Abstract
This review summarizes recent developments in the prevention and treatment of patients with early-stage breast cancer. The individual disease risk for different molecular subtypes was investigated in a large epidemiological study. With regard to treatment, new data are available from long-term follow-up of the Aphinity study, as well as new data on neoadjuvant therapy with atezolizumab in HER2-positive patients. Biomarkers, such as residual cancer burden, were investigated in the context of pembrolizumab therapy. A Genomic Grade Index study in elderly patients is one of a group of studies investigating the use of modern multigene tests to identify patients with an excellent prognosis in whom chemotherapy may be avoided. These and other aspects of the latest developments in the diagnosis and treatment of breast cancer are described in this review.
Key words: breast cancer, early stage, adjuvant treatment, neoadjuvant treatment, chemotherapy, endocrine therapy
Introduction
The majority of international congresses have been held online in the past two years, but this year the ASCO Congress 2022 was once again held in person. This Congress, as well as other events and current publications, are summarized in this review and placed in the context of current therapies.
With regard to prevention, interventions are becoming increasingly individualized. With regard to treatments, new drugs such as abemaciclib, olaparib and pembrolizumab are entering the clinical arena for the treatment of early-stage breast cancer patients. As these drugs become more widely used, biomarkers are being sought that can, on an individual basis, determine the effectiveness of new treatments or the patientʼs prognosis with conventional treatments. In this context, new data exist on multigene testing and chemotherapy in older patients. Understanding which patient groups would benefit most from immunotherapy with checkpoint inhibition could also assist in making individualized treatment decisions.
Prevention
Well-known but still a big unknown – reproductive traits as risk factors for breast cancer
As with the individualization of breast cancer treatment, prevention and early detection increasingly take account of individual risks not only for the disease itself but also for mortality after diagnosis. In this context, molecular characteristics often serve as surrogate markers for the relevant studies.
For example, in those at high risk of triple-negative disease, more extensive preventive measures may be warranted compared with women at increased risk of breast cancer but who have a good prognosis. Similarly, prevention could be individualized for different subtypes of breast cancer.
Several risk factors have already been studied from this perspective. For example, it has been clear for a long time that women with a BRCA1 germ line mutation are most likely to develop triple-negative breast cancer, and conversely women with triple-negative breast cancer have high rates of BRCA1/2 mutation 1 , 2 , 3 , 4 , 5 . However, other breast cancer risk genes such as BRCA2, BARD1 and PALB2 have also been associated with an increased risk of triple-negative breast cancer in particular 6 , 7 , 8 . Some low-penetrance risk genes were found to have an association with poor prognosis or specific molecular subtypes 9 , 10 , 11 , 12 , 13 , 14 , 15 .
Non-genetic risk factors focus on mammographic density 16 , 17 , 18 and reproductive factors 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 . In addition, in particular the age at menarche and at menopause, as well as the number of children, are well-established risk factors, as is the duration of breastfeeding 19 , 21 .
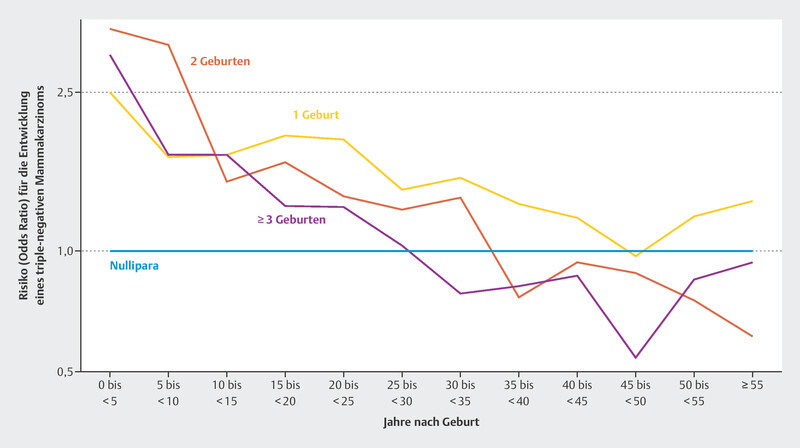
Regarding reproductive risk factors, a large study has now been published that examined reproductive factors in relation to risk for the various molecular subtypes of breast cancer 28 . This work examined more than 23 000 breast cancer patients and more than 71 000 healthy controls from 31 population-based studies. It was reported that women with at least one pregnancy had a lower risk of luminal like and HER2-positive breast cancer. However, this effect did not occur until about 10 years after the last birth. Pregnancies increased the risk of triple-negative breast cancer for decades after the last birth 28 , before approaching or falling below the risk of nulliparous women. Fig. 1 shows the risk development of a diagnosis of triple-negative breast cancer over time after delivery, compared to women who reported not given birth 28 .
Fig. 1.
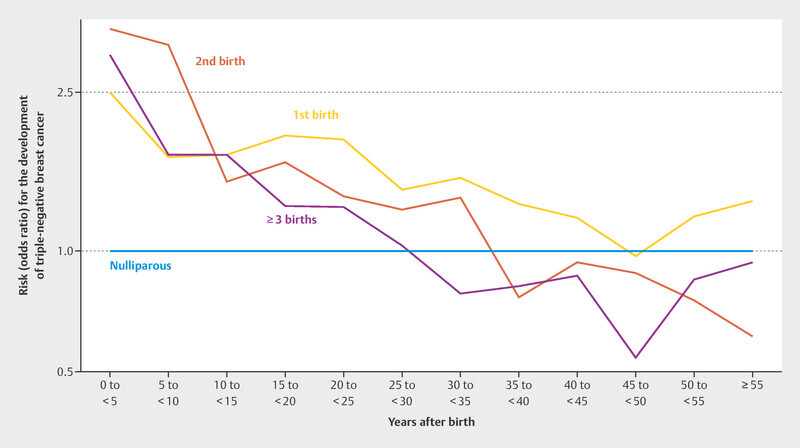
Odds ratio for developing triple-negative breast cancer for patients who have had one, two, and three deliveries relative to women who have not delivered a baby 28 .
The data from this large epidemiological study are significant because they disaggregate breast cancer risk over time. For centuries, pregnancy has been thought to reduce the risk of breast cancer 29 . While this is true for most postmenopausal patients and also for most molecular subtypes, the situation is different for triple-negative breast cancer 28 . In this case, the risk seems to remain elevated for many decades after pregnancy. This is particularly important because subsequent pregnancy may lead to a significant increase in the incidence of this subtype, which carries a poor prognosis.
Molecularly, the mechanisms leading to mammary gland transformation during pregnancy and lactation have also been associated with proliferation of epithelial stem cells in the breast 30 – 33 . The RANK/RANKL/OPG pathway appears to play a significant role not only in bone metabolism but also in the transformation of the mammary gland during pregnancy 33 , and is associated with other risk factors for the development of breast cancer 34 .
Future studies must show exactly which molecular mechanisms are responsible for these observations and whether these correlations can be utilized in breast cancer prevention.
Data on Ovarian Suppression in Combination with Tamoxifen
The choice of anti-hormonal adjuvant therapy in premenopausal patients is still under discussion. Simplified, national treatment recommendations call for patients at low risk of relapse to receive tamoxifen, and patients at intermediate risk of relapse to receive tamoxifen in combination with ovarian suppression. Patients at high risk of relapse may be treated with an aromatase inhibitor in combination with ovarian suppression 35 . Most of the evidence is drawn from the SOFT and TEXT studies 36 , 37 , 38 , 39 . The long-term follow-up data from the Korean ASTRRA study have now been published 40 .
Ovarian suppression in combination with tamoxifen – long-term data consolidate the evidence
The ASTRRA study enrolled patients who were under 46 years of age, had stage I to III disease at diagnosis, and had received (neo)adjuvant chemotherapy. A total of 1282 patients were randomized to treatment with tamoxifen for 5 years, or treatment with tamoxifen for 5 years and goserelin for 2 years. The median follow-up time in the recently reported analysis was 8.9 years. The previously observed disparity is again apparent in this analysis. Treatment with tamoxifen and ovarian suppression showed better disease-free survival with a hazard ratio of 0.67 (95% CI: 0.51 – 0.87). Absolute disease-free survival rates at 8 years were 80.2% for tamoxifen alone and 85.4% for patients with ovarian suppression, an absolute reduction of 5.2%. This difference did not translate into overall survival in a statistically significant manner, although it should be noted that survival in the recruited group of patients was excellent, with an OS rate at 8 years of 96.5% in the OFS group and 95.3% in the tamoxifen group (HR = 0.78; 95% CI: 0.49 – 1.25 40 . In subgroup analyses, the effects were more pronounced in patients aged 40 to 45 years and in HER2-negative patients.
Thus, the ASTRRA study contributes to the body of data that has emerged from the other studies in the treatment setting, namely that the addition of OFS improves disease-free survival, but probably not overall survival. The decision to treat the known side effects of OFS (goserelin in this case) should always be made individually in consultation with the patient.
Anti-HER2 Therapies in the Neoadjuvant and Adjuvant Setting
More than any other molecular subtype, treatment for HER2-positive early-stage breast cancer has improved the prognosis of affected patients with the introduction of new drugs. Not only trastuzumab, but also pertuzumab 41 , trastuzumab-emtansine (T-DM1) 42 , and neratinib 43 , 44 are approved for adjuvant treatment of patients with HER2-positive early-stage breast cancer.
Pertuzumab in long-term follow-up
Pertuzumab can be used in the neoadjuvant and adjuvant setting. In the neoadjuvant setting, the rate of pCR is increased by approximately 20% 45 , 46 , 47 . In the adjuvant setting, a disease-free survival (DFS) benefit was reported in the Aphinity study with a median follow-up of 45.4 months (HR in favor of combination therapy of 0.81; 95% CI: 0.66 – 1.00). Subgroup analysis by nodal status showed that patients with positive lymph node status in particular benefited from therapy (HR = 0.77; 95% CI 0.62 – 0.96) and patients with negative nodal status benefited less (HR = 1.13; 95% CI 0.68 – 1.86). Now, after a second interim analysis, the third interim analysis for overall survival has been published with a median follow-up of 8.4 years 48 . Just as in previous analyses, the evaluation in terms of overall survival did not achieve statistical significance with an HR of 0.83 (95% CI: 0.68 – 1.02), but there was a numerical advantage for the addition of pertuzumab. This effect was somewhat more pronounced in the nodal-positive patients (HR = 0.80, 95% CI: 0.63 – 1.00). In nodal-negative patients, an HR of 0.99 (0.64 – 1.55) indicates that pertuzumab has no effect on overall survival. Exploratory analyses of disease-free survival (DFS) showed very similar results to the previous studies, especially with regard to the greater treatment effect in nodal-positive patients.
Thus, the data on pertuzumab have not changed much and the current treatment recommendations 35 advising treatment in patients with nodal-positive disease and allowing individual treatment decisions in patients with nodal-negative disease remain valid after this analysis.
Atezolizumab in the neoadjuvant setting
While data exist from a large randomized study of pembrolizumab (KEYNOTE-522 study) for patients with early-stage triple-negative breast cancer 49 , 50 , and pembrolizumab is approved for neoadjuvant in combination with chemotherapy followed by adjuvant treatment, there are relatively few data for patients with hormone receptor-positive disease and patients with HER2-positive disease. Now, the results of IMpassion050 with reference to pCR have been published 51 . In the IMpassion050 study, 454 HER2-positive patients were enrolled and randomized to neoadjuvant therapy with either dose-dense chemotherapy with doxoribicin/cyclophosphamide followed by therapy with paclitaxel in combination with trastuzumab and pertuzumab, or to the same therapy in combination with atezolizumab. Overall, there was no difference in the pCR rate. It was 62.4% in patients with atezolizumab and slightly higher at 62.7% in patients without atezolizumab. Interestingly, in patients without atezolizumab, there was a significant difference between patients who were PD-L1-positive (pCR: 72.5%) and who were PD-L1-negative (pCR: 53.8). The difference was less in patients who received atezolizumab in addition to chemotherapy (64.2% in PD-L1 positivity and 60.7% in PD-L1 negativity) 51 .
This result is surprising. However, not all discussions about the accuracy of PD-L1 testing are over, and in the KEYNOTE-522 study, the CPS score was also not predictive of the efficacy of pembrolizumab. However, it is noteworthy that in the IMpassion050 study, treatment without atezolizumab had the highest overall pCR rates in the PD-L1-positive population. Surprisingly, a similar effect was seen in IMpassion131 52 with respect to overall survival. Patients with paclitaxel monotherapy had the best numerical overall survival in that study. There was no statistical difference. In breast cancer, there are now treatment scenarios where PD-L1 expression must be present in order to determine effectiveness (first-line advanced triple-negative breast cancer), whereas in patients undergoing neoadjuvant/adjuvant treatment, such a determination is not necessary. However, there may also be combination therapies for which PD-L1 determination is not necessary. More evidence is needed to understand these relationships 53 .
Pembrolizumab in Patients with Triple-Negative Early Breast Cancer – the Search for Biomarkers
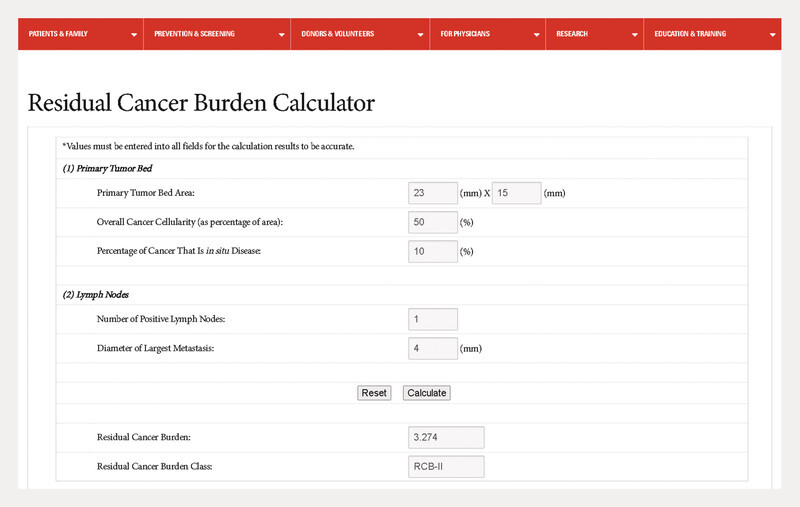
For the treatment of patients with early-stage triple-negative breast cancer at increased risk of recurrence, pembrolizumab was approved as a neoadjuvant treatment in combination with chemotherapy followed by monotherapy in the adjuvant setting to complete one year of treatment. In the KEYNOTE-522 study, it was shown that not only the pCR rate is increased, but that even patients without pCR drew some benefit in terms of event-free survival 49 , 50 , 54 , 55 . This was surprising because it was previously thought that the effect on prognosis was mainly mediated by pCR 56 – 59 . Similarly, in the KEYNOTE-522 study, patients with pCR had an excellent prognosis, which was only slightly better in patients treated with pembrolizumab (3-year event-free rate: 94.4 vs. 92.5%; HR = 0.73; 95% CI: 0.39 – 1.36). Given the side effects, there is often discussion as to whether, in light of this, there are patients who benefit more or less from adjuvant pembrolizumab therapy, or whether there are groups of patients for whom the adjuvant treatment can be omitted. In this context, a preliminary understanding of possible biomarkers is provided by an analysis of the KEYNOTE-522 study using the “Residual Cancer Burden” (RCB) score 60 . The RCB score 61 , 62 , 63 is calculated from several parameters that summarize the response to chemotherapy. For example, it can be determined with an online calculator 64 ( Fig. 2 ).
Fig. 2.
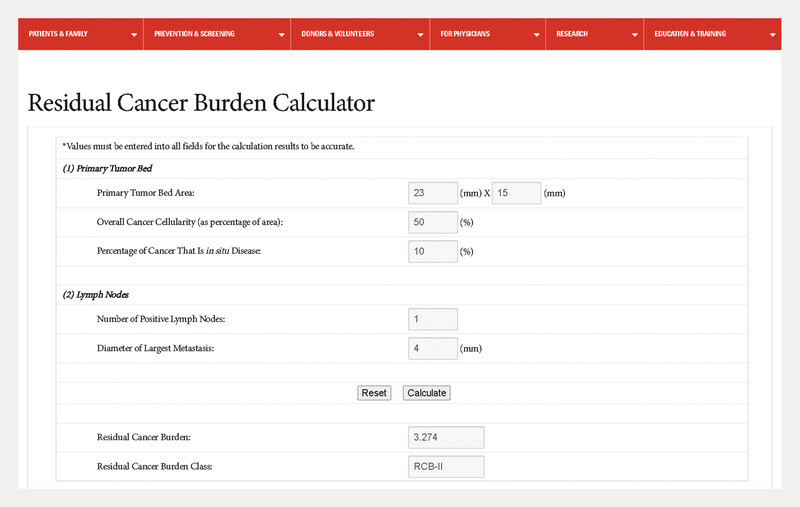
Online Residual Cancer Burden Calculator 64 .
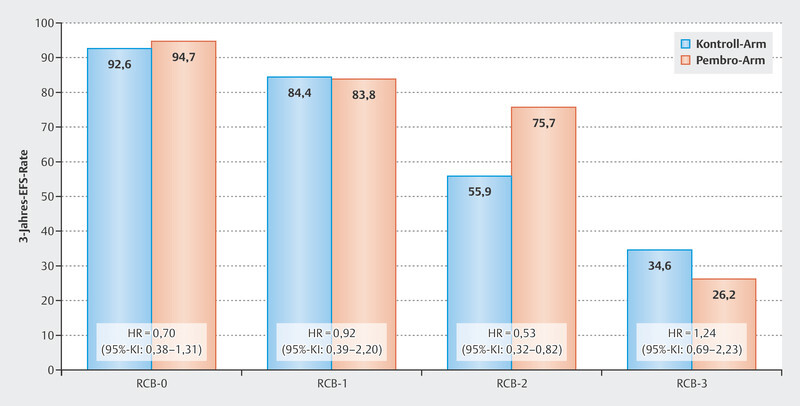
An analysis of efficacy in terms of event-free survival in the KEYNOTE-522 study demonstrated that the effect on prognosis may well differ between RCB groups. Patients with pCR (RCB-0) had excellent prognostic data, which was already known. In general, prognosis was worse depending on RCB category for patients in both the pembrolizumab arm and the control arm with increasing category (increasing residual tumor) ( Fig. 3 ). The clearest benefit for the addition of pembrolizumab was seen in the group of patients with RCB category 2. Here, the HR was 0.52 (95% CI: 0.32 – 0.82), and the 3-year event-free survival rates were 55.9% for the control arm, and 75.7% for the pembrolizumab arm. Patients in the worst category (RCB-3) did not appear to benefit from pembrolizumab therapy.
Fig. 3.
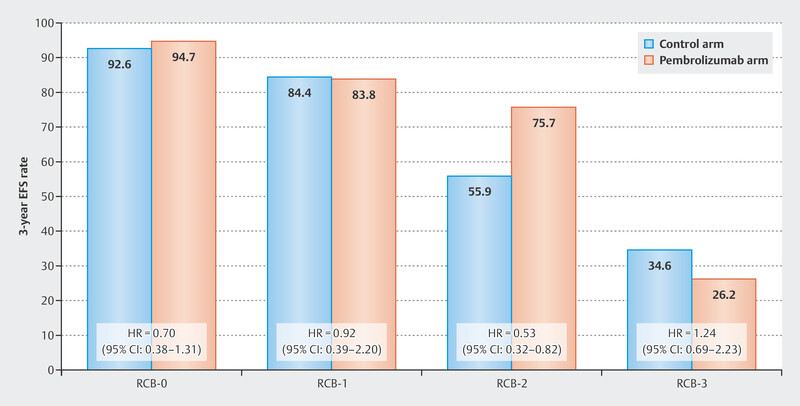
Event-free 3-year survival rates in the KEYNOTE-522 study by Residual Cancer Burden Group 60 .
The use of RCB in clinical practice is not part of a treatment recommendation. However, this examination indicates that this biomarker/score could be further reviewed in future studies to plan therapy after neoadjuvant therapy.
Biomarkers
To date, a few treatments for early and advanced disease have been mandatorily linked to certain biomarkers. These include anti-HER2 therapies (positive HER2 status), endocrine therapies (positive hormone receptor status), alpelisib (somatic PIK3CA tumor mutation), talazoparib/olaparib (germ line mutation in BRCA1/2 ), and pembrolizumab/atezolizumab (PD-L1 expression in metastatic triple-negative breast cancer). Other biomarkers have not been mandatorily established. Prognostic tests, such as multigene testing, can be used to identify patients with early-stage disease who have an excellent prognosis in order to avoid adjuvant chemotherapy. In the United States, one biomarker used in the adjuvant approval of the CDK4/6 inhibitor abemaciclib therapy is the well-known Ki-67 score.
Ki-67 and abemaciclib in patients with HR+ HER2− breast cancer
Ki-67 has been described as a proliferative marker since the 1980s 65 . Its role as a prognostic and predictive factor for pCR after neoadjuvant chemotherapy has been described in multiple studies 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 . However, its clinical use has not been mandatorily recommended to date. However, unlike in Europe, in 2021 the U. S. FDA determined that abemaciclib can be used as an adjuvant in patients with node-positive breast cancer and a Ki-67 ≥ 20%. This is not consistent with the regulatory situation in Europe, where patients with more than 3 affected lymph nodes but also patients with 1 – 3 affected lymph nodes can be prescribed abemaciclib if the tumor is ≥ 5 cm or is grade 3. This divergent approach is currently the subject of scientific debate 77 , 78 . Although it is undisputed that Ki-67 is a significant prognostic factor, the MonarchE study, which provided adjuvant data for abemaciclib treatment, did not demonstrate that Ki-67 has predictive value for the efficacy of abemaciclib, but did not confirm its prognostic relevance 79 . The concerns that oppose the use of Ki-67 are mainly the reproducibility and comparability of results with the risk of not selecting the right patients for therapy. This problem, as described, does not exist in Europe.
Multigene tests in elderly patients
Recent years have seen the accumulation of an extensive body of data that has led to the routine use of several multigene tests in Germany. All multigene tests are more or less capable of identifying HR+/HER2− patients with an excellent prognosis 80 – 85 . However, no clear results could be obtained with regard to predicting the efficacy of chemotherapy. In the RXponder study, the recurrence score was not able to predict the benefit of chemotherapy compared with adjuvant endocrine therapy without prior chemotherapy 86 . There is little data on this topic in older female patients.
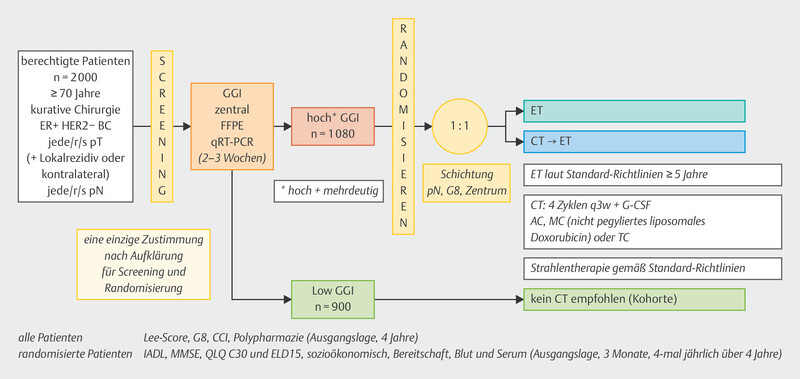
Against this background, the recently published ASTER-70s study provided new insights. In this study, the Genomic Grade Index (GGI) was determined 87 , 88 . The Genomic Grade Index was developed to characterize tumor grading with gene expression analyses. Quantitative PCR is used to determine 97 cell cycle and proliferation genes, and tumors are classified as high, intermediate (equivocal), and low.
The ASTER-70s study ( Fig. 4 ) 89 enrolled patients who were at least 70 years of age and had HR+ HER2− breast cancer without metastases, either as a new diagnosis or as local recurrence. After determination of the GGI, no further chemotherapy was recommended for patients with a low GGI, and randomization was performed for patients with an intermediate or high GGI. One treatment arm was treated with chemotherapy followed by adjuvant hormonal therapy. In the alternative treatment arm, patients received adjuvant endocrine treatment alone 89 . Nearly 1100 patients were randomized. With a median follow-up of almost 6 years, a trend could be seen in favor of therapy with chemotherapy (HR = 0.79, 95% CI: 0.60 – 1.03), which did not meet the threshold of statistical significance. Among older patients, lack of adherence to therapy was relatively high in the chemotherapy arm (20.5%) compared with the randomization arm without chemotherapy (0.6%) 89 . In such cases, a per protocol analysis is always useful, yielding an HR of 0.73 (95% CI: 0.55 – 0.98).
Fig. 4.
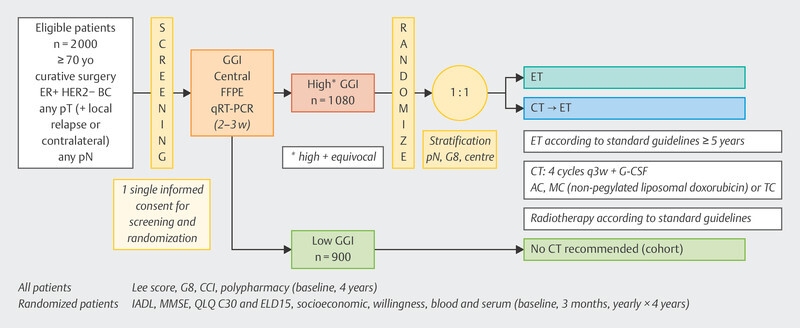
Study design of the ASTER-70s study.
Although the study was negative overall, it does provide evidence to suggest that there are older patients who may benefit from chemotherapy if they are at high risk of relapse (as determined here by GGI).
Outlook
In recent years, there has been a significant increase in data on multigene testing and treatment decisions for or against chemotherapy. Some studies such as the OPTIMA study (with PAM50) are currently still recruiting and will certainly supplement the data.
With respect to neoadjuvant/adjuvant therapy with pembrolizumab, biomarkers could help identify groups of patients who do not need adjuvant therapy. However, this needs to be addressed in future studies.
For patients with HER2-negative HR-positive breast cancer, the preliminary phase of the Natalee study, which is evaluating ribociclib in the adjuvant setting in patients at increased risk of recurrence, is awaited.
In the near future, these and other studies will expand the treatment options for patients in the early stages of the disease.
Acknowledgements
This work was done in part thanks to grants from onkowissen.de, Gilead, Novartis, Pfizer, Roche, and MSD. None of the companies had any part in the preparation and recommendations of this manuscript. The authors are solely responsible for the content of the manuscript.
Danksagung
Diese Arbeit entstand teilweise in Folge von Förderungen der Firmen onkowissen.de, Gilead, Novartis, Pfizer, Roche, und MSD. Keine der Firmen hatte einen Anteil an der Erstellung und den Empfehlungen dieses Manuskriptes. Für den Inhalt des Manuskriptes sind alleine die Autoren verantwortlich.
Footnotes
Conflict of Interest/Interessenkonflikt B. A. received honoria and travel grants from AstraZeneca, Gilead, Genomic Health, Roche, Novartis, Celgene, Lilly, MSD, Eisai, Teva, Tesaro, Daiichi Sankyo and Pfizer. C. K.-L. received honoraria from Roche, AstraZeneca, Celgene, Novartis, Pfizer, Lilly, Hexal, Amgen, Eisai, and SonoScape as well as honoraria for consultancy work from Phaon Scientific, Novartis, Pfizer, and Celgene as well as research assistance from Roche, Novartis, and Pfizer. Travel grant from Novartis and Roche, employment at Palleos Healthcare, and Managing Director and partner at Phaon Scientific. M. B.-P. received honoraria for lectures and advisory role from Roche, Novartis, Pfizer, pfm, Eli Lilly, Onkowissen, Seagen, AstraZeneca, Eisai, Amgen, Samsung, MSD, GSK, Daiichi Sankyo, Gilead, Sirius Pintuition, Pierre Fabre, and study support from Mammotome, Endomag and Merit Medical. E. B. received honoraria from Gilead, Ipsen, Sanofi, Sandoz, SunPharma, AstraZeneca, Novartis, Hexal, BMS, Lilly, Pfizer, Roche, MSD, BBraun and onkowissen.de for clinical research management and/or medical education activities. N. D. has received honoraria from MSD, Roche, AstraZeneca, Teva, Pfizer, Novartis, Seagen,Gilead, MCI Healthcare. P. A. F. reports personal fees from Novartis, grants from Biontech, personal fees from Pfizer, personal fees from Daiichi Sankyo, personal fees from AstraZeneca, personal fees from Eisai, personal fees from Merck Sharp & Dohme, grants from Cepheid, personal fees from Lilly, personal fees from Pierre Fabre, personal fees from SeaGen, personal fees from Roche, personal fees from Hexal, personal fees from Agendia, personal fees from Gilead. T. N. F. has participated on advisory boards for Amgen, Daiichi Sankyo, Novartis, Pfizer, and Roche and has received honoraria for lectures from Amgen, Celgene, Daiichi Sankyo, Roche, Novartis and Pfizer. A. D. H. received speaker and consultancy honoraria from AstraZeneca, Genomic Health, Roche, Novartis, Celgene, Lilly, MSD, Eisai, Teva, Tesaro, Daiichi Sankyo, Hexal and Pfizer. N. H. received honoraria for lectures and/or consulting from Amgen, AstraZeneca, Daiichi Sankyo, Exact Sciences, Gilead, Lilly, MSD, Mylan, Novartis, Pierre Fabre, Pfizer, Roche, Sandoz, Seagen. W. J. has received research Grants and/or honoraria from Sanofi-Aventis, Daiichi Sankyo, Novartis, Roche, Pfizer, Lilly, AstraZeneca, Chugai, GSK, Eisai, Cellgene and Johnson & Johnson. H.-C. K. has received honoraria from Pfizer, Novartis, Roche, Genomic Health/Exact Sciences, Amgen, AstraZeneca, Riemser, Carl Zeiss Meditec, Teva, Theraclion, Janssen-Cilag, GSK, LIV Pharma, Lily, SurgVision, Onkowissen, Gilead, Daiichi Sankyo and MSD, travel support from Carl Zeiss Meditec, LIV Pharma, Novartis, Amgen, Pfizer, Daiichi Sankyo, Tesaro and owns stock of Theraclion SA and Phaon Scientific GmbH. D. L. received honoraria from Amgen, AstraZeneca, Eli Lilly, High5md, Gilead, GSK, Loreal, MSD, Novartis, Onkowissen, Pfizer, Seagen, Teva. M. P. L. has participated on advisory boards for AstraZeneca, Lilly, MSD, Novartis, Pfizer, Eisai, Gilead, Exact Sciences, Pierre Fabre, Grünenthal, Daiichi Sankyo, PharmaMar and Roche and has received honoraria for lectures from MSD, Lilly, Roche, Novartis, Pfizer, Exact Sciences, Daiichi Sankyo, Grünenthal, Gilead, AstraZeneca, and Eisai. He is editorial board member of medactuell from medac. V. M. received speaker honoraria from Amgen, AstraZeneca, Daiichi Sankyo, Eisai, GSK, Pfizer, MSD, Medac, Novartis, Roche, Teva, Seagen, Onkowissen, high5 Oncology, Medscape, Gilead. Consultancy honoraria from Hexal, Roche, Pierre Fabre, Amgen, ClinSol, Novartis, MSD, Daiichi Sankyo, Eisai, Lilly, Sanofi, Seagen, Gilead. Institutional research support from Novartis, Roche, Seagen, Genentech. Travel grants: Roche, Pfizer, Daiichi Sankyo. E. S. received honoraria from Roche, Celgene, AstraZeneca, Novartis, Pfizer, Tesaro, Aurikamed GmbH, MCI Deutschland GmbH, bsh medical communications GmbH, Onkowissen TV. A. S. received research grants from Celgene, Roche, honoraria from Amgen, AstraZeneca, Aurikamed, Bayer, Celgene, Clinsol, Connectmedica, Gilead, GSK, I-MED, Lilly, MCI Deutschland, Metaplan, MSD, Nanostring, Novartis, Onkowissen.de, Promedicis, Pfizer, Pierre Fabre, Roche, Seagen, Streamedup, Teva, Tesaro, Thieme and travel support from Celgene, Pfizer, Roche. F. S. participated on advisory boards for Novartis, Lilly, Amgen and Roche and received honoraria for lectures from Roche, AstraZeneca, MSD, Novartis and Pfizer. H. T. received honoraria from Novartis, Roche, Celgene, Teva, Pfizer, Astra Zeneca and travel support from Roche, Celgene and Pfizer. C. T. received honoraria for advisory boards and lectures from Amgen, AstraZeneca, Celgene, Daiichi Sankyo, Eisai, Gilead, Lilly, MSD, Mylan, Nanostring, Novartis, Pfizer, Pierre Fabre, Puma, Roche, Seagen, Vifor. M. T. has participated on advisory boards for AstraZeneca, Clovis, Daiichi Sankyo, Eisai, Gilead Science, GSK, Lilly, MSD, Novartis, Organon, Pfizer, Pierre Fabre, Seagen and Roche and has received honoraria for lectures from Amgen, Clovis, Daiichi Sankyo, Eisai, GSK, Lilly, MSD, Roche, Novartis, Organon, Pfizer, Seagen, Exact Sciences, Viatris, Vifor and AstraZeneca and has received trial funding by Exact Sciences and Endomag Manuscript support was done by Amgen, ClearCut, pfm medical, Roche, Servier, Vifor. M. U. All honoraria went to the institution/employer: Abbvie, Amgen, AstraZeneca, Daiichi Sankyo, Eisai, Lilly, MSD, Myriad Genetics, Pfizer, Roche, Sanofi Aventis, Novartis, Pierre Fabre, Seagen; Gilead. M. W. has participated on advisory boards for AstraZeneca, Lilly, MSD, Novartis, Pfizer and Roche. I. W. has participated on advisory boards for Novartis, Daichii Sankyo, Lilly, Pfizer and received speaker honoraria from Astra Zeneca, Daichii Sankyo, MSD, Novartis, Pfizer, Roche. A. W. participated on advisory boards for Novartis, Lilly, Amgen, Pfizer, Roche, Tesaro, Eisai and received honoraria for lectures from Novartis, Pfizer, Aurikamed, Roche, Celgene./ B. A. hat von AstraZeneca, Gilead, Genomic Health, Roche, Novartis, Celgene, Lilly, MSD, Eisai, Teva, Tesaro, Daiichi Sankyo und Pfizer Honorare und Reisekostenzuschüsse erhalten. C. K.-L. hat folgende Honorare erhalten: Roche, AstraZeneca, Celgene, Novartis, Pfizer, Lilly, Hexal, Amgen, Eisai und SonoScape. Beraterhonorare von: Phaon Scientific, Novartis, Pfizer und Celgene. Forschungsuntertützung: Roche, Novartis und Pfizer. Reiseunterstützung: Novartis und Roche. Anstellung bei Palleos Healthcare. Managing Director und Partner bei Phaon Scientific. M. B.-P. hat von Roche, Novartis, Pfizer, pfm, Eli Lilly, Onkowissen, Seagen, AstraZeneca, Eisai, Amgen, Samsung, MSD, GSK, Daiichi Sankyo, Gilead, Sirius Pintuition und Pierre Fabre Honorare für Vorträge und beratende Tätigkeiten sowie von EndoMag, Mammotome und Merit Medical Studienunterstützung erhalten. E. B. hat von Gilead, Ipsen, Sanofi, Sandoz, SunPharma, AstraZeneca, Novartis, Hexal, BMS, Lilly, Pfizer, Roche, MSD, BBraun und onkowissen.de Honorare für klinisches Forschungsmanagement und/oder medizinische Aus- und Weiterbildung erhalten. N. D. hat von MSD, Roche, AstraZeneca, Teva, Pfizer, Novartis, Seagen, Gilead und MCI Healthcare Honorare erhalten. P. A. F. weist Folgendes aus: Honorare von Novartis, Zuwendungen von Biontech, Honorare von Pfizer, Honorare von Daiichi Sankyo, Honorare von AstraZeneca, Honorare von Eisai, Honorare von Merck Sharp & Dohme, Zuwendungen von Cepheid, Honorare von Lilly, Honorare von Pierre Fabre, Honorare von SeaGen, Honorare von Roche, Honorare von Hexal, Honorare von Agendia und Honorare von Gilead. T. N. F. hat in beratenden Gremien bei Amgen, Daiichi Sankyo, Novartis, Pfizer und Roche mitgewirkt und von Amgen, Celgene, Daiichi Sankyo, Roche, Novartis und Pfizer Honorare für Vorträge erhalten. A. D. H. hat als Referent und Berater von AstraZeneca, Genomic Health, Roche, Novartis, Celgene, Lilly, MSD, Eisai, Teva, Tesaro, Daiichi Sankyo, Hexal und Pfizer Honorare erhalten. N. H. hat für Vorträge und/oder Beratung von Amgen, AstraZeneca, Daiichi Sankyo, Exact Sciences, Gilead, Lilly, MSD, Mylan, Novartis, Pierre Fabre, Pfizer, Roche, Sandoz und Seagen Honorare erhalten. W. J. hat von Sanofi-Aventis, Daiichi-Sankyo, Novartis, Roche, Pfizer, Lilly, AstraZeneca, Chugai, GSK, Eisai, Cellgene und Johnson & Johnson Forschungsbeihilfen und/oder Honorare erhalten. H.-C. K. hat von Pfizer, Novartis, Roche, Genomic Health/Exact Sciences, Amgen, AstraZeneca, Riemser, Carl Zeiss Meditec, Teva, Theraclion, Janssen-Cilag, GSK, LIV Pharma, Lily, SurgVision, Onkowissen, Gilead, Daiichi Sankyo und MSD Honorare sowie von Carl Zeiss Meditec, LIV Pharma, Novartis, Amgen, Pfizer, Daiichi Sankyo und Tesaro Reisekostenzuschüsse erhalten und besitzt Aktien von Theraclion SA und Phaon Scientific GmbH. D. L. hat von Amgen, AstraZeneca, Eli Lilly, High5md, Gilead, GSK, Loreal, MSD, Novartis, Onkowissen, Pfizer, Seagen und Teva Honorare erhalten. M. P. L. hat in beratenden Gremien bei AstraZeneca, Lilly, MSD, Novartis, Pfizer, Eisai, Gilead, Exact Sciences, Pierre Fabre, Grünenthal, Daiichi Sankyo, PharmaMar und Roche mitgewirkt und von MSD, Lilly, Roche, Novartis, Pfizer, Exact Sciences, Daiichi Sankyo, Grünenthal, Gilead, AstraZeneca und Eisai Honorare für Vorträge erhalten. Er ist Mitglied der Redaktion von medactuell von medac. V. M. hat von Amgen, AstraZeneca, Daiichi Sankyo, Eisai, GSK, Pfizer, MSD, Medac, Novartis, Roche, Teva, Seagen, Onkowissen, high5 Oncology, Medscape und Gilead Honorare als Referent erhalten. Beraterhonorare von Hexal, Roche, Pierre Fabre, Amgen, ClinSol, Novartis, MSD, Daiichi Sankyo, Eisai, Lilly, Sanofi, Seagen, Gilead. Institutionelle Forschungsunterstützung von Novartis, Roche, Seagen, Genentech. Reisekostenzuschüsse von: Roche, Pfizer, Daiichi Sankyo. E. S. hat von Roche, Celgene, AstraZeneca, Novartis, Pfizer, Tesaro, Aurikamed GmbH, MCI Deutschland GmbH, bsh medical communications GmbH und Onkowissen TV Honorare erhalten. A. S. hat von Celgene und Roche Forschungszuschüsse, von Amgen, AstraZeneca, Aurikamed, Bayer, Celgene, Clinsol, Connectmedica, Gilead, GSK, I-MED, Lilly, MCI Deutschland, Metaplan, MSD, Nanostring, Novartis, Onkowissen.de, Promedicis, Pfizer, Pierre Fabre, Roche, Seagen, Streamedup, Teva, Tesaro und Thieme Honorare sowie von Celgene, Pfizer und Roche Reisekostenzuschüsse erhalten. F. S. hat in beratenden Gremien bei Novartis, Lilly, Amgen und Roche mitgewirkt und von Roche, AstraZeneca, MSD, Novartis und Pfizer Honorare für Vorträge erhalten. H. T. hat von Novartis, Roche, Celgene, Teva, Pfizer und AstraZeneca Honorare sowie von Roche, Celgene und Pfizer Reisekostenzuschüsse erhalten. C. T. hat für die Mitwirkung in beratenden Gremien und für Vorträge von Amgen, AstraZeneca, Celgene, Daiichi Sankyo, Eisai, Gilead, Lilly, MSD, Mylan, Nanostring, Novartis, Pfizer, Pierre Fabre, Puma, Roche, Seagen und Vifor Honorare erhalten. M. T. hat in beratenden Gremien bei AstraZeneca, Clovis, Daiichi Sankyo, Eisai, Gilead Science, GSK, Lilly, MSD, Novartis, Organon, Pfizer, Pierre Fabre, Seagen und Roche mitgewirkt und von Amgen, Clovis, Daiichi Sankyo, Eisai, GSK, Lilly, MSD, Roche, Novartis, Organon, Pfizer, Seagen, Exact Sciences, Viatris, Vifor und AstraZeneca Honorare für Vorträge sowie von Exact Sciences und Endomag finanzielle Mittel für Versuche erhalten. Manuskriptzuschüsse wurden von Amgen, ClearCut, pfm medical, Roche, Servier und Vifor geleistet. M. U. Alle Honorare gingen an die Institution/den Arbeitgeber: Abbvie, Amgen, AstraZeneca, Daiichi Sankyo, Eisai, Lilly, MSD, Myriad Genetics, Pfizer, Roche, Sanofi Aventis, Novartis, Pierre Fabre, Seagen, Gilead. M. W. hat in beratenden Gremien bei AstraZeneca, Lilly, MSD, Novartis, Pfizer und Roche mitgewirkt. I. W. hat in beratenden Gremien bei Novartis, Daichii Sankyo, Lilly und Pfizer mitgewirkt und von AstraZeneca, Daichii Sankyo, MSD, Novartis, Pfizer und Roche Honorare als Referent erhalten. A. W. hat in beratenden Gremien bei Novartis, Lilly, Amgen, Pfizer, Roche, Tesaro und Eisai mitgewirkt und von Novartis, Pfizer, Aurikamed, Roche und Celgene Honorare für Vorträge erhalten.
References/Literatur
- 1.Lakhani S R, Reis-Filho J S, Fulford L. Prediction of BRCA1 status in patients with breast cancer using estrogen receptor and basal phenotype. Clin Cancer Res. 2005;11:5175–5180. doi: 10.1158/1078-0432.CCR-04-2424. [DOI] [PubMed] [Google Scholar]
- 2.Couch F J, Hart S N, Sharma P. Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J Clin Oncol. 2015;33:304–311. doi: 10.1200/JCO.2014.57.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fasching P A, Loibl S, Hu C. BRCA1/2 Mutations and Bevacizumab in the Neoadjuvant Treatment of Breast Cancer: Response and Prognosis Results in Patients With Triple-Negative Breast Cancer From the GeparQuinto Study. J Clin Oncol. 2018;36:2281–2287. doi: 10.1200/JCO.2017.77.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fasching P A, Yadav S, Hu C. Mutations in BRCA1/2 and Other Panel Genes in Patients With Metastatic Breast Cancer-Association With Patient and Disease Characteristics and Effect on Prognosis. J Clin Oncol. 2021;39:1619–1630. doi: 10.1200/JCO.20.01200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimelis H, LaDuca H, Hu C. Triple-Negative Breast Cancer Risk Genes Identified by Multigene Hereditary Cancer Panel Testing. J Natl Cancer Inst. 2018;110:855–862. doi: 10.1093/jnci/djy106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breast Cancer Association Consortium . Mavaddat N, Dorling L. Pathology of Tumors Associated With Pathogenic Germline Variants in 9 Breast Cancer Susceptibility Genes. JAMA Oncol. 2022;8:e216744. doi: 10.1001/jamaoncol.2021.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoyer J, Vasileiou G, Uebe S. Addition of triple negativity of breast cancer as an indicator for germline mutations in predisposing genes increases sensitivity of clinical selection criteria. BMC Cancer. 2018;18:926. doi: 10.1186/s12885-018-4821-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kraus C, Hoyer J, Vasileiou G. Gene panel sequencing in familial breast/ovarian cancer patients identifies multiple novel mutations also in genes others than BRCA1/2. Int J Cancer. 2017;140:95–102. doi: 10.1002/ijc.30428. [DOI] [PubMed] [Google Scholar]
- 9.Escala-Garcia M, Guo Q, Dork T. Genome-wide association study of germline variants and breast cancer-specific mortality. Br J Cancer. 2019;120:647–657. doi: 10.1038/s41416-019-0393-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevens K N, Fredericksen Z, Vachon C M. 19p13.1 is a triple-negative-specific breast cancer susceptibility locus. Cancer Res. 2012;72:1795–1803. doi: 10.1158/0008-5472.CAN-11-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevens K N, Vachon C M, Lee A M. Common breast cancer susceptibility loci are associated with triple-negative breast cancer. Cancer Res. 2011;71:6240–6249. doi: 10.1158/0008-5472.CAN-11-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broeks A, Schmidt M K, Sherman M E. Low penetrance breast cancer susceptibility loci are associated with specific breast tumor subtypes: findings from the Breast Cancer Association Consortium. Hum Mol Genet. 2011;20:3289–3303. doi: 10.1093/hmg/ddr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fasching P A, Pharoah P D, Cox A. The role of genetic breast cancer susceptibility variants as prognostic factors. Hum Mol Genet. 2012;21:3926–3939. doi: 10.1093/hmg/dds159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Escala-Garcia M, Abraham J, Andrulis I L. A network analysis to identify mediators of germline-driven differences in breast cancer prognosis. Nat Commun. 2020;11:312. doi: 10.1038/s41467-019-14100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fagerholm R, Khan S, Schmidt M K. TP53-based interaction analysis identifies cis-eQTL variants for TP53BP2, FBXO28, and FAM53A that associate with survival and treatment outcome in breast cancer. Oncotarget. 2017;8:18381–18398. doi: 10.18632/oncotarget.15110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vachon C M, Scott C G, Tamimi R M. Joint association of mammographic density adjusted for age and body mass index and polygenic risk score with breast cancer risk. Breast Cancer Res. 2019;21:68. doi: 10.1186/s13058-019-1138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hack C C, Emons J, Jud S M. Association between mammographic density and pregnancies relative to age and BMI: a breast cancer case-only analysis. Breast Cancer Res Treat. 2017;166:701–708. doi: 10.1007/s10549-017-4446-7. [DOI] [PubMed] [Google Scholar]
- 18.Vachon C M, Pankratz V S, Scott C G. The contributions of breast density and common genetic variation to breast cancer risk. J Natl Cancer Inst. 2015 doi: 10.1093/jnci/dju397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collaborative Group on Hormonal Factors in Breast Cancer . Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13:1141–1151. doi: 10.1016/S1470-2045(12)70425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colditz G A, Bohlke K. Priorities for the primary prevention of breast cancer. CA Cancer J Clin. 2014;64:186–194. doi: 10.3322/caac.21225. [DOI] [PubMed] [Google Scholar]
- 21.Collaborative Group on Hormonal Factors in Breast Cancer . Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet. 2002;360:187–195. doi: 10.1016/S0140-6736(02)09454-0. [DOI] [PubMed] [Google Scholar]
- 22.Rudolph A, Song M, Brook M N. Joint associations of a polygenic risk score and environmental risk factors for breast cancer in the Breast Cancer Association Consortium. Int J Epidemiol. 2018;47:526–536. doi: 10.1093/ije/dyx242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brouckaert O, Rudolph A, Laenen A. Reproductive profiles and risk of breast cancer subtypes: a multi-center case-only study. Breast Cancer Res. 2017;19:119. doi: 10.1186/s13058-017-0909-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X R, Chang-Claude J, Goode E L. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst. 2011;103:250–263. doi: 10.1093/jnci/djq526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milne R L, Gaudet M M, Spurdle A B. Assessing interactions between the associations of common genetic susceptibility variants, reproductive history and body mass index with breast cancer risk in the breast cancer association consortium: a combined case-control study. Breast Cancer Res. 2010;12:R110. doi: 10.1186/bcr2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stickeler E, Aktas B, Behrens A. Update Breast Cancer 2021 Part 1 – Prevention and Early Stages. Geburtshilfe Frauenheilkd. 2021;81:526–538. doi: 10.1055/a-1464-0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huober J, Schneeweiss A, Hartkopf A D. Update Breast Cancer 2020 Part 3 – Early Breast Cancer. Geburtshilfe Frauenheilkd. 2020;80:1105–1114. doi: 10.1055/a-1270-7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung A Y, Ahearn T U, Behrens S. Distinct reproductive risk profiles for intrinsic-like breast cancer subtypes: pooled analysis of population-based studies. J Natl Cancer Inst. 2022 doi: 10.1093/jnci/djac117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramazzini B. De morbis artificium diatriba. 1700
- 30.Kiechl S, Schramek D, Widschwendter M. Aberrant regulation of RANKL/OPG in women at high risk of developing breast cancer. Oncotarget. 2017;8:3811–3825. doi: 10.18632/oncotarget.14013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sigl V, Jones L P, Penninger J M. RANKL/RANK: from bone loss to the prevention of breast cancer. Open Biol. 2016 doi: 10.1098/rsob.160230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sigl V, Owusu-Boaitey K, Joshi P A. RANKL/RANK control Brca1 mutation-driven mammary tumors. Cell Res. 2016;26:761–774. doi: 10.1038/cr.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wunderle M, Ruebner M, Haberle L. RANKL and OPG and their influence on breast volume changes during pregnancy in healthy women. Sci Rep. 2020;10:5171. doi: 10.1038/s41598-020-62070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mintz R, Wang M, Xu S. Hormone and receptor activator of NF-kappaB (RANK) pathway gene expression in plasma and mammographic breast density in postmenopausal women. Breast Cancer Res. 2022;24:28. doi: 10.1186/s13058-022-01522-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ditsch N, Wöcke A, Untch M. AGO Recommendations for the Diagnosis and Treatment of Patients with Early Breast Cancer: Update 2022. Breast Care. 2022 doi: 10.1159/000524879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Francis P A, Pagani O, Fleming G F. Tailoring Adjuvant Endocrine Therapy for Premenopausal Breast Cancer. N Engl J Med. 2018;379:122–137. doi: 10.1056/NEJMoa1803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Regan M M, Francis P A, Pagani O.Absolute improvements in freedom from distant recurrence with adjuvant endocrine therapies for premenopausal women with hormone receptor-positive (HR+) HER2-negative breast cancer (BC): Results from TEXT and SOFT J Clin Oncol 201836(Suppl.)Abstr.. 503 [Google Scholar]
- 38.Pagani O, Regan M M, Walley B A. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371:107–118. doi: 10.1056/NEJMoa1404037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Francis P A, Regan M M, Fleming G F. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372:436–446. doi: 10.1056/NEJMoa1412379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baek S Y, Noh W C, Ahn S-H. Adding ovarian function suppression to tamoxifen in young women with hormone-sensitive breast cancer who remain premenopausal or resume menstruation after chemotherapy: 8-year follow-up of the randomized ASTRRA trial. J Clin Oncol. 2022;40:506. doi: 10.1200/JCO.2022.40.16_suppl.506. [DOI] [Google Scholar]
- 41.von Minckwitz G, Procter M, de Azambuja E. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N Engl J Med. 2017;377:122–131. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von Minckwitz G, Huang C S, Mano M S. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med. 2019;380:617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 43.Chan A, Moy B, Mansi J. Final Efficacy Results of Neratinib in HER2-positive Hormone Receptor-positive Early-stage Breast Cancer From the Phase III ExteNET Trial. Clin Breast Cancer. 2021;21:80–9.1E8. doi: 10.1016/j.clbc.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 44.Martin M, Holmes F A, Ejlertsen B. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1688–1700. doi: 10.1016/S1470-2045(17)30717-9. [DOI] [PubMed] [Google Scholar]
- 45.Fasching P A, Hartkopf A D, Gass P. Efficacy of neoadjuvant pertuzumab in addition to chemotherapy and trastuzumab in routine clinical treatment of patients with primary breast cancer: a multicentric analysis. Breast Cancer Res Treat. 2019;173:319–328. doi: 10.1007/s10549-018-5008-3. [DOI] [PubMed] [Google Scholar]
- 46.Gianni L, Pienkowski T, Im Y H. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 47.Gianni L, Pienkowski T, Im Y H. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17:791–800. doi: 10.1016/S1470-2045(16)00163-7. [DOI] [PubMed] [Google Scholar]
- 48.Loibl S, Jassem J, Sonnenblick A. Updated Results of Aphinity at 8.4 years median follow up. ESMO Virtual Plenary 2022; July 14, 2022
- 49.Schmid P, Cortes J, Dent R. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N Engl J Med. 2022 doi: 10.1056/NEJMoa2112651. [DOI] [PubMed] [Google Scholar]
- 50.Schmid P, Cortes J, Pusztai L. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med. 2020;382:810–821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 51.Huober J, Barrios C H, Niikura N. Atezolizumab With Neoadjuvant Anti-Human Epidermal Growth Factor Receptor 2 Therapy and Chemotherapy in Human Epidermal Growth Factor Receptor 2-Positive Early Breast Cancer: Primary Results of the Randomized Phase III IMpassion050 Trial. J Clin Oncol. 2022 doi: 10.1200/JCO.21.02772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miles D, Gligorov J, Andre F. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol. 2021;32:994–1004. doi: 10.1016/j.annonc.2021.05.801. [DOI] [PubMed] [Google Scholar]
- 53.Jacob J B, Jacob M K, Parajuli P. Review of immune checkpoint inhibitors in immuno-oncology. Adv Pharmacol. 2021;91:111–139. doi: 10.1016/bs.apha.2021.01.002. [DOI] [PubMed] [Google Scholar]
- 54.Schmid P, Cortes J, Dent R. KEYNOTE-522: Phase III study of neoadjuvant pembrolizumab + chemotherapy vs. placebo + chemotherapy, followed by adjuvant pembrolizumab vs. placebo for early-stage TNBC. Ann Oncol. 2021 doi: 10.1016/j.annonc.2021.06.014. [DOI] [Google Scholar]
- 55.Schmid P, Cortes J, Dent R. KEYNOTE-522: Phase 3 Study of Pembrolizumab + Chemotherapy versus Placebo + Chemotherapy as Neoadjuvant Treatment, Followed by Pembrolizumab versus Placebo as Adjuvant Treatment for Early Triple-Negative Breast Cancer (TNBC) Ann Oncol. 2019 doi: 10.1093/annonc/mdz1394. [DOI] [Google Scholar]
- 56.Huang M, OʼShaughnessy J, Zhao J. Evaluation of Pathologic Complete Response as a Surrogate for Long-Term Survival Outcomes in Triple-Negative Breast Cancer. J Natl Compr Canc Netw. 2020;18:1096–1104. doi: 10.6004/jnccn.2020.7550. [DOI] [PubMed] [Google Scholar]
- 57.Huang M, OʼShaughnessy J, Zhao J. Association of Pathologic Complete Response with Long-Term Survival Outcomes in Triple-Negative Breast Cancer: A Meta-Analysis. Cancer Res. 2020;80:5427–5434. doi: 10.1158/0008-5472.CAN-20-1792. [DOI] [PubMed] [Google Scholar]
- 58.Cortazar P, Zhang L, Untch M. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 59.von Minckwitz G, Untch M, Blohmer J U. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 60.Pusztai L, Denkert C, OʼShaughnessy J. Event-free survival by residual cancer burden after neoadjuvant pembrolizumab + chemotherapy versus placebo + chemotherapy for early TNBC: Exploratory analysis from KEYNOTE-522. J Clin Oncol. 2022;40:503-503. doi: 10.1200/JCO.2022.40.16_suppl.503. [DOI] [Google Scholar]
- 61.Symmans W F, Yau C, Chen Y Y. Assessment of Residual Cancer Burden and Event-Free Survival in Neoadjuvant Treatment for High-risk Breast Cancer: An Analysis of Data From the I-SPY2 Randomized Clinical Trial. JAMA Oncol. 2021;7:1654–1663. doi: 10.1001/jamaoncol.2021.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Symmans W F, Wei C, Gould R. Long-Term Prognostic Risk After Neoadjuvant Chemotherapy Associated With Residual Cancer Burden and Breast Cancer Subtype. J Clin Oncol. 2017;35:1049–1060. doi: 10.1200/JCO.2015.63.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Symmans W F, Peintinger F, Hatzis C. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 64.MD Anderson Cancer Center Residual Cancer Burden CalculatorAccessed July 16, 2022 at:http://www3.mdanderson.org/app/medcalc/index.cfm?pagename=jsconvert3
- 65.Gerdes J, Lelle R J, Pickartz H. Growth fractions in breast cancers determined in situ with monoclonal antibody Ki-67. J Clin Pathol. 1986;39:977–980. doi: 10.1136/jcp.39.9.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Urruticoechea A, Smith I E, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol. 2005;23:7212–7220. doi: 10.1200/JCO.2005.07.501. [DOI] [PubMed] [Google Scholar]
- 67.Viale G, Giobbie-Hurder A, Regan M M. Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1-98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol. 2008;26:5569–5575. doi: 10.1200/JCO.2008.17.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheang M C, Chia S K, Voduc D. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yerushalmi R, Woods R, Ravdin P M. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11:174–183. doi: 10.1016/S1470-2045(09)70262-1. [DOI] [PubMed] [Google Scholar]
- 70.Fasching P A, Heusinger K, Haeberle L. Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment. BMC Cancer. 2011;11:486. doi: 10.1186/1471-2407-11-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heusinger K, Jud S M, Haberle L. Association of mammographic density with the proliferation marker Ki-67 in a cohort of patients with invasive breast cancer. Breast Cancer Res Treat. 2012;135:885–892. doi: 10.1007/s10549-012-2221-3. [DOI] [PubMed] [Google Scholar]
- 72.von Minckwitz G, Schmitt W D, Loibl S. Ki67 measured after neoadjuvant chemotherapy for primary breast cancer. Clin Cancer Res. 2013;19:4521–4531. doi: 10.1158/1078-0432.CCR-12-3628. [DOI] [PubMed] [Google Scholar]
- 73.Penault-Llorca F, Radosevic-Robin N. Ki67 assessment in breast cancer: an update. Pathology. 2017;49:166–171. doi: 10.1016/j.pathol.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 74.Fasching P A, Gass P, Haberle L. Prognostic effect of Ki-67 in common clinical subgroups of patients with HER2-negative, hormone receptor-positive early breast cancer. Breast Cancer Res Treat. 2019;175:617–625. doi: 10.1007/s10549-019-05198-9. [DOI] [PubMed] [Google Scholar]
- 75.Smith I, Robertson J, Kilburn L. Long-term outcome and prognostic value of Ki67 after perioperative endocrine therapy in postmenopausal women with hormone-sensitive early breast cancer (POETIC): an open-label, multicentre, parallel-group, randomised, phase 3 trial. Lancet Oncol. 2020;21:1443–1454. doi: 10.1016/S1470-2045(20)30458-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nielsen T O, Leung S CY, Rimm D L. Assessment of Ki67 in Breast Cancer: Updated Recommendations From the International Ki67 in Breast Cancer Working Group. J Natl Cancer Inst. 2021;113:808–819. doi: 10.1093/jnci/djaa201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dowsett M, Nielsen T O, Rimm D L. Ki67 as a Companion Diagnostic: Good or Bad News? J Clin Oncol. 2022 doi: 10.1200/JCO.22.00581. [DOI] [PubMed] [Google Scholar]
- 78.Tarantino P, Burstein H J, Lin N U. Should Ki-67 be adopted to select breast cancer patients for treatment with adjuvant abemaciclib? Ann Oncol. 2022;33:234–238. doi: 10.1016/j.annonc.2021.12.004. [DOI] [PubMed] [Google Scholar]
- 79.Harbeck N, Rastogi P, Martin M. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol. 2021;32:1571–1581. doi: 10.1016/j.annonc.2021.09.015. [DOI] [PubMed] [Google Scholar]
- 80.Buus R, Sestak I, Kronenwett R. Molecular Drivers of Oncotype DX, Prosigna, EndoPredict, and the Breast Cancer Index: A TransATAC Study. J Clin Oncol. 2021;39:126–135. doi: 10.1200/JCO.20.00853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cardoso F, vanʼt Veer L J, Bogaerts J. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N Engl J Med. 2016;375:717–729. doi: 10.1056/NEJMoa1602253. [DOI] [PubMed] [Google Scholar]
- 82.Paik S, Shak S, Tang G. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 83.Piccart M, van ʼt Veer L J, Poncet C. 70-gene signature as an aid for treatment decisions in early breast cancer: updated results of the phase 3 randomised MINDACT trial with an exploratory analysis by age. Lancet Oncol. 2021;22:476–488. doi: 10.1016/S1470-2045(21)00007-3. [DOI] [PubMed] [Google Scholar]
- 84.Sparano J A, Gray R J, Makower D F. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N Engl J Med. 2018;379:111–121. doi: 10.1056/NEJMoa1804710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sparano J A, Gray R J, Makower D F. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N Engl J Med. 2015;373:2005–2014. doi: 10.1056/NEJMoa1510764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kalinsky K, Barlow W E, Gralow J R. 21-Gene Assay to Inform Chemotherapy Benefit in Node-Positive Breast Cancer. N Engl J Med. 2021;385:2336–2347. doi: 10.1056/NEJMoa2108873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Toussaint J, Sieuwerts A M, Haibe-Kains B. Improvement of the clinical applicability of the Genomic Grade Index through a qRT-PCR test performed on frozen and formalin-fixed paraffin-embedded tissues. BMC Genomics. 2009;10:424. doi: 10.1186/1471-2164-10-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sotiriou C, Desmedt C. Gene expression profiling in breast cancer. Ann Oncol. 2006;17 10:x259–x262. doi: 10.1093/annonc/mdl270. [DOI] [PubMed] [Google Scholar]
- 89.Brain E, Viansone A A, Bourbouloux E. Final results from a phase III randomized clinical trial of adjuvant endocrine therapy ± chemotherapy in women ≥ 70 years old with ER+ HER2- breast cancer and a high genomic grade index: The Unicancer ASTER 70s trial. J Clin Oncol. 2022;40:500. doi: 10.1200/JCO.2022.40.16_suppl.500. [DOI] [Google Scholar]