Abstract
Surgical resection is a mainstay treatment for solid tumors. Yet, methods to distinguish malignant from healthy tissue are primarily limited to tactile and visual cues as well as the surgeon’s experience. As a result, there is a possibility that a positive surgical margin (PSM) or the presence of residual tumor left behind after resection may occur. It is well-documented that PSMs can negatively impact treatment outcomes and survival, as well as pose an economic burden. Therefore, surgical tumor imaging techniques have emerged as a promising method to decrease PSM rates. Nanoparticles (NPs) have unique characteristics to serve as optical contrast agents during image-guided surgery (IGS). Recently, there has been tremendous growth in the volume and types of NPs used for IGS, including clinical trials. Herein, we describe the most recent contributions of nanotechnology for surgical tumor identification.
This article is categorized under:
Therapeutic Approaches and Drug Discovery > Nanomedicine for Oncologic Disease
Implantable Materials and Surgical Technologies > Nanoscale Tools and Techniques in Surgery
Diagnostic Tools > in vivo Nanodiagnostics and Imaging
Keywords: fluorescence-guided surgery, image-guided surgery, imaging, nanoparticle, optical, surgical oncology
1 |. INTRODUCTION
Although the adoption of molecularly targeted therapies has improved cancer survival rates, surgical resection remains the most effective treatment option for cancer and is the primary treatment for the majority of solid tumors (Miller et al., 2016; Wyld, Audisio, & Poston, 2015). It is well-established that the ultimate cause of death in cancer is not from the primary tumor, but from metastases (Chaffer & Weinberg, 2011; Mehlen & Puisieux, 2006). As such, the complete removal of the primary tumor, or a negative surgical margin (NSM), is associated with decreased rates of local recurrence and improved survival (Haque, Contreras, McNicoll, Eckberg, & Petitti, 2006; Houssami, Macaskill, Luke Marinovich, & Morrow, 2014; Meric et al., 2003; Pawlik et al., 2005). Although surgery is potentially curative, the detection of malignant versus healthy tissue remains limited to the surgeon’s experience and visual/tactile cues. As a consequence, positive surgical margins (PSMs) occur in roughly 14–36% of tumor resection surgeries, presenting negative prognostic outcomes surgeries (Orosco et al., 2018; Tringale, Pang, & Nguyen, 2018). Additionally, PSMs necessitate further treatment, subjecting patients to additional side effects and economic burden (Tringale et al., 2018). As a result, image-guided surgery (IGS) has emerged as a promising solution for margin detection (Vahrmeijer, Hutteman, van der Vorst, van de Velde, & Frangioni, 2013).
We previously reported on the potential of nanotechnology for IGS in 2016 (Hill & Mohs, 2016). Since then, the use of nanoparticles (NPs) as contrast agents for IGS has experienced tremendous growth. Advancements in nanotechnology have allowed for improvements in IGS efficacy, the entrance of new NP classes into the IGS realm, and the initiation of several first-in-human clinical studies. Due to the ability to deliver a variety of therapeutic and imaging payload to solid tumors, multifunctional NPs hold high potential to provide imaging feedback to surgeons during all stages of treatment. Also, developments in optics and engineering have facilitated the growth of imaging modalities such as photoacoustic (PA) imaging, surface-enhanced Raman spectroscopy (SERS), and near-infrared (NIR) II fluorescence (Ding, Zhan, Lu, & Sun, 2018; Maloney et al., 2018). Nevertheless, in order to attain clinical translation, several challenges specific to nanotechnology must be addressed. Herein, we cover the progress in the use of NPs for IGS and outline their potential to improve outcomes in surgical oncology.
2 |. IMAGE-GUIDED SURGERY
Imaging modalities such as X-ray, magnetic resonance imaging (MRI), positron emission tomography (PET), computed tomography (CT), ultrasound (US), and single-photon emission computed tomography (SPECT) offer valuable information for diagnosis, staging, and preoperative planning. However, the prohibitive cost and sophisticated infrastructure of these imaging modalities limit translation to the operating room. Additionally, the resolution and contrast provided by these imaging modalities do not provide sufficient sensitivity and specificity to identify surgical margins and local metastasis (Alam et al., 2018; Frangioni, 2008). Intraoperative frozen section analysis (IFSA) of resected tissues is beneficial in reducing rates of local recurrence and is the gold standard for margin assessment (T. P. Olson, Harter, Muñoz, Mahvi, & Breslin, 2007). Nevertheless, IFSA requires an average of 24–27 min to obtain results, and final margin status is not definitive until after surgery (Chiappa et al., 2013; Jorns et al., 2012; Kennedy et al., 2015). Thus, there is a clinical need for an imaging modality that provides real-time feedback on surgical margin status.
IGS is emerging as a promising alternative for tumor margin assessment. IGS is defined, here, as surgery guided with real-time optical imaging feedback. The goals of IGS are locating the primary tumor, detection of residual disease in the resection margin, identification metastatic lymph nodes, and preservation of surrounding healthy tissue (Tummers, Warram, et al., 2018). Before surgery, the patient is injected with an exogenous contrast agent which accumulates in the tumor through passive or active targeting mechanisms while rapidly clearing from healthy tissues, providing contrast to malignant tissues. With the aid of an imaging system that is specific to the type of exogenous probe, the surgeon uses real-time image guidance to resect the primary tumor and identify any remaining cancer in the surgical cavity. As a result, the likelihood of attaining complete tumor resection, or NSM, is increased (Tipirneni et al., 2017).
The surgical tumor imaging methods with the most promise are techniques utilizing exogenous probes (Maloney et al., 2018). Although there are a variety of optical imaging modalities and exogenous IGS probes under development (M. T. Olson, Ly, & Mohs, 2019; Tipirneni et al., 2017; Tringale et al., 2018; Vahrmeijer et al., 2013), they generally fulfill the same criteria: Image of tumor is displayed in real-time; specific accumulation of contrast agent in the tumor and rapid clearance from healthy tissues, creating tumor contrast; the imaging system/contrast agent should identify tumors with high sensitivity and high resolution, including the detection of low signals and small tumors; image display and imaging system should not interfere with the surgical workflow (Mondal et al., 2014). Currently, several optical imaging modalities are compatible with tumor resection surgery. These include fluorescence, PA imaging, SERS, multimodal imaging, and theranostics, which integrates therapy with imaging.
2.1 |. Optical strategies for image-guided surgery
2.1.1 |. Fluorescence
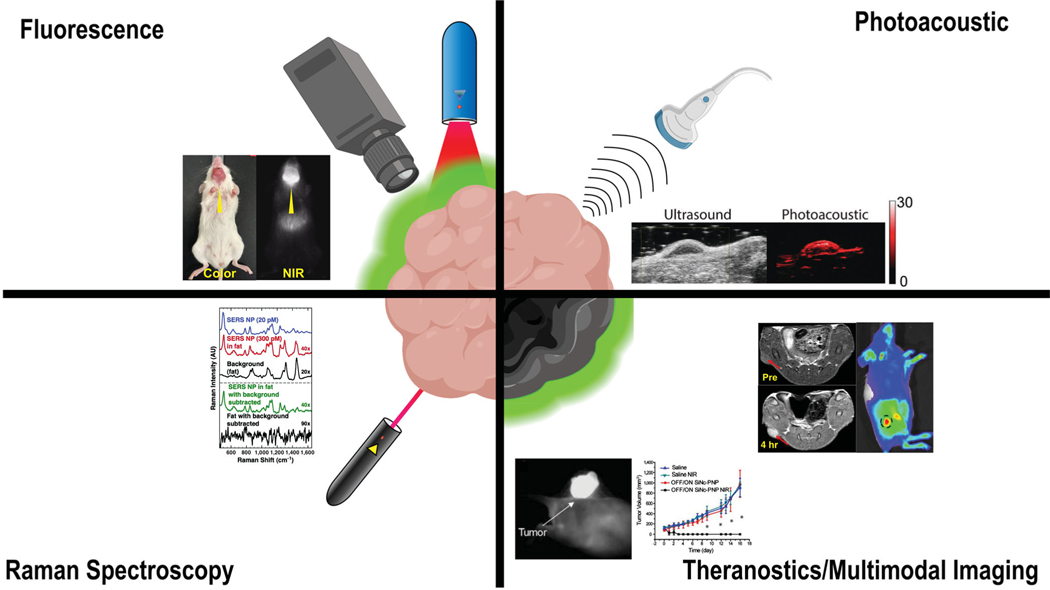
Fluorescence-guided surgery (FGS) is the most-commonly utilized optical modality for tumor margin detection (Figure 1). Fluorescence offers several advantages over traditional biomedical imaging modalities, namely, the absence of ionizing radiation and high spatial resolution (Hill & Mohs, 2016; Vahrmeijer et al., 2013). Fluorescence imaging in the NIR window (700–1,300 nm) is superior to visible wavelengths due to low scattering, negligible tissue autofluorescence, and relatively high tissue penetration. Imaging with light <600 nm is limited by scattering in biological tissues and hemoglobin absorbance, while imaging with light >1,300 nm is hindered by high water absorbance (G. Hong, Antaris, & Dai, 2017). The properties of NIR light allow for specific imaging of tumors with a high signal-to-background ratio (SBR), imparting surgeons the ability to use visual and tactile cues in combination with real-time fluorescence imaging to identify tumor margins.
FIGURE 1.
Optical imaging modalities used for IGS with examples of surgical tumor detection from the literature: fluorescence (Reprinted with permission from Zhao et al., 2017. Copyright 2016 Springer Nature), photoacoustic (Reprinted with permission from Thawani et al., 2017. Copyright 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim), Raman spectroscopy (Reprinted with permission from Mohs et al., 2010. Copyright 2010 American Chemical Society), theranostics (Reprinted with permission from Li et al., 2018. Copyright Ivy Spring International Publisher), and multimodal imaging (Reprinted with permission from Jin, Li, Yang, & Tian, 2019. Copyright Acta Materialia Inc. published by Elsevier Ltd.)
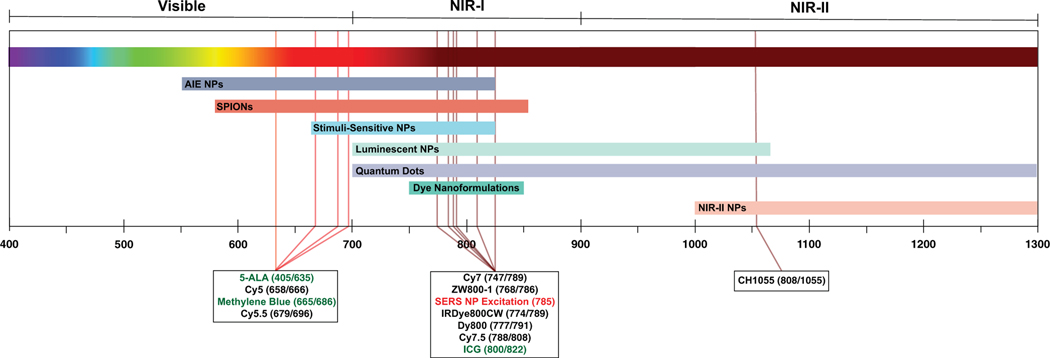
Although 5-aminolevulinic acid (5-ALA) improved complete resection and prolonged progression-free survival in a pivotal randomized phase 3 trial, leading to approval by the food and drug administration (FDA) in 2017 (Hadjipanayis & Stummer, 2019; Stummer et al., 2006), it suffered from inconsistencies in tumor contrast across the patient population, resulting in poor sensitivity and negative predictive value (NPV; Hadjipanayis, Widhalm, & Stummer, 2015; Moiyadi, Syed, & Srivastava, 2014). This is partially due to 5-ALA’s visible emission profile (ex. 405 nm; em. 635 nm). Aside from 5-ALA, there are two FDA-approved NIR fluorophores: Methylene blue (MB; ex. 665 nm; em. 686 nm) and indocyanine green (ICG; ex. 780 nm; em. 820 nm). ICG has garnered the most interest because its relatively lower tissue absorption and higher quantum yield (QY) are favorable for biomedical imaging (Matsui et al., 2010; Schaafsma et al., 2011). Although ICG is yet to be approved for FGS, it is under clinical investigation for intraoperative sentinel lymph node (SLN) mapping and tumor identification (Olson et al., 2019). Nevertheless, ICG still suffers from limitations such as poor aqueous stability, poor photostability, nonspecific binding to proteins, concentration-dependent aggregation, and reliance on the enhanced permeability and retention (EPR) effect for tumor accumulation. These characteristics result in low tumor signal and contrast (Alander et al., 2012; Schaafsma et al., 2011; H. Wang et al., 2018). Therefore, other NIR dyes such as cyanine-based and novel fluorophores are being investigated for FGS, and seek to improve tumor signal and contrast (Figure 2; Hernot, van Manen, Debie, Mieog, & Vahrmeijer, 2019; Olson et al., 2019).
FIGURE 2.
Emission profiles of commonly used dyes and NPs for surgical tumor detection and the emission wavelengths of NPs covered in this review. The majority of dyes fall into the NIR range. Fluorophores in the green text indicate FDA-approved compounds. Red text indicates the typical excitation wavelength for SERS NPs. The excitation and emission wavelength (nm) are reported in parenthesis after each dye
Finally, there are several FDA-approved and clinical-stage FGS systems that are on the market. Specific details of FGS systems are reviewed elsewhere (DSouza, Lin, Henderson, Samkoe, & Pogue, 2016). It is worth noting that these systems offer features that are complimentary with surgical workflow, maximizing the amenability of FGS with clinical practice. As novel fluorophores enter the clinical realm, the field must align on standardized system performance requirements for FGS systems (Pogue, 2018; Zhu, Rasmussen, & Sevick-Muraca, 2014).
2.1.2 |. Photoacoustic imaging
PA imaging combines the high contrast and specificity of spectroscopy with the spatial resolution capabilities of ultrasound to generate 2D and 3D images of organs and tissues (Beard, 2011). In PA imaging, laser pulses are directed toward biological tissues, where the energy is absorbed and converted to heat. The heat causes thermal expansion, which leads to the generation of ultrasonic waves that are ultimately detected by an ultrasound transducer (Xu & Wang, 2006). Endogenous molecules with high absorption coefficients, such as oxygenated and deoxygenated hemoglobin, can be visualized with high contrast using PA imaging. As a result, these properties can be leveraged to generate real-time images of blood flow and vascular networks in tissues of interest, including tumors (Figure 1; Ku, Wang, Xie, Stoica, & Wang, 2005; Lao, Xing, Yang, & Xiang, 2008; Laufer et al., 2012).
Additionally, exogenous compounds with a high molar extinction coefficient, sharp absorption peak in the NIR range, high photostability, and low QY, can be used as PA contrast agents (Weber, Beard, & Bohndiek, 2016). Interestingly, the properties of ICG make it a suitable PA contrast agent (C. Kim, Song, Gao, & Wang, 2010). However, due to the in vivo stability issues associated with ICG, others have sought to improve PA imaging of tumors with nanoformulations of ICG or the development of novel, tumor-targeted PA contrast agents (Weber et al., 2016).
To date, there are over two dozen PA imaging clinical trials that have been completed or are actively enrolling, with several investigating the use of PA for imaging of solid tumors and SLNs (NCT03931655, NCT03897270, NCT03630601, NCT03318107, and NCT03501823). Results from a study assessing excised thyroid tumors with PA imaging identified distinct differences in PA intensity (hemoglobin, lipid, and water) between malignant and healthy tissue (50 patients), thus demonstrating the potential application of PA imaging to surgical oncology (Dogra et al., 2014).
2.1.3 |. Surface-enhanced Raman spectroscopy
SERS and surface-enhanced resonance Raman scattering (SERRS) are spectroscopic techniques that are in development for tumor detection applications. SERS relies on the detection of Raman scattering, inelastically scattered light that is created upon the interaction of light generated by the Raman probe with the tissue of interest (Opilik, Schmid, & Zenobi, 2013). Each molecule within a tissue possesses a characteristic Raman scattering profile, otherwise known as a Raman fingerprint. Although label-free SERS can be used to identify tissue characteristics from endogenous molecules, it is complex and time-consuming. Alternatively, SERS NPs can be employed to provide a high-intensity signal during surgery (Cordero, 2018; Mohs et al., 2010; Zavaleta, Kircher, & Gambhir, 2011). As a result, SERS probes are utilized for the identification of biological structures, including tumors (Figure 1). Raman spectroscopy offers distinct advantages over fluorescence, namely resistance to photobleaching, the narrow peak of Raman signals (1–2 nm), multiplexing capabilities, and fM sensitivity (Lane, Qian, & Nie, 2015). Therefore, the use of SERS NPs is a highly sensitive method to simultaneously detect multiple tumor-associated biomarkers during tumor resection surgery and examination of resected tumors.
2.1.4 |. Multimodal imaging and theranostics
The previously mentioned imaging modalities offer unique benefits for tumor detection. However, each modality also suffers from a unique set of limitations as well. Due to these drawbacks, it is not possible to obtain complete information about a tumor using a single imaging modality. Multimodal imaging minimizes these limitations by imaging tumors with multiple, complementary imaging modalities (Figure 1; Ehlerding, Grodzinski, Cai, & Liu, 2018; Louie, 2010). For example, NIR fluorescence (NIRF) offers the benefit of high-contrast, real-time imaging of tumors. Relative to PET, NIRF has lower probe sensitivity and a low depth of detection. Nevertheless, PET is not well-suited for intraoperative imaging and is more useful for preoperative and postoperative settings (Frangioni, 2008; F.-M. Lu & Yuan, 2015). Therefore, the use PET and NIRF in tandem can bridge the gap between the high sensitivity and detection depth of preoperative PET imaging with the real-time, intraoperative imaging capabilities of NIRF (Christensen et al., 2017; Li, Zhang, et al., 2018). Multimodal imaging is not only limited to NIRF/PET. It can include any combination of clinically established (X-ray, MRI, PET, CT, or SPECT) and/or intraoperative (fluorescence, PA, Raman spectroscopy) imaging modalities. Due to their ability to simultaneously deliver multiple payloads, NPs are well-suited to serve as multimodal imaging probes, many of which are described in forthcoming sections.
There are several early examples of multimodal imaging. The first successful clinical demonstration was with the combination of the radiodiagnostic agent, 99mTc, with ICG for hybrid radio- and fluorescence-guided detection of SLNs in various solid malignancies. The lymphatic distribution pattern of 99mTc and ICG were proven to be identical, and thus, combined radio and fluorescence detection of SLNs was clinically feasible (Brouwer, Buckle, et al., 2012; Brouwer, Klop, et al., 2012; Buckle, Brouwer, Valdes Olmos, van der Poel, & van Leeuwen, 2012; van der Poel, Buckle, Brouwer, Valdés Olmos, & van Leeuwen, 2011). Other studies have followed with radio- and fluorescently-labeled multimodal imaging probes. The carcinoembryonic antigen (CEA)-targeted humanized antibody conjugate, 11In-diethylenetriaminepentaacetic acid (DTPA)-labetuzumab-IRDye800CW, was developed for combined SPECT/fluorescence imaging. 11In-DTPA-labetuzumab-IRDye800CW was proven to be effective at identifying invisible submillimeter lung tumors with preoperative SPECT and intraoperative fluorescence eye (Hekman et al., 2017). Another antibody-dye conjugate, epidermal growth factor receptor (EGFR)-targeted cetuximab-IRDye800CW, was utilized for FGS and ex vivo PA imaging of pancreas tumors in a phase 2 clinical study (NCT02736578; Tummers, Millers, et al., 2018). Finally, the silica-based 124I-cRGDY-PEG-Cornell dots (C dots) containing Cy5 were administered to patients with metastatic melanoma in a first-in-human clinical trial (NCT01266096). Although capable of dual PET-optical imaging, the pilot study only assessed the safety and feasibility of using 124I-cRGDY-PEG-C dots as a PET contrast agent. Due to favorable safety profile and evidence of preferential tumor uptake, 124I-cRGDY-PEG-C dots hold high potential as an optical-PET multimodal-imaging agent (Phillips et al., 2014).
NPs hold potential to further enhance drug biodistribution and pharmacokinetics (PK), maximizing the amount of drug delivered to the tumor, thus improving efficacy and limiting off-target toxicities (Tran, DeGiovanni, Piel, & Rai, 2017). Capitalizing on the multifunctional capabilities of NPs, nanotheranostics combine diagnostic agents with therapeutic agents. As a result, nanotheranostic agents allow the ability to noninvasively monitor treatment in real-time (Chen, Zhang, Zhu, Xie, & Chen, 2017). Nanotheranostics are applicable to surgical imaging and can serve as adjuvant therapy postsurgery.
Nanotheranostic agents that employ phototherapy are especially relevant to FGS. Phototherapy utilizes light and exogenous photosensitizers to elicit an antitumor effect, either through the generation of reactive oxygen species (ROS; photodynamic therapy, PDT) or heat (photothermal therapy, PTT). Due to the high tissue penetration of NIR light, NIR-responsive dyes that are used as contrast agents in FGS, such as ICG, are preferred as photosensitizers (Dolmans, Fukumura, & Jain, 2003; X. Huang, El-Sayed, Qian, & El-Sayed, 2006; Shanmugam, Selvakumar, & Yeh, 2014; Zou et al., 2016). Therefore, phototherapy is complementary to the surgical workflow, requiring minimal additional effort to induce phototherapeutic effects during surgery. As a result, phototherapy can be used in tandem with FGS, immediately eliminating any residual disease that may remain in the surgical cavity.
3 |. NANOTECHNOLOGY OVERVIEW
Recently, there has been tremendous growth in the volume and types of NPs used for IGS (Wang et al., 2019). NPs can be classified into two categories, hard and soft. Hard NPs are composed of inorganic materials and include quantum dots (QDs), noble metal, metal oxides, and lanthanide-based NPs. Optical features, such as strong absorption, photoluminescence, or magnetic properties, are inherent characteristics of the hard NPs, making them useful for bioimaging applications (Sangtani, Nag, Field, Breger, & Delehanty, 2017). Nevertheless, because hard NPs are composed of inorganic materials, the potential for toxicity and colloidal stability are significant concerns. Therefore, they are often surface-modified with biocompatible materials, such as polyethylene glycol (PEG), to improve in vivo safety and performance (Jiao et al., 2018; Sperling & Parak, 2010). On the contrary, soft NPs are composed of organic materials and include micelles, liposomes, and polymeric NPs. In addition to surface modification, soft NPs can be loaded with a diverse variety of cargo, such as fluorophores or therapeutic agents. Most soft NPs have can carry hydrophilic and amphiphilic cargo, such as ICG and other NIR dyes, providing in vivo stability to otherwise poorly soluble molecules. Interestingly, soft NPs can also be loaded with inorganic NPs as cargo, effectively producing hybrid NPs (Sangtani et al., 2017).
There are two primary mechanisms of NP uptake and retention in tumors: Passive and active targeting. Passive targeting of NPs is reliant upon the EPR effect, a phenomenon that allows for the elevated accumulation of macromolecules in solid tumors (Yashuhiro & Maeda, 1986). The EPR effect is the product of rapid tumor angiogenesis, which produces underdeveloped tumor vasculature possessing a defective endothelium with wide fenestrations (Danhier, 2016; Maeda, Nakamura, & Fang, 2013). As a result, molecules in the size range of 10–1,000 nm, or molecular weights >40 kDa, extravasate across the vascular epithelium and passively accumulate in solid tumors (Maeda et al., 2013; Torchilin, 2011). Additionally, NPs can be granted an extra degree of tumor specificity through conjugation to targeting ligands. Tumor-specific markers are leveraged for NP targeting during tumor imaging. Both hard and soft NPs are capable of conjugation to targeting ligands such as antibodies/antibody fragments, small molecules, peptides, and aptamers (Wang & Thanou, 2010). Unlike the conjugation of targeting ligands to small molecules, the functionalization of NPs is especially advantageous because the fluorophore is left chemically unmodified, limiting the possibility of altering optical performance (Kamaly, Xiao, Valencia, Radovic-Moreno, & Farokhzad, 2012). In order to ensure that adequate contrast is provided during surgery, it is essential that the target of interest is highly expressed while expression is minimal in surrounding healthy tissues (R. R. Zhang et al., 2017).
3.1 |. Challenges and critical barriers
To date, there is a substantial body of work that utilizes nanotechnology for cancer therapeutics and imaging. However, clinical outcomes of nanomedicine trials do not necessarily reflect the robust results that are observed preclinically (Maeda & Khatami, 2018). There are relatively few NP formulations have surpassed clinical hurdles and reached FDA-approval: ~50 FDA-approved NPs, while superparamagnetic iron oxide NPs (SPIONs) are the only nanoparticles approved for an imaging application, MRI (Agarwal, Bajpai, & Sharma, 2018; Han, Xu, Taratula, & Farsad, 2019; Ventola, 2017). Although the use of NPs for IGS is primarily in the preclinical development stage, it is necessary to discuss the critical challenges and barriers to successful clinical translation.
3.1.1 |. The EPR effect
The high clinical attrition rates of anti-cancer nanoparticles can be partially-attributed to tumor heterogeneity and tumor microenvironment (TME) factors that impact the efficacy of NP delivery (Hare et al., 2017). In nano-imaging, the EPR effect is often cited as the primary mechanism underlying tumor uptake and retention of NPs (Hare et al., 2017). However, the heterogeneity of human tumors is a challenge to this claim. Moreover, the EPR effect has only been reported for some tumor types, and is variable across different cancers, even among different tumors within the same patient (Maeda, 2015; Tanaka et al., 2018). In addition, the EPR effect is difficult to predict. It can be influenced by a complex set of TME factors, including tumor characteristics, vasculature, stroma, macrophages, lymphatics, and interstitial fluid pressure (Hare et al., 2017). Therefore, reliance on the EPR effect alone may be a poor predictor of tumor uptake and can result in unsatisfactory clinical outcomes. Although ongoing work is attempting to identify predictive EPR effect biomarkers and enhance EPR effect mediated tumor-targeting, the most popular solution is to bypass the EPR effect altogether and target tumor antigens (Golombek et al., 2018). As discussed in forthcoming sections, there is a strong pipeline of targeted NPs for IGS, which decreases the dependence on the EPR effect.
3.1.2 |. Challenges to translating multifunctional nanoparticles
Although multifunctional NPs can extend imaging and theranostic capabilities, they present several practical constraints. First, each added functionality of a NP requires additional synthetic steps for conjugation or loading of ligand. As a result, the synthetic yield is lowered with each step and requires more time to produce the final product (Cheng, Al Zaki, Hui, Muzykantov, & Tsourkas, 2012). Also, complex synthesis protocols for multifunctional NPs carry a high risk of batch-to-batch variability. Variations in size and ligand density could impact target binding, in vivo protein binding, and imaging performance/therapeutic efficacy. In fact, complexity in NP manufacture poses as a unique challenge to regulatory agencies, making it difficult to develop consistent guidelines for nanomedicines (Klein et al., 2019). These potential issues must be addressed before scale-up to clinical-grade certified good manufacturing practices (cGMP; Landesman-Milo & Peer, 2016).
Although the conjugation of multiple targeting ligands and contrast agents to NPs can improve performance, it can significantly increase the overall cost of a NP. For example, the annual price for a therapeutic monoclonal antibody (mAb) can be upwards of $100,000 (Hernandez et al., 2018; Shaughnessy, 2012). Although this is the annual cost for a therapeutic regiment, the NP conjugation efficiency can be less than 10%, and require a substantial amount for effective targeting (Thorek, Elias, & Tsourkas, 2009). Also, imaging systems required for NP detection can be prohibitively expensive (MRI: >$300,000, CT: $100,000–300,000, PET: >$300,000, optical imaging: $100,000–$300,000; Leary & Key, 2014), and the NP may require specific optimization for effective performance with each imaging modality (Louie, 2010; Payne et al., 2017). Taken together, the costs for the preclinical development of an IGS contrast agent are $10 million (Pogue et al., 2015), and added functionalities can further increase this number.
The most significant hurdle to multifunctional NPs is clinical development. Because FGS is in its relative infancy, there are a limited number of contrast agents that have reached FDA-approval. However, often looked to as a template for the development of FGS probes are molecularly targeted nuclear medicine contrast agents. It is estimated that these agents cost an average of ~$150 million for development in a single indication (Agdeppa & Spilker, 2009). If multifunctional NPs eventually reach the clinic, a clinical assessment dedicated to each feature of the NP would be required (i.e., preoperative imaging, intraoperative imaging, therapy; Box 1). Superiority over the standard of care would be necessary in each indication, thus setting a high bar for FDA-approval and multiplying development costs (Yu, Park, & Jon, 2012).
BOX 1. COMMONLY USED TERMS.
Intraoperative Imaging:
The imaging of anatomical structures and tissues during surgical procedures.
Image-Guided Surgery (IGS):
The use of real-time intraoperative imaging to identify and guide resection of primary tumor, local metastases, and metastatic lymph nodes. IGS includes, but is not limited to nuclear (MRI, PET, etc.) and optical (fluorescence, Raman, PA, etc.) imaging modalities.
Fluorescence-Guided Surgery (FGS):
The most commonly utilized method of IGS. FGS relies on fluorescence to identify and resect malignant tissue during surgery.
3.1.3 |. Safety concerns
Finally, concerns over safety and biocompatibility remain a critical challenge to the field. NP safety must be rigorously assessed alongside efficacy studies. Although there are safety challenges specific to each type of NP, safety, and biocompatibility issues generally can be attributed to a number of NP characteristics, including composition, shape, surface area, surface charge, catalytic activity, and/or the presence of bioreactive groups on the NP surface (Sukhanova et al., 2018). It has been well-documented that NPs have the potential to cause internal cellular damage and elicit unwanted immune responses, both of which can lead to tissue damage and other toxicities (Sukhanova et al., 2018). Another concern is the long-term biodistribution of NPs and the effect of nanomaterials on human health. This is difficult to assess in preclinical stages and may not ultimately be known until human studies are completed (Sahu & Hayes, 2017). Therefore, due to the lack of human NP exposure data, safety and biocompatibility remain a significant concern.
4 |. NANOPARTICLE STRATEGIES FOR IMAGE-GUIDED SURGERY
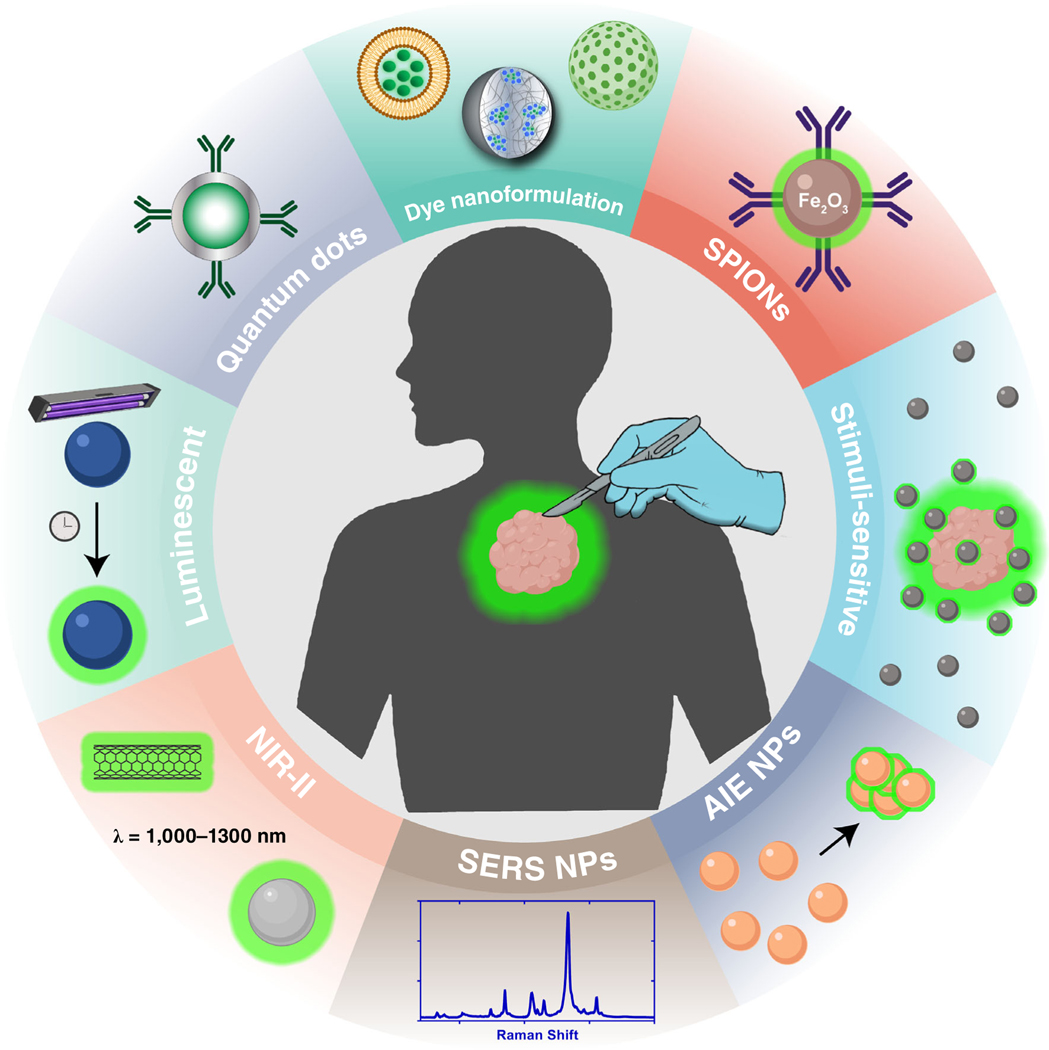
There have been great strides in the application of NPs for surgical tumor imaging. This review covers advancements in dye nanoformulations, SPIONs, stimuli-sensitive NPs, QDs, NIR-II NPs, persistent luminescence NPs (PLNPs), aggregation-induced emission (AIE) NPs, and SERS NPs as surgical contrast agents (Figure 3). Details of each study covered by this review are outlined in Table 1.
FIGURE 3.
Types of nanoparticles used for surgical image guidance
TABLE 1.
NPs for intraoperative tumor detection
| Nanoparticle | Class | Composition | Size (nm) | Ex/Em (nm) | Target (moiety) | Modality | References |
|---|---|---|---|---|---|---|---|
| TQ-BPN | AIE | TQ-BPN + Pluronic F-127 | 33 | 630/808 (SWIR) | FGS | J. Qi et al. (2018) | |
| L897 NPs | AIE | BPST + DSPE-PEG2000 | 34 | 711/888 | FGS | Wu et al. (2019) | |
| AIE NP | AIE | α-DTPEBBTD-C4 + DSPE-PEG2000 | 46 | 635/801 | FGS | Liu et al. (2017) | |
| Cor-AIE dots | AIE | Corannulene-PEG-TPP-TPA | 47 | 440/680 | FGS PDT |
Gu et al. (2018) | |
| HLZ-BTED dots | AIE | HLZ-BTED | 60 | 805/1,034 | FGS | Lin et al. (2019) | |
| DTE-TPECM NPs | AIE | DTE-TPECM + lipid-PEG2000 + YSA peptide | 68 | 410/550 | EphA2(YSA peptide) | FGS PA PDT |
J. Qi et al. (2018) |
| AGL AIE Nanodots | AIE | TPE-Ph-DCM + lipid-PEG2000 | 95 | 465/700 (10 hr) | FGS | Ni et al. (2019) | |
| JNP15 | AIE | Polyphorin + Bacteriopheophorbide NP | 100 | 750/820 | FGS PA |
Lovell et al. (2011); Shakiba et al. (2016) | |
| TPE-Th-B | AIE | TPE-Th-B + DSPE-PEG2000 | 110 | 363, 405/622 | FGS | H. Gao et al. (2019) | |
| Man-PRx800 | DN | PEG-α-cyclodextrin + ZW-800–1 | 7 | 772/788 | Mannose receptors (PEG/α-cyclodextrin) | FGS | Wada et al. (2018) |
| NIA | DN | PMLA + CTX + ICG + Tri-leucin | 12 | 730/795 | Various targets (CTX) | FGS | Patil et al. (2019) |
| LipImageTM 815 | DN | Liposomal IR786 | 50 | 793/815 | FGS | Cabon et al. (2016); Jacquart et al. (2013); Sayag et al. (2016) | |
| NanoICG | DN | HA-PBA + ICG | 80–150 | 780/800 | CD44 (HA) | FGS | Hill et al. (2015, 2016); Qi et al. (2018); Souchek et al. (2018); Wojtynek et al. (2019) |
| CF800 | DN | Liposomal ICG | 90.7 | 735/820 | FGS CT |
Patel et al. (2016); Zheng et al. (2015) | |
| SAMINs | DN | HA-PBA-Cy7.5 and HA-PBA-DTPAGd3+ | 97.8 | 98 | CD44 (HA) | FGS MRI |
Payne et al. (2017) |
| ICG/MSNs-RGD | DN | RGD + MSN + ICG | 100 | 770/837 | αvβ3 integrin (RGD) | FGS | Zeng et al. (2016) |
| NanoCy7.5 | DN | HA-PBA-Cy7.5 | 100 | 52 | CD44 (HA) | FGS | Hill et al. (2016); Kelkar, Hill, Marini, and Mohs (2016); Souchek et al. (2018) |
| LipoICG | DN | Liposomal ICG | 130 | 800/N/A | MSOT | Shi et al. (2015) | |
| LP-iDOPE | DN | Liposomal ICG | 191 | 780/811 | FGS PDT |
Suganami et al. (2015) | |
| RVG&IRDye800-Gd203 TNs | DN | Gd203 triangular Nanoplates-RVG-IRDye800 | Edge length = 37.5–42.5 | 775/806 | nAchR (RVG peptide) | FGS MRI |
Jin et al. (2019) |
| ZCG | PLNP | ZnGa2O4Cr0.004 | 10 | UV LED/696 (5 hr) | FGS CT |
Ai et al. (2018) | |
| NLPLNP | PLNP | Zn1.1Ga1.8Ge0.1 O4:Cr3+,Eu3+ @Si02 + FA | 50 | Red LED/696 (15 hr) | Folate receptor (FA) | FGS Theranostic |
Shi et al. (2015) |
| Luminescent UCNPs | PLNP | NaGdF4:Yb,tm,Ca@NaLuF4 + anti-HER2 mAb | 137 | 980/804 (24 hr) | HER2 (mAb) | FGS SPECT/CT |
Qiu et al. (2018) |
| ZGGO:Cr | PLNP | Zn1 + xGa2 − 2xGex04:Cr + Aptamer | 10–80 | 550/680 (5 hr) | (Aptamer) | FGS | J. Wang et al. (2017) |
| PDFT1032 polymer NPs | NIR-II | mPEG shell + DFT1032 | 68 | 809/1,032 | FGS | Shou et al. (2018) | |
| HI NPs | NIR-II | Liposomal HI | 80 | 808/110 | αvβ3 integrin (RGD) | FGS | Sun et al. (2017) |
| CH-4 T/SLB-MSN-Mdot/64Cu2+ | NIR-II | SLB-MSN-Mdot + CH-4 T + 64Cu2+ | 112 | 738/1,055 | FGS PET |
Q. Zhang et al. (2019) | |
| DCNP-L1-FSHβ | NIR-II | DCNPs (NaGdF4) + DNA + FSHβ | ~300 in vivo | 808/1,060 | FSHβ receptor (FSHβ peptide) | FGS | Wang, Li, et al. (2018) |
| UCNP@Azo + DCNP@β-CD | NIR-II | Nd@NaGdF4 DCNPs + Azo or βCD | 15 (UCNP@Azo), 8 (DCNP@β-CD) | 808/1,060 | FGS | M. Zhao et al. (2018) | |
| SCH1-SCH4 | NIR-II | Self-assembled PEG + CH1055 NPs | 170, 80, 30, 2 (SCH1–4) | 739–775/990–1,050 | FGS | Ding et al. (2018) | |
| RBCp | NIR-II | UCNPs@RB@RGD@avidin-RBC@ICG@biotin | 40 (UCNPs) 7 μm (RBCs) | ~808/~1,000 | FGS PDT |
Wang, Fan, et al. (2019) | |
| SWCNT-coated virus | NIR-II | SWNT + M13 virus (p8) + p3 + AF584 | 500 × 1 (SWNT) | 808/1,000–1,300 | SPARC (p3) | FGS | Ceppi et al. (2019) |
| HT@CDDP NPs | NIR-II | TQTPA + CDDP + HA | 60–70 | 760/1,016 | CD44 (HA) | FGS Theranostic |
Wang, Fan, et al. (2019) |
| Core-Shell Nanoparticles | NIR-II | PEO-b-PCL + NaYF4 + DiR | 60–90 | 980/1,175 | Folate receptor (Folate) | FGS | Tao et al. (2017) |
| RENPs@Lips | NIR-II | NaYF4/Nd 7%@NaYF4 + DPPC + Chol + PEG-lipid | 72 | 264, 545/1,064, 1,345 | FGS | Li et al. (2019) | |
| Ag2Se-cetuximab QDs | QD | Ag2Se QD-OPA-PEG-cetuximab | 2.8 | 720/930 | EGFR (cetuximab) | Theranostic | Zhu et al. (2017) |
| pRF-GQDs | QD | Graphene QD | 4 | 270/440 | FGS | Fan, Zhou, Garcia, Fan, and Zhou (2017) | |
| Bio CFQD® NPs | QD | In-based QD + ZnS + PEG | 12.2 | 405/615 | FGS | Yaghini et al. (2016); Yaghini, Turner, Pilling, Naasani, and MacRobert (2018) | |
| QD-apt | QD | ZnS QD-PEG-A32 | 20 | 330/605 | EGFRvIII (A32) | FGS | Tang et al. (2017) |
| Recombinant protein QDs | QD | EGFP-protein G-PbS QDs-anti HER2 mAb | 30 | 515/1,150 | HER2 (mAb) | FGS | Sasaki et al. (2015) |
| Gd-Ag2S Nanoprobe | QD | Gd3+-DOTA-Ag2S QDs | ~25 | 808/1,200 | FGS MRI |
C. Li et al. (2015) | |
| GERTs | SERS | Au + Si + BDT | 97 | 785, 808/N/A | SERS Theranostic |
Qiu et al. (2018) | |
| SERRS-MSOT-Nanostars | SERS | Au Nanostar + Si + PEG + IR-780 | 100 | 785/N/A | SERS MSOT |
Neuschmelting et al. (2018) | |
| SERS NPs | SERS | Au + Si + PEG + IR-780 | 108 | 785/N/A | SERS | Andreou et al. (2016) | |
| SERRS-NPs | SERS | Au + Si + PEG + IR-780 | 110 | 785/N/A | SERS | Harmsen et al. (2019) | |
| Multiplexed SERS NPs | SERS | Au + Si + mAb | 120 | 785/N/A | EGFR (mAb), HER2 (mAb), CD24 (mAb), CD44 (mAb), ER (mAb) | SERS | Kang, Wang, Reder, and Liu (2016); Wang, Ma, et al. (2017) |
| Multiplexed SERS NPs | SERS | Au + Si + PEG12 + DyLight 650 + mAb | 120 | 785/N/A | CA9 (mAb), CD47 (mAb), IgG4 (mAb) | SERS | Davis et al. (2018) |
| PEG-R-Si-au-NPs | SERS | Au + Si + PEG | 120 | 785/N/A | SERS | Karabeber et al. (2014); Thakor et al. (2011) | |
| αTF-SERRS NPs | SERS | Au + Si + IR-780 + ALT-836 | 122 | 785/N/A | TF (mAb) | SERS | Nayak et al. (2017) |
| PET-SERRS NPs | SERS | Au + Si + IR-780 + 68Ga | 132 | 785/N/A | SERS PET |
Wall et al. (2017) | |
| RGD SERRS | SERS | Au Nanostars + Si + IR-780 + RGDyK | 140 | 785/N/A | αvβ3 (RGD) | SERS | R. Huang et al. (2016) |
| CD47-targeted SERS-NPs | SERS | Au + Si + SM(PEG)12 + Dylight 650 + anti-CD47 mAb | 150 | 785/N/A | CD47 (mAb) | SERS | Davis et al. (2018) |
| αFR-NPs | SERS | Au Nanostar + Si + (1) αFR-Ab + IR780 or (2) PEG5000 + IR140 | 164 | 785/N/A | Folate receptor (ab3361 anti-folate binding protein Ab [LK26]) | SERS | Oseledchyk, Andreou, Wall, and Kircher (2017) |
| SERRS Nanostars | SERS | Au Nanostar + Si + PEG + IR-780 | 100–140 | 785/N/A | SERS | Harmsen et al. (2015); Spaliviero et al. (2016) | |
| F-SERS dots | SERS | Ag + Si + AF610 + mAb | 352–363 | 612, 785/628 (AF610) | EGFR (mAb), VEGF (mAb) | SERS FGS PTT |
Y. Kim et al. (2017) |
| FRNPs | SERS | Au Nanorod + Ag + Si + (PS)A6 DNA | 60–80 | 785/794 | SERS FGS PTT |
Pal et al. (2019) | |
| ICG@MCNPs | SPION | SPIONs + carbon NPs + ICG | 10 | 780/816 | FGS MRI PTT |
Song et al. (2017) | |
| ATF-PEG-IONP | SPION | SPION + PEG + ATF-NIR-830 + Dox or Cis | 25 | 800/830 | uPAR (ATF peptide) | FGS MRI Theranostic |
Gao et al. (2017) |
| NF-SION | SPION | SPION + oleic acid + PEG + Cy5.5-APTES | 38 | 675/705 | FGS | Lee et al. (2018) | |
| IONPs-ICG-HA | SPION | SPION + ICG + HA | 50 | 780/820 | H202; CD44 (HA) | FGS PTT/PDT PA |
Wang, Fan, et al. (2019) |
| ISCs | SPION | SPION + ICG | 97 | 850/N/A | MRI PA |
Thawani et al. (2017) | |
| 800ZW-sPION@dsiO2-YY146 | SPION | SPION + dSi02 + 800ZW + PEG + YY146 | 146 | 778/806 | CD146 (YY146 mAb) | FGS MRI |
Wang et al. (2015) |
| NIR-830-ZHER2:342-IONP-Cisplatin | SPION | SPION + NIR-830 + HER2 Affibody | 18–24 | 800/830 | HER-2 (Affibody) | FGS MRI Theranostic |
Satpathy et al. (2014, 2019) |
| P-GFLG-Cy5 | SS | HPMA copolymer-GFLG-Cy5 | 7 | 649/666 | Cysteine Cathespins | FGS | Blau et al. (2018) |
| PINS | SS | PEG-b-P(EPAlOO-r-ICGl) | 26 | 780/820 | pH < 6.9 | FGS | Zhao et al. (2017) |
| PNP | SS | SiNc + PEG-PCL | 40 | 750/780 | Self-quenching/de-quenching | FGS PDT |
Li, Zhang, et al. (2018) |
| LUM015 | SS | QSY21-GGRK-Cy5-PEG | Not reported | 650/675 | Cysteine Cathespins | FGS | Whitley et al. (2016) |
Abbreviations: AIE, Aggregation-induced emission; DN, dye nanoformulation; Em, emission; Ex, excitation; NIR-II, near-infrared-II; N/A, not applicable; PLNP, persistent luminescence nanoparticles; QD, quantum dot; SERS, surface-enhanced Raman spectroscopy; SPION, superparamagnetic iron oxide nanoparticle; SS, stimuli-sensitive.
4.1 |. Nanoparticle formulations of established fluorescent dyes
As mentioned above, several NIR dyes are well-suited for biomedical imaging (Figure 2). However, these dyes suffer from in vivo stability issues, undergo rapid clearance, lack tumor specificity, resulting in low tumor accumulation (G. Hong et al., 2017). As a result, several have developed nanoformulations of NIR dyes (liposomes, micelles, polymeric NPs, mesoporous silica NPS [MSNs], etc.) in order to improve the uptake and retention in tumors.
As the only FDA-approved NIR dye, ICG is the most commonly used fluorophore in NIR dye NPs. For example, we have sought to improve the tumor retention of ICG through nanoformulation via self-assembled hyaluronic acid (HA) NPs. As a result of conjugating HA polymers to the hydrophobic moiety, 1-pyrenebutanamide (PBA), hydrophobic pockets are formed during the NP self-assembly process. Hydrophobic and amphiphilic molecules, such as ICG, can be loaded into HA-PBA NPs and retained in the hydrophobic pockets through π–π stacking (Svechkarev, Kyrychenko, Payne, & Mohs, 2018). Compared to free ICG, NanoICG provided up to 2.9-fold higher intraoperative contrast and improved surgical outcomes in breast, pancreatic, and prostate tumors (Hill et al., 2015, 2016; Qi, Chen, et al., 2018; Souchek et al., 2018; Wojtynek et al., 2019). Others have improved the tumor uptake of ICG with active targeting ligands. ICG/MSNs-RGD are MSNs conjugated to an RGD peptide, targeting αvβ3 integrin in brain tumors. ICG/MSNs-RGD improved tumor signal 1.7-fold versus nontargeted ICG/MSNs. Although both were effective at identifying the primary tumor, ICG/MSNs-RGD demonstrated a propensity for identifying submillimeter tumors that would have gone unnoticed with other methods of tumor detection (Zeng, Shang, et al., 2016). Additionally, a NIR polymalic acid chlorotoxin nanoconjugate (NIA) provided high contrast to orthotopic brain tumors and improved the completeness of resection (Patil et al., 2019).
Although ICG is primarily used as a NIRF contrast agent, it is also effective at providing contrast in other modalities. A liposomal formulation of ICG, LipoICG, was developed as a multispectral optoacoustic tomography (MSOT) contrast agent. LipoICG provided sharp MSOT contrast to 4T1 tumors 24 hr after administration at a resolution of ~35 μm. Additionally, LipoICG provided a stronger MSOT signal than gold nanorods, a standard MSOT contrast agent, while still possessing high biocompatibility (Beziere et al., 2015). Others have developed multifunctional ICG-based NPs. CF800 is a liposomal formulation of ICG and iohexol, a combination of FDA-approved NIRF and CT contrast agents. This dual-modality probe was effective at providing preoperative contrast enhancement (>200 Hounsfield units) to lung tumors. The CT signal was consistent with the NIRF during intraoperative imaging, where CF800 provided a TBR >5 (Patel et al., 2016; Zheng et al., 2015). Another liposomal formulation of ICG, LP-iDOPE, provided strong contrast to orthotopic brain tumors. In addition to surgery, the authors also noted that LP-iDOPE could be used as a PDT photosensitizer to eliminate any remain tumor cells in the surgical cavity (Suganami et al., 2015).
Although the use of ICG in FGS is widespread, it is still limited by a relatively-low QY, lack of conjugatable functional groups, and poor in vivo stability. Other NIR dyes in preclinical and clinical development have desirable features that can be exploited to develop more effective FGS contrast agents (Figure 2; G. Hong et al., 2017). Man-PRx800 is a mannose receptor-targeted NP conjugated to ZW800–1. Man-PRx800 displayed preferential uptake in LNs and provided high contrast during surgery. Although the primary use of Man-PRx800 was not for primary tumor margin detection, it can be beneficial in the identification of metastatic LNs (Wada et al., 2018). Our group developed NanoCy7.5 through direct conjugation of Cy7.5 to HA NPs. Compared to free Cy7.5, NanoCy7.5 provided 14.8-fold higher contrast in breast tumors. This is a product of not only the high QY of Cy7.5 but also direct conjugation of the dye to the HA NP, minimizing the amount of free Cy7.5 escaping from the nanoformulation (Hill et al., 2016; Kelkar et al., 2016; Souchek et al., 2018). Also, NanoCy7.5 was combined with HA-PBA-GdDTPA in a mixed-micelle formulation, termed self-assembled multimodal imaging NPs (SAMINs), for dual-modality MRI-NIRF imaging of breast tumors. SAMINs provided greater T1 MRI contrast and % injected dose versus Magnevist (gadopentetate dimeglumine) with a 40× lower dose of gadolinium, while also retaining the ability to provide sufficient NIRF contrast during surgery (Payne et al., 2017). Finally, Jin et al. developed neuroblastoma-targeted Gd2O3 triangular nanoplates (TNs), conjugated to IRDye800CW, for dual-modality MRI-NIRF imaging. The neuroblastoma-targeted with Gd2O3 TNs provided a 3- to 5-fold higher MRI and NIRF signal to the tumor versus healthy surrounding tissue. As a result, resection guided Gd2O3 TNs improved 42-day survival versus controls and nontargeted Gd2O3 TNs (Jin, Li, Yang & Tian, 2019).
Due to their straightforward composition with FDA-approved or biocompatible materials, dye nanoformulations hold high potential as first-generation intraoperative contrast agents. As NIR dyes other than ICG enter the clinical realm, such as IRDye800 (Rosenthal et al., 2015), we expect to see an expansion in the use of dye nanoformulations for FGS.
4.2 |. Superparamagnetic Iron oxide nanoparticles
SPIONs are composed of either γ-Fe2O (maghemite), Fe3O4 (magnetite), or α-Fe2O3, with diameters ranging from 10 to 100 nm (Wahajuddin & Arora, 2012). Due to their superparamagnetic properties, SPIONs are useful as preoperative MRI contrast agents, capable of providing enhanced T2-weighted contrast to malignant tissues (Rosen, Chan, Shieh, & Gu, 2012). Furthermore, SPIONs possess an excellent biocompatibility profile (Corot, Robert, Idée, & Port, 2006). Past studies have indicated that SPIONs undergo biodegradation in vivo, limiting long-term cytotoxicity (Zimmer et al., 1995). Therefore, SPIONs are a favorable platform for biomedical imaging applications and are currently undergoing clinical assessment for MRI and preclinical development as contrast agents for FGS (Rosen et al., 2012; Zhu, Zhou, Mao, & Yang, 2017). In fact, the only FDA-approved SPION, ferumoxytol (treatment of iron-deficiency anemia in patients with chronic kidney disease), is also being used off-label as an MRI contrast agent in patients with renal failure who cannot be given gadolinium and is under clinical investigation for the detection of metastatic LNs and hepatic masses (Thakor et al., 2016). Thawani et al. developed a low-cost contrast agent termed ICG-coated SPIO-nanoparticle clusters (ISC), combining FDA-approved SPION and ICG. ISC is demonstrated enhanced contrast in preoperative T2-weighted MRI and a strong intraoperative PA signal in a flank model of GBM. When compared to microscopic surgery, PA-guided surgery improved complete tumor removal and prolonged survival (Thawani et al., 2017).
SPIONs can be further modified by the addition of targeting ligands and therapeutic agents (Rosen et al., 2012; Wahajuddin & Arora, 2012; Zhu, Zhou, et al., 2017). For example, Wang and coworkers coated SPIONs with dSiO2, 800ZW, and a CD-146-targeted antibody (800ZW–sPION@dSiO2–YY146). Compared to an untargeted NP, 800ZW–sPI-ON@dSiO2–YY146 generated an increase in NIRF signal and improved T2-weighted MRI contrast in a mouse model of gastric cancer (Wang et al., 2015). Another group developed a SPION platform for multimodal imaging and therapy of pancreatic and ovarian tumors. In both cases, PEGylated SPIONs conjugated to NIR-830 were functionalized with targeting ligands (ATF peptide for uPAR targeting in pancreatic cancer, and a HER-2-targeting affibody in ovarian cancer), enhancing T2-weighted MRI contrast and intraoperative NIRF contrast versus nontargeted SPIONs Additionally, the SPIONs were conjugated with chemotherapeutic agents and slowed tumor growth in comparison to free drug (N. Gao et al., 2017; Satpathy et al., 2014, 2019). Finally, Wang et al. demonstrated the use of HA/ICG-coated SPIONs (IONPs-ICG-HA) for combined FGS, PA, and PTT/PDT. IONPs-ICG-HA exploited TME features such as hypoxia, low pH, and excessive H2O2 production to trigger OH production by IONPs-ICG-HA. Also, ICG served as a photosensitizer to improve synergistic PDT/PTT and acted as a fluorescent imaging probe. This, combined with CD44 targeting provided by HA, resulted in the efficacious treatment of colorectal tumors and improved PA and NIRF contrast (Wang, Fan, et al., 2019). Although FGS was not the primary objective of the IONPs-ICG-HA study, it demonstrated the versatility of SPIONs for multimodal imaging/theranostics of solid tumors.
Due to the safety profile and efficacy of SPIONs, they remain an excellent platform for surgical imaging. Recent findings have made it clear that modifications to SPIONs (e.g., targeting, theranostics) have the potential to improve outcomes. However, further work is needed to assess the effects of active targeting on the pharmacokinetics and biodistribution of SPIONs (Rosen et al., 2012; Zhu, Zhou, et al., 2017).
4.3 |. Stimuli-sensitive nanoparticles
During tumor development, cellular processes become increasingly dysregulated to sustain proliferation and survival. As a result, tumors acquire hallmark characteristics that distinguish neoplastic disease from healthy tissue (Hanahan & Weinberg, 2011). These hallmarks manifest as abnormalities that are observed in the TME, such as acidic pH, hypoxia, or the upregulation of proteolytic enzymes. Consequently, these TME factors can be targeted by delivery systems and exploited by stimuli-responsive imaging probes for intraoperative imaging (Du, Lane, & Nie, 2015). With tumor-specific fluorescence activation, stimuli-sensitive NPs can maximize tumor signal and while minimizing fluorescence activation in background tissues.
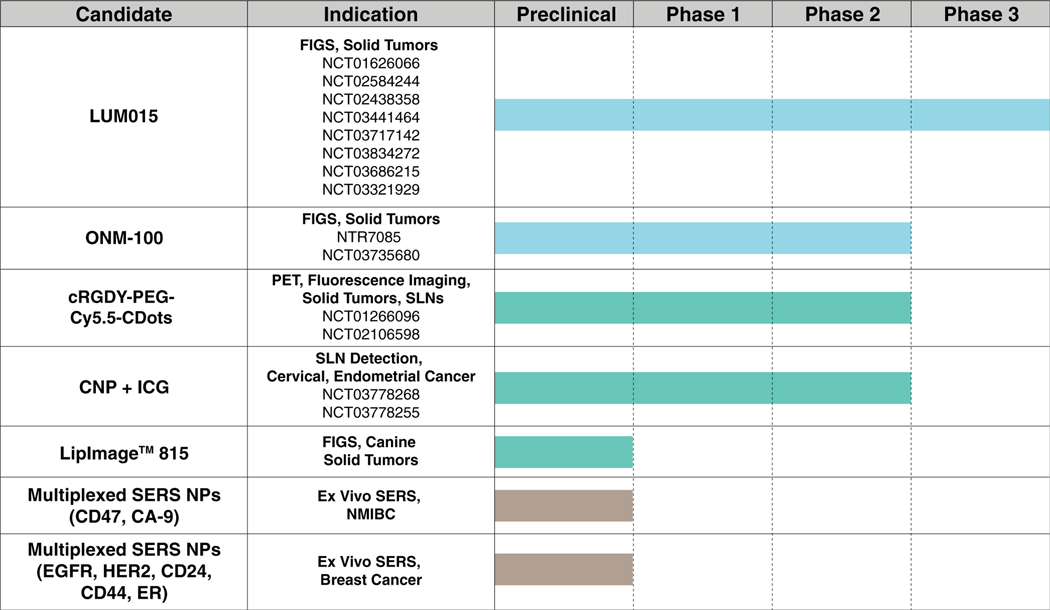
Low pH is a characteristic of the TME. The rapid growth and proliferation of cancer cells require a continuous source of energy. To maintain high energy requirements, cancer cells shift their metabolism from oxidative phosphorylation to aerobic glycolysis (Warburg effect), acidifying the TME (pHTumor = 6.5–6.9 vs. pHNormal Tissues = 7.4; Vander Heiden, Cantley, & Thompson, 2009). Recently, Zhao et al. developed a transistor-like ICG-based pH nanoprobe, termed PINS. At pH ≤6.9, PINS dissociates into protonated unimers, which are highly fluorescent, while at pH ≥7.0, PINS are retained in a nonfluorescent, micellar state. Effectiveness was demonstrated across a variety of orthotopic and metastatic tumors, in which PINS provided superior contrast versus a panel of NIR imaging probes. Also, PINS reduced the number of false positives versus fluorodeoxyglucose (FDG)-PET imaging. The surgical utility of PINS was demonstrated, dramatically improving survival after FGS in models of head and neck cancer and small occult breast nodules with a 100% confirmation rate (T. Zhao et al., 2017). Based on this robust preclinical data, PINS (ONM-100) is now under phase II clinical investigation in patients with solid tumors (Figure 4). Another group has developed a SiNc-based pH-activatable NP for combinatorial FGS/phototherapy. Upon internalization into cancer cells, the OFF/ON SiNc NPs were activated by pH-triggered dequenching of fluorescence, leading to sufficient tumor contrast and cytotoxicity (X. Li et al., 2018).
FIGURE 4.
Nanoparticles currently undergoing clinical investigation
Another characteristic of tumor cells is high expression of proteases. Proteases, such as cysteine cathepsins or matrix metalloproteinases (MMPs), have essential roles in facilitating tumor growth, invasion, and metastasis, chiefly through degradation of the extracellular matrix (Koblinski, Ahram, & Sloane, 2000). Therefore, proteases have been long sought-after as targets of cancer imaging probes (Mahmood & Weissleder, 2003). Cysteine cathepsin-activatable NPs have recently been reported (Blau et al., 2018; Whitley et al., 2016). LUM015 is a PEGylated dye-quencher conjugate linked with a cathepsin-degradable peptide sequence (QSY21-GGRK-Cy5-PEG). Upon degradation of the GGRK peptide by tumoral cathepsins, fluorescence is activated through a dequenching mechanism. After demonstrating outstanding efficacy in a genetically-engineered mouse model (GEMM) of soft tissue sarcoma (STS), LUM015 was administered to 15 patients that underwent surgical resection of STS or breast tumors and imaged ex vivo. Results demonstrated that that LUM015 was well-tolerated at three doses and provided sufficient contrast to tumors. In the majority of patients, LUM015 provided a tumor to normal tissue fluorescence ratio > 1, an indicator of strong potential as a contrast agent for FGS. Since this preliminary study, LUM015 has been undergoing various stages of clinical investigation in multiple indications, including a recently established multicenter phase 3 clinical trial (NCT03686215; Figure 4).
In addition to PINS and LUM015, other peptide-based activatable probes have initiated clinical trials (R. R. Zhang et al., 2017). Therefore, there is a high potential for the use of stimuli-sensitive probes for FGS. Nevertheless, heterogeneous expression levels of enzymes and conditions targeted by stimuli-sensitive NPs can potentially limit their efficacy (Bu, Shen, & Cheng, 2014; Fisher, Pusztai, & Swanton, 2013). To address these concerns, it is essential to stratify patients by stimuli expression levels to identify the full impact of tumor heterogeneity.
4.4 |. Quantum dots
QDs are semiconducting nanocrystals consisting of 200–10,000 atoms and are on the order of 2–10 nm in core diameter, while targeted and coated QDs can be much larger. Their composition imparts unique optical properties, including broad absorbance, large Stokes shift, high QY, long fluorescence lifetime, and long photostability. The most unique characteristic of QDs is size and composition-dependent tunable emission, allowing for the precise selection of fluorescence emission wavelength (Smith, Duan, Mohs, & Nie, 2008). The structure of a QD typically consists of a fluorescent core surrounded by a semiconductor shell. QDs can be conjugated to biocompatible ligands to improve in vivo stability, and functionalized with tumor-targeting ligands (McHugh et al., 2018). Although QDs offer optical properties that are desirable for intraoperative imaging, they also suffer from significant toxicity concerns. Until recently, the composition of QD core material has mainly consisted of heavy metals (e.g., Cd, Hg, Pb, and As), which can be released from the core during circulation. Cd toxicity is the most considerable concern because it is the most commonly used core material (Smith et al., 2008). The primary clinical manifestation of Cd toxicity is renal damage but can also cause bone, cardiovascular, immune, and endocrine dysfunction (Bernhoft, 2013). A solution to limit the risk of heavy metal toxicity is to optimize the QD shell composition to minimize the possibility of Cd2+ release (Oh et al., 2016). However, to eliminate the risk of heavy metal toxicity altogether, the field has been inclined to develop Cd-free QDs with biocompatible materials (In, Cu, Ag, Zn, S, and Se) that have comparable performance to Cd-containing QDs (Won et al., 2019; Zeng et al., 2016).
Yaghini and coworkers developed an In-based QD platform, termed bio CFQD®, for in vivo LN imaging. Bio CFQD® was shown to display high QY and colloidal stability in aqueous environments. After subcutaneous injection into rat paws, bio CFQD® accumulated in regional LNs located in the thoracic fat pads and exhibited fluorescence retention after LN dissection (Yaghini et al., 2016). Although a long-term biodistribution study indicated that bio CFQD® was retained in the liver and spleen after 90 days, no organ or hematological toxicity was observed (Yaghini et al., 2018). In the future, bio CFQD® can serve as an effective platform for tumor and SLN imaging.
Several groups have optimized QDs through functionalization with targeting ligands. Although it was only used for a theranostic application, Ag2Se-cetuximab nanoprobes were shown to accumulate in orthotopic oral tumors 13 hr after injection, and provide greater tumor signal than nontargeted QDs (Zhu, Chen, et al., 2017). An aptamer-based EGFRvIII-targeted QD, QD-Apt, was shown to cross the blood–brain barrier (BBB), providing sharp intraoperative contrast to orthotopic brain tumors. Interestingly, it was demonstrated that the EGFRvIII is highly expressed in 41.82% of human gliomas, and 0.00% in normal brain tissue, thus providing potential clinical relevance to QD-Apt (Tang et al., 2017).
There are several examples of QDs that have features of other NPs classes. Fan and coworkers developed pH-responsive fluorescent graphene quantum dots (pRF-GQDs), which displayed a fluorescence shift between green and blue at pH 6.8. Although fluorescence was emitted at a visible wavelength (em. = 440 nm), pRF-GQDs exploited tumor acidosis and provided contrast to pancreatic, liver, lung, and brain tumor xenograft models. Optimization of the optical properties (i.e., developing NIR-emitting QDs) could improve the intraoperative performance of these stimuli-sensitive QDs (Fan et al., 2017). NIR-II-emissive recombinant protein QDs were developed as a mAb-conjugatable QD platform based on recombinant enhanced green fluorescent protein (EGFP)-protein G. In a proof-of-concept study, the QD was conjugated to anti-HER-2 mAbs and provided distinct contrast to breast tumors. Although it is an effective tumor-targeting QD platform, the QD contained Pb, limiting its translational potential. Therefore, this targeting platform should be applied to biocompatible QDs to improve clinical relevance (Sasaki et al., 2015). Finally, Gd-Ag2S nanoprobes combined the high penetration depth of MRI with the real-time imaging capabilities of NIR-II fluorescence. The Gd-Ag2S nanoprobes were proven to be safe for up to 1 month, were detectable at concentrations as low as 0.025 × 10−3 M with T1-weighted MRI, and 4 × 10−6 M with NIR-II imaging. MR contrast-enhancement was observed up to 48 hr after administration to mice bearing orthotopic brain tumors. Gd-Ag2S was able to provide high intraoperative contrast (TBR ≈ 11) to tumors with minimal background interference. Histological analysis determined that Gd-Ag2S effectively reduced the quantity of residual tumors when compared to BLS (4.5% vs. 14%; Li et al., 2015).
4.5 |. NIR-II emitting nanoparticles
There is a large body of evidence suggesting that fluorescence in the NIR-I range (700–900 nm) is favorable for biomedical imaging. Although impacted by tissue scattering to a lesser degree than visible light, NIR-I fluorescence is still not entirely immune to scattering. In biological tissues, the magnitude of scattering is inversely proportional to the wavelength of light. Therefore, as wavelength increases, scattering decreases (Welsher, Sherlock, & Dai, 2011). Biomedical imaging in the NIR-II window (900–1,300 nm) is superior to the NIR-I window due to a higher resistance to scattering, and thus, deeper tissue penetration. Also, light in the NIR-II window is less susceptible to the absorption and autofluorescence that is observed in biological tissues and endogenous molecules (G. Hong et al., 2017; Smith, Mancini, & Nie, 2009). Consequently, NIR-II imaging has garnered interest for FGS due to its potential in generating high TBRs with minimal interference.
Nanoformulation of NIR-II fluorophores can improve in vivo stability and maximize the amount of dye delivered to the tumor. These contrast agents are typically soft NPs loaded with NIR-II fluorophores. For example, PDFT1032 polymeric NPs consisted of PDFT, a novel NIR-II-emitting polymer surrounded by a DSPE-mPEG shell. PDFT1032 generated high TBRs in osteosarcoma for up to 3 days after IV injection (Shou et al., 2018). The self-assembled, PEGylated Scheme NP platform consisted of four size-tunable NPs based on the NIR-II dye, CH1055. SCH4, the smallest NP (2 nm), exhibited rapid hepatic clearance and allowed for the identification of hepatic tumors with an excellent TBR (>7; Ding, Li, et al., 2018). H1 NPs, were functionalized with targeting ligands, allowing for the precise identification of glioblastoma and SLNs (Sun et al., 2017). A multifunctional NP termed HT@CDDP was capable as a theranostic agent for oral squamous cell carcinoma. HT@CDDP provided sufficient tumor contrast up to 24 hr after injection while also suppressing tumor growth with combinatorial chemo-PTT (Wang et al., 2019).
Another popular class of NIR-II fluorophores are lanthanide-based NPs. Although lanthanide NPs provide bright NIR-II emission that is favorable for bioimaging, they are retained in the reticuloendothelial system (RES) and give rise to long-term safety concerns. Recently, Li et al. reported on the development of a liposomally formulated NIR-II lanthanide NP. Liposomal formulation dramatically reduced the blood, liver, and spleen half-life of RENPs@Lips compared to other lanthanide NPs. As a result, more than 90% of RENPs were cleared from the hepatobiliary system 72 hr after administration. Additionally, RENPs@Lips provided sufficient interoperative contrast to identify primary tumors and SLNs in mouse models of melanoma and osteosarcoma. Taken together, these results demonstrated the potential benefits of using nanoformulation to improve PK profile, possibly expanding the use of lanthanide-based NPs to safe, in vivo use (Li et al., 2019).
In addition to simple nanoformulations, there are more complex systems that are utilized for the delivery of NIR-II contrast agents to solid tumors (Cai et al., 2019). Two unique examples include an SWNT-coated M13 bacteriophage (Ceppi et al., 2019) and red blood cell-based probe (RBCp; Wang, Fan, et al., 2019). Another innovative NP delivery strategy is in vivo assembly of up/downconversion NPs (U/DCNPs). This strategy reduces RES scavenging and background interference while maximizing tumor-specific NIR-II fluorescence signal. The Zhang group developed two such NIR-II NPs. The first, DCNP-L1-FSHβ, is a follicle-stimulating hormone receptor (FSHR)-targeted NP for the intraoperative detection of ovarian cancer. After dual injection of each component, the NP assembled at the tumor through DNA pairing. Intraoperative NIR-II imaging revealed that DCNP-L1-FSHβ identified tumors with a tumor-to-normal tissue ratio of 12.5 and provided enough contrast to distinguish submillimeter lesions in the peritoneal cavity (Wang, Li, et al., 2018). Similarly, the UCNP@Azo/β-CD NP system was able to increase tumor accumulation fourfold with in vivo assembly. It resulted in a 1.5-fold increase in SNR compared to ex vivo assembled NPs in peritoneal ovarian cancer metastases (M. Zhao et al., 2018).
Finally, Zhang et al. synthesized a dual-modality NIR-II/PET imaging probe, termed CH-4T/SLB–MSN–Mdot/64Cu2+ nanoprobe, consisting of the NIR-II fluorophore, CH-4T, and 64Cu2+ as a PET contrast agent. The hybrid MSN/supported lipid bilayer (SLB)/Mdot formulation maximized CH-4T NIR-II fluorescence while also serving as a chelator-free scaffold for 64Cu2+. As a result, NIR-II fluorescence was increased 4.27-fold versus CH-4T alone. These results were replicated in a model of squamous cell carcinoma (SCC), where the NPs provided high PET contrast before surgery as well as sufficient intraoperative NIR-II contrast for tumor recognition (Q. Zhang et al., 2019).
Although intraoperative imaging in the NIR-II range is an emerging field, we have observed tremendous growth due to the superior optical properties of the NIR-II window. Evidence of the first clinical study using NIR-II imaging emerged at the end of 2019. This study utilized ICG to image liver tumors in the NIR-I/II windows. Although off-peak ICG fluorescence was used, intraoperative NIR-II imaging demonstrated superior tumor detection sensitivity, tumor-to-normal liver tissue signal ratio, and tumor detection rate compared to imaging in the NIR-I window (Hu et al., 2019). Moving forward, it is likely that innovations in materials science and engineering will drive further breakthroughs. For example, InGaAs-based fluorescence detection cameras are most-commonly utilized in NIR-II imaging, however, as other NIR-II-sensitive materials, such as InSb or HgCdTe, are incorporated into cameras, the detection limit and depth will be improved, expanding the utility of NIR-II imaging (G. Hong et al., 2017). Nevertheless, because many of the NIR-II fluorophores are novel organic dyes or inorganic nanomaterials, comprehensive safety, and biocompatibility studies are necessary (Ding, Zhan, et al., 2018).
4.6 |. Luminescent nanoparticles
PLNPs are unique in that they do not require a continuous excitation source. Rather, PLNPs emit light several minutes to hours after the cessation of excitation. Because a continuous excitation source is not required during FGS, the risk of autofluorescence and background noise is minimized, allowing for high TBRs. Although there are over 200 chemical combinations reported in the literature, PLNPs for biomedical imaging applications are composed of host materials combined with rare earth (RE) and metal transition cations (dopant), emitting light in the red/NIR window (Liu et al., 2018). The underlying physics of PLNPs is complex and is still under investigation. However, persistent luminescence begins as excited electrons are generated by exposure to excitation light. Instead of falling back to relaxed energy levels, the electrons are captured by electron traps, which are characteristic of the PLNP composition. The energy stored in the trap is gradually released through a discharge process, and the end result is the continuous generation of light (Lécuyer et al., 2016).
Several groups have developed PLNPs for the purpose of FGS. Generally, PLNPs were based on either ZnGa (Ai et al., 2018; Shi et al., 2015; J. Wang, Ma, et al., 2017) or NaGd (Qiu, Zeng, et al., 2018), complexed with common nanomaterials, and functionalized with targeting ligands. In each example, PLNPs were exposed to excitation light before administration and emitted light in the NIR range, which could be observed up to 24 hr after initial excitation. Of note, Qui and coworkers developed NIR upconversion luminescent nanoprobes functionalized with anti-HER-2 mAbs. After IV administration, the PLNPs provided an excellent contrast to metastatic LNs that penetrated 7.7 mm. Interestingly, the PLNPs were tracked with SPECT/CT imaging, results from which demonstrated consistency with NIRF imaging (Qiu, Zeng, et al., 2018). Although each cited example showed enhanced tumor contrast provided by PLNPs, only one study reported the effects of PLNP administration on surgical efficacy. Ai et al. developed ZGC, which were ZnGa2O4Cr0.004-based PLNPs for the NIRF imaging of liver tumors. During surgery, ZGC provided high contrast with long-lasting NIRF liver tumors, and detected lesions that were smaller than 1 mm. Also, FGS with ZGC prolonged survival versus traditional surgery (Ai et al., 2018).
Finally, it is essential to address the questionable toxicological effects of RE metals. Several studies have demonstrated that long-term exposure to RE dust may lead to toxic effects in the lung and long-term disposition in the body (Rim, Koo, & Park, 2013). More specifically, it was found that RE oxides undergo biotransformation in macrophage lysosomes, ultimately resulting in inflammation and fibrosis (R. Li et al., 2014). In order to advance the clinical translation of PLNP, further investigation into their long-term biodistribution and toxicity are necessary.
4.7 |. Aggregation-induced emission nanoparticles
A characteristic of planar fluorophores, such as ICG, is aggregation-caused quenching (AQC) at high concentrations/ close proximity. In AQC, π–π stacking interactions between aromatic rings quenches fluorescence, limiting the fluorescence imaging capabilities of these dyes at high concentrations. However, organic nonplanar fluorophores exhibit a quality known as AIE. Their fluorescence is usually quenched by extensive intramolecular rotations and other motions in low concentrations. In high concentrations/close proximity, these motions are restricted due to π-π stacking, and the molecules regain fluorescence (Y. Hong, Lam, & Tang, 2011). Additionally, NIR-emitting AIEgens possess higher QY, broader Stokes shifts, and stronger resistance to photobleaching than conventional NIR dyes, while also being more biocompatible than QDs (Mei, Leung, Kwok, Lam, & Tang, 2015).
Improvements in AIEgen nanoformulations have allowed for their use as surgical contrast agents. AIE NPs are composed of AIEgens loaded into lipid NPs, which helps encourage aggregation and improve delivery to tumors. AIE NPs developed by Liu and coworkers were utilized for NIRF detection of peritoneal metastases. The AIE NPs provided an ultra-high TBR of ~7.2 to malignant tissues. As a result, the AIE NPs were able to identify several lesions that were 100–500 μm in size (Liu et al., 2017). TPE-Th-B NPs also displayed the ability to identify submillimeter pulmonary metastases of 4 T1 breast tumors with high NIRF contrast (H. Gao et al., 2019). Two AIE NPs used for surgical imaging demonstrated multimodal imaging capabilities. JNJ15 (Polyphorin + Bacteriopheophorbide NPs) effectively provided NIRF contrast to a rabbit model of head and neck SLNs. After the excision of SLNs, JNJ15 also provided contrast with PA imaging (Lovell et al., 2011; Shakiba et al., 2016). A more elaborate combination of multimodal imaging and PDT was demonstrated with UV-sensitive, DTE-TPECM smart function-transformable NPs. The design of DTE-TPECM NPs allowed for the unique ability to undergo reversible switching between closed- and opened-ring structures upon exposure to UV light, allowing for the production of robust PA and fluorescence/ROS signals, respectively. As a result, this versatile system offered presurgical imaging (PA), intraoperative imaging (fluorescence), and PDT capabilities, ultimately improving overall survival after surgical resection of 4T1 breast tumors (J. Qi, Chen, et al., 2018). Finally, two separate AIE NPs possessed characteristics of other NP classes including NIR-II capabilities (J. Qi, Sun, et al., 2018) and persistent luminescence (Ni et al., 2018) in a variety of tumor models. Of note, the NIR-II emitting AIE NPs (TQ-BPN), showcased the benefits of AIEgens with a superior NIR-II quantum yield (2.8%) versus common NIR-II nanomaterials such as SWCNTs (QY = 0.4%). Surprisingly, although the maximum emission of TQ-BPN is 808 nm, the off-peak, short-wave infrared (SWIR) fluorescence produced by TQ-BPN was still effective at providing NIR-II contrast.
There is a strong potential for the use of AIE NPs in FGS. As this NP class grows, it is likely that the performance of nanoformulations will be improved with higher loading capacities and functionalization with targeting moieties. Also, because all AIEgens are considered new molecular entities, it is necessary that rigorous preclinical drug metabolism, pharmacokinetics (DMPK), and toxicology studies be conducted.
4.8 |. Surface-enhanced Raman spectroscopy nanoparticles
SERS NPs are unique in that they rely on the Raman effect, rather than fluorescence, for image-guidance. SERS NPs consist of a multi-layered structure composed of a Raman reporter molecule adsorbed onto the surface of the metal core (Au or Ag NPs), falling into the 100–120 nm size range. The Raman reporter component is responsible for generating a Raman spectrum unique to the NP. Also, SERS NPs can be further modified with a biocompatible coating and/or targeting ligands to improve in vivo stability and tumor specificity (Y. Wang, Kang, Doerksen, Glaser, & Liu, 2016).
There are several methods of SERS NP-guided surgery: Nontargeted SERS, targeted SERS, multiplexed SERS, and multimodal imaging. Regardless of the imaging method, an enhanced SERS signal serves as tumor indicator and guides resection. Although nontargeted SERS NPs attribute tumor accumulation to the EPR effect and macropinocytosis, they are still effective and safe contrast agents (Andreou et al., 2016; Harmsen et al., 2015, 2019; Karabeber et al., 2014; Spaliviero et al., 2016; Thakor, Luong, et al., 2011). Notable highlights include the detection of microscopic foci as small as 100 μm with 1.5 fM sensitivity (Harmsen et al., 2015), and the detection of premalignant GI lesions with high sensitivity (93.1%) and positive predictive value (89%; Harmsen et al., 2019).
Tumor-targeting ligands improve the detection of cancer by SERS NPs. αTF-SERRS NPs were conjugated to an antiALT-836 mAb and were able to detect lung metastases as small as 200 μm (Nayak et al., 2017). Also, αvβ3 integrin-targeted SERRS NPs detected orthotopic brain tumors with fM sensitivity and allowed for the recognition of tiny lesions as small as five cells, outperforming nontargeted NPs (R. Huang et al., 2016).
SERS has the unique ability to employ multiplexed imaging for the detection of multiple tumor markers in a single sample. This is beneficial in cancer imaging, due to heterogeneous expression of tumor antigens. In order to detect multiple NP flavors, a ratiometric quantification method is required, comparing the signals of targeted SERS NPs to a nontargeted reference (Garai et al., 2013). Two imaging studies using multiplexed SERS nanoparticles were performed on resected human tumor samples. The first example applied multiplexed SERS to human nonmuscle invasive bladder cancer (NMIBC), using three flavors of topically administered SERS NPs (CD47, CA-9, and nontargeted) with a Raman endoscope system. It was found that both the targeted and nontarget NPs differentiated tumor from normal tissue with high accuracy (ROC AUC = 0.93). The nontargeted NPs exhibited fivefold deeper penetration and 3.3-fold higher tissue concentration than the targeted NPs, suggesting a strong EPR effect in NMIBC (Davis, Kiss, et al., 2018). The Liu group employed a panel of SERS NPs (EGFR, HER2, CD44, and ER) to perform multiplexed imaging of 57 human breast tumor samples 10–15 min after resection. The multiplexed SERS NPs enabled discrimination between tumor and healthy tissue, and multiple subtypes of breast cancer, achieving a sensitivity of 89.3% and a specificity of 92.1% (Wang, Ma, et al., 2017). Taken together, these studies showcased the high translational potential of multiplexed SERS imaging.
Although SERS offers high sensitivity and resolution in the detection of tumor margins, it is reliant on point spectroscopy, which requires long acquisition times and can result in residual disease being overlooked. Also, relative to other imaging modalities, SERS has a limited depth of penetration (Eberhardt, Stiebing, Matthäus, Schmitt, & Popp, 2015). Therefore, SERS has been combined with other imaging modalities that offer a wider field of view (NIRF, MSOT) or higher depth of penetration (PET) to maximize the effectiveness of SERS. Both F-SERS Dots and FRNPs combined NIRF with SERS. Combined imaging allowed for the quick recognition of tumor with NIRF, while SERS detected residual tumor with high sensitivity, including microscopic lesions that were not recognizable with NIRF (Y. Kim et al., 2017; Pal et al., 2019). SERRS-MSOT-nanostars combined SERS with multispectral optoacoustic tomography (MSOT) for the detection of GBM. MSOT allowed for the generation of 3D mapping while SERS allowed for the detection of GBM with high sensitivity and resolution (Neuschmelting et al., 2018). Finally, PET-SERRS NPs were conjugated to 68Ga to combine PET with SERS. PET was useful in the identification of tumor and metastatic LNs before surgery, while SERS detected malignant tissue during surgery (Wall et al., 2017).
Due to the high sensitivity of SERS along with multiplexing capabilities, SERS NPs hold high promise as effective contrast agents for surgical guidance. In order to break into the clinical realm, several hurdles must be surpassed. Because the primary components in SERS NPs are Au and Ag, there are concerns over potential long-term toxic effects of the NPs (Singh et al., 2018). Although previous studies have shown that Au NPs have negligible to mild toxic effects, further long-term biodistribution and toxicology studies are necessary prior to clinical investigation (Fraga et al., 2014; Thakor, Paulmurugan, et al., 2011). Additionally, advancements to SERS imaging systems such as wide-field SERS and endoscopic systems will improve the ease of use in the clinical setting (Lane et al., 2015).
4.9 |. Nanoparticles in the clinic
To date, NPs in clinical development include dye nanoformulations, stimuli-sensitive probes, and SERS NPs (Figure 4). More-sophisticated classes of NPs (QDs, luminescent, AIE, NIR-II, etc.) have not yet reached the clinic, but hold promise. Due to the commercial availability of FGS systems and compatibility with the surgical workflow, the majority of NPs undergoing clinical investigation for intraoperative tumor identification are for FGS indications (Tipirneni et al., 2017). However, it is worth noting that several of these NPs could be compatible with PA imaging, especially ICG-containing NPs. Nevertheless, PA imaging is more appropriate for applications other than surgery (i.e., applications that require high depth of detection) and is less amenable to the operating room setting (Steinberg et al., 2019). Although SERS NPs have been utilized for real-time tumor identification in resected patient samples, clinical hurdles lie within safety concerns associated with Au NPs and limited clinical applicability SERS systems (Kircher, 2017; Singh et al., 2018; Wang et al., 2016). For these reasons, there has been more clinical progress in the FGS space when compared to PA, and SERS.
The most clinically advanced NP is LUM015, which is undergoing eight clinical trials, including a pivotal phase 3 study (NCT03686215). As discussed in the stimuli-sensitive NP section, LUM015 is a pegylated cathepsin-activatable probe that is composed of Cy5 (ex. 658 nm; em. 696), a QSY21 quencher, and a GGRK peptide linker. As a consequence of protease overexpression in the TME, the probe is cleaved, separating dye and quencher, and activating fluorescence. The phase 3 trial is a multicenter, single-arm study with the primary objective of assessing the ability of LUM015 and the LUM2.6 FGS systems to detect residual tumor in 250 breast cancer patients undergoing lumpectomy. The secondary endpoint seeks to determine the rate of PSMs after FGS. This trial is expected to be completed in early 2020 and will be the first FGS NP to be tested on a large patient population. To date, the only results reported in the literature are from a dose-escalation study in 15 breast cancer patients, which reported tumor-to-normal tissue ratios of ~4.5 and an acceptable safety profile (B. L. Smith et al., 2018).
Because tumor acidosis is a relatively universal feature of tumors, ONM-100 (PINS), a pH-sensitive poly(ethyleneglycol)-b-poly(ethylpropylaminoethyl methacrylate) copolymer NP conjugated to ICG, is seeking FDA-approval as a tissue-agnostic FGS contrast agent. ONM-100 is currently undergoing two clinical trials, with the most advanced being a phase 2a study in 45 patients with breast, head and neck, colorectal, bladder, prostate, and ovarian cancers, and is estimated to be completed in early 2020 (NCT03735680). The primary endpoint of the study is to determine the mean fluorescence intensity of tumor versus nontumor in patients treated with ONM-100 while the secondary endpoint to establish the PK profile of ONM-100.
cRGDY-PEG-Cy5.5-CDots are integrin-targeted NPs that are undergoing clinical development as dual-modality PET/fluorescence contrast agents for SLN mapping (Bradbury et al., 2013). cRGDY-PEG-Cy5.5-CDots are composed of core-shell silica CDots, PEG, Cy5.5 (ex. 679 nm; em. 696 nm), and an integrin-targeting peptide, cRGDY. There are currently two active clinical trials for cRGDY-PEG-Cy5.5-CDots, with the most advanced being a phase 1/2 study in 105 patients with head and neck, breast, and colorectal tumors (NCT02106598). The investigators hope to utilize the cRGDY-PEG-Cy5.5-CDots to conduct preoperative SLN mapping with PET followed by fluorescence-guided removal of SLNs. The secondary objective of the study is to determine whether the cRGDY-PEG-Cy5.5-CDots can image breast tumors during surgery. The study is estimated to be completed by mid-2020.
The co-administration of carbon NPs (CNP) and ICG seeks to be established as a SLN mapping strategy. CNPs, 21 nm NPs consisting of activated carbon, accumulate in lymphatic vessels and LNs, and allow for their identification via visualization of black color (Y. Lu, Wei, Yao, Pan, & Yao, 2017). Addition of ICG could potentially improve SLN mapping through NIRF imaging. Currently, two large trials are assessing the feasibility of using CNP + ICG for the guided resection of SLNs in patients with cervical (NCT03778268; 1,143 patients) and endometrial (NCT03778255; 635 patients) tumors. The primary endpoint of the studies is to determine the predictive value of CNP + ICG versus CNP alone. The secondary endpoints seek to assess the SLN detection rate, sensitivity, and false-negative rates. The estimated study completion date is in mid-2020.
Three other NP candidates that have completed late-stage preclinical/translational studies. LipImage™ 815 is a lipid NP composed of Lipidot™ NPs (Gravier et al., 2011) and the NIR dye, IR780 (ex. 650 nm; em. 780 nm). In a prospective proof-of-concept study, LipImage™ 815 was administered to companion canines with spontaneous STS or subcutaneous tumors and underwent surgery shortly after. No adverse effects were observed as a result of LipImage™ 815 administration. Furthermore, LipImage™ 815 provided sufficient contrast to identify tumors in each dog (n = 9) and resulted in PSMs in only one case. Although a small study, this demonstrates the feasibility of using LipImage™ 815 for intraoperative tumor detection in spontaneous tumors, which have more heterogeneity than preclinical cell lines and GEMMs. Further studies on larger populations are needed to determine the sensitivity and specificity of the probe (Cabon et al., 2016). Finally, two sets of previously-discussed multiplexed SERS NPs demonstrated value as ex vivo contrast agents in identifying disease in resected NMIBC (Davis, Kiss, et al., 2018) and breast cancer (Wang, Ma, et al., 2017).
5 |. CONCLUSION AND FUTURE OUTLOOK
This review covered the current progress and advancements in the use of NPs for surgical tumor recognition. Since our prior report (Hill & Mohs, 2016), there has been a rapid development of nanotechnology for biomedical imaging with the entrance of several new technologies into the surgical tumor detection realm (i.e., NIR-II fluorescence, AIE NPs, etc.), as well the entry of first-generation NPs into the clinic. The use of NPs for surgical image guidance will likely continue to grow and play a significant role in the field.
However, several critical issues must be addressed to ensure successful clinical translation of NPs. The field must strictly adhere to rigid preclinical guidelines to maximize the translational potential of NPs and rule out under-performers. This includes testing NP candidates in clinically relevant models, such as orthotopic patient-derived xenograft tumors, which better recapitulate the heterogeneity and tissue-specific characteristics of human tumors. Additionally, strive to achieve clinical translation with efficacious, simple, and biocompatible (FDA-approved materials if possible) nanoformulations, while also minimizing NP complexity (Ioannidis, Kim, & Trounson, 2018; Park, 2019). Finally, because long-term biodistribution and safety of NPs are a significant concern, preclinical and clinical studies must assess these items with rigor (Han et al., 2019).
As more NPs enter the clinic, the IGS field must align on clinical trial design. This includes developing clinically relevant endpoints that seek to assess the effects of the contrast agent on patient benefit (i.e., survival or PSM rate) rather than merely correlating intraoperative signal with tumor location (Tummers, Miller, et al., 2018). Pogue and coworkers have established a cost-effective clinical trial framework that takes advantage of the FDA’s exploratory investigational new drug (eIND) pathway for the early clinical development of contrast agents with first-in-human phase 0 microdose trials (Pogue et al., 2015). Also, others have developed standard preclinical and clinical benchmark guidelines for the successful development of novel intraoperative contrast agents (Koller et al., 2018; Tummers, Warram, et al., 2018). In addition to contrast agents, several commercially-available imaging systems are used for surgical imaging. Each system varies in its dynamic range and sensitivity, which can lead to discrepancies in tumor recognition. Standard detection criteria must be developed to ensure consistent imaging effectiveness (DSouza et al., 2016).
Although there are several challenges to be addressed, the future outlook of the use of NPs for surgical tumor detection is bright, with the potential to create high value for cancer patients (Pogue, 2018). As advances are made at the intersection of engineering, materials science, and medicine, the in vivo performance of NPs for surgical tumor detection will continue to improve, and the benefits of NPs will be realized. For example, recent advancements in deep learning with improvements in neural networks have made substantial impacts in cancer diagnosis, proving to be a useful tool in digital pathology (Bera, Schalper, Rimm, Velcheti, & Madabhushi, 2019), radiology (Hosny, Parmar, Quackenbush, Schwartz, & Aerts, 2018), and the classification of skin lesions (Esteva et al., 2017). This technology can be applied to surgical image guidance as a tool to assist the surgeon in distinguishing between malignant and healthy tissue, increasing the likelihood of achieving NSMs. Additionally, computational methods leveraged for the development and optimization of NPs, improving their performance (Yamankurt et al., 2019). Given opportunities and the advancements highlighted by this review, we envision that NPs will become fundamental in the ultimate goal of reducing the incidence of PSMs in surgical oncology.
ACKNOWLEDGMENTS
We thank Madeline T. Olson for productive assistance in designing figures. We also thank Denis Svechkarev for editorial assistance. This work was supported in part by the National Institutes of Health grants [R01EB019449 and R21CA212500] and the Nebraska Research Initiative. NEW was funded by the University of Nebraska Medical Center Graduate Studies Fellowship and the John Borrlson Memorial Scholarship.
Funding information
National Cancer Institute, Grant/Award Number: R21CA212500; National Institute of Biomedical Imaging and Bioengineering, Grant/Award Number: R01EB019449; Nebraska Research Initiative
Footnotes
RELATED WIRES ARTICLE
Image-guided tumor surgery: will there be a role for fluorescent nanoparticles?
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
REFERENCES
- Agarwal V, Bajpai M, & Sharma A. (2018). Patented and approval scenario of Nanopharmaceuticals with relevancy to biomedical application, manufacturing procedure and safety aspects. Recent Patents on Drug Delivery & Formulation, 12(1), 40–52. 10.2174/1872211312666180105114644 [DOI] [PubMed] [Google Scholar]
- Agdeppa ED, & Spilker ME (2009). A review of imaging agent development. The AAPS Journal, 11(2), 286–299. 10.1208/s12248-009-9104-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai T, Shang W, Yan H, Zeng C, Wang K, Gao Y, … Tian J. (2018). Near infrared-emitting persistent luminescent nanoparticles for hepatocellular carcinoma imaging and luminescence-guided surgery. Biomaterials, 167, 216–225. 10.1016/j.biomaterials.2018.01.031 [DOI] [PubMed] [Google Scholar]
- Alam IS, Steinberg I, Vermesh O, van den Berg NS, Rosenthal EL, van Dam GM, … Rogalla S. (2018). Emerging intraoperative imaging modalities to improve surgical precision. Molecular Imaging and Biology, 20(5), 705–715. 10.1007/s11307-0181227-6 [DOI] [PubMed] [Google Scholar]
- Alander JT, Kaartinen I, Laakso A, Pätilä T, Spillmann T, Tuchin VV, … Välisuo P. (2012). A review of Indocyanine green fluorescent imaging in surgery. International Journal of Biomedical Imaging, 2012, 1–26. 10.1155/2012/940585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou C, Neuschmelting V, Tschaharganeh DF, Huang CH, Oseledchyk A, Iacono P, … Kircher MF (2016). Imaging of liver tumors using surface-enhanced Raman scattering nanoparticles. ACS Nano, 10(5), 5015–5026. 10.1021/acsnano.5b07200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard P. (2011). Biomedical photoacoustic imaging. Interface Focus, 1(4), 602–631. 10.1098/rsfs.2011.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bera K, Schalper KA, Rimm DL, Velcheti V, & Madabhushi A. (2019). Artificial intelligence in digital pathology — new tools for diagnosis and precision oncology. Nature Reviews Clinical Oncology, 16(11), 703–715. 10.1038/s41571-019-0252-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhoft RA (2013). Cadmium toxicity and treatment. The Scientific World Journal, 2013, 394652. 10.1155/2013/394652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beziere N, Lozano N, Nunes A, Salichs J, Queiros D, Kostarelos K, & Ntziachristos V. (2015). Dynamic imaging of PEGylated indocyanine green (ICG) liposomes within the tumor microenvironment using multi-spectral optoacoustic tomography (MSOT). Biomaterials, 37, 415–424. 10.1016/j.biomaterials.2014.10.014 [DOI] [PubMed] [Google Scholar]
- Blau R, Epshtein Y, Pisarevsky E, Tiram G, Israeli Dangoor S, Yeini E, … Satchi-Fainaro R. (2018). Image-guided surgery using near-infrared turn-ON fluorescent nanoprobes for precise detection of tumor margins. Theranostics, 8(13), 3437–3460. 10.7150/thno.23853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury MS, Phillips E, Montero PH, Cheal SM, Stambuk H, Durack JC, … Patel S. (2013). Clinically-translated silica nanoparticles as dual-modality cancer-targeted probes for image-guided surgery and interventions. Integrative Biology, 5(1), 74–86. 10.1039/c2ib20174g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer OR, Buckle T, Vermeeren L, Klop WMC, Balm AJM, van der Poel HG, … Valdes Olmos RA (2012). Comparing the hybrid fluorescent-radioactive tracer Indocyanine green-99mTc-Nanocolloid with 99mTc-Nanocolloid for sentinel node identification: A validation study using Lymphoscintigraphy and SPECT/CT. Journal of Nuclear Medicine, 53(7), 1034–1040. 10.2967/jnumed.112.103127 [DOI] [PubMed] [Google Scholar]
- Brouwer OR, Klop WMC, Buckle T, Vermeeren L, van den Brekel MWM, Balm AJM, … van Leeuwen FWB (2012). Feasibility of sentinel node biopsy in head and neck melanoma using a hybrid radioactive and fluorescent tracer. Annals of Surgical Oncology, 19(6), 1988–1994. 10.1245/s10434-011-2180-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu L, Shen B, & Cheng Z. (2014). Fluorescent imaging of cancerous tissues for targeted surgery. Advanced Drug Delivery Reviews, 76(1), 21–38. 10.1016/j.addr.2014.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckle T, Brouwer OR, Valdes Olmos RA, van der Poel HG, & van Leeuwen FWB (2012). Relationship between Intraprostatic tracer deposits and sentinel lymph node mapping in prostate Cancer patients. Journal of Nuclear Medicine, 53(7), 1026–1033. 10.2967/jnumed.111.098517 [DOI] [PubMed] [Google Scholar]
- Cabon Q, Sayag D, Texier I, Navarro F, Boisgard R, Virieux-Watrelot D, … Carozzo C. (2016). Evaluation of intraoperative fluorescence imaging-guided surgery in cancer-bearing dogs: A prospective proof-of-concept phase II study in 9 cases. Translational Research: The Journal of Laboratory and Clinical Medicine, 170, 73–88. 10.1016/j.trsl.2015.12.001 [DOI] [PubMed] [Google Scholar]
- Cai Y, Wei Z, Song C, Tang C, Han W, & Dong X. (2019). Optical nano-agents in the second near-infrared window for biomedical applications. Chemical Society Reviews, 48(1), 22–37. 10.1039/C8CS00494C [DOI] [PubMed] [Google Scholar]
- Ceppi L, Bardhan NM, Na Y, Siegel A, Rajan N, Fruscio R, … Birrer MJ (2019). Real-time single-walled carbon nanotube-based fluorescence imaging improves survival after Debulking surgery in an ovarian Cancer model. ACS Nano, 13(5), 5356–5365. 10.1021/acsnano.8b09829 [DOI] [PubMed] [Google Scholar]
- Chaffer CL, & Weinberg RA (2011). A perspective on cancer cell metastasis. Science (New York, N.Y.), 331(6024), 1559–1564. 10.1126/science.1203543 [DOI] [PubMed] [Google Scholar]
- Chen H, Zhang W, Zhu G, Xie J, & Chen X. (2017). Rethinking cancer nanotheranostics. Nature Reviews Materials, 2(7), 17024. 10.1038/natrevmats.2017.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Al Zaki A, Hui JZ, Muzykantov VR, & Tsourkas A. (2012). Multifunctional nanoparticles: Cost versus benefit of adding targeting and imaging capabilities. Science, 338(6109), 903–910. 10.1126/science.1226338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappa C, Rovera F, Corben AD, Fachinetti A, De Berardinis V, Marchionini V, … Dionigi R. (2013). Surgical margins in breast conservation. International Journal of Surgery, 11(S1), S69–S72. 10.1016/S1743-9191(13)60021-7 [DOI] [PubMed] [Google Scholar]
- Christensen A, Juhl K, Persson M, Charabi BW, Mortensen J, Kiss K, … Kjær A. (2017). uPAR-targeted optical near-infrared (NIR) fluorescence imaging and PET for image-guided surgery in head and neck cancer: Proof-of-concept in orthotopic xenograft model. Oncotarget, 8(9), 15407–15419. 10.18632/oncotarget.14282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero E. (2018). In-vivo Raman spectroscopy: From basics to applications. Journal of Biomedical Optics, 23(07), 1. 10.1117/1.JBO.23.7.071210 [DOI] [PubMed] [Google Scholar]
- Corot C, Robert P, Idée JM, & Port M. (2006, December 1). Recent advances in iron oxide nanocrystal technology for medical imaging. Advanced Drug Delivery Reviews, 58, 1471–1504. 10.1016/j.addr.2006.09.013 [DOI] [PubMed] [Google Scholar]
- Danhier F. (2016). To exploit the tumor micro environment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? Journal of Controlled Release, 244, 108–121. 10.1016/j.jconrel.2016.11.015 [DOI] [PubMed] [Google Scholar]
- Davis R, Campbell J, Burkitt S, Qiu Z, Kang S, Mehraein M, … Zavaleta C. (2018). A Raman imaging approach using CD47 antibody-Labeled SERS nanoparticles for identifying breast Cancer and its potential to guide surgical resection. Nanomaterials, 8(11), 953. 10.3390/nano8110953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RM, Kiss B, Trivedi DR, Metzner TJ, Liao JC, & Gambhir SS (2018). Surface-enhanced Raman scattering nanoparticles for multiplexed imaging of bladder Cancer tissue permeability and molecular phenotype. ACS Nano, 12, 9669–9679. 10.1021/acsnano.8b03217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding F, Li C, Xu Y, Li J, Li H, Yang G, & Sun Y. (2018). PEGylation regulates self-assembled small-molecule dye–based probes from single molecule to nanoparticle size for multifunctional NIR-II bioimaging. Advanced Healthcare Materials, 7(23), 1800973. 10.1002/adhm.201800973 [DOI] [PubMed] [Google Scholar]
- Ding F, Zhan Y, Lu X, & Sun Y. (2018). Recent advances in near-infrared II fluorophores for multifunctional biomedical imaging. Chemical Science, 9(19), 4370–4380. 10.1039/C8SC01153B [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogra VS, Chinni BK, Valluru KS, Moalem J, Giampoli EJ, Evans K, & Rao NA (2014). Preliminary results of ex vivo multi-spectral Photoacoustic imaging in the Management of Thyroid Cancer. American Journal of Roentgenology, 202(6), W552–W558. 10.2214/AJR.13.11433 [DOI] [PubMed] [Google Scholar]
- Dolmans DEJGJ, Fukumura D, & Jain RK (2003). Photodynamic therapy for cancer. Nature Reviews Cancer, 3(5), 380–387. 10.1038/nrc1071 [DOI] [PubMed] [Google Scholar]
- DSouza AV, Lin H, Henderson ER, Samkoe KS, & Pogue BW (2016). Review of fluorescence guided surgery systems: Identification of key performance capabilities beyond indocyanine green imaging. Journal of Biomedical Optics, 21(8), 080901. 10.1117/1.JBO.21.8.080901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Lane LA, & Nie S. (2015). Stimuli-responsive nanoparticles for targeting the tumor microenvironment. Journal of Controlled Release, 219, 205–214. 10.1016/j.jconrel.2015.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardt K, Stiebing C, Matthäus C, Schmitt M, & Popp J. (2015). Advantages and limitations of Raman spectroscopy for molecular diagnostics: An update. Expert Review of Molecular Diagnostics, 15(6), 773–787. 10.1586/14737159.2015.1036744 [DOI] [PubMed] [Google Scholar]
- Ehlerding EB, Grodzinski P, Cai W, & Liu CH (2018). Big potential from small agents: Nanoparticles for imaging-based companion diagnostics. ACS Nano, 12(3), 2106–2121. 10.1021/acsnano.7b07252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteva A, Kuprel B, Novoa RA, Ko J, Swetter SM, Blau HM, & Thrun S. (2017). Dermatologist-level classification of skin cancer with deep neural networks. Nature, 542(7639), 115–118. 10.1038/nature21056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z, Zhou S, Garcia C, Fan L, & Zhou J. (2017). PH-responsive fluorescent graphene quantum dots for fluorescence-guided cancer surgery and diagnosis. Nanoscale, 9(15), 4928–4933. 10.1039/c7nr00888k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R, Pusztai L, & Swanton C. (2013). Cancer heterogeneity: Implications for targeted therapeutics. British Journal of Cancer, 108(3), 479–485. 10.1038/bjc.2012.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga S, Brand~ao A, Soares ME, Morais T, Duarte JA, Pereira L, … Carmo H. (2014). Short- and long-term distribution and toxicity of gold nanoparticles in the rat after a single-dose intravenous administration. Nanomedicine: Nanotechnology, Biology, and Medicine, 10 (8), 1757–1766. 10.1016/j.nano.2014.06.005 [DOI] [PubMed] [Google Scholar]
- Frangioni JV (2008). New technologies for human cancer imaging. Journal of Clinical Oncology, 26(24), 4012–4021. 10.1200/JCO.2007.14.3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Bao P, Dai S, Liu R, Ji S, Zeng S, … Ding D. (2019). Far-red/near-infrared emissive (1,3-dimethyl)barbituric acid-based AIEgens for high-contrast detection of metastatic Tumors in the lung. Chemistry, An Asian Journal, 14(6), 871–876. 10.1002/asia.201801660 [DOI] [PubMed] [Google Scholar]
- Gao N, Bozeman EN, Qian W, Wang L, Chen H, Lipowska M, … Yang L. (2017). Tumor penetrating Theranostic nanoparticles for enhancement of targeted and image-guided drug delivery into peritoneal Tumors following Intraperitoneal delivery. Theranostics, 7(6), 1689–1704. 10.7150/thno.18125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garai E, Sensarn S, Zavaleta CL, Van de Sompel D, Loewke NO, Mandella MJ, … Contag CH (2013). High-sensitivity, real-time, ratiometric imaging of surface-enhanced Raman scattering nanoparticles with a clinically translatable Raman endoscope device. Journal of Biomedical Optics, 18(9), 096008. 10.1117/1.JBO.18.9.096008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golombek SK, May JN, Theek B, Appold L, Drude N, Kiessling F, & Lammers T. (2018). Tumor targeting via EPR: Strategies to enhance patient responses. Advanced Drug Delivery Reviews, 130, 17–38. 10.1016/j.addr.2018.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravier J, Navarro FP, Delmas T, Mittler F, Couffin A-C, Vinet F, & Texier I. (2011). Lipidots: Competitive organic alternative to quantum dots for in vivo fluorescence imaging. Journal of Biomedical Optics, 16(9), 096013. 10.1117/1.3625405 [DOI] [PubMed] [Google Scholar]
- Gu X, Zhang X, Ma H, Jia S, Zhang P, Zhao Y, … Tang BZ (2018). Corannulene-incorporated AIE Nanodots with highly suppressed Nonradiative decay for boosted Cancer Phototheranostics in vivo. Advanced Materials, 30(26), 1801065. 10.1002/adma.201801065 [DOI] [PubMed] [Google Scholar]
- Hadjipanayis CG, & Stummer W. (2019). 5-ALA and FDA approval for glioma surgery. Journal of Neuro-Oncology, 141(3), 479–486. 10.1007/s11060-019-03098-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjipanayis CG, Widhalm G, & Stummer W. (2015). What is the surgical benefit of utilizing 5-Aminolevulinic acid for fluorescence-guided surgery of malignant Gliomas? Neurosurgery, 77(5), 663–673. 10.1227/NEU.0000000000000929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Xu K, Taratula O, & Farsad K. (2019). Applications of nanoparticles in biomedical imaging. Nanoscale, 11, 799–819. 10.1039/c8nr07769j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, & Weinberg RA (2011). Hallmarks of cancer: The next generation. Cell, 144(5), 646–674. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Haque R, Contreras R, McNicoll MP, Eckberg EC, & Petitti DB (2006). Surgical margins and survival after head and neck cancer surgery. BMC Ear, Nose and Throat Disorders, 6, 1–6. 10.1186/1472-6815-6-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare JI, Lammers T, Ashford MB, Puri S, Storm G, & Barry ST (2017). Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Advanced Drug Delivery Reviews, 108, 25–38. 10.1016/j.addr.2016.04.025 [DOI] [PubMed] [Google Scholar]
- Harmsen S, Huang R, Wall MA, Karabeber H, Samii JM, Spaliviero M, … Kircher MF (2015). Surface-enhanced resonance Raman scattering nanostars for high-precision cancer imaging. Science Translational Medicine, 7(271), 271ra7. 10.1126/scitranslmed.3010633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen S, Rogalla S, Huang R, Spaliviero M, Neuschmelting V, Hayakawa Y, … Kircher MF (2019). Detection of premalignant gastrointestinal lesions using surface-enhanced resonance Raman scattering-nanoparticle endoscopy. ACS Nano, 13(2), 1354–1364. 10.1021/acsnano.8b06808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekman MCH, Rijpkema M, Bos DL, Oosterwijk E, Goldenberg DM, Mulders PFA, & Boerman OC (2017). Detection of micrometastases using SPECT/fluorescence dual-modality imaging in a CEA-expressing tumor model. Journal of Nuclear Medicine, 58 (5), 706–710. 10.2967/jnumed.116.185470 [DOI] [PubMed] [Google Scholar]
- Hernandez I, Bott SW, Patel AS, Wolf CG, Hospodar AR, Sampathkumar S, & Shrank WH (2018). Pricing of monoclonal antibody therapies: Higher if used for Cancer? American Journal of Managed Care, 24(2), 109–112. [PubMed] [Google Scholar]
- Hernot S, van Manen L, Debie P, Mieog JSD, & Vahrmeijer AL (2019). Latest developments in molecular tracers for fluorescence image-guided cancer surgery. The Lancet Oncology, 20(7), e354–e367. 10.1016/S1470-2045(19)30317-1 [DOI] [PubMed] [Google Scholar]
- Hill TK, Abdulahad A, Kelkar SS, Marini FC, Long TE, Provenzale JM, & Mohs AM (2015). Indocyanine green-loaded nanoparticles for image-guided tumor surgery. Bioconjugate Chemistry, 26(2), 294–303. 10.1021/bc5005679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill TK, Kelkar SS, Wojtynek NE, Souchek JJ, Payne WM, Stumpf K, … Mohs AM (2016). Near infrared fluorescent nanoparticles derived from hyaluronic acid improve tumor contrast for image-guided surgery. Theranostics, 6(13), 2314–2328. 10.7150/thno.16514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill TK, & Mohs AM (2016). Image-guided tumor surgery: Will there be a role for fluorescent nanoparticles? WIREs: Nanomedicine and Nanobiotechnology, 8(4), 498–511. 10.1002/wnan.1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong G, Antaris AL, & Dai H. (2017). Near-infrared fluorophores for biomedical imaging. Nature Biomedical Engineering, 1(1), 0010. 10.1038/s41551-016-0010 [DOI] [Google Scholar]
- Hong Y, Lam JWY, & Tang BZ (2011). Aggregation-induced emission. Chemical Society Reviews, 40(11), 5361–5388. 10.1039/c1cs15113d [DOI] [PubMed] [Google Scholar]
- Hosny A, Parmar C, Quackenbush J, Schwartz LH, & Aerts HJWL (2018). Artificial intelligence in radiology. Nature Reviews Cancer, 18(8), 500–510. 10.1038/s41568-018-0016-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houssami N, Macaskill P, Luke Marinovich M, & Morrow M. (2014). The association of surgical margins and local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy: A meta-analysis. Annals of Surgical Oncology, 21(3), 717–730. 10.1245/s10434-014-3480-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Fang C, Li B, Zhang Z, Cao C, Cai M, … Tian J. (2019). First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows. Nature Biomedical Engineering. 10.1038/s41551-019-0494-0 [DOI] [PubMed] [Google Scholar]
- Huang R, Harmsen S, Samii JM, Karabeber H, Pitter KL, Holland EC, & Kircher MF (2016). High precision imaging of microscopic spread of Glioblastoma with a targeted ultrasensitive SERRS molecular imaging probe. Theranostics, 6(8), 1075–1084. 10.7150/thno.13842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, El-Sayed IH, Qian W, & El-Sayed MA (2006). Cancer cell imaging and Photothermal therapy in the near-infrared region by using gold Nanorods. Journal of the American Chemical Society, 128(6), 2115–2120. 10.1021/ja057254a [DOI] [PubMed] [Google Scholar]
- Ioannidis JPA, Kim BYS, & Trounson A. (2018). How to design preclinical studies in nanomedicine and cell therapy to maximize the prospects of clinical translation. Nature Biomedical Engineering, 2(11), 797–809. 10.1038/s41551-018-0314-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquart A, Kéramidas M, Vollaire J, Boisgard R, Pottier G, Rustique E, … Texier I. (2013). LipImage™ 815: Novel dye-loaded lipid nanoparticles for long-term and sensitive in vivo near-infrared fluorescence imaging. Journal of Biomedical Optics, 18(10), 101311. 10.1117/1.jbo.18.10.101311 [DOI] [PubMed] [Google Scholar]
- Jiao M, Zhang P, Meng J, Li Y, Liu C, Luo X, & Gao M. (2018). Recent advancements in biocompatible inorganic nanoparticles towards biomedical applications. Biomaterials Science, 6(4), 726–745. 10.1039/C7BM01020F [DOI] [PubMed] [Google Scholar]
- Jin Y, Li Y, Yang X, & Tian J. (2019). Neuroblastoma-targeting triangular gadolinium oxide nanoplates for precise excision of cancer. Acta Biomaterialia, 87, 223–234. 10.1016/j.actbio.2019.01.042 [DOI] [PubMed] [Google Scholar]
- Jorns JM, Visscher D, Sabel M, Breslin T, Healy P, Daignaut S, … Wu AJ (2012). Intraoperative frozen section analysis of margins in breast conserving surgery significantly decreases reoperative rates: One-year experience at an ambulatory surgical center. American Journal of Clinical Pathology, 138(5), 657–669. 10.1309/AJCP4IEMXCJ1GDTS [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, & Farokhzad OC (2012). Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chemical Society Reviews, 41(7), 2971–3010. 10.1039/c2cs15344k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Wang Y, Reder NP, & Liu JTC (2016). Multiplexed molecular imaging of biomarker-targeted SERS nanoparticles on fresh tissue specimens with channel-compressed spectrometry. PLoS One, 11(9), e0163473. 10.1371/journal.pone.0163473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabeber H, Huang R, Iacono P, Samii JM, Pitter K, Holland EC, & Kircher MF (2014). Guiding brain tumor resection using surface-enhanced Raman scattering nanoparticles and a hand-held Raman scanner. ACS Nano, 8(10), 9755–9766. 10.1021/nn503948b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelkar SS, Hill TK, Marini FC, & Mohs AM (2016). Near infrared fluorescent nanoparticles based on hyaluronic acid: Self-assembly, optical properties, and cell interaction. Acta Biomaterialia, 36, 112–121. 10.1016/j.actbio.2016.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy GT, Okusanya OT, Keating JJ, Heitjan DF, Deshpande C, Litzky LA, … Singhal S. (2015). The optical biopsy: A novel technique for rapid intraoperative diagnosis of primary pulmonary adenocarcinomas. Annals of Surgery, 262(4), 602–609. 10.1097/SLA.0000000000001452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Song KH, Gao F, & Wang LV (2010). Sentinel lymph nodes and lymphatic vessels: Noninvasive dual-modality in vivo mapping by using indocyanine green in rats—Volumetric spectroscopic photoacoustic imaging and planar fluorescence imaging. Radiology, 255 (2), 442–450. 10.1148/radiol.10090281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Jeong S, Jung KO, Song MG, Lee C-H, Chung S, … Lee DS (2017). Simultaneous detection of EGFR and VEGF in colorectal cancer using fluorescence-Raman endoscopy. Scientific Reports, 7(1), 1035. 10.1038/s41598-017-01020-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher MF (2017). How can we apply the use of surface-enhanced Raman scattering nanoparticles in tumor imaging? Nanomedicine, 12 (3), 171–174. 10.2217/nnm-2016-0385 [DOI] [PubMed] [Google Scholar]
- Klein K, Stolk P, De Bruin ML, Leufkens HGM, Crommelin DJA, & De Vlieger JSB (2019). The EU regulatory landscape of non-biological complex drugs (NBCDs) follow-on products: Observations and recommendations. European Journal of Pharmaceutical Sciences, 133(March), 228–235. 10.1016/j.ejps.2019.03.029 [DOI] [PubMed] [Google Scholar]
- Koblinski JE, Ahram M, & Sloane BF (2000). Unraveling the role of proteases in cancer. Clinica Chimica Acta, 291(2), 113–135. 10.1016/S0009-8981(99)00224-7 [DOI] [PubMed] [Google Scholar]
- Koller M, Qiu S-Q, Linssen MD, Jansen L, Kelder W, de Vries J, … van Dam GM (2018). Implementation and bench marking of a novel analytical framework to clinically evaluate tumor-specific fluorescent tracers. Nature Communications, 9(1), 3739. 10.1038/s41467-018-05727-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku G, Wang X, Xie X, Stoica G, & Wang LV (2005). Imaging of tumor angiogenesis in rat brains in vivo by photoacoustic tomography. Applied Optics, 44(5), 770–775. 10.1364/AO.44.000770 [DOI] [PubMed] [Google Scholar]
- Landesman-Milo D, & Peer D. (2016). Transforming Nanomedicines from lab scale production to novel clinical modality. Bioconjugate Chemistry, 27(4), 855–862. 10.1021/acs.bioconjchem.5b00607 [DOI] [PubMed] [Google Scholar]
- Lane LA, Qian X, & Nie S. (2015). SERS nanoparticles in medicine: From label-free detection to spectroscopic tagging. Chemical Reviews, 115(19), 10489–10529. 10.1021/acs.chemrev.5b00265 [DOI] [PubMed] [Google Scholar]
- Lao Y, Xing D, Yang S, & Xiang L. (2008). Noninvasive photoacoustic imaging of the developing vasculature during early tumor growth. Physics in Medicine and Biology, 53(15), 4203–4212. 10.1088/0031-9155/53/15/013 [DOI] [PubMed] [Google Scholar]
- Laufer J, Johnson P, Zhang E, Treeby B, Cox B, Pedley B, & Beard P. (2012). In vivo preclinical photoacoustic imaging of tumor vasculature development and therapy. Journal of Biomedical Optics, 17(5), 056016. 10.1117/1.JBO.17.5.056016 [DOI] [PubMed] [Google Scholar]
- Leary J, & Key J. (2014). Nanoparticles for multimodal in vivo imaging in nanomedicine. International Journal of Nanomedicine, 9, 711–726. 10.2147/IJN.S53717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lécuyer T, Teston E, Ramirez-Garcia G, Maldiney T, Viana B, Seguin J, … Richard C. (2016). Chemically engineered persistent luminescence nanoprobes for bioimaging. Theranostics, 6(13), 2488–2523. 10.7150/thno.16589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Kim GR, Yoon J, Kim SE, Yoo JS, & Piao Y. (2018). In vivo delineation of glioblastoma by targeting tumor-associated macrophages with near-infrared fluorescent silica coated iron oxide nanoparticles in orthotopic xenografts for surgical guidance. Scientific Reports, 8(1), 11122. 10.1038/s41598-018-29424-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Cao L, Zhang Y, Yi P, Wang M, Tan B, … Wang Q. (2015). Preoperative detection and intraoperative visualization of brain Tumors for more precise surgery: A new dual-modality MRI and NIR Nanoprobe. Small, 11(35), 4517–4525. 10.1002/smll.201500997 [DOI] [PubMed] [Google Scholar]
- Li D, He S, Wu Y, Liu J, Liu Q, Chang B, … Cheng Z. (2019). Excretable lanthanide nanoparticle for biomedical imaging and surgical navigation in the second near-infrared window. Advanced Science, 6, 1902042. 10.1002/advs.201902042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Zhang J, Chi C, Xiao X, Wang J, Lang L, … Chen X. (2018). First-in-human study of PET and optical dual-modality image-guided surgery in glioblastoma using 68 Ga-IRDye800CW-BBN. Theranostics, 8(9), 2508–2520. 10.7150/thno.25599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Ji Z, Chang CH, Dunphy DR, Cai X, Meng H, … Xia T. (2014). Surface interactions with compartmentalized cellular phosphates explain rare earth oxide nanoparticle hazard and provide opportunities for safer design. ACS Nano, 8(2), 1771–1783. 10.1021/nn406166n [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Schumann C, Albarqi HA, Lee CJ, Alani AWG, Bracha S, … Taratula O. (2018). A tumor-activatable theranostic nanomedicine platform for NIR fluorescence-guided surgery and combinatorial phototherapy. Theranostics, 8(3), 767–784. 10.7150/th21209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Zeng X, Xiao Y, Tang L, Nong J, Liu Y, … Hong X. (2019). Novel near-infrared II aggregation-induced emission dots for: in vivo bioimaging. Chemical Science, 10(4), 1219–1226. 10.1039/c8sc04363a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lécuyer T, Seguin J, Mignet N, Scherman D, Viana B, & Richard C. (2018). Imaging and therapeutic applications of persistent luminescence nanomaterials. Advanced Drug Delivery Reviews, 138, 193–210. 10.1016/j.addr.2018.10.015 [DOI] [PubMed] [Google Scholar]
- Liu J, Chen C, Ji S, Liu Q, Ding D, Zhao D, & Liu B. (2017). Long wavelength excitable near-infrared fluorescent nanoparticles with aggregation-induced emission characteristics for image-guided tumor resection. Chemical Science, 8(4), 2782–2789. 10.1039/c6sc04384d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie A. (2010). Multimodality imaging probes: Design and challenges. Chemical Reviews, 110(5), 3146–3195. 10.1021/cr9003538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell JF, Jin CS, Huynh E, Jin H, Kim C, Rubinstein JL, … Zheng G. (2011). Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nature Materials, 10(4), 324–332. 10.1038/nmat2986 [DOI] [PubMed] [Google Scholar]
- Lu F-M, & Yuan Z. (2015). PET/SPECT molecular imaging in clinical neuroscience: Recent advances in the investigation of CNS diseases. Quantitative Imaging in Medicine and Surgery, 5(3), 433–447. 10.3978/j.issn.2223-4292.2015.03.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Wei J-Y, Yao D-S, Pan Z-M, & Yao Y. (2017). Application of carbon nanoparticles in laparoscopic sentinel lymph node detection in patients with early-stage cervical cancer. PLoS One, 12(9), e0183834. 10.1371/journal.pone.0183834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H. (2015). Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Advanced Drug Delivery Reviews, 91, 3–6. 10.1016/j.addr.2015.01.002 [DOI] [PubMed] [Google Scholar]
- Maeda H, & Khatami M. (2018). Analyses of repeated failures in cancer therapy for solid tumors: Poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clinical and Translational Medicine, 7(1), 11. 10.1186/s40169-018-0185-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H, Nakamura H, & Fang J. (2013). The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Advanced Drug Delivery Reviews, 65(1), 71–79. 10.1016/j.addr.2012.10.002 [DOI] [PubMed] [Google Scholar]
- Mahmood U, & Weissleder R. (2003). Near-infrared optical imaging of proteases in cancer. Molecular Cancer Therapeutics, 2(5), 489–496. [PubMed] [Google Scholar]
- Maloney BW, McClatchy DM, Pogue BW, Paulsen KD, Wells WA, & Barth RJ (2018). Review of methods for intraoperative margin detection for breast conserving surgery. Journal of Biomedical Optics, 23(10), 1–19. 10.1117/1.JBO.23.10.100901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui A, Tanaka E, Choi HS, Winer JH, Kianzad V, Gioux S, … Frangioni JV (2010). Real-time intra-operative near-infrared fluorescence identification of the extrahepatic bile ducts using clinically available contrast agents. Surgery, 148(1), 87–95. 10.1016/j.surg.2009.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh KJ, Jing L, Behrens AM, Jayawardena S, Tang W, Gao M, … Jaklenec A. (2018, May 3). Biocompatible semiconductor quantum dots as Cancer imaging agents. Advanced Materials, 30, 1706356. 10.1002/adma.201706356 [DOI] [PubMed] [Google Scholar]
- Mehlen P, & Puisieux A. (2006). Metastasis: A question of life or death. Nature Reviews Cancer, 6(6), 449–458. 10.1038/nrc1886 [DOI] [PubMed] [Google Scholar]
- Mei J, Leung NLC, Kwok RTK, Lam JWY, & Tang BZ (2015). Aggregation-induced emission: Together we Shine, united we soar! Chemical Reviews, 115(21), 11718–11940. 10.1021/acs.chemrev.5b00263 [DOI] [PubMed] [Google Scholar]
- Meric F, Mirza NQ, Vlastos G, Buchholz TA, Kuerer HM, Babiera GV, … Hunt KK (2003). Positive surgical margins and ipsilateral breast tumor recurrence predict disease-specific survival after breast-conserving therapy. Cancer, 97(4), 926–933. 10.1002/cncr.11222 [DOI] [PubMed] [Google Scholar]
- Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, … Jemal A. (2016). Cancer treatment and survivorship statistics, 2016. CA: A Cancer Journal for Clinicians, 66(4), 271–289. 10.3322/caac.21349 [DOI] [PubMed] [Google Scholar]
- Mohs AM, Mancini MC, Singhal S, Provenzale JM, Leyland-Jones B, Wang MD, & Nie S. (2010). Hand-held spectroscopic device for in vivo and intraoperative tumor detection: Contrast enhancement, detection sensitivity, and tissue penetration. Analytical Chemistry, 82(21), 9058–9065. 10.1021/ac102058k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiyadi A, Syed P, & Srivastava S. (2014). Fluorescence-guided surgery of malignant gliomas based on 5-aminolevulinic acid: Paradigm shifts but not a panacea. Nature Reviews Cancer, 14(2), 146. 10.1038/nrc3566-c1 [DOI] [PubMed] [Google Scholar]
- Mondal SB, Gao S, Zhu N, Liang R, Gruev V, & Achilefu S. (2014). Real-time fluorescence image-guided oncologic surgery. Advances in Cancer Research, 124, 171–211. 10.1016/B978-0-12-411638-2.00005-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak TR, Andreou C, Oseledchyk A, Marcus WD, Wong HC, Massagué J, & Kircher MF (2017). Tissue factor-specific ultrabright SERRS nanostars for Raman detection of pulmonary micrometastases. Nanoscale, 9(3), 1110–1119. 10.1039/c6nr08217c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuschmelting V, Harmsen S, Beziere N, Lockau H, Hsu HT, Huang R, … Kircher MF (2018). Dual-modality surface-enhanced resonance Raman scattering and multispectral Optoacoustic tomography nanoparticle approach for brain tumor delineation. Small, 14 (23), e1800740. 10.1002/smll.201800740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni X, Zhang X, Duan X, Zheng H, Xue X, & Ding D. (2019). Near-infrared afterglow luminescent aggregation-induced emission dots with ultrahigh tumor-to-liver signal ratio for promoted image-guided Cancer surgery. Nano Letters, 19(1), 318–330. 10.1021/acs.nanolett.8b03936 [DOI] [PubMed] [Google Scholar]
- Ni X, Zhang X, Duan X, Zheng H-L, Xue X-S, & Ding D. (2018). Near-infrared afterglow luminescent aggregation-induced emission dots with ultrahigh tumor-to-liver signal ratio for promoted image-guided Cancer surgery. Nano Letters, 19(1), 318–330. 10.1021/acs.nanolett.8b03936 [DOI] [PubMed] [Google Scholar]
- Oh E, Liu R, Nel A, Gemill KB, Bilal M, Cohen Y, & Medintz IL (2016). Meta-analysis of cellular toxicity for cadmium-containing quantum dots. Nature Nanotechnology, 11(5), 479–486. 10.1038/nnano.2015.338 [DOI] [PubMed] [Google Scholar]
- Olson MT, Ly QP, & Mohs AM (2019). Fluorescence guidance in surgical oncology: Challenges, opportunities, and translation. Molecular Imaging and Biology, 21(2), 200–218. 10.1007/s11307-018-1239-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson TP, Harter J, Muñoz A, Mahvi DM, & Breslin T. (2007). Frozen section analysis for intraoperative margin assessment during breast-conserving surgery results in low rates of re-excision and local recurrence. Annals of Surgical Oncology, 14(10), 2953–2960. 10.1245/s10434-007-9437-1 [DOI] [PubMed] [Google Scholar]
- Opilik L, Schmid T, & Zenobi R. (2013). Modern Raman imaging: Vibrational spectroscopy on the micrometer and Nanometer scales. Annual Review of Analytical Chemistry, 6(1), 379–398. 10.1146/annurev-anchem-062012-092646 [DOI] [PubMed] [Google Scholar]
- Orosco RK, Tapia VJ, Califano JA, Clary B, Cohen EEW, Kane C, … Nguyen QT (2018). Positive surgical margins in the 10 Most common solid cancers. Scientific Reports, 8(1), 5686. 10.1038/s41598-018-23403-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oseledchyk A, Andreou C, Wall MA, & Kircher MF (2017). Folate-targeted surface-enhanced resonance Raman scattering Nanoprobe Ratiometry for detection of microscopic ovarian Cancer. ACS Nano, 11(2), 1488–1497. 10.1021/acsnano.6b06796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Ray A, Andreou C, Zhou Y, Rakshit T, Wlodarczyk M, … Kircher MF (2019). DNA-enabled rational design of fluorescence-Raman bimodal nanoprobes for cancer imaging and therapy. Nature Communications, 10(1), 1926. 10.1038/s41467-019-09173-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K. (2019). The beginning of the end of the nanomedicine hype. Journal of Controlled Release, 305(June), 221–222. 10.1016/j.jconrel.2019.05.044 [DOI] [PubMed] [Google Scholar]
- Patel P, Kato T, Ujiie H, Wada H, Lee D, Hu H, … Yasufuku K. (2016). Multi-modal imaging in a mouse model of Orthotopic lung Cancer. PLoS One, 11(9), e0161991. 10.1371/journal.pone.0161991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil R, Galstyan A, Sun T, Shatalova ES, Butte P, Mamelak AN, … Holler E. (2019). Polymalic acid chlorotoxin nanoconjugate for near-infrared fluorescence guided resection of glioblastoma multiforme. Biomaterials, 206, 146–159. 10.1016/j.biomaterials.2019.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, … Vauthey JN (2005). Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Annals of Surgery, 241(5), 715–724. 10.1097/01.sla.0000160703.75808.7d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne WM, Hill TK, Svechkarev D, Holmes MB, Sajja BR, & Mohs AM (2017). Multimodal imaging nanoparticles derived from hyaluronic acid for integrated preoperative and intraoperative Cancer imaging. Contrast Media & Molecular Imaging, 2017, 9616791. 10.1155/2017/9616791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips E, Penate-Medina O, Zanzonico PB, Carvajal RD, Mohan P, Ye Y, … Bradbury MS (2014). Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Science Translational Medicine, 6(260), 260ra149. 10.1126/scitranslmed.3009524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue BW (2018). Perspective review of what is needed for molecular-specific fluorescence-guided surgery. Journal of Biomedical Optics, 23(10), 1–9. 10.1117/1.JBO.23.10.100601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue BW, Paulsen KD, Hull SM, Samkoe KS, Gunn J, Hoopes J, … Feldwisch J. (2015). Advancing molecular-guided surgery through probe development and testing in a moderate cost evaluation pipeline. Proceedings of SPIE—The International Society for Optical Engineering, 9311, 931112. 10.1117/12.2083224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi B, Crawford AJ, Wojtynek NE, Holmes MB, Souchek JJ, Almeida-Porada G, … Mohs AM (2018). Indocyanine green loaded hyaluronan-derived nanoparticles for fluorescence-enhanced surgical imaging of pancreatic cancer. Nanomedicine: Nanotechnology, Biology and Medicine, 14(3), 769–780. 10.1016/j.nano.2017.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Chen C, Zhang X, Hu X, Ji S, Kwok RTK, … Tang BZ (2018). Light-driven transformable optical agent with adaptive functions for boosting cancer surgery outcomes. Nature Communications, 9(1), 1–12. 10.1038/s41467-018-04222-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Sun C, Zebibula A, Zhang H, Kwok RTK, Zhao X, … Tang BZ (2018). Real-time and high-resolution bioimaging with bright aggregation-induced emission dots in short-wave infrared region. Advanced Materials, 30(12), 1706856. 10.1002/adma.201706856 [DOI] [PubMed] [Google Scholar]
- Qiu S, Zeng J, Hou Y, Chen L, Ge J, Wen L, … Gao M. (2018). Detection of lymph node metastasis with near-infrared upconversion luminescent nanoprobes. Nanoscale, 10(46), 21772–21781. 10.1039/C8NR05811C [DOI] [PubMed] [Google Scholar]
- Qiu Y, Zhang Y, Li M, Chen G, Fan C, Cui K, … Xiao Z. (2018). Intraoperative detection and eradication of residual microtumors with gap-enhanced Raman tags. ACS Nano, 12(8), 7974–7985. 10.1021/acsnano.8b02681 [DOI] [PubMed] [Google Scholar]
- Rim KT, Koo KH, & Park JS (2013). Toxicological evaluations of rare earths and their health impacts to workers: A literature review. Safety and Health at Work, 4(1), 12–26. 10.5491/SHAW.2013.4.1.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen JE, Chan L, Shieh D-B, & Gu FX (2012). Iron oxide nanoparticles for targeted cancer imaging and diagnostics. Nanomedicine: Nanotechnology, Biology and Medicine, 8(3), 275–290. 10.1016/j.nano.2011.08.017 [DOI] [PubMed] [Google Scholar]
- Rosenthal EL, Warram JM, De Boer E, Chung TK, Korb ML, Brandwein-Gensler M, … Zinn KR (2015). Safety and tumor specificity of cetuximab-IRDye800 for surgical navigation in head and neck cancer. Clinical Cancer Research, 21(16), 3658–3666. 10.1158/1078-0432.CCR-14-3284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu SC, & Hayes AW (2017). Toxicity of nanomaterials found in human environment. Toxicology Research and Application, 1, 1–13. 10.1177/2397847317726352 [DOI] [Google Scholar]
- Sangtani A, Nag OK, Field LD, Breger JC, & Delehanty JB (2017). Multifunctional nanoparticle composites: Progress in the use of soft and hard nanoparticles for drug delivery and imaging. WIREs: Nanomedicine and Nanobiotechnology, 9(6), 1–23. 10.1002/wnan.1466 [DOI] [PubMed] [Google Scholar]
- Sasaki A, Tsukasaki Y, Komatsuzaki A, Sakata T, Yasuda H, & Jin T. (2015). Recombinant protein (EGFP-protein G)-coated PbS quantum dots for in vitro and in vivo dual fluorescence (visible and second-NIR) imaging of breast tumors. Nanoscale, 7(12), 5115–5119. 10.1039/c4nr06480a [DOI] [PubMed] [Google Scholar]
- Satpathy M, Wang L, Zielinski R, Qian W, Lipowska M, Capala J, … Yang L. (2014). Active targeting using HER-2-affibody-conjugated nanoparticles enabled sensitive and specific imaging of orthotopic HER-2 positive ovarian tumors. Small, 10(3), 544–555. 10.1002/smll.201301593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpathy M, Wang L, Zielinski RJ, Qian W, Wang YA, Mohs AM, … Yang L. (2019). Targeted drug delivery and image-guided therapy of heterogeneous ovarian Cancer using HER2-targeted Theranostic nanoparticles. Theranostics, 9(3), 778–795. 10.7150/thno.29964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayag D, Cabon Q, Texier I, Navarro FP, Boisgard R, Virieux-Watrelot D, … Ponce F. (2016). Phase-0/phase-I study of dye-loaded lipid nanoparticles for near-infrared fluorescence imaging in healthy dogs. European Journal of Pharmaceutics and Biopharmaceutics, 100, 85–93. 10.1016/j.ejpb.2016.01.001 [DOI] [PubMed] [Google Scholar]
- Schaafsma BE, Mieog JSD, Hutteman M, Van Der Vorst JR, Kuppen PJK, Löwik CWGM, … Vahrmeijer AL (2011). The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. Journal of Surgical Oncology, 104(3), 323–332. 10.1002/jso.21943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakiba M, Ng KK, Huynh E, Chan H, Charron DM, Chen J, … Zheng G. (2016). Stable J-aggregation enabled dual photoacoustic and fluorescence nanoparticles for intraoperative cancer imaging. Nanoscale, 8(25), 12618–12625. 10.1039/c5nr08165c [DOI] [PubMed] [Google Scholar]
- Shanmugam V, Selvakumar S, & Yeh C-S (2014). Near-infrared light-responsive nanomaterials in cancer therapeutics. Chemical Society Reviews, 43(17), 6254–6287. 10.1039/C4CS00011K [DOI] [PubMed] [Google Scholar]
- Shaughnessy AF (2012). Monoclonal antibodies: Magic bullets with a hefty price tag. BMJ, 345, e8346. 10.1136/bmj.e8346 [DOI] [PubMed] [Google Scholar]
- Shi J, Sun X, Li J, Man H, Shen J, Yu Y, & Zhang H. (2015). Multifunctional near infrared-emitting long-persistence luminescent nanoprobes for drug delivery and targeted tumor imaging. Biomaterials, 37, 260–270. 10.1016/j.biomaterials.2014.10.033 [DOI] [PubMed] [Google Scholar]
- Shou K, Tang Y, Chen H, Chen S, Zhang L, Zhang A, … Cheng Z. (2018). Diketopyrrolopyrrole-based semiconducting polymer nanoparticles for in vivo second near-infrared window imaging and image-guided tumor surgery. Chemical Science, 9(12), 3105–3110. 10.1039/C8SC00206A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Pandit S, Mokkapati VRSS, Garg A, Ravikumar V, & Mijakovic I. (2018). Gold nanoparticles in diagnostics and therapeutics for human Cancer. International Journal of Molecular Sciences, 19(7), 1979. 10.3390/ijms19071979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Duan H, Mohs AM, & Nie S. (2008). Bioconjugated quantum dots for in vivo molecular and cellular imaging. Advanced Drug Delivery Reviews, 60(11), 1226–1240. 10.1016/j.addr.2008.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Mancini MC, & Nie S. (2009). Bioimaging: Second window for in vivo imaging. Nature Nanotechnology, 4(11), 710–711. 10.1038/nnano.2009.326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BL, Gadd MA, Lanahan CR, Rai U, Tang R, Rice-Stitt T, … Specht MC (2018). Real-time, intraoperative detection of residual breast cancer in lumpectomy cavity walls using a novel cathepsin-activated fluorescent imaging system. Breast Cancer Research and Treatment, 171(2), 413–420. 10.1007/s10549-018-4845-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Shen H, Yang T, Wang L, Fu H, Chen H, & Zhang Z. (2017). Indocyanine green loaded magnetic carbon nanoparticles for near infrared fluorescence/magnetic resonance dual-modal imaging and Photothermal therapy of tumor. ACS Applied Materials and Interfaces, 9(11), 9484–9495. 10.1021/acsami.7b00490 [DOI] [PubMed] [Google Scholar]
- Souchek JJ, Wojtynek NE, Holmes MB, Dutta S, Qi B, Datta K, … Mohs AM (2018). Optimized hyaluronic acid formulation of near infrared fluorophores for surgical detection of a prostate tumor xenograft. Acta Biomaterialia, 75, 1–28. 10.1016/j.actbio.2018.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaliviero M, Harmsen S, Huang R, Wall MA, Andreou C, Eastham JA, … Kircher MF (2016). Detection of lymph node metastases with SERRS nanoparticles. Molecular Imaging and Biology, 18(5), 677–685. 10.1007/s11307-016-0932-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, & Parak WJ (2010). Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 368(1915), 1333–1383. 10.1098/rsta.2009.0273 [DOI] [PubMed] [Google Scholar]
- Steinberg I, Huland DM, Vermesh O, Frostig HE, Tummers WS, & Gambhir SS (2019). Photoacoustic clinical imaging. Photoacoustics, 14(September 2018), 77–98. 10.1016/j.pacs.2019.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, & Reulen HJ (2006). Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncology, 7(5), 392–401. 10.1016/S1470-2045(06)70665-9 [DOI] [PubMed] [Google Scholar]
- Suganami A, Iwadate Y, Shibata S, Yamashita M, Tanaka T, Shinozaki N, … Tamura Y. (2015). Liposomally formulated phospholipid-conjugated indocyanine green for intra-operative brain tumor detection and resection. International Journal of Pharmaceutics, 496(2), 401–406. 10.1016/j.ijpharm.2015.10.001 [DOI] [PubMed] [Google Scholar]
- Sukhanova A, Bozrova S, Sokolov P, Berestovoy M, Karaulov A, & Nabiev I. (2018). Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Research Letters, 13, 44. 10.1186/s11671-018-2457-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Ding M, Zeng X, Xiao Y, Wu H, Zhou H, … Hong X. (2017). Novel bright-emission small-molecule NIR-II fluorophores for in vivo tumor imaging and image-guided surgery. Chemical Science, 8(5), 3489–3493. 10.1039/C7SC00251C [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svechkarev D, Kyrychenko A, Payne WM, & Mohs AM (2018). Probing the self-assembly dynamics and internal structure of amphiphilic hyaluronic acid conjugates by fluorescence spectroscopy and molecular dynamics simulations. Soft Matter, 14(23), 4762–4771. 10.1039/c8sm00908b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Kanatani S, Tomer R, Sahlgren C, Kronqvist P, Kaczynska D, … Uhlén P. (2018). Publisher correction: Whole-tissue biopsy phenotyping of three-dimensional tumours reveals patterns of cancer heterogeneity. Nature Biomedical Engineering, 2(1), 48–48. 10.1038/s41551-017-0162-1 [DOI] [PubMed] [Google Scholar]
- Tang J, Huang N, Zhang X, Zhou T, Tan Y, Pi J, … Cheng Y. (2017). Aptamer-conjugated PEGylated quantum dots targeting epidermal growth factor receptor variant III for fluorescence imaging of glioma. International Journal of Nanomedicine, 12, 3899–3911. 10.2147/IJN.S133166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Z, Dang X, Huang X, Muzumdar MD, Xu ES, Bardhan NM, … Ghoroghchian PP (2017). Early tumor detection afforded by in vivo imaging of near-infrared II fluorescence. Biomaterials, 134, 202–215. 10.1016/j.biomaterials.2017.04.046 [DOI] [PubMed] [Google Scholar]
- Thakor AS, Jokerst JV, Ghanouni P, Campbell JL, Mittra E, & Gambhir SS (2016). Clinically approved nanoparticle imaging agents. Journal of Nuclear Medicine, 57(12), 1833–1837. 10.2967/jnumed.116.181362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakor AS, Luong R, Paulmurugan R, Lin FI, Kempen P, Zavaleta C, … Gambhir SS (2011). The fate and toxicity of Raman-active silica-gold nanoparticles in mice. Science Translational Medicine, 3(79), 79ra33. 10.1126/scitranslmed.3001963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakor AS, Paulmurugan R, Kempen P, Zavaleta C, Sinclair R, Massoud TF, & Gambhir SS (2011). Oxidative stress mediates the effects of Raman-active gold nanoparticles in human cells. Small, 7(1), 126–136. 10.1002/smll.201001466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thawani JP, Amirshaghaghi A, Yan L, Stein JM, Liu J, & Tsourkas A. (2017). Photoacoustic-guided surgery with Indocyanine green-coated Superparamagnetic Iron oxide nanoparticle clusters. Small, 13(37), 1701300. 10.1002/smll.201701300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorek DLJ, Elias DR, & Tsourkas A. (2009). Comparative analysis of nanoparticle-antibody conjugations: Carbodiimide versus click chemistry. Molecular Imaging, 8(4), 221–229. 10.2310/7290.2009.00021 [DOI] [PubMed] [Google Scholar]
- Tipirneni KE, Warram JM, Moore LS, Prince AC, de Boer E, Jani AH, … Rosenthal E. (2017). Oncologic procedures amenable to fluorescence-guided surgery. Annals of Surgery, 266(1), 36–47. 10.1097/SLA.0000000000002127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchilin V. (2011). Tumor delivery of macromolecular drugs based on the EPR effect. Advanced Drug Delivery Reviews, 63(3), 131–135. 10.1016/j.addr.2010.03.011 [DOI] [PubMed] [Google Scholar]
- Tran S, DeGiovanni P-J, Piel B, & Rai P. (2017). Cancer nanomedicine: A review of recent success in drug delivery. Clinical and Translational Medicine, 6(1), 44. 10.1186/s40169-017-0175-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tringale KR, Pang J, & Nguyen QT (2018). Image-guided surgery in cancer: A strategy to reduce incidence of positive surgical margins. WIREs: Systems Biology and Medicine, 10(3), e1412. 10.1002/wsbm.1412 [DOI] [PubMed] [Google Scholar]
- Tummers WS, Miller SE, Teraphongphom NT, Gomez A, Steinberg I, Huland DM, … Rosenthal EL (2018). Intraoperative pancreatic Cancer detection using tumor-specific multimodality molecular imaging. Annals of Surgical Oncology, 25, 1880–1888. 10.1245/s10434-018-6453-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummers WS, Warram JM, van den Berg NS, Miller SE, Swijnenburg R-J, Vahrmeijer AL, & Rosenthal EL (2018). Recommendations for reporting on emerging optical imaging agents to promote clinical approval. Theranostics, 8(19), 5336–5347. 10.7150/thno.27384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahrmeijer AL, Hutteman M, van der Vorst JR, van de Velde CJH, & Frangioni JV (2013). Image-guided cancer surgery using near-infrared fluorescence. Nature Reviews. Clinical Oncology, 10(9), 507–518. 10.1038/nrclinonc.2013.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Poel HG, Buckle T, Brouwer OR, Valdés Olmos RA, & van Leeuwen FWB (2011). Intraoperative laparoscopic fluorescence guidance to the sentinel lymph node in prostate Cancer patients: Clinical proof of concept of an integrated functional imaging approach using a multimodal tracer. European Urology, 60(4), 826–833. 10.1016/j.eururo.2011.03.024 [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, & Thompson CB (2009). Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science, 324(5930), 1029–1033. 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventola CL (2017). Progress in nanomedicine: Approved and investigational nanodrugs. P & T: A Peer-Reviewed Journal for Formulary Management, 42(12), 742–755. [PMC free article] [PubMed] [Google Scholar]
- Wada H, Hyun H, Bao K, Lee JH, El Fakhri G, Choi Y, & Choi HS (2018). Multivalent mannose-decorated NIR nanoprobes for targeting pan lymph nodes. Chemical Engineering Journal, 340, 51–57. 10.1016/j.cej.2018.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahajuddin, & Arora S. (2012). Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. International Journal of Nanomedicine, 7, 3445. 10.2147/IJN.S30320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MA, Shaffer TM, Harmsen S, Tschaharganeh DF, Huang CH, Lowe SW, … Kircher MF (2017). Chelator-free radio-labeling of SERRS nanoparticles for whole-body PET and intraoperative Raman imaging. Theranostics, 7(12), 3068–3077. 10.7150/thno.18019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Fan W, Zhang Z, Wen Y, Xiong L, & Chen X. (2019). Advanced nanotechnology leading the way to multimodal imaging-guided precision surgical therapy. Advanced Materials, 31(49), e1904329. 10.1002/adma.201904329 [DOI] [PubMed] [Google Scholar]
- Wang H, Li X, Tse BW-C, Yang H, Thorling CA, Liu Y, … Liang X. (2018). Indocyanine green-incorporating nanoparticles for cancer theranostics. Theranostics, 8(5), 1227–1242. 10.7150/thno.22872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ma Q, Hu XX, Liu H, Zheng W, Chen X, … Tan W. (2017). Autofluorescence-free targeted tumor imaging based on luminous nanoparticles with composition-dependent size and persistent luminescence. ACS Nano, 11(8), 8010–8017. 10.1021/acsnano.7b02643 [DOI] [PubMed] [Google Scholar]
- Wang M, & Thanou M. (2010). Targeting nanoparticles to cancer. Pharmacological Research, 62(2), 90–99. 10.1016/j.phrs.2010.03.005 [DOI] [PubMed] [Google Scholar]
- Wang P, Qu Y, Li C, Yin L, Shen C, Chen W, … Fang D. (2015). Bio-functionalized dense-silica nanoparticles for MR/NIRF imaging of CD146 in gastric cancer. International Journal of Nanomedicine, 10, 749–763. 10.2147/IJN.S62837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kang S, Doerksen JD, Glaser AK, & Liu JTC (2016). Surgical guidance via multiplexed molecular imaging of fresh tissues Labeled with SERS-coded nanoparticles. IEEE Journal of Selected Topics in Quantum Electronics, 22(4), 154–164. 10.1109/JSTQE.2015.2507358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang W, Sun P, Cai Y, Xu W, Fan Q, … Han W. (2019). A novel multimodal NIR-II nanoprobe for the detection of metastatic lymph nodes and targeting chemo-photothermal therapy in oral squamous cell carcinoma. Theranostics, 9(2), 391–404. 10.7150/thno.30268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J, Beard PC, & Bohndiek SE (2016). Contrast agents for molecular photoacoustic imaging. Nature Methods, 13(8), 639–650. 10.1038/nmeth.3929 [DOI] [PubMed] [Google Scholar]
- Welsher K, Sherlock SP, & Dai H. (2011). Deep-tissue anatomical imaging of mice using carbon nanotube fluorophores in the second near-infrared window. Proceedings of the National Academy of Sciences of the United States America, 108(22), 8943–8948. 10.1073/pnas.1014501108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley MJ, Cardona DM, Lazarides AL, Spasojevic I, Ferrer JM, Cahill J, … Brigman BE (2016). A mouse-human phase 1 coclinical trial of a protease-activated fluorescent probe for imaging cancer. Science Translational Medicine, 8(320), 320ra4. 10.1126/scitranslmed.aad0293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtynek NE, Olson MT, Bielecki TA, An W, Bhat AM, Band H, … Mohs AM (2019). Nanoparticle formulation of Indocyanine green improves image-guided surgery in a murine model of breast Cancer. Molecular Imaging and Biology. 10.1007/s11307-019-01462-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won Y-H, Cho O, Kim T, Chung D-Y, Kim T, Chung H, … Jang E. (2019). Highly efficient and stable InP/ZnSe/ZnS quantum dot light-emitting diodes. Nature, 575(7784), 634–638. 10.1038/s41586-019-1771-5 [DOI] [PubMed] [Google Scholar]
- Wu W, Yang Y, Yang Y, Yang Y, Zhang K, Guo L, … Feng H. (2019). Molecular engineering of an organic NIR-II fluorophore with aggregation-induced emission characteristics for in vivo imaging. Small, 15, 1805549. 10.1002/smll.201805549 [DOI] [PubMed] [Google Scholar]
- Wyld L, Audisio RA, & Poston GJ (2015). The evolution of cancer surgery and future perspectives. Nature Reviews. Clinical Oncology, 12(2), 115–124. 10.1038/nrclinonc.2014.191 [DOI] [PubMed] [Google Scholar]
- Xu M, & Wang LV (2006). Photoacoustic imaging in biomedicine. Review of Scientific Instruments, 77(4), 041101. 10.1063/1.2195024 [DOI] [Google Scholar]
- Yaghini E, Turner H, Pilling A, Naasani I, & MacRobert AJ (2018). In vivo biodistribution and toxicology studies of cadmium-free indium-based quantum dot nanoparticles in a rat model. Nanomedicine: Nanotechnology, Biology, and Medicine, 14(8), 2644–2655. 10.1016/j.nano.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghini E, Turner HD, Le Marois AM, Suhling K, Naasani I, & MacRobert AJ (2016). In vivo biodistribution studies and ex vivo lymph node imaging using heavy metal-free quantum dots. Biomaterials, 104, 182–191. 10.1016/j.biomaterials.2016.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamankurt G, Berns EJ, Xue A, Lee A, Bagheri N, Mrksich M, & Mirkin CA (2019). Exploration of the nanomedicine-design space with high-throughput screening and machine learning. Nature Biomedical Engineering, 3(4), 318–327. 10.1038/s41551-019-0351-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashuhiro M, & Maeda H. (1986). Tumor-selective delivery of macromolecular drugs via the EPR effect: Background and future prospects. Cancer Research, 46, 6387–6392. 10.1021/bc100070g [DOI] [PubMed] [Google Scholar]
- Yu MK, Park J, & Jon S. (2012). Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics, 2(1), 3–44. 10.7150/thno.3463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavaleta CL, Kircher MF, & Gambhir SS (2011). Raman’s “effect” on molecular imaging. Journal of Nuclear Medicine, 52(12), 1839–1844. 10.2967/jnumed.111.087775 [DOI] [PubMed] [Google Scholar]
- Zeng C, Shang W, Wang K, Chi C, Jia X, Fang C, … Tian J. (2016). Intraoperative identification of liver cancer microfoci using a targeted near-infrared fluorescent probe for imaging-guided surgery. Scientific Reports, 6(1), 21959. 10.1038/srep21959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng S, Swihart MT, Zhang B, Prasad PN, Xu G, & Yong K-T (2016). New generation cadmium-free quantum dots for biophotonics and Nanomedicine. Chemical Reviews, 116(19), 12234–12327. 10.1021/acs.chemrev.6b00290 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Zhou H, Chen H, Zhang X, He S, Ma L, … Cheng Z. (2019). Hierarchically nanostructured hybrid platform for tumor delineation and image-guided surgery via NIR-II fluorescence and PET bimodal imaging. Small, 15(45), e1903382. 10.1002/smll.201903382 [DOI] [PubMed] [Google Scholar]
- Zhang RR, Schroeder AB, Grudzinski JJ, Rosenthal EL, Warram JM, Pinchuk AN, … Weichert JP (2017). Beyond the margins: Real-time detection of cancer using targeted fluorophores. Nature Reviews. Clinical Oncology, 14, 347–364. 10.1038/nrclinonc.2016.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Li B, Wang P, Lu L, Zhang Z, Liu L, … Zhang F. (2018). Supramolecularly engineered NIR-II and Upconversion nanoparticles in vivo assembly and disassembly to improve bioimaging. Advanced Materials, 30(52), 1804982. 10.1002/adma.201804982 [DOI] [PubMed] [Google Scholar]
- Zhao T, Huang G, Li Y, Yang S, Ramezani S, Lin Z, … Gao J. (2017). A transistor-like pH nanoprobe for tumour detection and image-guided surgery. Nature Biomedical Engineering, 1(1), 1–8. 10.1038/s41551-016-0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Muhanna N, De Souza R, Wada H, Chan H, Akens MK, … Jaffray D. (2015). A multimodal nano agent for image-guided cancer surgery. Biomaterials, 67, 160–168. 10.1016/j.biomaterials.2015.07.010 [DOI] [PubMed] [Google Scholar]
- Zhu B, Rasmussen JC, & Sevick-Muraca EM (2014). A matter of collection and detection for intraoperative and noninvasive near-infrared fluorescence molecular imaging: To see or not to see? Medical Physics, 41(2), 022105. 10.1118/1.4862514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C-N, Chen G, Tian Z-Q, Wang W, Zhong W-Q, Li Z, … Pang D-W (2017). Near-infrared fluorescent Ag 2 se-Cetuximab Nanoprobes for targeted imaging and therapy of Cancer. Small, 13(3), 1602309. 10.1002/smll.201602309 [DOI] [PubMed] [Google Scholar]
- Zhu L, Zhou Z, Mao H, & Yang L. (2017). Magnetic nanoparticles for precision oncology: Theranostic magnetic iron oxide nanoparticles for image-guided and targeted cancer therapy. Nanomedicine, 12(1), 73–87. 10.2217/nnm-2016-0316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer C, Weissleder R, Poss K, Bogdanova A, Wright S, & Enochs W. (1995). MR imaging of phagocytosis in experimental Gliomas. Radiology, 197, 533–538. [DOI] [PubMed] [Google Scholar]
- Zou L, Wang H, He B, Zeng L, Tan T, Cao H, … Li Y. (2016). Current approaches of Photothermal therapy in treating Cancer metastasis with Nanotherapeutics. Theranostics, 6(6), 762–772. 10.7150/thno.14988 [DOI] [PMC free article] [PubMed] [Google Scholar]
FURTHER READING
- Wang P, Fan Y, Lu L, Liu L, Fan L, Zhao M, … Zhang F. (2018). NIR-II nanoprobes in-vivo assembly to improve image-guided surgery for metastatic ovarian cancer. Nature Communications, 9(1), 2898. 10.1038/s41467-018-05113-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Wang X, Luo Q, Li Y, Lin X, Fan L, … Liu X. (2019). Fabrication of red blood cell-based multimodal Theranostic probes for second near-infrared window fluorescence imaging-guided tumor surgery and photodynamic therapy. Theranostics, 9(2), 369–380. 10.7150/thno.29817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Shi Y, Zhang S, Huang X, Zhang J, Zhang Y, … Dong X. (2019). Hydrogen peroxide responsive iron-based nanoplatform for multimodal imaging-guided cancer therapy. Small, 15(4), 1803791. 10.1002/smll.201803791 [DOI] [PubMed] [Google Scholar]
- Wang YW, Reder NP, Kang S, Glaser AK, Yang Q, Wall MA, … Liu JTC (2017). Raman-encoded molecular imaging with topically applied SERS nanoparticles for intraoperative guidance of lumpectomy. Cancer Research, 77(16), 4506–4516. 10.1158/0008-5472.CAN-17-0709 [DOI] [PMC free article] [PubMed] [Google Scholar]