Abstract
Background:
Individuals with Autism Spectrum Disorder (ASD) commonly show sensory over-responsivity (SOR), an impairing condition related to over-reactive brain and behavioral responses to aversive stimuli. While individuals with ASD often show atypically high physiological arousal, it is unclear how this relates to sensory reactivity. We therefore investigated how physiological arousal relates to brain and behavioral indices of SOR, to inform understanding of the biological mechanisms underlying SOR and to determine whether physiological measures are associated with SOR-related brain responses.
Methods:
Youth aged 8–18 (49 ASD; 30 age- and performance-IQ-matched typically developing (TD)) experienced mildly aversive tactile and auditory stimuli first during functional magnetic resonance imaging (N = 41 ASD, 26 TD) and then during skin conductance (SCR) (N = 48 ASD, 28 TD) and heart rate (HR) measurements (N = 48 ASD, 30 TD). Parents reported on their children’s SOR severity.
Results:
Autism Spectrum Disorder youth overall displayed greater SCR to aversive sensory stimulation than TD youth and greater baseline HR. Within ASD, higher SOR was associated with higher mean HR across all stimuli after controlling for baseline HR. Furthermore, the ASD group overall, and the ASD-high-SOR group in particular, showed reduced HR deceleration/greater acceleration to sensory stimulation compared to the TD group. Both SCR and HR were associated with brain responses to sensory stimulation in regions previously associated with SOR and sensory regulation.
Conclusions:
Autism Spectrum Disorder youth displayed heightened physiological arousal to mildly aversive sensory stimulation, with HR responses in particular showing associations with brain and behavioral measures of SOR. These results have implications for using psychophysiological measures to assess SOR, particularly in individuals with ASD who cannot undergo MRI.
Keywords: Autism spectrum disorders, physiology, fMRI, sensory over-responsivity
Introduction
Individuals with autism spectrum disorders (ASD) commonly display sensory processing differences, including atypical sensory discrimination (O’Riordan & Passetti, 2006), sensory seeking, sensory under-responsivity, and sensory over-responsivity (SOR; Liss, Saulnier, Fein, & Kinsbourne, 2006). Here, we focus on SOR, an extremely negative or avoidant response to sensory stimuli such as loud noises or being touched, as SOR is highly prevalent in autism (Ben-Sasson et al., 2009) and is particularly associated with greater impairment and distress, including more severe autism symptoms and anxiety (Horder, Wilson, Mendez, & Murphy, 2014; Pfeiffer, Kinnealey, Reed, & Herzberg, 2005), and reduced social functioning and adaptive skills (Ausderau et al., 2016; Glod, Riby, Honey, & Rodgers, 2015).
The underlying biology of SOR has, until recently, not been well understood. Biological reactions to sensory stimulation are commonly measured from peripheral indices of arousal such as cardiac and skin conductance responses (SCR). Studies using these measures have consistently shown heightened physiological responses in ASD participants compared to age-matched typically developing (TD) controls. For example, individuals with ASD have been shown to have higher heart rate (HR) when exposed to aversive sensory stimuli (Goodwin et al., 2006; Woodard et al., 2012) or completing a cognitive task with and without noise (Keith, Jamieson, & Bennetto, 2019). They have also shown higher SCR during an auditory tone presentation (Chang et al., 2012; Kuiper, Verhoeven, & Geurts, 2019). The few studies that failed to find differences in physiological arousal to sensory stimulation generally had small sample sizes or did not control for IQ differences (McCormick et al., 2014; Miller, Reisman, Mcintosh, & Simon, 2001). Taken together, extant data suggest atypically high peripheral arousal to sensory stimulation in ASD, but few studies have examined whether within-group variability in these measures is related to SOR behaviors (Schoen, Miller, Brett-Green, & Hepburn, 2008).
A growing body of research has begun to identify the underlying neurobiological mechanisms of SOR, indicating that SOR in ASD is related to over-reactive brain responses to aversive stimuli. Recent functional magnetic resonance imaging (fMRI) research has shown that SOR is related to greater brain activation in sensory and limbic regions in response to mildly aversive sensory stimulation as well as decreased habituation in these regions (Green et al., 2015, 2019). However, such fMRI studies are limited to older, verbal youth with cognitive functioning in the normal range, and, as such, there is a need to identify biological measures that can contribute to understanding of how SOR relates to brain function across ages and cognitive profiles. If strong associations exist between neural and peripheral physiological responses to sensory stimuli, then future research on the biological mechanisms underlying SOR will be less restricted by participant characteristics and cost. Physiological measures such as SCR and HR can be utilized across age, verbal ability, and cognitive level (e.g., Woodard et al., 2012) to measure arousal and attention (e.g., Bradley, 2009).
Therefore, our study aimed to examine how psychophysiological responses to aversive sensory stimulation relate to both brain and behavioral indices of SOR in youth with ASD compared to TD controls. We hypothesized that, consistent with prior findings, ASD youth would show higher HR and SCR responses to aversive sensory stimulation than TD youth. We further hypothesized that ASD youth with high SOR would have the highest physiological responses. Finally, we hypothesized that the psychophysiological measures would correlate with activation in brain areas previously shown to be related to SOR in children and adolescents with ASD.
Materials and methods
Participants
Participants were 49 youth with ASD (37 male) and 30 TD-matched controls (21 male), 8.2-18.0 years (M = 13.73; SD = 2.82). All participants had full-scale IQ (FSIQ) of 70 or above. See Appendix S1 for additional details on diagnosis and inclusion criteria. Groups did not differ significantly in age, motion during fMRI, or performance IQ, but they differed in full-scale and verbal IQs (Table 1), so FSIQ was tested as a covariate in all diagnostic group comparisons and included where significant at p < .10. Our study was approved by the UCLA Institutional Review Board, and informed consent and assent were obtained from the participants.
Table 1.
Descriptive statistics
| ASD-SOR-Low Group (N = 25) |
ASD-SOR-High Group (N = 24) |
Typically Developing Group (N = 30) |
|||||
|---|---|---|---|---|---|---|---|
| Measures | N | % | N | % | N | % | F or χ2 |
| Male | 18 | 72 | 19 | 79.17 | 21 | 70 | 0.61 |
| Right-handed | 24 | 96 | 23 | 95.83 | 26 | 86.67 | 2.27 |
| Medication status | 14 | 56 | 17 | 71 | 0 | 0 | 1.16f |
| Mean | SD | Mean | SD | Mean | SD | ||
| Age (years) | 13.99 | 2.78 | 13.56 | 3.04 | 13.65 | 2.76 | 0.16 |
| Full-scale IQ | 101.68b,c | 15.29 | 104.71 | 16.79 | 112.00b,c | 12.57 | 3.57* |
| Verbal IQ | 99.40d,e | 15.17 | 100.13d,e | 19.61 | 109.60d,e | 13.52 | 3.50* |
| Performance IQ | 104.56 | 17.64 | 108.88 | 15.21 | 111.50 | 11.67 | 1.50 |
| Sensory Over-Responsivity Inventory | |||||||
| Tactile count | 2.32 | 2.04 | 7.12 | 3.87 | 0.60 | 0.93 | 47.90*** |
| Auditory count | 2.04b,c,d,e | 1.86 | 8.79b,c | 4.90 | 0.30b,c,d,e | 0.65 | 60.60*** |
| Short Sensory Profile | |||||||
| Tactile sensitivity score | 30.12 | 3.94 | 23.13 | 5.29 | 34.03 | 1.61 | 55.82*** |
| Auditory score | 8.28 | 1.86 | 5.75 | 2.01 | 9.83 | 0.46 | 46.74*** |
| SOR composite score | −0.13 | 0.35 | 1.11 | 0.65 | −0.77 | 0.17 | 136.24*** |
| Scanner measuresa | |||||||
| Mean absolute motion | 0.38 | 0.22 | 0.46 | 0.31 | 0.42 | 0.22 | 0.51 |
| Mean relative motion | 0.15 | 0.15 | 0.17 | 0.13 | 0.16 | 0.16 | 0.09 |
| Volumes censored | 24.81 | 18.14 | 25.20 | 19.07 | 18.85 | 15.55 | 0.99 |
ASD, Autism Spectrum Disorder; IQ, Intelligence Quotient; SD, Standard Deviation; SOR, Sensory Over-Responsivity.
fMRI analysis Ns were: ASD-SOR-Low:21, ASD-SOR-High: 20, TD: 26
Compared between ASD-SOR-Low and ASD-SOR-High; p > .1
Paired groups are significantly different from each other (p < .05). If no superscript, all three groups are significantly different from each other.
Paired groups differ at p < .10.
p < .05.
p < .01.
p < .001.
Sensory paradigm
Participants received comparable sensory stimulation paradigms in two contexts: first while undergoing fMRI and afterward, outside of the scanner along with physiological (HR and SCR) measurement. The sensory paradigms included 6 blocks each of 15-sec mildly aversive auditory (various frequencies of pulsing colored (e.g., white) noise), tactile (scratchy materials rubbed on the left inner forearm), and joint (simultaneous auditory and tactile) stimulation. See Appendix S1 for additional sensory stimuli details. Participants focused on a central fixation cross during inter-trial intervals (ITIs), with 12.5-sec fixations before and between trials during the fMRI scan and 9-sec fixations during psychophysiological measurement. Psychophysiological measures were also collected during an initial 2-min baseline fixation period while sitting quietly.
Behavioral measures
Parents completed two sensory questionnaires about their child on the same day as the fMRI and psychophysiological sessions: the Short Sensory Profile (SSP; McIntosh, Miller, Shyu, & Dunn, 1999) and the Sensory Over-Responsivity (SensOR) Inventory (Schoen, Miller, & Green, 2008), which were used to generate an overall composite score by standardizing and averaging the subscales relevant to SOR (Table 1). Participants were divided into three SOR subgroups based on their sensory composite score (ASD-SOR-high, ASD-SOR-low, and TD without SOR). The ASD group was divided into high and low SOR based on a median split of the sensory composite as in prior work (Green et al., 2019). One TD participant with elevated SOR was excluded from data analysis. The SOR composite score had high internal consistency (TableS 5).
Physiological measurements
Skin conductance and heart rate (HR) were acquired continually throughout the physiological part of the experiment with the participant in a sitting position. Mean HR and skin conductance levels (SCL) were calculated for the experimental baseline phase and across each stimulus trial. Skin conductance response (SCR) for each trial was calculated as the maximum value 1–6 s after stimulus onset minus the mean value during the 2 s prior to stimulus onset. Additionally, for each stimulus trial, we calculated inter-beat intervals (IBIs), measured in milliseconds, where higher values indicate slower HR (more time between heart beats). IBI was calculated for each of the following intervals after stimulus onset: 0–1, 1–2, 2–3, 3–4, 4–5, 5–10, 10–15 s, minus the mean IBI 5 s before stimulus onset. This allowed us to examine both immediate changes in IBI after the stimulus onset (‘orienting’ phase; 0–1 to 1–2 s), subsequent HR acceleration (‘acceleration’ phase; 1–2 to 3–4 s), and sustained IBI across the remaining 11-s (‘habituation’ phase; 5–10 to 10–15 s). See Appendix S1 for additional details.
Psychophysiological data analysis
To test for group differences in mean SCR and HR as well as changes in these measures across the 18 trials, we ran repeated-measures ANOVAs with SOR group (ASD-SOR-high vs. ASD-SOR-low vs. TD) as a between-subjects factor and stimulus type (Joint, Auditory, Tactile) and trial (1–6) as within-subjects factors. If SOR group was non-significant, we replaced SOR with diagnostic group (ASD, TD). FSIQ, age, and sex were tested as covariates and included where significant at p < .10. Detailed descriptions of the effects of FSIQ, age, and sex on physiological arousal, as well as participant medication status and the effect of medication on main physiological findings, can be found in Appendices S1 and S2.
fMRI data acquisition and analysis
Complete information on fMRI acquisition, preprocessing, analysis, and motion correction can be found in Appendix S1. FSL’s fMRI Expert Analysis Tool (FEAT), version 6.0, was used for statistical analyses. Each experimental condition (auditory, tactile, joint) was modeled with respect to inter-trial fixation at the single-subject level. Overall, within- and between-group contrasts for this sensory paradigm were presented in Green et al. (2019). SCR, mean HR, IBI deceleration, and IBI acceleration scores were averaged across all auditory, tactile, and joint timepoints, de-meaned, and correlated with brain response averaged across all stimulus types. Within-group and between-group correlation maps were thresholded at Z > 2.3 (p < .01) and whole-brain cluster-corrected at p < .05. For all neuroimaging correlational analyses, parameter estimates were extracted from significant clusters to check for outliers, and only correlations that survived with outliers removed are reported.
Results
Baseline physiological arousal
A one-way ANOVA was conducted to compare diagnostic and SOR groups on mean SCL and HR during the initial 2-min baseline fixation period. There were no significant diagnostic (t(74) = .63, p = .53, d = .15) or SOR group (F(2,73) = .31, p = .74, η2=.01) differences in baseline SCL (FigureS 1a). There was a significant diagnostic group (t(76) = 2.20, p = .03, d = .51) but not SOR group (F(2,75) = 2.84, p = .06, η2=.07) difference in baseline HR (FigureS 1b), with the ASD group (M = 81.03, SD = 12.54) showing a higher mean baseline HR than the TD group (M = 74.79, SD = 11.59). Baseline HR was thus covaried in additional mean HR analyses described below.
Skin conductance response to sensory stimulation
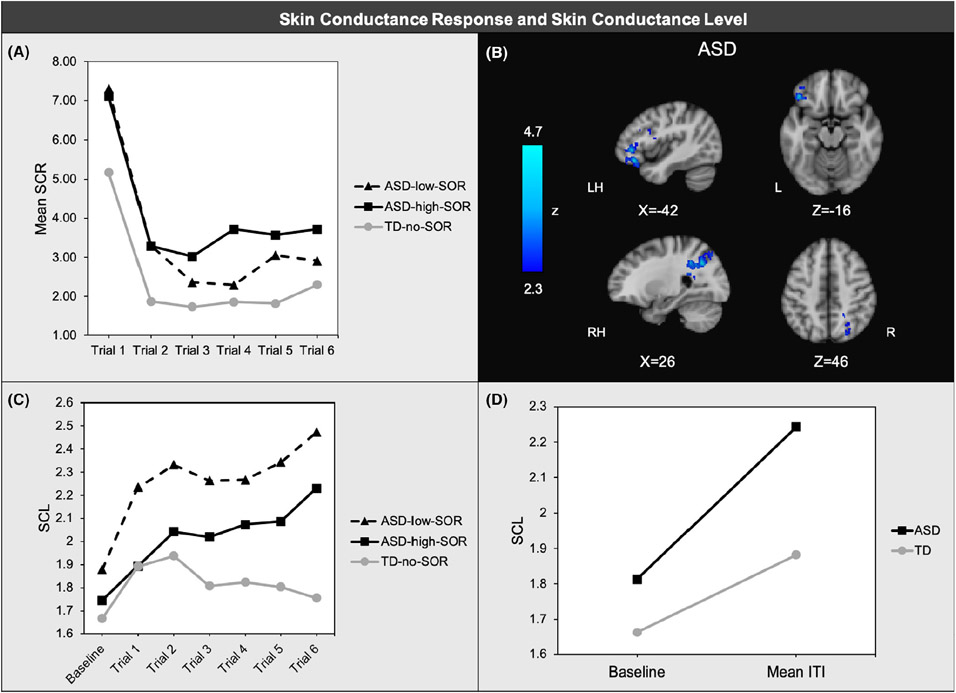
A repeated-measures ANOVA showed significant linear (F(1,72) = 5.64, p = .02, ηp2=.07) and quadratic (F(1,72) = 9.78, p < .01, ηp2=.12) main effects of trials, indicating that for all groups, SCR decreased over time and that the rate of change slowed over time. There was a main effect of SOR group (F(2,72) = 3.42, p = .04, ηp2=.09). A post hoc analysis revealed that the ASD group had higher mean SCR across all trials compared to the TD group (F(1,73) = 6.31, p = .01, ηp2=.08) (Figure 1a). There were no significant differences between ASD-SOR-high and ASD-SOR-low, and there were no other significant main effects or interactions. Thus, the ASD group had overall higher mean SCR to sensory stimulation than the TD group, but there were no group differences in habituation to the stimuli.
Figure 1.
Skin conductance response (SCR) averaged across joint, tactile, and auditory trials; (b) Region where brain response to aversive sensory stimulation was negatively associated with SCR within ASD; (c) Skin conductance level (SCL) across inter-trial intervals prior to joint trials ;(d)SCL change from 2-min baseline to inter-trial intervals averaged across all trials
Skin conductance level during inter-trial intervals (ITIs)
Because SCR specifically examines the change in skin conductance level after the onset of the stimulus compared to the 2-sec fixation prior to the stimulus, group differences in skin conductance levels (SCL) during the fixation period was also examined to analyze possible anticipatory arousal responses to the sensory stimuli. There was a significant trial*diagnostic group effect (F(1,72) = 7.10, p = .01, ηp2=.09) whereby the ASD group increased SCL during ITIs more quickly across the experiment compared to TDs. There was also a significant diagnostic group*trial*stimulus effect (F(1,72) = 5.46, p = .02, ηp2=.07), indicating that the diagnostic group difference in the ITIs was most pronounced prior to the joint aversive stimulus trials (Figure 1c). This is likely due to the fact that participants were asked rate stimulus aversiveness between the last joint stimulus and the last auditory and tactile stimuli, causing some dishabituation prior to the final auditory and tactile stimuli. There was no main effect of SOR or diagnostic group. Thus, overall, the ASD group showed increasing SCL during inter-trial fixation periods across the experiment in contrast to TD participants who showed decreasing SCL across the experiment. Finally, to determine whether the groups differed on how closely SCL returned to baseline during the fixation periods, a repeated-measures ANOVA was used to test for group differences in change in SCL from the 2-min baseline to the mean SCL across the ITIs. There was a significant diagnostic group*trial effect (F(1,72) = 7.52, p = .01, ηp2=.10) whereby the ASD group displayed a greater SCL increase between baseline and ITIs compared to the TD group, suggesting that the ASD group did not return as closely to baseline between stimuli (Figure 1d).
Heart rate during sensory stimulation.
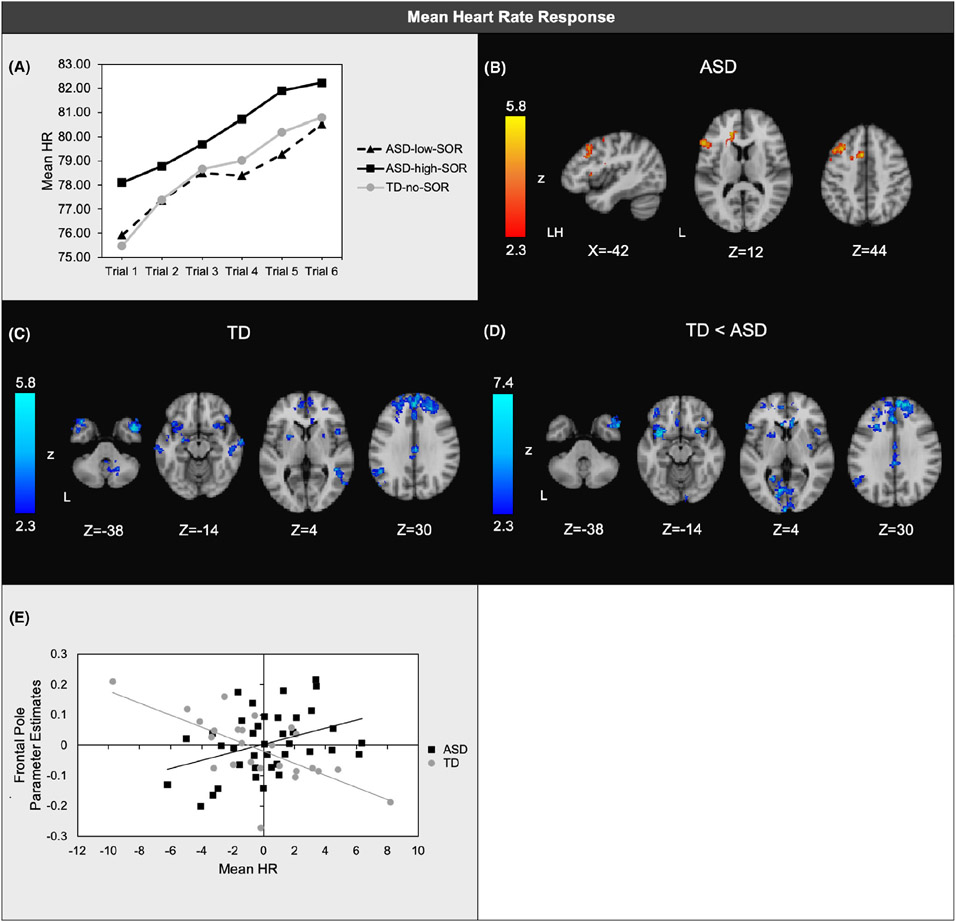
There was a significant main effect of quadratic slope across the six sets of trials (F(1,72) = 5.67, p = .02, ηp2=.07), indicating that for all groups, HR initially increased more quickly and then slowed the rate of change. There was no main effect of SOR group, but there was a main effect of baseline HR (F(1,72) = 533.33, p < .001, ηp2=.88). Higher baseline HR was associated with higher HR during sensory stimulation. After accounting for baseline HR, the ASD-SOR-high group had significantly higher HR than the ASD-SOR-low group during sensory stimulation (F(1,43) = 3.91, p = .05, ηp2=.08; Figure 2a).
Figure 2.
(a) Mean heart rate (HR) responses averaged across joint, tactile, and auditory trials; (b) Regions where brain responses to aversive sensory stimulation were positively associated with mean HR within ASD; (c) Regions where brain responses were positively associated with mean HR within TD; (d) Regions of significant diagnostic group differences in associations between HR and brain responses; (e) Scatterplot illustrating a representative correlation between mean HR and brain responses in each group. Horizontal axis: unstandardized residuals of mean HR. Vertical axis: parameter estimates extracted from areas of frontal pole shown to have significant ASD vs. TD group differences in the correlation between brain responses and HR orienting slopes. Two potential outliers were noted in the TD group; correlations within all clusters remained significant after removal of these outliers with the exception of left temporal pole and left angular gyrus (Table S2). All analyses covaried for baseline HR baseline
Heart rate acceleration/deceleration (IBI Results)
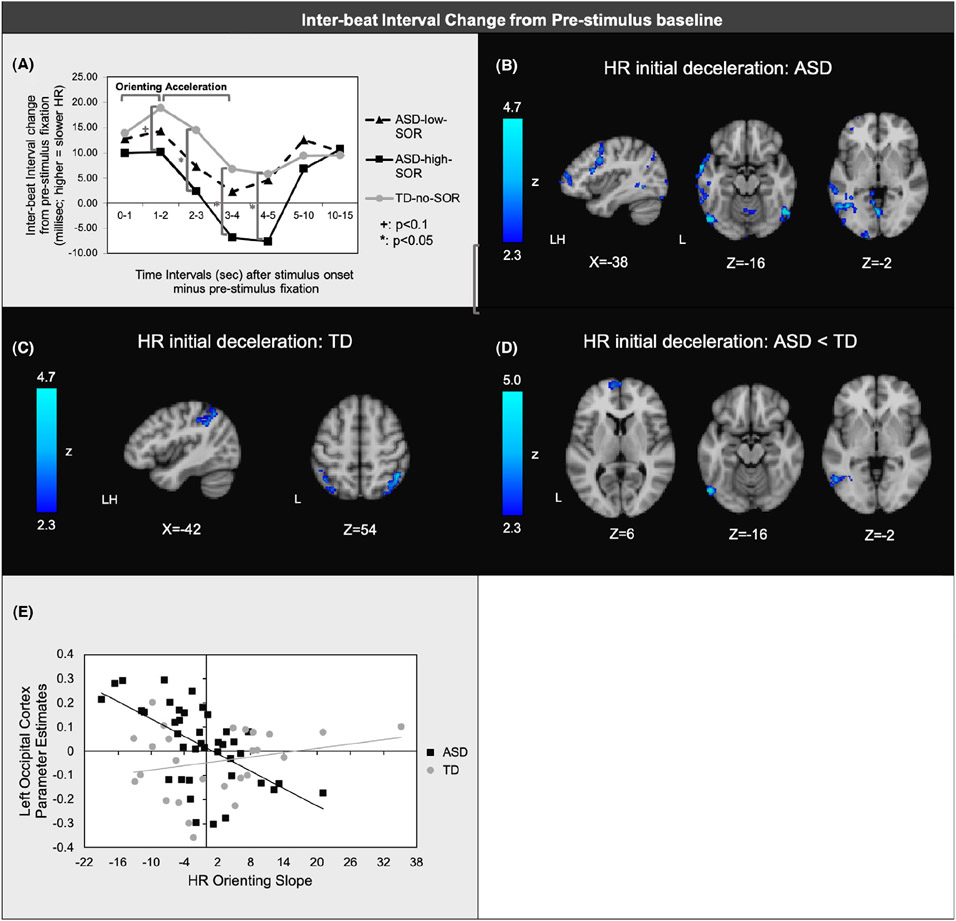
There was a significant SOR group difference in quadratic slope across the sensory trials (F(2,73) = 4.35, p = .02, ηp2=.11). Simple effects showed significantly greater acceleration for ASD-SOR-high than for TD from 2 to 3 (p = .03, ηp2=.07), 3-4 (p = .03, ηp2=.07), and 4-5 (p = .04, ηp2=.06) seconds after stimulus onset compared to stimulus baseline. The timepoint 1–2 s after stimulus onset showed a similar pattern of ASD-SOR-high greater than TD with a low-to-moderate effect size, but did not reach significance (p = .08, ηp2=.04). In contrast, effect sizes for group differences for the remaining timepoints were quite small (ηp2=.002–.01). Simple effects also showed a moderate effect size of greater HR acceleration for ASD-SOR-high than for ASD-SOR-low 4–5-s after stimulus onset, though this effect did not reach significance (p = .07; ηp2=.07). There were no significant differences between TD and ASD-SOR-low. There were also no effects of trial or stimulus type. Notably, the mean IBI for the TD and ASD-SOR-low groups never reached baseline levels, suggesting overall HR deceleration in these groups, whereas the ASD-SOR-high group did reduce IBI below baseline levels, suggesting HR acceleration (Figure 3a). In summary, results indicate that the ASD-SOR-high group showed a small-to-moderate effect of reduced HR deceleration during the orienting phase (though these effects did not reach statistical significance), showed significantly increased HR acceleration during the acceleration phase with moderate effect sizes, and showed no group differences during the habituation phase.
Figure 3.
(a)Mean inter-beat interval (∣B∣) change from the 5-sec prior to stimulus onset to each timepoint after stimulus onset (shown on horizontal axis) averaged across joint, auditory, and tactile trials. First 5 timepoints (orienting and acceleration phrases) show average ∣B∣ across 1-s periods; last 2 timepoints (sustained response/ habituation) show average across 5-s; (b) Regions of significant negative associations between ∣B∣ orienting slopes (0–1 to 1–2 s after stimulus onset) and brain responses to aversive sensory stimulation within ASD and (c) within TD; (d) Regions where orienting slopes showed greater negative correlations with brain responses in ASD vs. TD; (e) Scatterplot illustrating representative correlation between orienting slope and brain responses in each group (here, parameter estimates extracted from areas of lateral occipital cortex shown to have significant diagnostic group differences in the correlation between brain responses and orienting slopes)
fMRI findings
To determine whether peripheral measures of arousal to sensory stimuli were correlated with brain responses to matched sensory stimulation, we used the SCR and HR metrics that best differentiated diagnostic and SOR groups to correlate with fMRI activation. These metrics included mean SCR, mean HR, and IBI initial deceleration (‘orienting response”) and subsequent IBI acceleration. Psychophysiological measures were averaged across auditory, tactile, and joint stimuli and tested for correlations with brain responses to the comparable stimuli (also averaged across three stimulus types) using bottom-up, whole-brain analyses.
Mean SCR and HR.
SCR was negatively correlated with brain responses in the left orbital frontal cortex (OFC), left inferior frontal gyrus (IFG), and right lateral occipital cortex for ASD youth: ASD youth with greater mean SCR to sensory stimuli had reduced neural responses to comparable stimuli in these regions (Figure 1b; TableS 1). SCR was not significantly correlated with brain responses in TD youth, but there were no significant diagnostic group differences. In the ASD group, mean HR response was positively correlated with brain responses in the left middle frontal gyrus (MFG) and left inferior frontal gyrus (IFG) (Figure 2b; TableS 2). In contrast, for the TD group, mean HR was negatively correlated with brain responses in bilateral frontal cortex including dorsal and medial prefrontal cortex and OFC, as well as temporal regions, putamen, left angular gyrus, and the cerebellum (Figure 2c). The majority of these regions showed significant between-group differences in their correlations with mean HR (Figure 2d).
IBI Orienting response.
To determine how initial HR deceleration (‘orienting’ response) related to brain response to sensory stimulation, slopes were computed for average change in IBI from the 0-1sec to 1-2sec intervals of each stimulus trial. ASD youth who had reduced orienting slopes (i.e., flatter, less positive slopes) had greater activation in left frontal regions (MFG, frontal pole), precentral gyrus, left temporal regions, and occipital cortex (Figure 3b; TableS 3). For the TD group, reduced orienting slope was correlated with greater activation in the supramarginal gyrus and lateral occipital cortex (Figure 3c). The groups differed significantly such that activation in the left frontal pole and left lateral occipital cortex was more strongly anticorrelated with orienting slope in the ASD group than in the TD group (Figures 3d,e).
IBI acceleration.
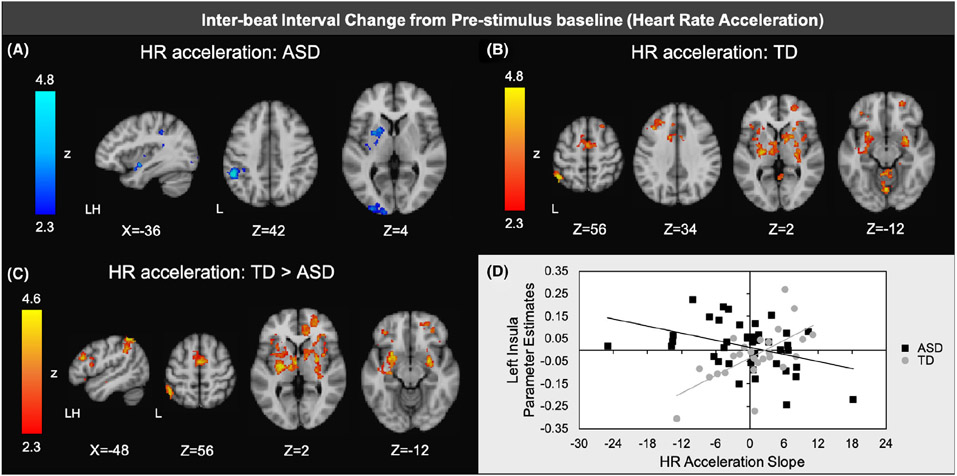
To determine how subsequent HR acceleration related to brain response to sensory stimulation, the slope was calculated for average change in IBI from the 1–2 s to 3–4 s intervals of each stimulus trial. ASD youth who had faster HR acceleration (i.e., steeper, more negative slopes) had greater activation in left insular cortex, left basal ganglia, and left sensory cortical regions (Figure 4a; TableS 4). In contrast, TD youth with faster HR acceleration showed less activation in a number of frontal regions (including left OFC, left MFG, and anterior cingulate cortex), regions related to salience detection (insular cortex, amygdala), basal ganglia, and sensory regions (thalamus, supramarginal gyrus, occipital cortex; Figure 4b). Most of the correlations also showed significant diagnostic group differences, indicating that the TD group had stronger positive correlations between IBI acceleration and these regions compared to the ASD group (Figures 4c,d).
Figure 4.
Regions of significant negative association between inter-beat interval (∣B∣) rate acceleration slopes (1–2 to 3–4 s after stimulus onset) and brain responses to aversive sensory stimulation within ASD and (b) regions of significant positive associations between acceleration slopes and brain response within TD; (c) Regions of significantly more positive correlations with acceleration slopes in TD vs. ASD; (d) Scatterplot illustrating representative correlation between acceleration slopes and brain responses in each group ( here, parameter estimates extracted from areas of insular cortex shown to have significant diagnostic group differences in correlation between brain responses and acceleration slopes)
Discussion
This study investigated how psychophysiological responses to mildly aversive sensory stimulation relate to neural and behavioral measures of sensory over-responsivity in youth with ASD. Using skin conductance and heart rate responses (SCR/HR), we demonstrated that ASD youth showed overall heightened arousal to mildly aversive sensory stimulation compared to typically developing youth. HR measures, including mean HR and inter-beat interval (IBI) acceleration/deceleration, were most sensitive in showing associations with ASD participants’ behavioral and neural measures of sensory reactivity.
The ASD group showed both higher resting HR and more heightened skin conductance and HR responses to aversive sensory stimuli than the TD group, which is consistent with prior research (e.g., Goodwin et al., 2006; Kuiper et al., 2019). SCR was elevated for the ASD group as a whole, regardless of parent-reported SOR, which may indicate an overall heightened physiological arousal to sensory stimuli across all ASD youth whether or not they display behavioral over-reactivity (e.g., covering ears, behavioral dysregulation, meltdowns) to sensory stimuli. Higher baseline HR in the ASD group is consistent with prior findings (e.g., Kushki et al., 2013) and could suggest that children with ASD have greater physiological arousal to novel contexts, are generally more anxious/hypervigilant, and/or experienced heightened anxiety in the lab setting. These results support prior findings that atypical neural sensory processing is common across ASD youth, but atypical behaviors displayed by ASD youth may depend on top–down regulation of these responses (Green et al., 2019).
Because SCR is dependent on a comparison with the final two seconds of the inter-trial-interval prior to the stimulus, group differences can be reduced if certain groups show a greater anticipatory response or increasing skin conductance just prior to the onset of the stimulus. This phenomenon was observed here, more so for the ASD than for the TD group. The TD group overall had a skin conductance level closer to their baseline during the two seconds prior to stimulus onset compared to the ASD group. This suggests that the ASD participants are either slower to return to baseline during rest periods or are anticipating the next stimulus to a greater extent than TD youth, which is possible given that each rest period was consistently 9-second long. Additionally, while the TD group showed decreasing skin conductance levels across inter-trial-intervals over the course of the experiment, the ASD group showed increasing skin conductance levels across these same intervals, suggesting increasing anticipation or sensitization. These results are consistent with prior findings that individuals with ASD have higher pain anticipation to aversive stimuli than matched controls (Gu et al., 2018). The ASD group could also be experiencing greater hypervigilance, particularly given the higher anxiety present in autism (Pfeiffer et al., 2005). From a methodological perspective, these findings show that typical SCR analysis may be overly conservative when comparing ASD to TD groups as the two groups are not equivalent in the inter-trial interval period used as a baseline for calculating SCR, which may have also contributed here to the lack of SOR group differences found in SCR. In other words, if one group but not the other is consistently anticipating the stimulus, they might show a rise in SCL right before the stimulus rather than right after the stimulus, which could reduce group differences in SCR (calculated as increase in SCL from the few seconds before to the few seconds after the stimulus). It may be beneficial in future studies to vary the inter-trial interval length to reduce anticipatory arousal, but further research should be conducted on group differences within the inter-trial periods to determine why they occur.
Compared to SCR, HR response better differentiated ASD youth with high compared to low SOR. The high-SOR group showed higher mean HR during the aversive sensory stimulation compared to the low-SOR group after controlling for baseline HR. Interbeat-interval HR analyses indicated that this overall higher HR was likely due to a combination of initially reduced HR deceleration and greater HR acceleration to the sensory stimulation observed in the high-SOR ASD group. Though it did not reach statistical significance (p = .08), the difference in HR deceleration between the high-SOR ASD group and the TD group had a small-to-moderate effect size and is consistent with the overall pattern of higher overall HR, suggesting that this effect is worth further examination with a larger sample. HR typically decreases directly after the onset of a novel stimulus, and this deceleration is generally interpreted as an orienting response which allows individuals to gather and process the information to determine how to respond to the stimulus (Bradley, 2009). This orienting response is usually followed by an acceleration in HR, which then levels off. Taken together, these results suggest that HR is a more sensitive indicator of parent-reported SOR severity than SCR, and that SOR may be related to both atypical orienting and HR acceleration responses to sensory stimulation. High-SOR ASD participants may be so overwhelmed by a stimulus perceived as both aversive and salient to them such that they have a reduced ability to process and respond efficiently to the stimulus, leading to increased arousal and decreased regulation. This pattern of reduced HR orienting responses has been observed in individuals with trauma histories in response to stimuli that evoke their trauma experiences (Elsesser, Sartory, & Tackenberg, 2004). Potentially, HR measures were more sensitive to picking up these SOR-related patterns of arousal because these measures took into account a longer portion of the stimulus period compared to SCR which measures the single highest response point during the first few seconds of the stimulus. This is consistent with previous fMRI studies showing that patterns of fMRI response across the 15-sec period of a stimulus are more related to SOR than the immediate response (Green et al., 2015, 2019). Moreover, higher baseline HR was associated with higher HR during sensory stimulation possibly because heightened alertness or anxiety led to increased HR reactivity.
We correlated physiological responses to the aversive sensory stimulation with brain responses to comparable sensory stimulation, toward the goal of using psychophysiological responses to measure SOR for participants who are unable to participate in MRI. In the ASD group, neither SCR nor HR was associated with activation in brain regions most consistently shown to be related to SOR reactivity (e.g., amygdala, primary sensory cortices), though both were associated with activation in other regions related to sensory processing and regulation in ASD (Green et al., 2015). Specifically, ASD youth with lower SCR showed greater activation in the left orbital frontal cortex—a region thought to regulate sensory reactivity for low-SOR ASD youth (Green et al., 2015, 2019). However, SCR was not correlated with parent-reported SOR, suggesting that skin conductance responses to sensory stimulation and parent-observed behavioral reactivity to sensory stimulation may independently be associated with neural regulation. Potentially, these two measures indicate different aspects of sensory processing, with physiological arousal more related to ‘state’ reactivity and parent report more related to ‘trait’ SOR, a phenotype that results from the interaction of multiple biological, behavioral, and environmental variables. It is possible that a combination of parent report and observed SCR could be used as a predictor of regulatory ability for children with ASD.
Mean HR response, initial HR deceleration slope (orienting response), and HR acceleration slope were all also associated with fMRI responses to aversive auditory and tactile stimulation. ASD and TD groups showed distinct relationships between these HR metrics and neural responses to sensory stimulation. Orienting response was negatively correlated with activation in visual cortex for both ASD and TD groups and with frontal regions for the ASD group only. Thus, reduced orienting response was correlated with increased activation in primary sensory cortex unrelated to the auditory and tactile sensory stimulation, consistent with Green et al. (2019), which showed that reduced inhibition of visual cortex during auditory/tactile stimulation is associated with higher SOR in ASD. Furthermore, ASD participants with reduced orienting responses are more likely to utilize frontal regions implicated in cognitive processing, such as working memory and episodic memory (Kim, Kroger, Calhoun, & Clark, 2015; Nyberg et al., 2003), during initial processing of the sensory stimulation. This could indicate effortful processing during a period that when processing is usually more automatic.
HR acceleration slope was also associated with brain responses in sensory processing areas for ASD and TD groups, but notably showed opposite directions of effect in each group. Within the ASD group, youth with greater HR acceleration showed greater activation in visual, tactile, and salience processing regions, whereas for the TD group, youth with lower HR acceleration showed greater activation in these same regions. The TD group further showed a relationship between reduced HR acceleration and greater activation in amygdala and frontal regions associated with inhibition (Sharp et al., 2010) and planning (Tanji & Hoshi, 2001). These findings could indicate increased frontal modulation of sensory-related networks in TD allowing for reduced HR acceleration, whereas in ASD youth, particularly those with high SOR, activation in the same regions, absent of frontal modulation, relates to increased HR acceleration. HR acceleration has been linked with a defensive response, which may limit one’s ability to habituate to intense stimuli (Goodwin et al., 2006; Sokolov, 1963). This is consistent with prior findings that ASD youth with high SOR show reduced neural habituation to sensory stimulation (Green et al., 2015, 2019).
Our study took a novel approach of relating psychophysiological and neural responses to mildly aversive sensory stimuli to investigate SOR in autism. However, the study did have a few limitations, one of which was our lack of measures for other types of sensory processing deficits (e.g., sensory under-responsivity, sensory seeking). Future research should also study how these additional sensory processing atypicalities relate to peripheral and neural responses. Also, it will be important for future studies to investigate how these neural and physiological responses relate to observable behavioral responses.
Furthermore, because one goal of this study was to determine whether physiological data collected outside of the scanner can be used as a proxy for imaging data for individuals who cannot participate in MRI, we chose to collect these data in two separate contexts. However, it is possible that the difference in environments and particularly the less aversive setting of the physiological data collection compared to the MRI scanner may have reduced the correlations between the data, and future studies might examine whether collecting the data simultaneously results in greater physiological–neurobiological associations. Moreover, while the environment in which we collected the physiological data was more generalizable than the MRI environment, it was still a laboratory setting with stimuli presented in a predictable and standardized manner. This was done to make it as consistent as possible with the MRI data collection. Given the greater flexibility in acquiring physiological measures, future studies should examine such physiological responses to more ecologically valid stimuli, particularly those that are less predictable. This could also include randomizing the order of MRI and psychophysiological assessments (in contrast to the design of our study in which MRI was always first). The design of our study exposed the participants to similar sensory paradigms twice in a row (approximately 75–90 min apart), leading to possible habituation or anticipation during the second, psychophysiological, paradigm. Given our prior findings that the ASD—especially ASD-SOR-high—participants show reduced habituation to sensory stimuli during MRI (Green et al., 2019), this might have increased group differences in physiological responses but likely could not have accounted for the lack of group differences in physiological habituation to the sensory stimuli.
Another possible limitation of our study was the administration of the tactile stimulation. While we established reliability on timing and pressure, there may still have been variations in the administration across scans. Given that we have replicated group differences in response to this tactile stimulus across participants and stimulus types, the block design likely averages out any slight variations in administration across participants (Green et al., 2015, 2019). However developing a standardized method of administering ecologically valid tactile stimulation is an important future direction for the field. Finally, future research should replicate this study with a larger sample to examine a wider range of age and cognitive abilities, as well as sex differences. Future studies should also control for hydration status, as dehydration can cause increases in heart rate (Kempton et al., 2011). Replication is also necessary to confirm trend-level findings and make conclusions about how to predict neural responses from physiological data.
Conclusions
We found that ASD youth overall experience more arousal to mildly aversive sensory stimuli with heart rate metrics in particular showing associations with parent-reported SOR. Notably, for youth with SOR, reduced orienting and greater defensive HR responses contributed to greater overall HR responses, indicating they may be overwhelmed by sensory stimulation, potentially causing deficits in normal stimulus processing and action planning. The ASD group also showed more physiological arousal during inter-trial intervals, which may suggest hypervigilance or anticipation of sensory stimulation. This has implications for how SCR is measured in autism. Both SCR and HR responses were associated with brain responses to similar aversive sensory stimulation, particularly in brain regions associated with higher-level sensory processing, regulation, and attention. Taken together, these findings indicate that psychophysiological metrics are associated with behavioral and neural measures of SOR, which has important implications for generalizing studies of the biological mechanisms underlying SOR to populations beyond those who can participate in MRI.
Supplementary Material
Appendix S1. Supplemental methods.
Appendix S2. Supplemental results
Figure S1. (a) Skin conductance level during the 2-minute baseline phase did not show any significant between-group differences. (b) Mean heart rate during the 2-minute baseline phase was significantly higher in the ASD compared to the TD group.
Table S1. Montreal Neurological Institute (MNI) Coordinates for Associations between Skin Conductance Responses and Brain Responses to Aversive Sensory Stimulation within the ASD Group.
Table S2. Montreal Neurological Institute (MNI) coordinates for associations between mean heart rate responses and brain responses to aversive sensory stimulation.
Table S3. Montreal Neurological Institute (MNI) coordinates for associations between inter-beat-interval orienting phase slopes and brain responses to aversive sensory stimulation.
Table S4. Montreal Neurological Institute (MNI) coordinates for associations between inter-beat-interval acceleration phase slopes and brain responses to aversive sensory stimulation.
Table S5. Correlation matrix of sensory over-responsivity composite score and parent-reported sensory measures within ASD participants.
Table S6. Descriptive table for stimuli pilot testing.
Key points.
Individuals with Autism Spectrum Disorder (ASD) commonly show both atypically high physiological arousal and sensory over-responsivity (SOR), extreme negative responses to aversive stimuli, but the relationship between the two is unclear.
In this study, youth with ASD showed higher physiological responses to aversive sensory stimulation compared to typically developing controls.
In particular, youth with SOR displayed atypical orienting and defensive heart rate responses, which suggests that SOR is related to abnormal stimulus processing and action planning.
Physiological responses were associated with fMRI brain responses to comparable sensory stimulation in regions previously associated with SOR and sensory regulation.
Using psychophysiology to correlate behavioral and neural measures of SOR has important implications for researching and treating SOR in populations who cannot undergo MRI.
Acknowledgments
This work was supported by grants from the Simons Foundation Autism Research Initiative (grant number 345389), National Institute of Child Health and Human Development (P50 HD055784), the National Institute of Mental Health (R01MH100028; K08 MH112871), and UCLA Friends of the Semel Institute (Scholar Grant to S.G.). The authors were also supported by the following training grants/fellowships: a National Research Service Award postdoctoral fellowship to S.G. (F32 MH105167). For generous support, the authors also wish to thank the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family and Northstar Fund. The project described was supported by Grant Numbers RR12169, RR13642, and RR00865 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH); its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCR or NIH. The funding sources and organizations listed above had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. These research efforts were conducted in part under the auspices of The Help Group-UCLA Autism Research Alliance, which contributed to participant recruitment. The authors have declared that they have no competing or potential conflicts of interest.
Footnotes
Supporting information
Additional supporting information may be found online in the Supporting Information section at the end of the article:
Conflict of interest statement: No conflicts declared.
References
- Ausderau KK, Sideris J, Little LM, Furlong M, Bulluck JC, & Baranek GT (2016). Sensory subtypes and associated outcomes in children with autism spectrum disorders. Autism Research, 9, 1316–1327. [DOI] [PubMed] [Google Scholar]
- Ben-Sasson A, Hen L, Fluss R, Cermak SA, Engel-Yeger B, & Gal E (2009). A Meta-Analysis of Sensory Modulation Symptoms in Individuals with Autism Spectrum Disorders. Journal of Autism and Developmental Disorders, 39, 1–11. [DOI] [PubMed] [Google Scholar]
- Bradley MM (2009). Natural selective attention: Orienting and emotion. Psychophysiology, 46, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MC, Parham LD, Blanche EI, Schell A, Chou C-P, Dawson M, & Clark F (2012). Autonomic and behavioral responses of children with autism to auditory stimuli. American Journal of Occupational Therapy, 66, 567–576. [DOI] [PubMed] [Google Scholar]
- Elsesser K, Sartoiy G, & Tackenberg A (2004). Attention, heart rate, and startle response during exposure to trauma-relevant pictures: a comparison of recent trauma victims and patients with posttraumatic stress disorder. Journal of Abnormal Psychology, 113, 289–301. [DOI] [PubMed] [Google Scholar]
- Glod M, Riby D, Honey E, & Rodgers J (2015). Psychological correlates of sensory processing patterns in individuals with autism spectrum disorder: a systematic review. Review Journal of Autism and Developmental Disorders, 2, 199–221. [Google Scholar]
- Goodwin MS, Groden J, Velicer WF, Lipsitt LP, Baron MG, Hofmann SG, & Groden G (2006). Cardiovascular arousal in individuals with autism. Focus on Autism and Other Developmental Disabilities, 21, 100–123. [Google Scholar]
- Green SA, Hernandez L, Lawrence KE, Liu J, Tsang T, Yeargin J, … & Bookheimer SY (2019). Distinct patterns of neural habituation and generalization in children and adolescents with autism with low and high sensory overresponsivity. American Journal of Psychiatry, 176, 1010–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Hernandez L, Tottenham N, Krasileva K, Bookheimer SY, & Dapretto M (2015). Neurobiology of sensory overresponsivity in youth with autism spectrum disorders. JAMA Psychiatry, 72, 778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Zhou TJ, Anagnostou E, Soorya L, Kolevzon A, Hof PR, & Fan J (2018). Heightened brain response to pain anticipation in high-functioning adults with autism spectrum disorder. The European Journal of Neuroscience, 47, 592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horder J, Wilson CE, Mendez MA, & Murphy DG (2014). Autistic traits and abnormal sensory experiences in adults. Journal of Autism and Developmental Disorders, 44, 1461–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith JM, Jamieson JP, & Bennetto L (2019). The influence of noise on autonomic arousal and cognitive performance in adolescents with autism spectrum disorder. Journal of Autism and Developmental Disorders, 49, 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempton MJ, Ettinger U, Foster R, Williams SCR, Calvert GA, Hampshire A, … & Smith MS (2011). Dehydration affects brain structure and function in healthy adolescents. Human Brain Mapping, 32, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Kroger JK, Calhoun VD, & Clark VP (2015). The role of the frontopolar cortex in manipulation of integrated information in working memory. Neuroscience Letters, 595, 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper MWM, Verhoeven EWM, & Geurts HM (2019). Stop making noise! auditory sensitivity in adults with an autism spectrum disorder diagnosis: physiological habituation and subjective detection thresholds. Journal of Autism and Developmental Disorders, 49, 2116–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushki A, Drumm E, Pla Mobarak M, Tanel N, Dupuis A, Chau T, & Anagnostou E (2013). Investigating the autonomic nervous system response to anxiety in children with autism spectrum disorders. PLoS One, 8, e59730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liss M, Saulnier C, Fein D, & Kinsbourne M (2006). Sensory and attention abnormalities in autistic spectrum disorders. Autism, 10, 155–172. [DOI] [PubMed] [Google Scholar]
- McCormick C, Hessl D, Macari SL, Ozonoff S, Green C, & Rogers SJ (2014). Electrodermal and behavioral responses of children with autism spectrum disorders to sensory and repetitive stimuli. Autism Research: Official Journal of the International Society for Autism Research, 7, 468–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh DN, Miller LJ, Shyu V, & Dunn W (1999). The sensory profile: examiner’s manual, San Antonio, TX: Psychological Corporation. [Google Scholar]
- Miller L, Reisman JE, Mcintosh D, & Simon J (2001). An ecological model of sensory modulation: Performance of children with fragile X syndrome, autistic disorder, attention-deficit/hyperactivity disorder, and sensory modulation dysfunction. In Smith-Roley S, Blanche E & Schaaf RC (Eds.), Understanding the nature of sensory integration with diverse populations (pp. 57–82). San Antonio, TX: Therapy Skill Builders. [Google Scholar]
- Nyberg L, Marklund P, Persson J, Cabeza R, Forkstam C, Petersson KM, &Ingvar M (2003). Common prefrontal activations during working memory, episodic memory, and semantic memory. Functional Neuroimaging of Memory, 41, 371–377. [DOI] [PubMed] [Google Scholar]
- O’Riordan M, & Passetti F (2006). Discrimination in autism within different sensory modalities. Journal of Autism and Developmental Disorders, 36, 665–675. [DOI] [PubMed] [Google Scholar]
- Pfeiffer B, Kinnealey M, Reed C, & Herzberg G (2005). Sensory modulation and affective disorders in children and adolescents with Asperger’s disorder. American Journal of Occupational Therapy, 59, 335–345. [DOI] [PubMed] [Google Scholar]
- Schoen SA, Miller LJ, Brett-Green B, & Hepburn SL (2008). Psychophysiology of children with autism spectrum disorder. Research in Autism Spectrum Disorders, 2, 417–429. [Google Scholar]
- Schoen SA, Miller LJ, & Green KE (2008). Pilot study of the sensory over-responsivity scales: assessment and inventory. American Journal of Occupational Therapy, 62, 393–406. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC, & Mehta MA (2010). Distinct frontal systems for response inhibition, attentional capture, and error processing. Proceedings of the National Academy of Sciences of the United States of America, 107, 6106–6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov EN (1963). Perception and the Conditioned Reflex. New York, NY: Macmillian. [Google Scholar]
- Tanji J, & Hoshi E (2001). Behavioral planning in the prefrontal cortex. Current Opinion in Neurobiology, 11, 164–170. [DOI] [PubMed] [Google Scholar]
- Woodard CR, Goodwin MS, Zelazo PR, Aube D, Scrimgeour M, Ostholthoff T, & Brickley M (2012). A comparison of autonomic, behavioral, and parent-report measures of sensory sensitivity in young children with autism. Research in Autism Spectrum Disorders, 6, 1234–1246. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplemental methods.
Appendix S2. Supplemental results
Figure S1. (a) Skin conductance level during the 2-minute baseline phase did not show any significant between-group differences. (b) Mean heart rate during the 2-minute baseline phase was significantly higher in the ASD compared to the TD group.
Table S1. Montreal Neurological Institute (MNI) Coordinates for Associations between Skin Conductance Responses and Brain Responses to Aversive Sensory Stimulation within the ASD Group.
Table S2. Montreal Neurological Institute (MNI) coordinates for associations between mean heart rate responses and brain responses to aversive sensory stimulation.
Table S3. Montreal Neurological Institute (MNI) coordinates for associations between inter-beat-interval orienting phase slopes and brain responses to aversive sensory stimulation.
Table S4. Montreal Neurological Institute (MNI) coordinates for associations between inter-beat-interval acceleration phase slopes and brain responses to aversive sensory stimulation.
Table S5. Correlation matrix of sensory over-responsivity composite score and parent-reported sensory measures within ASD participants.
Table S6. Descriptive table for stimuli pilot testing.