Abstract
The oncogenic potential of human polyomavirus JC (JCV), a ubiquitous virus that establishes infection during early childhood in approximately 70% of the human population, is unclear. As a neurotropic virus, JCV has been implicated in pediatric central nervous system tumors and has been suggested to be a pathogenic agent in pediatric acute lymphoblastic leukemia. Recent studies have demonstrated JCV gene sequences in pediatric medulloblastomas and among patients with colorectal cancer. JCV early protein T-antigen (TAg) can form complexes with cellular regulatory proteins and thus may play a role in tumorigenesis. Since JCV is detected in B-lymphocytes, a retrospective analysis of pediatric B-cell and non-B-cell malignancies as well as other HIV-associated pediatric malignancies was conducted for the presence of JCV gene sequences. DNA was extracted from 49 pediatric malignancies, including Hodgkin disease, non-Hodgkin lymphoma, large cell lymphoma and sarcoma. Polymerase chain reaction (PCR) was conducted using JCV specific nested-primer sets for the transcriptional control region (TCR), TAg, and viral capsid protein 1 (VP1) genes. Southern blot analysis and DNA sequencing were used to confirm specificity of the amplicons. A 215-bp region of the JCV VP1 gene was amplified from 26 (53%) pediatric tumor tissues. The JCV TCR and two JCV gene regions were amplified from a leiomyosarcoma specimen from an HIV-infected patient. The leiomyosarcoma specimen from the cecum harbored the archetype strain of JCV. Including the leiomyosarcoma specimen, three of five specimens sequenced were typed as JCV genotype 2. The failure to amplify JCV TCR, and TAg gene sequences in the presence of JCV VP1 gene sequence is surprising. Even though JCV TAg gene, which is similar to the SV40 TAg gene, is oncogenic in animal models, the presence of JCV gene sequences in pediatric malignancies does not prove causality. In light of the available data on the presence of JCV in normal and cancerous colon epithelial tissue and our data on amplification of JCV from the cecum of an HIV-infected pediatric patient, further studies are warranted on the role of colon epithelium in the pathogenesis of JCV infection.
Keywords: SV40, BKV, JCV, Medulloblastoma, HIV, AIDS, Leiomyosarcoma, Genotypes
INTRODUCTION
Human polyomavirus JC (JCV), a ubiquitous virus which infects a majority of children at an early age without any sequelae, has been implicated in several human diseases (1–4). JCV is the etiologic agent of the fatal neurological disease, progressive multifocal leukoencephalopathy (PML) (5, 6). JCV has been detected in the brains of PML patients and in the urine of healthy individuals. Data demonstrating the presence of JCV in lymphocytes, tonsils and lungs from non-PML patients and healthy individuals are inconsistent (7–9). Epidemiological and theoretical data have implicated JCV as a pathogenic agent in childhood acute lymphoblastic leukemia (ALL) (10). This latter suggestion has not been substantiated despite the rationale that the viral characteristics fulfill the criteria as a possible etiologic agent for B-cell precursor ALL in children (10–12). In other pediatric malignancies, more convincing data exist demonstrating the presence of JCV gene sequences in solid tumors, and implicating a pathogenic role of the virus, namely a subset of childhood cancers associated with the nervous system (13). JCV and the related polyomavirus BK (BKV), have been detected in childhood medulloblastoma and neuroblastoma, respectively (13–15).
In two studies involving childhood B-cell precursor ALL, no JCV DNA sequences were found in any of the specimens (11, 12). As a follow up to the theoretical considerations of JCV in B-cell ALL, we undertook the present study to look for JCV sequences in other childhood malignancies, specifically solid B-cell and non-B-cell tumors (10). Our data demonstrate high prevalence of JCV viral protein 1 (VP1) gene sequence in pediatric patients with malignancies, in the absence of transcriptional control region (TCR) and early protein gene sequences. Additionally, this study demonstrates for the first time the presence of the JCV genome in a leiomyosarcoma excised from the cecum of an HIV-positive pediatric patient.
MATERIALS AND METHODS
Patient Specimens and DNA Isolation
Tumor specimens from 40 HIV-negative and nine HIV-positive pediatric patients were obtained from the Pediatric Division of the National Cancer Institute-sponsored Cooperative Human Tissue Network (CHTN) in Columbus, OH, and from the NCI-sponsored AIDS Cancer and Specimen Bank (ACSB), Bethesda, MD, respectively, under guidelines approved by the University of Hawaii Committee on Human Subjects. The 49 solid tumors consist of non-Hodgkin lymphoma not otherwise specified (15), Hodgkin disease (15), leiomyosarcoma (4), large cell lymphoma (11), leukemia (2), Burkitt’s lymphoma (1) and undifferentiated tumor (1) (Table 1). Of the four leiomyosarcoma one each was from the cecum and bronchus and two were from the liver. The respective tissue bank pathologist centrally reviewed the histological diagnoses reported by the pathologists at the local institutions where the tissues were obtained. All HIV-positive specimens were paraffin-embedded, whereas HIV-negative specimens were frozen.
Table 1.
Prevalence of JCV Gene Sequences m Pediatric Tumors
| VP1 |
TAg |
TCR |
||||
|---|---|---|---|---|---|---|
| Tumor type | HIV+ | HIV− | HIV+ | HIV− | HIV+ | HIV− |
| Leiomyosarcoma (n=4) | 1/1* | 1/3* | 1/1 | 0/3 | 1/1/ | 0/3 |
| Large cell lymphoma (n=11) | 3/4 | 3/7 | 0/4 | 0/7 | 0/4 | 0/7 |
| Hodgkin’s lymphoma (n=15) | 1/1 | 9/14 | 0/1 | 0/14 | 0/1 | 0/14 |
| Non-Hodgkin’s lymphoma (n=15) | 0/0 | 7/15 | 0/0 | 0/15 | 0/0 | 0/15 |
| Burkitt’s lymphoma (n=1) | 1/1 | 0 | 0/1 | 0 | 0/1 | 0 |
| Leukemia (n=2) | 0/2 | 0 | 0/2 | 0 | 0/2 | 0 |
| Undifferentiated (n=l) | 0/0 | 0/1 | 0/0 | 0/1 | 0/0 | 0/1 |
Number HIV positive or negative/total number of HIV positive or negative tumors
Genomic DNA Extraction
Genomic DNA was extracted from frozen tissue or paraffin-embedded blocks using the protocols described below. Briefly, each tissue was minced with sterile instruments and placed in a conical tube with 2 mL digestion buffer (100 mM NaCl, 10 mM Tris-HCl pH 8.0, 25 mM EDTA pH 8.0, 0.5% SDS) and 100 μg/ml proteinase K/mL. The digestion mixture was incubated at 37°C overnight on a rocker and transferred to a 15 mL gel lock tube (5 Prime-3 Prime, Inc., Boulder, CO) for purification. An equal volume of buffered phenol was added to the tube and centrifuged at 2,000 rpm for 20 min followed by an equal volume of 1:1 phenol:chloroform and centrifuged again at 2,000 rpm for 20 min. An equal volume of chloroform: isoamyl alcohol (CIA; 24:1) was added and centrifuged for 20 min at 2,000 rpm. DNA was precipitated from the aqueous phase with 3 M sodium acetate and l00% ethanol, followed by a wash with 70% ethanol. The resulting DNA pellet was dried and reconstituted In H2O. For paraffin-embedded tissue, deparaffinization was performed with 400 μL xylene, centrifuged and repeated 3 times. The specimen was washed three times with 95% ethanol and was then lyophilized and digested. Digestion was performed in 100 mM Tris-Cl, 40 mM EDTA, 10 mM NaCl, pH 8.0, with 1% SDS and 500 μ/mL proteinase K at 50°C for 16 hr with occasional agitation and/or quick vortexing. Additional proteinase K and SDS were added at a concentration of 1 mg/mL and 2% respectively, and incubated for an additional 24 hr at 50°C. DNA was then extracted with phenol:CIA (25:24:1), ethanol precipitated and resuspended in H2O.
Positive control DNA was extracted from a urine cell pellet of a healthy individual excreting JCV. The positive control was verified by Southern hybridization and sequencing. A negative control sample, was prepared by substituting water for the DNA. Moreover, to avoid polymerase chain reaction (PCR) cross-contamination, pre-PCR and nested PCR were performed in a hood that was exposed to UV prior to the experiment. Additionally, aerosol-resistant tips were used throughout the PCR procedure, including gel loading and sequencing. Pre-PCR, PCR set up and electrophoresis experiments were performed in three physically separated rooms. Individuals, who had never performed JCV-related PCR assays, extracted genomic DNA in a different facility on campus.
The purity and integrity of the tumor tissue DNA was assessed by spectrophotometry, using 260/280 absorbance ratio and by PCR amplification of genomic DNA sequences using a primer pair specific for HLA DQ-α (Table 2) (16).
Table 2.
Prime Sequences and Cycling Conditions for Amplification and Direction of and HLADQα genes
| Gene | Primer Name | Primer Sequences | PCR Cycling Conditions | ||
|---|---|---|---|---|---|
|
Positions |
Temperature and Time |
Cycles | |||
| TAg* | Outer PCR | T1; 2580 – 2602 | 5’-CCAGCTTTACTTAACAGTTGCAG-3’ | 94°C 2 min | 1 |
| T3; 4368 – 4345 | 5’-GGGATGAAGACCTGGTTTGCCATG-3’ | 94°C 30 sec, 60°C 1 min 68°C 2 min | 40 | ||
| 68°C 7 min | 1 | ||||
| Nested PCR | JEP3; 3217 – 3233 | 5’-CCCTTGACTCTGCACCAGTGCC-3’ | 94°C 2 min | 1 | |
| JEP4; 3393 – 3274 | 5’-AGGGGCCAATAGACAGTGGC-3’ | 94°C 30 sec, 60°C 1 min 68°C 2 min | 40 | ||
| 68°C 7 min | 1 | ||||
| VP1* | Outer PCR | JLP15; 1710 – 1724 | 5’-ACAGTGTGGCCAGAATTCCACTACC-3’ | 94°C 1 min | 1 |
| JLP16; 1924 – 1902 | 5’-TAAAGCCTCCCCCCCAACAGAAA-3’ | 63°C 1 min, 94°C 1 min | 50 | ||
| 63°C 5 min | |||||
| Nested PCR | JLP1; 1769 – 1790 | 5’-CTCATGTGGGAGGCTGTKACCT-3’ | 94°C 2 min | 40 | |
| JLP4; 1897 – 1876 | 5’-ATGAAAGCTGGTGCCCTGCACT-3’ | 94°C 30 sec, 63°C 1 min | 1 | ||
| 65°C 5 min | 1 | ||||
| Probe | 1851 – 1868 | 5’-CTCATGACAATGGTGCAG-3’ | |||
| TCR* | Outer PCR | JRR27; 4990 – 5011 | 5’-CTCCCTATTCAGCACTTTGTCC-3’ | 94°C 2 min | 1 |
| JRR28; 331 – 307 | 5’-TCCAGGTTTTACTAACTTTCACAGA-3’ | 94°C 45 sec, 58°C 30 sec 72°C 1 min | 40 | ||
| 72°C 5 min | 1 | ||||
| Nested PCR | JRR1; 5086 – 5110 | 5’-CCTCCACGCCCTTACTACTTCTGAG-3’ | 94°C 2 min | 1 | |
| JRR2; 298 – 274 | 5’-GTGACAGCTGGCGAAGAACCATGGC-3’ | 94°C 45 sec, 58°C 30 sec 72°C 1 min | 40 | ||
| 72°C 5 min | 1 | ||||
| HLADQ-α* | HLA5; 34–51 | 5’-GGTGTAAACTTGTACCAG-3’ | 94°C 3 min | 1 | |
| HLA3; 255–237 | 5’-GGTAGCAGCGGTAGAGTTG-3’ | 94°C 30 sec, 55°C 30 sec 72°C 30 Sec | 40 | ||
| 72°C 5 min | 1 |
Detection of JCV Gene Sequences
JCV gene sequences in tumor DNA was detected using PCR, Southern hybridization, cloning and sequencing. Three JCV gene regions: early large T-antigen (TAg), VP1 and the transcriptional control region TCR, were amplified using nested-PCR (Table 2) (17–19). Genomic DNA extraction, pre-PCR reaction mixture preparation, and PCR and post-PCR analyses were conducted in separate rooms. All experiments were performed in a JCV plasmid DNA-free laboratory. Oligonucleotides were synthesized by the Great American Gene Company (Ramona, CA) (Table 2). Using a 9700 DNA thermal cycler (Perkin Elmer, Branchburg, NJ), outer PCR for JCV TCR, VP1, and TAg genes was performed in a 25-μL reaction mixture containing 0.1 μg genomic DNA, 10 mM Tris-HCl (pH 8.3), 50 mM KCI, 1.5 mM MgCl2, 0.01% (w/v) gelatin, 200 μM each dNTP, 0.6 units Taq Polymerase (Perkin-Elmer), and 200 nM each primer (Table 2). Nested PCR was performed using 1 μL of the outer PCR amplicon and appropriate primers (Table 2). Ten μL of each reaction were resolved on a 2.0% agarose gel (FMC Bioproducts, Rockland, ME) and visualized with ethidium-bromide staining. To ascertain the sensitivity of JCV amplification, JCV cell lines, Ml-HR and M1∆98-XR (20) containing known amounts of JCV plasmid DNA were diluted ten-fold, from 5 X 104 to 5 X 101 copies of JCV. Outer PCR and nested-PCR was conducted as described above using primers specific for JCV TAg, VP1 and TCR genes (Table 2). To avoid JCV plasmid contamination, JCV plasmid DNA was introduced in the laboratory after screening for JCV specific gene sequences from genomic DNA extracted from pediatric patients with malignancies.
Southern Blot Analysis
PCR products of‘ JCV VP1 and TAg coding regions were fractionated on agarose gels and transferred to nylon membranes (Boehringer Mannheim, Indianapolis, IN). DNA, fixed to the membrane by UV cross-linking at 254 nm for 3 min (Stratalinker, Stratagene, LaJolla, CA), was hybridized to a digoxigenin-ddUTP-labeled JCV VP1 or TAg genes specific probe (Table 2) at 54°C for 16 h. Membranes were then washed twice for 5 min each at 54°C with 2 X SSC (0.3 M NaCl, 30 mM Na-Citrate, pH 7.0) containing 0. 1% to SDS (w/v) and twice for 5 min each at 54°C with 0.1 X SSC containing 0.1% SDS. Detection of the digoxigenin-labeled probes with disodium 3-(4-methoxyspiro{1,2-dioxetane-3,2’-(5’-chloro)tricyc1o[3.3.1.13,7]decan}-4-y1)phenyl phosphate) (Boehringer Mannheim, Indianapolis, IN) was performed according to the manufacturer’s instructions. The hybridized membrane was exposed to X-ray film to detect the luminescent signal.
DNA Cloning and Sequencing
Amplified products were subjected to direct sequencing or were first cloned in a plasmid vector and then sequenced. PCR products were purified using the QIAmp PCR purification kit, (Qiagen Inc., Valencia, CA) and sequenced using the Dye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems Inc., Foster City, CA). Alternatively, the PCR products were gel purified using QIAmp PCR purification Kit (Qiagen Inc.), and cloned into vector pCR2.1 (Invitrogen, Carlsbad, CA). Both strands of the plasmid DNA were then sequenced using the BigDye™ Kit (Perkin-Elmer, Branchburg, NJ) in an automated DNA sequencer (Model 377, Applied Biosystems, Inc.).
Identification of JCV Genotypes
The methods for assigning JCV genotypes and subtypes have been previously described (21, 22). The 215-base pair fragment amplified with VP1 primers (Table 2) contains sites that distinguish the major JCV genotypes and subtypes. Sequence alignment was facilitated using the software package available on the VAX computer system, as part of the Genetics Computer Group (GCG).
RESULTS
Prevalence of JCV Gene Sequences
To determine the presence of JCV in a series of childhood solid tumors, we studied 49 pediatric malignancies (nine, 18% from HIV-infected children). UV spectrophotometry and HLA DQα screening of all specimens confirmed the adequate quality of DNA from all specimens. The pathology of the tumor specimens and prevalence of JCV TCR, and TAg, and VP1 gene sequences among pediatric tumors are summarized in Table 1. Amplification using nested VP1 primers resulted in the detection of JCV gene sequences in six of nine (67%) tumors from HIV-positive and 20 of 40 (50%) HIV-VP1-positive tumor specimens (21, 22) (Table 3). Due to lack of sufficient DNA, the VP1 gene was sequenced from six tumors; three each from HIV-negative and –positive patients. Nucleotide sequence analysis revealed that the JCV 107-bp VP1, and 136-bp TAg gene sequences from the negative patients (Fig. 1). To confirm the specificity of the PCR reaction, Southern hybridization was performed on all specimens and sequence analysis was conducted on six of 26 VP1 amplicons. A leiomyosarcoma tumor specimen was positive for JCV TCR and VP1 and TAg gene sequences. Remaining tumor specimens from HIV-positive and -negative patients were negative for the JCV TCR and the TAg gene sequence. Overall, 53% of the tumors were positive for JCV VP1 gene sequence. Of the 26 VP1-positive tumors, 67%, 47% and 54% were classified as Hodgkin disease, non-Hodgkin lymphoma not otherwise specified and large cell lymphoma, respectively (Table 1).
Table 3.
JCA genotyping based on nucleotide substitutions within the 179-bp gene region spanning the VP1 gene
| Malignancy |
HIV Status |
JCV Type |
Nucleotide Position Number |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1771 | 1788 | 1804 | 1818 | 1837 | 1843 | 1850 | 1869 | 1870 | |||
| Hodgkin Disease | Negative | 2 | C | T | T | C | T | T | A | G | A |
| Leiomyosarcoma | Negative | 4 | C | G | T | C | T | T | A | G | A |
| Follicular hyperpiesia | Negative | 1B | C | G | T | G | T | T | G | G | G |
| Hodgkin Disease | Positive | NC | C | G | T | C | T | T | G | G | A |
| Leiomyosarcoma | Positive | 2A | A | G | T | C | T | T | A | G | A |
| Burkitt’s Lymphoima | Positive | 2A | A | N/A | T | C | A | T | A | G | A |
N/A; Not available, NC; Not Classified
Figure 1.
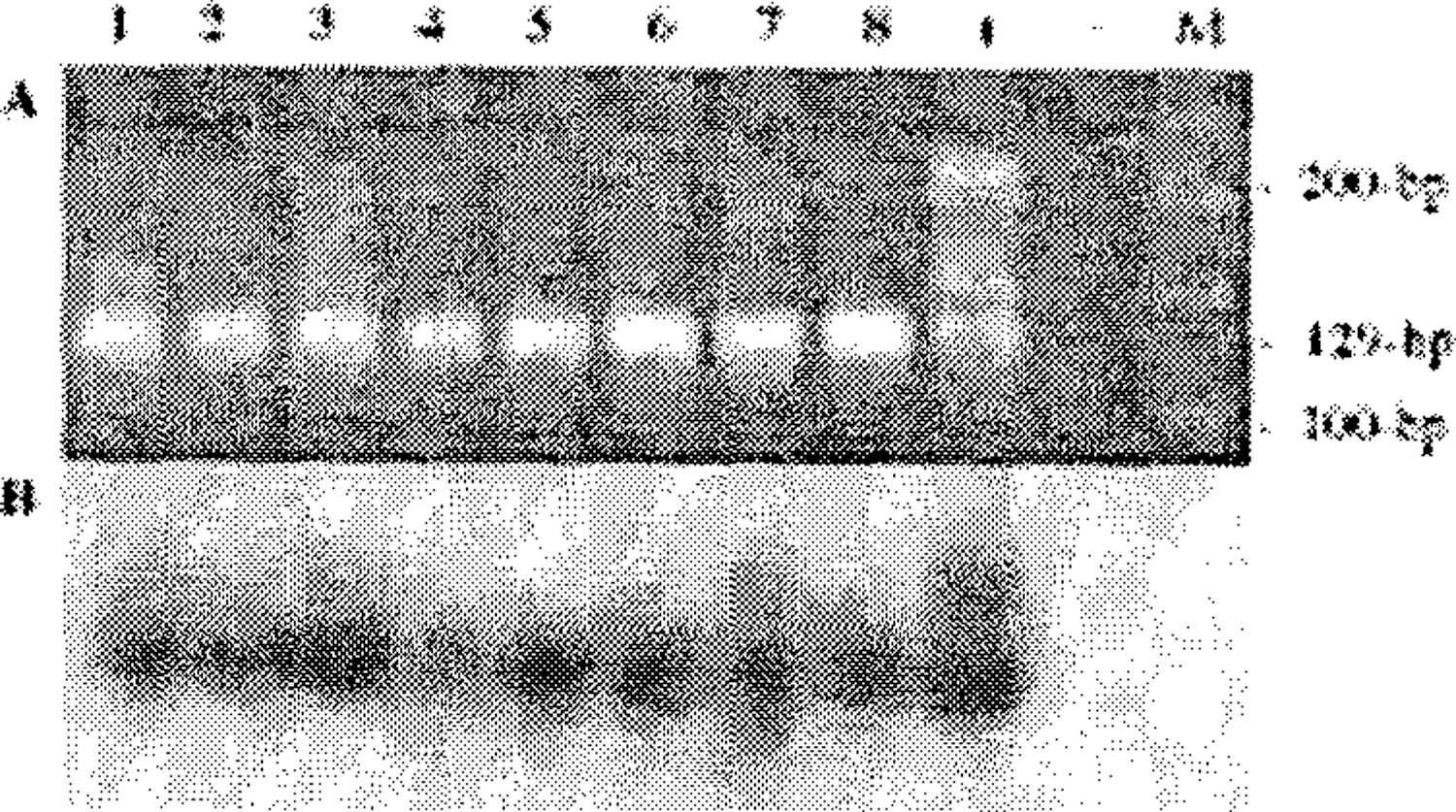
A, Representative ethidium bromide-stained agarose gel showing amplification of 129-bp VP1 mom HIV-l-positive and -negative pediatric patients with malignancies; B, Southern blot hybridization using JCV VP1-specific oligonucleotide probe. (+); DNA extracted from urine of a JCV-positive healthy individual, (-); water control, (M); 100-bp ladder.
Using cell lines containing known amounts of JCV plasmid DNA (control DNA), we were able to amplify 5 X 101 copies of JCV DNA using nested-PCR primers specific for JCV TCR and TAg and VP1 gene regions (Fig. 2). Nested-PCR data using control DNA is shown since all samples were analyzed for all three regions using nested-PCR (Fig. 1, Fig. 2 and Table 1). Similar results were also obtained using outer PCR primers and the aforementioned control DNA (data not shown).
Figure 2.
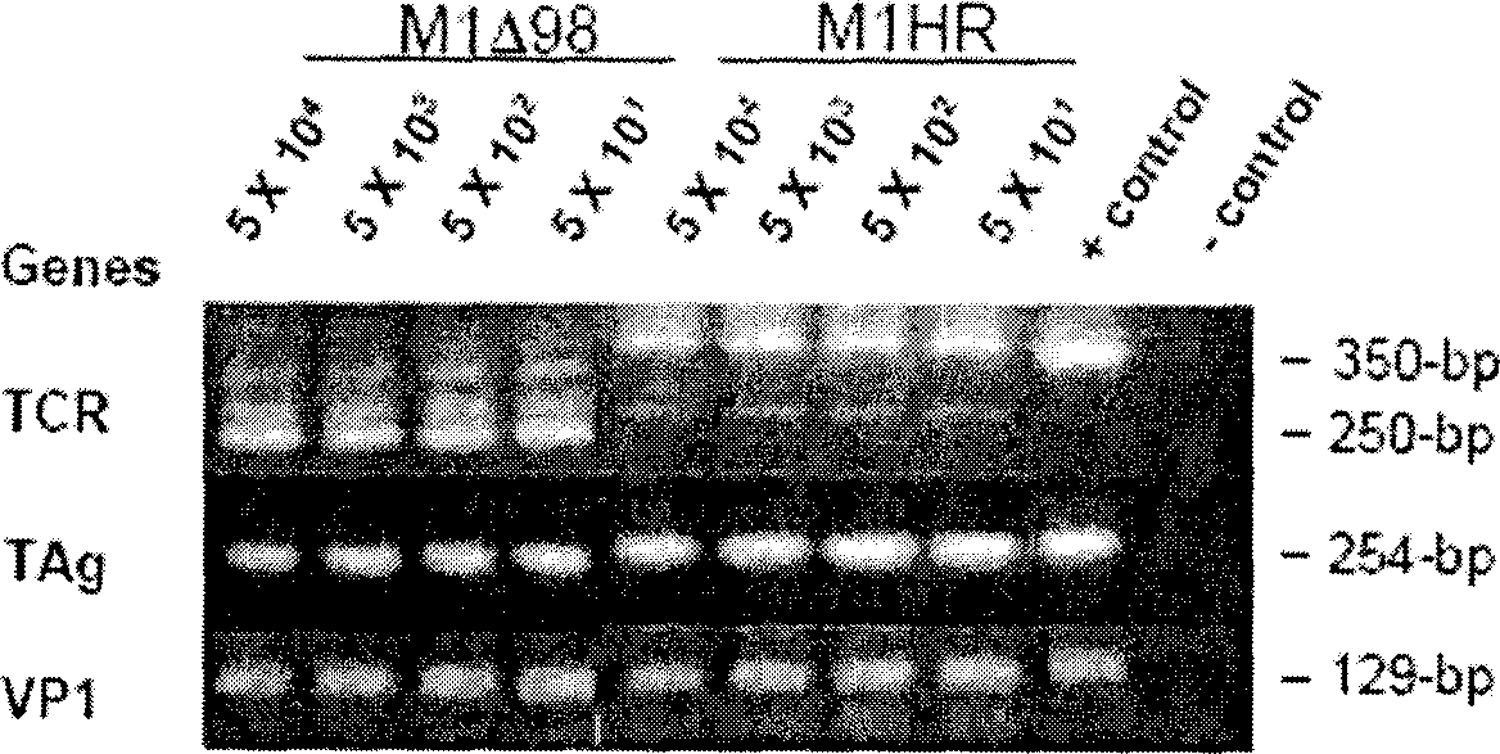
Serial dilutions of JCV plasmid DNA (5 X 104 to 5 X 101 copies) from cell lines M1∆98-XR and M1-HR (20) containing JCV plasmids, were subjected to nested PCR using primers specific for JCV TCR, TAg and VP1 (Table 2). Enzymatically amplified DNA was visualized by ethidium bromide staining on a 2% agarose gel. M1∆98-XR contains a 98-bp deletion in the promoter-enhancer region.
Nucleotide and Sequence Analysis of JCV TCR, and VP1 and TAg and Gene Sequences
Using previously established criteria, JCV VP1 gene region was employed for genotyping leiomyosarcoma specimen were 100% identical to the JCV Type 2A gene sequence (GenBank accession number AF015531). The JCV TCR sequence from the leiomyosarcoma specimen was classified as a JCV archetype sequence and was similar to JCV archetype sequences (GenBank accession numbers; AF2l8440, AF203616 and AF300964) that contain a 5-nucleotide deletion “GGGAA”, at position 227–231 (GenBank accession number NC_001699). Three tumors from HIV-negative patients were genotyped as JCV Type IB, 2 and 4. Of the other three tumors from HIV-positive patients, two were genotyped as JCV Type 2A and one was nontypeable (Table 3). The urine-derived positive control was JCV type 7.
DISCUSSION
The involvement of JCV in human oncogenesis is unclear. In vitro and in vivo animal and human data implicate the JCV TAg in the viral oncogenic process. JCV has been demonstrated to cause tumors after intracranial, intraoccular or intracerebral inoculation of Syrian hamsters, owl monkeys, squirrel monkeys and rats (23–26). A variety of CNS-derived tumors, including medulloblastoma, retinoblastoma, neuroblastoma, malignant astrocytoma, cerebral neuroectodermal tumors and adrenal neuroblastoma, have developed in these animals. JCV TAg is a multifunctional protein that mediates viral DNA replication and exhibits oncogenic activity in cultured cells and animal models (23–25). Several brief clinical reports have demonstrated the presence of JCV in human brain tumors, including oligodendroglioma, CNS lymphoma, glioma, astrocytoma, oligoastrocytoma, medulloblastoma, ependymomas, and choroid plexus papilloma (15, 23–27). Krynska and colleagues, using PCR, have demonstrated JCV early and late gene sequences in 11 of 23 DNA samples extracted from pediatric medulloblastomas (13). Moreover, they demonstrated JCV TAg in the nuclei of four tumor tissues. Recently, Del Valle and colleagues detected JCV TAg by immunohistochemistry in nuclei of 28 of 85 (33%), and JCV TAg gene sequence in 49 of 71 (69%) (28) human brain tumors, respectively.
In contrast, Hayashi and colleagues, using PCR and in-situ hybridization, were unable to detect JCV in medulloblastoma specimens from eight pediatric patients in Japan (29). All eight medulloblastoma specimens were screened using stringent PCR and Southern blotting conditions (29). Similarly, JCV or BKV gene sequences were not detected in five supratentorial primitive neuroectodermal tumors, and 15 primary medulloblastomas using PCR followed by Southern hybridization as well as in a large cohort of human brain tumors (27, 30, 31). Krynska and colleagues (13) demonstrated JCV VP1 gene sequences among 90% of pediatric patients with medulloblastomas. In the present study JCV VP1 gene sequences were found among 21 of 49 (43%) pediatric malignancy specimens (Table 1), although JCV TAg gene sequences were only identified in one leiomyosarcoma specimen from an HIV-positive patient. Del Valle and colleagues, using PCR, Southern hybridization and imunohistochemistry, were unable to detect JCV TAg in pediatric medulloblastoma tumor specimens containing the JCV agnoprotein (15). Similarly, Okamoto and colleagues, were unable to detect JCV DNA sequences in 32 medulloblastomas (27). Moreover, similar to our discordant result of presence of JCV VP1 gene in the absence of the TAg gene, of the five ependymomas positive for JCV DNA sequences, one ependymoma showed a discordant result; positive for the VP1 gene, and negative for the TAg gene. None of the ependymomas displayed immunostaining using JCV T-antigen and VP1 antibodies (27).
A hit-and-run mechanism may be responsible for the lack of JCV TAg gene sequences in our patient specimens, in which only JCV late protein gene sequences are detected. Such a mechanism is hypothesized in several cancers, and for JCV and SV40 oncogenesis (32, 33). As previously suggested, chromosomal instability during the process of tumorigenesis may be responsible for deletion of viral gene regions leading to the inability to detect all viral genes in the same tumor (15, 34–36). Alternatively, as demonstrated by Woods and colleagues, full-length expression of TAg is not a prerequisite for destabilization of the cell genome (37).
In this study, we found a high prevalence of JCV VP1 gene sequences in the absence of JCV TCR, and TAg gene sequences among pediatric malignancy specimens using the nested PCR primers JLP 15/16 and JLP1/4. The amplification of JCV VP1 gene sequence is specific since the primers employed for amplification do not cross-react with the related polyomaviruses, BKV and SV40. The presences of unique JCV VP1 gene sequences with no evidence of viral TCR, or TAg gene sequence is surprising. All oligonucleotide sequences and PCR conditions employed in this study for amplification of JCV are specific and sensitive (Fig. 1 and 2). It is unlikely that the lack of detection of JCV TCR, and TAg gene sequence from the majority of tumor tissues was due to the inability to extract good-quality viral DNA from tumor specimens and/or to detect viral DNA using PCR and Southern blot hybridization. We consistently amplified the housekeeping gene HLA DQ-α from all tumor tissues. Moreover, one leiomyosarcoma DNA specimen that was extracted using the same protocol used for other pediatric tumor specimens was positive for JCV TCR, and Tag and VP1 gene sequences. To test the sensitivity of our PCR primers, after analysis of the tumor specimens, we amplified one JCV genome per cell from JCV cell lines M1∆98-XR and M1-HR (20) using nested-PCR cycling conditions and primers for JCV VP1, TAg and TCR (Table 2 and Fig. 2).
In drawing an analogy to SV40 large tumor antigen which induces chromosomal damage prior to neoplastic transformation, Neel and colleagues speculated that the JCV TAg may induce polyploidy and chromosomal damage, setting the stage for oncogenesis (34). The recent “rouge cell” hypothesis suggests that the rouge cell phenomenon, ‘cultured lymphocytes exhibiting extreme chromosomal damage in the absence of any cause’, results from infection with JCV (34). In this regard, data suggest the presence of JCV TAg gene sequences in the mucosa of normal human colons, colorectal cancer xenografts raised in nude mice, and in the human colon cancer cell line SW480 (38). Laghi and colleagues reported high prevalence of JCV early coding region sequences among 23 pairs of normal and colorectal epithelium and adjacent cancerous tissues (38). Similarly, using PCR, immunohistochemistry and laser capture microdissection assay followed by PCR and Southern hybridization, Enam and colleagues, detected the presence of the JCV genome and viral proteins in 22 of 27 well-characterized epithelial malignant tumors of the large intestine (39). Ricciardiello and colleagues, found JCV TAg gene sequences in mucosal biopsy specimens collected from 25 (76%) patients, who underwent upper or lower gastrointestinal (GI) endoscopic examinations for routine clinical indications (40). Of the 129 specimens collected from the GI tract, 76 (59%) were positive for JCV TAg with no difference between the upper GI tract and colorectum. Moreover, JCV gene sequences were found in all patients with GI neoplasms. The authors suggested that JCV may play a role in chromosomal instability observed in colorectal carcinogenesis.
Our data on the presence of JCV TCR, and TAg, and VP1 gene regions, in the cecum of one HIV-positive pediatric patient with leiomyosarcoma is similar to previous data on the presence of JCV in the upper or lower gastrointestinal tract (38, 40). The pathology of the leiomyosarcoma was consistent with a lesion from the cecum. Previous studies have demonstrated the presence of EBV in leiomyosarcomas from pediatric patients with AIDS (41). This is the first study to show the presence of JCV in leiomyosarcoma tissue excised from the cecum of an HIV-positive pediatric patient. Detection of JCV in this tumor tissue is not sufficient evidence to state that JCV is the cause of the tumor. It is possible that the JCV present in normal colorectal epithelium may be infecting the tumor cells, rather than being the cause of the tumor.
JCV genotyping is based on the coding region that encompasses the early and late genes (42). Previous reports have demonstrated increased prevalence of JCV Type 2 among patients with PML suggesting a role for JCV Type 2 in the pathogenesis of PML (17, 22, 43, 44). Our finding of JCV Type 2 in three of five tumor specimens suggests that JCV Type 2 may be more oncogenic than other JCV types. Further analysis of JCV derived from the tumors will assist us in deciphering the role of JCV genotypes in tumor formation.
Based upon the structure of the TCR, two types of JCV have been identified: the archetypal form which is predominantly detected in kidney and urine and the rearranged form which is predominantly detected in the PML brains (23, 45–47). Ricciardiello and colleagues, have recently demonstrated exclusive presence of the Mad-1 strain (rearranged form) of JCV in human colon and a mutated Mad-1-like JCV in colon cancer tissues (40). In contrast, we have demonstrated the presence of the archetype form of JCV in the cecum of an HIV-positive pediatric patient diagnosed with leiomyosarcoma. Although JCV Tag is oncogenic in animal models, the presence of JCV sequences in pediatric malignancies does not prove that JCV is a human oncogenic virus. Taken together, these data suggest that JCV may play a role in pediatric brain tumors and possibly in colorectal carcinogenesis via chromosomal instability. Further studies, however, are needed to clarify the role of JCV in the pathogenesis of these malignancies.
Acknowledgements -
We thank Mr. Jay Fajardo and Fumiyuki Isami, for technical assistance and Dr. Richard Yanagihara for critical comments. The authors thank the CHTN, Columbus, OH; and ACSB, Bethesda, MD for the specimens. This work was supported by U.S. Public Health Service grants from the Research Centers in Minority Institutions Program, National Center for Research Resources (G12 RR003061), and the National Institute of Neurological Disorders and Stroke (S11 NS41833), National Institutes of Health.
Abbreviations:
- ACSB
AIDS Cancer and Specimen Bank
- ALL
Acute Lyinphoblastic Leukemia
- BKV
polyomavirus BK
- CHTN
Cooperative Human Tissue Network
- GCG
Genetics Computer Group
- PCR
Polymerase Chain Reaction
- PML
Progressive Multifocal Lcukocnccphalopathy
- JCV
Human Polyomavirus JC
- TAg
T-antigen
- TCR
Transcriptional Control Region
- VP1
Viral Protein 1
REFERENCES
- 1.Farge D, Herve R, Mikol J, Sauvaget F, Ingrand D, Singer B, Ferchal F, Auperin I, Gray F and Sudaka A, Simultaneous progressive multifocal leukoencephalopathy, Epstein-Barr virus (EBV) latent infection and cerebral parenchymal infiltration during chronic lymphocytic leukemia. Leukemia 1994, 8: 318–321. [PubMed] [Google Scholar]
- 2.Hogan TF, Padgett BL, Walker DL, Borden EC and Frias Z, Survey of human polyomavirus (JCV, BKV) infections in 139 patients with lung cancer, breast cancer, melanoma, or lymphoma. Prog. Clin. Biol. Res 1983, 105: 311–324. [PubMed] [Google Scholar]
- 3.Kira J, Focal brain lesions in acquired immunodeficiency syndrome: DNA diagnosis and further monitoring [editorial; comment]. Intern. Med 1999, 38: 521. [DOI] [PubMed] [Google Scholar]
- 4.Tenser RB, Sommerville KW, Mummaw JG and Frisque RJ, Isolation of JC virus capsomer-like structures from progressive multifocal leukoencephalopathy brain. J. Neurol. Sci 1986, 72: 243–254. [DOI] [PubMed] [Google Scholar]
- 5.Padgett BL and Walker DL, Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive multifocal leukoencephalopathy. J. Infect. Dis 1973, 127: 467–470. [DOI] [PubMed] [Google Scholar]
- 6.Padgett BL, Walker DL, ZuRhein GM, Hodach AE and Chou SM, JC Papovavirus in progressive multifocal leukoencephalopathy. J. Infect. Dis 1976, 133: 686–690. [DOI] [PubMed] [Google Scholar]
- 7.Monaco MC, Atwood WJ, Gravell M, Tornatore CS and Major EO, JC virus infection of hematopoietic progenitor cells, primary B lymphocytes, and tonsillar stromal cells: implications for viral latency. J virol 1996, 70: 7004–7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider EM and Dorries K, High frequency of polyomavirus infection in lymphoid cell preparations after allogeneic bone marrow transplantation. Transplant. Proc 1993, 25: 1271–1273. [PubMed] [Google Scholar]
- 9.Shimizu N, Irnamura A, Daimaru O, Mihara H, Kato Y, Kato R, Oguri T, Fukada M, Yokochi T, Yosliikawa K, Komatsu H, Ueda R and Nitta M, Distribution of JC virus DNA in peripheral blood lymphocytes of hematological disease cases. Intern. Med 1999, 38: 932–937. [DOI] [PubMed] [Google Scholar]
- 10.Smith M, Considerations on a possible viral etiology for B-precursor acute lymphoblastic leukemia of childhood [see comments]. J. Immunother 1997, 20: 59–100. [DOI] [PubMed] [Google Scholar]
- 11.Smith MA, Strickier HD, Granovsky M, Reaman G, Linct M, Daniel R and Shah KV, Investigation of leukemia cells from children with common acute lymphoblastic leukemia for genomic sequences of the primate polyomaviruses JC virus, BK virus, and Sinkan virus 40. Med. Pediatr. Oncol 1999, 33: 441–443. [DOI] [PubMed] [Google Scholar]
- 12.MacKenzie J, Perry J, Ford AM, Jarrett RF and Greaves M, JC and BK virus sequences are not detectable in leukaemic samples from children with common acute lymphoblastic leukaemia. Br. J. Cancer 1999, 81: 898–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krynska B, Del Valle L, Croul S, Gordon J, Katsetos CD, Carbone M, Giordano A and Khalili K, Detection of human neurotropic JC virus DNA sequence and express ion of the viral oncogenic protein in pediatric medulloblastomas. Proc, Natl. Acad. Sci. U. S. A 1999, 96: 11519–11524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flaegstad T, Andresen PA, Johnsen JI, Asomani SK, Jorgensen GE, Vignarajan S, Kjuul A, Kogner P and Traavik T, A possible contributory role of BK virus infection in neuroblastoma development. Cancer. Res 1999, 59: 1160–1163. [PubMed] [Google Scholar]
- 15.Del Valle L, Gordon J, Enam S, Delbue S, Croul S, Abraham S, Radhakrishnan S, Assimakopoulou M, Katsetos CD and Khalili K, Expression of human neurotropic polyomavirus JCV late gene product agnoprotein in human medulloblastoma. J. Natl. Cancer lnst 2002, 94: 267–273. [DOI] [PubMed] [Google Scholar]
- 16.Shiramizu B, Elbaggarp A, Foran T, Gofman I, Perin N and Schleimer S, Monitoring of cerebral spinal fluid by polymerase chain reaction in children with acute lymphoblastic leukemia. Int. J. Ped. Hema/Onc 1998, 5: 475–483. [Google Scholar]
- 17.Newman JT and Frisque RJ, Identification of JC virus variants in multiple tissues of pediatric and adult PML patients. J. Med. Virol 1999, 58: 79–86. [PubMed] [Google Scholar]
- 18.Agostini HT, Shishido-Hara Y, Baumhefiner RW, Singer EJ, Ryschkewitsch CF and Stoner GL, JC virus Type 2: definition of subtypes based on DNA sequence analysis of ten complete genomes. J. Gen. Virol 1998, 79: 1143–1151. [DOI] [PubMed] [Google Scholar]
- 19.Agostini HT, Ryschkewitsch CF, Mory R, Singer EJ and Stoner GL, JC virus (JCV) genotypes in brain tissue from patients with progressive multifocal leukoencephalopathy (PML) and in urine from controls without PML: increased frequency of JCV type 2 in PML. J. Infect. Dis 1997, 176: 1–8. [DOI] [PubMed] [Google Scholar]
- 20.Trowbridge PW and Frisque RJ, Analysis of G418-selected Rat2 cells containing prototype, variant, mutant, and chimeric JC virus and SV40 genomes. Virology 1993, 196: 458–474. [DOI] [PubMed] [Google Scholar]
- 21.Agostini HT, Ryschkewitsch CF and Stoner GL, Genotype profile of human polyomavirus JC excreted in urine of immunocompetent individuals. J. Clin. Microbiol. 1996, 34: 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agostini HT, Ryschkewitsch CF, Singer EJ, Baumhefner RW and Stoner GL, JC virus type 2B is found more frequently in brain tissue of progressive multifocal leukoencephalopathy patients than in urine from controls. J. Hum. Virol 1995, 1: 200–206. [PubMed] [Google Scholar]
- 23.Frisque RJ and White FA III., The molecular biology of JC virus, causative agent of progressive multifocal leukoencephalopathy. In: Molecular Neurovirology, Roos RP (ed.). Humana Press, Totowa, NJ, 1992, pp. 25–158. [Google Scholar]
- 24.Gallia GL, Gordon J and Khalili K, Tumor pathogenesis of human neurotropic JC virus in the CNS. J. Neurovirol 1998, 4: 175–181. [DOI] [PubMed] [Google Scholar]
- 25.Gordon J, Krynska B, Ottc J, Houff SA and Khalili K, Oncogenic potential of human neurotropic papovavirus, JCV, in CNS. Dev. Biol. Stand 1995, 94: 93–101. [PubMed] [Google Scholar]
- 26.Gordon J and Khalili K, The human polyomavirus, JCV, and neurological diseases. Int. J. Mol. Med 1998, 1: 647–655. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto H, Mineta T, Ueda S, Nakahara Y, Shiraishi T, Tamiya T and Tabuchi K, Detection of JC virus DNA sequences in brain tumors in pediatric patients. J. Neurosurg 2005, 102: 294–295. [DOI] [PubMed] [Google Scholar]
- 28.Del Valle L, Gordon J, Assimakopoulou M, Enam S, Geddes JF, Varakis JN, Katsctos CD, Croul S and Khalili K, Detection of JC virus DNA sequences and expression of the viral regulatory protein T-antigen in tumors of the central nervous system. Cancer Res 2001, 61: 4287–4293. [PubMed] [Google Scholar]
- 29., Hayashi H, Endo S, Suzuki S, Tanaka S, Sawa H, Ozaki Y, Sawainura Y and Nagashima K, JC virus large T protein transforms rodent cells but is not involved in human medulloblastoma. Neuropathology 2001, 21: 129–137. [DOI] [PubMed] [Google Scholar]
- 30.Kim JY, Koralnik IJ, LeFave M, Segal RA, Pfister LA and Pomeroy SL, Medulloblastomas and primitive neuroectodermal tumors rarely contain polyomavirus DNA sequences. Neuro-oncol 2002, 4: 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rollison DE, Utaipat U, Ryschkewitsch C, Hou J, Goldthwaite P, Daniel R, Helzlsouer KJ, Burger PC, Shah KV and Major EO, Investigation of human brain tumors for the presence of polyomavirus genome sequences by two independent laboratories. Int. J. Cancer 2005, 113: 769–774. [DOI] [PubMed] [Google Scholar]
- 32.Khalili K, Del Valle L, Otte J, Weaver M and Gordon J, Human neurotropic polyomavirus, JCV, and its role in carcinogenesis. Oncogene 2003, 22: 5181–5191. [DOI] [PubMed] [Google Scholar]
- 33.Weggen S, Bayer TA, von Deimling A, Reifenberger G, von Schweinitz D, Wiestler OD and Pictsch T, Low frequency of SV40, JC and BK polyomavirus sequences in human medulloblastomas, meningiomas and ependymomas. Brain Pathol 2000, 10: 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neel JV, Major EO, Awa AA, Glover T, Burgess A, Traub R, Curfman B and Satoh C, Hypothesis: "Rogue cell"-type chromosomal damage in lymphocytes is associated with infection with the JC human polyoma virus and has implications for oncopenesis. Proc Nail Acad Sci U S A 1996, 93:2690–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neel JV, An association, in adult Japanese, between the occurrence of rogue cells among cultured lymphocytes (JC virus activity) and the frequency of "simple" chromosomal damage among the lymphocytes of persons exhibiting these rogue cells. Am. J. Hum. Genet 1998, 63: 489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kappler R, Pietsch T, Weggen S, Wiestler OD and Scherthan H, Chromosomal imbalances and DNA amplifications in SV40 large T antigen-induced primitive neuroectodermal tumor cell lines of the rat. Carcinogenesis 1999, 20: 1433–1438. [DOI] [PubMed] [Google Scholar]
- 37.Woods C, LeFeuvre C, Stewart N and Bacchetti S, Induction of genomic instability in SV40 transformed human cells: sufficiency of the N-terminal 147 amino acids of large T antigen and role of pRB and p53. Oncogene 1994, 9: 2943–2950. [PubMed] [Google Scholar]
- 38.Laghi L, Randolph AE, Chauhan DP, Marra G, Major EO, Neel JV and Boland CR, JC virus DNA is present in the mucosa of the human colon and in colorectal cancers. Proc. Natl. Acad. Sci. U.S.A 1999, 96: 7484–7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Enam S, Del Valle L, Lara C, Gan DD, Ortiz-Hidalgo C, Palazzo JP and Khalili K, Association of human polyomavirus JCV with colon cancer: evidence for interaction of viral T-antigen and beta-catenin. Cancer Res 2002, 62: 7093–7101. [PubMed] [Google Scholar]
- 40.Ricciardiello L, Laghi L, Ramamirtham P, Chang CL, Chang DK, Randolph AE and Boland CR, JC virus DNA sequences are frequently present in the human upper and lower gastrointestinal tract. Gastroenterology 2000, 119: 1225–1235. [DOI] [PubMed] [Google Scholar]
- 41.McClain KL, Leach CT, Jenson HB, Joshi VV, Pollock BH, Parmley RT, DiCarlo FJ, Chadwick EG and Murphy SB, Association of Epstein-Barr virus with leiomyosarcomas in children with AIDS. N Engl J Med 1995, 332:12–18. [DOI] [PubMed] [Google Scholar]
- 42.Agostini HT, lobes DV and Stoner GL, Molecular evolution and epidemiology of JC virus. In. Human Polyomaviruses, Khalili K and Stoner GL (eds.). Wiley-Liss, Inc., New York, 2001, pp. 491–526. [Google Scholar]
- 43.Caldarelli-Stefano R, Vago L, Omodeo-Zorini E, Mediati M, Losciale L, Nebuloni M, Costanzi G and Ferrante P, Detection and typing of JC virus in autopsy brains and cxtraneural organs of AIDS patients and nonimmunocompromised individuals. J. Neurovirol 1999, 5: 125–133. [DOI] [PubMed] [Google Scholar]
- 44.Ferrante P, Mediati M, Caldarelli-Stefano R, Losciale L, Mancuso R, Cagni AE and Maserati R, Increased frequency of JC virus type 2 and of dual infection with JC virus type I and 2 in Italian progressive multifocal leukoencephalopathy patients. J. Neurovirol 2001, 7: 35–42. [DOI] [PubMed] [Google Scholar]
- 45.Martin JD, King DM, Slauch JM and Frisque RJ, Differences in regulatory sequences of naturally occurring JC virus variants. J Virol 1985, 53: 306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yogo Y, Kitarnura T, Sugimoto C, Ueki T, Aso Y, Hara K and Taguchi F, Isolation of a possible archetypal JC virus DNA sequence from nonimmunocompromised individuals. J. Virol 1990, 64: 3139–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White FA III., Ishaq M, Stoner GL and Frisque RJ, JC virus DNA is present in many human brain samples from patients without progressive multifocal leukoencephalopathy. J. Virol 1992, 66: 5726–5734. [DOI] [PMC free article] [PubMed] [Google Scholar]


