Abstract
Aims
The transition from hypertension to heart failure (HF) remains poorly understood. We hypothesized that insufficient perfusion to match global metabolic demand, reflected by a low ratio of myocardial blood flow to global myocardial mass, may be a HF risk marker.
Methods and results
A retrospective cohort (n = 346) of patients with hypertension who underwent clinical positron emission tomography (PET) myocardial perfusion imaging for chest pain and/or dyspnoea at Brigham and Women’s Hospital (Boston, MA, USA) were studied. Patients without obstructive coronary artery disease by history or PET perfusion (summed stress score <3), HF, cardiomyopathy, or ejection fraction (EF) <40% were followed for HF hospitalization (primary outcome), all-cause death, and their composite. Myocardial blood flow, left ventricular (LV) mass, volumes, and EF were obtained from PET, and a ‘flow/mass ratio’ was determined as hyperaemic myocardial blood flow over LV mass indexed to body surface area. A lower flow/mass ratio was independently associated with larger end-diastolic (β = −0.44, P < 0.001) and end-systolic volume (β = −0.48, P < 0.001) and lower EF (β = 0.33, P < 0.001). A flow/mass ratio below the median was associated with an adjusted hazard ratio of 2.47 [95% confidence interval (CI) 1.24–4.93; P = 0.01] for HF hospitalization, 1.95 (95% CI 1.12–3.41; P = 0.02) for death, and 2.20 (95% CI 1.39–3.49; P < 0.001) for the composite.
Conclusion
An integrated physiological measure of insufficient myocardial perfusion to match global metabolic demand identifies subclinical hypertensive heart disease and elevated risk of HF and death in symptomatic patients with hypertension but without flow-limiting coronary artery disease.
Keywords: Hypertensive heart disease, Hypertension, Heart failure
Graphical Abstract
See the editorial comment for this article ‘Transition to heart failure in hypertension: going to the heart of the matter’, by Antoni Bayés-Genís and Javier Díez, https://doi.org/10.1093/eurheartj/ehab651.
Introduction
Beyond its role as an atherosclerotic risk factor, hypertension contributes an estimated 40% to heart failure (HF) risk,1 making it a significant public health burden.2 While adverse left ventricular (LV) remodelling and LV hypertrophy (LVH) are well-recognized intermediate phenotypes for HF,3,4 overt myocardial structural changes are late-stage findings that are not universally present in all patients with hypertensive heart disease, thus highlighting the need for improved understanding of the pathophysiological mechanisms of hypertensive heart disease-associated HF.
Myocardial perfusion, even in the absence of obstructive epicardial coronary artery disease (CAD), has long been recognized as a central mechanism for the development of adverse myocardial remodelling and hypertensive heart disease-associated HF.5 Prior studies in hypertension have shown reduced coronary blood flow after adjusting for LV mass. In addition, there was a strong inverse relationship between coronary flow and LV mass, suggesting increased risk for myocardial ischaemia in this population.6 In hypertension, after hypertrophic adaptation to increased afterload, flow per gram of tissue can be normalized while global flow to the ventricle must increase to keep pace with demand.7,8 Myocardial blood flow that is inadequate to meet globally increased metabolic demand, especially in the vulnerable subendocardium, may lead to a cascade of alterations including subendocardial ischaemia, injury, diastolic dysfunction, fibrosis, and ultimately clinical HF. Recent studies in trained athletes with physiological LVH and hypertensive patients with pathological LVH demonstrated different patterns of myocardial blood flow when normalized to global LV mass to estimate whole organ perfusion.9 Unappreciated heterogeneity in the transmural distribution of myocardial blood flow or failure of myocardial perfusion to meet global changes in myocardial oxygen demand in pathological states of LV remodelling may be a potential explanation for why patients with symptoms in the absence of angiographically confirmed obstructive CAD still experience adverse cardiovascular outcomes.10,11 Whether imbalance between global myocardial perfusion and demand can be identified before the development of frank myocardial structural changes remains unknown.
To understand how myocardial perfusion as a function of global myocardial mass may be an early subclinical marker of HF risk, we leveraged a patient cohort with hypertension who underwent clinical stress testing with positron emission tomography (PET) myocardial perfusion imaging for anginal symptoms but had no obstructive epicardial CAD by history or by PET perfusion to generate a novel index of peak hyperaemic myocardial blood flow divided by LV mass, termed the ‘flow/mass ratio’. We hypothesized that the flow/mass ratio, accounting for global myocardial mass, would be associated with abnormal ventricular structure and function and with future HF risk, thus identifying a potential pathophysiological mechanism through which patients develop symptoms and hypertensive HF.
Methods
Study population
All patients referred for rest/stress myocardial PET imaging at the Brigham and Women’s Hospital (Boston, MA, USA) between 1 January 2006 and 31 October 2018 are included in an Institutional Review Board-approved clinical PET registry, from which the present cohort was generated (Figure 1). Patient medical history and medication use were obtained at the time of PET imaging by direct patient interview and review of electronic medical records. Blood pressure was measured with an automatic oscillometric cuff (GE Healthcare, Chicago, IL, USA) at rest and again at the time of vasodilator and radiotracer injection (peak stress) and serially in recovery. To isolate a population with symptoms potentially referable to hypertensive heart disease and at risk for future HF events, patients were included if they had a clinical history of hypertension and presented for evaluation of chest pain and/or dyspnoea. Patients were excluded for flow-limiting epicardial CAD, defined by (i) a clinical history of known CAD, prior myocardial infarction, percutaneous coronary intervention, or coronary artery bypass grafting, or (ii) the presence of abnormal myocardial perfusion by PET (summed stress score ≥ 3 consistent with ischaemia or scar). Patients with a clinical history of prevalent HF or cardiomyopathy, significant (>2+) valvular disease, or heart transplant were excluded, as were those with an LV ejection fraction (LVEF) < 40% by PET. In the case of repeat PET scans, only the earliest study was used. To enrich the study population for the risk of cardiovascular events, patients were required to have undergone clinical measurement of a circulating cardiac biomarker within 90 days of the PET study date (Figure 1). The study was approved by the Mass General Brigham Institutional Review Board and conducted in accordance with institutional guidelines.
Figure 1.
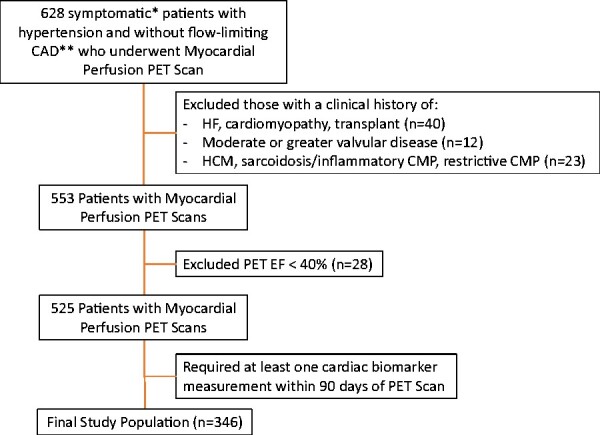
Patient flow diagram. *Study indications were limited to symptoms of chest pain and/dyspnoea. **Flow-limiting coronary artery disease was defined by either (i) a clinical history of known coronary artery disease, myocardial infarction, percutaneous coronary intervention, or coronary artery bypass grafting or (ii) the presence of abnormal perfusion defined by summed stress score ≥3. CAD, coronary artery disease; CMP, cardiomyopathy; EF, ejection fraction; HCM, hypertrophy cardiomyopathy; HF, heart failure; PET, positron emission tomography.
Circulating biomarker assessment
Serum creatinine and estimated glomerular filtration rate drawn within 90 days before or after the date of the PET study were obtained from the clinical medical record. To be included in the study cohort, all patients had either troponin T, troponin I, B-type natriuretic peptide, or N-terminal pro-B-type natriuretic peptide measured under clinical conditions within 90 days before or after PET. Cardiac biomarker assay details have been described previously.12
Quantification of myocardial blood flow and flow/mass ratio
Patients were imaged with a whole-body PET/computed tomography (CT) scanner (GE Discovery STE or DRX, Waukesha, WI, USA) with the CT being used for attenuation correction. Patients were imaged with rubidium-82 or ammonia-13, as described previously,13,14 at rest and after standard intravenous vasodilator infusion to achieve maximal coronary hyperaemia. Semiquantitative 17-segment visual assessment with a standard 5-point scoring system was used to evaluate myocardial scar and ischaemia. Global absolute myocardial blood flow was quantified at rest and at peak hyperaemia using a validated two-compartment kinetic model15 and is highly reproducible, with an intraclass correlation coefficient of 0.94 [95% confidence interval (CI) 0.88–0.98] in our laboratory. Left ventricular mass was determined from gated myocardial perfusion images with commercially available software (Corridor4DM, INVIA Medical Imaging Solutions, Ann Arbor, MI, USA) and indexed to body surface area (BSA). A ‘flow/mass ratio’ was determined for each patient as the ratio of hyperaemic myocardial blood flow divided by global LV mass index; thus, all references to the ‘flow/mass ratio’ herein are indexed to BSA. The median flow/mass ratio was established on a sex-specific basis, given the known sex differences in the typical distribution of LV mass: median flow/mass ratio = 0.0243 mL/g/min per g/m2 in men and 0.0352 mL/g/min per g/m2 in women, with values below the median termed ‘low’ and those above termed ‘preserved’. Myocardial flow reserve (MFR), an established metric of coronary vasodilatory capacity that does not account for total LV mass, was calculated as the ratio of hyperaemic to resting myocardial blood flow, as described previously.13 Coronary artery calcium scoring based on CT acquisition is described in the Supplementary material online.
Assessment of left ventricular structure and function
Left ventricular end-diastolic volume (EDV), end-systolic volume (ESV), and LVEF were determined from gated myocardial perfusion images at rest and peak hyperaemic stress during routine post-processing. Rest EDV and ESV were indexed to BSA. Left ventricular ejection fraction reserve was calculated as the difference between stress and rest LVEF. Transient ischaemic dilation was considered to be present if the ratio of stress to rest EDV was >1.13.16
Clinical outcomes
The primary outcome in longitudinal analyses was first incident HF hospitalization, which was determined by blinded two-physician review and adjudication of records of all hospitalizations from 30 days after the PET study through the end of study follow-up on 11 May 2020. On review, patients who were found to be admitted with HF at or within 30 days of PET were excluded. The secondary outcomes were all-cause death and the composite of HF hospitalization and all-cause death. Vital status was ascertained from the Social Security Death Index, National Death Index, and Partners Healthcare Research Patient Data Registry and verified by blinded two-physician review. Patients without clinical events were censored on the date of last contact in the electronic medical record after the date of the PET scan.
Statistical analysis
Continuous variables are reported as mean ± standard deviation or median (interquartile range), as appropriate. Demographic, medical history, haemodynamic, and imaging parameters were compared between those with flow/mass ratio above vs. below the median using Student’s t-test, Wilcoxon rank sum, and χ2, as appropriate.
The continuous cross-sectional relationship between continuous flow/mass ratio and PET-derived markers of LV structure and function was evaluated using linear regression with a series of three models: Model 1 comprised the unadjusted univariate association; Model 2 included adjustment for sex, age, and body mass index (BMI); and Model 3 further incorporated radiotracer, history of diabetes mellitus, estimated glomerular filtration rate, and number of antihypertensive medications at the time of the PET study, as a proxy for hypertension severity. Covariates were selected based on univariate screening and established biological relationships.
The relationship between dichotomized flow/mass ratio and clinical outcomes was assessed using Cox proportional hazards modelling, using the same three models as above with the addition of LVEF to Model 3. Proportional hazards assumptions were verified using graphical methods and Schoenfeld residuals. Poisson regression was used to compute adjusted annualized rates of HF hospitalization, all-cause death, and their composite, adjusted for all Model 3 covariates as above. We performed a sensitivity analysis using quintiles of flow/mass ratio rather than dichotomization at the median to evaluate the risk for clinical outcomes across the spectrum of flow/mass ratio.
Given the known association between HF risk and coronary microvascular dysfunction, defined by a reduction in MFR in the absence of epicardial obstruction, exploratory analyses examined whether flow/mass ratio could further risk stratify patients with preserved MFR. Cox proportional hazards modelling was used to compare the risk of HF hospitalization based on the presence of reduced MFR vs. preserved or reduced flow/mass ratio in the absence of reduced MFR.
Additional exploratory analyses aimed to identify whether the flow/mass ratio could further inform understanding of HF risk in the clinically ambiguous entity of ‘mid-range’ LVEF, which is known to encompass a range of heterogeneous pathogenesis and prognosis.17 Using Cox and Poisson regression models as above, we evaluated the association between flow/mass ratio, mid-range LVEF, and HF hospitalization.
Two-sided P-values <0.05 were considered statistically significant. All statistical analyses were performed with SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics
Baseline characteristics of the total cohort (n = 346) and stratified by median flow/mass ratio are shown in Table 1. The study cohort had a mean age of 62.7 (12.4) years, had a mean BMI in the obese range at 33.1 (8.2) kg/m2, and included 38.2% patients with diabetes. Patients with a low ratio of myocardial flow to global LV mass tended to have higher BMI, more frequent diabetes, and a non-significant trend towards a greater number of antihypertensive medications despite comparable systolic and diastolic blood pressure at the time of PET scan and similar utilization of cardiovascular medication classes (Table 1).
Table 1.
Baseline characteristics of the total cohort and by sex-specific mediana flow/mass ratio
| Total cohort (n = 346) | Flow/mass ratio < median (n = 173) | Flow/mass ratio ≥ median (n = 173) | P-value | |
|---|---|---|---|---|
| Age (years) | 62.7 (12.4) | 62.5 (12.8) | 63.0 (12.0) | 0.69 |
| Female sex | 254 (73.4) | 127 (73.4) | 127 (73.4) | 1.00 |
| BMI (kg/m2) | 33.1 (8.2) | 34.5 (8.7) | 31.6 (7.4) | 0.001 |
| Diabetes | 132 (38.2) | 77 (44.5) | 55 (31.8) | 0.01 |
| Race | ||||
| White | 134 (38.7) | 75 (43.4) | 59 (34.1) | 0.36 |
| Black | 118 (34.1) | 54 (31.2) | 64 (37.0) | |
| Hispanic/Latino | 63 (18.2) | 30 (17.3) | 33 (19.1) | |
| Other/unknown | 31 (9.0) | 14 (8.1) | 17 (9.8) | |
| eGFR (mL/min/1.73 m2) | 61 (56–62) | 61 (54–61) | 61 (57–65) | 0.19b |
| Symptoms at PET | ||||
| Chest pain | 303 (87.6) | 150 (86.7) | 153 (88.4) | 0.62 |
| Dyspnoea | 99 (28.6) | 50 (28.9) | 49 (28.3) | 0.91 |
| Medications | ||||
| ACEi/ARB | 116 (33.5) | 63 (36.4) | 53 (30.6) | 0.25 |
| CCB | 103 (29.8) | 57 (33.0) | 46 (26.6) | 0.20 |
| Beta-blockers | 183 (52.9) | 94 (54.3) | 89 (51.5) | 0.59 |
| Nitrates | 20 (5.8) | 10 (5.8) | 10 (5.8) | 1.00 |
| Diuretics | 136 (39.3) | 75 (43.4) | 61 (35.3) | 0.12 |
| Aspirin | 213 (61.6) | 111 (64.2) | 102 (59.0) | 0.32 |
| Lipid-lowering therapies | 184 (53.2) | 101 (58.4) | 83 (48.0) | 0.05 |
| Number of antihypertensives | ||||
| 0–1 | 185 (53.5) | 85 (49.1) | 100 (57.8) | 0.19 |
| 2 | 102 (29.5) | 52 (30.1) | 50 (28.9) | |
| 3 | 49 (14.2) | 31 (17.9) | 18 (10.4) | |
| 4+ | 10 (2.9) | 5 (2.9) | 5 (2.9) | |
| Non-invasive resting haemodynamics | ||||
| HR (b.p.m.) | 70.5 (13.6) | 68.2 (12.5) | 72.7 (14.3) | 0.002 |
| Systolic BP (mmHg) | 152.0 (27.4) | 150.2 (28.5) | 153.8 (26.2) | 0.22 |
| Diastolic BP (mmHg) | 76.0 (13.1) | 75.2 (13.3) | 76.9 (12.9) | 0.24 |
| Rate pressure product | 10 705 (2827) | 10 230 (2611) | 11 181 (2959) | 0.002 |
Values are mean ± standard deviation, n (%), or median (interquartile range).
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CCB, calcium channel blocker; eGFR, estimated glomerular filtration rate; HR, heart rate; PET, positron emission tomography.
The median flow/mass ratio was 0.0243 mL/g/min per g/m2 in men and 0.0352 mL/g/min per g/m2 in women.
P-value reflects Wilcoxon test. All others are t-tests for continuous variables and χ2 for categorical variables.
Perfusion and myocardial parameters at rest and peak hyperaemic stress are shown in Table 2. The study cohort had a mean resting LVEF of 62.2 (8.6%). As expected based on the construction of the flow/mass ratio, those with a low ratio of flow to global LV mass had both lower measures of myocardial blood flow, lower MFR, and larger LV mass. The proportion of patients with myocardial injury indicated by detectable circulating troponin was greater in those with a low flow/mass ratio; whereas the burden of non-obstructive calcified coronary plaque was minimal overall and did not differ by flow/mass ratio (Supplementary material online, Table S1).
Table 2.
Left ventricular perfusion, structure, function, and injury stratified by mediana flow/mass ratio
| Total cohort (n = 346) | Flow/mass ratio < median (n = 173) | Flow/mass ratio ≥ median (n = 173) | P-value | |
|---|---|---|---|---|
| Myocardial perfusion | ||||
| Rest MBF (mL/min/g) | 1.13 (0.49) | 0.93 (0.27) | 1.34 (0.57) | <0.001 |
| Stress MBF (mL/min/g) | 2.24 (0.92) | 1.62 (0.43) | 2.86 (0.86) | <0.001 |
| MFR | 2.09 (0.85) | 1.84 (0.57) | 2.35 (1.00) | <0.001 |
| Correctedb MFR | 2.22 (1.03) | 1.85 (0.68) | 2.58 (1.18) | <0.001 |
| Myocardial structure and function | ||||
| LV mass indexed to BSA (g/m2) | 65.0 (10.9) | 69.7 (11.2) | 60.2 (8.3) | <0.001 |
| Rest EDV indexed to BSA (mL/m2) | 46.0 (14.0) | 51.4 (14.7) | 40.5 (10.7) | <0.001 |
| Rest ESV indexed to BSA (mL/m2) | 18.0 (8.3) | 21.2 (8.9) | 14.7 (6.2) | <0.001 |
| Rest LVEF (%) | 62.2 (8.6) | 59.8 (8.3) | 64.5 (8.2) | <0.001 |
| Stress LVEF (%) | 67.8 (8.8) | 64.4 (8.5) | 71.1 (7.8) | <0.001 |
| LVEF reservec (%) | 5.6 (5.5) | 4.6 (5.5) | 6.5 (5.4) | 0.001 |
| TID, n (%) | 130 (37.6%) | 73 (42.2%) | 57 (33.0%) | 0.08 |
| Myocardial injury, n (%) | ||||
| Troponin detectedd | 25/322 (7.8%) | 17/155 (11.0%) | 8/167 (4.8%) | 0.04 |
| Natriuretic peptide elevatione | 15/115 (13.0%) | 10/67 (14.9%) | 5/48 (10.4%) | 0.48 |
BSA, body surface area; EDV, end-diastolic volume; ESV, end-systolic volume; LV, left ventricular; LVEF, left ventricular ejection fraction; MBF, myocardial blood flow; MFR, myocardial flow reserve (stress MBF/rest MBF; unitless); TID, transient ischaemic dilation.
The median flow/mass ratio was 0.0243 mL/g/min per g/m2 in men and 0.0352 mL/g/min per g/m2 in women.
Corrected MFR accounts for myocardial work as the ratio of stress MBF over rest MBF/rate pressure product × 10 000.
LVEF reserve = stress LVEF − rest LVEF.
Troponin values ascertained in a subset of 322 patients of the total cohort.
Natriuretic peptide values ascertained in a subset of 115 patients of the total cohort.
Association between flow/mass ratio and left ventricular structure and function
Left ventricular EDV, ESV, and rest and stress LVEF were all significantly associated with the flow/mass ratio (Table 3). A lower flow/mass ratio was associated with a more dilated ventricle at end-diastole and with a larger ESV suggestive of more impaired or incomplete chamber emptying. A lower flow/mass ratio was also associated with a lower LVEF. In addition, these measures of LV structure and function than were more strongly associated with the integrated ratio of flow to global LV mass than with either stress myocardial blood flow or MFR (Supplementary material online, Table S2).
Table 3.
Association of left ventricular structure and function with flow/mass ratio
| Model 1 (unadjusted) | Model 2a | Model 3b | ||||
|---|---|---|---|---|---|---|
| β (Δ per SD of flow/mass ratio) | P-value | β (Δ per SD of flow/mass ratio) | P-value | β (Δ per SD of flow/mass ratio) | P-value | |
| Rest EDVi (mL/m2) | −0.471 | <0.001 | −0.480 | <0.001 | −0.442 | <0.001 |
| Rest ESVi (mL/m2) | −0.517 | <0.001 | −0.519 | <0.001 | −0.481 | <0.001 |
| Rest LVEF (%) | 0.434 | <0.001 | 0.380 | <0.001 | 0.329 | <0.001 |
| Stress LVEF (%) | 0.518 | <0.001 | 0.474 | <0.001 | 0.430 | <0.001 |
| LVEF reservec (%) | 0.148 | 0.006 | 0.146 | 0.004 | 0.078 | 0.14 |
EDVi, end-diastolic volume indexed to body surface area; ESVi, end-systolic volume indexed to body surface area; LVEF, left ventricular ejection fraction; SD, standard deviation.
Adjusted for age, sex, and body mass index.
Adjusted for age, sex, body mass index, diabetes mellitus, estimated glomerular filtration rate, radiotracer, and number of antihypertensive medications.
LVEF reserve = stress LVEF − rest LVEF.
Association between flow/mass ratio and cardiovascular outcomes
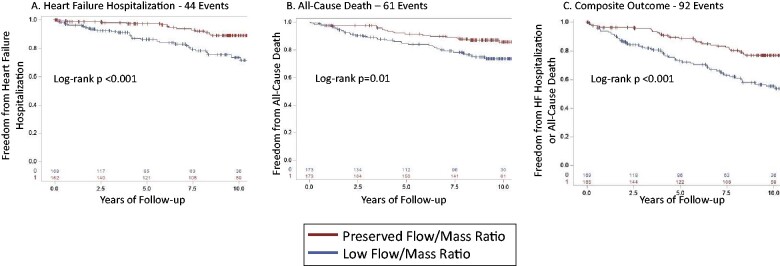
Given the association between the integrated flow/mass ratio and LV volumes and systolic function, we then assessed the relationship between the flow/mass ratio and risk of incident HF hospitalization and death. The primary outcome of HF hospitalization occurred in 44 patients with a median follow-up of 7.2 years. The secondary outcome of all-cause death occurred in 61 patients. The composite outcome of HF hospitalization or death occurred in 92 patients. Compared to a preserved flow/mass ratio (above the median), a low ratio of perfusion to total LV mass was associated with a greater cumulative incidence of HF hospitalization (Figure 2A), all-cause death (Figure 2B), and the composite of HF hospitalization and death (Figure 2C). Univariate and serial multivariable-adjusted hazard ratios (HRs) for HF hospitalization and death are shown in Table 4. Relative to a preserved ratio, a low flow/mass ratio was associated with an adjusted HR of 2.47 (95% CI 1.24–4.93) for HF hospitalization, 1.95 (95% CI 1.12–3.41) for death, and 2.20 (95% CI 1.39–3.49) for the composite outcome. Adjusted annualized event rates are shown in Supplementary material online, Figure S1. Similarly, when the flow/mass ratio was divided into quintiles (Supplementary material online, Table S3), the lowest quintile was associated with the highest hazard for HF hospitalization and death, although definitive conclusions regarding risk in the intermediate quintiles are limited by group size.
Figure 2.
Kaplan–Meier survival curves are shown for heart failure hospitalization (A), all-cause death (B), and the composite of heart failure hospitalization and all-cause death (C), with patients categorized by preserved vs. low flow/mass ratio (≥ vs. <sex-specific median) over a median follow-up of 7.2 years for heart failure hospitalization, 8.9 years for death, and 7.3 years for the composite outcome.
Table 4.
Association of flow/mass ratio and risk of heart failure hospitalization and all-cause mortality
| Model 1 (unadjusted) | Model 2a | Model 3b | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| HF hospitalization (44 events) | ||||||
| Flow/mass ratio | 2.94 (1.55–5.58) | <0.001 | 2.91 (1.52–5.57) | 0.001 | 2.47 (1.24–4.93) | 0.01 |
| All-cause death (61 events) | ||||||
| Flow/mass ratio | 1.92 (1.15–3.22) | 0.01 | 2.29 (1.35–3.88) | 0.002 | 1.95 (1.12–3.41) | 0.02 |
| Composite of HF hospitalization and all-cause death (92 events) | ||||||
| Flow/mass ratio | 2.35 (1.53–3.61) | <0.001 | 2.49 (1.62–3.84) | <0.001 | 2.20 (1.39–3.49) | <0.001 |
Relative to a preserved flow/mass ratio, a low flow/mass ratio below the sex-specific median is associated with an increased risk of HF hospitalization, all-cause death, and their composite. The median flow/mass ratio was 0.0243 mL/g/min per g/m2 in men and 0.0352 mL/g/min per g/m2 in women.
CI, confidence interval; HF, heart failure; HR, hazard ratio.
Adjusted for age, sex, and body mass index.
Adjusted for age, sex, body mass index, diabetes mellitus, estimated glomerular filtration rate, radiotracer, number of antihypertensive medications, and left ventricular ejection fraction.
Given previous evidence supporting an association of MFR, stress myocardial blood flow, and LV mass with clinical outcomes, these parameters were also dichotomized based on median value and evaluated in prediction of clinical outcomes but provided less effective risk stratification than the flow/mass ratio in this population of symptomatic hypertensive patients without epicardial CAD (Supplementary material online, Table S4 and Figure S2).
Furthermore, in an exploratory analysis to consider the potential role of the flow/mass ratio in the risk stratification of patients with normal MFR, a group typically thought to be at favourable prognostic risk, a reduced flow/mass ratio conferred increased risk of HF hospitalization (adjusted HR 5.96, 95% CI 2.02–17.57) despite normal MFR, comparable to the HF risk in those with low MFR, i.e. coronary microvascular dysfunction (adjusted HR 3.43, 95% CI 1.39–8.46) (Supplementary material online, Table S5 and Figures S3 and S4).
Prognostic value of flow/mass ratio in mid-range ejection fraction
Even in the absence of frankly reduced LVEF, subclinical abnormalities in systolic function have been demonstrated to confer HF risk13 and mid-range ejection fraction (EF) (LVEF 40–50%) has been increasingly recognized as a challenging clinical scenario of unclear prognostic and mechanistic significance.17 To explore whether concurrent abnormalities in global myocardial perfusion might further inform HF risk in mid-range LVEF, we stratified the population into three groups: those with preserved flow/mass ratio and LVEF >50%, an intermediate group with either low flow/mass ratio or LVEF 40–50%, and those with a reduced flow/mass ratio and mid-range LVEF 40–50%. The combination of mid-range LVEF and a low flow/mass ratio was associated with a significantly greater cumulative incidence of HF hospitalization (Figure 3) with an adjusted annualized rate of HF hospitalization of 13.1% (95% CI 4.9–35.4%) compared to 1.8% (95% CI 1.1–3.0%) in those with either low flow/mass ratio or mid-range LVEF and 1.0% (95% CI 0.5–1.8%) in those with preserved flow/mass ratio and LVEF >50% (Supplementary material online, Figure S5). Unadjusted and serially adjusted HR for HF hospitalization and all-cause death are shown in Supplementary material online, Table S6.
Figure 3.
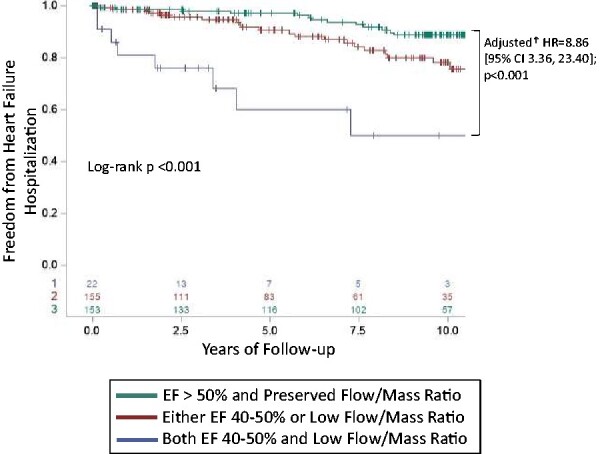
Freedom from heart failure hospitalization based on the integration of flow/mass ratio and mid-range ejection fraction (40–50% vs. ≥50%). Kaplan–Meier survival curves are shown for heart failure hospitalization with patients characterized by preserved vs. mid-range ejection fraction (ejection fraction > 50% vs. ejection fraction 40–50%) and preserved vs. low Flow/Mass ratio (≥ vs. <sex-specific median). Patients with both mid-range ejection fraction and low flow/mass ratio have the worst heart failure hospitalization prognosis. The displayed hazard ratio is derived from the Cox proportional hazards model, adjusted for age, sex, body mass index, history of diabetes, radiotracer, estimated glomerular filtration rate, and number of antihypertensive medications. CI, confidence interval; EF, ejection fraction; HF, heart failure; HR, hazard ratio.
Discussion
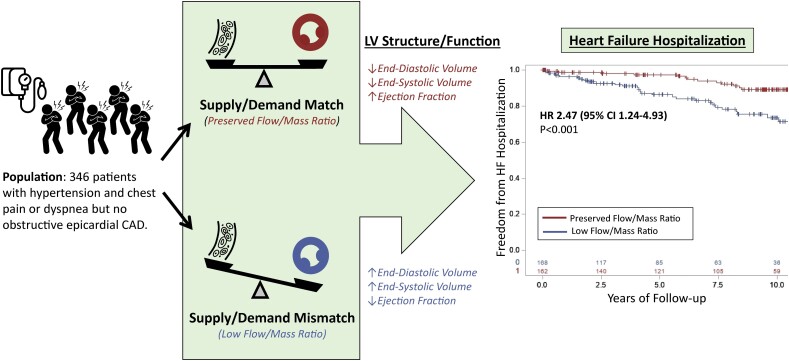
We have herein demonstrated that insufficient myocardial perfusion to match global metabolic demand, as measured by a reduced myocardial perfusion to global LV mass ratio, has functional and prognostic importance in symptomatic patients with hypertension but no obstructive epicardial CAD who presented for stress myocardial PET (Graphical Abstract). Low myocardial blood flow relative to global LV mass was associated with more adverse ventricular remodelling including more dilated LV volumes and lower systolic function in the form of EF, and with significantly greater risk of incident HF hospitalization. Interestingly, low myocardial blood flow relative to global LV mass concurrent with mid-range LVEF conferred higher risk of HF than either one alone. Given our prior findings that abnormalities in perfusion can predict HF risk despite the absence of apparent maladaptive remodelling,13 these findings extend our understanding of how inadequate myocardial perfusion to supply the hypertensive ventricle may promote the transition from symptomatic hypertension to clinical HF. Thus, this novel flow/mass ratio may serve as a biomarker that identifies a hypertensive endotype prone to the development of HF events.
Graphical Abstract.
Insufficient myocardial perfusion to match global metabolic demand, as measured by a myocardial perfusion to global left ventricular mass ratio, characterizes symptomatic patients with hypertension at risk of future heart failure and may help to understand the progression from hypertension to hypertensive heart disease. CAD, coronary artery disease; CI, confidence interval; HR, hazard ratio.
Prior studies have demonstrated the critical importance of myocardial perfusion and the imbalance between myocardial oxygen supply and demand in HF risk, in the absence of epicardial CAD or acute myocardial infarction.18 Studies using cardiac CT angiography have shown that epicardial coronary artery lumen volume indexed to LV myocardial mass associates with impaired fractional flow reserve, supporting the epicardial coronary artery structure–function relationship.19 Our results using PET-derived flow/mass ratio extend this concept by measuring total blood supply of the epicardial and microvascular coronary circulation indexed to LV mass. In the absence of obstructive epicardial CAD, coronary microvascular dysfunction is prevalent in hypertension and HF with preserved EF (HFpEF),12,20–22 and resulting subendocardial ischaemia has been implicated as one of the central pathways in HFpEF pathogenesis. Patients with HFpEF demonstrate higher troponin levels at rest and with exercise,23 and a positive troponin in the context of coronary microvascular dysfunction identifies patients at especially high risk for adverse cardiovascular outcomes.24 Beyond evidence of myocardial injury, abnormalities in myocardial perfusion have been linked to HF risk through associations with structural remodelling,14 diastolic dysfunction,12–14 and abnormal systolic mechanics13,14 in patients with hypertension, as well as those with chronic kidney disease and other cardiometabolic risk factors.
In systemic hypertension, chronic LV pressure overload induces not only structural adaptation in the form of myocyte hypertrophy but also changes in capillary density, microvascular resistance, and vascular stiffness due to vascular and interstitial remodelling and fibrosis,25 all of which contribute to abnormalities in myocardial perfusion. In early mechanistic studies in experimental animal models, changes in coronary artery luminal diameter were found not to increase proportionately with increases in LV mass,26,27 which has been attributed to increases in the coronary arteriolar wall-to-lumen ratio due to vascular smooth muscle hypertrophy.28 In the context of LVH, capillary density is reduced as a result of capillary rarefaction and of inadequate angiogenesis to meet the increased demand of the hypertrophying ventricle,29 a finding also present in patients with HFpEF.30 Endothelial dysfunction, manifested by impaired vasodilation and reduced nitric oxide availability, is found in patients with hypertension, LVH, and HFpEF and represents another mechanism of impairment in myocardial perfusion.31–33
Beyond the haemodynamic effects of chronically elevated blood pressure on ventricular wall stress, myocyte hypertrophy, and coronary vascular stiffening, non-haemodynamic factors may also contribute importantly to the development of impaired myocardial perfusion and resultant hypertensive heart disease. Hypertension, and its frequently associated constellation of cardiometabolic risk factors including obesity and diabetes, has been more recently understood as a complex metabolic risk state with inflammation, reactive oxygen species, and hormonal factors such as aldosterone implicated in the development of interstitial fibroblast differentiation, collagen accumulation, and perivascular fibrosis.25,31 Given the comparable blood pressures and antihypertensive regimens between those patients with preserved and impaired global myocardial flow/mass ratio, it is possible that these non-haemodynamic pathways account for differences in the adequacy of hyperaemic perfusion to meet global metabolic demand, higher rates of detectable myocardial injury, associated decrement in LV structure and systolic function, and higher downstream risk of HF events.
Patients with hypertension and anginal symptoms without obstructive epicardial CAD represent a clinical challenge,34 especially in the absence of overt structural remodelling or hypertrophy. By taking advantage of quantitative PET as a unique mechanistic tool in a symptomatic clinical cohort, we demonstrated a subclinical imbalance in the myocardial oxygen supply/demand relationship with functional and prognostic significance for hypertensive HF. While it would not be feasible or advisable that myocardial PET perfusion imaging and the flow/mass ratio be incorporated into routine clinical decision-making algorithms for patients with hypertension, the ability to simultaneously assess quantitative myocardial perfusion and structure provides a unique opportunity to expand the understanding of hypertensive HF pathogenesis.
Strengths and limitations
While this study incorporates an established technique for measuring myocardial blood flow in a core laboratory, detailed clinical event adjudication, and multiple complementary findings in support of the hypothesis, several limitations must be acknowledged. First, this is an observational study in a clinical registry population and may be subject to residual, uncontrolled confounding. However, despite the relatively small study population and few clinical events, there was adequate power to demonstrate a significant increased risk for both HF hospitalization and all-cause mortality among those patients with inadequate perfusion relative to LV mass. Second, while symptomatic, hypertensive patients without epicardial CAD represent only a fraction of the hypertensive population, this population may be enriched for abnormalities in myocardial perfusion and be at increased risk of remodelling and HF and thus represent a population in which early pathophysiological abnormalities truly referable to hypertensive heart disease can best be studied. Furthermore, patients with hypertension often have multiple comorbid cardiovascular risk factors with overlapping pathological mechanisms, such as inflammation, which may contribute to HF risk. Though the presence of diabetes and obesity was accounted for in the above adjusted analyses, dedicated future study is warranted to delineate their relative contributions. Third, while one could consider whether measurement of natriuretic peptides in symptomatic patients with hypertension suggests a clinical suspicion for HF, these measurements were almost all negative, validating that these patients were not already in subclinical HF at the time of PET. Fourth, in this study, quantification of LV structure and function parameters are confined to gated PET-CT images. However, reproducibility and accuracy of LV mass and LVEF assessment by PET have been previously reported,35–37 and the prognostic importance of abnormalities in myocardial perfusion in hypertensive HF risk have been demonstrated in patients without echocardiographic evidence of adverse remodelling.13 Finally, hypothesized transmural and/or regional heterogeneity in myocardial flow, including inadequate subendocardial perfusion and potential ischaemia and fibrosis, cannot be resolved by the current PET perfusion imaging technology. Future studies evaluating transmural perfusion gradients and LV tissue composition by cardiac magnetic resonance imaging and/or pathology would provide important complementary data to the current study.
Given the complex haemodynamic and metabolic pathways that characterize the transition from hypertension to hypertensive heart disease, further investigation that leverages detailed physiological and anatomic assessment will be essential. Future studies will be important to better understand the utility of this flow/mass ratio in elucidating HF risk in other conditions prone to LVH and adverse structural and functional changes, such as aortic stenosis, diabetes, and chronic kidney disease, and in apparently physiological hypertrophy in athletes. Extending the application of the flow/mass ratio to larger populations will also facilitate a more complete and nuanced understanding of HF risk across the full range of flow/mass ratio, which may provide further physiological insight into the HF transition.
Conclusions
Despite being one of the most critical and remediable cardiovascular risk factors, hypertension remains inadequately controlled and hypertensive heart disease insufficiently understood. Applying a physiology-oriented approach to integrate myocardial perfusion, structure, and function characterized symptomatic hypertensive patients with subclinical evidence of hypertensive heart disease and at elevated risk for HF and death. Investigation into the mechanisms by which ventricular perfusion adapts to meet metabolic demands imposed by the hypertensive ventricle can facilitate targeted risk assessment and intervention, with the aim of reducing the burden of hypertensive HF.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by the National Institutes of Health (T32HL007604 to J.M.B., 5T32HL094301 to W.Z., S.D., and B.W., K23HL135438 to V.R.T., and R01HL132021 to M.F.D.C) and a joint KL2/Catalyst Medical Research Investigator Training (CMeRIT) award from Harvard Catalyst and the Boston Claude D. Pepper Older Americans Independence Center (5P30AG031679-10 to S.D.).
Supplementary Material
Contributor Information
Jenifer M Brown, Heart and Vascular Center, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA; Cardiovascular Imaging Program, Division of Nuclear Medicine and Molecular Imaging, Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA.
Wunan Zhou, Cardiovascular Imaging Program, Division of Nuclear Medicine and Molecular Imaging, Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA; Cardiology Branch, Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health, 10 Center Drive, Bethesda, MD 20892, USA.
Brittany Weber, Heart and Vascular Center, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA; Cardiovascular Imaging Program, Division of Nuclear Medicine and Molecular Imaging, Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA.
Sanjay Divakaran, Heart and Vascular Center, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA; Cardiovascular Imaging Program, Division of Nuclear Medicine and Molecular Imaging, Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA.
Leanne Barrett, Cardiovascular Imaging Program, Division of Nuclear Medicine and Molecular Imaging, Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA.
Courtney F Bibbo, Cardiovascular Imaging Program, Division of Nuclear Medicine and Molecular Imaging, Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA.
Jon Hainer, Cardiovascular Imaging Program, Division of Nuclear Medicine and Molecular Imaging, Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA.
Viviany R Taqueti, Cardiovascular Imaging Program, Division of Nuclear Medicine and Molecular Imaging, Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA.
Sharmila Dorbala, Heart and Vascular Center, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA; Cardiovascular Imaging Program, Division of Nuclear Medicine and Molecular Imaging, Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA.
Ron Blankstein, Heart and Vascular Center, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA; Cardiovascular Imaging Program, Division of Nuclear Medicine and Molecular Imaging, Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA.
Marcelo F Di Carli, Heart and Vascular Center, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA; Cardiovascular Imaging Program, Division of Nuclear Medicine and Molecular Imaging, Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KD, Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD, Wright JT. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 2018;138:e426–e483. [DOI] [PubMed] [Google Scholar]
- 2. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; ESC Scientific Document Group . 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 3. Bluemke DA, Kronmal RA, Lima JAC, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events. J Am Coll Cardiol 2008;52:2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zile MR, Gaasch WH, Patel K, Aban IB, Ahmed A. Adverse left ventricular remodeling in community-dwelling older adults predicts incident heart failure and mortality. JACC Hear Fail 2014;2:512–522. [DOI] [PubMed] [Google Scholar]
- 5. Pichard AD, Gorlin R, Smith H, Ambrose J, Meller J. Coronary flow studies in patients with left ventricular hypertrophy of the hypertensive type. Am J Cardiol 1981;47:547–554. [DOI] [PubMed] [Google Scholar]
- 6. Rabkin SW. Considerations in understanding the coronary blood flow-left ventricular mass relationship in patients with hypertension. Curr Cardiol Rev 2017;13:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marcus ML. The Coronary Circulation in Health and Disease. New York, NY: McGraw-Hill; 1983. [Google Scholar]
- 8. Marcus ML, Mueller TM, Eastham CL. Effects of short- and long-term left ventricular hypertrophy on coronary circulation. Am J Physiol Circ Physiol 1981;241:H358–H362. [DOI] [PubMed] [Google Scholar]
- 9. Kjaer A, Meyer C, Wachtell K, Olsen MH, Ibsen H, Opie L, Holm S, Hesse B. Positron emission tomographic evaluation of regulation of myocardial perfusion in physiological (elite athletes) and pathological (systemic hypertension) left ventricular hypertrophy. Am J Cardiol 2005;96:1692–1698. [DOI] [PubMed] [Google Scholar]
- 10. Kenkre TS, Malhotra P, Johnson BD, Handberg EM, Thompson DV, Marroquin OC, Rogers WJ, Pepine CJ, Merz CNB, Kelsey SF. Ten-year mortality in the WISE study (Women’s Ischemia Syndrome Evaluation). Circ Cardiovasc Qual Outcomes 2017;10:e003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jordan KP, Timmis A, Croft P, van der Windt DA, Denaxas S, González-Izquierdo A, Hayward RA, Perel P, Hemingway H. Prognosis of undiagnosed chest pain: linked electronic health record cohort study. BMJ 2017;357:j1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taqueti VR, Solomon SD, Shah AM, Desai AS, Groarke JD, Osborne MT, Hainer J, Bibbo CF, Dorbala S, Blankstein R, Di Carli MF. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J 2018;39:840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou W, Brown JM, Bajaj NS, Chandra A, Divakaran S, Weber B, Bibbo CF, Hainer J, Taqueti VR, Dorbala S, Blankstein R, Adler D, O’Gara P, Di Carli MF. Hypertensive coronary microvascular dysfunction: a subclinical marker of end organ damage and heart failure. Eur Heart J 2020;41:2366–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bajaj NS, Singh A, Zhou W, Gupta A, Fujikura K, Byrne C, Harms HJ, Osborne MT, Bravo P, Andrikopolou E, Divakaran S, Bibbo CF, Hainer J, Skali H, Taqueti V, Steigner M, Dorbala S, Charytan DM, Prabhu SD, Blankstein R, Deo RC, Solomon SD, Di Carli MF. Coronary microvascular dysfunction, left ventricular remodeling and clinical outcomes in patients with chronic kidney impairment. Circulation 2020;141:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. El Fakhri G, Kardan A, Sitek A, Dorbala S, Abi-Hatem N, Lahoud Y, Fischman A, Coughlan M, Yasuda T, Di Carli MF. Reproducibility and accuracy of quantitative myocardial blood flow assessment with 82Rb PET: comparison with 13N-ammonia PET. J Nucl Med 2009;50:1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rischpler C, Higuchi T, Fukushima K, Javadi MS, Merrill J, Nekolla SG, Bravo PE, Bengel FM. Transient ischemic dilation ratio in 82Rb PET myocardial perfusion imaging: normal values and significance as a diagnostic and prognostic marker. J Nucl Med 2012;53:723–730. [DOI] [PubMed] [Google Scholar]
- 17. Rastogi A, Novak E, Platts AE, Mann DL. Epidemiology, pathophysiology and clinical outcomes for heart failure patients with a mid-range ejection fraction. Eur J Heart Fail 2017;19:1597–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Crea F, Merz CNB, Beltrame JF, Kaski JC, Ogawa H, Ong P, Sechtem U, Shimokawa H, Camici PG. The parallel tales of microvascular angina and heart failure with preserved ejection fraction: a paradigm shift. Eur Heart J 2017;38:473–477. [DOI] [PubMed] [Google Scholar]
- 19. Taylor CA, Gaur S, Leipsic J, Achenbach S, Berman DS, Jensen JM, Dey D, Bøtker HE, Kim HJ, Khem S, Wilk A, Zarins CK, Bezerra H, Lesser J, Ko B, Narula J, Ahmadi A, Øvrehus KA, St Goar F, De Bruyne B, Nørgaard BL. Effect of the ratio of coronary arterial lumen volume to left ventricle myocardial mass derived from coronary CT angiography on fractional flow reserve. J Cardiovasc Comput Tomogr 2017;11:429–436. [DOI] [PubMed] [Google Scholar]
- 20. Yang JH, Obokata M, Reddy YNV, Redfield MM, Lerman A, Borlaug BA. Endothelium-dependent and independent coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Eur J Heart Fail 2020;22:432–441. [DOI] [PubMed] [Google Scholar]
- 21. Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, Tan RS, Beussink-Nelson L, Faxén UL, Fermer ML, Broberg MA, Gan LM, Lund LH. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J 2018;39:3439–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rimoldi O, Rosen SD, Camici PG. The blunting of coronary flow reserve in hypertension with left ventricular hypertrophy is transmural and correlates with systolic blood pressure. J Hypertens 2014;32:2465–2471. [DOI] [PubMed] [Google Scholar]
- 23. Obokata M, Reddy YNV, Melenovsky V, Kane GC, Olson TP, Jarolim P, Borlaug BA. Myocardial injury and cardiac reserve in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol 2018;72:29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Taqueti VR, Everett BM, Murthy VL, Gaber M, Foster CR, Hainer J, Blankstein R, Dorbala S, Di Carli MF. Interaction of impaired coronary flow reserve and cardiomyocyte injury on adverse cardiovascular outcomes in patients without overt coronary artery disease. Circulation 2015;131:528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. González A, Ravassa S, López B, Moreno MU, Beaumont J, San José G, Querejeta R, Bayés-Genís A, Díez J. Myocardial remodeling in hypertension. Hypertension 2018;72:549–558. [DOI] [PubMed] [Google Scholar]
- 26. Stack RS, Rembert JC, Schirmer B, Greenfield JC. Relation of left ventricular mass to geometry of the proximal coronary arteries in the dog. Am J Cardiol 1983;51:1728–1731. [DOI] [PubMed] [Google Scholar]
- 27. Mueller TM, Marcus ML, Kerber RE, Young JA, Barnes RW, Abboud FM. Effect of renal hypertension and left ventricular hypertrophy on the coronary circulation in dogs. Circ Res 1978;42:543–549. [DOI] [PubMed] [Google Scholar]
- 28. Yamori Y, Mori C, Nishio T, Ooshima A, Horie R, Ohtaka M, Soeda T, Saito M, Abe K, Nara Y, Nakao Y, Kihara M. Cardiac hypertrophy in early hypertension. Am J Cardiol 1979;44:964–969. [DOI] [PubMed] [Google Scholar]
- 29. Rakusan K. Quantitative morphology of capillaries of the heart. Number of capillaries in animal and human hearts under normal and pathological conditions. Methods Achiev Exp Pathol 1971;5:272–286. [PubMed] [Google Scholar]
- 30. Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015;131:550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Camici PG, Tschöpe C, Di Carli MF, Rimoldi O, Van Linthout S. Coronary microvascular dysfunction in hypertrophy and heart failure. Cardiovasc Res 2020;116:806–816. [DOI] [PubMed] [Google Scholar]
- 32. Treasure CB, Klein JL, Vita JA, Manoukian SV, Renwick GH, Selwyn AP, Ganz P, Alexander RW. Hypertension and left ventricular hypertrophy are associated with impaired endothelium-mediated relaxation in human coronary resistance vessels. Circulation 1993;87:86–93. [DOI] [PubMed] [Google Scholar]
- 33. Hamasaki S, Suwaidi J, Al Higano ST, Miyauchi K, Holmes DR, Lerman A. Attenuated coronary flow reserve and vascular remodeling in patients with hypertension and left ventricular hypertrophy. J Am Coll Cardiol 2000;35:1654–1660. [DOI] [PubMed] [Google Scholar]
- 34. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ; ESC Scientific Document Group . 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407–477. [DOI] [PubMed] [Google Scholar]
- 35. Delbeke D, Lorenz CH, Votaw JR, Silveira ST, Frist WH, Atkinson JB, Kessler RM, Sandler MP. Estimation of left ventricular mass and infarct size from nitrogen-13-ammonia PET images based on pathological examination of explanted human hearts. J Nucl Med 1993;34:826–833. [PubMed] [Google Scholar]
- 36. Byrne C, Kjaer A, Forman JL, Hasbak P. Reproducibility of LVEF, LV volumes, and LV mass between Rubidium-82 PET/CT scans in young healthy volunteers using two commercially available software packages. J Nucl Cardiol 2020;27:1237–1245. [DOI] [PubMed] [Google Scholar]
- 37. Kiko T, Yoshihisa A, Yokokawa T, Misaka T, Yamada S, Kaneshiro T, Nakazato K, Takeishi Y. Direct comparisons of left ventricular volume and function by simultaneous cardiac magnetic resonance imaging and gated 13N-ammonia positron emission tomography. Nucl Med Commun 2020;41:383–388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.