Abstract
Background:
Some levothyroxine (LT4)-treated hypothyroid patients report a constellation of persistent and distressing cognitive symptoms that has been termed brain fog. This narrative review focuses on attempts to define and measure hypothyroid-associated brain fog, summarize possible etiologies and contributing factors, present treatment options, and propose avenues for future research.
Methods:
Published literature was reviewed to summarize available information on patient-reported symptoms associated with brain fog in hypothyroidism, as well as objective evidence of impairment based on neurocognitive testing and functional imaging studies. Given the limited information specific for hypothyroid-associated brain fog, relevant data from other medical conditions associated with brain fog were also reviewed and incorporated into recommendations for clinical care and future research areas.
Results:
Hypothyroid-associated brain fog has not been well defined or quantitated, and the underlying pathophysiology is unclear. Symptoms vary among patients but commonly include fatigue, depressed mood, and cognitive difficulties in the areas of memory and executive function. Symptoms often predate the diagnosis of hypothyroidism, and the magnitude of cognitive impairment can range from mild to severe. Regardless of severity, these symptoms are associated with impaired quality of life and cause dissatisfaction with treatment, so often lead to requests for alternate therapies. Disease-specific and psychological factors impact the experience of brain fog in complex ways, including potential limitations in LT4 monotherapy, self-knowledge of a disease state, and expectations for therapeutic effects.
Conclusions:
Brain fog is a variable symptom complex in people with hypothyroidism, causing significant distress and diminished quality of life. In the absence of proven therapies, individualized treatment plans are recommended, which incorporate thyroid-specific, general medical, and psychosocial approaches. In particular, cognitive rehabilitation is an underutilized technique that is beneficial in other medical conditions associated with brain fog and could improve symptoms in hypothyroid people. The limitations in our current knowledge and questions presented throughout this review highlight a major need for clinical research in this understudied area. Future research should include attention to standardization of survey instruments to quantitate brain fog in hypothyroid people, as well as rigorously designed intervention studies.
Keywords: brain fog, cognition, hypothyroidism, levothyroxine therapy
Introduction
Levothyroxine (LT4) monotherapy is standard of care for hypothyroidism, and most hypothyroid patients are satisfied with LT4 treatment. However, 10–15% report residual symptoms, poor quality of life, and dissatisfaction with LT4 treatment despite normal thyrotropin (TSH) levels (1–3). Common symptoms are fatigue, depressed mood, and cognitive difficulties, including problems with memory and word-finding, and these symptoms tend to cluster. Patients call this symptom constellation “brain fog” (4). Retrospective data suggest that many patients experience brain fog symptoms before the diagnosis of hypothyroidism (4) and are disappointed when LT4 therapy does not resolve all their symptoms (5,6).
Persistent brain fog symptoms are distressing to patients, may be associated with decreased adherence to LT4 therapy (7), and may lead patients to seek higher doses of LT4 or alternative therapies, including combined LT4/liothyronine (LT3) therapy or desiccated thyroid extract (DTE), despite lack of evidence supporting these alternate therapies. This narrative review summarizes the literature regarding (what we call) hypothyroid-associated brain fog in adult patients, data from other pertinent conditions, and areas for further research in this understudied condition.
What Is “Brain Fog”
The term brain fog is broadly used to describe what individuals experience when their cognitive functioning is not as sharp as usual, but there is no standard definition nor diagnostic criteria. The term is applied to a symptom complex that has been described in various ways in the literature but always involves some level of cognitive dysfunction. Cognitive dysfunction can be assessed through patient report (e.g., symptom questionnaire) or objective performance-based tests. These two approaches are complimentary, as they measure overlapping but nonidentical variables.
Brain fog symptoms are not unique to people with hypothyroidism. A variety of other clinical populations whose medical conditions do not directly involve the central nervous system have similar cognitive symptoms and dysfunction, including patients with cancer treated with chemotherapy (8), the menopausal transition (9), postural tachycardia syndrome (10), chronic fatigue syndrome (CFS) (11), celiac disease (12), non-celiac gluten sensitivity (12), systemic lupus erythematosus (13), hypoparathyroidism (14), and recently post-Covid-19 infection (15). Findings usually indicate deficits in focused and sustained attention, memory recall, and multitasking. In a recent joint conference held by the American, British, and European Thyroid Associations, thyroid-associated brain fog was defined as “mental cloudiness or lack of mental alertness” (16).
There is significant variation in how brain fog is studied and what outcome measures are used. This lack of consistency across studies is a challenge since little research has been conducted specifically in hypothyroid people reporting cognitive symptoms. Based on research in other areas, some reasonable parameters include cognitive involvement, persistence over some period of time, and association with the onset of disease or treatment (although there may be a delay). Unlike most other conditions which cause brain fog, a close temporal relation between symptom onset and the diagnosis of hypothyroidism is often not the case. This raises the question of how much of the symptom complex is actually due to hypothyroidism and/or inadequate treatment.
What Is Known About Hypothyroid-Associated Brain Fog
To date, only one published study has specifically investigated patient-reported brain fog in hypothyroidism (4). Ettleson et al. developed a survey, adapted from the validated ThyPRO thyroid symptom survey, to determine what symptoms participants associate with brain fog. The survey was distributed to the American Thyroid Association patient database and hypothyroid support websites and social media groups. Self-identified hypothyroid individuals were invited to complete the survey if they felt that they had brain fog.
Five thousand one hundred seventy responses were analyzed; 96% were women, with a mean age of 51 years. Seventy-nine percent of participants reported brain fog “frequently” or “all the time.” The most prominent associated symptoms, reported in more than 95%, were low energy/fatigue, forgetfulness, feeling sleepy, and difficulty focusing. Other symptoms included depressed mood, feeling anxious, mental confusion, and trouble making decisions. The authors concluded that “brain fog is a multi-symptom condition characterized by frequent fatigue, sleepiness, and forgetfulness, and that the overall combined symptom burden negatively impacts life.” They hypothesized that fatigue was driven by the sustained level of attention necessary to complete daily tasks in people with decreased neurocognitive reserves.
This study represents a laudable endeavor to characterize brain fog in people with hypothyroidism. Limitations include the survey instrument was not validated, it was not known whether symptoms were related to underlying hypothyroidism or other medical or psychiatric comorbidities, and lack of information on thyroid hormone levels.
Lessons from Other Disease States to Inform the Study of Hypothyroid-Associated Brain Fog
Given the paucity of information in hypothyroidism, can we draw lessons in how to measure brain fog from investigations in other disease states? There are two main categories of outcomes: patient-reported outcomes (PROs), ideally collected using validated survey instruments; and objective testing of behavior and cognitive domains using validated tests and sometimes functional imaging for corroboration.
Brain fog in survivors of cancer has been most studied in breast cancer, where it is commonly reported as “chemobrain” or “chemofog” by patients during or following chemotherapy (8). Animal and human studies provide evidence that specific cognitive domains dependent on the hippocampus and/or frontal lobes are affected, including attention, memory, processing speed, and executive function (17). This has been found in studies assessing patient-reported symptoms (18) and those using objective test measures assessing mean level performance as well as variability (19–23).
A meta-analysis of 14 functional magnetic resonance imaging (fMRI) studies in patients with cancer who had received chemotherapy, compared with chemotherapy-naive patients, found 2 clusters of lower activation in the frontoparietal attention network during cognitive tasks, which could reflect difficulty in mobilizing and/or sustaining attention, working memory, and executive function (24). Thus, the oncology literature provides extensive evidence of cancer-related brain fog, measured by objective and patient-reported measures, using standardized and validated instruments to test specific cognitive domains, as well as neuroimaging implicating abnormalities in the brain regions important in the cognitive domains impacted.
Another disease area where brain fog has been studied is myalgic encephalomyelitis (ME)/CFS. Jason et al. developed a validated patient-reported survey instrument that defined five specific types of fatigue in ME/CFS: post-exertional, wired, molasses, flu-like, and brain fog (11). Brain fog fatigue was defined as “cognitive impairment such as confusion and disorientation that impacts an individual's ability to perform daily activities.” Objective cognitive testing in ME/CFS patients has shown deficits in attention, working memory, and information processing speed (25), and structural and functional imaging studies have documented decreased white and subcortical gray matter volumes, decreased connectivity in neurocognitive networks (26), and neuroinflammation in widespread brain areas associated with the severity of neuropsychiatric symptoms (27).
These examples corroborate self-reports of symptoms that comprise brain fog in patients with cancer or ME/CFS, providing possible templates for advancing research in hypothyroid-associated brain fog. However, these examples describe significant variations in the severity of cognitive symptoms as measured through patient report or objective tests. Patients with cancer generally do not report the confusion and disorientation reported in ME/CFS, and within a population, some patients manifest more mild impairments than others. This suggests that brain fog is a heterogeneous symptom complex, with multifactorial etiology, so results from other disease states may not entirely apply to hypothyroid-associated brain fog. Developing and validating a disease-specific PRO such as brain fog is a lengthy and expensive process, and corroborating PROs with objective cognitive testing and functional imaging adds further complexity and cost. These challenges raise the question of whether adequate instruments already exist that reflect hypothyroid patients' reports of brain fog.
Need for Validated Hypothyroid-Specific Instruments to Measure Hypothyroid-Associated Brain Fog
The creation of a validated instrument to identify and quantitate hypothyroid-associated brain fog should utilize standard PRO development procedures (28). An iterative process is required since there is typically no “gold standard” against which to benchmark the PRO instrument. The minimal clinically important difference should be established. This procedure was followed by Watt et al. in the development of ThyPRO, a widely used thyroid disease-specific PRO (29–32).
Some individual ThyPRO items map well to anecdotal symptoms reported by patients with brain fog, including items asking about remembering, slow thinking, difficulty finding the right words, confusion, difficulty concentrating, difficulty learning something new, tiredness, exhaustion, feeling worn out, decreased motivation, sadness, coping, and mood swings. Seven of the 13 resulting ThyPRO scales appear relevant for brain fog: tiredness, cognitive impairment, anxiety, depressivity, emotional susceptibility, impaired social life, and impaired daily life. The short version of ThyPRO (ThyPRO-39) has 11 scales, including the same 7 scales relevant for measuring brain fog (33).
“Fatigue” was the top ranked domain identified by hypothyroid patients in the surveys underlying ThyPRO (general fatigue #1, physical fatigue #3, mental fatigue #4). This is interesting because Jason et al. concluded that ME/CFS-associated brain fog is a type of fatigue (11) and because fatigue was one of the most prominent symptoms in the study by Ettleson et al. (4). Could hypothyroidism-associated brain fog be considered a type of fatigue? It should be kept in mind that physical and mental fatigue may be associated, but are not identical, and many hypothyroid patients report both types of fatigue. Teasing these apart would be helpful when monitoring symptoms over time, testing interventions, and deciding how to treat patients with hypothyroid-associated brain fog.
This informs the question of whether the thyroid field already has validated tools to measure brain fog in treated hypothyroid patients, or whether we need to develop new disease-specific instruments. Many of the ThyPRO items and scales appear relevant for identifying and quantitating brain fog in hypothyroidism, but some of the scales are irrelevant (e.g., hyperthyroid symptoms, eye symptoms). Given the complexities of creating a new survey instrument, validation could begin with a focus on the ThyPRO-39 items contained in relevant subscales, as piloted by Ettleson et al. (4).
When patient and expert responses were compared for ThyPRO, a pattern emerged: experts focused on problems characteristic for the disease in question, while patients considered broader, nonspecific, psychological aspects of health-related quality of life (HRQoL) (29). Patients may report many overlapping symptoms that encompass mental fatigue, physical fatigue, memory, sleep, anxiety, and depression. Therefore, while it may be tempting to cast a wide net in trying to measure brain fog in hypothyroid patients, it will be prudent to try to maintain sufficient specificity for symptoms of hypothyroid-associated brain fog, and avoid labeling symptoms as brain fog when they are best addressed as separate issues.
Cognition and Mood in Hypothyroidism
Extending beyond patient-reported symptoms (4,5), there are numerous studies utilizing validated instruments that measure mood and/or cognitive function in hypothyroid people, although they have not specifically focused on people with significant cognitive symptoms. Cognition assessments have included tests of memory and executive function, the cognitive domains that best approximate patient-reported symptoms of brain fog, and which are known to be affected by thyroid hormone.
Overt hypothyroidism has been linked to a wide range of affective and cognitive dysfunction, measured using validated PRO instruments, cognitive testing, and structural and functional imaging studies (34–36). Memory is the most consistently affected cognitive domain. LT4 treatment is usually effective in treating these decrements, although there is not always complete reversal. However, in subclinical hypothyroidism (elevated TSH with normal free thyroxine [fT4] level), large cross-sectional and longitudinal epidemiological studies and a meta-analysis have demonstrated that a mildly elevated TSH level is not intrinsically associated with poor HRQoL, fatigue, depression, anxiety, or major cognitive deficits, including specific objective measures of memory and executive function (37).
Randomized, placebo-controlled blinded studies of LT4 therapy in people with subclinical hypothyroidism have failed to demonstrate improvement in HRQoL, mood, or cognitive measures (34,38–40). Based on these studies, a recent clinical practice guideline from an international panel of thyroid specialists concluded that treating subclinical hypothyroidism has no benefit on fatigue, depressive symptoms, or cognitive function (41). However, several smaller scale studies that utilized highly sensitive and labor-intensive measures of specific cognitive domains and/or neuroimaging have reported subtle effects of subclinical hypothyroidism on memory or executive function (34), which improved with treatment (42) or worsened when LT4 doses were decreased (43). These latter findings lend credence to the concept that slight decrements in memory and executive function exist in subclinical hypothyroidism but do not explain persistent and severe brain fog symptoms in LT4-treated people.
Focusing specifically on LT4-treated hypothyroid people, the literature regarding HRQoL, mood, and cognitive outcomes is divergent and inconclusive. Some studies have shown that people treated with LT4 have decreased psychological well-being or cognitive function compared with control groups, while other studies report no differences (34). Studies that focused on memory and executive function generally have failed to find decrements in these areas, although one functional imaging study reported decreased activity in brain areas relevant for memory and learning in treated Hashimoto's patients with normal TSH levels (44). In studies when LT4 doses were altered in a blinded manner in euthyroid LT4-treated people, maintaining TSH levels in the reference range, there were no changes in HRQoL, mood, or cognitive outcomes (45,46).
A recent systematic review and meta-analysis focused on cognitive function in long-term patients with thyroid cancer treated for hypothyroidism following cancer therapy (47). Patients had worse cognitive function in the areas of attention and concentration, processing speed, and language. Executive function impairment was less consistently found. Survivors of thyroid cancer are a distinct subgroup of treated hypothyroid people, since they have a history of cancer in addition to hypothyroidism, and are often treated with higher doses of LT4 than hypothyroid people with benign conditions. These issues make it difficult to generalize the results to people with benign causes of hypothyroidism, so should be studied separately.
Inclusion and Exclusion Criteria Considerations When Studying Hypothyroid-Associated Brain Fog
Based on the above summary, there is a glaring disconnect between brain fog symptoms reported by some people with apparently adequately treated hypothyroidism, which are severe enough to interfere with daily functioning, and unimpressive results of studies in mild hypothyroidism and LT4-treated patients that utilize validated PROs and objective testing. One explanation for this discrepancy is that most studies do not specifically recruit participants with these symptoms.
In this regard, the results of a recent randomized, double-blind crossover study of 75 hypothyroid patients are interesting (48). Patients underwent three treatment arms: LT4, LT4/LT3, or DTE, each for 22 weeks. TSH was in the reference range at the end of all three arms. There were no differences in validated measures of thyroid-related symptoms, HRQoL, depression, or memory. However, the top one-third most symptomatic patients at baseline had improved scores on all outcomes when therapies included LT3. This study was not designed to select people who were most symptomatic, and the results could reflect regression to the mean. An earlier trial conducted in patients with depressive symptoms (49) found no improvement in HRQoL or mood with LT4/LT3.
Future studies should clearly segregate symptomatic people to avoid diluting potential positive findings with large numbers of relatively asymptomatic people (16). While corroborative cognitive testing and functional imaging is labor-intensive, expensive, and not always available, efforts should be made to incorporate such techniques in subsets of symptomatic people in research trials.
Role of Tissue Hypothyroidism in the Brain
Numerous hypotheses have been proposed to explain cognitive decrements in hypothyroidism, including autoimmunity, inflammation, oxidative stress, and altered neurotransmission (50), although none have been proven. In patients with treated hypothyroidism, a leading hypothesis is the concept of “tissue hypothyroidism” in brain areas that subserve mood and cognitive functions. This has been shown most conclusively in animal experiments, but not yet proven in human studies. Triiodothyronine (T3) is the active thyroid hormone in the brain and other tissues, and most T3 in the brain is produced locally through regulated deiodination of thyroxine (T4) by the type 2 deiodinase (DIO2) (51).
It is possible that LT4 therapy does not lead to adequate intracellular levels of T3 in the brain. Supporting this hypothesis, thyroidectomized rats treated with LT4 to normalize TSH levels have relatively high brain T4 levels, which downregulate DIO2 activity in the cerebral cortex and hippocampus. Compared with placebo, hypothyroid rats have an altered pattern of T3-responsive gene expression, which only partially reverses with LT4. If the rats are treated with combined LT4/LT3, DIO2 activity in the cortex and hippocampus is restored, and T3-responsive gene expression normalizes (52).
While these rodent experiments are intriguing, what data do we have regarding tissue hypothyroidism in patients? LT4-treated hypothyroid people have lower mean serum T3 levels despite normal TSH levels and high–normal to mildly elevated free T4 levels, and 10% have levels below the reference range (16). LT4 monotherapy does not normalize an array of systemic biomarkers and metabolic parameters in hypothyroid people (53,54). There are 14 trials of LT4/LT3 or DTE in humans: 13 measured HRQoL and mood, and 11 showed no differences; 9 measured cognitive function, and 7 showed no differences (16). A recent systematic review and meta-analysis of benefits and harms of LT4/LT3 versus LT4 monotherapy for people with hypothyroidism (55) found no differences in treatment effect on clinical status, HRQoL, psychological distress, depressive symptoms, or fatigue.
These disappointing clinical trials indicate that the debate of whether or how tissue hypothyroidism in the brain contributes to cognitive dysfunction in humans is not resolved. Finally, when patients were queried regarding treatment preference in these trials, 43% preferred combined therapy, 23% preferred monotherapy, and 30% had no preference (16), suggesting that we may not be measuring everything that patients experience. For these reasons, future trials should incorporate treatment preference as an outcome, and further clinical research efforts should incorporate methods to assess tissue hypothyroidism in the central nervous system, perhaps utilizing functional imaging.
Genetic Polymorphisms and Other Mechanisms That Could Explain Brain Fog
Could most treated hypothyroid people have adequate tissue levels of T3 in the brain and are therefore asymptomatic, while a subset has tissue hypothyroidism in relevant brain areas, which manifest as brain fog? Lending credence to this hypothesis, there is a common polymorphism in the DIO2 gene, Thr92Ala, which may reduce catalytic activity of the enzyme and/or alter the intracellular Golgi apparatus (56). Mice with the Thr92Ala polymorphism show decreased physical activity, sleep more, and take more time to memorize objects, a measure of short-term memory. T3-responsive genes in brain areas show decreased T3 effects in these mice, and the behavioral deficits are improved more with LT4/LT3 than LT4 monotherapy (16,57).
In contrast to these elegant rodent experiments, there are few published studies regarding effects of the Thr92Ala polymorphism in humans, with inconclusive results. A Netherlands study found no impact of the polymorphism on response to LT4 monotherapy on HRQoL or executive function (58), and another study found no correlation between the Thy92Ala polymorphism and memory (48). In contrast, a U.K. study found that the polymorphism was associated with decreased HRQoL and predicted preference for combined therapy, although cognition was not assessed, and there were a number of limitations to the analysis, including limited power, lack of correction for multiple measures, and diminishing effects at 12 months compared with 3 months (59).
Another underexplored area is the possible association of thyroid autoimmunity with cognitive function. The literature focuses on HRQoL and mood, and findings have been inconsistent. In a population-based study of more than 30,000 people in Norway, no association between circulating antithyroid antibodies and depression or anxiety was found (60). Watt et al. administered ThyPRO to 199 people with autoimmune hypothyroidism and TSH levels that ranged from undetectable to 55 mU/L. Thyroid hormone levels were not associated with HRQoL scores, but thyroid peroxidase antibody (TPOAb) titers were associated with depressivity and anxiety (61).
In another study, 150 patients with treated hypothyroidism, normal TSH levels, markedly elevated TPOAb titers, persistent symptoms, and elevated fatigue and lower HRQoL scores were randomized to optimal medical therapy or thyroidectomy. TPOAbs decreased and fatigue and HRQoL improved markedly only in thyroidectomy group (62). Surgery may have conferred a large nonspecific effect in these patients, but the results are impressive and intriguing. A few small studies have reported mild cognitive decrements in treated Hashimoto's patients with normal TSH levels (63–65), although another study reported no cognitive decrements and normal fMRI imaging in Hashimoto's patients (66).
In a mouse model of Hashimoto's thyroiditis, affected euthyroid mice had decreased memory performance, and neuronal synaptic loss, impaired synaptic plasticity, and astrocyte loss in the hippocampus (67). These studies do not necessarily indicate that antithyroid antibodies directly impair brain function but do raise the question of whether autoimmune-mediated processes could contribute to brain fog. A similar process has been proposed for the extreme example of Hashimoto's encephalopathy (68). Is it possible that less extreme cases lead to brain fog? That possibility is entirely unexplored but would not explain brain fog symptoms in non-autoimmune hypothyroidism.
Additional Factors Related to Patient-Reported Brain Fog Symptoms in Treated Hypothyroidism
It is notable that 46% of participants in the study by Ettleson et al. reported that the onset of brain fog symptoms occurred before the diagnosis of hypothyroidism (4). This could reflect delays in diagnosis or indicate that cognitive symptoms are an early indicator of hypothyroidism. However, it raises the question of whether hypothyroidism was a red herring, diagnosed when an elevated TSH was measured in a patient with pre-existing unrelated symptoms. In that case, it would not be surprising that symptoms do not abate when hypothyroidism is treated. Another possibility is that long-standing undiagnosed hypothyroidism leads in some cases to more permanent changes in the brain, although to our knowledge, there are no imaging studies or pathological studies that address this hypothesis in adult-onset hypothyroidism.
Studies of brain fog in other clinical populations provide insights into additional contributing factors for these symptoms. In the study of females undergoing the menopausal transition, participants attributed their brain fog symptoms to a multifactorial process that included stress, getting older, physical health, changes in menstrual cycles, the burden of their roles, sleep disturbances, and emotional factors (9). In a study of people with postural tachycardia syndrome, the most frequent triggers for brain fog were fatigue and lack of sleep (10). Even in the general population, fatigue is common, with a prevalence of 22% and significant associations with obesity, insomnia, depression, anemia, low self-rated health status, and young age in one study (69).
Some of the surveys regarding brain fog in other clinical populations have asked what things improve their brain fog symptoms. The most common responses have included sleep, exercise, nutrition, and stress reduction (9). There are a limited number of intervention studies in other clinical populations regarding these factors; a recent systematic review in cancer-related cognitive impairment reported a beneficial effect of exercise on self-reported or objectively measured cognitive function (70), and a Cochrane review concluded that exercise probably reduces fatigue in ME/CFS (71). Finally, cognitive rehabilitation interventions, which include structured approaches to address cognitive deficits and patient-reported cognitive symptoms, are effective in improving cognitive function in a number of conditions (72–79).
To the best of our knowledge, there are only three intervention studies in hypothyroid patients that focused on nonthyroid hormone-related treatments, and none of them included specific measures of cognition: One study enrolled women with untreated subclinical hypothyroidism, who underwent an aerobic exercise program for 16 weeks. Those who exercised had improved HRQoL compared with those who did not (80). Two small studies reported that aromatherapy or a probiotic improved patient-reported measures of fatigue in treated hypothyroid patients (81,82). The mechanisms underlying these effects are unknown but appear to be relatively independent of changes in circulating thyroid hormone levels.
These data do not obviate a causative role of treated hypothyroidism itself in brain fog symptoms, but they raise cautionary issues regarding comorbidities or behavioral factors that may impact patient reports. They also suggest future areas for research and treatment in hypothyroid-associated brain fog, for example, inclusion of structured exercise programs. Additional caveats are the roles that self-knowledge of a disease state and expectations play in patients ascribing their symptoms to hypothyroidism, discussed in the following section.
Patients' Awareness of Hypothyroidism and Expectations for Treatment May Increase Reports of Brain Fog
Population-based studies consistently report that people who are unaware that they have an elevated TSH level report levels of fatigue and mood disturbances similar to people with normal thyroid function. In contrast, people who know they have thyroid disease report increased fatigue and decreased mood regardless of TSH levels, including normal levels. A population-based study of more than 25,000 people found no correlation between thyroid function as measured by TSH (including hyperthyroidism and hypothyroidism) and self-reported overall health, depression, or anxiety, but the subgroup with known thyroid disease had an increased risk of lower self-reported health and both mood disorders, despite normal TSH levels (83,84).
Another population-based study of almost 6000 people found that the prevalence of fatigue in respondents who were euthyroid with no known thyroid disease was 34%. Rates were similar in overt or subclinical hypothyroid respondents, who were unaware that they had an abnormal TSH level. In respondents with known thyroid disorders, the rate of reported fatigue increased to 50% and was independent of the TSH level (85). Another study reported that there was no correlation between subclinical hypothyroidism and depressive symptoms when people were unaware of their thyroid status (86). These studies suggest that patients who know they have hypothyroidism are more likely to notice symptoms and attribute them to their thyroid condition, even when they may be unrelated, termed diagnostic labeling or attribution bias.
In addition to knowledge regarding their thyroid status, hypothyroid people may be influenced by specific information they receive regarding cognitive issues. Although mainstream sources of information provide balanced perspectives, there are many sources such as the internet or social media that promulgate skewed information and opinions, which could affect reports of symptoms. In a relevant study, women with breast cancer were interviewed regarding cancer-related cognitive problems (87). Half of the women received a letter before the interview that described the occurrence of cognitive complaints in cancer, while the other half received a neutral letter. The women who received the letter describing this association reported significantly more cognitive symptoms, suggesting that they were primed to notice or report symptoms. This may also occur in people with hypothyroidism and should be considered when counseling patients and designing research studies.
There are models to help understand and predict the interplay of psychological adaptation and chronic medical conditions, which provide additional insights into possible relationships between hypothyroidism and psychological distress. “Illness representation” is a function of a patient's beliefs and perceptions about their illness, including ideas about causes and consequences of their illness, links between severity of illness and its impact on physical, social, and psychological function, and perceived control of their illness (88,89). Illness representation plays a critical role in adaptation to a chronic disease and is related to levels of depression and anxiety (90).
In a recent study, 354 hypothyroid women on LT4 were recruited via online Facebook forums and completed several measures including the Illness-Related Beliefs questionnaire. The intensity of negative beliefs about their hypothyroidism was directly related to levels of anxiety, depression, and anger symptoms. There was no relation between emotional distress and hormone levels or LT4 dose (91). These studies do not negate the reality of hypothyroid peoples' symptoms and distress, or imply that there are no organic processes involved in brain fog. However, they suggest that the level of distress associated with brain fog is modulated by illness-related beliefs.
Another potential bias is a tendency for hypothyroid patients to assume that higher doses of LT4, or perhaps alternate thyroid preparations, are intrinsically superior to standard LT4 therapy. In a randomized double-blinded study, LT4-treated hypothyroid people could not accurately identify how their LT4 doses had been altered, but the majority preferred whichever dose they perceived to be the higher dose, regardless of whether and in which direction their LT4 doses were altered (92).
In a recent online survey of hypothyroid patients, 969 patients were queried on potential issues related to treatment dissatisfaction (6). Seventy-eight percent were dissatisfied with treatment, 93% expected their hypothyroid-related symptoms to resolve within three months of starting therapy, and 26% expected all their symptoms to disappear with LT4. There was a negative correlation between treatment satisfaction and expectations for more support from their general practitioner. These results highlight the central roles of patient expectations and their relationship with their health care team in determining treatment satisfaction.
Highlighting this issue, there is a remarkable quote from a hypothyroid patient during the recent joint thyroid association conference (16). This patient expressed the opinion that “the goal of treating hypothyroidism should be the complete restoration of health, regardless of age, menopausal status, or any comorbidity.” Although this is not a universal sentiment, it shows that some patients have unreasonable expectations. Improved patient education could address these expectations and motivate patients to engage in other interventions that could improve their symptoms, as summarized in the next section.
Based on the above studies, it is reasonable to assume that factors such as attribution bias, illness representation, treatment bias, and expectations impact hypothyroid patient experiences of brain fog, and these need to be considered in clinical care and research in this patient group.
Treatment Options for Brain Fog in Hypothyroid Patients
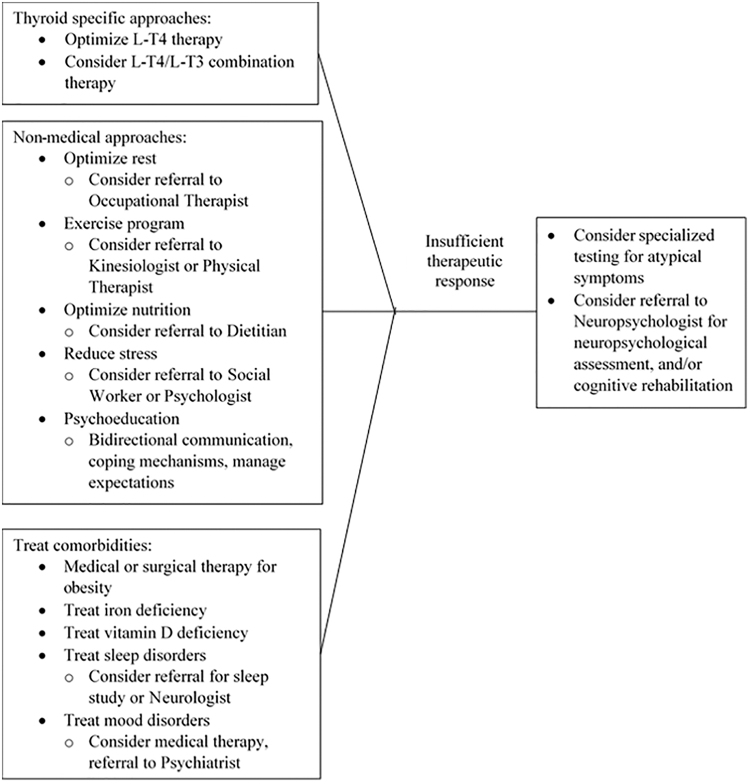
In the absence of proven treatments for brain fog symptoms in hypothyroid people, some commonsense approaches can be tried (Fig. 1). Optimizing LT4 doses and maintaining serum TSH levels in the reference range is an obvious first approach, although the unimpressive neurocognitive data in treating mild hypothyroidism suggest that this may have only minor effects. Many practitioners add LT3 to LT4 therapy, which can be done safely (93). Some patients with self-reported brain fog describe improvement with LT3 in online surveys (32,89). The preliminary data from Shakir et al. (48) suggest that the most symptomatic patients may derive the greatest benefit from LT4/LT3 combination therapy, although other intervention studies have been less impressive in unselected patients.
FIG. 1.
Recommended treatment algorithm for hypothyroid-associated brain fog.
Complementing these thyroid-targeted approaches, successful strategies from other disease states include optimizing rest, sleep, exercise, and nutrition, and reducing stress. In some cases, referrals to specialists in occupational therapy, physical therapy, nutrition, and/or stress management may be beneficial. Co-existing medical problems such as obesity, iron deficiency, vitamin D deficiency, and sleep and mood disorders, which are common in the general population and hypothyroid people, should be diagnosed and treated. Psychoeducation can be implemented early, which includes maintaining open bidirectional communication, developing compensatory strategies and coping skills, setting realistic goals, interventions aimed at influencing illness beliefs, and managing expectations.
Atypical symptoms that raise the question of alternate etiologies or structural neurological disorders should be evaluated as potential separate diagnoses. If symptoms are more severe, appear progressive, or are interfering with activities of daily living, referral to a neuropsychologist is an underutilized approach that should be considered. In addition to further psychoeducation, neuropsychologists can provide cognitive rehabilitation, which has been shown to be beneficial in a variety of clinical populations with cognitive symptoms, including attention, memory, and executive function (72–79).
Conclusions and Recommendations
Brain fog is a serious and distressing symptom complex for a significant number of people with hypothyroidism who ostensibly are adequately treated with LT4. It is difficult to precisely define and measure hypothyroid-associated brain fog, and the underlying pathophysiology is unclear. It is likely that disease-specific as well as psychological factors impact the experience of brain fog in complex ways. In the absence of proven pharmacological therapies, a multimodality and individualized approach is necessary. Importantly, all affective and neurocognitive symptoms in hypothyroid people should not automatically be attributed to the hypothyroidism. To try and treat persistent symptoms with increasing doses of LT4 or alternate thyroid hormone preparations may be doing patients a disservice, if other possible diagnoses are not adequately pursued. Refer Table 1 for key points.
Table 1.
Key Points Concerning Hypothyroid-Associated Brain Fog
| Approximately 10% of people with levothyroxine-treated hypothyroidism and normal TSH levels continue to experience distressing symptoms of fatigue, depressed mood, and cognitive difficulties, which they term “brain fog.” |
| There is no standard definition nor diagnostic criteria for hypothyroid-associated brain fog. |
| Brain fog is not unique to hypothyroidism but also occurs in other medical conditions. |
| The etiology of hypothyroid-associated brain fog is unclear, but the experience of brain fog may include thyroid-specific central nervous system effects, as well as medical comorbidities, lifestyle factors, attribution bias, and treatment expectations. |
| In the absence of proven treatments for hypothyroid-associated brain fog, an individualized approach that incorporates thyroid-specific therapies, diagnosis and treatment of other medical conditions, optimization of lifestyle and environmental factors is recommended. Cognitive rehabilitation is an underutilized treatment option for people with significant symptoms that persist despite these approaches. |
TSH, thyrotropin.
The limitations in our current knowledge and questions presented throughout this review highlight the major need for clinical research in this important area. Guidance for research includes areas listed in Table 2, as also discussed in the recent ATA/BTA/ETA joint conference (16).
Table 2.
Recommendations for the Design of Clinical Research Studies of Hypothyroidism-Associated Brain Fog
| Population-specific issues |
| Focus on dissatisfied patients with persistent cognitive symptoms |
| Consider only including patients with little endogenous thyroid function |
| Ensure that research participants have well-documented and persistent hypothyroidism |
| Include relevant demographic and clinical parameters in research design and analysis (e.g., age, sex, gender, BMI, mood, sleep disturbances, exercise patterns) |
| Include well-characterized and well-matched control groups (e.g., people without thyroid disorders, and/or treated hypothyroid people without cognitive symptoms) |
| Research design issues |
| Determine a standard definition of hypothyroid-associated brain fog |
| Agree on a validated PRO instrument to quantitate hypothyroid-associated brain fog, include fatigue as an outcome |
| Utilize PROs in large-scale studies, but conduct smaller scale, more intensive studies for objective corroboration of cognitive deficits using validated tests and functional imaging techniques that are sufficiently sensitive and linked to cognitive domains likely to be affected by thyroid dysfunction |
| Determine minimally clinically important differences in outcome measures and adequately power clinical studies based on this |
| Conduct longitudinal studies to determine the trajectory of cognitive symptoms |
| Include control group in repeated-measures designs to account for practice effects |
| Clinical trials must be blinded and placebo-controlled |
| Assess patient preference for type of treatment (or overall satisfaction with treatment) as an outcome measure in clinical trials |
| Power clinical studies to analyze outcomes by serum T3 levels, Thr92Ala polymorphisms, and TPOAb titers, or stratify by these factors. |
BMI, body mass index; PRO, patient-reported outcome; T3, triiodothyronine; TPOAb, thyroid peroxidase antibody.
Authors' Contributions
M.H.S.: conceptualization, methodology, writing—original draft, and writing—review and editing. L.J.B.: conceptualization, writing—original draft, and writing—review and editing.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Dr. Samuels is supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through grant award number UL1TR002369. Dr. Bernstein is supported by the Princess Margaret Cancer Centre Foundation.
References
- 1. Saravanan P, Chau W-F, Roberts N, Vedhara K, Greenwood R, Dayan CM. 2002. Psychological well-being in patients on ‘adequate’ doses of L-thyroxine: results of a large, controlled community-based questionnaire study. Clin Endocrinol 57:577–585. [DOI] [PubMed] [Google Scholar]
- 2. Wekking EM, Appelhof BC, Fliers E, Schene AH, Huyser T, Tijssen JGP, Wiersinga WM. 2005. Cognitive functioning and well-being in euthyroid patients on thyroxine replacement therapy for primary hypothyroidism. Eur J Endocrinol 153:747–753. [DOI] [PubMed] [Google Scholar]
- 3. Panicker V, Evans J, Bjoro T, Asvold BO, Dayan CM, Bjerkeset O. 2009. A paradoxical difference in relationship between anxiety, depression and thyroid function in subjects on and not on T4: findings from the HUNT study. Clin Endocrinol 71:574–580. [DOI] [PubMed] [Google Scholar]
- 4. Ettleson MD, Raine A, Batistuzzo A, Batista SP, McAninch E, Teixeira MCTV, Jonklaas J, Laiteerapong N, Ribeiro MO, Bianco AC. 2021. Brain fog in hypothyroidism: understanding the patient's perspective. Endocr Pract 28:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peterson SJ, Cappola AR, Castro MR, Dayan CM, Farwell AP, Hennessey JV, Kopp PA, Ross DS, Samuels MH, Sawka AM, Taylor PN, Jonklaas J, Bianco AC. 2018. An online survey of hypothyroid patients demonstrates prominent dissatisfaction. Thyroid 28:707–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mitchell AL, Hegedüs L, Žarković M, Hickey JL, Perros P. 2021. Patient satisfaction and quality of life in hypothyroidism: an online survey by the British Thyroid Foundation. Clin Endocrinol (Oxf) 94:513–520. [DOI] [PubMed] [Google Scholar]
- 7. Haskard-Zolnierek K, Wilson C, Pruin J, Deason R, Howard K. 2022. The relationship between brain fog and medication adherence for individuals with hypothyroidism. Clin Nurs Res 31:445–452. [DOI] [PubMed] [Google Scholar]
- 8. Bernstein LJ, McCreath GA, Komeylian Z, Rich JB. 2017. Cognitive impairment in breast cancer survivors treated with chemotherapy depends on control group type and cognitive domains assessed: a multilevel meta-analysis. Neurosci Biobehav Rev 83:417–428. [DOI] [PubMed] [Google Scholar]
- 9. Sullivan Mitchell E, Fugate Woods N. 2001. Midlife women's attributions about perceived memory changes: observations from the Seattle Midlife Women's Health Study. J Womens Health Gend Based Med 10:351–362. [DOI] [PubMed] [Google Scholar]
- 10. Ross AJ, Medow MS, Rowe PC, Stewart JM. 2013. What is brain fog? An evaluation of the symptom in postural tachycardia syndrome. Clin Auton Res 23:305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jason LA, Jessen T, Porter NS, Boulton A, Njoku M-G, Friedberg F. 2009. Examining types of fatigue in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Disabil Stud Q 2009:29. [Google Scholar]
- 12. Edwards George JB, Aideyan B, Yates K, Voorhees KN, O'Flynn J, Sweet K, Avery K, Ehrlich A, Bast A, Leffler DA 2021 Gluten-induced neurocognitive impairment: results of a nationwide study. J Clin Gastroenterol [Epub ahead of print]; DOI: 10.1097/MCG.0000000000001561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mackay M 2015. Lupus brain fog: a biologic perspective on cognitive impairment, depression, and fatigue in systemic lupus erythematosus. Immunol Res 63:26–37. [DOI] [PubMed] [Google Scholar]
- 14. Roszko KL, Hu TY, Guthrie LC, Brillante BA, Smith M, Collins MT, Gafni RI. 2022. PTH 1-34 Replacement therapy has minimal effect on quality of life in patients with hypoparathyroidism. J Bone Miner Res 37:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schou TM, Joca S, Wegener G, Bay-Richter C. 2021. Psychiatric and neuropsychiatric sequelae of COVID-19—a systematic review. Brain Behav Immun 97:328–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jonklaas J, Bianco AC, Cappola AR, Celi FS, Fliers E, Heuer H, McAninch EA, Moeller LC, Nygaard B, Sawka AM, Watt T, Dayan CM. 2021. Evidence-based use of levothyroxine/liothyronine combinations in treating hypothyroidism: a consensus document. Thyroid 31:156–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mounier NM, Abdel-Maged AE, Wahdan SA, Gad AM, Azab SS. 2020. Chemotherapy-induced cognitive impairment (CICI): an overview of etiology and pathogenesis. Life Sci 258:118071. [DOI] [PubMed] [Google Scholar]
- 18. Pullens MJ, De Vries J, Roukema JA. 2010. Subjective cognitive dysfunction in breast cancer patients: a systematic review. Psychooncology 19:1127–1138. [DOI] [PubMed] [Google Scholar]
- 19. Small BJ, Jim HSL, Eisel SL, Jacobsen PB, Scott SB. 2019. Cognitive performance of breast cancer survivors in daily life: role of fatigue and depressed mood. Psychooncology 28:2174–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yao C, Rich JB, Tannock IF, Seruga B, Tirona K, Bernstein LJ. 2106. Pretreatment differences in intraindividual variability in reaction time between women diagnosed with breast cancer and healthy controls. J Int Neuropsychol Soc 22:530–539. [DOI] [PubMed] [Google Scholar]
- 21. Collins B, Widmann G, Tasca GA. 2018. Effectiveness of intraindividual variability in detecting subtle cognitive performance deficits in breast cancer patients. J Int Neuropsychol Soc 24:724–734. [DOI] [PubMed] [Google Scholar]
- 22. Yao C, Rich JB, Tirona K, Bernstein LJ. 2017. Intraindividual variability in reaction time before and after neoadjuvant chemotherapy in women diagnosed with breast cancer. Psychooncology 26:2261–2268. [DOI] [PubMed] [Google Scholar]
- 23. Bernstein LJ, Catton PA, Tannock IF. 2014. Intra-individual variability in women with breast cancer. J Int Neuropsychol Soc 20:380–390. [DOI] [PubMed] [Google Scholar]
- 24. Bernstein LJ, Edelstein K, Sharma A, Alain C. 2021. Chemo-brain: an activation likelihood estimation meta-analysis of functional magnetic resonance imaging studies. Neurosci Biobehav Rev 130:314–325. [DOI] [PubMed] [Google Scholar]
- 25. Cockshell SJ, Mathias JL. 2010. Cognitive functioning in chronic fatigue syndrome: a meta-analysis. Psychol Med 40:1253–1267. [DOI] [PubMed] [Google Scholar]
- 26. Thapaliya K, Marshall-Gradisnik S, Staines D, Barnden L. 2020. Mapping of pathological change in chronic fatigue syndrome using the ratio of T1- and T2-weighted MRI scans. Neuroimage Clin 28:102366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nakatomi Y, Mizuno K, Ishii A, Wada Y, Tanaka M, Tazawa S, Onoe K, Fukuda S, Kawabe J, Takahashi K, Kataoka Y, Shiomi S, Yamaguti K, Inaba M, Kuratsune H, Watanabe Y. 2014. Neuroinflammation in patients with chronic fatigue syndrome/myalgic encephalomyelitis: an 11C-(R)-PK11195 PET study. J Nucl Med 55:945–950. [DOI] [PubMed] [Google Scholar]
- 28. Reeve BB, Wyrwich KW, Wu AW, Velikova G, Terwee CB, Snyder CF, Schwartz C, Revicki DA, Moinpour CM, McLeod LD, Lyons JC, Lenderking WR, Hinds PS, Hays RD, Greenhalgh J, Gershon R, Feeny D, Fayers PM, Cella D, Brundage M, Ahmed S, Aaronson NK, Butt Z. 2013. SOQOL recommends minimum standards for patient-reported outcome measures used in patient-centered outcomes and comparative effectiveness research. Qual Life Res 22:1889–1905. [DOI] [PubMed] [Google Scholar]
- 29. Watt T, Hegedüs L, Rasmussen AK, Groenvold M, Bonnema SJ, Bjorner JB, Feldt-Rasmussen U. 2007. Which domains of thyroid-related quality of life are most relevant? Patients and clinicians provide complementary perspectives. Thyroid 17:647–654. [DOI] [PubMed] [Google Scholar]
- 30. Watt T, Hegedüs L, Groenvold M, Bjorner JB, Rasmussen AK, Bonnema SJ, Feldt-Rasmussen U. 2010. Validity and reliability of the novel thyroid-specific quality of life questionnaire, ThyPRO. Eur J Endocrinol 162:161–167. [DOI] [PubMed] [Google Scholar]
- 31. Winther KH, Cramon P, Watt T, Bjorner JB, Ekholm O, Feldt-Rasmussen U, Groenvold M, Rasmussen ÅK, Hegedüs L, Bonnema SJ. 2016. Disease-specific as well as generic quality of life is widely impacted in autoimmune hypothyroidism and improves during the first six months of levothyroxine therapy. PLoS One 11:e0156925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nordqvist SF, Boesen VB, Rasmussen ÅK, Feldt-Rasmussen U, Hegedüs L, Bonnema SJ, Cramon PK, Watt T, Groenvold M, Bjorner JB. 2021. Determining minimal important change for the thyroid-related quality of life questionnaire ThyPRO. Endocr Connect 10:316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Watt T, Bjorner JB, Groenvold M, Cramon P, Winther KH, Hegedüs L, Bonnema SJ, Rasmussen ÅK, Ware JE Jr, Feldt-Rasmussen U. 2015. Development of a short version of the Thyroid-Related Patient-Reported Outcome ThyPRO. Thyroid 25:1069–1079. [DOI] [PubMed] [Google Scholar]
- 34. Schuff KG, Samuels MH 2019 Psychiatric and cognitive effects of hypothyroidism. In: Braverman LE, Cooper DS, Kopp PA (eds) Werner and Ingbar's the Thyroid: a Fundamental and Clinical Text, 11th ed. Wolters Kluwer, Philadelphia, PA, pp 599–604. [Google Scholar]
- 35. Chambers T, Anney R, Taylor PN, Teumer A, Peeters RP, Medici M, Caseras X, Rees DA. 2021. Effects of thyroid status on regional brain volumes: a diagnostic and genetic imaging study in UK Biobank. J Clin Endocrinol Metab 106:688–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yuan L, Luan D, Xu X, Yang Q, Huang X, Zhao S, Zhang Y, Zhou Z. 2020. Altered attention networks in patients with thyroid dysfunction: a neuropsychological study. Horm Behav 121:104714. [DOI] [PubMed] [Google Scholar]
- 37. van Vliet NA, van Heemst D, Almeida OP, Åsvold BO, Aubert CE, Bae JB, Barnes LE, Bauer DC, Blauw GJ, Brayne C, Cappola AR, Ceresini G, Comijs HC, Dartigues JF, Degryse JM, Dullaart RPF, van Eersel MEA, den Elzen WPJ, Ferrucci L, Fink HA, Flicker L, Grabe HJ, Han JW, Helmer C, Huisman M, Ikram MA, Imaizumi M, de Jongh RT, Jukema JW, Kim KW, Kuller LH, Lopez OL, Mooijaart SP, Moon JH, Moutzouri E, Nauck M, Parle J, Peeters RP, Samuels MH, Schmidt CO, Schminke U, Slagboom PE, Stordal E, Vaes B, Völzke H, Westendorp RGJ, Yamada M, Yeap BB, Rodondi N, Gussekloo J, Trompet S; Thyroid Studies Collaboration. 2021. Association of thyroid dysfunction with cognitive function: an individual participant data analysis. JAMA Intern Med 181:1440–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stuber MJ, Moutzouri E, Feller M, Del Giovane C, Bauer DC, Blum MR, Collet TH, Gussekloo J, Mooijaart SP, McCarthy VJC, Aujesky D, Westendorp R, Stott DJ, Glynn NW, Kearney PM, Rodondi N. 2020. Effect of thyroid hormone therapy on fatigability in older adults with subclinical hypothyroidism: a nested study within a randomized placebo-controlled trial. J Gerontol A Biol Sci Med Sci 75:e89–e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wildisen L, Feller M, Del Giovane C, Moutzouri E, Du Puy RS, Mooijaart SP, Collet TH, Poortvliet RKE, Kearney P, Quinn TJ, Klöppel S, Bauer DC, Peeters RP, Westendorp R, Aujesky D, Gussekloo J, Rodondi N. 2021. Effect of levothyroxine therapy on the development of depressive symptoms in older adults with subclinical hypothyroidism: an ancillary study of a randomized clinical trial. JAMA Netw Open 4:e2036645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stott DJ, Rodondi N, Kearney PM, Ford I, Westendorp RGJ, Mooijaart SP, Sattar N, Aubert CE, Aujesky D, Bauer DC, Baumgartner C, Blum MR, Browne JP, Byrne S, Collet TH, Dekkers OM, den Elzen WPJ, Du Puy RS, Ellis G, Feller M, Floriani C, Hendry K, Hurley C, Jukema JW, Kean S, Kelly M, Krebs D, Langhorne P, McCarthy G, McCarthy V, McConnachie A, McDade M, Messow M, O'Flynn A, O'Riordan D, Poortvliet RKE, Quinn TJ, Russell A, Sinnott C, Smit JWA, Van Dorland HA, Walsh KA, Walsh EK, Watt T, Wilson R, Gussekloo J; TRUST Study Group. 2017. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med 376:2534–2544. [DOI] [PubMed] [Google Scholar]
- 41. Bekkering GE, Agoritsas T, Lytvyn L, Heen AF, Feller M, Moutzouri E, Abdulazeem H, Aertgeerts B, Beecher D, Brito JP, Farhoumand PD, Singh Ospina N, Rodondi N, van Driel M, Wallace E, Snel M, Okwen PM, Siemieniuk R, Vandvik PO, Kuijpers T, Vermandere M. 2019. Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline. BMJ 365:l2006. [DOI] [PubMed] [Google Scholar]
- 42. Zhu DF, Wang ZX, Zhang DR, Pan ZL, He S, Hu XP, Chen XC, Zhou JN. 2006. fMRI revealed neural substrate for reversible working memory dysfunction in subclinical hypothyroidism. Brain 129:2923–2930. [DOI] [PubMed] [Google Scholar]
- 43. Göbel A, Göttlich M, Heldmann M, Georges R, Nieberding R, Rogge B, Sartorius A, Brabant G, Münte TF. 2019. Experimentally induced subclinical hypothyroidism causes decreased functional connectivity of the cuneus: a resting state fMRI study. Psychoneuroendocrinology 102:158–163. [DOI] [PubMed] [Google Scholar]
- 44. Bladowska J, Waliszewska-Prosół M, Ejma M, Sąsiadek M 2019 The metabolic alterations within the normal appearing brain in patients with Hashimoto's thyroiditis are correlated with hormonal changes. Metab Brain Dis 3453–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Walsh JP, Ward LC, Burke V, Bhagat CI, Shiels L, Henley D, Gillett MJ, Gilbert R, Tanner M, Stuckey BG. 2006. Small changes in thyroxine dosage do not produce measurable changes in hypothyroid symptoms, well-being, or quality of life: results of a double-blind, randomized clinical trial. J Clin Endocrinol Metab 91:2624–2630. [DOI] [PubMed] [Google Scholar]
- 46. Samuels MH, Kolobova I, Niederhausen M, Janowsky JS, Schuff KG. 2018. Effects of altering levothyroxine (L-T4) doses on quality of life, mood, and cognition in L-T4 treated subjects. J Clin Endocrinol Metab 103:1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saeed O, Bernstein LJ, Fazelzad R, Samuels M, Burmeister LA, Thabane L, Ezzat S, Goldstein DP, Jones J, Sawka AM. 2019. Cognitive functioning in thyroid cancer survivors: a systematic review and meta-analysis. J Cancer Surviv 13:231–243. [DOI] [PubMed] [Google Scholar]
- 48. Shakir MKM, Brooks DI, McAninch EA, Fonseca TL, Mai VQ, Bianco AC, Hoang TD. 2021. Comparative effectiveness of levothyroxine, desiccated thyroid extract, and levothyroxine+liothyronine in hypothyroidism. J Clin Endocrinol Metab 106:e4400–e4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sawka AM, Gerstein HC, Marriott MJ, MacQueen GM, Joffe RT. 2003. Does a combination regimen of thyroxine (T4) and 3,5,3'-triiodothyronine improve depressive symptoms better than T4 alone in patients with hypothyroidism? Results of a double-blind, randomized, controlled trial. J Clin Endocrinol Metab 88:4551–4555. [DOI] [PubMed] [Google Scholar]
- 50. Jurado-Flores M, Warda F, Mooradian A 2022 Pathophysiology and clinical features of neuropsychiatric manifestations of thyroid disease. J Endocr Soc 6:bvab194. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Russo SC, Salas-Lucia F, Bianco AC. 2021. Deiodinases and the metabolic code for thyroid hormone action. Endocrinology 162:bqab059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Werneck de Castro JP, Fonseca TL, Ueta CB, McAninch EA, Abdalla S, Wittmann G, Lechan RM, Gereben B, Bianco AC. 2015. Differences in hypothalamic type 2 deiodinase ubiquitination explain localized sensitivity to thyroxine. J Clin Invest 125:769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McAninch EA, Rajan KB, Miller CH, Bianco AC. 2018. Systemic thyroid hormone status during levothyroxine therapy In hypothyroidism: a systematic review and meta-analysis. J Clin Endocrinol Metab 103:4533–4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Peterson SJ, McAninch EA, Bianco AC. 2016. Is a normal TSH synonymous with “euthyroidism” in levothyroxine monotherapy? J Clin Endocrinol Metab 101:4964–4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Millan-Alanis JM, González-González JG, Flores-Rodríguez A, Singh Ospina N, Maraka S, Moreno-Peña PJ, Brito JP, González-Velázquez C, Rodríguez-Gutiérrez R. 2021. Benefits and harms of levothyroxine/L-triiodothyronine versus levothyroxine monotherapy for adult patients with hypothyroidism: systematic review and meta-analysis. Thyroid 31:1613–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bianco AC, Kim BS. 2018. Pathophysiological relevance of deiodinase polymorphism. Curr Opin Endocrinol Diabetes Obes 25:341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jo S, Fonseca TL, Bocco BMLC, Fernandes GW, McAninch EA, Bolin AP, Da Conceição RR, Werneck-de-Castro JP, Ignacio DL, Egri P, Németh D, Fekete C, Bernardi MM, Leitch VD, Mannan NS, Curry KF, Butterfield NC, Bassett JHD, Williams GR, Gereben B, Ribeiro MO, Bianco AC. 2019. Type 2 deiodinase polymorphism causes ER stress and hypothyroidism in the brain. J Clin Invest 129:230–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wouters HJ, van Loon HC, van der Klauw MM, Elderson MF, Slagter SN, Kobold AM, Kema IP, Links TP, van Vliet-Ostaptchouk JV, Wolffenbuttel BH. 2017. No effect of the Thr92Ala polymorphism of Deiodinase-2 on thyroid hormone parameters, health-related quality of life, and cognitive functioning in a large population-based cohort study. Thyroid 27:147–155. [DOI] [PubMed] [Google Scholar]
- 59. Panicker V, Saravanan P, Vaidya B, Evans J, Hattersley AT, Frayling TM, Dayan CM. 2009. Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients. J Clin Endocrinol Metab 94:1623–1629. [DOI] [PubMed] [Google Scholar]
- 60. Engum A, Bjøro T, Mykletun A, Dahl AA. 2005. Thyroid autoimmunity, depression and anxiety; are there any connections? An epidemiological study of a large population. J Psychosom Res 59:263–268. [DOI] [PubMed] [Google Scholar]
- 61. Watt T, Hegedüs L, Bjorner JB, Groenvold M, Bonnema SJ, Rasmussen AK, Feldt-Rasmussen U. 2012. Is thyroid autoimmunity per se a determinant of quality of life in patients with autoimmune hypothyroidism? Eur Thyroid J 1:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Guldvog I, Reitsma LC, Johnsen L, Lauzike A, Gibbs C, Carlsen E, Lende TH, Narvestad JK, Omdal R, Kvaløy JT, Hoff G, Bernklev T, Søiland H. 2019. Thyroidectomy versus medical management for euthyroid patients with Hashimoto Disease and persisting symptoms: a randomized trial. Ann Intern Med 170:453–464. [DOI] [PubMed] [Google Scholar]
- 63. Leyhe T, Müssig K, Weinert C, Laske C, Häring HU, Saur R, Klingberg S, Gallwitz B. 2008. Increased occurrence of weaknesses in attention testing in patients with Hashimoto's thyroiditis compared to patients with other thyroid illnesses. Psychoneuroendocrinology 33:1432–1436. [DOI] [PubMed] [Google Scholar]
- 64. Djurovic M, Pereira AM, Smit JWA, Vasovic O, Damjanovic S, Jemuovic Z, Pavlovic D, Miljic D, Pekic S, Stojanovic M, Asanin M, Krljanac G, Petakov M. 2018. Cognitive functioning and quality of life in patients with Hashimoto thyroiditis on long-term levothyroxine replacement. Endocrine 62:136–143. [DOI] [PubMed] [Google Scholar]
- 65. Samuels MH, Schuff KG, Carlson NE, Carello P, Janowsky JS. 2006. Health status, psychological symptoms, mood, and cognition in L-thyroxine-treated hypothyroid subjects. Thyroid 17:249–258. [DOI] [PubMed] [Google Scholar]
- 66. Quinque EM, Karger S, Arélin K, Schroeter ML, Kratzsch J, Villringer A. 2014. Structural and functional MRI study of the brain, cognition and mood in long-term adequately treated Hashimoto's thyroiditis. Psychoneuroendocrinology 42:188–198. [DOI] [PubMed] [Google Scholar]
- 67. Wang N, Sun Y, Yang H, Xu Y, Cai Y, Liu T, Xia Q, Zhu D, Wang F. 2021. Hashimoto's thyroiditis induces hippocampus-dependent cognitive alterations by impairing astrocytes in euthyroid mice. Thyroid 31:482–493. [DOI] [PubMed] [Google Scholar]
- 68. Laurent C, Capron J, Quillerou B, Thomas G, Alamowitch S, Fain O, Mekinian A. 2016. Steroid-responsive encephalopathy associated with autoimmune thyroiditis (SREAT): characteristics, treatment and outcome in 251 cases from the literature. Autoimmun Rev 15:1129–1133. [DOI] [PubMed] [Google Scholar]
- 69. Galland-Decker C, Marques-Vidal P, Vollenweider P. 2019. Prevalence and factors associated with fatigue in the Lausanne middle-aged population: a population-based, cross-sectional survey. BMJ Open 9:e027070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Campbell KL, Zadravec K, Bland KA, Chesley E, Wolf F, Janelsins MC. 2020. The effect of exercise on cancer-related cognitive impairment and applications for physical therapy: systematic review of randomized controlled trials. Phys Ther 100:523–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Larun L, Brurberg KG, Odgaard-Jensen J, Price JR. 2019. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev 10:CD003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cicerone KD, Goldin Y, Ganci K, Rosenbaum A, Wethe JV, Langenbahn DM, Malec JF, Bergquist TF, Kingsley K, Nagele D, Trexler L, Fraas M, Bogdanova Y, Harley JP. 2019. Evidence-based cognitive rehabilitation: systematic review of the literature from 2009 through 2014. Arch Phys Med Rehabil 100:1515–1533. [DOI] [PubMed] [Google Scholar]
- 73. Banningh JW, Prins JB, Vernooij-Dassen MJFJ, Wijnen HH, Olde Rikkert MGM, Kessels RPC. 2011. Group therapy for patients with mild cognitive impairment and their significant others: results of a waiting-list controlled trial. Gerontology 57:444–454. [DOI] [PubMed] [Google Scholar]
- 74. Ferguson RJ, Sigmon ST, Pritchard AJ, LaBrie SL, Goetze RE, Fink CM, Garrett AM. 2016. A randomized trial of videoconference-delivered cognitive behavioral therapy for survivors of breast cancer with self-reported cognitive dysfunction. Cancer 122:1782–1791. [DOI] [PubMed] [Google Scholar]
- 75. Ye M, Du K, Zhou J, Zhou Q, Shou M, Hu B, Jiang P, Dong N, He L, Liang S, Yu C, Zhang J, Ding Z, Liu Z. 2018. A meta-analysis of the efficacy of cognitive behavior therapy on quality of life and psychological health of breast cancer survivors and patients. Psychooncology 27:1695–1703. [DOI] [PubMed] [Google Scholar]
- 76. Richard NM, Bernstein LJ, Mason WP, Laperriere N, Maurice C, Millar BA, Shultz DB, Berlin A, Edelstein K. 2019. Cognitive rehabilitation for executive dysfunction in brain tumor patients: a pilot randomized controlled trial. J Neurooncol 142:565–575. [DOI] [PubMed] [Google Scholar]
- 77. Bernstein LJ, McCreath GA, Nyhof-Young J, Dissanayake D, Rich JB. 2018. A brief psychoeducational intervention improves memory contentment in breast cancer survivors with cognitive concerns: results of a single-arm prospective study. Support Care Cancer 26:2851–2859. [DOI] [PubMed] [Google Scholar]
- 78. Levine B, Stuss DT, Winocur G, Binns MA, Fahy L, Mandic M, Bridges K, Robertson IH. 2007. Cognitive rehabilitation in the elderly: effects on strategic behavior in relation to goal management. J Int Neuropsychol Soc 13:143–152. [DOI] [PubMed] [Google Scholar]
- 79. Bray VJ, Dhillon HM, Bell ML, Kabourakis M, Fiero MH, Yip D, Boyle F, Price MA, Vardy JL. 2017. Evaluation of a web-based cognitive rehabilitation program in cancer survivors reporting cognitive symptoms after chemotherapy. J Clin Oncol 35:217–225. [DOI] [PubMed] [Google Scholar]
- 80. Werneck FZ, Coelho EF, Almas SP, Garcia MMDN, Bonfante HLM, Lima JRP, Vigário PDS, Mainenti MRM, Teixeira PFDS, Vaisman M. 2018. Exercise training improves quality of life in women with subclinical hypothyroidism: a randomized clinical trial. Arch Endocrinol Metab 62:530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hawkins J, Hires CY, Dunne EW, Keenan LA 2019 Aromatherapy reduces fatigue among women with hypothyroidism: a randomized placebo-controlled clinical trial. J Complement Integr Med 17:/j/jcim.2019.17.issue-1/jcim-2018-0229/jcim-2018-0229.xml. [DOI] [PubMed] [Google Scholar]
- 82. Talebi S, Karimifar M, Heidari Z, Mohammadi H, Askari G. 2020. The effects of synbiotic supplementation on thyroid function and inflammation in hypothyroid patients: a randomized, double blind, placebo controlled trial. Complement Ther Med 48:102234. [DOI] [PubMed] [Google Scholar]
- 83. Engum A, Bjøro T, Mykletun A, Dahl AA. 2002. An association between depression, anxiety and thyroid function—a clinical fact or an artefact? Acta Psychiatr Scand 106:27–34. [DOI] [PubMed] [Google Scholar]
- 84. Jørgensen P, Langhammer A, Krokstad S, Forsmo S. 2015. Diagnostic labelling influences self-rated health. A prospective cohort study: the HUNT Study, Norway. Fam Pract 32:492–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. van de Ven AC, Netea-Maier RT, de Vegt F, Ross HA, Sweep FC, Kiemeney LA, Hermus AR, den Heijer M. 2012. Is there a relationship between fatigue perception and the serum levels of thyrotropin and free thyroxine in euthyroid subjects? Thyroid 22:1236–1243. [DOI] [PubMed] [Google Scholar]
- 86. Airaksinen J, Komulainen K, García-Velázquez R, Määttänen I, Gluschkoff K, Savelieva K, Jokela M. 2021. Subclinical hypothyroidism and symptoms of depression: evidence from the National Health and Nutrition Examination Surveys (NHANES). Compr Psychiatry 109:152253. [DOI] [PubMed] [Google Scholar]
- 87. Schagen SB, Das E, van Dam FS. 2009. The influence of priming and pre-existing knowledge of chemotherapy-associated cognitive complaints on the reporting of such complaints in breast cancer patients. Psychooncology 18:674–678. [DOI] [PubMed] [Google Scholar]
- 88. Leventhal H, Meyer D, Nerenz DR 1980 The common sense representation of illness danger. In: Rachman S (ed.) Contributions to Medical Psychology. Pergamon Press, New York, pp 17–30. [Google Scholar]
- 89. McAndrew LM, Martin JL, Friedlander M, Shaffer K, Breland J, Slotkin S, Leventhal H. 2018. The common sense of counseling psychology: introducing the Common-Sense Model of Self-Regulation. Couns Psychol Q 31:497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hagger MS, Orbell S. 2003. A meta-analytic review of the common-sense model of illness representations. Psychol Health 18:141–184. [Google Scholar]
- 91. Pankowski D, Wytrychiewicz-Pankowska K, Janowski K, Pisula E, Walicka M. 2021. The role of illness-related beliefs in depressive, anxiety, and anger symptoms: an on-line survey in women with hypothyroidism. Front Psychiatry 12:614361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Samuels MH, Kolobova I, Niederhausen M, Janowsky JS, Schuff KG. 2018. Effects of altering levothyroxine (L-T4) doses on quality of life, mood, and cognition in L-T4 treated subjects. J Clin Endocrinol Metab 103:1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Idrees T, Palmer S, Maciel RMB, Bianco AC. 2020. Liothyronine and desiccated thyroid extract in the treatment of hypothyroidism. Thyroid 30:1399–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]