Abstract
Background:
Allan-Herndon-Dudley syndrome (AHDS) is a severe psychomotor disability disorder that also manifests characteristic abnormal thyroid hormone (TH) levels. AHDS is caused by inactivating mutations in monocarboxylate transporter 8 (MCT8), a specific TH plasma membrane transporter widely expressed in the central nervous system (CNS). MCT8 mutations cause impaired transport of TH across brain barriers, leading to insufficient neural TH supply. There is currently no successful therapy for the neurological symptoms. Earlier work has shown that intravenous (IV), but not intracerebroventricular adeno-associated virus serotype 9 (AAV9) -based gene therapy given to newborn Mct8 knockout (Mct8−/y) male mice increased triiodothyronine (T3) brain content and partially rescued TH-dependent gene expression, suggesting a promising approach to treat this neurological disorder.
Methods:
The potential of IV delivery of AAV9 carrying human MCT8 was tested in the well-established Mct8−/y/Organic anion-transporting polypeptide 1c1 (Oatp1c1)−/ − double knockout (dKO) mouse model of AHDS, which, unlike Mct8−/y mice, displays both neurological and TH phenotype. Further, as the condition is usually diagnosed during childhood, treatment was given intravenously to P30 mice and psychomotor tests were carried out blindly at P120–P140 after which tissues were collected and analyzed.
Results:
Systemic IV delivery of AAV9-MCT8 at a juvenile stage led to improved locomotor and cognitive functions at P120–P140, which was accompanied by a near normalization of T3 content and an increased response of positively regulated TH-dependent gene expression in different brain regions examined (thalamus, hippocampus, and parietal cortex). The effects on serum TH concentrations and peripheral tissues were less pronounced, showing only improvement in the serum T3/reverse T3 (rT3) ratio and in liver deiodinase 1 expression.
Conclusion:
IV administration of AAV9, carrying the human MCT8, to juvenile dKO mice manifesting AHDS has long-term beneficial effects, predominantly on the CNS. This preclinical study indicates that this gene therapy has the potential to ameliorate the devastating neurological symptoms in patients with AHDS.
Keywords: gene therapy, MCT8, thyroid hormone cell transport
Introduction
Thyroid hormones (THs) are essential for the development and metabolic homeostasis of most organs and tissues (1). The major form of TH released in the blood from the thyroid gland is thyroxine (T4), which acts as a prohormone. T4 conversion to the active hormone, triiodothyronine (T3), or to the inactive form, reverse T3 (rT3), takes place intracellularly by iodothyronine deiodinases enzymes (2). The main mechanism of T3 action is achieved through binding to specific nuclear receptors, which, in turn, operate as regulators of gene transcription (3).
Since TH metabolism and action are intracellular events, they require the presence of TH-specific transporters mediating cellular TH uptake and efflux (4). The solute carrier family 16, member 2 (SLC16A2) gene, located on the X-chromosome, encodes for the monocarboxylate transporter 8 (MCT8) protein (5). MCT8 is well conserved throughout vertebrate evolution and is widely expressed in the body and central nervous system (CNS) (6). A key function of MCT8 is to facilitate TH transport across plasma membranes (5).
Inactivating MCT8 gene mutations in males cause a severe form of psychomotor disability (7–9), clinically described as Allan-Herndon-Dudley syndrome (AHDS) (10). Patients exhibit neurological impairments, including severe intellectual disability, truncal hypotonia, dystonia, and movement disorders. MCT8-deficiency also causes a TH phenotype, including elevated serum T3 levels, low rT3 and T4 with normal or slightly elevated thyroid stimulating hormone (TSH), resulting in markedly elevated free T3/T4 and T3/rT3 ratios (11).
Two independently generated Mct8-KO mouse models (12,13) closely recapitulate the TH phenotype observed in patients with AHDS, but they do not display expected neurological or behavioral phenotypes. This is due to a milder TH deprivation in mouse brains owing to a T4-specific transporter not present in the human blood brain barrier (BBB). Specifically, the Organic anion-transporting polypeptide 1c1 (Oatp1c1), encoded by the slco1c1 gene, was identified in mice, but not human, brain capillaries (14–16).
Double knockout (dKO) mct8−/y;oatp1c1−/− mice display disease-relevant phenotypes, including an impaired TH transport into the CNS and consequently a significantly decreased number of cortical parvalbumin-positive GABAergic interneurons, reduced myelination, and pronounced locomotor abnormalities (17). These results indicate that in mice Mct8 (together with Oatp1c1) plays a crucial role in the transport of THs into the CNS and, importantly, provides a robust disease model for human MCT8-deficiency (18,19).
To progress from an animal model to a human-based model, induced pluripotent stem cells were derived from AHDS patients and differentiated into brain microvascular endothelial cells, which showed MCT8-dependent transport of THs across the human BBB (16,20). However, MCT8 is not restricted to the brain endothelium, and it also affects TH transport across neural cell plasma membranes (21).
Gene therapy offers a promising approach to treat monogenic disorders. Spinal muscular atrophy type 1 patients, carrying deleterious mutations in the survival motor neuron 1 (SMN1) gene, were treated by a single intravenous (IV) infusion of adeno-associated virus serotype 9 (AAV9) containing DNA coding for SMN1, resulting in improved survival, as well as achievement of motor milestones and motor functions (22,23), with subsequent FDA approval of the gene therapy product Zolgensma. Additional examples for the beneficial gene therapy approach for monogenic disorders have been recently reported (24–26).
Although intracerebroventricular (ICV)-delivery directly targets the brain ventricles, thereby circumventing the BBB, IV-delivery offers systemic delivery, transducing primarily tissues outside the CNS, including blood vessels. Importantly, it has been shown that AAV9 IV-delivery can cross the BBB and efficiently infect CNS cells (27). In a recent proof-of-concept study, an AAV9-MCT8 construct was delivered by ICV or IV injections into neonatal Mct8-KO (Mct8−/y) mice (28), with an increase in brain TH signaling on IV, but not ICV, delivery. However, since the Mct8-KO mice do not display neurological impairments, it is unclear whether this approach results in rescue of the neurological symptoms.
Here, we tested the potential of IV delivery of AAV9-MCT8 in dKO mice. We chose to treat juvenile male mice at postnatal day 30 (P30) and tested the potential rescue of neurological and behavioral parameters at adulthood. This approach was espoused, as the diagnosis of MCT8 deficiency is usually made in childhood.
Materials and Methods
All procedures were approved by Cedars-Sinai Medical Center's Institutional Animal Care and Use Committee (IACUC No. 009128). mct8−/−/oatp1c1−/− females and mct8−/y/oatp1c1−/− males with C57BL/6 background were paired to generate dKO pups. Wild type (WT) C57BL/6 were used as controls. Only males were selected for all treatments. AAV9-MCT8 was administered to P30 juvenile dKO mice and controls by tail vein injection containing 50 × 1010 viral particles (vp)/g in a volume of 20 μL/g.
Behavioral as well as biochemical and molecular measurements were all performed on tissues and serum at P120–P140 and analyzed double blinded without the knowledge as to which group the mice belonged to. Mice identities were blinded by the person administering the AAAV9-MCT8 and were unknown to the technicians while performing the behavioral assays, dissection, tissue collection, and biochemical analysis. This was unblinded when results were assembled and for the statistical analysis. Animals were subjected to behavioral analysis before being sacrificed for tissue collection. Thus, results from the same mice are presented in all figures.
Details regarding virus preparation, behavioral and locomotor tests (rotarod, open field, gate analysis, Barnes maze, Y maze), tissue collection, and measurements of TH and TSH in serum are described (Supplementary Data).
Statistical analysis
All scatter plots were first tested for their normal distribution by using Kolmogorov-Smirnov test, with the Dallal-Wilkinson-Lilliefor corrected p value. Data sets that were normally distributed were tested by using one-way analysis of variance with Tukey's post hoc test for multiple comparisons. Data sets that were not normally distributed were compared by using Mann-Whitney non-parametric test for independent samples.
Behavior data collected over time including the rotarod test and Barnes maze were analyzed with mixed model regression with random intercept and the fixed factors of time (as a continuous variable), treatment group, and the interaction term of group with time. To compare learning curves between groups, the β coefficients were compared and differences were considered significant at the step-down Bonferroni-corrected alpha level of <0.05. Residuals were inspected to confirm the fit of the modeling. Data analysis was performed with GraphPad Prism v8.0.0 and SAS Enterprise Guide v8.2. All data are presented as mean ± standard error of the mean. p < 0.05 was considered statistically significant. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Results
IV delivery of AAV9-MCT8 at P30 improves locomotor performance of dKO mice
Given that patients with AHDS are often diagnosed during childhood, treatment feasibility should optimally be tested at a juvenile stage. Previous experience (28) indicated that IV delivery is more feasible compared with ICV delivery, as IV is a simpler route and would target not only the brain but also other body regions that express MCT8 such as the liver. Therefore, we tested IV delivery at the previously tested dose of 50 × 1010 vp/g of AAV9-MCT8 (28) to peri-pubertal P30 dKO mice (Fig. 1A).
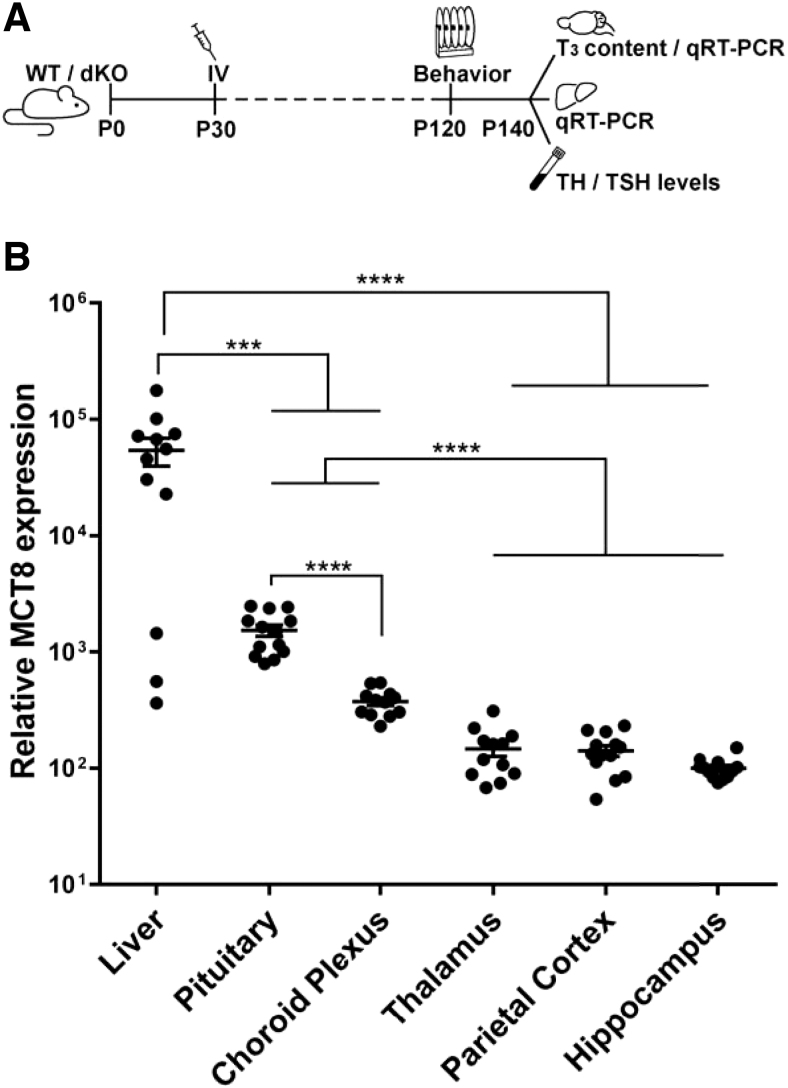
FIG. 1.
Study design and human MCT8 expression in the liver and brain regions after IV AAV9-MCT8 injection of P30 dKO mice. (A) Schematic of experimental design. dKO mice were treated at postnatal day 30 (P30) by tail vein (IV) delivery of AAV9-MCT8 at a dose of 50 × 1010 vp/g. (B) Quantification of MCT8 mRNA levels by qRT-PCR showed MCT8 re-expression relative to the three housekeeping genes Polr2a, Actb, and Gapdh in the liver and different brain regions of dKO treated animals. One-way ANOVA with Tukey's multiple comparisons. The data are presented as mean, and error bars represent SEM. (***p < 0.001, ****p < 0.0001). AAV9, adeno-associated virus serotype 9; ANOVA, analysis of variance; dKO, double knockout; IV, intravenous; MCT8, monocarboxylate transporter 8; mRNA, messenger RNA; qRT-PCR, quantitative real-time polymerase chain reaction; SEM, standard error of the mean; vp, viral particles.
To confirm that the viral delivery resulted in expression of human MCT8, liver and brain were collected at P140. As expected, human MCT8 messenger RNA (mRNA) expression was not detected in WT or dKO untreated mice (data not shown). MCT8 expression was observed in the liver and various brain regions of AAV9-MCT8 IV-treated dKO mice, with the highest levels seen in the liver. Pituitary and choroid plexus had higher MCT8 levels compared with the BBB-protected regions of the thalamus, parietal cortex, and hippocampus (Fig. 1B).
Mice underwent various behavioral analyses to assess locomotor function, which were performed at P120, when differences between WT and dKO were previously reported (17). Assessment by a rotarod test showed that untreated dKO mice had an overall reduced latency to fall and decreased learning curve compared with the WT group (Fig. 2A). After IV delivery of AAV9-MCT8, dKO mice showed a significant increase in the latency to fall and an increased learning curve, indicating that treatment improved locomotor performance and potentially cognitive function.
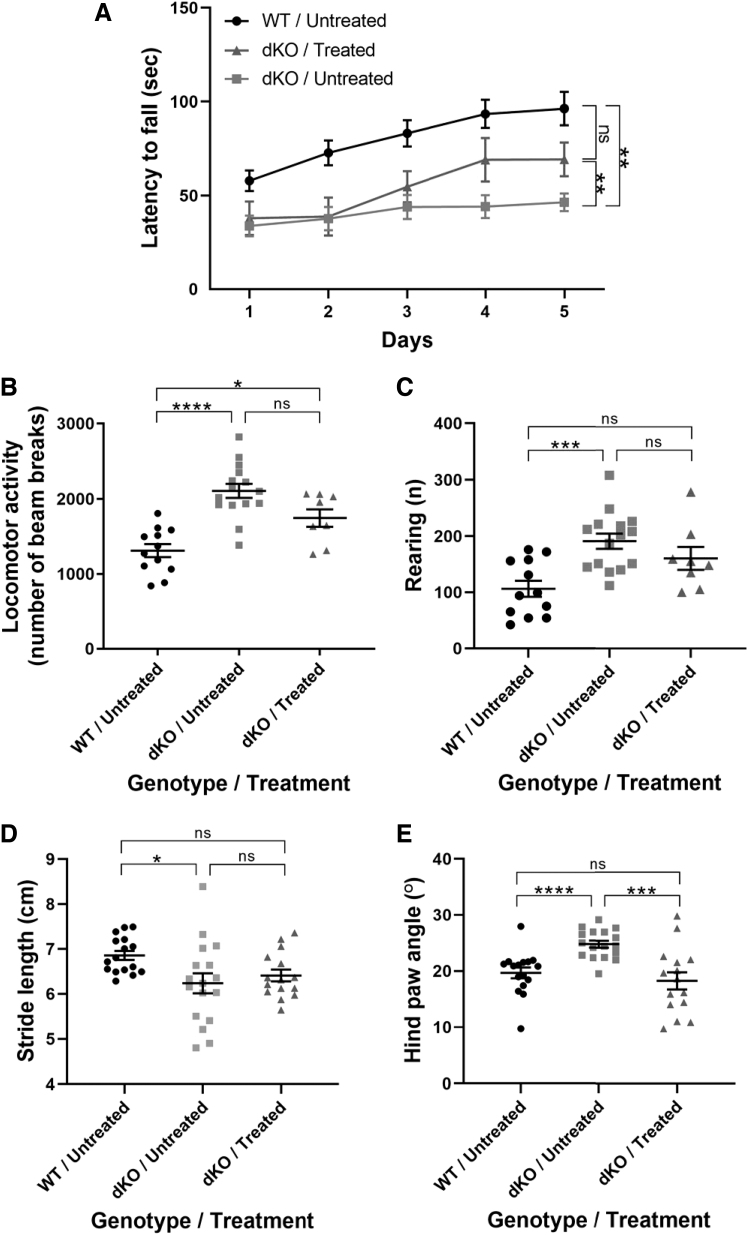
FIG. 2.
Locomotor performance is improved in dKO mice treated at P30. (A) Locomotor deficiencies were monitored by a rotarod test. Data were analyzed with mixed model regression with random intercept and the fixed factors of time, group, and the interaction term of group with time. To compare learning curves between groups, the β coefficients were compared. WT untreated mice (n = 16), treated dKO mice (n = 13), untreated dKO mice (n = 24). Open field tests included (B) horizontal locomotion and (C) vertical rearing. (D, E) Paw print assessment at P120 to determine (D) stride length and for (B–D) one-way ANOVA with Tukey's multiple comparisons was used. (E) Hind paw angle. For (E), Mann-Whitney non-parametric test for independent samples was used. The data are presented as mean, and error bars represent SEM. (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). WT, wild type.
Overall locomotor activity assessed by an open field test as well as rearing behavior were significantly higher in untreated dKO mice compared with WT mice, and this did not significantly change after treatment (Fig. 2B, C). Finally, hind paw analysis showed that there was no significant improvement in the stride length and a complete recovery in the hind paw angle (Fig. 2D, E). Collectively, data demonstrate that AAV9-MCT8 treatment at P30 provided recovery in some, but not all, locomotion parameters in dKO mice.
IV delivery of AAV9-MCT8 at P30 improves cognitive performance in dKO mice
Learning, spatial memory, and memory recall were assessed by using a Barnes maze (29). During the 4-day training period, treatment did not significantly improve the learning curve (Fig. 3A). After a 2-day break, untreated dKO mice required higher latency than untreated WT and treated dKO mice (Fig. 3B). The position of the escape hole was then moved during 2 days of training during which no significant differences were observed between all groups (Fig. 3C). These data suggest that the AAV9-MCT8 IV treatment of dKO mice at P30 resulted in a partial rescue in the learning and recall ability.
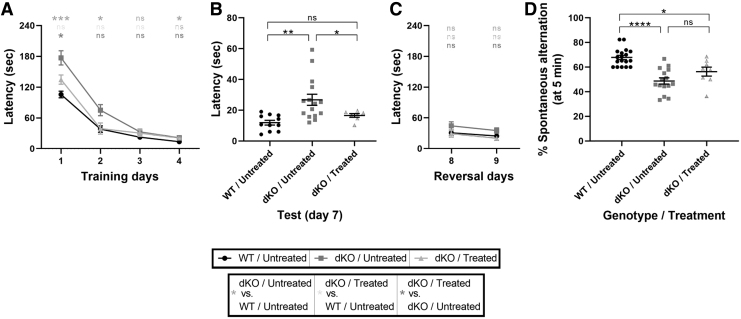
FIG. 3.
Improved cognitive performance in dKO mice treated at P30. The cognitive-related behavioral performance was assessed at P140. (A–C) In a Barnes maze test, the ability of mice to discover and then recall the location of an escape hole was evaluated during the learning phase (A, Training, days 1–4), after a 2-day break (B, Test, day 7), and after re-positioning of the escape hole (C, Reversal days 8 and 9). The latency to successful location of the escape hole was recorded. Data were analyzed with mixed model regression with random intercept and the fixed factors of time, group, and the interaction term of group with time. To compare learning curves between groups, the β coefficients were compared. (D) Spontaneous alternation between the arms of a Y-maze was assessed over a 5-minute period. One-way ANOVA with Tukey's multiple comparisons. The data are presented as mean, and error bars represent SEM. (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). All analyses were double blind.
A spontaneous alternation maze (Y-maze) test was used to further examine spatial memory. Untreated dKO mice demonstrated a significant decrease in the percent of spontaneous alterations compared with untreated WT mice, which was not significantly improved in the AAV9-MCT8-treated dKO mice (Fig. 3D). The results of both tests suggest that IV-treatment of P30 dKO mice with AAV9-MCT8 improves cognitive performance as well as restores some spatial and learning memory.
IV delivery of AAV9-MCT8 at P30 partially restores brain T3-content in dKO mice
To assess the effects on pathophysiology, the serum, liver, and brain were collected at P140 (Fig. 1A). Examining the T3 brain content, we showed that T3 levels in the thalamus (Fig. 4A) and hippocampus (Fig. 4B) of treated dKO mice were fully normalized, reaching WT levels. A significant increase in brain T3 content was also observed in the parietal cortex (Fig. 4C). Given the low brain levels of MCT8 expression after treatment, these results suggest that low MCT8 expression (Fig. 1B) is sufficient to normalize brain T3 content.
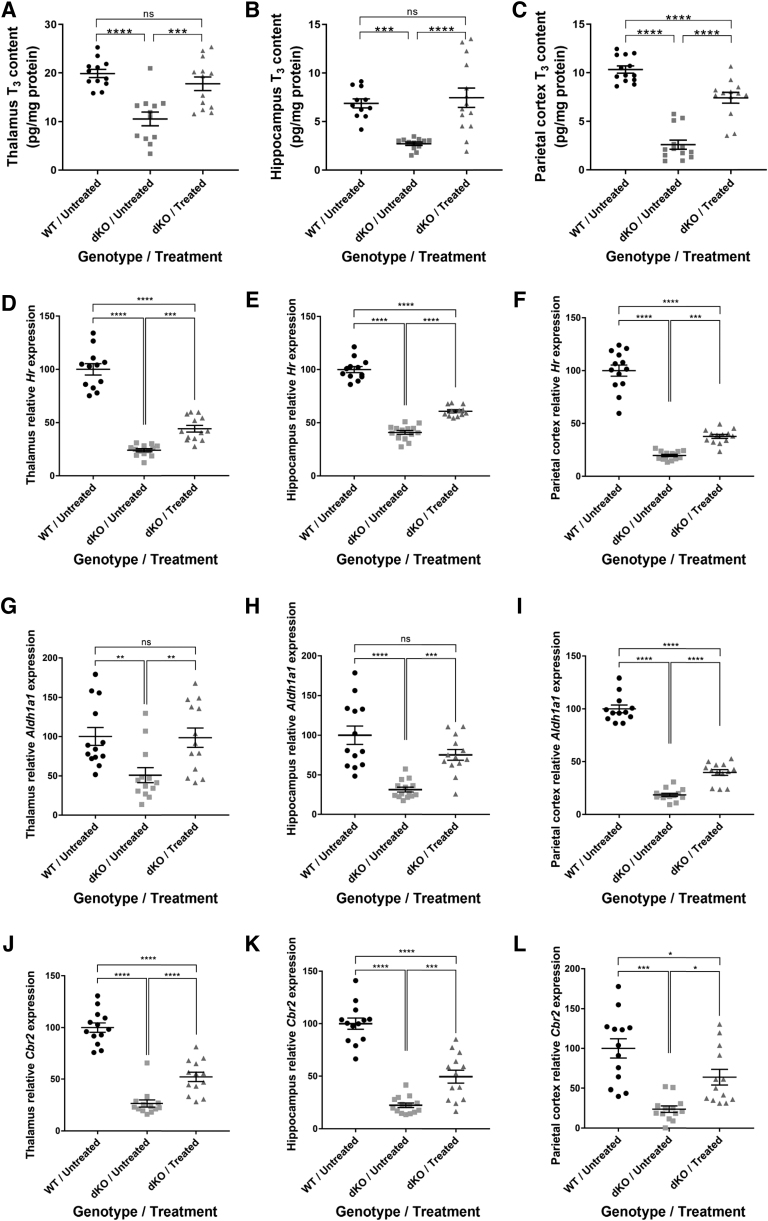
FIG. 4.
Brain T3 content and T3-induced gene expression in dKO mice treated at P30. T3 content measured in the (A) thalamus (B), hippocampus, and (C) parietal cortex. T3-induced genes were examined by qRT-PCR. Hr expression was measured in the (D) thalamus, (E) hippocampus, and (F) parietal cortex. Expression of Aldh1a1 was measured in the (G) thalamus, (H) hippocampus, and (I) parietal cortex. Expression of Cbr2 was measured in the (J) thalamus, (K) hippocampus, and (L) parietal cortex. The data are presented as mean, and error bars represent SEM. For (C, G, J), Mann-Whitney non-parametric test for independent samples was used. For (A, B, D–F, H, I, K, L), one-way ANOVA with Tukey's multiple comparisons was used. The data are presented as mean, and error bars represent SEM. (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). Aldh1a1, aldehyde dehydrogenase 1 family member a1; Cbr2, carbonyl reductase 2; Hr, hairless; T3, triiodothyronine.
IV delivery of AAV9-MCT8 at P30 corrects T3-inducible gene expression
To assess a T3 effect in the different brain regions, we next studied T3-inducible gene expression by quantitative real-time polymerase chain reaction (qRT-PCR). These genes were selected based on their known response to T3 (30). Hairless (Hr) levels were significantly improved in the thalamus (Fig. 4D), the hippocampus (Fig. 4E), and the parietal cortex (Fig. 4F) of treated dKO mice. Aldehyde dehydrogenase 1 family member a1 (Aldh1a1) levels were fully rescued in the thalamus (Fig. 4G) and hippocampus (Fig. 4H) and significantly improved in the parietal cortex (Fig. 4I).
Finally, Carbonyl reductase 2 (Cbr2) levels were also significantly improved in treated dKO compared with untreated dKO mice in the thalamus, the hippocampus, and the parietal cortex (Fig. 4J–L). These results suggest that IV delivery of AAV9-MCT8 to P30 dKO mice can substantially improve and maintain long-term brain T3 content and T3-inducible gene expression.
IV delivery of AAV9-MCT8 at P30 partially rescues phenotypes in the liver and minimally in serum of dKO mice
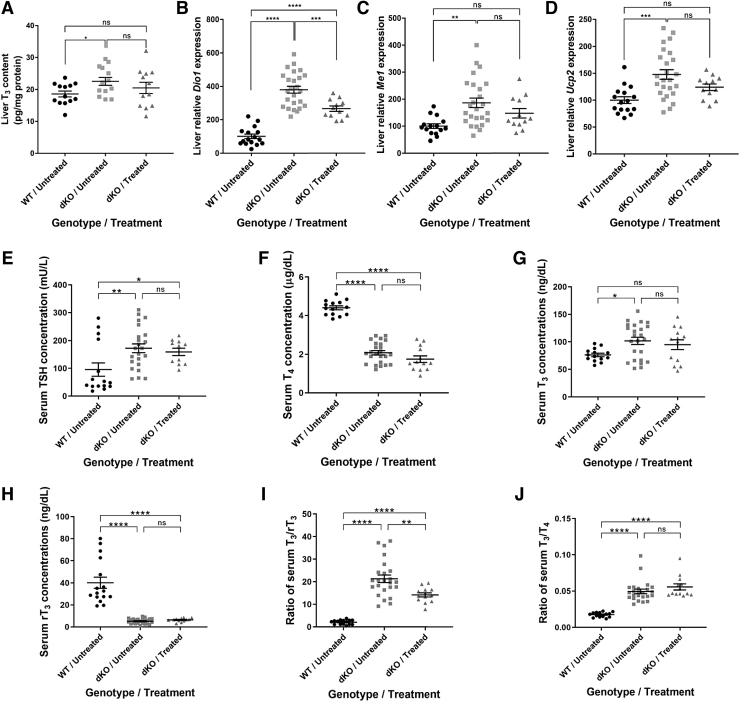
The significantly higher level of MCT8 expression in the liver (Fig. 1B) is expected with IV delivery, which can easily penetrate the blood vessels of the liver compared with the BBB capillaries (31). In contrast to the brain TH deficiency, patients with AHDS experience TH excess in peripheral tissues caused by the high serum T3 levels. We, therefore, measured liver T3 levels (Fig. 5A). The treatment did not result in a significant decrease of T3 levels in treated dKO mice. Analysis of liver T3-inducible genes was performed by qRT-PCR.
FIG. 5.
Liver T3 content, T3-induced gene expression, and serum TSH and TH concentrations in dKO mice treated at P30. Liver tissue was obtained for (A) measuring T3 concentrations and for qRT-PCR analysis of T3-induced gene expression, including (B) iodothyronine Dio1, (C) Me1, and (D) Ucp2. Concentrations of hormones in serum are shown, including (E) TSH, (F) T4, (G) T3, (H), and rT3. Ratios of (I) T3/rT3 and (J) T3/T4 were calculated. Data are presented as mean, and error bars represent SEM. For (A) no significant changes were found by one-way ANOVA. Student t-test was used to compare every two treatments. For (E, H, J), Mann-Whitney non-parametric test for independent samples was used. For (B–D, F, G, I), one-way ANOVA with Tukey's multiple comparisons was used. The data are presented as mean, and error bars represent SEM. (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). Dio1, deiodinase 1; Me1, malic enzyme 1; rT3, reverse T3; T4, thyroxine; TH, thyroid hormone; TSH, thyroid stimulating hormone; Ucp2, uncoupling protein 2.
The expression levels of deiodinase 1 (Dio1), malic enzyme 1 (Me1), and uncoupling protein 2 (Ucp2) (Fig. 5B–D), and the liver T3 level were all significantly elevated in the dKO untreated group compared with their WT littermates, confirming the effect of TH excess in the liver. AAV9-MCT8 delivery led to a significant decrease in Dio1 mRNA levels in treated dKO mice compared with untreated dKO mice. However, there was no significant reduction in Me1 and Ucp2 levels in response to treatment.
Patients with AHDS have abnormal serum TH levels, including elevated T3, low rT3 and T4 with normal or slightly elevated TSH, resulting in low T3/T4 and T3/rT3 ratios. The increased liver Dio1 enzymatic activity is one of the mechanisms responsible for these serum thyroid tests and a decrease in its expression is needed to ameliorate this phenotype (32). To test the effect of IV delivery of AAV9-MCT8 at P30 on the serum TH phenotype in dKO mice, blood was collected from P140 mice and serum TSH and TH levels were quantified. Serum levels of TSH, T4, T3, and rT3 as well as the T3/rT3 and the T3/T4 ratios were all significantly altered in dKO mice compared with their WT littermates (Fig. 5E–J).
Although AAV9-MCT8 treatment did not significantly alter serum levels of TSH, T4, T3, rT3, and T3/T4 ratio, the combination of a slight reduction in T3 and an increase in rT3 resulted in a significant decrease of the T3/rT3 ratio in agreement with the observed attenuation of Dio1 expression. This indicates that AAV9-MCT8 delivery at a juvenile stage can partially improve the abnormalities in serum.
Discussion
In this study, we tested the potential of IV delivery of AAV9-MCT8 to juvenile dKO mice. Our analysis shows the long-term expression of human MCT8 within the CNS and in peripheral tissue, suggesting that AAV9 can efficiently transduce cells with MCT8 in peripheral tissue and within the CNS. Re-expression of MCT8 resulted in improved locomotor and cognitive behavior as well as a substantial rescue of T3 content and associated gene expression in different areas of the brain.
MCT8-deficient patients suffer from a severe neuro-psychomotor phenotype and TH excess in peripheral tissues. Thus, an effective therapeutic strategy should account for deficient transport of THs across brain barriers and neural plasma membranes as well as the excess of TH in peripheral tissues. Being a rare disorder, AHDS is often misdiagnosed, resulting in later identification of the disease. Moreover, there are currently about 300 diagnosed cases, which mostly involve older children (11,33).
Thus, it is important to develop therapeutics that are effective at juvenile ages. We, therefore, tested the effect of tail vein IV delivery at P30, which is peri-pubertal when pathophysiological symptoms are apparent in both dKO mice and patients (17,34,35).
Endogenously, MCT8 is ubiquitously expressed and is prominently localized in the thyroid, liver, kidneys, and CNS (6,12,13). In the current study, we showed that IV-administration of AAV9-MCT8 to P30 dKO mice led to long-term MCT8 expression in the CNS and liver.
In the brain, MCT8 expression was observed in various regions, confirming the ability of AAV9 vectors to cross brain barriers and efficiently transduce brain cells during this period of development (27). Interestingly, higher expression was observed in brain regions that are not protected by the BBB such as the choroid plexus and pituitary (36,37) compared with the thalamus, hippocampus, and cortex. Strikingly, in the current study, this long-term brain expression resulted in a nearly complete normalization of T3 brain concentrations and associated gene expression in the thalamus, hippocampus, and parietal cortex.
MCT8-deficiency was previously suggested to be caused by both reduced transport of T3 across brain barriers and across neural cell membranes (16,17,21,38). These results, therefore, suggest that a rescue of the T3 brain content was achieved. However, future work is required to distinguish whether the improved brain content is caused by MCT8 expression in brain blood vessels or the choroid plexus, both of which can serve as gateways to the brain.
The improved brain content was accompanied by improved performance in the rotarod test and in gait analyses. Although it remains unclear why other locomotor functions did not change significantly, the observed improvements can be attributed to ameliorations in psychomotor functions. Treated animals also showed an improvement in the learning curve using the rotarod test, suggesting that treatment may have beneficial effects on cognitive and motor functions. Exploratory behavior, learning, and memory are believed to originate in the hippocampus (39). Notably, the mild rescue of hippocampus-dependent learning and memory in the Barnes maze test in response to treatment was observed to be correlated with the significant increase of T3 levels and T3-induced gene expression in the hippocampus.
MCT8 expression was significantly higher in the liver than in the brain. However, no significant rescue was observed in T3 levels in the liver. Nevertheless, a reduction was observed in liver Dio1 expression, a TH-regulated enzyme that generates T3 from T4 and is responsible in part for the T3 excess in serum (32). This effect on Dio1 expression resulted in a partial amelioration in serum T3/rT3 ratio, while other parameters were not significantly improved.
These results suggest a mild beneficial effect of systemic (IV) delivery of MCT8 on the peripheral tissues. Additional mechanisms contribute to the characteristic serum thyroid tests of AHDS, including decreased thyroidal secretion and altered negative feedback to the hypothalamus and pituitary, and thus have different TH availability (40–42). Thus, to augment the partial rescue, additional TH-normalizing treatments should be considered in conjunction with gene therapy.
There are several current treatment strategies that focus on TH analogs. These thyromimetic compounds are required to activate TH-induced transcriptional pathways via thyroid nuclear receptors, and they need to penetrate plasma membranes independent of MCT8. In animal models, the TH analogs DITPA (43,44), TRIAC (18), TETRAC (19), and Sobetirome (45) were able to restore some of the peripheral and central abnormalities; however, their effects on neurological symptoms remained limited or unknown due to the use of mice that were not neurologically affected. In patients with AHDS, DITPA (46) and TRIAC (47) reduced the high serum T3 concentration; however, there was no evidence for improvement in neurological symptoms. Thus, TH analogs can be considered to be used in combination with gene therapy.
Chemical and pharmaceutical chaperones have also been suggested as an alternative approach. These chaperones can restore the ability of some MCT8 gene mutations to transport TH across plasma membranes in animal models (48,49); however, their clinical effect has not been assessed to date. Moreover, the use of chemical chaperones is limited only to a handful of missense mutations, and it is therefore not applicable to the majority of patients.
Gene therapy, which emerged as a promising approach to treat monogenic developmental neurological disorders (22,23,25,26,50,51), can overcome these limitations and potentially target all mutations and patients. Moreover, restoration of a functional MCT8 has the potential to resolve TH transport, as well as unidentified MCT8 roles, such as the transport of additional potential substrates.
While this study shows a promising therapeutic direction, it has limitations. Although the dKO mice provide a useful model for AHDS, the symptoms are less severe than in patients. Further, additional disease-relevant features such as lack of language or the predisposition to death could not be tackled in this study due to limitations of the model.
Overall, this study shows that IV administration of AAV9-MCT8 to dKO mice provides substantial rescue of molecular and biochemical parameters in the brain, as well as amelioration of the TH excess effect in peripheral tissues. In addition, this treatment improves locomotor and behavioral performance. These findings support future clinical examination of AAV-based MCT8 gene therapy in patients with AHDS.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Soshana Svendsen for critical writing and editing. Biostatistical support was received from the Center for Research Support (“HALEV”), Faculty of Health Sciences, Ben Gurion University of the Negev and by the Samuel Oschin Comprehensive Cancer Institute at Cedars-Sinai.
Authors' Contributions
G.D.V., S.R., and C.N.S. provided the conceptual framework for the study and designed the experiments. X.-H.L. performed the brain dissections, qRT-PCR analyses, and the TH measurements. P.A. treated the animals. O.S. bred the colonies and collected tissues. O.S. and J.-P.V. performed behavioral analyses. R.O. analyzed the data. M.S. and C.B. performed the statistical analyses. S.L., B.K., and K.M. provided the virus. A.M.D. generated the human MCT8 insert used in the construction of the virus and provided intellectual input. H.H. provided the dKO mice and provided intellectual input. G.D.V. and C.N.S. wrote the article.
Author Disclosure Statement
The authors declare no conflict of interests.
Funding Information
This research was supported by grants from the Sherman Family Foundation, the Board of Governors Regenerative Medicine Institute at Cedars-Sinai Medical Center to C.N.S., the Israel Science Foundation grant 1621/18 and the Ministry of Science and Technology (MoST), Israel grant 3-15647 to G.D.V. This work was supported in part by grant DK15070 from the National Institutes of Health (USA) to S.R.
Supplementary Material
References
- 1. Yen PM 2001. Physiological and molecular basis of thyroid hormone action. Physiol Rev 81:1097–1142. [DOI] [PubMed] [Google Scholar]
- 2. Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeöld A, Bianco AC. 2008. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev 29:898–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harvey CB, Williams GR. 2002. Mechanism of thyroid hormone action. Thyroid 12:441–446. [DOI] [PubMed] [Google Scholar]
- 4. Groeneweg S, Van Geest FS, Peeters RP, Heuer H, Visser WE. 2019. Thyroid hormone transporters. Endocr Rev 41:1–55. [DOI] [PubMed] [Google Scholar]
- 5. Friesema ECH, Ganguly S, Abdalla A, Manning Fox JE, Halestrap AP, Visser TJ. 2003. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem 41:40128–40135. [DOI] [PubMed] [Google Scholar]
- 6. Vatine GD, Zada D, Lerer-Goldshtein T, Tovin A, Malkinson G, Yaniv K, Appelbaum L. 2013. Zebrafish as a model for monocarboxyl transporter 8-deficiency. J Biol Chem 288:169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Friesema ECH, Grueters PA, Biebermann H, Krude H, von Moers A, Reeser M, Barrett TG, Mancilla EE, Svensson J, Kester MHA, Kuiper GGJM, Balkassmi S, Uitterlinden AG, Koehrle J, Rodien P, Halestrap AP, Visser TJ. 2004. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet 364:1435–1437. [DOI] [PubMed] [Google Scholar]
- 8. Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S. 2004. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet 74:168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schwartz CE, May MM, Carpenter NJ, Rogers RC, Martin J, Bialer MG, Ward J, Sanabria J, Marsa S, Lewis JA, Echeverri R, Lubs HA, Voeller K, Simensen RJ, Stevenson RE. 2005. Allan-Herndon-Dudley syndrome and the monocarboxylate transporter 8 (MCT8) gene. Am J Hum Genet 77:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Allan W, Herndon CN, Dudley FC. 1944. Some examples of the inheritance of mental deficiency: apparently sex-linked idiocy and microcephaly. Am J Ment Defic 46:325–334. [Google Scholar]
- 11. Groeneweg S, van Geest FS, Abacı A, Alcantud A, Ambegaonkar GP, Armour CM, Bakhtiani P, Barca D, Bertini ES, van Beynum IM, et al. 2020. Disease characteristics of MCT8 deficiency: an international, retrospective, multicentre cohort study. Lancet Diabetes Endocrinol 8:594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dumitrescu AM, Liao XH, Weiss RE, Millen K, Refetoff S. 2006. Tissue-specific thyroid hormone deprivation and excess in monocarboxylate transporter (Mct) 8-deficient mice. Endocrinology 147:4036–4043. [DOI] [PubMed] [Google Scholar]
- 13. Trajkovic M, Visser TJ, Mittag J, Horn S, Lukas J, Darras VM, Raivich G, Bauer K, Heuer H. 2007. Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J Clin Invest 117:627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mayerl S, Visser TJ, Darras VM, Horn S, Heuer H. 2012. Impact of Oatp1c1 deficiency on thyroid hormone metabolism and action in the mouse brain. Endocrinology 153:1528–1537. [DOI] [PubMed] [Google Scholar]
- 15. Roberts LM, Woodford K, Zhou M, Black DS, Haggerty JE, Tate EH, Grindstaff KK, Mengesha W, Raman C, Zerangue N. 2008. Expression of the thyroid hormone transporters monocarboxylate transporter-8 (SLC16A2) and organic ion transporter-14 (SLCO1C1) at the blood-brain barrier. Endocrinology 149:6251–6261. [DOI] [PubMed] [Google Scholar]
- 16. Vatine GD, Al-Ahmad A, Barriga BK, Svendsen S, Salim A, Garcia L, Garcia VJ, Ho R, Yucer N, Qian T, Lim RG, Wu J, Thompson LM, Spivia WR, Chen Z, Van Eyk J, Palecek SP, Refetoff S, Shusta EV, Svendsen CN 2017 Modeling psychomotor retardation using iPSCs from MCT8-deficient patients indicates a prominent role for the blood-brain barrier. Cell Stem Cell 20:831.e5–843.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mayerl S, Müller J, Bauer R, Richert S, Kassmann CM, Darras VM, Buder K, Boelen A, Visser TJ, Heuer H. 2014. Transporters MCT8 and OATP1C1 maintain murine brain thyroid hormone homeostasis. J Clin Invest 124:1987–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kersseboom S, Horn S, Visser WE, Chen J, Friesema EC, Vaurs-Barrière C, Peeters RP, Heuer H, Visser TJ. 2015. In vitro and mouse studies support therapeutic utility of triiodothyroacetic acid in MCT8 deficiency. Mol Endocrinol 28:1961–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Horn S, Kersseboom S, Mayerl S, Müller J, Groba C, Trajkovic-Arsic M, Ackermann T, Visser TJ, Heuer H. 2013. Tetrac can replace thyroid hormone during brain development in mouse mutants deficient in the thyroid hormone transporter Mct8. Endocrinology 154:968–979. [DOI] [PubMed] [Google Scholar]
- 20. Vatine GD, Barrile R, Workman MJ, Sances S, Barriga BK, Rahnama M, Barthakur S, Kasendra M, Lucchesi C, Kerns J, Wen N, Spivia WR, Chen Z, Van Eyk J, Svendsen CN 2019 Human iPSC-derived blood-brain barrier chips enable disease modeling and personalized medicine applications. Cell Stem Cell 24:995.e6–1005.e6. [DOI] [PubMed] [Google Scholar]
- 21. Vatine GD, Shelest O, Barriga BK, Ofan R, Rabinski T, Mattis VB, Heuer H, Svendsen CN. 2021. Oligodendrocyte progenitor cell maturation is dependent on dual function of MCT8 in the transport of thyroid hormone across brain barriers and the plasma membrane. Glia 69:2146–2159. [DOI] [PubMed] [Google Scholar]
- 22. Mendell JR, Al-Zaidy S, Shell R, Arnold WD, Rodino-Klapac LR, Prior TW, Lowes L, Alfano L, Berry K, Church K, Kissel JT, Nagendran S, L'Italien J, Sproule DM, Wells C, Cardenas JA, Heitzer MD, Kaspar A, Corcoran S, Braun L, Likhite S, Miranda C, Meyer K, Foust KD, Burghes AHM, Kaspar BK. 2017. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med 377:1713–1722. [DOI] [PubMed] [Google Scholar]
- 23. Mendell JR, Al-Zaidy SA, Lehman KJ, McColly M, Lowes LP, Alfano LN, Reash NF, Iammarino MA, Church KR, Kleyn A, Meriggioli MN, Shell R. 2021. Five-year extension results of the phase 1 START trial of onasemnogene abeparvovec in spinal muscular atrophy. JAMA Neurol 78:834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Corti M, Liberati C, Smith BK, Lawson LA, Tuna IS, Conlon TJ, Coleman KE, Islam S, Herzog RW, Fuller DD, Collins SW, Byrne BJ. 2017. Safety of intradiaphragmatic delivery of adeno-associated virus-mediated alpha-glucosidase (rAAV1-CMV-hGAA) gene therapy in children affected by Pompe disease. Hum Gene Ther Clin Dev 28:208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mendell JR, Sahenk Z, Lehman K, Nease C, Lowes LP, Miller NF, Iammarino MA, Alfano LN, Nicholl A, Al-Zaidy S, Lewis S, Church K, Shell R, Cripe LH, Potter RA, Griffin DA, Pozsgai E, Dugar A, Hogan M, Rodino-Klapac LR. 2020. Assessment of systemic delivery of rAAVrh74.MHCK7.micro-dystrophin in children with duchenne muscular dystrophy: a nonrandomized controlled trial. JAMA Neurol 77:1122–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eichler F, Duncan C, Musolino PL, Orchard PJ, De Oliveira S, Thrasher AJ, Armant M, Dansereau C, Lund TC, Miller WP, Raymond GV, Sankar R, Shah AJ, Sevin C, Gaspar HB, Gissen P, Amartino H, Bratkovic D, Smith NJC, Paker AM, Shamir E, O'Meara T, Davidson D, Aubourg P, Williams DA. 2017. Hematopoietic stem-cell gene therapy for cerebral adrenoleukodystrophy. N Engl J Med 377:1630–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. 2009. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol 27:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iwayama H, Liao XH, Braun L, Bárez-López S, Kaspar B, Weiss RE, Dumitrescu AM, Guadaño-Ferraz A, Refetoff S. 2016. Adeno associated virus 9-based gene therapy delivers a functional monocarboxylate transporter 8, improving thyroid hormone availability to the brain of Mct8-deficient mice. Thyroid 26:1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Das MM, Godoy M, Chen S, Moser VA, Avalos P, Roxas KM, Dang I, Yáñez A, Zhang W, Bresee C, Arditi M, Liu GY, Svendsen CN, Goodridge HS. 2019. Young bone marrow transplantation preserves learning and memory in old mice. Commun Biol 2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bárez-López S, Grijota-Martínez C, Liao XH, Liao XH, Refetoff S, Guadaño-Ferraz A. 2019. Intracerebroventricular administration of the thyroid hormone analog TRIAC increases its brain content in the absence of MCT8. PLoS One 14:e0226017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sabbagh MF, Heng JS, Luo C, Castanon RG, Nery JR, Rattner A, Goff LA, Ecker JR, Nathans J. 2018. Transcriptional and epigenomic landscapes of CNS and non-CNS vascular endothelial cells. Elife 7:e36187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Di Cosmo C, Liao XH, Ye H, Ferrara AM, Weiss RE, Refetoff S, Dumitrescu AM. 2013. Mct8-deficient mice have increased energy expenditure and reduced fat mass that is abrogated by normalization of serum T3 levels. Endocrinology 154:4885–4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Geest FS, Groeneweg S, Visser WE. 2021. Monocarboxylate transporter 8 deficiency: update on clinical characteristics and treatment. Endocrine 71:689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bárez-López S, Grijota-Martínez C, Ausó E, Fernández-de Frutos M, Montero-Pedrazuela A, Guadaño-Ferraz A. 2019. Adult mice lacking Mct8 and Dio2 proteins present alterations in peripheral thyroid hormone levels and severe brain and motor skill impairments. Thyroid 29:1669–1682. [DOI] [PubMed] [Google Scholar]
- 35. López-Espíndola D, Morales-Bastos C, Grijota-Martínez C, Liao XH, Lev D, Sugo E, Verge CF, Refetoff S, Bernal J, Guadaño-Ferraz A. 2014. Mutations of the thyroid hormone transporter MCT8 cause prenatal brain damage and persistent hypomyelination. J Clin Endocrinol Metab 99:E2799–E2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anbalagan S, Gordon L, Blechman J, Matsuoka RL, Rajamannar P, Wircer E, Biran J, Reuveny A, Leshkowitz D, Stainier DYR, Levkowitz G. 2018. Pituicyte cues regulate the development of permeable neuro-vascular interfaces. Dev Cell 47:711.e5–726.e5. [DOI] [PubMed] [Google Scholar]
- 37. Ben-Zvi A, Liebner S. 2021. Developmental regulation of barrier- and non-barrier blood vessels in the CNS. J Intern Med. [Epub ahead of print]; DOI: 10.1111/joim.13263. [DOI] [PubMed] [Google Scholar]
- 38. Ceballos A, Belinchon MM, Sanchez-Mendoza E, Grijota-Martinez C, Dumitrescu AM, Refetoff S, Morte B, Bernal J. 2009. Importance of monocarboxylate transporter 8 for the blood-brain barrier-dependent availability of 3,5,3’-triiodo-L-thyronine. Endocrinology 150:2491–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Voss JL, Gonsalves BD, Federmeier KD, Tranel D, Cohen NJ. 2011. Hippocampal brain-network coordination during volitional exploratory behavior enhances learning. Nat Neurosci 14:115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Trajkovic-Arsic M, Visser TJ, Darras VM, Friesema EC, Schlott B, Mittag J, Bauer K, Heuer H. 2010. Consequences of monocarboxylate transporter 8 deficiency for renal transport and metabolism of thyroid hormones in mice. Endocrinology 151:802–809. [DOI] [PubMed] [Google Scholar]
- 41. Di Cosmo C, Liao XH, Dumitrescu AM, Philp NJ, Weiss RE, Refetoff S. 2010. Mice deficient in MCT8 reveal a mechanism regulating thyroid hormone secretion. J Clin Invest 120:3377–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fliers E, Unmehopa UA, Alkemade A. 2006. Functional neuroanatomy of thyroid hormone feedback in the human hypothalamus and pituitary gland. Mol Cell Endocrinol 151:1–8. [DOI] [PubMed] [Google Scholar]
- 43. Di Cosmo C, Liao XH, Dumitrescu AM, Weiss RE, Refetoff S. 2009. A thyroid hormone analog with reduced dependence on the monocarboxylate transporter 8 for tissue transport. Endocrinology 150:4450–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ferrara AM, Liao XH, Ye H, Weiss RE, Dumitrescu AM, Refetoff S. 2015. The thyroid hormone analog DITPA ameliorates metabolic parameters of male mice with Mct8 deficiency. Endocrinology 156:3889–3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bárez-López S, Hartley MD, Grijota-Martínez C, Scanlan TS, Guadaño-Ferraz A. 2018. Sobetirome and its amide prodrug Sob-AM2 exert thyromimetic actions in Mct8-deficient brain. Thyroid 28:1211–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Verge CF, Konrad D, Cohen M, Di Cosmo C, Dumitrescu AM, Marcinkowski T, Hameed S, Hamilton J, Weiss RE, Refetoff S. 2012. Diiodothyropropionic acid (DITPA) in the treatment of MCT8 deficiency. J Clin Endocrinol Metab 97:4515–4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Groeneweg S, Peeters RP, Moran C, et al. 2019. Effectiveness and safety of the tri-iodothyronine analogue Triac in children and adults with MCT8 deficiency: an international, single-arm, open-label, phase 2 trial. Lancet Diabetes Endocrinol 7:695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Braun D, Schweizer U. 2017. The chemical chaperone phenylbutyrate rescues MCT8 mutations associated with milder phenotypes in patients with Allan-Herndon-Dudley syndrome. Endocrinology 158:678–691. [DOI] [PubMed] [Google Scholar]
- 49. Braun D, Schweizer U. 2015. Efficient activation of pathogenic Δphe501 mutation in monocarboxylate transporter 8 by chemical and pharmacological chaperones. Endocrinology 156:4720–4730. [DOI] [PubMed] [Google Scholar]
- 50. White KA, Nelvagal HR, Poole TA, Lu B, Johnson TB, Davis S, Pratt MA, Brudvig J, Assis AB, Likhite S, Meyer K, Kaspar BK, Cooper JD, Wang S, Weimer JM. 2021. Intracranial delivery of AAV9 gene therapy partially prevents retinal degeneration and visual deficits in CLN6-Batten disease mice. Mol Ther Methods Clin Dev 20:497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pearson TS, Gupta N, San Sebastian W, et al. 2021. Gene therapy for aromatic L-amino acid decarboxylase deficiency by MR-guided direct delivery of AAV2-AADC to midbrain dopaminergic neurons. Nat Commun 12:4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.