Abstract
There are many benign anorectal disorders, which can make patients seek care. In low-resource settings, the incidence of those pathologies is not different from the industrialized and western world. However, an interesting difference colorectal surgeons and gastroenterologists can face is the fact that many patients do not seek help or are not aware and have little opportunities to be helped. Latin America population is estimated to be around 8% of the world population, with Brazil having the largest percentage. Infectious diseases, which were previously under control or were steadily declining, have emerged. For example, we have seen resurgence of dengue, malaria, and syphilis in pregnancy, as well as other sexually transmitted diseases that can affect the anorectal region. In this article, we will address the most common benign anorectal disorders.
Keywords: anorectal, benign, anal, abscess, fistula, incontinence
Anal Abscess
Etiology
Several hypotheses of the origin of anal abscesses have been published throughout the years. Generally, the most accepted hypothesis states that obstruction of the mucous anal gland outlet duct is the initial stage of anal abscess formation, which occurs due to fecal material or local trauma. This later leads to stasis and infection. 1 Afterward, pus may generate and traverse through the paths of least resistance along muscular fibers between the internal and external anal sphincters or through the external anal sphincter into the ischioanal fossa. 1 2 Parks published a study in 1961, which reported that 70% of specimens of anal fistula tracts that had been excised presented with anal gland epithelium and 25% of those samples showed cystic dilation of an anal gland. 3 Parks' cryptoglandular theory has been broadly accepted ever since. Less frequent noncryptoglandular causes of anorectal abscess occur in approximately 10% of cases and include inflammatory bowel disease (Crohn's disease and ulcerative colitis), infection (tuberculosis, actinomycosis, lymphogranuloma venereum [LGV]), trauma (impalement, surgery, foreign body), and malignancy (carcinoma, leukemia, lymphoma, radiation). 4
Classification
Anal abscesses are classified according to the anorectal space that they occupy 5 : (1) subcutaneous abscess (perianal), (2) intersphincteric abscess, (3) ischioanal abscess, and (4) supralevator abscess. Superficial types, including perianal and ischioanal abscesses, are the most common, consisting of approximately 80% of cases ( Table 1 ). 4 5 6
Table 1. Common benign anorectal disorders 54 .
| Benign anorectal disorders |
| • Anal abscess |
| • Anal fissure |
| • Pilonidal disease |
| • Sexually transmitted diseases |
| • Hemorrhoids |
| • Anal fistula |
| • Fecal incontinence |
| • Proctalgia |
Diagnosis and Barriers to Care
Although superficial forms of anorectal abscesses are usually easy to diagnose through patient's history and physical exam as there tends to be local swelling and pain, and less frequently fever and suppuration, 7 in low-income settings a prompt diagnosis may be delayed due to poor access to medical care, lack of patient's medical insurance due to economic limitations, and saturation of medical services in available local hospitals or clinics. Mexico, for example, is situated far behind other Organization for Economic Cooperation and Development (OECD) countries in health status and availability. 8 On the other hand, deeper abscesses (supralevator or high ischiorectal) may be more difficult to diagnose as swelling tends not to be readily visible on physical exam and pain may occur in a less specific pattern in the perineum, lower back, or buttocks. 9 10 11 Thus, these deeper abscesses may have a further delay in diagnosis and treatment. For those patients in whom diagnosis is not possible through physical exam, computed tomography (CT) scan, endoanal ultrasound (EAUS), or magnetic resonance imaging (MRI) should be considered. 7 However, these studies are commonly difficult to afford and sometimes not readily available for patients in the low-resource setting. In Mexico alone, there are only 2.9 MRI units and 6 CT scanners per 1,000,000 inhabitants, 12 13 far less than those available in many first-world countries.
Management and Outcomes
Antibiotics are indicated when cellulitis occurs if there is failure to improve after proper incision and drainage of the abscess or in immunosuppressed patients. 4 Otherwise, antibiotics are not routinely used, as some studies have found that they also do not prevent fistula formation. 14 Incision and drainage remains the mainstay of treatment for anal abscesses. Depending on the type of anal abscess, the site for drainage will be chosen; as a general rule, “outward” drainage is recommended whenever an abscess enters or passes through skeletal muscle (levator ani or external anal sphincter), whereas all other types must be drained internally through the rectum or anal canal. 15 Recurrence occurs in up to 44% of cases, most commonly during the first year after initial drainage. 16 17 18 19 20 21 An alternative method for ischioanal abscesses is to make an incision, drain the abscess, and place a Malecot or Pezzer drain under local anesthesia, which is later removed when suppuration ceases and there is cavity closure around the drain (usually 3–10 days). 22 Horseshoe abscesses, which most commonly originate in the deep postanal space and later extend to one or both ischioanal spaces, may be drained utilizing the modified Hanley technique entailing partial sphincterotomy combined with seton placement. 23 24 Abscess drainage with simultaneous fistulotomy may be considered. A Cochrane review of six randomized controlled trials showed that this approach was associated with a significant decrease in abscess recurrence, fistula or abscess persistence, and need for subsequent surgery (relative risk, 0.13; 95% confidence interval [CI], 0.07–0.24) without a statistically significant increase in incontinence. 25 All of the above-mentioned treatment options are usually readily available and not costly; thus, they are generally applicable in a low-income setting.
Anal Fissure
Definition and Etiology
An anal fissure is a tear in the epithelial lining of the distal anal canal. 26 Despite many theories, the exact cause remains uncertain. Although unclear if it is the cause or effect of anal fissures, sphincter hypertonicity, a common finding in this disease, has been documented by anal manometry in several studies and is the leading hypothesis behind the pathogenesis. 26 27 28 Another theory states that relative ischemia exists in the anoderm at the posterior midline. This has been demonstrated by arteriography and laser Doppler flow studies, and it is thought to be what generates the proper setting for fissure formation. 29 30 Although local trauma (large stool, anorectal intercourse, surgical procedures) has been associated with many cases of anal fissures, these can occur in the absence of trauma or constipation and may even occur in patients with diarrhea or sphincter hypotonia. 26
Classification and Clinical Features
Symptoms, especially in acute fissures, are sharp, burning, tearing pain or spasms that can last for hours after defecation and bright red bleeding with bowel movements. Fissures are classified as acute versus chronic and typical versus atypical.
Diagnosis and Barriers to Care
A thorough history and physical exam will usually suffice to establish the diagnosis of anal fissure in most patients. 31 The patient may lie in lateral decubitus or prone jackknife position, and by gently separating the buttocks, inspection should be done first at the posterior midline, where most fissures are found ( Fig. 1A, B ). Assessment of anal resting pressures through digital rectal exam may be performed, and if tolerated, office anoscopy may be performed selectively with previous application of anesthetic lubricant to confirm diagnosis when needed. 4 The above-mentioned methods usually lead to a straightforward diagnosis. Nevertheless, access to medical care and an experienced colorectal surgeon is not easily obtained in low-resource settings. In Latin America, this in part is related to the limited expenditure allotted to health care in many countries. For example, in Mexico and Brazil (the countries of residence of the authors), health spending amounts to $1,198 and $1,514 USD per capita, respectively, whereas in countries such as Japan, the United Kingdom, and the United States, health expenditure reaches $4,691, $5,268, and $10,948 USD per capita, respectively. 32 Fortunately, around 93% of acute fissures cure spontaneously. 33 However, chronic anal fissures represent approximately 10% of office visits in proctologic clinics 34 ; nevertheless, their real incidence might be underestimated because more than 80% of patients with benign anorectal diseases do not seek medical care. 35
Fig. 1.
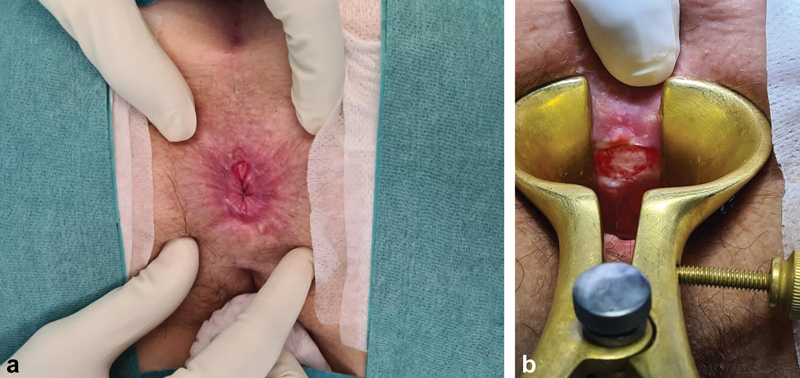
( A , B ) Anorectal fissure in the posterior midline of the anal verge.
Management and Outcomes
Nonoperative treatment is safe, with few side effects, and should be the first-line treatment. Nonoperative measures such as sitz baths and use of psyllium fiber or other bulking agents will resolve the symptoms of almost 50% of patients with an acute anal fissure. 36 Topical nitric oxide donors are associated with healing in approximately 50% of chronic anal fissures 37 ; however, a common side effect in up to 30% of patients is headache, 37 38 which may lead to treatment cessation in up to 20% of patients. 39 In some countries, nitroglycerine is not commercially available and may need to be created at a compounding pharmacy. An alternative for topical treatment is the use of calcium channel blocking agents, such as diltiazem or nifedipine, which have been associated with healing rates of anal fissures of 65 to 95%. 40 Some prospective studies have reported healing rates ranging from 18 to 71% with botulinum toxin application (20–60 units) within 9 weeks of treatment, with results comparable to or slightly better than topical therapies. 41 42 Thus, botulinum toxin application provides similar results compared with topical treatment as first-line therapy for chronic anal fissures; however, it provides only modest improvement in healing rates as second-line therapy following treatment with topical therapies. 36 Nevertheless, botulinum toxin might be expensive and difficult to obtain in certain areas. For example, in Mexico a bottle of 100U of botulinum toxin may range in cost from $3,600 to $4,600 pesos (the equivalent of $180 to $230 USD). Lateral internal sphincterotomy (LIS) is superior when compared with nitrates, calcium channel blockers, or botulinum toxin, with healing rates of 88 to 100% and with fecal incontinence rates ranging from 8 to 30%, as shown by multiple randomized trials; for this reason, current guidelines of the American Society of Colon and Rectal Surgeons (ASCRS) advocate LIS as the surgical treatment of choice for chronic anal fissures and state that it may be even offered in select patients without first confirming failure of pharmacological treatment. 36 Treatment of atypical fissures should focus in identifying the etiology to provide specific disease-directed therapy.
Pilonidal Disease
Etiology
It is thought that pilonidal disease (PD) occurs secondary to trauma of the skin and hair follicles in the natal cleft due to trapping of hairs, which are not necessarily those that originate in this area. Local friction, warmth and moisture, and possibly local hypoxia lead to local trauma secondary to the barbed texture of hair, which in time produces a granulomatous foreign body–type reaction. Initially, a sinus that may drain fluid occurs, but can later progress to multiple sinuses, cystic dilation, and even abscess formation. 4 Risk factors associated with the development of PD include body mass index > 25, poor hygiene, family history of PD, hirsutism, deep natal cleft, prolonged sitting, and excessive sweating. 43 44 45
Clinical Features and Diagnosis
During inspection, characteristic midline pits are almost always present in the natal cleft. In acute cases, patients may present with signs of infection including cellulitis or an abscess. On the other hand, chronic PD commonly presents with a draining sinus in the intergluteal fold ( Fig. 2 ). Usually, midline pits are connected to subcutaneous tracts that can be easily misdiagnosed as fistulas. 4 In the majority of cases, simple inspection and palpation during physical exam are enough to establish diagnosis. Thus, laboratory or radiology examinations are not routinely required. 46
Fig. 2.
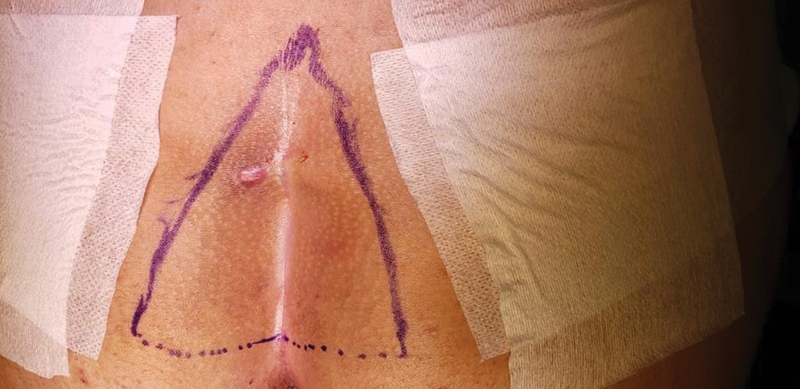
Chronic draining sinus in the intergluteal fold in patient with typical pilonidal disease.
Barriers to Care
PD affects mostly the working-age population and presents a significant disease burden worldwide. It is more common in Caucasian males with an incidence of 1.1%. 47 Costs are not widely reported; however, a recent Swedish study reported a cost of $6,222 EUR per patient for conventional wide excision and closure for pilonidal sinus, with a 32% recurrence rate at 5 years. 48 49 Additionally, the social impact of PD on young people significantly affects their interpersonal relationships, education, and social activity. 50 In low-resource settings, PD presents quite a challenge as it is a disease associated with considerable recurrence and complication rates, along with a probability of needing multiple treatment procedures before achieving adequate healing, which can be quite costly.
Management and Outcomes
In a low-resource setting, it is particularly important to offer patients a treatment approach that will effectively resolve the problem while shortening time of recovery. Thus, whenever excision is advised, primary closure/flap creation should be attempted (if technically feasible and infection is not present) as healing by secondary intention would imply a significant economic burden and necessity of reaching medical care for a longer period. In general, no single procedure is superior to the other. 49 If an abscess is encountered, incision and drainage is indicated. Around 10 to 15% of patients will have abscess recurrence, and 40 to 60% will progress to develop a pilonidal sinus requiring excision. 49 With the lay open technique, an incision is made over the pilonidal sinus tract of the PD and left open for closure under secondary intension. Rates of success for the initial operation can be as high as 97%, while recurrence may be as low as 8.8%. 51 52 Nevertheless, this will require more intense wound care sometimes with need of assistance and further expense. Primary closure has typically been regarded as having high risk for local infection; however, a Cochrane review including 26 trials and 2,530 patients found that there was faster healing with this technique and no difference in infection rates. In this review, off-midline primary closure reported faster healing and lower infection rates when compared with midline closure. 53 Primary closure with a flap may be considered, with the objective of excising the diseased tissue, covering the defect with healthy tissue, and raising the natal cleft anatomy. 54 A wide variety of flap techniques have been described. The Karydakis flap requires elliptical incision down to the sacral fascia with the ellipse corners 2 cm off the midline; after excision of the complete PD, a flap of skin and subcutaneous fat is created and raised to the opposite side of the midline, with closure in layers of the wound. 54 Another commonly used flap technique is the Bascom cleft lift, in which the natal cleft is elevated and closed off the midline ( Fig. 3 ). More complex flap techniques have been used especially in cases of wider disease, for example, the Limberg flap in which a rhomboid flap is created and rotated to cover larger defects after resection. 54 Other minimally invasive treatments including endoscopic/video-assisted ablation of pilonidal sinus, laser ablation of pilonidal sinus, and trephination have been described. In general, the specific resection technique should be chosen on a case-by-case basis and considering the individual surgeon's expertise. In addition to wound care, an adjunct to treatment is postoperative hair removal as it has been associated with a decrease in recurrence rates. 55
Fig. 3.
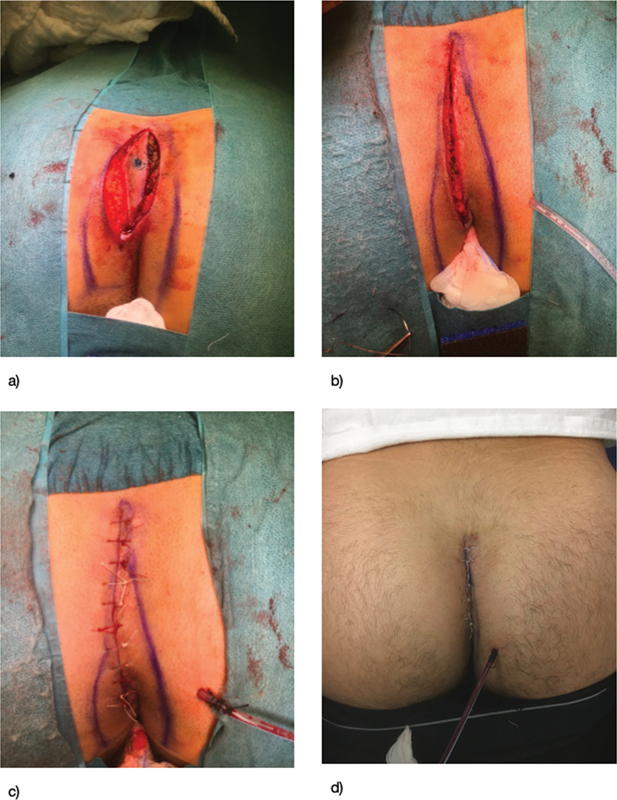
Bascom cleft repair following excision of a pilonidal cyst.
Sexually Transmitted Infections
Etiology
Anorectal sexually transmitted infections (STIs) are on the rise, possibly due to increased practice of receptive anal intercourse. Additionally, other risk factors are oral–anal sex and contiguous spread from genital infections. 54
Bacterial STIs
Chlamydia trachomatis
In the United States, it is the most commonly reported STI. 56 Males commonly present with urethritis, with symptoms of pyuria, dysuria, and urinary frequency, whereas women may commonly have urethritis or cervicitis with cervical discharge and bleeding. When the rectum is affected, patients present with proctitis frequently with tenesmus, pain, and discharge. 54 Diagnosis is performed using nucleic acid amplification test (NAAT) with 97% sensitivity and specificity. 57 Patients should be treated with 1 g of azithromycin or doxycycline 100 mg twice daily for 7 days. Alternatively, erythromycin or quinolones may be used.
Lymphogranuloma Venereum
It is caused by specific serotypes of C. trachomatis , namely serotypes L1–L3. Unlike other serotypes of C. trachomatis , LGV produces more invasive disease leading to severe proctitis with ulcers that can initiate the formation of abscesses, fistulas, chronic pain, and strictures. Since it also affects the lymphatic system, proctitis may be accompanied by usually unilateral inguinal and femoral lymphadenopathy. Rectal pain, tenesmus, discharge, fever, fatigue, and weight loss are common manifestations of proctitis. Diagnosis is made by clinical findings along with rectal or genital swab NAAT to detect C. trachomatis , followed by confirmatory LGV testing. Testing includes cultures, direct immunofluorescence, or nuclei acid detection. 54 Treatment is doxycycline 100 mg twice daily for 21 days. Erythromycin is an alternative for allergic patients. Infected lymph nodes may require aspiration or incision and drainage. 54
Gonorrhea
Neisseria gonorrhoeae , an intracellular diplococci, is the second most common STI in the United States. 56 Most cases are asymptomatic; however, infection may progress to cervicitis, urethritis, proctitis, and pelvic inflammatory disease in women and urethritis, epididymitis, or proctitis in men. Symptoms of urethritis include dysuria and urethral discharge, and in cases of proctitis tenesmus, hematochezia or purulent discharge may be present. 58 59 Anoscopy may show erythema and friable mucosa with mucopurulent discharge. Diagnosis is made with detection of gonococcus in urogenital, anorectal, pharyngeal, or conjunctival specimens or in first-catch urine. 60 Definitive testing should be done with NAAT as it has a sensitivity and specificity of 100%; however, it does not provide susceptibility testing for which culture may be required in some cases. 54 Treatment consists of ceftriaxone 250 mg given once intramuscularly plus 1 g of azithromycin. Patients should refrain from sexual intercourse for 7 days after completing therapy. 54
Syphilis
It is a systemic disease, caused by the spirochete Treponema pallidum . Syphilis is endemic in developing countries and especially common in patients with limited access to health care or economic resources. 61 Primary syphilis presents with a commonly painless ulcer 1 to 21 days after infection. Secondary syphilis presents weeks to months after inoculation with systemic symptoms such as fever, arthralgias, malaise, lymphadenopathy, a rectal mass, and a rash. 54 If untreated, a latent asymptomatic phase continues, which can last from 1 up to many years. Tertiary syphilis presents with cardiac affection, gummas, and necrotic and ulcerated center growths throughout the body. Neurosyphilis can occur at any stage of the disease. 54 Initial diagnosis is done with venereal disease research laboratory and rapid plasma reagin; however, confirmatory tests should be performed including antibody testing such as fluorescent treponemal absorption tests and other enzyme immunoassays and immunoblots. 57 Penicillin G is the treatment of choice for all stages of the disease, including a single intramuscular dose of benzathine penicillin G 2.4 million units for early-stage disease and the same dose once a week for 3 weeks in tertiary syphilis. 62
Chancroid
This anogenital disease, caused by Haemophilus ducreyi , is characterized by the presence of lesions that start as a papule and later progress to a pustule and open to form painful ulcerations. Ulcers may be multiple. Unilateral suppurative inguinal lymphadenopathy may occur. Diagnosis is mainly clinical and is made by the following criteria: presence of one or more painful genital ulcers, absence of syphilis infection, regional lymphadenopathy, and an ulcer exudate that is negative for herpes simplex virus (HSV). 57 63 Treatment consists of a single dose of either ceftriaxone 250 mg intramuscularly or azithromycin 1 g orally.
Donovanosis (Granuloma Inguinale)
Klebsiella granulomatis , an intracellular bacterium, is the cause of the disease. It is more prevalent in tropical areas of the world and in developing countries. Patients commonly present with genital papules or subcutaneous nodules that can progress to ulcers, which are usually painless, granulomatous, snakelike lesions that grow centrifugally. Lesions can easily bleed and be self-inoculated. Pseudobuboes may also appear. 64 They con also appear in the anorectum manifested as verrucous lesions and/or deep fissures with fibrotic ulcers. Diagnosis is achieved by identifying Donovan bodies in mononuclear cells with Giemsa-stained smears obtained from the ulcer. Polymerase chain reaction (PCR), where available, can aid in the diagnosis. 54 Recommended treatment is azithromycin 1 g orally once a week for 3 weeks or 500 mg orally once a day for 3 weeks. 57
Viral STIs
Herpes Simplex Virus
HSV, a DNA virus from the Herpesviridae family, is associated with genital lesions and proctitis especially in men who have sex with men (MSM). 57 65 In the anorectum, lesion are characterized by blistering in the mucosa with symptoms appearing in 4 to 21 days after receptive anal intercourse including burning sensation, pain, and pruritus. 66 Initially, lesions may develop as vesicles with surrounding erythema on perianal skin or in the anal canal. Lesions later rupture and become ulcerated ( Fig. 4 ). Proctoscopy may demonstrate a friable mucosa and diffuse ulcerations limited to the distal 10 cm of the rectum. 54 Serologic PCR assays with nucleic acid amplification of HSV DNA is the gold standard for diagnosis. Other methods for detection include cell growth culture and Tzanck preparation with direct immunofluorescence. Oral antiviral medications that have shown good results on randomized controlled trials and thus are currently used for treatment include acyclovir, valacyclovir, and famciclovir. 67 68 69 70 Intravenous acyclovir should be considered for severe HSV infection and systemic or neurologic disease that require hospitalization. 54
Fig. 4.
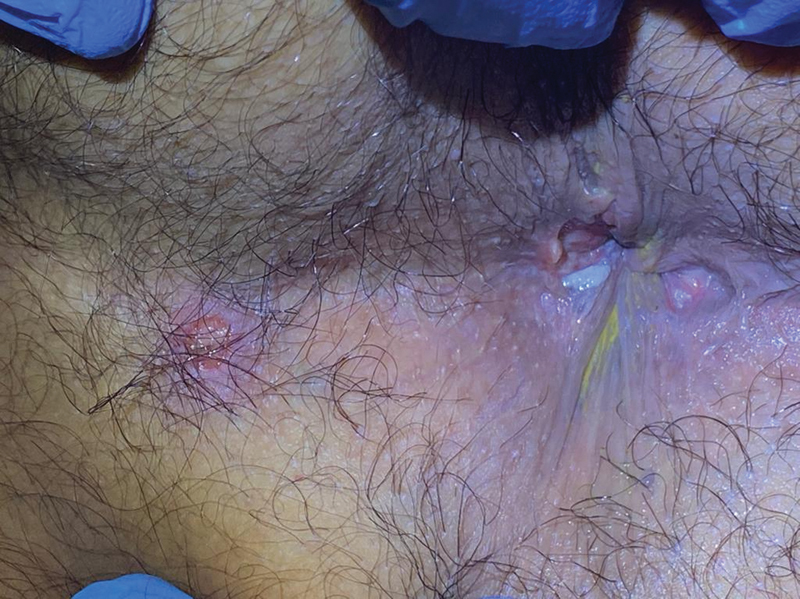
Ulcerated perianal lesions secondary to herpes simplex virus.
Human Papilloma Virus
Condylomas are common lesions among all sexual orientations; nevertheless, they are even more so among MSM. Several serotypes exist including subtypes 6 and 11, representing the most common ones and presenting as condylomas or warts of the genital region, anus, or rectum. On the other hand, subtypes 16 and 18 are most frequently associated with anal dysplasia and cancer. 71 Genital lesions appear as gray or pink cauliflowerlike growths that may lead to bleeding, pruritus, pain, or hygiene difficulty. Anoscopy should be performed to rule out lesions in the anal canal. 54 72 Diagnosis is usually made with physical exam. Dysplastic lesions may be detected with application of acetic acid, while high-resolution anoscopy is commonly utilized for ruling out intra-anal disease. Biopsy may be performed when diagnosis is not clear, whereas anal cytology has been recommended but with limited evidence. 72 A wide variety of treatment options exist, including topical medication, cryotherapy, fulguration, and excision; however, recurrence rates may range from 4 to 26%. 54 73 74
Molluscum Contagiosum
This virus from the poxvirus family produces characteristic pruritic or painful, contagious, flesh-colored or gray–white skin lesions after direct contact with skin or mucous membranes containing active lesions. It can also be self-inoculated to several locations of the body. The disease is commonly self-limiting in nonimmunocompromised patients. When needed, phenol, trichloroacetic acid, podophyllotoxin, or imiquimod is used for topical treatment. Additionally, ablation with electrocautery or cryotherapy has shown to be effective. 54 75 76 77
Human Immunodeficiency Virus
This RNA retrovirus affects CD4 receptors to enter the cell, which leads to compromised immune system activation and later to destruction of T lymphocytes. Anal manifestations include human immunodeficiency virus (HIV)-related anal ulcer, hemorrhoids, fissures, fistulas, abscesses, or as other conditions related to HIV status (anal condylomas, herpetic ulcers). When AIDS arises, potential anal manifestations are anal squamous cell carcinoma, lymphoma, and Kaposi's sarcoma. 78 79 Anorectal abscesses should be drained with an incision close to the anal verge, while fistulas should be managed with setons or sphincter-preserving surgeries. Treatment for anal fissure is similar as for non-HIV patients; however LIS should be used cautiously only in those in whom ulcerating disease has been ruled out. Intralesional steroids have been utilized for treatment of HIV-related anal ulcers with good results; however, if medical treatment fails, ulcer excision and mucosal advancement flap may be considered. 78 80
Parasitic STIs
Pediculosis Pubis
The parasite Pthirus pubis may produce infestation in the pubic hair and perianal area after sexual contact. Patients typically present with pruritus, and the infested skin may show flaky or crusted lesions. Diagnosis is mainly clinical; nevertheless, dermoscopy can be used to visually detect the parasites in the hair follicles. 81 Treatment with first-line options (permethrin 1% cream or pyrethrins with piperonyl butoxide) or alternative medications (malathion 0.5% lotion, ivermectin or lindane) is available. Additionally, combing the affected hair for removing nits and washing of clothing and bed sheets should be done.
Scabies
This disease is caused by the parasite Sarcoptes scabiei , which can be transmitted through fomites and commonly through sexual contact. 82 Pruritus is the main symptom, and it occurs predominantly nocturnally and is associated with papules, pustules, and excoriations that appear in a symmetric pattern most frequently in the interdigital webs, nipples, or genitals. 54 Diagnosis is made by identifying burrows, mites, eggs, or the mites' feces from affected areas. 83 Recommended treatment for scabies includes permethrin 5% cream or oral/topical ivermectin or alternatively lindane (lindane should only be used in failure or nontolerance to the above-mentioned medications due to risk of toxicity).
Limitations and Strategies in the Management of STIs in Low-Resource Settings
The use of state-of-the-art diagnostic tests to establish etiology to provide pathogen-directed treatment is widely accepted for high-income settings. 84 These include specialized, microscopic, cultural, and serological tests and, more recently, NAATs. Nevertheless, in a low-resource setting, the cost of setting up laboratory facilities that can provide such test might be prohibitive; furthermore, patients may not have access to hospitals or clinics with laboratory facilities at all. Thus, these constraints have led to empiric treatment approaches (syndromic management) for STIs based on clinician knowledge as a common form of management in low-resource areas. 84 For this purpose, point-of-care tests have been developed to provide prompt STI definitive diagnosis, reducing delay in treatment. 85 To promote the development of simple, accessible, and rapid point-of-care tests, the World Health Organization (WHO) developed the ASSURED (affordable, sensitive, specific, user-friendly, rapid and robust, equipment free, and delivered to end users) benchmark. 86 Point-of-care tests that meet the WHO ASSURED benchmark could bridge the gap for STI case management and control in these low-resource settings. 85
Hemorrhoids
Definition and Etiology
Anal cushions or hemorrhoids are part of the normal anatomy of the anal canal. They contribute 15% to the continence mechanism. 87 Those vessels are a mixture of arteries and veins. In fact, they are formed by anal mucosa, loose connective tissue, smooth muscle, and arterial and venous vessels, with the arterial component being more prominent. This anatomical particularity explains the occurrence of red blood bleeding, the most common symptom of this condition. The prevalence of hemorrhoids is unknown, although it is one of the most common benign condition for the colorectal surgeon. To date, the exact causes and the pathophysiology of this condition is poorly understood. Risk factors for hemorrhoids includes constipation and straining, and a diet poor in fiber and rich in spices. In addition, pregnancy and hereditariness are associated with this condition.
Classification
Hemorrhoids can be classified as external and internal hemorrhoids, for which different approaches are necessary. ( Fig. 5 ). External and internal hemorrhoids are located distal and proximal to the dentate line, respectively. External hemorrhoids are covered by the anoderm or skin.
Fig. 5.
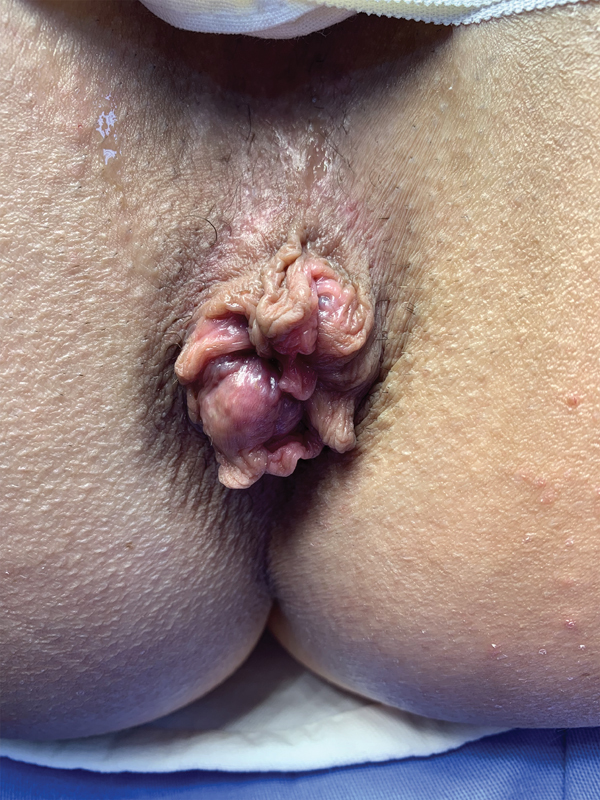
Internal and external hemorrhoidal disease.
External hemorrhoids can thrombose and can cause pain, itching, and bleeding, while internal hemorrhoids are classified and graded according to the size and relation to the anal verge 88 ( Table 2 ).
Table 2. Classification of hemorrhoids.
| Grade I | Nonprolapsing hemorrhoids |
| Grade II | Prolapsing hemorrhoids on straining but reduce spontaneously |
| Grade III | Prolapsing hemorrhoids requiring manual reduction |
| Grade IV | Nonreducible prolapsing hemorrhoids, which include acutely thrombosed, incarcerated hemorrhoids |
Diagnosis and Barriers to Care
Colorectal surgeons are usually well trained to realize the proctologic examination, which is the best way to establish the diagnosis. Patients can be examined in the lateral decubitus position or in the prone position. Inspection is performed with a good light source and after digital examination, patients underwent anoscopy with a side-viewing anoscope. However, all patients with a history of mucous discharge or rectal bleeding should have the colon evaluated through a complete colonoscopy. In low-income countries, colonoscopy is not available to all patients and many times a rigid or flexible sigmoidoscopy can be performed to exclude a rectal cancer, the most feared diagnosis.
Management and Outcomes
Management in any part of the world begins with correction of bowel habits, including fiber in the diet and fiber supplements to form the stool and facilitate evacuation. This simple management has level I evidence according to many gastroenterological colorectal societies. 89 Therefore, in low-resource settings, where a high-fiber diet is facilitated by abundance of fruits and vegetables, this first conservative measurement represents the main option, together with warm sitz baths and topical medications, which may be the only treatment required. In addition, in Brazil and other Latin American countries, coumarin and other phlebotonic medications are available and frequently utilized, especially for thrombosed hemorrhoids. 90 Office-based procedures such as sclerotherapy and especially rubber band ligation are the most utilized methods for grade I and II hemorrhoids. In Brazil, a macro rubber band equipment was introduced by Reis Neto, with good results 91 ( Fig. 6 ). The goal of a rubber band ligature is to promote fibrosis of the submucosa with subsequent fixation of the anal epithelium to the underlying sphincter. More than 1,500 patients have been treated with the macro ligation, and Reis and colleagues have demonstrated a better fibrosis and fixation by banding a bigger volume of mucosa. When grade III and IV piles with mucosal prolapse are present, surgical treatment is indicated. In fact, the most common excisional procedures, such as the Milligan-Morgan and the Ferguson operations, remain the first choice and the most indicated procedures for grade III and IV hemorrhoids. 92 The use of other more recent techniques, such as stapler and Doppler-guided hemorrhoidectomy, implies having additional cost due to the required equipment, and therefore, these recent techniques are less utilized in low-resource settings. However, because operations can cause significant pain, techniques of preemptive analgesia were implemented in several countries. In Latin America, spinal anesthesia is frequently utilized, and surgeons are being trained to utilized local anesthesia with pudendal block immediately after the procedure.
Fig. 6.

Equipment used for rubber band ligation.
Anal Fistula
Definition and Etiology
Anal fistula is a benign but frequently complex situation for the patients, due to both the symptoms associated with inflammation of the tracts and the implications in terms of preservation of continence mechanism. 93 In the majority of cases, it arises from a previous anal abscess. The prevalence and incidences of anal fistula are not different from other developed countries. The main mechanism is related to the cryptoglandular origin, and it is observed more in male and obese patients.
Classification
Anal fistulas are classified according to the anatomical site of the tracts in relation to the sphincter muscles. 94 Four main classes of fistulas are described in Table 3
Table 3. Anal fistula classification.
| Class | Description |
|---|---|
| Intersphincteric | The internal opening originates in the dentate line and the track in located between the internal and external sphincter with the external opening in the perianal region close to the anal verge |
| Transsphincteric | The tract traverses the internal and external sphincter with the opening in the ischiorectal fossa |
| Suprasphincteric | The tract originates at the dentate line more cephalad to the external sphincter mechanism before opening into the skin at the ischiorectal fossa |
| Extrasphincteric | The tract is more complex and traverses the muscles including the puborectalis with the opening proximally at the dentate line or in the lower rectal wall |
Diagnosis and Barriers to Care
Most patients report a history of previous anal abscess and development of an inflamed area with purulent discharge. Diagnosis is established with a good history and physical examination. Once the external opening is visualized, a gentle digital examination is performed to exclude residual abscess, muscle tenderness, and fecalomas or rectal tumors. If anal pain is not impeditive for anoscopy, anal canal evaluation can be performed to look for the internal opening, anal papillae, and associated hemorrhoids. Imaging methods such as MRI and EAUS are available in most of the important cities and referenced hospitals. 95 However, these methods are not utilized in small cities, as specialized colorectal units are not available. Therefore, many complex cases, especially those associated with inflammatory bowel disease, are sent to the big cities, where specialized surgeons are more experienced. Imaging methods are especially important in complex, extrasphincteric, or suprasphincteric fistulas. In South America, portable and more accessible ultrasound transducers were introduced recently. These transducers can be utilized in the operation room and fistula tracks can be well demonstrated using oxygen peroxide. 96 This is particularly interesting for cases where sphincter muscles are involved ( Fig. 7 ). When comparing MRI with EAUS, sensitivity of both methods is comparable (0.87; 95% CI, 0.70–0.95). 97 However, EAUS is a safe and less expensive preoperative investigation for fistula surgery. 98
Fig. 7.
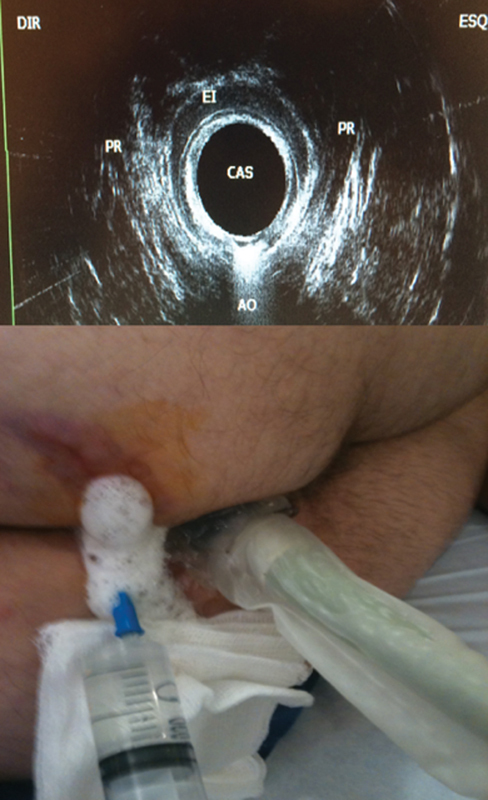
Endorectal ultrasound showing fistula involving sphincter complexes.
Management and Outcomes
Surgical treatment is usually necessary. Identification of the tract with probes under anesthesia is the most common approach, specially performed by general surgeons in small cities, which place a variety of materials that serves as setons. The most common technique is a fistulotomy, which allows cutting along the whole length of the fistula track, leaving it open to heal. 99 According to the Practice Parameters of the ASCRS, fistulotomy has grade of recommendation 1B. 7
For fistulas with long and complex tracks, when operations are not successful, those patients are sent to more specialized surgeons, when, in many situations, a fistulotomy has been already performed, with consequences to the sphincter mechanism. Anal deformities and asymmetric anus can be the result of a nonspecialized and trained surgeon dealing with this benign but tricky pathology. For persistent tracks with compromised sphincter muscles, more advanced techniques such as ligation of intersphincteric fistula tract and advancement flaps could be other options. 100 Because this pathology is complex, several procedures have been tried, including the use of glues, video-assisted anal fistula treatment (VAAFT), plugs, and, more recently, stem cells. 101 102 Better results appears to be related to procedures that allow opening of the tracks and removal of inflammatory tissue. The use of stem cells may improve the outcomes in the near future.
Anal Incontinence
Definition and Etiology
Anal incontinence is defined as the inability to control the passage of feces or gas in an individual older than 4 years. It is probably an underestimated condition as patients are usually embarrassed to report the symptom to the doctors. 103 However, the estimated prevalence in the general population ranges between 2 and 20%. 104 The consequences of losing sphincteric control can be devastating for the affected individuals, with loss of self-esteem and poor quality of life. The mechanisms that maintain anal continence are complex and multifactorial, but one of the most common causes are obstetric injury and surgical trauma to the sphincters. 105 In Brazil and Mexico, medical assistance to the pregnant women can be challenging, although the incidence of obstetric anal sphincter injuries (OASIS) is around 3.5%, very acceptable in comparison to the numbers that come from Europe and the United States. 106 The etiologies are listed in Table 4 .
Table 4. Etiologies of anal incontinence.
| Anal sphincter |
| Congenital anorectal malformations |
| Dysfunction radiation therapy |
| Obstetric anal sphincter injury |
| Anal surgery |
| Perianal fistulas |
| Sexual abuse |
| Rectal disorders |
| Inflammatory bowel disease |
| Radiation proctitis |
| Rectocele |
| Rectal intussusception |
| Rectal prolapse |
| Fecal impaction |
| Neurological disorders |
| Spinal cord lesions |
| Stroke |
| Multiple sclerosis |
| Spina bifida |
| Diabetic neuropathy |
| Obstetric nerve damage |
| Systemic scleroderma |
| Rapid colorectal transit |
| Chronic diarrhea |
| Time irritable bowel syndrome |
| Psychological encopresis |
| Dementia |
Classification
There is no widely acceptable classification system for anal incontinence. However, in a simplified way, we could consider three basic situations: (1) urgency—associated with damage on the voluntary muscles, especially the external sphincter; (2) passive incontinence—associated with damage in the internal sphincter; (3) soiling mechanism—when sensory motor or reflex alterations cause alterations in rectal perception.
Diagnosis and Barriers to Care
Patients should be evaluated with a meticulous and complete clinical examination providing important information regarding the severity and impact in quality of life. A complete and structured work-up of patients with fecal incontinence was proposed by Saldana Ruiz and Kaiser ( Table 5 ) 107 including some of the validated incontinence scores, such as the Cleveland Clinic Florida Scoring System (CCFSS). 108 This scoring system is the most utilized around the world and have helped us to objectively describe the type and severity of incontinence. It is an easy and simple way to assess incontinence and follow the results of the treatments. In low-income locations, clinical evaluation is the only way to evaluate patients. Although anal manometry and EAUS are more available than in the last decade, those methods are not the reality in many places in the undeveloped world. For patients with fecal incontinence, evaluation of anal pressures and specific parameters of anal manometry can help in the evaluation process. For example, in a 56-year-old female patient with incontinence and urgency, asymmetry and fatigue index are important to evaluate the indication for biofeedback or the use of a bulking agent 109 ( Fig. 8 ).
Table 5. Recommended work-up of fecal incontinence.
| Assessment tool | Details |
|---|---|
| History | • Onset |
| • Quantification: staining, soilage, seepage, accidents | |
| • Qualitative assessment: passive incontinence or urge incontinence | |
| • Obstetric history: pregnancies, vaginal deliveries | |
| • Previous surgeries: anorectal surgery, hysterectomy, bladder surgery, colon and rectal surgery, spinal surgery | |
| • Underlying diseases (diabetes, stroke, etc.) | |
| • Bowel function and stool quality | |
| • Incomplete evacuation | |
| • Stool/gas passage through vagina | |
| • Medications | |
| Scoring instruments | • Cleveland Clinic Florida Fecal Incontinence Score (CCF-FIS) |
| • Fecal Incontinence Quality of Life (FIQoL) score | |
| • Fecal Incontinence Severity Index (FISI) | |
| • St. Mark's Incontinence Score | |
| • EORTEC SF-36 | |
| • Other scoring instruments | |
| Physical examination | • Inspection: patulous anus, folds, perineal body, keyhole, skin irritation, perineal descent, prolapse, cloaca, rectovaginal fistula (stool in the vagina) |
| • Digital exam: sphincter integrity, tone (rest/squeeze), compensatory contraction/discoordination, rectocele, mass | |
| • Sensation/anal reflex | |
| • Instrumentation/visualization: rule out other pathologies (i.e., rectal tumor, proctitis) | |
| Anorectal physiology testing | • Manometry |
| • Anorectal sensation and volume tolerance | |
| • Compliance measurement | |
| • Nerve studies: PNTML, occasionally EMG | |
| • Placement of SNS trial electrode (phase I) | |
| Additional evaluation in select cases | • Imaging: anorectal and endovaginal ultrasound and dynamic MRI |
| • Defecating proctogram | |
| • Evaluation by other specialties (urogynecology, urology, gastroenterology, etc.) |
Abbreviation: MRI, magnetic resonance imaging; EORTEC, European Organization for Research and Treatment of Cancer; SF 36, The Short Form 36 Health Survey Questionnaire; PNTML, pudendal nerve terminal motor latency; EMG, electromyography; SNS, sacral nerve stimulation.
Source: Oliveira LCC, Povedano A, Fonseca R. Clinical Evaluation of Continence and Defecation. In: Oliveira LCC, ed. Anorectal Physiology Textbook: A Clinical and Surgical Perspective. Springer; 2020:47.
Fig. 8.
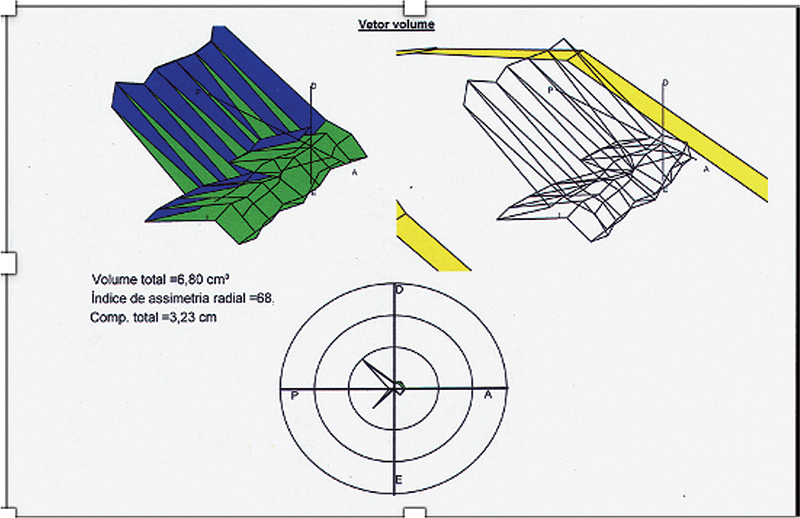
Measurement of asymmetry and fatigue index as part of work-up for fecal incontinence.
The evaluation of the anal canal with the three-dimensional ultrasound transducers brought important information regarding sphincter defects. 110 Those methods are complementary and are becoming more available, even in Latin America.
Management and Outcomes
Initial management includes bulking agents and fibers to modify stool consistency. In addition, loperamide and scopolamine are frequently utilized. When available and when patients are able to voluntary contract the sphincters, pelvic floor rehabilitation and biofeedback can be an option. The approach to the patients should be individualized taking in consideration the particularities of each one. For patients with a poor quality of life and an anatomical defect, such as a cloaca or patulous anus, some reconstruction is necessary, mainly with the technique of anterior sphincteroplasty. 111 Unfortunately, the denervated muscle do not sustain the effects of the repair for a long follow-up. 112
Therefore, since 2004, a minimally invasive procedure involving neuromodulation of afferent pathways that connect the pelvic floor with the brain has changed the way we have been treating patients with fecal incontinence. 113
Regardless of the etiology, the neurostimulation technique has brought a significant improvement in quality of life for those patients. The procedure is more expensive when compared with a sphincter repair, due to the equipment required. However, at long term, it is cost effective. In Latin America, we performed a multicenter trial including incontinent patients that were selected to neuromodulation and it was demonstrated a significant improvement in symptoms and quality of life. 114
In fact, the results of many series and prospective studies on sacral neuromodulation have changed the way we manage our patients with fecal incontinence. One of the advantages of sacral neuromodulation is that it is a reversible technique, with a predictive positive test and the possibility of improving both fecal and urinary incontinence. 115 Because it also has effects on neuroplasticity, sacral neuromodulation has changed the algorithm of treatment of fecal incontinence ( Fig. 9 ).
Fig. 9.
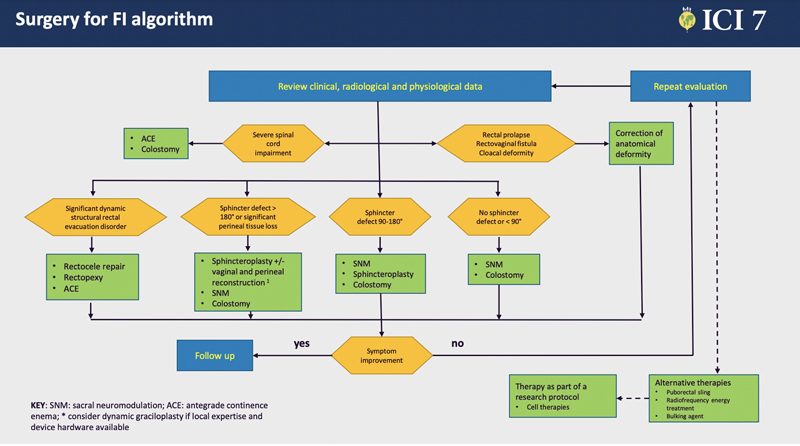
Last proposed fecal incontinence algorithm by the ICS task force on surgical treatment of fecal incontinence (Knowles et al, 2021)
Proctalgia
Anal and anorectal pain occurs in 7 to 24% of the general population, with most of the cases without a specific cause. 116 According to the Rome criteria for functional disorders, there are two major situations: levator ani syndrome and proctalgia fugax.
Levator Ani Syndrome
Levator ani syndrome is a chronic and recurrent anorectal pain with sensation of burning with irradiation to the gluteal region. It is recurrent and worsens in the seated position. Usually, it is idiopathic but can be associated with obesity, surgical procedures, trauma, or childbirth. Digital examination can cause pain and demonstrate a strained or rigid puborectalis. Treatment includes warm sitz baths, digital massage, analgesics, antidepressants, and physiotherapy. Surgical treatment is not indicated.
Proctalgia Fugax
It is a typical nocturnal pain, which originates from spastic contractions of the puborectalis. Located in the anus or lower rectum, it is related to the spasm of the anal sphincter, associated with stress. It lasts few minutes and is more common in women. Diagnosis is by exclusion criteria. Patients are treated with a multidisciplinary team with antispasmodics, oral diazepam, antidepressants, botulin toxin, warm sitz baths, and sphincter massage.
References
- 1.Gosselink M P, van Onkelen R S, Schouten W R. The cryptoglandular theory revisited. Colorectal Dis. 2015;17(12):1041–1043. doi: 10.1111/codi.13161. [DOI] [PubMed] [Google Scholar]
- 2.Seow-Choen F, Ho J MS. Histoanatomy of anal glands. Dis Colon Rectum. 1994;37(12):1215–1218. doi: 10.1007/BF02257784. [DOI] [PubMed] [Google Scholar]
- 3.Parks A G.Pathogenesis and treatment of fistula-in-ano BMJ 19611(5224):460–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steele S R, Hull T L, Read T E, Saclarides T J, Senagore A J, Whitlow C B. Springer International Publishing; 2016. The ASCRS Textbook of Colon and Rectal Surgery. [Google Scholar]
- 5.Ommer A, Herold A, Berg E, Fürst A, Sailer M, Schiedeck T. German S3 guideline: anal abscess. Int J Colorectal Dis. 2012;27(06):831–837. doi: 10.1007/s00384-012-1430-x. [DOI] [PubMed] [Google Scholar]
- 6.Ramanujam P S, Prasad M L, Abcarian H, Tan A B. Perianal abscesses and fistulas. A study of 1023 patients. Dis Colon Rectum. 1984;27(09):593–597. doi: 10.1007/BF02553848. [DOI] [PubMed] [Google Scholar]
- 7.Vogel J D, Johnson E K, Morris A M. Clinical practice guideline for the management of anorectal abscess, fistula-in-ano, and rectovaginal fistula. Dis Colon Rectum. 2016;59(12):1117–1133. doi: 10.1097/DCR.0000000000000733. [DOI] [PubMed] [Google Scholar]
- 8.Country profile: MexicoLibrary of Congress Federal Research Division. Published online July 2008. Accessed November 18, 2021 at:https://www.loc.gov/rr/frd/cs/profiles/Mexico.pdf
- 9.Herr C H, Williams J C. Supralevator anorectal abscess presenting as acute low back pain and sciatica. Ann Emerg Med. 1994;23(01):132–135. doi: 10.1016/s0196-0644(94)70020-6. [DOI] [PubMed] [Google Scholar]
- 10.Prasad M L, Read D R, Abcarian H. Supralevator abscess: diagnosis and treatment. Dis Colon Rectum. 1981;24(06):456–461. doi: 10.1007/BF02626783. [DOI] [PubMed] [Google Scholar]
- 11.Held D, Khubchandani I, Sheets J, Stasik J, Rosen L, Riether R. Management of anorectal horseshoe abscess and fistula. Dis Colon Rectum. 1986;29(12):793–797. doi: 10.1007/BF02555347. [DOI] [PubMed] [Google Scholar]
- 12.Azpiroz-Leehan J, Licona F M, Méndez M C. Imaging facilities for basic medical units: a case in the state of Guerrero, Mexico. J Digit Imaging. 2011;24(05):857–863. doi: 10.1007/s10278-010-9349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez F L, Azpiroz-Leehan J, Cadena M M, Duarte S Y, Teran A G. Knowledge Network for Medical Technology Management in Mexico. Int J Technol Assessment Health Care. 2009;25(04):564–569. doi: 10.1017/S0266462309990341. [DOI] [PubMed] [Google Scholar]
- 14.Sözener U, Gedik E, Kessaf Aslar A. Does adjuvant antibiotic treatment after drainage of anorectal abscess prevent development of anal fistulas? A randomized, placebo-controlled, double-blind, multicenter study. Dis Colon Rectum. 2011;54(08):923–929. doi: 10.1097/DCR.0b013e31821cc1f9. [DOI] [PubMed] [Google Scholar]
- 15.Zinicola R, Cracco N. Draining an anal abscess: the skeletal muscle rule. Colorectal Dis. 2014;16(07):562–562. doi: 10.1111/codi.12651. [DOI] [PubMed] [Google Scholar]
- 16.Cox S W, Senagore A J, Luchtefeld M A, Mazier W P. Outcome after incision and drainage with fistulotomy for ischiorectal abscess. Am Surg. 1997;63(08):686–689. [PubMed] [Google Scholar]
- 17.Yano T, Asano M, Matsuda Y, Kawakami K, Nakai K, Nonaka M. Prognostic factors for recurrence following the initial drainage of an anorectal abscess. Int J Colorectal Dis. 2010;25(12):1495–1498. doi: 10.1007/s00384-010-1011-9. [DOI] [PubMed] [Google Scholar]
- 18.Vasilevsky C A, Gordon P H. The incidence of recurrent abscesses or fistula-in-ano following anorectal suppuration. Dis Colon Rectum. 1984;27(02):126–130. doi: 10.1007/BF02553995. [DOI] [PubMed] [Google Scholar]
- 19.Onaca N, Hirshberg A, Adar R. Early reoperation for perirectal abscess: a preventable complication. Dis Colon Rectum. 2001;44(10):1469–1473. doi: 10.1007/BF02234599. [DOI] [PubMed] [Google Scholar]
- 20.Seow-En I, Ngu J. Routine operative swab cultures and post-operative antibiotic use for uncomplicated perianal abscesses are unnecessary. ANZ J Surg. 2017;87(05):356–359. doi: 10.1111/ans.12936. [DOI] [PubMed] [Google Scholar]
- 21.Schouten W R, van Vroonhoven T JMV. Treatment of anorectal abscess with or without primary fistulectomy. Results of a prospective randomized trial. Dis Colon Rectum. 1991;34(01):60–63. doi: 10.1007/BF02050209. [DOI] [PubMed] [Google Scholar]
- 22.Beck D E, Fazio V W, Lavery I C, Jagelman D G, Weakley F L. Catheter drainage of ischiorectal abscesses. South Med J. 1988;81(04):444–446. doi: 10.1097/00007611-198804000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Browder L K, Sweet S, Kaiser A M. Modified Hanley procedure for management of complex horseshoe fistulae. Tech Coloproctol. 2009;13(04):301–306. doi: 10.1007/s10151-009-0539-6. [DOI] [PubMed] [Google Scholar]
- 24.Ustynoski K, Rosen L, Stasik J, Riether R, Sheets J, Khubchandani I T. Horseshoe abscess fistula. Seton treatment. Dis Colon Rectum. 1990;33(07):602–605. doi: 10.1007/BF02052216. [DOI] [PubMed] [Google Scholar]
- 25.Malik A I, Nelson R L, Tou S. Incision and drainage of perianal abscess with or without treatment of anal fistula. Cochrane Database Syst Rev. 2010;(07):CD006827. doi: 10.1002/14651858.CD006827.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Herzig D O, Lu K C. Anal fissure. Surg Clin North Am. 2010;90(01):33–44. doi: 10.1016/j.suc.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Farouk R, Duthie G S, MacGregor A B, Bartolo D CC. Sustained internal sphincter hypertonia in patients with chronic anal fissure. Dis Colon Rectum. 1994;37(05):424–429. doi: 10.1007/BF02076185. [DOI] [PubMed] [Google Scholar]
- 28.Nothmann B J, Schuster M M. Internal anal sphincter derangement with anal fissures. Gastroenterology. 1974;67(02):216–220. [PubMed] [Google Scholar]
- 29.Schouten W R, Briel J W, Auwerda J JA, De Graaf E JR. Ischaemic nature of anal fissure. Br J Surg. 1996;83(01):63–65. doi: 10.1002/bjs.1800830120. [DOI] [PubMed] [Google Scholar]
- 30.Klosterhalfen B, Vogel P, Rixen H, Mittermayer C. Topography of the inferior rectal artery: a possible cause of chronic, primary anal fissure. Dis Colon Rectum. 1989;32(01):43–52. doi: 10.1007/BF02554725. [DOI] [PubMed] [Google Scholar]
- 31.Schlichtemeier S, Engel A. Anal fissure. Aust Prescr. 2016;39(01):14–17. doi: 10.18773/austprescr.2016.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lorenzoni L.Health systems characteristics: A survey of 21 Latin American and Caribbean countriesOECD Health Working Papers, No. 111, OECD Publishing, Paris. 2019. Accessed July 10, 2022 athttps://dx.doi.org/10.1787/0e8da4bd-en
- 33.Jost W H. Incidence of anal fissure in nonselected neurological patients. Dis Colon Rectum. 1999;42(06):828. doi: 10.1007/BF02236950. [DOI] [PubMed] [Google Scholar]
- 34.Banerjee A K.Treating anal fissure BMJ 1997314(7095):1638–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson R L, Abcarian H, Davis F G, Persky V. Prevalence of benign anorectal disease in a randomly selected population. Dis Colon Rectum. 1995;38(04):341–344. doi: 10.1007/BF02054218. [DOI] [PubMed] [Google Scholar]
- 36.Stewart D B, Sr, Gaertner W, Glasgow S, Migaly J, Feingold D, Steele S R. Clinical practice guideline for the management of anal fissures. Dis Colon Rectum. 2017;60(01):7–14. doi: 10.1097/DCR.0000000000000735. [DOI] [PubMed] [Google Scholar]
- 37.Berry S M, Barish C F, Bhandari R. Nitroglycerin 0.4% ointment vs placebo in the treatment of pain resulting from chronic anal fissure: a randomized, double-blind, placebo-controlled study. BMC Gastroenterol. 2013;13(01):106. doi: 10.1186/1471-230X-13-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ala S, Enayatifard R, Alvandipour M, Qobadighadikolaei R. Comparison of captopril (0.5%) cream with diltiazem (2%) cream for chronic anal fissure: a prospective randomized double-blind two-centre clinical trial. Colorectal Dis. 2016;18(05):510–516. doi: 10.1111/codi.13147. [DOI] [PubMed] [Google Scholar]
- 39.Fissure Study Group . Bailey H R, Beck D E, Billingham R P. A study to determine the nitroglycerin ointment dose and dosing interval that best promote the healing of chronic anal fissures. Dis Colon Rectum. 2002;45(09):1192–1199. doi: 10.1007/s10350-004-6392-9. [DOI] [PubMed] [Google Scholar]
- 40.Sanei B, Mahmoodieh M, Masoudpour H. Comparison of topical glyceryl trinitrate with diltiazem ointment for the treatment of chronic anal fissure: a randomized clinical trial. Acta Chir Belg. 2009;109(06):727–730. doi: 10.1080/00015458.2009.11680524. [DOI] [PubMed] [Google Scholar]
- 41.Lysy J, Israeli E, Levy S, Rozentzweig G, Strauss-Liviatan N, Goldin E. Long-term results of “chemical sphincterotomy” for chronic anal fissure: a prospective study. Dis Colon Rectum. 2006;49(06):858–864. doi: 10.1007/s10350-006-0510-9. [DOI] [PubMed] [Google Scholar]
- 42.Berkel A EM, Rosman C, Koop R, van Duijvendijk P, van der Palen J, Klaase J M. Isosorbide dinitrate ointment vs botulinum toxin A (Dysport) as the primary treatment for chronic anal fissure: a randomized multicentre study. Colorectal Dis. 2014;16(10):O360–O366. doi: 10.1111/codi.12615. [DOI] [PubMed] [Google Scholar]
- 43.Bolandparvaz S, Moghadam Dizaj P, Salahi R. Evaluation of the risk factors of pilonidal sinus: a single center experience. Turk J Gastroenterol. 2012;23(05):535–537. doi: 10.4318/tjg.2012.0381. [DOI] [PubMed] [Google Scholar]
- 44.Doll D, Matevossian E, Wietelmann K, Evers T, Kriner M, Petersen S. Family history of pilonidal sinus predisposes to earlier onset of disease and a 50% long-term recurrence rate. Dis Colon Rectum. 2009;52(09):1610–1615. doi: 10.1007/DCR.0b013e3181a87607. [DOI] [PubMed] [Google Scholar]
- 45.Harlak A, Mentes O, Kilic S, Coskun K, Duman K, Yilmaz F. Sacrococcygeal pilonidal disease: analysis of previously proposed risk factors. Clinics (São Paulo) 2010;65(02):125–131. doi: 10.1590/S1807-59322010000200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons . Johnson E K, Vogel J D, Cowan M L, Feingold D L, Steele S R. The American Society of Colon and Rectal Surgeons' clinical practice guidelines for the management of pilonidal disease. Dis Colon Rectum. 2019;62(02):146–157. doi: 10.1097/DCR.0000000000001237. [DOI] [PubMed] [Google Scholar]
- 47.Dwight R W, Maloy J K. Pilonidal sinus; experience with 449 cases. N Engl J Med. 1953;249(23):926–930. doi: 10.1056/NEJM195312032492303. [DOI] [PubMed] [Google Scholar]
- 48.Khodakaram K, Stark J, Höglund I, Andersson R E. Minimal excision and primary suture is a cost-efficient definitive treatment for pilonidal disease with low morbidity: a population-based interventional and a cross-sectional cohort study. World J Surg. 2017;41(05):1295–1302. doi: 10.1007/s00268-016-3828-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahmood F, Hussain A, Akingboye A. Pilonidal sinus disease: review of current practice and prospects for endoscopic treatment. Ann Med Surg (Lond) 2020;57:212–217. doi: 10.1016/j.amsu.2020.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harries R L, Alqallaf A, Torkington J, Harding K G. Management of sacrococcygeal pilonidal sinus disease. Int Wound J. 2019;16(02):370–378. doi: 10.1111/iwj.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gidwani A L, Murugan K, Nasir A, Brown R. Incise and lay open: an effective procedure for coccygeal pilonidal sinus disease. Ir J Med Sci. 2010;179(02):207–210. doi: 10.1007/s11845-009-0450-1. [DOI] [PubMed] [Google Scholar]
- 52.Garg P, Garg M, Gupta V, Mehta S K, Lakhtaria P. Laying open (deroofing) and curettage under local anesthesia for pilonidal disease: an outpatient procedure. World J Gastrointest Surg. 2015;7(09):214–218. doi: 10.4240/wjgs.v7.i9.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Al-Khamis A, McCallum I, King P M, Bruce J. Healing by primary versus secondary intention after surgical treatment for pilonidal sinus. Cochrane Database Syst Rev. 2010;2010(01):CD006213. doi: 10.1002/14651858.CD006213.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steele S R, Hull T L, Hyman N, Maykel J A, Read T E, Whitlow C B. Springer International Publishing; 2022. The ASCRS Textbook of Colon and Rectal Surgery. [Google Scholar]
- 55.Pronk A A, Eppink L, Smakman N, Furnee E JB. The effect of hair removal after surgery for sacrococcygeal pilonidal sinus disease: a systematic review of the literature. Tech Coloproctol. 2018;22(01):7–14. doi: 10.1007/s10151-017-1722-9. [DOI] [PubMed] [Google Scholar]
- 56.Centers for Disease Control and Prevention 2018 STD Surveillance ReportAccessed November 29, 2021 at:https://www.cdc.gov/nchhstp/newsroom/2019/2018-STD-surveillance-report.html
- 57.Centers for Disease Control and Prevention Workowski K A, Bolan G A.Sexually transmitted diseases treatment guidelines, 2015 MMWR Recomm Rep 201564(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 58.Kirkcaldy R D, Harvey A, Papp J R. Neisseria gonorrhoeae antimicrobial susceptibility surveillance - The Gonococcal Isolate Surveillance Project, 27 sites, United States, 2014. MMWR Surveill Summ. 2016;65(07):1–19. doi: 10.15585/mmwr.ss6507a1. [DOI] [PubMed] [Google Scholar]
- 59.Chan P A, Robinette A, Montgomery M. Extragenital infections caused by Chlamydia trachomatis and Neisseria gonorrhoeae: a review of the literature. Infect Dis Obstet Gynecol. 2016;2016:5.758387E6. doi: 10.1155/2016/5758387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meyer T, Buder S. The laboratory diagnosis of Neisseria gonorrhoeae : current testing and future demands . Pathogens. 2020;9(02):91. doi: 10.3390/pathogens9020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hook E W., IIISyphilis Lancet 2017389(10078):1550–1557. [DOI] [PubMed] [Google Scholar]
- 62.Clement M E, Okeke N L, Hicks C B. Treatment of syphilis: a systematic review. JAMA. 2014;312(18):1905–1917. doi: 10.1001/jama.2014.13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Copeland N K, Decker C F. Other sexually transmitted diseases chancroid and donovanosis. Dis Mon. 2016;62(08):306–313. doi: 10.1016/j.disamonth.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 64.Velho P ENF, Souza E M, Belda Junior W. Donovanosis. Braz J Infect Dis. 2008;12(06):521–525. doi: 10.1590/s1413-86702008000600015. [DOI] [PubMed] [Google Scholar]
- 65.Goodell S E, Quinn T C, Mkrtichian E, Schuffler M D, Holmes K K, Corey L. Herpes simplex virus proctitis in homosexual men. Clinical, sigmoidoscopic, and histopathological features. N Engl J Med. 1983;308(15):868–871. doi: 10.1056/NEJM198304143081503. [DOI] [PubMed] [Google Scholar]
- 66.Wexner S D, Smithy W B, Milsom J W, Dailey T H. The surgical management of anorectal diseases in AIDS and pre-AIDS patients. Dis Colon Rectum. 1986;29(11):719–723. doi: 10.1007/BF02555318. [DOI] [PubMed] [Google Scholar]
- 67.Leone P A, Trottier S, Miller J M. Valacyclovir for episodic treatment of genital herpes: a shorter 3-day treatment course compared with 5-day treatment. Clin Infect Dis. 2002;34(07):958–962. doi: 10.1086/339326. [DOI] [PubMed] [Google Scholar]
- 68.Aoki F Y, Tyring S, Diaz-Mitoma F, Gross G, Gao J, Hamed K. Single-day, patient-initiated famciclovir therapy for recurrent genital herpes: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2006;42(01):8–13. doi: 10.1086/498521. [DOI] [PubMed] [Google Scholar]
- 69.Chosidow O, Drouault Y, Leconte-Veyriac F. Famciclovir vs. aciclovir in immunocompetent patients with recurrent genital herpes infections: a parallel-groups, randomized, double-blind clinical trial. Br J Dermatol. 2001;144(04):818–824. doi: 10.1046/j.1365-2133.2001.04139.x. [DOI] [PubMed] [Google Scholar]
- 70.Wald A, Carrell D, Remington M, Kexel E, Zeh J, Corey L. Two-day regimen of acyclovir for treatment of recurrent genital herpes simplex virus type 2 infection. Clin Infect Dis. 2002;34(07):944–948. doi: 10.1086/339325. [DOI] [PubMed] [Google Scholar]
- 71.Lorincz A T, Temple G F, Kurman R J, Jenson A B, Lancaster W D. Oncogenic association of specific human papillomavirus types with cervical neoplasia. J Natl Cancer Inst. 1987;79(04):671–677. [PubMed] [Google Scholar]
- 72.Assi R, Reddy V, Einarsdottir H, Longo W E. Anorectal human papillomavirus: current concepts. Yale J Biol Med. 2014;87(04):537–547. [PMC free article] [PubMed] [Google Scholar]
- 73.Beck D E, Jaso R G, Zajac R A. Surgical management of anal condylomata in the HIV-positive patient. Dis Colon Rectum. 1990;33(03):180–183. doi: 10.1007/BF02134175. [DOI] [PubMed] [Google Scholar]
- 74.D'Ambrogio A, Yerly S, Sahli R. Human papilloma virus type and recurrence rate after surgical clearance of anal condylomata acuminata. Sex Transm Dis. 2009;36(09):536–540. doi: 10.1097/OLQ.0b013e3181a866a3. [DOI] [PubMed] [Google Scholar]
- 75.Skinner R B., JrTreatment of molluscum contagiosum with imiquimod 5% cream J Am Acad Dermatol 200247(4, Suppl):S221–S224. [DOI] [PubMed] [Google Scholar]
- 76.Syed T A, Lundin S, Ahmad S A. Topical 0.3% and 0.5% podophyllotoxin cream for self-treatment of condylomata acuminata in women. A placebo-controlled, double-blind study. Dermatology. 1994;189(02):142–145. doi: 10.1159/000246818. [DOI] [PubMed] [Google Scholar]
- 77.Hengge U R, Cusini M. Topical immunomodulators for the treatment of external genital warts, cutaneous warts and molluscum contagiosum. Br J Dermatol. 2003;149 66:15–19. doi: 10.1046/j.0366-077x.2003.05623.x. [DOI] [PubMed] [Google Scholar]
- 78.Safavi A, Gottesman L, Dailey T H. Anorectal surgery in the HIV+ patient: update. Dis Colon Rectum. 1991;34(04):299–304. doi: 10.1007/BF02050588. [DOI] [PubMed] [Google Scholar]
- 79.Weiss E G, Wexner S D. Surgery for anal lesions in HIV-infected patients. Ann Med. 1995;27(04):467–475. doi: 10.3109/07853899709002455. [DOI] [PubMed] [Google Scholar]
- 80.Nadal S R, Manzione C R, Horta S HC, Galvão V. Management of idiopathic ulcer of the anal canal by excision in HIV-positive patients. Dis Colon Rectum. 1999;42(12):1598–1601. doi: 10.1007/BF02236214. [DOI] [PubMed] [Google Scholar]
- 81.Salavastru C M, Chosidow O, Janier M, Tiplica G S. European guideline for the management of pediculosis pubis. J Eur Acad Dermatol Venereol. 2017;31(09):1425–1428. doi: 10.1111/jdv.14420. [DOI] [PubMed] [Google Scholar]
- 82.Bouvresse S, Chosidow O. Scabies in healthcare settings. Curr Opin Infect Dis. 2010;23(02):111–118. doi: 10.1097/QCO.0b013e328336821b. [DOI] [PubMed] [Google Scholar]
- 83.Workowski K A, Bachmann L H, Chan P A. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70(04):1–187. doi: 10.15585/mmwr.rr7004a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vargas S, Calvo G, Qquellon J.Point-of-care testing for sexually transmitted infections in low-resource settingsClin Microbiol Infect 2021:S1198-743X(21)00304-9 [DOI] [PubMed]
- 85.Unemo M, Bradshaw C S, Hocking J S. Sexually transmitted infections: challenges ahead. Lancet Infect Dis. 2017;17(08):e235–e279. doi: 10.1016/S1473-3099(17)30310-9. [DOI] [PubMed] [Google Scholar]
- 86.Kettler H, White K, Hawkes S. Geneva: World Health Organization; 2004. Mapping the Landscape of Diagnostics for Sexually Transmitted Infections: Key Findings and Recommendations. [Google Scholar]
- 87.Kaidar-Person O, Person B, Wexner S D. Hemorrhoidal disease: a comprehensive review. J Am Coll Surg. 2007;204(01):102–117. doi: 10.1016/j.jamcollsurg.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 88.Lohsiriwat V. Treatment of hemorrhoids: a coloproctologist's view. World J Gastroenterol. 2015;21(31):9245–9252. doi: 10.3748/wjg.v21.i31.9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Davis B R, Lee-Kong S A, Migaly J, Feingold D L, Steele S R. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Hemorrhoids. Dis Colon Rectum. 2018;61(03):284–292. doi: 10.1097/DCR.0000000000001030. [DOI] [PubMed] [Google Scholar]
- 90.Chiaretti M, Fegatelli D A, Pappalardo G, Venti M DS, Chiaretti A I. Comparison of centella with flavonoids for treatment of symptoms in hemorrhoidal disease and after surgical intervention: a randomized clinical trial. Sci Rep. 2020;10(01):8009. doi: 10.1038/s41598-020-64772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reis J A. High macro rubber band ligature [online] J Coloproctol. 2013;33(03):145–150. [Google Scholar]
- 92.Gallo G, Martellucci J, Sturiale A. Consensus statement of the Italian society of colorectal surgery (SICCR): management and treatment of hemorrhoidal disease. Tech Coloproctol. 2020;24(02):145–164. doi: 10.1007/s10151-020-02149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rizzo J A, Naig A L, Johnson E K. Anorectal abscess and fistula-in-ano: evidence-based management. Surg Clin North Am. 2010;90(01):45–68. doi: 10.1016/j.suc.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 94.Jimenez M, Mandava N. Treasure Island, FL: StatPearls Publishing; 2021. Anorectal fistula. [PubMed] [Google Scholar]
- 95.Balcı S, Onur M R, Karaosmanoğlu A D. MRI evaluation of anal and perianal diseases. Diagn Interv Radiol. 2019;25(01):21–27. doi: 10.5152/dir.2018.17499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim Y, Park Y J. Three-dimensional endoanal ultrasonographic assessment of an anal fistula with and without H(2)O(2) enhancement. World J Gastroenterol. 2009;15(38):4810–4815. doi: 10.3748/wjg.15.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Siddiqui M RS, Ashrafian H, Tozer P. A diagnostic accuracy meta-analysis of endoanal ultrasound and MRI for perianal fistula assessment. Dis Colon Rectum. 2012;55(05):576–585. doi: 10.1097/DCR.0b013e318249d26c. [DOI] [PubMed] [Google Scholar]
- 98.Brillantino A, Iacobellis F, Reginelli A. Preoperative assessment of simple and complex anorectal fistulas: tridimensional endoanal ultrasound? Magnetic resonance? Both? Radiol Med (Torino) 2019;124(05):339–349. doi: 10.1007/s11547-018-0975-3. [DOI] [PubMed] [Google Scholar]
- 99.Nottingham J M, Rentea R M. Treasure Island, FL: StatPearls Publishing; 2021. Anal fistulotomy. [PubMed] [Google Scholar]
- 100.Hussain T, Hughes B, Sharma A. LIFT combined with mucosal advancement flap for transphincteric fistulas. Tech Coloproctol. 2019;23(03):289–290. doi: 10.1007/s10151-019-01956-5. [DOI] [PubMed] [Google Scholar]
- 101.Sugrue J, Mantilla N, Abcarian A. Sphincter-sparing anal fistula repair: are we getting better? Dis Colon Rectum. 2017;60(10):1071–1077. doi: 10.1097/DCR.0000000000000885. [DOI] [PubMed] [Google Scholar]
- 102.Fitzpatrick D P, Kealey C, Brady D, Goodman M, Gately N. Treatments for the amelioration of persistent factors in complex anal fistula. Biotechnol Lett. 2022;44(01):23–31. doi: 10.1007/s10529-021-03207-w. [DOI] [PubMed] [Google Scholar]
- 103.Oliveira L CC. Springer; 2020. Anorectal Physiology: A Clinical and Surgical Perspective. [Google Scholar]
- 104.Brown H W, Wexner S D, Segall M M, Brezoczky K L, Lukacz E S. Accidental bowel leakage in the mature women's health study: prevalence and predictors. Int J Clin Pract. 2012;66(11):1101–1108. doi: 10.1111/ijcp.12018. [DOI] [PubMed] [Google Scholar]
- 105.O'Leary B D, Bholah T, Kalisse T, Hehir M P, Geary M P. Anal sphincter injury associated with vaginal twin delivery. Am J Perinatol. 2020;37(11):1134–1139. doi: 10.1055/s-0039-1692392. [DOI] [PubMed] [Google Scholar]
- 106.Peppe M V, Stefanello J, Infante B F, Kobayashi M T, Baraldi C O, Brito L GO. Perineal trauma in a low-risk maternity with high prevalence of upright position during the second stage of labor. Rev Bras Ginecol Obstet. 2018;40(07):379–383. doi: 10.1055/s-0038-1666810. [DOI] [PubMed] [Google Scholar]
- 107.Saldana Ruiz N, Kaiser A M. Fecal incontinence - challenges and solutions. World J Gastroenterol. 2017;23(01):11–24. doi: 10.3748/wjg.v23.i1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jorge J M, Wexner S D. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36(01):77–97. doi: 10.1007/BF02050307. [DOI] [PubMed] [Google Scholar]
- 109.Oliveira L C, Neves Jorge J M, Yussuf S, Habr-Gama A, Kiss D, Cecconello I. Anal incontinence improvement after silicone injection may be related to restoration of sphincter asymmetry. Surg Innov. 2009;16(02):155–161. doi: 10.1177/1553350609338374. [DOI] [PubMed] [Google Scholar]
- 110.Regadas F S, Murad-Regadas S M, Lima D M. Anal canal anatomy showed by three-dimensional anorectal ultrasonography. Surg Endosc. 2007;21(12):2207–2211. doi: 10.1007/s00464-007-9339-0. [DOI] [PubMed] [Google Scholar]
- 111.Oliveira L, Pfeifer J, Wexner S D. Physiological and clinical outcome of anterior sphincteroplasty. Br J Surg. 1996;83(04):502–505. doi: 10.1002/bjs.1800830421. [DOI] [PubMed] [Google Scholar]
- 112.Bravo Gutierrez A, Madoff R D, Lowry A C, Parker S C, Buie W D, Baxter N N.Long-term results of anterior sphincteroplasty Dis Colon Rectum 20044705727–731., discussion 731–732 [DOI] [PubMed] [Google Scholar]
- 113.Matzel K E, Stadelmaier U, Besendörfer M. Sacral nerve stimulation. Acta Chir Iugosl. 2004;51(02):49–51. doi: 10.2298/aci0402049m. [DOI] [PubMed] [Google Scholar]
- 114.Oliveira L, Hagerman G, Torres M L. Sacral neuromodulation for fecal incontinence in Latin America: initial results of a multicenter study. Tech Coloproctol. 2019;23(06):545–550. doi: 10.1007/s10151-019-02004-y. [DOI] [PubMed] [Google Scholar]
- 115.De Wachter S, Knowles C H, Elterman D S. New technologies and applications in sacral neuromodulation: an update. Adv Ther. 2020;37(02):637–643. doi: 10.1007/s12325-019-01205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bharucha A E, Lee T H. Anorectal and pelvic pain. Mayo Clin Proc. 2016;91(10):1471–1486. doi: 10.1016/j.mayocp.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


