Abstract
Mycobacterium smegmatis has been shown to contain two forms of polyprenyl phosphate (Pol-P), while Mycobacterium tuberculosis contains only one. Utilizing subcellular fractions from M. smegmatis and M. tuberculosis, we show that Pol-P synthesis is different in these species. The specific activities of the prenyl diphosphate synthases in M. tuberculosis are 10- to 100-fold lower than those in M. smegmatis. In M. smegmatis decaprenyl diphosphate and heptaprenyl diphosphate were the main products synthesized in vitro, whereas in M. tuberculosis only decaprenyl diphosphate was synthesized. The data from both organisms suggest that geranyl diphosphate is the allylic substrate for two distinct prenyl diphosphate synthases, one located in the cell membrane that synthesizes ω,E,Z-farnesyl diphosphate and the other present in the cytosol that synthesizes ω,E,E,E-geranylgeranyl diphosphate. In M. smegmatis, the ω,E,Z-farnesyl diphosphate is utilized by a membrane-associated prenyl diphosphate synthase activity to generate decaprenyl diphosphate, and the ω,E,E,E-geranylgeranyl diphosphate is utilized by a membrane-associated activity for the synthesis of the heptaprenyl diphosphate. In M. tuberculosis, however, ω,E,E,E-geranylgeranyl diphosphate is not utilized for the synthesis of heptaprenyl diphosphate. Thus, the difference in the compositions of the Pol-P of M. smegmatis and M. tuberculosis can be attributed to distinct enzymatic differences between these two organisms.
Polyprenyl phosphates (Pol-P) are involved in the biosynthesis of bacterial cell walls (14), and their availability is rate limiting for several aspects of cell wall synthesis in Staphylococcus aureus (15) and Bacillus spp. (2). It has also been suggested that the rate of synthesis of lipid I (in peptidoglycan synthesis) of Escherichia coli may be dependent on the pool level of Pol-P (26), and Baddiley (4) reported that Pol-P levels could regulate the rate of bacterial cell wall synthesis in vivo.
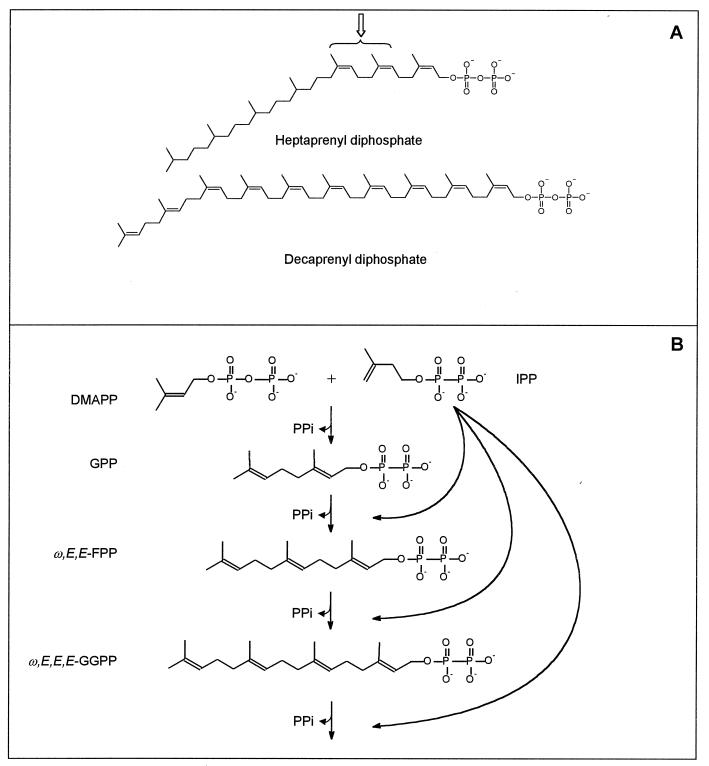
Mycobacterium smegmatis is known to contain two forms of Pol-P that are covalently attached to mannose (25). These are structurally unusual in that the decaprenyl phosphate contains one ω-isoprene unit, one E-isoprene unit, and eight Z-isoprene units (mono-E, poly-Z) (28) and the heptaprenyl phosphate consists of either four saturated isoprene units on the omega end of the molecule, two E-isoprene units, and one Z-isoprene unit (6) or four saturated and three Z-isoprene units (27) (Fig. 1A) (the stereochemical configuration of the isoprene units is always listed starting at the omega end of the molecule). It appears that Mycobacterium tuberculosis may be more typical than M. smegmatis, as a single predominant Pol-P (decaprenyl phosphate) was identified in this species, however; the stereochemistries of the individual isoprene units were not determined (24).
FIG. 1.
Structures of heptaprenyl diphosphate and decaprenyl diphosphate (A) and prenyl diphosphate synthesis scheme (B). The structure of the heptaprenyl diphosphate is drawn as described for heptaprenyl phosphoryl mannose by Wolucka and de Hoffmann (27). The stereochemistry of the two isoprene units indicated by the arrow is ambiguous, as they have been reported to be Z (27) (shown) or E (6). The structure of the decaprenyl diphosphate is drawn as described by Wolucka et al. (28). Panel B shows the chain elongation of E-prenyl diphosphates by head-to-tail condensation of various allylic diphosphates (used as reaction primers in this study) with IPP.
The fact that both forms of Pol-P in M. smegmatis are glycosylated suggested that both could be involved in the synthesis of cell wall polysaccharides. Our laboratory has shown that M. smegmatis utilizes its unusual Pol-P molecules in many stages of cell wall biosynthesis. Mature mycolic acids appear to be formed from precursors while attached to a heptaprenyl phosphate molecule (6). Decaprenyl-P-arabinose is a precursor of the arabinan portions of arabinogalactan, arabinomannan, and lipoarabinomannan (28). A polyprenyl diphosphate carrier lipid has been implicated in the synthesis of the linker unit galactan of M. smegmatis (17) and in the synthesis of linear forms of lipoarabinomannan (5).
Despite the crucial role of Pol-P in bacterial cell wall biogenesis, little is known about its biosynthesis, especially in Mycobacterium spp. Pol-P is typically synthesized by enzymes that catalyze the 1′-4 condensations of isopentenyl diphosphate (IPP) with allylic prenyl diphosphates (reaction primers) in order to generate longer, physiologically appropriate, allylic prenyl diphosphates (Fig. 1B). The diphosphates are subsequently dephosphorylated to form the appropriate Pol-P. The importance of this biosynthetic pathway in mycobacterial biology is demonstrated by the fact that all species of mycobacteria tested (reference 19 and our unpublished data) are susceptible to the antibiotic bacitracin, which specifically binds prenyl diphosphate intermediates in Pol-P synthesis (22). Prenyl diphosphate synthases are very widespread in nature, but of the hundreds in existence, only a few have been studied (20).
Thus, Pol-P synthesis is clearly important in the rate of bacterial growth and the synthesis of cell wall components essential for the viability of mycobacteria. The intriguing structural differences in Pol-P from M. smegmatis and M. tuberculosis prompted us to initiate an investigation of the biosynthesis of Pol-P in these two species to explain the enzymatic basis of these observations.
MATERIALS AND METHODS
Materials.
[14C]IPP (55 mCi/mmol) was purchased from Amersham Life Science Inc. (Arlington Heights, Ill.), potato acid phosphatase was purchased from Boehringer Mannheim (Indianapolis, Ind.), and dimethylallyl diphosphate (DMAPP), ω,E,E,E-geranylgeranyl diphosphate (ω,E,E,E-GGPP), farnesol (mixed stereoisomers), ω,E,E-farnesol, and ω,E-geraniol were purchased from Sigma (St. Louis, Mo.). ω,E,E-Farnesyl diphosphate (ω,E,E-FPP) and ω,E-geranyl diphosphate (GPP) were synthesized as described by Davisson et al. (8). Authentic prenols and prenyl phosphates of various chain lengths were obtained from the Institute of Biochemistry and Biophysics, Polish Academy of Sciences (Warsaw, Poland), and ω,E,E,Z-geranylgeraniol was a gift from C. J. Waechter (University of Kentucky). Kieselgel 60 F254 thin-layer chromatography (TLC) plates were from EM Science (Gibbstown, N.J.), and LKC18F reverse-phase TLC plates were from Whatman (Maidstone, England).
Subcellular fractionation.
M. tuberculosis (H37Rv) was grown to mid-log phase in glycerol-alanine-salts medium, washed with saline, and harvested by centrifugation. The resulting pellet was irradiated for 18 h at 2,315 rads/min using a JL Shepard instrument with a 137Cs source. This exposure was calculated to kill 100% of the bacteria but retain 90% of enzyme activity. M. smegmatis was grown to mid-log phase in nutrient broth (Difco, Detroit, Mich.). Cells were harvested by centrifugation, washed with a 0.9% saline solution, and centrifuged again. Some M. smegmatis cultures were harvested and subjected to the same irradiation protocol in order to confirm the effect of irradiation on prenyl diphosphate synthases. The prenyl diphosphate synthase activities in the irradiated preparations were 76% (average of 10 experiments) of those seen in nonirradiated controls.
The washed cell pellets from both species were resuspended in homogenization buffer containing 50 mM MOPS (morpholinepropanesulfonic acid) (pH 7.9), 0.25 M sucrose, 10 mM MgCl2, and 5 mM 2-mercaptoethanol and disrupted by probe sonication on ice with a Sanyo Soniprep 150 (10 cycles of 60 s on and 90 s off). The resulting suspension was centrifuged at 15,000 × g for 15 min. The pellet was discarded, and the supernatant was centrifuged at 200,000 × g for 1 h in a Beckman Ti70.1 rotor. The resulting supernatant (cytosol) was divided into 1-ml aliquots and frozen at −70°C until used. The 200,000 × g pellet (membranes) was resuspended in homogenization buffer, divided into aliquots, and frozen at −70°C. The protein concentrations of the fractions were estimated using a bicinchonchinic acid protein assay kit (Pierce, Rockford, Ill.).
Prenyl diphosphate synthase assays.
Prenyl diphosphate synthase activity was assayed in mixtures containing 50 mM MOPS (pH 7.9), 10 mM sodium orthovanadate, 5 mM MgCl2, 2.5 mM dithiothreitol, 0.3% Triton X-100, 100 μM allylic diphosphate, 30 μM [14C]IPP, and 200 to 400 μg protein in a final volume of 200 μl. After incubation at 37°C for 10 to 60 min (M. smegmatis) or 1 to 12 h (M. tuberculosis), the reaction was stopped by the addition of 1 ml of water saturated with NaCl. Reactions were assayed under conditions linear for both time and protein concentration. Radiolabeled products were extracted with butanol saturated with water, and an aliquot was taken for liquid scintillation spectrometry. The radiolabeled products were characterized by TLC before and after treatment with potato acid phosphatase.
Enzymatic dephosphorylation of reaction products.
Dephosphorylation of prenyl diphosphates to determine the length of the prenyl chain and the stereochemistry of the isoprene units was accomplished essentially as described by Fujii et al. (11). Samples were dried under a stream of nitrogen and dissolved in 5 ml of buffer containing 100 mM sodium acetate (pH 4.8), 0.1% Triton X-100, and 60% methanol. After a brief bath sonication, 1.5 U of potato acid phosphatase were added, and the mixture was incubated at room temperature overnight. Dephosphorylated products were extracted three times with 1 ml of n-hexane, the pooled extracts were washed with water, and the solvent was evaporated under nitrogen. Samples were redissolved in chloroform-methanol (2:1, vol/vol), and aliquots were taken for liquid scintillation spectrometry and analysis by TLC.
Product analysis.
Analysis of the chain length of the dephosphorylated products was accomplished by TLC on LKC18F plates developed in methanol-acetone (8:2, vol/vol). Radioactive spots were located with a System 200 Imaging Scanner (Bioscan Inc., Washington, D.C.) or by autoradiography. Standard polyprenols were located with an anisaldehyde spray reagent (9).
The radioactive spots derived from the dephosphorylated, enzymatically labeled products that were identified as either farnesol or geranylgeraniol were scraped from the reverse-phase TLC plates and extracted from the gel with chloroform-methanol (2:1, vol/vol). The extracts were pooled, dried under nitrogen, and dissolved in chloroform-methanol (2:1, vol/vol) containing authentic, nonradioactive ω,E,E- and ω,E,Z-farnesol or ω,E,E,Z- and ω,E,E,E-geranylgeraniol. The stereochemistry of the radiolabeled product when one isoprene unit was added to an allylic primer of known stereochemistry was determined by TLC on Silica Gel G60 plates developed with toluene-ethyl acetate (7:3, vol/vol).
RESULTS
Enzymatic transfer of radiolabeled prenyl groups from [14C]IPP into allylic prenyl diphosphates.
The cytosolic and membrane fractions prepared from M. smegmatis and M. tuberculosis contain enzymatic activities that incorporate radioactivity from [14C]IPP into longer allylic diphosphate products using DMAPP, GPP, ω,E,E-FPP, and ω,E,E,E-GGPP as reaction primers (Table 1). The cytosolic fractions from both organisms were most active in the presence of GPP. The membrane activities were also most active in the presence of GPP but could utilize the longer-chain primers ω,E,E-FPP and ω,E,E,E-GGPP more effectively, especially in M. smegmatis. When no allylic primer was added to the reaction mixtures containing either cytosol or membranes, no prenyl diphosphates were formed, even though a small amount of radioactivity was incorporated into butanol-extractable compounds in the case of M. smegmatis.
TABLE 1.
Incorporation of [14C]IPP into allylic diphosphates catalyzed by cytosol or membrane fractions prepared from M. tuberculosis or M. smegmatis in the presence of various allylic diphosphate primersa
| Allylic primer | Incorporation (pmol/mg/min) with:
|
|||
|---|---|---|---|---|
|
M. tuberculosis
|
M. smegmatis
|
|||
| Cytosol | Membranes | Cytosol | Membranes | |
| None | 0 | 0 | 0 | 0 |
| DMAPP | 4.0 | 0.6 | 45 | 15 |
| GPP | 25.4 | 32.2 | 215 | 188 |
| ω,E,E-FPP | 5.8 | 5.7 | 72 | 160 |
| ω,E,E,E-GGPP | 0.4 | 1.3 | 25 | 98 |
Reaction mixtures were as described in Materials and Methods.
Characterization of chain lengths of enzymatically labeled products synthesized by mycobacterial cytosol.
In order to determine the chain lengths of the radiolabeled products synthesized by the enzymatic reactions, they were extracted, dephosphorylated, and analyzed by reverse-phase TLC. Although the rates of the reactions were low in M. tuberculosis fractions, the incorporation of IPP into product was linear for up to 12 h. Analysis of the products showed that the ratios of prenyl diphosphates produced in each assay remained the same at all time points up to and including 12 h (data not shown). These properties allowed the generation of sufficient product for subsequent chain length and stereochemical analysis.
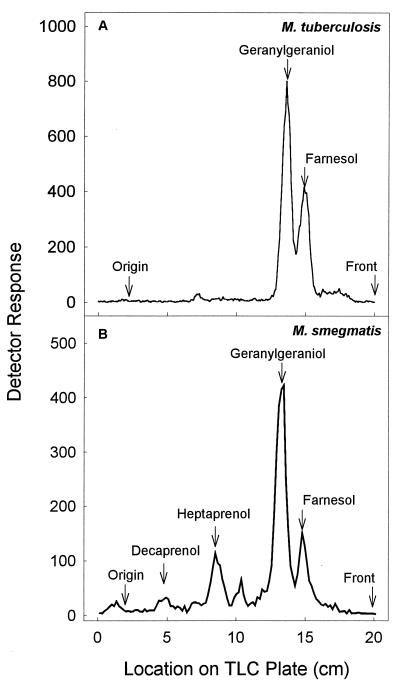
Figure 2 shows TLC analysis of 14C-labeled prenols generated by dephosphorylation of prenyl diphosphates synthesized by M. tuberculosis and M. smegmatis cytosol incubated in the presence of [14C]IPP and GPP. The major product was identified as geranylgeraniol (GGPP prior to dephosphorylation), with significantly smaller amounts of FPP being produced. When M. smegmatis cytosol was the enzyme source, relatively small amounts of heptaprenyl diphosphate and decaprenyl diphosphate were also detected (Fig. 2B).
FIG. 2.
TLC analysis of products synthesized by M. tuberculosis (A) or M. smegmatis (B) cytosol in the presence of [14C]IPP and GPP. Prenyl diphosphate synthase activity was assayed in mixtures containing 50 mM MOPS (pH 7.9), 10 mM sodium orthovanadate, 5 mM MgCl2, 2.5 mM dithiothreitol, 0.3% Triton X-100, 100 μM allylic diphosphate, 30 μM [14C]IPP, and 300 μg of protein in a final volume of 200 μl. Reaction mixtures were incubated at 37°C for 60 min (A) or 10 min (B). The reactions were stopped by the addition of 1 ml of water saturated with NaCl, and the product was extracted with n-butanol saturated with water. The resulting prenyl diphosphates were dephosphorylated with potato acid phosphatase, and equivalent amounts of radioactivity derived from dephosphorylated products were analyzed on LKC18F TLC plates developed in methanol-acetone (8:2, vol/vol). Radioactive spots were located with a System 200 Imaging Scanner (Bioscan Inc.), and standard polyprenols were located with an anisaldehyde spray reagent (9).
In other experiments, using enzymes from either organism, no detectable GPP was produced when DMAPP was used as the primer, and no radiolabeled FPP was produced when cold ω,E,E-FPP was used as the reaction primer (data not shown).
Characterization of chain lengths of enzymatically labeled products synthesized by mycobacterial membranes.
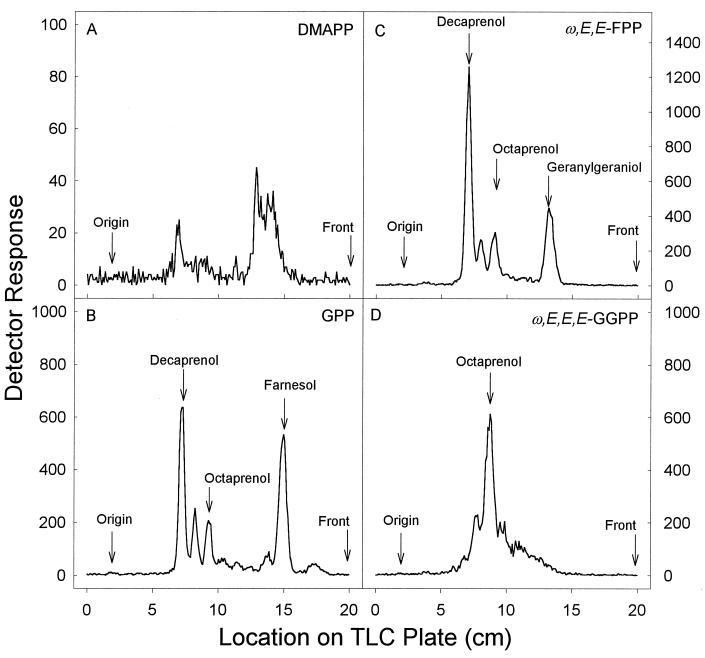
The low rate of synthesis of prenyl diphosphates by M. tuberculosis membranes from [14C]IPP and DMAPP resulted in very small amounts of product for analysis, thus preventing unequivocal identification (Fig. 3A). However, M. tuberculosis membranes incubated with GPP as the reaction primer resulted in FPP and decaprenyl diphosphate being the major products, but products that correspond to prenyl diphosphates having eight and nine isoprene units were also formed (Fig. 3B). Only geranylgeranyl diphosphate and octa-, nona-, and decaprenyl diphosphate were synthesized when ω,E,E-FPP was used as the reaction primer, and when ω,E,E,E-GGPP was used as the reaction primer, octaprenyl diphosphate was formed at a very low rate (∼1 pmol/mg/min).
FIG. 3.
TLC analysis of products synthesized by M. tuberculosis membranes in the presence of [14C]IPP and either DMAPP (A), GPP (B), ω,E,E-FPP (C), or ω,E,E,E-GGPP (D). Assay conditions were as described for Fig. 2. The radiolabeled prenyl diphosphates were dephosphorylated with potato acid phosphatase. Equivalent amounts of radioactivity derived from dephosphorylated products were analyzed on LKC18F TLC plates developed in methanol-acetone (8:2, vol/vol), except in panel A, where all of the radioactivity was loaded. Radioactive spots were located with a System 200 Imaging Scanner (Bioscan Inc.), and standard polyprenols were located with an anisaldehyde spray reagent (9).
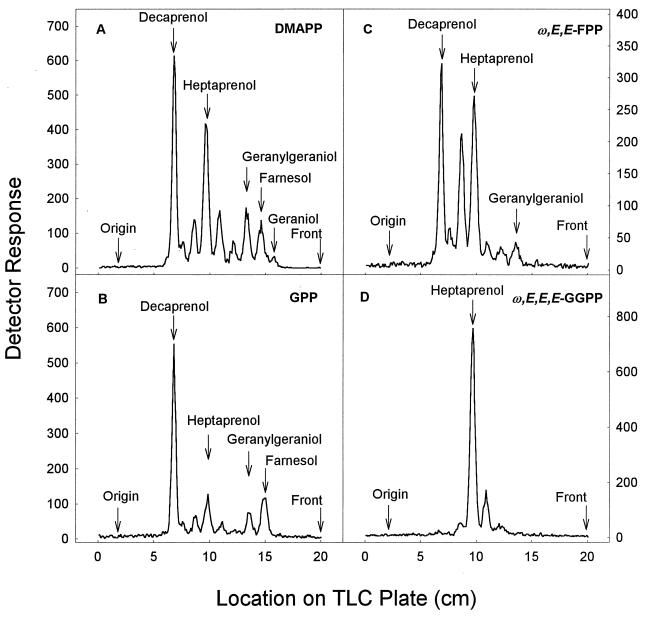
When M. smegmatis membranes were incubated with DMAPP as the primer and the products were subsequently dephosphorylated, a range of prenyl alcohols was seen, including geraniol, farnesol, geranylgeraniol, heptaprenol, and decaprenol, as well as peaks with mobilities consistent with pentaprenol, hexaprenol, and octaprenol (Fig. 4A). Similarly, when these membranes were incubated using GPP or ω,E,E-FPP as the reaction primer, decaprenyl diphosphate was the major product, but products that correspond to prenyl diphosphates of intermediate chain length were seen. When ω,E,E,E-GGPP was used as the reaction primer, heptaprenyl diphosphate was the major product. The specific activity of the membrane-associated heptaprenyl diphosphate synthesis from M. smegmatis membranes was 85 pmol/mg/min.
FIG. 4.
TLC analysis of products synthesized by M. smegmatis membranes in the presence of [14C]IPP and either DMAPP (A), GPP (B), ω,E,E-FPP (C), or ω,E,E,E-GGPP (D). Assay conditions were as described for Fig. 2. The radiolabeled prenyl diphosphates were dephosphorylated with potato acid phosphatase, and equivalent amounts of radioactivity were analyzed on LKC18F TLC plates developed in methanol-acetone (8:2, vol/vol). Radioactive spots were located with a System 200 Imaging Scanner (Bioscan Inc.), and standard polyprenols were located with an anisaldehyde spray reagent (9).
Characterization of stereochemistries of enzymatically labeled products.
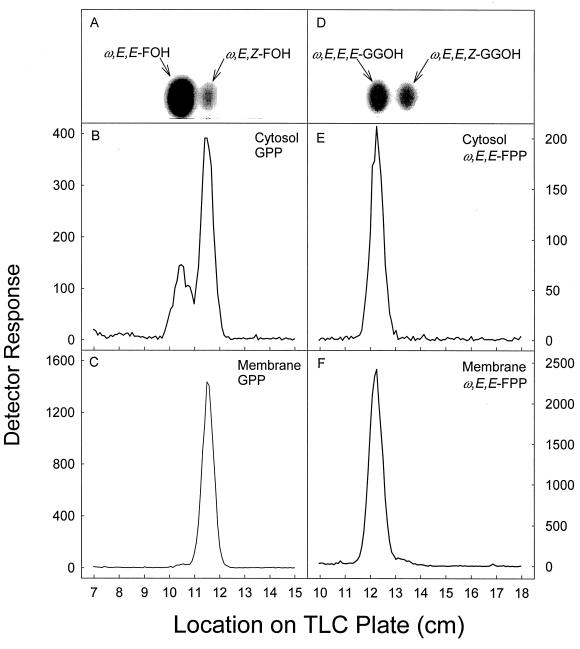
When the relatively small amount of FPP produced by M. tuberculosis cytosol incubated with either DMAPP or GPP as the primer (Fig. 2) was dephosphorylated and analyzed for stereochemistry by TLC, it was apparent that the ω,E,Z configuration was dominant (Fig. 5B). Small, but reproducible, amounts of ω,E,E-FPP were seen in these reactions.
FIG. 5.
Stereochemical analysis of FPP and GGPP enzymatically synthesized by M. tuberculosis cytosol or membranes using GPP (B and C) or ω,E,E-FPP (E and F) as the allyic primer. Assay conditions were as described for Fig. 2. The radiolabeled prenyl diphosphates were dephosphorylated with potato acid phosphatase. Dephosphorylated products were analyzed on LKC18F TLC plates as described for Fig. 2. Radioactive spots corresponding to farnesol and geranylgeraniol were located with a System 200 Imaging Scanner (Bioscan Inc.) and scraped from the plates. The radiolabeled material was extracted from the gel and applied to a Silica Gel G60 TLC plate, which was developed in toluene-ethyl acetate (7:3, vol/vol). Radioactive spots were located with a System 200 Imaging Scanner (Bioscan Inc.), and standard polyprenols (A and D) were located with an anisaldehyde spray reagent (9).
The majority of the radiolabeled GGPP produced when M. tuberculosis cytosol was incubated with ω,E,E-FPP was identified as ω,E,E,E-geranylgeraniol after it had been dephosphorylated (Fig. 5E). The farnesol that was produced by dephosphorylation of the radiolabeled products generated by incubation of M. tuberculosis membranes with [14C]IPP and GPP is virtually all ω,E,Z-farnesol (Fig. 5C). When the geranylgeraniol that was produced by the dephosphorylation of the radiolabeled products generated when M. tuberculosis membranes were incubated with ω,E,E-FPP as the primer was analyzed for stereoconformation, the material comigrated with authentic ω,E,E,E-geranylgeraniol (Fig. 5F).
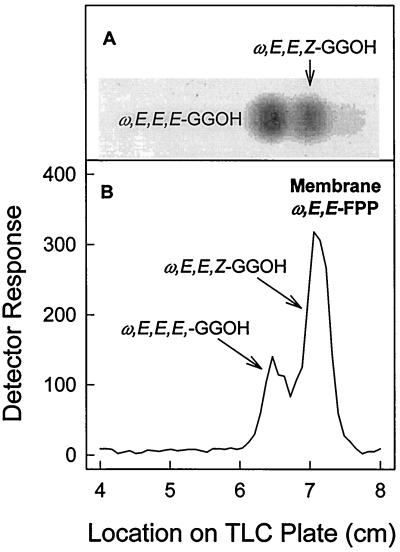
The stereochemistries of short-chain prenyl diphosphates synthesized by subcellular fractions derived from M. smegmatis were essentially the same as those described for the products synthesized by the fractions derived from M. tuberculosis. There was, however, one notable exception. When the geranylgeraniol that was produced by the dephosphorylation of the radiolabeled products generated when M. smegmatis membranes were incubated with ω,E,E-FPP as the reaction primer was analyzed, ω,E,E,Z-geranylgeraniol was dominant, although a significant amount of ω,E,E,E-GGPP was also produced (Fig. 6). Synthesis of ω,E,E,Z-GGPP was not observed in incubations in which M. tuberculosis membranes were used as the enzyme source.
FIG. 6.
Stereochemical analysis of GGPP enzymatically synthesized by M. smegmatis membranes using ω,E,E-FPP as the allyic primer (B). Assay conditions were as described for Fig. 2. The radiolabeled prenyl diphosphates were dephosphorylated with potato acid phosphatase, and the resulting geranylgeraniol was purified as described for Fig. 5. The radiolabeled geranylgeraniol was applied to a silica gel G60 TLC plate, which was developed in toluene-ethyl acetate (7:3, vol/vol). Radioactive spots were located with a System 200 Imaging Scanner (Bioscan Inc.), and standard polyprenols (A) were located with an anisaldehyde spray reagent (9).
DISCUSSION
The results from these experiments suggest that there are several prenyl diphosphate synthase activities found in association with the membrane and cytosol fractions derived from both M. smegmatis and M. tuberculosis. The data shown in Table 1 suggest that the cytosolic prenyl diphosphate synthases prefer short-chain allylic primers as substrates, whereas the membrane enzymes prefer longer primers. However, the specific activity of the prenyl diphosphate synthases in M. smegmatis was much higher than that in M. tuberculosis.
It is presently unclear whether the differences in the rates of synthesis of prenyl diphosphates in M. tuberculosis and M. smegmatis are due to relative differences in the abundances of the enzymes or in the catalytic efficiencies of the enzymes. It will be interesting to determine if the activities of these enzymes are limiting for the formation of cell wall in M. tuberculosis and M. smegmatis (slow growers and fast growers, respectively), as speculated earlier (2, 4, 15, 26). It is clear, however, that M. tuberculosis and M. smegmatis have Pol-P synthetic pathways that are significantly different both quantitatively and qualitatively.
Short-chain cytosolic prenyl diphosphate synthases in mycobacteria.
In both species the major product seen when cytosol was incubated with [14C]IPP and DMAPP, GPP, or ω,E,E-FPP was ω,E,E,E-GGPP. Interestingly, no radioactive GPP was synthesized from DMAPP, even though GPP is an obligate intermediate in the synthesis of all of the larger prenyl diphosphates. Similarly, only small amounts of ω,E,E-FPP were found, although it is also an obligate intermediate in the conversion of DMAPP and/or GPP to ω,E,E,E-GGPP. The majority of the FPP synthesized by both the cytosol and membranes was ω,E,Z-FPP, which is unlikely to be an intermediate in the synthesis of ω,E,E,E-GGPP. Since ω,E,E,E-GGPP was the only isomer of GGPP isolated from the cytosolic incubations and both isomers of FPP are water soluble (and hence available in the reaction mixtures), these results suggest that there may be a single multifunctional enzyme synthesizing ω,E,E,E-GGPP from DMAPP in mycobacterial cytosol. If this is the case, the enzyme would be analogous to several eukaryotic ω,E,E-FPP synthases that are also multifunctional. For example, purified and crystallized avian ω,E,E-FPP synthase synthesizes primarily ω,E,E-FPP when incubated with DMAPP as the allylic primer and releases only trace amounts of the GPP intermediate (18).
Membrane-associated prenyl diphosphate synthases in mycobacteria.
The prenyl diphosphate synthase activities derived from the membrane fractions of M. tuberculosis and M. smegmatis differed in several aspects. The membrane activities from M. smegmatis were capable of utilizing DMAPP as a reaction primer, whereas the membrane activities from M. tuberculosis could not. The predominant products of reactions using M. tuberculosis membranes and GPP as the reaction primer were ω,E,Z-FPP and decaprenyl diphosphate. However, when ω,E,E-FPP was used in the reaction, the primary products were decaprenyl diphosphate and ω,E,E,E-geranylgeranyl diphosphate; M. tuberculosis did not synthesize heptaprenyl diphosphate. In contrast, M. smegmatis membranes incubated with [14C]IPP and DMAPP, GPP, or ω,E,E-FPP, yielded a series of products ranging in size from GPP to decaprenyl diphosphate, with heptaprenyl diphosphate as a major product.
Both M. tuberculosis and M. smegmatis membranes synthesized products with chain lengths that are consistent with those of the mannose carriers previously reported (24, 25), indicating that the preparations were synthesizing physiologically relevant molecules.
Membranes from either bacterium incubated with ω,E,E,E-GGPP produced a single major product. M. tuberculosis membranes synthesized octaprenyl diphosphate (at a very low rate), while M. smegmatis membranes synthesized heptaprenyl diphosphate. It is unlikely that the octaprenyl diphosphate synthesized by M. tuberculosis is a precursor of decaprenyl diphosphate since very little decaprenyl diphosphate was synthesized in the presence of ω,E,E,E-GGPP but decaprenyl diphosphate was the primary product when GPP was the reaction primer. Since it is not possible to assign stereoconformations to molecules to which more than one isoprene unit is added (using the techniques reported here), it was not clear whether the octaprenyl diphosphate synthesized by M. tuberculosis membranes when GPP was used as a primer was identical to the octaprenyl diphosphate synthesized when ω,E,E,E-GGPP was used. It is also unclear what the function of the octaprenyl diphosphate is; the molecule is synthesized in much lower quantities than the heptaprenyl diphosphate of M. smegmatis and has not been reported as a mannose carrier (24).
Heptaprenyl diphosphate synthesis is particularly interesting due to its presence in M. smegmatis and absence in M. tuberculosis. Only a small amount of one intermediate (probably hexaprenyl diphosphate) can be seen in reaction mixtures in which M. smegmatis membranes are incubated with [14C]IPP and ω,E,E,E-GGPP (Fig. 4D). The synthetic activity appears to be due to the presence of a peripheral membrane enzyme(s) that can be released with 1 M KCl. Subsequent precipitation by ammonium sulfate and anion-exchange chromatography did not separate the activity into components (data not shown). Therefore, this activity appears to be due to a single multifunctional enzyme. This observation is more consistent with the structure of the heptaprenyl phosphoryl mannose reported by Wolucka and de Hoffmann (27) than with the structure reported by Besra et al. (6).
When ω,E,E-FPP was used in reactions with M. smegmatis membranes, both decaprenyl diphosphate and heptaprenyl diphosphate were synthesized, which is probably due to the fact that the membrane activities synthesize both ω,E,E,E-GGPP and ω,E,E,Z-GGPP (Fig. 6). However, when ω,E,E-FPP was incubated with the M. tuberculosis membranes, decaprenyl diphosphate was synthesized, even though we believe that ω,E,Z-FPP should be the precursor for decaprenyl diphosphate (assuming that the decaprenyl phosphate from this organism has the same stereochemistry as decaprenyl phosphate from M. smegmatis [28]), and there was no observable formation of ω,E,E,Z-GGPP (Fig. 5). This observation suggests that the enzyme that initiates the additions of IPP to FPP in order to form decaprenyl diphosphate is not strictly specific for one stereoisomer, as reported for other prenyl diphosphate synthases that are capable of utilizing allylic primers of various chain lengths and stereochemistries as substrates (7, 10, 16).
Isoprenoid chain elongation in mycobacteria.
Isoprenoid chain elongation in Mycobacterium spp. appears to be more complex than previously reported for eubacteria and for mammals. It has been reported that there are four prenyl diphosphate synthases that synthesize ω,E,E-FPP, ω,E,E,E-GGPP, all-E-octaprenyl diphosphate, and a mixed Z,E-polyprenyl diphosphate in E. coli (12) and Bacillus subtilis (23). Mammalian cells typically contain an ω,E,E-FPP synthase, an ω,E,E,E-GGPP synthase, an all-E-prenyl diphosphate synthase (for ubiquinone synthesis), and a Z-prenyl diphosphate synthase (for dolichyl phosphate synthesis) (13). In each of these examples, one of the products was a ubiquinone or menaquinone precursor. M. smegmatis could have as many as 10 prenyl diphosphate synthases. This is probably an overestimate, as it has been shown that some prenyl diphosphate synthase enzymes catalyze the addition of IPP to FPP to yield products of more than one chain length (3). However, it is also possible that the estimate of 10 enzymes is accurate, as some prenyl diphosphate synthases generate products with only one chain length (1, 23). The actual number of prenyl diphosphate synthases in M. smegmatis probably lies between 4 and 10.
We have identified seven open reading frames in the M. tuberculosis genome that encode proteins predicted to have significant homology to known prenyl diphosphate synthases. However, data presented here suggest that there could be as few as four active prenyl diphosphate synthases in this species. Cloning of the open reading frames and purification of the expressed enzymes will allow a careful characterization of their activities without a significant background of prenyl diphosphate synthesis generated by other enzymes. Two of these open reading frames, Rv1086 and Rv2361c, have been cloned and identified as encoding an ω,E,Z-FPP and a decaprenyl diphosphate synthase, respectively (21).
ACKNOWLEDGMENT
This work was supported by grant AI-18357 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
REFERENCES
- 1.Allen C M, Keenan M V, Sack J. Lactobacillus plantarum undecaprenyl pyrophosphate synthetase: purification and reaction requirements. Arch Biochem Biophys. 1976;175:236–248. doi: 10.1016/0003-9861(76)90504-x. [DOI] [PubMed] [Google Scholar]
- 2.Anderson R G, Hussey H, Baddiley J. The mechanism of wall synthesis in bacteria. The organization of enzymes and isoprenoid phosphates in the membrane. Biochem J. 1972;127:11–25. doi: 10.1042/bj1270011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baba T, Allen C M. Prenyl transferases from Micrococcus luteus: characterization of undecaprenyl pyrophosphate synthetase. Arch Biochem Biophys. 1980;200:474–484. doi: 10.1016/0003-9861(80)90379-3. [DOI] [PubMed] [Google Scholar]
- 4.Baddiley J. Teichoic acids in cell walls and membranes of bacteria. Essays Biochem. 1972;8:35–77. [PubMed] [Google Scholar]
- 5.Besra G S, Morehouse C B, Rittner C M, Waechter C J, Brennan P J. Biosynthesis of mycobacterial lipoarabinomannan. J Biol Chem. 1997;272:18460–18466. doi: 10.1074/jbc.272.29.18460. [DOI] [PubMed] [Google Scholar]
- 6.Besra G S, Sievert T, Lee R E, Slayden R A, Brennan P J, Takayama K. Identification of the apparent carrier in mycolic acid synthesis. Proc Natl Acad Sci USA. 1994;91:12735–12739. doi: 10.1073/pnas.91.26.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crick D C, Rush J S, Waechter C J. Characterization and localization of a long-chain isoprenyltransferase activity in porcine brain: proposed role in the biosynthesis of dolichyl phosphate. J Neurochem. 1991;57:1354–1362. doi: 10.1111/j.1471-4159.1991.tb08301.x. [DOI] [PubMed] [Google Scholar]
- 8.Davisson V J, Woodside A B, Poulter C D. Synthesis of allylic and homoallylic isoprenoid pyrophosphates. Methods Enzymol. 1985;110:130–144. doi: 10.1016/s0076-6879(85)10068-6. [DOI] [PubMed] [Google Scholar]
- 9.Dunphy P J, Kerr J D, Pennock J F, Whittle K J, Feeney J. The plurality of long chain isoprenoid alcohols (polyprenols) from natural sources. Biochim Biophys Acta. 1967;136:136–147. doi: 10.1016/0304-4165(67)90329-7. [DOI] [PubMed] [Google Scholar]
- 10.Ericsson J, Thelin A, Chojnacki T, Dallner G. Substrate specificity of cis-prenyltransferase in rat liver microsomes. J Biol Chem. 1992;267:19730–19735. [PubMed] [Google Scholar]
- 11.Fujii H, Koyama T, Ogura K. Efficient enzymatic hydrolysis of polyprenyl pyrophosphates. Biochim Biophys Acta. 1982;712:716–718. [PubMed] [Google Scholar]
- 12.Fujisaki S, Nishino T, Katsuki H. Isoprenoid synthesis in Escherichia coli. Separation and partial purification of four enzymes involved in the synthesis. J Biochem (Tokyo) 1986;99:1327–1337. doi: 10.1093/oxfordjournals.jbchem.a135600. [DOI] [PubMed] [Google Scholar]
- 13.Grunler J, Ericsson J, Dallner G. Branch-point reactions in the biosynthesis of cholesterol, dolichol, ubiquinone and prenylated proteins. Biochim Biophys Acta. 1994;1212:259–277. doi: 10.1016/0005-2760(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 14.Hemming F W. Lipids in glycan synthesis. In: Goodwin T W, editor. Biochemistry, series 1. Vol. 4. London, United Kingdom: Butterworths; 1974. pp. 39–97. [Google Scholar]
- 15.Higashi Y, Siewert G, Strominger J L. Biosynthesis of the peptidoglycan of bacterial cell walls. XIX. Isoprenoid alcohol phosphokinase. J Biol Chem. 1970;245:3683–3690. [PubMed] [Google Scholar]
- 16.Ishii K, Sagami H, Ogura K. A novel prenyltransferase from Paracoccus denitrificans. Biochem J. 1986;233:773–777. doi: 10.1042/bj2330773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mikusova K, Mikus M, Besra G S, Hancock I, Brennan P J. Biosynthesis of the linkage region of the mycobacterial cell wall. J Biol Chem. 1996;271:7820–7828. doi: 10.1074/jbc.271.13.7820. [DOI] [PubMed] [Google Scholar]
- 18.Reed B C, Rilling H C. Crystallization and partial characterization of prenyltransferase from avian liver. Biochemistry. 1975;14:50–54. doi: 10.1021/bi00672a009. [DOI] [PubMed] [Google Scholar]
- 19.Rieber M, Imaeda T, Cesari I M. Bacitracin action on membranes of mycobacteria. J Gen Microbiol. 1969;55:155–159. doi: 10.1099/00221287-55-1-155. [DOI] [PubMed] [Google Scholar]
- 20.Sacchettini J C, Poulter C D. Creating isoprenoid diversity. Science. 1997;277:1788–1789. doi: 10.1126/science.277.5333.1788. [DOI] [PubMed] [Google Scholar]
- 21.Schulbach M C, Brennan P J, Crick D C. Identification of a short (C15) chain Z-Isoprenyl diphosphate synthase and a homologous long (C50) chain isoprenyl diphosphate synthase in Mycobacterium tuberculosis. J Biol Chem. 2000;275:22876–22881. doi: 10.1074/jbc.M003194200. [DOI] [PubMed] [Google Scholar]
- 22.Storm D R, Strominger J L. Complex formation between bacitracin peptides and isoprenyl pyrophosphates. The specificity of lipid-peptide interactions. J Biol Chem. 1973;248:3940–3945. [PubMed] [Google Scholar]
- 23.Takahashi I, Ogura K. Prenyltransferases of Bacillus subtilis: undecaprenyl pyrophosphate synthetase and geranylgeranyl pyrophosphate synthetase. J Biochem (Tokyo) 1982;92:1527–1537. doi: 10.1093/oxfordjournals.jbchem.a134077. [DOI] [PubMed] [Google Scholar]
- 24.Takayama K, Goldman D S. Enzymatic synthesis of mannosyl-1-phosphoryl-decaprenol by a cell-free system of Mycobacterium tuberculosis. J Biol Chem. 1970;245:6251–6257. [PubMed] [Google Scholar]
- 25.Takayama K, Schnoes H K, Semmler E J. Characterization of the alkali-stable mannophospholipids of Mycobacterium smegmatis. Biochim Biophys Acta. 1973;316:212–221. doi: 10.1016/0005-2760(73)90011-8. [DOI] [PubMed] [Google Scholar]
- 26.van Heijenoort J. Murein synthesis. In: Neidhardt F C, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 1025–1034. [Google Scholar]
- 27.Wolucka B A, de Hoffmann E. Isolation and characterization of the major form of polyprenyl-phospho-mannose from Mycobacterium smegmatis. Glycobiology. 1998;8:955–962. doi: 10.1093/glycob/8.10.955. [DOI] [PubMed] [Google Scholar]
- 28.Wolucka B A, McNeil M R, de Hoffmann E, Chojnacki T, Brennan P J. Recognition of the lipid intermediate for arabinogalactan/arabinomannan biosynthesis and its relation to the mode of action of ethambutol on mycobacteria. J Biol Chem. 1994;269:23328–23335. [PubMed] [Google Scholar]