ABSTRACT
There are no licensed vaccines against enterotoxigenic Escherichia coli (ETEC), a leading cause of children’s diarrhea and travelers’ diarrhea. Recently, protein-based vaccine candidate MecVax was demonstrated to induce functional antibodies against both ETEC toxins (heat-stable toxin [STa] and heat-labile toxin [LT]) and seven ETEC adhesins (CFA/I and CS1 to CS6) and to protect against ETEC clinical diarrhea or intestinal colonization preclinically. Those studies used intraperitoneal, intramuscular, and intradermal routes, and a dose range for MecVax protein antigens, toxoid fusion 3xSTaN12S-mnLTR192G/L211A, and adhesin CFA/I/II/IV MEFA has not been investigated. Here, we further characterized MecVax broad immunogenicity, utilizing a subcutaneous route, and examined vaccine dose-dependent antibody response effects and also antibody functional activities against ETEC enterotoxicity and bacterial adherence. Data showed that mice immunized subcutaneously with MecVax developed robust IgG responses to seven ETEC adhesins (CFA/I, as well as CS1 to CS6) and two toxins (STa and LT). At a subcutaneous dose of 25, 20, or 10 μg or at an intramuscular dose of 12, 6, or 3 μg, MecVax induced similar levels IgG responses to the targeted toxins and adhesins, and these antibodies exhibited equivalent functional activities against ETEC toxin enterotoxicity and bacterial adherence. Once the intramuscular dose was decreased to 1 μg, vaccine-induced antibodies were significantly reduced and no longer neutralized STa enterotoxicity. The results indicated that MecVax administered subcutaneously is broadly immunogenic and, at an intramuscular dose of 3 μg, can induce functional antitoxin and anti-adhesin antibodies in mice, providing instructive information for future vaccine dose studies in humans and accelerating MecVax vaccine development.
IMPORTANCE Enterotoxigenic Escherichia coli (ETEC) is a leading cause of children’s diarrhea and the most common cause of travelers’ diarrhea. ETEC infections are responsible for >200 million diarrhea clinical cases and near 100,000 deaths annually. Currently, there are no licensed vaccines for ETEC diarrhea. The protein-based vaccine candidate MecVax unprecedentedly targets two ETEC toxins (STa and LT, produced by all ETEC strains) and seven ETEC adhesins (CFA/I, as well as CS1 to CS6, associated with >60% of ETEC clinical diarrhea cases) and has been demonstrated to be broadly immunogenic and cross protective; as such, it represents a potentially effective multivalent vaccine against ETEC-associated children’s and travelers’ diarrhea. This study further confirmed MecVax broad immunogenicity and evaluated the vaccine antigen dose effect on the induction of antigen-specific antibody responses in mice and on antibody functional activities against ETEC toxin enterotoxicity and bacterial adherence, yielding useful information for future human volunteer studies and the development of MecVax as an effective ETEC vaccine.
KEYWORDS: ETEC, enterotoxigenic Escherichia coli, diarrhea, vaccine, MecVax, dose-dependent effect, dose-dependent study
INTRODUCTION
Currently, there are no licensed vaccines against enterotoxigenic Escherichia coli (ETEC) infection, one of the top five causes of diarrhea in children aged <5 years in developing countries (1–3) and the most common cause of diarrhea in international travelers (4). Heterogeneity among ETEC pathovars, which produce different virulence factors: enterotoxins and adhesins, including colonization factor antigens (CFA) and coli surface antigens (CS), is a key obstacle for ETEC vaccine development (5, 6). Since an ETEC strain producing any one of >25 ETEC adhesins and one toxin (heat-stable toxin [STa] or heat-labile toxin [LT]) can cause children’s diarrhea or travelers’ diarrhea, an effective ETEC vaccine would need to be broadly immunogenic and to induce cross-protective antibodies against ETEC enterotoxins (STa and LT, which stimulate fluid hypersecretion in intestinal epithelial cells, and alone or together produced by all ETEC strains) and the most important ETEC adhesins at least (if not all adhesins), which mediate bacterial adherence to host receptors and colonization of host small intestines.
Different strategies have been attempted to solve ETEC heterogeneity challenge and to develop broadly protective ETEC vaccines. A cocktail whole-cell approach, in which a few killed or live attenuated strains are mixed together, led to oral vaccine candidates that carry three or six ETEC adhesins and LT toxin antigen (a nontoxic LT B subunit or a hybrid B subunit of LT and cholera toxin [CT]) for broad protection against ETEC stains expressing homologous adhesins (7–10). Another approach is based on conserved antigen(s). Aided by genomewide data mining, conserved antigens can be identified and explored for protection against heterogeneous ETEC strains (11–14). Both approaches are expected to broaden protection coverage and advance some vaccine candidates toward preclinical and clinical studies. Unfortunately, none of these products carries antigens to induce protective antibodies against STa, the key ETEC toxin that plays a more significant role in causing children’s diarrhea (1, 2) and travelers’ diarrhea (15).
In contrast to the other ETEC vaccine candidates, MecVax, a multivalent acellular product composed of two polyvalent proteins—toxoid fusion 3xSTaN12S-mnLTR192G/L211A and epitope- and structure-based CFA/I/II/IV multiepitope fusion antigen (MEFA)—carries antigens to unprecedentedly target both ETEC toxins (STa and LT) and the seven most important ETEC adhesins (CFA/I, CS1 to CS6). MecVax was recently demonstrated to be broadly immunogenic and to induce cross-protective antibodies against two ETEC toxins and seven adhesins (16, 17). This protein-based injectable subunit vaccine candidate, when administered intraperitoneally, intradermally, or intramuscularly, induced antibody responses to STa and LT toxins and seven adhesins (CFA/I, CS1 to CS6), and the vaccine-induced antibodies neutralized CT and STa enterotoxicity and inhibited adherence from ETEC bacteria expressing any of the seven targeted adhesins (16–18). More importantly, when administered intramuscularly MecVax passively protected neonatal pigs from ETEC toxin-mediated clinical diarrhea and actively prevented from ETEC bacterial colonization of the small intestines in adult rabbits (16, 19). This marks MecVax as the first ETEC vaccine candidate that potentially protects against ETEC strains producing any of the seven adhesins (CFA/I, CS1 to CS6) that are associated with 60 to 70% ETEC diarrhea clinical cases (20, 21), as well as ETEC strains producing STa and/or LT toxins, the bacteria associated with all ETEC diarrheal cases. Although MecVax’s broad immunogenicity has been demonstrated when administered intradermally or intramuscularly (16, 17), whether this vaccine candidate remains broadly immunogenic following subcutaneous injection has not been not examined. In addition, vaccine dose-dependent studies to determine the optimal doses for inducing protective antitoxin and anti-adhesin antibodies have not been conducted.
In this study, we first immunized mice with MecVax subcutaneously, at the same dose used previously in intramuscular or intradermal immunization studies, to examine vaccine candidate broad immunogenicity. Subsequently, we reduced the amounts of vaccine antigen components to examine the dose effect of MecVax induction of protective antitoxin and anti-adhesin antibodies. With the intramuscular route being the preferred method for MecVax administration in future clinical studies, we refocused on intramuscular immunization and examined the MecVax antigen dose-dependent effect on vaccine immunogenicity and protection against ETEC toxin enterotoxicity and bacterial adherence.
RESULTS
MecVax administered subcutaneously induced broad antigen-specific IgG antibody responses in mice.
Mice subcutaneously immunized with MecVax, at the dose of 25 μg of toxoid fusion 3xSTaN12S-mnLTR192G/L211A protein and 25 μg of CFA/I/II/IV MEFA protein (the same dose used previously in mouse intramuscular and intradermal immunization), adjuvanted with 0.2 μg of dmLT adjuvant, developed robust IgG responses to the targeted ETEC adhesins and toxins (Fig. 1). Anti-CFA/I, -CS1, -CS2, -CS3, -CS4, -CS5, and -STa and anti-LT IgG were detected at 3.7 ± 0.25, 3.5 ± 0.33, 2.8 ± 0.26, 2.7 ± 0.26, 3.5 ± 0.15, 3.3 ± 0.27, 3.5 ± 0.19, and 3.9 ± 0.19 log10, respectively, from the serum samples of the immunized mice. Anti-CS6 IgG responses were not tested at the time because of a lack of CS6 coating antigen. No antigen-specific IgG responses were detected among the control mouse serum samples. No antigen-specific IgA responses were detected from immunized or control mice.
FIG 1.

IgG titers (log10) from serum samples of the mice subcutaneously immunized with MecVax composed of 25 μg of CFA/I/II/IV MEFA protein and 25 μg of toxoid fusion 3xSTaN12S-mnLTR192G/L211A protein (●) or PBS as the control (○). dmLT adjuvant (0.2 μg) was used in the immunized group. Bars indicate antibody mean titers and standard deviations.
Mice subcutaneously immunized with MecVax at a reduced antigen dose developed similar levels of antitoxin and anti-adhesin IgG responses.
Five groups of mice subcutaneously immunized with toxoid fusion 3xSTaN12S-mnLTR192G/L211A and CFA/I/II/IV MEFA, at a dose ranging from 10 to 25 μg, developed similar levels of antigen-specific IgG responses (Table 1). Although group 4 (20 μg of toxoid fusion mixed with 25 μg of CFA/I/II/IV MEFA) had equal or greater IgG titers to CFA/I, CS2, CS4, CS5, LT, and STa, two-way analysis of variance (ANOVA) indicated that the differences among the five groups were not significant. No antigen-specific IgG from the control group was detected.
TABLE 1.
Anti-adhesin and antitoxin IgG antibody titers detected by ELISA with serum samples of the mice subcutaneously administered MecVaxa
| Groupb | Mean antibody titer (log10) ± SD |
|||||||
|---|---|---|---|---|---|---|---|---|
| CFA/1 | CS1 | CS2 | CS3 | CS4/6 | CS5/6 | LT | STa | |
| 1 | 3.4 ± 0.28 | 3.2 ± 0.25 | 2.8 ± 0.29 | 3.2 ± 0.27 | 2.5 ± 0.17 | 3.2 ± 0.19 | 3.7 ± 0.3 | 3.3 ± 0.22 |
| 2 | 3.8 ± 0.21 | 3.3 ± 0.16 | 2.4 ± 0.3 | 3.3 ± 0.15 | 3.5 ± 0.15 | 3.1 ± 0.16 | 3.8 ± 0.22 | 3.6 ± 0.09 |
| 3 | 3.4 ± 0.23 | 3.0 ± 0.18 | 2.6 ± 0.22 | 3.2 ± 0.24 | 3.3 ± 0.28 | 3.2 ± 0.27 | 3.6 ± 0.29 | 3.4 ± 0.25 |
| 4 | 3.8 ± 0.42 | 3.4 ± 0.22 | 2.8 ± 0.19 | 2.9 ± 0.11 | 3.9 ± 0.27 | 3.7 ± 0.25 | 3.9 ± 0.18 | 4.1 ± 0.2 |
| 5 | 3.7 ± 0.25 | 3.5 ± 0.33 | 2.8 ± 0.26 | 2.7 ± 0.26 | 3.5 ± 0.15 | 3.3 ± 0.27 | 3.9 ± 0.19 | 3.5 ± 0.19 |
| 6 (–) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
Five groups, groups 1 to 5, were subcutaneously immunized with MecVax combining toxoid fusion 3xSTaN12S-mnLTR192G/L211A and CFA/I/II/IV MEFA at different doses, with 0.2 μg of dmLT as the adjuvant. A control group, group 6, injected with PBS (no dmLT adjuvant) was used as the control. Serum samples from each mouse were examined in triplicates, and each titration assay was repeated.
Group 1, 10 μg of CFA/I/II/IV MEFA + 25 μg of toxoid fusion; group 2, 25 μg of CFA/I/II/IV MEFA + 10 μg of toxoid fusion; group 3, 20 μg of CFA/I/II/IV MEFA + 25 μg of toxoid fusion; group 4, 25 μg of CFA/I/II/IV MEFA + 20 μg of toxoid fusion; group 5, 25 μg of CFA/I/II/IV MEFA + 25 μg of toxoid fusion.
Antibodies from subcutaneously administered MecVax neutralized CT and STa enterotoxicity and inhibited the adherence of CFA/I and CS1 to CS6 adhesins.
Mouse serum samples from the five groups subcutaneously immunized with MecVax, at doses ranging from 10 to 25 μg of toxoid fusion and CFA/I/II/IV MEFA protein, equivalently neutralized CT (LT homologue) and STa enterotoxicity, shown by prevention of CT or STa from an elevation of cyclic GMP (cGMP) or cAMP. The cAMP levels (nM [pmol/mL]) in T-84 cells incubated with 10 ng of CT preexposed to the serum samples of five immunized groups were 19.8 ± 1.8 (group 1), 14 ± 2.0 (group 2), 12.1 ± 3.1 (group 3), 11.1 ± 0.8 (group 4), and 7.1 ± 0.5 (group 5). These cAMP levels were significantly lower than the levels in cells incubated with CT alone (178.2 ± 4.8; P < 0.01) or CT preexposed to the sera of control mice (159.2 ± 0.7; P < 0.01).
The cGMP levels (nM) in T-84 cells incubated with 2 ng of STa preexposed to the serum samples from the five immunized groups were 1.7 ± 0.1 (group 1), 1.2 ± 0.1 (group 2), 1.3 ± 0 (group 3), 0.8 ± 0.1 (group 4), and 1.4 ± 0.1 nM (group 5), respectively. These cGMP concentrations were significantly lower than the levels in T-84 cells incubated with 2 ng of STa alone (35.4 ± 2.5; P < 0.01) or 2 ng of STa toxin preexposed to the sera of control mice (34.5 ± 0.9; P < 0.01).
Mouse serum samples from the five subcutaneous immunization groups significantly inhibited the adherence of E. coli strains expressing CS1 or CS2 adhesin or ETEC field isolates producing CFA/I, CS3, CS4/CS6, or CS5/CS6 adhesin. After incubation with mouse serum samples from each of the five immunization groups, adherence to Caco-2 cells from the bacteria expressing CFA/I, CS1, CS2, CS3, CS4/CS6, or CS5/CS6 was reduced 59.4, 52, 70.5, 58.4, 62.7, and 53%, respectively, compared to adherence by the bacteria incubated with the control mouse serum (P < 0.01).
Mice intramuscularly immunized with MecVax at doses decreasing from 12 to 1 μg developed broad IgG responses to the targeted adhesins and toxins.
Mice intramuscularly immunized with MecVax, at a dose of 12, 6, 3, or 1 μg of CFA/I/II/IV MEFA protein and toxoid fusion 3xSTaN12S-mnLTR192G/L211A protein developed robust IgG responses to CFA/I, CS1-CS6, STa, and LT (Fig. 2). The group immunized with MecVax at a 1-μg dose of each protein showed significantly lower IgG titers to CFA/I, CS1-CS6, and STa. No antigen-specific IgG responses were detected from the serum samples of control mice.
FIG 2.
IgG (log10) titers from serum samples of the mice intramuscularly immunized with MecVax at different doses of CFA/I/II/IV MEFA and toxoid fusion 3xSTaN12S-mnLTR192G/L211A. Four groups of mice intramuscularly immunized with MecVax at various antigen doses—12 μg (♦), 6 μg (▾), 3 μg (▴), or 1 μg (■)—another group with PBS (●) served as the control. We included 0.2 μg of dmLT adjuvant in the four immunization groups. Each dot represents an IgG titer from a mouse in each group. Asterisks (*, **, and ***) represent P values of <0.05, <0.01, and <0.001, respectively. Bars indicate antibody titer means and standard deviations.
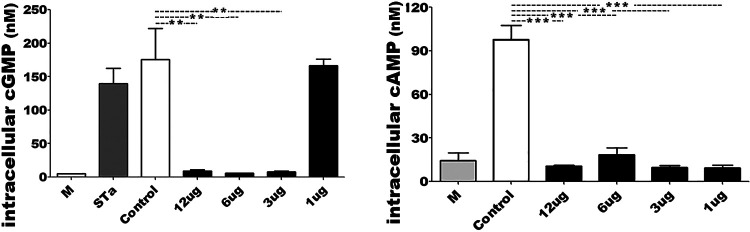
Mice intramuscularly immunized with MecVax at a dose of ≥3 μg of each protein developed antibodies that equally neutralized CT and STa enterotoxicity and inhibited the adherence of CFA/I and CS1 to CS6 adhesins.
Serum samples of the mice intramuscularly immunized with MecVax, at the doses of 3, 6, or 12 μg (of each protein) equally neutralized CT and STa enterotoxicity (Fig. 3). The cAMP or cGMP concentrations in T-84 cells exposed to CT or STa toxin pretreated with the serum samples from each immunization group showed no difference compared to the baseline cAMP or cGMP levels (in cells cultured with cultural medium, no toxin or serum). Mouse serum samples from these three groups also significantly inhibited adherence of bacteria expressing CFA/I, CS1, CS2, CS3, CS4/CS5, or CS5/CS6 to Caco-2 cells (Fig. 4).
FIG 3.
Intracellular cGMP or cAMP levels (nM [pmol/mL]) in T-84 cells to show the antibody neutralization activity against STa and CT enterotoxicity. T-84 cells exposed to STa or CT (LT homologue) pretreated with the serum sample from mice intramuscularly immunized with MecVax at different doses (■) or the control mice (□) were measured for cGMP or cAMP concentrations. T-84 cells incubated with culture medium (no toxin and no serum) were used to show T-84 cell baseline cGMP or cAMP. Boxes and bars indicate means and standard deviations of cGMP or cAMP levels. Asterisks (** and ***) indicate P values of <0.01 and <0.001, respectively.
FIG 4.
Antibody adherence inhibition assays were performed to show the number of ETEC or E. coli bacteria (CFU [%]) adherent to Caco-2 cells after incubation with serum samples of mice intramuscularly immunized with MecVax (■; at different doses) or PBS as the control (□). E. coli expressing CS1 or CS2 adhesin and ETEC isolates producing CFA/I, CS3, CS4/CS6, CS5/CS6, or CS6 adhesin were treated with mouse serum samples from the group intramuscularly immunized with MecVax at a dose of 12, 6, 3, 1, or 0 μg and then incubated with Caco-2 cells. Bacteria adhered to Caco-2 cells were collected, plated, and counted for CFU after overnight growth. Boxes and bars represent means and standard deviations of bacterial adherence (in %). Asterisks (*, **, and ***) indicate P values of <0.05, <0.01, and <0.001, respectively.
However, mouse serum samples from the group immunized with MecVax at doses of 1 μg of each protein, although they neutralized CT enterotoxicity and inhibited the adherence from adhesin CFA/I, CS1, CS2, CS3, CS4/CS5, or CS5/CS6 to Caco-2 cells, did not neutralize STa enterotoxicity (Fig. 3, left). Serum samples from control mice showed no in vitro protection against ETEC toxin enterotoxicity or bacterial adherence.
DISCUSSION
Previously, studies showed that MecVax, at a 25-μg dose of protein antigen (CFA/I/II/IV MEFA, toxoid fusion 3xSTaN12S-mnLTR192G/L211A), when administered intramuscularly or intradermally, induced functional antibodies against enterotoxicity of both ETEC toxins (LT and STa) and the adherence of seven ETEC adhesins (CFA/I, CS1 to CS6) (16, 17). Data from the present study indicated that MecVax at the same dose administered via a subcutaneous route also induced antibodies to neutralize LT and STa enterotoxicity and to inhibit the adherence of the seven targeted adhesins. This confirms that the ETEC vaccine candidate MecVax is broadly immunogenic and induces cross-functional antibodies against the seven most important ETEC adhesins and both toxins regardless of which parenteral immunization route is utilized. Since MecVax administered via any of three parenteral routes equivalently induces functional antibodies against the targeted toxins and adhesins, and since the intradermal and subcutaneous routes appear to offer no advantage for inducing antigen-specific IgA responses, the intramuscular route becomes the preferred route because of the simplicity of its clinical application and the feasibility for MecVax to be combined with other protein antigens or an intramuscularly administered vaccine product. Therefore, focus on vaccine dosage-dependent study was shifted to the intramuscular route. The data revealed that MecVax, when intramuscularly administered at the doses decreasing from 12 to 6 μg and further to 3 μg (toxoid fusion protein and CFA/I/II/IV MEFA protein) equally induced neutralizing antitoxin antibodies and functional anti-adhesin antibodies. Only after the dose was reduced to 1 μg did MecVax abolish the ability to induce neutralizing antibodies against STa toxin (although it was still effective against CT enterotoxicity and the adherence of the seven target adhesins). These data suggest 3 μg of each protein may represent a low-end dose for MecVax intramuscular administration in mice, providing instructive information for further vaccine dose optimization and future studies with human subjects.
MecVax carrying 1 μg of toxoid fusion protein was unable to induce neutralizing antitoxin antibodies against STa toxin enterotoxicity in intramuscularly immunized mice, suggesting that 1 μg is below the minimum dose. Although antibodies induced by MecVax at a 1-μg dose neutralized CT toxin, it is most likely that anti-LT antibodies induced by dmLT adjuvant synergistically contributed (with MecVax toxoid fusion antigen) to in vitro protection against CT enterotoxicity. The mucosal adjuvant dmLT elicits anti-LT antibodies and enhances parenterally administered CFA/I/II/IV MEFA for inducing IgG responses to the seven adhesins (CFA/I, CS1 to CS6) (22); thus, it is intended to be the adjuvant for MecVax future clinical studies. While 3 μg of 3xSTaN12S-mnLTR192G/L211A may represent a low-end dose, the dose for vaccine adhesin component antigen CFA/I/II/IV MEFA protein can be further reduced. Indeed, MecVax at 1 μg of each protein induced antibodies that equally inhibited bacterial adherence as MecVax with any higher doses (Fig. 4). This suggests that the optimal dose for CFA/I/II/IV MEFA protein in MecVax will be lower than 1 μg for mouse intramuscular immunization. This appears corelated to the molecule sizes of two protein antigens. The molecular weight of toxoid fusion protein (46 kDa) is three times that of the CFA/I/II/IV MEFA protein (15 kDa); thus, at the same mass, the ratio of CFA/I/II/IV MEFA to toxoid fusion in MecVax is 3:1. Additional mouse immunization studies with MecVax composed of 1 μg of CFA/I/II/IV MEFA protein and 3 μg of toxoid fusion, or even a dose lower than 3 μg but greater than 1 μg (but maintaining a 3:1 ratio for toxoid fusion versus CFA/I/II/IV), might further narrow down the optimal preclinical dose for MecVax.
It should be noted that the current dose-dependent study was carried out in a murine model. These results can be instructive for future preclinical studies with a rabbit colonization model and a pig challenge model, as well as pre-IND preparation to accelerate MecVax development. The MecVax intramuscular immunization doses for healthy adults and especially young children in developing countries, however, must be further optimized in future volunteer studies and clinical trials.
MATERIALS AND METHODS
E. coli bacterial strains.
Recombinant E. coli strains 9471 and 9472 (18) were used to expressi tagless toxoid fusion 3xSTaN12S-mnLTR192G/L211A and tagless adhesin CFA/I/II/IV MEFA recombinant proteins, the two protein antigens of MecVax. ETEC field isolates H10407 (CFA/I, LT, STa), E116 (CS3, LT, STa), E106 (CS4/CS6, LT, STa), and UM 75688 (CS5/CS6, LT, STa) were provided by David Sack (Johns Hopkins University) and Ann-Mari Svennerholm (Gothenburg University). 2423 ETP98066 (CS6, LT, and STa), provided by James Fleckenstein at Washington University at St. Louis (23), and recombinant E. coli strains expressing CS1 (24) or CS2 adhesin (25) (provided by June Scott at Emory University) were used for the extraction of adhesins (as ELISA coating antigens for anti-adhesin antibody titration) and in antibody adherence inhibition assays.
Mouse subcutaneous immunization with MecVax composed of 25 μg of CFA/I/II/IV MEFA protein and 25 μg of 3xSTaN12S-mnLTR192G/L211A toxoid fusion protein.
MecVax is demonstrated broadly immunogenic and induces functional antibodies against seven ETEC adhesins and both toxins at the dose of 25 μg of each antigen when administered intramuscularly (16) or intradermally (17). To test whether MecVax retains broad immunogenicity using the subcutaneous route, two groups of 8-week-old female BALB/c mice (Charles River Laboratories International, Inc., Wilmington, MA), at six mice per group, were included in the study. One group was subcutaneously immunized with MecVax, at doses of 25 μg of CFA/I/II/IV MEFA protein (9472) and 25 μg of 3xSTaN12S-mnLTR192G/L211A protein (9471), and adjuvanted with 0.2 μg of dmLT (double mutant AB5 LT, holotoxin-structured LTR192G/L211A; provided by PATH). Each immunized mouse received two booster injections, at the same dose as the primary, with an interval of 2 weeks. The other group of six mice injected with phosphate-buffered saline (PBS) was used as the negative control. Mouse serum samples collected before the primary and 2 weeks after the final injection were kept at −80°C until use.
Mouse subcutaneous immunization with MecVax at various doses of CFA/I/II/IV MEFA protein and 3xSTaN12S-mnLTR192G/L211A toxoid fusion protein.
To examine MecVax antigen dose-dependent effect under subcutaneous route, four groups (designated groups 1 to 4) of 8-week-old female BALB/c mice (six mice per group) were subcutaneously immunized with MecVax at various dose combinations of two protein antigens (Table 2) and adjuvanted with 0.2 μg of dmLT, followed by two boosters, as described above. Mouse serum samples were examined for antigen-specific responses and antibody functional activities against ETEC toxin enterotoxicity and bacterial adherence.
TABLE 2.
Different doses of MecVax protein antigens CFA/I/II/IV MEFA protein (strain 9472) and toxoid fusion 3xSTaN12S-mnLTR192G/L211A protein (strain 9471) used in mouse s.c. or i.m. immunization studiesa
| Mouse immunization route and MecVax antigen | MecVax dose (μg) |
||||
|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
| s.c. (n = 6) | |||||
| CFA/I/II/IV MEFA | 10 | 25 | 20 | 25 | 25 |
| 3xSTaN12S-mnLTR192G/L211A | 25 | 10 | 25 | 20 | 25 |
| i.m. (n = 10) | |||||
| CFA/I/II/IV MEFA | 12 | 6 | 3 | 1 | |
| 3xSTaN12S-mnLTR192G/L211A | 12 | 6 | 3 | 1 | |
dmLT adjuvant (0.2 μg) was included in the immunized groups. s.c., subcutaneous; i.m., intramuscular. Control animals received no MecVax.
Mouse intramuscular immunization with MecVax at doses of CFA/I/II/IV MEFA protein and 3xSTaN12S-mnLTR192G/L211A toxoid fusion protein that decreased from 12 to 1 μg.
To evaluate MecVax antigen dose effect using the intramuscular route, four groups of 8-week-old BALB/c mice were intramuscularly immunized with 12, 6, 3, or 1 μg of CFA/I/II/IV MEFA protein and 3xSTaN12S-mnLTR192G/L211A protein, respectively (Table 2), with 0.2 μg of dmLT adjuvant. Immunized mice received two boosters at the same dose of the primary with a 2-week interval. A group immunized with PBS was used as a control. Mice were sacrificed 2 weeks after the second booster.
Mouse immunization studies complied the 1996 National Research Council guidelines (26) and were supervised by a state attending veterinarian. Protocols were approved, respectively, by the Institutional Animal Care and Use Committee at Kansas State University and University of Illinois at Urbana-Champaign (protocol 19245).
Mouse serum antigen-specific antitoxin and anti-adhesin antibody titration.
Serum samples from each mouse subcutaneously or intramuscularly immunized with MecVax were titrated for anti-CFA/I, -CS1, -CS2, -CS3, -CS4 -CS5, -CS6, and -LT and for anti-STa IgG and IgA antibodies by ELISA as previously described (16, 18, 27–30). In brief, 100 ng of heat-extracted CFA/I, CS1, CS2, CS3, CS4, or CS5 fimbria; recombinant CS6 CssA subunit protein (for the intramuscularly immunized mice); or cholera toxin (CT; Sigma; CT is a homologue to LT and has been commonly used for anti-LT antibody titration) was coated onto each well of 2HB (Thermo Scientific, Rochester, NY) and used to titrate anti-adhesin and anti-LT antibodies. To titrate anti-STa antibodies, 10 ng of STa-ovalbumin conjugate was used to coat each well of Costar plates (Corning, Inc., Corning, NY). Serum samples from each immunized or control mouse were 2-fold diluted (from 200 to 25,600) and examined in triplicates. Horseradish peroxidase-conjugated goat anti-mouse IgG or IgA (1:5,000; Bethyl Laboratories, Montgomery, TX) was used as the secondary antibody. A 3,3′,5,5′-tetramethylbenzidine (TMB) Microwell peroxidase substrate system (2-C) (KPL, Gaithersburg, MD) was used as the substrate. Antibody titers were calculated by multiplying the adjusted optical density at 650 nm (OD650; i.e., row OD650 subtracted by background readings) by the highest serum dilution that produced an OD650 of >0.3 (above the background), and are presented as the log10 (28, 29, 31). IgM responses were not examined.
Antibody neutralization against STa or CT (LT homologue) toxin enterotoxicity.
Mouse serum samples were examined for neutralization activities against the enterotoxicity of STa and CT (commercially available CT was used to represent homologue LT) by measuring the intracellular cGMP or cAMP levels in T-84 cells with an enzyme immunoassay cAMP or cGMP kit (Enzo Life Sciences, Farmingdale, NY). As described previously (18, 28, 29, 31–33), a mouse serum sample (30 μL) from each immunization group or the control group was mixed with 10 ng of CT or 2 ng of STa, in duplicates, followed by incubation at room temperature for 30 min. Each serum-toxin mixture was added to T-84 cells, followed by incubation for 3 h (for CT in cAMP) or 1 h (for STa in cGMP) in a CO2 incubator. After a rinse with PBS (to remove extracellular cAMP or cGMP), T-84 cells were lysed (to release intracellular cAMP or cGMP), and the lysates were collected and measured for cGMP or cAMP levels (nM [pmol/mL]) according to the manufacturer’s protocols (Enzo Life Sciences).
Antibody adherence inhibition against bacteria expressing CFA/I, CS1, CS2, CS3, CS4/CS6, CS5/CS6, or CS6 adhesin.
Mouse serum samples from each group subcutaneously or intramuscularly immunized with MecVax were examined for antibody inhibition against adherence of bacteria producing CFA/I and CS1 to CS6 adhesins. As described previously (18, 27, 28, 34), ETEC or E. coli bacteria (3.5 × 106 CFU; multiplicity of infection of five bacteria per cell) pretreated with 10% mannose were mixed with 15 μL of mouse serum from each immunization or control group, in triplicates, followed by incubation on a shaker (50 rpm) at room temperature for 1 h. Each serum-bacterium mixture was added to confluent monolayer Caco-2 cells (ATCC, HTB-37; 7 × 105), which were cultured in a 24-well tissue culture plate containing Dulbecco modified Eagle medium–20% fetal bovine serum (Fisher Thermo Scientific, Pittsburg, PA). After incubation in a CO2 incubator (5% CO2) at 37°C for 1 h, Caco-2 cells were washed with PBS (to remove nonadherent bacteria) and then dislodged by incubation with 0.5% Triton X-100 (Sigma). Bacteria adherent to Caco-2 cells were collected, suspended in 1 mL of PBS, serially diluted, plated on LB plates, and counted (CFU) after overnight growth at 37°C.
Statistical analysis.
Antibody titration and antibody neutralization assays were carried out in triplicates and duplicates, respectively, with all assays repeated at least once. Differences at antibody titers (in log10) between treatments were analyzed by using a Student t test from SAS for Windows (v8; SAS Institute, Cary, NC), and differences in antibody neutralization activities were assessed using a nonparametric Mood median test. The antigen dose effect was examined using a Tukey’s multiple-comparison test (ANOVA) with a confidence interval of 95%. A P value of <0.05 was considered statistically significant.
ACKNOWLEDGMENTS
We thank James Fleckenstein (Washing University at St. Louis), Ann-Mari Svennerholm (University of Gothenburg, Gothenburg, Sweden), David Sack (Johns Hopkins University), and June Scott (Emory University) for sharing ETEC or E. coli strains. We also thank Donald Robertson (Kansas State University) for providing STa toxin and rabbit anti-STa antiserum, and we thank PATH for supplying dmLT.
Financial support for this study was provided by NIH R01AI121067 and University of Illinois at Urbana-Champaign.
Contributor Information
Weiping Zhang, Email: wpzhang@illinois.edu.
Christopher A. Elkins, Centers for Disease Control and Prevention
REFERENCES
- 1.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque ASG, Zaidi AKM, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O’Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 2.Platts-Mills JA, Liu J, Rogawski ET, Kabir F, Lertsethtakarn P, Siguas M, Khan SS, Praharaj I, Murei A, Nshama R, Mujaga B, Havt A, Maciel IA, McMurry TL, Operario DJ, Taniuchi M, Gratz J, Stroup SE, Roberts JH, Kalam A, Aziz F, Qureshi S, Islam MO, Sakpaisal P, Silapong S, Yori PP, MAL-ED Network Investigators , et al. 2018. Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: a reanalysis of the MAL-ED cohort study. Lancet Glob Health 6:E1309–E1318. doi: 10.1016/S2214-109X(18)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khalil IA, Troeger C, Blacker BF, Rao PC, Brown A, Atherly DE, Brewer TG, Engmann CM, Houpt ER, Kang G, Kotloff KL, Levine MM, Luby SP, MacLennan CA, Pan WK, Pavlinac PB, Platts-Mills JA, Qadri F, Riddle MS, Ryan ET, Shoultz DA, Steele AD, Walson JL, Sanders JW, Mokdad AH, Murray CJL, Hay SI, Reiner RC. 2018. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: the Global Burden of Disease Study 1990–2016. Lancet Infect Dis 18:1229–1240. doi: 10.1016/S1473-3099(18)30475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang ZD, DuPont HL. 2017. Etiology of travellers’ diarrhea. J Travel Med 24:S13–S16. doi: 10.1093/jtm/tax003. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W, Sack DA. 2012. Progress and hurdles in the development of vaccines against enterotoxigenic Escherichia coli in humans. Expert Rev Vaccines 11:677–694. doi: 10.1586/erv.12.37. [DOI] [PubMed] [Google Scholar]
- 6.Walker RI. 2015. An assessment of enterotoxigenic Escherichia coli and Shigella vaccine candidates for infants and children. Vaccine 33:954–965. doi: 10.1016/j.vaccine.2014.11.049. [DOI] [PubMed] [Google Scholar]
- 7.Akhtar M, Chowdhury MI, Bhuiyan TR, Kaim J, Ahmed T, Rafique TA, Khan A, Rahman SIA, Khanam F, Begum YA, Sharif MZ, Islam LN, Carlin N, Maier N, Fix A, Wierzba TF, Walker RI, Bourgeois AL, Svennerholm AM, Qadri F, Lundgren A. 2019. Evaluation of the safety and immunogenicity of the oral inactivated multivalent enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi adults in a double-blind, randomized, placebo-controlled Phase I trial using electrochemiluminescence and ELISA assays for immunogenicity analyses. Vaccine 37:5645–5656. doi: 10.1016/j.vaccine.2018.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qadri F, Akhtar M, Bhuiyan TR, Chowdhury MI, Ahmed T, Rafique TA, Khan A, Rahman SIA, Khanam F, Lundgren A, Wiklund G, Kaim J, Lofstrand M, Carlin N, Bourgeois AL, Maier N, Fix A, Wierzba T, Walker RI, Svennerholm AM. 2020. Safety and immunogenicity of the oral, inactivated, enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi children and infants: a double-blind, randomised, placebo-controlled phase 1/2 trial. Lancet Infect Dis 20:208–219. doi: 10.1016/S1473-3099(19)30571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darsley MJ, Chakraborty S, DeNearing B, Sack DA, Feller A, Buchwaldt C, Bourgeois AL, Walker R, Harro CD. 2012. The oral, live attenuated enterotoxigenic Escherichia coli vaccine ACE527 reduces the incidence and severity of diarrhea in a human challenge model of diarrheal disease. Clin Vaccine Immunol 19:1921–1931. doi: 10.1128/CVI.00364-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harro C, Louis Bourgeois A, Sack D, Walker R, DeNearing B, Brubaker J, Maier N, Fix A, Dally L, Chakraborty S, Clements JD, Saunders I, Darsley MJ. 2019. Live attenuated enterotoxigenic Escherichia coli (ETEC) vaccine with dmLT adjuvant protects human volunteers against virulent experimental ETEC challenge. Vaccine 37:1978–1986. doi: 10.1016/j.vaccine.2019.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riddle MS, Maciel M, Jr, Porter CK, Poole ST, Gutierrez RL, Gormley R, Laird RM, Sebeny PJ, Dori KE, Greenleaf ME, Hoq F, Turiansky GW, Jarell A, Hawk D, Tribble D, Savarino SJ. 2020. A first in human clinical trial assessing the safety and immunogenicity of transcutaneously delivered enterotoxigenic Escherichia coli fimbrial tip adhesin with heat-labile enterotoxin with mutation R192G. Vaccine 38:7040–7048. doi: 10.1016/j.vaccine.2020.09.025. [DOI] [PubMed] [Google Scholar]
- 12.O’Dowd A, Maciel M, Jr, Poole ST, Jobling MG, Rollenhagen JE, Woods CM, Sincock SA, McVeigh AL, Gregory MJ, Maves RC, Prouty MG, Holmes RK, Savarino SJ. 2020. Evaluation of the immunogenicity and protective efficacy of an enterotoxigenic Escherichia coli CFA/I adhesin-heat-labile toxin chimera. Infect Immun 88:e00252-20. doi: 10.1128/IAI.00252-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuhlmann FM, Martin J, Hazen TH, Vickers TJ, Pashos M, Okhuysen PC, Gomez-Duarte OG, Cebelinski E, Boxrud D, Del Canto F, Vidal R, Qadri F, Mitreva M, Rasko DA, Fleckenstein JM. 2019. Conservation and global distribution of non-canonical antigens in Enterotoxigenic Escherichia coli. PLoS Negl Trop Dis 13:e0007825. doi: 10.1371/journal.pntd.0007825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleckenstein JM, Rasko DA. 2016. Overcoming enterotoxigenic Escherichia coli pathogen diversity: translational molecular approaches to inform vaccine design. Methods Mol Biol 1403:363–383. doi: 10.1007/978-1-4939-3387-7_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turunen K, Antikainen J, Laaveri T, Kirveskari J, Svennerholm AM, Kantele A. 2020. Clinical aspects of heat-labile and heat-stable toxin-producing enterotoxigenic Escherichia coli: a prospective study among Finnish travellers. Travel Med Infect Dis 38:101855. doi: 10.1016/j.tmaid.2020.101855. [DOI] [PubMed] [Google Scholar]
- 16.Seo HGC, Ruan X, Duan Q, Sack DA, Zhang W. 2021. Preclinical characterization of immunogenicity and efficacy against diarrhea from MecVax, a multivalent enterotoxigenic E. coli vaccine candidate. Infect Immun 89:e00106-21. doi: 10.1128/IAI.00106-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia CY, Seo H, Sack DA, Zhang W. 2022. Intradermally administered enterotoxigenic Escherichia coli vaccine candidate MecVax induces functional serum immunoglobulin G antibodies against seven adhesins (CFA/I and CS1 through CS6) and both toxins (STa and LT). Appl Environ Microbiol 88:e02139-21. doi: 10.1128/AEM.02139-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan Q, Lu T, Garcia C, Yanez C, Nandre RM, Sack DA, Zhang W. 2018. Co-administered tag-less toxoid fusion 3xSTaN12S-mnLTR192G/L211A and CFA/I/II/IV MEFA (multiepitope fusion antigen) induce neutralizing antibodies to 7 adhesins (CFA/I, CS1-CS6) and both enterotoxins (LT, STa) of enterotoxigenic Escherichia coli (ETEC). Front Microbiol 9:1198. doi: 10.3389/fmicb.2018.01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones RM, Jr, Seo H, Zhang W, Sack DA. 2022. A multi-epitope fusion antigen candidate vaccine for enterotoxigenic Escherichia coli is protective against strain B7A colonization in a rabbit model. PLoS Negl Trop Dis 16:e0010177. doi: 10.1371/journal.pntd.0010177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isidean SD, Riddle MS, Savarino SJ, Porter CK. 2011. A systematic review of ETEC epidemiology focusing on colonization factor and toxin expression. Vaccine 29:6167–6178. doi: 10.1016/j.vaccine.2011.06.084. [DOI] [PubMed] [Google Scholar]
- 21.Svennerholm A-M. 2011. From cholera to enterotoxigenic Escherichia coli (ETEC) vaccine development. Indian J Med Res 133:188–196. [PMC free article] [PubMed] [Google Scholar]
- 22.Seo H, Lu T, Mani S, Bourgeois AL, Walker R, Sack DA, Zhang W. 2020. Adjuvant effect of enterotoxigenic Escherichia coli (ETEC) double-mutant heat-labile toxin (dmLT) on systemic immunogenicity induced by the CFA/I/II/IV MEFA ETEC vaccine: dose-related enhancement of antibody responses to seven ETEC adhesins (CFA/I, CS1-CS6. Hum Vaccin Immunother 16:419–425. doi: 10.1080/21645515.2019.1649555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo Q, Qadri F, Kansal R, Rasko DA, Sheikh A, Fleckenstein JM. 2015. Conservation and immunogenicity of novel antigens in diverse isolates of enterotoxigenic Escherichia coli. PLoS Negl Trop Dis 9:e0003446. doi: 10.1371/journal.pntd.0003446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez-Casal J, Swartley JS, Scott JR. 1990. Gene encoding the major subunit of CS1 pili of human enterotoxigenic Escherichia coli. Infect Immun 58:3594–3600. doi: 10.1128/iai.58.11.3594-3600.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Froehlich BJ, Karakashian A, Sakellaris H, Scott JR. 1995. Genes for CS2 pili of enterotoxigenic Escherichia coli and their interchangeability with those for CS1 pili. Infect Immun 63:4849–4856. doi: 10.1128/iai.63.12.4849-4856.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC. [Google Scholar]
- 27.Ruan X, Knudsen DE, Wollenberg KM, Sack DA, Zhang W. 2014. Multiepitope fusion antigen induces broadly protective antibodies that prevent adherence of Escherichia coli strains expressing colonization factor antigen I (CFA/I), CFA/II, and CFA/IV. Clin Vaccine Immunol 21:243–249. doi: 10.1128/CVI.00652-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruan X, Sack DA, Zhang W. 2015. Genetic fusions of a CFA/I/II/IV MEFA (multiepitope fusion antigen) and a toxoid fusion of heat-stable toxin (STa) and heat-labile toxin (LT) of enterotoxigenic Escherichia coli (ETEC) retain broad anti-CFA and antitoxin antigenicity. PLoS One 10:e0121623. doi: 10.1371/journal.pone.0121623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruan X, Robertson DC, Nataro JP, Clements JD, Zhang W, Group tSTVC . 2014. Characterization of heat-stable (STa) toxoids of enterotoxigenic Escherichia coli fused to a double mutant heat-labile toxin (dmLT) peptide in inducing neutralizing anti-STa antibodies. Infect Immun 82:1823–1832. doi: 10.1128/IAI.01394-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nandre R, Ruan X, Duan Q, Zhang W. 2016. Enterotoxigenic Escherichia coli heat-stable toxin and heat-labile toxin toxoid fusion 3xSTaN12S-dmLT induces neutralizing anti-STa antibodies in subcutaneously immunized mice. FEMS Microbiol Lett 363:fnw246. doi: 10.1093/femsle/fnw246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu M, Ruan X, Zhang C, Lawson SR, Knudsen DE, Nataro JP, Robertson DC, Zhang W. 2011. Heat-labile- and heat-stable-toxoid fusions (LTR192G-STaP13F of human enterotoxigenic Escherichia coli elicit neutralizing antitoxin antibodies. Infect Immun 79:4002–4009. doi: 10.1128/IAI.00165-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang W, Zhang C, Francis DH, Fang Y, Knudsen D, Nataro JP, Robertson DC. 2010. Genetic fusions of heat-labile (LT) and heat-stable (ST) toxoids of porcine enterotoxigenic Escherichia coli elicit neutralizing anti-LT and anti-STa antibodies. Infect Immun 78:316–325. doi: 10.1128/IAI.00497-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang C, Knudsen DE, Liu M, Robertson DC, Zhang W, Group tSTVC . 2013. Toxicity and immunogenicity of enterotoxigenic Escherichia coli heat-labile and heat-stable toxoid fusion 3xSTaA14Q-LTS63K/R192G/L211A in a murine model. PLoS One 8:e77386. doi: 10.1371/journal.pone.0077386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nandre RM, Ruan X, Duan Q, Sack DA, Zhang W. 2016. Antibodies derived from an enterotoxigenic Escherichia coli (ETEC) adhesin tip MEFA (multiepitope fusion antigen) against adherence of nine ETEC adhesins: CFA/I, CS1, CS2, CS3, CS4, CS5, CS6, CS21 and EtpA. Vaccine 34:3620–3625. doi: 10.1016/j.vaccine.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]