Abstract
Background
Trace metals are essential trace elements for the human body, but insufficient or excessive levels of metal ions can lead to the development of disease. The purpose of this study was to examine the relationship between dietary intake of copper and the prevalence of kidney stones in U.S. adult population.
Methods
We included data on dietary intake of trace metals from 28,623 adult participants in the National Health and Nutrition Examination Survey (NHANES) database between 2007 and 2018. Multivariate logistic regression and restricted cubic spline (RCS) dose-response curves were used to explore the association between trace metals and kidney stones, and 1:1 propensity score matching (PSM) was performed between the stone formers and non-stone formers to test the validity of the results.
Results
Dose-response curves showed a non-linear negative association between dietary copper intake and kidney stones, and an increase in copper intake reduced the risk of kidney stones. Multivariate logistic regression analysis showed that the odds ratio (95% confidence interval) for kidney stones in each quartile of copper intake compared to the lowest quartile were 0.905 (0.808–1.011, p = 0.075), 0.880 (0.785–0.987, p = 0.028) and 0.853 (0.756–0.959, p = 0.009). In addition, similar conclusions were reached after analysis of PSM in the stone formers and non-stone formers groups.
Conclusion
Dietary copper intake was negatively and non-linearly correlated with kidney stones, which is worthy of further research and application in clinical practice.
Keywords: kidney stones, dietary copper intake, dose-response curves, propensity score matching, NHANES database
Introduction
Kidney stones are a common urological condition caused by the deposition of mineral crystals in the urine in the kidneys (1, 2). Severe kidney stones can cause symptoms such as severe back pain, nausea, hematuria, and difficulty in urination. Bilateral urinary tract obstruction can lead to uremia, and the atrophy of kidney tissue caused by stones severely affects kidney function and may lead to loss of function of the entire kidney (3, 4). Kidney stones are a complex disease caused by environmental, dietary and genetic factors, with an incidence of over 6–12% and a 5-year recurrence rate of up to 50%, seriously affecting human health (5).
Trace metals are indispensable trace elements for living organisms, but insufficient or excessive levels of metal ions can lead to cell death, which in turn cause abnormalities or diseases in human physiology (6, 7). Some studies have found a relationship between trace metals and the occurrence and development of urinary stones (8–10). However, the role of trace metals in the pathogenesis of kidney stones is unclear. Some studies have reported that dietary zinc intake may be associated with an increased risk of kidney stones; whereas manganese intake may be associated with a reduced risk of kidney stones (11, 12). Copper, a trace metal, is an important cofactor in living organisms, and its redox properties make it both beneficial and toxic to cells (13, 14). As an essential trace element, copper is vital for the growth and development of the brain, bones, and other organs (15). Copper can only be consumed from dietary sources, and nut foods, animal offal, vegetables, and legumes contain high amounts of copper (16). Recent studies have found that intracellular copper accumulation can lead to a novel type of programmed cell death (PCD) called cuproptosis (17). However, the relationship between dietary copper intake and the risk of kidney stones is unclear.
In the current study, we used a large US population survey database, the National Health and Nutrition Examination Survey (NHANES) 2007–2018, to explore the dose-response relationship between copper intake and kidney stone risk, and validated it by propensity score matching (PSM) analysis.
Materials and methods
Data sources and preparation
The population for this study was drawn from the NHANES database, a cross-sectional survey designed to assess the health and nutritional status of non-institutionalized civilians in all 50 states and the District of Columbia in the United States (18). Approximately 5,000 individuals were sampled each year through a multilevel stratified probability design, and all participants receive a structured questionnaire at home and a physical examination at a mobile testing center. Data from this representative survey have been published online on a 2-year cycle since 1999. The data files published online are available for public use and can be found on the official website: https://www.cdc.gov/nchs/nhanes/index.htm.
In this study, we used publicly available data from NHANES for six cycles: 2007–2008, 2009–2010, 2011–2011, 2013–2014, 2015–2016, and 2017–2018. A total of 34,770 adults (≥20 years) participated in the survey during this 12-year period. We developed the following exclusion criteria (Figure 1): (a) incomplete stone survey (n = 91); (b) unknown/abnormal dietary trace metal intake (n = 4,025); (c) unknown body mass index (BMI) (n = 316); (d) unknown education level (n = 30); (e) unknown marital status (n = 11); (f) unknown hypertension/diabetes mellitus (n = 54); (g) unknown physical activities status (n = 7); (h) unknown renal function indicators (creatinine, urea nitrogen, and uric acid) (n = 1,613). A total of 6,147 individuals were excluded based on the above criteria, and a total of 28,623 study subjects were finally included in this study.
Figure 1.
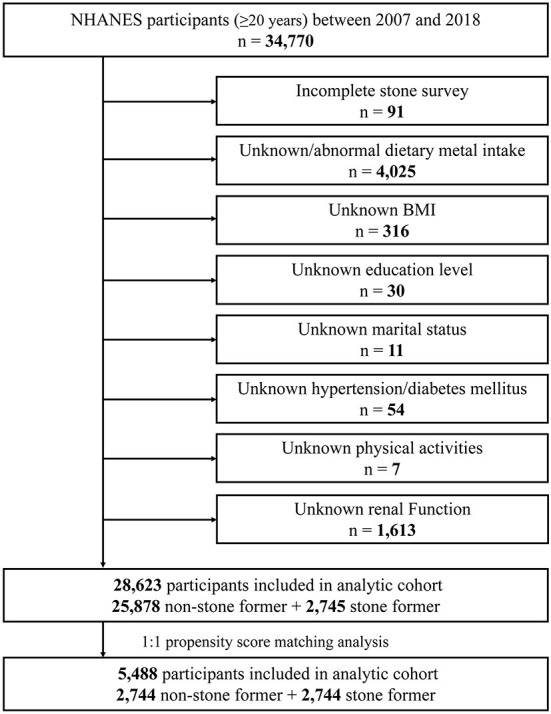
Schematic overview for patient identification.
Study variables
In NHANES, dietary protein, moisture and trace metals (phosphorus, magnesium, iron, zinc, copper, selenium) intake was assessed by the 24 h dietary review method, in which participants were asked by a professional technician about the types and amounts of foods and beverages consumed during 24 h and recorded in the NHANES computer-assisted dietary survey system. The intake of each food component was then estimated based on the University of Texas Food Intake Analysis System and the USDA Survey Nutrient Database.
In addition, we included other variables such as gender, age, race, marital status, family income, education level, BMI, hypertension, diabetes mellitus, smoking status, drinking, physical activities status (vigorous and moderate activities), blood urea nitrogen, creatinine, uric acid, and estimated glomerular filtration rate (eGFR). BMI was calculated as weight (kg)/[height (m2)*height (m2)]. Hypertension and diabetes mellitus were diagnosed by a physician or other health professional. The eGFR was calculated were followed as described previously (3, 19).
Kidney stones assessment
In the questionnaire, the participant was asked by a trained professional: “Have you ever had kidney stones”, and the participant was considered to have a history of kidney stones if he/she answered “Yes”.
Statistical analysis
All data and figures in this study were organized and analyzed using R software (version 3.5.3) and SPSS software (version 24.0). Sampling weights for interviews (WTSA2YR) and study design variables (SDMVPSU and SDMVSTRA) were used in the analysis of the data, and the weighting analysis was performed to consider the complex sampling design and to obtain the appropriate weights. Normally distributed continuous data were described by mean ± standard deviation (SD), and categorical data were described by frequency (n) and percentage (%). Trace metals were quadratically divided and multivariate logistic regression models were used to assess the association between different dietary trace metal intakes and kidney stones, with results presented as adjusted odds ratios (aORs) and 95% confidence intervals (CIs) intervals. For multivariate logistic regression analysis, we used two models to assess the effect of trace metals on kidney stones: the single-metal model: adjusted for individual trace metals and all other covariates; the multi-metal model: adjusted for all trace metals and all other covariates. Restricted cubic spline (RCS) functions were used to visually describe the dose-response relationship between dietary trace metal intake and kidney stones.
A 1:1 propensity score matching (PSM) analysis was used to balance the differences between the stone forming and non-stone formers, adjusted for confounding variables including: gender, age, race, marital status, family income, education level, BMI, hypertension, diabetes mellitus, smoking status, drinking, vigorous recreational activities and moderate recreational activities. The data after PSM were also reanalyzed to further test the correctness of the results. P-values were considered statistically significant when calculated at <0.05.
Results
A total of 28,623 participants were included in the study between 2007 and 2018, of which 2,745 (9.6%) were stone formers and 25,878 (90.4%) were non-stone formers. The clinicopathological characteristics of all participants are shown in Table 1. The chi-square test showed significant differences between stone formers and non-stone formers on the variables of gender, age, race, marital status, BMI, hypertension, diabetes mellitus, and physical activities variables (all P < 0.001). In addition, stone formers had higher levels of blood urea nitrogen, creatinine, and uric acid and lower eGFR compared to non-stone formers. Moreover, the dietary intakes of phosphorus, magnesium, copper and selenium were lower in stone formers compared to non-stone formers (all P < 0.05). Spearman's rank correlation coefficient analysis showed a positive correlation between dietary intakes of the six trace metals (Supplementary Figure S1).
Table 1.
Baseline characteristics of NHANES participants between 2007 and 2018.
| Characteristic | Total | None-stone formers | Stone formers | P -value |
|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | ||
| Total patients | 28,623 | 25,878 (90.4) | 2,745 (9.6) | |
| Gender | <0.001 | |||
| Male | 13,924 (48.6) | 12,390 (47.9) | 1,534 (55.9) | |
| Female | 14,699 (51.4) | 13,488 (52.1) | 1,211 (44.1) | |
| Age | <0.001 | |||
| <50 years | 14,402 (50.3) | 13,451 (52.0) | 951 (34.6) | |
| ≥50 years | 14,221 (49.7) | 12,427 (48.0) | 1,794 (65.4) | |
| Race | <0.001 | |||
| Non-Hispanic white | 12,098 (42.3) | 10,580 (40.9) | 1,518 (55.3) | |
| Non-Hispanic black | 5,891 (20.6) | 5,539 (21.4) | 352 (12.8) | |
| Mexican American | 4,381 (15.3) | 4,027 (15.6) | 354 (12.9) | |
| Other Hispanic | 3,005 (10.5) | 2,701 (10.4) | 304 (11.1) | |
| Other | 3,248 (11.3) | 3,031 (11.7) | 217 (7.9) | |
| Education level | 0.550 | |||
| Less than high school | 6,859 (24.0) | 6,178 (23.9) | 681 (24.8) | |
| High school or equivalent | 6,541 (22.9) | 5,922 (22.9) | 619 (22.6) | |
| College or above | 15,223 (53.2) | 13,778 (53.2) | 1,445 (52.6) | |
| Marital status | <0.001 | |||
| Married | 14,740 (51.5) | 13,166 (50.9) | 1,574 (57.3) | |
| Unmarried | 13,883 (48.5) | 12,712 (49.1) | 1,171 (42.7) | |
| Family income | 0.198 | |||
| < $20,000 | 6,572 (23.0) | 5,922 (22.9) | 650 (23.7) | |
| ≥$20,000 | 20,647 (72.1) | 18,669 (72.1) | 1,978 (72.1) | |
| Unknown | 1,404 (4.9) | 1,287 (5.0) | 117 (4.3) | |
| BMI (kg/m2) | <0.001 | |||
| <25.0 | 8,048 (28.1) | 7,519 (29.1) | 529 (19.3) | |
| ≥25.0 | 20,575 (71.9) | 18,359 (70.9) | 2,216 (80.7) | |
| Hypertension | <0.001 | |||
| Yes | 10,336 (36.1) | 8,943 (34.6) | 1,393 (50.7) | |
| No | 18,287 (63.9) | 16,935 (65.4) | 1,352 (49.3) | |
| Diabetes mellitus | <0.001 | |||
| Yes | 3,716 (13.0) | 3,099 (12.0) | 617 (22.5) | |
| No | 24,236 (84.7) | 22,202 (85.8) | 2,034 (74.1) | |
| Borderline | 671 (2.3) | 577 (2.2) | 94 (3.4) | |
| Smoking status | <0.001 | |||
| Never | 15,951 (55.7) | 14,608 (56.4) | 1,343 (48.9) | |
| Former | 6,900 (24.1) | 6,045 (23.4) | 855 (31.1) | |
| Current | 5,772 (20.2) | 5,225 (20.2) | 547 (19.9) | |
| Drinking | 0.216 | |||
| Yes | 20,334 (71.0) | 18,356 (70.9) | 1,978 (72.1) | |
| No/Unknown | 8,299 (29.0) | 7,522 (29.1) | 767 (27.9) | |
| Vigorous recreational activities | <0.001 | |||
| Yes | 6,306 (22.0) | 5,890 (22.8) | 416 (15.2) | |
| No | 22,317 (78.0) | 19,988 (77.2) | 2,329 (84.8) | |
| Moderate recreational activities | <0.001 | |||
| Yes | 11,574 (40.4) | 10,590 (40.9) | 984 (35.8) | |
| No | 17,049 (59.6) | 15,288 (59.1) | 1,761 (64.2) | |
| Blood urea nitrogen (mg/dL) | 13.76, 5.99 | 13.60, 5.81 | 15.29, 7.27 | <0.001 |
| Creatinine (mg/dL) | 0.90, 0.45 | 0.89, 0.41 | 0.98, 0.72 | <0.001 |
| Uric acid (mg/dL) | 5.45, 1.45 | 5.43, 1.44 | 5.65, 1.52 | <0.001 |
| eGFR [mL/(min·1.73 m2)] | 83.71, 25.80 | 84.63, 25.65 | 75.04, 25.61 | <0.001 |
| Daily intake (M, SD) | ||||
| Protein (mg) | 80.75, 42.16 | 81.01, 42.33 | 78.38, 40.49 | 0.002 |
| Moisture (g) | 2.88, 1.50 | 2.89, 1.50 | 2.83, 1.47 | 0.062 |
| Phosphorus (mg) | 1,340.9, 667.7 | 1,344.4, 670.2 | 1,307.5, 642.2 | 0.006 |
| Magnesium (mg) | 294.63, 146.58 | 295.88, 147.25 | 282.78, 139.54 | <0.001 |
| Iron (mg) | 14.42, 8.33 | 14.42, 8.33 | 14.41, 8.33 | 0.966 |
| Zinc (mg) | 11.04, 6.71 | 11.06, 6.74 | 10.87, 6.43 | 0.157 |
| Copper (mg) | 1.23, 0.74 | 1.24, 0.74 | 1.20, 0.69 | 0.007 |
| Selenium (mcg) | 112.36, 63.40 | 112.61, 63.50 | 110.03, 62.45 | 0.043 |
For categorical variables, P-values were analyzed by chi-square tests. For continuous variables, the t-test for slope was used in generalized linear models.
NHANES, National Health and Nutrition Examination Survey; BMI, body mass index; eGFR, estimated glomerular filtration rate; SD, standard deviation.
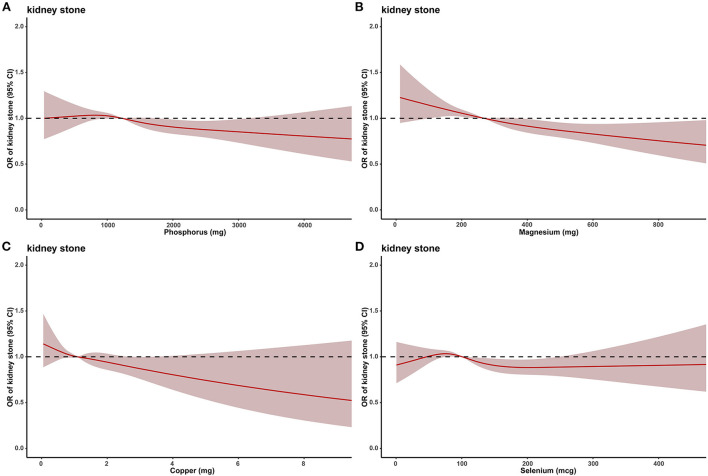
Dose-response curve analysis of the RCS was used to assess the relationship between dietary phosphorus, magnesium, copper, selenium intakes and kidney stones. The results showed that dietary phosphorus (p = 0.038), magnesium (p < 0.001), and copper (p = 0.002) intakes were negatively and non-linearly associated with kidney stone risk in all populations, and the risk of kidney stones decreased progressively with increasing phosphorus, magnesium, or copper intakes (Figure 2).
Figure 2.
The dose–response analysis between dietary metal intake and presence of kidney stones before propensity score matching. (A) Phosphorus. (B) Magnesium. (C) Copper. (D) Selenium.
To better assess the relationship between dietary phosphorus, magnesium, copper, selenium intake and kidney stones, we quadrupled-classified phosphorus, magnesium, copper, and selenium. After adjusting for all confounding variables, multivariate single-metal model logistic regression analysis revealed that dietary phosphorus, magnesium or copper intake was an independent risk factor for kidney stones. Compared with the lowest quartile, the ORs of kidney stones for each quartile of phosphorus intake were 0.977 (0.868–1.086, p = 0.605), 0.899 (0.801–1.010, p = 0.072) and 0.821 (0.727–0.929, p = 0.002), respectively; 0.874 (0.782–0.977, p = 0.018), 0.819 (0.730–0.919, p = 0.001), and 0.770 (0.683–0.868, p < 0.001) for each quartile of magnesium intake, respectively; 0.905 (0.808–1.011, p = 0.075), 0.880 (0.785–0.987, p = 0.028), and 0.853 (0.756–0.959, p = 0.009) for each quartile of copper intake, respectively (Table 2). However, in multivariate multi-metal logistic regression, only magnesium intake was associated with the risk of kidney stones (Supplementary Table S1).
Table 2.
Multivariate single-metal model logistic regression the relationship between the heavy metals and the presence of kidney stone.
| Metals | Phosphorus | Magnesium | Copper | Selenium | ||||
|---|---|---|---|---|---|---|---|---|
| aOR (95% CI) | P | aOR (95% CI) | P | aOR (95% CI) | P | aOR (95% CI) | P | |
| Overall | 0.008 | <0.001 | 0.041 | 0.133 | ||||
| Q1 | Reference | Reference | Reference | Reference | ||||
| Q2 | 0.971 (0.868–1.086) | 0.605 | 0.874 (0.782–0.977) | 0.018 | 0.905 (0.808–1.011) | 0.075 | 0.983 (0.879–1.100) | 0.769 |
| Q3 | 0.899 (0.801–1.010) | 0.072 | 0.819 (0.730–0.919) | 0.001 | 0.880 (0.785–0.987) | 0.028 | 0.952 (0.849–1.068) | 0.399 |
| Q4 | 0.821 (0.727–0.929) | 0.002 | 0.770 (0.683–0.868) | <0.001 | 0.853 (0.756–0.959) | 0.009 | 0.872 (0.772–0.986) | 0.029 |
| Male | 0.007 | 0.001 | 0.137 | 0.054 | ||||
| Q1 | Reference | Reference | Reference | Reference | ||||
| Q2 | 1.026 (0.864–1.217) | 0.773 | 0.901 (0.763–1.065) | 0.222 | 0.937 (0.793–1.106) | 0.441 | 0.972 (0.820–1.153) | 0.747 |
| Q3 | 0.991 (0.838–1.171) | 0.911 | 0.925 (0.787–1.088) | 0.347 | 0.919 (0.781–1.082) | 0.310 | 0.951 (0.807–1.122) | 0.554 |
| Q4 | 0.810 (0.684–0.958) | 0.014 | 0.736 (0.625–0.867) | <0.001 | 0.829 (0.704–0.976) | 0.024 | 0.821 (0.696–0.968) | 0.019 |
| Female | 0.063 | 0.001 | 0.343 | 0.670 | ||||
| Q1 | Reference | Reference | Reference | Reference | ||||
| Q2 | 0.929 (0.799–1.080) | 0.340 | 0.868 (0.746–1.010) | 0.066 | 0.897 (0.768–1.048) | 0.170 | 0.963 (0.828–1.120) | 0.624 |
| Q3 | 0.798 (0.675–0.943) | 0.008 | 0.701 (0.591–0.831) | <0.001 | 0.875 (0.740–1.034) | 0.116 | 0.917 (0.778–1.080) | 0.299 |
| Q4 | 0.958 (0.793–1.157) | 0.653 | 0.932 (0.776–1.119) | 0.449 | 0.968 (0.810–1.158) | 0.726 | 1.024 (0.845–1.241) | 0.809 |
| BMI < 25.0 kg/m2 | 0.412 | 0.136 | 0.623 | 0.403 | ||||
| Q1 | Reference | Reference | Reference | Reference | ||||
| Q2 | 0.919 (0.715–1.181) | 0.509 | 0.897 (0.698–1.153) | 0.396 | 0.924 (0.715–1.195) | 0.546 | 1.112 (0.869–1.422) | 0.399 |
| Q3 | 0.949 (0.735–1.225) | 0.688 | 0.842 (0.649–1.091) | 0.193 | 0.934 (0.722–1.209) | 0.604 | 0.922 (0.709–1.198) | 0.544 |
| Q4 | 0.794 (0.602–1.049) | 0.105 | 0.728 (0.556–0.953) | 0.021 | 0.837 (0.642–1.092) | 0.190 | 0.913 (0.695–1.200) | 0.515 |
| BMI ≥ 25.0 kg/m2 | 0.019 | 0.001 | 0.173 | 0.199 | ||||
| Q1 | Reference | Reference | Reference | Reference | ||||
| Q2 | 0.983 (0.866–1.114) | 0.784 | 0.867 (0.766–0.982) | 0.025 | 0.908 (0.801–1.030) | 0.135 | 0.952 (0.839–1.080) | 0.447 |
| Q3 | 0.884 (0.776–1.007) | 0.063 | 0.813 (0.715–0.924) | 0.002 | 0.885 (0.778–1.007) | 0.063 | 0.958 (0.843–1.089) | 0.513 |
| Q4 | 0.828 (0.722–0.949) | 0.007 | 0.781 (0.683–0.893) | <0.001 | 0.874 (0.765–0.998) | 0.047 | 0.864 (0.753–0.991) | 0.036 |
CI, confidence interval; aOR, adjusted odds ratio.
Adjusted to: gender, age, race, marital status, education level, BMI, hypertension, diabetes mellitus, vigorous physical activities, moderate physical activities, blood urea nitrogen, creatinine, uric acid, and estimated glomerular filtration rate (eGFR).
Dietary copper enters the body and is absorbed by the digestive system, synthesized by the liver into copper cyanobrotein and released into the blood, which is mainly involved in the physiological functions of the body in the form of plasma copper cyanobrotein (20). Most of the endogenous copper is excreted into the gastrointestinal tract along with unabsorbed copper, and a small amount of copper is excreted through other routes (21). One study found that increased exposure of serum copper was significantly associated with the risk of non-alcoholic fatty liver disease (NAFLD) and was particularly prominent in women, middle age and participants with improved insulin resistance status, with a 97% increased risk of NAFLD in the highest quartile of copper compared to the lowest quartile (22). Gao et al. (23) found that serum copper levels in the highest quartile were associated with urge incontinence (aOR = 1.74, 95% CI 1.11–2.74), especially in women over 50 years of age, serum copper levels in the highest quartile were associated with urge incontinence (aOR = 1.74, 95% CI 1.57–5.49), any urinary incontinence (aOR = 1.97, 95% CI 1.19–3.27), mixed incontinence (aOR = 2.43, 95% CI 1.19–4.97), and moderate/severe incontinence (aOR = 2.37, 95% CI 1.06–5.31). In addition, Bagheri et al. (24) found that serum copper levels were higher in patients with atherosclerosis and positively correlated with the severity of atherosclerosis (p = 0.001). The above findings suggest that excess serum copper levels can also adversely affect the organism and that excessive copper intake should be limited for normal individuals as well.
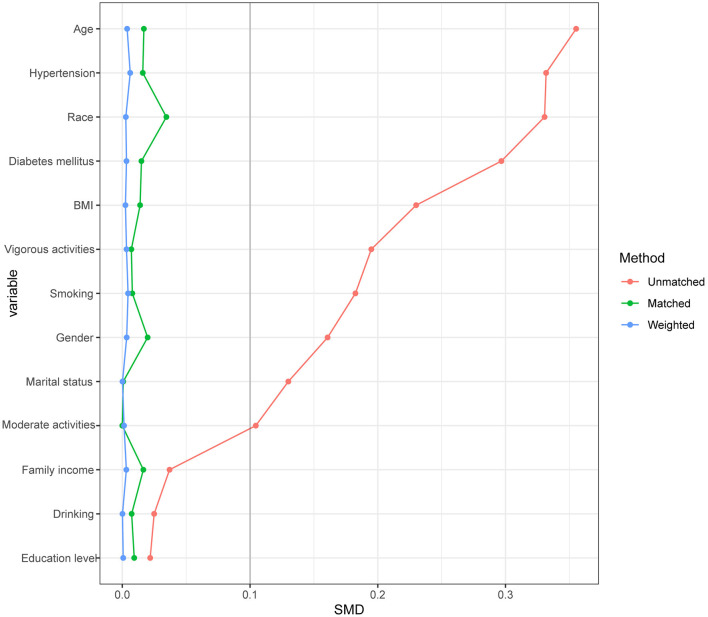
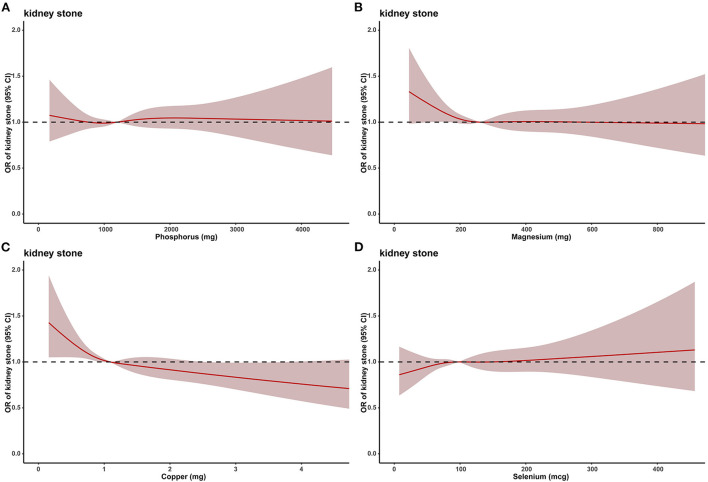
Due to the large difference in the number of stone formers and non-stone formers groups, we used a 1:1 PSM to correct for the difference between the two groups (Figure 3), 2,744 participants were included in both the stone formers and non-stone formers groups after the PSM. The clinicopathological characteristics of all participants after PSM are shown in Table 3. Subsequently, dose-response curves showed that only dietary copper (p = 0.001) intake was negatively and non-linearly associated with the risk of kidney stones (Figure 4). Multivariate single-metal model logistic regression results also demonstrated that dietary copper (p = 0.001) intake remained an independent risk factor for kidney stones, and the ORs for kidney stones in each quartile of copper intake compared to the lowest quartile were 0.877 (0.754–1.021, p = 0.092), 0.876 (0.751–1.022, p = 0.093) and 0.732 (0.626–0.856, p < 0.001) (Table 4). Moreover, multivariate multi-metal logistic regression showed that only copper intake was associated with the risk of kidney stones (Table 5).
Figure 3.
Propensity score matching analysis of the standardized mean difference results for the different variables.
Table 3.
Baseline characteristics of NHANES participants between 2007 and 2018 after PSM.
| Characteristic | Total | None-stone formers | Stone formers | P -value |
|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | ||
| Total patients | 5,488 | 2,744 (50.0) | 2,744 (50.0) | |
| Gender | 0.786 | |||
| Male | 3,056 (55.7) | 1,523 (55.5) | 1,533 (55.9) | |
| Female | 2,432 (44.3) | 1,221 (44.5) | 1,211 (44.1) | |
| Age | 0.459 | |||
| <50 years | 1,876 (34.2) | 925 (33.7) | 951 (34.7) | |
| ≥50 years | 3,612 (65.8) | 1,819 (66.3) | 1,793 (65.3) | |
| Race | 0.783 | |||
| Non-Hispanic white | 3,059 (55.7) | 1,542 (56.2) | 1,517 (55.3) | |
| Non-Hispanic black | 711 (13.0) | 359 (13.1) | 352 (12.8) | |
| Mexican American | 697 (12.7) | 343 (12.5) | 354 (12.9) | |
| Other Hispanic | 610 (11.1) | 306 (11.2) | 304 (11.1) | |
| Other | 411 (7.5) | 194 (7.1) | 217 (7.9) | |
| Education level | 0.991 | |||
| Less than high school | 1,363 (24.8) | 683 (24.9) | 680 (24.8) | |
| High school or equivalent | 1,240 (22.6) | 621 (22.6) | 619 (22.6) | |
| College or above | 2,885 (52.6) | 1,440 (52.5) | 1,445 (52.7) | |
| Marital status | 0.155 | |||
| Married | 3,200 (58.3) | 1,626 (59.3) | 1,574 (57.4) | |
| Unmarried | 2,288 (41.7) | 1,118 (40.7) | 1,170 (42.6) | |
| Family income | 0.786 | |||
| < $20,000 | 1,324 (24.1) | 668 (24.3) | 656 (23.9) | |
| ≥$20,000 | 3,945 (71.9) | 1,971 (71.8) | 1,974 (71.9) | |
| Unknown | 219 (4.0) | 105 (3.8) | 114 (4.2) | |
| BMI (kg/m2) | 0.891 | |||
| <25.0 | 1,062 (19.4) | 533 (19.4) | 529 (19.3) | |
| ≥25.0 | 4,426 (80.6) | 2,211 (80.6) | 2,215 (80.7) | |
| Hypertension | 0.666 | |||
| Yes | 2,768 (50.4) | 1,376 (50.1) | 1,392 (50.7) | |
| No | 2,720 (49.6) | 1,368 (49.9) | 1,352 (49.3) | |
| Diabetes mellitus | 0.096 | |||
| Yes | 1,219 (22.2) | 603 (22.0) | 616 (22.4) | |
| No | 4,107 (74.8) | 2,073 (75.5) | 2,034 (74.1) | |
| Borderline | 162 (3.0) | 68 (2.5) | 94 (3.4) | |
| Smoking status | 0.084 | |||
| Never | 2,684 (48.9) | 1,339 (48.8) | 1,345 (49.0) | |
| Former | 1,703 (31.0) | 882 (32.1) | 821 (29.9) | |
| Current | 1,101 (20.1) | 523 (19.1) | 578 (21.1) | |
| Drinking | 0.768 | |||
| Yes | 3,856 (70.3) | 1,933 (70.4) | 1,923 (70.1) | |
| No/Unknown | 1,632 (29.7) | 811 (29.6) | 821 (29.9) | |
| Vigorous recreational activities | 0.519 | |||
| Yes | 815 (14.9) | 399 (14.5) | 416 (15.2) | |
| No | 4,673 (85.1) | 2,345 (85.5) | 2,328 (84.8) | |
| Moderate recreational activities | 0.693 | |||
| Yes | 1,954 (35.6) | 970 (35.3) | 984 (35.9) | |
| No | 3,534 (64.4) | 1,774 (64.7) | 1,760 (64.1) | |
| Blood urea nitrogen (mg/dL) | 15.02, 6.96 | 14.74, 6.63 | 15.29, 7.27 | 0.003 |
| Creatinine (mg/dL) | 0.96, 0.60 | 0.95, 0.45 | 0.98, 0.72 | 0.057 |
| Uric acid (mg/dL) | 5.69, 1.50 | 5.73, 1.48 | 5.65, 1.52 | 0.042 |
| eGFR [mL/(min·1.73 m2)] | 75.36, 25.24 | 75.68, 24.85 | 75.05, 25.61 | 0.352 |
| Daily intake (M, SD) | ||||
| Protein (mg) | 79.46, 40.56 | 80.38, 41.18 | 78.53, 39.92 | 0.129 |
| Moisture (g) | 2.88, 1.48 | 2.88, 1.47 | 2.88, 1.50 | 0.959 |
| Phosphorus (mg) | 1,304.9, 642.0 | 1,301.9, 642.3 | 1,307.9, 641.9 | 0.727 |
| Magnesium (mg) | 285.38, 138.83 | 287.90, 138.12 | 282.86, 139.52 | 0.178 |
| Iron (mg) | 14.50, 8.26 | 14.58, 8.20 | 14.41, 8.33 | 0.461 |
| Zinc (mg) | 11.08, 6.68 | 11.30, 6.93 | 10.87, 6.43 | 0.018 |
| Copper (mg) | 1.23, 0.72 | 1.27, 0.75 | 1.20, 0.69 | <0.001 |
| Selenium (mcg) | 109.18, 61.61 | 108.30, 60.76 | 110.06, 62.44 | 0.290 |
For categorical variables, P-values were analyzed by chi-square tests. For continuous variables, the t-test for slope was used in generalized linear models.
NHANES, National Health and Nutrition Examination Survey; BMI, body mass index; eGFR, estimated glomerular filtration rate; SD, standard deviation; PSM, propensity score matching.
Figure 4.
The dose–response analysis between dietary metal intake and presence of kidney stones after propensity score matching. (A) Phosphorus. (B) Magnesium. (C) Copper. (D) Selenium.
Table 4.
Multivariate single-metal model logistic regression the relationship between the heavy metals and the presence of kidney stone after PSM.
| Metals | Phosphorus | Magnesium | Copper | Selenium | ||||
|---|---|---|---|---|---|---|---|---|
| aOR (95% CI) | P | aOR (95% CI) | P | aOR (95% CI) | P | aOR (95% CI) | P | |
| Overall | 0.929 | 0.307 | 0.001 | 0.297 | ||||
| Q1 | Reference | Reference | Reference | Reference | ||||
| Q2 | 1.051 (0.906–1.219) | 0.512 | 0.903 (0.779–1.047) | 0.177 | 0.877 (0.754–1.021) | 0.092 | 1.134 (0.977–1.315) | 0.098 |
| Q3 | 1.023 (0.878–1.193) | 0.770 | 0.903 (0.775–1.052) | 0.191 | 0.876 (0.751–1.022) | 0.093 | 1.113 (0.957–1.295) | 0.165 |
| Q4 | 1.016 (0.865–1.193) | 0.849 | 0.865 (0.739–1.014) | 0.073 | 0.732 (0.626–0.856) | <0.001 | 1.034 (0.881–1.213) | 0.684 |
| Male | 0.680 | 0.129 | <0.001 | 0.306 | ||||
| Q1 | Reference | Reference | Reference | Reference | ||||
| Q2 | 1.032 (0.825–1.291) | 0.783 | 0.884 (0.710–1.101) | 0.270 | 0.792 (0.632–0.992) | 0.042 | 1.135 (0.908–1.418) | 0.265 |
| Q3 | 1.024 (0.823–1.275) | 0.830 | 0.930 (0.751–1.153) | 0.510 | 0.822 (0.659–1.025) | 0.082 | 1.127 (0.909–1.396) | 0.276 |
| Q4 | 0.926 (0.744–1.152) | 0.491 | 0.784 (0.632–0.972) | 0.026 | 0.618 (0.497–0.768) | <0.001 | 0.975 (0.787–1.207) | 0.814 |
| Female | 0.265 | 0.261 | 0.827 | 0.562 | ||||
| Q1 | Reference | Reference | Reference | Reference | ||||
| Q2 | 1.052 (0.860–1.287) | 0.621 | 0.925 (0.756–1.133) | 0.452 | 0.948 (0.769–1.169) | 0.619 | 1.108 (0.906–1.356) | 0.318 |
| Q3 | 0.972 (0.778–1.214) | 0.802 | 0.836 (0.667–1.047) | 0.119 | 0.903 (0.723–1.128) | 0.371 | 1.075 (0.864–1.338) | 0.517 |
| Q4 | 1.268 (0.976–1.647) | 0.075 | 1.074 (0.838–1.378) | 0.572 | 0.927 (0.731–1.176) | 0.535 | 1.192 (0.917–1.550) | 0.190 |
| BMI <25.0 kg/m2 | 0.888 | 0.330 | 0.291 | 0.480 | ||||
| Q1 | Reference | Reference | Reference | Reference | ||||
| Q2 | 0.903 (0.642–1.269) | 0.556 | 1.097 (0.776–1.550) | 0.601 | 1.050 (0.737–1.496) | 0.788 | 1.244 (0.885–1.747) | 0.209 |
| Q3 | 0.892 (0.627–1.269) | 0.524 | 0.935 (0.654–1.336) | 0.711 | 1.024 (0.717–1.461) | 0.897 | 1.089 (0.762–1.554) | 0.641 |
| Q4 | 0.871 (0.592–1.280) | 0.481 | 0.784 (0.546–1.127) | 0.190 | 0.771 (0.538–1.104) | 0.155 | 0.961 (0.664–1.392) | 0.834 |
| BMI ≥ 25.0 kg/m2 | 0.784 | 0.356 | 0.003 | 0.474 | ||||
| Q1 | Reference | Reference | Reference | Reference | ||||
| Q2 | 1.091 (0.924–1.287) | 0.305 | 0.870 (0.738–1.025) | 0.097 | 0.843 (0.712–0.998) | 0.048 | 1.113 (0.943–1.313) | 0.207 |
| Q3 | 1.057 (0.891–1.254) | 0.526 | 0.898 (0.758–1.065) | 0.217 | 0.846 (0.712–1.005) | 0.057 | 1.125 (0.951–1.330) | 0.169 |
| Q4 | 1.051 (0.880–1.257) | 0.581 | 0.884 (0.741–1.055) | 0.172 | 0.716 (0.601–0.853) | <0.001 | 1.050 (0.879–1.255) | 0.593 |
CI, confidence interval; aOR, adjusted odds ratio; PSM, propensity score matching.
Adjusted to: gender, age, race, marital status, education level, BMI, hypertension, diabetes mellitus, vigorous physical activities, moderate physical activities, blood urea nitrogen, creatinine, uric acid, and estimated glomerular filtration rate (eGFR).
Table 5.
Multivariate multi-metal model logistic regression of the relationship between heavy metals and kidney stone after PSM.
| Metals | Phosphorus | Magnesium | Copper | Selenium | ||||
|---|---|---|---|---|---|---|---|---|
| aOR (95% CI) | P | aOR (95% CI) | P | aOR (95% CI) | P | aOR (95% CI) | P | |
| Overall | 0.504 | 0.599 | <0.001 | 0.246 | ||||
| Q1 | Reference | Reference | Reference | Reference | ||||
| Q2 | 1.109 (0.931–1.321) | 0.247 | 0.923 (0.768–1.110) | 0.394 | 0.827 (0.693–0.986) | 0.034 | 1.163 (0.990–1.366) | 0.067 |
| Q3 | 1.150 (0.924–1.430) | 0.210 | 0.989 (0.786–1.245) | 0.927 | 0.760 (0.616–0.938) | 0.011 | 1.185 (0.983–1.429) | 0.076 |
| Q4 | 1.224 (0.936–1.600) | 0.139 | 1.059 (0.800–1.402) | 0.689 | 0.581 (0.454–0.744) | <0.001 | 1.149 (0.922–1.433) | 0.216 |
CI, confidence interval; aOR, adjusted odds ratio; PSM, propensity score matching.
Adjusted to: gender, age, race, marital status, education level, BMI, hypertension, diabetes mellitus, vigorous physical activities, moderate physical activities, blood urea nitrogen, creatinine, uric acid, estimated glomerular filtration rate (eGFR), phosphorus, magnesium, copper and selenium.
Discussion
As a common kidney disease, the high incidence and recurrence rate of kidney stones bring serious impact on patients' life, and cause huge economic burden. Therefore, it is of great clinical and social significance to explore the factors associated with kidney stones and to identify risk factors and protective factors for kidney stones in advance. In this study, we first investigated the dietary trace metals associated with kidney stones, and found that increased dietary intake of phosphorus, magnesium, and copper were associated with lower odds of kidney stones, and this finding was confirmed by the dose-response curves. Subsequently, multivariate logistic regression also revealed that dietary phosphorus, magnesium, and copper were independent protective factors for kidney stones. To exclude as much as possible the influence of other confounding variables and to test the correctness of the results, we used PSM analysis to adjust for differences between the stone formers and non-stone formers groups. Subsequent dose-response curves as well as multivariate logistic regression confirmed that dietary copper intake may be a protective factor for kidney stones.
Copper, an essential trace element, is widely distributed in tissues and organs such as the liver, kidney and brain, and the body meets its requirement for copper mainly through daily dietary intake (25). Copper is involved in energy metabolism, immune function, antioxidant and energy metabolism in the body (26). Numerous studies have shown that deficiencies in dietary copper intake are associated with cardiovascular disease, central nervous system diseases, digestive system diseases, and skeletal system diseases (13, 14, 27). Li et al. (28) included 14,834 NHANES participants from 2009 to 2014 and found that dietary intake of zinc, iron, copper, and selenium were negatively associated with depression, and similar study has found that dietary intake of zinc, copper, and selenium was associated with cognitive performance (29). Yang et al. (30) found a non-linear negative association between dietary copper intake and stroke by analyzing clinical information from 10,550 participants in the NHANES database from 2013 to 2018. The above results suggest that increased dietary copper intake appears to be a protective factor against disease progression.
Recently, Tsvetkov et al. (17) found that intracellular copper accumulation can cause direct binding of copper to lipid acylated components of the tricarboxylic acid (TCA) cycle, which subsequently leads to aggregation of lipid acylated proteins and loss of Fe-S cluster proteins, ultimately leading to cell death. Tsvetkov.'s study has led researchers to pay increasing attention to the role of copper in living organisms. To date, there has been only one study of copper intake and risk of kidney stones in adults. Ferraro et al. (12) studied participants in the Health Professionals Follow-up Study (HPFS) and the Nurses' Health Study (NHS) I and II and found no association between dietary copper intake and kidney stone risk, but total copper intake was associated with a higher risk of kidney stones in the NHS I cohort (OR = 1.14, 95% CI 1.02–1.28). However, in our study, we found that dietary copper intake was associated with a lower risk of kidney stones, and the same results were also obtained after 1:1 PSM analysis. We speculate that the conflicting results may be due to the following reasons: first, copper has two aspects; it is both an antioxidant and a pro-oxidant. One study found that either excessive or insufficient copper intake increased susceptibility to atherosclerosis in rabbits, reflecting the bidirectional relationship between copper and atherosclerosis (31). Second, the year of sample collection, sample size, and type of variables adjusted for differed for each study. Therefore, further large samples are needed to elucidate the relationship between dietary copper intake and the risk of kidney stones.
There are several strengths deserve to be noted in our study. First, the sample of this study was large enough that the participants were representative of the national population of the United States, and the findings warrant generalization. Second, we used multivariate logistic regression analysis, to adjust for other confounding variables in our statistical analysis. Finally, to verify the correctness of the results, we also conducted a 1:1 PSM analysis, which was used to offset possible effects caused by gaps in the sample data. However, our analysis has some potential limitations. First, our study is a cross-sectional retrospective analysis with its inherent limitations. Second, although adjustments were made for some confounding factors, the effects of other unknown factors could not be excluded. Finally, the type of kidney stones was not available, and different stone types may have different results.
Conclusion
In conclusion, this study suggests that dietary copper intake was significantly and negatively associated with kidney stones in NHANES participants and that dietary copper intake may play an important role in the prevention of kidney stones in future clinical practice.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was reviewed and approved by the NHANES Committee. Written informed consent was obtained from all the patients/participants for their participation in this study and for the publication of any potentially identifiable images or data included in this article, following the Declaration of Helsinki.
Author contributions
SC, WM, YC, and MC designed the research. WZ, CW, JW, and WM performed the research and analyzed the results. WZ and CW wrote the paper. JW, SC, WM, YC, and MC edited the manuscript and provided the critical comments. All authors read and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China (82070773), Natural Science Foundation of Jiangsu Provincial (BK20201271), Jiangsu Provincial Key Research and Development Program (BE2019751), Innovative Team of Jiangsu Provincial (2017ZXKJQW07), and the National Key Research and Development Program of China (SQ2017YFSF090096).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.973887/full#supplementary-material
Spearman's rank correlation coefficients of six trace elements.
Multivariate multi-metal model logistic regression the relationship between the heavy metals and the presence of kidney stone.
References
- 1.Singh P, Harris PC, Sas DJ, Lieske JC. The genetics of kidney stone disease and nephrocalcinosis. Nat Rev Nephrol. (2022) 18:224–40. 10.1038/s41581-021-00513-4 [DOI] [PubMed] [Google Scholar]
- 2.Zisman AL, Coe FL, Cohen AJ, Riedinger CB, Worcester EM. Racial differences in risk factors for kidney stone formation. Clin J Am Soc Nephrol. (2020) 15:1166–73. 10.2215/CJN.12671019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao W, Hu Q, Chen S, Chen Y, Luo M, Zhang Z, et al. Polyfluoroalkyl chemicals and the risk of kidney stones in US adults: a population-based study. Ecotoxicol Environ Saf. (2021) 208:111497. 10.1016/j.ecoenv.2020.111497 [DOI] [PubMed] [Google Scholar]
- 4.Denburg MR, Koepsell K, Lee JJ, Gerber J, Bittinger K, Tasian GE. Perturbations of the gut microbiome and metabolome in children with calcium oxalate kidney stone disease. J Am Soc Nephrol. (2020) 31:1358–69. 10.1681/ASN.2019101131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Islam AK, Holt S, Reisch J, Nwariaku F, Antonelli J, Maalouf NM. What predicts recurrent kidney stone after parathyroidectomy in patients with primary hyperparathyroidism? J Am Coll Surg. (2020) 231:74–82. 10.1016/j.jamcollsurg.2020.04.015 [DOI] [PubMed] [Google Scholar]
- 6.Lohan MC, Tagliabue A. Oceanic micronutrients: trace metals that are essential for marine life. Elements. (2018) 14:385–90. 10.2138/gselements.14.6.385 [DOI] [Google Scholar]
- 7.Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol. (2014) 7:60–72. 10.2478/intox-2014-0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Komleh K, Hada P, Pendse AK, Singh PP. Zinc, copper and manganese in serum, urine and stones. Int Urol Nephrol. (1990) 22:113–8. 10.1007/BF02549826 [DOI] [PubMed] [Google Scholar]
- 9.Hofbauer J, Steffan I, Hobarth K, Vujicic G, Schwetz H, Reich G, et al. Trace elements and urinary stone formation: new aspects of the pathological mechanism of urinary stone formation. J Urol. (1991) 145:93–6. 10.1016/S0022-5347(17)38256-3 [DOI] [PubMed] [Google Scholar]
- 10.Atakan IH, Kaplan M, Seren G, Aktoz T, Gul H, Inci O. Serum, urinary and stone zinc, iron, magnesium and copper levels in idiopathic calcium oxalate stone patients. Int Urol Nephrol. (2007) 39:351–6. 10.1007/s11255-006-9050-4 [DOI] [PubMed] [Google Scholar]
- 11.Tang J, McFann K, Chonchol M. Dietary zinc intake and kidney stone formation: evaluation of NHANES III. Am J Nephrol. (2012) 36:549–53. 10.1159/000345550 [DOI] [PubMed] [Google Scholar]
- 12.Ferraro PM, Gambaro G, Curhan GC, Taylor EN. Intake of trace metals and the risk of incident kidney stones. J Urol. (2018) 199:1534–9. 10.1016/j.juro.2018.01.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ge EJ, Bush AI, Casini A, Cobine PA, Cross JR, DeNicola GM, et al. Connecting copper and cancer: from transition metal signalling to metalloplasia. Nat Rev Cancer. (2022) 22:102–13. 10.1038/s41568-021-00417-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cobine PA, Moore SA, Leary SC. Getting out what you put in: copper in mitochondria and its impacts on human disease. Biochim Biophys Acta Mol Cell Res. (2021) 1868:118867. 10.1016/j.bbamcr.2020.118867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bost M, Houdart S, Oberli M, Kalonji E, Huneau JF, Margaritis I. Dietary copper and human health: current evidence and unresolved issues. J Trace Elem Med Biol. (2016) 35:107–15. 10.1016/j.jtemb.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 16.El Sabry MI, Stino FKR, El-Ghany WAA. Copper: benefits and risks for poultry, livestock, and fish production. Trop Anim Health Prod. (2021) 53:487. 10.1007/s11250-021-02915-9 [DOI] [PubMed] [Google Scholar]
- 17.Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. (2022) 375:1254–61. 10.1126/science.abf0529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao W, Wu J, Zhang Z, Xu Z, Xu B, Chen M. Neutrophil-lymphocyte ratio acts as a novel diagnostic biomarker for kidney stone prevalence and number of stones passed. Transl Androl Urol. (2021) 10:77–86. 10.21037/tau-20-890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mao W, Wang K, Xu B, Zhang H, Sun S, Hu Q, et al. ciRS-7 is a prognostic biomarker and potential gene therapy target for renal cell carcinoma. Mol Cancer. (2021) 20:142. 10.1186/s12943-021-01443-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ndagi U, Mhlongo N, Soliman ME. Metal complexes in cancer therapy - an update from drug design perspective. Drug Des Devel Ther. (2017) 11:599–616. 10.2147/DDDT.S119488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dawood MAO. Dietary copper requirements for aquatic animals: a review. Biol Trace Elem Res. (2022). 10.1007/s12011-021-03079-1. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 22.Chen C, Zhou Q, Yang R, Wu Z, Yuan H, Zhang N, et al. Copper exposure association with prevalence of non-alcoholic fatty liver disease and insulin resistance among US adults (NHANES 2011-2014). Ecotoxicol Environ Saf. (2021) 218:112295. 10.1016/j.ecoenv.2021.112295 [DOI] [PubMed] [Google Scholar]
- 23.Gao Y, Liu Y, Wang P, Meng X, Zhang W, Sun Y. Serum copper and zinc levels and urinary incontinence in adult women. Biol Trace Elem Res. (2021) 199:842–9. 10.1007/s12011-020-02205-9 [DOI] [PubMed] [Google Scholar]
- 24.Bagheri B, Akbari N, Tabiban S, Habibi V, Mokhberi V. Serum level of copper in patients with coronary artery disease. Niger Med J. (2015) 56:39–42. 10.4103/0300-1652.149169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iakovidis I, Delimaris I, Piperakis SM. Copper and its complexes in medicine: a biochemical approach. Mol Biol Int. (2011) 2011:594529. 10.4061/2011/594529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arredondo M, Nunez MT. Iron and copper metabolism. Mol Aspects Med. (2005) 26:313–27. 10.1016/j.mam.2005.07.010 [DOI] [PubMed] [Google Scholar]
- 27.Witt B, Schaumloffel D, Schwerdtle T. Subcellular localization of copper-cellular bioimaging with focus on neurological disorders. Int J Mol Sci. (2020) 21:2341. 10.3390/ijms21072341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Z, Wang W, Xin X, Song X, Zhang D. Association of total zinc, iron, copper and selenium intakes with depression in the US adults. J Affect Disord. (2018) 228:68–74. 10.1016/j.jad.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 29.Li S, Sun W, Zhang D. Association of zinc, iron, copper, and selenium intakes with low cognitive performance in older adults: a cross-sectional study from national health and nutrition examination survey (NHANES). J Alzheimers Dis. (2019) 72:1145–57. 10.3233/JAD-190263 [DOI] [PubMed] [Google Scholar]
- 30.Yang L, Chen X, Cheng H, Zhang L. Dietary copper intake and risk of stroke in adults: a case-control study based on national health and nutrition examination survey 2013-2018. Nutrients. (2022) 14:409. 10.3390/nu14030409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamb DJ, Avades TY, Ferns GA. Biphasic modulation of atherosclerosis induced by graded dietary copper supplementation in the cholesterol-fed rabbit. Int J Exp Pathol. (2001) 82:287–94. 10.1046/j.1365-2613.2001.00200.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Spearman's rank correlation coefficients of six trace elements.
Multivariate multi-metal model logistic regression the relationship between the heavy metals and the presence of kidney stone.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.