ABSTRACT
Exploring the potential functions of nonconserved residues on the outer side of α-helices and systematically optimizing them are pivotal for their application in protein engineering. Based on the evolutionary structural conservation analysis of GH5_5 cellulases, a practical molecular improvement strategy was developed. Highly variable sites on the outer side of the α-helices of the GH5_5 cellulase from Aspergillus niger (AnCel5A) were screened, and 14 out of the 34 highly variable sites were confirmed to exert a positive effect on the activity. After the modular combination of the positive mutations, the catalytic efficiency of the mutants was further improved. By using CMC-Na as the substrate, the catalytic efficiency and specific activity of variant AnCel5A_N193A/T300P/D307P were approximately 2.0-fold that of AnCel5A (227 ± 21 versus 451 ± 43 ml/s/mg and 1,726 ± 19 versus 3,472 ± 42 U/mg, respectively). The half-life (t1/2) of variant AnCel5A_N193A/T300P/D307P at 75°C was 2.36 times that of AnCel5A. The role of these sites was successfully validated in other GH5_5 cellulases. Computational analyses revealed that the flexibility of the loop 6-loop 7-loop 8 region was responsible for the increased catalytic performance. This work not only illustrated the important role of rapidly evolving positions on the outer side of the α-helices of GH5_5 cellulases but also revealed new insights into engineering the proteins that nature left as clues for us to find.
IMPORTANCE A comprehensive understanding of the residues on the α-helices of the GH5_5 cellulases is important for catalytic efficiency and stability improvement. The main objective of this study was to use the evolutionary conservation and plasticity of the TIM-barrel fold to probe the relationship between nonconserved residues on the outer side of the α-helices and the catalytic efficiency of GH5_5 cellulases by conducting structure-guided protein engineering. By using a four-step nonconserved residue screening strategy, the functional role of nonconserved residues on the outer side of the α-helices was effectively identified, and a variant with superior performance and capability was constructed. Hence, this study proved the effectiveness of this strategy in engineering GH5_5 cellulases and provided a potential competitor for industrial applications. Furthermore, this study sheds new light on engineering TIM-barrel proteins.
KEYWORDS: nonconserved residues, α-helices, GH5_5 family cellulases, TIM-barrel fold, catalytic efficiency, lignocellulosic biomass
INTRODUCTION
Lignocellulosic biomass is a sustainable and environmentally friendly source for producing high-industrial-value products, such as biofuels and chemicals (1). Cellulases are a class of glycoside hydrolases (GHs) that efficiently degrade the internal β-1,4-glucosidic linkages of biopolymer fibers (2). Efficient and thermostable cellulases enable more cost-efficient and sustainable processes to improve degradation under industrially applied conditions (2–4). Moreover, utilizing potent cellulases thermostable at high temperatures can reduce microbial contamination and enhance cost competitiveness. Therefore, for modern cellulase research and industrial development, it is essential to improve enzyme catalytic efficiency and thermostability.
As one of the largest glycoside hydrolase (GH) families (http://www.cazy.org), GH5s are critical in biomass conversion cocktails (5). To date, 54 subfamilies of the GH5 family are included in the CAZy (Carbohydrate-Active EnZymes: http://www.cazy.org) database, with a total of 23,253 entries. GH5_5, the largest subfamily, contains only a single EC number, primarily comprised of secreted fungal and bacterial cellulases responsible for the cleavage of endo-β-1,4 glycosidic bonds of the cellulose structure (6). Most fungal GH5_5 cellulases are active at mild temperatures (50 to 70°C) and an acidic pH (4.0 to 6.0). The GH5_5 endoglucanase II (Cel5A) of Trichoderma reesei is widely used industrially due to its high catalytic efficiency (7). To date, six eukaryotic GH5_5 cellulases (5, 8–10) have been resolved, and AnCel5A from Aspergillus niger is regarded as a potential competitor for industrial applications because of its outstanding catalytic efficiency and thermostability (9).
GH5_5 cellulases consist of a classical TIM-barrel fold characterized by eight parallel β-strands that form a central protein core and are externally covered by α-helices (5). In all known TIM-barrel fold proteins, the catalytic residues are located at the “catalytic face,” which are at the C-terminal ends of the inner β-strands and the βα-loops (11, 12), while the “stability face” is located in the core and on the opposite end of the barrel (13, 14). As the skeleton structure of the TIM-barrel fold, the α-helix connects the catalytic face and the stability face and plays a vital role in maintaining the stability of the TIM-barrel (15, 16).
The amino acids on the outer side of the α-helices of the TIM-barrel fold protein have a dual identity, located on the outer side of the α-helices and the surface of the TIM-barrel, which are usually associated with enzyme stability (17, 18). However, it has also been shown that the activities of many enzymes are affected by substituting the amino acids on the α-helices (19, 20). For instance, by introducing ion-pair interactions on the outer side of the α-helices, the catalytic efficiencies of xylanase from Bacillus circulans and Bacillus sp. 30Y5 were improved about 2.6-fold (19) and 10% to 26% (20), respectively. The amino acids located on the outer side of the α-helix further improved the specific activity of glycosylase from Talaromyces leycettanus JCM12802 by optimizing the charges (21). Altogether, these reports provide insights into the specific positions involved in the catalytic activity of α-helices, especially on the protein surface; however, because different enzymes vary largely in their structure, general methods that govern catalytic activity are still not applicable to different enzyme classes.
This study developed a systematic protein engineering strategy to screen the functional sites on the outer side of the α-helices in GH5_5 cellulases. Highly variable amino acids on the outer side of the α-helices were selected and evaluated based on folding free energy (ΔΔG) conducted by FoldX algorithm (16, 22, 23). After recombination mutation, a significant increase in catalytic efficiency and thermostability of AnCel5A was observed, and two of the most effective AnCel5A variants were characterized and analyzed computationally. Moreover, these mutations can be effectively applied to other GH5_5 cellulases. Hence, this study proved the effectiveness of this strategy in engineering GH5_5 cellulases, revealed the potential function of rapidly evolving positions, and provided a new perspective for applying this strategy to other enzyme species.
RESULTS
Computational redesign and screening of AnCel5A mutants.
The amino acids on the outer side of the α-helices of the TIM-barrel fold protein are usually associated with the stability of GH5_5 cellulases (15, 16). AnCel5A from Aspergillus niger is a cellulase with a high-resolution structure and has favorable comprehensive properties, such as relatively higher catalytic efficiency and good thermostability, making it an excellent candidate for usage for the lignocellulosic biomass degradation (9). In this study, the nonconserved residues on the outer α-helices of AnCel5A (Fig. 1) were optimized by a four-stage (I to IV) strategy to improve its catalytic efficiency further.
FIG 1.
Schematic representation of the strategy used in this study. Our strategy was comprised of four phases (I to IV). In phase I, cyan variable sites were generated with ConSurf algorithms, red conserved sites were eliminated, the sites on the outside the α-helices were selected for ΔΔG validation, and the unstable mutants in orange were filtered out. In phase II, the effective mutations were identified by experimental verification (mutations with cyan words). In phase III, the favorable mutations were grouped by helices, followed by a multipoint combination to filter out the unstable mutations. Then the most effective multipoint combination at α8 was selected by experimental validation. Finally, in the fourth phase, mutations between different helices were combined with α8 to achieve the target function (α8 in gray, and α5 in light green).
The first phase was the computational predictions of variable positions. By using ConSurf software, 35 unique sites were identified as highly variable candidates (level 1 to 3) on the outer side of the α-helices of AnCel5A (Fig. 2), and the amino acids were mutated to the type with the highest frequency calculated by ConSurf software (Fig. S1). For example, at positions 43 and 85, T and D were the amino acids with the highest evolutionary frequency, so we mutated P43 and T85 to T and D, respectively. Subsequently, these potential candidates were inspected for thermal stability using FoldX to improve the quality of the library. According to the Computer-assisted Recombination (CompassR) rule (24), beneficial substitutions are recombined with ΔΔG values ranging from +0.36 to +7.52 kcal/mol. Still, there was study showed the protein probably destabilized when the ΔΔG values were above +3 kcal/mol (25). Compared to AnCel5A, the ΔΔG values of P43T and T85D were 1.85 and −0.17, respectively, both of which were in the acceptable range; therefore, P43 and T85 were mutated to P43T and T85D, respectively. The ΔΔG value of G305P was 6.62, which was probably unstable to the protein and is known as a typical pitfall to be eliminated (Fig. S1). Therefore, except for G305P, there were 34 alternative sites as potential candidates for experimental validation.
FIG 2.
Visualization of identified amino acid positions in each phase. (A) In phase I, the 34 positions were identified at the outer side of the α-helices. (B) In phase II, 14 identified amino acid positions in AnCel5A. (C) In phase III, two beneficial positions (T300P and D307P in α8) were identified by grouped helices. (D) Three beneficial positions were confirmed from phase IV (N193A in α5, T300P and D307P in α8).
In phase II, to identify the catalytic performance, we determined the specific activity of the mutants under optimum AnCel5A conditions (75°C, pH 4.0), according to the previous experiment. After experimental validation, 14 well-expressed mutants (T85D, K88N, D92A, N193A, T231A, D232A, Q235A, D239A, K241G, D255P, S262K, A269Q, T300P, and D307P) increased catalytic performance by a ≥10% increase in specific activity (Table S2).
The strategy of evolutionary structural conservation combined with FoldX effectively screened the mutants (Fig. 3). The screening results showed that the stability of the mutants was likely to be reduced at ΔΔG > 1, and the specific activities of the mutants were reduced even if the mutated amino acid was evolutionarily favorable, as in the cases of I50A, A89Y, K227V, and R258L. However, compared to AnCel5A, most of the mutants with ΔG <0 showed improved specific activity, such as T85D, D92A, G93K, D239A, S262K, T300P, and D307P (Fig. 3).
FIG 3.
Correlation between computational and experimental screening. The difference in the proportion of amino acids before and after mutation at the 34 candidate sites, with the proportion of amino acids from the computational screening in red and the proportion of original amino acids in AnCel5A in black (left). The differences in ΔΔG prediction before and after mutation at the 34 candidate sites; if the values were >0, this indicates that the mutation is negative to enzyme stability, and if the values were <0, this indicates that the mutation is beneficial to enzyme stability (middle). The difference in specific activity between the 34 mutants and AnCel5A, with AnCel5A in black and the mutant in red (right).
In the third phase, 14 mutants with an increased specific activity of more than 10% were grouped by helices, and FoldX was used for multipoint combination calculation to predict the stability of the mutants. The 14 selected sites were distributed on 5 different α-helices (Fig. 1 and Fig. 2B). According to Table S3, the 34 mutants remained stable after combination, and therefore, these 34 mutants were evaluated in subsequent experiments.
The specific activities of the 34 selected participants were determined, and the variant T300P/D307P revealed a 1.72-fold improvement in specific activity (Fig. 4A). These two confirmed beneficial positions were located in α8 (Fig. 2C).
FIG 4.
The specific activities of mutants in phase III and phase IV. (A) The specific activities of the 34 participants in phase III. (B) In phase IV, the specific activities of the seven combinations.
Finally, in the fourth phase, mutations between different helices were combined with α8 to achieve the target function. During the first three stages, the newly formed intramolecular interactions between the native residues and mutations positively impacted the AnCel5A catalytic efficiency. Hence, the best hit, T300P/D307P, was chosen as the template for the next crossover of mutations with other helices. In this phase, there were seven predicted combinational variants, which were all beneficial positions for recombination using FoldX prediction (Table S2).
After determining the specific activity of the variants, the triple AnCel5A variant N193A/T300P/D307P exhibited the greatest improvement in specific activity (2.01-fold) compared with AnCel5A-wild type (AnCel5A-WT) (Fig. 4B). Finally, the variants AnCel5A_T300P/D307P and AnCel5A_N193A/T300P/D307 were selected for the subsequent study.
Effects of pH and temperature on AnCel5A and the mutants.
AnCel5A was optimally active at pH 4.0 and 75°C using 1% CMC-Na as the substrate, and there were no apparent differences between AnCel5A and its mutants at optimal pH and temperature (Fig. 5).
FIG 5.
Enzymatic properties of AnCel5A and the mutants. (A) Effects of pH on the enzyme activities evaluated at 75°C. (B) Effects of temperature on the enzyme activities evaluated at the indicated temperatures and pH 4.0.
The half-life (t1/2) was investigated to determine the kinetic stability of AnCel5A and the mutants. When incubated at 75°C, the t1/2 of AnCel5A was 2.5 ± 0.2 min, while the T300P/D307P and N193A/T300P/D307P mutants had t1/2 values of 5.0 ± 0.6 and 5.9 ± 0.5 min, which were 1.0 and 1.36 times higher than those of AnCel5A, respectively (Table 1). Therefore, the thermal stability of T300P/D307P and N193A/T300P/D307P was improved compared with that of WT.
TABLE 1.
t1/2 values of AnCel5A, AiCel5A, AoCel5A and their mutations
| Enzyme |
t
1/2
|
|
|---|---|---|
| 75°C | 65°C | |
| AnCel5A | 2.5 ± 0.2 | 53 ± 1.5 |
| T300P/D307P | 5.0 ± 0.6 | 60 ± 2.0 |
| N193A/T300P/D307P | 5.9 ± 0.5 | 69 ± 1.5 |
| AiCel5A | 3.5 ± 0.5 | 40 ± 0.5 |
| D308P | 7.0 ± 1.0 | 68 ± 4.7 |
| AoCel5A | NDa | 3.3 ± 0.6 |
| N214A/V321P/D328P | ND | 12 ± 2.0 |
ND means too low to be determined.
Kinetic characterization of mutants.
The effects of mutations on kinetic characterization are shown in Table 2. The kcat/Km values were 438 ± 46 and 451 ± 43 ml/s/mg, respectively, representing 1.92- and 1.98-fold of the AnCel5A, which were greatly influenced by substituting specific residues, including decreased Km values and increased Vmax and kcat values.
TABLE 2.
Kinetic values of AnCel5A and mutants with CMC-Na as the substrate
| Enzyme | sp act (U/mg) | Km (mg/ml) | Vmax (μmol/min·mg) | Kcat (s–1) | Kcat/Km (ml/s/mg) |
|---|---|---|---|---|---|
| AnCel5A | 1,726 ± 19 | 7.64 ± 0.4 | 2,786 ± 79 | 1,625 ± 46 | 227 ± 21 |
| T300P/D307P | 2,969 ± 31 | 6.51 ± 0.3 | 4,899 ± 103 | 2,857 ± 76 | 438 ± 46 |
| N193A/T300P/D307P | 3,472 ± 42 | 6.56 ± 0.4 | 5,084 ± 106 | 2,965 ± 81 | 451 ± 43 |
MD simulation analysis of the improved catalysis and thermostability.
To explore how mutations may contribute to the increased catalytic efficiencies of mutants, MD simulations were carried out for the T300P/D307P and N193A/T300P/D307P models, as well as for AnCel5A. The 20-ns MD simulations were conducted at 300, and 340 K. As shown by the root-mean-square deviation (RMSD) (Fig. S3), both systems reached dynamic equilibrium in the last 5 ns of the simulation. We found a consistent increase of the variants T300P/D307P and N193A/T300P/D307P in fluctuation in the region of α7-loop 7 and loop 6, while the root-mean-square fluctuation (RMSF) in the region of loop 8 was significantly lower than that of AnCel5A (Fig. 6A and B). This trend was observed at 300 and 340 K, indicating that the change in interactions caused by the mutation was not influenced by temperature. Furthermore, these results implied that mutation might affect the local hydrogen or electrostatic bonding networks in the regions of loop 6, α7-loop 7, and loop 8.
FIG 6.
Comparative RMSFs across all trajectories and the structure schematic. (A) RMSFs computed from MD simulations for AnCel5A and the T300P/D307P and N193A/T300P/D307P mutants at 300 K. (B) RMSFs computed from MD simulations for AnCel5A and the T300P/D307P and N193A/T300P/D307P mutants at 340 K. To highlight the differences in RMSFs between structural units, loop 6, α7-loop 7, and loop 8 regions are highlighted in blue, yellow, and green, respectively. (C) Top view of the structure schematic of loop 6, α7-loop 7, and loop 8 regions, which are highlighted in blue, yellow, and green, respectively. (D) Side view of the structure schematic of loop 6, α7-loop 7, and loop 8 regions. Mutant residues are shown as cyan sticks.
As shown in Fig. 7A, Asp307 of AnCel5A, located in the middle region of α7, formed a salt bridge with Arg258, positioned at α7; the occupancy rate of the salt bridge was 90.2% (Table 3), suggesting a strong interaction between them. In the T300P/D307P and N193A/T300P/D307P variants, the salt bridges were disrupted by introducing the proline (Fig. 7B). The salt bridge between Arg258 and Asp307 had no occupancy rate (Table 3). In addition, other interactions found in the T300P/D307P or N193A/T300P/D307P variant did not form sufficient connections as strong as the salt bridge between α7 and α8 in AnCel5A (Fig. 7A). Therefore, the variants T300P/D307P and N193A/T300P/D307P may increase the freedom of α7 because of losing the salt bridge, eliciting the violent flap of α7-loop 7, ultimately enhancing the RMSF (Fig. 6A and B).
FIG 7.
Conformational analysis of α7, α8, and loop 8 in the MD trajectory of WT and the mutants. (A) Specific interactions around the D307 and T300 residues of AnCel5A. (B) Structural effects of T300P/D307P on α7 and α8. (C) Comparison of residue conformations around W281 in AnCel5A and T300P/D307P. (D) Comparison of residue conformations around W287 in AnCel5A and T300P/D307P.
TABLE 3.
Hydrogen bond or salt bridge propensities at 340K across all simulations for residues of AnCel5A, T300P/D307P, and N193A/T300P/D307P
| WT |
T300P/D307P |
N193A/T300P/D307P |
|||
|---|---|---|---|---|---|
| Acceptor/donor | % | Acceptor/donor | % | Acceptor/donor | % |
| D307/R258 | 90.20 | P307/T303 | 3.92 | P307/T303 | 1.89 |
| I291/T300 | 64.71 | P300/G304 | 19.61 | P300/G304 | 9.73 |
| T300/G304 | 56.86 | ||||
| T300/S293 | 3.92 | ||||
| Y290/W281 | 5.88 | Y290/W281 | 37.76 | Y290/W281 | 41.36 |
| Y249/W281 | 50.94 | Y249/W281 | 75.29 | Y249/W281 | 68.91 |
| W287/S293 | 54.12 | W287/S293 | 88.24 | W287/S293 | 89.67 |
| G284/W287 | 27.45 | G284/W287 | 39.22 | G284/W287 | 32.98 |
| W287/Y290 | 5.88 | W287/Y290 | 37.76 | W287/Y290 | 41.53 |
As shown in Fig. 6A and B, the RMSF values of loop 6 in the T300P/D307P and N193A/T300P/D307P variants were significantly higher than those of the WT, indicating that the motion of loop 6 was influenced by α7-loop7. Introducing N193A further improved loop 6 motion, enhanced the interactions between the enzyme and substrate, and ultimately increased the specific activity and the kinetic parameters of the N193A/T300P/D307P variant (26).
Thr300 of AnCel5A connects α8 and loop 8, forming four hydrogen bonds with Ile291, Ser293, Ala301, and Gly304, conforming to the regular motion of loop 8 (Fig. 7A). After introducing proline at position 300, there were sufficient changes to provide stronger Trp281 and Trp287 binding connections with Tyr249 and Tyr290, and the hydrogen bond occupancy rates of Trp281 and Trp287 were evidently higher than those of WT (Table 3), indicating that there were more residues forming hydrogen bonds with Trp281 and Trp287 to stabilize them, and thus the RMSF values were lower than those of WT.
Mutation verification on other GH5_5 cellulases.
Since these sites were screened mainly based on the structural conservation of GH5_5 family fungal cellulases, the screened residues may have a common effect on the other GH5_5 cellulases. Two GH5_5 cellulases, AoCel5A and AiCel5A, were selected to verify the common effect of the positive residues, and the corresponding sites of these two cellulases were replaced according to the results of AnCel5A. Five of 14 positive mutants (screened from phase II) were selected for experimental validation on AiCel5A and AoCel5A. When CMC-Na (1%) was used as the substrate, the optimal temperature and pH of AiCel5A were 75°C and 4.0, respectively, while AoCel5A exhibited maximal activity at 65°C and pH 4.0 (data not shown). There were no apparent differences between AiCel5A/AoCel5A and their mutants in terms of temperature and pH, consistent with the results of AnCel5A and its mutants. These results indicated that these specific mutations maybe not be optimal only for any given set of conditions.
The specific activity and kinetic parameters of AiCel5A/AoCel5A and their mutants were measured under their optimal conditions. Compared with the WT, obvious changes were detected in the catalytic activity of the variants (Table 4). Except for AoCel5A_T105D and AoCel5A_Q256A, the kinetic values of the other variants, Vmax and kcat/Km, increased. Double and triple combinations of the mutations were also constructed based on the results of AnCel5A, and the results showed that the specific activity and catalytic efficiency of the combined mutations were further improved (Table 4), which is also consistent with the results of AnCel5A. The catalytic efficiency of AiCel5A_D308P and AoCel5A_N214A/V321P/D328P increased 25.5% and 58.9%, respectively, compared with their WT proteins. The specific activities of variants AiCel5A_D308P and AoCel5A_N214A/V321P/D328P were improved by 31 and 75%, respectively, compared with the WT proteins.
TABLE 4.
Kinetic values of AoCel5A, AiCel5A, and mutants with CMC-Na as the substrate
| Enzyme | sp act (U/mg) | Km (mg/ml) | Vmax (μmol/min·mg) | Kcat (s–1) | Kcat/Km (ml/s/mg) |
|---|---|---|---|---|---|
| AiCel5A | 1,157 ± 17 | 5.74 ± 0.4 | 2,228 ± 77 | 1,188 ± 43 | 207 ± 21 |
| T85D | 1,379 ± 37 | 5.67 ± 0.3 | 2,413 ± 67 | 1,407 ± 38 | 248 ± 19 |
| Q236A | 1,281 ± 28 | 6.17 ± 0.7 | 2,328 ± 57 | 1,358 ± 32 | 220 ± 23 |
| D308P | 1,520 ± 16 | 4.98 ± 0.3 | 2,664 ± 87 | 1,420 ± 43 | 285 ± 29 |
| AoCel5A | 1,069 ± 21 | 7.94 ± 0.5 | 1,403 ± 61 | 818 ± 31 | 117 ± 19 |
| T105D | 905 ± 23 | 7.34 ± 0.9 | 1,333 ± 57 | 777 ± 30 | 105 ± 17 |
| N214A | 1,091 ± 27 | 7.51 ± 0.6 | 1,593 ± 64 | 870 ± 34 | 123 ± 18 |
| Q256A | 968 ± 21 | 7.37 ± 0.6 | 1,370 ± 77 | 799 ± 39 | 108 ± 19 |
| V321P | 1,112 ± 27 | 6.90 ± 0.5 | 1,522 ± 76 | 888 ± 39 | 128 ± 39 |
| D328P | 1,755 ± 18 | 6.74 ± 0.4 | 2,056 ± 83 | 1,199 ± 45 | 178 ± 31 |
| V321P/D328P | 1,839 ± 22 | 6.68 ± 0.4 | 2,173 ± 81 | 1,267 ± 49 | 189 ± 27 |
| N214A/V321P/D328P | 1,881 ± 31 | 6.93 ± 0.3 | 2,210 ± 71 | 1,289 ± 52 | 186 ± 31 |
Improvements were also observed in the degree of thermostability (Table 1). The t1/2 of AoCel5A_N214A/V321P/D328P and t1/2 of AiCel5A_D308P were 2-fold and 3.6-fold that of their WT proteins, respectively (Table 1). Combined with the previous results, these results further demonstrated that engineering based on this strategy could effectively improve the catalytic efficiency and thermostability of GH5_5 cellulases, and more importantly, such experimental results further confirmed the positive effect of the residues on the outer site of the α-helices on the catalytic efficiency of TIM-barrel structured cellulase.
Hydrolysis of PASC with cellulase.
The same amounts of AnCel5A, AnCel5A_N193A/T300P/D307P, and commercial cellulase TrCel5A were used to compare their ability to degrade PASC. Trichoderma reesei is an important strain for cellulase production (2), which produced efficient and thermotolerant cellulases for commercial use.
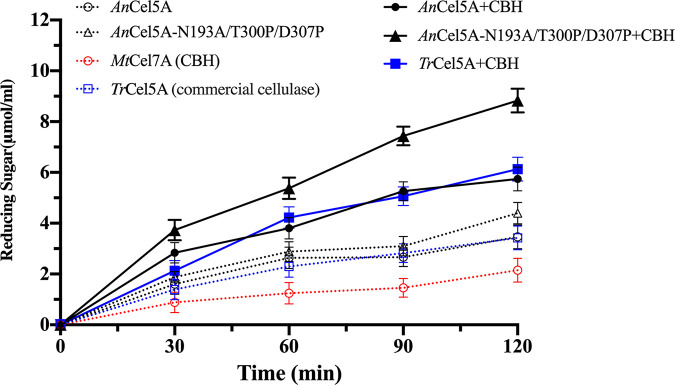
As shown in Fig. 8, compared to AnCel5A, the ability to degrade PASC of AnCel5A_N193A/T300P/D307P was improved. Furthermore, the enzyme combinations released more reducing sugars than the individual enzyme. The combination of AnCel5A, AnCel5A_N193A/T300P/D307P, TrCel5A, and MtCel7A released 5.74 mM, 8.83 mM, and 6.13 mM reducing sugars from PASC at 2 h, respectively, which were higher than that released by AnCel5A_N193A/T300P/D307P or MtCel7A individually from PASC. Hence, the results indicated that AnCel5A_N193A/T300P/D307P and MtCel7A have synergistic action on cellulose degradation. Meanwhile, AnCel5A_N193A/T300P/D307P showed a better ability to degrade PASC, the reducing sugar yields of AnCel5A_N193A/T300P/D307P with MtCel7A were 1.44 times that of TrCel5A with MtCel7A.
FIG 8.
Time-course hydrolysis of PASC. Time profiling of reducing sugars production was detected every 0.5 h for a total of 2 h under pH 5.0 and 50°C.
DISCUSSION
As a potential competitor in industrial applications, GH5_5 cellulases have high catalytic efficiency and outstanding thermostability. In the past few decades, there have been many studies on engineering the catalytic efficiency of GH5_5 cellulases, most of which have focused on the catalytic face of the TIM-barrel fold, especially loops. For instance, by site-directed mutagenesis in the loop near the active site, Zheng et al. (26) and Singh et al. (27) enhanced the catalytic activities of GtCel5 and EG5C-1 to 297 ± 8 ml/mg/s and 2.0 × 104 ml/mg/min, respectively. The α-helix is usually used to improve the thermostability of an enzyme (5, 16). However, the nonconserved residues on the outer side of the α-helices were identified to be associated with the catalysis of the TIM-barrel cellulases in this study, although located in the noncatalytic face. With a four-step optimization strategy, the catalytic efficiency of AnCel5A_N193A/T300P/D307P has been at a higher level of the GH5_5 family (451 ± 43 ml/mg/s), superior to the reported results (26–28). Furthermore, the mutant AnCel5A_N193A/T300P/D307P has a synergistic action on PASC degradation with CBH (MtCel7A), which released 1.44-fold as many reducing sugars as the commercial cellulase (from Trichoderma reesei) with CBH (MtCel7A).
The proposed strategy effectively accelerated the evolution of highly variable residues to produce more efficient enzymes, which has the universality for the GH5_5 family fungal cellulases. The enormous diversity of protein structures and functions can be regarded as the result of the enormous number of variable random processes that have occurred in nature in a sustained way for millions of years (29, 30). Conserved amino acids in proteins result from optimization processes during evolution (31, 32) which have more chance of increasing enzyme catalytic efficiency (26). Since the sites screened by the strategy resulted from evolutionary-based optimization, they not only improved the catalytic efficiency and thermostability of AnCel5A but also have universality. Meanwhile, the screened sites were not limited by the original amino acids nor the optimum condition of the cellulases. For instance, the corresponding T300P mutation in AnCel5A was in threonine, while in AoCel5A, it was in valine, and the optimum temperature of AoCel5A is 65°C, which is significantly different from the optimum temperature of AnCel5A at 75°C. However, the specific activity and the t1/2 of AoCel5A_N214A/V321P/D328P were 1.75-fold and 3.6-fold that of the WT when CMC-Na was used as the substrate. Therefore, at least for the TIM-barrel fold enzymes, this novel finding may help other researchers carry out subsequent work.
Modularity is a typical feature of TIM-barrel structures. Based on earlier studies of this kind of fold, the (βα)8-barrel evolved from duplication and fusion events of a half barrel or even smaller units (33). Recently, structure-based recombination has been carried out to fuse TIM-barrel fragments (15, 34, 35), and the recombinant chimeras showed different characteristics or advantages in activity (35) or temperature (36) from the parents. These results confirmed the inherently evolvable scaffold of the TIM-barrel and inferred that subdomain elements could evolve the TIM-barrel. Therefore, instead of directed evolution, the modular structure characteristics of the TIM-barrel structure were utilized to further accumulate the effect in this study. The α-helix was regarded as the basic evolutionary unit for combining the mutation sites, which greatly improved screening efficiency. It was a laboratory process that naturally evolved at an accelerated rate, and recombination of the α-helix significantly expanded the influence of a single point on variants, whose catalytic efficiency increased from approximately 10 or 20% to 72%.
Activity and thermostability are two important indicators for the industrial applications of enzymes. The goal of engineering a protein is to improve the thermal stability without losing catalytic activity (2, 37). Normally, mutants with increased catalytic efficiency usually decrease their stability because of the flexibility-stability trade-off enzymes (38), but some works have questioned this paradigm (39, 40), which also provides the opportunity for the engineering of proteins. In our study, AnCel5A_T300P/D307P and AnCel5A_N193A/T300P/D307P showed both improved catalytic efficiency and stability, which could reduce the cost required for industrial production. Comparative molecular dynamics (MD) simulations of AnCel5A and its variants were analyzed to reveal the reasons of improved catalytic efficiency and thermostability. We suspected that improved movement of loop 6 and α7-loop 7, the steady enhancement of key aromatic residues on loop 8, and the rigidity of proline were the primary reasons by which the mutants increased their catalytic performance without losing thermostability.
The GH5_5 cellulase active sites are formed by a shallow and wide groove lined with aromatic residues, including Trp281 and Trp287, which are located in the −2 binding site of AnCel5A (9) and provide π − π or σ − π packing interactions with the substrate to stabilize sugar moieties (5, 9). Prior evidence suggested that although GH5 enzymes have many aromatic residues along their substrate binding grooves, only two residues are strictly conserved, one of which is Trp287 located at the −2 subsite. This indicates the importance of Trp287 to the GH5 family cellulases (41). The improved stability of Trp287 could maintain the catalytic process and might be an avenue for improving catalytic efficiency. In addition, earlier studies have shown that the flapping of loop6 and loop7 is the most prominent feature for catalyzing the deprotonation of dihydroxyacetone phosphate (DHAP) or d-glyceraldehyde 3-phosphate (DGAP) (42, 43). Hence, the consistently increasing fluctuation in the α7-loop7 and loop6 regions may be another reason for the improved catalytic efficiency.
Salt bridges are important factors related to enzyme thermostability (44, 45). Since aspartic acid was mutated to proline, the salt bridge between Arg258 and Asp307 was destroyed, but the thermal stability of the T300P/D307P variant did not decrease (Table 1). Many studies have shown that proline has unique and rigid structural characteristics. The pyrrolidine ring of proline imposes rigid limits on the N-Cα rotation, giving proline less conformational freedom than other amino acids in any secondary structure (46). Two prolines were introduced at α8, which is a critical structural determinant for the thermostability of GH5 enzymes (16, 47). Therefore, we hypothesized that proline rigidity might compensate for the absence of a salt bridge at the C terminus of the protein. However, to the best of our knowledge, destroying salt bridges and introducing proline in α-helices to improve the catalytic performance of enzymes have rarely been reported (48, 49). Hence, such an evolution-guided result provided new insights into engineering proteins.
The optimum temperature for the thermophilic GH5 endoglucanase Egl5A (TeEgl5A) from Talaromyces emersonii, a typical thermophilic enzyme in the GH5 family, is 90°C (50). The sequence similarities of AnCel5A with TeEgl5A was just 49.6%, but one of the mutation residues D307P was identical to TeEgl5A, which may be involved in the maintenance of the thermal stability of the C terminus of GH5_5 cellulases (11, 47). Over billions of years, enzymes have been adapting and evolving in response to selection pressures from their environments (29). Enzymes evolve with greater stability to overcome thermal denaturation and maintain a folded structure with increased temperatures. The C terminus of GH5_5 cellulases is a key structural determinant to maintain the thermal stability of TIM-barrel fold cellulases (16, 47), which may also be a product of the thermal evolution of GH5_5.
In this study, a systematic protein engineering strategy was developed to screen the functional sites on the outer side of the α-helices in GH5_5 cellulases. Three of 34 sites were effective for evolving AnCel5A with a superior capability, which could be more competitive in industrial processes. This work illustrated that rapidly evolving positions at the TIM-barrel outer surface could play a role in increasing the catalytic performance of GH5_5 enzymes. Furthermore, such an unexpected result inspired us to infer that natural optimization would change views on rational design to a certain extent and provided new insights into engineering proteins that nature has left as clues for us to find.
MATERIALS AND METHODS
Strains, plasmids, and chemicals.
Escherichia coli TransI-T1 (TransGen Biotech Co., Ltd., Beijing, China) and Pichia pastoris GS115 (Invitrogen, Carlsbad, CA, USA) were used as the cloning and expression hosts, respectively. The plasmid pEASY-T3 (TransGen Biotech Co., Ltd.) and the shuttle vector pPIC9 (Invitrogen) were used for gene cloning and target gene expression, respectively. Restriction endonucleases (BglII, EcoRI, and NotI) were purchased from Thermo Fisher Scientific (Waltham, MA, USA). The Fast Mutagenesis System kit was purchased from TransGen Biotech Co., Ltd. Carboxymethyl cellulose-sodium (CMC-Na, medium viscosity) from Sigma-Aldrich (St. Louis, MO, USA) was used as the substrate. All chemicals were of analytical grade and were commercially available.
Gene cloning and site-directed mutagenesis.
The AnCel5A-encoding gene (GenBank: AF331518.1) was synthesized according to Yan et al. (9). To verify the common effect of the screened sites, two GH5_5 cellulases AoCel5A and AiCel5A sharing 77.1 and 81.2% identity with AnCel5A, respectively, were selected, and the corresponding sites of these two cellulases were replaced. The AoCel5A-encoding gene (GenBank: BAD72778.1) from Aspergillus oryzae and the AiCel5A-encoding gene (GenBank: PYI28634.1) from Aspergillus indologenus CBS 114.80 were synthesized with P. pastoris codon preference optimization. After NotI and EcoRI digestion, the gene without a signal peptide coding sequence was cloned into the pPIC9 vector. All mutants were constructed using plasmids pPIC9-AnCel5A, pPIC9-AoCel5A, and pPIC9-AiCel5A as templates. The primers are listed in the supplemental material.
Computational redesign of AnCel5A mutants.
A total of 5870 GH5_5 cellulases were included in the CAZy database, 93% of which were from bacteria, and the remaining approximately 500 sequences were from fungi. Although the sequence identity between the bacterial and fungal cellulase was only about 30%, both bacterial and fungal origins shared TIM barrel folds (5, 7). Compared to the bacterial cellulases, the GH5_5 fungal cellulases are known for high efficiency and versatile substrate utilization, in particular, strains of Aspergillus, Penicillium, and Trichoderma (51), so these 500 sequences were selected for statistical analysis.
The first phase of the strategy consisted of constructing a single mutation library using ConSurf software (http://consurf.tau.ac.il/) (52) and FoldX (22). The conservation scores were divided into nine grades for visualization and projected onto the three-dimensional structures of AnCel5A (Fig. 9A and B) (53). The frequency of grades 1 to 3 is less than 30%, representing the variable positions, colored turquoise in Fig. 9. In comparison, the frequency of grades 6 to 9 is more than 60%, representing the conserved positions, colored maroon in Fig. 9. The variable sites grade 1 to 3 on the outer side of the α-helices were selected as potential mutation positions. Subsequently, these potential candidates were inspected for thermal stability using FoldX to exclude unfavorable sites. The AnCel5A crystal structure (PDB: 5I77; chain A) was applied for ΔΔG calculation using FoldX with the following parameters: pH 4.0; temperature, 298 K; ionic strength, 0.05 M. By using the “Repair Object” command, the nonstandard torsion angles of residues were corrected. Three FoldX runs were calculated for each mutation. The ΔΔG (ΔΔG = ΔGfold, mut − ΔGfold, wt) values higher than 3.0 were excluded as destabilizing mutations (22, 24, 25).
FIG 9.

Schematic diagram of AnCel5A. (A) Top view of AnCel5A. The catalytic residues Glu142 and Glu248 are shown as sticks. The residues are colored according to the conservation scores as shown in the Color code. The most variable residues are colored in turquoise, and the frequency of which is approximately 0–30%, while the most conserved residues are colored in maroon, and the frequency of which is approximately 60–90%. (B) Side view of AnCel5A; the residues are colored according to the conservation scores. (C) Top view of AnCel5A; the residues are colored according to the conservation scores. (D) Side view of AnCel5A. The residues on the outer side of the α-helices are colored cyan.
Protein expression and purification.
The recombinant plasmids were linearized using BglII and introduced into P. pastoris-competent cells by electroporation. The positive transformants were screened on histidine-deficient minimal dextrose medium plates and then confirmed by their cellulase activity at the shake-tube level. Moreover, the potentially positive transformants were verified by colony PCR and sequencing. Subsequently, the transformants with the highest cellulase activity were selected for scaled-up cultivation. First, the positive transformants were inoculated in yeast extract peptone dextrose medium for 72 h at 30°C to obtain the seed fermentation broth. Then, the seed fermentation broth was inoculated into a 300-ml buffered glycerol-complex medium (BMGY) for cell propagation. After culturing at 30°C and 200 rpm for 48 h, the cells were transferred into a 200 ml of buffered methanol-complex medium (BMMY) containing 0.5% (vol/vol) methanol for protein expression.
The culture supernatants were harvested at 12,000 × g for 20 min at 4°C and were concentrated using an ultrafiltration membrane with a molecular weight cutoff of 10 kDa (Vivascience AG, Hannover, Germany). After overnight dialysis in 10 mM Tris-HCl (pH 8.0) (buffer A) at 4°C, the crude enzymes were applied to a HiTrap Q HP Anion Exchange Column (GE Healthcare, Uppsala, Sweden). The protein was eluted in a linear gradient of NaCl (0 to 1.0 M). Fractions exhibiting cellulase activity were collected and pooled, followed by SDS-PAGE analysis. N-linked glycosylation is one of the most prevalent posttranslational modifications in eukaryotes, resulting in a larger molecular weight than actual. Endo-β-N-acetyl-glucosaminidase H (Endo H; New England Biolabs, Ipswich, MA, USA) was used to remove N-glycans and the molecular mass was decreased to the predicted theoretical value after digestion. The concentrations of the purified recombinant enzymes were determined using the Bradford method.
Enzymatic characterization.
The 3,5-dinitrosalicylic acid (DNS) method (54) was used to determine the cellulase activity. Reaction mixtures contained 900 μL of 1% CMC-Na (wt/vol) in buffer (100 mM citric acid and 200 mM Na2HPO4, pH 4.0) and appropriately diluted enzyme (100 μl). The reaction mixtures were incubated for 10 min, followed by adding 1.5 ml DNS solution and boiling for 5 min to terminate the reactions. The amount of released reducing sugars was determined by measuring the absorbance at 540 nm. One unit of cellulase activity was defined as the amount of enzyme producing 1 μmol of reducing sugar per min.
The optimal pH of AnCel5A and its mutants was determined at 75°C in the pH range of 2.0 to 8.0. The temperature optima were determined at pH 4.0 at 30 to 90°C. Thermostability was investigated after incubation of the samples at 65 or 75°C (pH 4.0) for different periods, and pH stability was assessed after preincubating the enzymes (approximately 150 μg/ml) in various buffers (pH 3.0 to 10.0) at 37°C for 1 h. Residual enzyme activities were measured under optimal conditions.
The kinetic values were determined using 1 to 15 mg/ml CMC-Na as the substrate at pH 4.0 and 75°C for 5 min. GraphPad Prism 9.0 (GraphPad Software Inc., San Diego, CA, USA) was applied to calculate the values (Km and Vmax) using a nonlinear regression algorithm. The kcat and kcat/Km were calculated based on the Km and Vmax values. All reactions were performed in triplicate.
Structure analysis and molecular dynamics simulations.
Two homology models, AnCel5A_T300P/D307P and AnCel5A_N193A/T300P/D307P, were constructed using Discovery Studio 2017 (BIOVIA, San Diego, CA, USA) with PDB structure 5I77 from A. niger (9) as the structural template. The MD simulation was implemented by AMBER 14 along with the standard ff99SB force field (55), and each simulation was repeated in triplicate. Each system was simulated in a dodecahedral box filled with TIP3P explicit water within 1 nm from the protein edge. Na+ or Cl− were added to neutralize the charge in the system. A 10,000-step steepest descent was executed for energy minimization before the MD simulations. After energy minimization, the systems were gradually heated from 0 to 300 K over 100 ps, followed by 20-ns MD simulations using the Langevin algorithm with a 2-fs time step at 300 or 340 K and a pressure of 1.0 atm. Long-range electrostatic interactions were calculated using the particle mesh Ewald (PME) method (56), and bonds involving hydrogen atoms were constrained using the SHAKE algorithm (57).
Mutation verification on other GH5_5 cellulases.
To verify the universality of the strategy, the corresponding five positive mutations were performed on AiCel5A and AoCel5A. Ten mutants were constructed and expressed in P. pastoris GS115. For AoCel5A, including AoCel5A_T105D, AoCel5A_N214A, AoCel5A_Q256A, AoCel5A_V321P, AoCel5A_D328P, AoCel5A_V321P/D328P, and AoCel5A_N214A/V321P/D328P. For AiCel5A, three of the five variable sites were already consistent with the mutation types obtained from our strategy, so only three mutants were constructed, including AiCel5A_D308P, AiCel5A_T85D, AiCel5A_Q236A.
Enzymatic hydrolysis of phosphoric acid swollen cellulose (PASC).
To test the ability to degrade PASC, the commercial cellulase (TrCel5A, Sigma), AnCel5A, or the variant N193A/T300P/D307P was added to 10 ml 100 mM pH 5.0 phosphate buffer at the concentration of 0.1 mg/ml. The cellobiohydrolase (CBH) from Myceliophthora thermophila (58) was added at the concentration of 0.01 mg/ml to synergistic degradation of PASC. The mixtures were incubated at 50°C and 700 rpm for 2 h with 1% PASC. PASC was produced from Avicel (Sigma), as described previously (59). The production of reducing sugars was determined by the DNS method. Experiments were conducted in triplicate.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (grant no. 31872395), the State Key Laboratory of Animal Nutrition Project 2004DA125184G2101 and the earmarked fund for China Agriculture Research System.
We declare no conflict of interest.
J.Z., H.-Q.L., and H.-Q.H. conceived the study. J.Z. performed the experiments, analyzed the results, and wrote the manuscript. H.-Q.L. performed the simulations and analyzed the results. X.Q. analyzed the results. K.Y. performed the experiments. J.T. analyzed and explained the structure-function relationship. X.-L.W. and Y.-R.W. discussed the rational design of variants. Y.W. and B.Y. analyzed data. H.-Y.L. discussed the rational design of variants and revised the manuscript. The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript.
Footnotes
Supplemental material is available online only.
Contributor Information
Huo-qing Huang, Email: huanghuoqing@caas.cn.
Nicole R. Buan, University of Nebraska-Lincoln
REFERENCES
- 1.Bilal M, Wang Z, Cui J, Ferreira LFR, Bharagava RN, Iqbal HMN. 2020. Environmental impact of lignocellulosic wastes and their effective exploitation as smart carriers–a drive towards greener and eco-friendlier biocatalytic systems. Sci Total Environ 722:137903. 10.1016/j.scitotenv.2020.137903. [DOI] [PubMed] [Google Scholar]
- 2.Kuhad RC, Gupta R, Singh A. 2011. Microbial cellulases and their industrial applications. Enzyme Res 2011:280696. 10.4061/2011/280696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maleki M, Shahraki MF, Kavousi K, Ariaeenejad S, Hosseini Salekdeh G. 2020. A novel thermostable cellulase cocktail enhances lignocellulosic bioconversion and biorefining in a broad range of pH. Int J Biol Macromol 154:349–360. 10.1016/j.ijbiomac.2020.03.100. [DOI] [PubMed] [Google Scholar]
- 4.Olofsson J, Barta Z, Börjesson P, Wallberg O. 2017. Integrating enzyme fermentation in lignocellulosic ethanol production: life-cycle assessment and techno-economic analysis. Biotechnol Biofuels 10:51. 10.1186/s13068-017-0733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Payne CM, Knott BC, Mayes HB, Hansson H, Himmel ME, Sandgren M, Ståhlberg J, Beckham GT. 2015. Fungal Cellulases. Chem Rev 115:1308–1448. 10.1021/cr500351c. [DOI] [PubMed] [Google Scholar]
- 6.Aspeborg H, Coutinho PM, Wang Y, Brumer H, Henrissat B. 2012. Evolution, substrate specificity and subfamily classification of glycoside hydrolase family 5 (GH5). BMC Evol Biol 12:186. 10.1186/1471-2148-12-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neis A, da Silva Pinto L. 2021. Glycosyl hydrolases family 5, subfamily 5: relevance and structural insights for designing improved biomass degrading cocktails. Int J Biol Macromol 193:980–995. 10.1016/j.ijbiomac.2021.10.062. [DOI] [PubMed] [Google Scholar]
- 8.Liu G, Li Q, Shang N, Huang J-W, Ko T-P, Liu W, Zheng Y, Han X, Chen Y, Chen C-C, Jin J, Guo R-T. 2016. Functional and structural analyses of a 1,4-β-endoglucanase from Ganoderma lucidum. Enzyme Microb Technol 86:67–74. 10.1016/j.enzmictec.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Yan J, Liu W, Li Y, Lai H-L, Zheng Y, Huang J-W, Chen C-C, Chen Y, Jin J, Li H, Guo R-T. 2016. Functional and structural analysis of Pichia pastoris-expressed Aspergillus niger 1,4-β-endoglucanase. Biochem Biophys Res Commun 475:8–12. 10.1016/j.bbrc.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Vakhrusheva AV, Nemashkalov VA, Kravchenko OV, Tishchenko SV, Gabdulkhakov AG, Kljashtorny VG, Korotkova OG, Gusakov AV, Sinitsyn AP. 2017. Structural investigation of endoglucanase 2 from the filamentous fungus Penicillium verruculosum. Crystallogr Rep 62:254–259. 10.1134/S1063774517020304. [DOI] [Google Scholar]
- 11.Wierenga RK, Kapetaniou EG, Venkatesan R. 2010. Triosephosphate isomerase: a highly evolved biocatalyst. Cell Mol Life Sci 67:3961–3982. 10.1007/s00018-010-0473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richard JP, Zhai X, Malabanan MM. 2014. Reflections on the catalytic power of a TIM-barrel. Bioorg Chem 57:206–212. 10.1016/j.bioorg.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wierenga RK. 2001. The TIM-barrel fold: a versatile framework for efficient enzymes. FEBS Lett 492:193–198. 10.1016/S0014-5793(01)02236-0. [DOI] [PubMed] [Google Scholar]
- 14.Sterner R, Höcker B. 2005. Catalytic versatility, stability, and evolution of the (βα)8-barrel enzyme fold. Chem Rev 105:4038–4055. 10.1021/cr030191z. [DOI] [PubMed] [Google Scholar]
- 15.Zheng F, Vermaas JV, Zheng J, Wang Y, Tu T, Wang X, Xie X, Yao B, Beckham GT, Luo H. 2019. Activity and thermostability of GH5 endoglucanase chimeras from mesophilic and thermophilic parents. Appl Environ Microbiol 85:e02079-18. 10.1128/AEM.02079-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Contreras F, Thiele MJ, Pramanik S, Rozhkova AM, Dotsenko AS, Zorov IN, Sinitsyn AP, Davari MD, Schwaneberg U. 2020. KnowVolution of a GH5 cellulase from Penicillium verruculosum to improve thermal stability for biomass degradation. ACS Sustainable Chem Eng 8:12388–12399. 10.1021/acssuschemeng.0c02465. [DOI] [Google Scholar]
- 17.Foong FC, Doi RH. 1992. Characterization and comparison of Clostridium cellulovorans endoglucanases-xylanases EngB and EngD hyperexpressed in Escherichia coli. J Bacteriol 174:1403–1409. 10.1128/jb.174.4.1403-1409.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar S, Tsai CJ, Nussinov R. 2000. Factors enhancing protein thermostability. Protein Eng 13:179–191. 10.1093/protein/13.3.179. [DOI] [PubMed] [Google Scholar]
- 19.Pokhrel S, Joo JC, Lee H-S, Yoo YJ. 2013. Activity enhancement of a Bacillus circulans xylanase by introducing ion-pair interactions into an α-helix. Process Biochem 48:1495–1501. 10.1016/j.procbio.2013.07.008. [DOI] [Google Scholar]
- 20.Lai Z, Zhou C, Ma XC, Xue YF, Ma YH. 2021. Enzymatic characterization of a novel thermostable and alkaline tolerant GH10 xylanase and activity improvement by multiple rational mutagenesis strategies. Int J Biol Macromol 170:164–177. 10.1016/j.ijbiomac.2020.12.137. [DOI] [PubMed] [Google Scholar]
- 21.Tong LG, Zheng J, Wang X, Wang X, Huang H, Yang HM, Tu T, Wang YR, Bai YG, Yao B, Luo HH, Qin X. 2021. Improvement of thermostability and catalytic efficiency of glucoamylase from Talaromyces leycettanus JCM12802 via site-directed mutagenesis to enhance industrial saccharification applications. Biotechnol Biofuels 14:202. 10.1186/s13068-021-02052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buß O, Rudat J, Ochsenreither K. 2018. FoldX as protein engineering tool: better than random based approaches? Comput Struct Biotechnol J 16:25–33. 10.1016/j.csbj.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui Y, Chen Y, Liu X, Dong S, Tian Y, Qiao Y, Mitra R, Han J, Li C, Han X, Liu W, Chen Q, Wei W, Wang X, Du W, Tang S, Xiang H, Liu H, Liang Y, Houk KN, Wu B. 2021. Computational redesign of a PETase for plastic biodegradation under ambient condition by the GRAPE strategy. ACS Catal 11:1340–1350. 10.1021/acscatal.0c05126. [DOI] [Google Scholar]
- 24.Cui H, Cao H, Cai H, Jaeger K-E, Davari MD, Schwaneberg U. 2020. Computer-Assisted Recombination (CompassR) teaches us how to recombine beneficial substitutions from directed evolution campaigns. Chemistry 26:643–649. 10.1002/chem.201903994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen SV, Stein A, Dinitzen AB, Papaleo E, Tatham MH, Poulsen EG, Kassem MM, Rasmussen LJ, Lindorff-Larsen K, Hartmann-Petersen R. 2017. Predicting the impact of Lynch syndrome-causing missense mutations from structural calculations. PLoS Genet 13:e1006739. 10.1371/journal.pgen.1006739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng F, Tu T, Wang XY, Wang Y, Ma R, Su XY, Xie XM, Yao B, Luo HY. 2018. Enhancing the catalytic activity of a novel GH5 cellulase GtCel5 from Gloeophyllum trabeum CBS 900.73 by site-directed mutagenesis on loop 6. Biotechnol Biofuels 11 10.1186/s13068-018-1080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh S, Kumar K, Nath P, Goyal A. 2020. Role of glycine 256 residue in improving the catalytic efficiency of mutant endoglucanase of family 5 glycoside hydrolase from Bacillus amyloliquefaciens SS35. Biotechnol Bioeng 117:2668–2682. 10.1002/bit.27448. [DOI] [PubMed] [Google Scholar]
- 28.Akbarzadeh A, Pourzardosht N, Dehnavi E, Ranaei Siadat SO, Zamani MR, Motallebi M, Nikzad Jamnani F, Aghaeepoor M, Barshan Tashnizi M. 2018. Disulfide bonds elimination of endoglucanase II from Trichoderma reesei by site-directed mutagenesis to improve enzyme activity and thermal stability: an experimental and theoretical approach. Int J Biol Macromol 120:1572–1580. 10.1016/j.ijbiomac.2018.09.164. [DOI] [PubMed] [Google Scholar]
- 29.Pinney MM, Mokhtari DA, Akiva E, Yabukarski F, Sanchez DM, Liang R, Doukov T, Martinez TJ, Patricia C, Babbitt PC, Herschlag D. 2021. Parallel molecular mechanisms for enzyme temperature adaptation. Science 371:eaay2784. 10.1126/science.aay2784. [DOI] [PubMed] [Google Scholar]
- 30.Newton MS, Guo X, Söderholm A, Näsvall J, Lundström P, Andersson DI, Selmer M, Patrick WM. 2017. Structural and functional innovations in the real-time evolution of new (βα)8 barrel enzymes. Proc Natl Acad Sci USA 114:4727–4732. 10.1073/pnas.1618552114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davidson R, Baas B-J, Akiva E, Holliday GL, Polacco BJ, LeVieux JA, Pullara CR, Zhang YJ, Whitman CP, Babbitt PC. 2018. A global view of structure-function relationships in the tautomerase superfamily. J Biol Chem 293:2342–2357. 10.1074/jbc.M117.815340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang DY, Li Y, Wu WQ, Zhang H, Xu R, Xu H, Zhan R, Sun L. 2021. Identification and engineering on the nonconserved residues of metallo-β-lactamase-type thioesterase to improve the enzymatic activity. Biotech Bioengineering 118:4623–4634. 10.1002/bit.27921. [DOI] [PubMed] [Google Scholar]
- 33.Lang D, Thoma R, Henn-Sax M, Sterner R, Wilmanns M. 2000. Structural evidence for evolution of the β/α barrel scaffold by gene duplication and fusion. Science 289:1546–1546. 10.1126/science.289.5484.1546. [DOI] [PubMed] [Google Scholar]
- 34.Sharma P, Kaila P, Guptasarma P. 2016. Creation of active TIM barrel enzymes through genetic fusion of half-barrel domain constructs derived from two distantly related glycosyl hydrolases. FEBS J 283:4340–4356. 10.1111/febs.13927. [DOI] [PubMed] [Google Scholar]
- 35.Lapidoth G, Khersonsky O, Lipsh R, Dym O, Albeck S, Rogotner S, Fleishman SJ. 2018. Highly active enzymes by automated combinatorial backbone assembly and sequence design. Nat Commun 9:2780. 10.1038/s41467-018-05205-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mangiagalli M, Lotti M. 2021. Cold-Active β-Galactosidases: insight into cold adaption mechanisms and biotechnological exploitation. Marine Drugs 19:43. 10.3390/md19010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel AK, Singhania RR, Sim SJ, Pandey A. 2019. Thermostable cellulases: current status and perspectives. Bioresour Technol 279:385–392. 10.1016/j.biortech.2019.01.049. [DOI] [PubMed] [Google Scholar]
- 38.D’Amico S, Marx J-C, Gerday C, Feller G. 2003. Activity-stability relationships in extremophilic enzymes. J Biol Chem 278:7891–7896. 10.1074/jbc.M212508200. [DOI] [PubMed] [Google Scholar]
- 39.Oikawa T, Kazuoka T, Soda K. 2003. Paradoxical thermostable enzymes from psychrophile: molecular characterization and potentiality for biotechnological application. J Mol Catalysis B Enzymatic 23:65–70. 10.1016/S1381-1177(03)00073-0. [DOI] [Google Scholar]
- 40.Mangiagalli M, Lapi M, Maione S, Orlando M, Brocca S, Pesce A, Barbiroli A, Camilloni C, Pucciarelli S, Lotti M, Nardini M. 2021. The co-existence of cold activity and thermal stability in an Antarctic GH42 β-galactosidase relies on its hexameric quaternary arrangement. FEBS J 288:546–565. 10.1111/febs.15354. [DOI] [PubMed] [Google Scholar]
- 41.Lo Leggio L, Larsen S. 2002. The 1.62 A structure of Thermoascus aurantiacus endoglucanase: completing the structural picture of subfamilies in glycoside hydrolase family 5. FEBS Lett 523:103–108. 10.1016/S0014-5793(02)02954-X. [DOI] [PubMed] [Google Scholar]
- 42.Desamero R, Rozovsky S, Zhadin N, McDermott A, Callender R. 2003. Active site loop motion in triosephosphate isomerase: T-jump relaxation spectroscopy of thermal activation. Biochemistry 42:2941–2951. 10.1021/bi026994i. [DOI] [PubMed] [Google Scholar]
- 43.Zhai X, Amyes TL, Richard JP. 2015. Role of loop-clamping side chains in catalysis by Triosephosphate Isomerase. J Am Chem Soc 137:15185–15197. 10.1021/jacs.5b09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan CH, Yu TH, Wong KB. 2011. Stabilizing Salt-bridge enhances protein thermostability by reducing the heat capacity change of unfolding. PLoS One 6:e21624. 10.1371/journal.pone.0021624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watanabe K, Chishiro K, Kitamura K, Suzuki Y. 1991. Proline residues responsible for thermostability occur with high frequency in the loop regions of an extremely thermostable oligo-1,6-glucosidase from Bacillus thermoglucosidasius KP1006. J Biol Chem 266:24287–24294. 10.1016/S0021-9258(18)54226-5. [DOI] [PubMed] [Google Scholar]
- 46.Macarthur MW, Thornton JM. 1991. Influence of proline residues on protein conformation. J Mol Biol 218:397–412. 10.1016/0022-2836(91)90721-H. [DOI] [PubMed] [Google Scholar]
- 47.Liu W, Tu T, Gu Y, Wang Y, Zheng F, Zheng J, Wang Y, Su X, Yao B, Luo H. 2019. Insight into the thermophilic mechanism of a glycoside hydrolase family 5 β-Mannanase. J Agric Food Chem 67:473–483. 10.1021/acs.jafc.8b04860. [DOI] [PubMed] [Google Scholar]
- 48.Sun Z, Liu Q, Qu G, Feng Y, Reetz MT. 2019. Utility of B-Factors in protein science: interpreting rigidity, flexibility, and internal motion and engineering thermostability. Chem Rev 119:1626–1665. 10.1021/acs.chemrev.8b00290. [DOI] [PubMed] [Google Scholar]
- 49.Nestl BM, Hauer B. 2014. Engineering of flexible loops in enzymes. ACS Catal 4:3201–3211. 10.1021/cs500325p. [DOI] [Google Scholar]
- 50.Wang K, Luo H, Bai Y, Shi P, Huang H, Xue X, Yao B. 2014. A thermophilic endo-1,4-β-glucanase from Talaromyces emersonii CBS394.64 with broad substrate specificity and great application potentials. Appl Microbiol Biotechnol 98:7051–7060. 10.1007/s00253-014-5680-0. [DOI] [PubMed] [Google Scholar]
- 51.Passos D, Pereira N, Castro A. 2018. A comparative review of recent advances in cellulases production by Aspergillus, Penicillium and Trichoderma strains and their use for lignocellulose deconstruction. Curr Opin Green Sustain Chem 14:60–66. 10.1016/j.cogsc.2018.06.003. [DOI] [Google Scholar]
- 52.Armon A, Graur D, Ben-Tal N. 2001. ConSurf: identification of functional regions in proteins by surface-mapping of phylogenetic information. J Mol Biol 307:447–463. 10.1006/jmbi.2000.4474. [DOI] [PubMed] [Google Scholar]
- 53.Glaser F, Rosenberg Y, Kessel A, Pupko T, Ben-Tal N. 2005. The ConSurf-HSSP database: the mapping of evolutionary conservation among homologs onto PDB structures. Proteins 58:610–617. 10.1002/prot.20305. [DOI] [PubMed] [Google Scholar]
- 54.Miller GL. 1959. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. 10.1021/ac60147a030. [DOI] [Google Scholar]
- 55.Kirschner KN, Yongye AB, Tschampel SM, González-Outeiriño J, Daniels CR, Foley BL, Woods RJ. 2008. GLYCAM06: a generalizable biomolecular force field. Carbohydrates. J Comput Chem 29:622–655. 10.1002/jcc.20820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Darden T, York D, Pedersen L. 1993. Particle mesh Ewald: an N⋅log(N) method for Ewald sums in large systems. J Chem Physics 98:10089–10092. 10.1063/1.464397. [DOI] [Google Scholar]
- 57.Ryckaert JP, Ciccotti G, Berendsen HJC. 1977. Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J Comput Physics 23:327–341. 10.1016/0021-9991(77)90098-5. [DOI] [Google Scholar]
- 58.Kadowaki MAS, Higasi P, de Godoy MO, Prade RA, Polikarpov I. 2018. Biochemical and structural insights into a thermostable cellobiohydrolase from Myceliophthora thermophila. FEBS J 285:559–579. 10.1111/febs.14356. [DOI] [PubMed] [Google Scholar]
- 59.Aissa K, Novy V, Nielsen F, Saddler J. 2019. Use of Carbohydrate binding modules to elucidate the relationship between fibrillation, hydrolyzability, and accessibility of cellulosic substrates. ACS Sustainable Chem Eng 7:1113–1119. 10.1021/acssuschemeng.8b04780. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 to S3 and Fig. S1 to S5. Download aem.01046-22-s0001.pdf, PDF file, 4.3 MB (4.3MB, pdf)