Abstract
Traumatic brain injury (TBI) is a major cause of mortality and morbidity worldwide. Studies revealed that the pathogenesis of TBI involves upregulation of MMPs. MMPs form a large family of closely related zinc-dependent endopeptidases, which are primarily responsible for the dynamic remodulation of the extracellular matrix (ECM). Thus, they are involved in several normal physiological processes like growth, development, and wound healing. During pathophysiological conditions, MMPs proteolytically degrade various components of ECM and tight junction (TJ) proteins of BBB and cause BBB disruption. Impairment of BBB causes leakiness of the blood from circulation to brain parenchyma that leads to microhemorrhage and edema. Further, MMPs dysregulate various normal physiological processes like angiogenesis and neurogenesis, and also they participate in the inflammatory and apoptotic cascades by inducing or regulating the specific mediators and their receptors. In this review, we explore the roles of MMPs in various physiological/pathophysiological processes associated with neurological complications, with special emphasis on TBI.
Keywords: Traumatic brain injury, Matrix metalloproteinases, Extracellular matrix, Blood-brain barrier, Hemorrhage, Edema, Neuroinflammation, Neurodegeneration
Introduction
Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases with multifactorial functions in the physiology and pathology of animal body. MMPs are collectively called matrixins as they participate mainly in the degradation of the extracellular matrix (ECM) [1]. MMPs are implicated to have a significant role in normal growth, development, wound healing, angiogenesis, neurogenesis, bone remodeling, ovulation, and implantation. However, during pathophysiological conditions, MMPs cause blood-brain barrier (BBB) disruption, hemorrhage, neuroinflammation, and cell death in various neurological diseases [2]. They are secreted by different cell types including astrocytes, endothelial cells, neurons, fibroblasts, osteoblasts, etc. A fair amount of MMPs are also produced by circulating leukocytes, including neutrophils, monocytes, and lymphocytes that invade the brain during inflammation. These enzymes are produced as zymogens (pro-MMPs), and they are further processed by proteolytic enzymes such as serine proteases, furin, and plasmin to convert into active forms [1]. MMPs are finely tuned enzymes, which are strategically regulated at the level of transcription, maturation from precursor pro MMPs, interaction with various ECM components, and inhibition by endogenous inhibitors [1, 3]. Hormones, growth factors, and certain cytokines regulate the activation of MMPs. Endogenous MMP inhibitors (MMPIs) and tissue inhibitors of MMPs (TIMPs) specifically control the activities of MMPs. Under normal physiological conditions, MMPs are expressed at the modest level. Overexpression/activation of MMPs results in an imbalance between the activities of MMPs and TIMPs, which is a major mechanism in the pathophysiology of various diseases such as arthritis, cancer, atherosclerosis, aneurysms, nephritis, tissue ulcers, fibrosis, and several neurodegenerative diseases [4, 5]. Table 1 shows the classification and reported biological functions of MMPs with references.
Table 1.
Biological activity of MMPs
| MMP class | MMP | Enzyme | Biological activity | Reference |
|---|---|---|---|---|
| Gelatinases | MMP-2 | Gelatinase A | Neurite outgrowth | [187] |
| Mesenchymal cell differentiation with inflammatory phenotype | [188] | |||
| Epithelial cell migration | [189] | |||
| ECM and tight junction proteins degradation | [25, 29] | |||
| Cleavage of SDF-1α | [157] | |||
| MMP-9 | Gelatinase B | Cleavage of several chemokines | [190] | |
| Angiogenesis and neovascularization | [191] | |||
| Wound healing | [192] | |||
| Development of aortic aneurysms | [193] | |||
| Collagenases | MMP-1 | Collagenase-1 or interstitial collagenase | Keratinocyte migration and reepithelialization | [194] |
| Platelet aggregation | [188] | |||
| Involved in the process of HIV infection | [195] | |||
| MMP-8 | Neutrophil collagenase | Regulating lung inflammation | [196] | |
| Regulation of ovarian cancer, | [197] | |||
| MMP-13 | Collagenase-3 | Osteoclast activation | [198] | |
| Release of bFGF | [199] | |||
| MMP-18 | Collagenase-4 | Metamorphosis in Xenopus | [200] | |
| Stromelysins | MMP-3 | Stromelysin 1 | Mammary epithelial cell apoptosis | [201] |
| Mammary epithelial alveolar formation | [202] | |||
| Disrupted cell aggregation and increased cell invasion | [203] | |||
| MMP-10 | Stromelysin 2 | Skeletal development, wound healing, and vascular remodeling; its overexpression is implicated in lung tumorigenesis and tumor progression | [204] | |
| MMP-11 | Stromelysin 3 | Involved in aggressive basal cell carcinoma and in dermatofibroma | [205] | |
| MMP-27 | Homology to stromelysin 2 (51.6 %) | Tissue homeostasis and tissue repair. | [141] | |
| Matrilysins | MMP-7 | Matrilysin 1 (Pump-1) | Adipocyte differentiation | [206] |
| Disrupted cell aggregation and increased cell invasion | [203] | |||
| Fas receptor-mediated apoptosis | [207] | |||
| MMP-26 | Matrilysin 2 (endometase) | Regulation of chondrosarcoma invasion | [208] | |
| Involved in colorectal cancer and endometrial carcinoma | [209] | |||
| Membrane type | MMP-14 | MT1-MMP | Kidney tubulogenesis | [210] |
| Epithelial cell migration | [211] | |||
| Reduced cell adhesion and spreading | [212] | |||
| MMP-15 | MT2-MMP | Reduced cell adhesion and spreading | [212] | |
| MMP-16 | MT3-MMP | Reduced cell adhesion and spreading | [212] | |
| MMP-17 | MT4-MMP | Progression of breast cancer | [213] | |
| MMP-24 | MT5-MMP | Neuronal plasticity | [214] | |
| MMP-25 | MT6-MMP | Important role in colon cancer | [215] | |
| Others | MMP-12 | Macrophage metalloelastase | Generation of angiostatin-like fragment | [216] |
| MMP-19 | RASI-1 | Participating in rheumatoid arthritis (RA)-associated joint tissue destruction | [217] | |
| Degradation of aggrecan, COMP in arthritic disease | [218] | |||
| MMP-19 plays a role in cutaneous wound repair | [219] | |||
| MMP-20 | Enamelysin | Degradation of aggrecan, COMP in arthritic disease | [218] | |
| MMP-21 | (In chromosome 1) | Upregulates TGF-β during epithelial cancer | [220] | |
| MMP-23 | Femalysin | Role in potassium channel trafficking and are implicated in diseases including cancer and inflammatory disorders | [221] | |
| MMP-28 | Epilysin | Induces TGF-β-mediated epithelial to mesenchymal transition in lung carcinoma cells | [222] | |
| MMPs | Generation of endostatin-like fragment | [223] | ||
| All MMPs (three or more MMPs having the same function) | MMPs | Activation of VEGF | [224] | |
| MMP-2, | MMP-3, MMP-7 | Increased bioavailability of TGF-β | [225] | |
| MMPs | Increased bioavailability of IGF1 and cell proliferation | [226] | ||
| MMP-3, | MMP-7, MMP-9, MMP-12 | Generation of angiostatin-like fragment | [216, 227] | |
| MMP-1, | MMP-3, MMP-9 | Proinflammatory processing IL-1β from the precursor | [135] | |
| MMP-1, | MMP-2, MMP-3 | Cell migration | [228] | |
| MMP-1, | MMP-2, MMP-3 | Increased bioavailability of IGF1 and cell proliferation | [226] | |
| MMP-2, | MMP-3, MMP-9 | ECM and tight junction protein degradation | [25, 29] |
Studies have suggested that MMPs are upregulated during traumatic brain injury (TBI). TBI causes upregulation of MMPs, particularly MMP-2, MMP-3, and MMP-9, and MMPs are involved in the pathophysiology of TBI including neuroinflammation and cell death. Secretion of MMP-2 and MMP-9 (gelatinases) is significantly increased in rat cortical neuronal culture, which has been induced by mechanical stretch injury [6]. TBI mediated motor deficits have been compared in traumatically injured MMP-9 knockout mice and wild mice in which a significant reduction in motor deficits was observed in knockout mice when compared with wild mice [7]. Likewise, several studies have suggested that TBI activates MMPs, which might play a critical role in the degradation of the extracellular matrix, disruption of the BBB, facilitation of leukocyte infiltration, hemorrhage, synaptic plasticity, angiogenesis, edema formation, neuroinflammation, and neurodegeneration. Table 2 shows the various reports on the roles of MMPs in different models of TBI.
Table 2.
Role of MMPs in traumatic brain injury
| MMP class | MMP | Enzyme | Biological activity | Reference |
|---|---|---|---|---|
| Gelatinases | MMP-2 | Gelatinase A | Role of ERK MAPK pathway in inducing MMP-9 in CCI model | [6] |
| Tight junction proteins degradation, BBB dysfunction in blast model of TBI | [32] | |||
| Relationship between edema and MMP-2 in rodent surgical brain injury (SBI) model | [112] | |||
| MMP-9 | Gelatinase B | Apoptosis in infant brain, weight drop method in rat pups | [229] | |
| Role in edema and inflammation in weight drop model in mice | [230] | |||
| Role in edema formation in human TBI after automobile accident | [231] | |||
| Tight junction proteins degradation and BBB dysfunction in blast rat model | [32] | |||
| BBB dysfunction in weight drop model in rats | [35] | |||
| Involved in BBB dysfunction in cortical contusion in rats | [232] | |||
| Relationship between edema and MMP-9 in rodent surgical brain injury (SBI) model | [112] | |||
| Apoptosis in hippocampus in FPI injury, MMP-9 inhibitor attenuates | [233] | |||
| Role of ERK MAPK pathway in inducing MMP-9 in CCI model | [234] | |||
| Release of IL-1beta induced MMP-9 in in vitro mild stretch injury model | [235] | |||
| Expression of MMP-9 and related to body temperature in FPI model in rats | [233] | |||
| In association with the activation of IL-6 in humans | [138] | |||
| Role in BBB permeability and fibrinogen deposit in cortical contusion injury | [236] | |||
| Formation of edema is induced by VEGF and MMP-9 in humans | [231] | |||
| The potential association between the extracellular matrix metalloproteinase inducer (EMMPRIN), or CD147 and MMP-9 expression in FPI model in rats | [237] | |||
| Collagenases | MMP-8 | Neutrophil collagenase | Association of MMP-8 concentration and mortality in human brain microdialysate | [238] |
| Stromelysins | MMP-3 | Stromelysin 1 | Highly expressed during trauma-induced synaptogenesis in central FPI and combined central FPI and bilateral entorhinal cortical lesion (BEC) in rats | [86] |
| Level of MMP-3 to determine age of brain contusion in rats | [239] | |||
| Role in BBB dysfunction in blast TBI model in rats | [32] | |||
| Matrilysins | MMP-7 | Matrilysin 1 (Pump-1) | Relationship between cerebral inflammation (cytokines) and MMP-7 in human TBI | [238] |
| Membrane type | MMP-24 | MT5-MMP | Facilitate synapse reorganization in rat model of adaptive unilateral entorhinal cortical lesion (UEC) or maladaptive fluid percussion TBI + bilateral entorhinal cortical lesion (TBI + BEC) | [101] |
TBI is characterized by physical brain injury associated with a broad spectrum of symptoms and disabilities. TBI causes approximately 1.7 million deaths and hospitalizations every year in the USA alone. Based on the injury severity, TBI is typically categorized into mild, moderate, and severe by using the Glasgow Coma Scale, a system used to assess coma and impaired consciousness [8]. Three components such as eye opening, verbal response, and motor responses are usually added together to produce a total score in the Glasgow Coma Scale. A Glasgow Coma Scale score of 13–15 is defined as mild, 9–12 as moderate, and 3–8 as severe [8]. Based on the mechanisms of brain tissue injury, TBI is classified as primary and secondary. Primary injury is the result of mechanical forces applied to the skull and brain and leads to skull fractures, brain contusions, axonal injuries, rupturing of blood vessels, and intracranial hemorrhages [9]. A series of molecular, neurochemical, cellular, and pathophysiological mechanisms contributes to secondary injury that leads to elevated intracranial pressure, BBB disruption, neuroinflammation, brain edema, cerebral hypoxia, ischemia, and delayed neurodegeneration [10–12]. Secondary brain injury may be reversible; therefore, the therapeutic intervention can be targeted.
Classifications of MMPs
To date, 24 different vertebrate MMPs have been identified, of which 23 are found in humans and are grouped into different classes. MMPs are broadly divided into two general classes: the secreted MMPs and the membrane-type MMPs [13]. They can be grouped into collagenases, gelatinases, stromelysins, and matrilysin according to their substrate specificity and domain structure (Table 1).
Collagenases
Collagenases are principal neutral proteinases capable of degrading interstitial collagens I, II, and III at a specific site [13]. Collagen is a fibrillar protein, the most abundant structural component of human connective tissue. The collagenases include collagenase-1 (MMP-1, interstitial collagenase), collagenase-2 (MMP-8, neutrophil collagenase), and collagenase-3 (MMP-13). MMP-18 (Xenopus) is also included in this group.
Gelatinases
The two best studied gelatinases, gelatinase A (MMP-2) and gelatinase B (MMP-9), belong to this group. MMP-2 and MMP-9 are found in the extracellular matrix, cerebrospinal fluid, and serum. These enzymes digest collagens and gelatins. MMP-2 digests type I, II, and III collagens. The activity of MMP-2 and MMP-9 can be easily detected by using gelatin-substrate zymography assay.
Stromelysins
This group of stromelysin includes stromelysin 1 (MMP-3) and stromelysin 2 (MMP-10). Both have similar substrate specificities, but MMP-3 has a proteolytic efficiency higher than that of MMP-10. Besides digesting ECM components, MMP-3 activates a number of pro-MMPs. For example, its action on pro-MMP-1 is critical to form active MMP-1.
Matrilysins
MMP-7 (Matrilysin 1) and MMP-26 (Matrilysin 2) are included in this group. The matrilysins do not contain hemopexin domain [14]. Matrilysin is preferentially expressed by cells of the glandular epithelium, so it is distinct from other known MMPs that are expressed in connective tissues [15]. Matrilysin 2 also digests ECM compounds.
Membrane-Type MMPs
Membrane-type MMPs (MT-MMPs) have common structural domains of the MMP family, but they have an additional domain that is anchored to the plasma membrane, making them important effectors of pericellular ECM degradation and proteolytic activities [16]. There are six MT-MMPs, in which four are type I transmembrane proteins (MMP-14, MMP-15, MMP-16, and MMP-24) and two are glycosylphosphatidylinositol (GPI) anchored proteins (MMP-17 and MMP-25). Except MT4-MMP, all MT-MMPs are capable of activating pro-MMP-2. These enzymes can also digest ECM molecules and MT1-MMP is capable of degrading type I, II, and III collagens [17].
The actions of MMPs are strictly controlled by endogenous MMPIs and TIMPs. TIMPs are specific inhibitors of matrixins that participate in controlling the local activities of MMPs in tissues [18].
Role of MMPs in Brain Pathogenesis
MMPs are essential for the normal physiological functions as they are important in the dynamic remodulation of ECM. However, dysregulation of MMPs often leads to various pathophysiological complications such as BBB disruption, hemorrhage, neuroinflammation, and cell death [2]. High-level expression and activities of MMPs are reported in various neurological complications and diseases such as stroke, hemorrhage, Alzheimer’s disease, and TBI [19–24]. During brain injury or neurological diseases, MMPs are activated by various signaling mediators like reactive oxygen species (ROS), transforming growth factor-β (TGF-β), and inflammatory cytokines [25, 26]. MMPs in turn accelerate inflammation by activating inflammatory cytokines such IL-1β and TNF-α. MMP-mediated degradation of ECM proteins and tight junction (TJ) proteins of BBB leads to BBB disruption [27, 28]. MMPs play a key role in angiogenesis by stimulating the production of vascular endothelial growth factor (VEGF); however, augmented VEGF level in turn activates caspase-1 and causes apoptosis [29]. Further, caspase-1 matures pro-IL1β to active IL-1β and leads to neuroinflammation. The neurodegenerative effect of MMPs comes mainly through its role in converting pro-caspases to active caspases and paves the way for cell apoptosis. In addition, MMPs induce cellular and vasogenic edema during brain injury. Figure 1 depicts the schematic presentation of the roles of MMPs in different cellular signaling cascades. The following review sessions will discuss the major roles of MMPs in neurovascular dysfunction, hemorrhage, angiogenesis, synaptic plasticity, edema formation, neuroinflammation, and neurodegeneration in various neurological complications with special emphasis on TBI. In the context of TBI, we discuss the roles of MMPs in the pathophysiology of neurotrauma by including human cases and animal models.
Fig. 1.
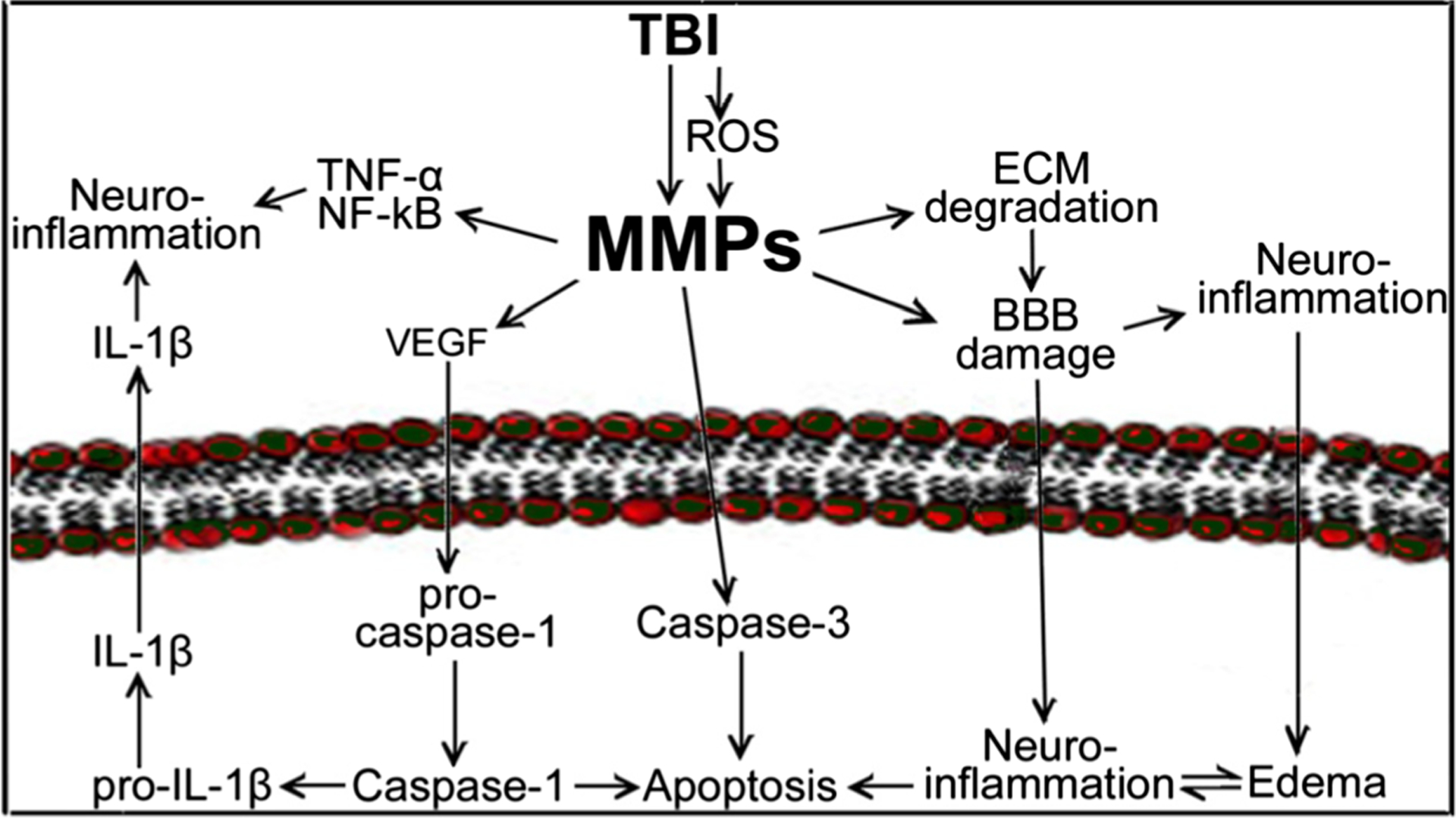
Schematic representation of the role of MMPs in traumatic brain injury. Brain injury activates the expression of MMPs either directly or via other mediators like oxidative stress (reactive oxygen species, ROS). MMPs exacerbate the inflammatory cascades by activating inflammatory cytokines such as IL-1β and TNF-α. MMPs degrade ECM proteins and tight junction proteins of BBB lead to BBB disruption. In addition, MMPs involve in neurodegeneration by activating cell death inducing caspase enzymes and causing apoptosis. Similarly, MMPs induce cellular and vasogenic edema during brain injury
Blood-Brain Barrier Disruption
During normal physiological conditions, MMPs help to sustain the dynamic integrity of BBB through its role in the remodulation of ECM. However, during pathophysiological conditions, its upregulation impairs the neurovascular system. Abnormal MMP activation causes degradation of microvascular basement membrane proteins, resulting in the loss of brain endothelium stability and increases BBB permeability in animal models as well as in vitro studies [27, 28]. Several studies reported that oxidative stress is the major causative factor that activates MMPs [25]. Inflammatory cytokines and chemokines are also reported as MMP activators [30, 31]. Enhanced MMP activity degrades extracellular matrix proteins and tight junction proteins such as occludin, claudin-5, ZO-1–5, and it exacerbate BBB permeability [1, 29]. Recently, we have reported that blast-induced mild TBI (mTBI) enhances oxidative radicals, which activates MMPs, that degrade perivascular units and lead to BBB disruption [32]. In this study, we analyzed the different types of MMPs and their specific role in the degradation of the BBB in blast-induced mild TBI. We studied three types of MMPs such as MMP-2, MMP-3, and MMP-9 and their activation in single or repeated mild shock wave exposure in brain microvessels. The levels of MMP-3/−9 protein increased gradually for up to 24 h of blast injury, while upregulation of MMP-2 seems to be short durable because the level of MMP-2 gradually decreased after 6 h. It has been suggested that MMP-2, MMP-3, and MMP-9 are involved in the degradation of perivascular units and TJ proteins, which leads to BBB leakiness and inflammation of cerebral vascular unit [32]. Figure 2 shows the expression of MMP-2 in the frontal cortex of rat brain exposed to mild primary blast-induced TBI and lateral fluid percussion injury (FPI). Blast experiments were conducted by exposing rats to 123 kPa blast wave pressure and subjected to analysis for different markers of oxidative stress, MMP expression, neuroinflammation, and cell death [32]. Studies show that single mild shock wave exposure elevates MMP-2 expression after 24 h of blast (Fig. 2, top row). As an Alternative experimental model of TBI, FPI also shows elevated MMP-2 expression in the cortical brain tissue after 24 h of injury with 15 psi pressure (Fig. 2, bottom row).
Fig. 2.
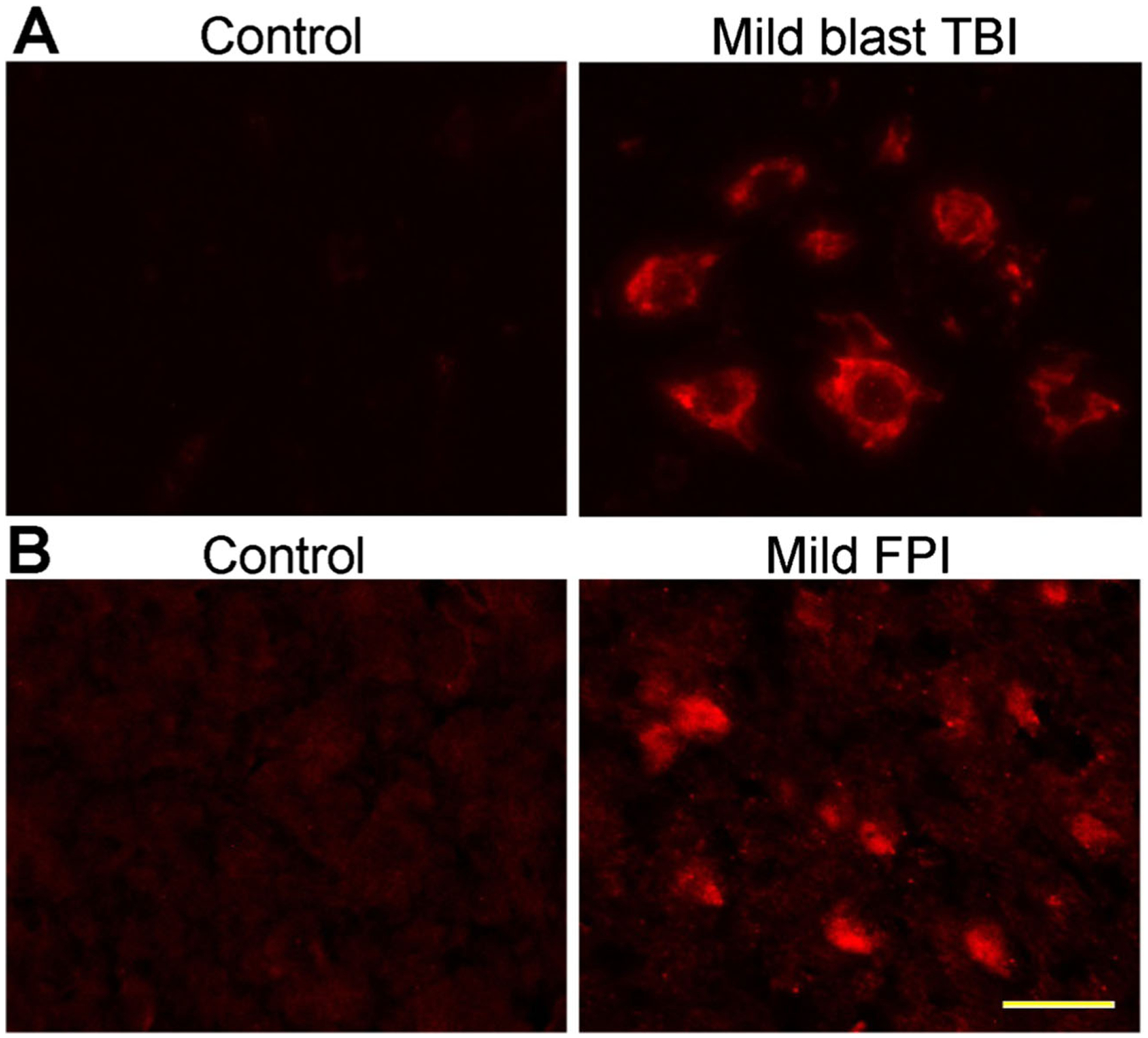
Activation of MMP-2 in mild traumatic brain injury: Immunofluorescent staining of MMP2 in rat brain cortex of primary blast (123-kPa peak overpressure) induced mTBI [32] (a) and mild fluid percussion injury (15 psi) and compared with control (b). Scale bar (yellow bar in the last panel)=20 μm in all panels
There are several other reports on the involvement of MMPs in the degradation of various types of ECM proteins and TJ proteins of BBB in brain injury [27, 28]. TBI causes upregulation of MMPs, particularly MMP-2, MMP-3, and MMP-9, and leads to disruption of BBB, edema formation, and cell death [33, 34]. The role of MMP-9 in BBB disruption has been substantiated as the pharmacological inhibitor minocycline significantly attenuated TBI-mediated BBB impairment in rats [35].
Cerebral Hemorrhage
Often TBI encompasses hemorrhage in and around the contusion. The BBB becomes greatly disrupted during intracerebral hemorrhage; as a result, macrophages and leucocytes infiltrate the brain parenchyma and their presence has been proposed to constitute a primary mechanism of neuronal death [36]. Activated microglia also aggravates neuronal cell death after intracerebral hemorrhage [37, 38]. MMPs play a pivotal role for causing hemorrhage in brain injury and stroke. They enhance vascular permeability, which leads to BBB disruption and several neurological complications such as brain edema, neuroinflammation, and neurodegeneration [39, 40]. A high-level MMP expression is believed to contribute to tissue destruction in brain injury and neuronal damage [20–24]. MMPs cause hemorrhage by proteolytic degradation of the extracellular matrix and tight junction proteins of BBB, which leads to BBB dysfunction and leakage of blood into the brain side in mild TBI (reviewed in [41, 42]). Horstmann et al. reported that the level of MMP-2 and MMP-9 in serum of patients suffering from subarachnoid hemorrhage was very high compared to normal healthy individuals [19]. Recently, we experimentally demonstrated vascular leakage due to BBB disruption and increase in vascular permeability in mild blast TBI by infusing high and small molecular weight dyes such as Evans blue and sodium fluorescein through the carotid artery [32]. During hemorrhagic transformation, MMP-9 participates in BBB dysfunction and exacerbates brain injury after cerebral ischemia. There are several other reports in which MMPs cause hemorrhage due to BBB damage [43, 44].
Upregulation of MMPs has been reported in various other pathophysiological conditions such as hypertension. Several authors reported that the hypertension-inducing hormone, angiotensin II (Ang II), is one of main factors leading to hemorrhage [45–47]. In several other biochemical mechanisms, Ang II has been reported as a mediator of oxidative stress via activation/induction of free radical-generating enzymes such NOX and NOS [48–55]. Ang II stimulates the production of ROS in endothelial cells by upregulating NADPH oxidases [56, 57]. These free radicals especially ROS activates TGF-β1 in brain microvessels. Ang II induced oxidative stress and TGF-β1 leads to the activation of MMPs [49]. The sustained activation of MMPs causes the degradation of TJ proteins such as occludin, claudin-5, and ZO-1, thereby impairing BBB and vascular wound healing process, that leads to chronic rupture of the capillaries leading to hemorrhagic stroke [58]. Degradation of basement membrane and TJ proteins of the BBB by MMPs enhances the BBB permeability and immune cell infiltration into the brain [59, 60], which is strongly associated with hemorrhagic and ischemic stroke [58, 61].
Angiogenesis
While reviewing the roles of MMPs in association with the pathophysiology of the brain, it is imperative to analyze its effect in angiogenesis. Angiogenesis is the physiological process in which new blood vessels emerge from existing endothelial-lined vessels [62]. Regarding the functional recovery after TBI, angiogenesis is a premier mechanism that includes mainly proliferation and migration of vascular endothelial cells. Being an invasive process, angiogenesis is always accompanied with degradation of basement membrane proteins [63, 64]. Here, the proteolytic activity of MMPs has greater roles to play. MMPs strategically degrade and remodulate various components of the extracellular matrix and pave the way for the emerging blood vessels [65]. MMP-3 and MMP-10 target proteoglycans, fibronectin, and laminin. MMP-8 and MMP-13 selectively target collagen I and II, respectively, whereas MMP-1 prefers collagen III. In addition, both MMP-2 and MMP-9 degrade denatured collagen (gelatin) [13]. By using both synthetic and endogenous MMP inhibitors, researchers inhibited angiogenic responses in vitro and in vivo [66–68]. In addition, the delayed or reduced angiogenic responses were reported in MMP-deficient mice during development [69, 70].
Apart from this, MMPs have a regulatory effect on the VEGF, one of the integral components of angiogenesis. But during pathophysiological conditions, MMPs impair angiogenesis through dysregulation of VEGF. VEGF is a ligand to the vascular endothelial growth factor receptor 1 or 2 (VEGFR-1 or VEGFR-2) and is a key regulator of angiogenic response and endothelium wound healing process [71]. When VEGF binds to VEGFR, the receptor dimerization and autophosphorylation occurs and promotes angiogenesis and repair damaged microvessels [72, 73]. Brain injury-associated upregulation of VEGF has been reported [74]. It has been reported that upregulation of VEGF or experimental administration of VEGF leads to induction of MMP-9 activity that causes BBB disruption [75]. In our previous study, we experimentally demonstrated that elevation of both MMP-3 and MMP-9 leads to upregulation of VEGF due to MMP-mediated proteolytic disruption of VEGFR-2 [29]. According to the study, sustained activation of MMPs leads to degradation of the VEGFR-2 protein, thereby making it difficult to repair injured capillaries. Exposure of hBECs to exogenous MMP-3 or MMP-9 leads to dimunition of VEGFR-2 protein, and the activation of MMP-3 or MMP-9 in brain microvessels was found to correlate with the downregulation of the VEGFR-2 protein [29]. Turk et al. reported the cleavage of VEGFR-2 by MMP-7 and MMP-9 at multiple positions (e.g., Leu-Ser/Met-Leu, Leu-Ser/Ile-Arg) [76]. In another study, it has been shown that the activation of plasma MMP cleaved the extracellular domain of VEGFR-2 on the endothelium [77]. As the receptor gets cleaved and becomes functionless, the level of ligand VEGF tends to increase. The upregulated VEGF is detrimental as it triggers caspases and aggravates apoptosis [77]. In our previous study, we demonstrated that elevated VEGF level could induce caspase-1 (interleukin converting enzyme) and cause apoptosis in alcohol-mediated vascular impairment in mice brain microvessels [29]. Treatment with the MMP inhibitor doxycycline attenuates VEGFR-2 cleavage as well as endothelial apoptosis [77]. In TBI, VEGFR-2 signaling blockage using the selective inhibitor SU5416 abrogates prosurvival response and induces high activation of caspase-3/7 leading to cell death [78]. However, in TBI, MMP-mediated regulation of VEGF and the associated biochemical cascades are elusive. Thus, it opens up a subject for future research. Angiogenic response is critical for the progression of wound healing in hemorrhage, stroke, and associated pathological conditions such as BBB impairment or inflammation. Therefore, a thorough understanding on the signaling events involved in angiogenesis and its molecular regulation has clinical implications.
Synaptic Plasticity
Impairment of cognitive function is one of the most devastating outcomes of TBI. Cognitive impairments often involve cell loss and vulnerability to different brain regions. Recovery of lost/impaired cognitive functions typically occurs via adaptive synaptic plasticity, which occurs in acute posttraumatic periods [79]. Though several factors contribute to the adaptive synaptic plasticity, MMPs have a significant role in posttraumatic synaptic reorganization through the dynamic modulation of ECM proteins ([80–84] and recently reviewed in [23, 85]). Here, we review the roles of MMPs in synaptic plasticity during the physiology and pathophysiology of the brain. MMPs play critical roles in neurite growth cone development, synaptic transmission, synaptic modification, and neuronal degeneration contributing to successful synaptic plasticity [3, 85–90]. In 2005, Mayer et al. reported MMP/substrate interaction during lesion-induced synaptogenesis [91]. MMPs influence axonal growth and synaptic modification with their regulatory effects on ECM [23, 85]. Redistribution of ECM proteins along deafferented dendrites leads to axon sprouting toward postsynaptic sites during cortical lesion synaptogenesis [92, 93]. Among the various MMPs, MMP-3 and MMP-9 are the most studied MMPs in synaptic plasticity. However, their effects in the adaptive synaptic plasticity during posttraumatic repair are not conclusively studied. MMP-3 targets ECM proteins, which are critical to neuronal growth and synaptic reorganization [84, 94–97]. Falo et al. reported significant elevation of MMP-3 in injury sites where active synaptogenesis is taking place [98]. High-level expression of MMP-2, MMP-3, and MMP-9 is observed in TBI models (our recent review [99]). An increase in MMP-3 activity has been reported as it persists toward the period of synapse regeneration [98]. MMP-9 is largely associated with modification of important synaptic receptors and altering the morphology of synapses [100]. In addition to these during synaptogenesis, the expression of MT5-MMP and ADAM-10 proteins elevates and supports a significant role in synaptic reorganization following TBI [101].
Upregulation of MMPs during various pathophysiological conditions of brain causes aberrant synoptic plasticity and spine dysmorphology [102, 103]. However, the signaling mechanisms involved in MMP-mediated synaptic dysfunction are largely unknown. Studies have suggested the roles of MMP-9 in mediating a typical synaptic plasticity in various neurological diseases like epilepsy and Alzheimer’s disease [102, 104]. Michaluk et al. reported that beta-dystroglycan (beta-DG), a transmembrane protein, is a synaptic target for MMP-9 [105]. They demonstrated it in neuronal cultures; glutamate/bicuculline-mediated stimulation of neuronal cultures caused an increase in MMP-9 activity which coincides with the cleavage of beta-DG, and this cleavage could be attenuated by the overexpression of TIMP-1. Philips et al. reported evidence of the role of MMP in TBI neuroplasticity based on studies with targeted hippocampal deafferentation [103]. Thus, it appears that an appropriate level of MMP is very critical for the posttraumatic adaptive neuroplasticity; thorough understanding would help to develop strategies that can favor for adaptive neuroplasticity over maladaptive neuroplasticity.
Edema Formation
Cerebral edema is extra accumulation of fluid in intraor extracellular spaces of the brain. It is one of the major factors that leads to high mortality and morbidity in TBI, and it accounts for up to half of the mortality in all victims of TBI [106]. Cerebral edema can be classified into two types: cellular and vasogenic [107]. Cellular edema is characterized by an increase in water content in intracellular compartments and will not be manifested as tissue swelling. Vasogenic edema allows intravascular proteins and fluid to penetrate into the parenchymal extracellular space and increases tissue water content and leads to swelling. This type of edema mainly results from the breakdown of BBB [108]. Usually, cellular edema appears after a few days of vasogenic edema, which develops in the first few hours after TBI. Cellular edema develops slowly and extends up to 2 weeks [109]. Usually in TBI, edema leads to swelling of brain tissue and elevates intracranial pressure (ICP) [110].
MMPs are one of the important mediators of edema formation in TBI. By using pharmacological inhibitors of MMPs such as minocycline or TIMP-1, the role of MMPs in edema formation has been demonstrated. The use of minocycline and TIMP-1 could abrogate TBI-mediated edema formation, BBB impairment, inflammatory responses, and cerebral ischemia [7, 43, 111]. In a surgical brain injury, edema is found around the surgical resection with simultaneous increases in MMP-9 and MMP-2 activity. In such injury, treatment with MMP inhibitor-1 decreased brain edema and attenuated the activation of MMP-9 and MMP-2 [112].
Several activators are involved in the formation of edema in TBI. Aquaporins (AQP) are one of the main activators that contribute to the development of cerebral edema [113]. Several investigators reported the role of AQPs in promoting edema formation after brain injury [114–116]. It is evident from the studies that inhibition of AQP4 using pharmacological inhibitors could mitigate the formation of edema in TBI [115, 117, 118]. AQPs are integral membrane proteins, which form pores in the membranes of mammalian cells; they selectively conduct water molecules in and out of the cells while preventing passage of ions and solutes [119, 120]. Among the known 13 AQPs, AQP1, AQP4, and AQP9 are highly expressed in the brain. AQP4 is mainly expressed in astrocytic end-feet [121]. In our previous blast-induced mild TBI study, we reported upregulation of AQP4 in the cerebral cortex of injured rats. This AQP4 co-expressed with the astrocytic marker GFAP in the perivascular area [32]. There are several reports in which MMPs induce or activate AQPs and lead to edema formation [35, 122]. The role of MMP-9 has been studied in the pathogenesis of brain edema and its associated neurological complications in ischemic and TBIs [21, 123, 124]. In 2000, Asashi et al. conducted a detailed study and reported that the brain extravasation significantly abrogated, and cognitive functions were protected when they conducted cerebral ischemia or trauma in MMP-9 knockout mice [125]. They also noticed similar protective effects when they treated the animals with the MMP inhibitor BB-94 [125]. These reports suggest that MMPs have a significant role in brain injury-mediated edema formation.
Neuroinflammation
Neuroinflammation is considered as a key secondary injury mechanism that sustains the damage long after TBI by exacerbating cerebral edema and intracranial pressure. Increasing amounts of evidence suggested that MMPs are instrumental in creating a proinflammatory microenvironment [126]. MMPs participate in the inflammatory cascade by inducing or regulating various inflammatory mediators and their receptors [127, 128]. These inflammatory mediators and receptors include cytokines, interferons, growth factors, and other regulatory proteins [128]. Studies show that MMP regulates the activation of inflammatory cytokines such as IL-1β, TNF-α, and TGF-β.
The cytokine, IL-1β, is a common inflammatory cytokine, which is significantly elevated during neurological complications such as Alzheimer’s disease, TBI, and stroke [129]. This is regarded as a major inflammatory biomarker. Activation of IL-1β involves proteolytic removal of the N-terminal part from its inactive precursor protein [130]. IL-1-converting enzyme (ICE), also called caspase-1, has been identified as the primary IL-1β activator [131]. There are several reports on the activation of IL-1β in caspase-1-independent pathways in vitro and in vivo [132, 133]. In 1996, Ito et al. first reported the MMP-mediated maturation of IL-1β [134]. Further studies suggested that MMPs like MMP-2, MMP-3, and MMP-9 are capable for the activation of IL-1β [135]. Converse to this finding when MMP-2-positive astrocytes were stimulated with IL-1β, they produced MMP-9 [136]. In another study, when IL-1β was injected to MMP-9 knockout mice, it showed reduced microglial activation [30] and attenuated BBB degradation [137]. Similarly, MMPs regulate the activation of other interleukins such as IL-6 and IL-18. Suehiro et al. reported that MMP-9 upregulated the level of IL-6 in systemic arterial and jugular venous blood from seven patients with TBI [138]. Thus, it appears that MMPs influence the inflammatory process by activating pro-IL or cleaving pro-IL to its active form.
TNF-α sheddase activity of MMPs provides another example on how MMPs influence inflammatory reaction by regulating cytokines. MMPs have the ability to release soluble TNF from its membrane-bound precursor. TNF-α is a pleiotropic proinflammatory cytokine produced by various cell types and is a central mediator of diverse cellular events. TNF-α is released from the cell surface by ADAM-17 (a disintegrin and metalloproteinase 17), which is also known as TNF-α-converting enzyme (TACE), the main TNF sheddase. It has been proven in ADAM-17 knockout mice, where it reduced the release of active TNF-α by 90 % [139]. Macrophage MMP-7 was found to be very important for TNF-α release [140]. In addition, several other MMPs such as MMP-1, MMP-2, MMP-3, MMP-9, MMP-12, MMP-14, and MMP-17 are reported as having role in releasing active TNF from the cell surface [141–143]. MMP-mediated activation of TNF-α has been reported in several signaling pathways associated with diseases and disorders. Xie et al. reported that MMP induced cerebral hemorrhage and involvement of TNF-α in subarachnoid hemorrhage patients [144]. Activated TNF-α in turn activates MMPs, and TNF-α-mediated activation of MMP-2 and MMP-9 is well documented. Lee et al. conducted a detailed study on the TNF-α-mediated activation of MMP-9 and reported that augmented MMP-9 gene expression was mediated through TNFR1/TRAF2/PKCalpha-dependent JNK1/2/c-Jun and c-Src/EGFR/PI3K/Akt signaling pathways in human A549 cells [145].
TGF-β is another cytokine with pleiotropic effect on which MMPs have regulatory roles. Several investigators reported the functional/regulatory interactions and interplay of MMPs with TGF-β. TGF-β has various physiological and pathological functions such as tissue wound healing, inflammation, cell proliferation, differentiation, migration, and ECM synthesis [146–148]. In addition, TGF-β has a significant role in regulating the immune system that controls both pro- and antiinflammatory effects depending on the cell type [146–148]. In TBI, increased levels of TGF-β have been reported [149, 150]. In initial days of head-injured patients, high levels of TGF-β in CSF have been detected [150]. MMP-2 and MMP-9 can activate TGF-β through proteolytic degradation of the latent TGF-β complex. TGF-β is translated as a precursor protein of 75 kDa, containing a signaling peptide, latencyassociated peptide (LAP), and the mature TGF-β. The precursor protein gets cleaved intracellularly by furin-type convertases, and upon secretion, the protein remains associated with ECM, still as latent TGF-β complex. ECM activation of TGF-β involves proteolytic cleavage by MMPs [151, 152]. Wang et al. demonstrated MMP-2-mediated activation of TGF-β1 in the arterial walls of aged rats. Recently, several studies have shown that TGF-β1 can upregulate MMP-9 expression and activity in different cell types [153, 154]; similarly, there are reports on the activation of MMP by TGF-β. Thus, MMPs play a key role in the regulation of TGF-β and thereby regulate inflammation, cell proliferation, and wound healing. Overall, MMPs modulate the process of neuroinflammation through their bidirectional interaction with cytokines.
Neurodegeneration
TBI almost always leads to progressive neurodegeneration depending on the severity. Proteases have a major role in cell death and it can trigger cell death by proteolytic damage [155]. MMPs upregulate apoptosis of several biologically active intra and/or extracellular molecules [156]. The level of MMPs or activation of MMPs, tissue specificity, and the balance between MMPs and TIMPs are other important factors which influence apoptosis [156]. Studies established the role of MMPs in neuronal apoptosis [156]. Apoptosis is a type of programmed cell death, which is triggered mainly by caspase activity [155]. In response to injury stimuli, MMPs get activated and induce apoptotic caspase enzymes [156]. In our recent study, in blast-induced mild TBI, we illustrated MMP-mediated induction of caspase-3 and apoptosis [32]. Using the MMP inhibitor, TIMP-1, the role of MMPs in apoptosis has been demonstrated. In another study, we demonstrated MMP-3 and MMP-9 as the main contributors for the caspase-1-dependent cell death in human endothelial cells [29].
MMPs have role in cleaving pro-IL-1β to active IL-1β [135], the key component of the cytokine network involved in apoptosis. Another class of nonmatrix proteins cleaved by MMP is the cleavage of stromal cell-derived factor-α (SDF-α), a chemokine converted to a neurotoxic protein causing neurodegeneration after precise processing by active MMP-2 [157]. In 2005, Copin et al. reported the roles of MMPs in cerebral ischemia-induced apoptosis and studied the specific role of MMP-9 in cell death mechanism. They also analyzed the pro and cleaved forms of PARP (poly (ADP) ribose polymerase) and α-spectrin and investigated DNA fragmentation in rats treated with MMP inhibitor and in MMP-9 knockout mice [158]. MMPs can inactivate PARP in a time-dependent manner similar to caspase-3 and cause harmful effects by hampering DNA strand break repair [159].
MMPs act on Proneurotrophins (i.e., nerve growth factor and brain-derived neurotrophic factor) and cleave extracellularly. Proneurotrophins are known growth factors that regulate cell survival but also promote neuronal cell death [160]. In the cerebellum and retinal ganglion cells, MMP-9 is involved in apoptosis through precise degradation of ECM proteins [161]. In addition, MMPs stimulate apoptosis by interacting with cell surface receptors. For instance, MMP-9 activates neuronal cell death through the association with lipoprotein receptor-related protein [162] and MMP-1 interacts with α1β2-, αvβ3-, and α5β1-integrins and is involved in apoptosis [163]. MMP-12 may cleave other cell surface receptors including proteinase-activated receptors and involve in the apoptosis of endothelial and epithelial cells [164].
Regarding TBI, the most reported MMPs are MMP-2 (gelatinase A) and MMP-9 (gelatinase B). In our recent work, we demonstrated the roles of MMP-2, MMP-3, and MMP-9 in neuroinflammation and neurodegeneration after blast-induced mild TBI in rats [32]. The other MMPs such as MMP-1 [163], MMP-7 [165], and MMP-11 [166] are also involved in apoptosis in various cell death mechanisms. Thus, the protein levels and activities of various MMPs have a regulatory role in the transition between physiology and pathophysiology.
Endogenous and Exogenous MMP Inhibitors
MMP inhibitors include natural endogenous tissue inhibitors and exogenous synthetic pharmacological inhibitors. Two major endogenous inhibitors of MMPs are TIMPS and α2 macroglobulin. TIMPs are the most thoroughly studied class of endogenous MMP inhibitors. Four TIMPs (TIMP-1, TIMP-2, TIMP-3, and TIMP-4) have been identified in vertebrates [18]. These inhibitors bind MMPs in a 1:1 stoichiometry. This family of inhibitors has been extensively reviewed by several authors [167–169]. TIMPs inhibit all MMPs tested so far, except that TIMP-1 fails to inhibit MT1-MMP [168]. The human TIMP proteins contain 184 to 194 amino acids that form an N-domain and a C-subdomain that are stabilized by six disulfide bonds [169]. TIMPs are 40 % identical in structure and have similar characters to inhibit individual MMPs. The expression of TIMPs is tissue-specific and is regulated at the level of transcription. TIMP-1 is widely expressed in many mammalian tissues, especially in the reproductive organs. In the brain, expression of TIMP-1 is restricted to the hippocampus, olfactory bulb, and cerebellum [170]. TIMP-2 is the most common tissue inhibitor and is expressed in most of the organs and tissues. TIMP-3 is expressed in many tissues and especially in the basement membranes of the eye and kidney. TIMP-4 is relatively restricted in the heart, kidney, ovary, pancreas, colon, testes, brain, and adipose tissue [171].
Plenty of exogenous synthetic inhibitors of MMP (MMPIs) have been developed and most of them were formulated by incorporating zinc-binding globulin (ZBG). ZGBs inactivate MMPs by displacing zinc-bound water molecule [172]. Classically, MMPIs include hydroxamic acids (ZBG1), carboxylates (ZBG2), thiols, and phosphonic acids (phosphorus-based ZBGs). Most of the first-generation MMPIs are broad spectrum inhibitors with nontargeted effects. Attempts are ongoing regarding the development of active site-directed MMPIs with high specificity. Apart from these, there are natural MMP inhibitors derived from various resources such as herbs, plants, fruits, and other agriculture products. These natural MMP inhibitors include long chain fatty acids, epigallocatechin gallate (EGCG) and other polyphenols, flavonoids, and a variety of other natural compounds. A detailed account of the various classes of MMPs and their chemical structure and effects was reviewed by several authors [167, 173].
MMPs as Therapeutic Targets
Regulation of MMPs is generally considered as an ideal target for developing therapeutic strategies against various diseases and disorders including brain injuries because of their role in mediating pathophysiology. Experimental lines of evidence suggested that MMP inhibition can be an effective strategy to treat such diseases. MMP inhibitor-mediated attenuation of brain edema resulting from the BBB disruption during postsurgical brain injury by using MMP inhibitor has been reported by several investigators [112, 174]. Lines of evidence on the involvement of MMP-2 and MMP-9 on various neurovascular complications including TBI persuaded to target these MMPs to develop a successful therapeutic strategy. MMP-2 inhibition reversed sepsis-induced BBB permeability and reduced brain inflammation and oxidative damage in an animal model of sepsis [175]. Similarly, treatment with MMP-9 and MMP-2 inhibitors and dual MMP-2 and MMP-9 inhibsitor could prevent BBB breakdown in Wistar rats subjected to pneumococcal meningitis [176]. A competitive, mechanism based dual inhibitor of MMP-2 and MMP-9, SB-3CT, successfully attenuated MMP-9 activity, reduced brain lesion volumes, and prevented neuronal loss and dendritic degeneration in an experimental mouse model of TBI [177]. A novel MMP inhibitor (Ro 28–2653) inhibits MT1-MMP (also known as MMP-14), MT3-MMP (also known as MMP-16), MMP-2, MMP-8, and MMP-9 and significantly reduced brain injury when administered in the first 2 days after focal cerebral ischemia [178]. The available animal data has shown that therapeutic MMP inhibition in acute brain injury and stroke has considerable potential [179]. Despite these promising results supporting MMP-based clinical trials, it is imperative to consider the apparent positive effects of MMP; both MMP-9 and MMP-2 are thought to be involved in repair and regeneration after nervous system injury [180]. Furthermore, the availability of a suitable noninvasive method of drug delivery through the BBB remains as a challenge for undertaking a successful MMP inhibitor-based clinical trial against brain injury and other disorders.
Although the use of pharmacological inhibitors against MMP seems a straightforward approach, there is only limited clinical success achieved so far. Regarding the clinical trials of MMP inhibitors, most of the attempts were carried out in the area of cancer therapy. Despite the fact that a number of preclinical data support the application of MMP inhibitors as anticancer drugs, all of these trials failed [179]. Failure of first-generation MMP inhibitors diminishes enthusiasm for MMP inhibitor-based drugs. Regardless of these disappointments, investigations on inhibiting MMP activity is still a rational therapeutic approach, particularly for inflammatory disorders in which MMPs often activate the signaling cascades leading to inflammation [181]. Several reasons are highlighted for the failure of clinical trials including poor knowledge of the complexity of MMP function, nonspecificity, and instability of inhibitors used. Broad spectrum MMP inhibitors like hydroxamate-based inhibitors are useful in a way because of their nontargeted undesirable effects. Investigating the precise isoform of MMP associated with specific pathological condition and spatiotemporal expression and functional diversity of the particular MMP involved is important in the design and development of highly specific inhibitors against the crucial MMP target.
Apart from the use of inhibitors, antibody-based biotherapy is also found to be promising as it can assure more selectivity and potency [182]. Devy et al. have identified a highly selective antibody-based MMP inhibitor (DX-2400) against MMP-14, which displays anti-invasive, antitumor, and antiangiogenic properties and blocks pro-MMP-2 processing and was suggested for breast cancer patients [183, 184]. The use of functional blocking antibodies often enables inhibition of specific functions of the MMP rather than their general proteolytic activity [179]. REGA-3G12 is a monoclonal antibody developed as a selective inhibitor against MMP-9 that specifically targets the catalytic domain, but the fibronectin or zinc-binding domains remain unaffected by it [182]. Similarly, the mouse 9E8 monoclonal antibody targets only the MMP-2 activating function of cellular MT1-MMP rather than the general proteolytic activity of this MMP [185].
Reestablishing the delicate normal balance between MMPs and their endogenous inhibitors to recoup the optimum MMP activity for normal physiological processes is another approach. Despite the disappointments from the clinical trials of first-generation MMP inhibitors, researchers are still hoping in the rise of MMP inhibition as a therapeutic approach by including more specific and precise inhibitory methods. Strategic upregulation of natural inhibitors, development of peptide inhibitors including functional blocking antibodies, optimization of available MMP inhibitors, and development of highly specific and stable pharmacological inhibitors with minimal adverse effects are the goals of future MMP targeted therapeutic trials. Third-generation and highly specific MMP inhibitors designed to block only the target MMPs are currently being evaluated [186].
Conclusion and Perspectives
MMPs play significant roles in various physiological processes primarily through their effects on ECM remodeling. However, their elevated levels and activities are largely associated with several neurological complications that include neurovascular dysfunction, hemorrhage, neuroinflammation, neurodegeneration, and impairment in angiogenesis and neurogenesis. The current review focused mainly on the role of MMPs in accomplishing the specific pathophysiology of TBI. Among the different types of MMPs studied so far, gelatinases (MMP-2 and MMP-9) are highly referred by associating with various neurological complications including TBI. Our previous findings on the augmented levels and activities of MMP-2, MMP-3, and MMP-9 in blast-injured rats and the critical roles of MMPs in various neuronal signaling mechanisms reported by several other investigations persuade us to undertake a more comprehensive study in this topic. A detailed study will delineate the molecular aspects of MMP regulation in post-TBI including the potential epigenetic changes that can bring about rapid modulation in the MMP levels and activities. By exploring the signaling mechanism that could regulate MMPs in TBI, we are anticipating the possibility of developing it as a valid therapeutic strategy against mTBI. The conventional therapeutic approaches using endogenous and synthetic MMP inhibitors are usually unsuccessful mainly due to the lack of efficacy and specificity or due to untoward side effects. Thus, gene therapy-based interventions seem more promising as they have the potential to ensure specificity and efficacy with minimum risk factors. We can accomplish this in two ways: either through the downregulation of MMPs or by the overexpression of TIMPs after deciphering the precise mechanisms of MMP regulation in mTBI. Since MMPs are indispensable in various normal physiological processes, regulatory strategies need to be adopted in a highly judicious manner.
Acknowledgments
The authors gratefully acknowledge the financial support by the US Department of Army Materials and Medical Command under award number W81XWH-15-1-0303.
References
- 1.Nagase H, Woessner JF Jr (1999) Matrix metalloproteinases. J Biol Chem 274:21491–21494 [DOI] [PubMed] [Google Scholar]
- 2.Yong VW (2005) Metalloproteinases: mediators of pathology and regeneration in the CNS. Nat Rev Neurosci 6:931–944 [DOI] [PubMed] [Google Scholar]
- 3.Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17:463–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engel CK, Pirard B, Schimanski S, Kirsch R, Habermann J, Klingler O, Schlotte V, Weithmann KU et al. (2005) Structural basis for the highly selective inhibition of MMP-13. Chem Biol 12:181–189 [DOI] [PubMed] [Google Scholar]
- 5.Visse R, Nagase H (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92:827–839 [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Mori T, Jung JC, Fini ME, Lo EH (2002) Secretion of matrix metalloproteinase-2 and −9 after mechanical trauma injury in rat cortical cultures and involvement of MAP kinase. J Neurotrauma 19:615–625 [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Jung J, Asahi M, Chwang W, Russo L, Moskowitz MA, Dixon CE, Fini ME et al. (2000) Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J Neurosci 20:7037–7042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teasdale G, Jennett B (1974) Assessment of coma and impaired consciousness. A practical scale. Lancet 2:81–84 [DOI] [PubMed] [Google Scholar]
- 9.Maas AI, Stocchetti N, Bullock R (2008) Moderate and severe traumatic brain injury in adults. Lancet Neurol 7:728–741 [DOI] [PubMed] [Google Scholar]
- 10.Lotocki G, de Rivero Vaccari JP, Perez ER, Sanchez-Molano J, Furones-Alonso O, Bramlett HM et al. (2009) Alterations in blood brain barrier permeability to large and small molecules and leukoscyte accumulation after traumatic brain injury: effects of post traumatic hypothermia. J Neurotrauma 26:1123–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pun PB, Lu J, Moochhala S (2009) Involvement of ROS in BBB dysfunction. Free Radic Res 43:348–364 [DOI] [PubMed] [Google Scholar]
- 12.Toklu HZ, Hakan T, Biber N, Solakoglu S, Ogunc AV, Sener G (2009) The protective effect of alpha lipoic acid against traumatic brain injury in rats. Free Radic Res 43:658–667 [DOI] [PubMed] [Google Scholar]
- 13.Nagase H, Visse R, Murphy G (2006) Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 69:562–573 [DOI] [PubMed] [Google Scholar]
- 14.Agnihotri R, Crawford HC, Haro H, Matrisian LM, Havrda MC, Liaw L (2001) Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin). J Biol Chem 276:28261–28267 [DOI] [PubMed] [Google Scholar]
- 15.Wilson CL, Matrisian LM (1996) Matrilysin: an epithelial matrix metalloproteinase with potentially novel functions. Int J Biochem Cell Biol 28:123–136 [DOI] [PubMed] [Google Scholar]
- 16.Hernandez-Barrantes S, Bernardo M, Toth M, Fridman R (2002) Regulation of membrane type-matrix metalloproteinases. Semin Cancer Biol 12:131–138 [DOI] [PubMed] [Google Scholar]
- 17.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG et al. (1999) MT1-MMP deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell 99:81–92 [DOI] [PubMed] [Google Scholar]
- 18.Brew K, Dinakarpandian D, Nagase H (2000) Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta 1477:267–283 [DOI] [PubMed] [Google Scholar]
- 19.Horstmann S, Su Y, Koziol J, Meyding-Lamade U, Nagel S, Wagner S (2006) MMP-2 and MMP-9 levels in peripheral blood after subarachnoid hemorrhage. J Neurol Sci 251:82–86 [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Lo EH (2003) Triggers and mediators of hemorrhagic transformation in cerebral ischemia. Mol Neurobiol 28:229–244 [DOI] [PubMed] [Google Scholar]
- 21.Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone FC (1998) Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke 29:1020–1030 [DOI] [PubMed] [Google Scholar]
- 22.Lo EH, Wang X, Cuzner ML (2002) Extracellular proteolysis in brain injury and inflammation: role for plasminogen activators and matrix metalloproteinases. J Neurosci Res 69:1–9 [DOI] [PubMed] [Google Scholar]
- 23.Yong VW, Power C, Forsyth P, Edwards DR (2001) Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci 2:502–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberg GA (2002) Matrix metalloproteinases in neuroinflammation. Glia 39:279–291 [DOI] [PubMed] [Google Scholar]
- 25.Haorah J, Ramirez SH, Schall K, Smith D, Pandya R, Persidsky Y (2007) Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction. J Neurochem 101:566–576 [DOI] [PubMed] [Google Scholar]
- 26.Hsieh HL, Wang HH, Wu WB, Chu PJ, Yang CM (2010) Transforming growth factor-beta1 induces matrix metalloproteinase-9 and cell migration in astrocytes: roles of ROS-dependent ERK- and JNK-NF-kappaB pathways. J Neuroinflammation 7:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA (2007) Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab 27:697–709 [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg GA, Cunningham LA, Wallace J, Alexander S, Estrada EY, Grossetete M et al. (2001) Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures. Brain Res 893:104–112 [DOI] [PubMed] [Google Scholar]
- 29.Abdul Muneer PM, Alikunju S, Szlachetka AM, Haorah J (2012) The mechanisms of cerebral vascular dysfunction and neuroinflammation by MMP-mediated degradation of VEGFR-2 in alcohol ingestion. Arterioscler Thromb Vasc Biol 32:1167–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svedin P, Hagberg H, Savman K, Zhu C, Mallard C (2007) Matrix metalloproteinase-9 gene knock-out protects the immature brain after cerebral hypoxia-ischemia. J Neurosci 27:1511–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Lint P, Libert C (2007) Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J Leukoc Biol 82:1375–1381 [DOI] [PubMed] [Google Scholar]
- 32.Abdul-Muneer PM, Schuetz H, Wang F, Skotak M, Jones J, Gorantla S, Zimmerman MC, Chandra N et al. (2013) Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in an animal model of mild traumatic brain injury induced by primary blast. Free Radic Biol Med 60:282–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayashi T, Kaneko Y, Yu S, Bae E, Stahl CE, Kawase T, van Loveren H, Sanberg PR et al. (2009) Quantitative analyses of matrix metalloproteinase activity after traumatic brain injury in adult rats. Brain Res 1280:172–177 [DOI] [PubMed] [Google Scholar]
- 34.Tejima E, Guo S, Murata Y, Arai K, Lok J, van Leyen K, Rosell A, Wang X et al. (2009) Neuroprotective effects of overexpressing tissue inhibitor of metalloproteinase TIMP-1. J Neurotrauma 26: 1935–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Higashida T, Kreipke CW, Rafols JA, Peng C, Schafer S, Schafer P, Ding JY, Dornbos D 3rd et al. (2011) The role of hypoxiainducible factor-1alpha, aquaporin-4, and matrix metalloproteinase-9 in blood-brain barrier disruption and brain edema after traumatic brain injury. J Neurosurg 114:92–101 [DOI] [PubMed] [Google Scholar]
- 36.Holmin S, Soderlund J, Biberfeld P, Mathiesen T (1998) Intracerebral inflammation after human brain contusion. Neurosurgery 42:291–298, discussion 298–299 [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Rogove AD, Tsirka AE, Tsirka SE (2003) Protective role of tuftsin fragment 1–3 in an animal model of intracerebral hemorrhage. Ann Neurol 54:655–664 [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Tsirka SE (2005) Tuftsin fragment 1–3 is beneficial when delivered after the induction of intracerebral hemorrhage. Stroke 36:613–618 [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg GA, Navratil M (1997) Metalloproteinase inhibition blocks edema in intracerebral hemorrhage in the rat. Neurology 48:921–926 [DOI] [PubMed] [Google Scholar]
- 40.Gasche Y, Fujimura M, Morita-Fujimura Y, Copin JC, Kawase M, Massengale J, Chan PH (1999) Early appearance of activated matrix metalloproteinase-9 after focal cerebral ischemia in mice: a possible role in blood-brain barrier dysfunction. J Cereb Blood Flow Metab 19:1020–1028 [DOI] [PubMed] [Google Scholar]
- 41.Lakhan SE, Kirchgessner A, Tepper D, Leonard A (2013) Matrix metalloproteinases and blood-brain barrier disruption in acute ischemic stroke. Front Neurol 4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shetty AK, Mishra V, Kodali M, Hattiangady B (2014) Blood brain barrier dysfunction and delayed neurological deficits in mild traumatic brain injury induced by blast shock waves. Front Cell Neurosci 8:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH (2001) Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci 21:7724–7732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barr TL, Latour LL, Lee KY, Schaewe TJ, Luby M, Chang GS, El-Zammar Z, Alam S et al. (2010) Blood-brain barrier disruption in humans is independently associated with increased matrix metalloproteinase-9. Stroke 41:e123–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adamski MG, Golenia A, Turaj W, Baird AE, Moskala M, Dziedzic T, Szczudlik A, Slowik A et al. (2014) The AGTR1 gene A1166C polymorphism as a risk factor and outcome predictor of primary intracerebral and aneurysmal subarachnoid hemorrhages. Neurol Neurochir Pol 48:242–247 [DOI] [PubMed] [Google Scholar]
- 46.Jusufovic M, Sandset EC, Bath PM, Berge E, Scandinavian Candesartan Acute Stroke Trial Study G (2014) Blood pressure lowering treatment with candesartan in patients with acute hemorrhagic stroke. Stroke 45:3440–3442 [DOI] [PubMed] [Google Scholar]
- 47.Villapol S, Saavedra JM (2015) Neuroprotective effects of angiotensin receptor blockers. Am J Hypertens 28:289–299 [DOI] [PubMed] [Google Scholar]
- 48.El Bekay R, Alvarez M, Monteseirin J, Alba G, Chacon P, Vega A, Martin-Nieto J, Jimenez J et al. (2003) Oxidative stress is a critical mediator of the angiotensin II signal in human neutrophils: involvement of mitogen-activated protein kinase, calcineurin, and the transcription factor NF-kappaB. Blood 102:662–671 [DOI] [PubMed] [Google Scholar]
- 49.Oudit GY, Kassiri Z, Patel MP, Chappell M, Butany J, Backx PH, Tsushima RG, Scholey JW et al. (2007) Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc Res 75:29–39 [DOI] [PubMed] [Google Scholar]
- 50.Landmesser U, Spiekermann S, Preuss C, Sorrentino S, Fischer D, Manes C, Mueller M, Drexler H (2007) Angiotensin II induces endothelial xanthine oxidase activation: role for endothelial dysfunction in patients with coronary disease. Arterioscler Thromb Vasc Biol 27:943–948 [DOI] [PubMed] [Google Scholar]
- 51.Wang HD, Xu S, Johns DG, Du Y, Quinn MT, Cayatte AJ, Cohen RA (2001) Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ Res 88:947–953 [DOI] [PubMed] [Google Scholar]
- 52.Tan Y, Li X, Prabhu SD, Brittian KR, Chen Q, Yin X, McClain CJ, Zhou Z et al. (2012) Angiotensin II plays a critical role in alcohol induced cardiac nitrative damage, cell death, remodeling, and cardiomyopathy in a protein kinase C/nicotinamide adenine dinucleotide phosphate oxidase-dependent manner. J Am Coll Cardiol 59: 1477–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW (1994) Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 74: 1141–1148 [DOI] [PubMed] [Google Scholar]
- 54.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG (1996) Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 97:1916–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mollnau H, Wendt M, Szocs K, Lassegue B, Schulz E, Oelze M, Li H, Bodenschatz M et al. (2002) Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res 90:E58–65 [DOI] [PubMed] [Google Scholar]
- 56.Rueckschloss U, Quinn MT, Holtz J, Morawietz H (2002) Dose dependent regulation of NAD(P)H oxidase expression by angiotensin II in human endothelial cells: protective effect of angioten sin II type 1 receptor blockade in patients with coronary artery disease. Arterioscler Thromb Vasc Biol 22:1845–1851 [DOI] [PubMed] [Google Scholar]
- 57.Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG (2002) Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension 40:511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosell A, Ortega-Aznar A, Alvarez-Sabin J, Fernandez-Cadenas I, Ribo M, Molina CA, Lo EH, Montaner J (2006) Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke 37:1399–1406 [DOI] [PubMed] [Google Scholar]
- 59.Alikunju S, Abdul Muneer PM, Zhang Y, Szlachetka AM, Haorah J (2011) The inflammatory footprints of alcohol-induced oxidative damage in neurovascular components. Brain Behav Immun 25(Suppl 1):S129–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gursoy-Ozdemir Y, Can A, Dalkara T (2004) Reperfusion induced oxidative/nitrative injury to neurovascular unit after focal cerebral ischemia. Stroke 35:1449–1453 [DOI] [PubMed] [Google Scholar]
- 61.Rosell A, Alvarez-Sabin J, Arenillas JF, Rovira A, Delgado P, Fernandez-Cadenas I, Penalba A, Molina CA et al. (2005) A matrix metalloproteinase protein array reveals a strong relation between MMP-9 and MMP-13 with diffusion-weighted image lesion increase in human stroke. Stroke 36:1415–1420 [DOI] [PubMed] [Google Scholar]
- 62.Risau W, Flamme I (1995) Vasculogenesis. Annu Rev Cell Dev Biol 11:73–91 [DOI] [PubMed] [Google Scholar]
- 63.Egeblad M, Werb Z (2002) New functions for the matrix metallo proteinases in cancer progression. Nat Rev Cancer 2:161–174 [DOI] [PubMed] [Google Scholar]
- 64.van Hinsbergh VW, Engelse MA, Quax PH (2006) Pericellular proteases in angiogenesis and vasculogenesis. Arterioscler Thromb Vasc Biol 26:716–728 [DOI] [PubMed] [Google Scholar]
- 65.Menzel-Severing J (2012) Emerging techniques to treat corneal neovascularisation. Eye (Lond) 26:2–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hiraoka N, Allen E, Apel IJ, Gyetko MR, Weiss SJ (1998) Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell 95:365–377 [DOI] [PubMed] [Google Scholar]
- 67.Benelli R, Adatia R, Ensoli B, Stetler-Stevenson WG, Santi L, Albini A (1994) Inhibition of AIDS-Kaposi’s sarcoma cell induced endothelial cell invasion by TIMP-2 and a synthetic peptide from the metalloproteinase propeptide: implications for an antiangiogenic therapy. Oncol Res 6:251–257 [PubMed] [Google Scholar]
- 68.Murphy AN, Unsworth EJ, Stetler-Stevenson WG (1993) Tissue inhibitor of metalloproteinases-2 inhibits bFGF-induced human microvascular endothelial cell proliferation. J Cell Physiol 157: 351–358 [DOI] [PubMed] [Google Scholar]
- 69.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM et al. (1998) MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 93:411–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S (1998) Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res 58:1048–1051 [PubMed] [Google Scholar]
- 71.Carmeliet P (2005) Angiogenesis in life, disease and medicine. Nature 438:932–936 [DOI] [PubMed] [Google Scholar]
- 72.Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller NP, Risau W, Ullrich A (1993) High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell 72:835–846 [DOI] [PubMed] [Google Scholar]
- 73.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC (1995) Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376:62–66 [DOI] [PubMed] [Google Scholar]
- 74.Morgan R, Kreipke CW, Roberts G, Bagchi M, Rafols JA (2007) Neovascularization following traumatic brain injury: possible evidence for both angiogenesis and vasculogenesis. Neurol Res 29: 375–381 [DOI] [PubMed] [Google Scholar]
- 75.Valable S, Montaner J, Bellail A, Berezowski V, Brillault J, Cecchelli R, Divoux D, Mackenzie ET et al. (2005) VEGF induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: both effects decreased by Ang-1. J Cereb Blood Flow Metab 25:1491–1504 [DOI] [PubMed] [Google Scholar]
- 76.Turk BE, Huang LL, Piro ET, Cantley LC (2001) Determination of protease cleavage site motifs using mixture-based oriented peptide libraries. Nat Biotechnol 19:661–667 [DOI] [PubMed] [Google Scholar]
- 77.Tran ED, DeLano FA, Schmid-Schonbein GW (2010) Enhanced matrix metalloproteinase activity in the spontaneously hypertensive rat: VEGFR-2 cleavage, endothelial apoptosis, and capillary rarefaction. J Vasc Res 47:423–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee C, Agoston DV (2009) Inhibition of VEGF receptor 2 increased cell death of dentate hilar neurons after traumatic brain injury. Exp Neurol 220:400–403 [DOI] [PubMed] [Google Scholar]
- 79.Levin HS, Mattis S, Ruff RM, Eisenberg HM, Marshall LF, Tabaddor K, High WM Jr, Frankowski RF (1987) Neurobehavioral outcome following minor head injury: a three center study. J Neurosurg 66:234–243 [DOI] [PubMed] [Google Scholar]
- 80.Garcia de Yebenes E, Ho A, Damani T, Fillit H, Blum M (1999) Regulation of the heparan sulfate proteoglycan, perlecan, by injury and interleukin-1alpha. J Neurochem 73:812–820 [DOI] [PubMed] [Google Scholar]
- 81.Oh LY, Larsen PH, Krekoski CA, Edwards DR, Donovan F, Werb Z, Yong VW (1999) Matrix metalloproteinase-9/gelatinase B is required for process outgrowth by oligodendrocytes. J Neurosci 19:8464–8475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Phillips LL, Reeves TM (2001) Interactive pathology following traumatic brain injury modifies hippocampal plasticity. Restor Neurol Neurosci 19:213–235 [PubMed] [Google Scholar]
- 83.Szklarczyk A, Lapinska J, Rylski M, McKay RD, Kaczmarek L (2002) Matrix metalloproteinase-9 undergoes expression and activation during dendritic remodeling in adult hippocampus. J Neurosci 22:920–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Muir EM, Adcock KH, Morgenstern DA, Clayton R, von Stillfried N, Rhodes K, Ellis C, Fawcett JW et al. (2002) Matrix metalloproteases and their inhibitors are produced by overlapping populations of activated astrocytes. Brain Res Mol Brain Res 100: 103–117 [DOI] [PubMed] [Google Scholar]
- 85.Dityatev A, Schachner M (2003) Extracellular matrix molecules and synaptic plasticity. Nat Rev Neurosci 4:456–468 [DOI] [PubMed] [Google Scholar]
- 86.Kim HJ, Fillmore HL, Reeves TM, Phillips LL (2005) Elevation of hippocampal MMP-3 expression and activity during trauma induced synaptogenesis. Exp Neurol 192:60–72 [DOI] [PubMed] [Google Scholar]
- 87.Nelson RB, Linden DJ, Hyman C, Pfenninger KH, Routtenberg A (1989) The two major phosphoproteins in growth cones are probably identical to two protein kinase C substrates correlated with persistence of long-term potentiation. J Neurosci 9:381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sheffield JB, Krasnopolsky V, Dehlinger E (1994) Inhibition of retinal growth cone activity by specific metalloproteinase inhibitors in vitro. Dev Dyn 200:79–88 [DOI] [PubMed] [Google Scholar]
- 89.Uhm JH, Dooley NP, Oh LY, Yong VW (1998) Oligodendrocytes utilize a matrix metalloproteinase, MMP-9, to extend processes along an astrocyte extracellular matrix. Glia 22:53–63 [DOI] [PubMed] [Google Scholar]
- 90.Vaillant C, Didier-Bazes M, Hutter A, Belin MF, Thomasset N (1999) Spatiotemporal expression patterns of metalloproteinases and their inhibitors in the postnatal developing rat cerebellum. J Neurosci 19:4994–5004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mayer J, Hamel MG, Gottschall PE (2005) Evidence for proteolytic cleavage of brevican by the ADAMTSs in the dentate gyrus after excitotoxic lesion of the mouse entorhinal cortex. BMC Neurosci 6:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brodkey JA, Laywell ED, O’Brien TF, Faissner A, Stefansson K, Dorries HU, Schachner M, Steindler DA (1995) Focal brain injury and upregulation of a developmentally regulated extracellular matrix protein. J Neurosurg 82:106–112 [DOI] [PubMed] [Google Scholar]
- 93.Yuan W, Matthews RT, Sandy JD, Gottschall PE (2002) Association between protease-specific proteolytic cleavage of brevican and synaptic loss in the dentate gyrus of kainate-treated rats. Neuroscience 114:1091–1101 [DOI] [PubMed] [Google Scholar]
- 94.Bejarano PA, Noelken ME, Suzuki K, Hudson BG, Nagase H (1988) Degradation of basement membranes by human matrix metalloproteinase 3 (stromelysin). Biochem J 256:413–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Imai K, Yokohama Y, Nakanishi I, Ohuchi E, Fujii Y, Nakai N, Okada Y (1995) Matrix metalloproteinase 7 (matrilysin) from human rectal carcinoma cells. Activation of the precursor, interaction with other matrix metalloproteinases and enzymic properties. J Biol Chem 270:6691–6697 [DOI] [PubMed] [Google Scholar]
- 96.Okada Y, Nagase H, Harris ED Jr (1987) Matrix metalloproteinases 1, 2, and 3 from rheumatoid synovial cells are sufficient to destroy joints. J Rheumatol 14 Spec No:41–42 [PubMed] [Google Scholar]
- 97.VanSaun M, Werle MJ (2000) Matrix metalloproteinase-3 removes agrin from synaptic basal lamina. J Neurobiol 44:369. [DOI] [PubMed] [Google Scholar]
- 98.Falo MC, Reeves TM, Phillips LL (2008) Agrin expression during synaptogenesis induced by traumatic brain injury. J Neurotrauma 25:769–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Abdul-Muneer PM, Chandra N, Haorah J (2015) Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol Neurobiol 51:966–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stawarski M, Rutkowska-Wlodarczyk I, Zeug A, Bijata M, Madej H, Kaczmarek L, Wlodarczyk J (2014) Genetically encoded FRET-based biosensor for imaging MMP-9 activity. Biomaterials 35:1402–1410 [DOI] [PubMed] [Google Scholar]
- 101.Warren KM, Reeves TM, Phillips LL (2012) MT5-MMP, ADAM-10, and N-cadherin act in concert to facilitate synapse reorganization after traumatic brain injury. J Neurotrauma 29:1922–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kamat PK, Swarnkar S, Rai S, Kumar V, Tyagi N (2014) Astrocyte mediated MMP-9 activation in the synapse dysfunction: an implication in Alzheimer disease. Ther Targets Neurol Dis 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Phillips LL, Chan JL, Doperalski AE, Reeves TM (2014) Time dependent integration of matrix metalloproteinases and their targeted substrates directs axonal sprouting and synaptogenesis following central nervous system injury. Neural Regen Res 9: 362–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Heuser K, Hoddevik EH, Tauboll E, Gjerstad L, Indahl U, Kaczmarek L, Berg PR, Lien S et al. (2010) Temporal lobe epilepsy and matrix metalloproteinase 9: a tempting relation but negative genetic association. Seizure 19:335–338 [DOI] [PubMed] [Google Scholar]
- 105.Michaluk P, Kolodziej L, Mioduszewska B, Wilczynski GM, Dzwonek J, Jaworski J, Gorecki DC, Ottersen OP et al. (2007) Beta-dystroglycan as a target for MMP-9, in response to enhanced neuronal activity. J Biol Chem 282:16036–16041 [DOI] [PubMed] [Google Scholar]
- 106.Marmarou A (2003) Pathophysiology of traumatic brain edema: current concepts. Acta Neurochir Suppl 86:7–10 [DOI] [PubMed] [Google Scholar]
- 107.Klatzo I (1987) Pathophysiological aspects of brain edema. Acta Neuropathol 72:236–239 [DOI] [PubMed] [Google Scholar]
- 108.Donkin JJ, Vink R (2010) Mechanisms of cerebral edema in traumatic brain injury: therapeutic developments. Curr Opin Neurol 23:293–299 [DOI] [PubMed] [Google Scholar]
- 109.Barzo P, Marmarou A, Fatouros P, Hayasaki K, Corwin F (1997) Contribution of vasogenic and cellular edema to traumatic brain swelling measured by diffusion-weighted imaging. J Neurosurg 87:900–907 [DOI] [PubMed] [Google Scholar]
- 110.Unterberg AW, Stroop R, Thomale UW, Kiening KL, Pauser S, Vollmann W (1997) Characterisation of brain edema following Bcontrolled cortical impact injurŷ in rats. Acta Neurochir Suppl 70:106–108 [DOI] [PubMed] [Google Scholar]
- 111.Candelario-Jalil E, Yang Y, Rosenberg GA (2009) Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience 158:983–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jadhav V, Yamaguchi M, Obenaus A, Zhang JH (2008) Matrix metalloproteinase inhibition attenuates brain edema after surgical brain injury. Acta Neurochir Suppl 102:357–361 [DOI] [PubMed] [Google Scholar]
- 113.Papadopoulos MC, Verkman AS (2008) Potential utility of aquaporin modulators for therapy of brain disorders. Prog Brain Res 170:589–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Qiu B, Li X, Sun X, Wang Y, Jing Z, Zhang X, Wang Y (2014) Overexpression of aquaporin1 aggravates hippocampal damage in mouse traumatic brain injury models. Mol Med Rep 9:916–922 [DOI] [PubMed] [Google Scholar]
- 115.Ding JY, Kreipke CW, Speirs SL, Schafer P, Schafer S, Rafols JA (2009) Hypoxia-inducible factor-1alpha signaling in aquaporin upregulation after traumatic brain injury. Neurosci Lett 453:68–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS (2000) Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med 6:159–163 [DOI] [PubMed] [Google Scholar]
- 117.Fazzina G, Amorini AM, Marmarou CR, Fukui S, Okuno K, Dunbar JG, Glisson R, Marmarou A et al. (2010) The protein kinase C activator phorbol myristate acetate decreases brain edema by aquaporin 4 downregulation after middle cerebral artery occlusion in the rat. J Neurotrauma 27:453–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Taya K, Gulsen S, Okuno K, Prieto R, Marmarou CR, Marmarou A (2008) Modulation of AQP4 expression by the selective V1a receptor antagonist, SR49059, decreases trauma-induced brain edema. Acta Neurochir Suppl 102:425–429 [DOI] [PubMed] [Google Scholar]
- 119.Agre P, Kozono D (2003) Aquaporin water channels: molecular mechanisms for human diseases. FEBS Lett 555:72–78 [DOI] [PubMed] [Google Scholar]
- 120.Pasantes-Morales H, Cruz-Rangel S (2010) Brain volume regulation: osmolytes and aquaporin perspectives. Neuroscience 168: 871–884 [DOI] [PubMed] [Google Scholar]
- 121.Nicchia GP, Nico B, Camassa LM, Mola MG, Loh N, Dermietzel R, Spray DC, Svelto M et al. (2004) The role of aquaporin-4 in the blood-brain barrier development and integrity: studies in animal and cell culture models. Neuroscience 129:935–945 [DOI] [PubMed] [Google Scholar]
- 122.Fukuda AM, Badaut J (2012) Aquaporin 4: a player in cerebral edema and neuroinflammation. J Neuroinflammation 9:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yong VW, Krekoski CA, Forsyth PA, Bell R, Edwards DR (1998) Matrix metalloproteinases and diseases of the CNS. Trends Neurosci 21:75–80 [DOI] [PubMed] [Google Scholar]
- 124.Hartung HP, Kieseier BC (2000) The role of matrix metallopro teinases in autoimmune damage to the central and peripheral nervous system. J Neuroimmunol 107:140–147 [DOI] [PubMed] [Google Scholar]
- 125.Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH (2000) Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab 20:1681–1689 [DOI] [PubMed] [Google Scholar]
- 126.Manicone AM, McGuire JK (2008) Matrix metalloproteinases as modulators of inflammation. Semin Cell Dev Biol 19:34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hu J, Van den Steen PE, Sang QX, Opdenakker G (2007) Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov 6:480–498 [DOI] [PubMed] [Google Scholar]
- 128.Konnecke H, Bechmann I (2013) The role of microglia and matrix metalloproteinases involvement in neuroinflammation and gliomas. Clin Dev Immunol 2013:914104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Allan SM, Tyrrell PJ, Rothwell NJ (2005) Interleukin-1 and neuronal injury. Nat Rev Immunol 5:629–640 [DOI] [PubMed] [Google Scholar]
- 130.Schmidt JA (1984) Purification and partial biochemical characterization of normal human interleukin 1. J Exp Med 160:772–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM et al. (1992) A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 356:768–774 [DOI] [PubMed] [Google Scholar]
- 132.Nylander-Lundqvist E, Back O, Egelrud T (1996) IL-1 beta activation in human epidermis. J Immunol 157:1699–1704 [PubMed] [Google Scholar]
- 133.Fantuzzi G, Ku G, Harding MW, Livingston DJ, Sipe JD, Kuida K, Flavell RA, Dinarello CA (1997) Response to local inflammation of IL-1 beta-converting enzyme-deficient mice. J Immunol 158:1818–1824 [PubMed] [Google Scholar]
- 134.Ito A, Mukaiyama A, Itoh Y, Nagase H, Thogersen IB, Enghild JJ, Sasaguri Y, Mori Y (1996) Degradation of interleukin 1beta by matrix metalloproteinases. J Biol Chem 271:14657–14660 [DOI] [PubMed] [Google Scholar]
- 135.Schonbeck U, Mach F, Libby P (1998) Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J Immunol 161: 3340–3346 [PubMed] [Google Scholar]
- 136.Gottschall PE, Yu X (1995) Cytokines regulate gelatinase A and B (matrix metalloproteinase 2 and 9) activity in cultured rat astrocytes. J Neurochem 64:1513–1520 [DOI] [PubMed] [Google Scholar]
- 137.Gidday JM, Gasche YG, Copin JC, Shah AR, Perez RS, Shapiro SD, Chan PH, Park TS (2005) Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol 289:H558–568 [DOI] [PubMed] [Google Scholar]
- 138.Suehiro E, Fujisawa H, Akimura T, Ishihara H, Kajiwara K, Kato S, Fujii M, Yamashita S et al. (2004) Increased matrix metalloproteinase-9 in blood in association with activation of interleukin-6 after traumatic brain injury: influence of hypothermic therapy. J Neurotrauma 21:1706–1711 [DOI] [PubMed] [Google Scholar]
- 139.Mohan MJ, Seaton T, Mitchell J, Howe A, Blackburn K, Burkhart W, Moyer M, Patel I et al. (2002) The tumor necrosis factor-alpha converting enzyme (TACE): a unique metalloproteinase with highly defined substrate selectivity. Biochemistry 41:9462–9469 [DOI] [PubMed] [Google Scholar]
- 140.Haro H, Crawford HC, Fingleton B, Shinomiya K, Spengler DM, Matrisian LM (2000) Matrix metalloproteinase-7-dependent release of tumor necrosis factor-alpha in a model of herniated disc resorption. J Clin Invest 105:143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lohi J, Wilson CL, Roby JD, Parks WC (2001) Epilysin, a novel human matrix metalloproteinase (MMP-28) expressed in testis and keratinocytes and in response to injury. J Biol Chem 276: 10134–10144 [DOI] [PubMed] [Google Scholar]
- 142.Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson AH, Drummond AH, Galloway WA et al. (1994) Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature 370:555–557 [DOI] [PubMed] [Google Scholar]
- 143.English WR, Puente XS, Freije JM, Knauper V, Amour A, Merryweather A, Lopez-Otin C, Murphy G (2000) Membrane type 4 matrix metalloproteinase (MMP17) has tumor necrosis factor-alpha convertase activity but does not activate pro MMP2. J Biol Chem 275:14046–14055 [DOI] [PubMed] [Google Scholar]
- 144.Xie X, Wu X, Cui J, Li H, Yan X (2013) Increase ICAM-1 and LFA-1 expression by cerebrospinal fluid of subarachnoid hemorrhage patients: involvement of TNF-alpha. Brain Res 1512:89–96 [DOI] [PubMed] [Google Scholar]
- 145.Lee IT, Lin CC, Wu YC, Yang CM (2010) TNF-alpha induces matrix metalloproteinase-9 expression in A549 cells: role of TNFR1/TRAF2/PKCalpha-dependent signaling pathways. J Cell Physiol 224:454–464 [DOI] [PubMed] [Google Scholar]
- 146.Flanders KC, Ren RF, Lippa CF (1998) Transforming growth factor-betas in neurodegenerative disease. Prog Neurobiol 54: 71–85 [DOI] [PubMed] [Google Scholar]
- 147.Massague J (2000) How cells read TGF-beta signals. Nat Rev Mol Cell Biol 1:169–178 [DOI] [PubMed] [Google Scholar]
- 148.Bottner M, Krieglstein K, Unsicker K (2000) The transforming growth factor-betas: structure, signaling, and roles in nervous system development and functions. J Neurochem 75:2227–2240 [DOI] [PubMed] [Google Scholar]
- 149.Rimaniol AC, Lekieffre D, Serrano A, Masson A, Benavides J, Zavala F (1995) Biphasic transforming growth factor-beta production flanking the pro-inflammatory cytokine response in cerebral trauma. Neuroreport 7:133–136 [PubMed] [Google Scholar]
- 150.Morganti-Kossmann MC, Rancan M, Stahel PF, Kossmann T (2002) Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr Opin Crit Care 8:101–105 [DOI] [PubMed] [Google Scholar]
- 151.Yu Q, Stamenkovic I (2000) Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev 14:163–176 [PMC free article] [PubMed] [Google Scholar]
- 152.Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC et al. (2002) The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol 157:493–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Han YP, Tuan TL, Hughes M, Wu H, Garner WL (2001) Transforming growth factor-beta- and tumor necrosis factor-alpha-mediated induction and proteolytic activation of MMP-9 in human skin. J Biol Chem 276:22341–22350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gordon GM, Ledee DR, Feuer WJ, Fini ME (2009) Cytokines and signaling pathways regulating matrix metalloproteinase-9 (MMP-9) expression in corneal epithelial cells. J Cell Physiol 221:402–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Thompson CB (1995) Apoptosis in the pathogenesis and treatment of disease. Science 267:1456–1462 [DOI] [PubMed] [Google Scholar]
- 156.Mannello F, Luchetti F, Falcieri E, Papa S (2005) Multiple roles of matrix metalloproteinases during apoptosis. Apoptosis 10:19–24 [DOI] [PubMed] [Google Scholar]
- 157.Zhang K, McQuibban GA, Silva C, Butler GS, Johnston JB, Holden J, Clark-Lewis I, Overall CM et al. (2003) HIV-induced metalloproteinase processing of the chemokine stromal cell derived factor-1 causes neurodegeneration. Nat Neurosci 6:1064–1071 [DOI] [PubMed] [Google Scholar]
- 158.Copin JC, Goodyear MC, Gidday JM, Shah AR, Gascon E, Dayer A, Morel DM, Gasche Y (2005) Role of matrix metalloproteinases in apoptosis after transient focal cerebral ischemia in rats and mice. Eur J Neurosci 22:1597–1608 [DOI] [PubMed] [Google Scholar]
- 159.Kwan JA, Schulze CJ, Wang W, Leon H, Sariahmetoglu M, Sung M, Sawicka J, Sims DE et al. (2004) Matrix metalloproteinase-2 (MMP-2) is present in the nucleus of cardiac myocytes and is capable of cleaving poly (ADP-ribose) polymerase (PARP) in vitro. FASEB J 18:690–692 [DOI] [PubMed] [Google Scholar]
- 160.Lee R, Kermani P, Teng KK, Hempstead BL (2001) Regulation of cell survival by secreted proneurotrophins. Science 294:1945–1948 [DOI] [PubMed] [Google Scholar]
- 161.Chintala SK, Zhang X, Austin JS, Fini ME (2002) Deficiency in matrix metalloproteinase gelatinase B (MMP-9) protects against retinal ganglion cell death after optic nerve ligation. J Biol Chem 277:47461–47468 [DOI] [PubMed] [Google Scholar]
- 162.Wang X, Lee SR, Arai K, Lee SR, Tsuji K, Rebeck GW, Lo EH (2003) Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med 9: 1313–1317 [DOI] [PubMed] [Google Scholar]
- 163.Meerovitch K, Bergeron F, Leblond L, Grouix B, Poirier C, Bubenik M, Chan L, Gourdeau H et al. (2003) A novel RGD antagonist that targets both alphavbeta3 and alpha5beta1 induces apoptosis of angiogenic endothelial cells on type I collagen. Vascul Pharmacol 40:77–89 [DOI] [PubMed] [Google Scholar]
- 164.Lee SR, Lo EH (2004) Induction of caspase-mediated cell death by matrix metalloproteinases in cerebral endothelial cells after hypoxia-reoxygenation. J Cereb Blood Flow Metab 24:720–727 [DOI] [PubMed] [Google Scholar]
- 165.Strand S, Vollmer P, van den Abeelen L, Gottfried D, Alla V, Heid H, Kuball J, Theobald M et al. (2004) Cleavage of CD95 by matrix metalloproteinase-7 induces apoptosis resistance in tumour cells. Oncogene 23:3732–3736 [DOI] [PubMed] [Google Scholar]
- 166.Boulay A, Masson R, Chenard MP, El Fahime M, Cassard L, Bellocq JP, Sautes-Fridman C, Basset P et al. (2001) High cancer cell death in syngeneic tumors developed in host mice deficient for the stromelysin-3 matrix metalloproteinase. Cancer Res 61:2189–2193 [PubMed] [Google Scholar]
- 167.Sang QX, Jin Y, Newcomer RG, Monroe SC, Fang X, Hurst DR, Lee S, Cao Q et al. (2006) Matrix metalloproteinase inhibitors as prospective agents for the prevention and treatment of cardiovascular and neoplastic diseases. Curr Top Med Chem 6:289–316 [DOI] [PubMed] [Google Scholar]
- 168.Will H, Atkinson SJ, Butler GS, Smith B, Murphy G (1996) The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiates autoproteolytic activation. Regulation by TIMP-2 and TIMP-3. J Biol Chem 271:17119–17123 [DOI] [PubMed] [Google Scholar]
- 169.Murphy G (2011) Tissue inhibitors of metalloproteinases. Genome Biol 12:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rivera S, Khrestchatisky M, Kaczmarek L, Rosenberg GA, Jaworski DM (2010) Metzincin proteases and their inhibitors: foes or friends in nervous system physiology? J Neurosci 30:15337–15357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Greene J, Wang M, Liu YE, Raymond LA, Rosen C, Shi YE (1996) Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. J Biol Chem 271:30375–30380 [DOI] [PubMed] [Google Scholar]
- 172.Rao BG (2005) Recent developments in the design of specific matrix metalloproteinase inhibitors aided by structural and computational studies. Curr Pharm Des 11:295–322 [DOI] [PubMed] [Google Scholar]
- 173.Benjamin MM, Khalil RA (2012) Matrix metalloproteinase inhibitors as investigative tools in the pathogenesis and management of vascular disease. EXS 103:209–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yamaguchi M, Jadhav V, Obenaus A, Colohan A, Zhang JH (2007) Matrix metalloproteinase inhibition attenuates brain edema in an in vivo model of surgically-induced brain injury. Neurosurgery 61:1067–1075, discussion 1075–1066 [DOI] [PubMed] [Google Scholar]
- 175.Dal-Pizzol F, Rojas HA, dos Santos EM, Vuolo F, Constantino L, Feier G, Pasquali M, Comim CM et al. (2013) Matrix metalloproteinase-2 and metalloproteinase-9 activities are associated with blood-brain barrier dysfunction in an animal model of severe sepsis. Mol Neurobiol 48:62–70 [DOI] [PubMed] [Google Scholar]
- 176.Barichello T, Generoso JS, Michelon CM, Simoes LR, Elias SG, Vuolo F, Comim CM, Dal-Pizzol F et al. (2014) Inhibition of matrix metalloproteinases-2 and −9 prevents cognitive impairment induced by pneumococcal meningitis in Wistar rats. Exp Biol Med (Maywood) 239:225–231 [DOI] [PubMed] [Google Scholar]
- 177.Hadass O, Tomlinson BN, Gooyit M, Chen S, Purdy JJ, Walker JM, Zhang C, Giritharan AB et al. (2013) Selective inhibition of matrix metalloproteinase-9 attenuates secondary damage resulting from severe traumatic brain injury. PLoS One 8:e76904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nagel S, Heinemann PV, Heiland S, Koziol J, Gardner H, Wagner S (2011) Selective MMP-inhibition with Ro 28–2653 in acute experimental stroke—a magnetic resonance imaging efficacy study. Brain Res 1368:264–270 [DOI] [PubMed] [Google Scholar]
- 179.Vandenbroucke RE, Libert C (2014) Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat Rev Drug Discov 13:904–927 [DOI] [PubMed] [Google Scholar]
- 180.Verslegers M, Lemmens K, Van Hove I, Moons L (2013) Matrix metalloproteinase-2 and −9 as promising benefactors in development, plasticity and repair of the nervous system. Prog Neurobiol 105:60–78 [DOI] [PubMed] [Google Scholar]
- 181.Khokha R, Murthy A, Weiss A (2013) Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat Rev Immunol 13:649–665 [DOI] [PubMed] [Google Scholar]
- 182.Martens E, Leyssen A, Van Aelst I, Fiten P, Piccard H, Hu J, Descamps FJ, Van den Steen PE et al. (2007) A monoclonal antibody inhibits gelatinase B/MMP-9 by selective binding to part of the catalytic domain and not to the fibronectin or zinc binding domains. Biochim Biophys Acta 1770:178–186 [DOI] [PubMed] [Google Scholar]
- 183.Devy L, Huang L, Naa L, Yanamandra N, Pieters H, Frans N, Chang E, Tao Q et al. (2009) Selective inhibition of matrix metalloproteinase-14 blocks tumor growth, invasion, and angiogenesis. Cancer Res 69:1517–1526 [DOI] [PubMed] [Google Scholar]
- 184.Devy L, Dransfield DT (2011) New strategies for the next generation of matrix-metalloproteinase inhibitors: selectively targeting membrane-anchored MMPs with therapeutic antibodies. Biochem Res Int 2011:191670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shiryaev SA, Remacle AG, Golubkov VS, Ingvarsen S, Porse A, Behrendt N, Cieplak P, Strongin AY (2013) A monoclonal antibody interferes with TIMP-2 binding and incapacitates the MMP-2-activating function of multifunctional, pro-tumorigenic MMP-14/MT1-MMP. Oncogenesis 2:e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Devel L, Czarny B, Beau F, Georgiadis D, Stura E, Dive V (2010) Third generation of matrix metalloprotease inhibitors: gain in selectivity by targeting the depth of the S1′ cavity. Biochimie 92: 1501–1508 [DOI] [PubMed] [Google Scholar]
- 187.Krekoski CA, Neubauer D, Graham JB, Muir D (2002) Metalloproteinase-dependent predegeneration in vitro enhances axonal regeneration within acellular peripheral nerve grafts. J Neurosci 22:10408–10415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sawicki G, Salas E, Murat J, Miszta-Lane H, Radomski MW (1997) Release of gelatinase A during platelet activation mediates aggregation. Nature 386:616–619 [DOI] [PubMed] [Google Scholar]
- 189.Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V (1997) Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science 277:225–228 [DOI] [PubMed] [Google Scholar]
- 190.Song J, Wu C, Zhang X, Sorokin LM (2013) In vivo processing of CXCL5 (LIX) by matrix metalloproteinase (MMP)-2 and MMP-9 promotes early neutrophil recruitment in IL-1beta-induced peritonitis. J Immunol 190:401–410 [DOI] [PubMed] [Google Scholar]
- 191.Rundhaug JE (2005) Matrix metalloproteinases and angiogenesis. J Cell Mol Med 9:267–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kyriakides TR, Wulsin D, Skokos EA, Fleckman P, Pirrone A, Shipley JM, Senior RM, Bornstein P (2009) Mice that lack matrix metalloproteinase-9 display delayed wound healing associated with delayed reepithelialization and disordered collagen fibrillogenesis. Matrix Biol 28:65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ikonomidis JS, Barbour JR, Amani Z, Stroud RE, Herron AR, McClister DM Jr, Camens SE, Lindsey ML et al. (2005) Effects of deletion of the matrix metalloproteinase 9 gene on development of murine thoracic aortic aneurysms. Circulation 112:I242–248 [DOI] [PubMed] [Google Scholar]
- 194.Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG, Parks WC (1997) The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. J Cell Biol 137:1445–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ragin AB, Wu Y, Ochs R, Scheidegger R, Cohen BA, McArthur JC, Epstein LG, Conant K (2009) Serum matrix metalloproteinase levels correlate with brain injury in human immunodeficiency virus infection. J Neurovirol 15:275–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Owen CA, Hu Z, Lopez-Otin C, Shapiro SD (2004) Membrane bound matrix metalloproteinase-8 on activated polymorphonuclear cells is a potent, tissue inhibitor of metalloproteinase-resistant collagenase and serpinase. J Immunol 172:7791–7803 [DOI] [PubMed] [Google Scholar]
- 197.Stadlmann S, Pollheimer J, Moser PL, Raggi A, Amberger A, Margreiter R, Offner FA, Mikuz G et al. (2003) Cytokine regulated expression of collagenase-2 (MMP-8) is involved in the progression of ovarian cancer. Eur J Cancer 39:2499–2505 [DOI] [PubMed] [Google Scholar]
- 198.Holliday LS, Welgus HG, Fliszar CJ, Veith GM, Jeffrey JJ, Gluck SL (1997) Initiation of osteoclast bone resorption by interstitial collagenase. J Biol Chem 272:22053–22058 [DOI] [PubMed] [Google Scholar]
- 199.Whitelock JM, Murdoch AD, Iozzo RV, Underwood PA (1996) The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin, and heparanases. J Biol Chem 271:10079–10086 [DOI] [PubMed] [Google Scholar]
- 200.Stolow MA, Bauzon DD, Li J, Sedgwick T, Liang VC, Sang QA,Shi YB (1996) Identification and characterization of a novel collagenase in Xenopus laevis: possible roles during frog development. Mol Biol Cell 7:1471–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Boudreau N, Sympson CJ, Werb Z, Bissell MJ (1995) Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science 267:891–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sympson CJ, Talhouk RS, Alexander CM, Chin JR, Clift SM,Bissell MJ, Werb Z (1994) Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J Cell Biol 125:681–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Noe V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, Bruyneel E, Matrisian LM et al. (2001) Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci 114:111–118 [DOI] [PubMed] [Google Scholar]
- 204.Gill JH, Kirwan IG, Seargent JM, Martin SW, Tijani S, Anikin VA, Mearns AJ, Bibby MC et al. (2004) MMP-10 is overexpressed, proteolytically active, and a potential target for therapeutic intervention in human lung carcinomas. Neoplasia 6: 777–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Unden AB, Sandstedt B, Bruce K, Hedblad M, Stahle-Backdahl M (1996) Stromelysin-3 mRNA associated with myofibroblasts is overexpressed in aggressive basal cell carcinoma and in dermatofibroma but not in dermatofibrosarcoma. J Invest Dermatol 107:147–153 [DOI] [PubMed] [Google Scholar]
- 206.Fukai F, Ohtaki M, Fujii N, Yajima H, Ishii T, Nishizawa Y, Miyazaki K, Katayama T (1995) Release of biological activities from quiescent fibronectin by a conformational change and limited proteolysis by matrix metalloproteinases. Biochemistry 34: 11453–11459 [DOI] [PubMed] [Google Scholar]
- 207.Powell WC, Fingleton B, Wilson CL, Boothby M, Matrisian LM (1999) The metalloproteinase matrilysin proteolytically generates active soluble Fas ligand and potentiates epithelial cell apoptosis. Curr Biol 9:1441–1447 [DOI] [PubMed] [Google Scholar]
- 208.Xu X, Ma J, Li C, Zhao W, Xu Y (2015) Regulation of chondrosarcoma invasion by MMP26. Tumour Biol 36:365–369 [DOI] [PubMed] [Google Scholar]
- 209.Hu Q, Yan C, Xu C, Yan H, Qing L, Pu Y, He Z, Li X (2014) Matrilysin-2 expression in colorectal cancer is associated with overall survival of patients. Tumour Biol 35:3569–3574 [DOI] [PubMed] [Google Scholar]
- 210.Kadono Y, Okada Y, Namiki M, Seiki M, Sato H (1998) Transformation of epithelial Madin-Darby canine kidney cells with p60(v-src) induces expression of membrane-type 1 matrix metalloproteinase and invasiveness. Cancer Res 58:2240–2244 [PubMed] [Google Scholar]
- 211.Koshikawa N, Giannelli G, Cirulli V, Miyazaki K, Quaranta V (2000) Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J Cell Biol 148:615–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Belkin AM, Akimov SS, Zaritskaya LS, Ratnikov BI, Deryugina EI, Strongin AY (2001) Matrix-dependent proteolysis of surface transglutaminase by membrane-type metalloproteinase regulates cancer cell adhesion and locomotion. J Biol Chem 276:18415–18422 [DOI] [PubMed] [Google Scholar]
- 213.Chabottaux V, Sounni NE, Pennington CJ, English WR, van den Brule F, Blacher S, Gilles C, Munaut C et al. (2006) Membrane-type 4 matrix metalloproteinase promotes breast cancer growth and metastases. Cancer Res 66:5165–5172 [DOI] [PubMed] [Google Scholar]
- 214.Komori K, Nonaka T, Okada A, Kinoh H, Hayashita-Kinoh H, Yoshida N, Yana I, Seiki M (2004) Absence of mechanical allodynia and Abeta-fiber sprouting after sciatic nerve injury in mice lacking membrane-type 5 matrix metalloproteinase. FEBS Lett 557:125–128 [DOI] [PubMed] [Google Scholar]
- 215.Sun Q, Weber CR, Sohail A, Bernardo MM, Toth M, Zhao H, Turner JR, Fridman R (2007) MMP25 (MT6-MMP) is highly expressed in human colon cancer, promotes tumor growth, and exhibits unique biochemical properties. J Biol Chem 282:21998–22010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lijnen HR, Ugwu F, Bini A, Collen D (1998) Generation of an angiostatin-like fragment from plasminogen by stromelysin-1 (MMP-3). Biochemistry 37:4699–4702 [DOI] [PubMed] [Google Scholar]
- 217.Sedlacek R, Mauch S, Kolb B, Schatzlein C, Eibel H, Peter HH, Schmitt J, Krawinkel U (1998) Matrix metalloproteinase MMP-19 (RASI-1) is expressed on the surface of activated peripheral blood mononuclear cells and is detected as an autoantigen in rheumatoid arthritis. Immunobiology 198:408–423 [DOI] [PubMed] [Google Scholar]
- 218.Stracke JO, Fosang AJ, Last K, Mercuri FA, Pendas AM, Llano E, Perris R, Di Cesare PE et al. (2000) Matrix metalloproteinases 19 and 20 cleave aggrecan and cartilage oligomeric matrix protein (COMP). FEBS Lett 478:52–56 [DOI] [PubMed] [Google Scholar]
- 219.Hieta N, Impola U, Lopez-Otin C, Saarialho-Kere U, Kahari VM (2003) Matrix metalloproteinase-19 expression in dermal wounds and by fibroblasts in culture. J Invest Dermatol 121:997–1004 [DOI] [PubMed] [Google Scholar]
- 220.Ahokas K, Skoog T, Suomela S, Jeskanen L, Impola U, Isaka K, Saarialho-Kere U (2005) Matrilysin-2 (matrix metalloproteinase-26) is upregulated in keratinocytes during wound repair and early skin carcinogenesis. J Invest Dermatol 124:849–856 [DOI] [PubMed] [Google Scholar]
- 221.Galea CA, Nguyen HM, George Chandy K, Smith BJ, Norton RS (2014) Domain structure and function of matrix metalloprotease 23 (MMP23): role in potassium channel trafficking. Cell Mol Life Sci 71:1191–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Illman SA, Lehti K, Keski-Oja J, Lohi J (2006) Epilysin (MMP-28) induces TGF-beta mediated epithelial to mesenchymal transition in lung carcinoma cells. J Cell Sci 119:3856–3865 [DOI] [PubMed] [Google Scholar]
- 223.Lin HC, Chang JH, Jain S, Gabison EE, Kure T, Kato T, Fukai N, Azar DT (2001) Matrilysin cleavage of corneal collagen type XVIII NC1 domain and generation of a 28-kDa fragment. Invest Ophthalmol Vis Sci 42:2517–2524 [PubMed] [Google Scholar]
- 224.Hashimoto G, Inoki I, Fujii Y, Aoki T, Ikeda E, Okada Y (2002) Matrix metalloproteinases cleave connective tissue growth factor and reactivate angiogenic activity of vascular endothelial growth factor 165. J Biol Chem 277:36288–36295 [DOI] [PubMed] [Google Scholar]
- 225.Imai K, Hiramatsu A, Fukushima D, Pierschbacher MD, Okada Y (1997) Degradation of decorin by matrix metalloproteinases: identification of the cleavage sites, kinetic analyses and transforming growth factor-beta1 release. Biochem J 322(Pt 3):809–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Fowlkes JL, Enghild JJ, Suzuki K, Nagase H (1994) Matrix metalloproteinases degrade insulin-like growth factor-binding protein-3 in dermal fibroblast cultures. J Biol Chem 269:25742–25746 [PubMed] [Google Scholar]
- 227.Patterson BC, Sang QA (1997) Angiostatin-converting enzyme activities of human matrilysin (MMP-7) and gelatinase B/type IV collagenase (MMP-9). J Biol Chem 272:28823–28825 [DOI] [PubMed] [Google Scholar]
- 228.Kajita M, Itoh Y, Chiba T, Mori H, Okada A, Kinoh H, Seiki M (2001) Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J Cell Biol 153:893–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sifringer M, Stefovska V, Zentner I, Hansen B, Stepulak A, Knaute C, Marzahn J, Ikonomidou C (2007) The role of matrix metalloproteinases in infant traumatic brain injury. Neurobiol Dis 25:526–535 [DOI] [PubMed] [Google Scholar]
- 230.Homsi S, Federico F, Croci N, Palmier B, Plotkine M, Marchand-Leroux C, Jafarian-Tehrani M (2009) Minocycline effects on cerebral edema: relations with inflammatory and oxidative stress markers following traumatic brain injury in mice. Brain Res 1291:122–132 [DOI] [PubMed] [Google Scholar]
- 231.Hirose T, Matsumoto N, Tasaki O, Nakamura H, Akagaki F, Shimazu T (2013) Delayed progression of edema formation around a hematoma expressing high levels of VEGF and mmp-9 in a patient with traumatic brain injury: case report. Neurol Med Chir (Tokyo) 53:609–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Shigemori Y, Katayama Y, Mori T, Maeda T, Kawamata T (2006) Matrix metalloproteinase-9 is associated with blood-brain barrier opening and brain edema formation after cortical contusion in rats. Acta Neurochir Suppl 96:130–133 [DOI] [PubMed] [Google Scholar]
- 233.Jia F, Pan YH, Mao Q, Liang YM, Jiang JY (2010) Matrix metalloproteinase-9 expression and protein levels after fluid percussion injury in rats: the effect of injury severity and brain temperature. J Neurotrauma 27:1059–1068 [DOI] [PubMed] [Google Scholar]
- 234.Mori T, Wang X, Aoki T, Lo EH (2002) Downregulation of matrix metalloproteinase-9 and attenuation of edema via inhibition of ERK mitogen activated protein kinase in traumatic brain injury. J Neurotrauma 19:1411–1419 [DOI] [PubMed] [Google Scholar]
- 235.Ralay Ranaivo H, Zunich SM, Choi N, Hodge JN, Wainwright MS (2011) Mild stretch-induced injury increases susceptibility to interleukin-1beta-induced release of matrix metalloproteinase-9 from astrocytes. J Neurotrauma 28:1757–1766 s [DOI] [PubMed] [Google Scholar]
- 236.Muradashvili N, Benton RL, Saatman KE, Tyagi SC, Lominadze D (2015) Ablation of matrix metalloproteinase-9 gene decreases cerebrovascular permeability and fibrinogen deposition post traumatic brain injury in mice. Metab Brain Dis 30:411–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wei M, Li H, Shang Y, Zhou Z, Zhang J (2014) Increased CD147 (EMMPRIN) expression in the rat brain following traumatic brain injury. Brain Res 1585:150–158 [DOI] [PubMed] [Google Scholar]
- 238.Roberts DJ, Jenne CN, Leger C, Kramer AH, Gallagher CN, Todd S, Parney IF, Doig CJ et al. (2013) Association between the cerebral inflammatory and matrix metalloproteinase responses after severe traumatic brain injury in humans. J Neurotrauma 30: 1727–1736 [DOI] [PubMed] [Google Scholar]
- 239.Li RB, Guo XC, Liang HX, Wang FY, Zhu BL (2009) Study on changes of MMP-3 expression after brain contusion in rats. Leg Med (Tokyo) 11 Suppl 1:S176–179 [DOI] [PubMed] [Google Scholar]


