Abstract
Objectives
(1) To investigate sex differences in molar wear in known‐age Cayo Santiago rhesus macaques (Macaca mulatta) and, (2) To explore sex differences in body weight and molar eruption timing as factors influencing sex differences in molar wear.
Materials and methods
Data set I comprises wear scores, ages and body weights of 212 living monkeys included in the 1985 roundup. Data set II consists of molar wear measurements taken on 2D images of 103 of these monkeys' dental remains. Ordinal logistic regression was used to analyze the first data set. General linear models were used to analyze the second.
Results
Males generally exhibited more wear than females at equivalent chronological ages, though results varied by tooth type for the second data set. Male body weight in the full 1985 living sample was significantly related to dental wear, when age was taken into account; however, when males less than 8 years of age were eliminated from the sample, the association between dental wear and weight became statistically insignificant. Analysis of the second data set suggested no statistically significant sex difference in dental wear for third molars, despite the approximately 2 year sex difference in eruption age for this tooth type.
Discussion
This study suggests that body weight in males might be a predictor of dental wear and that if it is, body weight might also influence sex differences in dental wear. Sex differences in dental eruption timing do not appear to explain sex differences in dental wear in this sample.
Keywords: abrasion, attrition, monkeys, teeth
1. INTRODUCTION
Dental wear can be defined as the physical loss of tooth substance from attrition (contact between teeth) and/or abrasion (contact between teeth and food, dust, and/or grit) (Hillson, 1986; Lucas, 2004). As animals age, they naturally accumulate dental wear (Cuozzo et al., 2010; Hillson, 1986). Beyond age, other factors that affect tooth wear have been categorized as “animal” and “food” factors (Pérez‐Barbería, 2019). The former include behaviors such as time spent masticating food and chewing efficiency (Pérez‐Barbería, 2019), but can be expanded to encompass differences in enamel and dentine thickness and/or hardness as well as dental eruption timing, both of which could potentially affect dental wear. “Food” factors primarily include abrasive properties of food (Pérez‐Barbería, 2019), but hard foods (Teaford & Oyen, 1989) as well as dust ingested with food (Schulz‐Kornas et al., 2019) have also been implicated in causing dental wear.
Understanding factors affecting tooth wear is relevant to research that (1) analyzes tooth wear to reconstruct past diets (e.g., Fiorenza et al., 2011; Górka et al., 2016; Masotti et al., 2017), (2) uses tooth wear to age adult remains (e.g., Caspari & Lee, 2004; Mays et al., 1995), and (3) evaluates the effect of dental senescence on survival and reproduction in living primates (e.g., Altmann et al., 2010; King et al., 2005). The present study aims to further investigate sex differences in molar tooth wear, a topic germane to all three of these research areas. As molars function to shear, crush, and grind food (Lucas, 2004), sex differences in molar tooth wear may indicate sex differences in diet that are revealing about differential resource use or behavior in the past (e.g., Masotti et al., 2017; Molnar, 1971). The use of dental wear to age human remains requires attention to sex, as sex differences in dental wear can complicate age at death estimates (Fernée et al., 2021).
Finally, since males and females often have different life‐history strategies, they may also differ in rates and fitness consequences of dental senescence, defined as the loss of tooth function with age (Carranza et al., 2004). Many female mammals have the ability to reproduce throughout their lifetimes, while males, especially in social groups with high pre‐copulatory inter‐male competition, have their greatest reproductive success when they are in prime physical condition (e.g., Clutton‐Brock, 1988). Thus, for example, in red deer, males in their prime consume more forage than females in support of their greater body sizes, resulting in greater rates of tooth wear in males over time (Pérez‐Barbería, Carranza, et al., 2015). In essence males appear to trade off long‐term chewing functionality for a shorter‐term gain in body size. It is not clear if a similar trade‐off occurs in non‐human primates with high pre‐copulatory inter‐male competition for mates. For female non‐human primates, dental wear in old age has been associated with reduced infant survival during dry seasons in sifakas (King et al., 2005), but infant survival was not affected by tooth wear in aged ring‐tailed lemur females (Cuozzo et al., 2010).
Greater wear in male versus female molars at comparable chronological ages has been noted in several non‐primate mammals (red deer: Loe et al., 2003; Carranza et al., 2004; large herbivores: Gaillard et al., 2015), though other studies have not found such differences (roe deer: Veiberg, Mysterud, Gaillard, et al., 2007; Weddell seal: Stirling, 1969). In large, polygynous hypsodont herbivores sex differences in wear at equivalent chronological ages have been attributed to (1) the greater body masses of males versus females supported by processing larger quantities of food (Pérez‐Barbería, Carranza, et al., 2015) and/or lower quality food (Loe et al., 2003), and (2) the smaller relative molar sizes of males at eruption (Carranza et al., 2004).
In support of the view that body mass can affect dental wear, age‐controlled within‐sex positive correlations have been found between body weight and molar wear (reindeer: Veiberg, Mysterud, Bjørkvoll, et al., 2007) and between mandible length (a proxy for body size) and molar wear (red deer: Pérez‐Barbería, Ramsay, et al., 2015). The opposite prediction, that body mass and dental wear might actually correlate negatively due to reduced chewing efficiency in animals with very worn dentitions, was not borne out in a study of red deer (Loe et al., 2003). A recent study (Carranza et al., 2020) found that under conditions of low intra‐sexual competition, male red deer had lower rates of wear over time than did females when controlling for age; however, as expected, under conditions of high‐intrasexual competition when body mass is at a premium, males had higher rates of tooth wear than did females.
For non‐human primates, there have been few age‐controlled studies of dental wear. Butler and Bernstein (1974) found that known age Macaca nemestrina females at the Yerkes Primate Research Center had greater wear than comparably aged M. nemestrina males, from the age of eight onwards. These authors suggested that the cause of this difference could be that large male canines might “impede” lateral molar grinding movements. Nass (1981), however, found that male and female Macaca fuscata had almost “identical” age‐specific molar wear, but that males showed “a perceptible increase over females with age” as evidenced by a slightly higher slope of dental wear versus age. Cuozzo et al. (2012) and Cuozzo et al. (2014) found that in one microhabitat, male ring‐tailed lemurs in the two to four year‐old age range had greater molar wear than comparably aged females. They suggested that this difference may relate to sex differences in diet: female ring‐tailed lemurs have priority of access at food sources, with males feeding more on non‐preferred resources (especially tamarind fruit). No sex differences in molar wear were found in known‐age Amboseli baboons (Altmann et al., 2010; Galbany et al., 2011; Galbany et al., 2014) or in mandrills from Gabon (Galbany et al., 2014). Known‐aged Cayo Santiago Macaca mulatta males appeared to exhibit greater wear than females at older ages (Wang et al., 2016), a finding we explore in more detail in the present study. Thus, for non‐human primates, there does not appear to be a consistent pattern of greater wear in males relative to females. These inconsistencies may relate to a variety of factors, including the degree to which sexes rely on different resources (e.g., Cuozzo et al., 2012; Cuozzo et al., 2014) sex differences in body weight (as per studies cited above of non‐primate mammals, and/or methodological factors (e.g., use of different methods for quantifying wear).
Data from the Cayo Santiago rhesus colony and associated skeletal collection provide a known‐age sample for further exploring sex differences in dental wear in a sexually dimorphic, provisioned non‐human primate population. There are two data sets used in the present study: qualitative wear score data referenced in Wang et al. (2016) that was collected on Cayo Santiago rhesus monkeys in the 1985 roundup (data set 1), and quantitative wear score data collected from these monkeys' dental remains (data set 2). The present study uses these data sets to ask the overarching question: Is there a difference in dental wear between males and females of the 1985 roundup when age is taken into account and does that difference persist until the end of these monkeys' lives?
Beyond the empirical expectation of a sex difference in dental wear from Wang et al.'s (2016) study on dental pathology and eruption in the Cayo Santiago monkeys of the 1985 roundup, the theoretical reasons for expecting a sex difference are not entirely clear. It does not seem that Cayo Santiago males could be expected to “trade‐off” long term chewing functionality for rapid growth in body size (as do some red deer populations (Carranza et al., 2020)) because there appears to be relatively low male–male competition for fertile females (Dubuc et al., 2014) and male body size does not predict reproductive success in this population (Kimock et al., 2019). Cayo Santiago monkeys are, however, sexually dimorphic in body weight, with males weighing approximately 44% more than females (Kimock et al., 2019). With this sex difference in body weight, males might be expected to process larger quantities of food over their lifetimes, accumulating more dental wear over time. However, females must process more food than their body weights alone would suggest owing to the energetic demands of pregnancy and lactation. Indeed, lactating females in this populations consumed more chow per kg body weight than other age/sex classes (Marriott et al., 1989). Adult males processed the most vegetative matter (Marriott et al., 1989) relative to other age/sex classes, while adult females were found to be more geophagic than males (Knezevich, 1998). The exact sex difference in the quantity and quality of food—and soil—ingested over these monkeys' lifespans is not known, but sex differences in food quality might be expected to be less in this provisioned population than they would be in the wild.
There are other factors that could affect sex differences in dental wear in Cayo Santiago monkeys. It is not known if there are enamel thickness differences between the sexes in this population, although one study did not find sex differences in relative enamel thickness in macaques (Kato et al., 2014). Finally, third molars (but not first or second molars) erupt significantly later in females than they do males in this population (Wang et al., 2016): nearly all males erupt their third molars by 7 years of age, but females do not reach that same milestone until the age of nine. Thus, male third molars, specifically, could be expected to begin to wear earlier, leading to greater wear than is present in comparably aged females.
Using the two data sets referred to above, the present study tests the hypothesis that males will have greater wear than females at comparable ages, as might be expected from previous study (Wang et al., 2016) as well as by such factors as greater male body weight and earlier third molar eruption timing. Using data set 1 (qualitative wear score and body weight data collected during the 1985 roundup), this study further tests the hypothesis that within males, body size is related to tooth wear, such that males of greater weight are expected to have more worn molars than lower‐weight males of the same chronological age. Because female weight fluctuates with reproductive status, female body weights collected at a point in time (the 1985 roundup) irrespective of their reproductive status are not expected to relate to cumulative dental wear. A relationship between body weight in males and dental wear at age of dental examination would support the possibility that sex differences in body mass could be affecting sex differences in wear in this population. Lastly, using data set 2, this study tests the hypothesis that sex differences in dental wear are related to dental eruption timing. If dental eruption timing is the sole factor responsible for sex differences in dental wear, then there should be significant sex differences in dental wear for third molars but not for other molar types.
2. MATERIALS AND METHODS
The rhesus monkey colony on the island of Cayo Santiago (lying 1 km off the southeastern coast of Puerto Rico) was founded in 1938 (Kessler & Rawlins, 2016; Rawlins & Kessler, 1986). At that time, primatologist Clarence Carpenter transferred 450 rhesus macaques from the Lucknow region of India to Cayo Santiago, where their descendants remain to this day. Regular provisioning of the colony with monkey chow began in 1956 (Rawlins & Kessler, 1986). In 1970, the University of Puerto Rico established the Caribbean Primate Research Center (CPRC) to meet the increasing demands of managing the colony. At this time, CPRC Science‐in‐Charge Donald Sade started a skeletal collection of monkeys' remains. Jean E. Turnquist and Mathew J. Kessler began a program of routine carcass collection from the island in the 1980's. In 1982, the CPRC Museum, now the Laboratory of Primate Morphology was established at the School of Medicine to house all of the monkeys' skeletal remains.
In 1985, there was a “round‐up” of nearly the entire colony for the purpose of tetanus immunization (Wang et al., 2016). At this time, physical examinations of all of the monkeys occurred, which included measurement and recording of weight and dental wear (Wang et al., 2016) used in the present study. Data set 1 consists of ages, weights and dental wear scores from a cross‐sectional sample of 212 rhesus monkey adults (74 females, 138 males) examined during the 1985 “round‐up.” Wear scores were taken from Wang et al. (2016). Only adults with erupted third molars were scored for dental wear. The wear score is a qualitative summary of degree of wear for the entire molar row, according to Murphy's (1959) ratings of dentine exposure: 0 (no wear), 1 (slight), 2 (moderate), 3 (much), 4 (severe), and 5 (extreme). Scoring was performed by two observers. Consensus was reached on any wear scores that differed between observers (Wang et al., 2016).
Data set 2 consists of dental wear measurements taken in the CPRC Skeletal Collection on monkeys included in Data set 1. Tattoo numbers were used to match dental remains to animals included in the 1985 roundup. In total, dental remains of 103 individuals were identified (39 females and 64 males). Sixty‐six percent of the female sample remained on the island for the entirety of their lifespans, while 63% of the males did. The remaining 34% of females and 37% of males were transferred from Cayo Santiago to a facility located in Sabana Seca after 1985 and remained there for the rest of their lives.
On Cayo Santiago, monkeys eat not only monkey chow, but also some forage (Marriott et al., 1989), which is not as readily available to them in Sabana Seca. Marriott et al. (1989) found that the time spent eating on Cayo Santiago was 10.8% of overall time. Of that, the diet was 50.2% monkey chow or other non‐naturally occurring food versus 49.8% natural vegetation, and all monkeys ate soil. Thus, the a priori expectation is that teeth would wear more slowly at Sabana Seca than on Cayo Santiago. To assess this possibility, percent of wear for each tooth type was modeled in a general linear model in SAS 9.4 (SAS Institute Inc., 2017), as a function of age‐at‐death, sex, and whether an animal was transferred to Sabana Seca. Percent of wear refers to the percentage of dentine exposure relative to the occlusal area of a tooth (more details of this measure are given below). For all six molar types, there was no statistically significant difference in percent of wear for whether an animal was or was not transferred to Sabana Seca. The number of males in the sample that spent more than 5% of their lifespans at Sabana Seca and the number of females that did so were also not significantly different (Chi‐square = 0.745, p < 0.388). Finally, there was no statistically significant difference in the numbers of males versus females transferred to Sabana Seca (Chi‐square = 0.122, df = 1, p < 0.727) in this sample. For all of these reasons, the analysis of sex differences in tooth wear was conducted on the full data set (including both transferred and non‐transferred individuals).
To measure wear on dental remains, photographs were taken with a Nikon D5600 digital camera mounted on a tripod and using a bubble level to orient the camera with its optical axis orthogonal to the occlusal surfaces of molar rows (as per Clement & Hillson, 2012). To orient the occlusal surfaces of teeth, mandibles, and maxillae were propped up with clay or in a container of uncooked rice grains until occlusal surfaces appeared, when viewed through the camera, to be as horizontal as possible (see Figure 1). All images were taken with a scale that was used later to perform measurements on the images in ImageJ (Schneider et al., 2012). To quantify wear, all areas of dentine exposure on each molar were measured with ImageJ's polygon tool and their area summed (similar to Clement & Hillson, 2012; Galbany et al., 2014). The dentine exposure sum for each molar was then divided by each molar's occlusal area, also measured with the polygon tool (Figure 1). The ratio of the sum total of dentine exposure on a tooth to its occlusal area served as the measure of wear. When multiplied by 100, this measure is referred to as the percent of wear. Only one tooth per antimeric pair was measured, with the choice of right or left depending primarily on the occlusal surface that was best oriented. If both antimeres were equally well oriented, then choice of right or left was alternated between antimeric pairs.
FIGURE 1.
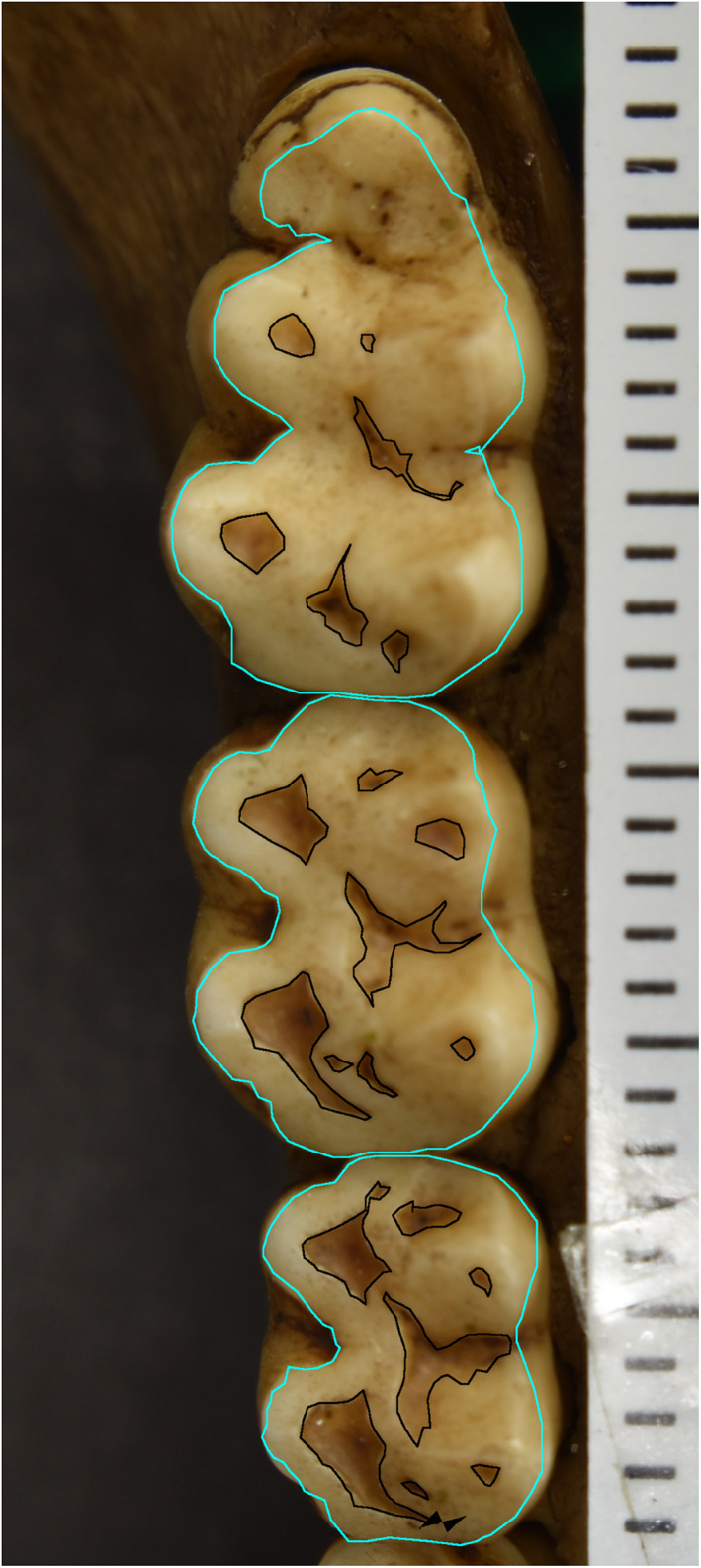
This is the lower right molar row of specimen 1639. Areas of dentine exposure are outlined (black), as is the occlusal area of the tooth (cyan)
We acknowledge that in some areas, it is difficult to distinguish between dentine exposure and very thin or stained enamel from 2D images. More specifically, some talonid basins appeared worn when on further inspection of the images it was clear that they did not match other areas of clear dentine exposure at cusp tips in terms of their shade and color. In these instances, we subtracted these reinspected areas from our original dentine exposure totals to obtain our final data set.
Measurement error in the wear ratio (sum of area of dentine exposure divided by occlusal area) was assessed on a set of 10 dentitions for six molar types (upper and lower, first, second, and third molars); thus, error was assessed on a sample of 60 teeth. Measurements were taken 1 week apart. Measurement error was calculated as percent error as follows: 100 times the absolute value of the difference in measurements taken at time one and time two, divided by the average of the two measurements. Average measurement error in the wear ratio over all teeth was 7.3%. We note a tendency for smaller patches of dentin exposure to be associated with greater percentage measurement error, even though absolute error for these patches is small.
To analyze data set 1, with ordinal wear scores, we used PROC LOGISTIC in SAS 9.4 to perform ordinal logistic regression. This type of regression analysis is appropriate when the dependent variable is ordinally scaled, given that the proportional odds assumption is met (parallel slopes for transitions across the dependent variable (SAS Institute Inc., 2017). When the proportional odds assumption is not met, SAS support recommends using the “unequal slopes” option in PROC LOGSITIC, which we used in one set of analyses (see Results below). The model with the lowest Akaike Information Criterion (AIC) was selected. To increase sample sizes in each wear category, we combined wear scores of 0 and 1 into category 0, wear scores of 2 and 3 into wear category 1, and wear scores of 4 and 5 into wear category 2. Spearman correlations between age and weight were low (−0.042 for females and 0.279 for males). Because age and weight were minimally correlated, we included them as independent predictors in our models.
To analyze data set 2, we used PROC GLM in SAS 9.4. In these analyses we tested the percent of wear (dependent variable) as a function of sex, age at death, the interaction of sex and age at death, age at death squared, and the interaction of sex and age at death squared. All combinations of these variables were tried as candidate models. The model with the lowest AIC was selected. Whether the model with the best fit (lowest AIC) was linear, quadratic, and/or included interactions differed for each analysis (see Results below).
Data sets 1 and 2 are available as supplementary files.
3. RESULTS: DATA SET 1
For data set 1, we tested the following predictions: (1) In comparably aged animals, males will exhibit greater molar wear than females; (2) Males with larger body masses will exhibit greater wear than males with smaller body masses; and (3) There will be no relationship between body mass and molar wear in females, as many females were pregnant or lactating at the time of dental examination. Prediction three assumes that because female weight fluctuates with reproductive status, female body weights collected at a point in time, irrespective of their reproductive status, will not relate to cumulative dental wear. Prior to performing statistical analyses of these predictions, we verified that males had greater body mass than females in this sample: mean weight of males (11.2 kg) differed from that of females (9.4 kg) (t = −7.580, p < 0.000).
Prediction 1: Logistic regressions of wear category (0, 1, or 2) on sex, age, and the interaction of sex and age in the full sample (n = 212 individuals) revealed that the model with the lowest AIC included just two predictors: sex and age. Both age (Wald Chi‐square 75.5, p < 0.0001) and sex (Wald Chi‐square 10.1, p < 0.0015) were statistically significant predictors of wear category (see Table 1 for details). Thus, results support prediction 1: males had greater wear than females across age categories. Figure 2 plots medians of raw wear scores (0–5) as a function of age cohort in males versus females. Note that for most age cohorts, male median wear scores are greater than those of females.
TABLE 1.
Summary of SAS PROC logistic results for data set I
| Sample | Entire sample (n = 212) | Males only (n = 138) | Males ≥8 years of age (n = 104) | Females (n = 74) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test | Wald Chi‐Square | df | p‐value | Wald Chi‐Square | df | p‐value | Wald Chi‐Square | df | p‐value | Wald Chi‐Square | df | p‐value |
| Global null hypothesis (tests significance of model) | 75.8664 | 2 | <0.0001 | 51.7249 | 2 | <0.0001 | 35.4373 | 2 | <0.0001 | 22.9855 | 3 | <0.0001 |
| Analysis of effects: age at exam | 75.5088 | 1 | <0.0001 | 49.4485 | 1 | <0.0001 | 35.1498 | 1 | <0.0001 | 21.3031 | 1 | <0.0001 |
| Analysis of effects: sex | 10.1242 | 1 | 0.0015 | – | – | – | – | – | – | – | – | – |
| Analysis of effects: weight | – | – | – | 4.3035 | 1 | 0.0380 | 3.6778 | 1 | 0.0551 | – | – | – |
| Analysis of effect of weight for transition between wear combination 1 and 2 | – | – | – | – | – | – | – | – | – | 1.6890 | 1 | 0.1937 |
| Analysis of effects of weight for transition between wear combination 0 and 1 | – | – | – | – | – | – | – | – | – | 2.6548 | 1 | 0.1032 |
Note: Statistically significant values are indicate in boldface.
FIGURE 2.
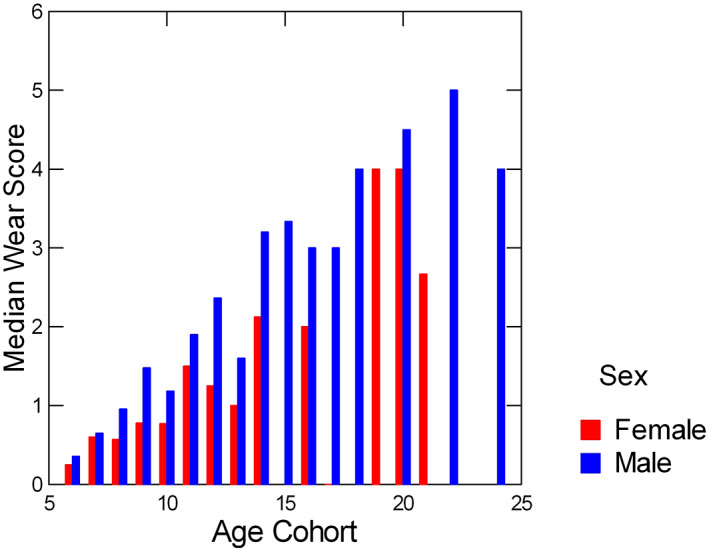
Median wear scores for females and males in each age cohort; age cohorts were constructed by rounding up increments of half a year or more to the nearest year. Ages that fell below half‐year increments were rounded down
Prediction 2: For males (n = 138), wear category was regressed against age, weight and the interaction of age and weight. The model with just age and weight had a lower AIC than the model that included the interaction. Thus, only the first model (with no interaction term) is reported in Table 1. Both age (Wald Chi‐square 49.5, p < 0.0001) and weight (Wald Chi‐square 4.3, p < 0.0380) were statistically significant predictors of wear category. These relationships can be seen in Figure 3 in which median wear category (z‐axis) is plotted against both age‐cohort (years) and weight group (kg). Note that within most age cohorts, there appears to be a positive relationship between median wear score and weight group, with the most obvious exception being the oldest animals (20 years of age or more), with very low body weights and high molar wear.
FIGURE 3.
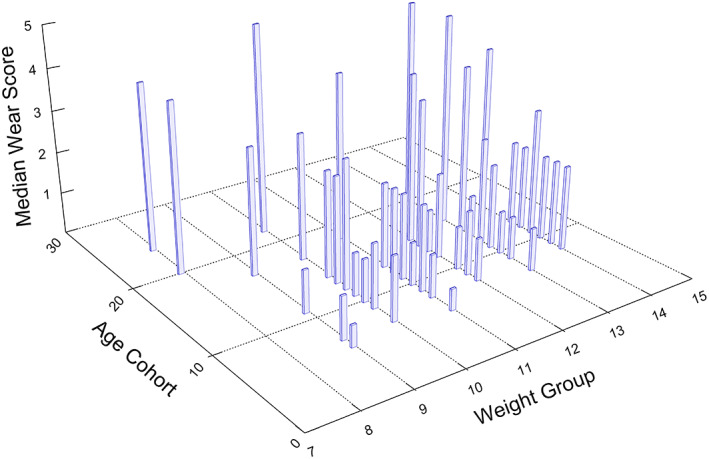
Median wear scores as a function of age cohort and weight group. Age cohorts were constructed by rounding up half years (or greater) to the nearest year. Ages that fell below a half‐year increment were rounded down. Weight groups were constructed similarly, with weight increments equal to or greater than 0.5 kg rounded up to the nearest kg, and weight increments below 0.5 kg rounded down. Note that within most age groups, there appears to be a positive relationship between median wear score and weight group, with the most obvious exception being the oldest animals, with very low weights and high molar wear
Because of the positive, though low correlation between weight and age in males, we performed an additional regression analysis excluding males that had not reached 8 years of age, the age at which most males have completed skeletal growth (Bercovitch & Goy, 1990). By excluding these younger‐aged males, the Spearman correlation between age and weight dropped to −0.044. In this reduced sample (n = 104), age continued to be a statistically significant predictor of wear (Wald Chi‐square = 35.2, p < 0.0001) but the p‐value for weight rose to just beyond the threshold of statistical significance (Wald Chi‐square = 3.7, p < 0.0551). These results are summarized in Table 1.
Prediction 3: For females (n = 74), there was no interaction effect for weight and age when wear category was regressed against, weight, age, and the interaction of weight and age. Thus, the regression model was run with only weight and age as predictors. In this model, we used the unequal slopes option, as the proportional odds assumption was not met, running two tests for the effect of weight: one for the transition between wear combination scores 0 and 1, and one for the transition between 1 and 2 (See Table 1). As predicted, for females age was a significant predictor of wear (Wald Chi‐square = 21.3031, p < 0.0001), but weight was not for either of the weight tests (Wald Chi‐square = 2.6548, p < 0.1032 for the first transition, and, Wald Chi‐square 1.6890, p < 0.1937 for the second). The lack of association between wear and weight in females is evident in Figure 4.
FIGURE 4.
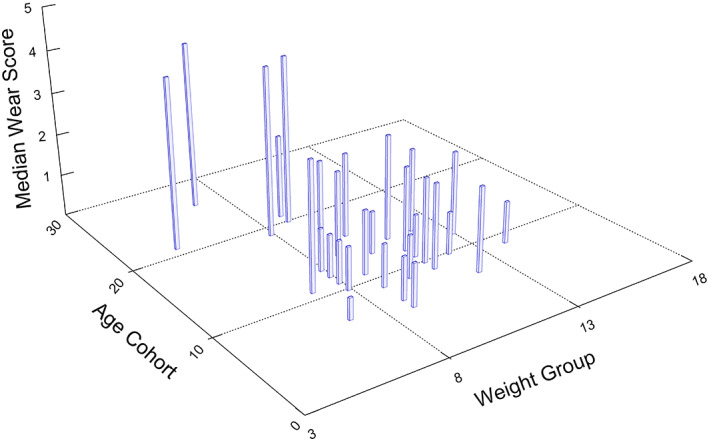
Median wear scores as a function of age cohort and weight group in females. Age cohorts were constructed by rounding up half years (or greater) to the nearest year. Ages that fell below a half‐year increment were rounded down. Weight groups were constructed similarly, with weight increments equal to or greater than 0.5 kg rounded up to the nearest kg, and weight increments below 0.5 kg rounded down. Note that there does not appear to be a pattern of greater median wear scores in relation to weight group within each age cohort
4. RESULTS: DATA SET 2
For data set 2, we tested these predictions: (1) Males will have greater wear than females when age is statistically controlled, and (2) If a sex difference in eruption timing is the sole cause of sex differences in dental wear, then there will be systematic significant differences in third molar wear between males and females, but no significant sex differences for other molar types.
To analyze sex differences in tooth wear, percent of wear for each tooth type was regressed in candidate models that included different combinations of age, sex, the interaction of age and sex, age at death squared, and the interaction of sex and age at death squared. Models with the lowest AICs for each tooth type are presented in Tables 2 (upper molars) and 3 (lower molars), as are sample sizes for each analysis. Figure 5 plots the analysis of covariance from these GLMs for each tooth type. The statistically significant results from these regressions can be summarized as follows. For UM1, there was a statistically significant effect for age at death and the interaction of age at death and sex. For UM2, there were statistically significant effects for sex, the interaction of age at death and sex, and for the interaction of sex by age at death squared. For UM3, LM1, LM2, and LM3, there were statistically significant effects only for age at death. Note, however, that for LM3, the effect of sex just exceeded the threshold for statistical non‐significance (p = 0.053).
TABLE 2.
SAS GLM results for data set II: upper molars
| Sample | Upper M1 (n = 38 f, 62 m) | Upper M2 (n = 38 f, 63 m) | Upper M3 (n = 37 f, 63 m) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Test | F‐value | df | p‐value | F‐value | df | p‐value | F‐value | df | p‐value |
| Model | 30.89 | 3 | <0.001 | 26.14 | 5 | <0.001 | 6.41 | 2 | 0.0024 |
| Sex | 2.01 | 1 | 0.1592 | 5.87 | 1 | 0.0173 | 0.06 | 1 | 0.8045 |
| Age at death | 88.45 | 1 | <0.001 | 2.23 | 1 | 0.1385 | 12.58 | 1 | 0.0006 |
| Age at death interaction with sex | 4.13 | 1 | 0.0450 | 7.87 | 1 | 0.0061 | – | – | – |
| Age at death squared | – | – | – | 0.09 | 1 | 0.7612 | – | – | – |
| Sex by age at death squared interaction | – | – | – | 10.63 | 1 | 0.0015 | – | – | – |
Note: Statistically significant values are indicate in boldface.
TABLE 3.
SAS GLM results for data set II: Lower molars
| Sample | Lower M1 (n = 39 f, 62 m) | Lower M2 (n = 39 f, 64 m) | Lower M3 (n = 38 f, 63 m) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Test | F‐value | df | p‐value | F‐value | df | p‐value | F‐value | df | p‐value |
| Model | 22.05 | 3 | <0.001 | 40.76 | 3 | <0.001 | 40.07 | 2 | <0.001 |
| Sex | 0.10 | 1 | 0.7504 | 0.31 | 1 | 0.5776 | 3.84 | 1 | 0.053 |
| Age at death | 61.73 | 1 | <0.001 | 116.24 | 1 | <0.001 | 80.05 | 1 | <0.001 |
| Age at death interaction with sex | 0.87 | 1 | 0.3534 | 1.64 | 1 | 0.2033 | – | – | – |
Note: Statistically significant values are indicate in boldface.
FIGURE 5.
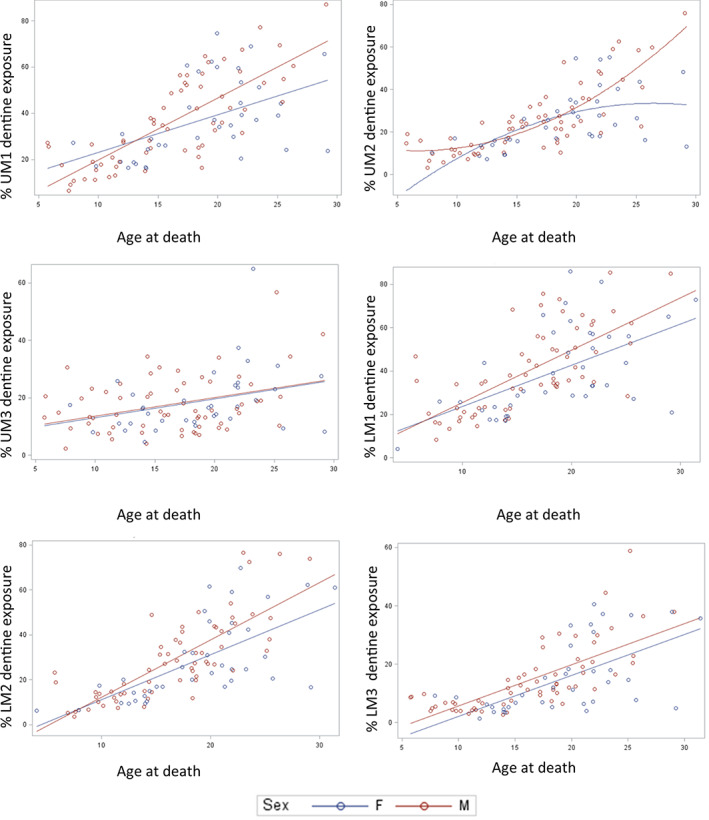
Graphs of percent of dental wear (exposed dentine area over occlusal area multiplied by 100), as a function of sex and age. In each graph, regression lines from PROC GLM models are displayed. Note that in most of these graphs, male molars tend to be more worn than those of females especially at later chronological ages
Thus, for two of six tooth types, neither of them third molars, there was a significant effect of sex (UM2) and significant interaction effects between age and sex (UM1 and UM2). The nature of the interaction between age and sex is such that for the UM1 and UM2, the difference in wear between males and females increases with age (Figure 5). The interaction suggests that males and females may be wearing their UM1s and UM2s at different rates.
5. DISCUSSION
Analyses conducted in this study suggest that sex differences in molar wear in individuals scored during the 1985 roundup persisted until these animals' deaths. However, not all molar tooth types revealed a statistically significant sex difference in wear in these animals' dental remains. Data set 1 was used to test the hypothesis that within males, body weights would relate to the degree of dental wear they exhibited at the age of dental examination, but that this would not be the case for females, whose body weight is expected to fluctuate (in relation to reproductive status) more so than that of males. Analyses support a relationship between body weight and dental wear for the full sample of males, but the relationship became statistically insignificant for a reduced sample consisting only of males equal to or greater than 8 years of age. Overall, there is some indication, though not a particularly strong one, that body weight in males may be related to dental wear when age is taken into account. For females, there was no indication of a relationship between body weight and dental wear, when age was taken into account.
Data set 2 was used to test the hypothesis that if sex differences in dental wear were purely a function of sex differences in dental eruption ages, then third molars would systematically exhibit significant sex differences in dental wear, but other molar types would not. This, however, was not the case. As noted above, only the UM1 and UM2 exhibited significant sex differences (either as a main effect or in interactions with age). For the LM3, the sex difference in dental wear was close to statistical significance, but this was not the case for the UM3. Given that the numbers of teeth for each molar type were approximately equal (see Tables 2 and 3), the power for detecting statistically significant differences in each of these tooth types, assuming equivalent effect sizes, is nearly equivalent. It may be the case that there is simply more time for a sex difference to manifest in M1s and M2s, which erupt at earlier ages than M3s. Nevertheless, there is no evidence for a systematic sex difference in wear in third molars relative to first and second molars owing to the pronounced sex difference in third molar eruption ages.
Some of non‐significant results in this study could have to do with noise introduced by methodological limitations. These include 7.3% measurement error for 2D measurements, the difficulties of differentiating dentine exposure from areas of thinning or discolored enamel on some teeth, and the use of 2D rather than 3D measurements. Teeth wear in three dimensions. MicroCT studies of dental wear (e.g., Pampush et al., 2018) can take this fact into account as well as definitively differentiate between true dentine exposure and thinning enamel.
Despite its limitations, the present study suggests that male Cayo Santiago rhesus monkey molars tend to exhibit greater wear than those of females at comparable ages. Yet, male–male physical competition for mating is low (Dubuc et al., 2014) and male body size does not predict reproductive success (Kimock et al., 2019) in this population. This situation differs from that recently described in red deer, where male dental wear exceeded that of females under conditions of high inter‐male mating competition, but not under conditions when mating competition was low (Carranza et al., 2020). Given that male Cayo Santiago monkeys weigh more than females and that there is some indication in the present study that body weight within males is related to dental wear, it could be that the sex difference in Cayo Santiago monkey dental wear is simply a function of males processing more food with their molars over their lifetimes than females do. In addition, if adult males eat more vegetation than females do (Marriott et al., 1989), and if vegetation is more abrasive than monkey chow, then a sex difference in diet quality may be a contributing factor to the sex difference in dental wear found here. It is also worth noting that males in this population exhibit not only more dental wear, but also exhibit greater frequencies of breakage and antemortem tooth loss than do females (Wang et al., 2016). Higher frequencies of antemortem tooth loss in males might be related to their tendency to develop elevated levels of molar wear relative to females.
In humans, when sex differences in dental wear have been found, it is usually the case that males exhibit greater wear and/or wear rates than females, in both modern (e.g., Cunha‐Cruz et al., 2010; Hugoson et al., 1988; Johansson, 1992; Knight et al., 1997; Molnar et al., 1983; Tomenchuk & Mayhall, 1979) and ancient population samples (e.g., Fernée et al., 2021; Kaifu, 1998; Masotti et al., 2017). Sex differences in molar wear have been attributed to a variety of factors, including sex differences in dietary abrasiveness (Masotti et al., 2017; Molnar et al., 1983), male‐biased bruxism (Tomenchuk & Mayhall, 1979), and greater enamel thickness in females relative to males (Fernée et al., 2021). In contrast to what appears to be a tendency for human males to wear their molars more rapidly than human females, Molnar (1971) reported significantly greater wear in the female molars of California Native American skeletal remains relative to those of males, which he argued was in part related to females' greater consumption of abrasive plant foods. The present study suggests that one more factor may contribute to a tendency for human males to wear their molars more rapidly than human females—an overall sex difference in body weight that results in sex differences in how much food molars must process over time.
Body weight, however, is clearly not the most important factor affecting dental wear in all instances where sex differences in dental wear are found. A diet consisting predominantly of monkey chow would, for the Cayo Santiago population, homogenize the diets of males and females to a greater degree than exists in many non‐human primates in the wild or in some modern human population groups. Indeed, Molnar's (1971) study suggests that sex differences in dietary abrasiveness overrides any effect of larger body size on dental wear. Similarly, male and female ring‐tailed lemurs are comparable in body size, but in one micro‐habitat, males relied more than females on non‐preferred tamarind fruit, which appeared to result in greater molar wear in males (Cuozzo et al., 2012; Cuozzo et al., 2014). Finally, and perhaps most challenging to the idea that sex differences in body size could be behind sex differences in molar wear, is that in Amboseli baboons (Altmann et al., 2010; Galbany et al., 2011) and mandrills from Gabon (Galbany et al., 2014), which are highly sexually dimorphic, no sex differences in first molar wear were found in known‐age samples (Altmann et al., 2010). What is needed to more fully evaluate the potential effect of body size on cumulative dental wear, either within or between the sexes, is control for differences among individuals and between sexes in dietary abrasiveness and hardness. Finally, the effect of female reproductive status on cumulative lifetime dental wear might be assessed in the Cayo Santiago macaque population by examining correlations between parity and dental wear in relation to age at death.
6. CONCLUSION
This study investigated sex differences in molar wear in Cayo Santiago monkeys included in the 1985 roundup. Wear scores were greater for males than for females when age was taken into account. The male bias in molar dental wear persisted in some tooth types for a subsample from the roundup whose skeletal remains are curated in the Primate Morphology Laboratory of the Caribbean Primate Research Center. Despite the fact that third molars erupt earlier in males than in females, male third molars did not systematically evince more wear than those of females, with age taken into account. A relationship between body weight and molar wear with age taken into account was found in males, but that relationship became statistically insignificant when the male sample was limited to males equal to or greater than 8 years of age (the age at which skeletal maturation is complete in males). Body size has been related to dental wear in non‐primate mammals, but this is the first study to offer evidence of a possible relationship between dental wear and body size in non‐human primates. Taken together, these results suggest that in this provisioned population, sex differences in body weight might have an influence on sex differences in dental wear, but that sex differences in molar eruption timing do not.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
AUTHOR CONTRIBUTIONS
Debbie Guatelli‐Steinberg: Conceptualization (lead); data curation (equal); formal analysis (lead); funding acquisition (equal); investigation (equal); methodology (equal); project administration (equal); supervision (lead); validation (lead); visualization (lead); writing – original draft (lead); writing – review and editing (lead). Taylor Guerrieri: Data curation (supporting); validation (supporting). Terry Kensler: Data curation (supporting); resources (supporting); writing – review and editing (supporting). Elizabeth Maldonado: Data curation (supporting); resources (supporting). George Francis: Methodology (supporting); writing – review and editing (supporting). Luci Kohn: Funding acquisition (equal); writing – review and editing (supporting). Martin Zhao: Funding acquisition (equal); writing – review and editing (supporting). Jean E. Turnquist: Data curation (supporting); investigation (supporting); methodology (supporting); resources (supporting); writing – review and editing (supporting). Qian Wang: Conceptualization (supporting); data curation (equal); funding acquisition (equal); methodology (supporting); project administration (equal); resources (equal); writing – review and editing (supporting).
Supporting information
Appendix S1: Supporting Information
Appendix S2: Supporting Information
Appendix S3: Supporting Information
ACKNOWLEDGMENTS
The CPRC Skeletal Collection has been supported by National Institutes of Health NIH contracts NIH 5P40OD012217. This project is supported by NSF grants to D.G.‐S., M.Q.Z., L.K, and Q.W. (NSF 1926528, NSF 1926402, 1926481, 1926601). Tooth wear scores were assigned during the 1985 roundup by Dr. Jean E. Turnquist and Dr. Matthew Kessler. Dr. Melween I. Martinez Rodriguez (Current CPRC Director), Mr. Bonn V. Liong Aure, Dr. Angelina Ruiz‐Lambides, and other CPRC staff members for their support and help. Emma Lagan and Dan Steinberg are thanked for their advice on photography. The authors thank Drs. Frank Cuozzo, Chris Deter, and Javier Pérez‐Barbería for discussion. Finally, we thank the AABA reviewers whose insightful comments helped us improve this work.
Guatelli‐Steinberg, D. , Guerrieri, T. , Kensler, T. B. , Maldonado, E. , Francis, G. , Kohn, L. A. P. , Zhao, M. Q. , Turnquist, J. E. , & Wang, Q. (2022). Male Cayo Santiago rhesus macaques (Macaca mulatta) tend to have greater molar wear than females at comparable ages: Exploring two possible reasons why. American Journal of Biological Anthropology, 178(3), 437–447. 10.1002/ajpa.24519
Funding information National Institutes of Health, Grant/Award Number: 5P40OD012217; National Science Foundation, Grant/Award Numbers: 1926402, 1926481, 1926528, 1926601
DATA AVAILABILITY STATEMENT
We are including the data sets in the form of excel sheets in the Supplementary Material that anyone can access.
REFERENCES
- Altmann, J. , Gesquiere, L. , Galbany, J. , Onyango, P. O. , & Alberts, S. C. (2010). Life history context of reproductive aging in a wild primate model. Annals of the New York Academy of Sciences, 1204, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercovitch, F. B. , & Goy, R. W. (1990). The socioendocrinology of reproductive development and reproductive success in macaques. In Ziegler T. & Bercovitch F. B. (Eds.), Socioendocrinology of primate reproduction (pp. 59–93). Wiley‐Liss, Inc. [Google Scholar]
- Butler, R. J. , & Bernstein, I. S. (1974). Canine role in dental wear patterns: Macaca nemestrina . American Journal of Physical Anthropology, 40(3), 391–395. [DOI] [PubMed] [Google Scholar]
- Carranza, J. , Alarcos, S. , Sánchez‐Prieto, C. B. , Valencia, J. , & Mateos, C. (2004). Disposable‐soma senescence mediated by sexual selection in an ungulate. Nature, 432(7014), 215–218. [DOI] [PubMed] [Google Scholar]
- Carranza, J. , Pérez‐Barbería, J. , Mateos, C. , Alarcos, S. , Torres‐Porras, J. , Pérez‐González, J. , Sánchez‐Prieto, C. B. , Valencia, J. , Castillo, L. , De la Peña, E. , Barja, I. , Seoane, J. M. , Reglero, M. M. , Flores, A. , & Membrillo, A. (2020). Social environment modulates investment in sex trait versus lifespan: Red deer produce bigger antlers when facing more rivalry. Scientific Reports, 10(1), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspari, R. , & Lee, S. H. (2004). Older age becomes common late in human evolution. Proceedings of the National Academy of Sciences, 101(30), 10895–10900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement, A. F. , & Hillson, S. W. (2012). Intrapopulation variation in macro tooth wear patterns—A case study from Igloolik, Canada. American Journal of Physical Anthropology, 149(4), 517–524. [DOI] [PubMed] [Google Scholar]
- Clutton‐Brock, T. H. (1988). Reproductive success. In Clutton‐Brock T. H. (Ed.), Reproductive success: Studies of individual variation in contrasting breeding systems (pp. 472–485). University of Chicago Press. [Google Scholar]
- Cunha‐Cruz, J. , Pashova, H. , Packard, J. D. , Zhou, L. , & Hilton, T. J. (2010). Tooth wear: prevalence and associated factors in general practice patients. Community Dentistry and Oral Epidemiology, 38((3)), 228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuozzo, F. P. , Head, B. R. , Sauther, M. L. , Ungar, P. S. , & O'Mara, M. T. (2014). Sex and habitat as a source of dental wear variation early in life among known‐aged wild ring‐tailed lemurs (Lemur catta). American Journal of Primatology., 76, 1037–1048. [DOI] [PubMed] [Google Scholar]
- Cuozzo, F. P. , Sauther, M. L. , Gould, L. , Sussman, R. W. , Villers, L. M. , & Lent, C. (2010). Variation in dental wear and tooth loss among known‐aged, older ring‐tailed lemurs (Lemur catta): A comparison between wild and captive individuals. American Journal of Primatology, 72(11), 1026–1037. [DOI] [PubMed] [Google Scholar]
- Cuozzo, F. P. , Sauther, M. L. , & Ungar, P. S. (2012). Variation in rates of tooth wear in a single primate population: Effects of sex and microhabitat. American Journal of Physical Anthropology, 147(S54), 123. [Google Scholar]
- Dubuc, C. , Ruiz‐Lambides, A. , & Widdig, A. (2014). Variance in male lifetime reproductive success and estimation of the degree of polygyny in a primate. Behavioral Ecology, 25(4), 878–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernée, C. , Zakrzewski, S. , & Robson Brown, K. (2021). Dimorphism in dental tissues: Sex differences in archaeological individuals for multiple tooth types. American Journal of Physical Anthropology, 175(1), 106–127. [DOI] [PubMed] [Google Scholar]
- Fiorenza, L. , Benazzi, S. , Tausch, J. , Kullmer, O. , Bromage, T. G. , & Schrenk, F. (2011). Molar macrowear reveals Neanderthal eco‐geographic dietary variation. PLoS One, 6(3), e14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard, J. M. , Berger, V. , Tidière, M. , Duncan, P. , & Lemaître, J. F. (2015). Does tooth wear influence ageing? A comparative study across large herbivores. Experimental Gerontology, 71, 48–55. [DOI] [PubMed] [Google Scholar]
- Galbany, J. , Altmann, J. , Pérez‐Pérez, A. , & Alberts, S. C. (2011). Age and individual foraging behavior predict tooth wear in Amboseli baboons. American Journal of Physical Anthropology, 144(1), 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbany, J. , Romero, A. , Mayo‐Alesón, M. , Itsoma, F. , Gamarra, B. , Pérez‐Pérez, A. , Willaume, E. , Kappeler, P. M. , & Charpentier, M. J. (2014). Age‐related tooth wear differs between Forest and Savanna primates. PLoS One, 9(4), e94938. 10.1371/journal.pone.0094938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Górka, K. , Romero, A. , & Pérez‐Pérez, A. (2016). Dental‐macrowear and diet of Tigara foragers from point Hope, northern Alaska. Anthropologischer Anzeiger, 73(3), 257–264. [DOI] [PubMed] [Google Scholar]
- Hillson, S. (1986). Teeth. Cambridge University Press. [Google Scholar]
- Hugoson, A. , Bergendal, T. , Ekfeldt, A. , & Helkimo, M. (1988). Prevalence and severity of incisal and occlusal tooth wear in an adult Swedish population. Acta Odontologica Scandinavica, 46(5), 255–265. [DOI] [PubMed] [Google Scholar]
- Johansson, A. (1992). A cross‐cultural study of occlusal tooth wear. Swedish Dental Journal. Supplement, 86, 1–59. [PubMed] [Google Scholar]
- Kaifu, Y. (1998). Sex differences in tooth wear in the Japanese. Bulletin of National Science Museum, 24, 49–59. [Google Scholar]
- Kato, A. , Tang, N. , Borries, C. , Papakyrikos, A. M. , Hinde, K. , Miller, E. , Kunimatsu, Y. , Hirasaki, E. , Shimizu, D. , & Smith, T. M. (2014). Intra‐and interspecific variation in macaque molar enamel thickness. American Journal of Physical Anthropology, 155(3), 447–459. [DOI] [PubMed] [Google Scholar]
- Kessler, M. J. , & Rawlins, R. G. (2016). A 75‐year pictorial history of the Cayo Santiago rhesus monkey colony. American Journal of Primatology, 78(1), 6–43. 10.1002/ajp.22381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimock, C. M. , Dubuc, C. , Brent, L. J. , & Higham, J. P. (2019). Male morphological traits are heritable but do not predict reproductive success in a sexually‐dimorphic primate. Scientific Reports, 9(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, S. J. , Arrigo‐Nelson, S. J. , Pochron, S. T. , Semprebon, G. M. , Godfrey, L. R. , Wright, P. C. , & Jernvall, J. (2005). Dental senescence in a long‐lived primate links infant survival to rainfall. Proceedings of the National Academy of Sciences, 102(46), 16579–16583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knezevich, M. (1998). Geophagy as a therapeutic mediator of endoparasitism in a free‐ranging group of rhesus macaques Macaca mulatta . American Journal of Primatology, 44, 71–82. [DOI] [PubMed] [Google Scholar]
- Knight, D. J. , Leroux, B. G. , Zhu, C. , Almond, J. , & Ramsay, D. S. (1997). A longitudinal study of tooth wear in orthodontically treated patients. American Journal of Orthodontics and Dentofacial Orthopedics, 112(2), 194–202. [DOI] [PubMed] [Google Scholar]
- Loe, L. E. , Mysterud, A. , Langvatn, R. , & Stenseth, N. C. (2003). Decelerating and sex‐dependent tooth wear in Norwegian red deer. Oecologia, 135(3), 346–353. [DOI] [PubMed] [Google Scholar]
- Lucas, P. W. (2004). Dental functional morphology: How teeth work. Cambridge University Press. [Google Scholar]
- Marriott, B. M. , Roemer, J. , & Sultana, C. (1989). An overview of the food intake patterns of the Cayo Santiago Rhesus monkeys (Macaca mulatta): Report of a pilot study. Puerto Rico Health Sciences Journal, 8, 87–94. [PubMed] [Google Scholar]
- Masotti, S. , Bogdanic, N. , Arnaud, J. , Cervellati, F. , & Gualdi‐Russo, E. (2017). Tooth wear pattern analysis in a sample of Italian early bronze age population. Proposal of a 3‐D sampling sequence. Archives of Oral Biology, 74, 37–45. [DOI] [PubMed] [Google Scholar]
- Mays, S. , de la Rua, C. , & Molleson, T. (1995). Molar crown height as a means of evaluating existing dental wear scales for estimating age at death in human skeletal remains. Journal of Archaeological Science, 22(5), 659–670. [Google Scholar]
- Molnar, S. (1971). Human tooth wear, tooth function and cultural variability. American Journal of Physical Anthropology, 34(2), 175–189. [DOI] [PubMed] [Google Scholar]
- Molnar, S. , McKee, J. K. , Molnar, I. M. , & Przybeck, T. R. (1983). Tooth wear rates among contemporary Australian aborigines. Journal of Dental Research, 62(5), 562–565. [DOI] [PubMed] [Google Scholar]
- Murphy, T. (1959). The changing patterns of dentine exposure in human teeth attrition. American Journal of Physical Anthropology, 17, 167–178. [DOI] [PubMed] [Google Scholar]
- Nass, G. G. (1981). Sex differences in tooth wear of Macaca fuscata, the Arashiyama‐a troop in Texas. Primates, 22(2), 266–276. [Google Scholar]
- Pampush, J. D. , Spradley, J. P. , Morse, P. E. , Griffith, D. , Gladman, J. T. , Gonzales, L. A. , & Kay, R. F. (2018). Adaptive wear‐based changes in dental topography associated with atelid (Mammalia: Primates) diets. Biological Journal of the Linnean Society, 124, 584–606. [Google Scholar]
- Pérez‐Barbería, F. J. (2019). Tooth wear as a practical indicator of sexual differences in senescence and mastication investment in ecology studies. Ecological Indicators, 103, 735–744. [Google Scholar]
- Pérez‐Barbería, F. J. , Carranza, J. , & Sánchez‐Prieto, C. (2015). Wear fast, die young: More worn teeth and shorter lives in Iberian compared to Scottish red deer. PLoS One, 10(8), e0134788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez‐Barbería, F. J. , Ramsay, S. L. , Hooper, R. J. , Pérez‐Fernández, E. , Robertson, A. H. J. , Aldezabal, A. , Goddard, P. , & Gordon, I. J. (2015). The influence of habitat on body size and tooth wear in Scottish red deer (Cervus elaphus). Canadian Journal of Zoology, 93(1), 61–70. [Google Scholar]
- Rawlins, R. G. , & Kessler, M. J. (Eds.). (1986). The Cayo Santiago macaques: History, behavior, and biology. SUNY Press. [Google Scholar]
- SAS Institute Inc . (2017). SAS/STAT® 14.3 User's guide. SAS Institute Inc. [Google Scholar]
- Schneider, C. A. , Rasband, W. S. , & Eliceiri, K. W. (2012). NIH image to ImageJ: 25 years of image analysis. Nature Methods, 9(7), 671–675. 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz‐Kornas, E. , Stuhlträger, J. , Clauss, M. , Wittig, R. M. , & Kupczik, K. (2019). Dust affects chewing efficiency and tooth wear in forest dwelling Western chimpanzees (Pan troglodytes verus). American Journal of Physical Anthropology, 169(1), 66–77. [DOI] [PubMed] [Google Scholar]
- Stirling, I. (1969). Tooth wear as a mortality factor in the Weddell seal, Leptonychotes weddelli . Journal of Mammalogy, 50(3), 559–565. [Google Scholar]
- Teaford, M. F. , & Oyen, O. J. (1989). Differences in the rate of molar wear between monkeys raised on different diets. Journal of Dental Research, 68(11), 1513–1518. [DOI] [PubMed] [Google Scholar]
- Tomenchuk, J. , & Mayhall, J. T. (1979). A correlation of tooth wear and age among modern Igloolik Eskimos. American Journal of Physical Anthropology, 51(1), 67–77. [DOI] [PubMed] [Google Scholar]
- Veiberg, V. , Mysterud, A. , Bjørkvoll, E. , Langvatn, R. , Loe, L. E. , Irvine, R. J. , Bonenfant, C. , Couweleers, F. , & Stenseth, N. C. (2007). Evidence for a trade‐off between early growth and tooth wear in Svalbard reindeer. Journal of Animal Ecology, 76(6), 1139–1148. [DOI] [PubMed] [Google Scholar]
- Veiberg, V. , Mysterud, A. , Gaillard, J. M. , Delorme, D. , Laere, G. V. , & Klein, F. (2007). Bigger teeth for longer life? Longevity and molar height in two roe deer populations. Biology Letters, 3(3), 268–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q. , Turnquist, J. E. , & Kessler, M. J. (2016). Free‐ranging Cayo Santiago rhesus monkeys (Macaca mulatta): III. Dental eruption patterns and dental pathology. American Journal of Primatology, 78(1), 127–142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Appendix S2: Supporting Information
Appendix S3: Supporting Information
Data Availability Statement
We are including the data sets in the form of excel sheets in the Supplementary Material that anyone can access.


