Abstract
Introduction:
Adolescents and young adults with intellectual and developmental disabilities (IDD) have high rates of obesity and low levels of physical activity. This analysis examined changes in light, moderate-to-vigorous physical activity (MVPA) and sedentary time, and the association between changes in MVPA and weight loss in adolescents and young adults with IDD and overweight and obesity participating in a 6-month multi-component weight loss intervention.
Methods:
Adolescents and young adults with IDD and overweight or obesity (BMI ≥85 percentile, n = 110, age ~16 yrs., 52.7% female) and a parent were randomized to one of 3 intervention groups: face-to-face (FTF) delivery/conventional reduced energy diet (CD) (n=36), remote delivery (RD)/CD (n=39), or RD/reduced energy enhanced stop light diet (eSLD) (n=35.) Participants were asked to engage in 60 min./day of MVPA on 5 or more days/wk. Participants and a parent attended twice monthly education/behavioral counseling sessions with a health educator to assist participants in complying with dietary and MVPA recommendations. Education/counseling in the RD arms was delivered remotely using video conferencing, and self-monitoring of MVPA and daily steps was completed using a wireless activity tracker. Education/counseling in the FTF arm was delivered during home-visits and self-monitoring of MVPA and daily steps was completed by self-report using paper tracking forms designed for individuals with IDD. MVPA, light activity, and sedentary time were assessed over 7 days at baseline and 6 months using a portable accelerometer (ActiGraph wGT3x-BT).
Results:
Mixed modeling analysis completed using participants with valid accelerometer data (i.e., ≥ 4 - 10 hr. days) at baseline (n=68) and 6 months (n=30) revealed no significant changes in light, moderate-MVPA, or sedentary time across the 6-month intervention (all p>0.05). Participants obtained 15.2 ± 21.5 mins/day of MVPA at baseline and 19.7 ± 19.7 mins/day at 6 months (p=0.119). Mixed modeling indicated no significant effects of group (p=0.79), time (p=0.10), or group-by-time interaction (p=0.21) on changes in MVPA from baseline to 6 months. Correlational analysis conducted on participants with valid accelerometer data at both baseline and 6 months (n=24) revealed no significant associations between baseline sedentary time (r=0.10, p=0.40) and baseline MVPA (r=−0.22, p =0.30) and change in MVPA across the 6-month intervention. Additionally, attendance at education/counseling sessions (r=0.26, p =0.22) and frequency of self-monitoring of MVPA were not significantly associated with change in MVPA from baseline to 6 months (r= 0.26, p =0.44). Baseline MVPA (r=0.02, p =0.92) and change in MVPA from baseline to 6 months (r=0.13, p =0.30) were not associated with changes in body weight across the 6-month intervention.
Conclusion:
We observed a non-significant increase in MVPA (30%) which was not associated with the magnitude of weight loss in a sample of adolescents and young adults with IDD who participated in a 6-month multi-component weight loss intervention. Additional strategies to increase MVPA in adolescents and young adults with IDD participating in weight loss interventions need to be developed and evaluated.
Keywords: Accelerometer, Down syndrome, Autism, Weight Loss, Exercise, Youth
INTRODUCTION
Adolescents with intellectual and developmental disabilities (IDD) have higher rates of obesity and lower levels of moderate-to-vigorous physical activity (MVPA) compared with their typically developing peers. The risk of obesity in adolescents with IDD (age ≥ 11-18 yrs.) is 1.8 times greater than their typically developing peers (Maïano et al., 2016). The prevalence of overweight is especially high in adolescents with Down Syndrome and autism spectrum disorder (Grondhuis and Aman, 2014, Healy et al., 2019, Bertapelli et al., 2016). Daily MVPA is lower in adolescents with IDD compared to their typically developing peers (Troiano et al., 2008, Frey et al., 2008). A number of studies have reported that no adolescents with IDD achieve the 60 min./day of MVPA as recommended by the U.S. Department of Health and Human Services for all youth, including those with IDD (Piercy et al., 2018) when MVPA was assessed objectively by accelerometer (Matute-Llorente et al., 2013, Esposito et al., 2012, Phillips and Holland, 2011). However, a study by Izquierdo-Gomez et al (Izquierdo-Gomez et al., 2014), reported that 43/100 (43%) of adolescents with Down Syndrome meet the 60-min./day recommendation when MVPA was measured by accelerometer.
Increased MVPA (≥150 min/wk.) in conjunction with a reduced energy diet and behavioral counseling to assist participants with adherence to the diet and MVPA, is an important component of current multicomponent weight loss recommendations (Jensen et al., 2014, Donnelly et al., 2009). However, data on the changes in objectively assessed MVPA in adolescents/young adults with IDD participating in weight management interventions is limited and equivocal (Hinckson et al., 2013, Ptomey et al., 2015, Gephart and Loman, 2013, Curtin et al., 2013). For example, Curtin et al (Curtin et al., 2013) reported no change in accelerometer assessed MVPA in a small sample of adolescents/young adults with Down syndrome (age 13-26 yrs.) randomized to a 6-month weight loss intervention with (n=11) or without (n=10) parentally education/behavioral support. Mean MVPA increased ~18 min/day in the parental supported group and deceased ~7 min./day in the group without parental support (p=0.006). Our group reported minimal increases in MVPA (< 2 min./day) assessed by accelerometer in a small sample of adolescents (n=16, age = ~15 yrs.) who completed a 2-month parental supported weight loss intervention (Ptomey et al., 2015). Physical activity assessed by accelerometer during a recent weight management trial by our group, provided an opportunity to evaluate changes in light, MVPA, and sedentary time, and the association between changes in MVPA and weight loss in adolescents and young adults with IDD and overweight and obesity participating in a 6-month multi-component weight loss intervention.
METHODS
Overview
The rationale, design, and methods and results for weight loss at 6-months for this trial have been described in previous publications(Donnelly et al., 2016, Ptomey et al., 2021). Briefly, this trial was designed to compare the effectiveness of two diets (enhanced Stop Light Diet (eSLD) vs. conventional diet (CD)), and two delivery strategies (face-to-face (FTF)) vs. remote-virtual delivery (RD)) for weight loss in adolescents and young adults with IDD. Adolescents and young adults (n = 110, age ~ 16 yrs.) with mild-to-moderate IDD (IQ 40-74) and a BMI ≥85th percentile and a parent were randomized to one of 3 groups: FTF/CD (n=36), RD/CD (n=39), or RD/eSLD (n=35). Participants and a parent attended 30-45 min. education/behavioral counseling sessions with a health educator twice per month, and were asked to follow one of two diets (CD or eSLD), and to increase their MVPA to 60 min./day least 5 days/wk. The RD arms were delivered using FaceTime™ on an iPad® tablet (Apple Inc., Cupertino, CA) and daily self-monitoring of MVPA and steps was completed using Fitbit® wireless activity monitors (Google LLC, Mountain View, CA). The FTF intervention was delivered during home-visit meetings and daily self-monitoring of pedometer steps and self-reported minutes of MVPA was completed using pencil and paper records. Results indicated significantly greater 6-month weight loss in the eSLD (−6.4%) compared with the CD group (−2.4%, p =0.01) and no significant differences in weight loss between FTF (−0.2%) and RD groups (−2.4%, p =0.20). This trial, which was approved by the University’s Institutional Review Board and registered on clinicaltrails.gov (NCT02561754), was conducted in the local metropolitan area from November 2015 to May 2020.
Participants
Inclusion criteria:
Age 13-21 years, a diagnosis of mild-to-moderate IDD verified by a primary care physician, BMI ≥ 85th percentile on CDC growth charts or waist circumference to height ratio > 0.5, sufficient functional ability to understand directions, communicate through spoken language, living at home with a parent or guardian, and internet access in the home.
Exclusion criteria:
Insulin dependent diabetes, participation in a weight management program involving diet and MVPA in the past 6 months, diagnosed eating disorder, serious food allergies, aversions to common foods (e.g., unwilling to consume dairy products, vegetables), consuming special diets (e.g., vegetarian, Atkins etc.), diagnosis of Prader-Willi Syndrome; or unable to participate in MVPA.
Recruitment:
Participants were recruited using flyers and social media posts by local organizations that serve adolescents with IDD in the community. Potential participants were asked to contact the study coordinator who answered questions about the study and administered an initial participant eligibility screener. A home visit was scheduled with those remaining interested and potentially eligible to determine final eligibility, and to obtain parental consent and adolescent assent. Consented participants were stratified by BMI percentile (<95th percentile, ≥95th percentile) and randomized to the RD/CD, RD/eSLD, or FTF/CD arms.
Intervention
Orientation.
Health educators conducted home visits with each participant and a parent prior to initiating the intervention. These sessions included detailed descriptions of the dietary and MVPA components of the intervention, and the respective delivery and self-monitoring formats (FTF/RD). Participants in the RD arms were provided a Fitbit® Charge HR wireless activity tracker (size 35.5 x 28 mm) which monitors daily steps and minutes of MVPA and an iPad® tablet, which was pre-loaded with the Fitbit® app. Participants in the FTF arm were provided with a pedometer (Omron HJ-320, Lake Forest, IL) to self-monitor daily steps and shown how to self-monitor minutes of MVPA using paper records specifically designed for use in individuals with IDD.
Physical Activity.
Participants in all intervention arms were asked to attain 60 min./day of MVPA on 5 or more days/wk (Piercy et al., 2018). Participants were asked to progress from 3 days/wk. at 15 min/day during week one (or current activity level if higher) to 5 days/wk. at 60 min/day by week 12, remaining at this level through the 6-month intervention. Participants were also asked to increase their daily steps by 10% each week from their current level until reaching a goal of 10,000 steps/day. Study personnel provided the participants with resources and ideas for a variety of activities including walking, swimming, biking, active video games, and recreational sports outside of school using guidelines and suggestions from the Special Olympics “Train at Home” program.
Self-Monitoring of Physical Activity.
Self-monitoring of MVPA and daily steps in the RD arms was completed using the Fitbit® wrist-worn activity tracker. Real-time data from the Fitbit®was automatically transferred to the Fitbit® app loaded on the iPad® which provided a graphic display of daily steps, minutes of sedentary time, time spent in light, moderate and vigorous PA, and heart rate relative to pre-set goals. Participants in the FTF arm were provided with paper tracking sheets to record their daily pedometer steps and self-reported minutes of daily planned MVPA. Activity tracking data from participants in all intervention arms was available to their health educator for use in providing participant feedback and guidance during the twice monthly education/behavioral counseling sessions. Data on MVPA obtained from the Fitbit® and self-reports were used only in the context of participant feedback and not to assess change in MVPA across the 6-month intervention as the validity of MVPA assessed by Fitbit® or self-reports in adolescents with IDD has not been established (Ptomey et al., 2017).
Education/behavioral counseling sessions.
Participants and parents in all intervention groups were asked to attend ~30-45 min. sessions with a health educator once every two weeks. During each session health educators provided strategies for increasing support and decreasing barriers for participation in MVPA. Health educators reviewed self-monitoring data for MVPA with each participant and assisted them in devising realistic strategies to meet their weekly MVPA goals (e.g., I will walk the dog after school for 30 minutes on Monday, Wednesday, and Friday). Four of the 12 sessions discussed topics specific to MVPA which included the importance of MVPA for health and function, how to implement MVPA in the daily schedule, reducing barriers to MVPA, and the importance of hydration during exercise.
Assessments
Physical Activity by Accelerometer
Accelerometer protocol.
Physical activity and sedentary time were assessed at baseline and 6 months using an ActiGraph model wGT3x-BT tri-axial accelerometer (3.3 x 4.6 x 3.5 cm, wt. = 19 g., dynamic range ± 8 g) (Archimed Inc, Lyon, France). ActiGraphs provide valid and reliable assessments of MVPA in adolescents (Freedson et al., 2005, Hanggi et al., 2013, Rowlands et al., 2014) and adults (Butte et al., 2012, Trost et al., 2005, Hendelman et al., 2000), and have been widely used to describe physical activity in typically developing children/adolescents (Sherar et al., 2011, Borde et al., 2017) and children/adolescents with IDD (McGarty et al., 2014, Hinckson et al., 2013, Pan et al., 2015). Participants were asked to wear the ActiGraph on a belt over their non-dominant hip at the anterior axillary line during waking hours for 7 consecutive days, with the exception of bathing, swimming, and contact sports. A 7-day monitoring period provides a reliable estimate of physical activity (Ward et al., 2005, Kang et al., 2014, Cain et al., 2013). The hip rather than the wrist location was used due to the lack of comparable data and established protocols for the assessment of physical activity using wrist worn ActiGraphs (Crouter et al., 2015, Hildebrand et al., 2014, Chandler et al., 2016). ActiGraphs were initialized to collect raw data from all 3 axes at 60 Hz and downloaded using ActiLife Software version 6.13.3 (Archimed Inc, Lyon, France). ActiGraph data for a minimum of four 10-hour days was required for inclusion in the analysis (Troiano et al., 2008).
Accelerometer data processing.
We are aware that accelerometer activity intensity cut points developed and validated in samples of typically developing/developed adolescents and adults may not generalize to adolescents or adults with IDD due to differences in gait patterns, energy expenditure of exercise and decreased exercise capacity between typically developing/developed adolescents and adults and those with IDD (McGarty et al., 2014, Pitetti et al., 2001, Agiovlasitis et al., 2011) However, activity intensity cut-points developed and validated specifically for adolescents (13 - ≤18 yrs.) and young adults with IDD, such as those in the current trial (18-21 yrs.) are currently unavailable. Therefore, intensity cut points i.e., sedentary (≤ 1.0 MET), light (1.01-2.99 METs) and MVPA ( ≥ 3.0 METs) developed and validated for typically developing/developed adolescents and adults were used for this analysis. The cut points employed in our analysis, described below, used accelerometer data from the vertical axis. Accelerometer data processing was completed using custom SAS/R programs.
Participants age ≤ 18 yrs.
We used the age specific intensity cut points for children/adolescents (6-18 yrs.) developed by Freedson et al. which have demonstrated acceptable validity for classification of MVPA (≥ 3 METs) (METs =2.757 + (0.0015 *accelerometer counts/min.) - (0.08957 * age (yr.)) – (0.000038 * accelerometer counts/min. * age (yr.)) (Freedson et al., 2005, Freedson et al., 1997). Sedentary time was defined as ≤ 100 counts/min. (Trost et al., 2011) and non-wear time was considered to be 20 or more consecutive 60-second epochs of zero counts (Cain et al., 2013, Madsen et al., 2015, Carson et al., 2013).
Participants age > 18 yrs.
Accelerometer data was processed with the protocol used for adults in the 2003-2004 and 2005-2006 cycles of NHANES (Troiano et al., 2008, Matthews et al., 2008). Activity intensity cut points were as follows: sedentary ( ≤ 100 accelerometer counts/min.), light (101-2019 accelerometer counts/min.), MVPA (≥ 2020 accelerometer counts/min.) (Troiano et al., 2008, Matthews et al., 2008). Non-wear time was defined ≥ 60 consecutive minutes of zero counts, with an allowance for 1-2 min. of counts between 0 and 100.
Statistical Analysis
Mixed modeling was conducted separately for accelerometer outcomes including counts/min., sedentary, light, and MVPA. Models estimated overall group difference across time (i.e., group effect), change from baseline to 6 months (i.e., time effect), and group difference in change (i.e., group-by-time interaction), while accounting for the clustering of measurements (level-1) repeated for participants (level-2) as well as age, sex, and diagnosis (i.e., Down syndrome, Autism Spectrum Disorder (ASD), or other). Bivariate tests (independent-samples t-test, chi-square or Fisher’s exact test) were conducted to identify participant characteristics that were significantly associated with providing valid accelerometer data (i.e., ≥4 days with at least 10 hours of wear time [yes/no]) or wearing the device (i.e., ≥2 days with at least 1 hour of wear time [yes/no]) at baseline and 6 months. Pearson correlation coefficients were calculated to examine the association of the following variables with change in MVPA from baseline to 6 months: Baseline sedentary time, baseline MVPA, attendance at education/counseling sessions and frequency of self-monitoring of MVPA and steps. Pearson correlations were also used to examine the association between change in MVPA and change in body weight across 6 months. All analyses were conducted with SAS 9.4 (Cary, NC).
RESULTS
Participants
Baseline participant characteristics and the consort diagram are presented in Table 1 and Figure 1, respectively. Participants were ~16 years of age, 53% female, and 81 % non-Hispanic white. Forty-eight percent of participants were diagnosed with Down syndrome while 38% were diagnosed with ASD. Participants attended ~83% of the twice monthly education/counseling sessions and self-monitored MVPA and steps on ~73% of total study days. Ninety five percent of those randomized completed the 6-month intervention.
Table 1.
Baseline characteristics of adolescents and young adults with intellectual and developmental disabilities by intervention group.
| Face-to-face Delivery/Conventional Diet (n=36) | Remote Delivery/Conventional Diet (n=39) | Remote Delivery/Enhanced Stop Light Diet (n=35) | |
|---|---|---|---|
|
| |||
| M ± SD / n (%) | M ± SD / n (%) | M ± SD / n (%) | |
| Age (yrs.) | 16.3 ± 2.7 | 15.6 ± 1.7 | 16.7 ± 2.5 |
| Sex | |||
| Male | 20 (56%) | 15 (39%) | 17 (49%) |
| Female | 16 (44%) | 24 (62%) | 18 (51%) |
| Race | |||
| White | 30 (83%) | 38 (97%) | 29 (83%) |
| Black | 3 (8%) | 0 (0%) | 4 (11%) |
| Two or More Races | 3 (8%) | 1 (3%) | 2 (6%) |
| Ethnicity | |||
| Not Hispanic/Latino | 34 (94%) | 37 (95%) | 31 (89%) |
| Hispanic/ Latino | 2 (6%) | 2 (5%) | 4 (11%) |
| Diagnosis | |||
| Autism Spectrum Disorder | 5 (42%) | 14 (36%) | 13 (37%) |
| Down Syndrome | 17 (47%) | 21 (54%) | 15 (43%) |
| Other | 4 (11%) | 4 (10%) | 7 (20%) |
| Weight (kg) | 88.4 ± 29.5 | 74.9 ± 16.5 | 83.6 ± 26.4 |
| BMI (kg/m2) | 34.1 ± 8.3 | 31.3 ± 5.8 | 32.7 ± 7.1 |
Figure 1.
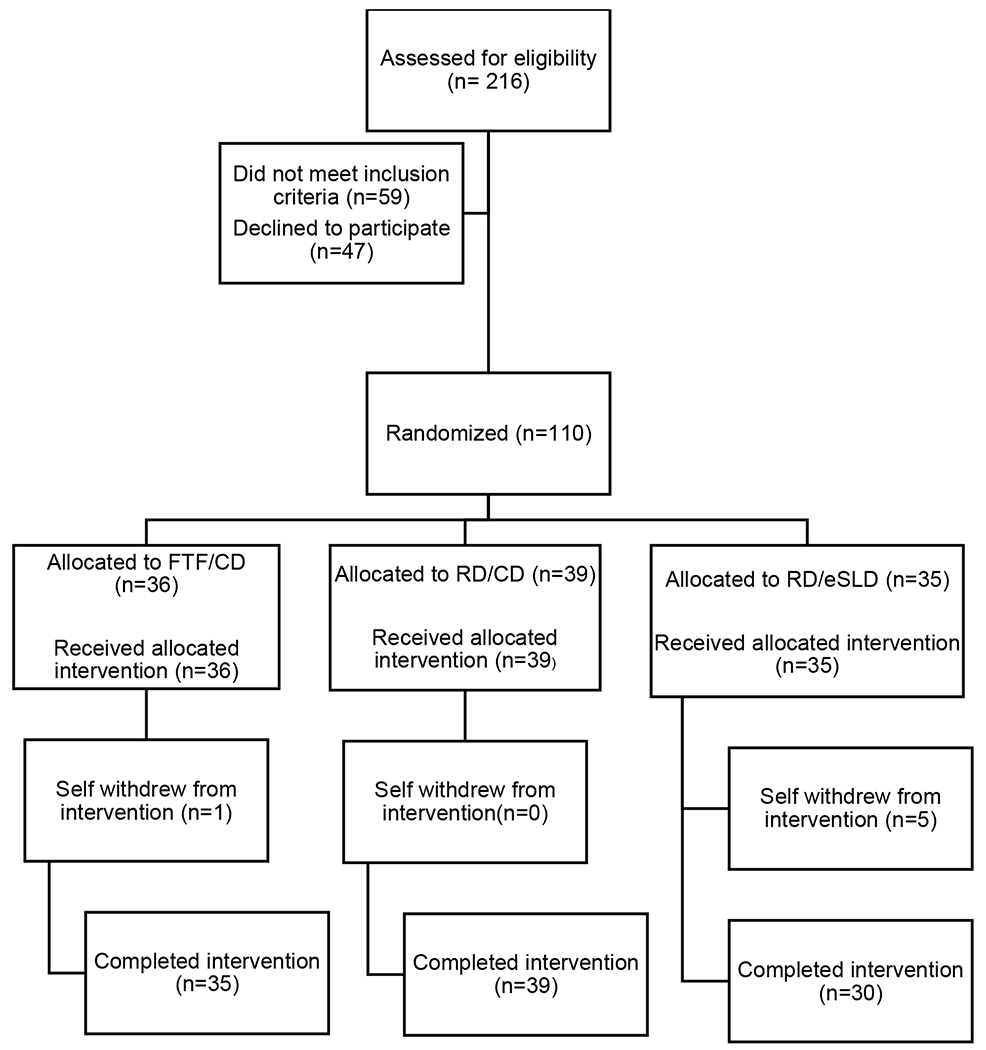
Consort Diagram.
FTF/CD=Face-to-face delivery/Conventional Diet, RD/CD = Remote delivery/Conventional Diet, RD/eSLD= Remote Delivery/Enhanced Stop Light Diet
Compliance with the accelerometer protocol
Accelerometers were worn by 90 participants at baseline (82% of baseline sample) and 44 participants at 6 months (42% of 6-month sample). Mixed modeling analysis was completed using data from participants with valid accelerometer data (i.e., ≥ 4 - 10 hr. days) at baseline (n=68) and at 6 months (n=30). The average days/minutes per day of wear time in this sample were 5.8 days/792 min./day and 5.9 days/765 min./day at baseline and 6 months, respectively. Participant characteristics (i.e., age, sex, race, ethnicity, IDD diagnosis, and intervention group) were not significantly associated with accelerometer wear (yes/no) or providing valid accelerometer data (yes/no) at baseline or at 6 months (all p>0.05).
Physical Activity Across the 6-month Intervention
The estimated marginal means adjusted for participants’ age, sex race, and IDD diagnosis for all physical activity outcomes by intervention group are shown in Table 2. Mixed modeling revealed a non-significant increase in MVPA (4.5 min./day, ~30%) from 15.2 ± 21.5 mins/day at baseline to 19.7 ± 19.7 mins/day at 6 months (p=0.119). The recommended 60 min./day of MVPA was achieved by 4.4% (3/68) and 6.7% (2/30) of participants at baseline and 6 months, respectively. Mixed modeling of changes in MVPA from baseline to 6 months indicated no significant effects of group (p=0.79), time (p=0.10), or group-by-time interaction (p=0.21). Mixed modeling also indicated no significant effect of group, time, or group-by-time interactions for change in accelerometer counts/min., and daily minutes of light activity or sedentary time across 6 months (all p>0.05). Correlational analysis conducted on participants with valid accelerometer data at both baseline and 6 months (n=24) revealed no significant associations between baseline sedentary time (r=0.10, p=0.40) and baseline MVPA (r=−0.22, p =0.30) and change in MVPA across the 6-month intervention. Additionally, attendance at education/counseling sessions (r=0.26, p =0.22) and frequency of self-monitoring of MVPA were not significantly associated with change in MVPA from baseline to 6 months (r= 0.26, p =0.44).
Table 2.
Marginal means for accelerometer counts/min., and daily minutes of sedentary, light and moderate-to-vigorous physical activity (MVPA) in adolescents and young adults with intellectual and developmental disabilities at baseline and 6 months, adjusted for age, sex, and IDD diagnosis.
| Face-to-face Delivery/ Conventional Diet | Remote Delivery/ Conventional Diet | Remote Delivery/ Enhanced Stop Light Diet | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (n=23) | 6 Months (n=9) | Baseline (n=23) | 6 Months (n=9) | Baseline (n=22) | 6 Months (n=12) | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Counts/min | 328.0 | 31.5 | 341.9 | 36.1 | 378.4 | 34.4 | 346.7 | 38.9 | 329.4 | 31.0 | 332.2 | 34.1 |
| Sedentary (mins/day) | 487.7 | 28.1 | 500.4 | 33.3 | 472.7 | 30.7 | 484.2 | 35.8 | 457.0 | 27.7 | 469.8 | 31.2 |
| Light (mins/day) | 299.7 | 16.7 | 281.3 | 19.9 | 307.7 | 18.2 | 277.3 | 21.4 | 280.6 | 16.4 | 257.7 | 18.7 |
| MVPA (mins/day) | 18.6 | 3.5 | 23.2 | 4.2 | 18.4 | 3.9 | 16.7 | 4.5 | 16.0 | 3.5 | 21.6 | 4.0 |
Individual variability in changes in MVPA (Figure 2)
Figure 2.
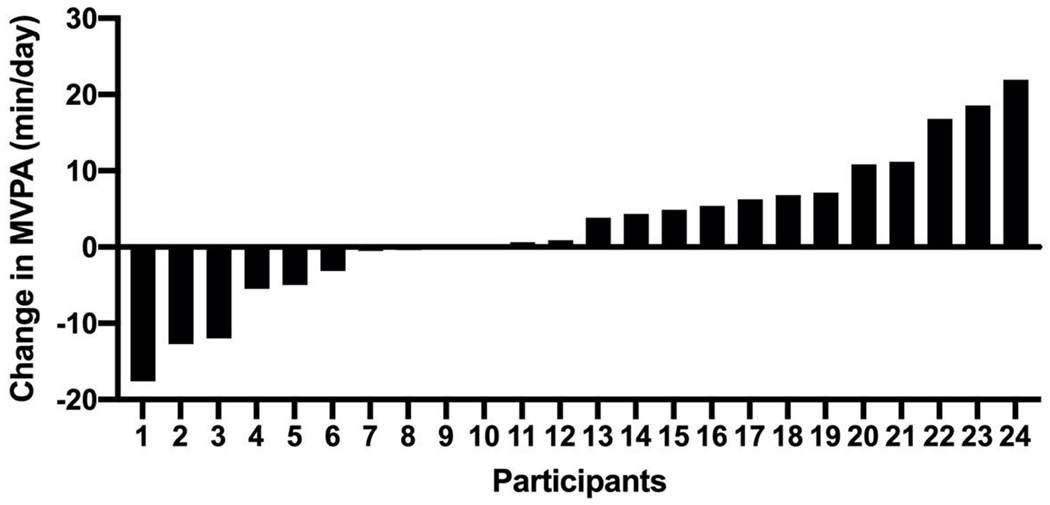
Individual changes in MVPA/ (min/day) in adolescents and young adults with intellectual and developmental disabilities across a 6-month weight loss intervention.
Change in MVPA across 6 months ranged from – 17.6 to + 21.9 min./day in the 24 participants who provided valid accelerometer data at both the baseline and 6 months. MVPA increased in 50% (12/24) of participants, was essentially unchanged (± 5 min./day) in 25% (6/24) and decreased in 25% of participants. MVPA increased by ≥10 mins/day in ~21% (5/24) of participants.
Association of MVPA with Weight Loss
Baseline sedentary time (r=−0.11, p=0.36), baseline MVPA (r=0.02, p =0.92), and change in MVPA from baseline to 6 months (r=0.13, p =0.30) were not associated with changes in weight across the 6-month intervention in the 24 participants with valid accelerometer data at both baseline and 6 months.
DISCUSSION
This analysis demonstrated a non-significant increase in MVPA (4.5 min/day) in a sample of adolescents and young adults with IDD and overweight and obesity who participated in a 6-month multicomponent weight loss intervention. MVPA at baseline was low (~15 min./day) with only 4.4% and 6.7% of participants achieving the recommended 60 min./day of MVPA at baseline and 6 months, respectively. Additionally, we found no association between participant attendance at education/counseling sessions or frequency of self-monitoring of MVPA and change in MVPA from baseline to 6 months.
Our results for change in MVPA are agreement with the limited previously published data which have reported limited success in increasing MVPA in adolescents and young adults with IDD participating in weight management interventions using both objective (accelerometer) and self-report measures of MVPA. For example, we previously reported minimal increases in MVPA (< 2 min./day) assessed by accelerometer in a small sample of adolescents with IDD (n=16, age = ~15 yrs.) who completed a 2-month parental supported weight loss intervention (Ptomey et al., 2015). Hinckson et al (Hinckson et al., 2013) reported minimal changes in weekly physical activity (walking, swimming and active play) assessed by questionnaire at the completion of a 10-wk. family supported, 18 session school-based weight management program which included an activity component (dancing, walking games, and family exercise) and at a 24-week follow-up in 22 adolescents with IDD and overweight and obesity. Gephart and Lohman (Gephart and Loman, 2013) reported minimal changes in self-reported physical activity in 40 individuals with IDD and overweight and obesity (age 8-20 yrs.) residing in group homes who completed a 10-wk. intervention designed to improve diet quality and increase physical activity and delivered by care providers. Two reports suggest increased physical activity in adolescents with IDD participating in weight management interventions. For example, Curtin et al (Curtin et al., 2013) assessed MVPA in a small sample of adolescents/young adults with Down syndrome (age 13-26 yrs.) randomized to a 6-month weight loss intervention with (n=11), or without parental education and behavioral support (n=10). Change in MVPA was significantly greater in group that received parental education and support (+18 min./day) compare to the group with no parental support (−7 min./day) (p=0.006). An et al (An et al., 2019) reported a 22 min/day in self-reported exercise time in 14 adolescents with IDD (age 12-15 yrs.) who completed a 4-month ( 2 days/wk.), school-based pilot trial using the I Can Do It! national health promotion model designed to develop health awareness relative to healthy eating and participation in physical activity.
The difficulty in increasing MVPA in adolescents and young adults with IDD participating in weight management interventions is highlighted by the general lack of success as previously described and suggests that alternative strategies for increasing MVPA, which more specifically address barriers to participation in physical activity in this population, should be developed and evaluated. Barriers to physical activity in adolescents with mild-to-moderate IDD, who typically live at home with a parent, relative to both the adolescent and their parents have been identified. For example, adolescents with IDD are frequently unaware of the potential health benefits of physical activity, they perceive that physical activities difficult to learn, they often lack a supportive partner to join them in physical activity, and are dependent on parents to provide transportation to exercise facilities (Stanish et al., 2015). Parental barriers include family structure (marital status, other siblings etc.), lack of self-efficacy for encouraging activity in their adolescent, time constraints, and lack of affordable/accessible transportation (McGarty and Melville, 2018). Research evaluating the effectiveness of addressing any of these barriers to increasing physical activity in adolescents with IDD is basically non-existent. McGarty et al. (McGarty et al., 2018) suggested that education of parents/caregivers may reduce barriers to participation in physical activity in children and adolescents with IDD. However, empirical support for this hypothesis is limited to the small trial reported by Curtin et al. al (Curtin et al., 2013) in adolescents with Down syndrome as previously described. We have developed a strategy which uses video conferencing (Zoom®) to deliver instructor directed exercise sessions to groups of adolescents with IDD in their home, thus eliminating any transportation concerns and providing an opportunity for social interaction between participants and participants and the instructor. We completed a pilot trial in 31 adolescents with IDD who were asked to attend 30-min. group exercise sessions 3 times/wk. over 12 weeks, delivered via Zoom® on a tablet computer to participants in their homes (Ptomey et al., 2017b). Participants attended ~77% of scheduled sessions and averaged ~ 27 min./session of total activity with ~12 min./session of MVPA assessed by accelerometer. These results demonstrate the feasibility of the group remote delivery approach for delivery of physical activity over a short 12-week time frame. However, the effectiveness of this approach to deliver physical activity over a longer time frame (6-12 months), to promote social support and interaction between participants, to increase MVPA outside the remotely delivered sessions, and the impact of family participation are unknown and are being evaluated in an on-going trial (Ptomey et al., 2019).
Research on physical activity in adolescents with IDD is hampered by issues relative to the assessment of physical activity in this population. Self-report instruments (diaries, questionnaires) which are commonly used to assess physical activity in health-related research in typically developing/developed individuals (Trost, 2007), are impractical in individuals with IDD due to limitations in cognitive abilities. Accelerometers, typically worn on a belt at the waist, provide an objective measure of physical activity (Troiano et al., 2008); however, their use in individuals with IDD hampered by two major issues. First, compliance with accelerometer protocols in individuals with IDD, in terms of forgetting or refusing to wear the accelerometer and/or meeting wear time criteria designed to adequately quantify the level of typical daily physical activity, has been problematic. In the current trial 90 of 110 of participants wore the accelerometer at baseline; however, only 68 participants provided valid data (≥ 4-10 hr. days). At 6 months only 44 of 104 participants wore the accelerometer with valid data available for only 30 participants. A systematic review of 17 studies that measured physical activity in individuals with IDD reported compliance with accelerometers protocols ranged widely from 45% to 100% (Leung et al., 2017). Poor compliance with accelerometer protocols in individuals with IDD has been frequently observed (Melville et al., 2011, Spanos et al., 2015, Ptomey et al., 2017). Reducing the criteria for valid accelerometer data from the typical ≥ 4-10 hr. days to ≥ 3– 6-hr. days may improve compliance as demonstrated in 2 studies which obtained valid accelerometer data on 61% to 83% of adults with IDD using the ≥ 3– 6-hr, day criteria (Melville et al., 2011, Spanos et al., 2015). However, the validity of the ≥ 3– 6-hr. day criteria to quantify daily physical activity in either adults or adolescents with IDD has not been established. The use of wrist worn devices has also been suggested to improve compliance with objective physical activity assessment protocols. Although not an outcome assessment, participants in the RD groups in the current trial (n=74) were asked self-monitor daily physical activity using a Fitbit® worn on the wrist. Results indicated participants wore the Fitbit® on ~72% of days across the 6-month intervention. This suggests that wrist-worn devices may improve compliance with objective activity assessment protocols in adolescents with IDD compared with devices worn at the waist. However, the validity of accelerometers, the Fitbit®, or other devices worn at the wrist for the assessment of physical activity in adolescents with IDD has not been established.
The second major issue associated with the use of accelerometers is the lack of validated cut points to quantify activity intensity, e.g., light, moderate, and vigorous. Validated activity intensity cut points for both adolescents and adults with IDD are currently unavailable. ActiGraph intensity cut points have been developed for 8-to 11-year-old children (McGarty et al., 2016); however, these cut-points are limited to a narrow age range, and were developed and validated using direct observation (SOFIT) of physical activity in a free-living environment rather than using physiological data (e.g., oxygen consumption) in a controlled lab environment. As described previously, activity intensity cut points validated in typically developing/developed individuals, although widely used in studies of individuals with IDD (McGarty and Melville, 2018, Leung et al., 2017) may not be appropriate for this purpose. Additionally, the use of cut points validated for typically developing/developed individuals to describe the levels of physical activity in individuals with IDD or to make comparisons of the absolute amount of MVPA or the percentage of participants who achieve a specific criterion of MVPA (e.g., 60 min./day) may be problematic. Thus, the validation of activity intensity cut points for both adolescents and adults with IDD are warranted.
In summary, this analysis demonstrated that a 6-month weight loss intervention which included twice monthly education/behavioral sessions and daily self-monitoring of physical activity had a minimal impact on objectively assessed MVPA in a sample of adolescents and young adults with mild-to-moderate IDD and overweight and obesity. Although the randomized sample for this trial was large (n=110) valid accelerometer data (i.e., ≥ 4-10 hr. days) was available for only 68 participants at baseline and 30 participants at 6 months, with 24 participants providing valid accelerometer data at both baseline and 6 months. Thus, the inability to obtain valid accelerometer data merits consideration in the interpretation of our results. Additionally, as all participants were from a sample of adolescents with mild to moderate IDD who had overweight or obesity and were motivated to lose weight, and thus, the results are not generalizable to all adolescents with IDD. Increased physical activity has the potential to facilitate weight management and improve cardiovascular fitness (Izquierdo-Gomez et al., 2015), muscular strength and endurance (Shields et al., 2013), and reduce chronic disease risk (Wallen et al., 2013) in individuals with IDD. However, the lack of success in increasing MVPA in adolescents with IDD in general (McGarty et al., 2018, Hassan et al., 2019) and specifically for adolescents with IDD participating in a weight loss program argues for the evaluation of additional strategies for increasing physical activity in adolescents with IDD such as increased parental education and involvement, peer social support, behavioral techniques/incentive systems to improve motivation for physical activity.
Funding:
National Institutes of Child Health and Development (R01HD079642)
Footnotes
Conflict of Interest: No authors declare any conflicts of interest
Clinical Trials Number: NCT02561754
Data Sharing Statement: Deidentified individual participant data (including data dictionaries) will be made available, in addition to study protocols, the statistical analysis plan, and the informed consent form. The data will be made available upon publication to researchers who provide a methodologically sound proposal for use in achieving the goals of the approved proposal. Proposals should be submitted to the corresponding author at lptomey@kumc.edu
REFERENCES
- Agiovlasitis S, Motl RW, Fahs CA, Ranadive SM, Yan H, Echols GH, Rossow L & Fernhall B 2011. Metabolic rate and accelerometer output during walking in people with Down syndrome. Med Sci Sports Exerc, 43, 1322–7. [DOI] [PubMed] [Google Scholar]
- An J, Dubose KD, Decker JT & Hatala LE 2019. A school-based mentoring program developing healthy behaviors of adolescents with intellectual and developmental disabilities: A pilot feasibility study. Disability and Health Journal, 12, 727–731. [DOI] [PubMed] [Google Scholar]
- Bertapelli F, Pitetti K, Agiovlasitis S & Guerra-Junior G 2016. Overweight and obesity in children and adolescents with Down syndrome—prevalence, determinants, consequences, and interventions: A literature review. Research in developmental disabilities, 57, 181–192. [DOI] [PubMed] [Google Scholar]
- Borde R, Smith JJ, Sutherland R, Nathan N & Lubans DR 2017. Methodological considerations and impact of school-based interventions on objectively measured physical activity in adolescents: a systematic review and meta-analysis. Obes Rev. [DOI] [PubMed] [Google Scholar]
- Butte NF, Ekelund U & Westerterp KR 2012. Assessing physical activity using wearable monitors: measures of physical activity. Med Sci Sports Exerc, 44, S5–12. [DOI] [PubMed] [Google Scholar]
- Cain KL, Sallis JF, Conway TL, Van Dyck D & Calhoon L 2013. Using accelerometers in youth physical activity studies: a review of methods. J Phys Act Health, 10, 437–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson V, Salmon J, Arundell L, Ridgers ND, Cerin E, Brown H, Hesketh KD, Ball K, Chinapaw M, Yildirim M, Daly RM, Dunstan DW & Crawford D 2013. Examination of mid-intervention mediating effects on objectively assessed sedentary time among children in the Transform-Us! cluster-randomized controlled trial. Int J Behav Nutr Phys Act, 10, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler JL, Brazendale K, Beets MW & Mealing BA 2016. Classification of physical activity intensities using a wrist-worn accelerometer in 8-12-year-old children. Pediatr Obes, 11, 120–7. [DOI] [PubMed] [Google Scholar]
- Crouter SE, Flynn JI & Bassett DR Jr. 2015. Estimating physical activity in youth using a wrist accelerometer. Med Sci Sports Exerc, 47, 944–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin C, Bandini LG, Must A, Gleason J, Lividini K, Phillips S, Eliasziw M, Maslin M & Fleming RK 2013. Parent Support Improves Weight Loss in Adolescents and Young Adults with Down Syndrome. The Journal of Pediatrics, 163, 1402-1408. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly J, Ptomey L, Goetz J, Sullivan D, Gibson C, Greene J, Lee R, Mayo M, Honas J & Washburn R 2016. Weight management for adolescents with intellectual and developmental disabilities: Rationale and design for an 18 month randomized trial. Contemporary clinical trials, 51, 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW & Smith BK 2009. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Medicine and science in sports and exercise, 41, 459–471. [DOI] [PubMed] [Google Scholar]
- Esposito PE, Macdonald M, Hornyak JE & Ulrich DA 2012. Physical activity patterns of youth with Down syndrome. Intellect Dev Disabil, 50, 109–19. [DOI] [PubMed] [Google Scholar]
- Freedson PS, Pober D & Janz KF 2005. Calibration of accelerometer output for children. Medicine & Science in Sports & Exercise, 37, S523–S530. [DOI] [PubMed] [Google Scholar]
- Freedson PS, Sirard J, Debold E, Pate R, Dowda M, Trost S & Sallis J 1997. Calibration of the Computer Science and Applications, Inc. (CSA) accelerometer. Med Sci Sports Exerc, 29, Supplement (p. 45).9000155 [Google Scholar]
- Frey GC, Stanish HI & Temple VA 2008. Physical activity of youth with intellectual disability: review and research agenda. Adapt Phys Activ Q, 25, 95–117. [DOI] [PubMed] [Google Scholar]
- Gephart EF & Loman DG 2013. Use of prevention and prevention plus weight management guidelines for youth with developmental disabilities living in group homes. Journal of Pediatric Health Care, 27, 98–108. [DOI] [PubMed] [Google Scholar]
- Grondhuis S & Aman M 2014. Overweight and obesity in youth with developmental disabilities: a call to action. Journal of Intellectual Disability Research, 58, 787–799. [DOI] [PubMed] [Google Scholar]
- Hanggi JM, Phillips LR & Rowlands AV 2013. Validation of the GT3X ActiGraph in children and comparison with the GT1M ActiGraph. J Sci Med Sport, 16, 40–4. [DOI] [PubMed] [Google Scholar]
- Hassan N, Landorf K, Shields N & Munteanu S 2019. Effectiveness of interventions to increase physical activity in individuals with intellectual disabilities: a systematic review of randomised controlled trials. Journal of Intellectual Disability Research, 63, 168–191. [DOI] [PubMed] [Google Scholar]
- Healy S, Aigner CJ & Haegele JA 2019. Prevalence of overweight and obesity among US youth with autism spectrum disorder. Autism, 23, 1046–1050. [DOI] [PubMed] [Google Scholar]
- Hendelman D, Miller K, Baggett C, Debold E & Freedson P 2000. Validity of accelerometry for the asssesment of moderate intensity physical activity in the field. Med Sci Sports Exerc, 32 (Suppl), S442–S449. [DOI] [PubMed] [Google Scholar]
- Hildebrand M, Vt V. a. N. H., Hansen BH & Ekelund U 2014. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med Sci Sports Exerc, 46, 1816–24. [DOI] [PubMed] [Google Scholar]
- Hinckson EA, Dickinson A, Water T, Sands M & Penman L 2013. Physical activity, dietary habits and overall health in overweight and obese children and youth with intellectual disability or autism. Res Dev Disabil, 34, 1170–8. [DOI] [PubMed] [Google Scholar]
- Izquierdo-Gomez R, Martínez-Gómez D, Acha A, Veiga OL, Villagra A & Diaz-Cueto M 2014. Objective assessment of sedentary time and physical activity throughout the week in adolescents with Down syndrome. The UP&DOWN study. Res Dev Disabil, 35, 482–9. [DOI] [PubMed] [Google Scholar]
- Izquierdo-Gomez R, Martinez-Gomez D, Fernhall B, Sanz A & Veiga OL 2015. The role of fatness on physical fitness in adolescents with and without Down syndrome: The UP&DOWN study. Int J Obes (Lond). [DOI] [PubMed] [Google Scholar]
- Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM & Kushner RF 2014. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Journal of the American college of cardiology, 63, 2985–3023. [DOI] [PubMed] [Google Scholar]
- Kang M, Bjornson K, Barreira TV, Ragan BG & Song K 2014. The minimum number of days required to establish reliable physical activity estimates in children aged 2-15 years. Physiol Meas, 35, 2229–37. [DOI] [PubMed] [Google Scholar]
- Leung W, Siebert EA & Yun J 2017. Measuring physical activity with accelerometers for individuals with intellectual disability: A systematic review. Research in Developmental Disabilities, 67, 60–70. [DOI] [PubMed] [Google Scholar]
- Madsen K, Linchey J, Gerstein D, Ross M, Myers E, Brown K & Crawford P 2015. Energy Balance 4 Kids with Play: Results from a Two-Year Cluster-Randomized Trial. Child Obes. [DOI] [PubMed] [Google Scholar]
- Maïano C, Hue O, Morin AJ & Moullec G 2016. Prevalence of overweight and obesity among children and adolescents with intellectual disabilities: a systematic review and meta‐analysis. Obesity Reviews, 17, 599–611. [DOI] [PubMed] [Google Scholar]
- Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR & Troiano RP 2008. Amount of time spent in sedentary behaviors in the United States, 2003-2004. American Journal of Epidemiology, 167, 875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute-Llorente A, Gonzalez-Aguero A, Gomez-Cabello A, Vicente-Rodriguez G & Casajus JA 2013. Physical activity and cardiorespiratory fitness in adolescents with Down syndrome. Nutr Hosp, 28, 1151–5. [DOI] [PubMed] [Google Scholar]
- Mcgarty AM, Downs SJ, Melville CA & Harris L 2018. A systematic review and meta-analysis of interventions to increase physical activity in children and adolescents with intellectual disabilities. Journal of Intellectual Disability Research, 62, 312–329. [DOI] [PubMed] [Google Scholar]
- Mcgarty AM & Melville CA 2018. Parental perceptions of facilitators and barriers to physical activity for children with intellectual disabilities: A mixed methods systematic review. Research in Developmental Disabilities, 73, 40–57. [DOI] [PubMed] [Google Scholar]
- Mcgarty AM, Penpraze V & Melville CA 2014. Accelerometer use during field-based physical activity research in children and adolescents with intellectual disabilities: a systematic review. Res Dev Disabil, 35, 973–81. [DOI] [PubMed] [Google Scholar]
- Mcgarty AM, Penpraze V & Melville CA 2016. Calibration and cross-validation of the ActiGraph wGT3X+ accelerometer for the estimation of physical activity intensity in children with intellectual disabilities. PloS one, 11, e0164928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melville CA, Boyle S, Miller S, Macmillan S, Penpraze V, Pert C, Spanos D, Matthews L, Robinson N & Murray H 2011. An open study of the effectiveness of a multi-component weight-loss intervention for adults with intellectual disabilities and obesity. British Journal of Nutrition, 105, 1553–1562. [DOI] [PubMed] [Google Scholar]
- Pan CY, Liu CW, Chung IC & Hsu PJ 2015. Physical activity levels of adolescents with and without intellectual disabilities during physical education and recess. Res Dev Disabil, 36c, 579–586. [DOI] [PubMed] [Google Scholar]
- Phillips AC & Holland AJ 2011. Assessment of objectively measured physical activity levels in individuals with intellectual disabilities with and without Down’s syndrome. PLoS One, 6, e28618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM & Olson RD 2018. The physical activity guidelines for Americans. Jama, 320, 2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitetti KH, Yarmer DA & Fernhall B 2001. Cardiovascular fitness and body composition of youth with and without mental retardation. Adapted Physical Activity Quarterly, 18, 127–141. [Google Scholar]
- Ptomey L, Washburn R, Lee J, Greene J, Szabo-Reed A, Sherman J, Danon J, Osborne L, Little T & Donnelly J 2019. Individual and family-based approaches to increase physical activity in adolescents with intellectual and developmental disabilities: Rationale and design for an 18 month randomized trial. Contemporary clinical trials, 84, 105817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptomey L, Willis E, Lee J, Washburn R, Gibson C, Honas J & Donnelly J 2017. The feasibility of using pedometers for self‐report of steps and accelerometers for measuring physical activity in adults with intellectual and developmental disabilities across an 18‐month intervention. Journal of Intellectual Disability Research, 61, 792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptomey LT, Sullivan DK, Lee J, Goetz JR, Gibson C & Donnelly JE 2015. The use of technology for delivering a weight loss program for adolescents with intellectual and developmental disabilities. Journal of the Academy of Nutrition and Dietetics, 115, 112–118. [DOI] [PubMed] [Google Scholar]
- Ptomey LT, Washburn RA, Goetz JR, Sullivan DK, Gibson CA, Mayo MS, Krebill R, Gorczyca AM, Montgomery RN & Honas JJ 2021. Weight Loss Interventions for Adolescents With Intellectual Disabilities: An RCT. Pediatrics, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands AV, Rennie K, Kozarski R, Stanley RM, Eston RG, Parfitt GC & Olds TS 2014. Children’s physical activity assessed with wrist- and hip-worn accelerometers. Med Sci Sports Exerc, 46, 2308–16. [DOI] [PubMed] [Google Scholar]
- Sherar LB, Griew P, Esliger DW, Cooper AR, Ekelund U, Judge K & Riddoch C 2011. International children’s accelerometry database (ICAD): design and methods. BMC Public Health, 11, 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields N, Taylor NF, Wee E, Wollersheim D, O’shea SD & Fernhall B 2013. A community-based strength training programme increases muscle strength and physical activity in young people with Down syndrome: a randomised controlled trial. Research in developmental disabilities, 34, 4385–4394. [DOI] [PubMed] [Google Scholar]
- Spanos D, Hankey CR & Melville CA 2015. The Effectiveness of a Weight Maintenance Intervention for Adults with Intellectual Disabilities and Obesity: A Single Stranded Study. J Appl Res Intellect Disabil. [DOI] [PubMed] [Google Scholar]
- Stanish HI, Curtin C, Must A, Phillips S, Maslin M & Bandini LG 2015. Physical Activity Enjoyment, Perceived Barriers, and Beliefs Among Adolescents With and Without Intellectual Disabilities. J Phys Act Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T & Mcdowell M 2008. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc, 40, 181–8. [DOI] [PubMed] [Google Scholar]
- Trost SG 2007. State of the art reviews: measurement of physical activity in children and adolescents. American Journal of lifestyle medicine, 1, 299–314. [Google Scholar]
- Trost SG, Loprinzi PD, Moore R & Pfeiffer KA 2011. Comparison of accelerometer cut points for predicting activity intensity in youth. Med Sci Sports Exerc, 43, 1360–8. [DOI] [PubMed] [Google Scholar]
- Trost SG, Mciver KL & Pate RR 2005. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc, 37, S531–43. [DOI] [PubMed] [Google Scholar]
- Wallen EF, Mullersdorf M, Christensson K & Marcus C 2013. A school-based intervention associated with improvements in cardiometabolic risk profiles in young people with intellectual disabilities. J Intellect Disabil, 17, 38–50. [DOI] [PubMed] [Google Scholar]
- Ward DS, Evenson KR, Vaughn A, Rodgers AB & Troiano RP 2005. Accelerometer use in physical activity: best practices and research recommendations. Med Sci Sports Exerc, 37, S582–8. [DOI] [PubMed] [Google Scholar]


