Abstract
The study of proteins circulating in blood offers tremendous opportunities to diagnose, stratify or possibly prevent diseases. With recent technological advances and the urgent need to understand the effects of COVID-19, proteomic analysis of blood-derived serum and plasma have become even more important for studying human biology and pathophysiology. Here, we provide views and perspectives about technological developments and possible clinical applications that use mass spectrometry or affinity-based methods. We discuss examples where plasma proteomics contributed with valuable insights about SARS-CoV-2 infections, aging, hemostasis, and the opportunities offered by combining proteomics with genetic data. As a contribution to the Human Proteome Organization (HUPO) Human Plasma Proteome Project (HPPP), we present the Human Plasma PeptideAtlas build 2021-07 that comprises 4395 canonical and 1482 additional non-redundant human proteins detected in 240 MS-based experiments. In addition, we report the new Human Extracellular Vesicle PeptideAtlas 2021-06, which comprises five studies and 2757 canonical proteins detected in extracellular vesicles circulating in blood, of which 74% (2047) are in common with the plasma PeptideAtlas. Our overview summarizes the recent advances, impactful applications, and ongoing challenges for translating plasma proteomics into utility for precision medicine.
Keywords: proteomics, mass spectrometry, blood, plasma, Human Proteome Project, Human Plasma Proteome Project, PeptideAtlas, proximity extension assays (PEA by Olink), DNA aptamers (Somascan)
Graphical Abstract
Note to editors: some artwork used in this figure are created with BioRender.com and are provided under BioRender’s Academic License.
Introduction
Human blood provides an easily obtainable, extensive, and sensitive diagnostic material for assessment of wellness and disease in individuals and populations. The abundance of proteins, their primary isoforms, alternative splice isoforms, post-translational modifications, and protein sequence variants provide a distinct snapshot into the current function of the circulatory system and all organs with which blood comes in contact. However, the extent, precision, and ease with which measurements of the complete protein content can be made, as well as the interpretation of obtained measurements, remain a major challenge1. The extremely high abundance of a small set of plasma proteins (20 proteins account for 99% of the protein by weight), a wide dynamic range of protein levels, as well as the high sequence variability of immunoglobulins, continue to make plasma protein measurements a complex task2.
Many different technologies are currently deployed to generate protein measurements in serum or plasma, from highly optimized single-protein assays commonly used in clinical laboratories, to exploratory and more flexible mass-spectrometry (MS) based workflows and affinity-based assays used as part of research workflows. Even though modern MS-based assay systems allow for rapid development of custom measurements for specific targets of interest, their substantial complexity still hinders their widespread deployment as proteome analyzers in clinical settings. In the past few years, highly multiplexed affinity-based platforms have emerged as complementary methods, offering attractive high throughput assays that can rapidly provide abundance measurements for panels plasma proteins in thousands of samples.
In this article, we provide a perspective on efforts emerging in the community as a contribution from the HUPO3 Human Plasma Proteome Project (HPPP)4, one of the key initiatives of the HUPO Human Proteome Project (HPP)5–9. We begin by reviewing recent technology advancements in plasma proteomics, especially in the context of affinity-based platforms and MS workflows. We then present the latest results from the compendium of reanalyzed MS datasets in the PeptideAtlas resource, which has been split into plasma samples and blood-derived extracellular vesicle samples. We finish with a review of timely progress in applications related to plasma proteomics, including COVID-19 diagnostics, genetics, proteogenomics, hemostasis, aging research, and ethical considerations for proteome biomarker applications.
Recent Advances
Improvements to the plasma proteomics pipeline can enable the community to make progress across a wide spectrum of areas, including sample throughput and preparation, analytical depth and analysis time, bioinformatics tools for data processing, and the identification of post-translational modifications (PTMs) or protein sequence variants. Yet, there is no single application, instrumentation setup, or software package that have combined all these pipeline advancements. In addition to optimizing the elements of the plasma proteomics analytical procedures, we recently raised awareness about the importance of other quality measures such as sample integrity and associated metadata, all of which together are crucial for interpreting the experimental outcome(s)1.
In contrast to cellular studies of proteomes, investigating blood plasma poses unique challenges related to the composition and the high dynamic abundance range of the analytes. Circulating proteins span a very large concentration range, with a small number of these making up over 99% of the bulk of the circulating proteome (as compared to 2500 proteins for human cells). Of these, 14 proteins contribute the largest fraction of all proteins in blood, with a further 6 proteins representing the next tier of concentrations, for a total of the 20 most abundant proteins in blood10. For MS-based approaches, the top-down nature of the technology can make blood protein analysis difficult, usually only identifying and quantifying a few hundred proteins per sample. Depletion of the top 14 proteins (i.e. ALB, HP, TF, IgG, IgA, IgM, SERPINA1, A2M, ORM, APOA1, APOA2, C3, TTR, and FGA) using antibody affinity approaches and analyzing the remainder of the sample by LC-MS usually extends the coverage to 500-800 proteins. With the addition of fractionation and sophisticated mass spectrometry scanning methods, several thousand proteins can be detected at high quantitative accuracy but at the cost of analysis time. The need for depletion in MS studies is still controversial, as the fear of co-extracting other proteins is thought to occur, but there has been little credible evidence to support this notion. As for the current state of the art of LC-MS/MS-based analyses of plasma or serum, approaches using either top abundant protein depletion or fractionation, or combinations of both, allow for in-depth blood protein identification, quantification, and the determination of post-translational modifications or even interactions. Mass spectrometry is currently the only technology that will allow a versatile detection of these features as compared to predefined content offered by affinity-based approaches. For example, it is possible to identify 400-500 proteins when using undepleted plasma and a single LC-MS/MS run11, as compared to 2600 proteins when subjecting undepleted plasma to multiple fractionation steps12–14. Additionally, LC-MS/MS approaches also allow for the detection of protein isoforms (i.e. sequence trimming) and post-translational modifications. Combining these approaches enables LC-MS/MS based analyses to detect more than 4000 proteins12,13. Although such protocols are not routinely performed in many academic laboratories due to extensive sample preparation and the need for highly reproducible workflows, commercial companies have stepped in by providing end-to-end or semi-automated workflows, including, but not exclusively, Biognosys, BGI, Proteome Sciences, and OmicEra Diagnostics.
In addition to these significant advances enabling in-depth characterization of the human plasma and serum proteome, other technological developments have focused on improving the reproducibility, throughput, and sample preparation prior to LC-MS/MS analyses. In order to circumvent lengthy, error prone and variable manual handling, sophisticated chips or microfluidic devices integrating biochemical steps of pre-analytical protocols have been developed15–17. Some of these microfluidic devices integrate plasma preparation, depletion of abundant proteins, digestion and peptide desalting for a fully automated processing of blood samples18. Others developed highly parallelized preanalytical workflows based on automation using the 96-well plate format19–22. In line with such progress and to avoid bias in the distribution of samples before sample processing, dedicated applications have been developed to randomize samples23, supporting the reliability of the entire workflow. Additionally, MS-based proteomics is able to move to very fast data acquisition as recently shown by Messner et al.24. Scanning SWATH allowed them to acquire plasma proteomes in just 1 min gradients (3.5 min run-to-run time), which resulted in just 180 proteins, but this still included clinical useful information from 47 FDA-approved biomarkers.
Recently, the maturation of affinity-based assays for protein identification has accelerated the development and adoption of these technologies for high-throughput plasma and serum proteomics. Most prominent and offering the largest content are the Proximity Extension Assays (PEA by Olink) using paired antibodies and either qPCR25,26,27 or sequencing as a readout28, as well as an analysis based on a large library of modified slow off-rate DNA aptamers (SomaScan by SomaLogic)29,30. Both commercial platforms provide access to profiling of proteins in a multiplexed and plate-based format, utilize DNA to report protein levels and over the last decade have gradually and successfully increased the content of their offered products. For the near future, a ~3000-protein assay from Olink (2940 unique human proteins) and a ~7000-protein assay from SomaLogic (6377 unique human proteins) with even greater content can be expected. Between these platform technologies there is quite a high overlap in protein targets, with 2033 unique proteins detectable across both platforms. When compared to the current Human Plasma PeptideAtlas, constructed using MS, only 1484 unique proteins were listed for all 3 approaches (i.e., PEA, SomaScan and MS). However, by combining all three platforms, over 8000 proteins could be targeted for potential detection in plasma, providing the opportunity for the largest blood protein analysis to date.
However, no systematic study has yet been performed that - with a common set of samples analyzed at the same time - compares the accuracy and precision of all three platforms. Instead, there are only very few studies that perform broad comparisons between the protein levels reported by different affinity proteomics platforms, observing a wide range of correlation between paired protein levels determined by each of the technologies31,32,33,34 . SomaLogic and Olink data has been used more widely to confirm the identification of Quantitative Trait Loci (pQTLs) in genome wide associations studies (GWAS)35,36. Currently, efforts to compare affinity proteomics and MS-based data in head-to head experiments are rare. There is one systematic study involving MS-based data (734 proteins with DIA and 368 proteins with DDA) with data from Olink assays (8 panels, 736 proteins). In this work, Petrera et al. reported a limited overlap of just 35 proteins but argued for the complementarity of the methods37. In another study by Ruffieux et al., two sample cohorts were analyzed by SomaScan, detecting 1096 proteins in both cohorts, and MS-based assays using immuno-depleted and 6-plex isobaric labelling38, detecting 133 proteins in both cohorts39. In the common set of 72 proteins detected by both methods, they found agreement between some of the reported protein levels (e.g. SHBG, CAH1, or CXCL7) and another 25 proteins with concordant effects associated with pQTLs (e.g. CFB, CO7, or FETUA). Consequently, there are factors other than analytical sensitivity that need to be considered. This can include how pre-analytical effects or genetic variation influence peptide identification and recognition of the epitope40. Combinations of these effects may either concern the protein of interest directly or be influenced indirectly by other sample components. To better understand the susceptibility and robustness of the assays and platforms, it will become increasingly important to annotate the circulating proteins in terms of tissue origin and the processes leading to their presence in blood. Towards an increased use for clinical phenotyping, other characteristics such as physiological protein function, and the detection of donor-specific variants, sample-specific isoforms or proteoforms will have to be considered.
As with all technologies, the capabilities and data accuracy need to be tested and standardized to ensure false discoveries are kept to a minimum with high consistency of the true positives. In 2021, both Olink and SomaLogic attended the Proteomics Standards Initiative41,42 Spring Workshop to present their technology and discuss as a group pathways to ensure standards in assay correctness, accuracy, and precision to provide the community with confidence that the assays produced by each company adhere to agreed performance and standards. Regardless, the power of affinity-based proteomics technologies such as Olink and SomaLogic now provides singular platforms enabling large scale comparison of sample cohorts with highly consistent assay performance amongst each platform. For Olink, a consortium was developed allowing access to over 50,000 patient samples across many different biological contexts to enable comparison of singular cohorts against a vast open platform collection of cohorts analyzed with the same types of assays. The SCALLOP consortium (Systematic and Combined AnaLysis of Olink Proteins) is a collaborative framework for discovery and follow-up of genetic associations with proteins on the Olink Proteomics platform41. Similarly, SomaLogic has developed a consortium for sharing data, but, unlike SCALLOP, the SomaScan Proteomics Consortium relies on locally stored data rather than a central repository, making easy open access unavailable. These affinity-based technologies that are constrained in their sample analysis processes also provide an attractive format for other large consortia of like-minded researchers to join forces and develop large-scale cohort analyses. Here, the UK Biobank Pharma Proteomics Project (UKB-PPP Consortium), a partnership between the UK Biobank with, and funded by, 15 biopharmaceutical companies is conducting a proteomics study of the world’s largest scientific studies of blood protein biomarkers using 56,000 samples from 53,000 participants deposited in the UK Biobank. The study aims to enable better understanding of disease biology and support innovative drug development for more effective therapies. The 56,000 patient samples will undergo both the Olink-1536 and −3072 platform in 2021 with the goal to have the data fully shared with the public once completed.
In the last several years there has been a resurgence in technology development by commercial companies to take novel technologies developed in academic labs to commercial realization. Starting with sample preparation, the use of nanoparticle-based protein corona fractionation has been developed by the company Seer. They offer a fully automated sample processing solution to enable plasma protein analysis without top abundant protein depletion, but by expanding the sample analysis time, resulting in the ability to detect ~2600 plasma proteins21. Next, for innovations in sequencing methods, a number of technologies have emerged in the massively parallel protein sequencing and quantification space, though most are still in early to late development stages. Many of these emerging technologies begin with peptides (like bottom-up proteomics using LC-MS/MS) and then perform Edman degradation-based (or an equivalent) sequential amino acid cleavage followed by single molecule (i.e., amino acid) detection, resulting in either full or partial peptide sequences. Examples of some companies taking this approach include Erisyon43, which tags specific amino acids with unique fluorophores; Quantum Si, which uses semiconductor-based photon detection; and Encodia, which uses a hybrid DNA-Edman-like sequence analysis with next-generation DNA sequencing outputs44. An alternative single molecule sequencing technique uses the more established nanopore sensing platform, such as Oxford Nanopore Technology’s use of nanopores for long-read nucleic acid sequencing, which can be repurposed for protein sequencing45,46. Finally, a novel sequencing method from Nautilus Biotechnology uses intact proteins and multi-cycle imaging with tagged binding reagents to generate patterns that can be deconvoluted into protein identities at the single molecule level; this is similar to the use of short peptide motif specific antibodies47,48 but on a massive array. As with other protein analysis technologies, it remains to be seen how these approaches will be affected by the wide dynamic range and high abundance of a few proteins in plasma and serum. However, if these technologies prove to be successful, and become validated, viable, and cost-effective solutions, they could enable a broad range of applications in health and basic research.
Human Plasma PeptideAtlas Build in 2021
PeptideAtlas has been collecting public human plasma DDA datasets since 200449 and producing human plasma-specific builds50–52 based on a uniform reprocessing of those data with the Trans-Proteomic Pipeline53–55. Our publication on the state of the Human Plasma PeptideAtlas in 201756 provides a historical summary of the HUPO Human Plasma Proteome Project4, as well as a comparison between the 2017 build and previous builds.
The same basic protocol for the data reanalysis as described in the 2017 build is followed here with the following updates. The data processing used the Trans-Proteomic Pipeline (TPP) pre-release version 6.0. The human protein sequence reference database is the Tiered Human Integrated Search Proteome (THISP)57 version 2020-10. The full list of datasets used has been updated as described below. The overall workflow for processing datasets remains as before: searching with Comet58 and post-processing with PeptideProphet59 and iProphet60. The PeptideAtlas build process also remains the same. The process aims for a protein-level false discovery rate (FDR) of approximately 1% by setting the PSM-level FDR far below 1%, as described below.
A new development for this update is that there are now two separate builds, one for true plasma and serum datasets, and a separate build for datasets that are primarily extracellular vesicles (EVs) separated from plasma. The previous 2017 plasma build accidentally contained two datasets (PXD002950 and PXD003935) that were EV enrichments, and did add proteins to that build that were unique to the EV datasets and may not reflect the proteins of pure plasma. This has been rectified in the 2021 build by removing these EV datasets from the plasma build and creating a new EV-specific build that contains these two datasets as well as additional datasets that have since been made publicly available. First, we describe the plasma-only build, and then return to the EV-specific build.
The Human Plasma PeptideAtlas 2021-07 build contains the combined results from 240 experiments (increasing by 35% from 178 since 2017), yielding 206,001 distinct peptide sequences (peptide-level FDR 0.002 with 358 decoy peptides) at a PSM-level FDR of 0.0002 (12,036 decoy PSMs) from nearly 60 million identified PSMs out of 247 million MS/MS spectra searched. Figure 1 depicts the increase in the number of canonical proteins as experiments are added to the build in roughly chronological order. The 2017 build contained ~43 million PSMs, and thus experiments after the ~42 million PSM mark (recalling that 2 EV datasets were removed) represent new data added to the 2021 build. Whereas the 2017 build had 3672 proteins from 178 experiments (including the 2 EV datasets), the 2021 build now reaches 4395 canonical proteins (there are 0 decoys among the canonical proteins, thus implying an FDR < 0.0002, computed assuming one error). There were 218 decoy entries among the 16,257 non-redundant protein entries (including many non-neXtProt sequences such as alternative isoforms and immunoglobulins) implying a protein-level FDR of ~0.01 for all protein mappings. The full set of non-redundant proteins includes 4395 canonical, 321 indistinguishable representative, 3321 non-core canonical, 1603 representative, 5544 marginally distinguished, 1059 weak, and 14 insufficient evidence proteins; the definitions of these categories were recently described in detail61.
Figure 1. Canonical proteins detected as a function of MS/MS spectra added to the Human Plasma PeptideAtlas build.
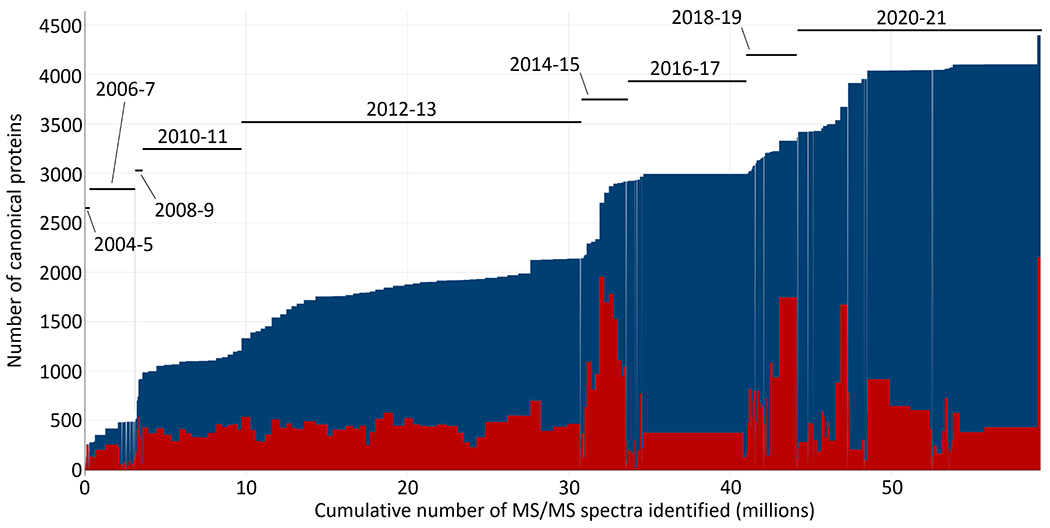
Each rectangle represents one experiment; the width of the rectangle is the number of identified PSMs in the experiment. The upper blue extent indicates the total number of canonical proteins assembled in the build. The lower red rectangles represent the total number of canonical proteins in each experiment. Most experiments contain only ~450 canonical proteins, but some experiments reach as high as ~2000 canonical proteins. An approximate timescale for when datasets were added to the build is overlaid in two-year extents since 2004. An interactive version of the figure, where individual experiments can be identified, is available at the PeptideAtlas build summary web page.
We have also updated the criteria for categorizing a protein as canonical. Since the development of the HPP Data Interpretation Guidelines 2.162, we defined canonical proteins as those that have at least two non-nested (not fully contained within the other) peptides of nine or more residues with at least 18 amino acids of protein coverage that map uniquely to the reference proteome. We have updated the definition to limit the mapping to the 20,379 primary isoforms contained in neXtProt. Proteins outside this space (such as additional isoforms, immunoglobulin sequences found in UniProtKB/TrEMBL, etc.) that fulfill the above criteria are instead categorized as noncore-canonical. By these updated rules, the 2017 build would have had only 2991 canonical proteins, so the gain is more substantial than it would have been under the old rules. Plasma is especially sensitive to this new method because of the many variable UniProtKB/TrEMBL immunoglobulin sequences that are detected in plasma samples.
The Proteome of Plasma-derived Extracellular Vesicles
In parallel to the efforts of studying the proteins circulating in blood, there are a number of publications reporting the use of MS-based and affinity-based methods to study extracellular vesicles isolated from human plasma63–66. Important for a common understanding about the difference and overlap between the plasma proteome and the one composed of extracellular vesicles, we discuss the protocols and data analysis for these types of samples. EVs refer to a broad and heterogeneous category of vesicles, defined by their origin in the cell and their size, including: apoptotic bodies (50-1000 nm in diameter), which are released from cells undergoing apoptosis; microvesicles (MVs), which bud off from the plasma membrane (100-1000 nm); and exosomes (~50 nm), of endosomal origin, which are released in the extracellular environment upon fusion of multivesicular bodies with the plasma membrane67. EVs have indeed been linked to multiple physiological and pathological processes and hence comprise important extracellular signaling components. Thus, analyzing the EV component of human plasma offers a valuable source for biomarkers, but also presents various challenges such as sensitivity and specificity of the enrichment, quantitative and qualitative reproducibility, and the lack of a genetically defined blueprint that categorizes proteins into a circulating EV proteome.
Traditionally EVs have been enriched from clarified cell media and body fluids through centrifugation, size-separation, affinity enrichment, or combinations thereof68–72. EVs overlap in terms of diameter and density with other soluble plasma proteins72 and are of low abundance (~1010 particles/mL)73. Consequently, unintended co-enrichment of high abundant plasma proteins poses a challenge. Vice versa, if not specifically removed, plasma samples contain proteins derived from EVs, albeit in relatively low concentrations. In fact, circulating lipoproteins outnumber plasma-derived EVs by six to seven orders of particle concentration (particles/ml)73. When defining the protein content of a plasma sample, it is helpful to consider the different components: the soluble and secreted proteins circulating in plasma, EVs, platelets, and other proteins of cellular origin.
Experience decomposing the circulating proteome, much of the quality assurance related to sample preparation, experimental design, and reporting of findings, already performed by the plasma proteomics community, should be adapted to studies with EVs. This includes, but is not limited to, avoiding pooling of samples, reporting the details and steps of plasma collection and preparation, and implementing quality assurance marker panels to assess the degree of contamination74.
The Human Extracellular Vesicle PeptideAtlas 2021-06 comprises 23 experiments from just five datasets derived from studies reporting MS-based analysis of plasma EVs samples, all using different centrifugation approaches to enrich for EVs75–79. The build contains ~4 million PSMs (PSM-level FDR 0.0002 with 735 decoy PSMs) and 94,831 distinct peptides (peptide-level FDR 0.0003 with 31 decoy peptides), resulting in 2750 canonical proteins (zero decoys in the canonicals thus a canonical protein FDR < 0.0004 computed with 1 error) as shown in Figure 2. The overall protein-level FDR including both canonical and lesser proteins is 0.003 (30 decoys out of 11,379 entries).
Figure 2. Number of canonical proteins as a function of MS/MS spectra added to the Human Blood Extracellular Vesicle PeptideAtlas build.
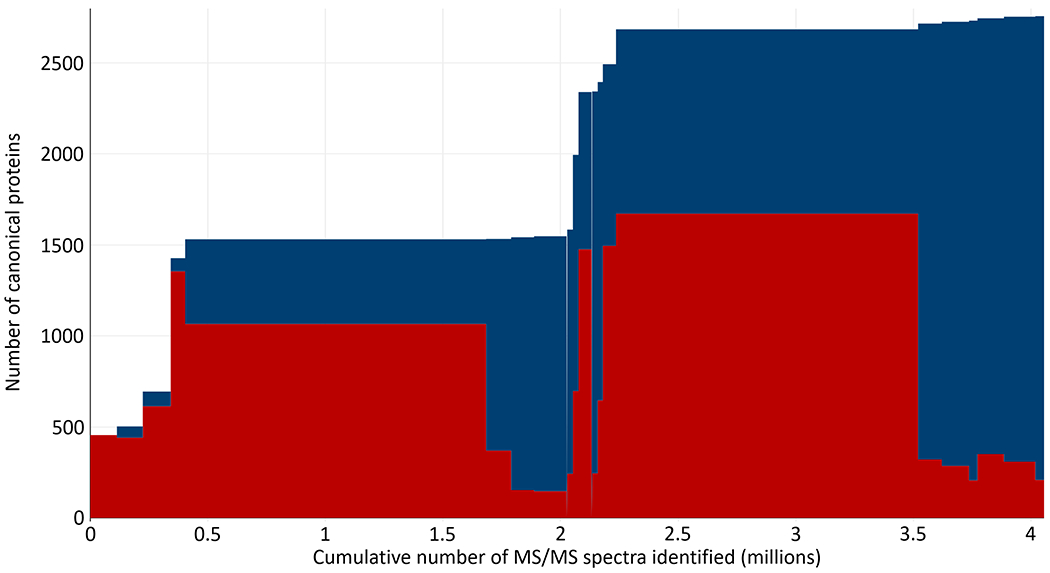
Each rectangle represents one experiment; the width of the rectangle is the number of identified PSMs in the experiment. The upper blue extent indicates the total number of canonical proteins assembled in the build. The lower red rectangles represent the total number of canonical proteins in each experiment.
In Figure 3 we compare the plasma and EV builds at the protein level. Figure 3A depicts the correlation between protein abundances for the 2047 canonical proteins in common between the two builds. Abundances are computed based on the spectral counting of the total number of PSM events associated with each protein from all supporting peptides. Levels are expressed after log10 transformation and scaling between the two builds so that the most abundant proteins have similar values. The diagonal in Figure 3A shows the line of equal levels and not fitted to the distribution. The distribution is suggestive of a population of high abundance proteins that are relatively more abundant in plasma, and a population of lower abundance proteins that are relatively more abundant in EVs, but the scatter and uncertainties are high. Figure 3B depicts a Venn-like diagram that is stratified by the same abundance scale as panel A, where the higher of the two abundances is used in the overlap. The panel indicates that 40% of all proteins are shared between the two builds, with 46% occurring only in plasma and 14% only in the EV build. The excess plasma proteins occur primarily at lower abundances, as would be expected since the plasma build probes much deeper into the proteome with ten times as many PSMs.
Figure 3. Comparison of the Plasma and EV build protein abundances.
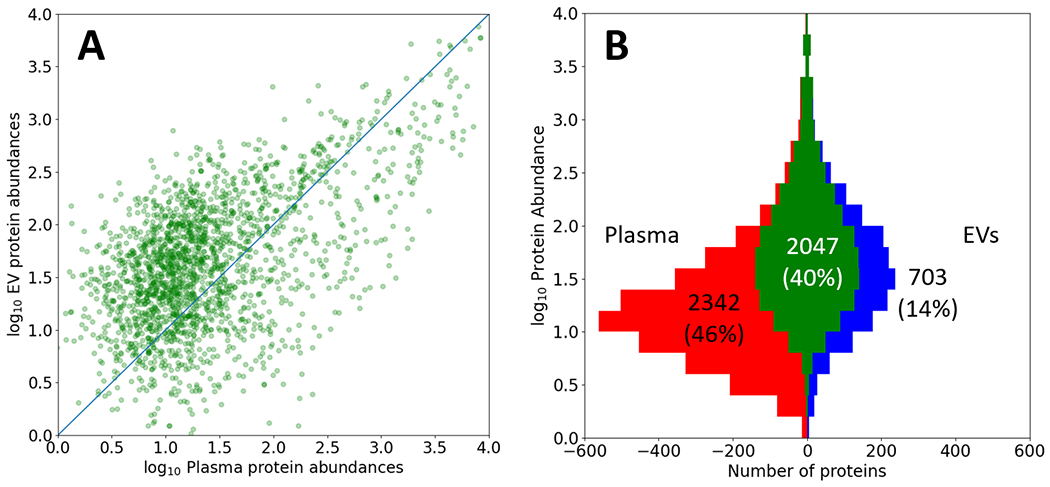
A) Comparison of log10 EV protein abundances vs log10 Plasma protein abundances for the 2047 proteins in common between the two builds. The abundance estimates are based on overall PSM counts and are arbitrarily scaled. A diagonal line of equivalent abundance is overlaid. B) A Venn-like diagram depicting the overlap (central 40%) of proteins in the Plasma (left) and EV (right) builds, stratified by log10 protein abundance estimates based on PSM counts on the y-axis. The plasma build has 10 times as many PSMs and therefore more proteins detectable at lower abundance.
We provide Supplemental Table 1 that contains the union of proteins in both the plasma and EV builds, along with the crudely estimated log10 abundances as described above, as well as annotations from neXtProt80 and the HPA81. Columns one and two are the UniProtKB identifiers and corresponding gene symbols, respectively. Columns three and four provide the plasma build abundance and EV build abundance; column five is the greater of columns three and four (as used in Figure 3B). Columns six and seven provide the typical blood cell type82 and overall tissue type/organ in which the protein is typically most abundant as provided by HPA81. These annotations are derived by combining proteomics and transcriptomics information about protein expression. This has recently been extended to categorize the 2500+ secreted proteins of which about 730 are blood-bound83. The final column eight provides the full protein name as provided by neXtProt. In Supplementary Table 2 we provide a very small excerpt from Supplemental Table 1 for the ten most abundant proteins in the EV build that are not detected at all in the far more sensitive plasma build. These all occur between 2.5 and 3.1 in the abundance scale in Figure 3 and originate from different organ systems. The plasma and maximum abundance columns described in Supplementary Table 1 are excluded in Supplementary Table 2. Together the five studies contribute 910 proteins that had previously not been reported in studies primarily aiming at characterizing the soluble plasma proteome. We do note that “classical” or “commonly used” EV markers including CD81, CD63, CD9, Alix, and several tetraspanins had been reported already in previous builds of the plasma proteome database67,84,85 perhaps due to leakage or contamination.
Plasma proteomics efforts investigating COVID-19
The Corona Virus Disease 2019 (COVID-19) spread around the world with devastating effect on mankind, infecting and killing millions86. The scientific community responded rapidly by investigating the biology of the virus and its effects on humans87. Novel markers for pathogen detection, treatment, and even new types of vaccines were developed and applied within months. Proteomics as a generic tool has been widely applied in the pandemic, from drafting protein interaction maps of the SARS-CoV-2 virus and human proteins, to the identification of previously unknown viral epitopes, and the detailed description of the host response by investigating the human plasma proteome upon SARS-CoV-2 infection88,89. To provide a detailed overview of the plasma or serum proteome literature investigating COVID-19, we searched PubMed and the preprint servers bioRxiv and medRxiv and summarized the results below. For PubMed, we used the title and abstract terms “(Serum OR Plasma) AND (Proteomics OR Proteomic OR Proteome) AND COVID-19”. To consider the latest research results, we performed several searches on bioRxiv and medRxiv, reflecting the logic of PubMed. The searches were conducted on the 6th and 7th of June 2021. This ended after closer investigation of the search results in 50 manuscripts, which investigated 48 different cohorts (one dataset was investigated in three manuscripts). The manuscripts included 14 non-peer reviewed publications. In total, the published studies analyzed more than 8785 samples corresponding to 3494 COVID-19 patients, 1579 controls and their longitudinal samples. Five studies investigated large-scale cohorts with more than 500 samples90–93 and 22 cohorts measured 100 or more samples (Figure 4A). Technologies used in these efforts included MS-based proteomics (24 manuscripts), the Olink platform (20), classic immunoassays (5) and the SomaLogic platform (4) (Figure 4B).
Figure 4: Literature search of manuscripts investigating serum or plasma alterations in COVID-19.
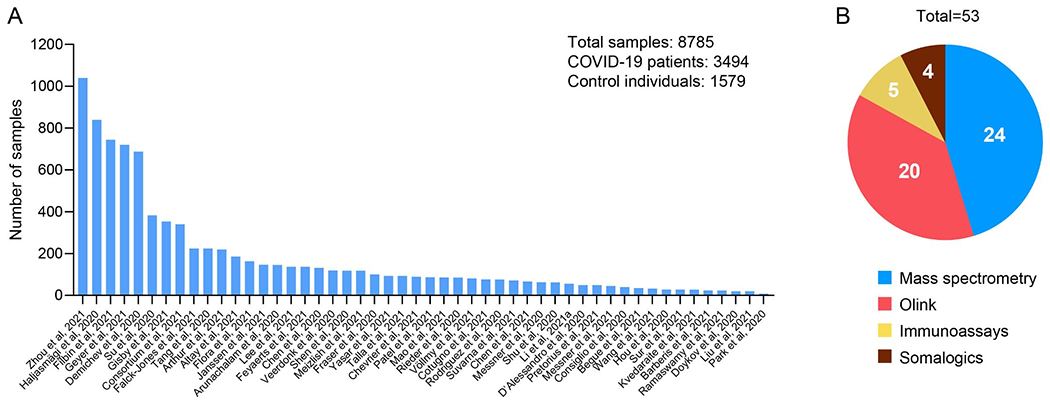
(A) Studies ranked according to the analyzed number of samples. (B) Portion of manuscripts applying different technologies to investigate the proteome of COVID-19 patients. Of note, in some studies more than one technology has been applied.
Understanding the mechanism of COVID-19 pathogenesis
The majority of projects focused on the comparison of two or more groups, including the classical cases-vs.-control comparisons, and the differences between COVID-19 disease severity groups, which ranged from mild to severe symptoms and fatal outcome94–98. In most studies, the controls consisted of healthy individuals, but several studies used symptomatic patients that turned out to be PCR-negative or specialized control-disease cohorts such as influenza patients99,100. The proteome-wide analysis of functional proteins in the plasma or serum is critical to understand the changes of mechanism in COVID-19 patients100–103. Studies applying MS-based proteomics to investigate differences between COVID-19 patients and controls showed a very consistent picture. In general, they pointed towards altered protein levels of the inflammation system, antibody response, and coagulation system. Coagulopathies are one of the main complications in COVID-19, which makes altered coagulation factors and coagulation regulators such as F13, HRG, KNG1, APOH, FN1 and PLG interesting candidates for therapeutic targets or to investigate pathological mechanisms. The inflammation system was activated in COVID-19 patients, shown by increased levels of inflammation proteins in COVID-19 patients compared to healthy individuals or COVID-19 negative but symptomatic patients. These proteins were further elevated in severe patients compared to patients with milder symptoms. Inflammation proteins that were frequently reported by these studies included ORM1, ORM2, SAA2, LBP, LGALS3BP, the complement system, and the classical inflammation biomarkers CRP and SAA1. The highly detailed picture of dozens of acute phase proteins identified in these studies are nicely complemented by investigations using affinity reagents, which target selected and typically lower abundant plasma proteins such as cytokines. Utilizing a high-density antibody microarray, Hou et al. measured 532 proteins in the serum of early COVID-19 (n=15) and influenza (n=13) patients, and identified a landscape of inflammation and immune signaling in the patients with early SARS-CoV-2 infection, in which CCL2 and CXCL10 signaling cascades were identified as the potential targets for COVID-19 therapy100. In another study, using the Olink platform, Janssen et al. measured 269 proteins (Inflammation, Cardiometabolic, and Cardiovascular II panels) in 147 COVID-19 patients. Hepatocyte growth factor (HGF) and stem cell factor (SCF) were identified as the biomarkers that are associated with the disease severity. Functional mononuclear cytokine assays revealed the abrogated adaptive cytokine production and prominent T-cell exhaustion in critically ill patients101. Filbin et al. measured 1400+ proteins using Olink’s assays to study COVID-19 patients with severe symptoms93. Using the same assays, a study from Zhong et al. analyzed plasma from patients with mild to moderate symptoms and highlighted, among many cytokines and immune-related proteins, elevated plasma levels of the membrane-bound receptors SCARB2 and SIGLEC1104. Su et al. chose a multi-omics approaches where circulating proteins and other types of molecular data obtained from blood samples were used to phenotype different levels of COVID-19 severity105. Zhou et al. used SomaScan data and Mendelian randomization to find that elevated levels of the circulating neanderthal OAS1 isoform were protective against COVID-19 susceptibility and severity106 This highlights the complementary information that can be extracted by different methodologies and this synergy of information can also be seen in studies describing longitudinal patient trajectories.
In total, 21 of the 53 proteomics studies benefitted from the power of longitudinal cohorts. This included highly detailed protein trajectories in some cases, even with day-to-day resolution, and studies investigating sample sets which were obtained over several weeks from hospitalized patients91,98,107–109. The longitudinal studies have been applied to describe the clinical trajectory of patient responses to COVID-19, confirming dysregulated proteins from cases-vs.-controls comparisons and shedding light on complexly regulated proteins such as GSN. This protein has been reported only in a subset of studies, but there it has been shown to be one of the most significantly regulated proteins95,96. Moreover, longitudinal trajectories have been applied to track the antibody response in a highly detailed manner by MS-based proteomics, showing an individual-specific but also very broad response in terms of the number of different antibody regions that were produced. Such longitudinal trajectories to confirm seroconversion can be seen as medically relevant information in addition to biomarkers identifying COVID-19 patients or predicting their disease course. This exemplifies the large potential of plasma proteomics as a multi-parameter readout in medicine.
The extensive burden on the medical system and shortage of equipment have been major problems. Hence, biomarkers with the potential to stratify patients into disease severity groups would be very valuable, and might be one reason why many groups focused on potential single biomarker or panels of biomarkers, combined in machine learning models98,99,107–111. Using proteomic and metabolomic data from 18 non-severe and 13 severe patients, Shen et al. achieved a model reaching an area under the curve (AUC) of a receiver-operating characteristic (ROC) of 0.957, wherein the model consisted of 22 proteins and 7 metabolites99. Demichev et al. investigated a larger cohort, characterizing COVID-19 disease progression and predicting the outcome according to WHO grades107. This resulted in an AUC of 0.81 to predict the survival of critically ill patients from early time points. Both models included proteins of the inflammation and the coagulation systems. Several other efforts reported potential predictive markers of mortality that were regulated early on in the disease course of COVID-19 patients, such as ITIH4, which has been reported in two different studies98,108. Chevrier et al. found that a comparison of data between patients with mild (n=28) and severe (n=38) COVID-19 showed that severe patients had higher levels of M-CSF, IL-6, and TNF. These cytokines are often present in high concentrations throughout the course of patients with severe diseases. In patients with mild symptoms, these cytokines will decrease as the disease progresses. However, CCL3 and CCL4 significantly increase in the later stages of patients with severe diseases, a finding that might help to better stratify patients by disease severity. Altay et al.112 measured the cytokine levels of mild to moderate COVID-19 patients treated with Combined Metabolic Activators (CMA) and placebo in Open-Label Phase-2 Clinical Trial and Double-Blinded Phase-3 Clinical Trial. It was found that CMA is an effective treatment to mild and moderate COVID-19 patients, which might play its functions through the regulation of proinflammatory proteins112. All these results may provide comprehensive insight into the COVID-19 infection across patients of different disease severity, which could facilitate patient outcomes. Still, many of the highlighted results require further validation in more diverse and larger study sets. Nonetheless, plasma proteomics data provided valuable and imminent insight into the clinical phenotype of the disease.
Multiplexed COVID-19 serology
Besides the efforts to determine circulating proteins related to the SARS-CoV-2 infection, studies of the virus-specific humoral response have played critical roles in understanding the effects of the pandemic, COVID-19 pathogenesis, disease severity and recovery. Thus, the proteome-wide profiling of circulating antibodies against SARS-CoV-2 has assisted the community to understand the seroprevalence, host immunity and possibly identify and test biomarkers for COVID-19 therapy. The community has developed and applied different multiplexed approaches to capture the antibody response and diversity against the virus and its variants of concern. Among others, Tao and colleagues fabricated a SARS-CoV-2 proteome microarray that covers 21 purified structural and nonstructural proteins113–115. Using the microarray, the antibodies in a large set of serum samples (~2000) obtained from COVID-19 patients of different severity were profiled. The results not only reveal the immunogenicity of both the structural Spike (S), Nucleocapsid (N) proteins, and the nonstructural proteins (e. g., ORF9b and NSP5.1). More importantly, circulating antibodies that indicate the early viral infection and COVID-19 severity were identified114,115. In addition to planar protein arrays, bead-based systems determined levels of IgG, IgM or IgA in a variety of large scale studies that analyzed different sample types to assess the immune response against a number of different SARS-CoV-2 antigens and variants of concern, as well as antigens representing other viruses116–119.
An alternative protein-based detection of circulating antibodies uses arrays of peptides, which can be produced in a short time using the viral genome sequence120–130. In the early COVID-19 pandemic, Yu et al.122 developed a microarray with 966 15-mer peptides, which fully cover all the SARS-CoV-2 proteins, with which the landscape of B-cell epitopes for SARS-CoV-2 IgM and IgG antibodies in early COVID-19 infection were constructed120. Shrock et al. developed a VirScan phage-display platform containing an oligopeptide library across the proteomes of all known pathogenic human viruses (~400 species and strains). The serum of 232 COVID-19 patients and 190 pre-COVID-19 era controls were screened using VirScan, identifying more than 800 epitopes and highly sensitive biomarkers that indicate the SARS-CoV-2 exposure history by machine learning128.
In the near future, these multiplexed serological studies will assist the community beyond COVID-19, enable a proteome-wide dive into the diversity and repertoire of the immune system131,132, and allow a highly sensitive and specific assessment of the immune response in clinical use. Together with proteomics studies of the circulating proteome, serological analyses will provide valuable insights into inter-individual diversity133 and offer possibilities to stratify or monitor patients over time.
Proteomics, Genomics, and Protein Quantitative Trait Loci
The capability of analyzing large numbers of proteins in large sample sets have created new opportunities for plasma proteomics together with other types of molecular data. In particular, the use of genetic data to inform about protein abundance levels has surged and delivered important insights about human biology40. In essence, these efforts determine the association between genetic variation and protein expression to pinpoint causal mechanisms affecting the proteomic signatures. These associations are reported as protein Quantitative Trait Loci (pQTLs) and represent a locus with one or more genetic variations. Since pQTLs can influence a wide range of biological processes, studying circulating protein levels as intermediate phenotypes can reveal time-resolved molecular insights. As illustrated in Figure 5, pQTLs can localize with regions affecting the expression of the respective protein directly (association in cis), as well as other regions in the genome that influence protein expression indirectly (association in trans). In this manner, genetic variation can alter a protein’s sequence, affect protein expression and influence its regulation. Co-localization of the identified pQTLs can then, for example, be used to explore available genetic information for known genetic risk factors, and thereby reveal insights about mechanistic relationships between protein levels and a disease phenotype. Intriguingly, there are pQTLs reported for about 30% of the currently listed FDA-approved protein biomarkers 40.
Figure 5: Genome wide associations of circulating protein levels.
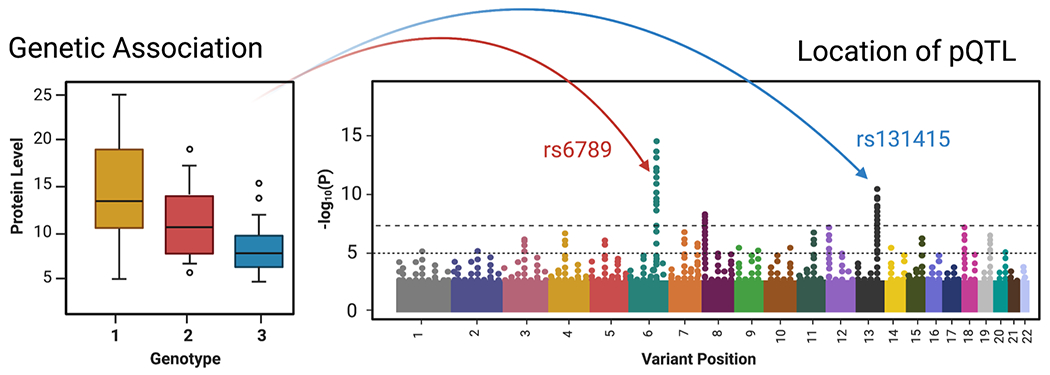
Simplified illustration to depict how genetic variation can influence protein levels. Projecting the results of regression analyses onto the genome can identify regions of genetic variation, so called protein Quantitative Trait Loci (pQTLs), that associate with protein levels. These regions can either localize with the proteins of interest (cis-pQTLs, red) or relate to other regions in the genome (trans-pQTLs, blue).
Therefore, the availability of population-wide studies with sequencing data offers tremendous possibilities for the plasma proteomics community and technology providers. Until today, most of these genome wide association studies (GWAS) that use proteomics data from plasma or serum have been conducted on affinity proteomics platforms. First described in 2014 for 90+ proteins and 1000 samples134, recent GWAS studies have reported information of 90 proteins from 30,000 subjects135 or even on 4700 proteins in nearly 5500 subjects136. Reassuringly, many of the identified cis-pQTLs could be replicated across different studies35,36 when using SomaLogic’s and Olink’s affinity-based platforms. Recent efforts have now also focused on comparing the hits identified across platforms in order to determine how different assays, phenotypes and genetic variation influence the protein detectability and the reporting of pQTL137. In comparison to the affinity proteomics studies, there are currently not a substantial number of GWAS studies that are based on MS-derived plasma data138–140. Thus, there is a huge potential of adding sequence resolved MS-based proteomics data to these studies. Eventually, studying the complexity and effects of combining many different genetic variants present exciting opportunities for proteomics to inform about cellular mechanisms on a molecular level141. This is exemplified by GWAS-anchored studies connecting multi-omic signatures of mRNA expression and levels of circulating protein and metabolites142.
Proteogenomics
Proteogenomics is a collective and generic term for several analytical approaches where DNA and/or RNA sequence data and proteomics data are combined to support, or show the effect, of each other143. In MS-based proteomics sample specific protein sequence databases based on RNA or DNA sequence information can be generated and thereby enable the identification and quantification of coding protein sequence alterations. Such proteogenomics applications include methods to detect single amino acid variants (SAAV), mutations, or splice variants initially discovered by RNA/DNA analysis at the protein level. In addition, by mapping protein sequence data to regions of the genome believed to be non-coding, novel, previously unknown genes and gene variants can be identified143.
One of the first reported applications of plasma proteogenomics was to detect coding germline variants i.e. coding single nucleotide polymorphisms (cSNP) or single amino acid variants (SAAV) in plasma proteins. These data can be obtained by creating sample specific databases from SNP arrays or DNA sequencing of white blood cells collected in parallel with the plasma sample preparation, or by using publicly available repositories of SNPs, such as dbSNP (https://www.ncbi.nlm.nih.gov/snp/). As an example, the apolipoprotein E allele APOE4 in the homozygous form is a known major risk factor for late-onset Alzheimer’s disease with a higher risk compared with the homozygous APOE3 form144. APOE4 has an arginine at position 112 instead of a cysteine in APOE2 and APOE3, and has been detected both using targeted and global MS22,145. SAAV analysis has also been used to pinpoint proteins transferred across the placenta during pregnancy. Here the SAAV is used as a proof-of-origin, by tracing SAAVs unique to either mother or child in the bloodstream of the mother or baby, respectively146.
In genomics, the term ‘liquid biopsy’ is used to describe the detection of cell-free nucleic acids, mainly circulating free DNA (cfDNA), in body fluids such as blood. Liquid biopsies take advantage of the specific genetic differences between tumoral and normal DNA to identify highly specific biomarkers without invasive biopsies of the tumors themselves. On the tissue level several proteogenomics studies have been published defining the proteogenomics landscape in cancer tissues, including from colon cancers147, renal cell carcinomas148, and breast cancers149. In many of these studies, sample specific databases for proteomics are created based on parallel RNAseq or DNAseq analyses on the same sample. In parallel, applying proteogenomics analysis of plasma could provide an additional dimension of information in biomarker discovery or tumor origin, as proteins containing mutated sequences would by default be produced by the tumor. Few studies have so far explored this application. Mutated protein sequences (e.g., PTEN, KIT, and B2M) in plasma have been described from patients with malignant melanoma, based on sequences matching using a database containing known mutations from COSMIC150.
Overall, the use of sample-specific sequence databases in MS-based plasma proteomics is expected to increase and will most likely require novel quality assurance steps, including false discovery rate control and inspection of individual spectra reflecting HPP Guidelines for Interpretation of Mass Spectrometry Data v3.0151. More studies using liquid samples will emerge.
A major application of proteogenomics is the sensitive detection of circulating tumor cells (CTCs), cell-free DNA, and single-cell analyses of transcripts and proteins, including cell-surface antigens from primary and metastatic tumors. One platform is the microfluidics Genesis System from Celsee. Tumor protein markers, such as the EGFRv3 tumor neoantigen, can be used to isolate cancer-specific exosomes.
Hemostasis
Hemostasis represents the fine balance between bleeding and clotting, as one of the core functions of blood in repairing the effect of injuries. The proteins engaged in hemostasis are highly abundant in blood and are also involved in other processes such as immunity and inflammation. The use of proteomics in the setting of thrombosis and hemostasis is growing, as the search for blood markers associated with patient outcomes such as venous thromboembolism (VTE) and stroke intensifies. In the context of plasma proteomics and specifically over the past 5 years we identified 8 major studies focusing on hemostasis, and specifically the imbalance between bleeding and clotting.
Two studies focused on improving the fundamental understanding of the composition of blood clots152,153 used approaches to prepare plasma clots in vitro with samples from healthy adults, using LC-MS/MS to investigate the composition of those clots. This approach has been useful in determining that close to 500 proteins are included in the fibrin clot structure153, and identified specific substrates for FXIIIa, a protein responsible for the fibrin cross links in blood clots, as well as the mechanism for the cross links themselves152. Additional findings, important in the context of precision medicine, include the impacts of BMI and age on the fibrin clot structure153. Thrombin is known as the “master regulator” protein in hemostasis, playing a key role in the fine balance between bleeding and clotting. Bhagwat et al. used LC-MS/MS to identify thrombin substrates154. While this is an n=1 study, it provides a proof of principle for future studies in this space, identifying 54 substrates for thrombin, opening the door for studies in clinical settings which can identify whether the thrombin substrates change depending on the health status, age, etc. of individuals. Using LC-MS/MS, Stachowicz et al.155 investigated the differences in the ex vivo plasma clot composition in patients with thrombotic antiphospholipid syndrome (APS) compared with VTE, identifying differences in up to 63 proteins. The importance of this study lies in the fact that not all clots are the same and that the underlying disease plays an important role in the mechanism and composition of blood clots in different clinical settings, knowledge that is crucial for practice of precision medicine.
While VTE is a major cause of morbidity and mortality, affecting 10 million individuals globally each year156, we have identified only one study that focused on identifying the plasma protein markers for VTE157. This comprehensive study based on extensively phenotyped Venous thromboembolism BIOmarker Study (VEBIOS) and FARIVE cohorts in Sweden and France, respectively, utilized LC-MS and performed validation using ELISA. This study identified PDGFB as a novel VTE-associated plasma protein and as a potential novel biomarker for VTE.
Over the past 5 years there has been an increased focus on stroke and specifically on understanding the mechanisms of disease, as well as on identifying blood markers associated with specific clinical outcomes. Three studies of interest utilized a plasma proteomics approach158–160. Penn et al. provided evidence for the applicability of MS, and specifically LC/MRM-MS, in the setting of Transient Ischemic Attack (TIA), identifying that TIA was driven by coagulation, inflammation, cell adhesion, and atrial fibrillation and that 30 proteins were differentially abundant between individuals with TIA and healthy controls158. This group then validated a plasma protein signature identified in the proof-of-concept study in a large cohort of >500 individuals with TIA, with individuals with non-cerebrovascular emergency department patients serving as controls. Whilst the original 16 protein signature was not validated due to quality control (QC) issues, IGFBP-3 and PON3 were identified as predictors of TIA. LC-ESI-MS was used to specifically study the sex-based differences in the mechanism of ischemic stroke, identifying corticosteroid signaling as the main mechanism differentiating between male and female stroke patients, and circulating corticosteroid-binding globulin (CBG) as the marker for sex-based differences of stroke outcomes and severity160.
Aging
The concept of aging of the plasma proteome is well known, with age-specific differences in the plasma proteome shown in newborns and children compared to adults, as well as within the aging adult population. Over the past 5 years, multiple studies focusing on plasma proteomics of aging have been conducted; we highlight several161–164.
Starting with the youngest and most vulnerable population, the preterm infants, Suski et al. used Proteominer, iTRAQ, 2D-cation exchange/RP-HPLC and LC-MS/MS to study the differences in the plasma proteome between preterm infants (born <30 weeks’ gestation) and healthy full-term newborns; 137 proteins were quantified163. Inflammation, immunomodulation, complement activation, and coagulation were identified as major pathways associated with prematurity. Despite the difficulty in collecting blood samples from preterm babies, this important study aids our understanding on how preterm birth affects multiple pathways with associated proteins that are differentially expressed during early development, such as gestational age-dependent hemopexin induction. In addition to MS-based approaches, the low sample volume required for some affinity proteomics procedures has made these attractive for studying the changes in the circulating proteome during early development165,166 or to explore the possibilities for predicting future diseases167.
On the opposite end of human lifespan are individuals with extreme longevity (healthy aging). Populations with disproportionate numbers of extremely long-lived humans exist in several geographical locations which provide unique samples and opportunities to study aging and longevity. Identifying specific proteins, and their networks, can also lead to the identification of interactors and their effects upon other molecules (e.g., metabolites, DNA binding) related to extreme longevity and might reveal a molecular basis for these long-lived individuals. These molecular sentinels of aging may provide opportunities for intervention to extend healthy longevity and slow down aging168,169.
Multiple large studies have focused on the changes in the plasma proteome across chronological age, covering wide age ranges from subjects in their early 20’s to centenarians, utilizing ELISA-like plate-based assays such as SomaScan162, antibody assays based on suspension bead arrays170 or LC-MS/MS161. For the study by Tanaka et al., proteomic profiling was conducted on serum samples of 240 healthy men and women using the SomaScan 1.3K protein assay system to examine samples from the Baltimore Longitudinal Study of Aging (BLSA) or Genetic and Epigenetic Signatures of Translational Aging Laboratory Testing (GESTALT) cohorts; 217 proteins were associated (20 negatively associated, 197 positively associated) with age (p < 3.83×10−5) in the basic model adjusted for sex, and further refinement by body mass index (BMI), and serum creatinine resulted in 210 (22 negative, 188 positive) age-associated proteins. Growth differentiation factor 15 (GDF15) had the strongest, positive association with age, which is supported by several other studies171,172. Another 10 other proteins (metalloproteases, etc.) comprised the most significantly associated proteins. In a recent proteomic analysis using the SomaLogic 4K panel (4387 unique proteins) coupled to genetic variants of the New England Centenarian Study (median age = 104 years), protein signatures associated with rare variants in chromosomes 9 and 13 suggest potentially interesting biomarkers of longevity that are genetically regulated171. The protein signature results from a cohort of 224 participants was replicated in a subsequent analysis of 1000 Ashkenazi Jewish participants associated with APOE genotypes and confirmed a strong overexpression of BIRC2 and reduced expression of APOB in carriers of the APOE2 allele171. The analysis also discovered and replicated associations between longevity variants and slower changes of protein biomarkers of aging, including a novel protein signature of a variant of the CDKN2A/CDKN2B gene in chromosome 9, suggesting genetic regulation of GDF15.
However, many humans that grow old will not reach centenarian status and the natural aging process is related to a decline in overall health in most cases. These biological hallmarks of aging have been extensively defined elsewhere173; their phenotypic measurements can be used to assess aging populations. General fluid cognitive ability can be quantified with standardized tests and brain size can easily be measured by MRI. In a recent paper, Harris et al. looked for associations between these measurements and the plasma proteome (via Olink assays) in the Lothian Birth Cohorts of 1921 (n=165) and 1936 (n=798), as well as the INTERVAL BioResource (n=4451)174. Several proteins related to neurological processes were associated with general fluid cognitive ability and could lead to the biological factors correlating with late life cognitive function. In a different study, a Malaysian aging cohort (Towards Useful Aging (TUA); n=160) was used to identify the correlation of cognitive function with differential protein abundance levels175. Over 200 proteins were identified and quantified by mass spectrometry; 24 proteins were significantly different between the age groups. The most affected processes involved the immune system, hemostasis and neurodegenerative pathways.
Several studies have used the SomaScan technology to determine which proteins are associated with frailty. In the LonGenity cohort (n=206+519) 143 proteins were significantly associated with frailty176. Most of the frailty-associated proteins are higher in females compared to males, which confirms the higher prevalence of frailty amongst women. Specific pathways involved are lipid metabolism, and networks related to tissue, skeletal and muscular system development. Association of plasma proteome with frailty was assessed with SomaScan in the InCHIANTI cohort (n=752, Italy) in a similar way177.
The SomaScan platform has also been used extensively to look at overall changes in the plasma proteome across lifespan and to try to define a proteomic signature of healthy aging in other large cohorts, e.g. TwinUK (n=206)178, combined cohorts of Whitehall II, Fenland, HUNT3, Covenance, and Heritage (n=16894)179, LonGenity (n=1025)180, InCHIANTI (n=997)181 and others. Lehallier et al. used the INTERVAL and LonGenity cohorts (18-95 years old) (N=4263) to find the plasma proteins with the most prominent changes associated with age (sclerostin (SOST), ERFIP2 and GDF15) in addition to several proteins that differ with sex (such as CGA FSHB)182. Additionally, they determined that most of the plasma proteome changes across lifespan are non-linear, but rather grouped into clusters of protein trajectories that change with age. These data were used to determine a proteomic aging clock183,184 based on the systematic review by Johnson et al.185.
So far, few studies have used mass spectrometry to assess the plasma proteome of large aging cohorts, but the continuous advances in instrumentation as well as in computational tools will surely lead to exciting new publications. Recently, the plasma proteome of centenarians has been assessed by mass spectrometry186. Here, proteome analysis of plasma samples from a small cohort (n=9) of healthy centenarians were compared with a similar sized control group of older people (aged 67-81 years old). LC-MS/MS analysis revealed almost 50 plasma proteins with significant differences in abundance between the study cohort and the control group. Overall, the ‘proteomic hallmark’ of healthy centenarians was mainly attenuation of inflammaging and improved immune function by lowering the pro-inflammatory status and preservation of humoral immune response. Proteins involved in successful aging are CLEC3B, CRISP3, IGFALS, TAS1R3 and TGFBI and in unsuccessful aging: AOPEP, CD14, CDKL1 and CRTAC1. Another study that used MS-based approaches looked at age-dependent changes in the plasma proteome in 118 individuals in 3 age groups (21-30, 41-50 and 60+) in China161. Their findings included defining “youth factors” and “anti-aging factors”. Downregulation of “youth factors” might lead to decline in biological function and result in disease, whereas upregulation of “anti-aging factors” might be beneficial for health.
To identify plasma proteins associated with longevity with mass spectrometry approaches, Wang et al. used TMT peptide labelling and LC-MS/MS to study individuals from longevous families (a family with at least two long-lived (≥85 years old) immediate family members) and compared their plasma to age and gender matched individuals from non-longevous families. The Bama Yao Autonomous County in China is one of the well-known longevity regions. Multiple genome and population studies have been carried out in the past, and recently proteomics studies are being pursued164,187. Specifically the plasma proteomic and autoantibody profile of offspring of long-lived families and age-related controls were compared187. TMT-labelling enabled quantification of over 500 proteins, out of which 12 proteins were differentially expressed between the two groups (proteins with increased levels: LPA, DEFA3, immunoglobulin, BLVRB and PDIA3; proteins with reduced levels: TPM, GBA, DSG2, SRC, CHGA, ITGB3 and TAGLN2). In a second study, 175 proteins were identified to be differentially abundant between the groups, and a panel of five proteins (MMP2, CCL5, PF4, IGFBP2 and C9) may serve as biomarkers to predict healthy aging and longevity164. Tanaka et al. performed a study of gender-based differences in the plasma proteome of the InCHIANTI cohort (n=997, Italy) using an LC-MS/MS approach to identify 10 proteins that were associated with age181.
Ethical Considerations
The plasma proteome is the key medium for developing predictive biomarker tests or test panels for diagnosis, prognosis, and response to therapy in precision medicine. A large majority of the still-limited number of FDA-approved biomarkers are proteins188. Plasma proteomes may contain sensitive personally-identifiable information, a parallel to the much-discussed ethical issues from genome-based diagnostics189. Proteomic profiles can reveal identity, biological gender, ethnicity, pregnancy status, and a wide range of health conditions (“incidental findings”) beyond the clinical question or suspected organ damage that motivated the testing. Protein sequence variants reflect corresponding variants in the genome. Of course, protein concentrations often change during growth and development and pathogenesis of diseases, in contrast to the static inherited genome. Machine learning/deep learning and artificial intelligence techniques can enhance data extraction and correlations from complex assays. An informative case study is the re-examination189 of a plasma proteome study of 42 individuals in a weight-loss protocol with 7 samples over a 12-month period190. Inter-individual variation was significantly greater than intra-individual variation over the year. Essentially every individual was identified across time from the Pearson correlation coefficients of their plasma proteins and 89% were identified from a set of 53 peptide allele sequences.
Porsdam Mann et al. introduced the four core principles of bioethics — autonomy, justice, non-maleficence, and beneficence191. From a systematic literature review of “ethics and clinical proteomics”, they identified 10 normative themes — standards and quality control, integration of new laboratory and computer technologies, privacy, discrimination, conflicting rights and duties, beneficence and justice, incidental findings, regulation, international guidance, and clarity of goals. They emphasize a dichotomy between actionable and non-actionable test results. Finally, they propose a path to maximizing individual and overall public benefit and human dignity from progressively wider application of proteomic and multi-omic profiling of patients and healthy individuals.
One strategy to protect personally-identifiable data is to routinely withhold all laboratory results of incidental findings and depend upon the patient, presumably through the ordering physician, to “opt-in” for certain kinds of information beyond the tests specifically directed at a current clinical management decision. A common example is the detection of Apolipoprotein E allele E4, signifying enhanced risk for Alzheimer disease, for which there currently is no effective therapy. Deidentified data could still be made available for secondary analyses. A complementary strategy is to put the immediate clinical decision in a broader context and view the testing as opening a path to more knowledge about the patient and the patient’s health risks; the aim is to improve long-term general health and well-being of individuals and populations, explicitly including underrepresented populations191. Healthy individuals may want proteomics results to support scientific wellness goals192–194. Regular proteomic profiling may become informative about environmental and dietary influences on health, especially if coupled with measures of exposures. With regards to privacy, in the United States, GINA, the Genetic Information Nondiscrimination Act of 2008 (42 U.S.C. § 2000ff) protects individuals and families from misuse of genetic information in the spheres of employment and health insurance. More generally, HIPAA, the Health Insurance Portability and Accountability Act of 1996, holds healthcare organizations accountable for any release of personal health information (PHI) without the patient’s consent or knowledge. In Europe the General Data Protection Regulation (GDPR) Act protects privacy of patient data and cross-national data flow195.
In either approach, the study participant or patient needs to be informed about the nature of the assays and the kinds of information that will be generated before making a decision. Any requirement for extensive discussion may undermine desired uses of proteomics tests in busy clinical practices. Output of targeted MS or affinity-based methods may be more readily understood than DDA or DIA methods. Methods to minimize laboratory errors and confusion in communication, as well as identifiability of proteomic data196 are essential.
Stimulating the proteomics community and our clinical colleagues to discuss and address these ethical dimensions of proteomic testing could follow the notable examples of the 1975 Asilomar Conference in the early days of recombinant DNA research and the 2017 international guidelines limiting human gene editing to somatic cells.
Conclusions and Future Directions
This review of a broad array of studies of the human plasma proteome shows robust progress on many fronts. The HUPO Human Plasma Proteome Project (HPPP) was one of the first four initiatives of HUPO during 2001-20023, along with the Human Brain Proteome Project, the Human Liver Proteome Project, and the Proteomics Standards Initiative6,9. Periodically the Human Plasma Proteome PeptideAtlas has been updated, as in this article, including application of stringent Guidelines for Interpretation of Mass Spectrometry Data v2.162 and v3.0151 and comparison of plasma, kidney, and urine proteomes50. Now there are 4395 plasma proteins and 2750 proteins in extracellular vesicles confidently identified by mass spectrometry, and large numbers being identified with affinity-based platforms as illustrated in the section about longevity
Proteogenomics has become a major research field, developed most extensively by the U.S. National Cancer Institute Clinical Proteomic Tumor Analysis Consortium (CPTAC), with special emphasis on phosphoproteomics as a guide to mechanism-based targeted therapies. Such post-translational modifications cannot be identified or predicted from genomic or transcriptomic analyses alone. This approach is applicable to all organ- and disease-focused research. This paper highlights research on pQTLs, a special feature of proteogenomics reflected in quantitation of protein expression and the role of extracellular vesicles isolated from plasma, as a type of liquid biopsy197. We also delve deeply into timely research on the SARS-CoV-2 virus and the COVID-19 pandemic, the broad topic of hemostasis and coagulopathies, and differences in the plasma proteome among individuals of different ages and on different trajectories for aging and longevity, using longitudinal sample collections and integrative analyses192–194.
A particular priority for the near-term is cross-comparison of the findings of differential abundance in the human plasma proteome as detected by the three major platforms: DDA and DIA mass spectrometry, SomaLogic’s aptamers, and Olink’s antibody-based proximity extension assays. Performing multi-platform studies on the same samples would enhance our understanding of the advantages and limitations of each; hopefully, such studies will advance the acceptance of the findings, the progress toward clinically applied biomarkers, and the identification of mechanisms and specific targets for precision medicine with chemotherapy and immunotherapy. The HPP MS Data Interpretation Guidelines are an excellent model for quality assurance for each of these platforms. The HPPP in 2005 illustrated the large impact of criteria for protein identification, with subsets based on reference proteome sequences with a single peptide (“one-hit wonders”) or two peptides or three or more peptides4; current HPP MS Guidelines require two non-nested peptides of at least 9 amino acids in length, based on high-quality spectra identified by their Universal Spectrum Identifier198, and shown not to be accounted for by known sequence variants or isobaric PTMs in abundant proteins upon search with the neXtProt uniqueness checker199 or ProteoMapper200. Collaborations among laboratories specializing in individual platforms would be an expeditious approach, as would a single laboratory using all three platforms.
Proteomics is a major contributor to “Big Data” in biology and medicine. Bioinformatics tools and workflows are critical to leverage and exploit omics datasets in response to specific biological question, and to prioritize serological biomarker candidates201. One of the major issues is that bioinformatics solutions are often fragmented and distributed under different forms (e.g., stand-alone programs, web tools and databases)202. A noteworthy solution is the web-based Galaxy framework203, which has combined tools into tailored workflows to mine transcriptomics and proteomics databases along with plasma proteomics datasets, to select tissue-leakage candidate biomarkers204 and to prioritize blood biomarkers for early pancreatic cancer diagnosis205.
Much deeper, more quantitative analyses of pathways, networks, and protein-protein, protein-nucleic acid, and protein-lipid complexes will benefit from machine learning, deep learning, and artificial intelligence techniques. An example of synergy is the bridge between structural biology of proteins and proteomics206, now applied to Cryo-EM207. Moreover, protein analyses are feasible at the level of single cells, an area of integration with RNA-Seq through use of mass cytometry, live-cell imaging, and computational tools208. NIH has issued an RFA for single-cell protein sequencing and single-cell proteomics (RFA-HG-21-001)44. There remains uncertainty about translation products from the “non-coding” portion (98%) of the human genome sequence; however, it is clear from ATAC-Seq and other methods that tissue-specific enhancers and super-enhancers from the non-protein-coding DNA greatly influence transcription and protein expression. Proteomics can add functional depth to the biological interpretation of the expanding knowledge of cell and tissue repertoires of transcripts, chromatin structure, DNA methylation, chromatin looping, and occupancy by transcription factors and RNA-binding proteins as revealed by the Encyclopedia of DNA Elements (ENCODE) phase III7,209. In challenges such as the Maps to Mechanisms to Medicine announced by the International Common Disease Alliance (www.icda.bio) data from plasma and extracellular vesicle proteomes will reflect organ-based processes that can be monitored across large population biobanks.
Finally, plasma proteomics and multi-omics biomarker development will surely play an increasing role in precision medicine and precision health, including personal dense, dynamic data clouds that connect systems biomedicine to “scientific wellness”192,210, diagnose disease processes at early stages, predict patient-specific targets for small molecule, protein, or antibody therapeutics, and serve as companion tests for monitoring response to therapy. As always, advances in instrumentation, sample handling, and informatics will be critical in addressing new biological questions and creating effective preventive and clinical interventions.
Supplementary Material
Supplementary Table S1. Union of proteins in both the plasma and blood extracellular vesicle PeptideAtlas builds, along with the crudely estimated log10 abundances and functional annotations.
Supplementary Table S2. Top 10 most abundant proteins in the EV build without a detection in the plasma build. Additional information about the proteins and their RNA expression were derived for the Human Protein.
Acknowledgments
This work was partially funded by the National Institutes of Health, National Institute of General Medical Sciences grant R01GM087221 (EWD and RLM), R24GM127667 (EWD), the Office of the Director S10OD026936 (RLM), and the National Institute on Aging grant U19AG023122 (RLM); National Institute of Environmental Health Sciences grant P30ES017885 and National Cancer Institute grant U24CA210967 (GSO), National Science Foundation grant DBI-1933311 (EWD), the French Investissement Avenir Infra-structures Nationales en Biologie et Santé grant ANR-10-INBS-08 (VB and YV). JMS acknowledges grants from the Knut and Alice Wallenberg Foundation for the Human Protein Atlas, the Erling-Persson Family Foundation for the KTH Centre for Applied Precision Medicine, and the Stockholm County Council (HMT 20190962).
Footnotes
Conflict of interest
The authors declare the following competing financial interest(s): Krishnan K. Palaniappan is an employee of Freenome. Philipp E. Geyer is an employee of OmicEra Diagnostics GmbH. All other authors declare no competing financial interest.
Supporting Information
The following supporting information is available free of charge at ACS website http://pubs.acs.org
References
- (1).Ignjatovic V; Geyer PE; Palaniappan KK; Chaaban JE; Omenn GS; Baker MS; Deutsch EW; Schwenk JM Mass Spectrometry-Based Plasma Proteomics: Considerations from Sample Collection to Achieving Translational Data. J. Proteome Res 2019, 18 (12), 4085–4097. 10.1021/acs.jproteome.9b00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Loo JA; Yan W; Ramachandran P; Wong DT Comparative Human Salivary and Plasma Proteomes. J. Dent. Res 2010, 89 (10), 1016–1023. 10.1177/0022034510380414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Hanash S; Celis JE The Human Proteome Organization: A Mission to Advance Proteome Knowledge. Mol. Cell. Proteomics MCP 2002, 1 (6), 413–414. [DOI] [PubMed] [Google Scholar]
- (4).Omenn GS; States DJ; Adamski M; Blackwell TW; Menon R; Hermjakob H; Apweiler R; Haab BB; Simpson RJ; Eddes JS; Kapp EA; Moritz RL; Chan DW; Rai AJ; Admon A; Aebersold R; Eng J; Hancock WS; Hefta SA; Meyer H; Paik Y-K; Yoo J-S; Ping P; Pounds J; Adkins J; Qian X; Wang R; Wasinger V; Wu CY; Zhao X; Zeng R; Archakov A; Tsugita A; Beer I; Pandey A; Pisano M; Andrews P; Tammen H; Speicher DW; Hanash SM Overview of the HUPO Plasma Proteome Project: Results from the Pilot Phase with 35 Collaborating Laboratories and Multiple Analytical Groups, Generating a Core Dataset of 3020 Proteins and a Publicly-Available Database. Proteomics 2005, 5 (13), 3226–3245. 10.1002/pmic.200500358. [DOI] [PubMed] [Google Scholar]
- (5).Legrain P; Aebersold R; Archakov A; Bairoch A; Bala K; Beretta L; Bergeron J; Borchers CH; Corthals GL; Costello CE; Deutsch EW; Domon B; Hancock W; He F; Hochstrasser D; Marko-Varga G; Salekdeh GH; Sechi S; Snyder M; Srivastava S; Uhlén M; Wu CH; Yamamoto T; Paik Y-K; Omenn GS The Human Proteome Project: Current State and Future Direction. Mol. Cell. Proteomics 2011, 10 (7), M111.009993. 10.1074/mcp.M111.009993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Adhikari S; Nice EC; Deutsch EW; Lane L; Omenn GS; Pennington SR; Paik Y-K; Overall CM; Corrales FJ; Cristea IM; Van Eyk JE; Uhlén M; Lindskog C; Chan DW; Bairoch A; Waddington JC; Justice JL; LaBaer J; Rodriguez H; He F; Kostrzewa M; Ping P; Gundry RL; Stewart P; Srivastava S; Srivastava S; Nogueira FCS; Domont GB; Vandenbrouck Y; Lam MPY; Wennersten S; Vizcaino JA; Wilkins M; Schwenk JM; Lundberg E; Bandeira N; Marko-Varga G; Weintraub ST; Pineau C; Kusebauch U; Moritz RL; Ahn SB; Palmblad M; Snyder MP; Aebersold R; Baker MS A High-Stringency Blueprint of the Human Proteome. Nat. Commun 2020, 11 (1), 5301. 10.1038/s41467-020-19045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Omenn GS; Lane L; Overall CM; Cristea IM; Corrales FJ; Lindskog C; Paik Y-K; Van Eyk JE; Liu S; Pennington SR; Snyder MP; Baker MS; Bandeira N; Aebersold R; Moritz RL; Deutsch EW Research on the Human Proteome Reaches a Major Milestone: >90% of Predicted Human Proteins Now Credibly Detected, According to the HUPO Human Proteome Project. J. Proteome Res 2020, 19 (12), 4735–4746. 10.1021/acs.jproteome.0c00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Omenn GS; Lane L; Overall CM; Paik Y-K; Cristea IM; Corrales FJ; Lindskog C; Weintraub S; Roehrl MHA; Liu S; Bandeira N; Srivastava S; Chen Y-J; Aebersold R; Moritz RL; Deutsch EW Progress Identifying and Analyzing the Human Proteome: 2021 Metrics from the HUPO Human Proteome Project. J. Proteome Res 2021, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Omenn GS Reflections on the HUPO Human Proteome Project, the Flagship Project of the Human Proteome Organization, at 10 Years. Mol. Cell. Proteomics 2021, 20, 100062. 10.1016/j.mcpro.2021.100062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Anderson NL Counting the Proteins in Plasma. Clin. Chem 2010, 56 (11), 1775–1776. 10.1373/clinchem.2010.146167. [DOI] [PubMed] [Google Scholar]
- (11).Bruderer R; Muntel J; Müller S; Bernhardt OM; Gandhi T; Cominetti O; Macron C; Carayol J; Rinner O; Astrup A; Saris WHM; Hager J; Valsesia A; Dayon L; Reiter L Analysis of 1508 Plasma Samples by Capillary-Flow Data-Independent Acquisition Profiles Proteomics of Weight Loss and Maintenance. Mol. Cell. Proteomics MCP 2019, 18 (6), 1242–1254. 10.1074/mcp.RA118.001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Keshishian H; Burgess MW; Gillette MA; Mertins P; Clauser KR; Mani DR; Kuhn EW; Farrell LA; Gerszten RE; Carr SA Multiplexed, Quantitative Workflow for Sensitive Biomarker Discovery in Plasma Yields Novel Candidates for Early Myocardial Injury. Mol. Cell. Proteomics 2015, 14 (9), 2375–2393. 10.1074/mcp.M114.046813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Keshishian H; Burgess MW; Specht H; Wallace L; Clauser KR; Gillette MA; Carr SA Quantitative, Multiplexed Workflow for Deep Analysis of Human Blood Plasma and Biomarker Discovery by Mass Spectrometry. Nat. Protoc 2017, 12 (8), 1683–1701. 10.1038/nprot.2017.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Tognetti M; Sklodowski K; Mueller S; Kamber D; Muntel J; Bruderer R; Reiter L Biomarker Candidates for Tumors Identified from Deep-Profiled Plasma Stem Predominantly from the Low Abundant Area; preprint in bioRxiv; Cancer Biology, 2021. 10.1101/2021.10.05.463153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Klatt J-N; Dinh TJ; Schilling O; Zengerle R; Schmidt F; Hutzenlaub T; Paust N Automation of Peptide Desalting for Proteomic Liquid Chromatography - Tandem Mass Spectrometry by Centrifugal Microfluidics. Lab. Chip 2021, 21 (11), 2255–2264. 10.1039/d1lc00137j. [DOI] [PubMed] [Google Scholar]
- (16).Klatt J-N; Depke M; Goswami N; Paust N; Zengerle R; Schmidt F; Hutzenlaub T Tryptic Digestion of Human Serum for Proteomic Mass Spectrometry Automated by Centrifugal Microfluidics. Lab. Chip 2020, 20 (16), 2937–2946. 10.1039/d0lc00530d. [DOI] [PubMed] [Google Scholar]
- (17).Skjærvø Ø; Halvorsen TG; Reubsaet L All-in-One Paper-Based Sampling Chip for Targeted Protein Analysis. Anal. Chim. Acta 2019, 1089, 56–65. 10.1016/j.aca.2019.08.043. [DOI] [PubMed] [Google Scholar]
- (18).Gilquin B; Cubizolles M; Den Dulk R; Revol-Cavalier F; Alessio M; Goujon C-E; Echampard C; Arrizabalaga G; Adrait A; Louwagie M; Laurent P; Navarro FP; Couté Y; Cosnier M-L; Brun V PepS: An Innovative Microfluidic Device for Bedside Whole Blood Processing before Plasma Proteomics Analyses. Anal. Chem 2021, 93 (2), 683–690. 10.1021/acs.analchem.0c02270. [DOI] [PubMed] [Google Scholar]
- (19).Fu Q; Johnson CW; Wijayawardena BK; Kowalski MP; Kheradmand M; Van Eyk JE A Plasma Sample Preparation for Mass Spectrometry Using an Automated Workstation. J. Vis. Exp. JoVE 2020, No. 158, e59842. 10.3791/59842. [DOI] [PubMed] [Google Scholar]
- (20).Kotol D; Hober A; Strandberg L; Svensson A-S; Uhlén M; Edfors F Targeted Proteomics Analysis of Plasma Proteins Using Recombinant Protein Standards for Addition Only Workflows. BioTechniques 2021, 71 (3), 473–483. 10.2144/btn-2021-0047. [DOI] [PubMed] [Google Scholar]
- (21).Blume JE; Manning WC; Troiano G; Hornburg D; Figa M; Hesterberg L; Platt TL; Zhao X; Cuaresma RA; Everley PA; Ko M; Liou H; Mahoney M; Ferdosi S; Elgierari EM; Stolarczyk C; Tangeysh B; Xia H; Benz R; Siddiqui A; Carr SA; Ma P; Langer R; Farias V; Farokhzad OC Rapid, Deep and Precise Profiling of the Plasma Proteome with Multi-Nanoparticle Protein Corona. Nat. Commun 2020, 11 (1), 3662. 10.1038/s41467-020-17033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Geyer PE; Kulak NA; Pichler G; Holdt LM; Teupser D; Mann M Plasma Proteome Profiling to Assess Human Health and Disease. Cell Syst. 2016, 2 (3), 185–195. 10.1016/j.cels.2016.02.015. [DOI] [PubMed] [Google Scholar]
- (23).Borges H; Hesse A-M; Kraut A; Yohann Couté; Brun V; Burger T Well Plate Maker: A User-Friendly Randomized Block Design Application to Limit Batch Effects in Largescale Biomedical Studies. Bioinforma. Oxf. Engl 2021, 37 (17), 2770–2771. 10.1093/bioinformatics/btab065. [DOI] [PubMed] [Google Scholar]
- (24).Messner CB; Demichev V; Bloomfield N; Yu JSL; White M; Kreidl M; Egger A-S; Freiwald A; Ivosev G; Wasim F; Zelezniak A; Jürgens L; Suttorp N; Sander LE; Kurth F; Lilley KS; Mülleder M; Tate S; Ralser M Ultra-Fast Proteomics with Scanning SWATH. Nat. Biotechnol 2021, 39 (7), 846–854. 10.1038/s41587-021-00860-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Assarsson E; Lundberg M; Holmquist G; Björkesten J; Thorsen SB; Ekman D; Eriksson A; Rennel Dickens E; Ohlsson S; Edfeldt G; Andersson A-C; Lindstedt P; Stenvang J; Gullberg M; Fredriksson S Homogenous 96-Plex PEA Immunoassay Exhibiting High Sensitivity, Specificity, and Excellent Scalability. PloS One 2014, 9 (4), e95192. 10.1371/journal.pone.0095192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Gullberg M; Gústafsdóttir SM; Schallmeiner E; Jarvius J; Bjarnegård M; Betsholtz C; Landegren U; Fredriksson S Cytokine Detection by Antibody-Based Proximity Ligation. Proc. Natl. Acad. Sci. U. S. A 2004, 101 (22), 8420–8424. 10.1073/pnas.0400552101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Blokzijl A; Nong R; Darmanis S; Hertz E; Landegren U; Kamali-Moghaddam M Protein Biomarker Validation via Proximity Ligation Assays. Biochim. Biophys. Acta 2014, 1844 (5), 933–939. 10.1016/j.bbapap.2013.07.016. [DOI] [PubMed] [Google Scholar]
- (28).Zhong W; Edfors F; Gummesson A; Bergström G; Fagerberg L; Uhlén M Next Generation Plasma Proteome Profiling to Monitor Health and Disease. Nat. Commun 2021, 12 (1), 2493. 10.1038/s41467-021-22767-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Rohloff JC; Gelinas AD; Jarvis TC; Ochsner UA; Schneider DJ; Gold L; Janjic N Nucleic Acid Ligands With Protein-like Side Chains: Modified Aptamers and Their Use as Diagnostic and Therapeutic Agents. Mol. Ther. Nucleic Acids 2014, 3, e201. 10.1038/mtna.2014.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Brody EN; Willis MC; Smith JD; Jayasena S; Zichi D; Gold L The Use of Aptamers in Large Arrays for Molecular Diagnostics. Mol. Diagn. J. Devoted Underst. Hum. Dis. Clin. Appl. Mol. Biol 1999, 4 (4), 381–388. 10.1016/s1084-8592(99)80014-9. [DOI] [PubMed] [Google Scholar]
- (31).Raffield LM; Dang H; Pratte KA; Jacobson S; Gillenwater LA; Ampleford E; Barjaktarevic I; Basta P; Clish CB; Comellas AP; Cornell E; Curtis JL; Doerschuk C; Durda P; Emson C; Freeman CM; Guo X; Hastie AT; Hawkins GA; Herrera J; Johnson WC; Labaki WW; Liu Y; Masters B; Miller M; Ortega VE; Papanicolaou G; Peters S; Taylor KD; Rich SS; Rotter JI; Auer P; Reiner AP; Tracy RP; Ngo D; Gerszten RE; O’Neal WK; Bowler RP; NHLBI Trans-Omics for Precision Medicine (TOPMed) Consortium. Comparison of Proteomic Assessment Methods in Multiple Cohort Studies. Proteomics 2020, 20 (12), e1900278. 10.1002/pmic.201900278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Pietzner M; Wheeler E; Carrasco-Zanini J; Kerrison ND; Oerton E; Koprulu M; Luan J; Hingorani AD; Williams SA; Wareham NJ; Langenberg C Cross-Platform Proteomics to Advance Genetic Prioritisation Strategies; bioRxiv; 2021. 10.1101/2021.03.18.435919. [DOI] [Google Scholar]
- (33).Liu RX; Thiessen-Philbrook HR; Vasan RS; Coresh J; Ganz P; Bonventre JV; Kimmel PL; Parikh CR Comparison of Proteomic Methods in Evaluating Biomarker-AKI Associations in Cardiac Surgery Patients. Transl. Res. J. Lab. Clin. Med 2021, S1931–5244(21)00162–6. 10.1016/j.trsl.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Xu M; Deng J; Xu K; Zhu T; Han L; Yan Y; Yao D; Deng H; Wang D; Sun Y; Chang C; Zhang X; Dai J; Yue L; Zhang Q; Cai X; Zhu Y; Duan H; Liu Y; Li D; Zhu Y; Radstake TRDJ; Balak DMW; Xu D; Guo T; Lu C; Yu X In-Depth Serum Proteomics Reveals Biomarkers of Psoriasis Severity and Response to Traditional Chinese Medicine. Theranostics 2019, 9 (9), 2475–2488. 10.7150/thno.31144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Emilsson V; Ilkov M; Lamb JR; Finkel N; Gudmundsson EF; Pitts R; Hoover H; Gudmundsdottir V; Horman SR; Aspelund T; Shu L; Trifonov V; Sigurdsson S; Manolescu A; Zhu J; Olafsson O; Jakobsdottir J; Lesley SA; To J; Zhang J; Harris TB; Launer LJ; Zhang B; Eiriksdottir G; Yang X; Orth AP; Jennings LL; Gudnason V Co-Regulatory Networks of Human Serum Proteins Link Genetics to Disease. Science 2018, 361 (6404), 769–773. 10.1126/science.aaq1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Sun BB; Maranville JC; Peters JE; Stacey D; Staley JR; Blackshaw J; Burgess S; Jiang T; Paige E; Surendran P; Oliver-Williams C; Kamat MA; Prins BP; Wilcox SK; Zimmerman ES; Chi A; Bansal N; Spain SL; Wood AM; Morrell NW; Bradley JR; Janjic N; Roberts DJ; Ouwehand WH; Todd JA; Soranzo N; Suhre K; Paul DS; Fox CS; Plenge RM; Danesh J; Runz H; Butterworth AS Genomic Atlas of the Human Plasma Proteome. Nature 2018, 558 (7708), 73–79. 10.1038/s41586-018-0175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Petrera A; von Toerne C; Behler J; Huth C; Thorand B; Hilgendorff A; Hauck SM Multiplatform Approach for Plasma Proteomics: Complementarity of Olink Proximity Extension Assay Technology to Mass Spectrometry-Based Protein Profiling. J. Proteome Res 2021, 20 (1), 751–762. 10.1021/acs.jproteome.0c00641. [DOI] [PubMed] [Google Scholar]
- (38).Oller Moreno S; Cominetti O; Núñez Galindo A; Irincheeva I; Corthésy J; Astrup A; Saris WHM; Hager J; Kussmann M; Dayon L The Differential Plasma Proteome of Obese and Overweight Individuals Undergoing a Nutritional Weight Loss and Maintenance Intervention. Proteomics Clin. Appl 2018, 12 (1). 10.1002/prca.201600150. [DOI] [PubMed] [Google Scholar]
- (39).Ruffieux H; Carayol J; Popescu R; Harper M-E; Dent R; Saris WHM; Astrup A; Hager J; Davison AC; Valsesia A A Fully Joint Bayesian Quantitative Trait Locus Mapping of Human Protein Abundance in Plasma. PLoS Comput. Biol 2020, 16 (6), e1007882. 10.1371/journal.pcbi.1007882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Suhre K; McCarthy MI; Schwenk JM Genetics Meets Proteomics: Perspectives for Large Population-Based Studies. Nat. Rev. Genet 2021, 22 (1), 19–37. 10.1038/s41576-020-0268-2. [DOI] [PubMed] [Google Scholar]
- (41).Orchard S; Kersey P; Hermjakob H; Apweiler R The HUPO Proteomics Standards Initiative Meeting: Towards Common Standards for Exchanging Proteomics Data. Comp. Funct. Genomics 2003, 4 (1), 16–19. 10.1002/cfg.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Deutsch EW; Orchard S; Binz P-A; Bittremieux W; Eisenacher M; Hermjakob H; Kawano S; Lam H; Mayer G; Menschaert G; Perez-Riverol Y; Salek RM; Tabb DL; Tenzer S; Vizcaíno JA; Walzer M; Jones AR Proteomics Standards Initiative: Fifteen Years of Progress and Future Work. J. Proteome Res 2017, 16 (12), 4288–4298. 10.1021/acs.jproteome.7b00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Swaminathan J; Boulgakov AA; Hernandez ET; Bardo AM; Bachman JL; Marotta J; Johnson AM; Anslyn EV; Marcotte EM Highly Parallel Single-Molecule Identification of Proteins in Zeptomole-Scale Mixtures. Nat. Biotechnol 2018, 36, 1076–1082. 10.1038/nbt.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Kagan J; Moritz RL; Mazurchuk R; Lee JH; Kharchenko PV; Rozenblatt-Rosen O; Ruppin E; Edfors F; Ginty F; Goltsev Y; Wells JA; LaCava J; Riesterer JL; Germain RN; Shi T; Chee MS; Budnik BA; Yates JR; Chait BT; Moffitt JR; Smith RD; Srivastava S National Cancer Institute Think-Tank Meeting Report on Proteomic Cartography and Biomarkers at the Single-Cell Level: Interrogation of Premalignant Lesions. J. Proteome Res 2020, 19 (5), 1900–1912. 10.1021/acs.jproteome.0c00021. [DOI] [PubMed] [Google Scholar]
- (45).Huang G; Voet A; Maglia G FraC Nanopores with Adjustable Diameter Identify the Mass of Opposite-Charge Peptides with 44 Dalton Resolution. Nat. Commun 2019, 10 (1), 835. 10.1038/s41467-019-08761-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Ouldali H; Sarthak K; Ensslen T; Piguet F; Manivet P; Pelta J; Behrends JC; Aksimentiev A; Oukhaled A Electrical Recognition of the Twenty Proteinogenic Amino Acids Using an Aerolysin Nanopore. Nat. Biotechnol 2020, 38 (2), 176–181. 10.1038/s41587-019-0345-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Poetz O; Hoeppe S; Templin MF; Stoll D; Joos TO Proteome Wide Screening Using Peptide Affinity Capture. Proteomics 2009, 9 (6), 1518–1523. 10.1002/pmic.200800842. [DOI] [PubMed] [Google Scholar]
- (48).Wingren C; James P; Borrebaeck CA Strategy for Surveying the Proteome Using Affinity Proteomics and Mass Spectrometry. Proteomics 2009, 9 (6), 1511–1517. 10.1002/pmic.200800802. [DOI] [PubMed] [Google Scholar]
- (49).Desiere F; Deutsch EW; Nesvizhskii AI; Mallick P; King NL; Eng JK; Aderem A; Boyle R; Brunner E; Donohoe S; Fausto N; Hafen E; Hood L; Katze MG; Kennedy KA; Kregenow F; Lee H; Lin B; Martin D; Ranish JA; Rawlings DJ; Samelson LE; Shiio Y; Watts JD; Wollscheid B; Wright ME; Yan W; Yang L; Yi EC; Zhang H; Aebersold R Integration with the Human Genome of Peptide Sequences Obtained by High-Throughput Mass Spectrometry. Genome Biol. 2005, 6 (1), R9. 10.1186/gb-2004-6-1-r9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Farrah T; Deutsch EW; Omenn GS; Sun Z; Watts JD; Yamamoto T; Shteynberg D; Harris MM; Moritz RL State of the Human Proteome in 2013 as Viewed through PeptideAtlas: Comparing the Kidney, Urine, and Plasma Proteomes for the Biology- and Disease-Driven Human Proteome Project. J. Proteome Res 2014, 13 (1), 60–75. 10.1021/pr4010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Farrah T; Deutsch EW; Omenn GS; Campbell DS; Sun Z; Bletz JA; Mallick P; Katz JE; Malmström J; Ossola R; Watts JD; Lin B; Zhang H; Moritz RL; Aebersold R A High-Confidence Human Plasma Proteome Reference Set with Estimated Concentrations in PeptideAtlas. Mol. Cell. Proteomics MCP 2011, 10 (9), M110.006353. 10.1074/mcp.M110.006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Deutsch EW; Eng JK; Zhang H; King NL; Nesvizhskii AI; Lin B; Lee H; Yi EC; Ossola R; Aebersold R Human Plasma PeptideAtlas. Proteomics 2005, 5 (13), 3497–3500. 10.1002/pmic.200500160. [DOI] [PubMed] [Google Scholar]
- (53).Keller A; Eng J; Zhang N; Li X; Aebersold R A Uniform Proteomics MS/MS Analysis Platform Utilizing Open XML File Formats. Mol. Syst. Biol 2005, 1 (1), E1–E8. 10.1038/msb4100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Deutsch EW; Shteynberg D; Lam H; Sun Z; Eng JK; Carapito C; von Haller PD; Tasman N; Mendoza L; Farrah T; Aebersold R Trans-Proteomic Pipeline Supports and Improves Analysis of Electron Transfer Dissociation Data Sets. Proteomics 2010, 10 (6), 1190–1195. 10.1002/pmic.200900567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Deutsch EW; Mendoza L; Shteynberg D; Slagel J; Sun Z; Moritz RL Trans-Proteomic Pipeline, a Standardized Data Processing Pipeline for Large-Scale Reproducible Proteomics Informatics. PROTEOMICS - Clin. Appl 2015, 9 (7–8), 745–754. 10.1002/prca.201400164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Schwenk JM; Omenn GS; Sun Z; Campbell DS; Baker MS; Overall CM; Aebersold R; Moritz RL; Deutsch EW The Human Plasma Proteome Draft of 2017: Building on the Human Plasma PeptideAtlas from Mass Spectrometry and Complementary Assays. J. Proteome Res 2017, 16 (12), 4299–4310. 10.1021/acs.jproteome.7b00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Deutsch EW; Sun Z; Campbell DS; Binz P-A; Farrah T; Shteynberg D; Mendoza L; Omenn GS; Moritz RL Tiered Human Integrated Sequence Search Databases for Shotgun Proteomics. J. Proteome Res 2016, 15 (11), 4091–4100. 10.1021/acs.jproteome.6b00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Eng JK; Jahan TA; Hoopmann MR Comet: An Open-Source MS/MS Sequence Database Search Tool. Proteomics 2013, 13 (1), 22–24. 10.1002/pmic.201200439. [DOI] [PubMed] [Google Scholar]
- (59).Keller A; Nesvizhskii AI; Kolker E; Aebersold R Empirical Statistical Model to Estimate the Accuracy of Peptide Identifications Made by MS/MS and Database Search. Anal. Chem 2002, 74 (20), 5383–5392. 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- (60).Shteynberg D; Deutsch EW; Lam H; Eng JK; Sun Z; Tasman N; Mendoza L; Moritz RL; Aebersold R; Nesvizhskii AI IProphet: Multi-Level Integrative Analysis of Shotgun Proteomic Data Improves Peptide and Protein Identification Rates and Error Estimates. Mol. Cell. Proteomics MCP 2011, 10 (12), M111.007690. 10.1074/mcp.M111.007690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).van Wijk KJ; Leppert T; Sun Q; Boguraev SS; Sun Z; Mendoza L; Deutsch EW The Arabidopsis PeptideAtlas: Harnessing Worldwide Proteomics Data to Create a Comprehensive Community Proteomics Resource. Plant Cell 2021, koab211. 10.1093/plcell/koab211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Deutsch EW; Overall CM; Van Eyk JE; Baker MS; Paik Y-K; Weintraub ST; Lane L; Martens L; Vandenbrouck Y; Kusebauch U; Hancock WS; Hermjakob H; Aebersold R; Moritz RL; Omenn GS Human Proteome Project Mass Spectrometry Data Interpretation Guidelines 2.1. J. Proteome Res 2016, 15 (11), 3961–3970. 10.1021/acs.jproteome.6b00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Wu D; Yan J; Shen X; Sun Y; Thulin M; Cai Y; Wik L; Shen Q; Oelrich J; Qian X; Dubois KL; Ronquist KG; Nilsson M; Landegren U; Kamali-Moghaddam M Profiling Surface Proteins on Individual Exosomes Using a Proximity Barcoding Assay. Nat. Commun 2019, 10 (1), 3854. 10.1038/s41467-019-11486-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Hoshino A; Kim HS; Bojmar L; Gyan KE; Cioffi M; Hernandez J; Zambirinis CP; Rodrigues G; Molina H; Heissel S; Mark MT; Steiner L; Benito-Martin A; Lucotti S; Di Giannatale A; Offer K; Nakajima M; Williams C; Nogués L; Pelissier Vatter FA; Hashimoto A; Davies AE; Freitas D; Kenific CM; Ararso Y; Buehring W; Lauritzen P; Ogitani Y; Sugiura K; Takahashi N; Alečković M; Bailey KA; Jolissant JS; Wang H; Harris A; Schaeffer LM; García-Santos G; Posner Z; Balachandran VP; Khakoo Y; Raju GP; Scherz A; Sagi I; Scherz-Shouval R; Yarden Y; Oren M; Malladi M; Petriccione M; De Braganca KC; Donzelli M; Fischer C; Vitolano S; Wright GP; Ganshaw L; Marrano M; Ahmed A; DeStefano J; Danzer E; Roehrl MHA; Lacayo NJ; Vincent TC; Weiser MR; Brady MS; Meyers PA; Wexler LH; Ambati SR; Chou AJ; Slotkin EK; Modak S; Roberts SS; Basu EM; Diolaiti D; Krantz BA; Cardoso F; Simpson AL; Berger M; Rudin CM; Simeone DM; Jain M; Ghajar CM; Batra SK; Stanger BZ; Bui J; Brown KA; Rajasekhar VK; Healey JH; de Sousa M; Kramer K; Sheth S; Baisch J; Pascual V; Heaton TE; La Quaglia MP; Pisapia DJ; Schwartz R; Zhang H; Liu Y; Shukla A; Blavier L; DeClerck YA; LaBarge M; Bissell MJ; Caffrey TC; Grandgenett PM; Hollingsworth MA; Bromberg J; Costa-Silva B; Peinado H; Kang Y; Garcia BA; O’Reilly EM; Kelsen D; Trippett TM; Jones DR; Matei IR; Jarnagin WR; Lyden D Extracellular Vesicle and Particle Biomarkers Define Multiple Human Cancers. Cell 2020, 182 (4), 1044–1061.e18. 10.1016/j.cell.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Skog J; Würdinger T; van Rijn S; Meijer DH; Gainche L; Curry WT; Carter BS; Krichevsky AM; Breakefield XO Glioblastoma Microvesicles Transport RNA and Proteins That Promote Tumour Growth and Provide Diagnostic Biomarkers. Nat. Cell Biol 2008, 10 (12), 1470–1476. 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Peinado H; Alečković M; Lavotshkin S; Matei I; Costa-Silva B; Moreno-Bueno G; Hergueta-Redondo M; Williams C; García-Santos G; Ghajar CM; Nitadori-Hoshino A; Hoffman C; Badal K; Garcia BA; Callahan MK; Yuan J; Martins VR; Skog J; Kaplan RN; Brady MS; Wolchok JD; Chapman PB; Kang Y; Bromberg J; Lyden D Melanoma Exosomes Educate Bone Marrow Progenitor Cells toward a Pro-Metastatic Phenotype through MET. Nat. Med 2012, 18 (6), 883–891. 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).van Niel G; D’Angelo G; Raposo G Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol 2018, 19 (4), 213–228. 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- (68).Takov K; Yellon DM; Davidson SM Comparison of Small Extracellular Vesicles Isolated from Plasma by Ultracentrifugation or Size-Exclusion Chromatography: Yield, Purity and Functional Potential. J. Extracell. Vesicles 2019, 8 (1), 1560809. 10.1080/20013078.2018.1560809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Zhang X; Borg EGF; Liaci AM; Vos HR; Stoorvogel W A Novel Three Step Protocol to Isolate Extracellular Vesicles from Plasma or Cell Culture Medium with Both High Yield and Purity. J. Extracell. Vesicles 2020, 9 (1), 1791450. 10.1080/20013078.2020.1791450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Stranska R; Gysbrechts L; Wouters J; Vermeersch P; Bloch K; Dierickx D; Andrei G; Snoeck R Comparison of Membrane Affinity-Based Method with Size-Exclusion Chromatography for Isolation of Exosome-like Vesicles from Human Plasma. J. Transl. Med 2018, 16 (1), 1. 10.1186/s12967-017-1374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Boriachek K; Islam Md. N.; Möller A; Salomon C; Nguyen N; Hossain Md. S. A.; Yamauchi Y; Shiddiky MJA Biological Functions and Current Advances in Isolation and Detection Strategies for Exosome Nanovesicles. Small 2018, 14 (6), 1702153. 10.1002/smll.201702153. [DOI] [PubMed] [Google Scholar]
- (72).Ayala-Mar S; Donoso-Quezada J; Gallo-Villanueva RC; Perez-Gonzalez VH; González-Valdez J Recent Advances and Challenges in the Recovery and Purification of Cellular Exosomes. ELECTROPHORESIS 2019, 40 (23–24), 3036–3049. 10.1002/elps.201800526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Johnsen KB; Gudbergsson JM; Andresen TL; Simonsen JB What Is the Blood Concentration of Extracellular Vesicles? Implications for the Use of Extracellular Vesicles as Blood-Borne Biomarkers of Cancer. Biochim. Biophys. Acta BBA - Rev. Cancer 2019, 1871 (1), 109–116. 10.1016/j.bbcan.2018.11.006. [DOI] [PubMed] [Google Scholar]
- (74).Geyer PE; Voytik E; Treit PV; Doll S; Kleinhempel A; Niu L; Mueller JB; Bader J; Teupser D; Holdt LM; Mann M Plasma Proteome Profiling to Detect and Avoid Sample-Related Biases in Biomarker Studies. bioRxiv 2018, 478305. 10.1101/478305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Braga-Lagache S; Buchs N; Iacovache M-I; Zuber B; Jackson CB; Heller M Robust Label-Free, Quantitative Profiling of Circulating Plasma Microparticle (MP) Associated Proteins. Mol. Cell. Proteomics 2016, 15 (12), 3640–3652. 10.1074/mcp.M116.060491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Cheow ESH; Cheng WC; Lee CN; de Kleijn D; Sorokin V; Sze SK Plasma-Derived Extracellular Vesicles Contain Predictive Biomarkers and Potential Therapeutic Targets for Myocardial Ischemic (MI) Injury. Mol. Cell. Proteomics 2016, 15 (8), 2628–2640. 10.1074/mcp.M115.055731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Tunset ME; Haslene-Hox H; Van Den Bossche T; Vaaler AE; Sulheim E; Kondziella D Extracellular Vesicles in Patients in the Acute Phase of Psychosis and after Clinical Improvement: An Explorative Study. PeerJ 2020, 8, e9714. 10.7717/peerj.9714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Chen I-H; Xue L; Hsu C-C; Paez JSP; Pan L; Andaluz H; Wendt MK; Iliuk AB; Zhu J-K; Tao WA Phosphoproteins in Extracellular Vesicles as Candidate Markers for Breast Cancer. Proc. Natl. Acad. Sci 2017, 114 (12), 3175–3180. 10.1073/pnas.1618088114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Fel A; Lewandowska AE; Petrides PE; Wiśniewski JR Comparison of Proteome Composition of Serum Enriched in Extracellular Vesicles Isolated from Polycythemia Vera Patients and Healthy Controls. Proteomes 2019, 7 (2), 20. 10.3390/proteomes7020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Lane L; Argoud-Puy G; Britan A; Cusin I; Duek PD; Evalet O; Gateau A; Gaudet P; Gleizes A; Masselot A; Zwahlen C; Bairoch A NeXtProt: A Knowledge Platform for Human Proteins. Nucleic Acids Res. 2012, 40 (D1), D76–D83. 10.1093/nar/gkr1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Uhlén M; Fagerberg L; Hallström BM; Lindskog C; Oksvold P; Mardinoglu A; Sivertsson Å; Kampf C; Sjöstedt E; Asplund A; Olsson I; Edlund K; Lundberg E; Navani S; Szigyarto CA-K; Odeberg J; Djureinovic D; Takanen JO; Hober S; Alm T; Edqvist P-H; Berling H; Tegel H; Mulder J; Rockberg J; Nilsson P; Schwenk JM; Hamsten M; von Feilitzen K; Forsberg M; Persson L; Johansson F; Zwahlen M; von Heijne G; Nielsen J; Pontén F Proteomics. Tissue-Based Map of the Human Proteome. Science 2015, 347 (6220), 1260419. 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- (82).Uhlen M; Karlsson MJ; Zhong W; Tebani A; Pou C; Mikes J; Lakshmikanth T; Forsström B; Edfors F; Odeberg J; Mardinoglu A; Zhang C; von Feilitzen K; Mulder J; Sjöstedt E; Hober A; Oksvold P; Zwahlen M; Ponten F; Lindskog C; Sivertsson Å; Fagerberg L; Brodin P A Genome-Wide Transcriptomic Analysis of Protein-Coding Genes in Human Blood Cells. Science 2019, 366 (6472), eaax9198. 10.1126/science.aax9198. [DOI] [PubMed] [Google Scholar]
- (83).Uhlén M; Karlsson MJ; Hober A; Svensson A-S; Scheffel J; Kotol D; Zhong W; Tebani A; Strandberg L; Edfors F; Sjöstedt E; Mulder J; Mardinoglu A; Berling A; Ekblad S; Dannemeyer M; Kanje S; Rockberg J; Lundqvist M; Malm M; Volk A-L; Nilsson P; Månberg A; Dodig-Crnkovic T; Pin E; Zwahlen M; Oksvold P; von Feilitzen K; Häussler RS; Hong M-G; Lindskog C; Ponten F; Katona B; Vuu J; Lindström E; Nielsen J; Robinson J; Ayoglu B; Mahdessian D; Sullivan D; Thul P; Danielsson F; Stadler C; Lundberg E; Bergström G; Gummesson A; Voldborg BG; Tegel H; Hober S; Forsström B; Schwenk JM; Fagerberg L; Sivertsson Å The Human Secretome. Sci. Signal 2019, 12 (609), eaaz0274. 10.1126/scisignal.aaz0274. [DOI] [PubMed] [Google Scholar]
- (84).Mathivanan S; Simpson RJ ExoCarta: A Compendium of Exosomal Proteins and RNA. Proteomics 2009, 9 (21), 4997–5000. 10.1002/pmic.200900351. [DOI] [PubMed] [Google Scholar]
- (85).Andreu Z; Yáñez-Mó M Tetraspanins in Extracellular Vesicle Formation and Function. Front. Immunol 2014, 5, 442. 10.3389/fimmu.2014.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data https://covid19.who.int/ (accessed 2021 -06 -13).
- (87).COVID-19 Data Portal - accelerating scientific research through data https://www.covid19dataportal.org/ (accessed 2021 -06 -13).
- (88).Gordon DE; Jang GM; Bouhaddou M; Xu J; Obernier K; White KM; O’Meara MJ; Rezelj VV; Guo JZ; Swaney DL; Tummino TA; Hüttenhain R; Kaake RM; Richards AL; Tutuncuoglu B; Foussard H; Batra J; Haas K; Modak M; Kim M; Haas P; Polacco BJ; Braberg H; Fabius JM; Eckhardt M; Soucheray M; Bennett MJ; Cakir M; McGregor MJ; Li Q; Meyer B; Roesch F; Vallet T; Mac Kain A; Miorin L; Moreno E; Naing ZZC; Zhou Y; Peng S; Shi Y; Zhang Z; Shen W; Kirby IT; Melnyk JE; Chorba JS; Lou K; Dai SA; Barrio-Hernandez I; Memon D; Hernandez-Armenta C; Lyu J; Mathy CJP; Perica T; Pilla KB; Ganesan SJ; Saltzberg DJ; Rakesh R; Liu X; Rosenthal SB; Calviello L; Venkataramanan S; Liboy-Lugo J; Lin Y; Huang X-P; Liu Y; Wankowicz SA; Bohn M; Safari M; Ugur FS; Koh C; Savar NS; Tran QD; Shengjuler D; Fletcher SJ; O’Neal MC; Cai Y; Chang JCJ; Broadhurst DJ; Klippsten S; Sharp PP; Wenzell NA; Kuzuoglu-Ozturk D; Wang H-Y; Trenker R; Young JM; Cavero DA; Hiatt J; Roth TL; Rathore U; Subramanian A; Noack J; Hubert M; Stroud RM; Frankel AD; Rosenberg OS; Verba KA; Agard DA; Ott M; Emerman M; Jura N; von Zastrow M; Verdin E; Ashworth A; Schwartz O; d’Enfert C; Mukherjee S; Jacobson M; Malik HS; Fujimori DG; Ideker T; Craik CS; Floor SN; Fraser JS; Gross JD; Sali A; Roth BL; Ruggero D; Taunton J; Kortemme T; Beltrao P; Vignuzzi M; García-Sastre A; Shokat KM; Shoichet BK; Krogan NJ A SARS-CoV-2 Protein Interaction Map Reveals Targets for Drug Repurposing. Nature 2020, 583 (7816), 459–468. 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Weingarten-Gabbay S; Klaeger S; Sarkizova S; Pearlman LR; Chen D-Y; Gallagher KME; Bauer MR; Taylor HB; Dunn WA; Tarr C; Sidney J; Rachimi S; Conway HL; Katsis K; Wang Y; Leistritz-Edwards D; Durkin MR; Tomkins-Tinch CH; Finkel Y; Nachshon A; Gentili M; Rivera KD; Carulli IP; Chea VA; Chandrashekar A; Bozkus CC; Carrington M; MGH COVID-19 Collection & Processing Team; Bhardwaj N; Barouch DH; Sette A; Maus MV; Rice CM; Clauser KR; Keskin DB; Pregibon DC; Hacohen N; Carr SA; Abelin JG; Saeed M; Sabeti PC Profiling SARS-CoV-2 HLA-I Peptidome Reveals T Cell Epitopes from out-of-Frame ORFs. Cell 2021, 184 (15), 3962–3980.e17. 10.1016/j.cell.2021.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).High-Resolution Serum Proteome Trajectories in COVID-19 Reveal Patient-Specific Seroconversion. EMBO Mol. Med 2021, n/a (n/a), e14167. 10.15252/emmm.202114167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Haljasmägi L; Salumets A; Rumm AP; Jürgenson M; Krassohhina E; Remm A; Sein H; Kareinen L; Vapalahti O; Sironen T; Peterson H; Milani L; Tamm A; Hayday A; Kisand K; Peterson P Longitudinal Proteomic Profiling Reveals Increased Early Inflammation and Sustained Apoptosis Proteins in Severe COVID-19. Sci. Rep 2020, 10 (1), 20533. 10.1038/s41598-020-77525-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Demichev V; Tober-Lau P; Lemke O; Nazarenko T; Thibeault C; Whitwell H; Röhl A; Freiwald A; Szyrwiel L; Ludwig D; Correia-Melo C; Aulakh SK; Helbig ET; Stubbemann P; Lippert LJ; Grüning N-M; Blyuss O; Vernardis S; White M; Messner CB; Joannidis M; Sonnweber T; Klein SJ; Pizzini A; Wohlfarter Y; Sahanic S; Hilbe R; Schaefer B; Wagner S; Mittermaier M; Machleidt F; Garcia C; Ruwwe-Glösenkamp C; Lingscheid T; Bosquillon de Jarcy L; Stegemann MS; Pfeiffer M; Jürgens L; Denker S; Zickler D; Enghard P; Zelezniak A; Campbell A; Hayward C; Porteous DJ; Marioni RE; Uhrig A; Müller-Redetzky H; Zoller H; Löffler-Ragg J; Keller MA; Tancevski I; Timms JF; Zaikin A; Hippenstiel S; Ramharter M; Witzenrath M; Suttorp N; Lilley K; Mülleder M; Sander LE; PA-COVID-19 Study group; Ralser M; Kurth F A Time-Resolved Proteomic and Prognostic Map of COVID-19. Cell Syst. 2021, 12 (8), 780–794.e7. 10.1016/j.cels.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Filbin MR; Mehta A; Schneider AM; Kays KR; Guess JR; Gentili M; Fenyves BG; Charland NC; Gonye ALK; Gushterova I; Khanna HK; LaSalle TJ; Lavin-Parsons KM; Lilley BM; Lodenstein CL; Manakongtreecheep K; Margolin JD; McKaig BN; Rojas-Lopez M; Russo BC; Sharma N; Tantivit J; Thomas MF; Gerszten RE; Heimberg GS; Hoover PJ; Lieb DJ; Lin B; Ngo D; Pelka K; Reyes M; Smillie CS; Waghray A; Wood TE; Zajac AS; Jennings LL; Grundberg I; Bhattacharyya RP; Parry BA; Villani A-C; Sade-Feldman M; Hacohen N; Goldberg MB Longitudinal Proteomic Analysis of Severe COVID-19 Reveals Survival-Associated Signatures, Tissue-Specific Cell Death, and Cell-Cell Interactions. Cell Rep. Med 2021, 2 (5), 100287. 10.1016/j.xcrm.2021.100287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Consortium, Co.−19 M. B. At. (COMBAT); Ahern DJ; Ai Z; Ainsworth M; Allan C; Allcock A; Ansari A; Arancibia-Carcamo CV; Aschenbrenner D; Attar M; Baillie JK; Barnes E; Bashford-Rogers R; Bashyal A; Beer S; Berridge G; Beveridge A; Bibi S; Bicanic T; Blackwell L; Bowness P; Brent A; Brown A; Broxholme J; Buck D; Burnham KL; Byrne H; Camara S; Ferreira IC; Charles P; Chen W; Chen Y-L; Chong A; Clutterbuck E; Coles M; Conlon CP; Cornall R; Cribbs AP; Curion F; Davenport EE; Davidson N; Davis S; Dendrou C; Dequaire J; Dib L; Docker J; Dold C; Dong T; Downes D; Drakesmith A; Dunachie SJ; Duncan DA; Eijsbouts C; Esnouf R; Espinosa A; Etherington R; Fairfax B; Fairhead R; Fang H; Fassih S; Felle S; Mendoza MF; Ferreira R; Fischer R; Foord T; Forrow A; Frater J; Fries A; Sanchez VG; Garner L; Geeves C; Georgiou D; Godfrey L; Golubchik T; Vazquez MG; Green A; Harper H; Harrington HA; Heilig R; Hester S; Hill J; Hinds C; Hird C; Ho L-P; Hoekzema R; Hollis B; Hughes J; Hutton P; Jackson M; Jainarayanan A; James-Bott A; Jansen K; Jeffery K; Jones E; Jostins L; Kerr G; Kim D; Klenerman P; Knight JC; Kumar V; Sharma PK; Kurupati P; Kwok A; Lee A; Linder A; Lockett T; Lonie L; Lopopolo M; Lukoseviciute M; Luo J; Marinou S; Marsden B; Martinez J; Matthews P; Mazurczyk M; McGowan S; McKechnie S; Mead A; Mentzer AJ; Mi Y; Monaco C; Montadon R; Napolitani G; Nassiri I; Novak A; O’Brien D; O’Connor D; O’Donnell D; Ogg G; Overend L; Park I; Pavord I; Peng Y; Penkava F; Pinho MP; Perez E; Pollard AJ; Powrie F; Psaila B; Quan TP; Repapi E; Revale S; Silva-Reyes L; Richard J-B; Rich-Griffin C; Ritter T; Rollier CS; Rowland M; Ruehle F; Salio M; Sansom SN; Delgado AS; Sauka-Spengler T; Schwessinger R; Scozzafava G; Screaton G; Seigal A; Semple MG; Sergeant M; Karali CS; Sims D; Skelly D; Slawinski H; Sobrinodiaz A; Sousos N; Stafford L; Stockdale L; Strickland M; Sumray O; Sun B; Taylor C; Taylor S; Taylor A; Thongjuea S; Thraves H; Todd JA; Tomic A; Tong O; Trebes A; Trzupek D; Tucci FA; Turtle L; Udalova I; Uhlig H; Grinsven E. van; Vendrell I; Verheul M; Voda A; Wang G; Wang L; Wang D; Watkinson P; Watson R; Weinberger M; Whalley J; Witty L; Wray K; Xue L; Yeung HY; Yin Z; Young RK; Youngs J; Zhang P; Zurke Y-X A Blood Atlas of COVID-19 Defines Hallmarks of Disease Severity and Specificity. medRxiv 2021, 2021.05.11.21256877. 10.1101/2021.05.11.21256877. [DOI] [Google Scholar]
- (95).D’Alessandro A; Thomas T; Dzieciatkowska M; Hill RC; Francis RO; Hudson KE; Zimring JC; Hod EA; Spitalnik SL; Hansen KC Serum Proteomics in COVID-19 Patients: Altered Coagulation and Complement Status as a Function of IL-6 Level. J. Proteome Res 2020, 19 (11), 4417–4427. 10.1021/acs.jproteome.0c00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Messner CB; Demichev V; Wendisch D; Michalick L; White M; Freiwald A; Textoris-Taube K; Vernardis SI; Egger A-S; Kreidl M; Ludwig D; Kilian C; Agostini F; Zelezniak A; Thibeault C; Pfeiffer M; Hippenstiel S; Hocke A; von Kalle C; Campbell A; Hayward C; Porteous DJ; Marioni RE; Langenberg C; Lilley KS; Kuebler WM; Mülleder M; Drosten C; Suttorp N; Witzenrath M; Kurth F; Sander LE; Ralser M Ultra-High-Throughput Clinical Proteomics Reveals Classifiers of COVID-19 Infection. Cell Syst. 2020, 11 (1), 11–24.e4. 10.1016/j.cels.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Chen Y-M; Zheng Y; Yu Y; Wang Y; Huang Q; Qian F; Sun L; Song Z-G; Chen Z; Feng J; An Y; Yang J; Su Z; Sun S; Dai F; Chen Q; Lu Q; Li P; Ling Y; Yang Z; Tang H; Shi L; Jin L; Holmes EC; Ding C; Zhu T-Y; Zhang Y-Z Blood Molecular Markers Associated with COVID-19 Immunopathology and Multi-Organ Damage. EMBO J. 2020, 39 (24), e105896. 10.15252/embj.2020105896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Völlmy F; van den Toorn H; Zenezini Chiozzi R; Zucchetti O; Papi A; Volta CA; Marracino L; Vieceli Dalla Sega F; Fortini F; Demichev V; Tober-Lau P; Campo G; Contoli M; Ralser M; Kurth F; Spadaro S; Rizzo P; Heck AJ A Serum Proteome Signature to Predict Mortality in Severe COVID-19 Patients. Life Sci. Alliance 2021, 4 (9), e202101099. 10.26508/lsa.202101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Shen B; Yi X; Sun Y; Bi X; Du J; Zhang C; Quan S; Zhang F; Sun R; Qian L; Ge W; Liu W; Liang S; Chen H; Zhang Y; Li J; Xu J; He Z; Chen B; Wang J; Yan H; Zheng Y; Wang D; Zhu J; Kong Z; Kang Z; Liang X; Ding X; Ruan G; Xiang N; Cai X; Gao H; Li L; Li S; Xiao Q; Lu T; Zhu Y; Liu H; Chen H; Guo T Proteomic and Metabolomic Characterization of COVID-19 Patient Sera. Cell 2020, 182 (1), 59–72.e15. 10.1016/j.cell.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Hou X; Zhang X; Wu X; Lu M; Wang D; Xu M; Wang H; Liang T; Dai J; Duan H; Xu Y; Yu X; Li Y Serum Protein Profiling Reveals a Landscape of Inflammation and Immune Signaling in Early-Stage COVID-19 Infection. Mol. Cell. Proteomics MCP 2020, 19 (11), 1749–1759. 10.1074/mcp.RP120.002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Janssen NAF; Grondman I; de Nooijer AH; Boahen CK; Koeken VACM; Matzaraki V; Kumar V; He X; Kox M; Koenen HJPM; Smeets RL; Joosten I; Brüggemann RJM; Kouijzer IJE; van der Hoeven HG; Schouten JA; Frenzel T; Reijers MHE; Hoefsloot W; Dofferhoff ASM; van Apeldoorn MJ; Blaauw MJT; Veerman K; Maas C; Schoneveld AH; Hoefer IE; Derde LPG; van Deuren M; van der Meer JWM; van Crevel R; Giamarellos-Bourboulis EJ; Joosten LAB; van den Heuvel MM; Hoogerwerf J; de Mast Q; Pickkers P; Netea MG; van de Veerdonk FL Dysregulated Innate and Adaptive Immune Responses Discriminate Disease Severity in COVID-19. J. Infect. Dis 2021, 223 (8), 1322–1333. 10.1093/infdis/jiab065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Ramaswamy A; Brodsky NN; Sumida TS; Comi M; Asashima H; Hoehn KB; Li N; Liu Y; Shah A; Ravindra NG; Bishai J; Khan A; Lau W; Sellers B; Bansal N; Guerrerio P; Unterman A; Habet V; Rice AJ; Catanzaro J; Chandnani H; Lopez M; Kaminski N; Dela Cruz CS; Tsang JS; Wang Z; Yan X; Kleinstein SH; van Dijk D; Pierce RW; Hafler DA; Lucas CL Immune Dysregulation and Autoreactivity Correlate with Disease Severity in SARS-CoV-2-Associated Multisystem Inflammatory Syndrome in Children. Immunity 2021, 54 (5), 1083–1095.e7. 10.1016/j.immuni.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Cotugno N; Ruggiero A; Bonfante F; Petrara MR; Zicari S; Pascucci GR; Zangari P; De Ioris MA; Santilli V; Manno EC; Amodio D; Bortolami A; Pagliari M; Concato C; Linardos G; Campana A; Donà D; Giaquinto C; CACTUS Study Team; Brodin P; Rossi P; De Rossi A; Palma P Virological and Immunological Features of SARS-CoV-2-Infected Children Who Develop Neutralizing Antibodies. Cell Rep. 2021, 34 (11), 108852. 10.1016/j.celrep.2021.108852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Zhong W; Altay O; Arif M; Edfors F; Mardinoglu A; Uhlén M; Fagerberg L Next Generation Plasma Proteome Profiling of COVID-19 Patients with Mild to Moderate Symptoms; preprint; Infectious Diseases (except HIV/AIDS), 2021. 10.1101/2021.06.15.21258940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Su Y; Chen D; Yuan D; Lausted C; Choi J; Dai CL; Voillet V; Duvvuri VR; Scherler K; Troisch P; Baloni P; Qin G; Smith B; Kornilov SA; Rostomily C; Xu A; Li J; Dong S; Rothchild A; Zhou J; Murray K; Edmark R; Hong S; Heath JE; Earls J; Zhang R; Xie J; Li S; Roper R; Jones L; Zhou Y; Rowen L; Liu R; Mackay S; O’Mahony DS; Dale CR; Wallick JA; Algren HA; Zager MA; Wei W; Price ND; Huang S; Subramanian N; Wang K; Magis AT; Hadlock JJ; Hood L; Aderem A; Bluestone JA; Lanier LL; Greenberg PD; Gottardo R; Davis MM; Goldman JD; Heath JR Multi-Omics Resolves a Sharp Disease-State Shift between Mild and Moderate COVID-19. Cell 2020, 183 (6), 1479–1495.e20. 10.1016/j.cell.2020.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Zhou S; Butler-Laporte G; Nakanishi T; Morrison DR; Afilalo J; Afilalo M; Laurent L; Pietzner M; Kerrison N; Zhao K; Brunet-Ratnasingham E; Henry D; Kimchi N; Afrasiabi Z; Rezk N; Bouab M; Petitjean L; Guzman C; Xue X; Tselios C; Vulesevic B; Adeleye O; Abdullah T; Almamlouk N; Chen Y; Chassé M; Durand M; Paterson C; Normark J; Frithiof R; Lipcsey M; Hultström M; Greenwood CMT; Zeberg H; Langenberg C; Thysell E; Pollak M; Mooser V; Forgetta V; Kaufmann DE; Richards JB A Neanderthal OAS1 Isoform Protects Individuals of European Ancestry against COVID-19 Susceptibility and Severity. Nat. Med 2021, 27 (4), 659–667. 10.1038/s41591-021-01281-1. [DOI] [PubMed] [Google Scholar]
- (107).Demichev V; Tober-Lau P; Lemke O; Nazarenko T; Thibeault C; Whitwell H; Röhl A; Freiwald A; Szyrwiel L; Ludwig D; Correia-Melo C; Aulakh SK; Helbig ET; Stubbemann P; Lippert LJ; Grüning N-M; Blyuss O; Vernardis S; White M; Messner CB; Joannidis M; Sonnweber T; Klein SJ; Pizzini A; Wohlfarter Y; Sahanic S; Hilbe R; Schaefer B; Wagner S; Mittermaier M; Machleidt F; Garcia C; Ruwwe-Glösenkamp C; Lingscheid T; Bosquillon de Jarcy L; Stegemann MS; Pfeiffer M; Jürgens L; Denker S; Zickler D; Enghard P; Zelezniak A; Campbell A; Hayward C; Porteous DJ; Marioni RE; Uhrig A; Müller-Redetzky H; Zoller H; Löffler-Ragg J; Keller MA; Tancevski I; Timms JF; Zaikin A; Hippenstiel S; Ramharter M; Witzenrath M; Suttorp N; Lilley K; Mülleder M; Sander LE; Ralser M; Kurth F; Kleinschmidt M; Heim KM; Millet B; Meyer-Arndt L; Hübner RH; Andermann T; Doehn JM; Opitz B; Sawitzki B; Grund D; Radünzel P; Schürmann M; Zoller T; Alius F; Knape P; Breitbart A; Li Y; Bremer F; Pergantis P; Schürmann D; Temmesfeld-Wollbrück B; Wendisch D; Brumhard S; Haenel SS; Conrad C; Georg P; Eckardt K-U; Lehner L; Kruse JM; Ferse C; Körner R; Spies C; Edel A; Weber-Carstens S; Krannich A; Zvorc S; Li L; Behrens U; Schmidt S; Rönnefarth M; Dang-Heine C; Röhle R; Lieker E; Kretzler L; Wirsching I; Wollboldt C; Wu Y; Schwanitz G; Hillus D; Kasper S; Olk N; Horn A; Briesemeister D; Treue D; Hummel M; Corman VM; Drosten C; von Kalle C A Time-Resolved Proteomic and Prognostic Map of COVID-19. Cell Syst. 2021, S2405471221001605. 10.1016/j.cels.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Geyer PE; Arend FM; Doll S; Louiset M-L; Virreira Winter S; Müller-Reif JB; Torun FM; Weigand M; Eichhorn P; Bruegel M; Strauss MT; Holdt LM; Mann M; Teupser D High-Resolution Serum Proteome Trajectories in COVID-19 Reveal Patient-Specific Seroconversion. EMBO Mol. Med 2021, 13 (8), e14167. 10.15252/emmm.202114167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Lee J-S; Han D; Kim SY; Hong KH; Jang M-J; Kim MJ; Kim Y-G; Park JH; Cho SI; Park WB; Lee KB; Shin HS; Oh HS; Kim TS; Park SS; Seong M-W Longitudinal Proteomic Profiling Provides Insights into Host Response and Proteome Dynamics in COVID-19 Progression. Proteomics 2021, 21 (11–12), e2000278. 10.1002/pmic.202000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Tang S; Sun R; Xiao Q; Mao T; Ge W; Huang C; Luo M; Qian L; Chen H; Zhang Q; Li S; Liu W; Li S; Xu X; Li H; Wu L; Dai J; Gao H; Li L; Lu T; Liang X; Cai X; Ruan G; Liu K; Xu F; Li Y; Zhu Y; Huang J; Guo T Proteomics Uncovers Immunosuppression in COVID-19 Patients with Long Disease Course. medRxiv 2020, 2020.06.14.20131078. 10.1101/2020.06.14.20131078. [DOI] [Google Scholar]
- (111).Shu T; Ning W; Wu D; Xu J; Han Q; Huang M; Zou X; Yang Q; Yuan Y; Bie Y; Pan S; Mu J; Han Y; Yang X; Zhou H; Li R; Ren Y; Chen X; Yao S; Qiu Y; Zhang D-Y; Xue Y; Shang Y; Zhou X Plasma Proteomics Identify Biomarkers and Pathogenesis of COVID-19. Immunity 2020, 53 (5), 1108–1122.e5. 10.1016/j.immuni.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Altay O; Arif M; Li X; Yang H; Aydın M; Alkurt G; Kim W; Akyol D; Zhang C; Dinler-Doganay G; Turkez H; Shoaie S; Nielsen J; Borén J; Olmuscelik O; Doganay L; Uhlén M; Mardinoglu A Combined Metabolic Activators Accelerates Recovery in Mild-to-Moderate COVID-19. medRxiv 2021, 2020.10.02.20202614. 10.1101/2020.10.02.20202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Jiang H; Li Y; Zhang H; Wang W; Yang X; Qi H; Li H; Men D; Zhou J; Tao S SARS-CoV-2 Proteome Microarray for Global Profiling of COVID-19 Specific IgG and IgM Responses. Nat. Commun 2020, 11 (1), 3581. 10.1038/s41467-020-17488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Lei Q; Li Y; Hou H; Wang F; Ouyang Z; Zhang Y; Lai D; Banga Ndzouboukou J; Xu Z; Zhang B; Chen H; Xue J; Lin X; Zheng Y; Yao Z; Wang X; Yu C; Jiang H; Zhang H; Qi H; Guo S; Huang S; Sun Z; Tao S; Fan X Antibody Dynamics to SARS-CoV-2 in Asymptomatic COVID-19 Infections. Allergy 2021, 76 (2), 551–561. 10.1111/all.14622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Li Y; Xu Z; Lei Q; Lai D; Hou H; Jiang H; Zheng Y; Wang X; Wu J; Ma M; Zhang B; Chen H; Yu C; Xue J; Zhang H; Qi H; Guo S; Zhang Y; Lin X; Yao Z; Sheng H; Sun Z; Wang F; Fan X; Tao S Antibody Landscape against SARS-CoV-2 Reveals Significant Differences between Non-Structural/Accessory and Structural Proteins. Cell Rep. 2021, 36 (2), 109391. 10.1016/j.celrep.2021.109391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Roxhed N; Bendes A; Dale M; Mattsson C; Hanke L; Dodig-Crnković T; Christian M; Meineke B; Elsässer S; Andréll J; Havervall S; Thålin C; Eklund C; Dillner J; Beck O; Thomas CE; McInerney G; Hong M-G; Murrell B; Fredolini C; Schwenk JM Multianalyte Serology in Home-Sampled Blood Enables an Unbiased Assessment of the Immune Response against SARS-CoV-2. Nat. Commun 2021, 12 (1), 3695. 10.1038/s41467-021-23893-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Hober S; Hellström C; Olofsson J; Andersson E; Bergström S; Jernbom Falk A; Bayati S; Mravinacova S; Sjöberg R; Yousef J; Skoglund L; Kanje S; Berling A; Svensson A-S; Jensen G; Enstedt H; Afshari D; Xu LL; Zwahlen M; von Feilitzen K; Hanke L; Murrell B; McInerney G; Karlsson Hedestam GB; Lendel C; Roth RG; Skoog I; Svenungsson E; Olsson T; Fogdell-Hahn A; Lindroth Y; Lundgren M; Maleki KT; Lagerqvist N; Klingström J; Da Silva Rodrigues R; Muschiol S; Bogdanovic G; Arroyo Mühr LS; Eklund C; Lagheden C; Dillner J; Sivertsson Å; Havervall S; Thålin C; Tegel H; Pin E; Månberg A; Hedhammar M; Nilsson P Systematic Evaluation of SARS-CoV-2 Antigens Enables a Highly Specific and Sensitive Multiplex Serological COVID-19 Assay. Clin. Transl. Immunol 2021, 10 (7), e1312. 10.1002/cti2.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).Becker M; Strengert M; Junker D; Kaiser PD; Kerrinnes T; Traenkle B; Dinter H; Häring J; Ghozzi S; Zeck A; Weise F; Peter A; Hörber S; Fink S; Ruoff F; Dulovic A; Bakchoul T; Baillot A; Lohse S; Cornberg M; Illig T; Gottlieb J; Smola S; Karch A; Berger K; Rammensee H-G; Schenke-Layland K; Nelde A; Märklin M; Heitmann JS; Walz JS; Templin M; Joos TO; Rothbauer U; Krause G; Schneiderhan-Marra N Exploring beyond Clinical Routine SARS-CoV-2 Serology Using MultiCoV-Ab to Evaluate Endemic Coronavirus Cross-Reactivity. Nat. Commun 2021, 12 (1), 1152. 10.1038/s41467-021-20973-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Becker M; Dulovic A; Junker D; Ruetalo N; Kaiser PD; Pinilla YT; Heinzel C; Haering J; Traenkle B; Wagner TR; Layer M; Mehrlaender M; Mirakaj V; Held J; Planatscher H; Schenke-Layland K; Krause G; Strengert M; Bakchoul T; Althaus K; Fendel R; Kreidenweiss A; Koeppen M; Rothbauer U; Schindler M; Schneiderhan-Marra N Immune Response to SARS-CoV-2 Variants of Concern in Vaccinated Individuals. Nat. Commun 2021, 12 (1), 3109. 10.1038/s41467-021-23473-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Wang H; Wu X; Zhang X; Hou X; Liang T; Wang D; Teng F; Dai J; Duan H; Guo S; Li Y; Yu X SARS-CoV-2 Proteome Microarray for Mapping COVID-19 Antibody Interactions at Amino Acid Resolution. ACS Cent. Sci 2020, 6 (12), 2238–2249. 10.1021/acscentsci.0c00742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Li Y; Lai D; Zhang H; Jiang H; Tian X; Ma M; Qi H; Meng Q; Guo S; Wu Y; Wang W; Yang X; Shi D; Dai J; Ying T; Zhou J; Tao S Linear Epitopes of SARS-CoV-2 Spike Protein Elicit Neutralizing Antibodies in COVID-19 Patients. Cell. Mol. Immunol 2020, 17 (10), 1095–1097. 10.1038/s41423-020-00523-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (122).Li Y; Lai D; Lei Q; Xu Z; Wang F; Hou H; Chen L; Wu J; Ren Y; Ma M; Zhang B; Chen H; Yu C; Xue J; Zheng Y; Wang X; Jiang H; Zhang H; Qi H; Guo S; Zhang Y; Lin X; Yao Z; Pang P; Shi D; Wang W; Yang X; Zhou J; Sheng H; Sun Z; Shan H; Fan X; Tao S Systematic Evaluation of IgG Responses to SARS-CoV-2 Spike Protein-Derived Peptides for Monitoring COVID-19 Patients. Cell. Mol. Immunol 2021, 18 (3), 621–631. 10.1038/s41423-020-00612-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Li Y; Ma M; Lei Q; Wang F; Hong W; Lai D; Hou H; Xu Z; Zhang B; Chen H; Yu C; Xue J; Zheng Y; Wang X; Jiang H; Zhang H; Qi H; Guo S; Zhang Y; Lin X; Yao Z; Wu J; Sheng H; Zhang Y; Wei H; Sun Z; Fan X; Tao S Linear Epitope Landscape of the SARS-CoV-2 Spike Protein Constructed from 1,051 COVID-19 Patients. Cell Rep. 2021, 34 (13), 108915. 10.1016/j.celrep.2021.108915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Schwarz T; Heiss K; Mahendran Y; Casilag F; Kurth F; Sander LE; Wendtner C-M; Hoechstetter MA; Müller MA; Sekul R; Drosten C; Stadler V; Corman VM SARS-CoV-2 Proteome-Wide Analysis Revealed Significant Epitope Signatures in COVID-19 Patients. Front. Immunol 2021, 12, 629185. 10.3389/fimmu.2021.629185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (125).Heidepriem J; Dahlke C; Kobbe R; Santer R; Koch T; Fathi A; Seco B; Ly M; Schmiedel S; Schwinge D; Serna S; Sellrie K; Reichardt N-C; Seeberger P; Addo M; Loeffler F; on behalf of the ID-UKE COVID-19 Study Group. Longitudinal Development of Antibody Responses in COVID-19 Patients of Different Severity with ELISA, Peptide, and Glycan Arrays: An Immunological Case Series. Pathogens 2021, 10 (4), 438. 10.3390/pathogens10040438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).Holenya P; Lange PJ; Reimer U; Woltersdorf W; Panterodt T; Glas M; Wasner M; Eckey M; Drosch M; Hollidt J; Naumann M; Kern F; Wenschuh H; Lange R; Schnatbaum K; Bier FF Peptide Microarray-based Analysis of Antibody Responses to SARS-CoV-2 Identifies Unique Epitopes with Potential for Diagnostic Test Development. Eur. J. Immunol 2021, 51 (7), 1839–1849. 10.1002/eji.202049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Heffron AS; McIlwain SJ; Amjadi MF; Baker DA; Khullar S; Armbrust T; Halfmann PJ; Kawaoka Y; Sethi AK; Palmenberg AC; Shelef MA; O’Connor DH; Ong IM The Landscape of Antibody Binding in SARS-CoV-2 Infection. PLOS Biol. 2021, 19 (6), e3001265. 10.1371/journal.pbio.3001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Shrock E; Fujimura E; Kula T; Timms RT; Lee I-H; Leng Y; Robinson ML; Sie BM; Li MZ; Chen Y; Logue J; Zuiani A; McCulloch D; Lelis FJN; Henson S; Monaco DR; Travers M; Habibi S; Clarke WA; Caturegli P; Laeyendecker O; Piechocka-Trocha A; Li JZ; Khatri A; Chu HY; MGH COVID-19 Collection & Processing Team16‡; Villani A-C; Kays K; Goldberg MB; Hacohen N; Filbin MR; Yu XG; Walker BD; Wesemann DR; Larman HB; Lederer JA; Elledge SJ Viral Epitope Profiling of COVID-19 Patients Reveals Cross-Reactivity and Correlates of Severity. Science 2020, 370 (6520), eabd4250. 10.1126/science.abd4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Qi H; Ma M; Hu C; Xu Z; Wu F; Wang N; Lai D; Li Y; Zhang H; Jiang H; Meng Q; Guo S; Kang Y; Zhao X; Li H; Tao S Antibody Binding Epitope Mapping (AbMap) of Hundred Antibodies in a Single Run. Mol. Cell. Proteomics 2021, 20, 100059. 10.1074/mcp.RA120.002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Qi H; Ma M; Jiang H; Ling J; Chen L; Zhang H; Lai D; Li Y; Guo Z; Hu C; Guo S; Meng Q; Ren Y; Yang X; Wang W; Zhou J; Zhao X; Li H; Tao S Systematic Profiling of SARS-CoV-2-Specific IgG Epitopes at Amino Acid Resolution. Cell. Mol. Immunol 2021, 18 (4), 1067–1069. 10.1038/s41423-021-00654-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Hu C; Huang W; Chen H; Song G; Li P; Shan Q; Zhang X; Zhang F; Zhu H; Wu L; Li Y Autoantibody Profiling on Human Proteome Microarray for Biomarker Discovery in Cerebrospinal Fluid and Sera of Neuropsychiatric Lupus. PloS One 2015, 10 (5), e0126643. 10.1371/journal.pone.0126643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Zandian A; Forsstrom B; Haggmark-Manberg A; Schwenk JM; Uhlen M; Nilsson P; Ayoglu B Whole-Proteome Peptide Microarrays for Profiling Autoantibody Repertoires within Multiple Sclerosis and Narcolepsy. J Proteome Res 2017, 16 (3), 1300–1314. 10.1021/acs.jproteome.6b00916. [DOI] [PubMed] [Google Scholar]
- (133).Neiman M; Hellström C; Just D; Mattsson C; Fagerberg L; Schuppe-Koistinen I; Gummesson A; Bergström G; Kallioniemi O; Achour A; Sallinen R; Uhlén M; Nilsson P Individual and Stable Autoantibody Repertoires in Healthy Individuals. Autoimmunity 2019, 52 (1), 1–11. 10.1080/08916934.2019.1581774. [DOI] [PubMed] [Google Scholar]
- (134).Enroth S; Johansson A; Enroth SB; Gyllensten U Strong Effects of Genetic and Lifestyle Factors on Biomarker Variation and Use of Personalized Cutoffs. Nat. Commun 2014, 5, 4684. 10.1038/ncomms5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Folkersen L; Gustafsson S; Wang Q; Hansen DH; Hedman ÅK; Schork A; Page K; Zhernakova DV; Wu Y; Peters J; Eriksson N; Bergen SE; Boutin TS; Bretherick AD; Enroth S; Kalnapenkis A; Gådin JR; Suur BE; Chen Y; Matic L; Gale JD; Lee J; Zhang W; Quazi A; Ala-Korpela M; Choi SH; Claringbould A; Danesh J; Davey Smith G; de Masi F; Elmståhl S; Engström G; Fauman E; Fernandez C; Franke L; Franks PW; Giedraitis V; Haley C; Hamsten A; Ingason A; Johansson Å; Joshi PK; Lind L; Lindgren CM; Lubitz S; Palmer T; Macdonald-Dunlop E; Magnusson M; Melander O; Michaelsson K; Morris AP; Mägi R; Nagle MW; Nilsson PM; Nilsson J; Orho-Melander M; Polasek O; Prins B; Pålsson E; Qi T; Sjögren M; Sundström J; Surendran P; Võsa U; Werge T; Wernersson R; Westra H-J; Yang J; Zhernakova A; Ärnlöv J; Fu J; Smith JG; Esko T; Hayward C; Gyllensten U; Landen M; Siegbahn A; Wilson JF; Wallentin L; Butterworth AS; Holmes MV; Ingelsson E; Mälarstig A Genomic and Drug Target Evaluation of 90 Cardiovascular Proteins in 30,931 Individuals. Nat. Metab 2020, 2 (10), 1135–1148. 10.1038/s42255-020-00287-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (136).Emilsson V; Gudmundsdottir V; Gudjonsson A; Karim MA; Ilkov M; Staley JR; Gudmundsson EF; Jonsson BG; Launer LJ; Lindeman JH; Morton NM; Aspelund T; Lamb JR; Jennings LL; Gudnason V Coding and Regulatory Variants Affect Serum Protein Levels and Common Disease; preprint; Genetics, 2020. 10.1101/2020.05.06.080440. [DOI] [Google Scholar]
- (137).Pietzner M; Wheeler E; Carrasco-Zanini J; Kerrison ND; Oerton E; Koprulu M; Luan J; Hingorani AD; Williams SA; Wareham NJ; Langenberg C Cross-Platform Proteomics to Advance Genetic Prioritisation Strategies; preprint; Genetics, 2021. 10.1101/2021.03.18.435919. [DOI] [Google Scholar]
- (138).Solomon T; Lapek JDJ; Jensen SB; Greenwald WW; Hindberg K; Matsui H; Latysheva N; Braekken SK; Gonzalez DJ; Frazer KA; Smith EN; Hansen J-B Identification of Common and Rare Genetic Variation Associated With Plasma Protein Levels Using Whole-Exome Sequencing and Mass Spectrometry. Circ. Genomic Precis. Med 2018, 11 (12), e002170. 10.1161/CIRCGEN.118.002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Johansson Å; Enroth S; Palmblad M; Deelder AM; Bergquist J; Gyllensten U Identification of Genetic Variants Influencing the Human Plasma Proteome. Proc. Natl. Acad. Sci. U. S. A 2013, 110 (12), 4673–4678. 10.1073/pnas.1217238110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).de Vries PS; Yu B; Feofanova EV; Metcalf GA; Brown MR; Zeighami AL; Liu X; Muzny DM; Gibbs RA; Boerwinkle E; Morrison AC Whole-Genome Sequencing Study of Serum Peptide Levels: The Atherosclerosis Risk in Communities Study. Hum. Mol. Genet 2017, 26 (17), 3442–3450. 10.1093/hmg/ddx266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (141).Molendijk J; Parker BL Proteome-Wide Systems Genetics to Identify Functional Regulators of Complex Traits. Cell Syst. 2021, 12 (1), 5–22. 10.1016/j.cels.2020.10.005. [DOI] [PubMed] [Google Scholar]
- (142).Viñuela A; Brown AA; Fernandez J; Hong M-G; Brorsson CA; Koivula RW; Davtian D; Dupuis T; Forgie IM; Adam J; Allin KH; Caiazzo R; Cederberg H; Masi FD; Elders PJM; Giordano GN; Haid M; Hansen T; Hansen T; Hattersley AT; Heggie AJ; Howald C; Jones AG; Kokkola T; Laakso M; Mahajan A; Mari A; McDonald TJ; McEvoy D; Mourby M; Musholt P; Nilsson B; Pattou F; Penet D; Raverdy V; Ridderstrale M; Romano L; Rutters F; Sharma S; Teare H; T’Hart LM; Tsirigos K; Vangipurapu J; Vestergaard H; Brunak S; Franks PW; Frost G; Grallert H; Jablonka B; McCarthy MI; Pavo I; Pedersen O; Ruetten H; Walker M; Consortium, the D.; Adamski J; Schwenk JM; Pearson ER; Dermitzakis ET Genetic Analysis of Blood Molecular Phenotypes Reveals Regulatory Networks Affecting Complex Traits: A DIRECT Study. medRxiv 2021, 2021.03.26.21254347. 10.1101/2021.03.26.21254347. [DOI] [Google Scholar]
- (143).Nesvizhskii AI Proteogenomics: Concepts, Applications and Computational Strategies. Nat. Methods 2014, 11 (11), 1114–1125. 10.1038/nmeth.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (144).Tanzi RE The Genetics of Alzheimer Disease. Cold Spring Harb. Perspect. Med 2012, 2 (10), a006296–a006296. 10.1101/cshperspect.a006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).Martínez-Morillo E; Nielsen HM; Batruch I; Drabovich AP; Begcevic I; Lopez MF; Minthon L; Bu G; Mattsson N; Portelius E; Hansson O; Diamandis EP Assessment of Peptide Chemical Modifications on the Development of an Accurate and Precise Multiplex Selected Reaction Monitoring Assay for Apolipoprotein E Isoforms. J. Proteome Res 2014, 13 (2), 1077–1087. 10.1021/pr401060x. [DOI] [PubMed] [Google Scholar]
- (146).Pernemalm M; Sandberg A; Zhu Y; Boekel J; Tamburro D; Schwenk JM; Björk A; Wahren-Herlenius M; Åmark H; Östenson C-G; Westgren M; Lehtiö J In-Depth Human Plasma Proteome Analysis Captures Tissue Proteins and Transfer of Protein Variants across the Placenta. eLife 2019, 8. 10.7554/eLife.41608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (147).Vasaikar S; Huang C; Wang X; Petyuk VA; Savage SR; Wen B; Dou Y; Zhang Y; Shi Z; Arshad OA; Gritsenko MA; Zimmerman LJ; McDermott JE; Clauss TR; Moore RJ; Zhao R; Monroe ME; Wang Y-T; Chambers MC; Slebos RJC; Lau KS; Mo Q; Ding L; Ellis M; Thiagarajan M; Kinsinger CR; Rodriguez H; Smith RD; Rodland KD; Liebler DC; Liu T; Zhang B; Pandey A; Paulovich A; Hoofnagle A; Mani DR; Chan DW; Ransohoff DF; Fenyo D; Tabb DL; Levine DA; Boja ES; Kuhn E; White FM; Whiteley GA; Zhu H; Zhang H; Shih I-M; Bavarva J; Whiteaker J; Ketchum KA; Clauser KR; Ruggles K; Elburn K; Hannick L; Watson M; Oberti M; Mesri M; Sanders ME; Borucki M; Gillette MA; Snyder M; Edwards NJ; Vatanian N; Rudnick PA; McGarvey PB; Mertins P; Townsend RR; Thangudu RR; Rivers RC; Payne SH; Davies SR; Cai S; Stein SE; Carr SA; Skates SJ; Madhavan S; Hiltke T; Chen X; Zhao Y; Wang Y; Zhang Z Proteogenomic Analysis of Human Colon Cancer Reveals New Therapeutic Opportunities. Cell 2019, 177 (4), 1035–1049.e19. 10.1016/j.cell.2019.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148).Clark DJ; Dhanasekaran SM; Petralia F; Pan J; Song X; Hu Y; da Veiga Leprevost F; Reva B; Lih T-SM; Chang H-Y; Ma W; Huang C; Ricketts CJ; Chen L; Krek A; Li Y; Rykunov D; Li QK; Chen LS; Ozbek U; Vasaikar S; Wu Y; Yoo S; Chowdhury S; Wyczalkowski MA; Ji J; Schnaubelt M; Kong A; Sethuraman S; Avtonomov DM; Ao M; Colaprico A; Cao S; Cho K-C; Kalayci S; Ma S; Liu W; Ruggles K; Calinawan A; Gümüş ZH; Geiszler D; Kawaler E; Teo GC; Wen B; Zhang Y; Keegan S; Li K; Chen F; Edwards N; Pierorazio PM; Chen XS; Pavlovich CP; Hakimi AA; Brominski G; Hsieh JJ; Antczak A; Omelchenko T; Lubinski J; Wiznerowicz M; Linehan WM; Kinsinger CR; Thiagarajan M; Boja ES; Mesri M; Hiltke T; Robles AI; Rodriguez H; Qian J; Fenyö D; Zhang B; Ding L; Schadt E; Chinnaiyan AM; Zhang Z; Omenn GS; Cieslik M; Chan DW; Nesvizhskii AI; Wang P; Zhang H; Hashimi AS; Pico AR; Karpova A; Charamut A; Paulovich AG; Perou AM; Malovannaya A; Marrero-Oliveras A; Agarwal A; Hindenach B; Pruetz B; Kim B-J; Druker BJ; Newton CJ; Birger C; Jones CD; Tognon C; Mani DR; Valley DR; Rohrer DC; Zhou DC; Tansil D; Chesla D; Heiman D; Wheeler D; Tan D; Chan D; Demir E; Malc E; Modugno F; Getz G; Hostetter G; Wilson GD; Hart GW; Zhu H; Liu H; Culpepper H; Sun H; Zhou H; Day J; Suh J; Huang J; McDermott J; Whiteaker JR; Tyner JW; Eschbacher J; Chen J; McGee J; Zhu J; Ketchum KA; Rodland KD; Clauser K; Robinson K; Krug K; Hoadley KA; Um KS; Elburn K; Holloway K; Wang L-B; Blumenberg L; Hannick L; Qi L; Sokoll LJ; Cornwell M; Loriaux M; Domagalski MJ; Gritsenko MA; Anderson M; Monroe ME; Ellis MJ; Dyer M; Anurag M; Burke MC; Borucki M; Gillette MA; Birrer MJ; Lewis M; Ittmann MM; Smith M; Vernon M; Chaikin M; Chheda MG; Khan M; Roche N; Edwards NJ; Vatanian N; Tignor N; Beckmann N; Grady P; Castro P; Piehowski P; McGarvey PB; Mieczkowski P; Hariharan P; Gao Q; Dhir R; Kothadia RB; Thangudu RR; Montgomery R; Jayasinghe RG; Smith RD; Edwards R; Zelt R; Bremner R; Liu R; Hong R; Mareedu S; Payne SH; Cottingham S; Markey SP; Jewell SD; Patel S; Satpathy S; Richey S; Davies SR; Cai S; Boca SM; Patil S; Sengupta S; Carter S; Gabriel S; Thomas SN; De Young S; Stein SE; Carr SA; Foltz SM; Hilsenbeck S; Krubit T; Liu T; Skelly T; Westbrook T; Borate U; Velvulou U; Petyuk VA; Bocik WE; Chen X; Shi Y; Geffen Y; Lu Y; Wang Y; Maruvka Y; Li Z; Shi Z; Tu Z Integrated Proteogenomic Characterization of Clear Cell Renal Cell Carcinoma. Cell 2019, 179 (4), 964–983.e31. 10.1016/j.cell.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).Mertins P; Mani DR; Ruggles KV; Gillette MA; Clauser KR; Wang P; Wang X; Qiao JW; Cao S; Petralia F; Kawaler E; Mundt F; Krug K; Tu Z; Lei JT; Gatza ML; Wilkerson M; Perou CM; Yellapantula V; Huang K; Lin C; McLellan MD; Yan P; Davies SR; Townsend RR; Skates SJ; Wang J; Zhang B; Kinsinger CR; Mesri M; Rodriguez H; Ding L; Paulovich AG; Fenyö D; Ellis MJ; Carr SA Proteogenomics Connects Somatic Mutations to Signalling in Breast Cancer. Nature 2016, 534 (7605), 55–62. 10.1038/nature18003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (150).Babačić H; Lehtiö J; Pico de Coaña Y; Pernemalm M; Eriksson H In-Depth Plasma Proteomics Reveals Increase in Circulating PD-1 during Anti-PD-1 Immunotherapy in Patients with Metastatic Cutaneous Melanoma. J. Immunother. Cancer 2020, 8 (1), e000204. 10.1136/jitc-2019-000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (151).Deutsch EW; Lane L; Overall CM; Bandeira N; Baker MS; Pineau C; Moritz RL; Corrales F; Orchard S; Van Eyk JE; Paik Y-K; Weintraub ST; Vandenbrouck Y; Omenn GS Human Proteome Project Mass Spectrometry Data Interpretation Guidelines 3.0. J. Proteome Res 2019, 18 (12), 4108–4116. 10.1021/acs.jproteome.9b00542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (152).Schmitt LR; Henderson R; Barrett A; Darula Z; Issaian A; D’Alessandro A; Clendenen N; Hansen KC Mass Spectrometry-Based Molecular Mapping of Native FXIIIa Cross-Links in Insoluble Fibrin Clots. J. Biol. Chem 2019, 294 (22), 8773–8778. 10.1074/jbc.AC119.007981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (153).Ząbczyk M; Stachowicz A; Natorska J; Olszanecki R; Wiśniewski JR; Undas A Plasma Fibrin Clot Proteomics in Healthy Subjects: Relation to Clot Permeability and Lysis Time. J. Proteomics 2019, 208, 103487. 10.1016/j.jprot.2019.103487. [DOI] [PubMed] [Google Scholar]
- (154).Bhagwat SR; Hajela K; Bhutada S; Choudhary K; Saxena M; Sharma S; Kumar A Identification of Unexplored Substrates of the Serine Protease, Thrombin, Using N-Terminomics Strategy. Int. J. Biol. Macromol 2020, 144, 449–459. 10.1016/j.ijbiomac.2019.12.137. [DOI] [PubMed] [Google Scholar]
- (155).Stachowicz A; Zabczyk M; Natorska J; Suski M; Olszanecki R; Korbut R; Wiśniewski JR; Undas A Differences in Plasma Fibrin Clot Composition in Patients with Thrombotic Antiphospholipid Syndrome Compared with Venous Thromboembolism. Sci. Rep 2018, 8 (1), 17301. 10.1038/s41598-018-35034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).ISTH Steering Committee for World Thrombosis Day. Thrombosis: A Major Contributor to the Global Disease Burden. J. Thromb. Haemost 2014, 12 (10), 1580–1590. 10.1111/jth.12698. [DOI] [PubMed] [Google Scholar]
- (157).Bruzelius M; Iglesias MJ; Hong M-G; Sanchez-Rivera L; Gyorgy B; Souto JC; Frånberg M; Fredolini C; Strawbridge RJ; Holmström M; Hamsten A; Uhlén M; Silveira A; Soria JM; Smadja DM; Butler LM; Schwenk JM; Morange P-E; Trégouët D-A; Odeberg J PDGFB, a New Candidate Plasma Biomarker for Venous Thromboembolism: Results from the VEREMA Affinity Proteomics Study. Blood 2016, 128 (23), e59–e66. 10.1182/blood-2016-05-711846. [DOI] [PubMed] [Google Scholar]
- (158).Penn AM; Saly V; Trivedi A; Lesperance ML; Votova K; Jackson AM; Croteau NS; Balshaw RF; Bibok MB; Smith DS; Lam KK; Morrison J; Lu L; Coutts SB; Borchers CH Differential Proteomics for Distinguishing Ischemic Stroke from Controls: A Pilot Study of the SpecTRA Project. Transl. Stroke Res 2018, 9 (6), 590–599. 10.1007/s12975-018-0609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (159).Penn AM; Bibok MB; Saly VK; Coutts SB; Lesperance ML; Balshaw RF; Votova K; Croteau NS; Trivedi A; Jackson AM; Hegedus J; Klourfeld E; Yu AYX; Zerna C; Modi J; Barber PA; Hoag G; Borchers CH; SpecTRA study group. Validation of a Proteomic Biomarker Panel to Diagnose Minor-Stroke and Transient Ischaemic Attack: Phase 2 of SpecTRA, a Large Scale Translational Study. Biomark. Biochem. Indic. Expo. Response Susceptibility Chem 2018, 23 (8), 793–803. 10.1080/1354750X.2018.1499130. [DOI] [PubMed] [Google Scholar]
- (160).O’Connell GC; Walsh KB; Burrage E; Adeoye O; Chantler PD; Barr TL High-Throughput Profiling of the Circulating Proteome Suggests Sexually Dimorphic Corticosteroid Signaling Following Ischemic Stroke. Physiol. Genomics 2018, 50 (10), 876–883. 10.1152/physiolgenomics.00058.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (161).Xu R; Gong CX; Duan CM; Huang JC; Yang GQ; Yuan JJ; Zhang Q; Xiong XY; Yang QW Age-Dependent Changes in the Plasma Proteome of Healthy Adults. J. Nutr. Health Aging 2020, 24 (8), 846–856. 10.1007/s12603-020-1392-6. [DOI] [PubMed] [Google Scholar]
- (162).Tanaka T; Biancotto A; Moaddel R; Moore AZ; Gonzalez-Freire M; Aon MA; Candia J; Zhang P; Cheung F; Fantoni G; CHI consortium; Semba RD; Ferrucci L Plasma Proteomic Signature of Age in Healthy Humans. Aging Cell 2018, 17 (5), e12799. 10.1111/acel.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (163).Suski M; Bokiniec R; Szwarc-Duma M; Madej J; Bujak-Giżycka B; Kwinta P; Borszewska-Kornacka MK; Revhaug C; Baumbusch LO; Saugstad OD; Pietrzyk JJ Prospective Plasma Proteome Changes in Preterm Infants with Different Gestational Ages. Pediatr. Res 2018, 84 (1), 104–111. 10.1038/s41390-018-0003-2. [DOI] [PubMed] [Google Scholar]
- (164).Wang Z; Zhang R; Liu F; Jiang P; Xu J; Cao H; Du X; Ma L; Lin F; Cheng L; Zhou X; Shi Z; Liu Y; Huang Y; Ye S; Li C TMT-Based Quantitative Proteomic Analysis Reveals Proteomic Changes Involved in Longevity. Proteomics Clin. Appl 2019, 13 (4), e1800024. 10.1002/prca.201800024. [DOI] [PubMed] [Google Scholar]
- (165).Zhong W; Danielsson H; Tebani A; Karlsson MJ; Elfvin A; Hellgren G; Brusselaers N; Brodin P; Hellström A; Fagerberg L; Uhlén M Dramatic Changes in Blood Protein Levels during the First Week of Life in Extremely Preterm Infants. Pediatr. Res 2021, 89 (3), 604–612. 10.1038/s41390-020-0912-8. [DOI] [PubMed] [Google Scholar]
- (166).Danielsson H; Tebani A; Zhong W; Fagerberg L; Brusselaers N; Hård A-L; Uhlén M; Hellström A Blood Protein Profiles Related to Preterm Birth and Retinopathy of Prematurity. Pediatr. Res 2021. 10.1038/s41390-021-01528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (167).Stockfelt M; Hong M-G; Hesselmar B; Adlerberth I; Wold AE; Schwenk JM; Lundell A-C; Rudin A Circulating Proteins Associated with Allergy Development in Infants-an Exploratory Analysis. Clin. Proteomics 2021, 18 (1), 11. 10.1186/s12014-021-09318-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (168).Earls JC; Rappaport N; Heath L; Wilmanski T; Magis AT; Schork NJ; Omenn GS; Lovejoy J; Hood L; Price ND Multi-Omic Biological Age Estimation and Its Correlation With Wellness and Disease Phenotypes: A Longitudinal Study of 3,558 Individuals. J. Gerontol. A. Biol. Sci. Med. Sci 2019, 74 (Suppl_1), S52–S60. 10.1093/gerona/glz220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (169).Sebastiani P; Federico A; Morris M; Gurinovich A; Tanaka T; Chandler KB; Andersen SL; Denis G; Costello CE; Ferrucci L; Jennings L; Glass DJ; Monti S; Perls TT Protein Signatures of Centenarians and Their Offspring Suggest Centenarians Age Slower than Other Humans. Aging Cell 2021, 20 (2), e13290. 10.1111/acel.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (170).Hong M-G; Dodig-Crnković T; Chen X; Drobin K; Lee W; Wang Y; Edfors F; Kotol D; Thomas CE; Sjöberg R; Odeberg J; Hamsten A; Silveira A; Hall P; Nilsson P; Pawitan Y; Uhlén M; Pedersen NL; Hägg S; Magnusson PK; Schwenk JM Profiles of Histidine-Rich Glycoprotein Associate with Age and Risk of All-Cause Mortality. Life Sci. Alliance 2020, 3 (10), e202000817. 10.26508/lsa.202000817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (171).Gurinovich A; Song Z; Zhang W; Federico A; Monti S; Andersen SL; Jennings LL; Glass DJ; Barzilai N; Millman S; Perls TT; Sebastiani P Effect of Longevity Genetic Variants on the Molecular Aging Rate. GeroScience 2021, 43 (3), 1237–1251. 10.1007/s11357-021-00376-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (172).Fujita Y; Taniguchi Y; Shinkai S; Tanaka M; Ito M Secreted Growth Differentiation Factor 15 as a Potential Biomarker for Mitochondrial Dysfunctions in Aging and Age-Related Disorders. Geriatr. Gerontol. Int 2016, 16 Suppl 1, 17–29. 10.1111/ggi.12724. [DOI] [PubMed] [Google Scholar]
- (173).López-Otín C; Blasco MA; Partridge L; Serrano M; Kroemer G The Hallmarks of Aging. Cell 2013, 153 (6), 1194–1217. 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (174).Harris SE; Cox SR; Bell S; Marioni RE; Prins BP; Pattie A; Corley J; Muñoz Maniega S; Valdés Hernández M; Morris Z; John S; Bronson PG; Tucker-Drob EM; Starr JM; Bastin ME; Wardlaw JM; Butterworth AS; Deary IJ Neurology-Related Protein Biomarkers Are Associated with Cognitive Ability and Brain Volume in Older Age. Nat. Commun 2020, 11 (1), 800. 10.1038/s41467-019-14161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (175).Abu Bakar ZH; Damanhuri HA; Makpol S; Wan Kamaruddin WMA; Abdul Sani NF; Amir Hamzah AIZ; Nor Aripin KN; Mohd Rani MD; Noh NA; Razali R; Mazlan M; Abdul Hamid H; Mohamad M; Wan Ngah WZ Effect of Age on the Protein Profile of Healthy Malay Adults and Its Association with Cognitive Function Competency. J. Alzheimers Dis. JAD 2019, 70 (s1), S43–S62. 10.3233/JAD-180511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (176).Sathyan S; Ayers E; Gao T; Milman S; Barzilai N; Verghese J Plasma Proteomic Profile of Frailty. Aging Cell 2020, e13193. 10.1111/acel.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (177).Landino K; Tanaka T; Fantoni G; Candia J; Bandinelli S; Ferrucci L Characterization of the Plasma Proteomic Profile of Frailty Phenotype. GeroScience 2021, 43 (2), 1029–1037. 10.1007/s11357-020-00288-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (178).Menni C; Kiddle SJ; Mangino M; Viñuela A; Psatha M; Steves C; Sattlecker M; Buil A; Newhouse S; Nelson S; Williams S; Voyle N; Soininen H; Kloszewska I; Mecocci P; Tsolaki M; Vellas B; Lovestone S; Spector TD; Dobson R; Valdes AM Circulating Proteomic Signatures of Chronological Age. J. Gerontol. Ser. A 2015, 70 (7), 809–816. 10.1093/gerona/glu121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (179).Williams SA; Kivimaki M; Langenberg C; Hingorani AD; Casas JP; Bouchard C; Jonasson C; Sarzynski MA; Shipley MJ; Alexander L; Ash J; Bauer T; Chadwick J; Datta G; DeLisle RK; Hagar Y; Hinterberg M; Ostroff R; Weiss S; Ganz P; Wareham NJ Plasma Protein Patterns as Comprehensive Indicators of Health. Nat. Med 2019, 25 (12), 1851–1857. 10.1038/s41591-019-0665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (180).Sathyan S; Ayers E; Gao T; Weiss EF; Milman S; Verghese J; Barzilai N Plasma Proteomic Profile of Age, Health Span, and All-Cause Mortality in Older Adults. Aging Cell 2020, e13250. 10.1111/acel.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (181).Tanaka T; Basisty N; Fantoni G; Candia J; Moore AZ; Bioancotto A; Schilling B; Bandinelli S; Ferrucci L Plasma Proteomic Biomarker Signature of Age Predicts Health and Life Span. eLife 2020, 9. 10.7554/eLife.61073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (182).Lehallier B; Gate D; Schaum N; Nanasi T; Lee SE; Yousef H; Moran Losada P; Berdnik D; Keller A; Verghese J; Sathyan S; Franceschi C; Milman S; Barzilai N; Wyss-Coray T Undulating Changes in Human Plasma Proteome Profiles across the Lifespan. Nat. Med 2019, 25 (12), 1843–1850. 10.1038/s41591-019-0673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (183).Lehallier B; Shokhirev MN; Wyss-Coray T; Johnson AA Data Mining of Human Plasma Proteins Generates a Multitude of Highly Predictive Aging Clocks That Reflect Different Aspects of Aging. Aging Cell 2020, e13256. 10.1111/acel.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (184).Johnson AA; Shokhirev MN; Lehallier B The Protein Inputs of an Ultra-Predictive Aging Clock Represent Viable Anti-Aging Drug Targets. Ageing Res. Rev 2021, 70, 101404. 10.1016/j.arr.2021.101404. [DOI] [PubMed] [Google Scholar]
- (185).Johnson AA; Shokhirev MN; Wyss-Coray T; Lehallier B Systematic Review and Analysis of Human Proteomics Aging Studies Unveils a Novel Proteomic Aging Clock and Identifies Key Processes That Change with Age. Ageing Res. Rev 2020, 60, 101070. 10.1016/j.arr.2020.101070. [DOI] [PubMed] [Google Scholar]
- (186).Santos-Lozano A; Valenzuela PL; Llavero F; Lista S; Carrera-Bastos P; Hampel H; Pareja-Galeano H; Gálvez BG; López JA; Vázquez J; Emanuele E; Zugaza JL; Lucia A Successful Aging: Insights from Proteome Analyses of Healthy Centenarians. Aging 2020, 12 (4), 3502–3515. 10.18632/aging.102826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (187).Ye S; Ma L; Zhang R; Liu F; Jiang P; Xu J; Cao H; Du X; Lin F; Cheng L; Zhou X; Shi Z; Liu Y; Huang Y; Wang Z; Li C Plasma Proteomic and Autoantibody Profiles Reveal the Proteomic Characteristics Involved in Longevity Families in Bama, China. Clin. Proteomics 2019, 16, 22. 10.1186/s12014-019-9242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (188).Anderson NL; Ptolemy AS; Rifai N The Riddle of Protein Diagnostics: Future Bleak or Bright? Clin. Chem 2013, 59 (1), 194–197. 10.1373/clinchem.2012.184705. [DOI] [PubMed] [Google Scholar]
- (189).Geyer PE; Mann SP; Treit PV; Mann M Plasma Proteomes Can Be Re-Identifiable and Potentially Contain Personally Sensitive and Incidental Findings. Mol. Cell. Proteomics MCP 2021, 100035. 10.1074/mcp.RA120.002359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (190).Geyer PE; Wewer Albrechtsen NJ; Tyanova S; Grassl N; Iepsen EW; Lundgren J; Madsbad S; Holst JJ; Torekov SS; Mann M Proteomics Reveals the Effects of Sustained Weight Loss on the Human Plasma Proteome. Mol. Syst. Biol 2016, 12 (12), 901. 10.15252/msb.20167357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (191).Mann SP; Treit PV; Geyer PE; Omenn GS; Mann M Ethical Principles, Constraints and Opportunities in Clinical Proteomics. Mol. Cell. Proteomics MCP 2021, 100046. 10.1016/j.mcpro.2021.100046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (192).Price ND; Magis AT; Earls JC; Glusman G; Levy R; Lausted C; McDonald DT; Kusebauch U; Moss CL; Zhou Y; Qin S; Moritz RL; Brogaard K; Omenn GS; Lovejoy JC; Hood L A Wellness Study of 108 Individuals Using Personal, Dense, Dynamic Data Clouds. Nat. Biotechnol 2017, 35 (8), 747–756. 10.1038/nbt.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (193).Dodig-Crnković T; Hong M-G; Thomas CE; Häussler RS; Bendes A; Dale M; Edfors F; Forsström B; Magnusson PKE; Schuppe-Koistinen I; Odeberg J; Fagerberg L; Gummesson A; Bergström G; Uhlén M; Schwenk JM Facets of Individual-Specific Health Signatures Determined from Longitudinal Plasma Proteome Profiling. EBioMedicine 2020, 57, 102854. 10.1016/j.ebiom.2020.102854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (194).Tebani A; Gummesson A; Zhong W; Koistinen IS; Lakshmikanth T; Olsson LM; Boulund F; Neiman M; Stenlund H; Hellström C; Karlsson MJ; Arif M; Dodig-Crnković T; Mardinoglu A; Lee S; Zhang C; Chen Y; Olin A; Mikes J; Danielsson H; von Feilitzen K; Jansson P-A; Angerås O; Huss M; Kjellqvist S; Odeberg J; Edfors F; Tremaroli V; Forsström B; Schwenk JM; Nilsson P; Moritz T; Bäckhed F; Engstrand L; Brodin P; Bergström G; Uhlen M; Fagerberg L Integration of Molecular Profiles in a Longitudinal Wellness Profiling Cohort. Nat. Commun 2020, 11 (1), 4487. 10.1038/s41467-020-18148-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (195).Critselis E Impact of the General Data Protection Regulation on Clinical Proteomics Research. Proteomics Clin. Appl 2019, 13 (2), e1800199. 10.1002/prca.201800199. [DOI] [PubMed] [Google Scholar]
- (196).Bandeira N; Deutsch EW; Kohlbacher O; Martens L; Vizcaíno JA Data Management of Sensitive Human Proteomics Data: Current Practices, Recommendations, and Perspectives for the Future. Mol. Cell. Proteomics 2021, 20, 100071. 10.1016/j.mcpro.2021.100071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (197).Yu W; Hurley J; Roberts D; Chakrabortty SK; Enderle D; Noerholm M; Breakefield XO; Skog JK Exosome-Based Liquid Biopsies in Cancer: Opportunities and Challenges. Ann. Oncol 2021, 32 (4), 466–477. 10.1016/j.annonc.2021.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (198).Deutsch EW; Perez-Riverol Y; Carver J; Kawano S; Mendoza L; Van Den Bossche T; Gabriels R; Binz P-A; Pullman B; Sun Z; Shofstahl J; Bittremieux W; Mak TD; Klein J; Zhu Y; Lam H; Vizcaíno JA; Bandeira N Universal Spectrum Identifier for Mass Spectra. Nat. Methods 2021, 18 (7), 768–770. 10.1038/s41592-021-01184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (199).Schaeffer M; Gateau A; Teixeira D; Michel P-A; Zahn-Zabal M; Lane L The NeXtProt Peptide Uniqueness Checker: A Tool for the Proteomics Community. Bioinforma. Oxf. Engl 2017, 33 (21), 3471–3472. 10.1093/bioinformatics/btx318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (200).Mendoza L; Deutsch EW; Sun Z; Campbell DS; Shteynberg DD; Moritz RL Flexible and Fast Mapping of Peptides to a Proteome with ProteoMapper. J. Proteome Res 2018, 17 (12), 4337–4344. 10.1021/acs.jproteome.8b00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (201).Prassas I; Chrystoja CC; Makawita S; Diamandis EP Bioinformatic Identification of Proteins with Tissue-Specific Expression for Biomarker Discovery. BMC Med. 2012, 10, 39. 10.1186/1741-7015-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (202).Zhou Y; Zhou B; Pache L; Chang M; Khodabakhshi AH; Tanaseichuk O; Benner C; Chanda SK Metascape Provides a Biologist-Oriented Resource for the Analysis of Systems-Level Datasets. Nat. Commun 2019, 10 (1), 1523. 10.1038/s41467-019-09234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (203).Afgan E; Baker D; Batut B; van den Beek M; Bouvier D; Cech M; Chilton J; Clements D; Coraor N; Grüning BA; Guerler A; Hillman-Jackson J; Hiltemann S; Jalili V; Rasche H; Soranzo N; Goecks J; Taylor J; Nekrutenko A; Blankenberg D The Galaxy Platform for Accessible, Reproducible and Collaborative Biomedical Analyses: 2018 Update. Nucleic Acids Res. 2018, 46 (W1), W537–W544. 10.1093/nar/gky379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (204).Nguyen L; Brun V; Combes F; Loux V; Vandenbrouck Y Designing an In Silico Strategy to Select Tissue-Leakage Biomarkers Using the Galaxy Framework. Methods Mol. Biol. Clifton NJ 2019, 1959, 275–289. 10.1007/978-1-4939-9164-8_18. [DOI] [PubMed] [Google Scholar]
- (205).Vandenbrouck Y; Christiany D; Combes F; Loux V; Brun V Bioinformatics Tools and Workflow to Select Blood Biomarkers for Early Cancer Diagnosis: An Application to Pancreatic Cancer. Proteomics 2019, 19 (21–22), e1800489. 10.1002/pmic.201800489. [DOI] [PubMed] [Google Scholar]
- (206).Menon R; Roy A; Mukherjee S; Belkin S; Zhang Y; Omenn GS Functional Implications of Structural Predictions for Alternative Splice Proteins Expressed in Her2/Neu–Induced Breast Cancers. J. Proteome Res 2011, 10 (12), 5503–5511. 10.1021/pr200772w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (207).Zhou X; Li Y; Zhang C; Zheng W; Zhang G; Zhang Y Progressive and Accurate Assembly of Multi-Domain Protein Structures from Cryo-EM Density Maps. BioRxiv Prepr. Serv. Biol 2020, 2020.10.15.340455. 10.1101/2020.10.15.340455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (208).Lun X-K; Bodenmiller B Profiling Cell Signaling Networks at Single-Cell Resolution. Mol. Cell. Proteomics 2020, 19 (5), 744–756. 10.1074/mcp.R119.001790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (209).ENCODE Project Consortium; Moore JE; Purcaro MJ; Pratt HE; Epstein CB; Shoresh N; Adrian J; Kawli T; Davis CA; Dobin A; Kaul R; Halow J; Van Nostrand EL; Freese P; Gorkin DU; Shen Y; He Y; Mackiewicz M; Pauli-Behn F; Williams BA; Mortazavi A; Keller CA; Zhang X-O; Elhajjajy SI; Huey J; Dickel DE; Snetkova V; Wei X; Wang X; Rivera-Mulia JC; Rozowsky J; Zhang J; Chhetri SB; Zhang J; Victorsen A; White KP; Visel A; Yeo GW; Burge CB; Lécuyer E; Gilbert DM; Dekker J; Rinn J; Mendenhall EM; Ecker JR; Kellis M; Klein RJ; Noble WS; Kundaje A; Guigó R; Farnham PJ; Cherry JM; Myers RM; Ren B; Graveley BR; Gerstein MB; Pennacchio LA; Snyder MP; Bernstein BE; Wold B; Hardison RC; Gingeras TR; Stamatoyannopoulos JA; Weng Z Expanded Encyclopaedias of DNA Elements in the Human and Mouse Genomes. Nature 2020, 583 (7818), 699–710. 10.1038/s41586-020-2493-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (210).Omenn GS; Magis AT; Price ND; Hood LE Personal Dense Dynamic Data Clouds Connect Systems Bio-Medicine to Scientific Wellness. In Systems Medicine; Springer Nature Methods in Molecular Biology series; 2021; p in press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Union of proteins in both the plasma and blood extracellular vesicle PeptideAtlas builds, along with the crudely estimated log10 abundances and functional annotations.
Supplementary Table S2. Top 10 most abundant proteins in the EV build without a detection in the plasma build. Additional information about the proteins and their RNA expression were derived for the Human Protein.


