Abstract
Background:
Every year, around 2 million people suffer from hospital-acquired infections worldwide. Aerosols are produced while performing ultrasonic scaling. They are potential source of infection and cross contamination. It can result in spread of several infections including hepatitis, HIV, SARS CoV 2, etc. Preprocedural rinse before scaling is considered an effective measure to reduce the microbial load in aerosols.
Materials and Methods:
This study was a triple-blinded randomized controlled trial performed on 90 participants diagnosed with chronic periodontitis. Random allocation of participants into three groups, i.e., Group-1, Group-2, and Group-3, was done, wherein 0.12% chlorhexidine (CHX), 1.5% hydrogen peroxide (HP), and distilled water (DW) were used, respectively, as preprocedural mouthrinse. The participants of each group were instructed to perform mouthrinse for 60 s before the start of ultrasonic scaling sessions. Blood agar plates were kept at three locations, i.e., operator's chest area, patient's chest area, and assistant's chest area for aerosol collection after scaling. Colony-forming units (CFUs) were counted for microbiological analysis.
Results:
Least number of CFUs was found in the CHX group, followed by HP and DW groups. Location wise, the patient's chest area had the highest CFU count and the least was at the assistant's chest area. CFU count between the groups was statistically significant.
Conclusion:
Preprocedural rinse using HP can effectively be used as a method to reduce dental aerosols generated during ultrasonic scaling.
KEYWORDS: Aerosols, chlorhexidine, hydrogen peroxide, mouthrinse, preprocedural rinse, ultrasonic scaling
INTRODUCTION
Dental aerosols are any solid or liquid particles suspended in air by human, animal, machine, or instrument. Aerosol contains small particle size of 1–5 μm.[1] Such particles can remain viable for several hours after they are dispersed in the air and can transport long distances over a range of 1 meter. They can cause several infections such as respiratory, ophthalmic, skin, and hepatitis.[2] Zemouri et al. in a review showed that as many as 38 types of microorganisms could be found in the air of a dental clinic.[3] Ultrasonic scaling produces a significant amount of aerosols which could lead to cross-infection as well as spread of infection. The Centers for Disease Control and Prevention recommends the use of rubber dam placement, high-velocity air evacuation, and proper patient positioning as methods of reducing aerosols during ultrasonic scaling.[4] The most commonly used mouthrinse for preprocedural rinsing is chlorhexidine (CHX). CHX of concentration of 0.2% or 0.12% has broad-spectrum antimicrobial activity. The disadvantages Chlorhexidine rinses are unpleasant taste, enhanced supragingival calculus formation, altered taste sensation, mucosal dryness, etc.[5] This gives us a scope to search for a better preprocedural rinse having similar antimicrobial spectrum and lesser side effects.
Hydrogen peroxide (HP) is a potent oxidizing agent. It helps in plaque control by exerting antimicrobial effects against Gram-positive and Gram-negative microorganisms.[6] It kills the microorganism by release of oxygen. Apart from its antiplaque action, at 3% concentration, it is used in the treatment of necrotizing ulcerative gingivitis. It was also introduced to reduce microorganisms in the periodontal pocket as a combination of HP with baking soda and sodium chloride irrigation.[7] A systematic review on effectiveness of preprocedural rinses suggested that regular use of preprocedural rinses can reduce aerosol load during dental procedures.[8] Reis et al. stated that HP mouthrinse may be beneficial in reducing SARS-CoV-2 viral load.[9] Ramesh et al. in a study showed that the combination of HP with CHX is effective compared to CHX alone in reducing aerosol contamination.[10] However, there is very limited literature available wherein HP and CHX were compared. Therefore, the present study was designed to compare the antimicrobial efficacy of HP and CHX mouth as preprocedural mouthrinse.
MATERIALS AND METHODS
Participants of this triple-blinded, randomized clinical trial were selected from the Outpatient Department of Periodontology, Haldia Institute of Dental Sciences and Research, Haldia, West Bengal, from October 2020 to June 2021. The protocol of the study was reviewed and approved by the Institutional Ethics Committee. Inclusion criteria were (i) systemically healthy individuals of 25–55 years of age and are diagnosed with mild-to-moderate chronic periodontitis with at least 20 natural teeth present in the oral cavity, (ii) mean plaque index (PI)[11] score of 2.0–3.0, and (iii) the presence of four or more sites with probing pocket depth ≥4 mm [Table 1]. Participants with a history of allergy or hypersensitivity to any ingredients of CHX gluconate and HP, smoking, lactation, pregnancy, and intake of any topical or systemic antibiotics in the past 6 months were excluded from the study.
Table 1.
Baseline demographic and clinical characteristics of each Group
| Group 1 (0.12% CHX) | Group 2 (1.5% HP) | Group 3 (DW) | P | |
|---|---|---|---|---|
| Age | 38.42±5.13 | 40.32±5.32 | 39.12±6.32 | NS |
| Male/female | 14/16 | 17/13 | 17/13 | NS |
| Number of teeth | 27.22±1.23 | 26.12±1.47 | 26.34±1.32 | NS |
| PI | 2.53±0.04 | 2.62±0.08 | 2.57±0.12 | NS |
PI: Plaque index, CHX: Chlorhexidine, HP: Hydrogen peroxide, NS: Nonsignificant, DW: Distilled water
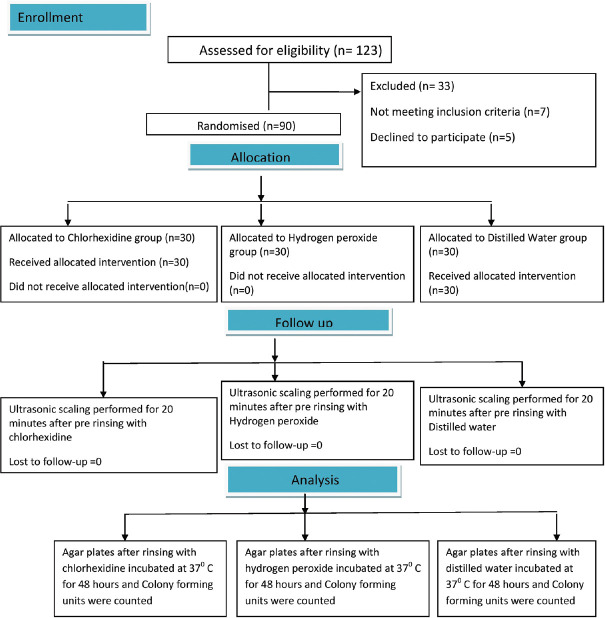
The sample size was determined based on previous studies with power 80% and at 5% significance level, 25 participants per group were required.[12] Taking attrition rate of 20% into consideration, a total of 30 participants in each group were selected. Randomization of the patients was done using a computer-generated random sequence table. Participants were then provided with informed written consent and were randomly allocated to three parallel treatment groups as (1) Group 1 (CHX), (2) Group 2 (HP), (3) Group 3 (Control) wherein 0.12% CHX (Periogard®, Colgate Palmolive Co., USA), 1.5% hydrogen peroxide (Peroxyl Colgate, Colgate-Palmolive Co., U.S.A), and control as distilled water (DW) were used, respectively. The study method is shown in Figure 1.
Figure 1.
Flowchart of the study participants
The study was performed in a closed dental clinic having no source of contamination by other dental procedures.
Treatment protocol
First, patients in each group were advised to rinse for a minute with 15 ml of either 0.12% CHX or 1.5% HP or DW. After 10 min, randomly selected quadrants from each patient's mouth were subjected to single sitting ultrasonic scaling with high-volume saliva ejector placed in the mouth. Blood agar plates were used for microbiological culture. The patients' mouth was considered a reference point and blood agar plates were placed at three different locations, i.e., patient's chest, operator's chest, and dental assistant's chest area. Three different color codings were used for the allocation concealment of agar plates, which were not known to patients or clinicians performing the treatment. After completion of the procedure, any aerosolized particles remaining suspended in air were allowed to collect for the next 30 min on uncovered agar plates. After the scaling, the blood agar plates were incubated at 37°C for 48 h. The number of colony-forming units (CFUs) on each plate was counted.
Statistical analysis
Statistical analysis was done using the SPSS software (SPSS for Windows, Version 16.0, SPSS Inc., Chicago, IL, USA). Age, number of teeth, and PI were expressed as mean and standard deviation of each group [Table 1]. CFUs among the three groups at all the three locations were derived using the analysis of variance. Mean differences between the groups were determined using independent t-test. P < 0.05 was considered statistically significant.
RESULTS
There was no adverse effect reported for any of the materials used in the study. A total of 90 participants participated in the study. The demographic details are presented in Table 1. Both the test groups showed significant microbial reduction when compared to the control group. At all the three locations, the CHX group showed a statistically significant reduction in CFUs count followed by the HP group and DW group [Table 2]. The difference in CFU count between the locations was found to be statistically significant [Table 3]. The highest and the lowest number of CFUs was found at the patient's chest area and at the assistant's chest area, respectively [Figures 2-4].
Table 2.
Colony-forming units count in relation to treatment and location
| Location of agar plate | Number of CFUs (mean±SD) | ||
|---|---|---|---|
|
| |||
| Group 1 | Group 2 | Group 3 | |
| Doctor’s chest | 27.5±2.80 | 42.8±3.20 | 62.17±3.75 |
| Assistant’s chest | 11.12±1.45 | 21.12±2.23 | 37.16±3.17 |
| Patient’ chest | 83.23±5.21 | 168.7±4.32 | 201.52±7.13 |
CFUs: Colony-forming units, SD: Standard deviation
Table 3.
Comparison of colony-forming units count between the three groups (n=30 in each group) at all three locations
| Location | Rinse | Mean±SD | F | P |
|---|---|---|---|---|
| Doctor’s chest | CHX | 27.5±2.80 | 845.281 | <0.001 |
| HP | 42.8±3.20 | |||
| DW | 62.17±3.75 | |||
| Patient’s chest | CHX | 83.23±5.21 | 3472.787 | <0.001 |
| HP | 168.7±4.32 | |||
| DW | 201.52±7.13 | |||
| Assistant’s chest | CHX | 11.12±1.45 | 901.256 | <0.001 |
| HP | 21.12±2.23 | |||
| DW | 37.16±3.17 |
Statistically significant: P<0.05. SD: Standard deviation, CHX: Chlorhexidine, HP: Hydrogen peroxide, DW: Distilled water
Figure 2.
Colony-forming units after chlorhexidine use
Figure 4.
Colony-forming units after distilled water use
Figure 3.
Colony-forming units after hydrogen peroxide use
DISCUSSION
Infection control is an essential element in dental practice. Results from several studies have shown that aerosols contain highly virulent pathogens and they can travel more than 6′ of distance.[13] Moreover, in the pandemic situation with COVID-19 disease breakdown, special emphasis has been given to regulate infection control and minimize aerosol production since SARS-CoV-2 is shown to have high transmissibility through bioaerosols.[14] Dental procedures such as ultrasonic scaling generate high volume of aerosol which holds the potential to spread several infectious diseases. Methods to reduce aerosols contamination include use of high-suction evacuators, ultraviolet (UV) chamber, and use of UV. These methods are expensive and sometimes difficult to incorporate in regular dental practices. Therefore, use of preprocedural rinses is an economical method to reduce aerosol contamination. A meta-analysis concluded that the use of preprocedural mouthrinse using CHX, cetylpyridinium chloride, or essential oils could result in as much as 68.4% reduction of CFUs in dental aerosol.[15] In a study by Sharma et al., it was shown that both CHX and HP could significantly reduce gingival inflammation but CHX being more effective compared to the latter.[16]
However, contradictory results were also found in several studies. Hossainian et al., in their systematic review on the effects of HP mouthwashes on plaque control, concluded that it does not consistently prevent plaque accumulation as short-term therapy as it lacks antimicrobial property.[17] In the present study, the efficacy of HP as preprocedural rinse was evaluated and it showed a statistically significant reduction in microbial count as compared to DW. This dissimilarity in the results could be due to the heterogeneity among the studies such as concentration of the materials used, follow-up period, treatment procedures performed, microorganisms tested, and location of the culture media. Recently, a study on international guidance of aerosol generation mitigation suggested that preprocedural mouthrinse with HP reduces intraoral viral load of SARS-CoV-2 and is an effective way to reduce aerosol contamination.[18] Nevertheless, rinsing with HP could result in reduced plaque formation and colonization of the microorganisms.
CONCLUSION
It can be concluded from the present study that both CHX, as a gold standard antiplaque agent, had the highest amount of antimicrobial properties. However, HP, having minimal side effects compared to CHX, could be used as an alternative to CHX as a preprocedural mouthrinse.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kumar PS, Subramanian K. Demystifying the mist: Sources of microbial bioload in dental aerosols. J Periodontol. 2020;91:1113–22. doi: 10.1002/JPER.20-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otter JA, Yezli S, Salkeld JA, French GL. Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am J Infect Control. 2013;41:S6–11. doi: 10.1016/j.ajic.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Zemouri C, de Soet H, Crielaard W, Laheij A. A scoping review on bio-aerosols in healthcare and the dental environment. PLoS One. 2017;12:e0178007. doi: 10.1371/journal.pone.0178007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Dental Settings: Interim Infection Prevention and Control Guidance for Dental Settings during the COVID-19 Response. [Last accessed on accessed 2021 Aug 24]. Available from: http://cdc.gov/coronavirus/2019-ncov/hcp/dental-settings.html .
- 5.Tartaglia GM, Tadakamadla SK, Connelly ST, Sforza C, Martín C. Adverse events associated with home use of mouthrinses: a systematic review. Ther Adv Drug Saf. 2019;10:1–16. doi: 10.1177/2042098619854881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Omidbakhsh N, Sattar SA. Broad-spectrum microbicidal activity, toxicologic assessment, and materials compatibility of a new generation of accelerated hydrogen peroxide-based environmental surface disinfectant. Am J Infect Control. 2006;34:251–7. doi: 10.1016/j.ajic.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ortega KL, Rech BO, El Haje GL, Gallo CB, Pérez-Sayáns M, Braz-Silva PH. Do hydrogen peroxide mouthwashes have a virucidal effect? A systematic review. J Hosp Infect. 2020;106:657–62. doi: 10.1016/j.jhin.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohd-Said S, Mohd-Dom TN, Suhaimi N, Rani H, McGrath C. Effectiveness of pre-procedural mouth rinses in reducing aerosol contamination during periodontal prophylaxis: A systematic review. Front Med (Lausanne) 2021;8:600769. doi: 10.3389/fmed.2021.600769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reis IN, do Amaral GC, Mendoza AA, das Graças YT, Mendes-Correa MC, Romito GA, et al. Can preprocedural mouthrinses reduce SARS-CoV-2 load in dental aerosols? Med Hypotheses. 2021;146:110436. doi: 10.1016/j.mehy.2020.110436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramesh A, Thomas JT, Muralidharan NP, Varghese SS. Efficacy of adjunctive usage of hydrogen peroxide with chlorhexidine as preprocedural mouthrinse on dental aerosol. Natl J Physiol Pharm Pharmacol. 2015;5:431–5. [Google Scholar]
- 11.Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38:l610–6. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 12.Nisha S, Shivmallu AB, Gujjari SK, Shashikumar P, Ali NM, Kulkarni M. Efficacy of preprocedural boric acid mouthrinse in reducing viable bacteria in dental aerosols produced during ultrasonic scaling. Contem Clin Dent. 2021;12:282–8. doi: 10.4103/ccd.ccd_374_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kutter JS, Spronken MI, Fraaij PL, Fouchier RA, Herfst S. Transmission routes of respiratory viruses among humans. Curr Opin Virol. 2018;28:142–51. doi: 10.1016/j.coviro.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J. Pathogenicity and transmissibility of 2019-nCoV-A quick overview and comparison with other emerging viruses. Microbes Infect. 2020;22:69–71. doi: 10.1016/j.micinf.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marui VC, Souto ML, Rovai ES, Romito GA, Chambrone L, Pannuti CM. Efficacy of preprocedural mouthrinses in the reduction of microorganisms in aerosol: A systematic review. J Am Dent Assoc. 2019;150:1015–26.e1. doi: 10.1016/j.adaj.2019.06.024. [DOI] [PubMed] [Google Scholar]
- 16.Sharma K, Acharya S, Verma E, Singhal D, Singla N. Efficacy of chlorhexidine, hydrogen peroxide and tulsi extract mouthwash in reducing halitosis using spectrophotometric analysis: A randomized controlled trial. J Clin Exp Dent. 2019;11:e457–63. doi: 10.4317/jced.55523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hossainian N, Slot DE, Afennich F, Van der Weijden GA. The effects of hydrogen peroxide mouthwashes on the prevention of plaque and gingival inflammation: A systematic review. Int J Dent Hyg. 2011;9:171–81. doi: 10.1111/j.1601-5037.2010.00492.x. [DOI] [PubMed] [Google Scholar]
- 18.Robertson C, Clarkson JE, Aceves-Martins M, Ramsay CR, Richards D, Colloc T CoDER Working Group. A Review of Aerosol Generation Mitigation in International Dental Guidance. Int Dent J. 2021:1–6. doi: 10.1016/j.identj.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]