Abstract
Background
The ECHO trial randomized women to intramuscular depot medroxyprogesterone acetate (DMPA-IM), levonorgestrel implant (LNG-implant), or copper intrauterine device (Cu-IUD). In a substudy of the ECHO trial, we tested the hypothesis that contraceptives influence genital inflammation by comparing cervicovaginal cytokine changes following contraception initiation. In addition, we compared cytokine profiles in women who acquired HIV (cases) versus those remaining HIV negative (controls).
Methods
Women (n = 251) from South Africa and Kenya were included. Twenty-seven cervicovaginal cytokines were measured by Luminex at baseline, and 1 and 6 months after contraceptive iTanko et alnitiation. In addition, cytokines were measured preseroconversion in HIV cases (n = 25) and controls (n = 100).
Results
At 6 months after contraceptive initiation, women using Cu-IUD had increased concentrations of 25/27 cytokines compared to their respective baseline concentrations. In contrast, women initiating DMPA-IM and LNG-implant did not experience changes in cervicovaginal cytokines. Preseroconversion concentrations of IL-1β, IL-6, and TNF-α, previously associated with HIV risk, correlated with increased HIV risk in a logistic regression analysis, although not significantly after correcting for multiple comparisons. Adjusting for contraceptive arm did not alter these results.
Conclusions
Although Cu-IUD use broadly increased cervicovaginal cytokine concentrations at 6 months postinsertion, these inflammatory changes were found not to be a significant driver of HIV risk.
Clinical Trials Registration
Keywords: reproductive tract, DMPA-IM, LNG-implant, Cu-IUD, inflammation
Cervicovaginal cytokines were significantly elevated in women randomized to Cu-IUD 6 months after insertion, but not with DMPA-IM or LNG-implant use. However, these elevated cytokines in Cu-IUD users were not significantly associated with increased HIV risk.
Over 800 million women of reproductive age use modern contraceptive methods [1], including long-acting progestin-only injectables, implants, and intrauterine devices (IUDs) [2, 3] In sub-Saharan Africa, the most commonly used contraceptives are the intramuscular injectable depot medroxyprogesterone acetate (DMPA-IM) and the progestin-only levonorgestrel (LNG) implant, which both suppress ovulation, cause cervical mucus thickening, and endometrial atrophy [2, 4]. These contraceptives are highly effective in preventing unintended pregnancies [5]. However, the observed link between DMPA-IM use and increased human immunodeficiency virus (HIV) risk warranted urgent attention [6–8]. The Evidence for Contraceptive Options and HIV Outcomes (ECHO) trial was conducted to compare the relative HIV incidence rates in women randomly assigned to receive DMPA-IM, LNG-implant, and copper IUD (Cu-IUD) [9]. This found no significant difference in incidence of HIV acquisition among the contraceptive methods tested.
Prior to the ECHO trial, pointing to mechanisms underlying contraceptive impact on HIV risk, several observational studies showed that some hormonal contraceptives (primarily DMPA-IM but also combined oral contraceptives) induce genital inflammation, evidenced by increased production of inflammatory cytokines and chemokines [10, 11], decreased antiviral immunity or increased mucosal HIV target cell frequencies [12–14], and disruption/thinning of the squamous epithelial barrier in the lower reproductive tract [15, 16]. Others have demonstrated an inflammatory response to the Cu-IUD in the lower genital tract and endometrium, possibly directed at Cu [17, 18]. In this mucosal substudy within the ECHO trial, we hypothesized that these contraceptive methods would differentially influence the genital immune environment, with a focus on inflammation. Thus, we assessed longitudinal cytokine profiles in matched cervicovaginal samples collected before, and at 1 and 6 months after contraception initiation. We further compared cervicovaginal cytokine profiles in preseroconversion samples from women who subsequently acquired HIV (cases) and those who remained HIV negative (controls) to evaluate cytokine association with HIV risk.
METHODS
Study Participants
Of the 12 study sites of the ECHO trial, 3 sites with capacity for genital sample collection and storage were selected for this mucosal substudy. The ECHO trial was an open-label randomized trial assessing the incidence of HIV among women assigned to use DMPA-IM, LNG-implant, or the Cu-IUD (Clinicaltrials.gov NCT02550067) [9]. The parent trial enrolled women between December 2015 and September 2017 who were aged 16–35 years, HIV negative, sexually active, desiring effective contraception, and willing to be randomized to 1 of 3 study contraceptive methods. Women were excluded if they reported having used injectable contraception (DMPA or norethisterone enanthate), an implant, or IUD in the last 6 months, or if they had any medical contraindication to the study contraceptive methods [9]. Ethical approvals for this nested substudy were obtained from the Human Research Ethics Committees at the University of Cape Town (HREC 371/2015), University of Witwatersrand (HREC PRC 141112), Kenya Medical Research Institute (KEMRI; SERU/CMR/P0014/3109), University of Washington (STUDY00000261), and FHI360 (523201). All participants provided written informed consent.
Design of Pre-/Post-Contraceptive–Initiation Substudy
Following ethics approvals, we consecutively offered enrolment into this pre-/post-contraceptive–initiation mucosal substudy to all women participating in the ECHO trial at Wits Reproductive Health and HIV Institute (Wits RHI; Johannesburg, South Africa; n = 109), Desmond Tutu Health Foundation Emavundleni (Cape Town, South Africa; n = 151), and KEMRI (Kisumu, Kenya; n = 170). Approximately 20 women per arm per study site were randomly selected and included if they had initiated their randomized contraceptive method and used it for at least the first month.
Design of Case-Control Substudy
Available cervicovaginal secretions (CVS) samples from all women across the 3 study sites who subsequently acquired HIV were included for a case-control study of cytokines and HIV risk. To ensure sufficient statistical power of the study, a random selection of women who did not seroconvert were selected as controls at a ratio of 4 controls to 1 case, matched on study site, visit, and age. Samples from the visit prior to HIV seroconversion (median of 6 months; interquartile range [IQR], 3.0–12.0 months) were assayed. For controls, the sample from the same time period (median, 6 months; IQR, 3.0–12.0 months) in follow-up was selected. Baseline samples for all women in the case-control study were also included, if available.
Mucosal Sample Collection and Processing
CVS were collected from study participants using a disposable menstrual cup (Softcup; Evofem), placed by the participant or study clinician and worn for approximately 1 hour prior to the collection of other mucosal samples. Upon removal, the menstrual cup was immediately placed into a sterile 50-mL Falcon tube and transported on ice to the laboratory within 6 hours of collection. At the laboratory, the tube was centrifuged at 1500 rpm for 10 minutes at 4°C, after which the menstrual cup was carefully removed and discarded. The tube containing the secretion was weighed and the volume of the secreted fluid calculated (weight of tube with secretion − weight of empty tube). The secretion was diluted 5-fold with sterile phosphate buffer saline (Sigma-Aldrich) and stored in cryovials at −80°C until use [19, 20].
Diagnosis of Sexually Transmitted Infections and Bacterial Vaginosis
Testing for Chlamydia trachomatis and Neisseria gonorrhoeae was performed at screening using endocervical swabs and treatment was provided upon etiologic diagnosis or when a woman presented with symptoms, according to national algorithms in each country at the time. Treatment for sexually transmitted infections (STIs) was provided on site as soon as possible after diagnosis and that for infections diagnosed via laboratory testing on screening samples; this often resulted in treatment being delivered at the enrolment visit. For C. trachomatis/N. gonorrhoeae testing, GeneXpert Instrument Systems platform (Cepheid) with the Abbott Real Time PCR assay (Abbott Molecular) were used at Wits RHI and Emavundleni sites, while the Panther System (Hologic) was used at the KEMRI site. Herpes simplex virus type 2 (HSV-2) serology was performed for HSV-2 gG2 IgG using the enzyme-linked immunosorbent assay (EIA; HerpeSelect, Focus Diagnostics) at baseline for all 3 sites [9]. For HSV-2 seropositive and indeterminate EIA results, confirmatory testing was performed via western blot at the University of Washington Virology laboratory. For bacterial vaginosis (BV) diagnosis, lateral vaginal swabs were rolled onto a glass slide, fixed, and kept at room temperature. Nugent scoring was conducted by the National Institute for Communicable Diseases laboratory in Johannesburg, South Africa [21]. BV was treated syndromically, as per national guidelines in each participating country.
Measurement of Cytokines
CVS were assayed using Luminex (Bio-Plex Pro Human cytokine 27-plex; Bio-Rad Laboratories) to measure the concentrations of 27 cytokines, including inflammatory cytokines (interleukin 1β [IL-1β], IL-6, IL-12p70, tumor necrosis factor-α [TNF-α]); chemokines (IL-8, Eotaxin, IFN-γ inducible protein-10 [IP-10], monocyte chemoattractant protein-1 [MCP-1], macrophage inflammatory protein-1α [MIP-1α], MIP-1β, regulated on activation, normal T-cell expressed and secreted [RANTES]); adaptive cytokines (IL-2, IL-4, IL-5, IL-13, IL-15, IL-17A, interferon-γ [IFN-γ]); growth factors (IL-7, IL-9, platelet-derived growth factor-BB [PDGF-BB], fibroblast growth factor [FGF]-basic, granulocyte-colony stimulating factor [G-CSF], granulocyte-macrophage colony stimulating factor [GM-CSF], vascular endothelial growth factor [VEGF]); and regulatory cytokines (IL-1 receptor agonist [IL-1RA], IL-10), according to the manufacturer’s protocol. CVS samples were thawed and filtered using SPIN-X 0.2-μm cellulose acetate filters (Sigma). A total of 1046 CVS samples (both pre- and postcontraceptive and HIV case-control samples) were run on 14 plates over 10 days. To control for inter- and intraplate variation, 5 CVS samples were run in duplicate on each plate (intraplate controls) and a panel of 5 samples were run on all 14 plates (interplate controls; Supplementary Table 1). To enable comparison across plates, matched CVS samples from baseline and 1 month after contraceptive initiation were first assayed on the same plate, and then samples from 6 months after contraceptive initiation with their corresponding baseline samples and case-control samples were assayed on the same plate. Thus, the results generated from 1- and 6-month post-contraceptive–initiation samples are not represented on the same figures. The lower limit of detection for cytokines ranged from 1.4 to 92.6 pg/mL. Data were collected using a Bio-Plex Suspension Array Reader (Bio-Rad Laboratories). A Bio-Plex manager software (version 4) was used to analyze the data and a 5-parameter logistic regression formula was used to calculate cytokine concentrations from standard curves. Cytokine concentrations below the lower limit of detection of the assay were reported as the midpoint between zero and the lowest concentration measured. Cytokines that were undetectable in > 40% of samples assayed were excluded for all analyses.
Statistical Analyses
Unsupervised hierarchical clustering from log10-transformed cytokine data was performed to group women according to the relatedness of their cytokine expression profiles using the R package “ComplexHeatmap” [22]. The difference in cytokine concentration between contraceptive arms was determined using the R package “vegan” [23] by permutational multivariate analysis of variance (PERMANOVA) using euclidean distance matrices and the adonis function. A sparse partial least-squares discriminant analysis (sPLSDA) was used to determine cytokine profiles that differ between contraceptive groups using the R package “mixOmics” [24]. The Wilcoxon signed rank test was used for paired samples in the pre-/post-contraception–initiation analyses. The relationship between contraception and HIV acquisition risk was assessed in an intention-to-treat and per-protocol analyses. Both analyses gave similar results. For HIV cases and controls, differences in baseline characteristics were evaluated using the Fisher exact, χ2, and Mann-Whitney U tests, while differences in cytokine levels were determined using the Mann-Whitney U test (for unpaired samples). Multivariate logistic regression analyses were used to determine the association between HIV acquisition and cytokine concentrations and adjustment for contraceptive arm. Intraplate correlations were determined by the nonparametric Spearman rank correlation test. A false discovery rate step-down procedure was used to adjust P values to decrease false-positive results due to multiple comparisons [25]. P values < .05 were considered significant. GraphPad Prism version 8.3 (GraphPad software) and R version 3.6 (R Core Team) were used for statistical analyses and data visualization.
RESULTS
Comparison of CVS Cytokine Concentrations Before and After Contraceptive Initiation
To compare CVS cytokine changes associated with contraceptive initiation, a total of 149 women with samples available at baseline, and 1 month and 6 months postinitiation were included. Five women did not have samples at 1 month. Of these 149 women, 52/149 were randomized to the Cu-IUD arm, 47/149 to the DMPA-IM arm, and 50/149 to the LNG-implant arm (Supplementary Figure 1A). None of the participants switched contraceptive arms prior to the 6-month visit. At baseline, no significant differences in demographics, sexual risk behaviors, and STI prevalence were evident among women randomized to different contraceptive arms (Table 1). No significant differences in baseline CVS cytokine concentrations were noted across the 3 contraceptive arms (Supplementary Table 2).
Table 1.
Baseline Characteristics of Participants in the Pre-/Post-Contraceptive–Initiation Study
| Characteristic | Randomization Arm | ||
|---|---|---|---|
| Cu-IUD | DMPA-IM | LNG-implant | |
| n | 52 | 47 | 50 |
| Age, y, median (25th–75th percentile) | 24 (19.3–28.8) | 24 (21.0–28.0) | 23 (20.8–26.0) |
| BMI, kg/m2, median (25th–75th percentile) | 24 (21.2–30.3) | 24 (21.7–28.7) | 23 (20.4–29.7) |
| Genital infection, n (%) | |||
| Chlamydia trachomatis | 14 (26.9) | 7 (14.9) | 5 (10.0) |
| Neisseria gonorrhoeae | 4 (7.7) | 3 (6.4) | 2 (4.0) |
| Bacterial vaginosis | |||
| Negative, Nugent 0–3 | 25 (48.1) | 32 (68.1) | 27 (54.0) |
| Intermediate, Nugent 4–6 | 8 (15.4) | 5 (10.6) | 6 (12.0) |
| Positive, Nugent 7–10 | 19 (36.5) | 10 (21.3) | 17 (34.0) |
| Herpes simplex virus type 2 | |||
| Negative | 23 (44.2) | 15 (31.9) | 21 (42.0) |
| Indeterminate | 5 (9.6) | 6 (12.8) | 7 (14.0) |
| Positive | 24 (46.2) | 26 (55.3) | 21 (42.0) |
| Demographics/sexual risk behavior, n (%) | |||
| Condomless sex in previous 3 mo | 37 (71.2) | 33 (70.2) | 36 (72.0) |
| Condom usage with last vaginal sex among women who had sex | 22 (42.3) | 25 (53.2) | 23 (46.0) |
| Vaginal bleeding during sex | 8 (15.4) | 6 (12.8) | 7 (14.0) |
| >1 partner in previous 3 mo | 9 (17.3) | 5 (10.6) | 3 (6.0) |
| Marital status | |||
| Never married | 37 (71.2) | 32 (68.1) | 31 (62.0) |
| Married | 15 (28.8) | 15 (31.9) | 19 (38.0) |
| Education | |||
| No schooling | 1 (1.9) | 0 (0.0) | 0 (0.0) |
| Primary school education | 16 (30.8) | 11 (23.4) | 8 (16.0) |
| Secondary education | 30 (57.7) | 34 (72.3) | 37 (74.0) |
| Tertiary education | 5 (9.6) | 2 (4.3) | 5 (10.0) |
No record of an HSV-2, chlamydia, and gonorrhea tests was reported for 1 woman randomized to LNG-implant. No BMI measurement was available for 1 woman randomized to DMPA-IM arm. Twenty-five women included in this table were part of the case-control study (2 cases and 23 controls).
Abbreviations: BMI, body mass index; Cu-IUD, copper intrauterine device; DMPA-IM, intramuscular depot medroxyprogesterone acetate; LNG, levonorgestrel.
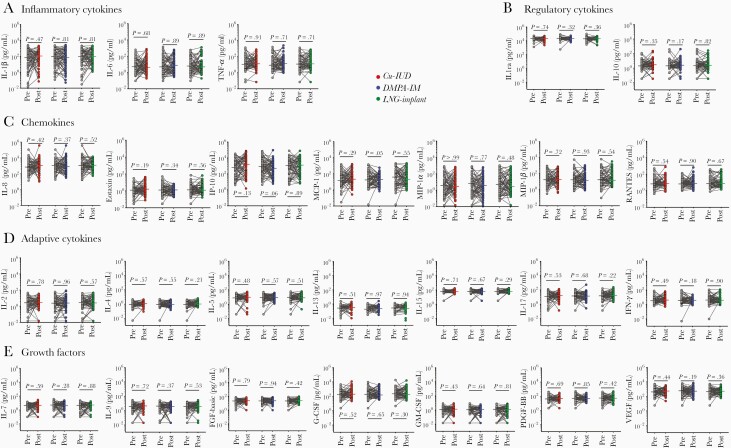
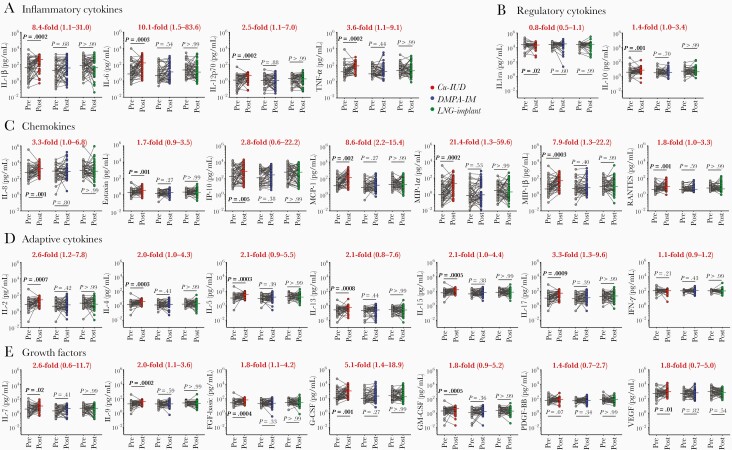
At 1 month after contraceptive initiation, no significant changes in CVS cytokine concentrations were found compared to their respective baseline samples in any of the contraceptive arms (Figure 1). In contrast, at 6 months after contraceptive initiation, major shifts in CVS cytokine profiles were noted in women randomized to the Cu-IUD arm, with 25/27 of the cytokines being significantly elevated compared to matched baseline samples, after adjusting for multiple comparisons (Figure 2). Cytokines that were significantly elevated in the Cu-IUD arm included all 4 of the inflammatory cytokines that were measured (IL-1β, IL-6, IL-12p70, TNF-α), all 7 chemokines (IL-8, Eotaxin, IP-10, MCP-1, MIP-1α, MIP-1β, RANTES), 6/7 adaptive cytokines (IL-2, IL-4, IL-5, IL-13, IL-15, IL-17A, but not IFN-γ), 6/7 growth factors (IL-7, IL-9, FGF-basic, G-CSF, GM-CSF, VEGF, but not PDGF-BB), and 1/2 regulatory cytokines (IL-10 but not IL-1RA; Figure 2). Of these, MIP-1α (median, 21.4-fold increase; IQR, 1.3–59.6; adjusted P = .0002), MIP-1β (7.9-fold increase; IQR, 1.3–22.2; adjusted P = .0003), IL-6 (10.1-fold increase; IQR, 1.5–83.6; adjusted P = .0003), MCP-1 (8.6-fold increase; IQR, 2.2–15.4; adjusted P = .002), and IL-1β (8.4-fold increase; IQR, 1.1–31.0; adjusted P = .0002) were the most strongly upregulated. While the regulatory cytokine IL-10 was moderately elevated in women using Cu-IUD (1.4-fold increased; IQR, 1.0–3.4; adjusted P = .001), IL-1RA concentrations were suppressed (0.8-fold decrease; IQR, 0.5–1.1; adjusted P = .02), suggesting dysregulation of the cervicovaginal inflammatory environment. Changes in CVS cytokine profiles were not observed in women randomized to the LNG-implant or DMPA-IM arms, compared to baseline.
Figure 1.
A–E, Effect of 1-month contraceptive use on cervicovaginal cytokines. Concentrations of each cytokine before (Pre) and 1 month after (Post) contraceptive initiation. Grey dots indicate precontraceptive cytokine concentrations. Cu-IUD (n = 60), DMPA-IM (n = 67), and LNG-implant (n = 63) users are shown in red, blue, and green, respectively. Horizontal lines indicate the median. Statistical significance was calculated using a Wilcoxon signed rank for paired samples with adjustment for multiple comparisons using the false discovery rate step-down procedure. A P value < .05 was considered significant. Abbreviations: Cu-IUD, copper intrauterine device; DMPA-IM, intramuscular injectable depot medroxyprogesterone acetate; FGF, fibroblast growth factor; G-CSF, granulocyte-colony stimulating factor; GM-CSF, granulocyte-macrophage colony stimulating factor; IFN-γ, interferon-γ; IL, interleukin; IL-1ra, IL-1 receptor agonist; IP-10, IFN-γ inducible protein-10; LNG, levonorgestrel; MCP-1, monocyte chemoattractant protein-1; MIP-1α, macrophage inflammatory protein-1α; PDGF-BB, platelet-derived growth factor-BB; RANTES, regulated on activation, normal T-cell expressed and secreted; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor.
Figure 2.
A–E, Effect of 6-months contraceptive use on cervicovaginal cytokines. Concentrations of each cytokine before and 6 months after contraceptive initiation. Grey dots indicate precontraceptive cytokine concentrations. Cu-IUD (n = 52), DMPA-IM (n = 47), and LNG-implant (n = 50) users are shown in red, blue, and green, respectively. Horizontal lines indicate the median. Median fold change and interquartile range is represented in red for the Cu-IUD arm. Statistical significance was calculated using a Wilcoxon signed rank for paired samples with adjustment for multiple comparisons using the false discovery rate step-down procedure. A P value < .05 was considered significant. Abbreviations: Cu-IUD, copper intrauterine device; DMPA-IM, intramuscular injectable depot medroxyprogesterone acetate; FGF, fibroblast growth factor; G-CSF, granulocyte-colony stimulating factor; GM-CSF, granulocyte-macrophage colony stimulating factor; IFN-γ, interferon-γ; IL, interleukin; IL-1ra, IL-1 receptor agonist; IP-10, IFN-γ inducible protein-10; LNG, levonorgestrel; MCP-1, monocyte chemoattractant protein-1; MIP-1α, macrophage inflammatory protein-1α; PDGF-BB, platelet-derived growth factor-BB; RANTES, regulated on activation, normal T-cell expressed and secreted; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor.
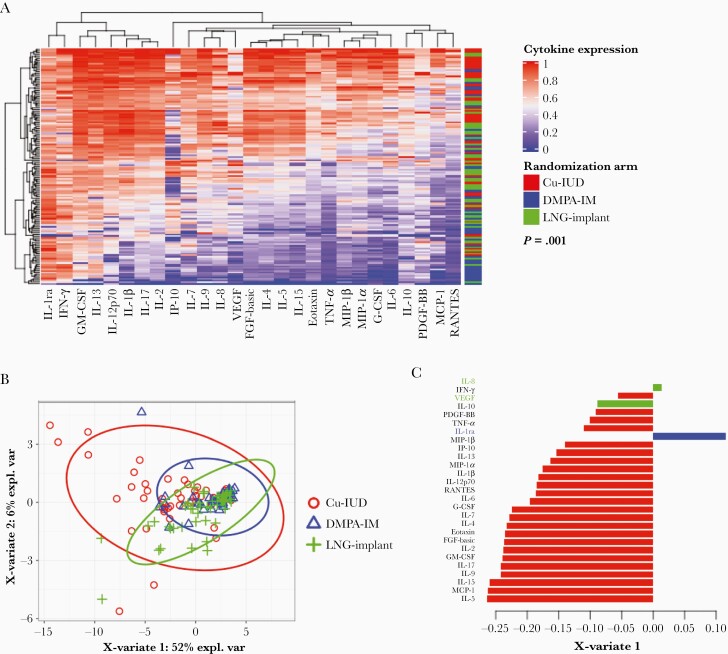
Unsupervised hierarchical clustering of the 6-month cytokine concentrations showed that women in the Cu-IUD arm clustered distinctly from those in the DMPA-IM and LNG-implant arms (Figure 3A). In a sparse partial least squares discriminant analysis (sPLSDA), differences in cytokine profiles between the 3 contraceptive groups were significant (PERMANOVA P = .001; Figure 3B). Women randomized to the Cu-IUD arm had the majority of CVS cytokines (24/27) negatively loaded on X variate 1 in the sPLSDA, compared to DMPA-IM and LNG-implant initiation, which clustered distinctly, associated with only IL-1RA or the combination of IL-8 and VEGF loadings on X variate 1, respectively (Figure 3C). This suggests a differential effect of contraceptives on cytokines.
Figure 3.
Visualization of clustering of cytokines 6 months after contraceptive initiation. A, Unsupervised hierarchical clustering showing the variation in cytokine concentrations in individual women and clustering women according to the similarities of their cytokine expression profiles and their contraceptive method 6 months after initiation of contraceptive. Cytokine concentrations are indicated using a color scale that ranges from blue (low) through white to red (high). The dendrogram above the heat map indicates degrees of relatedness between cytokine profiles within the study participants. The dendrogram on the left of the heat map depicts relationships between the cytokine expression levels across all the study participants. P value for statistical significance was generated using PERMANOVA. B, sPLSDA of cytokines 6 months after contraceptive initiation. C, Barplots indicate the loadings of X-variate 1 from the sPLSDA analysis. The absolute value and the negative or positive sign represent the importance of the cytokine and the correlations between the variables, respectively. Red, blue, and green represent Cu-IUD (n = 52), DMPA-IM (n = 47), and LNG-implant (n = 50), respectively. Abbreviations: Cu-IUD, copper intrauterine device; DMPA-IM, intramuscular injectable depot medroxyprogesterone acetate; expl. var, explained variation; FGF, fibroblast growth factor; G-CSF, granulocyte-colony stimulating factor; GM-CSF, granulocyte-macrophage colony stimulating factor; IFN-γ, interferon-γ; IL, interleukin; IL-1ra, IL-1 receptor agonist; IP-10, IFN-γ inducible protein-10; LNG, levonorgestrel; MCP-1, monocyte chemoattractant protein-1; MIP-1α, macrophage inflammatory protein-1α; PDGF-BB, platelet-derived growth factor-BB; RANTES, regulated on activation, normal T-cell expressed and secreted; sPLSDA, sparse partial least-squares discriminant analysis; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor.
Evaluation of CVS Cytokine Profiles Prior to HIV Seroconversion
Twenty-five women acquired HIV during follow-up in this mucosal substudy. CVS cytokine profiles in preseroconversion samples from these women were compared to 100 women who did not seroconvert (Supplementary Figure 1B). Two cases switched contraceptive arms prior to the preseroconversion sample and 2 controls switched contraceptive arms at the matched time point (either to DMPA-IM or no contraceptive). No differences in baseline demographics, sexual risk behaviors, and genital infections were noted between HIV cases and controls (Table 2). Compared to controls, women who subsequently acquired HIV had higher concentrations of 12/27 cytokines across various functional classes in CVS samples collected preseroconversion in an intention-to-treat analysis(Table 3). These included IL-1β, IL-6, TNF-α, Eotaxin, RANTES, IL-17, GM-CSF, IL-15, IL-2, IL-4, IL-5, and FGF-basic. However, none of these differences remained significant after adjusting for multiple comparisons.
Table 2.
Baseline Characteristics of Participants in the Case-Control Study
| Characteristic | HIV Cases | Controls | P Value |
|---|---|---|---|
| n | 25 | 100 | |
| Age, y, median (25th–75th percentile) | 22 (20.0–26.5) | 23 (20.0–26.0) | .78a |
| BMI, kg/m2, median (25th–75th percentile) | 26 (21.1–27.9) | 26 (22.8–30.8) | .22a |
| Time point for sample collection prior to HIV seroconversion, mo, median (25th–75th percentile) | 6 (3.0–12.0) | 6 (3.0–12.0) | >.99a |
| Genital infection, n (%) | |||
| Chlamydia trachomatis | 8 (32.0) | 20 (20.4) | .28b |
| Neisseria gonorrhoeae | 3 (12.0) | 6 (6.1) | .39b |
| Herpes simplex virus type 2 | |||
| Negative | 5 (20.0) | 42 (42.9) | .11c |
| Indeterminate | 4 (16.0) | 11 (11.2) | |
| Positive | 16 (64.0) | 45 (45.9) | |
| Demographics/sexual risk behaviors, n (%) | |||
| Condomless sex in previous 3 mo | 20 (80.0) | 61 (61.0) | .10b |
| Condom usage with last vaginal sex among women who had sex | 12 (52.2) | 50 (55.0) | .82b |
| Vaginal bleeding during sex | 2 (11.1) | 3 (4.8) | .31b |
| >1 partner in previous 3 mo | 3 (12.0) | 4 (4.0) | .14b |
| Marital status | |||
| Never married | 16 (64.0) | 75 (75.0) | .32b |
| Married | 9 (36.0) | 25 (25.0) | |
| Education | |||
| Primary school education | 7 (28.0) | 18 (18.0) | .27b |
| Secondary and tertiary education | 18 (18.0) | 82 (82.0) | |
No record of an HSV-2, chlamydia, and gonorrhea tests was reported for 2 women in the control group. P value < .05 is considered significant. Cases are HIV seroconverters and controls are HIV nonseroconverters.
Abbreviations: BMI, body mass index; HIV, human immunodeficiency virus; HSV, herpes simplex virus.
Mann-Whitney t test.
Fisher exact test.
χ2 test.
Table 3.
Cytokine Concentrations of HIV Cases and Controls in an Intention-to-Treat Analysis
| Cytokine | HIV Cases (n = 25) | Controls (n = 100) | Unadjusted P Value |
Adjusted P Value |
||
|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | |||
| Inflammatory | ||||||
| IL-1β | 260.7 | 45.2–1005. 0 | 52.0 | 5.7–454.4 | .03 | .09 |
| IL-6 | 93. 5 | 3.6–399.0 | 11.4 | 2.6–82.0 | .04 | .09 |
| IL-12p70 | 3.5 | 0.7–6.6 | 1.8 | 0.6–4.3 | .09 | .13 |
| TNF-α | 59. 3 | 11.0–151.0 | 15.1 | 6.6–67. 4 | .02 | .16 |
| Chemokines | ||||||
| IL-8 | 1783.0 | 356.5–3985.0 | 533.3 | 206.8–2121.0 | .07 | .14 |
| Eotaxin | 2.6 | 1.5–4.4 | 1.6 | 1.1–3.3 | .02 | .20 |
| IP-10 | 252.9 | 54.6–3122.0 | 141.6 | 20.8–1156.0 | .14 | .19 |
| MCP-1 | 26.9 | 8.0–81.1 | 14.7 | 5.0–61.3 | .16 | .21 |
| MIP-1α | 3.2 | 0.8–28.3 | 1.6 | 0.3–16.7 | .18 | .22 |
| MIP-1β | 14.5 | 3.5–149.8 | 8.1 | 2.7–32.7 | .21 | .23 |
| RANTES | 8.2 | 4.3–19.3 | 4.7 | 3.4–9.7 | .02 | .13 |
| Adaptive | ||||||
| IL-2 | 14.5 | 4.5–36.1 | 5.5 | 2.6–21.0 | .03 | .08 |
| IL-4 | 3.0 | 1.4–5.0 | 1.4 | 1.0–3.8 | .03 | .08 |
| IL-5 | 26.2 | 11.0–46.5 | 13.5 | 6.9–34.3 | .02 | .11 |
| IL-13 | 0.4 | 0.3–0.6 | 0.2 | 0.1–0.4 | .07 | .12 |
| IL-15 | 97.8 | 51.0–204.9 | 57.3 | 34.9–140.5 | .02 | .11 |
| IL-17 | 39.1 | 17.1–81.1 | 12.2 | 5.5–47.7 | .01 | .22 |
| IFN-γ | 121.2 | 88.8–134.6 | 109.4 | 84.9–127.2 | .43 | .45 |
| Growth factors | ||||||
| IL-7 | 5.4 | 2.3–12.5 | 3.5 | 0.6–7.7 | .10 | .14 |
| IL-9 | 28.5 | 16.2–42.4 | 18.7 | 11.4–38.5 | .07 | .13 |
| FGF-basic | 44.7 | 21.4–92.4 | 22.5 | 15.0–57.4 | .02 | .10 |
| G-CSF | 414.0 | 102.1–1307.0 | 166.2 | 55.0–863.9 | .20 | .23 |
| GM-CSF | 3.43 | 1.57–5.71 | 1.7 | 0.8– 4.4 | .04 | .08 |
| PDGF-BB | 63.5 | 32.6–103.2 | 50.5 | 35.3–74.5 | .41 | .44 |
| VEGF | 919.2 | 505.2–1752.0 | 717.4 | 263.9–1241.0 | .06 | .13 |
| Regulatory | ||||||
| IL-1RA | 25 029.0 | 19 944.0–34 390.0 | 23 765.0 | 16 291.0–31 878.0 | .51 | .51 |
| IL-10 | 6.3 | 2.2–11.6 | 3.5 | 2.2–6.9 | .07 | .12 |
Cases are HIV seroconverters and controls are HIV nonseroconverters. P values were calculated using the nonparametric Mann-Whitney t test before adjusting for multiple comparisons using the false discovery rate. P value < .05 is considered significant.
Abbreviations: FGF, fibroblast growth factor; G-CSF, granulocyte-colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon-γ; IL, interleukin; IP-10, IFN-γ inducible protein-10; IQR, interquartile range; MCP-1, monocyte chemoattractant protein-1; PDGF, platelet-derived growth factor; RANTES, regulated on activation, normal T cell expressed and secreted; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor.
Logistic regression analysis also suggested that preseroconversion concentrations of several cytokines were positively associated with subsequent HIV acquisition in the intention-to-treat analysis, including IL-1β (odds ratio [OR], 1.59; 95% confidence interval [CI], 1.06–2.53; unadjusted P = .035), IL-6 (OR, 1.58; 95% CI, 1.04–2.43; unadjusted P = .034), TNF-α (OR, 2.15; 95% CI, 1.15–4.16; unadjusted P = .019), Eotaxin (OR, 3.19; 95% CI, 1.04–10.65; unadjusted P = .048), IL-2 (OR, 2.32; 95% CI, 1.11–5.29; unadjusted P = .034), IL-4 (OR, 3.35; 95% CI, 1.18–10.44; unadjusted P = .028), IL-5 (OR, 3.01; 95% CI, 1.14–8.80; unadjusted P = .033), IL-17 (OR, 2.59; 95% CI, 1.25–5.87; unadjusted P = .015), FGF-basic (OR, 3.48; 95% CI, 1.16–11.49; unadjusted P = .032), and GM-CSF (OR, 2.45; 95% CI, 1.08–6.47; unadjusted P = .048; Table 4). None of these associations remained significant after correcting for multiple comparisons. Controlling for contraceptive arm in the model did not have a substantial impact on these associations.
Table 4.
Relationship Between HIV Seroconversion and Cytokine Concentrations in an Intention-to-Treat Analysis
| Cytokine | Unadjusted OR (95% CI) |
P Value | Adjusted for
Contraceptive Arm OR (95% CI) |
P Value |
|---|---|---|---|---|
| Inflammatory | ||||
| IL-1β | 1.59 (1.06–2.53) | .035 | 1.55 (.99–2.55) | .068 |
| IL-6 | 1.58 (1.04–2.43) | .034 | 1.53 (.97–2.46) | .071 |
| IL-12p70 | 2.05 (.96–4.83) | .078 | 1.83 (.81–4.57) | .168 |
| TNF-α | 2.15 (1.15–4.16) | .019 | 2.08 (1.06–4.22) | .035 |
| Chemokines | ||||
| IL-8 | 1.53 (.90–2.62) | .114 | 1.51 (.86–2.63) | .142 |
| Eotaxin | 3.19 (1.04–10.65) | .048 | 2.83 (.84–10.34) | .100 |
| IP-10 | 1.34 (.88–2.07) | .174 | 1.32 (.85–2.05) | .220 |
| MCP-1 | 1.32 (.78–2.21) | .284 | 1.19 (.66–2.10) | .560 |
| MIP-1α | 1.29 (.87–1.94) | .207 | 1.23 (.80–1.89) | .341 |
| MIP-1β | 1.38 (.84–2.26) | .197 | 1.29 (.76–2.18) | .335 |
| RANTES | 2.12 (.90–4.98) | .081 | 1.93 (.78–4.68) | .146 |
| Adaptive | ||||
| IL-2 | 2.32 (1.11–5.29) | .034 | 2.20 (1.01–5.34) | .062 |
| IL-4 | 3.35 (1.18–10.44) | .028 | 3.10 (1.00–10.60) | .058 |
| IL-5 | 3.01 (1.14–8.80) | .033 | 2.82 (.98–9.01) | .065 |
| IL-13 | 1.68 (.80–3.99) | .199 | 1.49 (.69–3.60) | .336 |
| IL-15 | 2.99 (1.00–9.73) | .057 | 2.73 (.82–9.86) | .110 |
| IL-17 | 2.59 (1.25–5.87) | .015 | 2.55 (1.16–6.12) | .026 |
| IFN-γ | 1.66 (.21–14.02) | .627 | 1.31 (.16–11.42) | .800 |
| Growth factors | ||||
| IL-7 | 2.07 (.99–4.71) | .065 | 1.92 (.88–4.48) | .111 |
| IL-9 | 2.85 (.87–10.50) | .096 | 2.50 (.71–9.84) | .166 |
| FGF-basic | 3.48 (1.16–11.49) | .032 | 3.26 (.98–12.01) | .063 |
| G-CSF | 1.41 (.80–2.50) | .229 | 1.30 (.70–2.42) | .399 |
| GM-CSF | 2.45 (1.08–6.47) | .048 | 2.23 (.96–6.12) | .089 |
| PDGF-BB | 1.75 (.45–6.34) | .398 | 1.41 (.34–5.36) | .620 |
| VEGF | 2.44 (.93–6.94) | .079 | 2.23 (.83–6.47) | .123 |
| Regulatory | ||||
| IL-1RA | 1.34 (.50–4.42) | .593 | 1.34 (.49–4.50) | .601 |
| IL-10 | 2.06 (.94–4.67) | .073 | 1.88 (.84–4.32) | .124 |
Logistic regression was performed and the P values shown are unadjusted for multiple comparisons; P value < .05 is considered significant.
Abbreviations: CI, confidence interval; FGF, fibroblast growth factor; G-CSF, granulocyte-colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon-γ; IL, interleukin; IP-10, IFN-γ inducible protein-10; MCP-1, monocyte chemoattractant protein-1; OR, odds ratio; PDGF, platelet-derived growth factor; RANTES, regulated on activation, normal T cell expressed and secreted; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor.
DISCUSSION
Access to safe and effective contraception has brought substantial improvements to maternal and child health [26]. Despite these important public health gains for women and their children, certain contraceptive methods have been associated with increased genital inflammation, raising concerns about unintended consequences for disease susceptibility, including a small risk for acquisition of HIV and other STIs [10, 27, 28]. In this mucosal substudy within the ECHO trial, we show that women randomized to the Cu-IUD experienced significant and broad increases in cervicovaginal cytokine concentrations 6 months after Cu-IUD insertion while those using DMPA-IM and LNG-implant did not. In addition, although women who subsequently acquired HIV during follow-up had higher CVS concentrations of several inflammatory cytokines and chemokines (including IL-1β, IL-6, TNF-α, Eotaxin, IL-2, IL-4, IL-5, IL-17, GM-CSF, and FGF-basic) compared to women who remained HIV negative, this was not significant after correcting for multiple comparisons. Correcting for contraceptive arm did not significantly impact the relationship between CVS cytokines and HIV risk, after adjusting for multiple comparisons. This is consistent with the finding that contraceptive arm was not a driver of HIV risk in the ECHO trial, despite being associated with inflammatory changes in the pre-/post-contraceptive–initiation analysis. Combined with data from the parent ECHO trial results, our findings suggest that the broad changes in genital cytokine profiles associated with Cu-IUD insertion were either of insufficient magnitude, duration, or nature to impact HIV risk significantly over the other 2 study arms.
Previous observational studies, including those from our group, suggested that DMPA-IM use either increased or decreased CVS cytokine concentrations [10, 11, 29–31]. In this substudy from the ECHO trial, comparing before contraceptive initiation to after initiation CVS cytokine concentrations in women randomized to contraceptives, we did not find any change in cytokines in women randomized to DMPA-IM or LNG-implant in the 6 months after contraceptive initiation.
Insertion of Cu-IUDs has been demonstrated to have a significant histological, biochemical, and inflammatory immune impact on the female reproductive tract [32–34]. Early studies, focusing on tissues collected from the upper reproductive tract, suggested that inflammatory changes were restricted to the uterus and endometrium [32–34]. It is now clear that these changes are also easily detectable in cervicovaginal secretions collected in the lower reproductive tract [35, 36]. While we did not detect inflammatory changes at 1 month after Cu-IUD insertion across our 3 study sites, another substudy within the ECHO trial that recruited from different clinical sites in South Africa (Pretoria and Durban) found elevated cytokines at 1 month after Cu-IUD insertion [37]. Others have similarly reported increased inflammatory biomarkers as early as 2–4 weeks after Cu-IUD insertion [35, 36]. The reason for the delayed inflammatory signatures in the Cu-IUD arm across our sites is unclear, although it would be important to determine whether this enhanced genital inflammation associated with insertion of the Cu-IUD is sustained beyond 6 months or transient, to fully understand the relationship between Cu-IUD, inflammation, and HIV risk.
While it is tempting to assume that cytokines measured in cervicovaginal fluid originate from the lower reproductive tract, it is likely the Cu-IUD induces cytokine production by cells in both the upper and lower reproductive tract [38]. It is also clear from the earliest studies that both anatomical and inflammatory changes observed following insertion of a Cu-IUD occur in the upper reproductive tract, because the device is intrauterine [32–34]. Because CVS collected in menstrual cups likely reflects a mixture of fluids from both the upper and lower reproductive tracts, the location of inflammation within the female genital tract is important to consider in trying to understand the relationship between inflammation and HIV risk, particularly because the dogma for decades has been that HIV infects target cells located in the lower reproductive tract of women [39], although this has recently been disputed [40].
Increases in cervicovaginal cytokine concentrations have also been linked with disrupted epithelial barrier integrity [41, 42], which increases HIV risk. BV and some STIs are thought to concurrently disrupt mucosal epithelial barrier integrity and induce inflammatory cytokines [43–45]. Vaginal microbial communities dominated by certain Lactobacillus spp. have been associated with improved epithelial barrier integrity, while those dominated by Gardnerella vaginalis, common in BV, have been associated with compromised epithelial barrier function, linked to their production of soluble metabolites that inhibit wound healing [44]. Women using a Cu-IUD have been shown to be more susceptible to BV, Candida, and Mycoplasma infections [46, 47], suggesting that differences in the vaginal bacteriome and mycobiome of women randomized to the Cu-IUD in this trial need to be determined urgently. Indeed, women in the Cu-IUD arm in this mucosal substudy did have marked changes in vaginal microbiota compared to their respective baseline samples and the other contraceptive arms [48].
Our study had some limitations. The ECHO trial did not include a no contraceptive group, so we were not able to compare CVS cytokine changes over 6 months in noncontracepting versus contracepting women. We did not determine long-term persistence of CVS cytokine concentrations. In addition, we could not perform longitudinal analyses using a generalized estimating equation model with repeated measures due to our assaying schema and lack of sufficient matching Luminex data with all time points. The case-control analysis was limited by the small number of women who HIV seroconverted in this substudy, representing only a subset of women (3/12 clinical sites) who participated in the ECHO trial. Future research is needed to confirm our findings in the full ECHO cohort by increasing the sample size and/or including a noncontraceptive control group. Finally, many factors that were not measured in this substudy may influence genital inflammation, including behavioral factors (condom use, partner HIV status) and infections with common STIs (chlamydia, gonorrhea, HSV-2, and trichomoniasis [49, 50]).
Our findings from this mucosal mechanisms substudy within the first large, randomized, and longitudinal trial of contraceptive methods in a setting of high HIV prevalence, that includes 3 clinical trial sites from South Africa and Kenya, importantly demonstrates that the Cu-IUD is significantly more inflammatory than hormonal contraceptives (DMPA-IM and LNG-implant), although the Cu-IUD was included as the nonhormonal inert alternative. Despite being a strong influence on cervicovaginal inflammation, Cu-IUD did not increase HIV acquisition risk more than DMPA-IM or LNG-implant in women randomized to this study arm. While this supports the overall finding of the ECHO trial, our results suggest that hormonal and nonhormonal contraceptives likely influence immune mechanisms and HIV risk in the female genital tract distinctly and that “one mechanism does not explain all.”
In conclusion, we found that Cu-IUD, but not DMPA-IM nor LNG-implant, broadly increased concentrations of cervicovaginal inflammatory, chemotactic, and adaptive cytokines and growth factors. Furthermore, we found that CVS cytokines were not significantly associated with HIV acquisition in the HIV case-control analysis. We conclude that genital inflammation caused by insertion of the Cu-IUD was not sufficient to increase HIV risk compared to DMPA-IM and LNG-implant in the ECHO trial. While genital inflammation induced by Cu-IUD did not appear to significantly impact HIV risk in this substudy, these effects may indicate the potential for susceptibility to other pathogens that warrant further investigation.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank all the women who participated in this study for their devotion to the study and the time they committed to research procedures.
Members of the R01HD089831 Evidence for Contraceptive Options and HIV Outcomes (ECHO) Biological Mechanisms Ancillary Study Team. Coordinating center (University of Washington): Renee Heffron, Heather Jaspan (principal investigators); Jared Baeten, Caitlin Scoville, Kate Heller, Harald Haugen, and Colin Pappajohn. Study sites: Emavundleni Research Centre, University of Cape Town, Desmond Tutu HIV Centre (Cape Town, South Africa), Gonasagrie Nair (principal investigator), Banzi Bam, Elaine Sebastian, and Ebrahiema Jacobs; Kenya Medical Research Institute, Research Care and Training Program (Kisumu, Kenya), Maricianah Onono (principal investigator), Lizzie Kabete, and Imeldah Wakhungu; Wits Reproductive Health and HIV Institute, University of Witswatersrand (Johannesburg, South Africa), Thesla Palanee-Phillips (principal investigator), Krishnaveni Reddy, Emily Kekana, Cecelia Mokoena, Nomsa Morudu, and Kerushini Moodley. Laboratories: Center for Global Health and Diseases, Case Western Reserve University (Cleveland, USA), Adam Burgener (principal investigator); Emory University (Atlanta, USA), Steven Bosinger (principal investigator), Prachi Gupta, and Sydney Nelson; Seattle Children’s Research Institute (Seattle, USA), Heather Jaspan (principal investigator), and Bryan Brown; University of Cape Town (Cape Town, South Africa), Jo-Ann Passmore (principal investigator), Heather Jaspan, Shameem Jaumdally, Hoyam Gamieldien, Rubina Bunjun, Tanko Fatime Ramla, Smritee Dabee, Rushil Harryparsad, Anna-Ursula Happel, Masalula Sinkala, Kathryn Norman, Yamkela Qumbelo, Trishana Nundalall, Agano Kiravu, Denzhe Singo, and Vernon Plaatjies; University of Manitoba (Manitoba, Canada), Adam Burgener (principal investigator), Laura Noel-Romas, Hossaena Ayele, Kenzie Birse, and Samantha Hornes.
Disclaimer. The study funders had no role in the study design, data collection, analysis, and interpretation, as well as in the preparation of the manuscript. The content is the sole responsibility of the authors and does not necessarily represent the official views of the study funders.
Financial support. This work was supported within the by the Bill and Melinda Gates Foundation (grant number OPP1032115); United States Agency for International Development (USAID; grant number AID-OAA-A-15–00045); Swedish International Development Cooperation Agency (grant number 2017/762965-0); South Africa Medical Research Council; and United Nations Population Fund. Support for the ancillary study of biological mechanisms was from the National Institutes of Health, National Institute of Child Health and Human Development. Contraceptive supplies were donated by the Government of South Africa and USAID.
Contributor Information
Ramla F Tanko, Institute of Infectious Disease and Molecular Medicine, Department of Pathology, University of Cape Town, Cape Town, South Africa; Medical Research Centre, Institute of Medical Research and Medicinal Plant Studies, Ministry of Scientific Research and Innovation, Yaoundé, Cameroon.
Rubina Bunjun, Institute of Infectious Disease and Molecular Medicine, Department of Pathology, University of Cape Town, Cape Town, South Africa.
Smritee Dabee, Seattle Children’s Research Institute, Seattle, Washington, USA.
Shameem Z Jaumdally, Institute of Infectious Disease and Molecular Medicine, Department of Pathology, University of Cape Town, Cape Town, South Africa.
Maricianah Onono, Kenya Medical Research Institute, Kisumu, Kenya.
Gonasagrie Nair, Desmond Tutu HIV Foundation, Cape Town, South Africa.
Thesla Palanee-Phillips, Wits Reproductive Health and HIV Institute, University of the Witwatersrand, Johannesburg, South Africa.
Rushil Harryparsad, Institute of Infectious Disease and Molecular Medicine, Department of Pathology, University of Cape Town, Cape Town, South Africa.
Anna Ursula Happel, Institute of Infectious Disease and Molecular Medicine, Department of Pathology, University of Cape Town, Cape Town, South Africa.
Hoyam Gamieldien, Institute of Infectious Disease and Molecular Medicine, Department of Pathology, University of Cape Town, Cape Town, South Africa.
Yamkela Qumbelo, Institute of Infectious Disease and Molecular Medicine, Department of Pathology, University of Cape Town, Cape Town, South Africa.
Musalula Sinkala, Institute of Infectious Disease and Molecular Medicine, Department of Pathology, University of Cape Town, Cape Town, South Africa.
Caitlin W Scoville, University of Washington, Seattle, Washington, USA.
Kate Heller, University of Washington, Seattle, Washington, USA.
Jared M Baeten, University of Washington, Seattle, Washington, USA; Gilead Sciences, Foster City, California, USA.
Steven E Bosinger, Emory University, Atlanta, Georgia, USA; Yerkes National Primate Research Center, Atlanta, Georgia, USA.
Adam Burgener, Center for Global Health and Diseases, Case Western Reserve University, Cleveland, USA; Department of Obstetrics and Gynecology and Medical Microbiology, University of Manitoba, Winnipeg, Canada; Department of Medicine Solna, Center for Molecular Medicine, Karolinska Institute, Stockholm, Sweden.
Renee Heffron, University of Washington, Seattle, Washington, USA.
Heather B Jaspan, Institute of Infectious Disease and Molecular Medicine, Department of Pathology, University of Cape Town, Cape Town, South Africa; Seattle Children’s Research Institute, Seattle, Washington, USA.
Jo Ann S Passmore, Institute of Infectious Disease and Molecular Medicine, Department of Pathology, University of Cape Town, Cape Town, South Africa; National Research Foundation - Department of Science and Technology Centre for the AIDS Programme of Research in South Africa Centre of Excellence in HIV Prevention, Durban, South Africa; National Health Laboratory Service, Cape Town, South Africa.
Data Availability
Access to the data from this ancillary study of the ECHO Study may be requested through submission of a research concept to: rheffron@uw.edu. The concept must include the research question, data requested, analytic methods, and steps taken to ensure ethical use of the data. Access will be granted if the concept is evaluated to have scientific merit and if sufficient data protections are in place. As of the time of publication, data access applications are in process with the governing institutional review boards of the ECHO Study to make deidentified data from the primary ECHO dataset publicly available.
References
- 1. United Nations, Department of Economic and Social Affairs, Population Division. Contraceptive use by method 2019. Data Booklet (ST/ESA/SER.A/435). United Nations, 2019. [Google Scholar]
- 2. Mishell DR. Intrauterine devices: mechanisms of action, safety, and efficacy. Contraception 1998; 58(Suppl 3):45–53S. [DOI] [PubMed] [Google Scholar]
- 3. Steiner MJ, Lopez LM, Grimes DA, et al. Sino-implant (II)—a levonorgestrel-releasing two-rod implant: systematic review of the randomized controlled trials. Contraception 2010; 81:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Regidor PA. The clinical relevance of progestogens in hormonal contraception: present status and future developments. Oncotarget 2018; 9:34628–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tsui AO, Brown W, Li Q.. Contraceptive practice in sub-Saharan Africa. Popul Dev Rev 2017; 43:166–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baeten JM, Benki S, Chohan V, et al. Hormonal contraceptive use, herpes simplex virus infection, and risk of HIV-1 acquisition among Kenyan women. AIDS 2007; 21:1771–7. [DOI] [PubMed] [Google Scholar]
- 7. Morrison CS, Chen PL, Kwok C, et al. Hormonal contraception and HIV acquisition: reanalysis using marginal structural modeling. AIDS 2010; 24:1778–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heffron R, Donnell D, Rees H, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis 2012; 12:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ahmed K, Baeten JM, Beksinska M, et al. HIV incidence among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: a randomised, multicentre, open-label trial. Lancet 2019; 394:303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deese J, Masson L, Miller W, et al. Injectable progestin-only contraception is associated with increased levels of pro-inflammatory cytokines in the female genital tract. Am J Reprod Immunol 2015; 74:357–67. [DOI] [PubMed] [Google Scholar]
- 11. Morrison CS, Fichorova R, Chen PL, et al. A longitudinal assessment of cervical inflammation and immunity associated with HIV-1 infection, hormonal contraception, and pregnancy. AIDS Res Hum Retroviruses 2018; 34:889–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sciaranghella G, Tong N, Mahan AE, Suscovich TJ, Alter G.. Decoupling activation and exhaustion of B cells in spontaneous controllers of HIV infection. AIDS 2013; 27:175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Byrne EH, Anahtar MN, Cohen KE, et al. Association between injectable progestin-only contraceptives and HIV acquisition and HIV target cell frequency in the female genital tract in South African women: a prospective cohort study. Lancet Infect Dis 2016; 16:441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ray RM, Maritz MF, Avenant C, et al. The contraceptive medroxyprogesterone acetate, unlike norethisterone, directly increases R5 HIV-1 infection in human cervical explant tissue at physiologically relevant concentrations. Sci Rep 2019; 9:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Engel RM, Morris M, Henning T, et al. Evaluation of pigtail macaques as a model for the effects of copper intrauterine devices on HIV infection. J Med Primatol 2014; 43:349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murphy K, Irvin SC, Herold BC.. Research gaps in defining the biological link between HIV risk and hormonal contraception. Am J Reprod Immunol 2014; 72:228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ortiz ME, Croxatto HB, Bardin CW.. Mechanisms of action of intrauterine devices. Obstet Gynecol Surv 1996; 51:42–51S. [DOI] [PubMed] [Google Scholar]
- 18. O’Brien PA, Kulier R, Helmerhorst FM, Usher-Patel M, d’Arcangues C.. Copper-containing, framed intrauterine devices for contraception: a systematic review of randomized controlled trials. Contraception 2008; 77:318–27. [DOI] [PubMed] [Google Scholar]
- 19. Jaumdally SZ, Jones HE, Hoover DR, et al. Comparison of sampling methods to measure HIV RNA viral load in female genital tract secretions. Am J Reprod Immunol 2017; 77:e12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jaumdally SZ, Masson L, Jones HE, et al. Lower genital tract cytokine profiles in South African women living with HIV: influence of mucosal sampling. Sci Rep 2018; 8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nugent RP, Krohn MA, Hillier SL.. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 1991; 29:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gu Z, Eils R, Schlesner M.. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016; 32:2847–9. [DOI] [PubMed] [Google Scholar]
- 23. Oksanen J, Blanchet FG, Friendly M, et al. Vegan: community ecology package, 2019. https://cran.r-project.org/web/packages/vegan/index.html. Accessed 14 December 2020. [Google Scholar]
- 24. Rohart F, Gautier B, Singh A, Lê Cao K-A.. mixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Comput Biol 2017; 13:e1005752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Columb MO, Sagadai S.. Multiple comparisons. Curr Anaesth Crit Care 2006; 17:233–6. [Google Scholar]
- 26. Smith JA, Beacroft L, Abdullah F, et al. Responding to the ECHO trial results: modelling the potential impact of changing contraceptive method mix on HIV and reproductive health in South Africa. J Int AIDS Soc 2020; 23:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morrison CS, Chen P-L, Kwok C, et al. Hormonal contraception and the risk of HIV acquisition: an individual participant data meta-analysis. PLoS Med 2015; 12:e1001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Polis CB, Curtis KM, Hannaford PC, et al. An updated systematic review of epidemiological evidence on hormonal contraceptive methods and HIV acquisition in women. AIDS 2016; 30:2665–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Francis SC, Hou Y, Baisley K, et al. Immune activation in the female genital tract: expression profiles of soluble proteins in women at high risk for HIV infection. PLoS One 2016; 11:e0143109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ngcapu S, Masson L, Sibeko S, et al. Lower concentrations of chemotactic cytokines and soluble innate factors in the lower female genital tract associated with the use of injectable hormonal contraceptive. J Reprod Immunol 2015; 110:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Molatlhegi RP, Liebenberg LJ, Leslie A, et al. Plasma concentration of injectable contraceptive correlates with reduced cervicovaginal growth factor expression in South African women. Mucosal Immunol 2020; 13:449–59. [DOI] [PubMed] [Google Scholar]
- 32. Chang CC, Tatum HJ.. A study of the antifertility effect of intrauterine copper. Contraception 1970; 1:265–70. [Google Scholar]
- 33. Ämmälä M, Nyman T, Strengell L, Rutanen E-M.. Effect of intrauterine contraceptive devices on cytokine messenger ribonucleic acid expression in the human endometrium. Fertil Steril 1995; 63:773–8. https://programme.ias2019.org/Abstract/Abstract/4816. Accessed 20 September 2021. [DOI] [PubMed] [Google Scholar]
- 34. Ortiz ME, Croxatto HB.. Copper-T intrauterine device and levonorgestrel intrauterine system: biological bases of their mechanism of action. Contraception 2007; 75:S16–30. [DOI] [PubMed] [Google Scholar]
- 35. Shobokshi A, Shaarawy M.. Cervical mucus granulocyte macrophage colony stimulating factor and interleukin-2 soluble receptor in women using copper intrauterine contraceptive devices. Contraception 2002; 66:129–32. [DOI] [PubMed] [Google Scholar]
- 36. Sharma P, Shahabi K, Spitzer R, Farrugia M, Kaul R, Yudin M.. Cervico-vaginal inflammatory cytokine alterations after intrauterine contraceptive device insertion: A pilot study. PLoS One 2018; 13:e02072666-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Deese J, Radzey N, Meyer B, et al. Copper IUD and levonorgestrel implant increase genital inflammation in the ECHO trial. CROI Conference, Boston, MA, 2020. https://www.croiconference.org/abstract/copper-iud-and-levonorgestrel-implant-increase-genital-inflammation-in-the-echo-trial/. Accessed 20 September 2021.
- 38. Lee SK, Kim CJ, Kim D-J, Kang J.. Immune cells in the female reproductive tract. Immune Netw 2015; 15:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shattock RJ, Moore JP.. Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol 2003; 1:25–34. [DOI] [PubMed] [Google Scholar]
- 40. Stieh DJ, Maric D, Kelley ZL, et al. Vaginal challenge with an SIV-based dual reporter system reveals that infection can occur throughout the upper and lower female reproductive tract. PLoS Pathog 2014; 10:e1004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thurman AR, Doncel GF.. Innate immunity and inflammatory response to trichomonas vaginalis and bacterial vaginosis: relationship to HIV acquisition. Am J Reprod Immunol 2011; 65:89–98. [DOI] [PubMed] [Google Scholar]
- 42. Arnold KB, Burgener A, Birse K, et al. Increased levels of inflammatory cytokines in the female reproductive tract are associated with altered expression of proteases, mucosal barrier proteins, and an influx of HIV-susceptible target cells. Mucosal Immunol 2016; 9:194–205. [DOI] [PubMed] [Google Scholar]
- 43. Borgdorff H, Gautam R, Armstrong SD, et al. Cervicovaginal microbiome dysbiosis is associated with proteome changes related to alterations of the cervicovaginal mucosal barrier. Mucosal Immunol 2016; 9:621–33. [DOI] [PubMed] [Google Scholar]
- 44. Zevin AS, Xie IY, Birse K, et al. Microbiome composition and function drives wound-healing impairment in the female genital tract. PLoS Pathog 2016; 12:e10058891-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McKinnon LR, Achilles SL, Bradshaw CS, et al. The evolving facets of bacterial vaginosis: implications for HIV transmission. AIDS Res Hum Retroviruses 2019; 35:219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Erol O, Simavlı S, Derbent AU, Ayrım A, Kafalı H.. The impact of copper-containing and levonorgestrel-releasing intrauterine contraceptives on cervicovaginal cytology and microbiological flora: a prospective study. Eur J Contracept Reprod Health Care 2014; 19:187–93. [DOI] [PubMed] [Google Scholar]
- 47. Achilles SL, Austin MN, Meyn LA, Mhlanga F, Chirenje ZM, Hillier SL.. Impact of contraceptive initiation on vaginal microbiota. Am J Obstet Gynecol 2018; 218:622.e1–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jaspan H, Bunjun R, Brown B, et al. Contraceptive-induced changes in genital tract HIV-1 cellular targets and microbiota among women enrolled in the ECHO trial. International AIDS Society Conference, 2019, Mexico City, Mexico.
- 49. Jewkes RK, Dunkle K, Nduna M, Shai N.. Intimate partner violence, relationship power inequity, and incidence of HIV infection in young women in South Africa: a cohort study. Lancet 2010; 376:41–8. [DOI] [PubMed] [Google Scholar]
- 50. Masson L, Mlisana K, Little F, et al. Defining genital tract cytokine signatures of sexually transmitted infections and bacterial vaginosis in women at high risk of HIV infection: a cross-sectional study. Sex Transm Infect 2014; 90:580–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Access to the data from this ancillary study of the ECHO Study may be requested through submission of a research concept to: rheffron@uw.edu. The concept must include the research question, data requested, analytic methods, and steps taken to ensure ethical use of the data. Access will be granted if the concept is evaluated to have scientific merit and if sufficient data protections are in place. As of the time of publication, data access applications are in process with the governing institutional review boards of the ECHO Study to make deidentified data from the primary ECHO dataset publicly available.