Objectives:
Protein overfeeding in infants can have negative effects, such as diabetes and childhood obesity; key to reducing protein intake from formula is improving protein quality. The impact of a new infant formula [study formula (SF)] containing alpha-lactalbumin, lactoferrin, partially hydrolyzed whey, and whole milk on growth and tolerance compared to a commercial formula (CF) and a human milk reference arm was evaluated.
Methods:
This randomized, double-blind trial included healthy, singleton, term infants, enrollment age ≤14 days. Primary outcome was mean daily weight gain. Secondary outcomes were anthropometrics, formula intake, serum amino acids, adverse events, gastrointestinal characteristics, and general disposition.
Results:
Non-inferiority was demonstrated. There were no differences between the formula groups for z scores over time. Formula intake [−0.33 oz/kg/day, 95% confidence interval (CI): −0.66 to −0.01, P = 0.05] and mean protein intake (−0.13 g/kg/day, 95% CI: −0.26 to 0.00, P = 0.05) were lower in the SF infants, with higher serum essential amino acid concentrations (including tryptophan) compared to the CF infants. Energetic efficiency was 14.0% (95% CI: 8.3%, 19.7%), 13.0% (95% CI: 6.0%, 20.0%), and 18.1% (95% CI: 9.4%, 26.8%) higher for weight, length, and head circumference, respectively, in SF infants compared to the CF infants. SF infants had significantly fewer spit-ups and softer stool consistency than CF infants.
Conclusions:
The SF resulted in improved parent-reported gastrointestinal tolerance and more efficient growth with less daily formula and protein intake supporting that this novel formula may potentially reduce the metabolic burden of protein overfeeding associated with infant formula.
Keywords: alpha-lactalbumin, growth, nutrition, tryptophan
What Is Known
Protein levels in infant formula often exceed human milk.
Protein overfeeding in early infancy is hypothesized to impact endocrine and metabolic programming contributing to negative outcomes including diabetes and childhood obesity.
Trials confirm that feeding infants formulas with lower protein content supports adequate growth; however, protein quality must be considered.
What Is New
Results demonstrate that macronutrient profile favorably impacts energetic efficiency, amino acid levels, and parent-reported gastrointestinal tolerance.
Infants fed formula with a high-quality protein blend and whole milk grew adequately with less formula and protein intake compared to a standard formula.
See Invited Commentary: “Welcome to a New Infant Formula” by Baker and Merritt on page 389.
Human milk (HM), the recommended source of nutrition, is not available for all infants. HM research guides infant formula (IF) design, yet gaps in composition and outcomes remain. Formula-fed infants consume more energy and protein and gain more weight than HM-fed infants (1–3), increasing the risk of adverse short- and long-term outcomes (4–6). Protein quality and content in IF have a significant role (7,8). To compensate for the lower bioavailability of amino acids (AA), the established minimum protein content of IF is higher than are levels in mature HM to ensure the provision of sufficient essential AA to support protein synthesis (9–12). However, excessive protein intake in early infancy is hypothesized to change endocrine and metabolic programming with potential impacts on obesity and disease risk (5,6,13). Therefore, it is essential for IF to supply the necessary essential AA without exceeding the quantity of protein infants can utilize. Trials examining the safety of lower protein formulas to reduce the risks of protein overfeeding have confirmed adequate growth, yet recent research suggests that protein quality must be considered in addition to protein quantity (6,9,14–16).
Informed by HM research, a new IF that contains a high-quality protein blend with alpha-lactalbumin, lactoferrin, partially hydrolyzed whey proteins, and whole bovine milk was developed. This trial evaluated the growth, safety, and tolerance of infants fed this new formula compared to those fed a commercial formula and an HM-fed reference group.
METHODS
Participants
Infants were recruited from 35 US locations. Healthy, singleton, term (≥37 and ≤42 weeks) infants with a birth weight of ≥2500 g, ≤14 days, between fifth and 95th percentile of World Health Organization (WHO) Growth Standards (17) whose parent(s)/legal guardian(s) had already decided to formula feed and were exclusively consuming and tolerating cow’s milk-based IF before enrollment, or HM for the reference group, were eligible. Exclusion criteria were anatomic or physiologic conditions or use of medications that would interfere with normal growth, development, or feeding, maternal history with known adverse effects on the fetus or newborn infant, or history of cow’s milk protein or soy intolerance/allergy.
Design and Procedures
The protocol was approved by an independent institutional review board and was conducted according to International Council for Harmonisation Good Clinical Practice Guidelines and in compliance with the principles of the Declaration of Helsinki. The trial was conducted as a 16-week trial with an 8-week extension and was registered at www.clinicaltrials.gov (NCT04218929 and NCT04389606 January 6, 2020 and May 15, 2020, respectively). Written informed consent was obtained from all participating infant’s parents(s)/legal guardian(s).
The study was a multisite, randomized, double-blind, controlled, non-inferiority trial. Enrolled infants were randomized to receive either the study formula (SF) or a commercial formula (CF) for a total of 24 weeks. A statistician, with no other involvement in the trial, generated the randomization sequence (stratified by sex). Site randomization occurred via Interactive Web-Based Randomization System (IWRS). Investigators and parent(s)/legal guardian(s) were blinded throughout the trial.
Following enrollment, participants were evaluated at 11 visits (8 in person and 3 by telephone). Infants in the HM reference group completed identical study procedures.
Trial Formulas
Both the SF (ByHeart, Inc, New York, NY) and the CF (Enfamil, Mead Johnson, LLC, Chicago, IL) were isocaloric (100 kcal/5 fluid oz/150 mL), contained identical amounts of protein (2 g/100 kcal), and similar amounts of lipids (5.6 g/100 kcal ByHeart; 5.3 g/100 kcal Enfamil), carbohydrates (10 g/100 kcal ByHeart; 11.3 g/100 kcal Enfamil), and micronutrients (Table 1, Supplemental Digital Content, http://links.lww.com/MPG/C828). The SF differed from the CF in that it contained whole milk (bovine), alpha-lactalbumin enriched whey, lactoferrin, partially hydrolyzed whey protein, and a single prebiotic [galacto-oligosaccharide (GOS)]. The CF contained skim milk (bovine), whey protein concentrate, and a combination of GOS/polydextrose. Infants were fed ad libitum.
Measurements
The primary objective was to demonstrate non-inferiority in mean daily weight gain of infants fed the SF compared to CF between baseline and 24 weeks of age. Secondary objectives were to evaluate additional anthropometric measurements, serum AA concentrations, formula intake volume, adverse events (AE), gastrointestinal characteristics, and general disposition.
At the baseline visit, following informed consent and randomization, a physical exam and anthropometric evaluation [weight, length, and head circumference (HC)] occurred. Maternal and infant history, demographics, and prior and concomitant medications were reviewed, and instruction was provided for formula preparation and how to complete the 3-day formula intake diary. At each in-person follow-up visit, anthropometric measurements were obtained by 2 trained study personnel per standardized protocol. Two measurements were taken for weight (without clothing on a calibrated digital electronic scale), length (in the recumbent position by calibrated length board), and HC (by measuring tape), and in the case of a predefined difference, a third was obtained.
Parent(s)/legal guardian(s) recorded formula intake on a 3-day formula/diet record. Consistent with recommendations from the American Academy of Pediatrics, parent(s)/legal guardian(s) were discouraged from feeding their infant foods other than assigned formula during the first 16 weeks of the trial. At each visit, investigators monitored feeding compliance, complementary food intake, AE, medications, tolerance, and general disposition. AE were clinically assessed for severity and relatedness. Gastrointestinal tolerance outcomes reported were stool frequency (number/d), stool consistency (5-point scale), spit-up (number/d), and amount of gas (3-point scale). General disposition included fussiness (4-point scale) and total crying time (h/d).
Amino Acid Analysis
At approximately 16 and 24 weeks of life, venous blood (1 mL) was drawn, and serum was stored frozen until analysis. Blood draw was delayed in the case of illness until the illness was resolved for 48 hours. A subgroup of infants in the per-protocol (PP) population without any complementary food intake were identified, equally distributed by sex between groups, for analysis. Serum AA were analyzed on a Hitachi L-8900Amino Acid Analyzer.
Calculation of Energetic Efficiency
The energetic efficiency (EE) was calculated as a ratio of mean daily weight, length, or head circumference gain (g/d or mm/d) to average energy intake in kilocalories and grams of protein [converted from mean formula intake (oz/d) by 20 kcal/oz or 2 g/5 oz].
Statistics
Sample size was based on a non-inferiority hypothesis that weight gain velocity (g/d) in SF infants was non-inferior to CF infants based on the established margin of −3 g/d (18). Assuming a standard deviation in weight gain of 6.0 g/d and 90% power, 70 participants per formula group would demonstrate non-inferiority with a one-sided type I error of 0.025. Assuming a 25% attrition rate and up to 5% of participants with a major protocol violation, a target sample size of 200 formula-fed infants (100 per arm) was determined. Both sample sizes and statistical analyses were determined a priori.
All analyses were conducted in the PP population, a subset of infants who completed the 24-week trial with no more than 9 days during the trial where all feedings were non-study formula and consumed no more than 24 single non-study feedings. Infants in the HM group were included for analysis if they were receiving HM as the predominant source of nourishment.
All mixed-effect model for repeated measures (MMRM) used sex, age at enrollment, formula, site, visit, formula by site interaction, and formula by visit interaction as factors (additional factors noted by model). Models for growth and parent-reported tolerance and disposition outcomes included SF, CF, and HM; models for formula intake and EE included only SF and CF. From these models, contrast effect estimates, and P values were calculated to evaluate comparisons between SF and CF, and SF and HM groups, where applicable. When both formula and HM comparisons were tested, a Bonferroni corrected alpha level of 0.025 was used. EE was compared between SF and CF as percent difference based on raw values and in MMRM models. Serum AA concentrations were compared between SF and CF using Wilcoxon rank-sum test. Days of gastrointestinal AE were calculated for each infant and compared between SF and CF, and SF and HM using the Wilcoxon rank-sum test.
RESULTS
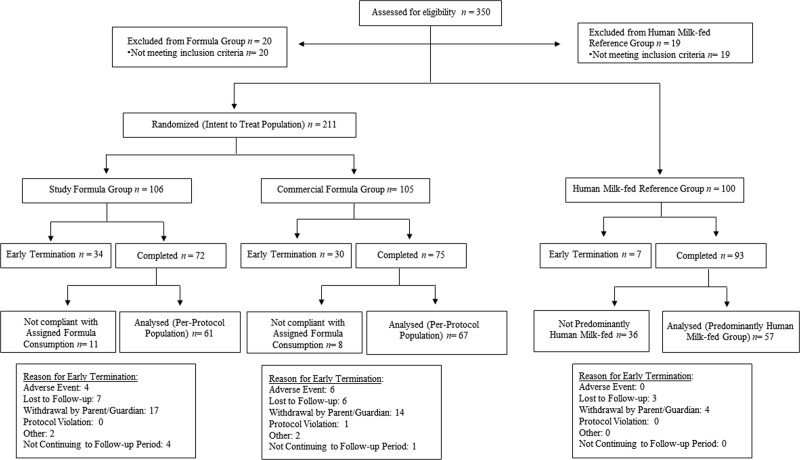
Between April 2019 and June 2021, 350 infants were screened, with 211 infants randomized (106 in the SF group, 105 in the CF group) and 100 enrolled in the HM group (Fig. 1). The coronavirus disease 2019 (COVID-19) pandemic resulted in slowed enrollment but with limited impact on study completion (9 participants in the PP population with a missed visit, similarly distributed among the formula groups). For analysis, there were 61 (57.5%) infants in the PP population in the SF group and 67 (63.8%) in the CF group. Fifty-seven (57.0%) infants were included in the HM reference group.
FIGURE 1.
Flow diagram of study progression. Flow diagram of participants from study enrollment to completion.
Baseline demographic data between formula groups were similar (Table 2, Supplemental Digital Content, http://links.lww.com/MPG/C828 and Table 3, Supplemental Digital Content, http://links.lww.com/MPG/C828).
Growth Outcomes
Mean daily weight gain velocity (g/d) between trial enrollment and 24 weeks was 26.7 [standard deviation (SD) = 5.2] in the SF group and 26.1 (SD = 4.7) in the CF group. In the PP population, non-inferiority at 24 weeks was demonstrated with the lower limit of the 95% confidence interval of the difference in model-based mean daily weight gain between formula groups of −0.99 g/d. Non-inferiority was also confirmed in the intention to treat (ITT) population.
No differences were observed over time between SF and CF for weight-for-age, length-for-age, HC-for-age, and weight-for-length z scores (Fig. 1A–D, Supplemental Digital Content, http://links.lww.com/MPG/C828). Differences of limited clinical significance were intermittently detected between the groups at individual visits. Growth in the SF group was different from HM, consistent with established literature, and as expected (2) (Table 4, Supplemental Digital Content, http://links.lww.com/MPG/C828).
Formula Intake and Energetic Efficiency
Formula intake by weight (oz/kg/d) was estimated to be lower in the SF group (−0.33 oz/kg/d, 95% CI: −0.66 to −0.01, P = 0.05) than in the CF group. This translates to a decreased protein intake in the SF group of −0.13 g/kg/d (95% CI: −0.26 to 0.00, P = 0.05) compared to the CF group.
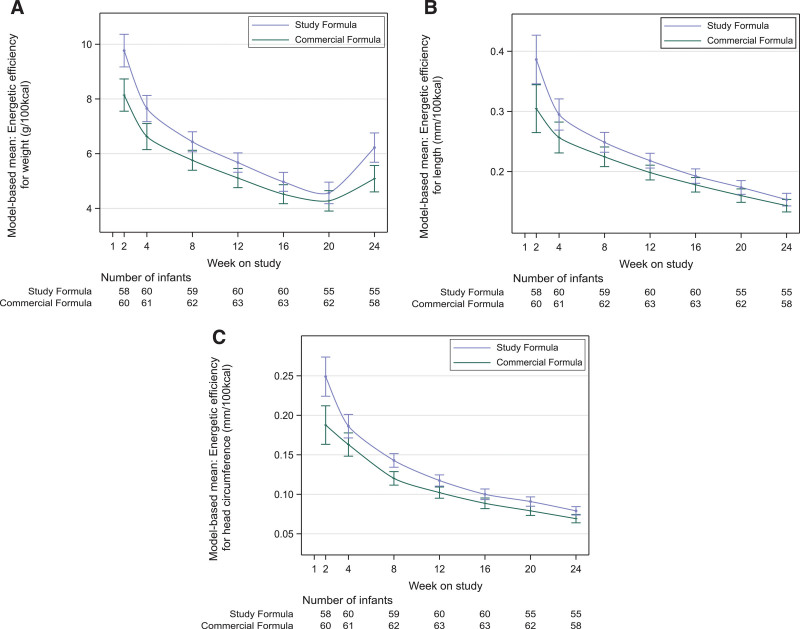
EE by weight was different between treatment groups over time (interaction P = 0.02; Fig. 2A). EE was also higher in the SF group for length (P = 0.002; Fig. 2B) and for HC (P < 0.001; Fig. 2C). EE per oz/mL of formula was 14.0% (95% CI: 8.3%–19.7%), 13.0% (95% CI: 6.0%–20.0%), and 18.1% (95% CI: 9.4%–26.8%) higher for weight, length, and HC, respectively, in SF group compared to CF group.
FIGURE 2.
Model-based mean energetic efficiency by feeding group and study visit in the PP population. Results from MMRM using previously described factors plus weight, length or head circumference at baseline, and food intake to account for introduction of supplementary solid food introduced during the study period; weight (A), length (B), HC (C). Error bars represent standard error of the mean. HC = head circumference; MMRM = model for repeated measures; PP = per-protocol.
Serum Amino Acid Analysis
In a subgroup of 74 infants, the combination of the essential and conditionally essential AA was greater in the SF group compared to the CF group (P = 0.048). Tryptophan and threonine concentrations, the ratios of tryptophan to large neutral amino acids (LNAA) concentrations and of tryptophan to branched-chain amino acid (BCAA) concentrations were higher in the SF group compared to the CF group (P = 0.019, P = 0.042, P = 0.007, P = 0.026, respectively). There was no difference in concentrations of the other essential and conditionally essential AA, the BCAA, and LNAA between the 2 formula groups. Infants fed the SF had greater concentrations of some AA compared to HM-fed infants; however, a majority (90%) were within one standard deviation of the HM group (Table 1, Table 5, Supplemental Digital Content, http://links.lww.com/MPG/C828).
TABLE 1.
Serum essential and conditionally essential amino acid concentrations and ratios (µmol/L) in a subgroup of the PP population at week 16 by feeding group
| Study formula (n = 21) | Commercial formula (n = 28) | Human milk (n = 25) | |
|---|---|---|---|
| Cysteine | 7 (8) | 5 (7) | 7 (5) |
| Histidine | 120 (43) | 104 (32) | 151 (72) |
| Isoleucine | 105 (24) | 97 (20) | 89 (29) |
| Leucine | 150 (29) | 152 (33) | 142 (42) |
| Lysine | 211 (51) | 201 (44) | 191 (53) |
| Methionine | 32 (8) | 33 (8) | 27 (7) |
| Phenylalanine | 76 (14) | 78 (18) | 75 (18) |
| Threonine | 282 (88)* | 234 (70) | 221 (83) |
| Tryptophan | 96 (15)* | 86 (17) | 78 (17) |
| Valine | 237 (33) | 243 (47) | 209 (58) |
| Total essential and conditional amino acids† | 1344 (236)* | 1197 (206) | 1229 (348) |
| Total BCAA‡ | 493 (81) | 492 (98) | 440 (128) |
| Total LNAA§ | 667 (110) | 666 (130) | 616 (163) |
| Tryptophan to LNAA ratio | 0.146 (0.026)* | 0.130 (0.018) | 0.130 (0.030) |
| Tryptophan to BCAA ratio | 0.20 (0.04)* | 0.18 (0.03) | 0.19 (0.05) |
Mean (SD). BCAA = branched-chain amino acid; CF = commercial formula; LNAA = large neutral amino acids; PP = per-protocol; SD = standard deviation; SF = study formula.
Significant difference between SF and CF, P < 0.05.
The essential and conditionally essential amino acid group was the sum of cysteine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, valine.
The BCAA group was the sum of isoleucine, leucine, and valine.
The LNAA group was the sum of isoleucine, leucine, phenylalanine, tyrosine, valine.
Gastrointestinal Tolerance
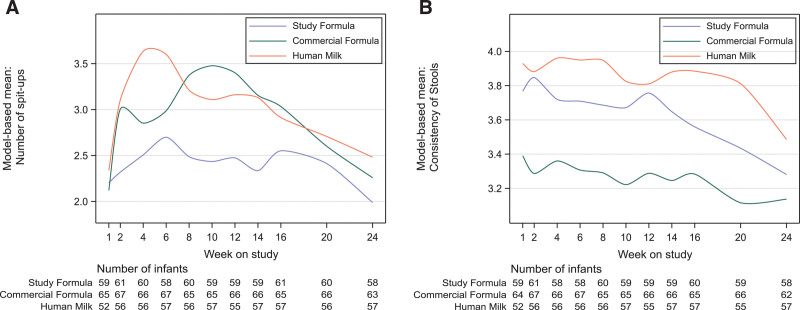
MMRM demonstrated significantly fewer mean number of spit-ups over time in the SF group compared to the CF group (P = 0.01). The SF group experienced fewer spit-ups at weeks 8-14 than the CF group (Bonferroni corrected P < 0.025). No statistically significant differences were observed in mean number of spit-ups between the SF group and the HM group at any time point except week 4 (Fig. 3A, Table 6, Supplemental Digital Content, http://links.lww.com/MPG/C828).
FIGURE 3.
Model-based mean number of spit-ups and stool consistency by feeding group and study visit in the PP population. Results from MMRM using previously described factors plus stool consistency or number of spit-ups reported at baseline; number of spit-ups (A), stool consistency on a 5-point scale; 1=hard, 5=watery (B). MMRM = model for repeated measures; PP = per-protocol.
Mean stool consistency was softer in the SF group compared to the CF group (mean score difference 0.39, 95% CI: 0.27–0.52, P < 0.001). No statistically significant differences were observed in mean stool consistency between the SF group and the HM group at any timepoint except weeks 16 and 20 (Fig. 3B). No differences were observed in mean number of stools per day between the SF and CF groups, and the occurrence of moderate or excessive gas was less in the SF group, although not statistically significant (Table 6, Supplemental Digital Content, http://links.lww.com/MPG/C828).
General Disposition
There was no difference between SF and CF groups for level of fussiness or average number of hours of crying per day.
Adverse Events
At least one AE was reported in 52 infants (85%) in the SF group, compared to 54 (81%) in the CF group, and 48 infants (84%) in the HM group (Table 7, Supplemental Digital Content, http://links.lww.com/MPG/C828). Investigators classified all AEs as mild or moderate. Most (86%) AEs were designated as not related to formula/HM. At least one possibly related AE was reported in 10 infants (16%) in the SF group, compared to 12 infants (18%) in the CF group and to 2 infants (4%) in the HM group (Table 7, Supplemental Digital Content, http://links.lww.com/MPG/C828). The number of days of gastrointestinal AEs was less in the SF group than in the CF group [median: 34 d, interquartile range (IQR): 9–138 vs median: 80 d, IQR: 41–152, P = 0.08]. The SF group received less medications for gastrointestinal and metabolism conditions than did the CF group (22, 36% vs 28, 42%) (Table 8, Supplemental Digital Content, http://links.lww.com/MPG/C828).
DISCUSSION
Infants in the SF group grew as well as infants in the CF group; there were no differences in z scores over time. Interestingly, infants fed the SF consumed less average daily formula and less total protein, had higher essential AA concentrations, and had a higher EE, demonstrating that this novel IF, made with a high-quality protein blend and whole milk, results in improved protein utilization and more efficient growth per oz of formula than a commercial IF. Parents of infants in the SF group reported gastrointestinal benefits, less spit-up, and softer stool.
EE is a measure of the impact of macronutrient components on growth, enabling the comparison per equivalent intake between sources of nutrition (16). Fledderman et al (16) found that the quality of protein ingredients (particularly whey predominance and alpha-lactalbumin and, thus, a higher tryptophan content) in addition to more palmitic acid in the sn-2 position (improved fat absorption) in IF are drivers of EE (16,19). The inclusion of these components in the SF resulted in 13-18% more efficient growth per oz of formula consumed, similar to what has been reported in HM-fed infants (16). As a result of this improved EE, infants in the SF group consumed less daily protein while maintaining adequate growth. This decrease is equivalent to a reduction in formula protein content from 2 g/100 kcal to 1.85–1.9 g/100 kcal based on established typical formula intake for an infant at the 50th percentile for weight.
Growth is limited by the availability of essential nutrients, including AA, necessary for protein synthesis. HM contains a relatively high proportion of these AA relative to total protein compared to bovine milk (20,21). IF has traditionally been formulated with protein levels that exceed those of HM to ensure adequate provision of essential AA (9–12). Previous studies demonstrated that reduced protein formulas with extensively and partially hydrolyzed proteins (22,23), whey predominance (24–26), alpha-lactalbumin (27), or with a modified AA profile (9) could result in adequate growth.
Alpha-lactalbumin, the predominant protein in mature HM, is recognized for its high proportion of essential AA, notably tryptophan, cysteine, and BCAA (20,28). Multiple trials have shown that the enrichment of alpha-lactalbumin in IF supports reduced protein content without negative impact on growth (19,27,29–31) and higher levels of serum essential AA including tryptophan (19,30–32). Due to the abundance of alpha-lactalbumin, HM contains double the amount of tryptophan as bovine milk (proportion of total protein) (20,21). In the current trial, the SF was enriched with alpha-lactalbumin at levels consistent with mature HM. Infants fed the SF had a higher level of total essential and conditionally essential AA, higher levels of tryptophan, and a higher tryptophan to LNAA ratio than infants in the CF group. As tryptophan is usually the most limiting AA for infants, adequate provision is essential for growth; levels of tryptophan have been suggested as a measure of protein adequacy in IF (20,28,33). Additionally, tryptophan is a precursor to serotonin, known for its regulation of central nervous system activity, including sleep (34). Animal studies have shown the tryptophan to LNAA ratio is a reliable indicator of brain tryptophan and serotonin concentrations, and human trials have shown that a higher tryptophan to LNAA ratio decreases sleep latency in infants (35–37).
The outcomes of reduced spit-ups and softer stools in the SF were expected based on previously reported outcomes from multiple components included in the SF. Alpha-lactalbumin improves formula digestion tolerability (29,31). Hydrolyzed proteins and alpha-lactalbumin reduce regurgitation in infants (29,31,38–41). The whey predominance of the SF likely further contributes, as gastric emptying of whey protein is faster than casein (42). In several trials, infants fed a GOS-containing formula had a softer stool consistency (43–45). Palmitic acid is better absorbed when esterified to the sn-2 position of a triglyceride; poorer absorption of palmitic acid in sn-1 and sn-3 positions results in calcium-fatty acid soaps in the intestine, contributing to harder stool (46,47). In HM, 70%–88% of the palmitic acid is in the sn-2 position, which is greater than found in vegetable oils (5%–20%) typically used in IF (46,48). Bovine milk has approximately 40% of palmitic acid in the sn-2 position (48). Manios et al (48) observed an improvement in stool consistency in infants fed a formula with whole bovine milk. In addition to the use of whole milk, omission of palm oil in the SF likely also contributed to softer stools. Palm oil, due to the high proportion of palmitic acid in the sn-1 and sn-3 positions, results in harder stools (49).
This study has several notable strengths. First is its design, which resulted in a generalizable demographic distribution. The inclusion of the HM-fed reference group allows for comparison to the recommended nutrition standard. Lastly, concordance of serum analysis, formula intake data, EE calculations, and anthropometrics validate the conclusion.
There are also several limitations. Enrollment of the HM group occurred before the formula-fed infants, the latter coinciding with the start of the COVID-19 pandemic. However, this should not have impacted growth and tolerance outcomes. It was not possible to calculate EE for the HM group (volume was not measured), and the finding of similar reported EE in this population should be verified. Another limitation is the comparison of the SF to only one control formula; however, this formula is considered a standard in the field. The conclusion regarding protein quality was based on previous literature on the SF proteins and the trial outcomes, not on the Digestible Indispensable Amino Acid Score (DIAAS). It is important to note that the gastrointestinal tolerance and general disposition data are subjective. In addition, the plasma AA analysis was performed in a subgroup of infants in the PP population; however, the effect size was large. Collection of data on infant sleep patterns may have illustrated the impact of the higher tryptophan to LNAA ratio in the SF.
Finally, although the SF did not impact one of the known early risk factors in infants for the development of obesity and disease risk, weight gain, it is possible that the reduction in protein intake and improved EE may have positively impacted other outcomes, including metabolic and endocrine programming and improvements in body composition. Previous literature has shown that differences in body composition were observed in infants despite no differences in measured weight (50). A longer study period may have demonstrated weight differences as beneficial effects of lower protein provision on weight have been observed to emerge after 6 months of age (6). Additionally, the proteins in the SF, and particularly alpha-lactalbumin, contribute high proportions of BCAA, increasing serum concentrations in the SF infants and potentially counter-balancing a decrease in these concentrations that may have been seen from a lower protein intake.
CONCLUSIONS
The macronutrient composition of the SF, including a whey-predominant protein blend with alpha-lactalbumin, lactoferrin, partially hydrolyzed proteins, and whole milk, was chosen to reflect advances in HM research. The outcomes of this trial support the conclusion that this novel formula decreases parent-reported gastrointestinal discomfort and allows for less total protein consumed with more efficient growth, potentially reducing the metabolic burden of protein overfeeding associated with IF.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jpgn.org).
This article has been developed as a Journal CME and MOC Part II Activity by NASPGHAN. Visit https://learnonline.naspghan.org/ to view instructions, documentation, and the complete necessary steps to receive CME and MOC credits for reading this article.
S.Z. receives an honorarium as part of the ByHeart Scientific Advisory Board. Bruce German receives an honorarium as part of the ByHeart Scientific Advisory Board. C.F. receives an honorarium as part of the ByHeart Scientific Advisory Board. S.D. receives an honorarium as part of the ByHeart Scientific Advisory Board and has received research grants from ByHeart. In addition, S.D. serves on Scientific Advisory Boards for Austnutria, Danone North America, Danone Institute International, and the National Dairy Council. She currently receives grant funding from General Mills, International Foods and Fragrances, Kyowa Hakka Bio, the National Dairy Council, National Institutes of Health (NIH), and the U.S. Department of Agriculture. B.L. receives an honorarium as part of the ByHeart Scientific Advisory Board and has received research grants from ByHeart. The remaining authors report no conflicts of interest.
The study was registered at clinicaltrials.gov registration numbers: NCT04218929 and NCT04389606.
Sources of Funding: This trial was funded by ByHeart, Inc. No funding was received from National Institutes of Health (NIH), Wellcome Trust, Howard Hughes Medical Institute, or other sources.
REFERENCES
- 1.Heinig MJ, Nommsen LA, Peerson JM, et al. Energy and protein intakes of breast-fed and formula-fed infants during the first year of life and their association with growth velocity: the darling study. Am J Clin Nutr. 1993;58:152–61. [DOI] [PubMed] [Google Scholar]
- 2.Azad MB, Vehling L, Chan D, et al. Infant feeding and weight gain: Separating breast milk from breastfeeding and formula from food. Pediatrics. 2018;142:e20181092. [DOI] [PubMed] [Google Scholar]
- 3.Hester SN, Hustead DS, MacKey AD, et al. Is the macronutrient intake of formula-fed infants greater than breast-fed infants in early infancy? J Nutr Metab. 2012;2012:891201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Escribano J, Luque V, Ferre N, et al. Increased protein intake augments kidney volume and function in healthy infants. Kidney Int. 2011;79:783–90. [DOI] [PubMed] [Google Scholar]
- 5.Luque V, Closa-Monasterolo R, Escribano J, et al. Early programming by protein intake: the effect of protein on adiposity development and the growth and functionality of vital organs. Nutr Metab Insights. 2015;8:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koletzko B, Demmelmair H, Grote V, et al. Optimized protein intakes in term infants support physiological growth and promote long-term health. Semin Perinatol. 2019;43:151153. [DOI] [PubMed] [Google Scholar]
- 7.Koletzko B, Broekaert I, Demmelmair H, et al. Protein intake in the first year of life: a risk factor for later obesity? The E.U. childhood obesity project. Adv Exp Med Biol. 2005;569:69–79. [DOI] [PubMed] [Google Scholar]
- 8.Lönnerdal B, Zetterström R. Protein content of infant formula--how much and from what age? Acta Paediatr Scand. 1988;77:321–5. [DOI] [PubMed] [Google Scholar]
- 9.Kouwenhoven SMP, Antl N, Finken MJJ, et al. A modified low-protein infant formula supports adequate growth in healthy, term infants: a randomized, double-blind, equivalence trial. Am J Clin Nutr. 2020;111:962–74. [DOI] [PubMed] [Google Scholar]
- 10.Koletzko B, Baker S, Cleghorn G, et al. Global standard for the composition of infant formula: recommendations of an ESPGHAN coordinated international expert group. J Pediatr Gastroenterol Nutr. 2005;41:584–99. [DOI] [PubMed] [Google Scholar]
- 11.Administration USF and D. Code of Federal Regulations Title 21. Section 107.100. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=107.100
- 12.Food and Agriculture Organization of the United Nations WHO. Standard for Infant Formula and Formulas for Special Medical Purposes Intended for Infants. CXS 72-1981. Published 2007. Accessed November 22, 2021. https://www.fao.org/fao-who-codexalimentarius/en/
- 13.Socha P, Grote V, Gruszfeld D, et al. Milk protein intake, the metabolic-endocrine response, and growth in infancy: data from a randomized clinical trial. Am J Clin Nutr. 2011;94:1776–84. [DOI] [PubMed] [Google Scholar]
- 14.Abrams SA, Hawthorne KM, Pammi M. A systematic review of controlled trials of lower-protein or energy-containing infant formulas for use by healthy full-term infants. World Rev Nutr Diet. 2016;114:61–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patro-Gołab B, Zalewski BM, Kouwenhoven SMP, et al. Protein concentration in milk formula, growth, and later risk of obesity: a systematic review. J Nutr. 2016;146:551–64. [DOI] [PubMed] [Google Scholar]
- 16.Fleddermann M, Demmelmair H, Koletzko B. Energetic efficiency of infant formulae: a review. Ann Nutr Metab. 2014;64:276–83. [DOI] [PubMed] [Google Scholar]
- 17.Onis M. WHO child growth standards based on length/height, weight and age. Acta Paediatr. 2007;95:76–85. [DOI] [PubMed] [Google Scholar]
- 18.Nelson SE, Rogers RR, Ziegler EE, et al. Gain in weight and length during early infancy. Early Hum Dev. 1989;19:223–39. [DOI] [PubMed] [Google Scholar]
- 19.Fleddermann M, Demmelmair H, Grote V, et al. Infant formula composition affects energetic efficiency for growth: the BeMIM study, a randomized controlled trial. Clin Nutr. 2014;33:588–95. [DOI] [PubMed] [Google Scholar]
- 20.Layman DK, Lönnerdal B, Fernstrom JD. Applications for a-lactalbumin in human nutrition. Nutr Rev. 2018;76:444–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lien EL. Infant formulas with increased concentrations of alpha-lactalbumin. Am J Clin Nutr. 2003;77:1555S–8S. [DOI] [PubMed] [Google Scholar]
- 22.Ahrens B, Hellmuth C, Haiden N, et al. Hydrolyzed formula with reduced protein content supports adequate growth: a randomized controlled noninferiority trial. J Pediatr Gastroenterol Nutr. 2018;66:822–30. [DOI] [PubMed] [Google Scholar]
- 23.Rigo J, Schoen S, Verghote M, et al. Partially hydrolysed whey-based formulae with reduced protein content support adequate infant growth and are well tolerated: Results of a randomised controlled trial in healthy term infants. Nutrients. 2019;11:1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexander DD, Yan J, Bylsma LC, et al. Growth of infants consuming whey-predominant term infant formulas with a protein content of 1.8 g/100 kcal: a multicenter pooled analysis of individual participant data. Am J Clin Nutr. 2016;104:1083–92. [DOI] [PubMed] [Google Scholar]
- 25.Ziegler EE, Fields DA, Chernausek SD, et al. Adequacy of infant formula with protein content of 1.6 g/100 kcal for infants between 3 and 12 months. J Pediatr Gastroenterol Nutr. 2015;61:596–603. [DOI] [PubMed] [Google Scholar]
- 26.Turck D, Grillon C, Lachambre E, et al. Adequacy and safety of an infant formula with a protein/energy ratio of 1.8 g/100 kcal and enhanced protein efficiency for term infants during the first 4 months of life. J Pediatr Gastroenterol Nutr. 2006;43:364–71. [DOI] [PubMed] [Google Scholar]
- 27.Petersen H, Nomayo A, Zelenka R, et al. Adequacy and safety of α-lactalbumin–enriched low-protein infant formula: a randomized controlled trial. Nutrition. 2020:4. [DOI] [PubMed] [Google Scholar]
- 28.Lönnerdal B, Lien EL. Nutritional and physiologic significance of α-Lactalbumin in infants. Nutr Rev. 2003;61:295–305. [DOI] [PubMed] [Google Scholar]
- 29.Lien EL, Davis AM, Euler AR. Growth and safety in term infants fed reduced-protein formula with added bovine alpha-lactalbumin. J Pediatr Gastroenterol Nutr. 2004;38:170–6. [DOI] [PubMed] [Google Scholar]
- 30.Trabulsi J, Capeding R, Lebumfacil J, et al. Effect of an α-lactalbumin-enriched infant formula with lower protein on growth. Eur J Clin Nutr. 2011;65:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis AM, Harris BJ, Lien EL, et al. α-Lactalbumin-rich infant formula fed to healthy term infants in a multicenter study: plasma essential amino acids and gastrointestinal tolerance. Eur J Clin Nutr. 2008;62:1294–301. [DOI] [PubMed] [Google Scholar]
- 32.Heine W, Radke M, Wutzke K, et al. α-Lactalbumin-enriched low-protein infant formulas: a comparison to breast milk feeding. Acta Paediatr. 1996;85:1024–8. [DOI] [PubMed] [Google Scholar]
- 33.Sidransky H, Sarma DS, Bongiorno M, et al. Effect of dietary tryptophan on hepatic polyribosomes and protein synthesis in fasted mice. J Biol Chem. 1968;243:1123–32. [PubMed] [Google Scholar]
- 34.Saidi O, Rochette E, Doré E, et al. Randomized double-blind controlled trial on the effect of proteins with different tryptophan/large neutral amino acid ratios on sleep in adolescents: the protmorpheus study. Nutrients. 2020;12:18851–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinberg LA, Connell NCO, Hatch TF, et al. Human and clinical nutrition tryptophan intake influences infants’ sleep latency. Pediatrics. 1992:1781–91. [DOI] [PubMed] [Google Scholar]
- 36.Yogman MW, Zeisel SH, Roberts C. Assessing effects of serotonin precursors on newborn behavior. J Psychiatr Res. 1982;17:123–33. [DOI] [PubMed] [Google Scholar]
- 37.Yogman MW, Zeisel SH. Diet and sleep patterns in newborn infants. N Engl J Med. 1983;309:1147–9. [DOI] [PubMed] [Google Scholar]
- 38.Corvaglia L, Mariani E, Aceti A, et al. Extensively hydrolyzed protein formula reduces acid gastro-esophageal reflux in symptomatic preterm infants. Early Hum Dev. 2013;89:453–5. [DOI] [PubMed] [Google Scholar]
- 39.Vandenplas Y, Devreker T, Hauser B. Double-blind trial of formula in distressed and regurgitating infants. Pediatrics. 2008;121(Supplement 2):S113S113.1–S113. [Google Scholar]
- 40.Vandenplas Y, Leluyer B, Cazaubiel M, et al. Double-blind comparative trial with 2 antiregurgitation formulae. J Pediatr Gastroenterol Nutr. 2013;57:389–93. [DOI] [PubMed] [Google Scholar]
- 41.Vivatvakin B, Estorninos E, Lien R, et al. Clinical response to two formulas in infants with parent-reported signs of formula intolerance: a multi-country, double-blind, randomized trial. Glob Pediatr Heal. 2020:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyer R, Foong RXM, Thapar N, et al. Systematic review of the impact of feed protein type and degree of hydrolysis on gastric emptying in children. BMC Gastroenterol. 2015;15:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sierra C, Bernal MJ, Blasco J, et al. Prebiotic effect during the first year of life in healthy infants fed formula containing GOS as the only prebiotic: a multicentre, randomised, double-blind and placebo-controlled trial. Eur J Nutr. 2015;54:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fanaro S, Marten B, Bagna R, et al. Galacto-oligosaccharides are bifidogenic and safe at weaning: A double-blind randomized multicenter study. J Pediatr Gastroenterol Nutr. 2009;48:82–8. [DOI] [PubMed] [Google Scholar]
- 45.Williams T, Choe Y, Price P, et al. Tolerance of formulas containing prebiotics in healthy, term infants. J Pediatr Gastroenterol Nutr. 2014;59:653–8. [DOI] [PubMed] [Google Scholar]
- 46.Bar-Yoseph F, Lifshitz Y, Cohen T, et al. SN2-palmitate reduces fatty acid excretion in Chinese formula-fed infants. J Pediatr Gastroenterol Nutr. 2016;62:341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kennedy K, Fewtrell MS, Morley R, et al. Double-blind, randomized trial of a synthetic triacylglycerol in formula-fed term infants: effects on stool biochemistry, stool characteristics, and bone mineralization. Am J Clin Nutr. 1999;70:920–7. [DOI] [PubMed] [Google Scholar]
- 48.Manios Y, Karaglani E, Thijs-Verhoeven I, et al. Effect of milk fat-based infant formulae on stool fatty acid soaps and calcium excretion in healthy term infants: two double-blind randomised cross-over trials. BMC Nutr. 2020;6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Padial-Jaudenes M, Castanys-Munoz E, Ramirez M, et al. Physiological impact of palm olein or palm oil in infant formulas: a review of clinical evidence. Nutrients. 2020;12:36761–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tahir MJ, Ejima K, Li P, et al. Associations of breastfeeding or formula feeding with infant anthropometry and body composition at 6 months. Matern Child Nutr. 2021;17:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.