: 10.1046/j.1365-2958.1999.01641.x. [DOI] [PubMed] [Google Scholar]
- 2.Christianson T W, Sikorski R S, Dante M, Shero J H, Hieter P. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992;110:119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- 3.Damagnez V, Cottarel G. Candida albicans CDK1 and CYB1: cDNA homologues of the cdc2/CDC28 and cdc13/CLB1/CLB2 cell cycle control genes. Gene. 1996;172:137–141. doi: 10.1016/0378-1119(95)00893-4. [DOI] [PubMed] [Google Scholar]
- 4.Davis D, Wilson R B, Mitchell A P. RIM101-dependent and -independent pathways govern pH responses in Candida albicans. Mol Cell Biol. 2000;20:971–978. doi: 10.1128/mcb.20.3.971-978.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douglas C M, D'Ippolito J A, Shei G J, Meinz M, Onishi J, Marrinan J A, Li W, Abruzzo G K, Flattery A, Bartizal K, Mitchell A, Kurtz M B. Identification of the FKS1 gene of Candida albicans as the essential target of 1,3-beta-d-glucan synthase inhibitors. Antimicrob Agents Chemother. 1997;41:2471–2479. doi: 10.1128/aac.41.11.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng Q, Summers E, Guo B, Fink G. Ras signaling is required for serum-induced hyphal differentiation in Candida albicans. J Bacteriol. 1999;181:6339–6346. doi: 10.1128/jb.181.20.6339-6346.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fonzi W A, Irwin M Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberg D, Marbach I, Gross E, Levitzki A, Simchen G. A Candida albicans homolog of CDC25 is functional in Saccharomyces cerevisiae. Eur J Biochem. 1993;213:195–204. doi: 10.1111/j.1432-1033.1993.tb17748.x. [DOI] [PubMed] [Google Scholar]
- 9.Gow N A, Robbins P W, Lester J W, Brown A J, Fonzi W A, Chapman T, Kinsman O S. A hyphal-specific chitin synthase gene (CHS2) is not essential for growth, dimorphism, or virulence of Candida albicans. Proc Natl Acad Sci USA. 1994;91:6216–6220. doi: 10.1073/pnas.91.13.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffman C S, Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- 11.Iwaguchi S I, Kanbe T, Tohne T, Magee P T, Suzuki T. High-frequency occurrence of chromosome translocation in a mutant strain of Candida albicans by a suppressor mutation of ploidy shift. Yeast. 2000;16:411–422. doi: 10.1002/(SICI)1097-0061(20000330)16:5<411::AID-YEA532>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 12.Janbon G, Sherman F, Rustchenko E. Appearance and properties of l-sorbose-utilizing mutants of Candida albicans obtained on a selective plate. Genetics. 1999;153:653–664. doi: 10.1093/genetics/153.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janbon G, Sherman F, Rustchenko E. Monosomy of a specific chromosome determines l-sorbose utilization: a novel regulatory mechanism in Candida albicans. Proc Natl Acad Sci USA. 1998;95:5150–5155. doi: 10.1073/pnas.95.9.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaiser C, Michaelis S, Mitchell A. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 15.Lai C C, Boguski M, Broek D, Powers S. Influence of guanine nucleotides on complex formation between Ras and CDC25 proteins. Mol Cell Biol. 1993;13:1345–1352. doi: 10.1128/mcb.13.3.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lo H J, Kohler J R, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink G R. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 17.Loeb J D, Sepulveda-Becerra M, Hazan I, Liu H. A G1 cyclin is necessary for maintenance of filamentous growth in Candida albicans. Mol Cell Biol. 1999;19:4019–4027. doi: 10.1128/mcb.19.6.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma H, Kunes S, Schatz P J, Botstein D. Plasmid construction by homologous recombination in yeast. Gene. 1987;58:201–216. doi: 10.1016/0378-1119(87)90376-3. [DOI] [PubMed] [Google Scholar]
- 19.Maeda T, Mochizuki N, Yamamoto M. Adenylyl cyclase is dispensable for vegetative cell growth in the fission yeast Schizosaccharomyces pombe. Proc Natl Acad Sci USA. 1990;87:7814–7818. doi: 10.1073/pnas.87.20.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magee P T. Variations in chromosome size and organization in Candida albicans and Candida stellatoidea. Trends Microbiol. 1993;1:338–342. doi: 10.1016/0966-842x(93)90074-2. [DOI] [PubMed] [Google Scholar]
- 21.Mendenhall M D, Hodge A E. Regulation of Cdc28 cyclin-dependent protein kinase activity during the cell cycle of the yeast Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1998;62:1191–1243. doi: 10.1128/mmbr.62.4.1191-1243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mio T, Adachi-Shimizu M, Tachibana Y, Tabuchi H, Inoue S B, Yabe T, Yamada-Okabe T, Arisawa M, Watanabe T, Yamada-Okabe H. Cloning of the Candida albicans homolog of Saccharomyces cerevisiae GSC1/FKS1 and its involvement in beta-1,3-glucan synthesis. J Bacteriol. 1997;179:4096–4105. doi: 10.1128/jb.179.13.4096-4105.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moir D T, Shaw K J, Hare R S, Vovis G F. Genomics and antimicrobial drug discovery. Antimicrob Agents Chemother. 1999;43:439–446. doi: 10.1128/aac.43.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montelone B A, Hoekstra M F, Malone R E. Spontaneous mitotic recombination in yeast: the hyper-recombinational rem1 mutations are alleles of the RAD3 gene. Genetics. 1988;119:289–301. doi: 10.1093/genetics/119.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navarro-Garcia F, Perez-Diaz R M, Magee B B, Pla J, Nombela C, Magee P. Chromosome reorganization in Candida albicans 1001 strain. J Med Vet Mycol. 1995;33:361–366. [PubMed] [Google Scholar]
- 26.Odds F C. Candida and candidosis. Philadelphia, Pa: Bailliere Tindall; 1988. [Google Scholar]
- 27.Perepnikhatka V, Fischer F J, Niimi M, Baker R A, Cannon R D, Wang Y K, Sherman F, Rustchenko E. Specific chromosome alterations in fluconazole-resistant mutants of Candida albicans. J Bacteriol. 1999;181:4041–4049. doi: 10.1128/jb.181.13.4041-4049.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez-Martin J, Uria J A, Johnson A D. Phenotypic switching in Candida albicans is controlled by a SIR2 gene. EMBO J. 1999;18:2580–2592. doi: 10.1093/emboj/18.9.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petter R, Chang Y C, Kwon-Chung K J. A gene homologous to Saccharomyces cerevisiae SNF1 appears to be essential for the viability of Candida albicans. Infect Immun. 1997;65:4909–4917. doi: 10.1128/iai.65.12.4909-4917.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pla J, Gil C, Monteoliva L, Navarro-Garcia F, Sanchez M, Nombela C. Understanding Candida albicans at the molecular level. Yeast. 1996;12:1677–1702. doi: 10.1002/(SICI)1097-0061(199612)12:16%3C1677::AID-YEA79%3E3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 31.Poulter R T. Natural auxotrophic heterozygosity in Candida albicans. Crit Rev Microbiol. 1987;15:97–101. doi: 10.3109/10408418709104452. [DOI] [PubMed] [Google Scholar]
- 32.Ramanan N, Wang Y. A high-affinity iron permease essential for Candida albicans virulence. Science. 2000;288:1062–1064. doi: 10.1126/science.288.5468.1062. [DOI] [PubMed] [Google Scholar]
- 33.Rattray A J, Symington L S. Use of a chromosomal inverted repeat to demonstrate that the RAD51 and RAD52 genes of Saccharomyces cerevisiae have different roles in mitotic recombination. Genetics. 1994;138:587–595. doi: 10.1093/genetics/138.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santos M A, Ueda T, Watanabe K, Tuite M F. The non-standard genetic code of Candida spp.: an evolving genetic code or a novel mechanism for adaptation? Mol Microbiol. 1997;26:423–431. doi: 10.1046/j.1365-2958.1997.5891961.x. [DOI] [PubMed] [Google Scholar]
- 35.Sherlock G, Bahman A M, Mahal A, Shieh J C, Ferreira M, Rosamond J. Molecular cloning and analysis of CDC28 and cyclin homologues from the human fungal pathogen Candida albicans. Mol Gen Genet. 1994;245:716–723. doi: 10.1007/BF00297278. [DOI] [PubMed] [Google Scholar]
- 36.Smith H E, Su S S, Neigeborn L, Driscoll S E, Mitchell A P. Role of IME1 expression in regulation of meiosis in Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:6103–6113. doi: 10.1128/mcb.10.12.6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Staab J F, Bradway S D, Fidel P L, Sundstrom P. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science. 1999;283:1535–1538. doi: 10.1126/science.283.5407.1535. [DOI] [PubMed] [Google Scholar]
- 38.Stoldt V R, Sonneborn A, Leuker C E, Ernst J F. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 1997;16:1982–1991. doi: 10.1093/emboj/16.8.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thevelein J M, de Winde J H. Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol Microbiol. 1999;33:904–981. doi: 10.1046/j.1365-2958.1999.01538.x. [DOI] [PubMed] [Google Scholar]
- 40.Whelan W L, Magee P T. Natural heterozygosity in Candida albicans. J Bacteriol. 1981;145:896–903. doi: 10.1128/jb.145.2.896-903.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whelan W L, Soll D R. Mitotic recombination in Candida albicans: recessive lethal alleles linked to a gene required for methionine biosynthesis. Mol Gen Genet. 1982;187:477–485. doi: 10.1007/BF00332632. [DOI] [PubMed] [Google Scholar]
- 42.White T C. The presence of an R467K amino acid substitution and loss of allelic variation correlate with an azole-resistant lanosterol 14alpha demethylase in Candida albicans. Antimicrob Agents Chemother. 1997;41:1488–1494. doi: 10.1128/aac.41.7.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson R B, Davis D, Enloe B M, Mitchell A P. A recyclable Candida albicans URA3 cassette for PCR product-directed gene disruptions. Yeast. 2000;16:65–70. doi: 10.1002/(SICI)1097-0061(20000115)16:1<65::AID-YEA508>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 44.Wilson R B, Davis D, Mitchell A P. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J Bacteriol. 1999;181:1868–1874. doi: 10.1128/jb.181.6.1868-1874.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]