Abstract
Background
Cardiac tumours are rare but affected patients may present with symptoms mimicking other cardiac diseases. The most frequent symptoms include heart failure, arrhythmias, or embolic phenomena.
Case summary
A 39-year-old man with a history of extranodal NK/T-cell lymphoma of the nasal type (ENKTL-NT) in clinical remission presented at our department with incessant ventricular tachycardia. The arrhythmia could only be controlled with a combination of intravenously administered beta-blockers, ajmaline, and amiodarone. Diagnostic workup excluded ischaemia, but imaging revealed a tumour located in the apex of the left ventricle. Endomyocardial biopsy confirmed the diagnosis of cardiac relapse of ENKTL-NT. Upon chemotherapy no further arrhythmias developed.
Discussion
Many malignancies can metastasize into the heart. Multimodal imaging including echocardiography, cardiac magnetic resonance imaging, and a positron-emission tomography computed tomography paved the way to the diagnosis that was finally established by endomyocardial biopsy. In the present case, a cardiac metastasis from an ENKTL-NT presented with incessant ventricular tachycardia.
Keywords: Incessant ventricular tachycardia, Lymphoma, Cardiac metastasis, Extranodal NK/T-cell lymphomas, Case report
Learning points.
In rare cases, a cardiac tumour may be the underlying aetiology for a ventricular tachycardia. Relapse of extra-cardiac malignant lymphoma in the heart after complete clinical remission is possible and necessitates fast multimodal imaging.
Cardiac tumours may be fragile and endomyocardial biopsy should be performed with caution.
Introduction
Tumours of the heart are rare. Data based on unspecific autopsy series show a prevalence of approximately 0.02% for primary cardiac tumours.1 The majority of these primary tumours consists of myxoma located most likely in the left atrium.2 Secondary cardiac tumours show a much higher prevalence of 0.7–3.5% in biopsy series3 with much higher rates in patients affected by malignancy.4 Autopsy studies comparing the prevalence of primary and secondary cardiac tumours report between 22-times5 and 132-times6 higher rates of secondary cardiac tumours. The most common secondary cardiac tumours are metastases from melanomas or the adjacent mediastinum. Cardiac tumours are often asymptomatic, they may present in rare cases with symptoms of heart failure most likely due to pericardial effusion or obstruction, arrhythmias, or embolic phenomena.7 We report the rare case of a patient presenting with incessant tachycardia due to a cardiac relapse of an extranodal NK/T-cell lymphoma of the nasal type. The patient gave written informed consent for a publication of a case report.
Timeline
| January 2021 | Diagnosis of an extranodal NK/T-cell lymphoma of the nasal type (ENKTL-NT) |
| February–March 2021 | Polychemotherapy with dexamethasone, etoposide, ifosfamide, and carboplatin and local radiation of the nasal region (DeVIC) |
| July 2021 | ‘clinical remission’ |
| 20 August 2021 | Admission to our emergency department, electrocardiogram (ECG) shows a ventricular tachycardia (VT), electrical storm leads to ventricular fibrillation (VF) with the need for cardiopulmonary resuscitation, and three defibrillations. Haemodynamic stabilization but ongoing incessant VT. Continuous antiarrhythmic therapy with landiolol and ajmaline. Transthoracic echocardiography (TTE) shows a tumour in the apex of the left ventricle and a hypokinesia in this region. Coronary angiography rules out ischaemia |
| 21 August 2021 | Termination of incessant VT with a bolus of ajmaline, continuation of the intravenous therapy with ajmaline and landiolol. Addition of continuous intravenous amiodarone due to recurrent VTs. |
| 23 August 2021 | Cardiac magnetic resonance imaging (MRI) revealing 5.5 × 2.5 × 3.5 cm–sized tumour. |
| 24 August 2021 | Positron-emission tomography computed tomography (PET-CT) scan revealing increased tracer uptake in the apical region. Endomyocardial biopsy of the apical region of the left ventricle is performed. |
| 25 August 2021 | Histological confirmation of a relapse of the ENKTL-NT as a cardiac metastasis |
| 27 August 2021 | Start with dexamethasone as part of the induction therapy. Five events of VF. Rapid termination with defibrillation. Application of a transvenous pacer for overdrive pacing. |
| 30 August 2021 | Removal of the transvenous pacer |
| 2 September 2021 | Start with methotrexate as part of the chemotherapy |
| 3 September 2021 | Start with pegaspargase as part of the chemotherapy |
| 7 September 2021 | Transfer to the haematology ward with a wearable defibrillator |
| 10 September 2021 | Discharge from hospital with a wearable defibrillator, antiarrhythmic medication included only amiodarone |
| 10 December 2021 | TTE shows full remission of the apical tumour |
| 22 February 2022 | Electrophysiological (EP) examination: no induction of VT, amiodarone was stopped |
| 22 March 2022 | Cardiac MRI shows late gadolinium enhancement in the apical region without evidence of a tumour |
| 7 April 2022 | No rhythmic events occurred after hospital discharge. EP examination, TTE, and cardiac MRI do not support an indication for an implantable cardioverter defibrillator (ICD). Further, the wearable defibrillator was removed. |
Case presentation
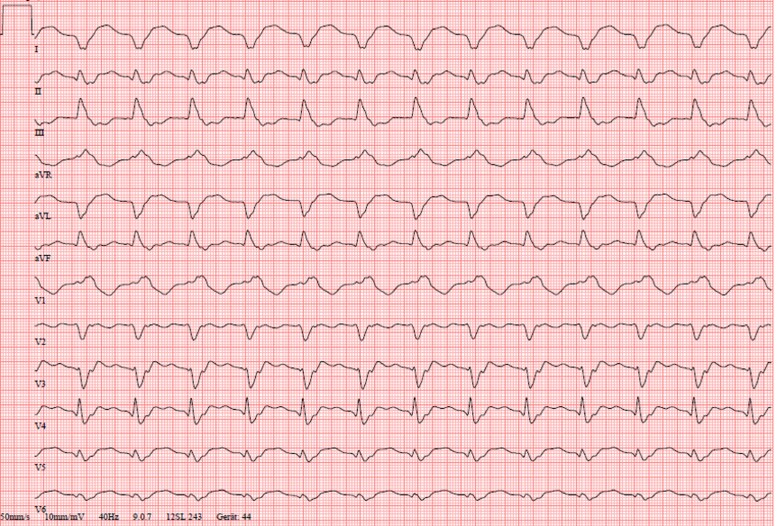
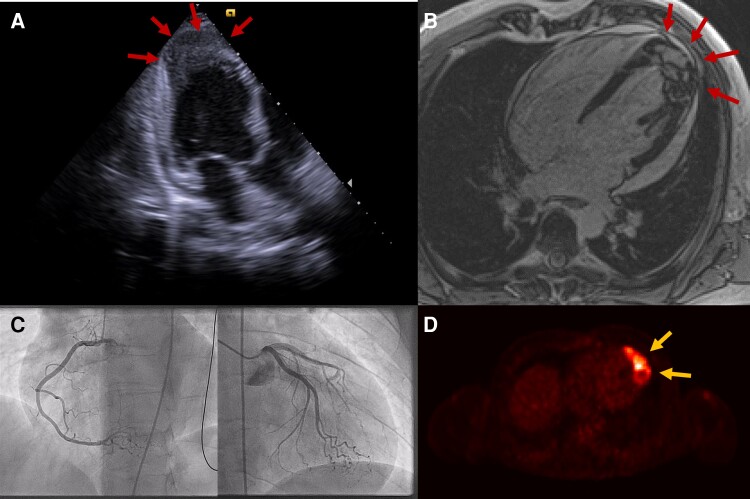
We report a 39-year-old man who presented without specific symptoms at the general practitioner for a routine check-up due to an extranodal NK/T-cell lymphoma of the nasal type (ENKTL-NT) in clinical remission. During the physical examination, the general physician detected tachycardia. The ECG showed a rhythmic wide QRS complex tachycardia with a heart rate of 155 beats per minute that was interpreted as haemodynamically stable VT with an apical-anterior-lateral exit (Figure 1). An immediate transfer to our emergency department was organized. As the patient arrived with the still ongoing VT, the haemodynamic situation deteriorated towards cardiogenic shock with the need for electrical cardioversion. One synchronized shock (biphasic) with 100 J was not successful and induced VF that was followed by chest compressions for only a few seconds. Three defibrillations lead to an instable sinus rhythm that degenerated into a haemodynamically tolerated VT again. An endotracheal intubation could be avoided due to the short period of the resuscitation manoeuvres. An intravenous antiarrhythmic therapy regime consisting of continuous ajmaline, landiolol, and electrolyte substitution kept the ventricular rate at 120–150 beats per minute for 20 h with only short periods of sinus rhythm. TTE revealed a hyperechogenic zone of 3 × 2 cm in the apex of the left ventricle of unknown aetiology as well as a hypokinesia in this region and a small pericardial effusion (Figure 2A, see Supplementary material online, Video S1). An ischaemic cause of the rhythmic instability was ruled out by coronary angiography (Figure 2C). Urgent electrophysiological examination with VT ablation was discussed but rejected due to the apical tumour. The VT could finally be terminated with a bolus of ajmaline 24 h after its onset. It was followed by a bradycardic sinus rhythm with short interruptions caused by VTs and VFs with the need of recurrent delivery of electrical shocks, addition of intravenous amiodarone as triple antiarrhythmic therapy, and intermittent overdrive pacing with a transvenous external pacer.
Figure 1.
Twelve-lead electrocardiogram (50 mm/s) at the presentation in the emergency department showing broad QRS complex tachycardia with 155 beats per minute.
Figure 2.
Multimodal cardiac imaging. (A) Transthoracic echocardiogram (apical three-chamber view) showing a tumour in the apex of the left ventricle (red arrows) and a small pericardial effusion. (B) Cardiac magnetic resonance imaging showing an inhomogeneous tumour in the apex of the left ventricle (red arrows). (C) Coronary angiography rules out stenosing coronary artery disease. (D) 18-FDG PET-CT scan showing increased tracer uptake in the apex of the left ventricle (yellow arrows).
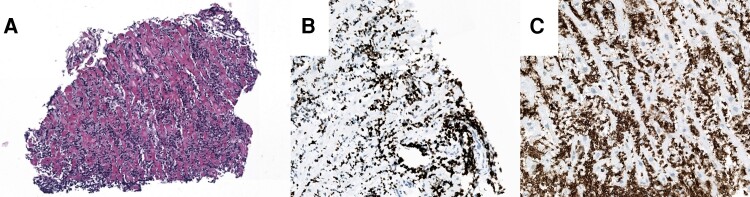
Blood tests showed slightly increased levels of NT-proBNP and high-sensitive cardiac troponin T (Table 1). Diagnostic workup revealed a 5.5 × 2.5 × 3.5 cm–sized tumour with cardiac MRI (Figure 2B, see Supplementary material online, Video S2) and PET-CT scan showed increased tracer uptake in the apical region (Figure 2D), both highly suspicious of a metastatic relapse of the ENKTL-NT. Histological workup of an endomyocardial biopsy of the left-ventricular apical region confirmed this diagnosis (Figure 3). Of note, the biopsy forceps found little resistance in the tumorous tissue and perforated the apical region (see Supplementary material online, Video S3). Luckily, transthoracic echocardiograms showed no increase of the pericardial effusion and the patient did not develop tamponade.
Table 1.
Blood tests
| Admission | Day + 7 | Day + 18 | Month + 6 | |
|---|---|---|---|---|
| NT-proBNP (pg/mL) | 527 | 108 | ||
| hsTnT (pg/mL) | 158 | 147 | 51 | 6 |
| CK (IU/L) | 165 | 50 | 61 | 35 |
| LDH (IU/L) | 326 | 256 | 177 | 221 |
| Creatinine (mg/dL) | 1.4 | 1.1 | 1.2 | 1.2 |
| eGFR (mL/min/1.73 m²) | 64 | 82 | 77 | 75 |
| Total bilirubin(mg/dL) | 1.59 | 0.87 | 2.3 | 1.1 |
| Hb (g/dL) | 13.3 | 12.2 | 11.3 | 10.6 |
| Leucocytes (×109/L) | 3.9 | 7.1 | 2.8 | 7.2 |
| hsCRP (mg/L) | 3.9 | 6.7 | 0.8 | 1.5 |
| Lactate (mmol/L) | 1.6 | 1.3 |
hsTnT, high-sensitive troponin T; CK, creatine kinase; LDH, lactate dehydrogenase; eGFR, estimated glomerular filtration rate; Hb, haemoglobin; hsCRP, high-sensitive C-reactive protein.
Figure 3.
Histological workup reveals relapse of the ENKTL-NT. (A) Endomyocardial biopsy: haematoxylin–eosin stain, dense lymphocytic infiltration. (B) Endomyocardial biopsy: Ki67 (proliferation marker): highly proliferative cells. (C) Endomyocardial biopsy: CD3 (T cells): lymphocytic infiltrate consists of T cells.
Seven days after the hospital admission a chemotherapy consisting of a 6-day run-in phase with dexamethasone followed by methotrexate and pegaspargase was initiated by our haematology department. Thereafter, no further arrhythmias were detectable, and the antiarrhythmic therapy regimen was stepwise deescalated to low-dose bisoprolol and amiodarone. The patient was supplied with a wearable defibrillator and was transferred to the haematology ward 18 days after admission and left our hospital in a good condition 3 days later. Antiarrhythmic medication to this time point included only amiodarone. Follow-ups at our department showed complete remission of the apical tumour and a normal systolic and diastolic function on TTE and cardiac MRI (see Supplementary material online, Videos S4 and S5). To evaluate the indication for an ICD, we performed an EP examination. Prior to the EP examination, amiodarone was stopped, and no arrhythmias were inducible. Therefore, we did not re-instate amiodarone and removed the wearable ICD.
Discussion
Many malignancies can metastasize into the heart. This is particularly the case for melanomas and cancers primarily adjacent to the heart. Here, we report a case of a metastasis from an ENKTL-NT. The patient presented with incessant VT that was not responsive to electrical shocks or combined antiarrhythmic drugs. Yet, the haemodynamic situation was tolerated. According to the guidelines of the European Society of Cardiology regarding the treatment of VTs,8 a potential next escalation level in case of a deterioration would have been the induction of deep sedo-analgesia to reduce sympathetic drive. Further, temporary use of extracorporeal life support such as veno-arterial extracorporeal membrane oxygenation or Impella devices is an option. In our case, episodes of VTs were interrupted by phases of symptomatic bradycardia, likely induced by the combination of antiarrhythmic drugs. We performed intermittent transvenous overdrive pacing and kept potassium and magnesium levels high. Differential diagnosis in a young male that was otherwise healthy and considered in clinical remission of ENKTL-NT included ischaemia or idiopathic VT. After the exclusion of an ischaemia urgent VT ablation was discussed as a bailout strategy even in the absence of ischaemia9 but rejected due to possible complications associated with the apical tumour-like perforation or thromboembolism. Although rare, lymphomas are reported to metastasize into the heart.4,5 Multimodal imaging including echocardiography, cardiac MRI, and a PET-CT paved the way to the diagnosis that could finally be established by the histological workup of an endomyocardial biopsy. In case of a cardiac metastasis, specific chemotherapy is probably the most reasonable therapeutic option. Surgery may only be considered in individual cases with a solitary tumour strictly limited to the heart.10 In our case, guideline directed chemotherapy for ENKTL-NT led to a rapid regression of the tumour and rhythmic stabilization. The implantation of an ICD was rejected in favour of a wearable defibrillator with a planned re-evaluation within months given the possible reversible aetiology of the VT.8 Finally, there was no indication for an ICD at the follow up.
Conclusions
In conclusion, cardiac tumours may cause acute and severe arrhythmias, especially in young patients without obvious cardiovascular risk factors and with a recent history of malignancy. Cardiac imaging paves the way to correct diagnosis and allows appropriate treatment.
Supplementary Material
Acknowledgements
The authors thank all colleagues who were involved in the clinical care of the patient.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Funding: None declared.
Contributor Information
Ewald Kolesnik, Division of Cardiology, University Heart Center, Medical University of Graz, Auenbruggerplatz 15, Graz 8036, Austria.
Verena Stangl, Diagnostic and Research Institute of Pathology, Medical University of Graz, Graz, Austria.
Bernhard Haring, Division of Cardiology, University Heart Center, Medical University of Graz, Auenbruggerplatz 15, Graz 8036, Austria.
Daniel Scherr, Division of Cardiology, University Heart Center, Medical University of Graz, Auenbruggerplatz 15, Graz 8036, Austria.
Peter P Rainer, Division of Cardiology, University Heart Center, Medical University of Graz, Auenbruggerplatz 15, Graz 8036, Austria; BioTechMed Graz, Graz, Austria.
Lead author biography
 Born 28 August 1989 in Klagenfurt (Austria), Ewald Kolesnik studied human medicine at the Medical University of Graz. After his graduation in 2015, he joined the department of cardiology at the Medical University of Graz, a tertiary referral centre serving a population of over 2 million. His clinical focus lies on echocardiography, heart failure, and intensive care medicine. His research field is the investigation of modern antidiabetic concepts and their impact on myocardial function in basic science and as subinvestigator of clinical trials.
Born 28 August 1989 in Klagenfurt (Austria), Ewald Kolesnik studied human medicine at the Medical University of Graz. After his graduation in 2015, he joined the department of cardiology at the Medical University of Graz, a tertiary referral centre serving a population of over 2 million. His clinical focus lies on echocardiography, heart failure, and intensive care medicine. His research field is the investigation of modern antidiabetic concepts and their impact on myocardial function in basic science and as subinvestigator of clinical trials.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
References
- 1. Reynen K. Frequency of primary tumors of the heart. Am J Cardiol 1996;77:107. [DOI] [PubMed] [Google Scholar]
- 2. Butany J, Nair V, Naseemuddin A, Nair GM, Catton C, Yau T. Cardiac tumours: diagnosis and management. Lancet Oncol 2005;6:219–228. [DOI] [PubMed] [Google Scholar]
- 3. Al-Mamgani A, Baartman L, Baaijens M, de Pree I, Incrocci L, Levendag PC. Cardiac metastases. Int J Clin Oncol 2008;13:369–372. [DOI] [PubMed] [Google Scholar]
- 4. Bussani R, De-Giorgio F, Abbate A, Silvestri F. Cardiac metastases. J Clin Pathol 2007;60:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lam KY, Dickens P, Chan AC. Tumors of the heart. A 20-year experience with a review of 12,485 consecutive autopsies. Arch Pathol Lab Med 1993;117:1027–1031. [PubMed] [Google Scholar]
- 6. Butany J, Leong SW, Carmichael K, Komeda M. A 30-year analysis of cardiac neoplasms at autopsy. Can J Cardiol 2005;21:675–680. [PubMed] [Google Scholar]
- 7. Casavecchia G, Lestuzzi C, Gravina M, Corrado G, Tusa M, Brunetti ND, Manuppelli V, Monte IP. Cardiac tumors. J Cardiovasc Echogr 2020;30:S45–S53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez-Madrid A, Nikolaou N, Norekvål TM, Spaulding C, Van Veldhuisen DJ, ESC Scientific Document Group . 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 9. Dinov B, Fiedler L, Schonbauer R, Bollmann A, Rolf S, Piorkowski C, Hindricks G, Arya A. Outcomes in catheter ablation of ventricular tachycardia in dilated nonischemic cardiomyopathy compared with ischemic cardiomyopathy: results from the Prospective Heart Centre of Leipzig VT (HELP-VT) study. Circulation 2014;129:728–736. [DOI] [PubMed] [Google Scholar]
- 10. Kumar N, Agarwal S, Ahuja A, Das P, Airon B, Ray R. Spectrum of cardiac tumors excluding myxoma: experience of a tertiary center with review of the literature. Pathol Res Pract 2011;207:769–774. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.