Abstract
Background
Clinical practice guidelines discourage routine preoperative screening tests for patients undergoing low-risk procedures. This study sought to determine the frequency and costs of potentially low-value preoperative screening tests in Veterans Health Administration (VA) patients undergoing low-risk procedures.
Methods
Using the VA Corporate Data Warehouse, we identified Operative Stress Score class 1 procedures (“very minor”) performed without general anesthesia in VA during fiscal year 2019 and calculated the overall national and facility-level rates and costs of nine common tests received in the 30 preoperative days. Patient factors associated with receiving at least one screening test, and the number of tests received, were examined.
Results
Eighty-six thousand three hundred twenty-seven of 178,775 low-risk procedures (49.3%) were preceded by 321,917 potentially low-value screening tests representing $11,505,170 using Medicare average costs. Complete blood count was the most common (33.2% of procedures), followed by basic metabolic profile (32.0%), urinalysis (26.3%), electrocardiography (18.9%), and pulmonary function test (12.4%). Older age, female sex, Black race, and having more comorbidities were associated with higher odds of low-value testing. Transthoracic echocardiogram occurred prior to only 4.5% of the procedures but accounted for 47.8% of the total costs ($5,499,860). In 129 VA facilities, the facility-level proportion of procedures preceded by at least one test ranged from 0 to 81.2% and facility-level costs ranged from $0 to $388,476.
Conclusions
Routine preoperative screening tests for very low-risk procedures are common and costly in some VA facilities. These results highlight a potential target to improve quality and value by reducing unnecessary care. Measures of low-value perioperative care could be integrated into VA’s extensive quality monitoring and improvement infrastructure.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13741-022-00265-0.
Keywords: Preoperative testing, Surgical care, Value, Efficiency, Implementation
Background
Screening tests prior to surgical and other medical procedures are justified if they produce actionable data that might alter clinical management or patient outcomes. However, for patients undergoing low-risk procedures, preoperative screening tests often do not change clinical management, sometimes lead to unnecessary follow-up testing and interventions, and can cause delays in receiving surgery (Schein et al. 2000; Lira et al. 2001; Cavallini et al. 2004; Keay et al. 2012; Saver 2015; Kirkham et al. 2016; Kirkham et al. 2015). To address this problem, the American Society of Anesthesiologists’ (ASA) Choosing Wisely Top-5 list of activities to avoid states: “Don’t obtain baseline laboratory studies in patients without significant systemic disease (ASA I or II) undergoing low-risk surgery - specifically complete blood count, basic or comprehensive metabolic panel, coagulation studies when blood loss (or fluid shifts) is/are expected to be minimal.” (Onuoha et al. 2014) National and international healthcare agencies have also issued similar guidance to avoid preoperative screening tests for low-risk procedures (Balk et al. 2014; National Institute for Health and Care Excellence 2016; Fleisher et al. 2015).
The existence of prominent guidance to avoid preoperative screening tests for low-risk procedures does not mean testing is never justified. For patients with infrequent contact with the healthcare system, surgical procedures can sometimes serve as opportunities to provide overdue screening and preventive care (Hambright et al. 2016; Wilson et al. 2012). For some patients with certain comorbidities or frailty, there may be anesthetic risks that justify testing even for low-risk procedures (Chassot et al. 2002). Thus, the recommendations to avoid preoperative testing for low-risk procedures should be interpreted to mean that testing should not be routine for all patients, not that testing should never be done. Nonetheless, except for these relatively rare situations, preoperative screening tests performed prior to minor surgery are often low value and should be avoided.
Even though guidance to avoid preoperative testing has been available for years, low-value preoperative tests continue to be a common and major contributor to unnecessary health care spending in the USA and Canada (Kirkham et al. 2016; Kirkham et al. 2015; Onuoha et al. 2015; Mafi et al. 2017; Schwartz et al. 2014). We previously found that 47.0% of generally healthy patients (ASA-PS I or II) undergoing a carpal tunnel release in the Veterans Health Administration (VA), the largest integrated healthcare system in the USA, had at least one low-value preoperative screening test within 30 days before surgery (Harris et al. 2019). We also found that 49% of 50,106 cataract surgeries performed in VA in 2017 were preceded by one or more preoperative screening test with an overall annual cost of $2.6 million (Mudumbai et al. 2021). In each of these studies, substantial variability existed between facilities, with some ordering almost zero tests and others routinely ordering tests for almost all patients undergoing these procedures. Although these studies highlight the need to better understand the drivers of low-value preoperative testing and what strategies might be used to improve practice, the data only involve two common procedures. It is unknown if these patterns of low-value testing generalize to a variety of other minor procedures.
To address this gap, this study had the following goals: (1) determine the overall and facility-level rates of receiving any of nine common low-value preoperative tests in the 30 days prior to any Operative Stress Score (OSS) I (“very minor”) procedures performed without general anesthesia in the VA in fiscal year 2019; (2) examine the patient factors that are associated with receiving at least one low-value test and the number of tests received; and (3) estimate the overall and facility-level costs of low-value preoperative testing. Knowing more about the overall burden of potentially unnecessary preoperative testing, as well as associated patient factors, might inform and motivate the development of interventions to reduce low-value care, especially in locations where the burden is highest.
Methods
Data source and cohort
All data were derived from the VA Corporate Data Warehouse (CDW), a nationwide database of all VA healthcare records. The cohort consisted of VA patients undergoing low-risk procedures in fiscal year 2019. More than one procedure per patient was included if the surgery dates were separated by at least 30 days.
Defining low-risk procedures
The expanded Operative Stress Score (OSS) was developed to classify 5753 Category I Current Procedural Terminology (CPT) codes into five categories of physiologic stress (Yan et al. 2021; Shinall Jr et al. 2020). OSS 1-5 procedures are termed very low stress, low stress, moderate stress, high stress, and very high stress, respectively. The methodology of the expanded OSS and mapping of CPT codes to OSS categories are presented in the supplemental material of Yan et al. (Yan et al. 2021) OSS 1 includes 463 CPT codes for very low-stress procedures (e.g., wrist ganglion cyst excision, fasciotomy of foot and/or toe, carpal tunnel release). Due to the inherent risk of more sedating anesthesia that might justify use of screening tests, we excluded procedures performed with general anesthesia and included only those performed with monitored anesthesia care, spinal or regional block, or local anesthesia.
Defining preoperative screening tests
Preoperative tests were identified using CPT codes recorded in the CDW in the 30 days before the OSS1 procedure. Tests included complete blood count (CBC); basic metabolic panel (BMP); coagulation tests; urinalysis; electrocardiography (EKG); pulmonary function tests (PFT); trans-thoracic echocardiograms (TTE); cardiac stress tests, and chest x-rays. Tests were excluded if they occurred within 30 days prior to an OSS 2–5 procedure that may have justified it.
Patient characteristics
Patient characteristics included age at time of the procedure, gender, race/ethnicity, marital status, service connection status (a measure of socio-economic status and medical need tied to military service), and 30 diagnoses included in the Elixhauser Comorbidity Index (Southern et al. 2004) recorded in the preoperative year.
Estimating costs
To provide an approximation of the financial impact of testing, Centers for Medicare Services (CMS) reimbursement Fee Schedule for physician fees, facility fees, and the CMS clinical laboratory fee schedule for 2019 were used to assign a cost to each preoperative screening test.
Statistical analysis
Overall and facility-level rates of OSS 1 procedures that were preceded by at least one low-value test were calculated. Rates of OSS 1 procedures preceded by each of the nine tests were also calculated. Mixed-effects logistic regression was used to examine associations between patient characteristics (e.g., demographics, comorbidities) and receipt of at least one low-value test, with random intercepts for the VA facility where the procedure was performed (Pinheiro and Bates 2000). Mixed-effects negative binomial regression was used to examine associations between the same patient, procedure, and facility characteristics and the number of low-value tests received. Odds ratios, 95% confidence intervals, and p values were produced for all regression model coefficients.
Results
In fiscal year 2019, 148,728 VA patients received 178,775 OSS I procedures without general anesthesia, of which 86,327 (49.3%) involved at least one screening test in the 30 preoperative days. Among the 44,545 OSS I procedures that were excluded involving general anesthesia, 82.5% were preceded by at least one screening test. Characteristics of the cohort, stratified by receipt of any preoperative screening test, are presented in Table 1.
Table 1.
Characteristics of Veterans Health Administration patient receiving OSS 1 procedures without general anesthesia in fiscal year 2019
| Patient characteristics (%) | Any preop-test | ||
|---|---|---|---|
| No 92,448 (51.7) |
Yes 86,327 (48.3) |
Total 178,775a |
|
| Age, mean (SD) | 65.5 (15.0) | 68.3 (12.3) | 66.9 (13.8) |
| Gender, No. (%) | |||
| Male | 83,029 (50.9) | 80,236 (49.1) | 163,265 (91.3) |
| Female | 9419 (60.7) | 6091 (39.3) | 15,510 (8.7) |
| Race/ethnicity, No. (%) | |||
| Native American | 676 (53.9) | 579 (46.1) | 1254 (0.7) |
| Asian | 663 58.0) | 480 (42.0) | 1143 (0.6) |
| Black | 16,371 (49.4) | 16,789 (50.6) | 33,160 (18.5) |
| Hawaiian, Pacific Islander | 724 (50.8) | 700 (49.2) | 1424 (0.8) |
| White | 69,240 (52.1) | 63,662 (47.9) | 132,902 (74.3) |
| Missing | 4774 (53.7) | 4118 (46.3) | 8892 (5.0) |
| Marital status, No. (%) | |||
| Married | 47,703 (51.9) | 44,263 (48.1) | 91,966 (51.4) |
| Not married | 44,745 (51.5) | 42,064 (48.5) | 86,806 (48.6) |
| Service connection | |||
| Less than 50% | 52,707 (51.6) | 49,358 (48.4) | 102,065 (57.1) |
| More than 50% | 39,741 (51.8) | 36,969 (48.2) | 76,710 (42.9) |
| Past year elixhauser comorbidities, No. (%) | |||
| Congestive heart failure | 6732 (31.4) | 14,728 (68.6) | 21,460 (12.0) |
| AIDS | 495 (39.1) | 772 (60.9) | 1267 (0.7) |
| Cardiac arrhythmia | 13,972 (37.4) | 23,340 (62.6) | 37,312 (20.9) |
| Pulmonary circulation disorders | 1631 (28.1) | 4183 (71.9) | 5814 (3.2) |
| Peripheral vascular disease | 10,434 (39.2) | 16,197 (60.8) | 26,631 (14.9) |
| Hypertension | 49,644 (46.2) | 57,685 (53.7) | 107,329 (60.0) |
| Hypertension with complications | 8221 (31.4) | 17,926 (68.6) | 26,147 (14.6) |
| Paralysis | 1587 (42.8) | 2123 (57.2) | 3710 (2.1) |
| Other neurological disorders | 5821 (40.0) | 8766 (60.0) | 14,587 (8.2) |
| Chronic pulmonary disease | 17243 (41.1) | 24,665 (28.9) | 41,908 (23.4) |
| Diabetes mellitus | 22,651 (43.8) | 29,070 (56.2) | 51,721 (28.9) |
| Diabetes mellitus with complications | 17,726 (40.5) | 26,055 (59.5) | 43,781 (24.5) |
| Hypothyroidism | 6928 (44.4) | 8664 (55.6) | 15,592 (8.7) |
| Renal failure | 10,285 (33.5) | 20,390 (66.5) | 30,675 (17.2) |
| Liver disease | 5815 (38.0) | 9479 (62.0) | 15,294 (8.6) |
| Peptic ulcer | 502 (32.1) | 1064 (67.9) | 1566 (0.9) |
| Lymphoma | 748 (24.5) | 2309 (75.5) | 3057 (1.7) |
| Metastatic cancer | 1506 (21.9) | 5379 (78.1) | 6885 (3.9) |
| Solid tumor w/o metastasis | 25,998 (47.7) | 28,483 (52.3) | 54,481 (30.5) |
| Rheumatoid arthritis | 2191 (42.9) | 2912 (57.1) | 5103 (2.9) |
| Coagulopathy | 1950 (27.9) | 5040 (72.1) | 6990 (3.9) |
| Obesity | 15,790 (46.2) | 18,363 (53.8) | 34,153 (19.1) |
| Weight loss | 2912 (29.6) | 6932 (70.4) | 9844 (5.5) |
| Fluid and electrolyte disorders | 6378 (26.8) | 17,383 (73.2) | 23,761 (13.3) |
| Blood loss anemia | 731 (25.9) | 2087 (74.1) | 2818 (1.6) |
| Deficiency anemia | 4904 (32.9) | 9998 (67.1) | 14,902 (8.2) |
| Alcohol abuse | 2257 (35.4) | 4114 (64.6) | 6371 (3.6) |
| Drug abuse | 4445 (45.8) | 5253 (54.1) | 9698 (5.4) |
| Psychosis | 1602 (44.0) | 2040 (56.0) | 3642 (2.0) |
| Depression | 22,404 (48.0) | 24,295 (52.0) | 46,699 (26.1) |
aTotal represents 178,775 procedures (separated by at least 30 days) in 148,728 patients.
As presented in Table 2, complete blood count was the most common (33.2% of procedures), followed by basic metabolic profile (32.0%), urinalysis (26.3%), electrocardiogram (18.9%), and pulmonary function test (12.4%). The least common screening tests were cardiac stress tests (2.0% of procedures), coagulation tests (<1.0%), and chest x-rays (0.0%). Overall, we identified 321,917 preoperative screening tests in the 30 days prior to the 178,775 OSS 1 procedures, representing $11,505,170 in Medicare Average Costs. Transthoracic echocardiogram occurred prior to only 4.5% of the procedures but accounted for 47.8% of the total costs ($5,499,860).
Table 2.
Low-value preoperative testing prior to 178,775 OSS 1 procedures in Veterans Health Administration during fiscal year 2019
| Preoperative test | Percent received >=1 tests | Total number | Total cost |
|---|---|---|---|
| Complete blood count | 33.2% | 77,497 | $805,438 |
| Basic metabolic profile | 32.0% | 70,240 | $912,317 |
| Urinalysis | 26.3% | 55,122 | $200,930 |
| Electrocardiography | 18.9% | 54,310 | $742,460 |
| Pulmonary function tests | 12.4% | 52,311 | $852,580 |
| Transthoracic echocardiogram | 4.5% | 8424 | $5,499,860 |
| Cardiac stress test | 2.0% | 3759 | $2,490,125 |
| Coagulation test | <1% | 254 | $1458 |
| Chest x-ray | 0% | 0 | 0 |
| Total | 48.3% | 321,917 | $11,505,170 |
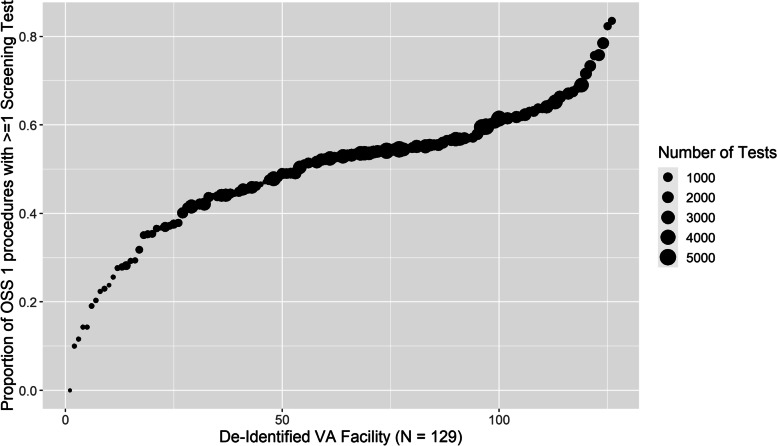
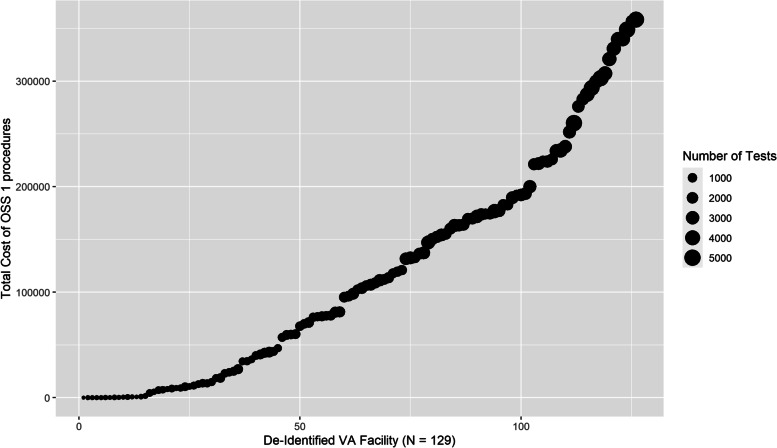
In 129 VA facilities, the facility-level proportion of low-risk procedures preceded by at least one test ranged from 0 to 81.2% (Fig. 1), and facility-level total costs ranged from $0 to $388,476 with a median total cost of $69,786 (Fig. 2). The top quartile of facilities with the highest testing cost accounted for 57% of total costs. In mixed effect logistic regression (Table 3), older age, being female, not married, Black, or having service-connected status greater than 50% were associated with higher odds of preoperative testing. Every comorbidity in the Elixhauser Comorbidity Index except peptic ulcer was associated with higher odds of testing. The intraclass correlation coefficient (ICC)—the proportion of outcome variance attributable to facility rather than patient factors—was 0.189. The beta-binomial regression model examining factors associated with the number of preoperative tests received found the same pattern of results (Supplemental Table 1) and had an ICC = 0.02.
Fig. 1.
The proportion of OSS 1 procedures proceeded by at least 1 low-value test in 129 VA facilities
Fig. 2.
Total cost low-value screening test prior to OSS 1 procedures in 129 VA facilities
Table 3.
Patient characteristics associated with odds of receiving at least one preoperative screening test
| Factor | OR | LL | UL | p value |
|---|---|---|---|---|
| Intercept | 0.43 | 0.33 | 0.53 | 0.00 |
| Age | 1.01 | 1.00 | 1.01 | 0.00 |
| Female | 1.06 | 1.02 | 1.10 | 0.00 |
| Not married | 1.03 | 1.01 | 1.05 | 0.01 |
| Service connected >50% | 1.02 | 1.00 | 1.04 | 0.05 |
| Native American (ref = white) | 1.05 | 0.93 | 1.17 | 0.40 |
| Asian | 1.01 | 0.88 | 1.13 | 0.92 |
| Black | 1.04 | 1.01 | 1.07 | 0.00 |
| Hawaiian | 1.07 | 0.95 | 1.18 | 0.26 |
| Race missing | 0.98 | 0.93 | 1.03 | 0.44 |
| Peptic ulcer | 1.08 | 0.96 | 1.20 | 0.19 |
| AIDS | 1.42 | 1.30 | 1.54 | 0.00 |
| Lymphoma | 2.66 | 2.57 | 2.75 | 0.00 |
| Metastatic cancer | 3.42 | 3.36 | 3.48 | 0.00 |
| Solid tumor no metastasis | 0.94 | 0.91 | 0.96 | 0.00 |
| Rheumatoid arthritis | 1.25 | 1.19 | 1.31 | 0.00 |
| Coagulopathy | 1.42 | 1.36 | 1.48 | 0.00 |
| Obesity | 1.09 | 1.06 | 1.11 | 0.00 |
| Weight loss | 1.41 | 1.36 | 1.46 | 0.00 |
| Fluid and electrolyte disorders | 1.69 | 1.66 | 1.73 | 0.00 |
| Blood loss anemia | 1.39 | 1.29 | 1.48 | 0.00 |
| Deficiency anemia | 1.19 | 1.14 | 1.23 | 0.00 |
| Alcohol abuse | 1.12 | 1.06 | 1.18 | 0.00 |
| Drug abuse | 1.06 | 1.02 | 1.11 | 0.01 |
| Psychosis | 1.10 | 1.02 | 1.17 | 0.01 |
| Depression | 1.06 | 1.04 | 1.09 | 0.00 |
| Congestive heart failure | 1.22 | 1.18 | 1.26 | 0.00 |
| Cardiac arrhythmia | 1.32 | 1.29 | 1.34 | 0.00 |
| Valvular disorder | 1.23 | 1.18 | 1.28 | 0.00 |
| Pulmonary circulation disorder | 1.24 | 1.17 | 1.30 | 0.00 |
| Peripheral vascular disorder | 1.07 | 1.04 | 1.10 | 0.00 |
| Hypertension | 1.18 | 1.16 | 1.21 | 0.00 |
| Hypertension with complications | 1.12 | 1.08 | 1.15 | 0.00 |
| Paralysis | 1.19 | 1.12 | 1.27 | 0.00 |
| Other neurological disorder | 1.09 | 1.05 | 1.13 | 0.00 |
| Chronic pulmonary disease | 1.27 | 1.24 | 1.29 | 0.00 |
| Diabetes melitus | 1.07 | 1.04 | 1.10 | 0.00 |
| Diabetes melitus with complications | 1.14 | 1.11 | 1.17 | 0.00 |
| Hypothyroidism | 1.06 | 1.02 | 1.09 | 0.00 |
| Renal failure | 1.37 | 1.33 | 1.40 | 0.00 |
| Liver disease | 1.33 | 1.29 | 1.37 | 0.00 |
Discussion
High-value, patient-centered health care includes selectively ordering preoperative tests that may inform clinical management or improve patient outcomes. Tests unlikely to meet these criteria may cause avoidable harm, inconvenience, and waste of resources that could be used for higher-value services. For years, government healthcare agencies and professional organizations have been recommending the avoidance of routine preoperative testing for low-risk procedures (Balk et al. 2014; National Institute for Health and Care Excellence 2016; Fleisher et al. 2015). However, we found that almost half of OSS 1 procedures received by VA patients in FY19 were preceded by at least one potentially low-value preoperative test. Using Medicare Average Costs, we estimated that the 321,917 preoperative screening tests prior to OSS 1 procedures may represent up to $11,505,170 in low-value care.
As noted, some portion of this testing may represent high-quality care (e.g., opportunities for overdue screening) or tests that are unrelated to the OSS1 procedures. If the distribution of testing was uniformly modest (e.g., 5 or 10%), the case for investments in quality improvement might be easier to dismiss. However, we can see in Figs. 1 and 2 that there exist many VA facilities that routinely test the vast majority of patients prior to low-risk procedures, and 27 facilities with estimated associated costs over $200,000. For these facilities, these results highlight a significant opportunity to improve quality by providing less unnecessary care.
Beyond describing the magnitude and distribution of opportunities for quality improvement, another purpose of this study was to identify the patient characteristics associated with preoperative testing. We found older age, being female, not married, black, and having comorbidities were all associated with higher odds of testing. The large ICC in the model predicting receipt of at least one test (0.189) suggests that much of the variance in preoperative testing is at the facility-level. In other words, the likelihood of getting tested is as much a function of where you are treated as your specific medical profile.
Although excluded from our primary analyses, general anesthesia was received for 16.5% of OSS1 procedures in FY19, of which 82.2% underwent preoperative testing. Anesthetic risk, not just procedural risk, needs to be factored into the decision to order preoperative tests. We previously found that much of the variance in using general anesthesia for a low-risk procedure (carpal tunnel release) is driven by clinician or facility factors rather than patient characteristics or preferences (Harris et al. 2020). Therefore, although preoperative testing may be justified for patients undergoing general anesthesia, there may be quality improvement opportunities in facilities that commonly or routinely use general anesthesia even for low-risk procedures.
Several limitations are worth noting. First, there is no way to be sure that tests in the 30 days prior to OSS1 procedures were ordered for preoperative screening purposes. Some of the tests we identified may be justified by factors independent of the upcoming low-risk procedure. However, we have no reason to expect that such justifications for the tests differ systematically between facilities. In our previous work on cataract and carpal tunnel release surgery, we excluded 10% of preoperative tests because they were not preceded by a ‘plausible ordering visit, such as ophthalmology or anesthesia consult. As we could not implement this methodology in this study due to the diversity of procedures, it is possible that our estimates of low-value testing are 10% too high due to this limitation. Also, it is unknown to what extent these results might generalize outside of the VA system.
Conclusions
In summary, low-value preoperative screening tests for patients undergoing low-risk procedures without general anesthesia appear to be common and costly in the VA system, with the burden of this low-value care concentrated in certain facilities. One obvious way to begin to address the burden of low-value preoperative testing is to develop quality measures of low-value perioperative care that could be integrated into VA’s extensive quality monitoring and improvement infrastructure. By identifying facilities with the highest burden of low-value care, then seeking to identify its root causes, interventions (e.g., educational, informatics, behavioral) can be designed and implemented to improve the quality of care by providing less of it.
Supplementary Information
Additional file 1: Supplemental Table 1. Patient Characteristics Associated with Number of Preoperative Screening Tests Received.
Acknowledgements
The views expressed do not reflect those of the US Department of Veterans Affairs (VA) or other institutions.
Abbreviations
- VA
Veterans Health Administration
- ASA
American Society of Anesthesiologists
- OSS
Operative Stress Score
- CDW
Corporate Data Warehouse
- CPT
Current Procedural Terminology
- CBC
Complete blood count
- BMP
Basic metabolic panel
- EKG
Electrocardiography
- PFT
Pulmonary function tests
- TTE
Trans-thoracic echocardiograms
- CMS
Centers for Medicare Services
- ICC
Intraclass correlation coefficient
- FY
Fiscal year
Authors’ contributions
AH and SM conceived the study. All authors contributed to the study design. AH and TB undertook the data analysis. AH drafted the manuscript which underwent revision by all other authors. The authors read and approved the final manuscript.
Funding
This work was funded by grants from the VA HSR&D Service (IIR 16-216; RCS14-232).
Availability of data and materials
VA patient data are not publicly. Please contact author for aggregate data requests.
Declarations
Ethics approval and consent to participate
The study was approved by the Stanford University Institutional Review Board and the VA Palo Alto Research & Development Committee with a waiver of informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Cavallini GM, Saccarola P, D'Amico R, Gasparin A, Campi L. Impact of preoperative testing on ophthalmologic and systemic outcomes in cataract surgery. Eur J Ophthalmol. 2004;14(5):369–374. doi: 10.1177/112067210401400502. [DOI] [PubMed] [Google Scholar]
- Chassot PG, Delabays A, Spahn DR. Preoperative evaluation of patients with, or at risk of, coronary artery disease undergoing non-cardiac surgery. Br J Anaesth. 2002;89(5):747–759. doi: 10.1093/bja/89.5.747. [DOI] [PubMed] [Google Scholar]
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Developed in collaboration with the American College of Surgeons, American Society of Anesthesiologists, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Anesthesiologists, and Society of Vascular Medicine Endorsed by the Society of Hospital Medicine. J Nucl Cardiol. 2015;22(1):162–215. doi: 10.1007/s12350-014-0025-z. [DOI] [PubMed] [Google Scholar]
- Hambright G, Agrawal V, Sladek PL, Slonim SM, Truitt MS. Acute care surgery: trauma, critical care, emergency general surgery … and preventative health? Am J Surg. 2016;212(5):803–806. doi: 10.1016/j.amjsurg.2016.07.006. [DOI] [PubMed] [Google Scholar]
- Harris AHS, Meerwijk EL, Kamal RN, et al. Variability and costs of low-value preoperative testing for carpal tunnel release surgery. Anesth Analg. 2019;129(3):804–811. doi: 10.1213/ANE.0000000000004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AHS, Meerwijk EL, Kamal RN, et al. Variation in Surgeons' requests for general anesthesia when scheduling carpal tunnel release. Hand (N Y) 2020;15(5):608–614. doi: 10.1177/1558944719828006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay L, Lindsley K, Tielsch J, Katz J, Schein O. Routine preoperative medical testing for cataract surgery. Cochrane Database Syst Rev. 2012;3:CD007293. doi: 10.1002/14651858.CD007293.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham KR, Wijeysundera DN, Pendrith C, et al. Preoperative testing before low-risk surgical procedures. CMAJ. 2015;187(11):E349–E358. doi: 10.1503/cmaj.150174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham KR, Wijeysundera DN, Pendrith C, et al. Preoperative laboratory investigations: rates and variability prior to low-risk surgical procedures. Anesthesiology. 2016;124(4):804–814. doi: 10.1097/ALN.0000000000001013. [DOI] [PubMed] [Google Scholar]
- Lira RP, Nascimento MA, Moreira-Filho DC, Kara-Jose N, Arieta CE. Are routine preoperative medical tests needed with cataract surgery? Revista panamericana de salud publica=Pan Am J Public Health. 2001;10(1):13–17. doi: 10.1590/S1020-49892001000700003. [DOI] [PubMed] [Google Scholar]
- Mafi JN, Russell K, Bortz BA, Dachary M, Hazel WA, Jr, Fendrick AM. Low-cost, high-volume health services contribute the Most to unnecessary health spending. Health Aff (Millwood) 2017;36(10):1701–1704. doi: 10.1377/hlthaff.2017.0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudumbai SC, Pershing S, Bowe T, et al. Variability and costs of low-value preoperative testing for cataract surgery within the veterans health administration. JAMA Netw Open. 2021;4(5):e217470. doi: 10.1001/jamanetworkopen.2021.7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence . Routine preoperative tests for elective surgery. 2016. [PubMed] [Google Scholar]
- Onuoha OC, Arkoosh VA, Fleisher LA. Choosing wisely in anesthesiology: the gap between evidence and practice. JAMA Intern Med. 2014;174(8):1391–1395. doi: 10.1001/jamainternmed.2014.2309. [DOI] [PubMed] [Google Scholar]
- Onuoha OC, Hatch MB, Miano TA, Fleisher LA. The incidence of un-indicated preoperative testing in a tertiary academic ambulatory center: a retrospective cohort study. Perioper Med. 2015;4:14. doi: 10.1186/s13741-015-0023-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D. Mixed effects models in S and S-plus. New York, NY: Springer; 2000. [Google Scholar]
- Saver BG. Preoperative testing in patients undergoing cataract surgery. N Engl J Med. 2015;373(3):285. doi: 10.1056/NEJMc1506125. [DOI] [PubMed] [Google Scholar]
- Schein OD, Katz J, Bass EB, et al. The value of routine preoperative medical testing before cataract surgery. Study of medical testing for cataract surgery. N Engl J Med. 2000;342(3):168–175. doi: 10.1056/NEJM200001203420304. [DOI] [PubMed] [Google Scholar]
- Schwartz AL, Landon BE, Elshaug AG, Chernew ME, McWilliams JM. Measuring low-value care in Medicare. JAMA Intern Med. 2014;174(7):1067–1076. doi: 10.1001/jamainternmed.2014.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinall MC, Jr, Arya S, Youk A, et al. Association of Preoperative Patient Frailty and Operative Stress with Postoperative Mortality. JAMA Surg. 2020;155(1):e194620. doi: 10.1001/jamasurg.2019.4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern DA, Quan H, Ghali WA. Comparison of the Elixhauser and Charlson/Deyo methods of comorbidity measurement in administrative data. Med Care. 2004;42(4):355–360. doi: 10.1097/01.mlr.0000118861.56848.ee. [DOI] [PubMed] [Google Scholar]
- Wilson H, Bostock N, Phillip N, Shannon P, Payne D, Kennedy A. Opportunistic adolescent health screening of surgical inpatients. Arch Dis Child. 2012;97(10):919–921. doi: 10.1136/archdischild-2012-301835. [DOI] [PubMed] [Google Scholar]
- Balk E, Earley A, Hadar N, Shah N, Trikalinos T. Benefits and harms of routine preoperative testing:comparative effectiveness review no. 130. Rockville: Agency for Healthcare Research and Quality; 2014. [PubMed]
- Yan Q, Kim J, Hall DE, et al. Association of Frailty and the expanded operative stress score with preoperative acute serious conditions, complications and mortality in males compared to females: a retrospective observational study. Ann Surg. 2021. https://pubmed.ncbi.nlm.nih.gov/34183515/. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplemental Table 1. Patient Characteristics Associated with Number of Preoperative Screening Tests Received.
Data Availability Statement
VA patient data are not publicly. Please contact author for aggregate data requests.