ABSTRACT
Flavobacterium columnare causes columnaris disease in wild and aquaculture-reared freshwater fish. F. columnare virulence mechanisms are not well understood, and current methods to control columnaris disease are inadequate. Iron acquisition from the host is important for the pathogenicity and virulence of many bacterial pathogens. F. columnare iron acquisition has not been studied in detail. We identified genes predicted to function in siderophore production for ferric iron uptake. Genes predicted to encode the proteins needed for siderophore synthesis, export, uptake, and regulation were deleted from F. columnare strain MS-FC-4. The mutants were examined for defects in siderophore production, for growth defects in iron-limited conditions, and for virulence against zebrafish and rainbow trout. Mutants lacking all siderophore activity were obtained. These mutants displayed growth defects when cultured under iron-limited conditions, but they retained virulence against zebrafish and rainbow trout similar to that exhibited by the wild type, indicating that the F. columnare MS-FC-4 siderophores are not required for virulence under the conditions tested.
IMPORTANCE Columnaris disease, which is caused by Flavobacterium columnare, is a major problem for freshwater aquaculture. Little is known regarding F. columnare virulence factors, and control measures are limited. Iron acquisition mechanisms such as siderophores are important for virulence of other pathogens. We identified F. columnare siderophore biosynthesis, export, and uptake genes. Deletion of these genes eliminated siderophore production and resulted in growth defects under iron-limited conditions but did not alter virulence in rainbow trout or zebrafish. The results indicate that the F. columnare strain MS-FC-4 siderophores are not critical virulence factors under the conditions tested but may be important for survival under iron-limited conditions in natural aquatic environments or aquaculture systems.
KEYWORDS: columnaris disease, fish pathogen, siderophore
INTRODUCTION
Flavobacterium columnare causes columnaris disease in many species of freshwater fish (1, 2). This includes important aquaculture fish such as rainbow trout, channel catfish, tilapia, and many others (3, 4). Columnaris epidemics result in high mortality rates and have a large economic impact on aquaculture facilities. F. columnare virulence mechanisms remain poorly understood, and current control measures are inadequate, relying heavily on antibiotics that can lead to the spread of antibiotic resistance.
Genomic and physiological studies revealed some potential virulence factors (2, 5–7). These include proteases, chondroitin-sulfate lyases, adhesins, and secretion systems that deliver these proteins to the outside of the cell. Genetic experiments are beginning to determine which of the predicted virulence factors are important for disease. Such studies demonstrated that the type IX protein secretion system (T9SS) is critical for F. columnare virulence (8, 9). Proteins secreted by the T9SS include predicted adhesins, chondroitin-sulfate lyases, proteases, and nucleases. However, individual secreted proteins that have been examined thus far, including chondroitinases and several proteases, appear to be less critical for virulence (9, 10).
The ability to acquire iron in vivo is essential for most infectious bacterial pathogens. Host animals produce high-affinity iron-binding proteins such as transferrin and lactoferrin that sequester extracellular iron (11). Salmonids, for example, produce transferrin proteins (12) that restrict the iron available to pathogens such as F. columnare. Bacteria evolved efficient mechanisms to acquire ferric iron and compete with host iron sequestration. Ferric iron-uptake systems are critical virulence factors in Gram-negative pathogens such as Escherichia coli, Pseudomonas aeruginosa, and the fish pathogen Edwardsiella ictaluri (13–15).
Siderophores are common components of bacterial ferric iron acquisition systems. These small, high-affinity iron chelators are secreted by the bacteria to chelate the generally insoluble ferric iron. The siderophore-iron complexes are taken up by specific receptors on the bacterial cell surface (16). The high affinity of siderophores for ferric iron allows bacterial pathogens to compete for iron with transferrins and other components of the host iron-sequestration system. Siderophores are known virulence factors of some fish pathogens, such as Vibrio anguillarum (17). Bacterial siderophores are diverse in structure and are typically assembled either by large multienzyme nonribosomal peptide synthetases (NRPSs), by smaller Iuc (iron-uptake chelate) enzymes, or by a combination of the two (16, 18, 19).
Aerobactin is a well studied siderophore produced by E. coli and many other Gram-negative bacteria (20, 21). It is not synthesized by a NRPS but rather relies on four Iuc enzymes, IucA, IucB, IucC, and IucD, for its synthesis. IucB and IucD acetylate and hydroxylate lysine, respectively, to form acetyl-hydroxylysine (ahLys). IucA and IucC attach ahLys to citrate to form aerobactin (22). The outer membrane receptor IutA (iron-uptake transport) is used to take up the siderophore-iron complex.
Several studies of iron acquisition mechanisms of F. columnare and related fish pathogens have been reported (23–26). Genome analyses revealed F. columnare genes predicted to be involved in iron acquisition (5, 6). F. columnare responds to iron deprivation by altering expression of some of these genes and by altering virulence (27). Studies of Flavobacterium psychrophilum revealed that iron deprivation increased expression of some antigenic proteins and increased the effectiveness of attenuated vaccine strains (28, 29). F. columnare produces siderophores under iron-limited conditions (23). Exposure to fish mucus resulted in increased expression of genes predicted to be involved in siderophore production (30). However, the roles played by F. columnare siderophores in disease remain to be determined.
In this study, we identified genes of F. columnare strain MS-FC-4 (31) predicted to be involved in siderophore production, export, uptake, and regulation. This wild-type strain was selected because it was isolated from an outbreak in rainbow trout, is virulent, and is amenable to genetic manipulation (9, 31, 32). F. columnare MS-FC-4 belongs to genomovar 1 and genetic group 1 (31) and is thus a bona fide F. columnare strain, whereas members of other genetic groups of F. columnare fish pathogens have recently been assigned different Flavobacterium species names (33). Using the efficient genetic techniques developed for F. columnare strain MS-FC-4 (9), we constructed siderophore-deficient mutants and used them to investigate the importance of the F. columnare siderophores for growth in iron-limited conditions and for the ability to cause disease in zebrafish and in rainbow trout.
RESULTS
Identification of siderophore-related genes in F. columnare strain MS-FC-4.
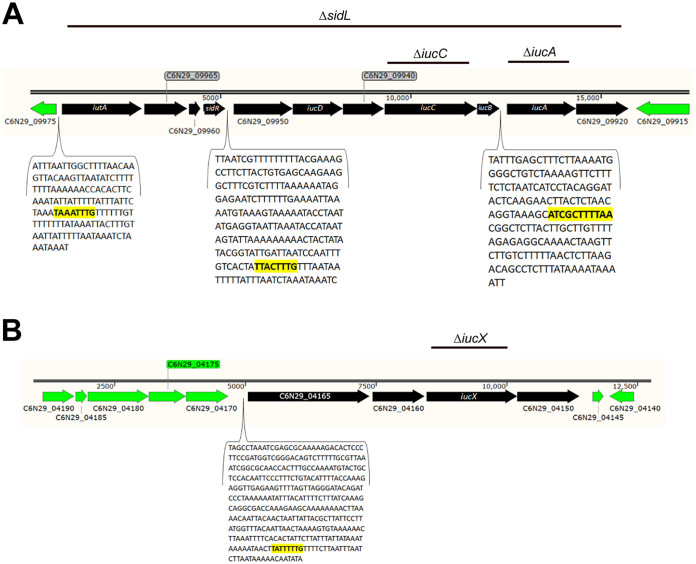
We examined the F. columnare MS-FC-4 genome and identified 15 genes encoding proteins predicted to be involved in siderophore synthesis, export, uptake, and regulation (Table 1). Of these genes, 11 were clustered in a region spanning 14 kilobase pairs (kbp) (Fig. 1A). Among these 11 were genes encoding orthologs of the siderophore biosynthesis enzymes IucA, IucB, IucC, and IucD, a predicted siderophore efflux transporter (C6N29_09920), the outer membrane receptor IutA, and a predicted regulatory protein that we named SidR. The genes of this sidL (siderophore locus) cluster (C6N29_09970 to C6N29_09920) are transcribed in the same direction and appear to be organized in three operons, spanning C6N29_09970 to C6N29_09955, C6N29_09950 to C6N29_09930, and C6N29_09925 to C6N29_09920. Before the first two predicted operons, sequences similar to Bacteroidota (previously Bacteroidetes) σA-dependent “housekeeping” promoters (TTG/TAnnTTTG) (34) were detected. The region upstream of the third predicted operon has a sequence resembling the recently described F. psychrophilum extracellular function sigma factor (σECF) binding motif SM6 (35).
TABLE 1.
F. columnare genes encoding proteins predicted to function in siderophore biosynthesis, export, and uptakea
| Locus tag | Protein name | NCBI product name | Conserved domainsb | Predicted functionc | Protein localizationd |
|---|---|---|---|---|---|
| C6N29_04150 | MFS transporter | Macrolide efflux protein A and similar major facilitator superfamily transporters (cd06173, pfam07690) | Siderophore export | Cytoplasmic membrane | |
| C6N29_04155 | IucX | Hypothetical protein | RhbC (rhizobactin IucA/IucC-like siderophore biosynthesis protein) super family (cl34746, COG4264, pfam04183, pfam06276) | Siderophore biosynthesis | Cytoplasm |
| C6N29_04160 | Hypothetical protein | PRK14016 super family, cyanophycin synthetase, provisional (cl36324), RimK-like ATP grasp domain (pfam08443) | Siderophore biosynthesis | Cytoplasm | |
| C6N29_04165 | TonB-dependent siderophore receptor | OM channel superfamily, possible TonB-dependent siderophore receptor (cl21487 cl21470, TIGR01783, pfam00593, pfam07715) | Siderophore uptake | Outer membrane | |
| C6N29_09920 | MATE family efflux transporter | MATE_like_14 (cd13139, TIGR00797, pfam01554) | Siderophore export | Cytoplasmic membrane | |
| C6N29_09925 | IucA | IucA/IucC family siderophore biosynthesis protein | IucA/IucC family siderophore biosynthesis protein (COG4264, pfam04183, pfam06276) | Siderophore biosynthesis | Cytoplasm |
| C6N29_09930 | IucB | N-Acetyltransferase | Acetyltransferase (GNAT) domain (pfam13523) | Siderophore biosynthesis | Cytoplasm |
| C6N29_09935 | IucC | GNAT family N-acetyltransferase | Acetyltransferase (GNAT) domain (pfam13523), IucA/IucC family siderophore biosynthesis protein (COG 4264, pfam04183, pfam06276) | Siderophore biosynthesis | Cytoplasm |
| C6N29_09940e | DUF1624 domain-containing protein | Acyl_transf_3 super family (cl21495, pfam07786) | Cytoplasmic membrane | ||
| C6N29_09945 | IucD | Alcaligin biosynthesis protein | Lysine/ornithine N-monooxygenase (COG3486, pfam13434) | Siderophore biosynthesis | Cytoplasm |
| C6N29_09950e | Aspartate aminotransferase family protein | Glutamate or tyrosine decarboxylase or related PLP-dependent protein (COG0076, pfam00282) | Cytoplasm | ||
| C6N29_09955 | SidR | LuxR family transcriptional regulator | HTH_LUXR (smart00421, COG2197, pfam00196) | Regulation of siderophore synthesis | Cytoplasm |
| C6N29_09960e | Hypothetical protein | Cytoplasm | |||
| C6N29_09965e | Peptidase M4 | PepSY_TM (pfam03929) | Cytoplasmic membrane | ||
| C6N29_09970 | IutA | TonB-dependent siderophore receptor | TonB-dependent siderophore receptor (cl21487, TIGR01783, pfam00593, pfam07715) | Siderophore uptake | Outer membrane |
MATE, multidrug and toxic compound extrusion; MFS, major facilitator superfamily; OM, outer membrane; GNAT, Gcn5-related N-acetyltransferase.
Conserved domains as assigned by NCBI and by the Joint Genome Institute Integrated Microbial Genomes and Microbiomes (IMG/M version 6.0, https://img.jgi.doe.gov/m) (53). TIGRFAM, pfam, smart, cl, cd, or COG numbers are indicated.
Function predicted based on conserved domains and gene organization.
Location of each protein was predicted using psortb 3.0 (65).
C6N29_09940, C6N29_09950, C6N29_09960, and C6N29_09965 did not have predicted functions but were included in this table because they were part of the sidL locus and were conserved in the seven genomes analyzed in Table S1.
FIG 1.
Map of region containing predicted F. columnare MS-FC-4 siderophore biosynthesis, export, uptake, and regulation genes. Black arrows indicate siderophore-related genes and the directions in which they are transcribed. Green arrows indicate genes flanking the siderophore gene regions. Numbers above the genes refer to the number of kilobase pairs (kbp) of sequence. Regions deleted in mutants are indicated by lines above each map. (A) The sidL locus spans 11 genes predicted to be involved in siderophore synthesis, export, uptake, or regulation. The gaps between genes containing predicted promoter sequences suggest organization as three operons. The sequence within each bracket is the region between the two coding regions, with Bacteroidota promoters shown in bold and highlighted yellow. (B) iucA/iucC-like gene, iucX, and nearby genes. The five genes in green shown upstream of C6N29_04165 appear unrelated to siderophores and instead form an apparent ParB-related, ThiF-related cassette (PRTRC) system, encoding proteins with TIGR03736, TIGR03737, TIGR03738, and TIGR03741 domains.
The remaining four genes predicted to be related to siderophore synthesis or function (C6N29_04150, C6N29_04155 [iucX], C6N29_04160, and C6N29_04165) form an apparent operon 1.2 Mbp away from the sidL locus (Fig. 1B). IucX is similar in sequence to IucA and IucC of the sidL locus and may be involved in siderophore synthesis, C6N29_04150 encodes a predicted transporter, and C6N29_04165 encodes a protein with a TonB-dependent siderophore receptor domain (TIGR01783).
E. coli IucA and IucC are related proteins that are critical for production of the hydroxamate siderophore, aerobactin, and contribute to pathogenicity (36–38). The F. columnare IucA, IucC, and IucX proteins each contain the conserved domains pfam04183 and pfam06276, which define members of the IucA/IucC siderophore-synthase component domain family (Table 1). These are the only predicted F. columnare proteins that have either of these domains. F. columnare IucA and IucC are 45.9% identical to each other, and IucX is 18.8% identical to IucA and 21.0% identical to IucC (Fig. S1). The F. columnare IucA, IucC, and IucX proteins exhibit regions of similarity to those from E. coli (Fig. S2 to S4). F. columnare IucA is 22% identical to E. coli IucA over 448 amino acids, F. columnare IucC is 28% identical to E. coli IucC over 583 amino acids, and IucX exhibits 20% identity to E. coli IucA over 430 amino acids and 22% amino acid identity to E. coli IucC over 324 amino acids. F. columnare IucX is also 28% identical to the Staphylococcus aureus siderophore-synthesis protein SfaB, an IucA/IucC-like protein that produces the carboxylate siderophore staphyloferrin A (39, 40).
Siderophores are exported by efflux transporters (41). E. coli contains at least seven genes encoding cytoplasmic membrane proteins related to multidrug efflux transporters that could export siderophores (42). A predicted F. columnare multidrug and toxic compound extrusion (MATE) family efflux transporter (C6N29_09920) was encoded within the sidL region. This transporter may export the siderophore produced by the enzymes encoded by the sidL locus. Similarly, a gene encoding a predicted major facilitator transporter (C6N29_04150) was immediately downstream of iucX and may be involved in exporting a siderophore produced by IucX.
When siderophores bind ferric iron, they form a ferri-siderophore complex that is transported into the cell by specific membrane proteins. E. coli IutA is an outer membrane receptor involved in uptake of iron-bound siderophores (43), and the distantly related F. columnare protein C6N29_09970 (20% amino acid identity to E. coli IutA over 171 amino acids) may have a similar role. The F. columnare and E. coli IutA proteins each contain a conserved TonB-dependent siderophore receptor domain (TIGR01783). C6N29_04165, which encodes another protein with a predicted TonB-dependent siderophore receptor domain, was located two genes upstream of iucX (Fig. 1B).
Genome analyses of F. columnare MS-FC-4 revealed no other predicted siderophore biosynthetic genes. Many bacterial siderophores are synthesized by NRPSs (16, 18), but F. columnare MS-FC-4 appears to lack NRPSs of any type. Its genome lacks genes encoding proteins with domains that belong to pfam00668 (condensation domain) and TIGR01720 (nonribosomal peptide synthase domain) that are characteristic of NRPSs. Two other protein domains associated with siderophore-synthesis NRPSs were also absent: TIGR02275 (2,3-dihydroxybenzoate-AMP ligase) and TIGR04316 (2,3-dihydro-2,3-dihydroxybenzoate dehydrogenase).
The genomes of six other F. columnare strains (CSF-298-10, ATCC 49512, Pf1, TC 1691, C#2, and 94-081) were also examined. Each of the genomes contained a region similar to sidL, with genes encoding orthologs of each of the proteins encoded by the F. columnare MS-FC-4 sidL locus arranged in the same order (Table S1). Each of the 11 proteins encoded by the sidL locus from these six strains were similar to or identical to the F. columnare MS-FC-4 proteins. Unlike F. columnare MS-FC-4, which has three genes encoding IucA/IucC-like proteins (iucA and iucC within the sidL locus and iucX 1.2 Mbp away), the other six strains lacked iucX and thus had only two genes encoding IucA/IucC-like proteins (iucA and iucC). Examination of the genomes revealed an apparent insertion of 39 genes (including iucX) in the F. columnare MS-FC-4 genome extending from C6N29_04030 (encoding conjugal transfer protein TraG) to C6N29_04220 (encoding a predicted phage integrase protein) that was not present in the other genomes. This region contained many genes encoding predicted conjugative proteins and phage-related proteins, suggesting a foreign origin. Similar to strain MS-FC-4, each of the other six genomes lacked genes encoding proteins with domains that belong to pfam00668 (condensation domain) and TIGR01720 (nonribosomal peptide synthase domain) that are characteristic of NRPSs, and they also lacked genes encoding NRPS proteins belonging to TIGRFAM families TIGR02275 and TIGR04316.
Mutants defective in siderophore production.
The region spanning the sidL gene cluster (C6N29_09920 to C6N29_09970) was deleted, and the ΔsidL mutant was examined. A chrome azurol S (CAS) assay was used to detect siderophore production by the wild-type and mutant strains (Fig. 2). The presence of siderophores is indicated by an orange halo around the F. columnare growth. Compared to the wild type, the ΔsidL mutant exhibited greatly reduced siderophore activity. Single deletions of iucA and iucC, which are important for aerobactin synthesis in E. coli, were also constructed. The halos formed by cells of the F. columnare ΔiucA and ΔiucC mutants were smaller than the wild-type halo, suggesting that these genes are each involved in siderophore synthesis (Fig. 2). A double mutant lacking iucA and iucC produced a halo that was smaller than was observed for either single iuc deletion but larger than observed for ΔsidL. This result and the similarity in sequence between IucA and IucC (Fig. S1) suggest that these proteins may have overlapping functions and may exhibit partial redundancy.
FIG 2.

Siderophore activity for F. columnare wild type (WT), siderophore synthesis gene deletion mutants, and complemented mutants. Midexponential cultures were spotted onto chrome azurol S (CAS) plates and incubated at 28°C for 72 h. Siderophore presence is indicated by an orange halo around F. columnare growth. pRC48 is a complementation plasmid carrying iucA, and pRC56 is a complementation plasmid carrying iucX. This figure also includes chromosomal complementation strains in which genes labeled with “CC” were inserted at their original position in the chromosome. For the ΔiucX ΔiucA ΔiucC mutant, iucA was inserted back into the chromosome, and for the ΔsidL ΔiucX mutant, iucX was inserted back into the chromosome.
The ΔiucA ΔiucC double mutant retained some siderophore activity (Fig. 2). To determine whether the remotely located iucA/iucC-like gene iucX (C6N29_04155) contributed to this residual activity, we deleted iucX and analyzed the mutant for siderophore production. Deletion of iucX from wild-type cells did not cause a recognizable defect in siderophore production (Fig. 2). In contrast, the triple mutant, ΔiucA ΔiucC ΔiucX exhibited a dramatic decrease in siderophore production similar to that seen for the ΔsidL mutant. Complementation of the ΔiucX ΔiucA ΔiucC triple mutant with the multicopy plasmid pRC48, which carries iucA expressed from a plasmid promoter, restored siderophore production. The halo for this complemented strain was similar in size to that exhibited by the wild type. The ability of iucA alone to complement for the loss of the three iucA/iucC-like genes might be explained by its presence on a multicopy plasmid and its expression from a plasmid promoter. To examine complementation by iucA expressed from its native promoter, we integrated it into its original position on the chromosome of the ΔiucX ΔiucA ΔiucC mutant. This strain, which we refer to as ΔiucX ΔiucACC ΔiucC (where the subscript letters CC indicate chromosomal complementation by integration of iucA), exhibited partial restoration of siderophore production (Fig. 2). Together, the results presented above suggest partial redundancy of function between the related IucA, IucC, and IucX proteins.
In an attempt to eliminate all siderophore synthesis, we deleted sidL and iucX, generating the ΔsidL ΔiucX mutant. The ΔsidL ΔiucX mutant exhibited the greatest defect in siderophore production (Fig. 2). Complementation with pRC56, containing iucX, partially restored siderophore production to the low level observed for the ΔsidL mutant. Chromosomal complementation with iucX demonstrated similar results. The decreased siderophore production of the ΔsidL ΔiucX mutant compared to the ΔsidL mutant and the complementation results support a minor role for iucX in siderophore production.
Siderophore production mutants exhibit growth defects in iron-limited and chelated iron conditions.
Wild-type F. columnare and siderophore mutants were grown in iron-rich tryptone yeast extract salts (TYES) (44) and iron-limited (TS) media to examine how siderophore production affects growth (Fig. 3). TS medium is TYES medium without yeast extract, which contains various forms of iron (45). Wild-type cells grew nearly as well in TS as in TYES (Fig. 3). The ΔiucA, ΔiucC, and ΔiucX mutants grew similar to the wild type in TYES or TS media. The triple mutant lacking all iucA/iucC-like genes (ΔiucX ΔiucA ΔiucC) exhibited a growth defect in TYES and in TS media. Complementation of the ΔiucX ΔiucA ΔiucC triple mutant with pRC48, which carries iucA, or by chromosomal complementation with iucA restored growth in limited-iron conditions to near wild-type levels (Fig. 4).
FIG 3.
Growth of F. columnare strains in iron-rich tryptone yeast extract salt (TYES) and iron-limited (TYES medium without yeast extract [TS]) media. (A) Wild type (WT) and single iuc gene deletion mutants grown in TYES. (B) Wild type and single iuc gene deletion mutants grown in TS. (C) Multiple siderophore gene deletion mutants grown in TYES. A significant difference in peak cell density was seen between the WT and ΔsidL ΔiucX mutant (P < 0.05). (D) Multiple deletion mutants grown in TS. Significant differences in peak cell density were seen between the WT and ΔsidL mutants, between the WT and ΔiucX ΔiucA ΔiucC mutants, and between the WT and ΔsidL ΔiucX mutants (P < 0.05). Strains were grown at 28°C with shaking (200 rpm), and measurements were taken every 2 h for 36 h. The error bars represent standard error of the mean.
FIG 4.

Growth of F. columnare wild-type, siderophore mutants, and complemented mutant strains in iron-limited (TS) medium. (A) Wild type (WT), ΔiucX ΔiucA ΔiucC mutant, ΔiucX ΔiucA ΔiucC mutant complemented with a plasmid pRC48, which carries iucA, and ΔiucX ΔiucA ΔiucC mutant chromosomally complemented by inserting iucA back into the chromosome. A significant difference in peak cell density was seen between the WT and the ΔiucX ΔiucA ΔiucC mutant (P < 0.05). (B) WT, ΔsidL ΔiucX mutant, ΔsidL ΔiucX mutant complemented with plasmid pRC56, which carries iucX, and ΔsidL ΔiucX chromosomally complemented by inserting iucX back into the chromosome. A significant difference in peak cell density was seen between the WT and ΔsidL ΔiucX mutant (P < 0.05). Strains were grown at 28°C with shaking (200 rpm), and measurements were taken every 2 h for 36 h. The error bars represent standard error of the mean.
The ΔsidL mutant grew nearly as well as the wild type in TYES medium but declined more rapidly once the stationary phase was reached (Fig. 3). In TS, the ΔsidL mutant showed a growth defect similar to the ΔiucX ΔiucA ΔiucC mutant. The ΔsidL ΔiucX double mutant, which did not produce siderophores, exhibited a lag in growth and a significantly lower peak cell density compared to the wild type when grown in either TYES or TS. Introduction of iucX into the ΔsidL ΔiucX mutant either on plasmid (pRC56) or by integration into the chromosome partially restored growth in limited-iron conditions (Fig. 4).
The results presented above indicate that growth defects of F. columnare siderophore mutants were seen in iron-limited conditions. In fish tissues, even less ferric iron could be available because of sequestration by iron-binding proteins such as transferrin. Under those conditions, siderophores could be important to allow F. columnare to acquire ferric iron and mount an infection.
To decrease iron availability further and mimic the expected iron-restricted environment of fish tissues, we grew F. columnare in the presence of the iron chelator deferiprone (46). Deferiprone at 728 μM prevented growth of wild-type F. columnare (Fig. S5). Supplementation with ferric chloride restored growth, suggesting that the inhibitory effects of deferiprone were the result of iron chelation rather than of some other toxic effect. The minimum concentration of chelator needed to prevent growth was determined for each F. columnare strain (Table 2). The wild-type strain required the highest deferiprone concentration (728 μM) to prevent growth. Deletion of iucA, iucC, or iucX resulted in modest reductions in the amount of deferiprone required to prevent growth. Deletion of both iucA and iucC or deletion of iucA, iucC, and iucX resulted in a larger reduction in the amount of deferiprone needed to prevent growth, suggesting the importance of IucA/IucC-like proteins in iron uptake. The ΔsidL and ΔsidL ΔiucX mutants required only 50 μM deferiprone to prevent growth, indicating the importance of siderophores for F. columnare growth under conditions where free iron levels are low because of sequestration.
TABLE 2.
F. columnare growth in TYES broth with iron chelatora
| Strain | Amt of chelator (deferiprone) needed to prevent growth, μMb,c | Amt of iron (FeCl3) needed to restore turbid growthc,d |
|---|---|---|
| Wild type | 728 | 100 |
| ΔiucA | 345 | 40 |
| ΔiucC | 345 | 40 |
| ΔiucX | 586 | 60 |
| ΔiucA ΔiucC | 96 | 10 |
| ΔiucX ΔiucA ΔiucC | 75 | 10 |
| ΔsidL | 50 | 10 |
| ΔsidL ΔiucX | 50 | 10 |
TYES, tryptone yeast extract salts.
The amounts listed indicate the minimum concentration of deferiprone that prevented F. columnare growth.
Tubes were incubated in a rotator for 24 h at 28°C.
The minimum concentration of ferric chloride added to restore turbid F. columnare growth in the presence of chelator.
F. columnare MS-FC-4 siderophores are not required for virulence in zebrafish and rainbow trout.
Wild-type F. columnare and the ΔsidL ΔiucX siderophore-deficient mutant were examined for virulence against zebrafish in a pilot challenge experiment (Fig. 5). Previous studies demonstrated that the ΔgldN mutant, which lacks a functional T9SS, was avirulent in zebrafish (9), so this strain was used as a negative control. The ΔsidL ΔiucX siderophore mutant did not exhibit a dramatic decrease in virulence compared to the wild-type strain, and the slight apparent decrease in virulence seen in Fig. 5B was not statistically significant. The results suggest that the F. columnare MS-FC-4 siderophores are not essential for virulence under the conditions used. Zebrafish infected with the ΔsidL ΔiucX mutant showed signs of columnaris disease, including external lesions and lethargy, during the 10-day study. TYES plates (containing tobramycin) streaked from the gills, fins, and skin of dead fish displayed F. columnare colonies, suggesting that the fish died from columnaris infections.
FIG 5.
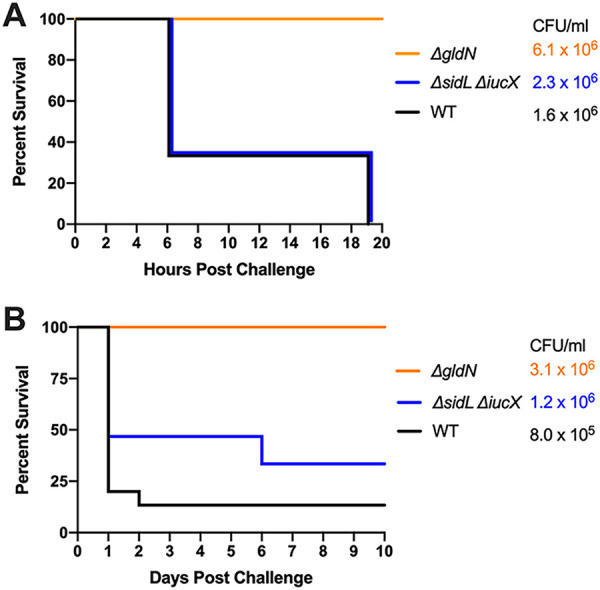
Challenges of zebrafish with F. columnare wild-type and siderophore mutant strains at two doses. A total of 15 fish were challenged with each strain by immersion, and the percent survival was recorded. The avirulent ΔgldN mutant was used instead of a media control. (A) Adult zebrafish were exposed to wild-type MS-FC-4 (1.6 × 106 CFU/mL), siderophore-deficient (2.3 × 106 CFU/mL), and ΔgldN (6.1 × 106 CFU/mL) strains. (B) Adult zebrafish were exposed to WT (8.0 × 105 CFU/mL), siderophore-deficient (1.2 × 106 CFU/mL), and ΔgldN (3.1 × 106 CFU/mL) strains. The CFU values shown are the final challenge concentrations. The percent survival for fish challenged with the wild-type and ΔsidL ΔiucX mutant strains were not significantly different in either panel.
Wild-type F. columnare and the ΔsidL ΔiucX siderophore-deficient mutant were also examined for virulence against rainbow trout fry in a larger-scale challenge experiment. No virulence defect was seen for ΔsidL ΔiucX (Fig. 6), similar to the situation with zebrafish. F. columnare was reisolated from all rainbow trout fry challenge mortalities, suggesting that the morbidity and mortality observed were due to F. columnare infections. 16S rRNA genes were amplified, digested as described in the Materials and Methods section, and analyzed. In each case, the isolated bacteria belonged to genomovar 1, as expected for F. columnare strain MS-FC-4.
FIG 6.

Challenge of rainbow trout with F. columnare wild-type and siderophore mutant strains. Rainbow trout fry were challenged with each strain by immersion, and the percent survival was recorded. The avirulent ΔgldN mutant was used instead of a media control. The strains examined were wild-type MS-FC-4 (3.4 × 107 CFU/mL), siderophore-deficient mutant (2.2 × 107 CFU/mL), and ΔgldN mutant (1.8 × 107 CFU/mL). The CFU values shown are the final challenge concentrations. The percent survival for fish challenged with the wild-type and ΔsidL ΔiucX mutant strains were not significantly different.
DISCUSSION
F. columnare is an important freshwater pathogen that was first described 100 years ago (1). It continues to cause outbreaks of columnaris disease in wild and aquaculture-reared fish today. A more complete understanding of the critical virulence factors involved in columnaris disease may result in improved strategies to control outbreaks. Iron-uptake systems have been linked to virulence in other fish pathogens (13, 17), and F. columnare produces an iron-chelating siderophore (23). Here, we identified the critical F. columnare siderophore biosynthesis genes and examined the roles of F. columnare siderophores as possible virulence factors.
Genomic analyses identified genes that encode proteins predicted to be involved in siderophore biosynthesis, export, uptake, and regulation. The encoded proteins included five Iuc (iron-uptake chelate) proteins involved in siderophore synthesis, efflux transporters, and outer membrane receptors (Table 1). The F. columnare iuc genes are distantly related to E. coli iuc genes involved in the synthesis of the siderophore aerobactin.
F. columnare ΔiucA and ΔiucC mutants exhibited partial defects in siderophore production compared to the wild type. The double mutant (ΔiucA ΔiucC) was more defective in siderophore production, and the triple mutant (ΔiucX ΔiucA ΔiucC) lacked all iucA/iucC-like genes and was almost completely deficient in siderophore activity. Siderophore activity was restored to near wild-type levels in the ΔiucX ΔiucA ΔiucC mutant by introduction of a multicopy plasmid expressing ΔiucA from a plasmid promoter. These results suggest partial redundancy between these three genes. The presence of a low level of apparent siderophore activity in the ΔiucX ΔiucA ΔiucC mutant suggests that additional genes may be involved. A mutant lacking iucX and lacking the large siderophore locus sidL (which includes iucA, iucC, and nine additional genes) produced no detectable siderophore activity. This suggests that additional genes in the sidL locus contribute to siderophore activity. Overall, our results demonstrated that F. columnare genes distantly related to those involved in E. coli aerobactin synthesis are needed for siderophore production. The chemical structure of the F. columnare siderophore(s) and their relationship to E. coli aerobactin remain to be determined.
Siderophore mutants were examined for growth defects in iron-limited conditions. Single deletions of iucA, iucC, and iucX resulted in similar growth to wild type, supporting the idea of redundancy between these related genes. In strains in which multiple genes were deleted, such as the ΔsidL and the ΔiucX ΔiucA ΔiucC mutants, growth defects were seen in iron-limited conditions. Complementation of the ΔiucX ΔiucA ΔiucC mutant with pRC48, which carries iucA, restored growth in iron-limited media, and complementation by insertion of iucA on the chromosome partially restored growth. pRC48 is a multicopy plasmid and uses a plasmid promoter to express iucA. It may overexpress IucA, which could mask the absence of IucC and IucX, allowing siderophore production and growth similar to the wild type. These data support the idea of partial redundancy between the iucA/iucC-like genes. The greatest growth defects were seen in the ΔsidL ΔiucX siderophore-deficient mutant. This strain showed defects when grown in iron-rich and iron-limited media, suggesting that siderophores are important for cells to acquire ferric iron for optimal growth.
To mimic the potentially lower iron availability that could occur in fish tissues during an infection, we grew F. columnare with the iron chelator deferiprone. Strains missing most siderophore-synthesis genes (ΔsidL) or all siderophore-synthesis genes (ΔsidL ΔiucX) failed to grow in the presence of as little as 50 μM deferiprone. In contrast, approximately 15 times this amount was needed to prevent the growth of wild-type cells. Fish produce transferrin proteins that sequester free iron available to pathogens (12). To survive in the host, many pathogens need mechanisms to compete with such host proteins for iron. Our data indicate that F. columnare siderophores can compete with an iron chelator, allowing growth in an iron-restricted environment. Siderophores may thus be important for F. columnare to survive in fish tissues and in other restrictive environments. Overall, the results demonstrate the role of F. columnare siderophores in growth under conditions of limited iron, which may be important for survival in natural aquatic environments and in aquaculture systems.
The ΔsidL ΔiucX mutant lacked all siderophore activity but retained virulence against zebrafish and rainbow trout comparable to the wild type, indicating that siderophores produced by F. columnare MS-FC-4 are not required for virulence under the conditions of our challenge experiments. F. columnare cells may have other mechanisms other than production of siderophores to obtain iron. For example, they could use TonB-dependent outer membrane receptors to bind siderophores produced by other bacteria. The nonsiderophore-producing bacterium Pseudomonas fragi uses this strategy to survive during periods of iron starvation (47). In the infection challenge experiments presented here, other bacteria were present in the tank water and on the fish, and these bacteria could have contributed siderophores that F. columnare could use. The F. columnare MS-FC genome encodes 23 proteins that have TonB-dependent receptor domains (pfam00593). Three of these (IutA, C6N29_04165, and C6N29_10625) belong to protein family TIGR01783 (TonB-dependent siderophore receptor) and thus are predicted to function specifically in siderophore uptake. Some of these may allow uptake of siderophores produced by other organisms, and some of the 20 other TonB-dependent receptors may also contribute to iron uptake. Iron is present in multiple forms, and siderophores may not be needed to access all of these. F. columnare secretes proteases and other digestive enzymes (2, 8, 9, 48). Some of these may digest iron-containing fish proteins, releasing heme and other forms of iron for F. columnare. The human pathogen Porphyromonas gingivalis, a distant relative of F. columnare within the phylum Bacteroidota, releases heme in this way and requires heme uptake for virulence (49). F. columnare iron acquisition systems are underexplored. Future studies are needed to characterize other F. columnare components involved in iron uptake and to determine their roles in virulence.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
F. columnare strain MS-FC-4 (31) was the wild-type strain used in this study. F. columnare strains were grown at 28 to 30°C in tryptone yeast extract salts (TYES) medium (50, 51), which contains per L, 4 g tryptone, 0.4 g yeast extract, 0.5 g MgSO4·7H2O, and 0.5 g CaCl2·2H2O, with the pH level adjusted to 7.2. F. columnare cultures used for rainbow trout challenges were grown in TYES-2×Mg, which is identical to TYES except that it contains twice as much MgSO4. E. coli strains were grown at 37°C in lysogeny broth (LB) (52). A total of 100 μg/mL ampicillin was used to select for plasmids in E. coli, 5 μg/mL tetracycline (agar culture) or 2.5 μg/mL tetracycline (liquid cultures) was used to select for plasmids in F. columnare, and 1 μg/mL tobramycin was used to counterselect against E. coli for conjugation experiments and eliminate most bacteria when isolating F. columnare from infected zebrafish. All F. columnare strains were derived from the wild-type strain MS-FC-4 (32), and all F. columnare DNA fragments cloned in plasmids came from this wild-type strain. The strains and plasmids used in this study are listed in Table 3, and the primers are listed in Table 4.
TABLE 3.
Strains and plasmids used in this studya
| Strain or plasmid | Descriptionb | Source or reference |
|---|---|---|
| E. coli strains | ||
| DH5αMCR | Strain used for general cloning | Life Technologies (Grand Island, NY) |
| S17-1 λ pir | Strain used for conjugation | 66 |
| F. columnare strains | ||
| MS-FC-4 | Wild type | 31, 32 |
| FCB 115 | ΔsidL, siderophore synthesis region (C6N29_09920, C6N29_09925, C6N29_09930, C6N29_09935, C6N29_09940, C6N29_09945, C6N29_09950, C6N29_09955, C6N29_09960, C6N29_09965, C6N29_09970) | This study |
| FCB161 | ΔsidL, ΔiucX | This study |
| FCB164 | ΔiucX | This study |
| FCB166 | ΔiucA | This study |
| FCB175 | ΔiucX, ΔiucA | This study |
| FCB199 | ΔiucX, ΔiucA, ΔiucC | This study |
| FCB208 | ΔiucC | This study |
| FCB219 | ΔiucA, ΔiucC | This study |
| FCB231 | ΔsidL, ΔiucX complemented by iucX inserted into the chromosome | This study |
| FCB232 | ΔiucX, ΔiucA, ΔiucC complemented by iucA inserted into the chromosome | This study |
| Plasmids | ||
| pCP23 | E. coli-F. columnare shuttle plasmid; Apr (Tcr) | 67 |
| pMS75 | Suicide vector carrying sacB; Apr (Tcr) | 10 |
| pRC44 | 2.2-kbp region downstream of the siderophore biosynthesis genes amplified using primers 2465 and 2466 and cloned into KpnI and BamHI sites of pMS75; Apr (Tcr) | This study |
| pRC45 | 2.1-kbp region upstream of the siderophore biosynthesis genes amplified using primers 2467 and 2468 and cloned into BamHI and SalI sites of RC44; Apr (Tcr) | This study |
| pRC48 | 1.9-kbp fragment containing iucA amplified using primers 2546 and 2547 and cloned into KpnI and PstI sites of pCP23; Apr (Tcr) | This study |
| pRC49 | 2.2-kbp region downstream of iucA amplified using primers 2542 and 2543 and cloned into KpnI and BamHI sites pMS75; Apr (Tcr) | This study |
| pRC50 | 2.0-kbp region upstream of iucA amplified using primers 2544 and 2545 and cloned into BamHI and SalI sites of pRC49; Apr (Tcr) | This study |
| pRC56 | 1.8-kbp fragment containing iucX amplified using primers 2564 and 2565 and cloned into KpnI and PstI sites of pCP23; Apr (Tcr) | This study |
| pRC57 | 2.2-kbp region downstream of iucX amplified using primers 2560 and 2561 and cloned into BamHI and SalI sites of pMS75; Apr (Tcr) | This study |
| pRC58 | 2.6-kbp region upstream of iucX amplified using primers 2562 and 2563 and cloned into SalI and SphI sites of pRC57; Apr (Tcr) | This study |
| pRC62 | 2.7-kbp fragment containing iucC amplified using primers 2587 and 2588 and cloned into KpnI and PstI sites of pCP23; Apr (Tcr) | This study |
| pRC63 | 2.1-kbp region upstream of iucC amplified using primers 2585 and 2586 and cloned into BamHI and SalI sites of pMS75; Apr (Tcr) | This study |
| pRC64 | 2.3-kbp region downstream of iucC amplified using primers 2583 and 2584 and cloned into KpnI and BamHI sites of pRC63; used to delete iucC from FCB166 and FCB175, generating FCB219 and FCB232; Apr (Tcr) | This study |
| pRC67 | 2.1-kbp region downstream of iucC amplified using primers 2599 and 2600 and cloned into KpnI and BamHI sites of pRC63; used to delete iucC, generating FCB208; Apr (Tcr) | This study |
| pRC69 | 6.3-kbp region containing upstream, iucX, and downstream amplified using primers 2560 and 2563 and cloned into BamHI and SphI sites of pMS75; Apr (Tcr) | This study |
| pRC71 | 6.8-kbp region containing upstream, iucA, and downstream amplified using primers 2601 and 2586 and cloned into BamHI and SalI sites of pMS75; Apr (Tcr) | This study |
kbp, kilobase pair.
Antibiotic resistance phenotypes: ampicillin (Apr) and tetracycline (Tcr). Unless indicated otherwise, the antibiotic resistance phenotypes are those expressed in E. coli. The antibiotic resistance phenotypes given in parentheses are those expressed in F. columnare but not in E. coli.
TABLE 4.
Primers used in this study
| Primer | Sequence (5′ to 3′)a | Plasmid constructed using this primer |
|---|---|---|
| 2465 | GCTAGGGTACCACCGCAGAGTTTTGGTTGAA | pRC44 |
| 2466 | GCTAGGGATCCCTTGGTTTTTGGGTTTTTCAG | pRC44 |
| 2467 | GCTAGGGATCCAGCAAATTTGTTTGCAGTCCC | pRC45 |
| 2468 | GCTAGGTCGACTGCATCGCCGTGTGTACTAT | pRC45 |
| 2542 | GCTAGGGTACCCATACGCCCGAAGGAGAAA | pRC49 |
| 2543 | GCTAGGGATCCGGAACACTGGCTAATCCTATTCAT | pRC49 |
| 2544 | GCTAGGGATCCTGTAAGAATACGAGCAGTTGATGT | pRC50 |
| 2545 | GCTAGGTCGACCGTCAGGAATTGGGTGATG | pRC50 |
| 2546 | GCTAGGGTACCTTACTTGCTTGTTTTAGAGAGG | pRC48 |
| 2547 | GCTAGCTGCAGCTTTGATAGTTTTTACCATACAGG | pRC48 |
| 2560 | GCTAGGGATCCAACGATTTCGTTGCTTCAGG | pRC57, RC69 |
| 2561 | GCTAGGTCGACATGCGGGTAGAAGACAAAACC | pRC57 |
| 2562 | GCTAGGTCGACGATGTTTGTTCTTAAATCTTTCCA | pRC58 |
| 2563 | GCTAGGCATGCCACAGCAACCTTATCCGT | pRC58, RC69 |
| 2564 | GCTAGGGTACCATGCAAGTAAAAGCGACATCC | pRC56 |
| 2565 | GCTAGCTGCAGAGTGCCATGTATTCACCCAAA | pRC56 |
| 2583 | GCTAAGGTACCCATTCCGACAAAAGGAGGAA | pRC64 |
| 2584 | GCTAAGGATCCAACCCTATTGCTCCGTACAAAAAA | pRC64 |
| 2585 | GCTACGGATCCTTTGCCTATCATACCCCAATACTG | pRC63 |
| 2586 | GCTAAGTCGACATTCCCAAATCATGCAGACC | pRC63, RC71 |
| 2587 | GCTATGGTACCTTTACCGTACTAACCCTACTAACG | pRC62 |
| 2588 | GCTAACTGCAGGCATATTCTTTTTGAACCCACT | pRC62 |
| 2599 | GCTATGGTACCGGCCTTCATAGTCTAGATGAAG | pRC67 |
| 2600 | GCTATGGATCCTCTAACCCTATTGCTCCGTAC | pRC67 |
| 2601 | GCTAGGGATCCCATACGCCCGAAGGAGAAA | pRC71 |
Underlined sequences indicate added restriction enzyme sites.
Bioinformatic analysis.
The F. columnare wild-type strain MS-FC-4 sidL locus and the iucX gene were identified by examining the genome sequence NCBI GenBank ID PVLU00000000 (31) for genes encoding proteins predicted to be involved in siderophore synthesis, export, and uptake. This was accomplished using the Joint Genome Institute’s Integrated Microbial Genomes and Microbiomes (IMG/M version 6.0 [53]) function profile tool to search for sequences that encode proteins that have domains that belong to the families TIGR01783, TIGR01720, TIGR02275, TIGR04316, pfam04183, pfam06276, and pfam00668. For TIGRFAMs, the trusted cutoffs assigned by the J. Craig Venter Institute (JCVI) that allow identification of the vast majority of family members with few false positives (54) were used. All proteins encoded by the sidL locus and by iucX and nearby genes were also examined for conserved domains using both IMG/M and the National Center for Biotechnology Information (NCBI) conserved domain searches (55–58). The six other complete or nearly complete F. columnare genomes in the IMG/M database that were analyzed for genes involved in siderophore synthesis, with NCBI GenBank IDs in parentheses, were strains CSF-298-10 (MUAW00000000), ATCC 49512 (CP003222), Pf1 (CP016277), TC 1691 (CP018912), C#2 (CP015107), and 94-081 (CP013992). Note that a recent publication (33) indicated that two of these strains, F. columnare strains C#2 and 94-081, be assigned to the new species Flavobacterium covae.
Conjugative transfer of plasmids in F. columnare strains.
Plasmids were transferred from E. coli S17-1λpir into F. columnare strain MS-FC-4 by conjugation essentially as previously described (9). In brief, 1 mL of E. coli overnight culture was inoculated into 9 mL LB, 5 mL of F. columnare overnight culture was inoculated into 25 mL TYES, and the cultures were incubated with shaking at 37 and 28°C, respectively, until the optical density at 600 nm (OD600) reached 0.5. The cells were centrifuged at 3,440 × g for 15 min. The E. coli and F. columnare pellets were washed with TYES and suspended in TYES. E. coli and F. columnare suspensions were combined and centrifuged at 4,600 × g for 3 min. Excess medium was removed, and the mixture was spotted on TYES agar and incubated for 24 h at 30°C. The cells were scraped off the agar and suspended in 1.5 mL of TYES medium. Then, 100-μL aliquots were spread on TYES agar containing 1 μg/mL tobramycin and 5 μg/mL tetracycline and incubated at 30°C for 72 h.
Construction of iron utilization mutants.
In-frame deletion mutants were constructed as previously described (8, 9). For example, to delete iucA, a 2.2-kbp region downstream of iucA was amplified by PCR using Phusion DNA polymerase (New England Biolabs) and primers 2542 (adding a KpnI site) and 2543 (adding a BamHI site). The product was digested with KpnI and BamHI and ligated into pMS75 that had been digested with the same enzymes to produce pRC49. A 2.0-kbp region upstream of iucA was amplified using primers 2544 (adding a BamHI site) and 2545 (adding a SalI site). The product was digested with BamHI and SalI and ligated into pRC49 that had been digested with the same enzymes, to generate pRC50. pRC50 was transferred to F. columnare MS-FC-4 by conjugation, and colonies with the plasmid integrated into the chromosome by recombination were obtained by selecting for tetracycline resistance. Resistant colonies were streaked for isolation on antibiotic plates, and isolated colonies were grown in liquid without tetracycline to allow loss of the plasmid. The cells were plated on TYES media containing 5% sucrose, and the mutant was obtained by selecting for sucrose resistance. PCR was performed to confirm the deletion. Other iron utilization genes were disrupted in a similar way using the plasmids described in Table 3 and the primers listed in Table 4. Most deletion plasmids used the restriction enzyme pairs BamHI/SalI and KpnI/BamHI to digest the vector and inserts except for pRC58, which used BamHI/SalI and SalI/SphI pairs.
Plasmid complementation of iron utilization mutants.
A 1.9-kbp fragment spanning iucA was amplified using primers 2546 (adding a KpnI site) and 2547 (adding a PstI site). The product was digested with KpnI and PstI and ligated into the shuttle vector pCP23 that had been digested with the same enzymes to produce pRC48. The plasmid was transferred into the F. columnare ΔiucA mutant by conjugation. Plasmid complementation of other mutants was performed in a similar way using the plasmids described in Table 3 and the primers listed in Table 4.
Chromosomal complementation of iron utilization mutants.
A 6.3-kbp product spanning iucX and adjacent upstream and downstream regions was amplified using primers 2560 (adding a BamHI site) and 2563 (adding a SphI site). The product was digested with BamHI and SphI and ligated into pMS75 that had been digested with the same enzymes to generate pRC69. The plasmid was transferred into the F. columnare ΔsidL ΔiucX mutant by conjugation. Complementation of the ΔiucX ΔiucA ΔiucC mutant was performed in a similar way using the plasmids described in Table 3 and the primers listed in Table 4.
Siderophore detection.
Siderophore synthesis was examined using a chrome azurol S (CAS) assay. CAS plates were prepared as described (59, 60) with growth media modifications described by Guan et al. (23), and 30 μL of mid-log-phase (OD600 0.5) F. columnare cultures were spotted on CAS plates and incubated at 30°C for 72 h. Siderophore production is indicated by an orange halo around F. columnare growth.
F. columnare growth in iron-limited conditions.
Stocks of F. columnare in TYES broth with glycerol added to 17.5%, stored at −80°C, were used to inoculate 20 mL of TYES broth, and these mixtures were incubated for 14 h at 28°C with shaking at 200 rpm. This was done to minimize the amount of cell clumping before inoculating the microtiter plate. Overnight cultures were standardized to an OD600 of 0.5, and 40 μL of culture was added to 960 μL of medium (TYES or TS) per well in a 48-well microtiter plate. The cells were incubated in a CLARIOstar microplate reader (BMG Labtech, Ortenberg, Germany) at 28°C with shaking at 200 rpm. Readings were taken every 2 h for 36 h. The cultures were measured in triplicate in the microtiter plates, and growth experiments were performed twice for each strain.
F. columnare growth in chelated iron conditions.
F. columnare strains were streaked from the freezer onto TYES plates and incubated at 30°C for 24 h, and 5 mL of TYES was inoculated and incubated overnight at 28°C with rotation. Fifteen μL of overnight culture was used to inoculate 3 mL of TYES. Deferiprone (46) was added, and the cultures were incubated at 28°C with rotation. Growth was observed after 24 h. A range of 0 μM to 1 mM deferiprone was tested in increments of 25 μM, and the minimum concentrations that prevented growth of each strain are listed in Table 2. Experiments were performed twice in test tubes and once in a flask to confirm the inhibitory chelator concentrations.
Ferric chloride (FeCl3) was added to TYES medium containing deferiprone to determine the minimum amount of supplemental iron needed to restore turbid F. columnare growth. F. columnare strains were streaked from the freezer onto TYES plates and incubated at 30°C for 24 h. Then, 5 mL of TYES was inoculated and incubated overnight at 28°C with rotation, and 15 μL of overnight culture was used to inoculate 3 mL of TYES containing the appropriate deferiprone concentration to prevent growth. FeCl3 was added, and the cultures were incubated at 28°C with rotation. A control tube (no F. columnare cells added) demonstrated that FeCl3 did not precipitate when added to medium containing deferiprone. A range of 0 μM to 200 μM FeCl3 was tested in increments of 10 μM, and the minimum concentrations that restored turbid growth for each strain are listed in Table 2. The experiments were performed twice in test tubes and once in a flask to confirm the concentrations.
Zebrafish challenges.
Adult zebrafish were challenged with F. columnare wild-type and mutant strains as previously described (9) with slight modifications. Bacterial strains were grown in TYES medium for 14 h at 28°C. Five mL of overnight culture was diluted into 25 mL of fresh TYES and incubated until the OD600 reached 0.5. The cultures were serially diluted, plated on TYES agar, and incubated for 2 days at 28°C to determine the number of live cells per mL. To test the virulence of each strain, naive adult Ekkwill zebrafish were immersed in a solution of 0.5 mL F. columnare cells and 99.5 mL dechlorinated tap water for 30 min. No signs of disease were observed prior to challenge, and no indications of F. columnare or columnaris disease were observed in the uninfected control tanks or in the maintenance tanks at any time. Control fish were exposed to a solution of 0.5 mL of the avirulent F. columnare ΔgldN mutant (9) and 99.5 mL of water. After exposure, the fish were moved to tanks containing 2 liters of fresh water at 28°C and observed for up to 10 days for signs of infection. Each treatment was performed in triplicate tanks, with each tank containing five zebrafish. Mortalities were recorded daily. A minimum of 20% of fish that died were examined for bacteria phenotypic of F. columnare (yellow, rhizoid, tobramycin-resistant colonies) by swabbing the gills, fins, and skin and streaking on TYES plates containing tobramycin (1 μg/mL) and incubating for 2 days at 30°C.
Rainbow trout challenges.
Commercially available certified disease-free rainbow trout (Oncorhynchus mykiss) eggs were acquired from Troutlodge Inc. (Sumner, WA). Viable hatched trout were hand fed daily to satiation using a commercially available trout feed (Ziegler Inc., Gardners, PA). The trout were maintained at the U.S. Department of Agriculture (USDA)-Agricultural Research Service (ARS) National Center for Cool and Cold Water Aquaculture (NCCCWA) research facility in Kearneysville, WV, in flow-through water at a rate of 1 liter/min at 12.5°C until the challenge weight of ~1.3 g was met. The fish in this facility are checked yearly for multiple diseases, including columnaris disease, and except for fish in the challenge room, they are certified disease-free. No signs of disease were observed prior to the challenge, and no indications of F. columnare or columnaris disease were observed in the uninfected control tanks or in the maintenance tanks at any time. Fish were moved to the challenge aquaria 1 week prior to immersion challenge to acclimate to the elevated water temperature of 16°C.
Wild-type (strain MS-FC-4), mutant, and complemented strains were all used for immersion challenges. Frozen bacterial stocks were stored at −80°C in 75% TYES-2×Mg broth and 25% glycerol. Bacterial cultures for challenges were grown as previously described with slight modifications (61). Briefly, 10 μL of each frozen stock was inoculated into 10 mL TYES-2×Mg broth and incubated overnight at 30°C with shaking at 150 rpm. These starter cultures were used to inoculate 10-mL cultures (1:20 dilution) that were grown to an OD540 of 0.4. Then, for each culture, ~6 μL was inoculated into 1 L TYES-2×Mg broth in a 2.8-L Fernbach flask. These cultures were incubated at 30°C with shaking at 150 rpm until an OD540 of 0.5 to 0.6 was reached, at which point the cells were used for the challenge.
Challenges of fry were performed using triplicate 3-L tanks with restricted water flows (~200 mL/min) at 16°C. Each tank contained 40 fish of approximately 1.35 g each. Water flows were stopped for the immersion challenge, and the tanks were inoculated with bacterial cultures and incubated for 0.5 h, after which the water flows were resumed. Control tanks were inoculated with sterile TYES-2×Mg broth. Serial dilutions of water samples from each tank after inoculation were plated on TYES-2×Mg agar to determine CFU/mL. The final challenge concentrations for each experiment are listed in the figures. Mortalities were removed and counted daily. The data for triplicate tanks of each strain were pooled, and the survivor fractions for each strain were calculated. Approximately 16% of mortalities were randomly tested by homogenizing gill tissue and streaking on TYES-2×Mg agar plates to determine whether F. columnare was present. Confirmation of F. columnare was determined by morphological observation of yellow, rhizoid, adherent colonies and by amplifying 16S rRNA genes and confirming the genomovar by enzymatic digestion (HaeIII) and gel electrophoresis as previously described (62–64). F. columnare was detected in all mortalities tested, and all were genomovar I (and genetic group 1), as expected for strain MS-FC-4. The challenges continued for 21 days or until 3 days without recorded mortalities postexposure.
Statistical analyses.
For growth curve assays a one-way analysis of variance (ANOVA) with Tukey’s post-test was used to analyze differences between strains, unless otherwise noted. The error bars represent the standard error of the mean (SEM). GraphPad Prism (version 9.1.2) was used to analyze infection challenges. A value of P < 0.05 was considered significant.
Ethics statements.
The experiments with zebrafish were performed at the University of Wisconsin-Milwaukee and followed protocols approved by the University of Wisconsin-Milwaukee Institutional Animal Care and Use Committee. The rainbow trout challenges were performed as described in protocol 176, which was approved by the NCCCWA Institutional Animal Care and Use Committee.
Data availability.
All of the data associated with this work are included either in the paper or in the online supplemental materials.
ACKNOWLEDGMENTS
This work was funded in part by U.S. Department of Agriculture-ARS CRIS projects 8082-32000-006-00-D and 5090-31320-004-00D, by cooperative agreements 5090-31320-004-03S and 58-5090-1-022, and by grant NA18OAR4170097 with project R/SFA-20 from the University of Wisconsin Sea Grant Institute under grants from the National Sea Grant College Program, National Oceanic and Atmospheric Administration, U.S. Department of Commerce, and the State of Wisconsin. The views contained in this document are those of the authors and should not be interpreted as necessarily representing the official policies, either expressed or implied, of the U.S. Government. Mention of trade name, proprietary product, or specific equipment does not constitute a guarantee or warranty by the USDA and does not imply its approval to the exclusion of other products that may be suitable.
Footnotes
Supplemental material is available online only.
Contributor Information
Mark J. McBride, Email: mcbride@uwm.edu.
Charles M. Dozois, INRS
REFERENCES
- 1.Davis HS. 1922. A new bacterial disease of freshwater fishes. Bull US Bureau Fish 38:261–280. [Google Scholar]
- 2.Declercq AM, Haesebrouck F, Van den Broeck W, Bossier P, Decostere A. 2013. Columnaris disease in fish: a review with emphasis on bacterium-host interactions. Vet Res 44:27. 10.1186/1297-9716-44-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner BA, Wise DJ, Khoo LH, Terhune JS. 2002. The epidemiology of bacterial diseases in food-size channel catfish. J Aquat Anim Health 14:263–272. . [DOI] [PubMed] [Google Scholar]
- 4.Bullock GL, Hsu TC, Shotts EB. 1986. Columnaris disease of fishes. Fish Disease Leaflet 72; U.S. Fish and Wildlife Service, U.S. Department of the Interior, Washington, DC. [Google Scholar]
- 5.Zhang Y, Zhao L, Chen W, Huang Y, Yang L, Sarathbabu V, Wu Z, Li J, Nie P, Lin L. 2017. Complete genome sequence analysis of the fish pathogen Flavobacterium columnare provides insights into antibiotic resistance and pathogenicity related genes. Microb Pathog 111:203–211. 10.1016/j.micpath.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 6.Tekedar HC, Karsi A, Reddy JS, Nho SW, Kalindamar S, Lawrence ML. 2017. Comparative genomics and transcriptional analysis of Flavobacterium columnare strain ATCC 49512. Front Microbiol 8:588. 10.3389/fmicb.2017.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kayansamruaj P, Dong HT, Hirono I, Kondo H, Senapin S, Rodkhum C. 2017. Comparative genome analysis of fish pathogen Flavobacterium columnare reveals extensive sequence diversity within the species. Infect Genet Evol 54:7–17. 10.1016/j.meegid.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Li N, Zhu Y, LaFrentz BR, Evenhuis JP, Hunnicutt DW, Conrad RA, Barbier P, Gullstrand CW, Roets JE, Powers JL, Kulkarni SS, Erbes DH, Garcia JC, Nie P, McBride MJ. 2017. The type IX secretion system is required for virulence of the fish pathogen Flavobacterium columnare. Appl Environ Microbiol 83:e01769-17. 10.1128/AEM.01769-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thunes NC, Conrad RA, Mohammed HH, Zhu Y, Barbier P, Evenhuis JP, Perez-Pascual D, Ghigo JM, Lipscomb RS, Schneider JR, Li N, Erbes DH, Birkett C, LaFrentz BR, Welch TJ, McBride MJ. 2022. Type IX secretion system effectors and virulence of the model Flavobacterium columnare strain MS-FC-4. Appl Environ Microbiol 88:e0170521. 10.1128/AEM.01705-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li N, Qin T, Zhang XL, Huang B, Liu ZX, Xie HX, Zhang J, McBride MJ, Nie P. 2015. Gene deletion strategy to examine the involvement of the two chondroitin lyases in Flavobacterium columnare virulence. Appl Environ Microbiol 81:7394–7402. 10.1128/AEM.01586-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Payne SM. 1994. Detection, isolation, and characterization of siderophores. Methods Enzymol 235:329–344. 10.1016/0076-6879(94)35151-1. [DOI] [PubMed] [Google Scholar]
- 12.Hirono I, Aoki T. 1995. Characteristics and genetic-analysis of fish transferrin. Fish Pathol 30:167–174. 10.3147/jsfp.30.167. [DOI] [Google Scholar]
- 13.Abdelhamed H, Lu J, Lawrence ML, Karsi A. 2016. Ferric hydroxamate uptake system contributes to Edwardsiella ictaluri virulence. Microb Pathog 100:195–200. 10.1016/j.micpath.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Meyer JM, Neely A, Stintzi A, Georges C, Holder IA. 1996. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect Immun 64:518–523. 10.1128/iai.64.2.518-523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams PH. 1979. Novel iron uptake system specified by ColV plasmids: an important component in the virulence of invasive strains of Escherichia coli. Infect Immun 26:925–932. 10.1128/iai.26.3.925-932.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheldon JR, Laakso HA, Heinrichs DE. 2016. Iron acquisition strategies of bacterial pathogens. Microbiol Spectr 4: VMBF-0010-2015. 10.1128/microbiolspec.VMBF-0010-2015. [DOI] [PubMed] [Google Scholar]
- 17.Wolf MK, Crosa JH. 1986. Evidence for the role of a siderophore in promoting Vibrio anguillarum infections. J Gen Microbiol 132:2949–2952. 10.1099/00221287-132-10-2949. [DOI] [PubMed] [Google Scholar]
- 18.Crosa JH, Walsh CT. 2002. Genetics and assembly line enzymology of siderophore biosynthesis in bacteria. Microbiol Mol Biol Rev 66:223–249. 10.1128/MMBR.66.2.223-249.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Challis GL. 2005. A widely distributed bacterial pathway for siderophore biosynthesis independent of nonribosomal peptide synthetases. Chembiochem 6:601–611. 10.1002/cbic.200400283. [DOI] [PubMed] [Google Scholar]
- 20.Gibson F, Magrath DI. 1969. The isolation and characterization of a hydroxamic acid (aerobactin) formed by Aerobacter aerogenes 62-I. Biochim Biophys Acta 192:175–184. 10.1016/0304-4165(69)90353-5. [DOI] [PubMed] [Google Scholar]
- 21.Bindereif A, Neilands JB. 1985. Aerobactin genes in clinical isolates of Escherichia coli. J Bacteriol 161:727–735. 10.1128/jb.161.2.727-735.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mydy LS, Bailey DC, Patel KD, Rice MR, Gulick AM. 2020. The siderophore synthetase IucA of the aerobactin biosynthetic pathway uses an ordered mechanism. Biochemistry 59:2143–2153. 10.1021/acs.biochem.0c00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan L, Santander J, Mellata M, Zhang Y, Curtiss R, 3rd.. 2013. Identification of an iron acquisition machinery in Flavobacterium columnare. Dis Aquat Organ 106:129–138. 10.3354/dao02635. [DOI] [PubMed] [Google Scholar]
- 24.Moller JD, Ellis AE, Barnes AC, Dalsgaard I. 2005. Iron acquisition mechanisms of Flavobacterium psychrophilum. J Fish Dis 28:391–398. 10.1111/j.1365-2761.2005.00639.x. [DOI] [PubMed] [Google Scholar]
- 25.Avendano-Herrera R, Toranzo AE, Romalde JL, Lemos ML, Magarinos B. 2005. Iron uptake mechanisms in the fish pathogen Tenacibaculum maritimum. Appl Environ Microbiol 71:6947–6953. 10.1128/AEM.71.11.6947-6953.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruce TJ, Ma J, Sudheesh PS, Cain KD. 2021. Quantification and comparison of gene expression associated with iron regulation and metabolism in a virulent and attenuated strain of Flavobacterium psychrophilum. J Fish Dis 44:949–960. 10.1111/jfd.13354. [DOI] [PubMed] [Google Scholar]
- 27.Beck BH, Li C, Farmer BD, Barnett LM, Lange MD, Peatman E. 2016. A comparison of high- and low-virulence Flavobacterium columnare strains reveals differences in iron acquisition components and responses to iron restriction. J Fish Dis 39:259–268. 10.1111/jfd.12343. [DOI] [PubMed] [Google Scholar]
- 28.LaFrentz BR, LaPatra SE, Call DR, Wiens GD, Cain KD. 2009. Proteomic analysis of Flavobacterium psychrophilum cultured in vivo and in iron-limited media. Dis Aquat Organ 87:171–182. 10.3354/dao02122. [DOI] [PubMed] [Google Scholar]
- 29.Long A, Fehringer TR, Swain MA, LaFrentz BR, Call DR, Cain KD. 2013. Enhanced efficacy of an attenuated Flavobacterium psychrophilum strain cultured under iron-limited conditions. Fish Shellfish Immunol 35:1477–1482. 10.1016/j.fsi.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Lange MD, Farmer BD, Abernathy J. 2018. Catfish mucus alters the Flavobacterium columnare transcriptome. FEMS Microbiology Lett 365. 10.1093/femsle/fny244. 10.1093/femsle/fny244. [DOI] [PubMed] [Google Scholar]
- 31.Bartelme RP, Barbier P, Lipscomb RS, LaPatra SE, Newton RJ, Evenhuis JP, McBride MJ. 2018. Draft genome sequence of the fish pathogen Flavobacterium columnare strain MS-FC-4. Genome Announc 6:e00429-18. 10.1128/genomeA.00429-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evenhuis JP, Mohammed H, LaPatra SE, Welch TJ, Arias CR. 2016. Virulence and molecular variation of Flavobacterium columnare affecting rainbow trout in Idaho, USA. Aquaculture 464:106–110. 10.1016/j.aquaculture.2016.06.017. [DOI] [Google Scholar]
- 33.LaFrentz BR, Kralova S, Burbick CR, Alexander TL, Phillips CW, Griffin MJ, Waldbieser GC, Garcia JC, de Alexandre Sebastiao F, Soto E, Loch TP, Liles MR, Snekvik KR. 2022. The fish pathogen Flavobacterium columnare represents four distinct species: Flavobacterium columnare, Flavobacterium covae sp. nov., Flavobacterium davisii sp. nov. and Flavobacterium oreochromis sp. nov., and emended description of Flavobacterium columnare. Syst Appl Microbiol 45:126293. 10.1016/j.syapm.2021.126293. [DOI] [PubMed] [Google Scholar]
- 34.Chen S, Kaufman MG, Bagdasarian M, Bates AK, Walker ED. 2010. Development of an efficient expression system for Flavobacterium strains. Gene 458:1–10. 10.1016/j.gene.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guérin C, Lee B-H, Fradet B, van Dijk E, Mirauta B, Thermes C, Bernardet J-F, Repoila F, Duchaud E, Nicolas P, Rochat T. 2021. Transcriptome architecture and regulation at environmental transitions in flavobacteria: the case of an important fish pathogen. ISME Commun 1:33. 10.1038/s43705-021-00029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ford S, Cooper RA, Williams PH. 1986. Biochemical genetics of aerobactin biosynthesis in Escherichia coli. FEMS Microbiol Lett 36:281–285. 10.1111/j.1574-6968.1986.tb01710.x. [DOI] [Google Scholar]
- 37.de Lorenzo V, Neilands JB. 1986. Characterization of iucA and iucC genes of the aerobactin system of plasmid ColV-K30 in Escherichia coli. J Bacteriol 167:350–355. 10.1128/jb.167.1.350-355.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ling J, Pan H, Gao Q, Xiong L, Zhou Y, Zhang D, Gao S, Liu X. 2013. Aerobactin synthesis genes iucA and iucC contribute to the pathogenicity of avian pathogenic Escherichia coli O2 strain E058. PLoS One 8:e57794. 10.1371/journal.pone.0057794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beasley FC, Vines ED, Grigg JC, Zheng Q, Liu SY, Lajoie GA, Murphy MEP, Heinrichs DE. 2009. Characterization of staphyloferrin A biosynthetic and transport mutants in Staphylococcus aureus. Mol Microbiol 72:947–963. 10.1111/j.1365-2958.2009.06698.x. [DOI] [PubMed] [Google Scholar]
- 40.Cotton JL, Tao JS, Balibar CJ. 2009. Identification and characterization of the Staphylococcus aureus gene cluster coding for staphyloferrin A. Biochemistry 48:1025–1035. 10.1021/bi801844c. [DOI] [PubMed] [Google Scholar]
- 41.Miethke M, Marahiel MA. 2007. Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev 71:413–451. 10.1128/MMBR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sulavik MC, Houseweart C, Cramer C, Jiwani N, Murgolo N, Greene J, DiDomenico B, Shaw KJ, Miller GH, Hare R, Shimer G. 2001. Antibiotic susceptibility profiles of Escherichia coli strains lacking multidrug efflux pump genes. Antimicrob Agents Chemother 45:1126–1136. 10.1128/AAC.45.4.1126-1136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carbonetti NH, Williams PH. 1984. A cluster of five genes specifying the aerobactin iron uptake system of plasmid ColV-K30. Infect Immun 46:7–12. 10.1128/iai.46.1.7-12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farmer BD. 2004. Improved methods for the isolation and characterization of Flavobacterium columnare. MS thesis. Louisiana State University, Baton Rouge, LA. [Google Scholar]
- 45.Bridson EY, Brecker A. 1970. Design and formulation of microbial culture media, p 229–295. In Norris JR, Ribbons DW (ed), Methods in microbiology, vol. 3. Academic Press, Cambridge, MA. [Google Scholar]
- 46.Hider RC, Hoffbrand AV. 2018. The role of deferiprone in iron chelation. N Engl J Med 379:2140–2150. 10.1056/NEJMra1800219. [DOI] [PubMed] [Google Scholar]
- 47.Champomier-Verges MC, Stintzi A, Meyer JM. 1996. Acquisition of iron by the non-siderophore-producing Pseudomonas fragi. Microbiology 142:1191–1199. 10.1099/13500872-142-5-1191. [DOI] [PubMed] [Google Scholar]
- 48.Newton JC, Wood TM, Hartley MM. 1997. Isolation and partial characterization of extracellular proteases produced by isolates of Flavobacterium columnare derived from catfish. J Aquat Anim Health 9:75–85. . [DOI] [Google Scholar]
- 49.Olczak T, Simpson W, Liu X, Genco CA. 2005. Iron and heme utilization in Porphyromonas gingivalis. FEMS Microbiol Rev 29:119–144. 10.1016/j.femsre.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Cain KD, LaFrentz BR. 2007. Laboratory maintenance of Flavobacterium psychrophilum and Flavobacterium columnare. Curr Protoc Microbiol 6:13B.1.1–13B.1.12. [DOI] [PubMed] [Google Scholar]
- 51.Holt RA, Rohovec JS, Fryer JL. 1993. Bacterial coldwater disease, p 3–23. In Inglis V, Roberts RJ, Bromage NR (ed), Bacterial diseases of fish. Blackwell Scientific Publications, Oxford, England. [Google Scholar]
- 52.Bertani G. 1951. Studies on lysogenesis: I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol 62:293–300. 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen IMA, Chu K, Palaniappan K, Ratner A, Huang JH, Huntemann M, Hajek P, Ritter S, Varghese N, Seshadri R, Roux S, Woyke T, Eloe-Fadrosh EA, Ivanova NN, Kyrpides NC. 2021. The IMG/M data management and analysis system v.6.0: new tools and advanced capabilities. Nucleic Acids Res 49:D751–D763. 10.1093/nar/gkaa939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haft DH, Selengut JD, Richter RA, Harkins D, Basu MK, Beck E. 2013. TIGRFAMs and genome properties in 2013. Nucleic Acids Res 41:D387–D395. 10.1093/nar/gks1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marchler-Bauer A, Bo Y, Han LY, He JE, Lanczycki CJ, Lu SN, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Lu F, Marchler GH, Song JS, Thanki N, Wang ZX, Yamashita RA, Zhang DC, Zheng CJ, Geer LY, Bryant SH. 2017. CDD/SPARCLE: functional classification of proteins via subfamily domain architectures. Nucleic Acids Res 45:D200–D203. 10.1093/nar/gkw1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manfredi P, Renzi F, Mally M, Sauteur L, Schmaler M, Moes S, Jeno P, Cornelis GR. 2011. The genome and surface proteome of Capnocytophaga canimorsus reveal a key role of glycan foraging systems in host glycoproteins deglycosylation. Mol Microbiol 81:1050–1060. 10.1111/j.1365-2958.2011.07750.x. [DOI] [PubMed] [Google Scholar]
- 57.Marchler-Bauer A, Bryant SH. 2004. CD-Search: protein domain annotations on the fly. Nucleic Acids Res 32:W327–W331. 10.1093/nar/gkh454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marchler-Bauer A, Derbyshire MK, Gonzales NR, Lu SN, Chitsaz F, Geer LY, Geer RC, He J, Gwadz M, Hurwitz DI, Lanczycki CJ, Lu F, Marchler GH, Song JS, Thanki N, Wang ZX, Yamashita RA, Zhang DC, Zheng CJ, Bryant SH. 2015. CDD: NCBI’s conserved domain database. Nucleic Acids Res 43:D222–D226. 10.1093/nar/gku1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Louden BC, Haarmann D, Lynne AM. 2011. Use of blue agar CAS assay for siderophore detection. J Microbiol Biol Educ 12:51–53. 10.1128/jmbe.v12i1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwyn B, Neilands JB. 1987. Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56. 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 61.Evenhuis JP, LaFrentz BR. 2016. Virulence of Flavobacterium columnare genomovars in rainbow trout Oncorhynchus mykiss. Dis Aquat Organ 120:217–224. 10.3354/dao03027. [DOI] [PubMed] [Google Scholar]
- 62.LaFrentz BR, Waldbieser GC, Welch TJ, Shoemaker CA. 2014. Intragenomic heterogeneity in the 16S rRNA genes of Flavobacterium columnare and standard protocol for genomovar assignment. J Fish Dis 37:657–669. 10.1111/jfd.12166. [DOI] [PubMed] [Google Scholar]
- 63.Triyanto H, Wakabayashi H. 1999. Genotypic diversity of strains of Flavobacterium columnare from diseased fishes. Fish Pathol 34:65–71. 10.3147/jsfp.34.65. [DOI] [Google Scholar]
- 64.Olivares-Fuster O, Shoemaker CA, Klesius PH, Arias CR. 2007. Molecular typing of isolates of the fish pathogen, Flavobacterium columnare, by single-strand conformation polymorphism analysis. FEMS Microbiol Lett 269:63–69. 10.1111/j.1574-6968.2006.00605.x. [DOI] [PubMed] [Google Scholar]
- 65.Yu NY, Wagner JR, Laird MR, Melli G, Rey S, Lo R, Dao P, Sahinalp SC, Ester M, Foster LJ, Brinkman FS. 2010. PSORTb 3.0: improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 26:1608–1615. 10.1093/bioinformatics/btq249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Lorenzo V, Timmis KN. 1994. Analysis and construction of stable phenotypes in gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol 235:386–405. 10.1016/0076-6879(94)35157-0. [DOI] [PubMed] [Google Scholar]
- 67.Agarwal S, Hunnicutt DW, McBride MJ. 1997. Cloning and characterization of the Flavobacterium johnsoniae (Cytophaga johnsonae) gliding motility gene, gldA. Proc Natl Acad Sci USA 94:12139–12144. 10.1073/pnas.94.22.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 and Fig. S1 to S5. Download aem.00948-22-s0001.pdf, PDF file, 1.1 MB (1.1MB, pdf)
Data Availability Statement
All of the data associated with this work are included either in the paper or in the online supplemental materials.