Abstract
Driveline infection (DLI) is common after left ventricular assist device (LVAD). Limited data exist on DLI prevention and management. We investigated the impact of standardized driveline care initiatives, specific pathogens, and chronic antibiotic suppression (CAS) on DLI outcomes. 591 LVAD patients were retrospectively categorized based on driveline care initiatives implemented at our institution (2009–2019). Era (E)1: nonstandardized care; E2: standardized driveline care protocol; E3: addition of marking driveline exit site; E4: addition of “no shower” policy. 87(15%) patients developed DLI at a median (IQR) of 403(520) days. S. aureus and P. aeruginosa were the most common pathogens. 31 (36%) of DLI patients required incision and drainage (I&D) and 5 (5.7%) device exchange. P. aeruginosa significantly increased risk for initial I&D (HR 2.7, 95% CI, 1.1–6.3) and recurrent I&D or death (HR 4.2, 95% CI, 1.4–12.5). Initial I&D was associated with a significant increased risk of death (HR 2.92 (1.33–6.44); P = 0.008) when compared to patients who did not develop DLI. Implementation of standardized driveline care protocol (E2) was associated with increased 2-year freedom from DLI compared to nonstandardized care (HR 0.36, 95% CI, 0.2–0.6, P < 0.01). Additional preventive strategies (E3&E4) showed no further reduction in DLI rates. 57(65%) DLI patients received CAS, 44% of them required escalation to intravenous antibiotics and/or I&D. Presence of P. aeruginosa DLI markedly increased risk for I&D or death. Conditional survival of patients progressing to I&D is diminished. Standardized driveline care protocol was associated with a significant reduction in DLI, while additional preventive strategies require further testing.
Keywords: left ventricular assist device, mechanical circulatory support, driveline infection, chronic antibiotic suppression
Left ventricular assist device (LVAD) is an established therapy for advanced heart failure (HF). Despite improvements in device technology, infections after LVAD remain among the most common complications.1 Infections occur in up to 50% of patients, negatively impacting thrombotic and bleeding complications, rehospitalization rates, overall survival, and possible outcomes after heart transplant (HT).2
The International Society for Heart and Lung Transplantation (ISHLT) has grouped LVAD infections into three categories: non-VAD infections, VAD-related infections, and VAD-specific infections.3 VAD-specific infections include infections that are related to LVAD components, such as the pump, cannula, pocket, or percutaneous driveline (DLI).3 This distinction among VAD-specific infections is important, as DLIs that extend deeper towards the pump may become refractory to standard antimicrobial therapy, require surgical intervention and are associated with reduced survival when compared to more superficial DLIs.4
Several risk factors for DLI have been proposed, including obesity, female sex, diabetes, and poor psychosocial support; yet none of these are readily modifiable.5,6 Thus, prevention of DLIs appears critical. However, only a few studies have investigated standardized prevention strategies with specific driveline care protocols,7,8 and none have reported on contemporary cohorts that include HeartMate 3 (HM3) support.
The most common microorganisms causing DLIs are biofilm-producing bacteria such as Staphylococcus aureus and Pseudomonas aeruginosa.5,9,10 P. aeruginosa DLIs are particularly difficult to treat due to limited antibiotic susceptibility and tendency to develop resistance to antibacterial agents.11,12 P. aeruginosa is a waterborne pathogen,12,13 thus preventing water contamination of the driveline exit site might effectively reduce this infection. Importantly, limited data exist on how prognosis for VAD-specific infections differs based on the pathogen type.
Over the past decade, our center has sequentially implemented initiatives aimed at improving driveline care and preventing DLIs. We have transitioned from a nonstandardized to a standardized driveline care protocol, then focused on proper driveline positioning by marking the driveline exit site preoperatively, and, more recently, instituted a “no-shower” policy. Concurrently, management of established DLIs has evolved over time, with more consistent use of chronic antibiotic suppression (CAS) and surgical interventions, such as incision and drainage (I&D).
Herein, we uniquely divided our cohort of LVAD patients based on temporal changes in driveline care that occurred at our institution and aimed to: (1) provide an in-depth analysis of DLI onset, risk factors, antimicrobial and surgical management; (2) describe microbiological profiles; (3) identify clinical and microbial predictors of worse clinical outcomes as defined by need for I&D or death; and lastly, (4) investigate safety and efficacy of CAS.
Methods
Study Population and Data Collection
This study was approved by Columbia University Irving Medical Center (CUIMC) Institutional Review Board. We enrolled 591 adult patients implanted with LVADs at CUIMC between February 2009 and May 2019 and followed them through June 30th, 2020.
Definitions and Study Design
DLI data were prospectively collected with informed consent and retrospectively adjudicated by an infectious disease (ID) specialist (JA), utilizing ISHLT 2011 criteria for VAD-specific DLI. All DLIs met criteria for at least possible superficial DLI.13 The investigation conforms with the principles outlined in the Declaration of Helsinki.
Time to initial DLI was the time between LVAD implant and first positive driveline wound culture. Polymicrobial infections had ≥2 organisms identified by wound culture within 30 days of DLI diagnosis. Data on antibiotic class, route of administration (oral vs. intravenous (IV)), and duration were collected. CAS was defined as no interruption in antibiotic therapy during follow-up. Escalation in CAS was defined as need to change from oral to IV antibiotics and/or requirement for I&D. Decision to initiate or escalate CAS was at the discretion of ID and surgical consultants. Recurrent positive wound culture among patients not on CAS was defined as any positive wound culture after discontinuation of antibiotics. Driveline Care Strategies (details in Supplemental Digital Content http://links.lww.com/ASAIO/A789).
Beginning in April 2011, LVAD patients were transitioned to a standardized driveline care protocol, as previously described,8 which was later expanded for HM3 care, including pump-specific:(1) driveline dressing kit; (2) educational videos; (3) detailed standardized operative procedure (SOP) for dressing change.
Starting in April 2016, driveline exit sites were marked preoperatively, above the umbilicus and along the midclavicular line. The goal of this initiative was to minimize trauma, by avoiding patients’ belt line, and to facilitate self-care and application of the anchor(s).
Starting in July 2017, a “no-shower” policy was implemented, advising against complete submersion in water and recommending handheld showers for lower body/head and sponge baths for the torso. All patients were educated about bathing routine with the help of occupational therapy and written instructions were provided before discharge.
Thus, the cohort was divided into four eras (Es): E1: nonstandardized driveline care (01/09–03/11); E2: standardized care protocol (04/11–03/16); E3: E2 and marking of the driveline exit site (04/16–06/17); E4: E3 and “no-shower” policy (07/17–05/19). All patients received perioperative antibiotics for 48 hours as per institutional protocol (rifampin, fluconazole, cefazolin, mupirocin nasal ointment). Vancomycin was used as an alternative for patients with penicillin allergies. This strategy has not changed over the study period. Patients who were colonized with MRSA/MSSA did not undergo a formal decolonization process.
The LVAD selection criteria have not changed over the study period and are applied to all types of devices equally. Nearly all LVADs were implanted by the same surgeon (YN), utilizing a full sternotomy approach and standardized driveline tunneling technique (Supplemental Digital Content, http://links.lww.com/ASAIO/A789).
Statistical Analysis
Statistical analysis was conducted using R version 4.0.3. Descriptive statistics are presented as mean ± standard deviation for continuous variables and percentage for categorical variables. Where continuous variables were not normally distributed, data are presented as median, interquartile range (IQR). Differences in means or proportions of baseline characteristics were determined using one-way ANOVA for continuous variables and Pearson’s χ2/Fischer exact test for categorical variables.
Cox proportional hazard models were fit to determine 2-year survival and freedom from DLI using the following variables: era, age, sex, body mass index (BMI) (≥30 vs. <30), HF etiology, diabetes, serum creatinine, albumin, white blood cell count, implant strategy, INTERMACS profile (≤2 vs. >2), pump type. In the analysis of 2-year survival, occurrence of DLI was also accounted for as a time-varying predictor. Cox proportional hazard models were fit to determine 2-year freedom from initial I&D and recurrent I&D or death using the following variables: age, sex, BMI, diabetes, pathogen type (P. aeruginosa vs. others), and presence of bacteremia. Multivariable models for each of these analyses were fit by incorporating any variables with P ≤ 0.2 in the univariable model. The proportional hazards assumption was checked utilizing Schoenfeld Residuals Testing. Kaplan–Meier survival analysis was used to examine: (1) 2-year survival by era and pump type; (2) freedom from DLI by era. Further, 2-year survival post-implant was compared to 2-year survival post-DLI and post-I&D with Cox proportional hazard models with robust estimation of standard errors to account for the time-varying nature of these exposures. This analysis was conditioned on 3-month survival post-LVAD, a timeframe chosen based on the delayed nature of DLI development, making it unlikely that any early death (within the first 3 months) is attributed to DLI complications. Follow-up was censored in case of transplant, loss of follow-up/transferred care, or death. Statistical significance was set at P < 0.05.
Results
Baseline Clinical Characteristics Stratified by Era
Table 1 shows baseline characteristics of 591 patients stratified by era: 93 (15.7%) E1, 305 (51.6%) E2, 82 (13.9%) E3, 111 (18.8%) E4. HMII was implanted in 370 (62.6%), HM3 in 160 (27.1%) and HVAD in 61 (10.3%) patients. As time progressed (from E1 to E4), patients were older, had higher BMI, and were more likely to receive LVAD as DT. Short-term mechanical circulatory support (MCS), HM3 use, bypass time, and intensive care unit length of stay increased over time, while INTERMACS profile and total length of stay remained unchanged.
Table 1.
Baseline Characteristics
| Total Cohort | Era 1 | Era 2 | Era 3 | Era 4 | P value | |
|---|---|---|---|---|---|---|
|
| ||||||
| Number of patients | 591 | 93 | 305 | 82 | 111 | |
| Preoperative characteristics | ||||||
| Age, years | 55.99 ± 13.88 | 51.42 ± 13.26 | 56.35 ± 13.64 | 57.58 ± 13.83 | 57.64 ± 14.44 | 0.0046 |
| Male, n (%) | 476 (80.54) | 71 (76.34) | 249 (81.64) | 66 (80.49) | 90 (81.08) | 0.7289 |
| Race, n (%) | 0.0002 | |||||
| White | 376 (63.95) | 53 (56.99) | 190 (62.5) | 53 (64.63) | 80 (73.39) | |
| Black | 141 (23.98) | 16 (17.2) | 84 (27.63) | 18 (21.95) | 23 (21.10) | |
| Other | 71 (12.07) | 24 (25.81) | 30 (9.87) | 11 (13.41) | 6 (5.50) | |
| BMI, kg/m2 | 27.13 ± 5.83 | 26.05 ± 5.50 | 26.46 ± 5.39 | 27.73 ± 6.75 | 29.41 ± 5.97 | <0.0001 |
| Etiology, Ischemic, n (%) | 269 (45.52) | 44 (47.52) | 134 (43.93) | 38 (46.34) | 53 (47.75) | 0.8793 |
| HTN, n (%) | 355 (56.68) | 47 (50.54) | 167 (54.75) | 49 (59.76) | 72 (64.86) | 0.1554 |
| A fib/flutter, n (%) | 302 (51.10) | 49 (52.69) | 168 (55.08) | 37 (45.12) | 48 (43.24) | 0.1144 |
| Stroke, n (%) | 66 (11.17) | 10 (10.75) | 35 (10.75) | 6 (7.32) | 15 (13.51) | 0.5963 |
| Dialysis, n (%) | 13 (2.22) | 0 (0.00) | 7 (2.32) | 1 (1.22) | 5 (4.50) | 0.1719 |
| Smoking, n (%) | 306 (52.22) | 49 (53.85) | 176 (58.28) | 33 (40.24) | 48 (43.24) | 0.0050 |
| Diabetes, n (%) | 227 (38.41) | 32 (34.43) | 123 (40.33) | 29 (35.37) | 43 (38.74) | 0.6985 |
| INTERMACS Profile, n (%) | 0.8669 | |||||
| ≤2 | 429 (72.59) | 65 (69.89) | 221 (72.46) | 62 (75.61) | 81 (72.97) | |
| >2 | 162 (27.41) | 28 (30.11) | 84 (27.54) | 20 (24.39) | 30 (27.03) | |
| Treatment strategy | 262 (44.33) | 69 (74.19) | 153 (50.16) | 14 (17.07) | 26 (23.42) | <0.0001 |
| BTT, n (%) | ||||||
| MCS support pre-LVAD, n (%)† | 272 (46.02) | 34 (36.56) | 133 (43.61) | 50 (60.98) | 55 (49.55) | <0.01 |
| Laboratory Data | ||||||
| Serum creatinine, mg/dL* | 1.46 ± 0.73 | 1.45 ± 0.59 | 1.47 ± 0.78 | 1.36 ± 0.72 | 1.53 ± 0.68 | 0.4364 |
| Serum albumin, g/dL* | 3.58 ± 0.58 | 3.52 ± 0.52 | 3.58 ± 0.59 | 3.56 ± 0.56 | 3.63 ± 0.62 | 0.6145 |
| Serum total bilirubin, mg/dL* | 1.28 ± 1.05 | 1.41 ± 1.01 | 1.35 ± 1.13 | 1.13 ± 0 .95 | 1.11 ± 0.90 | 0.0682 |
| INR* | 1.31 ± 0.33 | 1.28 ± 0.27 | 1.35 ± 0.39 | 1.27 ± 0.23 | 1.24 ± 0.20 | 0.0125 |
| Platelet count (1000/μL)* | 195.68 ± 74.57 | 200.94 ± 72.96 | 195.08 ± 74.63 | 199.89 ± 83.38 | 189.78 ± 69.23 | 0.6980 |
| Hemoglobin, g/dL* | 11.16 ± 2.17 | 10.65 ± 1.81 | 11.25 ± 2.12 | 11.56 ± 2.22 | 11.04 ± 2.48 | 0.0321 |
| WBC (1000/μL)* | 8.80 ± 3.60 | 8.72 ± 3.60 | 8.62 ± 3.35 | 9.24 ± 5.27 | 9.02 ± 3.19 | 0.4992 |
| Intraoperative Data | ||||||
| Device type | <0.0001 | |||||
| HM II, n (%) | 370 (62.61) | 93 (100.00) | 233 (76.39) | 36 (43.90) | 8 (7.21) | |
| HM 3, n (%) | 160 (27.07) | 0 (0.00) | 25 (8.20) | 39 (47.56) | 96 (86.49) | |
| HVAD, n (%) | 61 (10.32) | 0 (0.00) | 47 (15.41) | 7 (8.54) | 7 (3.61) | |
| Bypass time, min | 102.08 ± 49.84 | 88.18 ± 38.86 | 103.26 ± 50.35 | 97.84 ± 42.40 | 113.62 ± 58.43 | 0.0028 |
| Postoperative data | ||||||
| ICU length of stay, days* | 15.27 ± 18.92 | 12.70 ± 15.32 | 13.47 ± 14.11 | 18.75 ± 30.55 | 19.77 ± 20.59 | 0.0055 |
| Total length of stay, days* | 49.66 ± 37.73 | 49.87 ± 33.20 | 48.61 ± 38.26 | 50.96 ± 44.31 | 51.54 ± 34.39 | 0.9012 |
MCS support includes intraaortic balloon pump, Impella, extracorporeal membrane oxygenation (ECMO), and CentriMag biventricular assist device.
Data presented as mean ± standard deviation.
BMI, body mass index; BTT, bridge to transplantation; HM, HeartMate; HTN, hypertension; HVAD, Heartware ventricular assist device; ICU, intensive care unit; INR, international normalized ratio; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; LVAD, left ventricular assist device; MCS, mechanical circulatory support; WBC, white blood cell count.
Postoperative Survival Stratified by Era
One- and 2-year post-implant survival proportions for the entire cohort were 84.4% and 78.0%, respectively (see Figure S1, Supplemental Digital Content, http://links.lww.com/ASAIO/A789). No significant difference in 2-year survival was noted among patients across eras (see Figure S2, Supplemental Digital Content, http://links.lww.com/ASAIO/A789).
In unadjusted Cox proportional hazards analysis, diabetes, ischemic etiology, higher creatinine, lower albumin, and pump type other than HM3 were associated with increased mortality. After multivariable adjustment, the above variables, except diabetes, remained significant predictors. Notably, the presence of DLI and individual eras did not affect 2-year postimplant survival (Table 2).
Table 2.
Univariable and Multivariable Analysis for Two-Year Survival (N = 590*)
| Univariate HR (95% CI) | P value | Multivariate HR (95% CI) | P value | |
|---|---|---|---|---|
|
| ||||
| E2 vs. E1 | 0.94 (0.55–1.61) | 0.8100 | 0.90 (0.51–1.57) | 0.7103 |
| E3 vs. E2 | 0.60 (0.30–1.17) | 0.1300 | 0.80 (0.39–1.61) | 0.5233 |
| E4 vs. E2 | 0.96 (0.58–1.60) | 0.8850 | 1.69 (0.83–3.46) | 0.1500 |
| Age, years | 1.01 (0.99–1.02) | 0.3900 | ||
| Male | 0.75 (0.48–1.17) | 0.2000 | 0.66 (0.41–1.07) | 0.0938 |
| BMI ≥30 | 1.33 (0.88–2.00) | 0.1710 | 1.53 (0.99–2.34) | 0.0524 |
| Diabetes mellitus | 1.49 (1.02, 2.18) | 0.0398 | 1.29 (0.86–1.92) | 0.2171 |
| Etiology, Ischemic | 1.73 (1.18–2.55) | 0.0054 | 1.72 (1.15–2.58) | 0.0088 |
| INTERMACS Profile ≤2 | 1.15 (0.75, 1.79) | 0.5210 | ||
| Device Intent, DT | 0.90 (0.61,1.34) | 0.6080 | ||
| Serum creatinine, mg/dL | 1.36 (1.16–1.60) | 0.0002 | 1.34 (1.13–1.58) | 0.0006 |
| Serum albumin, g/dL | 0.62 (0.45–0.86) | 0.0037 | 0.67 (0.48–0.92) | 0.0137 |
| WBC (1000/μL) | 1.02 (0.98, 1.07) | 0.2860 | ||
| Pump type (HMII vs. HM3) | 1.67 (1.01,2.79) | 0.0472 | 2.25 (1.08–4.70) | 0.0311 |
| Pump type (HVAD vs. HM3) | 2.97 (1.57, 5.61) | 0.0008 | 4.11 (1.87–9.06) | 0.0005 |
| DLI1 | 1.42 (0.60, 3.34) | 0.4220 | ||
One patient died the day of implant and was excluded from this analysis. Further, three patients did not have serum albumin data, being excluded from the albumin-only model and multivariate model (N = 587).
Utilized a time-varying covariate approach, to account for exposure (DLI) occurring through follow-up.
BMI, body mass index; DLI, driveline infection; DT, destination therapy; E1, Era 1; E2, Era 2; E3, Era 3, E4, Era 4; HM3, HeartMate 3; HMII, HeartMate II; HVAD, HeartWare; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; WBC, white blood cell count.
Incidence, Predictors, and Microbiological Profile of DLIs
The cumulative incidence of DLI occurred in 87 (14.7%) of patients at a median (IQR) of 403 (520) days after LVAD. Baseline characteristics of patients with vs. without incident DLI were overall similar (see Table S1, Supplemental Digital Content, http://links.lww.com/ASAIO/A789). Two-year freedom from DLI differed among eras: 58.7% E1; 83.6% E2; 85.9% E3; 85.9% E4 (P < 0.01) (Figure 1). Notably, device type did not influence DLI incidence (see Figure S3, Supplemental Digital Content, http://links.lww.com/ASAIO/A789).
Figure 1.
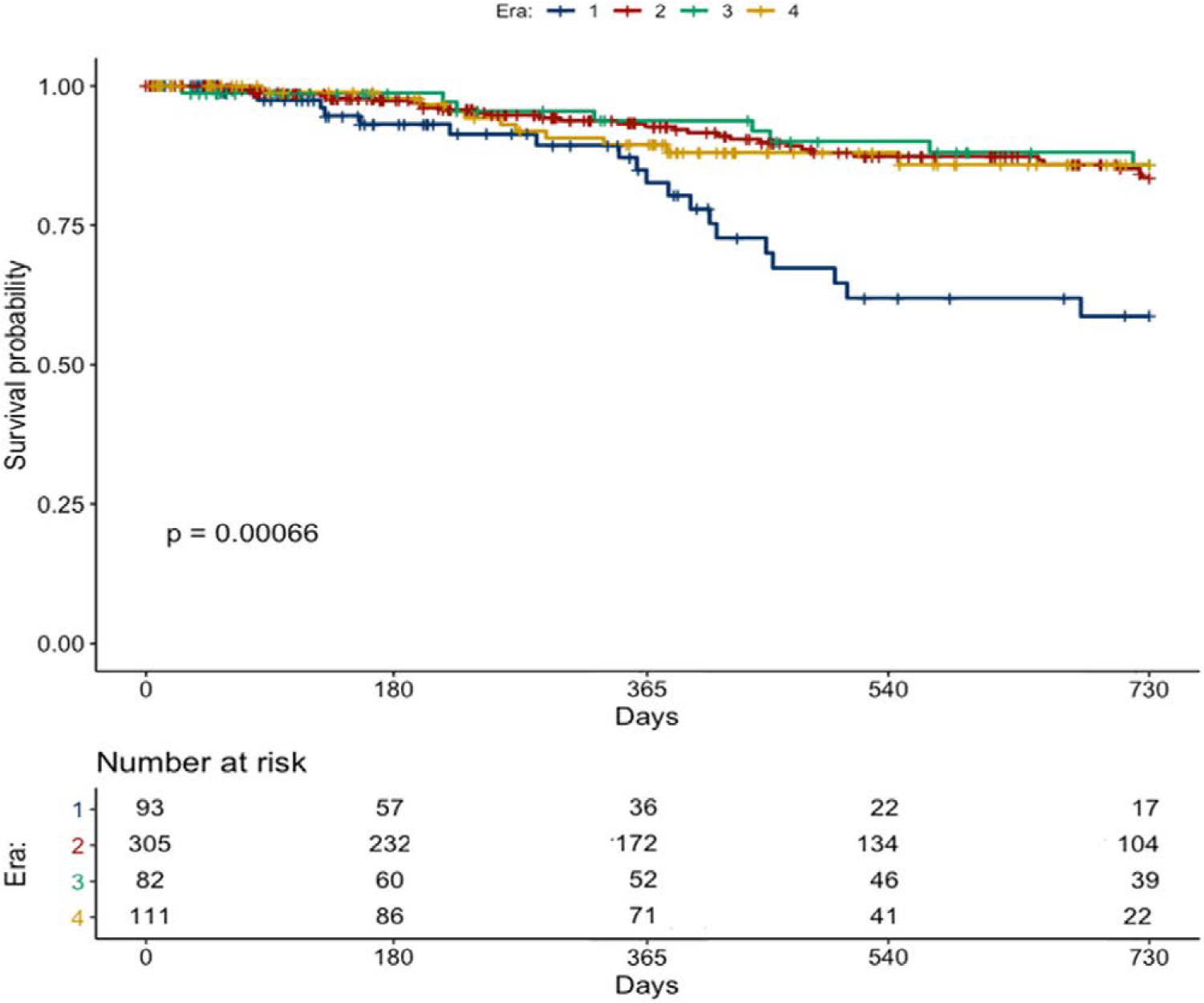
Two-year freedom from driveline infection stratified by era. (Era 1: nonstandardized driveline care protocol; Era 2: standardized driveline care protocol; Era 3: Era 2 and marking of the positioning of driveline exit site; Era 4: Era 3 and “no shower” policy).
In unadjusted analysis, E1 and female sex were independent predictors of DLI at 2 years. After multivariable adjustments, only implant during E1 remained a significant predictor (Table 3).
Table 3.
Univariable and Multivariable Analysis for 2-Year Freedom from Driveline Infection (N = 590*)
| Univariate HR (95% CI) | P value | Multivariate HR (95% CI) | P value | |
|---|---|---|---|---|
|
| ||||
| E2 vs. E1 | 0.35 (0.20–0.62) | 0.0003 | 0.36 (0.20–0.64) | 0.0006 |
| E3 vs. E2 | 0.86 (0.40–1.87) | 0.7046 | 0.89 (0.41–1.95) | 0.7731 |
| E4 vs. E2 | 1.04 (0.52–2.07) | 0.9130 | 1.01 (0.51–2.01) | 0.9847 |
| Age, years | 0.99 (0.97–1.01) | 0.1650 | 1.00 (0.98–1.01) | 0.8053 |
| Male | 0.55 (0.33–0.94) | 0.0280 | 0.59 (0.46–1.30) | 0.0578 |
| BMI ≥30 | 0.90 (0.52–1.55) | 0.6920 | ||
| Diabetes mellitus | 0.81 (0.49–1.33) | 0.4040 | ||
| Etiology, Ischemic | 0.69 (0.42–1.13) | 0.1380 | 0.77 (0.46–1.30) | 0.3226 |
| INTERMACS profile ≤2 | 1.16 (0.68–1.99) | 0.5790 | ||
| Device intent, DT | 1.35 (0.84–2.19) | 0.2170 | ||
| Serum creatinine, mg/dL | 0.92 (0.62–1.36) | 0.6750 | ||
| Serum albumin, g/dL | 1.39 (0.92–2.11) | 0.1220 | 1.37 (0.89–2.10) | 0.1510 |
| WBC (1000/μL) | 0.98 (0.92–1.05) | 0.5760 | ||
| Pump type (HMII vs. HM3) | 1.19 (0.70–2.03) | 0.5280 | ||
| Pump type (HVAD vs. HM3) | 0.65 (0.22–1.90) | 0.4270 | ||
One patient died the day of implant and was excluded from this analysis. Further, three patients did not have serum albumin data, being excluded from the albumin-only model and multivariate model (N = 587).
E1, Era 1; E2, Era 2; E3, Era 3, E4, Era 4; BMI, body mass index; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; DT, destination therapy; WBC, white blood cell count; HM3, HeartMate 3; HVAD, HeartWare; HMII, HeartMate II; DLI, driveline infection.
There were 109 different organisms cultured from 87 DLI patients, the most common were S. aureus (methicillin-sensitive 28.4%, methicillin-resistant 8.3%) and P. aeruginosa (16.5%) (see Table S3, Supplemental Digital Content, http://links.lww.com/ASAIO/A789). Distribution of Gram-positive, Gram-negative and polymicrobial infections was similar across eras (see Figure S4, Table S4, Supplemental Digital Contents, http://links.lww.com/ASAIO/A789).
DLI Management and Outcomes
Time from implant to DLI development was similar across eras (Table 4). Median time to initiation of antibiotics was 0 (3) days, and initial antibiotic strategy was oral in 54 (62.1%) and IV in 33 (37.9%) patients, with no significant changes across eras. CAS use numerically increased over time, with 83% of patients treated in E4. Escalation to surgical management with early (≤30 days) and late (>30 days) I&D was required in 13 (14.9%) and 25 (28.7%) patients, respectively. I&D and wound vacuum-assisted closure were more commonly used in later eras. Device exchange due to DLI occurred in 5 (5.8%) patients, all during early eras (Table 4).
Table 4.
Driveline Infection: Incidence and Management Strategies by Era (N = 87)
| Entire cohort | Era 1 | Era 2 | Era 3 | Era 4 | P value | |
|---|---|---|---|---|---|---|
|
| ||||||
| Number of patients | 87 | 21 | 42 | 12 | 12 | |
| Time to DLI development, days* | 403 [530] | 442 [618] | 397.5 [463.2] | 404.5 [464.2] | 378 [303.7] | 0.8557 |
| Time to initiation of antibiotics, days* | 0 [3] | 0 [7] | 0 [3.25] | 0 [1] | 0 [0.5] | 0.2617 |
| Initial antibiotic strategy | 0.0647 | |||||
| Oral, n (%) | 54 (62.07) | 18 (85.71) | 23 (54.76) | 7 (58.33) | 6 (50.00) | |
| IV, n (%) | 33 (37.93) | 3 (14.29) | 19 (45.24) | 5 (41.67) | 6 (50.00) | |
| CAS, n (%) | 57 (65.52) | 11 (52.38) | 27 (64.29) | 9 (75.00) | 10 (83.33) | 0.5076 |
| Abdominal CT scan within 30 days of DLI diagnosis, n (%) | 33 (37.93) | 4 (19.05) | 19 (45.24) | 5 (41.67) | 5 (41.67) | 0.7454 |
| Incision and drainage ≤30 days from DLI diagnosis, n (%) | 13 (14.94) | 1 (4.76) | 6 (14.29) | 3 (25.00) | 3 (25.00) | 0.4496 |
| Wound VAC, ≤30 days from DLI diagnosis, n (%) | 12 (13.79) | 1 (4.76) | 6 (14.28) | 2 (16.67) | 3 (25.00) | 0.5385 |
| Incision and drainage >30 days after DLI diagnosis, n (%) | 25 (28.74) | 6 (28.57) | 7 (16.67) | 6 (50.00) | 6 (50.00) | 0.3926 |
| Wound VAC >30 days from DLI diagnosis, n (%) | 23 (26.44) | 4 (19.05) | 7 (16.67) | 6 (50.00) | 6 (50.00) | 0.1500 |
| Driveline re-routing, n (%) | 2 (2.30) | 2 (9.52) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0.0690 |
| Device exchange due to DLI, n (%) | 5 (5.75) | 1 (4.76) | 4 (9.52) | 0 (0.00) | 0 (0.00) | 0.3007 |
Data presented as median (interquartile range).
CAS, chronic antibiotic suppression; CT, computed tomography; DLI, driveline infection; LVAD, left ventricular assist device; VAC, vacuum-assisted closure.
Among 87 DLI patients, 27 (31.0%) died, 37 (42.5%) had HT, 19 (21.8%) remained on support, one (1.2%) was explanted, and 3 (3.4%) had transferred care at the end of follow-up. Causes of death for the above patients are provided in Table S5.
Among 87 DLI patients, 15 (17.2%) suffered a stroke during the study period: 9 (10.3%) had a stroke after DLI diagnosis: (5 (55.6%) ischemic, 4 (44.4%) ischemic with hemorrhagic conversion, 1 (11.1%) hemorrhagic), at a median time of 89 (155, 471) days; and 6 (6.9%) had a stroke before the onset DLI: (3 (50.0%) ischemic, 1 (16.7%) ischemic with hemorrhagic conversion, and 2 (33.3%) hemorrhagic). Among the 504 patients with no DLI, 86 (17.4%) suffered a stroke during the study period: 51 (59.3%) ischemic, 23 (26.7%) ischemic with hemorrhagic conversion, and 12 (14.0%) hemorrhagic.
Thirty-one (35.6%) DLI patients required I&D at a median of 77 (380) days after DLI diagnosis. Baseline characteristics of patients with vs. without I&D were comparable, except for higher BMI and prevalence of diabetes in the I&D group (Table S2, Supplemental Digital Content, http://links.lww.com/ASAIO/A789). One- and 2-year survival, conditioned upon survival to 3 months among patients with no DLI, DLI, and DLI requiring I&D is shown in Figure 3D. The presence of DLI was associated with an increased, albeit nonsignificant risk of death (HR 1.76 (0.98–3.14) P = 0.058), while requirement for I&D was associated with a significantly increased risk of death (HR 2.92 (1.33–6.44) P = 0.008) when compared to no DLI patients.
Figure 3.
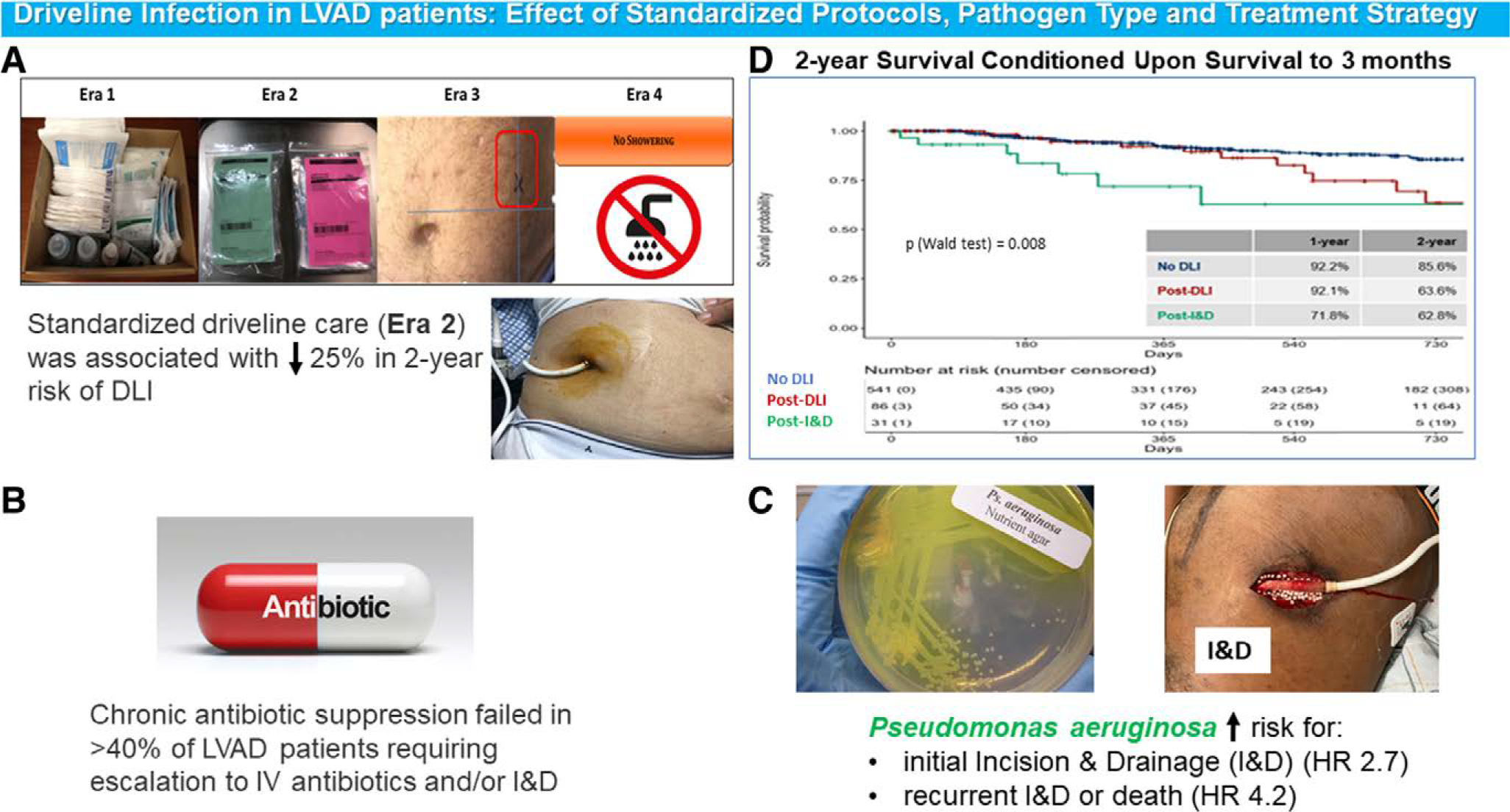
(A) Driveline infection prevention strategies implemented over time stratified by Era. (B) Impact of chronic antibiotic suppression on DLI outcomes. (C) Impact of pathogen type on DLI outcomes. (D) Two-year Survival Conditioned Upon Survival to 3 months post-LVAD, stratified by clinical course (no DLI, DLI without I&D, DLI with I&D)
Among 31 patients with I&D, 12 (38.7%) required recurrent I&D and 11 (35.5%) died at the end of follow-up. Initial I&D was required in 40.0% of P. aeruginosa vs. 13.5% S. aureus DLIs. Recurrent I&D was required in 33.3% of P. aeruginosa vs. 10.8% S. aureus DLIs.
In adjusted analyses, P. aeruginosa DLI was associated with HR (IQR) 2.7 (1.1–6.3) for initial I&D (Table 5), and HR (IQR) 4.2 (1.4–12.5) for recurrent I&D or death (Table 6), when compared to all other microorganisms. Additionally, diabetes was associated with HR (IQR): 2.4 (1.0–5.7) for initial I&D (Table 5).
Table 5.
Univariable and Multivariable Analysis for Predictors of Initial Incision and Drainage (N = 84A)
| Univariate HR (95% CI) | P value | Multivariate HR (95% CI) | P value | |
|---|---|---|---|---|
|
| ||||
| Age, years | 0.99 (0.96–1.02) | 0.5980 | ||
| Male | 0.68 (0.28–1.62) | 0.3800 | 0.49 (0.20–1.25) | 0.1372 |
| BMI ≥ 30 | 2.09 (0.94–4.67) | 0.0707 | 1.65 (0.72–3.77) | 0.2348 |
| Diabetes mellitus | 1.96 (0.89–4.30) | 0.0927 | 2.43 (1.04–5.67) | 0.0408 |
| Pseudomonas vs. others* | 2.55 (1.12–5.83) | 0.0264 | 2.66 (1.12–6.30) | 0.0261 |
| Presence of Bacteremia | 1.65 (0.45–5.60) | 0.4220 | ||
Three patients underwent incision and drainage (I&D) at the time of driveline infection diagnosis and were excluded from this analysis.
Other organisms included Achromobacter xylosoxidans, Acinetobacter baumannii complex, Acinetobacter Pittii, Burkholderia cepacia complex, coagulase-negative staphylococcus, Corynebacterium striatum, Diphtheroids, Enterobacter aerogenes, Enterobacter Cloacae, Enterococcus faecalis, Escherichia coli, Klebsiella oxytoca, Klebsiella pneumoniae, Serratia Liquefaciens, Serratia marcescens, Staphylococcus epidermidis, Staphylococcus hominus, Staphylococcus lugdunensis, Stenotrophomonas maltophilia, Streptococcus constellatus subspecies, Streptococcus pyogenes, Streptococcus viridans.
BMI, body mass index.
Table 6.
Univariable and Multivariable Analysis for Predictors of Recurrent Incision and Drainage or Death (N = 30A)
| Univariate HR (95% CI) | P value | Multivariate HR (95% CI) | P value | |
|---|---|---|---|---|
|
| ||||
| Age, years | 1.00 (0.96–1.05) | 0.8890 | ||
| Male | 1.01 (0.33–3.11) | 0.9890 | ||
| BMI ≥30 | 2.32 (0.86–6.26) | 0.0982 | 2.08 (0.77–5.68) | 0.1511 |
| Diabetes mellitus | 0.93 (0.35–2.47) | 0.8820 | ||
| Pseudomonas vs. others* | 4.52 (1.54–13.27) | 0.0060 | 4.24 (1.44–12.49) | 0.0088 |
| Presence of Bacteremia | 1.90 (0.61–5.89) | 0.2690 | ||
One patient was lost to follow-up after the initial incision and drainage (I&D) and was excluded from this analysis.
Other organisms included Achromobacter xylosoxidans, Acinetobacter baumannii complex, Acinetobacter Pittii, Burkholderia cepacia complex, coagulase-negative staphylococcus, Corynebacterium striatum, Diphtheroids, Enterobacter aerogenes, Enterobacter Cloacae, Enterococcus faecalis, Escherichia coli, Klebsiella oxytoca, Klebsiella pneumoniae, Serratia Liquefaciens, Serratia marcescens, Staphylococcus epidermidis, Staphylococcus hominus, Staphylococcus lugdunensis, Stenotrophomonas maltophilia, Streptococcus constellatus subspecies, Streptococcus pyogenes, Streptococcus viridans
BMI, body mass index.
Outcomes of CAS
Fifty-seven (65.5%) DLI patients were placed on CAS, 26 (28.9%) were not and 4 were excluded due to HT, death, or device exchange soon after DLI diagnosis. See Table S6, Supplemental Digital Content, http://links.lww.com/ASAIO/A789 shows baseline characteristics of the two groups. Time from implant to initial DLI was shorter among CAS patients vs. those not on CAS. Median duration of CAS was 336 (493) days. Distribution of Gram-positive, Gram-negative, and polymicrobial infections of initial DLI was similar between patients with vs. without CAS (Figure 2, see Table S6, Supplemental Digital Content, http://links.lww.com/ASAIO/A789). S. aureus and P. aeruginosa DLIs were numerically, but not significantly, more frequent among CAS patients. The most frequently used CAS were doxycycline (25%) and cephalexin (16%) (Figure 2).
Figure 2.
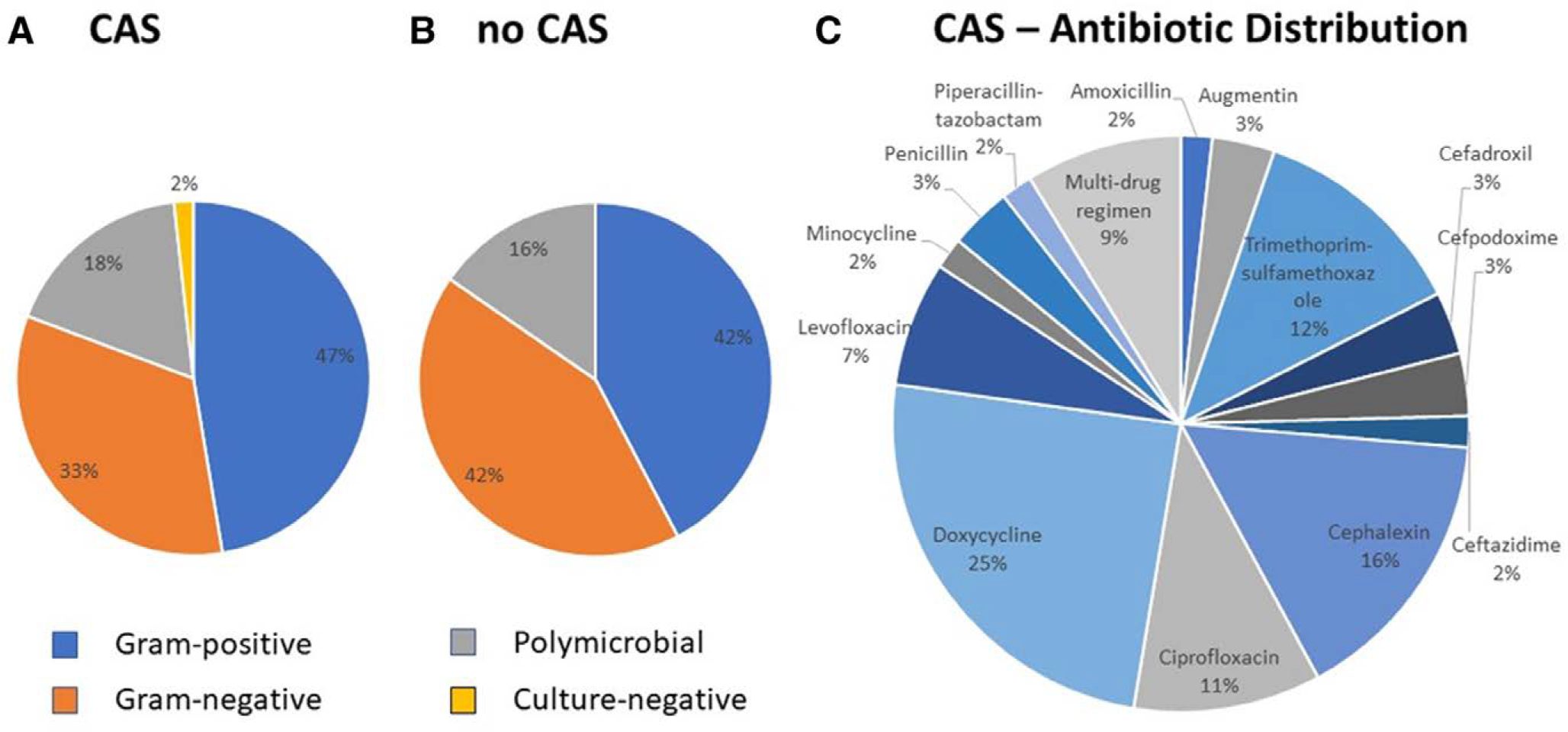
Distribution of initial pathogen (gram-positive, gram-negative, polymicrobial, culture-negative) stratified by: (A) chronic antibiotic suppression (CAS) use vs. (B) not (No CAS). (C) Antibiotic distribution among patients treated with CAS. CAS, chronic antibiotic suppression: Gram-positive: 21 methicillin-sensitive Staphylococcus aureus, 7 methicillin-resistant Staphylococcus aureus, 2 Diphtheroids, 1 Enterococcus faecalis, 3 coagulase-negative staphylococcus. Gram-negative: 15 Pseudomonas aeruginosa, 6 Serratia marcescens, 4 Klebsiella pneumoniae, 2 Stenotrophomonas maltophilia, 1 Achromobacter xylosoxidans, 1 Acinetobacter baumannii complex, 1 Burkholderia cepacia complex, 1 Enterobacter cloacae, 1 Escherichia coli, 1 Serratia liquefaciens. No CAS: Not on Chronic Antibiotic Suppression: Gram-positive: 7 methicillin-sensitive Staphylococcus aureus, 2 methicillin-resistant Staphylococcus aureus, 1 coagulase-negative staphylococcus, 1 Corynebacterium striatum, 1 Streptococcus viridans. Gram negative: 5 Serratia marcescens, 3 Pseudomonas aeruginosa, 2 Enterobacter cloacae, 2 Enterobacter aerogenes, 2 Stenotrophomonas maltophilia, 1 Acinetobacter baumannii complex, 1 Acinetobacter oitti, 1 Klebsiella pneumoniae
Escalation of CAS therapy was required in 25 (43.9%) patients: 5 (8.8%) required IV antibiotics alone and 20 (35.1%) I&D at a median of 169 (334) days after CAS initiation. Pathogens among patients undergoing I&D were: S. aureus in 11 patients, P. aeruginosa in 5, Serratia marcescens in 2, Enterobacter cloacae, and Burkholderia cepacia in 1 each. Among CAS patients, 4 (7.0%) required device exchange (3 methicillin-sensitive S. aureus and 1 P. aeruginosa),17 (29.8%) died, 26 (45.6%) had HT and 12 (21.1%) remained on support and on CAS at the end of follow-up. Clostridium Difficile (C. Difficile) infection developed in 7 (12.3%) patients, with no difference across eras (P = 0.93).
Among 26 patients not on CAS, 10 (38.5%) completely cleared their DLI while 16 (61.5%) had a recurrent positive wound culture after stopping antibiotics for treatment. Recurrent infections were caused by the same organism in 15 (93.7%) and a different organism in 1 (6.3%) patient. Ten patients were restarted on oral antibiotics, 6 required escalation to IV antibiotics, and 4 to I&D. Among 16 recurrent DLI patients, 6 (37.5%) died, 5 (31.3%) had HT, 4 (25.0%) remained on support, and 1 (6.2%) transferred for care at the end of follow-up.
Discussion
In contrast to prior work, which has mainly described the incidence and predictors of DLI, we uniquely divided our cohort of LVAD patients into four separate eras based on temporal changes in driveline care at our institution and focused on identifying bacterial pathogens that are associated with poor prognosis. As such, the current study has several important findings summarized in Figure 3: (1) P. aeruginosa DLI was associated with 2.7-fold increased risk of initial I&D and 4.2-fold increased risk of recurrent I&D or death, when compared to all other microorganisms; (2) progression to I&D was associated with 2.9-fold increased risk of death when compared to patients without DLI; (3) implementation of a standardized driveline dressing protocol (E2) resulted in a 25% absolute reduction in 2-year rate of DLI, compared to nonstandardized care (E1), while additional preventive initiatives (E3 and E4) did not lead to further reduction; and (4) CAS did not translate into long-term suppression of infection in >40% of patients.
At our institution, DLI incidence was 14.7%, which is similar to previously published reports.15,16 LVAD implant during E1 was an independent predictor of DLI at 2 years. In contrast to prior reports, which showed younger age and BMI being associated with higher risk of DLI,5,17 these variables were not predictors of DLI in our cohort. Although initial reports raised concern for higher DLI risk in HM3, due to larger driveline diameter and overall stiffness secondary to the modular driveline connector,16 we did not find any difference in 2-year freedom from DLI among studied devices. Our results are in agreement with ENDURANCE18 and MOMENTUM 36 trials, demonstrating no difference in DLI rates between HVAD (19.6%) and HMII (15.4%), and HMII (19.4%) and HM3 (23.3%), respectively. Our results are also in agreement with the recently published CLEAR-LVAD19 study, demonstrating the superior survival of HM3 compared to HVAD and HM II devices.
While DLI did not adversely impact 2-year survival postimplant, only a minority (12%) of DLI patients had complete eradication of the infection after antimicrobial treatment. The best option for a definitive cure of DLI remains removing all LVAD components at the time of HT. In our cohort, 42.5% of DLI patients ultimately underwent HT. Historically, DLI has been an indication for prioritization on the United Network for Organ Sharing (UNOS) HT waitlist. However, in October 2018, a new heart allocation system took effect with appropriate goals to prioritize the sickest patients and reduce waitlist mortality.20 These changes translated into a less favorable environment for LVAD patients, with longer wait times and preferential organ allocation to those with more severe complications.21 Infections that would now meet the criteria for higher priority must be extensive (e.g., deep, systemic infections requiring surgical interventions), resulting in a more compromised LVAD patient undergoing HT. Thus, as the new reiterations of the allocation system are being considered, these and other LVAD related complications must be further reviewed, allowing these patients to receive a heart in a timely manner. For HT ineligible patients, options to eradicate DLI are limited to more morbid procedures, such as device exchange, with unknown long-term results.22–24 Thus, prevention of DLI is of the utmost importance.
Current ISHLT guidelines do not provide detailed instructions for driveline care.3 Thus, over the past decade, many VAD centers have developed expert-driven, site-specific protocols. More recently, a European consensus document was published, addressing, in part, the unmet need for standardized driveline care.25 At our institution, we created pump-specific standards that include: (1) driveline dressing kit; (2) educational videos; (3) detailed SOPs for dressing changes (Supplemental Digital Content, http://links.lww.com/ASAIO/A789). These standards were established during E2 and have recently become invaluable telemedicine tools during the COVID-19 pandemic. In 2016, our group published the results of this strategy showing an absolute 1-year reduction in DLI of 11%.8 The present work expands on this initial report by presenting 2-year results (inclusive of HM3) and investigates the impact of two subsequent initiatives: marking of the driveline exit site (E3) and “no shower” policy (E4). We demonstrated a sustained benefit of the standardized protocol (E2), with an absolute 2-year reduction in DLI of 25%, while the latter two initiatives did not result in additional improvements.
The best approach for DLI treatment has not been clearly established. Preventing progression is critical. In our cohort, escalation of care to I&D occurred in 36% of patients, with increased frequency across eras; 39% of these patients required multiple procedures. Based on our conditional survival analysis (Figure 3D), initial I&D was associated with 2.9-fold increased risk of death when compared to patients with no DLI. Given the staggering number of patients that remain on support for prolonged periods of time, our results further highlight the necessity of ongoing vigilant care to prevent this devastating complication.
Data supporting effectiveness of CAS has been conflicting, with wide range of reported relapses.26–30 The majority (65.5%) of our DLI patients were placed on CAS. Notably, >40% of CAS patients required escalation to either IV antibiotics or I&D, thus proving this approach not uniformly successful. Overall, 17 (29.8%) CAS patients died, 26 (45.6%) had HT and 7 (12.3%) developed C. difficile colitis. CAS also carries additional hazards associated with drug side effects, drug interactions, in particular warfarin, and antimicrobial resistance.
With respect to microbial pathogens, P. aeruginosa DLI was an independent predictor of an initial I&D, and of recurrent I&D or death, despite the majority (83%) treated with CAS. These findings suggest perhaps a different, more aggressive management approach is needed early on in the diagnosis of P. aeruginosa DLI. No general recommendations regarding showering are presently available, thus practice patterns vary among institutions. One prior single-center study reported reductions in DLI rates due to P. aeruginosa after instructing patients to stop conventional showering.31 Although we were not able to demonstrate reductions in P. aeruginosa infections in E4 when compared to E1–3 (see Table S3, Supplemental Digital Content, http://links.lww.com/ASAIO/A789), this could be potentially attributed to insufficient duration of follow-up, as DLI is a delayed complication of LVAD therapy.
This study has several limitations. The single-center retrospective design presents inherent limitations to the analysis and generalizability of our results. However, for the same reasons, (1) more granular data were collected allowing in-depth characterization of microbial species responsible for DLIs, and (2) medical care was largely uniform (the same primary surgeon, ID specialist, and no significant changes to the LVAD selection criteria over the study period) among patients strengthening the quality of our outcome data. The multivariable analysis identified a lack of standardized protocol (E1) as the only predictor of 2-year risk of DLI. The relatively small sample size may account for the lack of significance of other risk factors that have been previously identified and of the additional preventive strategies (E3 and E4) studied. Patients in E2-E4 had prolonged CPB time when compared to E1, potentially because of the evolved surgical complexity. However, data on concomitant procedures during LVAD surgery was not collected over the 10-year of this observation study (2009–2019). Serial changes in C-reactive protein and white blood cell count pre-, post-DLI diagnosis, and in response to therapy were not available for this analysis, thus their impact on the clinical outcomes remains unknown. Lastly, assessment of compliance with “no shower” policy was not formally performed, as there is no objective way to monitor adherence other than reliance on patients’ reporting and continuous reinforcement.
In conclusion, P. aeruginosa DLI was associated with a markedly increased need for surgical interventions and higher mortality. Conditional survival of patients who required I&D was diminished when compared to patients who did not develop DLI or required I&D. Implementation of a comprehensive standardized dressing protocol (E2) led to a 25% absolute reduction in the 2-year rates of DLI. CAS was widely used in our cohort but failed to suppress infection in a large proportion of patients. Whether new prevention and treatment strategies, including rigorous reinforcement of a strict “no shower” policy, could further mitigate risk and improve outcomes of DLIs requires additional prospective studies.
Supplementary Material
Acknowledgments
This research has been supported by funds from the Lisa and Mark Schwartz Program to Reverse Heart Failure at New York-Presbyterian Hospital/Columbia University.
Footnotes
Disclosure: P.C.C is recipient of a research grant from Abbott; he also serves as a consultant for the same company. Y.N. serves as a consultant for Abbott, CryoLife, and Zimmer-Biomet, and as a speaker for Nipro Co. G.T.S. serves as a consultant for Abbott. N.U. serves on advisory boards for Leviticus and Livemetric/Cormetric; he also serves as a consultant for Abbott and Medtronic. The remaining authors have no conflicts of interest to report.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML and PDF versions of this article on the journal’s Web site (www.asaiojournal.com).
References
- 1.Teuteberg JJ, Cleveland JC Jr, Cowger J, et al. : The Society of Thoracic Surgeons Intermacs 2019 Annual Report: The Changing Landscape of Devices and Indications. Ann Thorac Surg 109: 649–660, 2020. [DOI] [PubMed] [Google Scholar]
- 2.Quader MA, Wolfe LG, Kasirajan V: Heart transplantation outcomes in patients with continuous-flow left ventricular assist device-related complications. J Heart Lung Transplant 34: 75–81, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Kusne S, Mooney M, Danziger-Isakov L, et al. : An ISHLT consensus document for prevention and management strategies for mechanical circulatory support infection. J Heart Lung Transplant 36: 1137–1153, 2017. [DOI] [PubMed] [Google Scholar]
- 4.Tattevin P, Flécher E, Auffret V, et al. : Risk factors and prognostic impact of left ventricular assist device-associated infections. Am Heart J 214: 69–76, 2019. [DOI] [PubMed] [Google Scholar]
- 5.Pavlovic NV, Randell T, Madeira T, Hsu S, Zinoviev R, Abshire M: Risk of left ventricular assist device driveline infection: A systematic literature review. Heart Lung 48: 90–104, 2019. [DOI] [PubMed] [Google Scholar]
- 6.Patel CB, Blue L, Cagliostro B, et al. : Left ventricular assist systems and infection-related outcomes: A comprehensive analysis of the MOMENTUM 3 trial. J Heart Lung Transplant 39: 774–781, 2020. [DOI] [PubMed] [Google Scholar]
- 7.Lander MM, Kunz N, Dunn E, et al. : Substantial reduction in drive-line infection rates with the modification of driveline dressing protocol. J Card Fail 24: 746–752, 2018. [DOI] [PubMed] [Google Scholar]
- 8.Cagliostro B, Levin AP, Fried J, et al. : Continuous-flow left ventricular assist devices and usefulness of a standardized strategy to reduce drive-line infections. J Heart Lung Transplant 35: 108–114, 2016. [DOI] [PubMed] [Google Scholar]
- 9.Rahal A, Ruch Y, Meyer N, et al. : Left ventricular assist device-associated infections: Incidence and risk factors. J Thorac Dis 12: 2654–2662, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lines TH, Sabato LA, Nesbitt WJ, Moretz JD, Brinkley DM, Satyanarayana G: Minimum inhibitory concentration changes in relapsed left ventricular assist device driveline infections. Int J Artif Organs 43: 494–499, 2020. [DOI] [PubMed] [Google Scholar]
- 11.Lister PD, Wolter DJ, Hanson ND: Antibacterial-resistant Pseudomonas aeruginosa: Clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev 22: 582–610, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerr KG, Snelling AM: Pseudomonas aeruginosa: A formidable and ever-present adversary. J Hosp Infect 73: 338–344, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Mena KD, Gerba CP. Risk Assessment of Pseudomonas aeruginosa in Water. In: Whitacre DM, (ed), Reviews of Environmental Contamination and Toxicology, Vol 201, Boston, MA, Springer US, 2009, pp. 71–115. [DOI] [PubMed] [Google Scholar]
- 14.Hannan MM, Husain S, Mattner F, et al. ; International Society for Heart and Lung Transplantation: Working formulation for the standardization of definitions of infections in patients using ventricular assist devices. J Heart Lung Transplant 30: 375–384, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Topkara VK, Kondareddy S, Malik F, et al. : Infectious complications in patients with left ventricular assist device: Etiology and outcomes in the continuous-flow era. Ann Thorac Surg 90: 1270–1277, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Schlöglhofer T, Michalovics P, Riebandt J, et al. : Left ventricular assist device driveline infections in three contemporary devices. Artif Organs 45: 464–472, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imamura T, Kinugawa K, Nitta D, et al. : Readmission due to drive-line infection can be predicted by new score by using serum albumin and body mass index during long-term left ventricular assist device support. J Artif Organs 18: 120–127, 2015. [DOI] [PubMed] [Google Scholar]
- 18.Rogers JG, Pagani FD, Tatooles AJ, et al. : Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med 376: 451–460, 2017. [DOI] [PubMed] [Google Scholar]
- 19.Pagani FD, Mehra MR, Cowger JA, et al. : Clinical outcomes and healthcare expenditures in the real world with left ventricular assist devices - The CLEAR-LVAD study. J Heart Lung Transplant 40: 323–333, 2021. [DOI] [PubMed] [Google Scholar]
- 20.Goff RR, Uccellini K, Lindblad K, et al. : A change of heart: Preliminary results of the US 2018 adult heart allocation revision. Am J Transplant 20: 2781–2790, 2020. [DOI] [PubMed] [Google Scholar]
- 21.Cogswell R, John R, Estep JD, et al. : An early investigation of outcomes with the new 2018 donor heart allocation system in the United States. J Heart Lung Transplant 39: 1–4, 2020. [DOI] [PubMed] [Google Scholar]
- 22.Takeda K, Takayama H, Sanchez J, et al. : Device exchange from HeartMate II to HeartMate 3 left ventricular assist device. Interact Cardiovasc Thorac Surg 29: 430–433, 2019. [DOI] [PubMed] [Google Scholar]
- 23.Hanke JS, Rojas SV, Dogan G, et al. : First series of left ventricular assist device exchanges to HeartMate 3. Eur J Cardiothorac Surg 51: 887–892, 2017. [DOI] [PubMed] [Google Scholar]
- 24.Chou BP, Lamba HK, Cheema FH, et al. : Outcomes of repeat left ventricular assist device exchange. ASAIO J 66: 64–68, 2020. [DOI] [PubMed] [Google Scholar]
- 25.Bernhardt AM, Schlöglhofer T, Lauenroth V, et al. ; Driveline Expert STagINg and carE DESTINE study group, a Ventricular Assist Device Driveline Infection Study Group: Prevention and early treatment of driveline infections in ventricular assist device patients - The DESTINE staging proposal and the first standard of care protocol. J Crit Care 56: 106–112, 2020. [DOI] [PubMed] [Google Scholar]
- 26.Jennings DL, Chopra A, Chambers R, Morgan JA: Clinical outcomes associated with chronic antimicrobial suppression therapy in patients with continuous-flow left ventricular assist devices. Artif Organs 38: 875–879, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Radcliffe C, Doilicho N, Niu YS, Grant M: Efficacy and safety of chronic antimicrobial suppression therapy for left ventricular assist device driveline infections: A single-center descriptive experience. Transpl Infect Dis 22: e13379, 2020. [DOI] [PubMed] [Google Scholar]
- 28.Ekkelenkamp MB, Vervoorn MT, Bayjanov JR, Fluit AC, Benaissa-Trouw BJ, Ramjankhan FZ: Therapy and outcome of staphylococcus aureus infections of intracorporeal ventricular assist devices. Artif Organs 42: 983–991, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levy DT, Guo Y, Simkins J, et al. : Left ventricular assist device exchange for persistent infection: A case series and review of the literature. Transpl Infect Dis 16: 453–460, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Nienaber JJ, Kusne S, Riaz T, et al. ; Mayo Cardiovascular Infections Study Group: Clinical manifestations and management of left ventricular assist device-associated infections. Clin Infect Dis 57: 1438–1448, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aburjania N, Sherazi S, Tchantchaleishvili V, Alexis JD, Hay CM: Stopping conventional showering decreases Pseudomonas infections in left ventricular assist device patients. Int J Artif Organs 40: 282–285, 2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


