INTRODUCTION
Altered mental status describes a nonspecific change in baseline level of awareness, cognition, attention, or consciousness. There are many synonyms for altered mental status, and its imprecision can complicate both communication across providers and review of the literature for workup or management recommendations. Common synonyms for altered mental status include “acute confusional state” or “confusion,” “acute brain failure,” “encephalopathy,” and “disorientation.”1,2 Although often used as a synonym, delirium is a more specific descriptor for an acute, usually fluctuating altered mental status characterized by a decline in attention, and an additional cognitive deficit or altered level of arousal.3 Both altered mental status and delirium serve as important phenotypes but are not in themselves diagnoses. Rather, they are symptoms resulting from a host of illnesses with varying time courses and levels of severity. The differential diagnosis of altered mental status includes conditions that are both life-threatening and reversible, and so a prompt systematic approach to the patient is key. This article provides an advanced overview for the neurologist evaluating and managing hospitalized adults with altered mental status.
EPIDEMIOLOGY
Altered mental status is commonly observed in hospitalized adults. Approximately 5% to 10% of adults presenting to the emergency department exhibit altered mental status, especially older adults, although some studies describe a bimodal age distribution with a high incidence also observed in very young children.4,5 Altered mental status is a common reason for hospital admission and is associated with poor outcomes, including mortality in approximately 10% of patients.6 The substantial adverse outcomes associated with altered mental status are best characterized in studies of older adults with inpatient delirium, which is present in an estimated 10% to 23% of patients admitted to a general medical service, 15% to 50% of postoperative patients, and as many as 85% of patients in the intensive care unit.7–11 However, unless there is a standardized screening protocol, most recognized cases will exhibit agitation, as opposed to hypoactivity, which is a cause for underdiagnosis. Like altered mental status, delirium has traditionally been viewed as a transient condition with a benign prognosis. However, there is a strong body of literature documenting both the protracted course and significant clinical consequences experienced by many with delirium. Delirium is associated with prolonged hospital stay and readmission, loss of independence, and new or accelerated cognitive impairment.12–18 Although delirium may result in a significant physical and psychological cost, it is also associated with a substantial economic burden, with an estimated attributable cost of $38 billion to $152 billion annually in the United States alone, or more than $182 billion annually in a combined population of 18 European countries.9,19
Although the need for swift identification and management of altered mental status is clear, complete understanding of its epidemiology is limited due to underdiagnosis by both physicians and nurses.20,21 For instance, delirium may be unrecognized because of lack of screening, its overlap with cognitive impairment, its fluctuating course, and the expectation that this may be normal behavior for a hospitalized older adult.22 As neurologists, we have the opportunity to identify altered mental status in our patients, especially in patients for whom we are consulted about an issue other than altered mental status, by performing a careful neurologic assessment.
INITIAL EVALUATION
The evaluation of a patient with altered mental status begins with a focused history (Table 1). As the patient may be unable to provide a history, it will likely be necessary to collect collateral information from family, friends, or the primary medical team. The first step is to determine the timeline for the mental status change, and the circumstances surrounding it, such as medication/drug use or trauma. An acute alteration in mental status is a medical emergency that requires a prompt, standardized evaluation. Airway, breathing, and circulation (“ABC’s”) should be assessed in tandem with an updated set of complete vital signs and finger-stick blood glucose. Tachycardia may be a sign of systemic infection, pulmonary embolism, or atrial fibrillation with rapid ventricular rate. Hypoxemia and fever can each result in altered mental status and both shape the differential. Naloxone should be administered if there is a high index of suspicion for opiate overdose, which should not be overlooked in postoperative and hospitalized patients. If hypoglycemic, glucose should be administered, but only after thiamine supplementation to reduce the risk for developing Wernicke encephalopathy.
Table 1.
Initial approach to altered mental status
| Initial evaluation | Vital signs; airway, breathing, and circulation Blood glucose level Consider naloxone Electrolyte panel, including sodium, potassium, chloride, bicarbonate, calcium, magnesium, phosphorus Complete blood count Urine toxicology Renal function tests Liver function tests including albumin Urine analysis with culture Chest radiograph Focused history: baseline cognitive function, time course Physical examination, looking especially for focal neurologic deficits |
| Subsequent evaluation | Brain imaging with computed tomography (CT) and/or MRI Lumbar puncture (performed after CT; should be performed initially if high suspicion for infection) Serum ammonia, thyroid function tests, vitamin B12 Autoimmune serologies Blood cultures Electroencephalography (should be performed initially if high suspicion of status epilepticus) |
A focused neurologic examination, discussed in more detail later in this article, may reveal a focal finding that will guide initial management. An adult with acute altered mental status will frequently present as a stroke activation, in which case a National Institutes of Health Stroke Scale will be performed followed by a decision regarding acute stroke management. However, a general physical examination and detailed neurologic examination should follow, as described later in this article.
Additional tests should be obtained in tandem with the initial assessment. These include a complete blood count and comprehensive metabolic panel to look for aberrances in electrolytes, especially sodium, calcium, and magnesium, as well as renal and liver function. An arterial blood gas may be necessary if there is concern for hypoxemia or hypercarbia. Troponin and electrocardiogram should be considered to rule out myocardial infarction. Human immunodeficiency virus may be tested early to stratify immune status. Urine should be collected to look for infection and recreational substances; a postvoid residual can be measured if there is concern for urinary retention. A nasopharyngeal swab for Severe Acute Respiratory Syndrome Coronavirus 2 infection and a chest radiograph may be helpful if there is concern for lung infection.
History
Additional pieces of the history can be collected once the patient is stabilized. In addition to timeline, it is important to understand the patient’s baseline cognitive function and the trajectory of the altered mental status; for example, does the mental status fluctuate (and if so, are there periods when the patient is close to baseline), or is the patient persistently altered? A fluctuating mental status oscillating between being altered and being at baseline may be more likely to be due to sleep deprivation or medications, whereas persistently altered mental status that may fluctuate but not return to baseline, may represent a more static process, such as an infection or stroke. Premorbid cognitive dysfunction is an important risk factor for the development of altered mental status, especially delirium, and may consist of features that can mimic delirium, such as hallucinations in Lewy body dementia. A wide array of comorbid medical conditions may also contribute to altered mental status, including epilepsy, known structural brain abnormalities (eg, tumor, ventriculoperitoneal shunt), chronic kidney disease, and liver disease. Immune status should be investigated both in medical history and medication review. New or recently discontinued medications or herbal supplements, and recreational substance use may also provide clues to the cause of altered mental status. A detailed review of systems should focus on symptoms referable to a systemic infection, such as headache, stiff neck, fever, cough, shortness of breath, or dysuria. Because history and review of systems may be limited, a through physical examination, in addition to a targeted battery of laboratory tests or imaging, will likely be warranted.
Physical Examination
The systematic general physical examination may offer clues to non-neurological causes of altered mental status and is crucial for guiding a targeted workup. One should examine the head and neck for evidence of trauma, tenderness, and meningismus. The cardiac examination should include a volume status assessment with inspection of jugular venous pulsations and extremities, and auscultation of the heart to assess for murmurs that may hint at endocarditis. Auscultation of the lungs may reveal evidence of volume overload or infection. Palpation of the back may cause point tenderness over the spinous processes, as in epidural abscesses, or over the kidneys, as in pyelonephritis. An abdominal examination may demonstrate ascites or tenderness. A careful skin examination may reveal stigmata of injection drug use or liver disease, or rash.
The primary goal of the neurologic evaluation is to assess for focal abnormalities. The mental status examination begins during the initial assessment of the patient, which may reveal the patient’s level of alertness, attention, and thought organization through the course of obtaining the history and initial general physical examination. Although altered mental status may be viewed as a global cerebral dysfunction, the neurologist can identify underlying focal pathology that may be missed by the non-neurologist. Focal lesions, such as from stroke or tumor, may result in Wernicke aphasia (dominant superior temporal gyrus), abulia (frontal lobe, anterior cingulate cortex, basal ganglia), agitation (nondominant parietal lobe), and coma (reticular activating system). Lesions in these locations often produce colocalizing signs, such as a homonymous hemianopsia, pyramidal weakness, neglect, or loss of brainstem reflexes, respectively. Although most patients with acute altered mental status do not have stroke, altered mental status is common in patients with stroke: one recent meta-analysis found that delirium occurred in 25% of patients within 6 weeks of a stroke.23
The mental status examination may result in a clinical diagnosis of delirium, although the remainder of the examination and workup must be completed to uncover its etiology. The mental status examination may include targeted delirium assessments using validated scales. The “gold standard” research criteria for a diagnosis of delirium are found in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM5).24 One of the most widely used and validated, and simpler, scales is the Confusion Assessment Method (CAM), which includes 4 fundamental features of delirium: (1) acute onset and fluctuating course, (2) inattention, and either (3) disorganized thinking, or (4) altered level of consciousness.25 Compared with the DSM, this scale has a high specificity for delirium diagnosis, is relatively efficient, and can be administered by a variety of health care professionals. The CAM has several adaptations, including for intensive care unit (ICU) patients (CAM-ICU).14 Additional commonly used scales for non-ICU patients include the Delirium Rating Scale, Delirium Observation Screening Scale, and the Nursing Delirium Screening Scale.26,27 Each scale has advantages and disadvantages, including complexity and requirement for training.28 One recently developed delirium screening instrument that is brief and does not require additional training is the 4AT, which assesses for alertness, acute/fluctuating course, orientation, and attention (reciting the months of the year backward).29 Not only are these scales useful to confirm the phenotype of delirium, they can also be used as daily screening tests for all hospitalized patients.
In the process of completing a thorough neurologic examination on a patient with altered mental status, one should remain vigilant for several key features associated with altered mental status. Thiamine deficiency may result in gaze palsies, nystagmus, and ataxia. Myoclonus or asterixis may be caused by uremia, hyperammonemia, or an offending medication (eg, cefepime, benzodiazepines). One should also evaluate for evidence of parkinsonism, which may suggest an underlying neurodegenerative disease such as Parkinson disease or Lewy body dementia. Identifying a focal deficit will guide subsequent management, which must include neuroimaging if a focal deficit is found, as well as consideration of blood tests, lumbar puncture, and/or electroencephalogram (EEG).
SUBSEQUENT EVALUATION
If the cause of altered mental status is not found with the initial workup, additional testing may be warranted. Mnemonics exist to organize the many causes of altered mental status, such as “AEIOU TIPS,” and “VITAMIN E.” Here, we will focus on the latter, which stands for: vascular, infectious, traumatic/toxic, autoimmune, metabolic, iatrogenic, neoplastic/neurodegenerative, and epileptic (Table 2).30 As the differential for altered mental status is extensive, the workup can feel like an expensive, potentially harmful fishing expedition. For this reason, a focused algorithmic approach based on clinical suspicion is key.
Table 2.
Common precipitants of altered mental status (“VITAMIN E”)
| Vascular | Stroke (ischemic or hemorrhagic) Subarachnoid hemorrhage Hypertensive emergency and posterior reversible encephalopathy syndrome (PRES) Cerebral amyloid angiopathy (CAA) Vasculitis |
| Infectious | Urinary tract infection Pneumonia Encephalitis, meningitis Sepsis |
| Traumatic | Posttraumatic encephalopathy Subdural hemorrhage |
| Toxic | Intoxication or overdose Withdrawal Medications (prescription, over-the-counter, supplements) |
| Autoimmune | Vasculitis Systemic lupus erythematosus or Sjogren syndrome Steroid-responsive encephalopathy with autoimmune thyroiditis (SREAT), or Hashimoto encephalitis Acute disseminated encephalomyelitis Autoimmune limbic encephalitis |
| Metabolic | Electrolyte abnormalities (eg, hypo/hypernatremia, hypercalcemia, hypermagnesemia) Endocrine abnormalities (eg, hypo/hyperglycemia, hypo/hyperthyroidism, adrenal crisis, Cushing syndrome) Uremic encephalopathy Hepatic encephalopathy Thiamine or cobalamin deficiency Hypoxia, hypercarbia |
| Iatrogenic | Day/night dysregulation, sleep deprivation Sensory deprivation Limited mobility (use of restraints, urinary catheters) Surgery Untreated pain Polypharmacy, especially with certain medications (eg, anticholinergics, antihistamines, benzodiazepines, steroids, fluoroquinolone and cephalosporin antibiotics) |
| Neoplastic | Intracranial neoplasm (primary or metastatic) Paraneoplastic encephalitis Carcinomatous meningitis |
| Neurodegenerative | Alzheimer disease Lewy body dementia Prion disease |
| Epileptic | Nonconvulsive status epilepticus Postictal state |
The next step should be to obtain neuroimaging, especially if there is a focal finding on examination. A noncontrast computed tomography (CT) scan of the brain is an appropriate screen for intracranial hemorrhage, hydrocephalus, or a large mass, and vascular imaging of the head and neck can be included if there is concern for stroke. If the CT does not reveal an obvious precipitant for the altered mental status, then MRI brain with gadolinium should be pursued.
The utility of a lumbar puncture as part of an altered mental status workup is a common question for consulting neurologists. A head CT should be obtained before lumbar puncture in patients with altered mental status.31 As nosocomial meningitis is uncommon, a lumbar puncture is most useful in patients who present to the emergency department with altered mental status, although it should still be considered in hospitalized patients with recent neurosurgery or skull fracture, medical devices or surgical hardware implanted in the central nervous system, or immunocompromise.32 It is reasonable to have a low threshold to start empiric treatment for bacterial and viral meningitis given their high morbidity and mortality. In the setting of infection, cerebrospinal fluid (CSF) may be only mildly abnormal within 24 hours of symptom onset, or even normal, as can be seen in herpes simplex virus encephalitis.33,34 For this reason, if one has a high suspicion for meningitis or encephalitis, the lumbar puncture should be repeated 72 hours later. CSF testing should be especially broad in those who are immunocompromised, including for fungal (especially Cryptococcus) and other viral (eg, cytomegalovirus) etiologies.
Abnormal CSF may also point toward autoimmune, paraneoplastic, or neoplastic etiologies. The detection of autoimmune encephalitis is increasing over time with more frequent testing and identification of new autoantibodies. Indeed, the prevalence and incidence of autoimmune encephalitis is similar to that of infectious encephalitis.35 It can be helpful to test for oligoclonal bands and immunoglobulin G index (both in CSF and serum), as this may point to inflammation even if there is no pleocytosis; CSF protein may also be elevated in these cases. Additional workup with systemic imaging (CT chest, abdomen, and pelvis) and testing for autoantibodies on serum and CSF should be considered if inflammation is found. Carcinomatous meningitis is also a consideration, which may present with focal findings, such as cranial nerve deficits and ataxia, and is more likely to be found in patients with lymphoma, leukemia, melanoma, or lung and breast cancer.36 Although MRI may show abnormal enhancement, including of the cranial nerves or nerve roots, obtaining both CSF cytology and flow cytometry increases the sensitivity of detection, especially if repeated.37 The treatment of malignancy with chimeric antigen receptor T-cell immunotherapy or check point inhibitors may also result in meningitis or encephalitis, either as an adverse event from the medication or due to susceptibility to infection, and so testing is often warranted if altered mental status develops in these patients.38,39
Focal seizures with impaired awareness must be ruled out with EEG in every patient with altered mental status without a clear etiology, as the yield is high in patients with unexplained altered mental status on general medical wards.40 Risk factors for seizure include history of prior seizure, mass, and infection, and so it is very reasonable to obtain EEG in patients with altered mental status.41
Additional serum studies beyond those described previously are warranted in certain clinical contexts. A comprehensive/extended toxicology screen may helpful but is time-sensitive. If there is a history of liver disease, presence of liver enzyme abnormalities, or use of hepatically cleared medications such as valproic acid, then an ammonia should be checked; hyperammonemia of unclear etiology may be due to a portal-systemic shunt.42 In addition to the initial workup, other metabolic abnormalities, such as hypo/hypernatremia (especially if rapidly fluctuating), and vitamin B12 deficiency, should be considered. Endocrinopathies, such as hypo/hyperthyroidism, and autoimmune diseases, such as steroid-responsive encephalopathy with autoimmune thyroiditis (SREAT), or Hashimoto encephalitis, should be considered if antithyroid antibodies are present in the right clinical context. Additional autoimmune diseases such as systemic lupus erythematosus or Sjogren syndrome, can present with altered mental status; in those with an existing diagnosis of systemic lupus erythematosus, the presence of active disease should be screened for with antinuclear antibodies, double-stranded DNA, and complement levels.41
EVALUATION AND MANAGEMENT OF DELIRIUM
More extensive testing may not be necessary for a patient with a nonfocal neurologic examination, a clinical diagnosis of delirium, and clear risk factor(s) for delirium (Box 1). Delirium should improve once the precipitant is removed; if the patient does not improve, then additional workup is warranted. Once the diagnosis of delirium is established, the goals of the neurologic evaluation are to identify both risk factors (underlying vulnerabilities) and inciting events, which will then frame management recommendations. Some of the most common risk factors for delirium include advanced age, cognitive impairment, and hearing and vision impairments.10,43–48 Indeed, in one prospective study, older adults with known dementia were 40% more likely to develop delirium; for general medicine service patients, those with dementia are 2.3 to 4.7 times more likely to develop delirium compared with those without dementia.9,10 Given this association, older adults without a known diagnosis of cognitive impairment who develop delirium while hospitalized should undergo an outpatient neurocognitive evaluation. There are many precipitants for delirium, which, in addition to the causes of altered mental status discussed previously, include immobility (eg, physical restraints), dehydration, polypharmacy (especially the use of narcotics, benzodiazepines, or anticholinergics), bladder catheters, and surgery.43,48,49 The postoperative period features several risk factors that exemplify the delicate balance of clinical care in older adults who are susceptible to delirium. For instance, both postoperative pain medication use, and inadequate pain management, are associated with the development of delirium.50
Box 1. Delirium risk factors.
| Age >65 |
| Cognitive impairment or dementia |
| Prior history of delirium |
| Sensory impairment (vision, hearing) |
| Immobility |
| Impairment in activities of daily living |
| Dehydration |
| Malnutrition |
| History of alcohol use or substance use disorder |
| Multiple comorbid conditions |
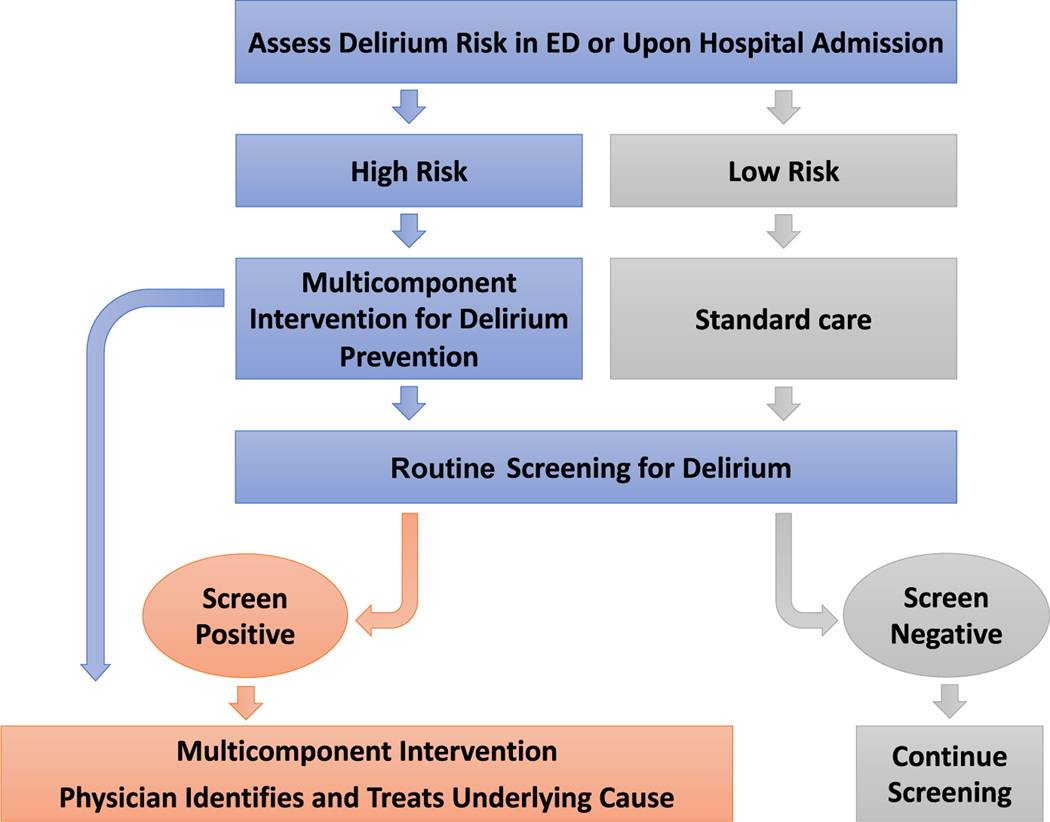
Once considered an inevitability for older adults in the hospital, clinical studies conducted over the past 30 years have demonstrated improved clinical outcomes, including a reduction in incident delirium by as much as 30% to 40% in both non-ICU medical and surgical patients through implementation of largely nonpharmacologic, clinical pathways (Fig. 1).51–55 The first multicomponent delirium prevention pathway used several nursing interventions to mitigate delirium risk factors and provokers, including cognitive impairment (through cognitive stimulation, frequent reorientation), immobility, hearing impairment (through providing amplification devices), and making fluids more available to avoid dehydration. This protocol was inexpensive and was associated with a reduction of delirium incidence.53 Similar evidence-based protocols are increasingly used in a variety of hospital settings with excellent results. Indeed, one Cochrane systematic review of 39 trials encompassing 16,082 patients found that multicomponent delirium interventions reduced delirium incidence when compared with usual care in both medical and surgical populations.56 These protocols include both screening for delirium, as discussed previously, as well as calculating delirium risk on admission. The AWOL score, which incorporates age, spelling “world” backward (or serial 7s in non-English speakers), orientation, and illness severity, is an example of an efficient, validated delirium prediction scale for people admitted to the hospital from the emergency department.57 Use of delirium risk prediction scores are especially helpful in settings in which delirium prevention resources are limited.
Fig. 1.
Delirium care pathway.
Pharmacology has largely been unhelpful, and at times harmful, when applied to delirium management. Indeed, a key intervention in the prevention and management of delirium is tapering or discontinuing medications that increase its risk or have a change in pharmacokinetics due to illness. Benzodiazepines should be avoided unless delirium is due to alcohol or sedative withdrawal. A recent Cochrane review of 9 trials demonstrated no reduction in delirium severity or duration in patients receiving antipsychotics of different classes compared with those who did not receive antipsychotics.58 At this time, antipsychotics should be reserved for patients who are a danger to themselves or to staff. When used, the lowest dose should be used, and cardiac complications (eg, prolonged QT) should be monitored.59 Use of antipsychotics should be reserved for extenuating circumstances because of both lack of effectiveness and risk of harm, as demonstrated in US Food and Drug Administration warnings of increased mortality in older adults with their use.60
There is increasing interest in other pharmacologic interventions, although current evidence does not support the use of any medication explicitly for delirium prevention or treatment. One Cochrane review of 14 trials of pharmacologic interventions (encompassing 6 drug classes) in critically ill patients found no difference between placebo and any drug with regard to delirium-free and coma-free days, or length of stay, although it did find that dexmedetomidine may shorten delirium duration.61 In recently published sweeping practice guidelines for clinical management of ICU patients, no pharmacologic agent was recommended for delirium prevention or treatment, except for dexmedetomidine for when agitation from delirium may be precluding extubation.62 Given mixed results, further research regarding the use of dexmedetomidine for delirium treatment is warranted. Although alterations in sleep-wake cycle are implicated in the development of delirium, administration of melatonin, or melatonin receptor agonist ramelteon, has been associated with delirium reduction in some, but not all, randomized controlled trials.63–66 Melatonin is commonly used as the one pharmacologic intervention in multidisciplinary delirium care pathways given potential benefit and benign side-effect profile.
KEY POINTS.
Altered mental status is a common occurrence in patients who present to the emergency department or who are hospitalized, and is associated with increased mortality.
Altered mental status is descriptor, not a diagnosis, which requires a prompt and careful evaluation to exclude life-threatening precipitants.
A thorough neurologic examination should be performed to exclude a focal deficit in someone with altered mental status.
Delirium is not inevitable for older adults or those with cognitive impairment; rather, it can be prevented in 30% to 40% of cases by using multicomponent delirium prevention pathways.
Delirium prevention and management should center on nonpharmacologic measures with reservation of medications (eg, antipsychotics) only to those who are at risk of harming themselves or others.
SUMMARY.
Altered mental status is common in people presenting to the hospital, especially in older adults and those with cognitive impairment. Altered mental status is a helpful description that should result in a rapid search for the underlying diagnosis, which includes broad differential of life-threatening and reversible precipitants, to provide a targeted treatment plan.
CLINICS CARE POINTS.
Acute altered mental status should be evaluated as quickly as possible in order to identify and treat reversible causes.
Altered mental status may be caused by neurologic and non-neurologic etiologies and so a broad work-up is warranted.
Pharmacology has largely been unhelpful, and at times harmful, when applied to delirium management. Antipsychotics should only be reserved for patients who are a danger to themselves or to staff.
DISCLOSURE
Dr LaHue has nothing to disclose. Funding for Dr Douglas: Sara & Evan Williams Foundation Endowed Neurohospitalist Chair.
REFERENCES
- 1.Smith AT, Han JH. Altered mental status in the emergency department. Semin Neurol 2019;39(1):5–19. [DOI] [PubMed] [Google Scholar]
- 2.Wijdicks EFM. Metabolic encephalopathy: behind the name. Neurocrit Care 2018;29(3):385–7. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Desk reference to the diagnostic criteria from DSM-5. Arlington, Va: American Psychiatric Association; 2013. [Google Scholar]
- 4.Wofford JL, Loehr LR, Schwartz E. Acute cognitive impairment in elderly ED patients: etiologies and outcomes. Am J Emerg Med 1996;14(7):649–53. [DOI] [PubMed] [Google Scholar]
- 5.Hustey FM, Meldon SW. The prevalence and documentation of impaired mental status in elderly emergency department patients. Ann Emerg Med 2002;39(3):248–53. [DOI] [PubMed] [Google Scholar]
- 6.Kanich W, Brady WJ, Huff JS, et al. Altered mental status: evaluation and etiology in the ED. Am J Emerg Med 2002;20(7):613–7. [DOI] [PubMed] [Google Scholar]
- 7.Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med 2017; 377(15):1456–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Geriatrics Society Expert Panel on Postoperative Delirium in OlderAdults. Postoperative delirium in older adults: best practice statement from the American Geriatrics Society. J Am Coll Surg 2015;220(2):136–148 e131. [DOI] [PubMed] [Google Scholar]
- 9.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet 2014;383(9920):911–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNicoll L, Pisani MA, Zhang Y, et al. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc 2003;51(5):591–8. [DOI] [PubMed] [Google Scholar]
- 11.Gibb K, Seeley A, Quinn T, et al. The consistent burden in published estimates of delirium occurrence in medical inpatients over four decades: a systematic review and meta-analysis study. Age Ageing 2020;49(3):352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francis J, Martin D, Kapoor WN. A prospective study of delirium in hospitalized elderly. JAMA 1990;263(8):1097–101. [PubMed] [Google Scholar]
- 13.Salluh JI, Soares M, Teles JM, et al. Delirium epidemiology in critical care (DECCA): an international study. Crit Care 2010;14(6):R210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 2004;291(14):1753–62. [DOI] [PubMed] [Google Scholar]
- 15.McCusker J, Cole MG, Dendukuri N, et al. Does delirium increase hospital stay? J Am Geriatr Soc 2003;51(11):1539–46. [DOI] [PubMed] [Google Scholar]
- 16.Inouye SK, Rushing JT, Foreman MD, et al. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med 1998;13(4): 234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing 2006;35(4):350–64. [DOI] [PubMed] [Google Scholar]
- 18.LaHue SC, Douglas VC, Kuo T, et al. Association between inpatient delirium and hospital readmission in patients >/5 65 years of age: a retrospective cohort study. J Hosp Med 2019;14(4):201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leslie DL, Marcantonio ER, Zhang Y, et al. One-year health care costs associated with delirium in the elderly population. Arch Intern Med 2008;168(1):27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gustafson Y, Brannstrom B, Norberg A, et al. Underdiagnosis and poor documentation of acute confusional states in elderly hip fracture patients. J Am Geriatr Soc 1991;39(8):760–5. [DOI] [PubMed] [Google Scholar]
- 21.Inouye SK, Foreman MD, Mion LC, et al. Nurses’ recognition of delirium and its symptoms: comparison of nurse and researcher ratings. Arch Intern Med 2001; 161(20):2467–73. [DOI] [PubMed] [Google Scholar]
- 22.Inouye SK. Delirium in older persons. N Engl J Med 2006;354(11):1157–65. [DOI] [PubMed] [Google Scholar]
- 23.Shaw RC, Walker G, Elliott E, et al. Occurrence rate of delirium in acute stroke settings: systematic review and meta-analysis. Stroke 2019;50(11):3028–36. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association., American psychiatric association. DSM-5 task force. Diagnostic and statistical manual of mental disorders : DSM-5. 5th edition. Arlington, VA Washington, D.C.: American Psychiatric Association; 2013. [Google Scholar]
- 25.Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990;113(12):941–8. [DOI] [PubMed] [Google Scholar]
- 26.Gaudreau JD, Gagnon P, Harel F, et al. Fast, systematic, and continuous delirium assessment in hospitalized patients: the nursing delirium screening scale. J Pain Symptom Manage 2005;29(4):368–75. [DOI] [PubMed] [Google Scholar]
- 27.Helfand BKI, D’Aquila ML, Tabloski P, et al. Detecting delirium: a systematic review of identification instruments for non-ICU settings. J Am Geriatr Soc 2021; 69(2):547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De J, Wand AP. Delirium screening: a systematic review of delirium screening tools in hospitalized patients. Gerontologist 2015;55(6):1079–99. [DOI] [PubMed] [Google Scholar]
- 29.Bellelli G, Morandi A, Davis DH, et al. Validation of the 4AT, a new instrument for rapid delirium screening: a study in 234 hospitalised older people. Age Ageing 2014;43(4):496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown EG, Douglas VC. Moving beyond metabolic encephalopathy: an update on delirium prevention, workup, and management. Semin Neurol 2015;35(6): 646–55. [DOI] [PubMed] [Google Scholar]
- 31.Hasbun R, Abrahams J, Jekel J, et al. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med 2001;345(24): 1727–33. [DOI] [PubMed] [Google Scholar]
- 32.Metersky ML, Williams A, Rafanan AL. Retrospective analysis: are fever and altered mental status indications for lumbar puncture in a hospitalized patient who has not undergone neurosurgery? Clin Infect Dis 1997;25(2):285–8. [DOI] [PubMed] [Google Scholar]
- 33.Onorato IM, Wormser GP, Nicholas P. ‘Normal’ CSF in bacterial meningitis. JAMA 1980;244(13):1469–71. [PubMed] [Google Scholar]
- 34.Koskiniemi M, Vaheri A, Taskinen E. Cerebrospinal fluid alterations in herpes simplex virus encephalitis. Rev Infect Dis 1984;6(5):608–18. [DOI] [PubMed] [Google Scholar]
- 35.Dubey D, Pittock SJ, Kelly CR, et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol 2018;83(1):166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clarke JL, Perez HR, Jacks LM, et al. Leptomeningeal metastases in the MRI era. Neurology 2010;74(18):1449–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott BJ, Douglas VC, Tihan T, et al. A systematic approach to the diagnosis of suspected central nervous system lymphoma. JAMA Neurol 2013;70(3):311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brahmer JR, Lacchetti C, Thompson JA. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: american society of clinical oncology clinical practice guideline summary. J Oncol Pract 2018;14(4):247–9. [DOI] [PubMed] [Google Scholar]
- 39.Gust J, Ponce R, Liles WC, et al. Cytokines in CAR Tcell-associated neurotoxicity. Front Immunol 2020;11:577027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Betjemann JP, Nguyen I, Santos-Sanchez C, et al. Diagnostic yield of electroencephalography in a general inpatient population. Mayo Clin Proc 2013;88(4): 326–31. [DOI] [PubMed] [Google Scholar]
- 41.Douglas VC, Josephson SA. Altered mental status. Continuum (Minneap Minn) 2011;17(5 Neurologic Consultation in the Hospital):967–83. [DOI] [PubMed] [Google Scholar]
- 42.Raskin NH, Price JB, Fishman RA. Portal-systemic encephalopathy due to congenital intrahepatic shunts. N Engl J Med 1964;270:225–9. [DOI] [PubMed] [Google Scholar]
- 43.Inouye SK. Predisposing and precipitating factors for delirium in hospitalized older patients. Dement Geriatr Cogn Disord 1999;10(5):393–400. [DOI] [PubMed] [Google Scholar]
- 44.Inouye SK, Zhang Y, Jones RN, et al. Risk factors for delirium at discharge: development and validation of a predictive model. Arch Intern Med 2007;167(13): 1406–13. [DOI] [PubMed] [Google Scholar]
- 45.LaHue SC, Liu VX. Loud and clear: sensory impairment, delirium, and functional recovery in critical illness. Am J Respir Crit Care Med 2016;194(3):252–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryan DJ, O’Regan NA, Caoimh RO, et al. Delirium in an adult acute hospital population: predictors, prevalence and detection. BMJ Open 2013;3(1):e001772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.LaHue SC, Douglas VC, Miller BL. The one-two punch of delirium and dementia during the COVID-19 pandemic and beyond. Front Neurol 2020;11:596218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.LaHue SC, James TC, Newman JC, et al. Collaborative delirium prevention in the age of COVID-19. J Am Geriatr Soc 2020;68(5):947–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA 1996;275(11):852–7. [PubMed] [Google Scholar]
- 50.Wang Y, Sands LP, Vaurio L, et al. The effects of postoperative pain and its management on postoperative cognitive dysfunction. Am J Geriatr Psychiatry 2007; 15(1):50–9. [DOI] [PubMed] [Google Scholar]
- 51.Young J, Murthy L, Westby M, et al. Diagnosis, prevention, and management of delirium: summary of NICE guidance. BMJ 2010;341:c3704. [DOI] [PubMed] [Google Scholar]
- 52.Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med 2015;175(4): 512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inouye SK, Bogardus ST Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999;340(9): 669–76. [DOI] [PubMed] [Google Scholar]
- 54.Strijbos MJ, Steunenberg B, van der Mast RC, et al. Design and methods of the Hospital Elder Life Program (HELP), a multicomponent targeted intervention to prevent delirium in hospitalized older patients: efficacy and cost-effectiveness in Dutch health care. BMC Geriatr 2013;13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.LaHue SC, Maselli J, Rogers S. Outcomes following implementation of a hospital-wide multicomponent delirium care pathway. J Hosp Med 2021;16(7):397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev 2016;3:CD005563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Douglas VC, Hessler CS, Dhaliwal G, et al. The AWOL tool: derivation and validation of a delirium prediction rule. J Hosp Med 2013;8(9):493–9. [DOI] [PubMed] [Google Scholar]
- 58.Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev 2018;6:CD005594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nikooie R, Neufeld KJ, Oh ES, et al. Antipsychotics for treating delirium in hospitalized adults: a systematic review. Ann Intern Med 2019;171(7):485–95. [DOI] [PubMed] [Google Scholar]
- 60.Kuehn BM. FDA warns antipsychotic drugs may be risky for elderly. JAMA 2005; 293(20):2462. [DOI] [PubMed] [Google Scholar]
- 61.Burry L, Hutton B, Williamson DR, et al. Pharmacological interventions for the treatment of delirium in critically ill adults. Cochrane Database Syst Rev 2019; 9:CD011749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Devlin JW, Skrobik Y, Gelinas C, et al. Executive summary: clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 2018;46(9):1532–48. [DOI] [PubMed] [Google Scholar]
- 63.Hatta K, Kishi Y, Wada K, et al. Preventive effects of ramelteon on delirium: a randomized placebo-controlled trial. JAMA Psychiatry 2014;71(4):397–403. [DOI] [PubMed] [Google Scholar]
- 64.Al-Aama T, Brymer C, Gutmanis I, et al. Melatonin decreases delirium in elderly patients: a randomized, placebo-controlled trial. Int J Geriatr Psychiatry 2011; 26(7):687–94. [DOI] [PubMed] [Google Scholar]
- 65.de Jonghe A, van Munster BC, Goslings JC, et al. Effect of melatonin on incidence of delirium among patients with hip fracture: a multicentre, double-blind randomized controlled trial. CMAJ 2014;186(14):E547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nishikimi M, Numaguchi A, Takahashi K, et al. Effect of administration of ramelteon, a melatonin receptor agonist, on the duration of stay in the ICU: a single-center randomized placebo-controlled trial. Crit Care Med 2018;46(7):1099–105. [DOI] [PMC free article] [PubMed] [Google Scholar]