Abstract
Inactivation of ccpA in Enterococcus faecalis leads to reduction of the growth rate, derepression of the galKETR operon in the presence of a mixture of glucose and galactose, and reduction of transcription of ldh in the presence of glucose. Moreover, the E. faecalis ccpA gene fully complements a Bacillus subtilis ccpA mutant, arguing for similar functions of these two homologous proteins. Protein comparison on two-dimensional gels from the wild-type cells and the ccpA mutant cells revealed a pleiotropic effect of the mutation on gene expression. The HPr protein of the carbohydrate-phosphotransferase system was identified by microsequencing, and a modification of its phosphorylation state was observed between the wild-type and the mutant strains. Moreover, at least 16 polypeptides are overexpressed in the mutant, and 6 are repressed. Interestingly, 13 of the 16 polypeptides whose synthesis is enhanced in the mutant were also identified as glucose starvation proteins. The N-terminal amino acid sequences of four of them match sequences deduced from genes coding for l-serine dehydratase, dihydroxyacetone kinase (two genes), and a protein of unknown function from Deinococcus radiodurans.
In their natural surroundings, microorganisms are usually subjected to environmental fluctuations, i.e., in the composition and abundance of carbon and energy sources. Bacteria have a high adaptability potential against these modifications. In many cases, the presence of a rapidly metabolizable carbon source leads to the reduction of expression of genes involved in the metabolism of other carbon substrates. This regulation by preferential nutrients has been named catabolite repression (CR). Conversely, carbon starvation leads to the entry of cells into stationary phase. Some bacteria, like Bacillus species, form endospores to survive nutrient-poor conditions. However, this morphological differentiation is not encountered in the vast majority of microorganisms. Nevertheless, nondifferentiating bacteria exhibit a variety of alterations in genetic regulation and physiological changes that ensure survival during periods of prolonged starvation and resistance to multiple environmental stresses (12, 15, 22, 23). In Escherichia coli, two classes of genes encoding starvation proteins have been defined: cst genes, subjected to activation by the cyclic AMP-cyclic AMP receptor protein complex, and pex genes, independent of catabolite repression (31). Carbon starvation (Cst) proteins are involved in escape from starvation, whereas postexponential (Pex) proteins are implicated in cross protection against exogenous stresses (37). Many of these Pex proteins are known to be regulated by the transcriptional factor ςS (16, 26). In Bacillus subtilis and numerous other gram-positive bacteria, the transcriptional factor ςB is involved in the stationary-phase response. In these microorganisms, the distinction between Pex and Cst is not yet established. It has been shown that CR in low-G+C-content gram-positive bacteria is mediated via a negative regulatory mechanism (38) involving at least three components: a trans-acting factor called catabolite control protein A (CcpA), cis-acting sequences termed catabolite responsive elements (cres), and the HPr protein of the phosphoenolpyruvate-sugar-phosphotransferase system (PTS). CcpA is a DNA binding protein that belongs to the LacI/GalR family of transcriptional regulators (41) and was first identified in B. subtilis as a gene responsible for the catabolite repression of amyE, encoding α-amylase (18). Its action is mediated via binding to cre sequences, located within or near the promoter of the targeted genes. Weickert and Chambliss (42) proposed a consensus sequence for this 14-bp region of dyad symmetry on the basis of point-mutationa1 analysis in the amyE promoter region: TG(T/A)NANCGNTN(T/A)CA. The specific binding of CcpA to cres requires an additional factor, the HPr protein of the PTS. In addition to the phosphorylation site at histidine 15 implicated in the sugar transport process, HPr of gram-positive bacteria can be phosphorylated at the serine 46 residue by an ATP-dependent HPr kinase (7). HPr(Ser-P), but not free HPr, can bind to CcpA in vitro, and this interaction is stimulated by high concentrations of fructose-1,6-bisphosphate (FBP), one of the intermediates of the glycolytic pathway (6). Repression of targeted genes results from the fixation of CcpA with its cofactors to cres, which blocks transcription initiation by RNA polymerase (19). cres confer not only repression of genes but also glucose-mediated transcriptional activation of the acetate kinase gene (ackA) in B. subtilis (14) and the las operon, encoding pyruvate kinase, phosphofructokinase, and lactate dehydrogenase, in Lactococcus lactis (29). The involvement of CcpA in catabolite repression has also been established in other low-G+C-content gram-positive bacteria, including Bacillus megaterium, Staphylococcus xylosus, Lactobacillus casei, and L. lactis (9, 20, 29, 33).
Enterococcus faecalis is a nonsporulant low-G+C-content gram-positive bacterium which is able to develop a multiresistant state when deprived of glucose (12). This multiresistance is correlated with the synthesis of at least 42 glucose starvation proteins (Glsp) (13). In a previous paper, we reported the cloning and sequencing of an E. faecalis gene homologous to ccpA of B. subtilis (27). In this report, we analyze its role in CR and, using a two-dimensional (2-D) gel electrophoresis approach, we attempt to distinguish Pex and Cst proteins among the glucose starvation proteins.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Cultures of E. faecalis JH2-2 (21, 43) were grown at 37°C without shaking in 20-ml glass tubes containing 10 ml of semisynthetic medium (for the composition, see Bacto Folic AOAC Medium [Difco, Detroit, Mich.]) supplemented with various carbon sources. E. coli XL1Blue (Stratagene, La Jolla, Calif.) was used as a plasmid host and was cultivated under vigorous agitation at 37°C in 2TY medium (32) with ampicillin (100 μg/ml) as required. B. subtilis QB7144 [trpC2 amyE::(pA ynaJ′-lacZ+ cat)] and QB7147 [trpC2 ccpA::Tn917 spc amyE::(pA ynaJ′-lacZ+ cat)] (11) were used for complementation experiments and were cultivated in CSK medium (11) at 37°C under vigorous agitation.
Analysis of mRNA transcription by Northern blotting.
Total RNA of E. faecalis was isolated by using the RNeasy Midi Kit (Qiagen, Inc., Valencia, Calif.). After DNase treatment, samples were precipitated and the amount of RNA was determined by spectrophotometry. Northern blots of exactly 10 μg of electrophoresed RNA were prepared by using Hybond N+ membranes and standard procedures (36). For quantification of the relative intensities of the hybridizing bands in the Northern blots, rRNA bands observed after ethidium bromide staining of gels were used as an internal standard for each sample. For this purpose, the stained 23S and 16S rRNA bands were scanned and quantified by densitometry with OptiQuant image analysis software (Packard Instrument Company, Canberra, Australia). The sizes of transcripts were estimated by comparing the band mobilities of standards in an RNA ladder (0.56 to 9.4 kb) (Amersham International, Little Chalfont, United Kingdom). Oligonucleotide primers were used in PCRs to generate specific fragments of genes: ldh, 5′-GGAATGGTACACATGACTGC-3′ and 5′-CGTCAGGATTATTTTTCACC-3′; pfk, 5′-GCATTGGTATTTTAACCAGC-3′ and 5′-TCACCATGTGAAAAGTTCAA-3′; galK, 5′-TTGGTGAGAAAGGGACAGCC-3′ and 5′-GCAGGATAAAAATCAGCAGC-3′; gls27, 5′-AATAATGCACTAGATGCTGC-3′ and 5′-TAAAAGACATTCAAACATGG-3′; and gls17, 5′-GAAGAATTTATCGATAAAGC-3′ and 5′-GGCCATCGCTGAAGCACTGC-3′. These PCR fragments were then used to generate specific probes by PCR, using 200 pmol of the reverse primers; 2 μM of dGTP, dCTP, and dTTP; 1.5 mM MgCl2; 1 μl of purified PCR product; 1× PCR buffer (Amersham); 5 U of Taq DNA polymerase (Amersham); and 20 μCi of [α32P]dATP (Amersham Pharmacia Biotech). Reactions were run for 10 cycles. Prehybridization and hybridization of membrane-bound RNA with single-stranded DNA probes were performed at 60°C with gentle agitation.
Mapping the transcriptional start sites.
Primer CCPA3 (5′-TAGATACATTTGCCTCTCTAGC-3′), complementary to nucleotides +33 to +53 of ccpA, was labeled with 10 U of polynucleotide kinase (Roche Molecular Biochemicals) and 2 μCi of [γ32P]ATP (Amersham International; 10 mCi/ml) and then mixed with 10 μg of total RNA in 14 μl of the reverse transcriptase buffer containing 40 U of RNase inhibitor (Roche Molecular Biochemicals). After the mixture was heated at 65°C for 5 min, annealing was obtained by a slow decrease of the temperature to 25°C. The extension reaction was then performed in a 20-μl final volume with 50 U of avian myeloblastosis virus reverse transcriptase (Roche Molecular Biochemicals) and 0.5 mM deoxynucleoside triphosphates at 42°C for 1 h. After heat denaturation, 2-μl samples were loaded onto a 6% polyacrylamide-urea sequencing gel for electrophoresis, together with a sequencing reaction performed with the same primer (T7 sequencing kit; Pharmacia Biotech), and the bands were detected after exposure to a storage phosphor screen (Packard Instrument Company).
General molecular methods.
Restriction endonucleases, alkaline phosphatase, and ligase were obtained from Roche Molecular Biochemicals and Amersham International and used according to the furnished instructions. PCR was carried out in a reaction volume of 25 μl with 100 ng of chromosomal DNA of E. faecalis using Ready To Go PCR beads (Pharmacia Biotech). PCR products were purified with the QIAquick kit (Qiagen). DNA and amino acid sequences were analyzed using the Mac Vector (Kodak Scientific Imaging Systems) program, and database searches were performed with the BLAST program (1). Other standard techniques were carried out as described by Sambrook et al. (36). Competent B. subtilis cells were used for transformation (2). E. faecalis and E. coli were transformed by electroporation with a Gene-pulser apparatus (Bio-Rad Laboratories, Richmond, Calif.).
Construction of the ccpA insertional mutant.
To construct an insertional mutant with a disruption in the E. faecalis ccpA gene, a 440-bp internal E. faecalis ccpA fragment was amplified from chromosomal DNA with primers CCPA1 (5′-GTGTTGTCCATCGGTAATCC-3′) and CCPA1rev (5′-GCAGAATTCGTTGCTTCTGTGTAATC-3′) and, after being polished with Pfu polymerase (Stratagene), ligated with the insertional vector pUCB300 (10) previously digested with SmaI. The resulting plasmid, pCCPA1, obtained after transformation of E. coli XL1Blue, was used to transform competent cells of E. faecalis JH2-2. Erythromycin-resistant colonies were selected on agar plates containing 15 μg of erythromycin per ml. Integrations were verified by PCR and Southern blot analysis, and the disappearance of CcpA was confirmed by Western blotting with antibodies raised against CcpA from B. megaterium (25).
Western blot analysis.
E. faecalis JH2-2 and CL14 strains were grown to an optical density at 600 nm (OD600) of 0.4 in 10 ml of semisynthetic medium supplemented with 0.15% glucose. Crude extracts were prepared by vortexing the pellets in 500 μl of extraction buffer (Tris [pH 7], 50 mM; EDTA, 2 mM; β-mercaptoethanol, 0.74% [vol/vol]) with glass beads (0.1- to 0.25-mm diameter) and subsequent removal of cell debris by centrifugation. The proteins of cell extracts were separated by nondenaturing polyacrylamide gel electrophoresis on a 14% polyacrylamide gel and transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore) by electroblotting (MilliBlot-Graphite electroblotter; Millipore). HPr was detected with a rabbit polyclonal antiserum raised against HPr of Staphylococcus carnosus. HPr antibodies were visualized by using the ECL Western blot analysis system (Amersham).
Complementation of a B. subtilis ccpA mutant.
To express ccpA from E. faecalis in B. subtilis, the gene was amplified by PCR with primers CCPA2for (5′-GGACAAGATCTTATTTATAGGAGGAGAACATGG-3′) and CCPA2rev (5′-CAATGCATGCCGGACTGATTTACTTAATCAAC-3′). These primers changed the ribosome-binding site of ccpA to a more appropriate sequence for Bacillus and introduced BglII and SphI sites that were used to clone the gene under the control of the xynCB promoter in pHTxyn, resulting in pCCPA2. Plasmid pHTxyn was obtained by introducing a 1.5-kbp EcoRI/HindIII fragment from pHM12 (H. Putzer, unpublished data) containing the xynCB promoter and the regulator xynR in plasmid pHT315 (3), a shuttle vector with 15 copies/chromosome in B. subtilis, digested by the same restriction enzymes.
Two-dimensional protein gel electrophoresis.
Cells were cultured in semisynthetic medium supplemented with 0.15% glucose. Culture aliquots of 5 ml were pulse labeled between OD600s of 0.2 and 0.4 with 250 μCi of [35S]methionine and [35S]cysteine protein-labeling mix (New England Nuclear Co.; 1,000 Ci/mmol). Protein extraction and 2-D electrophoresis were performed as previously described (13). The dried gels were exposed to a storage phosphor screen (Packard Instrument Company) for 48 h, and the intensity of synthesis of proteins was determined by the quantification of the corresponding spot using the OptiQuant image analysis software. For the preparative electrophoresis, 50 ml of bacterial culture was used. After separation, the gel was transferred onto a polyvinylidene difluoride membrane (Immobilon-P) by electroblotting (MilliBlot-Graphite electroblotter) according to the manufacturer's instructions. After Coomassie blue staining of the membrane, the interesting spots were cut off and proteins were sequenced by the Institut für Biochemie (University of Vienna, Austria). Preliminary sequence data were obtained from The Institute for Genomic Research (TIGR) (website at http://www.tigr.org).
RESULTS
Transcriptional analysis of the E. faecalis ccpA gene.
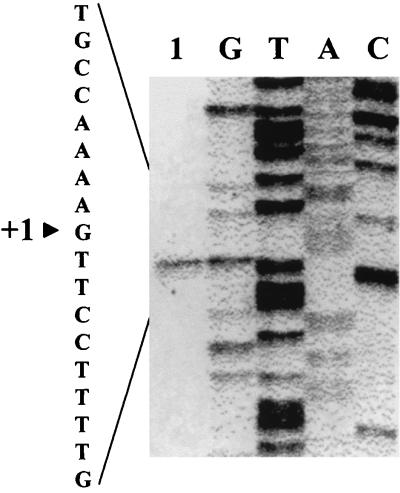
Northern blot analysis revealed a unique transcript of approximately 1.2 kb, indicating that the E. faecalis ccpA gene was expressed as a monocistronic mRNA (data not shown). A potential rho-independent terminator structure, with a ΔG° value of −25.6 kcal/mol, was identified downstream of ccpA. Primer extension analysis performed on total RNA extracted from cells grown in glucose-supplemented semisynthetic medium and harvested in mid-exponential growth phase suggested that the transcriptional initiation site was a guanine (G) located 139 bp upstream of the ccpA open reading frame (ORF) translational initiation codon (Fig. 1). No obvious sequences corresponding to a −10/−35 hexanucleotide pair was identified at the correct position upstream from this transcriptional initiation site. Another putative promoter deduced from the sequence has been previously described 48 bp upstream of the translational start site (27), but it did not seem to be active, at least under the culture conditions used for primer extension.
FIG. 1.
Determination of the 5′ end of the ccpA transcript by primer extension. A DNA-sequencing preparation was run in parallel using the same primer. The arrowhead corresponds to the point within the sequence representing the apparent 5′ end.
Physiological impact of ccpA mutation in E. faecalis.
To determine the function of CcpA in E. faecalis, the chromosomal ccpA gene was disrupted by integration of a nonreplicative vector carrying an internal ccpA. The mutant strain obtained was designated CL14. The fermentative pattern of 50 carbohydrates by the API 50-CH (Biomerieux) series was identical for both wild-type and mutant strains. To determine phenotypic alteration(s) in the mutant, the growth of cultures was monitored in semisynthetic medium containing glucose, galactose, mannitol, mannose, sucrose, fructose, or lactose as a carbon source. The doubling times of CL14 were clearly affected (Table 1). They were higher than that of the wild type on the seven carbohydrates tested. For instance, in semisynthetic medium supplemented with 0.15% glucose or mannose, the ccpA mutation led to a 45% increase in the doubling time.
TABLE 1.
Doubling times of E. faecalis strains JH2-2 and CL14 with different carbon sourcesa
| Semisynthetic medium + sugar (0.15%) | Doubling time (min)
|
|
|---|---|---|
| Wild type | ccpA mutant | |
| Glucose | 44.6 ± 4.2 | 64.6 ± 5.9 |
| Lactose | 52.7 ± 2.8 | 68.1 ± 8.8 |
| Fructose | 55.1 ± 1.1 | 71.3 ± 1.7 |
| Galactose | 83.6 ± 9.2 | 98.4 ± 1.2 |
| Sucrose | 54.6 ± 0.8 | 66.7 ± 1.3 |
| Mannitol | 66.2 ± 0.7 | 85.5 ± 11.7 |
| Mannose | 42.0 ± 0.3 | 64.6 ± 1.9 |
Results presented correspond to the mean value of three experiments, and standard deviations are indicated.
Complementation of a B. subtilis ccpA mutant.
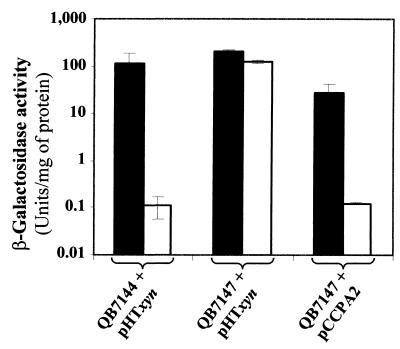
The similarities between ccpA of Bacillus species and E. faecalis prompted us to test the complementation of a B. subtilis ccpA mutant with the E. faecalis gene. For this purpose, we used two strains of B. subtilis: QB7144 and QB7147 (11). The QB7147 strain carries a fusion of the xynB promoter with the lacZ gene (ynaJ′-lacZ) as well as a ccpA mutation. In this strain, the expression of lacZ is induced by xylose and is not repressed by glucose, while in strain QB7144, which contains a wild-type ccpA gene, addition of glucose induces a strong repression of the β-galactosidase gene. Cloning the E. faecalis ccpA gene downstream of the B. subtilis xynCB promoter in a replicative plasmid and changing its ribosome-binding site to adapt it to its new host (resulting in plasmid pCCPA2) permitted the expression of E. faecalis CcpA in B. subtilis. With this construct, the glucose-specific repression of the ynaJ′-lacZ fusion was restored (Fig. 2).
FIG. 2.
β-Galactosidase activities of B. subtilis strains QB7144 and QB7147 containing different plasmids. The specific activities of β-galactosidase were determined in extracts prepared from exponentially growing cells (OD600 = 0.5). The mean values of three independent experiments are presented. Cells were grown in CSK medium supplemented with 0.2% xylose (solid bars) or with 0.2% xylose and 1% glucose (open bars). The method of Miller (32) was used for the determination of β-galactosidase activity.
CcpA-mediated transcriptional regulation of galactose utilization genes.
Using the E. faecalis genome sequence provided by TIGR, the potential galK gene of E. faecalis was identified as part of an operon comprising three other genes: galETR. The deduced amino acid products of these genes are 76% homologous to galactokinase of Streptococcus thermophilus, 80% homologous to UDP-galactose 4-epimerase of L. lactis, 71% homologous to galactose-1-P-uridyl transferase of Streptococcus mutans, and 56% homologous to the galactose operon repressor of S. thermophilus. A potential rho-independent terminator was identified downstream of the galR gene, and a potential cre box (TGTACACGTTTTCA) with only one mismatch (boldface) with the consensus cre sequence, was localized 94 bp upstream of the putative translational start codon of the first gene, galK.
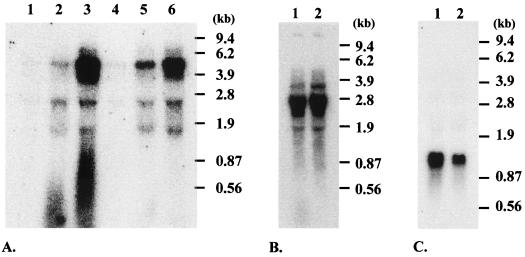
Northern blots of total RNA extracted from strains JH2-2 and CL14 grown in semisynthetic medium supplemented with 0.15% glucose, 0.15% glucose plus 0.15% galactose, or 0.15% galactose were performed with a galK-specific probe (Fig. 3A). No or weak bands were detected when the two strains were grown on glucose (Fig. 3A, lanes 1 and 4), while a strong signal corresponding to a 4.8-kb transcript was detected, suggesting transcriptional regulation, when cultures were performed in the presence of galactose (Fig. 3A, lanes 3 and 6). The size of this transcript corresponded to that expected for the putative galKETR operon. Analysis of total RNA extracted from strains cultured on a mixture of glucose and galactose revealed a partial derepression of the galK transcription in strain CL14 compared to that in the wild-type strain (Fig. 3A, lanes 2 and 5). Repression factors, corresponding to the ratio between the amount of transcript under nonrepressive and repressive conditions, were about 4.5 and 17.5 for the ccpA mutant and the wild-type strains, respectively.
FIG. 3.
(A) Northern blot analysis of the expression of the galK gene in the E. faecalis strains JH2-2 (lanes 1, 2, and 3) and CL14 (lanes 4, 5, and 6) grown with 0.15% glucose (lanes 1 and 4), 0.15% glucose plus 0.15% galactose (lanes 2 and 5), or 0.15% galactose (lanes 3 and 6). (B) Northern blot analysis of the expression of the pfk gene in E. faecalis strains JH2-2 (lane 1) and CL14 (lane 2) grown with glucose. (C) Northern blot analysis of the expression of the ldh gene in E. faecalis strains JH2-2 (lane 1) and CL14 (lane 2) grown with glucose.
Effects of CcpA on regulation of transcription of glycolysis enzymes.
Recently, Luesink et al. (29) reported that the las operon of L. lactis, encoding the glycolytic enzymes lactate dehydrogenase, pyruvate kinase, and phosphofructokinase, was transcriptionally activated by CcpA in the presence of glucose. In order to determine whether such regulation was effective in E. faecalis, we first searched for the corresponding genes in the partially determined genome sequence at the TIGR database. Three genes whose deduced amino acid sequences share 88% homology with that of L. casei lactate dehydrogenase (ldh), 80% homology with that of Bacillus stearothermophilus phosphofructokinase (pfk), and 81% homology with that of Bacillus licheniformis pyruvate kinase (pyk) were identified. While in L. lactis the three genes form an operon, the organization of genes found in E. faecalis was different: pfk and pyk seemed to form an operon, whereas ldh was monocistronic. A potential cre-box (TGAAAACTGTATCA), with one mismatch (boldface) with the consensus sequence, was identified 114 bp upstream of the ATG start codon of the ldh gene, whereas no sequence matching this consensus was identified near the putative promoter region of the pfk-pyk operon.
Northern blot analyses performed with total RNA extracted from exponentially growing cells in semisynthetic medium supplemented with glucose and hybridized with a pfk-specific probe (Fig. 3B) showed no significant differences in the amounts of transcript between the wild-type and the ccpA mutant strains. The size of the transcript corresponds to that expected for the pfk-pyk operon. Similar results were obtained when a pyk-specific probe was used (data not shown). Northern blotting carried out with an ldh-specific probe showed one unique transcript of 1.3 kb (Fig. 3C), corresponding to the expected size for ldh. The amount of transcript was 2.2-fold lower in the ccpA mutant than in the wild-type strain, suggesting a role for CcpA in a weak activation of ldh transcription.
Pleiotropic effect on protein synthesis and influence on phosphorylation state of HPr of the ccpA mutation.
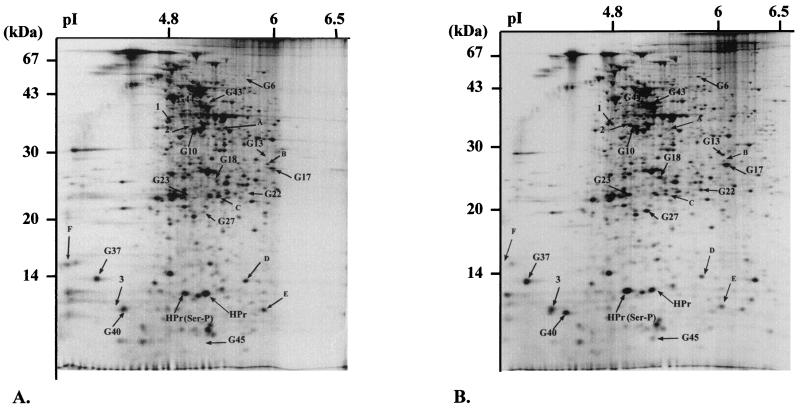
To determine whether CcpA would affect the synthesis of other E. faecalis proteins, a 2-D polyacrylamide gel electrophoresis approach was used. Several differences were observed in the 2-D protein patterns of strains CL14 and JH2-2 when cells were harvested in mid-growth phase (Fig. 4). Indeed, the synthesis of at least 16 polypeptides is obviously enhanced in the ccpA mutant, whereas 6 are repressed. Interestingly, most of the polypeptides with enhanced synthesis had already been identified in a previous work as glucose starvation-inducible proteins (Glsp) in E. faecalis (13).
FIG. 4.
The 2-D electrophoresis protein pattern of E. faecalis strains JH2-2 (A) and CL14 (B) grown on glucose and harvested in the exponential growth phase. The arrows indicate proteins showing modified expression in the ccpA mutant strain. The spots indicated by a G and a number and those indicated only by numbers are polypeptides under negative control by CcpA, whereas proteins indicated by letters are under positive control by CcpA. The majority of the G proteins have been identified in a previous study (13) as inducible upon glucose starvation (Gls proteins). Three additional Gls proteins (G43, G44, and G45) have been identified since that time (unpublished results). The proteins indicated by numbers are specific to the ccpA mutant. N-terminal microsequencing of proteins isolated from two spots showed that they correspond to the HPr protein of E. faecalis. The more acidic spot seems to be the form of this protein phosphorylated on serine 46. For more details, see the text.
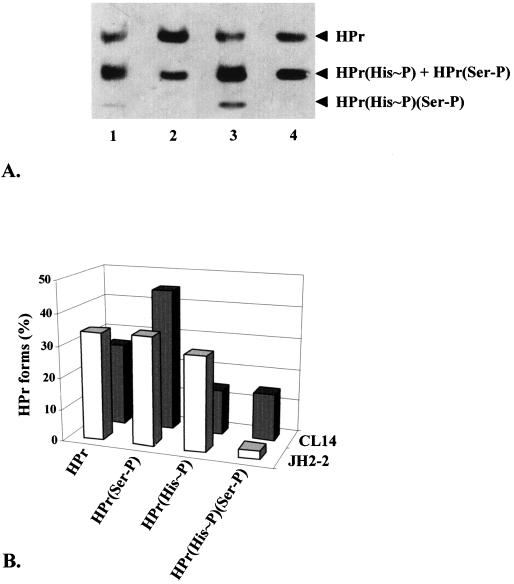
In addition to these variations in the amount of protein synthesis, the case of the HPr protein seems special. This protein was identified on the 2-D gels by microsequencing and by cross reaction with antibodies raised against S. carnosus HPr. Two spots corresponded to this protein; these are likely due to nonphosphorylated and Ser(46)-phosphorylated forms of HPr, the His15∼P being heat unstable (30). The amounts of total HPr in the two strains did not show significant differences but displayed variations in the phosphorylation states. CL14 shows a twofold amplification of the HPr(Ser-P) form, whereas the unphosphorylated form of HPr decreased by the same factor. This modification of the phosphorylation state was verified by Western blotting of native proteins (Fig. 5). This analysis confirmed a larger amount of HPr(Ser-P) but also revealed that the level of the double-phosphorylated form of this protein in particular is increased in the ccpA mutant. On the other hand, unphosphorylated HPr and, more significantly, the HPr(His∼P) fraction showed reduced levels (Fig. 5).
FIG. 5.
(A) Western blot analysis of HPr of E. faecalis strains JH2-2 (lanes 1 and 2) and CL14 (lanes 3 and 4). The extracts in lanes 1 and 3 correspond to native proteins, and the extracts in lanes 2 and 4 were boiled for 5 min before loading, leading to the dephosphorylation of histidine. The positions of the different forms of HPr are indicated by arrowheads. (B) Percentages of the different HPr forms in E. faecalis wild-type and ccpA mutant strains were deduced from the results of the Western blot analysis after scanning and densitometry analysis by OptiQuant image analysis Software.
Analysis of some CcpA-dependent proteins.
Among the 16 polypeptides with enhanced synthesis in the ccpA mutant strain, the N-terminal parts of 4 of them were determined by microsequencing as follows: for Gls10, MKKIINEP; for Gls17, YLXIEEFI; for Gls27, MELTVKDI, and for Gls40, MKADILLV. The corresponding genes were found in the genome sequence provided by TIGR, and adjacent regions were analyzed. The results of sequence analyses indicated that gls40, gls27, and gls10 are part of an operon of four genes, which would terminate at a potential rho-independent terminator. ORF1 shares 38% identity with glycerol dehydrogenase from E. coli, Gls10 and Gls27 share 42 and 40% identity with putative dihydroxyacetone kinase from E. coli, and Gls40 shares 35% identity with a protein of unknown function from Deinococcus radiodurans. Upstream of this operon, three potential cres with two mismatches (boldface) in comparison to the consensus sequence were identified. The first (TATCAACGATGTTA) is located 437 nucleotides upstream of the potential translational start site of orf1, and the two degenerations conserved the symmetry. The two others are located 36 (TGAAAGCGTTTTAT) and 70 (AGAAAACGATACCA) nucleotides upstream of this translational start site.
Analysis of adjacent regions of gls17 indicates that this gene is part of an operon of four genes. ORF1 shares 42% identity with the regulatory protein PfoR from Clostridium perfringens, and ORF2 and Gls17 share 39 and 51% identity with probable l-serine dehydratase beta and alpha chains from B. subtilis, respectively. Finally, ORF4 is 60% identical with seryl-tRNA synthetase from B. subtilis. A perfect cre box (TGAAAACGTTATCA) was identified 1 nucleotide after the translational start site of orf1.
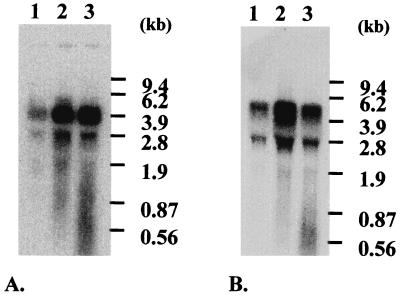
The obvious induction of these proteins in the ccpA mutant strain and at the onset of glucose starvation of strain JH2-2 has been verified at the transcriptional level. Northern blot analyses were performed with total RNA extracted from growing cells of strain CL14 and growing and starved cells of strain JH2-2 (Fig. 6). The results of hybridization with a gls27-specific probe showed one band, the size of which corresponds to that of the entire operon (Fig. 6A). This approximately 4.2-kb transcript showed a 4.5-fold increase in the ccpA mutant strain and at the onset of glucose starvation compared to the level in growing cells of JH2-2. The results of hybridization with a gls17-specific probe are shown in Fig. 6B. A transcript of approximately 4.9 kb was detected, which corresponds to the size of the putative operon. This transcript was strongly induced (7.4-fold) in the mutant cells during growth phase compared to the JH2-2 strain. Moreover, the mRNA level was 2.2-fold higher at the onset of glucose starvation compared to that in growing cells of JH2-2.
FIG. 6.
Northern blot analysis of the E. faecalis gls27 and gls17 genes. Total RNA was isolated from strains JH2-2 (lanes 1 and 3) and CL14 (lane 2) exponentially grown on glucose (lanes 1 and 2) or at the onset of glucose starvation (lane 3). Hybond N+ membranes were hybridized with a gls27-specific probe (A) or a gls17-specific probe (B).
DISCUSSION
In this communication, we report the characterization and functional analysis of a ccpA homologue from E. faecalis. Our transcriptional analyses indicated that transcription is monocistronic and takes place from a promoter located 139 bp upstream from the ccpA reading frame. In the next step, we tried to determine the putative regulatory role of CcpA in E. faecalis and its implication in carbon metabolism. Analysis of galK transcription in a ccpA mutant strain of E. faecalis indicated that transcription of the corresponding operon is partially derepressed in the presence of a mixture of glucose and galactose (Fig. 3A). This phenomenon could be correlated with the presence of a putative cre sequence in the promoter region of the galKETR operon. Similarly, in L. lactis, disruption of the ccpA gene did not result in a complete derepression of gal operon transcription (29), suggesting that either the induction of the gal transcription is reduced by the disruption of the ccpA gene or an additional system of glucose repression might be active. Processes such as inducer exclusion and inducer expulsion, which have been demonstrated in E. faecalis (44), or other control mechanisms involved in the regulation of the gal operon may also account for the observed residual glucose repression in the E. faecalis ccpA mutant. The E. faecalis ccpA gene could also restore glucose repression of a ynaJ′-lacZ fusion in a B. subtilis ccpA mutant, showing that the sequence conservation of ccpA between E. faecalis and B. subtilis was paralleled by similar functions in these microorganisms. These data clearly demonstrate the implication of CcpA in CR of E. faecalis.
Inactivation of the E. faecalis ccpA gene also resulted in a reduction of the growth rate on different sugars, a phenomenon generally observed for ccpA mutants of other bacteria (4, 9, 19). This suggests that, in addition to its role in CR, CcpA could also be involved in other regulatory processes. Indeed, CcpA is responsible for glucose-mediated transcriptional activation of alsS, ackA, and some glycolytic enzymes in B. subtilis (14, 17, 39). Moreover, Luesink et al. (29) reported the transcriptional activation of the las operon by CcpA in the presence of glucose in L. lactis. Our observations indicated that the organization of these genes in E. faecalis was different. Genes encoding pyruvate kinase and phosphofructokinase form an operon whose transcription seemed independent of CcpA, whereas the gene encoding lactate dehydrogenase is monocistronic and its transcription is 2.2-fold reduced in the ccpA mutant strain (Fig. 3). In B. subtilis, pfk and pyk are also CcpA independent (39). However, in that microorganism, an activation of the gap gene and the pgk operon by glucose, which seems to be dependent on CcpA, has been reported (39). In E. faecalis, Northern blot experiments showed that transcription of the operon comprising ygaP, gap, pgk, and tpi seems independent of CcpA (data not shown). This result is further strengthened by the absence of a cre-like sequence in the promoter region of this operon. A potential cre was identified in the promoter region of ldh in E. faecalis and could be implicated in the CcpA-mediated activation of this gene. This result suggests that CcpA in E. faecalis could also act as a transcriptional activator, as in B. subtilis and L. lactis, which is further supported by the 2-D gel analysis indicating that six proteins showed reduced expression in the ccpA mutant. However alternative explanations, such as indirect effects on transcription or changes in RNA stability, cannot be excluded. The probable lower glycolytic capacity of the E. faecalis ccpA mutant, due to the lack of activation of at least ldh in the presence of glucose, might be one of several factors explaining the growth deficiency. Among these are the unbalanced expression of catabolic enzymes that might be a burden to the cells, the lack of ammonium assimilation, as demonstrated for the B. subtilis ccpA mutant, and an accumulation of glycolytic intermediates that cannot be excreted (17, 40).
In order to identify other proteins that may belong to the CcpA regulon, we used a 2-D electrophoresis approach. A comparison of the protein pattern of the wild-type and ccpA mutant cells harvested in mid-exponential growth phase revealed that several proteins were affected by the ccpA mutation. Among them, a variation in the phosphorylation states of HPr was observed. One might hypothesize that this phenomenon is correlated with the observed smaller amount of ldh transcript in the ccpA mutant, which could provoke higher levels of glycolytic intermediates, such as FBP, required for activation of the HPr kinase of B. subtilis (34). Such results were obtained for the HPr kinase of E. faecalis (5), but a recent study indicated that for highly purified recombinant E. faecalis HPr kinase, FBP could also be omitted in vitro (24), suggesting that its implication is not so clear as for B. subtilis.
In addition to its effects on HPr, the ccpA mutation leads to an obviously enhanced synthesis of at least 16 polypeptides. This number certainly does not reflect the totality of proteins that belong to the CcpA regulon. Indeed, genes and operons required for the utilization of specific carbon sources are in most cases subjected to CR and to substrate induction; thus, even in a ccpA mutant, they will be expressed only if the carbon sources are present in the medium (for a review, see reference 35). In this way, the 16 polypeptides with enhanced synthesis in a ccpA mutant genetic background probably do not need any inducer or corresponding inducers are already present in the culture medium.
Four of the polypeptides with enhanced synthesis in the E. faecalis ccpA mutant were microsequenced, and the corresponding genes were found in the unfinished genome sequence of E. faecalis. One of the microsequences obtained corresponds to a gene whose product shares high identity with the putative l-serine dehydratase alpha subunit of B. subtilis. l-Serine dehydratase (or l-serine deaminase) catalyzes the conversion of l-serine to pyruvate, the first step of the degradative pathway of this amino acid.
The other microsequences obtained correspond to genes that were part of an operon encoding glycerol dehydrogenase, an ORF coding for a putative protein of unknown function, and two polypeptides corresponding to putative dihydroxyacetone kinases. As in other bacteria, this result suggests that glycerol dissimilation in E. faecalis can be achieved by two biochemical pathways. Following uptake via the glycerol facilitator, glycerol may be first phosphorylated by glycerol kinase and subsequently oxidized to dihydroxyacetone phosphate by a flavin-linked glycerol-3-phosphate dehydrogenase. Corresponding enzymatic activities have been identified in E. faecalis (8). Alternatively, glycerol is first oxidized by an NAD-linked glycerol dehydrogenase to dihydroxyacetone (DHA) and subsequently phosphorylated to DHA-phosphate by an ATP-dependent DHA kinase (28). The roles of these activities in the starvation stress response remain to be analyzed.
ACKNOWLEDGMENTS
This work was partly supported by financial aid from the Agence de l'Eau Seine-Normandie. C. Leboeuf is the recipient of an award from the Ministère de la Recherche et de l'Enseignement Supérieur of France.
We thank I. Martin-Verstraete for kindly providing us strains QB7144 and QB7147, H. Putzer for plasmid pHM12, E. Küster and W. Hillen for CcpA antibodies, and W. Hengstenberg for HPr antibodies. The expert advice of C. Karmazyn-Campelli for the B. subtilis experimentations were greatly appreciated.
REFERENCES
- 1.Altshul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Anagnostopolous C, Spizizen J. Requirements for transformation in B. subtilis. J Bacteriol. 1961;81:741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arantes O, Lereclus D. Construction of cloning vectors for Bacillus thuringiensis. Gene. 1991;108:115–119. doi: 10.1016/0378-1119(91)90495-w. [DOI] [PubMed] [Google Scholar]
- 4.Behari J, Youngman P. A homolog of CcpA mediates catabolite control in Listeria monocytogenes but not carbon source regulation of virulence genes. J Bacteriol. 1998;180:6316–6324. doi: 10.1128/jb.180.23.6316-6324.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deutscher J, Engelmann R. Purification and characterization of an ATP-dependent protein kinase from Streptococcus faecalis. FEMS Microbiol Lett. 1984;23:157–162. [Google Scholar]
- 6.Deutscher J, Küster E, Bergstedt U, Charrier V, Hillen W. Protein kinase-dependent HPr/CcpA interaction links glycolytic activity to carbon catabolite repression in Gram-positive bacteria. Mol Microbiol. 1995;15:1049–1053. doi: 10.1111/j.1365-2958.1995.tb02280.x. [DOI] [PubMed] [Google Scholar]
- 7.Deutscher J, Saier M H J. ATP-dependent protein kinase-catalyzed phosphorylation of a seryl residue in HPr, a phosphate carrier protein of the phosphotransferase system in Streptococcus pyogenes. Proc Natl Acad Sci USA. 1983;80:6790–6794. doi: 10.1073/pnas.80.22.6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deutscher J, Sauerwald H. Stimulation of dihydroxyacetone and glycerol kinase activity in Streptococcus faecalis by phosphoenolpyruvate-dependent phosphorylation catalyzed by enzyme I and HPr of the phosphotransferase system. J Bacteriol. 1986;166:829–836. doi: 10.1128/jb.166.3.829-836.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egeter O, Brückner R. Catabolite repression mediated by the catabolite control protein CcpA in Staphylococcus xylosus. Mol Microbiol. 1996;21:739–749. doi: 10.1046/j.1365-2958.1996.301398.x. [DOI] [PubMed] [Google Scholar]
- 10.Frère J, Benachour A, Novel M, Novel G. Identification of the theta-type minimal replicon of the Lactococcus lactis spp. lactis CNRZ270 lactose protease plasmid pUCL22. J Basic Microbiol. 1993;27:97–102. [Google Scholar]
- 11.Galinier A, Deutscher J, Martin-Verstraete I. Phosphorylation of either Crh or HPr mediates binding of CcpA to the Bacillus subtilis xyn cre and catabolite repression of the xyn operon. J Mol Biol. 1999;286:307–314. doi: 10.1006/jmbi.1998.2492. [DOI] [PubMed] [Google Scholar]
- 12.Giard J-C, Hartke A, Flahaut S, Benachour A, Boutibonnes P, Auffray Y. Starvation-induced multiresistance in Enterococcus faecalis JH2-2. Curr Microbiol. 1996;148:264–271. doi: 10.1007/s002849900048. [DOI] [PubMed] [Google Scholar]
- 13.Giard J-C, Hartke A, Flahaut S, Boutibonnes P, Auffray Y. Glucose starvation response in Enterococcus faecalis JH2-2: survival and protein analysis. Res Microbiol. 1997;148:27–35. doi: 10.1016/S0923-2508(97)81897-9. [DOI] [PubMed] [Google Scholar]
- 14.Grundy F J, Waters D A, Allen S H G, Henkin T M. Regulation of the Bacillus subtilis acetate kinase gene by CcpA. J Bacteriol. 1993;175:7348–7355. doi: 10.1128/jb.175.22.7348-7355.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartke A, Bouche S, Gansel X, Boutibonnes P, Auffray Y. Starvation-induced stress resistance in Lactococcus lactis subsp. lactis IL1403. Appl Environ Microbiol. 1994;60:3474–3478. doi: 10.1128/aem.60.9.3474-3478.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hengge-Aronis R. Survival or hunger and stress: the role of rpoS in stationary phase gene regulation in Escherichia coli. Cell. 1993;72:165–168. doi: 10.1016/0092-8674(93)90655-a. [DOI] [PubMed] [Google Scholar]
- 17.Henkin T M. The role of the CcpA transcriptional regulator in carbon metabolism in Bacillus subtilis. FEMS Microbiol Lett. 1996;135:9–15. doi: 10.1111/j.1574-6968.1996.tb07959.x. [DOI] [PubMed] [Google Scholar]
- 18.Henkin T M, Grundy F J, Nicholson W L, Chambliss G H. Catabolite repression of α-amylase gene expression in Bacillus subtilis involves a trans-acting gene product homologous to the Escherichia coli lacI and galR repressors. Mol Microbiol. 1991;5:575–584. doi: 10.1111/j.1365-2958.1991.tb00728.x. [DOI] [PubMed] [Google Scholar]
- 19.Hueck C J, Hillen W. Catabolite repression in Bacillus subtilis: a global regulatory mechanism for the Gram-positive bacteria? Mol Microbiol. 1995;15:395–401. doi: 10.1111/j.1365-2958.1995.tb02252.x. [DOI] [PubMed] [Google Scholar]
- 20.Hueck C J, Kraus A, Schmiedel D, Hillen W. Cloning, expression and functional analyses of the catabolite control protein CcpA from Bacillus megaterium. Mol Microbiol. 1995;16:855–864. doi: 10.1111/j.1365-2958.1995.tb02313.x. [DOI] [PubMed] [Google Scholar]
- 21.Jacob A E, Hobbs S J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974;117:360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenkins D E, Chaisson S A, Matin A. Starvation-induced cross protection against osmotic challenge in Escherichia coli. J Bacteriol. 1990;172:2779–2781. doi: 10.1128/jb.172.5.2779-2781.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jouper-Jaan A, Goodman A E, Kjelleberg S. Bacteria starved for prolonged periods develop increased protection against lethal temperatures. FEMS Microbiol Ecol. 1992;101:229–236. [Google Scholar]
- 24.Kravanja M, Engelmann R, Dossonnet V, Bluggel M, Meyer H E, Frank R, Galinier A, Deutscher J, Schnell N, Hengstenberg W. The hprK gene of Enterococcus faecalis encodes a novel bifunctional enzyme: the HPr kinase/phosphatase. Mol Microbiol. 1999;31:59–66. doi: 10.1046/j.1365-2958.1999.01146.x. [DOI] [PubMed] [Google Scholar]
- 25.Küster E, Luesink E J, de Vos W M, Hillen W. Immunological crossreactivity to the catabolite control protein CcpA from Bacillus megaterium is found in many Gram-positive bacteria. FEMS Microbiol Lett. 1996;139:109–115. doi: 10.1111/j.1574-6968.1996.tb08188.x. [DOI] [PubMed] [Google Scholar]
- 26.Lange R, Hengge-Aronis R. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol Microbiol. 1991;5:49–59. doi: 10.1111/j.1365-2958.1991.tb01825.x. [DOI] [PubMed] [Google Scholar]
- 27.Leboeuf C, Auffray Y, Hartke A. Cloning, sequencing and characterization of the ccpA gene from Enterococcus faecalis. Int J Food Microbiol. 2000;55:109–113. doi: 10.1016/s0168-1605(00)00185-9. [DOI] [PubMed] [Google Scholar]
- 28.Lin E C. Glycerol dissimilation and its regulation in bacteria. Annu Rev Microbiol. 1976;30:535–578. doi: 10.1146/annurev.mi.30.100176.002535. [DOI] [PubMed] [Google Scholar]
- 29.Luesink E J, van Herpen R E M A, Grossiord B P, Kuipers O P, de Vos W M. Transcriptional activation of the glycolytic las operon and catabolite repression of the gal operon in Lactococcus lactis are mediated by the catabolite control protein CcpA. Mol Microbiol. 1998;30:789–798. doi: 10.1046/j.1365-2958.1998.01111.x. [DOI] [PubMed] [Google Scholar]
- 30.Martensen T M. Chemical properties, isolation, and analysis of O-phosphates in proteins. In: Wold F, Moldave K, editors. Methods in Enzymology. Vol. 107. San Diego, Calif: Academic Press; 1984. pp. 3–23. [DOI] [PubMed] [Google Scholar]
- 31.Matin A. The molecular basis of carbon-starvation-induced general resistance in Escherichia coli. Mol Microbiol. 1991;5:3–10. doi: 10.1111/j.1365-2958.1991.tb01819.x. [DOI] [PubMed] [Google Scholar]
- 32.Miller J H. Experiments in molecular genetics. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 33.Monedero V, Gosalbes M J, Perez-Martinez G. Catabolite repression in Lactobacillus casei ATCC 393 is mediated by CcpA. J Bacteriol. 1997;179:6657–6664. doi: 10.1128/jb.179.21.6657-6664.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reizer J, Hoischen C, Titgemeyer F, Rivolta C, Rabus R, Stülke J, Karamata D, Saier M H J, Hillen W. A novel protein kinase that controls carbon catabolite repression in bacteria. Mol Microbiol. 1998;27:1157–1169. doi: 10.1046/j.1365-2958.1998.00747.x. [DOI] [PubMed] [Google Scholar]
- 35.Saier M H, Chauvaux S, Cook G M, Deutscher J, Paulsen I T, Reizer J, Ye J J. Catabolite repression and inducer control in Gram-positive bacteria. Microbiology. 1996;142:217–230. doi: 10.1099/13500872-142-2-217. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 37.Schultz J E, Matin A. Molecular and functional characterization of a carbon starvation gene of Escherichia coli. J Mol Biochem. 1991;218:129–140. doi: 10.1016/0022-2836(91)90879-b. [DOI] [PubMed] [Google Scholar]
- 38.Stewart G C. Catabolite repression in the Gram-positive bacteria: generation of negative regulators of transcription. J Cell Biochem. 1993;51:25–28. doi: 10.1002/jcb.240510106. [DOI] [PubMed] [Google Scholar]
- 39.Tobisch S, Zuhlke D, Bernhardt J, Stulke J, Hecker M. Role of CcpA in regulation of the central pathways of carbon catabolism in Bacillus subtilis. J Bacteriol. 1999;181:6996–7004. doi: 10.1128/jb.181.22.6996-7004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turinsky A J, Grundy F J, Kim J-H, Chambliss G H, Henkin T M. Transcriptional activation of the Bacillus subtilis ackA gene requires sequences upstream of the promoter. J Bacteriol. 1998;180:5961–5967. doi: 10.1128/jb.180.22.5961-5967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weickert M J, Adhya S. A family of bacterial regulators homologous to Gal and Lac repressors. J Biol Chem. 1992;267:15869–15874. [PubMed] [Google Scholar]
- 42.Weickert M J, Chambliss G H. Site-directed mutagenesis of a catabolite repression operator sequence in Bacillus subtilis. Proc Natl Acad Sci USA. 1990;87:6238–6242. doi: 10.1073/pnas.87.16.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yagi Y, Clewell D B. Recombination-deficient mutant of Streptococcus faecalis. J Bacteriol. 1980;143:966–970. doi: 10.1128/jb.143.2.966-970.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye J J, Minarcik J, Saier M H. Inducer expulsion and the occurrence of an HPr(Ser-P)-activated sugar-phosphate phosphatase in Enterococcus faecalis and Streptococcus pyogenes. Microbiology. 1996;142:585–592. doi: 10.1099/13500872-142-3-585. [DOI] [PubMed] [Google Scholar]