Abstract
In heart failure with reduced ejection fraction (HFrEF), nitric oxide-soluble guanylyl cyclase (sGC) pathway dysfunction impairs skeletal muscle arteriolar vasodilation and thus capillary hemodynamics, contributing to impaired oxygen uptake kinetics. Targeting this pathway with sGC activators offers a new treatment approach to HFrEF. We tested the hypotheses that sGC activator administration would increase the O2 delivery ratio in the skeletal muscle interstitial space (PO2is) of HFrEF rats during twitch contractions due, in part, to increases in red blood cell (RBC) flux (fRBC), velocity (VRBC), and capillary hematocrit (Hctcap). HFrEF was induced in male Sprague-Dawley rats via myocardial infarction. After 3 weeks, rats were treated with 0.3 mg/kg of the sGC activator BAY 60–2770 (HFrEF + BAY; n = 11) or solvent (HFrEF; n = 9) via gavage b.i.d for 5 days prior to phosphorescence quenching (PO2is, in contracting muscle) and intravital microscopy (resting) measurements in the spinotrapezius muscle. Intravital microscopy revealed higher fRBC (70 ± 9 vs 25 ± 8 RBC/s), VRBC (490 ± 43 vs 226 ± 35 μm/s), Hctcap (16 ± 1 vs 10 ± 1%) and a greater number of capillaries supporting flow (91 ± 3 vs 82 ± 3%) in HFrEF + BAY vs HFrEF (all P < 0.05). Additionally, PO2is was especially higher during 12–34s of contractions in HFrEF + BAY vs HFrEF (P < 0.05). Our findings suggest that sGC activators improved resting via increased fRBC, VRBC, and Hctcap allowing for better matching during the rest-contraction transient, supporting sGC activators as a potential therapeutic to target skeletal muscle vasomotor dysfunction in HFrEF.
Keywords: Microcirculation, Nitric oxide, Exercise, Oxygen transport
1. Introduction
Heart failure (HF) is a prevalent chronic disease with high morbidity and mortality [1]. Following myocardial infarction (MI), multiple organ system dysfunction coalesces to constrain skeletal muscle oxygen delivery [2]. Thus, HF severity is often classified by the impaired O2 transport measured via peak oxygen uptake tests ( peak) [3,4]. Within skeletal muscle in HF with reduced ejection fraction (HFrEF) there is decreased nitric oxide (NO) bioavailability, impaired endothelium-dependent arteriolar dilation, capillary involution, and compromised capillary hemodynamics at rest and during muscle contractions, resulting in decreased skeletal muscle perfusive and diffusive O2 transport [5-7]. Accordingly, skeletal muscle in HFrEF has a limited ability to match spatially and temporally resulting in greater deoxygenation (i.e., decreased interstitial O2 partial pressures, PO2is) following the onset of contractions [8].
Ferreira and colleagues [9] found that the NO-donor sodium nitroprusside could acutely improve the microvascular O2 driving pressure (PO2mv) in contracting skeletal muscle of HFrEF rats. However, there has been substantial interest in stimulating the soluble guanylyl cyclase (sGC) vasodilation cascade (i.e., downstream of NO). sGC activators have shown efficacy for increasing enzymatic activity of sGC to generate cyclic guanosine monophosphate (cGMP) independently of NO [10]. sGC activators preferentially target the NO-insensitive, oxidized, pathological, and heme-deficient form of sGC present in HFrEF [10]. In fact, long term treatment with the sGC activator, Ataciguat, in HFrEF-induced rats, reduced mitochondrial superoxide production, enhanced cardiac capillary density, reduced left ventricular filling pressures, and overall showed more effective cardioprotection in comparison to angiotensin-converting enzyme therapy [11]. sGC activators have greater pharmacological activity in diseased (heme-deficient) versus healthy isolated vascular smooth muscle cells by inducing endothelium-independent smooth muscle vasodilation, thereby demonstrating the potential for improving skeletal muscle oxygenation and improved capillary hemodynamics in HFrEF [10]. Therefore, we hypothesized that treatment with a sGC activator would increase the ratio in the skeletal muscle interstitial space (PO2is) of HFrEF rats at rest and during twitch contractions due, in part, to increases in red blood cell (RBC) flux (fRBC), velocity (VRBC), and capillary tube hematocrit (Hctcap).
2. Methods
2.1. Animals
All procedures were approved by the Institutional Animal Care and Use Committee of Kansas State University and conducted according to the National Research Council Guide for the Care and Use of Laboratory Animals. Experiments were performed on male Sprague-Dawley rats (n = 20; 3–4 mo old, Charles River Laboratories, Wilmington, MA). Upon arrival animals were maintained in accredited (Association for the Assessment and Accreditation of Laboratory and Animal Care) animal facilities under a 12:12 h light:dark cycle with food and water provided ad libitum.
2.2. Myocardial infarction (MI) procedure
MI was induced via surgical ligation of the left main coronary artery [12]. Rats were anesthetized with a 2.5% isoflurane-O2 mixture and intubated for mechanical ventilation with a rodent respirator (model 680, Harvard Apparatus, Holliston, MA) for the duration of the surgical procedure. The heart was exposed through a left thoracotomy in the fifth intercostal space and the left main coronary artery was ligated with 6–0 silk suture ~1–2 mm distal to the edge of the left atrium. Ampicillin (50 mg kg −1 i.m.) was injected into the closed incision to reduce susceptibility to infection. Thereafter, analgesic agents bupivacaine (1.5 mg kg −1 s.c.) and buprenorphine (0.01–0.05 mg/kg i.m.) were administered. All rats were then monitored closely for ~6 h post-MI for the development of arrhythmias and signs of stress. Rats were assessed daily for signs of distress (appetite, weight loss/gain, gait/posture, etc.) and care was administered as appropriate according to an intensive 14-day post-operative plan conducted in conjunction with the university veterinary staff.
2.3. Echocardiography
21 days following MI echocardiography was performed to assess left ventricular (LV) dysfunction. Transthoracic echocardiography was performed using a commercially available system (Logiq S8; GE Health Care, Milwaukee, WI) with an 18 MHz linear transducer (L8-18i) as previously described [8]. Rats were initially anesthetized with a 2.5% isoflurane-O2 mixture and placed on a heating pad to maintain core temperature. Standard two-dimensional and M-mode images from the midpapillary level were obtained with frame rates of >50 frames/s. Ventricular dimensions and wall thicknesses were obtained from M-mode measurements over four consecutive cardiac cycles. LV internal dimensions and posterior wall (PW) thicknesses were measured at end systole (LVIDs; PWs) and end diastole (LVIDd; PWd). Fractional shortening (FS) was calculated from the measurements of LV chamber diameters: FS = [(LVIDd − LVIDs)/LVIDd] × 100. LV end-systolic (LVESv) and end-diastolic (LVEDv) volumes were calculated using the Teichholz formula: LV volume = (7.0/2.4 + LV dimension) × LV dimension3. Stroke volume was calculated as: SV = LVEDv- LVESv. Ejection fraction (EF) was calculated as: EF = [LVEDv- LVESv/LVEDv] × 100.
2.4. Drug dosing
Following echocardiography rats were randomly assigned to activator (HFrEF + BAY; n = 11) or control HFrEF (HFrEF; n = 9) groups. The sGC activator BAY 60–2770 (Bayer AG, Pharmaceuticals, Wuppertal, Germany) was weighed and mixed with 10% Transcutol, 20% Cremophor, (Sigma Aldrich, St. Louis, MO), and 70% water to obtain a dose with the final concentration of 0.3 mg/kg BAY 60–2770 in a 1 ml volume. The HFrEF group was given solvent only. Either BAY 60–2770 or solvent was administered via oral gavage. To mimic clinical protocols, the drug/solvent was administered b.i.d. for 5 days prior to the following post-MI experiments.
2.5. Surgical preparations
After completion of the dosing regimen rats were anesthetized initially with a 5% isoflurane-O2 mixture and subsequently maintained on 2.5% isoflurane-O2 while positioned on a heating pad to maintain core temperature at ~37 °C as measured via rectal thermometer. The carotid artery was isolated and cannulated and a 2-French catheter-tip pressure transducer (Millar Instruments, Houston, TX, USA) was advanced into the LV for measurements of systolic and diastolic pressures and changes in LV pressure over time (LV dp/dt). Upon completion of the LV measurements the transducer was removed and the carotid artery was cannulated with a catheter (PE-10 connected to PE-50, Intra-Medic polyethylene tubing, Clay Adams Brand, Benton, Dickson and Company, Sparks, MD, USA) for measurement of mean arterial pressure (MAP) and heart rate (HR) (Digi-Med BPA; Model 400). The caudal artery was catheterized for administration of pentobarbital sodium anesthesia (50 mg/ml) and arterial blood sampling. Rats were then transitioned to pentobarbital sodium anesthesia (20 mg/kg body wt.) given intra-arterially while concentrations of isoflurane were decreased and subsequently discontinued. The depth of anesthesia was regularly monitored via toe pinch and palpebral reflex, with pentobarbital anesthesia supplemented (3.5–7.0 mg/kg) as necessary.
The overlying skin and fascia were carefully removed from the mid-dorsal-caudal region of the rat and the left spinotrapezius muscle was exposed in a manner that did not disrupt vascular supply to the muscle [13]. This muscle was chosen for its similar fiber-type composition and oxidative capacity to the untrained human quadriceps muscle [14]. Silver wire electrodes were then sutured (6-0 silk) to the rostral (cathode) and caudal (anode) regions of the muscle to induce twitch contractions. The exposed muscle was continuously superfused with Krebs-Henseleit bicarbonate-buffered solution (4.7 mM KCl, 2.0 mM CaCl2, 2.4 mM MgSO4, 131 mM NaCl, and 22 mM NaHCO3; pH = 7.4; equilibrated with 5% CO2–95% N2 at 38 °C) and the surrounding tissue was covered with Saran Wrap (Dow Industries, Indianapolis, IN) to minimize dehydration.
2.6. Experimental protocol
Following surgical preparations phosphorescence quenching was used to determine PO2is at rest and during contractions using a frequency domain phosphorimeter (PMOD 500; Oxygen Enterprises, Philadelphia, PA) as previously described [15,16]. Oxyphore G4 (Pd-meso-tetra-(3,5-dicarboxyphenyl)-tetrabenzo-porphyrin) was injected locally into the muscle (3–4 injections at 10-μM concentration) using a 29-G needle. Care was taken to avoid damaging any visible vasculature. This probe allows dynamic visualization of the tissue PO2is levels of the spinotrapezius muscle [17]. Following injections, the spinotrapezius muscle was covered with Saran wrap to protect the muscle from dehydration during a 15-min period which allows for diffusion of Oxyphor G4 throughout the muscle into the interstitial space. A bifurcated light guide was positioned ~3–4 mm above the surface of the exposed muscle in a field absent of large vessels. To minimize any phosphorescent interference all measurements were performed in a dark room with minimal light exposure.
2.7. Contractions protocol
Twitch contractions were electrically evoked (1Hz, 6–7 V, 2-ms pulse duration) with a Grass S88 Stimulator (Quincy, MA) for 180 s PO2is was measured and recorded at 2 s intervals throughout the duration of the protocol. This stimulation protocol elicits a 4-5-fold increase in blood flow and a 7-fold increase in metabolic rate consistent with moderate intensity exercise [18,19]. Immediately following PO2is measurements approximately 0.3 ml of blood was sampled from the caudal artery catheter for the determination of arterial blood lactate ([La−]), pH, PCO2, %O2 saturation, and systemic hematocrit (Nova Stat Profile M; Nova Biomedical, Waltham, MA).
2.8. Analysis of interstitial PO2 kinetics
The kinetics analyses of the PO2is response was measured utilizing the Stern-Volmer relationship. Direct measurement of phosphorescence lifetime yielded PO2is via the following equation:
where is the quenching constant and τ and τ0 are the phosphorescence lifetimes at the ambient O2 concentration and in the absence of O2, respectively. In tissues at 32.3 °C (mean spinotrapezius muscle surface temperature was ~32 °C) the parameters for G4 are as follows: kQ of 258 mmHg−1 s−1 and τ0 of 226 μs [17]. As these parameters are dependent on pH and temperature of the muscle, neither of which change sufficiently to impact the PO2is measurement during the contraction protocol, the resolved phosphorescence lifetime is determined by the PO2is. The kinetics analyses of the PO2is responses were conducted using 10 s of resting data and the 180-s contraction bout using a monoexponential plus time delay model:
or a monoexponential plus time delay using a secondary component when required:
where PO2 (t) represents the PO2is at any point in time, PO2 (BL) is the baseline before the onset of contractions, Δ1 and Δ2 are the primary and secondary amplitudes, TD and TD2 are the time delays for each component, and τ and τ2 are the time constants (i.e., the time required to reach 63% of the amplitude) for the primary and secondary amplitudes. The mean response time was calculated as the sum of the model-derived TD and τ. When the secondary component model was necessary, the primary amplitude was constrained to not exceed the nadir value to maximize accuracy of the primary response kinetics. The goodness of model fit was determined using the following criteria: 1) the coefficient of determination; 2) sum of squared residuals; 3) visual inspection and analysis of the model fits to the data and the residuals. Since the second amplitude (Δ2PO2, undershoot of the PO2) was often monoexponential in nature, it was manually calculated by subtracting the difference between the PO2is at the end of contractions minus the nadir value of PO2is during contractions.
2.9. Intravital microscopy
Following PO2is measurements, electrode sutures were gently removed. The caudal end of the spinotrapezius was carefully exteriorized and sutured (6-0 silk) to a thin wire horseshoe-shaped manifold at five or six equidistant points around the perimeter. The rat was placed on a water-heated Lucite platform with the spinotrapezius positioned such that a microvascular field within the midcaudal and dorsal surface was observed using an intravital microscope (Nikon, Eclipse E600-FN; × 40 objective; 0.8 numerical aperture) equipped with a noncontact illuminated lens and a high-resolution color monitor (total viewing area = 270 × 210 μm; Sony Trinitron PVM-1954Q, Ichinonya, Japan). The muscle was transilluminated to ensure clear resolution of the sarcomere A-bands. The final magnification ( × 1184) was confirmed by initial calibration of the system using a stage micrometer (MA285, Meiji Techno). This magnification is adequate for measuring all essential structural and hemodynamic variables [6,20]. The spinotrapezius muscle was maintained at physiological sarcomere length (~2.7 μm) throughout the subsequent observation period, and any exposed tissue was continuously superfused with the Krebs-Henseleit solution. Once the spinotrapezius muscle was positioned on the platform, 5–10 microvascular viewing fields were each recorded for ~1–1.5 min. Recordings were time referenced and stored for subsequent offline analysis.
2.10. Capillary data analysis
5-7 fields were chosen randomly from each rat for analysis on the basis of clear visualization of sarcomeres, fibers, and capillaries. Capillaries supporting RBC flow were assessed in real time, and each capillary was placed into one of two categories: 1) normal flow = 30 s of continuous, or 2) impeded flow or stopped flow for >10 of 30 s. These criteria were used for determination of percentage of flowing capillaries [i.e. (number of capillaries supporting RBC flow/total number of visible capillaries per area), × 100]. For all capillaries in which hemodynamics were assessed and where the capillary endothelium was clearly visible on both sides of the lumen, capillary luminal diameter was measured (2–4 measurements/capillary) with calipers accurate to ± 0.25 mm (±0.17 μm at × 1184 magnification). Capillary lineal density was determined as the number of capillaries crossing a line drawn perpendicular to the fiber axis.
Examination of the microvascular fields was conducted by frame-by-frame analysis techniques (Dartfish Video Software, Fribourg, Switzerland). Sarcomere length was determined from sets of 10 consecutive in-register sarcomeres (i.e., distance between 11 consecutive A-bands) measured parallel to the muscle fiber longitudinal axis. This measurement was repeated 3–4 times where sarcomeres were visible to obtain a mean sarcomere length for each viewing field. VRBC was determined in all capillaries that were continuously perfused by following the RBC path length. fRBC was measured by counting the number of RBCs in a capillary passing an arbitrary point for several frames. For each capillary in which hemodynamic data were measured, Hctcap was calculated by the following equation:
where volumeRBC is red blood cell volume, which was taken to be 61 μm3 [21] and capillaries were approximated as circular in cross section [22]. Following intravital microscopy the animals were euthanized with an overdose of pentobarbital sodium (>100 mg/kg).
2.11. Heart failure classification
Following experimentation, the heart was excised and the chambers were separated. The LV was opened by making an incision through the interventricular septum, from the base to the apex and was spread out onto a wax sheet. A digital photograph of the endocardium was taken to demarcate between the remodeled (luminous) from healthy (dark red) myocardium. The image was printed and infarct size determined by planimetry as previously described [8,23].
2.12. Western immunoblotting
sGC is a heterodimer consisting of two subunits, α and β [24]. In this study we only investigated the concentration of sGCβ1 within the spinotrapezius muscle, because the literature suggests the β1 subunit is the common dimerizing subunit for all isoforms of sGC and its deletion results in loss of both isoforms [25] making it key to the NO-sGC cascade. To quantify the sGCβ1 subunit expression, samples were homogenized in RIPA buffer (Sigma-Aldrich, St. Louis, MO) containing a protease inhibitor cocktail (1:100 vol:vol; Sigma-Aldrich). A BeadBug 6 homogenizer (Benchmark Scientific, Edison, NJ) with 3.0 mm zirconium beads was used to homogenize the tissues. Although sGCβ1 expression was measured in the entire spinotrapezius muscle, which may be influenced by varying cell types, we expect this to be largely representative of sGCβ1 expression in the myocytes. Following 3–30 s cycles of 4350 rpm with 30 rests, homogenates were centrifuged at 1500 g for 10 min. The supernatant was collected and protein concentration determined following Qubit protocol. Jess Simple Western System (Protein Simple, San Jose, CA, USA) was performed following the instruments protocol. Proteins were loaded at 2 mg/ml with primary antibody (sGCβ1, Novus, NBP1-89784) concentration of 1:50, which is the appropriate antibody concentration with the Jess system. GAPDH (Novus, NB300-221) served as a housekeeping protein. Compass simple western software (V 4.1.0) was used to calculate heights, area, and signal/noise ratio. All samples were run in duplicate and repeated at least twice.
2.13. Statistical analysis
All PO2is curve fitting and statistical analyses were performed using a commercially available software package (SigmaPlot 12.5, Systat Software, San Jose, CA). Student’s paired and unpaired t-tests were performed, when appropriate, to determine differences in morphometric, pressure, and microscopy measurements followed by Pearson correlation tests. A student’s unpaired t-test was used to detect differences in PO2is kinetic parameters between HFrEF + BAY and HFrEF. A two-way repeated measures ANOVA was used to assess temporal interactions (Group x Time). Holm-sidak post hoc tests were used for multiple comparisons when significant differences were detected. Data are presented as means ± SE. Significance was accepted at P < 0.05.
3. Results
3.1. Hemodynamic and morphometric data
20 rats (HFrEF + BAY, n = 11; HFrEF, n = 9) were analyzed for hemodynamic and morphometric data. Prior to dosing the rats, measurements of LV function were assessed via transthoracic echocardiography. No differences were found between groups in any echocardiographic measurements (fractional shortening 20.5 ± 3.0 vs. 21.0 ± 2.9%; ejection fraction 44.7 ± 5.1 vs. 46.3 ± 4.8%; stroke volume 0.8 ± 0.1 vs. 0.9 ± 0.1 ml/beat, HFrEF + BAY vs HFrEF; all p > 0.05). Importantly, LV end-diastolic pressure (LVEDP) (taken immediately prior to terminal experiments) and the index of MI (% of endocardial surface area infarcted; assessed via planimetry) are indicative of moderate HFrEF [23,26] (Table 1) and did not differ between groups.
Table 1.
Hemodynamic and morphometric data.
| HFrEF | HFrEF + BAY | P-value | |
|---|---|---|---|
| Infarct Size (%) | 28 ± 3 | 29 ± 2 | 0.83 |
| LVEDP (mmHg) | 14 ± 1 | 16 ± 1 | 0.46 |
| LV + dP/dt (mmHg/s) | 8082 ± 264 | 7709 ± 397 | 0.60 |
| LV/BW (mg/g) | 2.00 ± 0.04 | 1.96 ± 0.07 | 0.90 |
| RV/BW (mg/g) | 0.62 ± 0.04 | 0.62 ± 0.03 | 0.91 |
| Lung/BW (mg/g) | 3.17 ± 0.10 | 3.75 ± 0.12* | 0.01 |
| Lung Weight (g) | 1.58 ± 0.05 | 1.70 ± 0.08 | 0.16 |
| Body Weight (g) | 500 ± 12 | 454 ± 10* | 0.02 |
| Initial Body Weight (g) | 370 ± 11 | 356 ± 11 | 0.41 |
Data are means ± SE. Analyzed via student’s unpaired t-test. LVEDP, left ventricular end-diastolic blood pressure; LV, left ventricle; +dP/dt, rise in blood pressure over time; RV, right ventricle; BW, body weight.
P < 0.05 vs HFrEF; HFrEF, n = 9; HFrEF + BAY, n = 11.
3.2. Phosphorescence quenching
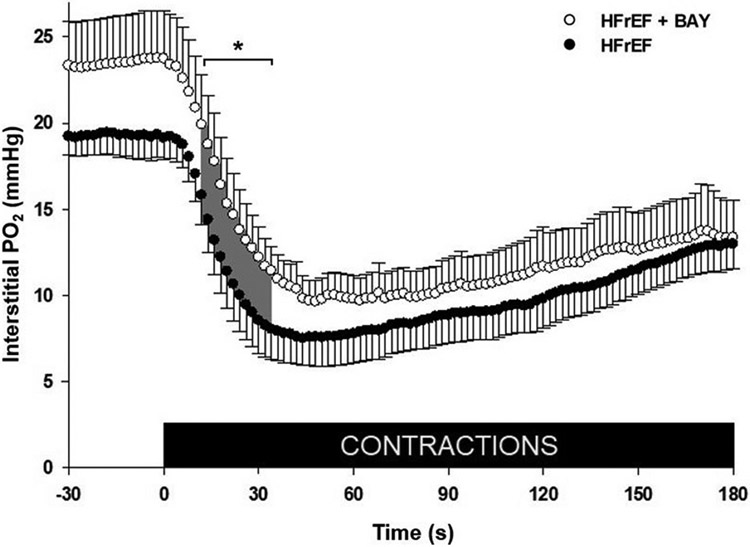
19 rats were assessed for the PO2is measurements due to one not completing the protocol (HFrEF + BAY n = 10; HFrEF n = 9). Mean PO2is profiles at rest and during contractions are presented in Fig. 1. There were no significant differences in MAP or HR during contractions (Table 2). Following contractions there was a lower lactate concentration in HFrEF + BAY (1.06 ± 0.07 vs 1.34 ± 0.12 mmol/L), but no differences in blood gases (PaO2: 86.9 ± 2.5 vs 87.2 ± 1.8 mmHg; SaO2: 95.8 ± 0.4 vs 95.6 ± 0.4%) or pH (7.4 ± 0.01, both groups) were observed, HFrEF + BAY vs HFrEF, respectively. No significant differences were identified for the kinetics parameters between HFrEF + BAY and HFrEF (P > 0.05; Table 3). However, PO2is values were particularly higher in HFrEF + BAY rats (P < 0.05; Fig. 1) during 12–34 s of contractions.
Fig. 1. Interstitial PO2 from rest to contractions in spinotrapezius muscle.
Data are means ± SE. Analyzed via two-way ANOVA (Group x Time). Group average spinotrapezius interstitial PO2 (PO2is) at rest and during electrically induced contractions for HFrEF + BAY (open circles; n = 10) and HFrEF (filled circles; n = 9); contractions beginning at 0s. *P < 0.05 (shaded area; 12–34 s).
Table 2.
Central hemodynamics during phosphorescence quenching.
| HFrEF | HFrEF + BAY | P-value | |
|---|---|---|---|
| Heart Rate (bpm) | 359 ± 6 | 357 ± 5 | 0.80 |
| MAP (mmHg) | 107 ± 5 | 105 ± 2 | 0.53 |
Data are means ± SE. Analyzed via student’s unpaired t-test. MAP, mean arterial pressure. HFrEF, n = 9; HFrEF + BAY, n = 11.
Table 3.
Interstitial PO2 kinetics parameters of spinotrapezius at rest and during 180 s of contractions.
| HFrEF | HFrEF + BAY | P-value | |
|---|---|---|---|
| PO2(BL), mmHg | 23.0 ± 1.9 | 19.2 ± 1.2 | 0.08 |
| Δ1PO2, mmHg | 11.9 ± 1.2 | 13.0 ± 2.1 | 0.61 |
| T, S | 12 ± 1 | 15 ± 1 | 0.09 |
| TD, S | 9 ± 1 | 6 ± 1 | 0.12 |
| MRT, S | 26 ± 4 | 24 ± 4 | 0.73 |
| PO 2(Nadir) | 7.4 ± 1.3 | 9.9 ± 1.3 | 0.12 |
| Δ2PO2, mmHg | 5.1 ± 1.2 | 3.4 ± 0.6 | 0.46 |
| PO2(End), mmHg | 12.9 ± 1.7 | 13.3 ± 1.6 | 0.57 |
| AUC, mmHg | 1100 ± 123 | 1321 ± 114 | 0.26 |
| ΔPO2/ T, mmHg/S | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.31 |
Data are means ± SE. PO2(BL), baseline interstitial PO2; Δ1PO2, primary amplitude; τ, timepoint of τ; TD, time delay, MRT, mean response time; PO2(nadir), lowest response of PO2 profile; Δ2PO2, secondary amplitude, PO2(End), interstitial PO2 at end of contractions; AUC, area under the curve, total PO2 throughout experimentation; ΔPO2/τ, change in PO2 for a given τ. Compared via two-tailed unpaired t tests.
3.3. RBC hemodynamics
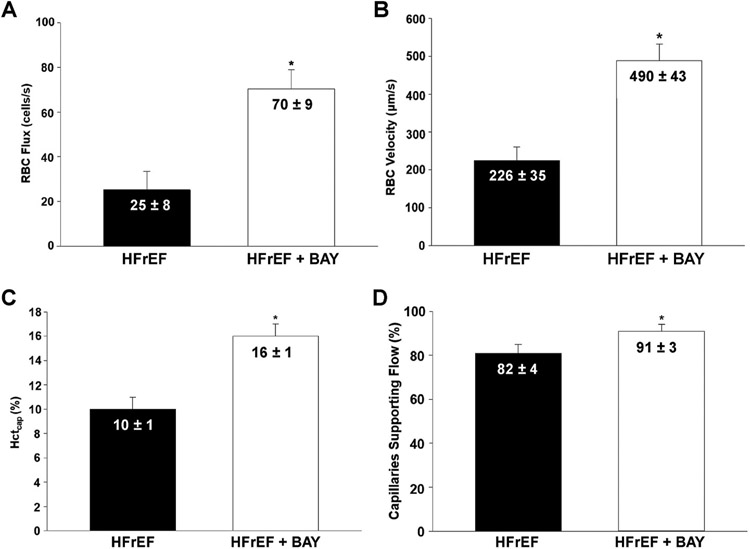
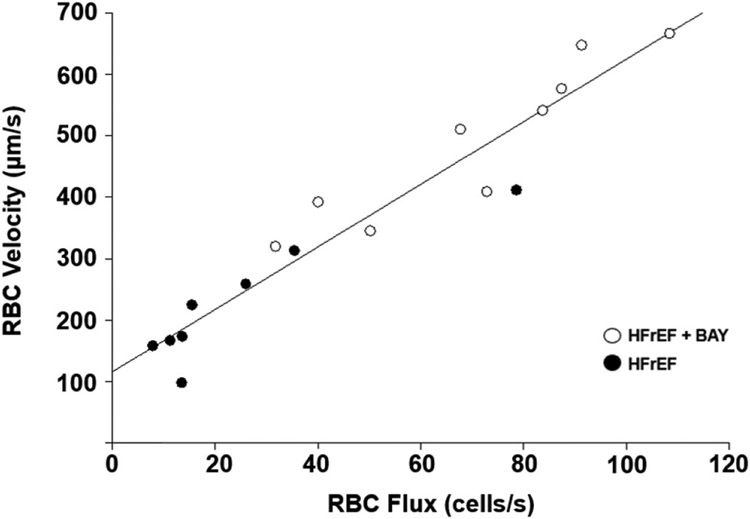
17 rats (HFrEF + BAY; n = 9, HFrEF; n = 8) were utilized for intravital capillary hemodynamic measurements due to two animals losing cardiovascular stability during measurements. Sarcomere length was not different across groups and conditions, ranging from 2.4 to 2.7 μm. No differences in capillary lineal density for all capillaries were seen in HFrEF + BAY vs HFrEF (P = 0.33). Increased fRBC, VRBC. Hctcap, and % of capillaries with RBC flow were observed in HFrEF + BAY vs HFrEF (P < 0.05; Fig. 2). VRBC was positively and linearly correlated with fRBC (R = 0.958; Fig. 3).
Fig. 2. Capillary hemodynamics in spinotrapezius muscle at rest.
Data are means ± SE. Analyzed via student’s unpaired t-test. A. RBC flux in skeletal muscle capillaries supporting flow. B. RBC velocity in skeletal muscle capillaries supporting flow. C. Capillary hematocrit in skeletal muscle capillaries. D. Percentage of capillaries supporting RBC flow. HFrEF + BAY n = 9; HFrEF n = 8; all * P < 0.05.
Fig. 3. Relationship between average RBC velocity and flux in capillaries supporting continuous flow in HFrEF + BAY and HFrEF.
(P < 0.05).
3.4. Western immunoblotting
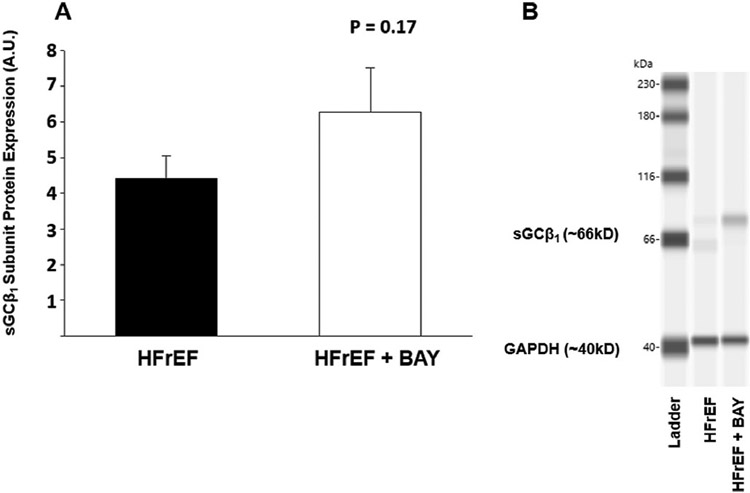
18 rats (HFrEF + BAY; n = 10, HFrEF; n = 8) were used to assess sGCβ1 expression within the spinotrapezius muscle. Group means were not statistically significant (P = 0.17); however, group averages show BAY 60–2770 allowing for an increase in sGCβ1 expression compared to the HFrEF group (Fig. 4).
Fig. 4. Western immunoblotting.
Data are means ± SE. A. Normalized protein expression in HFrEF (n = 8) vs HFrEF + BAY (n = 10) (P > 0.05). B. Representative blotting of sGCβ1 expression in HFrEF vs HFrEF + BAY.
4. Discussion
This is the first investigation, to our knowledge, to study the effects of sGC activation (via BAY 60–2770) on capillary hemodynamics and PO2is in the skeletal muscle of HFrEF rats. sGC activator increased PO2is at the onset of contractions (12–34 s; Fig. 1) and improved capillary hemodynamics (increased fRBC, VRBC, and Hctcap; Fig. 2A-D). This supports the notion that sGC activators may target dysfunctional sGC to allow for improved matching in the skeletal muscle of HFrEF rats at rest and following the onset of contractions. As previously demonstrated in HFrEF rats, there is a significantly lower PO2is [8], capillary fRBC, VRBC and percentage of capillaries flowing [6] which impairs and likely contributes to exercise intolerance. Novel therapeutic agents, such as BAY 60–2770, have been developed to increase sGC bioactivity >200-fold, regardless of redox state. However, western immunoblotting data herein show no significant differences in sGCβ1 expression in the spinotrapezius in HFrEF + BAY rats (P = 0.17).
4.1. Resting hemodynamic measurements
Endothelial dysfunction and diminished skeletal muscle blood flow in HFrEF are due, in part, to decreased NO bioavailability via endothelial NO synthase (eNOS) uncoupling, increased reactive O2 species, and ultimately sGC oxidation [27,28]. Determining the temporal and spatial distribution of RBCs, which is impaired in HFrEF [6,7], is imperative to understanding the effect of sGC activation on PO2is and therefore blood-myocyte O2 exchange. Importantly, capillary hemodynamics at rest in HFrEF rats exhibit a decreased fRBC and VRBC, which has been shown to improve with dietary nitrate supplementation [29,30]. Therefore, we hypothesized that sGC activator administration would increase the proportion of capillaries supporting flow and RBC dynamics (fRBC, VRBC, and Hctcap). Indeed, in the skeletal muscle of HFrEF rats, following sGC activator administration, more capillaries supported RBC flow and there was an augmented capillary fRBC (Fig. 2). The particulate nature of blood dictates that the skeletal muscle surface area per capillary available for O2 exchange is a function of Hctcap and capillary length in RBC-flowing capillaries [31,32]. In HFrEF + BAY there were ~10% more capillaries supporting RBC flow, which could contribute to an increased with contractions. Crucially, in HFrEF those capillaries not supporting RBC flow at rest do not start flowing with contractions [7]. More capillaries supporting RBC flow in HFrEF with a greater Hctcap allows for greater perfusive and diffusive O2 delivery (DO2) with higher VRBC, also promoting longitudinal recruitment of capillary surface area, thereby allowing better matching of .
Interestingly, the 3–4 mmHg higher PO2is in HFrEF + BAY rats at rest prior to contractions was not statistically different. It is possible that this reflects, in part, an increased as permitted by the greater . Another potential explanation for this finding resides in the spatial heterogeneity of among individual fiber fields [6,20,31], where it is possible to have an increased fRBC, VRBC, and Hctcap in a smaller region of skeletal muscle, whereas changes in PO2is reflect a larger sample composed as the sum of smaller regions with variable ratios. Notably, the elevated fRBC, VRBC, and Hctcap in HFrEF + BAY at rest were associated with increased dynamic matching as metabolic demands dramatically increase (i.e. 12-34 s during contractions) (Fig. 1).
In accordance with our findings, other studies have substantiated the pharmaceutical potency of sGC activators and stimulators in improving endothelial function following MI [11,33]. Importantly, in vivo, it was shown that the use of sGC activator BAY 58–2667, an analogue of BAY 60–2770, increased whole protein expression of the heme-binding sGCβ1 subunit in pathological vessels [34]. However, we measured sGCβ1 subunit expression in the spinotrapezius and documented no significant increase in sGCβ1 among groups.
4.2. Contracting skeletal muscle measurements
sGC activators, which have been previously under-studied in skeletal muscle, can decrease progressive cardiac remodeling following MI [11, 34] and improve vascular smooth muscle function throughout the pulmonary vasculature [35,36]. Much of the literature supporting sGC activators as a therapeutic in HFrEF has focused on improving cardiac and pulmonary function [11,35], however, improving the peripheral vascular function in HFrEF via sGC activators offers a potential therapeutic avenue to improve exercise tolerance and promote effective exercise rehabilitation.
Skeletal muscle PO2is kinetics reflect the dynamic matching between and within the interstitial space during transitions in metabolic demand [8,15,16]. In health, with the onset of muscular contractions there is a rapid increase in (initial phase ~20 s) to meet mitochondrial elevated demands [32,37-39]. This elicits shear-stress mediated vasodilation in locomotory skeletal muscle [40], followed by the secondary phase which reflects, in part, feedback control to maintain matching [41]. However, peripheral derangements in rats with moderate HFrEF reduce capillary fRBC and VRBC [6]. This decrease in shear-stress on the luminal side of the endothelium hampers eNOS expression [9] and thereby impairs perfusive and diffusive O2 delivery across the rest-to-contraction transition [6]. Notably, a more precipitous fall in PO2is following the onset of contractions in HFrEF, compared to health, is indicative of a slower increase in relative to [8]. Prior studies from our laboratory demonstrated that 5 days of dietary nitrate supplementation in HFrEF rats enhances matching (i.e., PO2is, 29), potentially via increased percentage of capillaries supporting blood flow and greater fRBC at rest and during contractions [30]. Within the capillary bed, there is a vast surface area for O2 exchange, but diminished bulk blood flow and altered RBC distribution among capillaries limits matching in skeletal muscles at rest [6] and during contractions in HFrEF [7,8].
Increased PO2is in HFrEF + BAY compared to HFrEF demonstrated herein is expected to speed kinetics, thereby decreasing the O2 deficit and reliance upon anaerobic pathways for ATP regeneration. This will help support sustained exercise bouts by reducing intracellular metabolic perturbations that accelerate glycogenolysis and contribute to exhaustion [42,43]. Evidence supporting this notion, including the lower blood lactate concentration following twitch contractions in HFrEF + BAY compared to HFrEF herein indicate either a reduced glycolysis during contractions, improved lactate removal, or both [2,42,44]. Improving metabolism is imperative in HFrEF, because previous studies have shown accelerated glycolysis and glycogenolysis in response to an increased proportion and recruitment of type II fibers in HFrEF skeletal muscle [44-46] therefore decreasing their exercise capacity. Moreover, as DO2 is determined by the number of RBCs adjacent to the contracting skeletal muscle fibers [31], the ~60% Hctcap increase with sGC activation will elevate DO2. This, combined with the enhanced perfusive O2 delivery, may allow the HFrEF + BAY rats to speed their kinetics further increasing exercise tolerance.
4.3. Clinical implications
Improving exercise tolerance is imperative to decreasing the morbidity and mortality in HFrEF patients. This study indicates that sGC activators may be beneficial to combating the exercise intolerance characteristic of HFrEF patients by increasing skeletal muscle via activation of oxidized sGC. Although this study utilizes single muscle twitch contractions, evidence suggests that targeting individual muscles or muscle groups in patient populations can improve peripheral perfusive and diffusive O2 transport and exercise capacity [47].
HFrEF patients function largely in the O2-delivery-dependent region with respect to kinetics (to the left of the “tipping point”, 2). Thus, any elevated muscle O2 delivery has the potential to speed kinetics and enhance exercise tolerance [2]. With increased sodium, and thus fluid retention, the progression and severity of HFrEF worsens. Interestingly, in HFrEF, sGC activators have recently been shown to decrease sympathetic outflow and increase renal perfusion (via increases in cGMP) [48]. There was a decreased body weight with HFrEF + BAY which may be due to decreased fluid retention or appetite in the BAY group, or potentially increased production of brown adipose tissue in place of the less metabolically active white adipose tissue [49].
4.4. Experimental considerations
The present investigation assessed the use of sGC activators in male rats. Women have a lower risk of developing heart disease until post-menopausel, when the incidence increases dramatically. Recent data suggests that women rely on greater basal NO bioavailability in HFrEF [15], therefore, future studies warrant investigating PO2is and capillary hemodynamics in female rats and ovariectomized female rats receiving the sGC activator. In addition, assessing capillary hemodynamics microscopically presents challenges regarding small sampling area/volume and variability among sampled fields. These were overcome partially by using rigorous techniques on several different locations per muscle to limit errors in assessing capillary hemodynamics. Furthermore, participation of multiple independent blinded investigators provided confidence in quality control. Lastly, sGC activators are capable of activating the oxidized heme on sGC, therefore, in order to assess the entirety of its effects in the microcirculation a larger MI%, (i.e., >40% of LV damage) resulting in greater increases in ROS (i.e., more pathological sGC), is necessary in order to assess the utility of sGC activators across the broader range of HFrEF severity possible in the rat post-MI model [50].
5. Conclusions
This study is the first to demonstrate efficacy of administration of a sGC activator in a HFrEF model in the skeletal muscle microcirculation and its ability to increase relative to at rest and during the exercise (muscle contraction) transient. Specifically, at rest the sGC activator increased the proportion of capillaries supporting flow, and elevated fRBC, VRBC, and Hctcap. PO2is was also higher during the rest-contraction transient suggesting improved matching during submaximal exercise. The increased components of perfusive and diffusive O2 transport in HFrEF + BAY demonstrated herein have significant implications for sGC activators as a potential therapeutic avenue in HFrEF to improve exercise tolerance and quality of life.
Funding
This work was supported in part by Grant #BG6342 awarded to D.C.P. by Bayer AG and the Sustained Momentum for Investigators with Laboratories Established grant from Kansas State University, College of Veterinary Medicine.
Footnotes
Declaration of competing interest
Peter Sandner is an employee of Bayer AG, Pharmaceuticals.
References
- [1].Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain A, Chang A, Chang S, Das S, Delling F, Djousse L, Ferguson J, Fornage M, Jordan L, Khan S, Kissela B, Knutson K, Kwan T, Lackland D, Lewis T, Lichtman J, Longenecker C, Loop M, Lutsey P, Martin S, Matsushita K, Moran A, Mussolino M, O’Flaherty M, Pandry A, Perak A, Rosamond W, Roth G, Sampson U, Satou G, Schroeder E, Shah S, Spartano N, Stokes A, Tirschwell D, Tsao C, Turakhia M, VanWagner L, Wilkins J, Wong S, Virani S, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee, Heart disease and stroke statistics—2019 update: a report from the American Heart Association, Circulation 139 (10) (2019) e56–e528, 2019. [DOI] [PubMed] [Google Scholar]
- [2].Poole DC, Hirai DM, Copp SW, Musch TI, Muscle oxygen transport and utilization in heart failure: implications for exercise (in) tolerance, Am. J. Physiol. Heart Circ. Physiol 302 (5) (2012) H1050–H1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Weber KT, Kinasewitz GT, Janicki JS, Fishman AP, Oxygen utilization and ventilation during exercise in patients with chronic cardiac failure, Circulation 65 (6) (1982) 1213–1223. [DOI] [PubMed] [Google Scholar]
- [4].Crespo-Leiro MG, Metra M, Lund LH, Milicic D, Costanzo MR, Filippatos G, Gustafsson F, Tsui S, Barge-Caballero E, De Jonge N, Frigerio M, Hamdan R, Hasin T, Hülsmann M, Nalbantgil S, Potena L, Bauersachs J, Gkouziouta A, Ruhparwar A, Ristic AD, Straburzynska-Migaj E, Mcdonagh T, Serferovic P, Ruschitzka F, Advanced heart failure: a position statement of the heart failure association of the European society of cardiology, Eur. J. Heart Fail 20 (11) (2018) 1505–1535. [DOI] [PubMed] [Google Scholar]
- [5].Drexler H, Hayoz D, Münzel T, Hornig B, Just H, Brunner HR, Zelis R, Endothelial function in chronic congestive heart failure, Am. J. Cardiol 69 (19) (1992) 1596–1601. [DOI] [PubMed] [Google Scholar]
- [6].Kindig CA, Musch TI, Basaraba RJ, Poole DC, Impaired capillary hemodynamics in skeletal muscle of rats in chronic heart failure, J. Appl. Physiol 87 (2) (1999) 652–660. [DOI] [PubMed] [Google Scholar]
- [7].Richardson TE, Kindig CA, Musch TI, Poole DC, Effects of chronic heart failure on skeletal muscle capillary hemodynamics at rest and during contractions, J. Appl. Physiol 95 (3) (2003) 1055–1062. [DOI] [PubMed] [Google Scholar]
- [8].Craig JC, Colburn TD, Caldwell JT, Hirai DM, Tabuchi A, Baumfalk DR, Behnke BJ, Ade CJ, Musch TI, Poole DC, Central and peripheral factors mechanistically linked to exercise intolerance in heart failure with reduced ejection fraction, Am. J. Physiol. Heart Circ. Physiol 317 (2) (2019) H434–H444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ferreira LF, Hageman KS, Hahn SA, Williams J, Padilla DJ, Poole DC, Musch TI, Muscle microvascular oxygenation in chronic heart failure: role of nitric oxide availability, Acta Physiol. 188 (1) (2006) 3–13. [DOI] [PubMed] [Google Scholar]
- [10].Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TYHS, Arun Meurer S., Deile M, Taye A, Knorr A, Lapp H, Müller H, Turgay Y, Rothkegel C, Tersteegen A, Kemp-Harper B, Müller-Esterl W, Schmidt HH, Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels, J. Clin. Invest 116 (9) (2006) 2552–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fraccarollo D, Galuppo P, Motschenbacher S, Ruetten H, Schäfer A, Bauersachs J, Soluble guanylyl cyclase activation improves progressive cardiac remodeling and failure after myocardial infarction: cardioprotection over ACE inhibition, Basic Res. Cardiol 109 (4) (2014) 421. [DOI] [PubMed] [Google Scholar]
- [12].Musch TI, Terrell JA, Skeletal muscle blood flow abnormalities in rats with a chronic myocardial infarction: rest and exercise, Am. J. Physiol. Heart Circ. Physiol 262 (2) (1992) H411–H419. [DOI] [PubMed] [Google Scholar]
- [13].Bailey JK, Kindig CA, Behnke BJ, Musch TI, Schmid-Schoenbein GW, Poole DC, Spinotrapezius muscle microcirculatory function: effects of surgical exteriorization, Am. J. Physiol. Heart Circ. Physiol 279 (2000) H3131–H3137. [DOI] [PubMed] [Google Scholar]
- [14].Delp MD, Duan C, Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle, J. Appl. Physiol 80 (1) (1996) 261–270. [DOI] [PubMed] [Google Scholar]
- [15].Craig JC, Colburn TD, Hirai DM, Musch TI, Poole DC, Sexual dimorphism in the control of skeletal muscle interstitial PO2 of heart failure rats: effects of dietary nitrate supplementation, J. Appl. Physiol 126 (5) (2019) 1184–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Colburn TD, Hirai DM, Craig JC, Ferguson SK, Weber RE, Schulze KM, Behnke BJ, Musch TI, Poole DC, Transcapillary PO2 Gradients in contracting muscles across the fibre type and oxidative continuum, J. Physiol 598 (15) (2020) 3187–3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Esipova TV, Karagodov A, Miller J, Wilson DF, Busch TM, Vinogradov SA, Two new “protected” oxyphors for biological oximetry: properties and application in tumor imaging, Anal. Chem 83 (22) (2011) 8756–8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Behnke BJ, Barstow TJ, Kindig CA, McDonough P, Musch TI, Poole DC, Dynamics of oxygen uptake following exercise onset in rat skeletal muscle, Respir. Physiol. Neurobiol 133 (3) (2002) 229–239. [DOI] [PubMed] [Google Scholar]
- [19].Hirai DM, Copp SW, Holdsworth CT, Ferguson SK, McCullough DJ, Behnke BJ, Musch TI, Poole DC, Skeletal muscle microvascular oxygenation dynamics in heart failure: exercise training and nitric oxide-mediated function, Am. J. Physiol. Heart Circ. Physiol 306 (5) (2014) H690–H698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Poole DC, Musch TI, Kindig CA, In vivo microvascular structural and functional consequences of muscle length changes, Am. J. Physiol. Heart Circ. Physiol 272 (5) (1997) H2107–H2114. [DOI] [PubMed] [Google Scholar]
- [21].Altman PL, Dittmer DS, Biology Data Book, second ed., FASEB, Bethesda, MD, 1964, pp. 1598–1613. [Google Scholar]
- [22].Kindig CA, Sexton WL, Fedde MR, Poole DC, Skeletal muscle microcirculatory structure and hemodynamics in diabetes, Respir. Physiol 111 (1998) 163–175. [DOI] [PubMed] [Google Scholar]
- [23].Pfeffer MA, Pfeffer JM, Fishbein MC, Fletcher PJ, Spadaro J, Kloner RA, Braunwald E, Myocardial infarct size and ventricular function in rats, Circ. Res 44 (4) (1979) 503–512. [DOI] [PubMed] [Google Scholar]
- [24].Sandner P, Zimmer DP, Milne GT, Follmann M, Hobbs A, Stasch JP, Soluble Guanylate Cyclase Stimulators and Activators: Handbook of Experimental Pharmacology, 2021, pp. 355–394. [DOI] [PubMed] [Google Scholar]
- [25].Friebe A, Mergia E, Dangel O, Lange A, Koesling D, Fatal gastrointestinal obstruction and hypertension in mice lacking nitric oxide-sensitive guanylyl cyclase, Proc. Natl. Acad. Sci. Unit. States Am 104 (18) (2007) 7699–7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Musch TI, Elevated diaphragmatic blood flow during submaximal exercise in rats with chronic heart failure, Am. J. Physiol. Heart Circ. Physiol 265 (5) (1993) H1721–H1726. [DOI] [PubMed] [Google Scholar]
- [27].Hirai T, Zelis R, Musch TI, Effects of nitric oxide synthase inhibition on the muscle blood flow response to exercise in rats with heart failure, Cardiovasc. Res 30 (3) (1995) 469–476. [PubMed] [Google Scholar]
- [28].Münzel T, Daiber A, Ullrich V, Mülsch A, Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinase, Arterioscler. Thromb. Vasc. Biol 25 (8) (2005) 1551–1557. [DOI] [PubMed] [Google Scholar]
- [29].Ferguson SK, Holdsworth CT, Colburn TD, Wright JL, Craig JC, Fees A, Jones A, Allen J, Musch TI, Poole DC, Dietary nitrate supplementation: impact on skeletal muscle vascular control in exercising rats with chronic heart failure, J. Appl. Physiol 121 (3) (2016) 661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ferguson S, Holdsworth C, Wright J, Fees A, Musch TI, Poole DC, Impact of nitrate supplementation via beetroot juice on capillary hemodynamics in skeletal muscle of rats in chronic heart failure (1106.16), Faseb. J (2014). [Google Scholar]
- [31].Federspiel WJ, Popel AS, A theoretical analysis of the effect of the particulate nature of blood on oxygen release in capillaries, Microvasc. Res 32 (2) (1986) 164–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kindig CA, Richardson TE, Poole DC, Skeletal muscle capillary hemodynamics from rest to contractions: implications for oxygen transfer, J. Appl. Physiol 92 (6) (2002) 2513–2520. [DOI] [PubMed] [Google Scholar]
- [33].Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam C, Ponikowski P, Voors A, Jia G, McNulty S, Patel M, Roessig L, Koglin J, O’Connor CM, Vericiguat in patients with heart failure and reduced ejection fraction, N. Engl. J. Med 382 (20) (2020) 1883–1893. [DOI] [PubMed] [Google Scholar]
- [34].Irvine JC, Ganthavee V, Love JE, Alexander AE, Horowitz JD, Stasch JP, Kemp-Harper BK, Ritchie RH, The soluble guanylyl cyclase activator bay 58-2667 selectively limits cardiomyocyte hypertrophy, PLoS One 7 (11) (2012), e44481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Deruelle P, Grover TR, Storme L, Abman SH, Effects of BAY 41–2272, a soluble guanylate cyclase activator, on pulmonary vascular reactivity in the ovine fetus, Am. J. Physiol. Lung Cell Mol. Physiol 288 (4) (2005) L727–L733. [DOI] [PubMed] [Google Scholar]
- [36].Pankey EA, Bhartiya M, Badejo AM Jr., Haider U, Stasch J, Murthy SN, Nossaman BD, Kadowitz P, Pulmonary and systemic vasodilator responses to the soluble guanylyl cyclase activator, BAY 60-2770, are not dependent on endogenous nitric oxide or reduced heme, Am. J. Physiol. Heart Circ. Physiol (2011) H792–H802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jones AM, Poole DC, Oxygen uptake dynamics: from muscle to mouth—an introduction to the symposium, Med. Sci. Sports Exerc 37 (9) (2005) 1542–1550. [DOI] [PubMed] [Google Scholar]
- [38].Koga S, Rossiter HB, Heinonen I, Musch TI, Poole DC, Dynamic heterogeneity of exercising muscle blood flow and O2 utilization, Med. Sci. Sports Exerc 46 (5) (2014) 860. [DOI] [PubMed] [Google Scholar]
- [39].Walsh B, Howlett R, Stary C, Kindig C, Hogan M, Determinants of oxidative phosphorylation onset kinetics in isolated myocytes, Med. Sci. Sports Exerc 37 (9) (2005) 1551–1558. [DOI] [PubMed] [Google Scholar]
- [40].Pohl U, Herlan K, Huang A, Bassenge E, EDRF-mediated shear induced dilation opposes myogenic vasoconstriction in small rabbit arteries, Am. J. Physiol. Heart Circ. Physiol 261 (1991) H2016–H2023. [DOI] [PubMed] [Google Scholar]
- [41].Wunsch SA, Muller-Delp J, Delp MD, Time course of vasodilatory responses in skeletal muscle arterioles: role in hyperemia at onset of exercise, Am. J. Physiol. Heart Circ. Physiol 279 (4) (2000) H1715–H1723. [DOI] [PubMed] [Google Scholar]
- [42].Richardson RS, Noyszewski EA, Leigh JS, Wagner PD, Lactate efflux from exercising human skeletal muscle: role of intracellular PO2, J. Appl. Physiol 85 (2) (1998) 627–634. [DOI] [PubMed] [Google Scholar]
- [43].Massie BM, Conway M, Rajagopalan B, Yonge R, Frostick S, Ledingham J, Sleight P, Radda G, Skeletal muscle metabolism during exercise under ischemic conditions in congestive heart failure: evidence for abnormalities unrelated to blood flow, Circulation 78 (2) (1988) 320–326. [DOI] [PubMed] [Google Scholar]
- [44].Drexler H, Riede U, Münzel T, König H, Funke E, Just H, Alterations of skeletal muscle in chronic heart failure, Circulation 85 (5) (1992) 1751–1759. [DOI] [PubMed] [Google Scholar]
- [45].Sullivan MJ, Green HJ, Cobb FR, Altered skeletal muscle metabolic response to exercise in chronic heart failure. Relation to skeletal muscle aerobic enzyme activity, Circulation 84 (4) (1991) 1597–1607. [DOI] [PubMed] [Google Scholar]
- [46].Lipkin DP, Jones DA, Round JM, Poole-Wilson PA, Abnormalities of skeletal muscle in patients with chronic heart failure, Int. J. Cardiol 18 (2) (1988) 187–195. [DOI] [PubMed] [Google Scholar]
- [47].Esposito F, Reese V, Shabetai R, Wagner PD, Richardson RS, Isolated quadriceps training increases maximal exercise capacity in chronic heart failure: the role of skeletal muscle convective and diffusive oxygen transport, J. Am. Coll. Cardiol 58 (13) (2011) 1353–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Frees A, Assersen KB, Jensen M, Hansen PB, Vanhoutte PM, Madsen K, Federlein A, Lund L, Toft A, Jensen BL, Natriuretic peptides relax human intrarenal arteries through natriuretic peptide receptor type-A recapitulated by soluble guanylyl cyclase agonists, Acta Physiol. (2020), e13565. [DOI] [PubMed] [Google Scholar]
- [49].Hoffmann LS, Etzrodt J, Willkomm L, Sanyal A, Scheja L, Fischer AW, Stasch JP, Bloch W, Friebe A, Heeren J, Pfeifer A, Stimulation of soluble guanylyl cyclase protects against obesity by recruiting brown adipose tissue, Nat. Commun 6 (1) (2015) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Delp MD, Duan C, Mattson JP, Musch TI, Changes in skeletal muscle biochemistry and histology relative to fiber type in rats with heart failure, J. Appl. Physiol 83 (4) (1997) 1291–1299. [DOI] [PubMed] [Google Scholar]