Abstract
BACKGROUND & AIMS –
Radiofrequency ablation (RFA) is an effective treatment for Barrett’s esophagus (BE). However, recurrence of BE after initially successful RFA is common, and outcomes following recurrence not well-described. We report the outcomes associated with recurrence following initially successful RFA.
METHODS –
We performed a retrospective cohort study of 306 patients treated with RFA for dysplastic BE. Complete eradication of intestinal metaplasia (CE-IM) was defined as complete histologic and endoscopic remission of IM. Recurrence was defined as any presence of IM or dysplasia in the tubular esophagus or dysplasia in the gastric cardia subsequent to CE-IM. We examined rates and risk factors for recurrence, dysplastic recurrence and invasive adenocarcinoma after CE-IM. We also describe the clinical course of patients following recurrence.
RESULTS –
Of 306 eligible patients undergoing RFA, 218 achieved CE-IM and also had subsequent surveillance endoscopy. Of these, 52 (24%) experienced recurrence of IM or Barrett’s-associated neoplasia over 540.6 person-years (incidence rate 9.6%/year). Thirty (58%) of these achieved second CEIM; four (1.8% of total, 7.7% of recurrences) ultimately progressed to invasive adenocarcinoma (incidence rate 0.65%/year). Longer Prague M was a strong risk factor for invasive adenocarcinoma (rate ratio of 1.34/centimeter). Most dysplastic recurrences were in the cardia, and the majority were not visible, but detected on random biopsies.
CONCLUSION –
Most patients with recurrent BE after initially successful RFA achieve second CE-IM, however 1.8% progressed to invasive adenocarcinoma. Longer Prague M was predictive of invasive adenocarcinoma. Four-quadrant random biopsy of the cardia is advisable during surveillance endoscopy after CE-IM.
Background
Barrett’s esophagus (BE) is a precancerous condition in which the distal esophagus undergoes a metaplastic change to intestinal metaplasia, associated with an increased risk of developing esophageal adenocarcinoma.1 Radiofrequency ablation (RFA) has been associated with high rates of complete eradication of dysplasia and intestinal metaplasia and a decreased risk of cancer development.2–5 While RFA is a safe and effective treatment for dysplastic BE,6, 7 the durability of the neosquamous epithelium is not fully understood, and continued endoscopic surveillance after RFA is necessary to monitor potential disease recurrence.8
The incidence of recurrence and esophageal adenocarcinoma following complete eradication of dysplasia (CE-D) and complete eradication of intestinal metaplasia (CE-IM) in BE has been reported in prior studies,5, 8–13 but limited literature exists on the clinical outcomes of patients following recurrence. It also remains unknown whether recurrence predicts the ultimate failure of ablative therapy or is correctable with further ablative treatment. An additional gap in knowledge exists regarding features of recurrence or baseline characteristics that predict the clinical course following recurrence.
We aimed to describe the clinical and endoscopic characteristics of patients with recurrence of intestinal metaplasia after initial RFA resulting in CE-IM, and to describe the clinical outcomes associated with recurrence following CE-IM in BE treated with RFA. We further sought to analyze histologic and endoscopic features of recurrence, along with baseline clinical and endoscopic characteristics, for utility as risk factors to predict the clinical course following recurrence.
Methods
Study Design
We performed a retrospective cohort study of 306 adult patients at the University of North Carolina (UNC) Hospitals who were treated once or more with RFA for dysplastic BE between March, 2006 and June, 2015. Initial diagnosis of BE required two criteria (9). First, inspection by upper endoscopy revealed an abnormal, salmon-colored epithelium extending above the gastroesophageal junction (GEJ), demarcated by the top of the gastric folds. Second, at least one biopsy from the tubular esophagus was interpreted as containing intestinalized epithelium with goblet cells. Patients with active pregnancy, those who underwent prior treatment with other ablative modalities, those with non-dysplastic BE, and those with invasive adenocarcinoma at baseline were excluded.
Patient data were extracted from electronic medical records, and a standardized form was used to assess potential risk factors and demographics. Baseline data collected included gender, age, body mass index (BMI), smoking and drinking habits, circumferential and maximal length of baseline BE, and histologic grade of BE. Follow-up visits were recorded including esophageal endoscopic findings, histology results, and treatments administered.
CE-IM was defined as complete histologic and endoscopic remission of IM after a single endoscopy with biopsies taken throughout the prior extent of BE. Complete eradication of dysplasia (CE-D) was defined as complete histologic and endoscopic remission of dysplasia after a single endoscopy with biopsies taken throughout the prior extent of BE. Recurrence was defined as dysplastic or non-dysplastic IM in the esophagus or any dysplasia in the cardia on histology subsequent to CE-IM. Because non-dysplastic intestinal metaplasia of the cardia is a common finding in subjects with chronic GERD,14 a finding of non-dysplastic IM of the cardia alone was not considered disease recurrence. Second CE-IM was defined as CE-IM achieved after recurrence. Because of a lack of consensus in the literature regarding the definition of CE-IM and CE-D, sensitivity analyses were also performed in which CE-IM and CE-D were defined as two negative endoscopies as above.
Treatment
An expert gastrointestinal pathologist reviewed original pathology specimens of all patients to confirm dysplasia and to determine the worst baseline histologic grade of BE prior to endoscopic therapy. All patients received proton pump inhibitor twice daily prior to and throughout treatment. Systematic endoscopic examination was performed with high-definition white light and narrow band imaging, and any endoscopically visible mucosal abnormalities identified in the field of BE were treated with endoscopic mucosal resection prior to RFA for the full duration of the cohort. RFA treatments were performed as previously described.4 Patients received follow-up RFA treatment every 2–3 months until CE-IM was achieved. After CE-IM, patients were scheduled for continued endoscopic surveillance. Endoscopic surveillance intervals were 3–6 months in year one after CE-IM, and annually thereafter. Endoscopic surveillance consisted of systematic biopsies in 4 quadrants every one centimeter of the previous BE, as well as systematic 4 quadrant sampling of the gastric cardia just below the Z line (5–10 mm below the top of the gastric folds). Any visualized mucosal abnormality of the esophagus or cardia was biopsied separately.
Outcomes and Statistical Analysis
Descriptive statistics were reported as number and column percent, with categories chosen for continuous variables. Mean and standard deviation were additionally reported for continuous variables. Recurrence, dysplastic recurrence, and invasive adenocarcinoma progression among patients who achieved CE-IM, as well as second CE-IM among patients who recurred, were reported as rates per 100 person-years with censoring at the last visit where histology was recorded. These outcomes were also presented as Kaplan-Meier plots with statistical comparison using the log-rank test. We calculated unadjusted and mutually adjusted rate ratios (RR) and 95% profile likelihood confidence limits (CL) for all outcomes by baseline characteristics and prior surveillance events using Poisson rates regression with maximum likelihood estimation. We estimated rate ratios for second CEIM using exact Markov-Chain Monte Carlo estimation for small sample size.
Results
Of the 306 eligible patients with BE treated with RFA therapy, 260 (85.0%) achieved CE-IM and 272 (88.9%) achieved CE-D. Among the patients that achieved CE-IM, 218 had at least one additional endoscopy visit with histologic sampling and were included for analysis of first recurrence; the remainder received endoscopic surveillance by their local gastroenterologist.
Of patients receiving surveillance endoscopy at UNC, 52 (24%) experienced recurrence of IM or Barrett’s-associated neoplasia over 540.6 person-years (incidence rate 9.6% per year). The mean time to recurrence was 1.88 years (SD 1.42) after CE-IM and the mean number of surveillance endoscopies to recurrence was 2.32 (SD 1.35). At any given surveillance endoscopy, the proportion of patients displaying recurrence was similar (Figure 1a and 1b). Stratified Kaplan Meier analyses were consistent with a constant rate of recurrence over time (Figure 2a). Initial recurrences were isolated to the esophagus in 33 (63%), isolated to the cardia in 17 (33%), and found in both the esophagus and cardia in 2 (4%) patients. Baseline characteristics of included patients are shown in Table 1. The mean age at the time of first RFA treatment among included patients was 69.9 (SD 10.4), the mean Prague M length of BE was 4.58 (SD 3.00), and the mean circumferential length (Prague C) of BE was 3.18 (SD 3.11). Most patients (86%) were overweight or obese, and most (51%) were receiving treatment for HGD.
Figure 1.
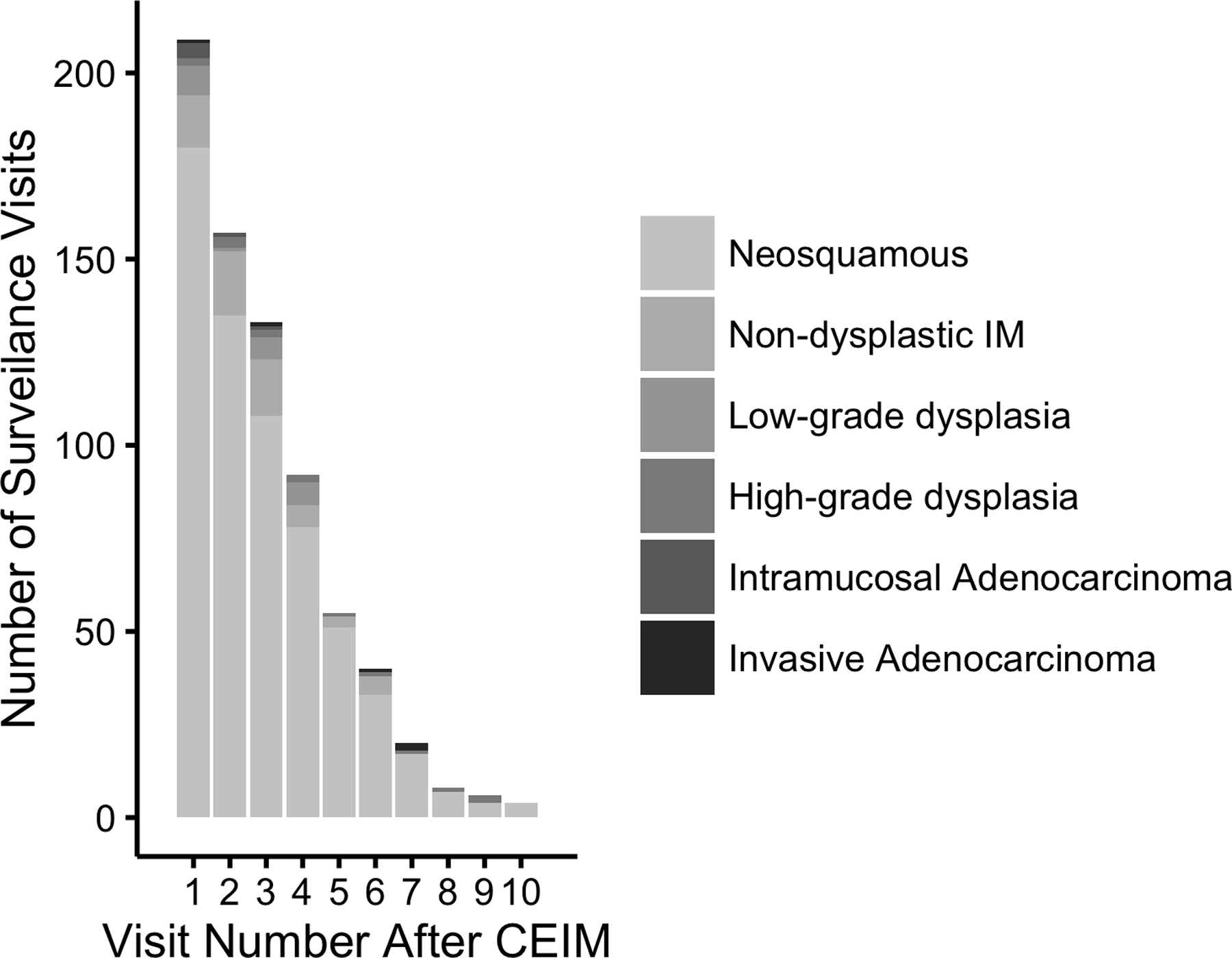
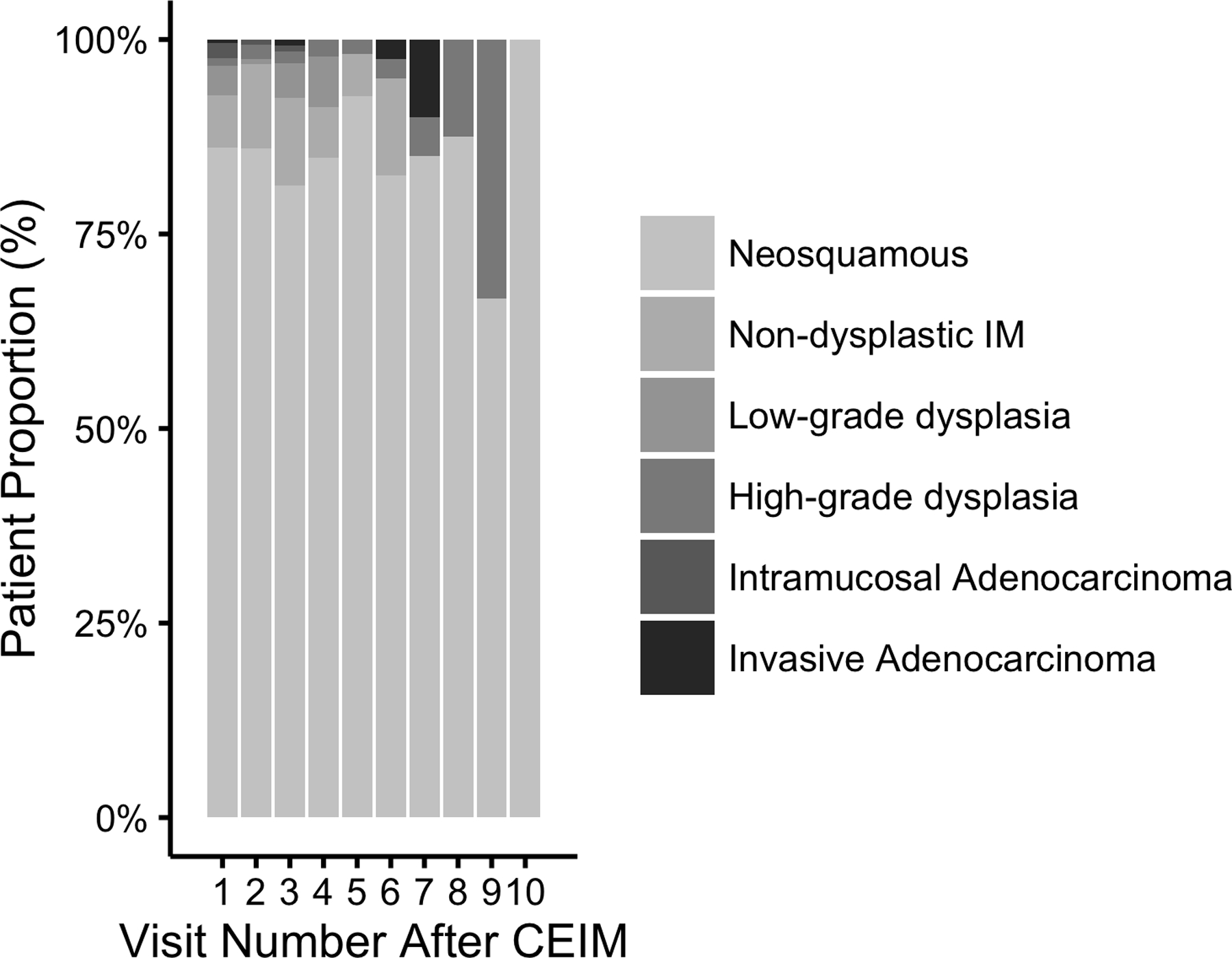
Bar Graph of Number and Proportion of Surveillance Visits Among 218 Patients With Complete Eradication of Intestinal Metaplasia (CEIM) with Surveillance Histologic Grade as a Function of Number of Visits After CEIM. Figure 1a shows absolute numbers of procedures, while Figure 1b shows proportions with various histologies by visit number.
Figure 2.
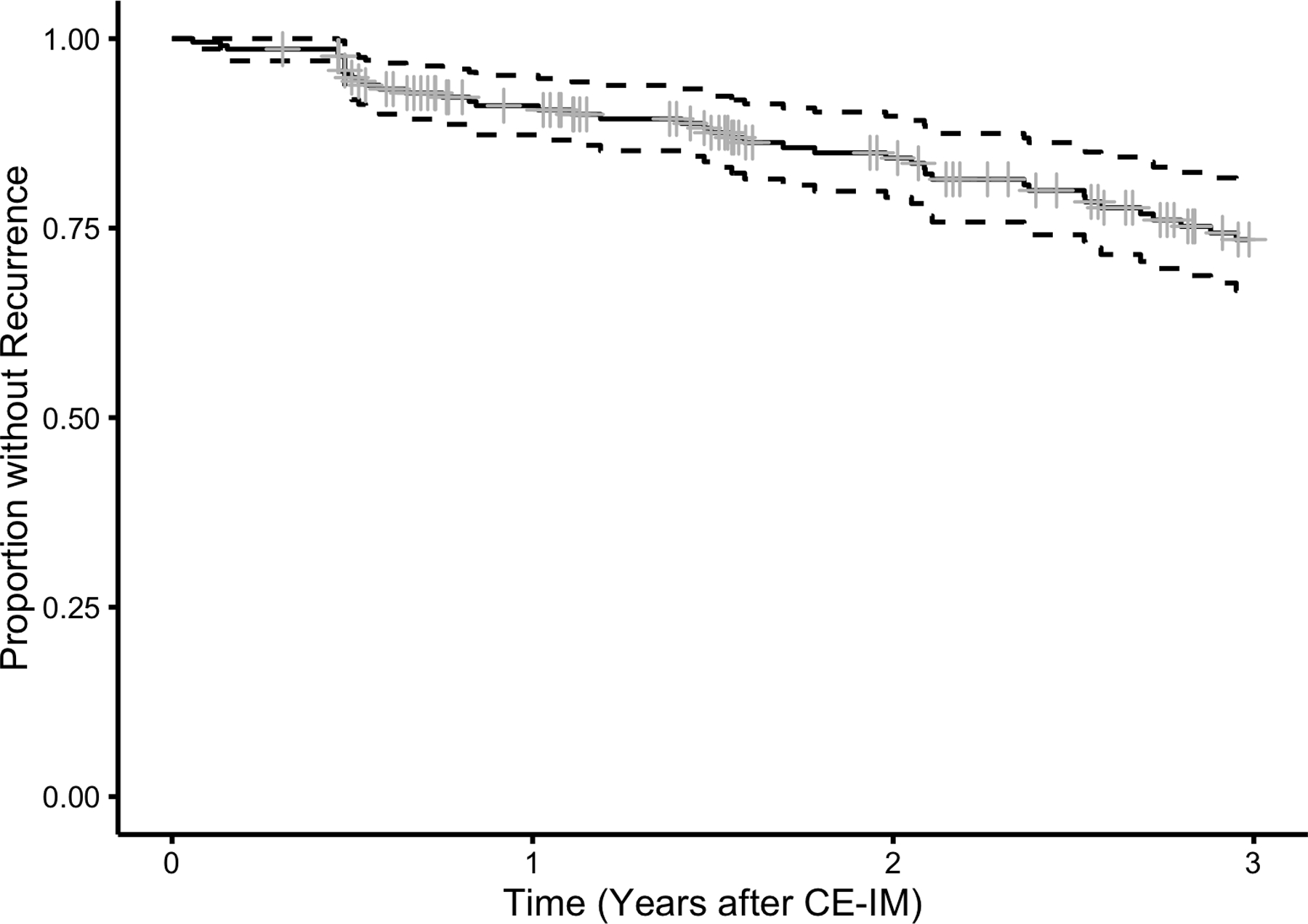
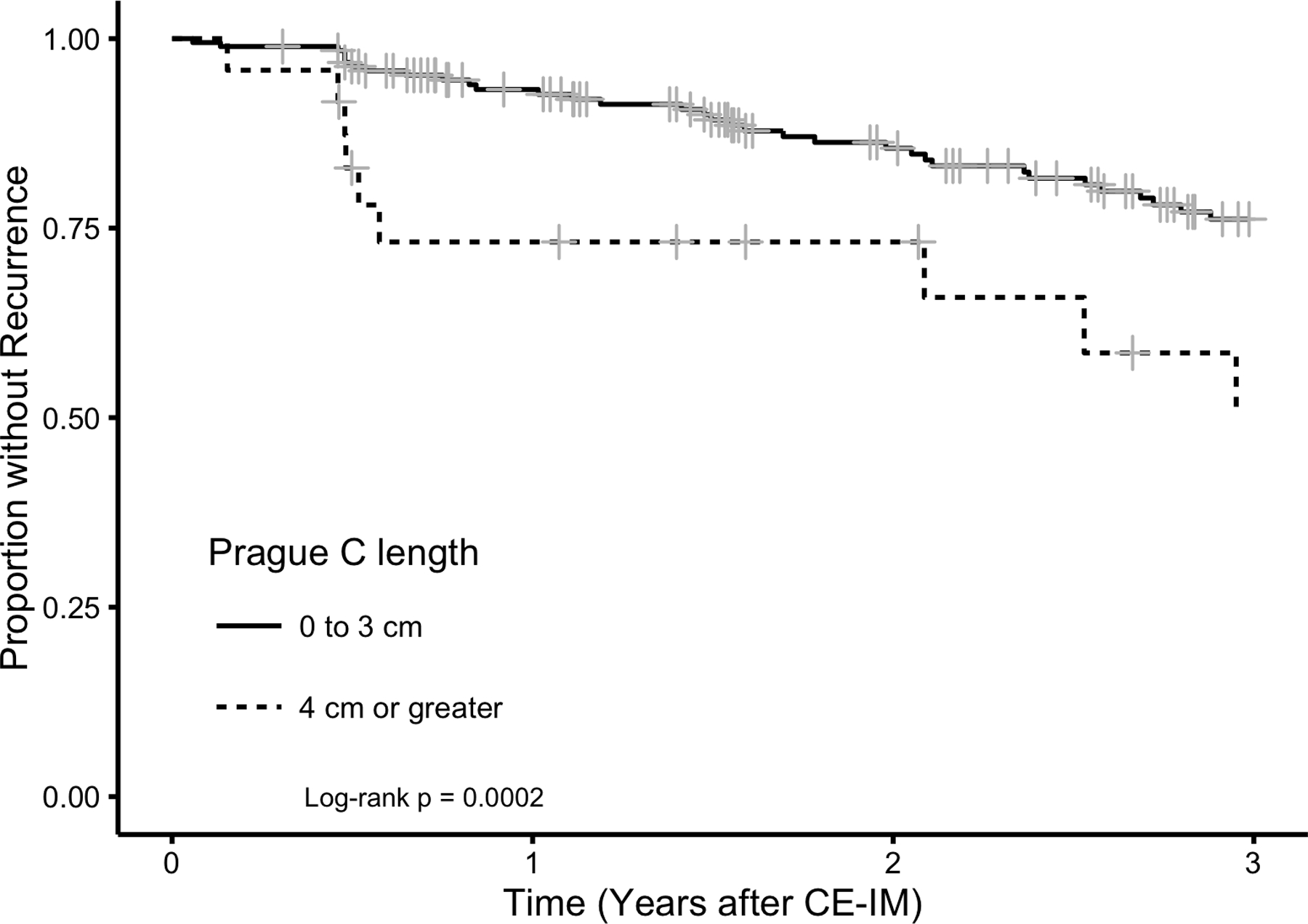
Kaplan-Meier plot of estimated proportion of 218 Patients who Achieved CEIM without a First Recurrence of IM or Barrett’s-associated Neoplasia by time following CE-IM. Figure 2a demonstrates overall recurrence for the cohort, while Figure 2b stratifies by segment length. Longer Prague C length was a strong risk factor for recurrence.
Table 1.
Baseline Characteristics of 218 Included Patients who Achieved Complete Eradication of Intestinal Metaplasia (CE-IM) and had an additional surveillance examination.
| Baseline Characteristic | All eligible patients (306) - N (%) | Patients with CE-IM (260) - N (%) | Patients with CE-IM in surveillance (218) - N (%) |
|---|---|---|---|
| Age (years) | |||
| - 25 to 60 | 53 (17.3) | 44 (17.3) | 36 (16.7) |
| - 61 to 80 | 194 (63.4) | 168 (66.1) | 145 (67.4) |
| - 81 to 101 | 52 (17.0) | 42 (16.5) | 34 (15.8) |
| - Mean [SD] | 70.2 [11.2] | 70.0 [11.0] | 69.9 [10.4] |
| Low-grade dysplasia | 98 (32.0) | 84 (32.3) | 75 (34.3) |
| High-grade dysplasia | 188 (61.4) | 158 (50.8) | 128 (58.7) |
| Intramucosal adenocarcinoma | 20 (6.5) | 18 (6.9) | 15 (6.9) |
| BMI (mg/kg2) | |||
| - Normal (18.5 – 24.9) | 53 (17.5) | 40 (15.6) | 30 (13.9) |
| - Overweight (25.0 – 29.9) | 119 (39.4) | 108 (42.2) | 96 (44.4) |
| - Obesity (>=30) | 130 (43.0) | 108 (42.2) | 90 (41.7) |
| - Mean [SD] | 29.9 [5.9] | 30.0 [6.0] | 30.0 [5.7] |
| Female | 106 (34.6) | 89 (34.2) | 68 (31.2) |
| Male | 200 (65.4) | 171 (65.8) | 150 (68.8) |
| No history of alcohol use | 127 (42.8) | 107 (42.5) | 89 (42.0) |
| - Current alcohol use | 134 (45.1) | 115 (45.6) | 96 (45.3) |
| - Past alcohol use | 36 (12.1) | 30 (11.9) | 27 (12.7) |
| No history of tobacco use | 131 (43.5) | 117 (45.7) | 104 (48.4) |
| - Current tobacco use | 52 (17.3) | 41 (16.0) | 33 (15.3) |
| - Past tobacco use | 118 (39.2) | 98 (38.3) | 78 (36.3) |
| Prague M length (cm) | |||
| - 0 to 3 | 119 (39.7) | 108 (42.2) | 89 (41.6) |
| - 4 or more | 181 (60.3) | 148 (57.8) | 125 (58.4) |
| - Mean [SD] | 5.0 [3.5] | 4.6 [3.1] | 4.6 [3.0] |
| Prague C length (cm) | |||
| - 0 to 3 | 262 (85.6) | 230 (88.5) | 194 (89.0) |
| - 4 or more | 44 (14.4) | 30 (11.5) | 24 (11.0) |
| - Mean [SD] | 3.7 [3.8] | 3.0 [3.1] | 3.2 [3.1] |
| GERD symptoms at diagnosis | 123 (43.0) | 109 (44.7) | 91 (44.0) |
| - no GERD symptoms | 163 (57.0) | 135 (55.3) | 116 (56.0) |
| ASA use | 127 (41.8) | 109 (42.1) | 91 (44.0) |
| - no use | 177 (58.2) | 150 (57.9) | 116 (56.0) |
| NSAID use | 45 (14.8) | 39 (15.1) | 36 (16.6) |
| - no use | 259 (85.2) | 220 (84.9) | 181 (83.4) |
| Nissen Fundoplication | 15 (5.0) | 13 (5.0) | 12 (5.6) |
| - no Nissen | 288 (95.5) | 245 (95.0) | 204 (94.4) |
BMI, body mass index.
Of the 52 patients who experienced recurrence, 30 (58%) achieved second CE-IM, one (2%) presented on recurrence with invasive adenocarcinoma, two (4%) failed endoscopic re-treatment after recurrence and progressed to invasive adenocarcinoma, 19 (37%) had experienced neither event and were receiving ongoing endoscopic treatment for their recurrence at the time of this report, and one (2%) was lost to follow-up. Baseline characteristics did not predict second CE-IM (Table 2).
Table 2.
Unadjusted rate ratios (RR) for achieving a second CE-IM following recurrence by baseline characteristics and recurrence grade among 36 patients who experienced recurrence and had at least one additional surveillance endoscopy.
| Characteristic | Second CE-IM - N (%) | No Second CE-IM - N (%) | Unadjusted RR (95% CL) |
|---|---|---|---|
| Age (years) - 40 to 59 | 21 (70.0) | 5 (83.3) | Ref. |
| - 60 to 79 | 4 (13.3) | 1 (16.7) | 0.72 (0.24 – 2.88) |
| - 80 to 99 | 5 (16.7) | 0 (0.0) | 1.42 (0.31 – 7.14) |
| BMI (mg/kg2) - Normal (18.5 – 24.9) | 6 (20.0) | 0 (0.0) | Ref. |
| - Overweight (25.0 – 29.9) | 8 (26.7) | 3 (50.0) | 0.78 (0.24 – 2.73) |
| - Obesity (>=30) | 16 (53.3) | 3 (50.0) | 1.10 (0.41 – 3.44) |
| Female | 10 (33.3) | 1 (16.7) | Ref. |
| Male | 20 (66.7) | 5 (83.3) | 0.82 (0.37 – 1.96) |
| Prague M length (cm) - 0 to 3 | 6 (20.0) | 2 (33.3) | Ref. |
| - 4 or greater | 24 (80.0) | 4 (66.7) | 1.05 (0.42 – 3.13) |
| Prague C length (cm) - 0 to 3 | 24 (80.0) | 5 (83.3) | Ref. |
| - 4 or greater | 6 (20.0) | 1 (16.7) | 2.22 (0.74 – 5.58) |
| Baseline grade - Low-grade dysplasia | 8 (26.7) | 2 (33.3) | Ref. |
| - High-grade dysplasia | 21 (70.0) | 4 (66.7) | 1.58 (0.67 – 4.13) |
| - Intramucosal adenocarcinoma | 1 (3.3) | 0 (0.0) | 2.52 (0.06 – 18.82) |
| Recurrence grade – non-dysplastic | 11 (36.7) | 1 (16.7) | Ref. |
| - Low-grade dysplasia | 12 (40.0) | 3 (50.0) | 0.70 (0.28 – 1.76) |
| - High-grade dysplasia | 4 (13.3) | 1 (16.7) | 0.88 (0.20 – 2.96) |
| - Intramucosal Adenocarcinoma | 3 (10.0) | 1 (16.7) | 1.31 (0.23 – 4.97) |
| Non-dysplastic recurrence | 11 (36.7) | 1 (16.7) | Ref. |
| - Dysplastic recurrence | 19 (63.3) | 5 (83.3) | 0.79 (0.36 – 1.85) |
BMI, body mass index.
Among the 30 patients who achieved second CE-IM, four (13%) later experienced second recurrence (incidence rate 2.1% per year): one with non-dysplastic intestinal metaplasia, one with low-grade dysplasia, and two with high-grade dysplasia. Of these four, one progressed to invasive adenocarcinoma (this patient had a first recurrence of low-grade dysplasia and a second recurrence of high-grade dysplasia), another was receiving endoscopic treatment at the time of this study, and two of the four achieved a third CE-IM.
Longer Prague C length was strongly associated with recurrence (Table 3), especially in the period of time immediately following CE-IM (Figure 2b). Though baseline histologic grade was not a risk factor for recurrence overall, HGD was a strong risk factor for dysplastic recurrence (Table 4). Among patients who recurred, recurrences were more often of lower than baseline histologic grade (Figure 3). However, four cases of invasive adenocarcinoma occurred after CE-IM, an incidence rate of 0.65% per year (Figure 4). One of the four invasive adenocarcinoma progressions occurred after second CE-IM was achieved. Three of these four were preceded by a dysplastic, but non-cancer, recurrence. Longer Prague M length was a risk factor for invasive adenocarcinoma progression with a rate ratio of 1.34 (95% CL 1.00 – 1.73) per additional centimeter of length.
Table 3.
Unadjusted and mutually adjusted rate ratios (RR) for any recurrence by baseline characteristics following complete eradication of intestinal metaplasia (CE-IM) among patients with at least one surveillance endoscopy after achieving CE-IM.
| Characteristic | Recurrence - N (%) | No Recurrence - N (%) | Unadjusted RR (95% CL) | Adjusted RR (95% CL) |
|---|---|---|---|---|
| Age (years) - 25 to 60 | 8 (15.4) | 26 (16.1) | 0.89 (0.39 – 1.82) | 1.03 (0.43 – 2.20) |
| - 61 to 80 | 36 (69.2) | 109 (67.7) | Ref. | Ref. |
| - 81 to 101 | 8 (15.4) | 26 (16.1) | 0.90 (0.39 – 1.84) | 1.09 (0.45 – 2.38) |
| BMI (mg/kg2) - Normal (18.5 – 24.9) | 9 (17.3) | 20 (12.3) | Ref. | Ref. |
| - Overweight (25.0 – 29.9) | 19 (36.5) | 77 (47.5) | 0.57 (0.27 – 1.33) | 0.48 (0.21 – 1.16) |
| - Obesity (>=30) | 24 (46.2) | 65 (40.1) | 0.66 (0.32 – 1.50) | 0.65 (0.30 – 1.52) |
| Female | 13 (25.0) | 55 (33.5) | Ref. | Ref. |
| Male | 39 (75.0) | 109 (66.5) | 1.17 (0.64 – 2.29) | 1.23 (0.69 – 2.52) |
| Prague M length (cm) - 0 to 3 | 13 (25.0) | 74 (46.3) | Ref. | Ref. |
| - 4 or greater | 39 (75.0) | 86 (53.8) | 2.33 (1.28 – 4.55) | 2.08 (1.09 – 4.18) |
| Prague C length (cm) - 0 to 3 | 41 (78.8) | 151 (92.1) | Ref. | Ref. |
| - 4 or greater | 11 (21.2) | 13 (7.9) | 2.93 (1.43 – 5.49) | 2.55 (1.18 – 5.25) |
| Baseline grade – Low-grade dysplasia | 19 (36.5) | 56 (34.1) | Ref. | Ref. |
| - High-grade dysplasia | 31 (59.6) | 95 (59.9) | 1.15 (0.65 – 2.06) | 1.36 (0.75 – 2.51) |
| - Intramucosal adenocarcinoma | 2 (3.8) | 13 (7.9) | 0.51 (0.08 – 1.75) | 0.73 (0.11 – 2.76) |
BMI, body mass index.
Table 4.
Unadjusted and mutually adjusted rate ratios (RR) for dysplastic recurrence by baseline characteristics following complete eradication of intestinal metaplasia (CE-IM) among patients with at least one surveillance endoscopy after achieving CE-IM.
| Characteristic | Recur - N (%) | No Recurrence - N (%) | Unadjusted RR (95% CL) | Adjusted RR (95% CL) |
|---|---|---|---|---|
| Age (years) - 25 to 60 | 3 (10.7) | 31 (16.8) | 0.63 (0.15 – 1.86) | 0.77 (0.17 – 2.42) |
| - 61 to 80 | 19 (67.9) | 126 (68.1) | Ref. | Ref. |
| - 81 to 101 | 6 (21.4) | 28 (15.1) | 1.28 (0.47 – 3.03) | 1.67 (0.56 – 4.39) |
| BMI (mg/kg2) - Normal (18.5 – 24.9) | 3 (14.3) | 25 (13.4) | Ref. | Ref. |
| - Overweight (25.0 – 29.9) | 11 (39.3) | 85 (45.7) | 0.75 (0.26 – 2.69) | 0.72 (0.23 – 2.69) |
| - Obesity (>=30) | 13 (46.4) | 76 (40.9) | 0.80 (0.28 – 2.86) | 1.03 (0.34 – 3.84) |
| Female | 10 (35.7) | 58 (30.9) | Ref. | Ref. |
| Male | 18 (64.3) | 130 (69.1) | 0.70 (0.33 – 1.59) | 0.65 (0.23 – 2.69) |
| Prague M length (cm) - 0 to 3 | 7 (25.0) | 80 (43.5) | Ref. | Ref. |
| - 4 or greater | 21 (75.0) | 104 (56.5) | 2.33 (1.04 – 5.93) | 2.34 (0.99 – 6.14) |
| Prague C length (cm) - 0 to 3 | 23 (82.1) | 169 (89.9) | Ref. | Ref. |
| - 4 or greater | 5 (17.9) | 19 (10.1) | 2.37 (0.80 – 5.75) | 1.86 (0.59 – 5.04) |
| Baseline grade – Low-grade dysplasia | 6 (21.4) | 69 (36.7) | Ref. | Ref. |
| - High-grade dysplasia | 21 (75.0) | 105 (55.9) | 2.46 (1.05 – 6.70) | 2.91 (1.21 – 8.15) |
| - Intramucosal adenocarcinoma | 1 (3.6) | 14 (7.4) | 0.80 (0.04 – 4.70) | 0.78 (0.04 – 5.08) |
BMI, body mass index.
Figure 3.
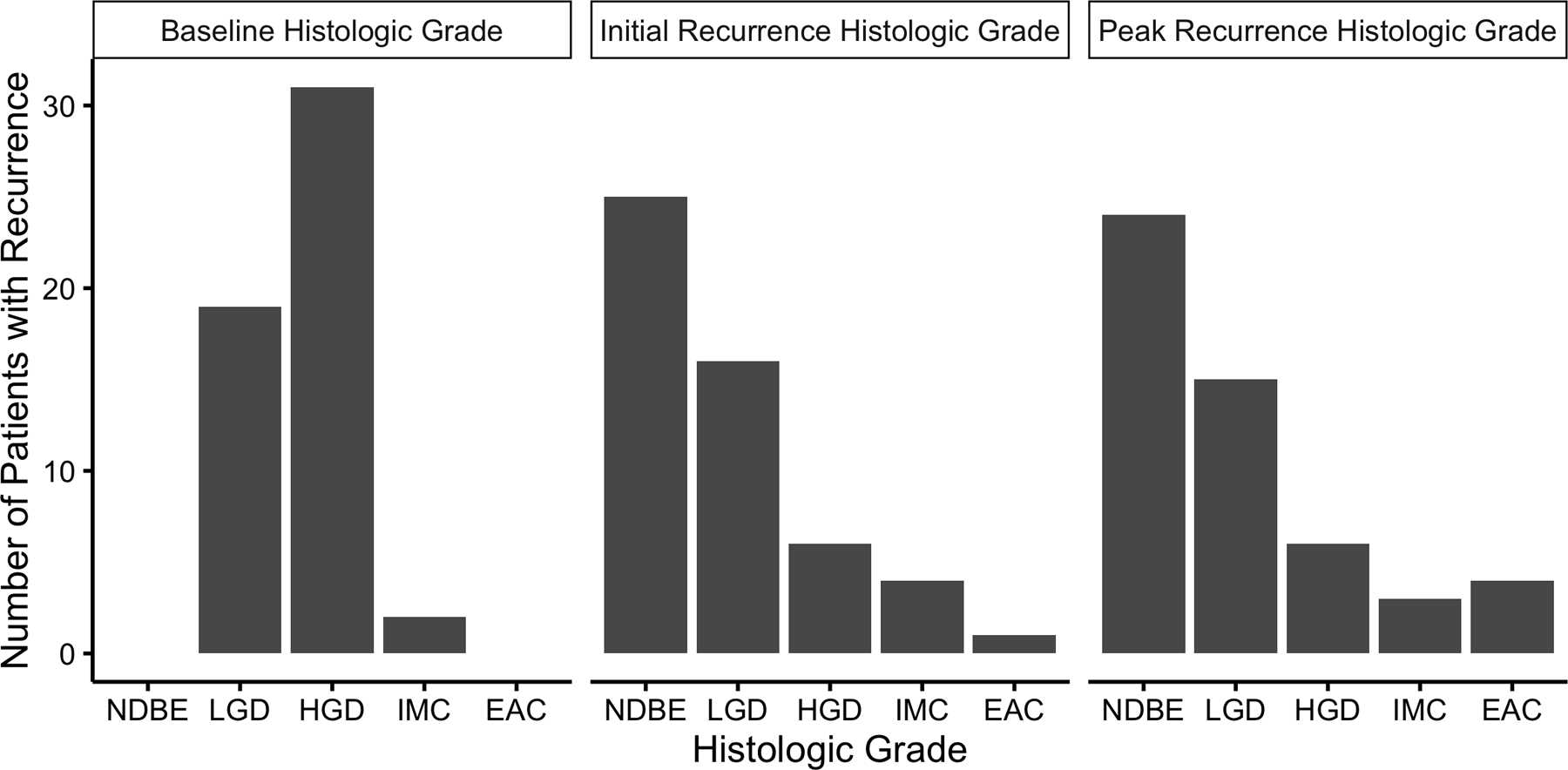
Number of 52 Patients with Recurrence by Highest Histologic Grade at Baseline and Recurrence. Most recurrences were of lower histologic grade than baseline worst histologic grade. NDBE, non-dysplastic Barrett’s esophagus; LGD, low-grade dysplasia; HGD, high-grade dysplasia; IMC, intramucosal adenocarcinoma; EAC, invasive esophageal adenocarcinoma.
Figure 4.
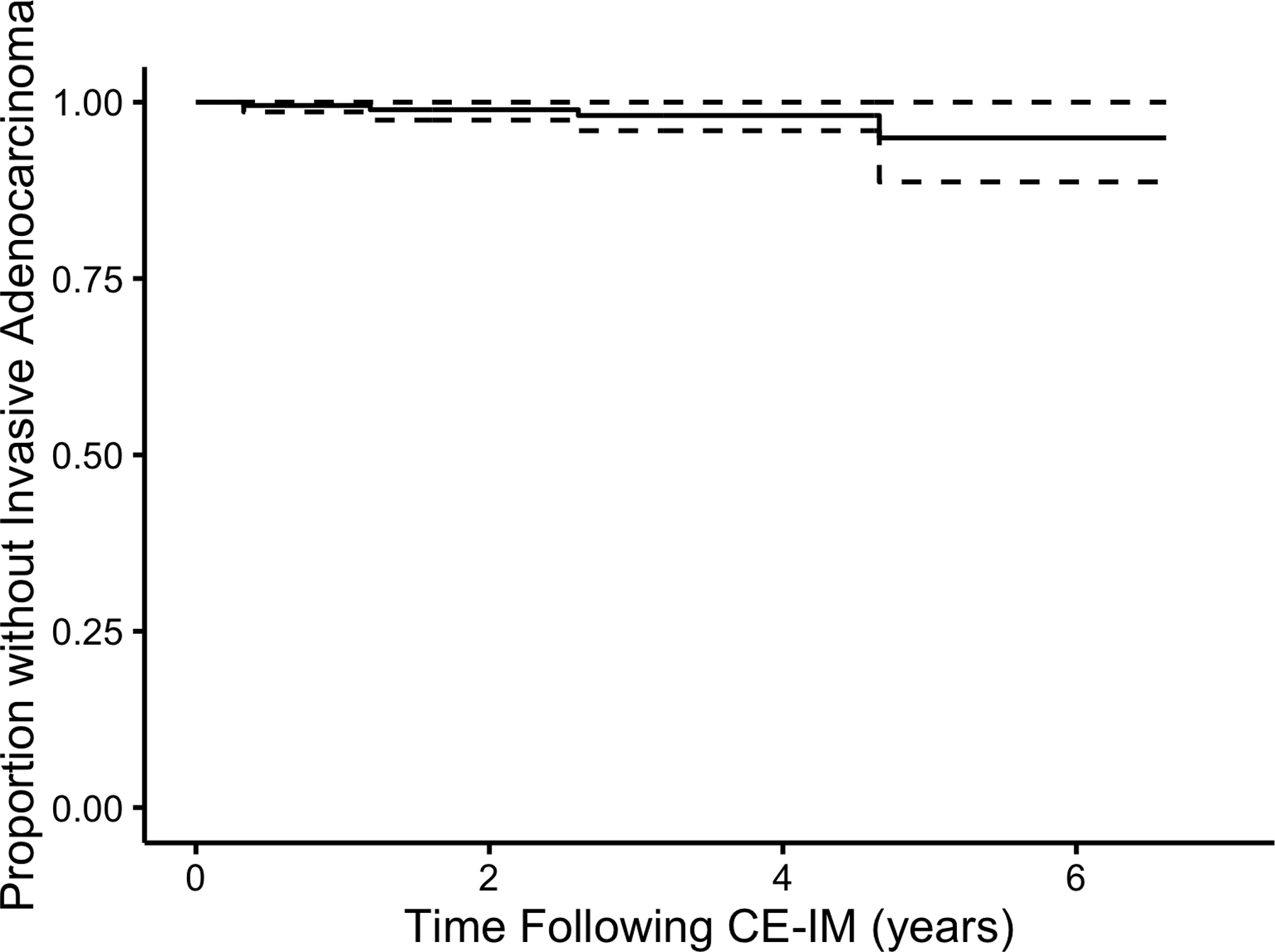
Kaplan-Meier plot of estimated Proportion of Patients without Progression to Invasive Esophageal Adenocarcinoma by Time Following CE-IM. At five years after CE-IM 5.1% (95% CL 0.0 – 11.3%) of patients were estimated to experience invasive cancer progression.
Recurrence overall was more common in the esophagus than in the gastric cardia, but dysplastic recurrence was more actually more common in the gastric cardia (Figure 5). Recurrence was visible in 8 of the 10 (80%) dysplastic recurrences in the esophagus, but only in 4 of the 17 (23.5%) dysplastic recurrences in the gastric cardia. No cardia recurrence was noted to be > 1 cm distal to the post-treatment GEJ. While non-dysplastic intestinal metaplasia isolated to the gastric cardia was not considered recurrence for the present study, it was commonly observed in surveillance, occurring in 42 (21.5%) of 200 patients with cardia biopsies.
Figure 5.
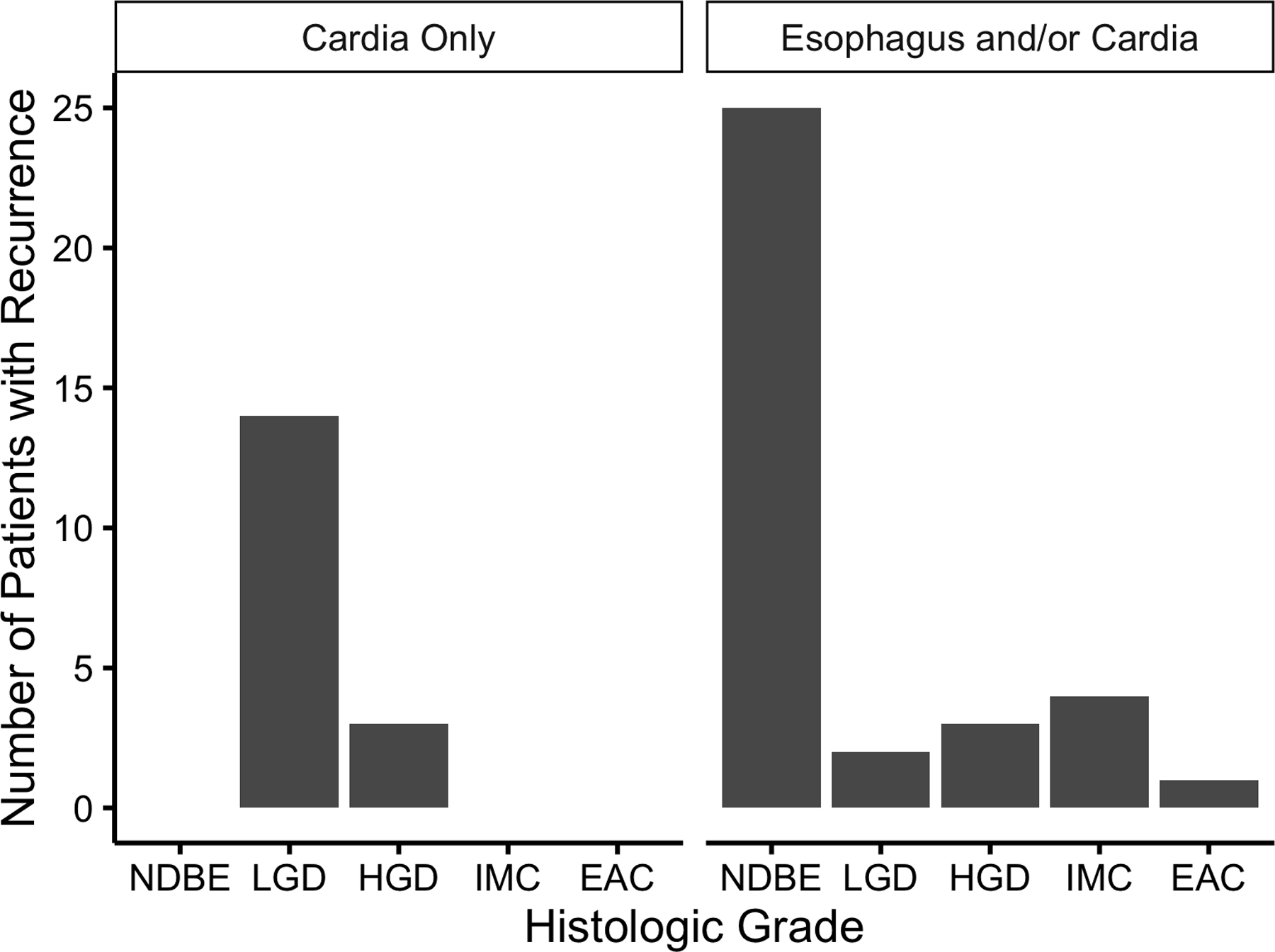
Number of Recurrences by Histologic Grade at Recurrence in the Tubular Esophagus or in the Gastric Cardia
Supplemental analyses with the definition of CE-IM as two consecutive endoscopies without Barrett’s-associated neoplasia or any treatment given are presented in supplemental tables S-1 to S-3. The precision of estimates in the supplemental analysis was less than in the primary analysis due to fewer patients with CE-IM and fewer patient-years at risk in surveillance because of the additional confirming endoscopy. However, point estimates of risk factors for recurrence were similar for both definitions of CE-IM. The rate of invasive adenocarcinoma progression using the supplemental definition of CE-IM was also similar to that of the primary definition, at 0.96% per year.
Discussion
Although RFA has been shown to successfully eradicate dysplasia and IM and to reduce the risk of progression to EAC, recurrence of IM in the esophagus is common, and there are limited data on the clinical outcomes of patients following this recurrence. In this large single-center cohort of patients with dysplastic BE, recurrence was common, with almost a quarter of our patients eventually experiencing recurrence. A small number of patients (4, 1.8%) did experience invasive adenocarcinoma after CEIM, requiring surgical resection. These outcomes emphasize an important point – that patients undergoing endoscopic eradication therapies are never “out of the woods,” and that both the endoscopist and the patient should consider this a lifelong health concern. On a brighter note, these recurrences were generally of lower histological grade than the primary lesion, and were generally amenable to further ablative treatment. Longer circumferential and maximal length (Prague C or M ≥ 4cm) was predictive of recurrence, but other patient characteristics such as age, BMI, and gender, did not predict recurrence.
This study expands on prior research regarding the durability of CE-IM after treatment with RFA by describing patients’ course after recurrence. Our findings are consistent with prior smaller studies showing most recurrences are non-dysplastic and endoscopically manageable,12 and among those with recurrent IM, most had a benign clinical course.9 Risk factors previously reported as predictors of recurrence include increasing age, non-Caucasian race, higher grades of dysplasia, and longer lengths of BE.9, 13 Our findings suggest circumferential (Prague C) rather than maximal (Prague M) length is most strongly associated with recurrence. Patient characteristics in another study did not predict recurrence, consistent with our findings for characteristics other than circumferential length.12 In one single center study, a large hiatal hernia and histology grade of HGD or IMC after the first ablation treatment were associated with an increased risk of recurrence or progression of adenocarcinoma after ablation.11 Our findings are consistent with previous, smaller studies in which recurrence of IM or dysplasia has been observed at the gastroesophageal junction without visible BE.8, 15
The strengths of this study include the largest single-center U.S. cohort of patients with recurrence of IM or Barrett’s-associated neoplasia studied to date. This study allowed more consistent and complete reporting than is available in multicenter studies, including details about visual endoscopic impression in addition to histologic data. The study also has limitations. The single-center nature of this data may limit generalizability, and sample size following recurrence may have limited the ability to detect protective factors or risk factors for second CE-IM. The present study has few patients with follow-up after CE-IM greater than five years such that direct inference about the risk of recurrence or progression later in these patients’ course is not possible. Detection of recurrence and CE-IM are limited by techniques that sample a minute proportion of the surface area of BE, making sampling error possible. Inference about the biologic mechanisms of recurrence from clinical studies such as this is limited. The underlying mechanism of recurrence as reported in clinical studies is unknown and may represent a de novo process or sampling error. Forty-two of 260 (16%) of patients who achieved CE-IM underwent surveillance endoscopy at other centers and were excluded from analysis of recurrence, which presents the possibility of missing data bias.
The findings from our study further emphasize the importance of thorough and ongoing endoscopic surveillance following CE-IM in patients treated with RFA therapy for BE. Routine measurement of Prague C and M length is indicated to better identify and manage patients with a higher risk for recurrence. Since a substantial proportion (63.8%) of recurrences can be successfully retreated and second CE-IM can be re-established, endoscopic surveillance offers the potential to detect and treat recurrence before progression to invasive cancer. An additional important conclusion from this work is that dysplastic recurrence in the cardia actually represented the majority of dysplastic recurrences detected during endoscopic surveillance. Whether these lesions represented de novo occurrences, or were prevalent lesions that existed prior to ablation but only discovered in post-ablation surveillance, is unclear. These dysplastic recurrences were particularly difficult to identify visibly, and most would have been missed without routine sampling. The lack of a visible lesion in the majority of these cases of dysplasia of the cardia suggests thorough histologic 4 quadrant sampling of the cardia is advisable after CE-IM.
Supplementary Material
Study Highlights.
What is current knowledge?
Recurrence of Barrett’s esophagus following ablative therapies is not uncommon.
Surveillance endoscopy is required following treatment to identify recurrence.
What is new here?
Among patients with recurrence, most respond to recurrent ablative therapy, and re-attain complete eradication of intestinal metaplasia.
Progression to cancer was rare, and usually preceded by detection of dysplastic recurrence.
Dysplastic recurrence was most often detected in 4-quadrant ‘random’ biopsies of the gastric cardia, making these biopsies advisable as part of the surveillance examination.
Acknowledgments
This research was funded by NIH/NIDDK T32 DK07634, K24 DK100548 and T35 DK007386.
Footnotes
Relevant Financial Disclosures: Dr. Shaheen receives research funding from Covidien Medical, CSA Medical, NeoGenomics, Takeda Pharmaceuticals, CDx Medical, and Interpace Diagnostics.
References
- 1.Shaheen NJ, Richter JE. Barrett’s oesophagus. Lancet 2009;373:850–61. [DOI] [PubMed] [Google Scholar]
- 2.Orman ES, Li N, Shaheen NJ. Efficacy and durability of radiofrequency ablation for Barrett’s Esophagus: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013;11:1245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. Jama 2014;311:1209–17. [DOI] [PubMed] [Google Scholar]
- 4.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med 2009;360:2277–88. [DOI] [PubMed] [Google Scholar]
- 5.Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett’s esophagus with dysplasia. Gastroenterology 2011;141:460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyday WD, Corbett FS, Kuperman DA, et al. Radiofrequency ablation of Barrett’s esophagus: outcomes of 429 patients from a multicenter community practice registry. Endoscopy 2010;42:272–8. [DOI] [PubMed] [Google Scholar]
- 7.Vassiliou MC, von Renteln D, Wiener DC, et al. Treatment of ultralong-segment Barrett’s using focal and balloon-based radiofrequency ablation. Surg Endosc 2010;24:786–91. [DOI] [PubMed] [Google Scholar]
- 8.Vaccaro BJ, Gonzalez S, Poneros JM, et al. Detection of intestinal metaplasia after successful eradication of Barrett’s Esophagus with radiofrequency ablation. Dig Dis Sci 2011;56:1996–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orman ES, Kim HP, Bulsiewicz WJ, et al. Intestinal metaplasia recurs infrequently in patients successfully treated for Barrett’s esophagus with radiofrequency ablation. Am J Gastroenterol 2013;108:187–95; quiz 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleischer DE, Overholt BF, Sharma VK, et al. Endoscopic radiofrequency ablation for Barrett’s esophagus: 5-year outcomes from a prospective multicenter trial. Endoscopy 2010;42:781–9. [DOI] [PubMed] [Google Scholar]
- 11.Yasuda K, Choi SE, Nishioka NS, et al. Incidence and predictors of adenocarcinoma following endoscopic ablation of Barrett’s esophagus. Dig Dis Sci 2014;59:1560–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta M, Iyer PG, Lutzke L, et al. Recurrence of esophageal intestinal metaplasia after endoscopic mucosal resection and radiofrequency ablation of Barrett’s esophagus: results from a US Multicenter Consortium. Gastroenterology 2013;145:79–86 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasricha S, Bulsiewicz WJ, Hathorn KE, et al. Durability and predictors of successful radiofrequency ablation for Barrett’s esophagus. Clin Gastroenterol Hepatol 2014;12:1840–7.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spechler SJ, Zeroogian JM, Antonioli DA, et al. Prevalence of metaplasia at the gastro-oesophageal junction. Lancet 1994;344:1533–6. [DOI] [PubMed] [Google Scholar]
- 15.Korst RJ, Santana-Joseph S, Rutledge JR, et al. Patterns of recurrent and persistent intestinal metaplasia after successful radiofrequency ablation of Barrett’s esophagus. J Thorac Cardiovasc Surg 2013;145:1529–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


