Author's summary
Direct cell reprogramming refers to the conversion of cell identity, without transition through an induced pluripotent state. Owing to the ability to directly reprogram into numerous cell types, this reprogramming strategy has received enormous attention for regenerative therapy. Particularly, direct reprogramming into functional endothelial cells, which are crucial for neovascularization and vascular regeneration, holds promising therapeutic potential for treating patients with ischemic cardiovascular diseases. This review provides a summary of the-state-of-art in direct endothelial reprograming strategies and highlights their potential applications. It also discusses key questions and challenges that will help guiding future research and development of this emerging area.
Keywords: Cardiovascular disease, Direct cell reprogramming, Endothelial cells, Neovascularization, Cell differentiation and regenerative medicine
Abstract
Cell-based therapy has emerged as a promising option for treating advanced ischemic cardiovascular disease by inducing vascular regeneration. However, clinical trials with adult cells turned out disappointing in general. As a newer approach, direct reprogramming has emerged to efficiently generate endothelial cells (ECs), which can promote neovascularization and vascular regeneration. This review provides recent updates on the direct endothelial reprogramming. In general, directly reprogrammed ECs can be generated by two approaches: one by transitioning through a plastic intermediate state and the other in a one-step transition without any intermediate states toward pluripotency. Moreover, the methods to deliver reprogramming factors and chemicals for the fate conversion are highlighted. Next, the therapeutic effects of the directly reprogrammed ECs on animal models are reviewed in detail. Other applications using directly reprogrammed ECs, such as tissue engineering and disease modeling, are also discussed. Lastly, the remaining questions and foremost challenges are addressed.
INTRODUCTION
Cardiovascular disease (CVD) is the global leading cause of morbidity and mortality.1) Moreover, there are a great number of patients who suffer from ischemic CVD, such as myocardial infarction (MI) and peripheral artery disease. When conventional therapeutic options such as surgery, intervention and medication are exhausted, such patients have no other options but to undertake cardiac transplantation or lower extremity amputation.2),3),4) Accordingly, newer approaches have emerged to treat these diseases via biologically inducing neovascularization through gene therapy or cell therapy, resulting in re-establishment of functional blood vessels, proper blood perfusion and tissue repair. For inducing therapeutic neovascularization, supplying functional endothelial cells (ECs) has been a key element.
After general failure of clinical trials with adult cell therapy, pluripotent stem cells (PSCs) such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) have emerged as the beneficial sources of cell therapy for vascular regeneration. Since the early studies on human iPSCs (hiPSCs) reprogramming from somatic cells, the development of differentiation techniques to generate vascular cells for clinical application have been accelerated.5),6),7),8) Particularly, hiPSC-EC application in an ischemic murine model has shown the first step towards vascular regeneration. In addition, hiPSC-derived ECs have shown their potentials for stimulating neovascularization in vivo, resulting in the recovery of blood flow in animal models of ischemic CVDs.9),10),11),12) However, long-term culture to generate the full-pluripotent state of iPSCs before EC differentiation and potential concerns for side effects associated with the use of pluripotent stem cells have delayed clinical development.
Successful use of transcription factors to reprogram into iPSCs hinted that more direct routes to lineage-specific cell fate conversion were possible. During this conversion, the pluripotent step is omitted. This novel approach is called “direct reprogramming” or “transdifferentiation.”13) In early 2010, mouse fibroblasts were directly reprogrammed into neurons by defined factors.14) In consequence of screening 19 candidates, they identified three transcription factors, which were sufficient to generate induced neurons (iNs) via a direct reprogramming strategy. Additionally, transdifferentiation of somatic cells into various cell types, such as cardiomyocytes (CMs), human blood progenitors, hepatocyte-like cells, or human neurons was reported one after another within a year.15),16),17),18),19) Furthermore, direct reprogramming into vascular ECs from human somatic cells in order to promote neovascularization have also been demonstrated since early 2010s.20),21)
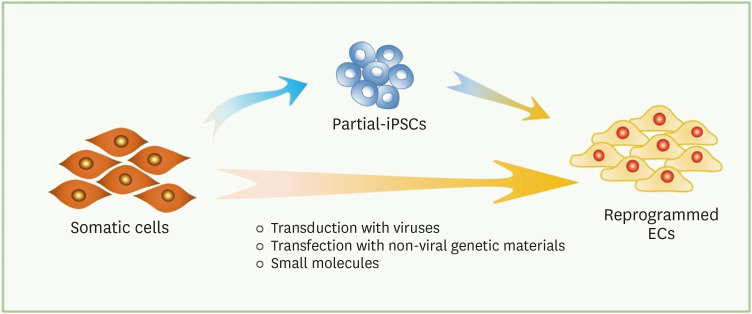
In this review, we summarize key studies on direct reprogramming into ECs. We highlight differences between the techniques performed in each study and categorize them into two subgroups by the cell fate change during the transition: direct reprogramming through partial-iPSCs or direct transdifferentiation (Figure 1). Moreover, key factors, delivery system, and chemicals supplements in culture media applied to promote EC transdifferentiation are summarized in the tables (Tables 1 and 2). We further describe the therapeutic applications of directly reprogrammed ECs in animal models and possible therapeutic applications.
Figure 1. Schematic of direct endothelial reprogramming. Somatic cells can be reprogrammed into iPSCs, which possess the full pluripotent state, and be differentiated into ECs with lineage-specific factors. In direct reprogramming, somatic cells can be transdifferentiated into ECs via two different processes: (a) transit through a plastic state or (b) direct conversion. In (a), brief exposure to reprogramming factors followed by conditioned media allows the somatic cells to be converted into ECs without achieving the pluripotency. In (b), introduction of endothelial lineage-specific transcription factors and small molecules allows the somatic cells into ECs.
EC = endothelial cell; iPSC = induced pluripotent stem cell.
Table 1. Endothelial reprogramming via partial-iPSC.
| Reprogrammed cell name | Source cell type | Reprogramming factors | Delivery method | Reprogramming culture condition | Culture duration | Reference |
|---|---|---|---|---|---|---|
| PiPS-EC | Human fibroblast | OCT4, SOX2, KLF4, and c-MYC | Lentivirus infection or plasmid transfection | EGM-2 | 10–14 days | Margariti et al., Proc Natl Acad Sci U S A (2012)21) |
| iEC (via angioblast-like progenitor cell) | Human embryonic and neonatal fibroblast | 4 Factors/miRs: OCT4, SOX2, KLF4, c-MYC, miR302, and miR367 | Retroviral infection or episomal nucleofection | MIM: Insulin, transferrin, bFGF, VEGF, BMP4, and MTG | 24 days | Kurian et al., Nat Methods (2013)22) |
| 6 Factors/miR: OCT4, SOX2, KLF4, LMYC, LIN28, shP53, miR302, and miR367 | EC media: EGM-2 | |||||
| iEnd | Human neonatal fibroblast | OCT4 and KLF4 | Lentiviral infection | EC Media I: BMP4, VEGF, and bFGF | 28 days | Li et al., Arterioscler Thromb Vasc Biol (2013)23) |
| EC Media II: 8-Br-cAMP, VEGF, bFGF, and SB431542 |
EGM-2 = Endothelial Cell Growth Medium-2; iEC = induced endothelial cell; iEnd cell = induced endothelial cell; iPSC = induced pluripotent stem cell; MIM = mesodermal induction medium; miRs = microRNAs; MTG = 1-Thioglycerol; PiPS-EC = partial-induced pluripotent stem cell-derived endothelial cell; shP53 = p53 short hairpin RNA.
Table 2. Direct endothelial reprogramming with lineage-specific factors.
| Reprogrammed cell name | Source cell type | Key factors (chemicals) | Delivery method | Culture condition | Culture duration | Reference |
|---|---|---|---|---|---|---|
| rAC-VEC | Human amniotic fluid-derived cell | ETV2, FLI1, and ERG1 (SB431542) | Lentiviral infection | EM: SB431542, EC supplement, and Heparin | 28 days | Ginsberg et al., Cell (2012)20) |
| iEC | Mouse skin and tail-tip fibroblast | Etv2, Foxo1, Klf2, Tal1 and Lmo2 | Lentiviral infection | EBM-2 | 12 days | Han et al., Circulation (2014)24) |
| iEC* | Human neonatal dermal fibroblast | (Poly I:C) | Chemical stimulation | Activation of innate immunity: Poly I:C | 7 days + 7 days + 14 days and more | Sayed et al., Circulation (2015)37) |
| Transdifferentiation medium I: bFGF, VEGF, and BMP4 | ||||||
| Transdifferentiation medium II: EGM-2, bFGF, VEGF, BMP4, and 8-Br-cAMP | ||||||
| After sorting: EGM-2 and SB431542 | ||||||
| ETVEC | Human adult fibroblast | ETV2 (VEGF and bFGF) | Lentiviral infection | EGM-2, VEGF, and bFGF | 25 days (beyond 50 days) | Morita et al., Proc Natl Acad Sci U S A (2015)25) |
| iEC | Human neonatal fibroblast | ETV2, FLI1, GATA2, and KLF4 (BMP4, VEGF, bFGF, and SB431542) | Lentiviral infection | Differentiation medium: BMP4, VEGF, and bFGF | 3 days + 25 days | Wong and Cooke, J Tissue Eng (2016)26) |
| EC growth medium: EGM-2 MV and SB431542 | ||||||
| rEC (early vs. late) | Human dermal fibroblast | ETV2 (VEGFA and VPA) | Lentiviral infection | Early rEC: EGM-2 and VEGFA | 7 days for early rEC and 3 months for late rEC | Lee et al., Circ Res (2017)27) |
| Late rEC: EGM-2, temporal treatment of VPA, and VEGFA | ||||||
| EiEC | Human adipose-derived stem cell and human umbilical mesenchymal stem cells | ETV2 (SB431542, VEGF, bFGF, and EGF) | Lentiviral infection | EIM: Insulin, ascorbic acid, Heparin, VEGF, bFGF, EGF, SB431542, CHIR99021 and BMP4 | 10 days (up to 2 months) | Cheng et al., Stem Cell Res Ther (2018)28) |
| EMM: SB431542, VEGF, bFGF, and EGF | ||||||
| iEC | Human embryonic lung fibroblast | DKK3 (VEGF) | Adenoviral infection | EGM-2 and VEGF | 10 days or more | Chen et al., Arterioscler Thromb Vasc Biol (2019)29) |
| Fsk-iEC | Human fibroblast and UCB-MSC | ETV2 (forskolin) | Lentiviral or retroviral infection | EGM-2 and Forskolin | 14 days | Kim et al., Mol Ther (2020)30) |
| iVEC | Human dermal fibroblast | ETV2 | Retroviral infection | EGM-2 MV | N/A | Bersini et al., Elife (2020)31) |
| iEC | Human adult dermal fibroblast | ETV2, KLF2 and TAL1 with siTWIST1 (rosiglitazone) | Lentiviral infection | 1st stage: EGM-2 MV and rosiglitazone | 4 weeks + 2 weeks | Han et al., Biomaterials (2021)32) |
| 2nd stage: EGM-2 MV |
EBM-2 = Endothelial Cell Growth Basal Medium-2; EC = endothelial cell; EGM-2 MV = Microvascular Endothelial Cell Growth Medium-2; EiEC = ETV2-induced endothelial cell; EIM = endothelial induction medium; EM = endothelial growth media; EMM = endothelial maintenance medium; Fsk = forskolin; iEC = induced endothelial cell; iEnd cell = induced endothelial cell; iVEC = induced vascular endothelial cell; N/A = not applicable; PiPSC-EC = partial-induced pluripotent stem cell-derived endothelial cell; rAC-VEC = reprogrammed amniotic fluid-derived cell-vascular endothelial cell; rEC = reprogrammed endothelial cell; siTWIST = small interfering RNA of TWIST1; UCB-MSC = umbilical cord blood-derived mesenchymal stem cell; VEGF = vascular endothelial growth factor; VPA = valproic acid.
*Chemically driven direct reprogramming towards ECs.
DIRECT REPROGRAMMING INTO ENDOTHELIAL CELL VIA PARTIAL-INDUCED PLURIPOTENT STEM CELLS
To challenge EC generation from somatic cells yet bypassing the pluripotent state, EC differentiation approach under the concept of direct reprogramming was first developed a decade ago. In 2012, Margariti et al.21) developed a method to generate ECs through partial-induced pluripotent stem (PiPS) cells by introducing four reprogramming factors (OCT4, SOX2, KLF4 and c-MYC) to human fibroblasts and with subsequent culture in Endothelial Cell Growth Media-2 (EGM-2). They demonstrated that generated ECs, PiPS-ECs, exhibit EC-specific characteristics such as mRNA and protein marker expressions whereas PiPS cells did not form tumors in vivo 2 months after subcutaneous injection in severe combined immunodeficiency (SCID) mice. A year later, Kurian et al.22) reported a mesodermal progenitor cell differentiation method, in which somatic cells transit through a plastic intermediate state. They converted human fibroblasts into plastic state cells, then CD34+ mesodermal progenitor cells to further differentiate into endothelial and smooth muscle lineages. They transduced four reprogramming factors (OCT4, SOX2, KLF4 and c-MYC) in combination with two microRNAs (miRNAs), miR302 and miR367, using a retrovirus. Alternatively, they transfected human fibroblasts with six reprogramming factors (OCT4, SOX2, KLF4, LMYC, LIN28 and p53 short hairpin RNA (shP53) with the two miRNAs using episomal plasmids. They cultured the cells for eight days to first induce a plastic state before mesodermal reprogramming. They transferred the intermediate cells in mesodermal induction medium (MIM) and cultured for eight more days, followed by endothelial differentiation in EGM-2 for another eight days. Although the converted ECs acquired characteristics of primary ECs, such as gene expression and DNA methylation profiles, they noted that residual expression of the transgenes especially with retroviral transduction was detectable. On the other hand, episomal vectors were rapidly cleared in the differentiated ECs and expression of the pluripotency markers TRA-1-60 and TRA-1-81 was undetectable even after the plastic state induction. Additionally, Li et al.23) promoted an induction of ECs from human neonatal fibroblasts, which were transduced with only two reprogramming factors, OCT4 and KLF4, then cultured in chemically defined media for 28 days before EC-specific marker selection. They noted that the conversion was a gradual process bypassing iPSC generation, confirmed with the lack of iPSC-specific marker expression and the induction of EC-specific markers at mRNA, protein and histone modification levels. To enhance the efficiency, they included bone morphogenetic protein 4 (BMP4), transforming growth factor (TGF)-β superfamily of proteins, in the media only for early 7 days then sequential treatment with cAMP-dependent protein kinase A (8-Br-cAMP) for 7 days and a TGF-β receptor inhibitor (SB431542) for another 14 days. They validated that induced endothelial (iEnd) cells displayed EC characteristics in vitro and in vivo comparable to primary human ECs.
Together these studies demonstrated that a brief introduction of two or more reprogramming factors in somatic cells, such as embryonic and neonatal fibroblasts, allowed a direct endothelial conversion while simultaneously preventing a significant expression of definite pluripotency markers. Elaborately, however, these procedures still involve pluripotency reprogramming factors to induce the partially pluripotent stem cell state. Endothelial differentiation in this process is promoted by the culture media including growth factors and small molecules and cell culture matrices.
DIRECT REPROGRAMMING INTO ENDOTHELIAL CELL WITH LINEAGE-SPECIFIC FACTORS
More straightforward direct reprogramming strategy was developed by introducing lineage-specific transcription factors into the somatic cells instead of general reprogramming factors. Ginsberg et al.20) described that direct reprogramming of human amniotic fluid-derived cells (ACs) into vascular ECs was feasible by transient expression of ETV2 and co-expression of FLI1 and ERG1 in combination with TGF-β pathway inhibition. They observed that the reprogrammed vascular ECs, rAC-VECs, are durable, highly proliferative, and able to form tube-like structure during liver regeneration in vivo. Clearly, the source cell they used, ACs, were negative for OCT4, SOX2 and NANOG expression before endothelial conversion, bypassing a pluripotent step. Unfortunately, this pioneering method of direct reprogramming was not valid with postnatal fibroblasts. In another study, Han et al. separately screened 11 candidates of EC lineage transcription factors and demonstrated that lentiviral transduction of five transcription factors, Etv2, Foxo1, Klf2, Tal1 and Lmo2, into adult mouse dermal fibroblasts led to direct conversion into ECs, referred to as induced endothelial cells (iECs).24) They validated the function of iECs in vitro and in vivo. This strategy did not involve pluripotency induction because Oct4 and Nanog were undetectable in their cells during the reprograming. Nevertheless, this endothelial conversion with the five factors was not effective for all cell types, such as bone marrow mononuclear cells. Indeed, direct reprogramming was shown with mouse dermal fibroblasts only. In another study, Morita et al.25) also screened 18 transcription factors and found that a single factor ETV2 is sufficient for endothelial transdifferentiation both from human neonatal and adult skin fibroblasts. Transient expression of ETV2, in the aid of endogenous FOXC2, promotes direct reprogramming of fibroblast into ECs via FOX:ETS motif interactions and enhance FLI1 and ERG1 expression for further EC conversion. This group demonstrated that basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) supplemented in EGM-2, improved the efficiency of the ETV2-mediated direct reprogramming and proliferation of these converted ECs. This study, however, lacks the downregulation of ETV2 after reprogramming and referred to these cells as ETVECs. In parallel, another screening was also performed by Wong and Cooke demonstrating that transduction of four factors, ETV2, FLI1, GATA2, and KLF4, successfully reprogrammed human fibroblasts toward ECs.26) They confirmed that ETV2 is the most critical factor in the combination. Removing ETV2 substantially reduced PECAM1 expression in the directly reprogrammed cells. In fact, Lee et al.27) most clearly reported that ETV2 is the master regulator of the direct reprogramming into ECs and it alone is sufficient for the process. Their study emphasized that transient expression of ETV2 in human fibroblasts is critical for efficient conversion of human fibroblasts into reprogrammed ECs (rECs), which, like human mature ECs, do not constantly express ETV2. In addition, they generated two types of rECs, early and late rECs, depending on the maturity levels. After initial ETV2 transduction, KDR+ cells were sorted at day seven, which were called early rECs. After another round of overexpression of ETV2 in early rECs after 2 weeks and further culture for 3 months resulted in more efficient and stable rEC generation, referred to as late rECs. Both types of rECs exhibited similar neovascularization and vascular regenerative effects, while late rECs have more mature EC characteristics including higher PECAM1 expression. Another study by Cheng et al. reported that human adipose-derived stem cells can also be directly reprogrammed into ECs via short-term ETV2 expression and TGF-β inhibition.28) However, their transdifferentiation protocol includes two-stage induction steps, comprising many small molecules of angiogenic growth factors, modulators for signaling pathways in addition to human insulin and ascorbic acid. With the ETV2-induced endothelial cells (EiECs), they showed 60-day-expandability with normal karyotypes and human EC (HUVEC)-like molecular features via microarray analysis. Chen et al.29) converted human embryonic lung fibroblasts into functional ECs. They demonstrated that participation of mesenchymal-to-epithelial transition (MET), activated by DKK3 overexpression, stimulated KDR expression under a defined condition through miR-125a-5p/Stat3 axis pathway. Recently, Kim et al.30) provided molecular mechanism underlying transdifferentiated ECs with via RNA-seq and ChIP-seq analyses. Moreover, they showed that forskolin treatment facilitates the direct reprogramming efficiency, by stimulating cAMP signal transduction, with stronger effects than 8-Br-cAMP and 8-CTP-cAMP, which are cAMP analogs. Furthermore, they examined therapeutic effects not only in the ischemic hindlimb model but also in rat liver scaffold to validate long-term effects. Intriguingly, Bersini et al.31) generated induced vascular endothelial cells (iVECs) and smooth muscle cells (iSMCs) via direct reprogramming of fibroblasts donated from young (19- to 30-year-olds) and old (62- to 87-year-olds) healthy individuals and Hutchinson-Gilford Progeria Syndrome (HGPS) patients.31) Unfortunately, they only monitored transcriptomic and functional differences of iSMCs between healthy donors and HGPS patients but not iVECs. More recently, Han et al.32) demonstrated direct conversion of human fibroblast into functional ECs by lentiviral transduction of 3 transcription factors, ETV2, KLF2, and TAL1 in combination with EMT inhibitors, siTWIST1 and Rosiglitazone treatment, and second-stage culture for two more weeks. Importantly, the authors scrutinized all possible EC characteristics with the iECs, which are defined by CDH5+PECAM1+ double-positive cells. Interestingly, it was discussed that their source cell, adult fibroblasts, that are less plastic than amniotic cells or neonatal dermal fibroblasts that were used by other groups, required longer duration of ETV2 expression.
Altogether, studies have demonstrated that ETV2 is the master regulator of EC-lineage induction and that its transient expression is sufficient for direct reprogramming into ECs. Furthermore, all the studies mentioned above aimed to enhance the reprogramming efficiency by adding other transcription factors, growth factors, small molecules, and regulators of signal transduction and histone modification.
VEHICLES TO DELIVER DIRECT-REPROGRAMMING FACTORS
Most of the reprogramming studies involve a viral transfection approach to promote ectopic expression of the key reprogramming factors in somatic cells (Tables 1 and 2). Retroviral, lentiviral, adenoviral, and adeno-associated viral (AAV) vectors have been shown for the efficient gene transfer. For example, Ginsberg et al.20) cloned ETV2, ERG1 and FLI1 cDNAs into lentivirus vector for direct endothelial reprogramming. After the viral infection, they observed adequate levels of corresponding mRNAs and proteins in transduced ACs for several months. Moreover, the transduction, especially with ETV2, led to a robust induction of EC marker expressions and a rapid increase in cell numbers compared to untransduced cells. Kim et al. induced direct reprogramming of ECs from fibroblasts and umbilical cord blood-derived mesenchymal stem cells (UBC-MSC) with lentivirus and retrovirus, respectively.30) On the other hand, to avoid genome integration caused by the application of retroviral or lentiviral transduction, recent studies employ more with adenovirus and AAV as the delivery system. For example, Chen et al.29) used adenoviral vectors to introduce DKK3 in human embryonic lung fibroblasts. They reported that transient expression of DKK3 facilitated by adenovirus infection, activated KDR, a marker of ECs. Currently, there is no research reported for AAV use for direct endothelial reprogramming, yet the feasibility has been verified with direct reprogramming of other target cells. Rezvani et al.33) showed in vivo hepatic reprogramming of myofibroblast using AAV6 vectors expressing hepatic transcription factors. Altogether, multiple studies have shown that virus-mediated transduction of desired genetic material into human somatic cells is very effective. However, viral delivery systems generally have issues associated with cytotoxicity, immunogenicity, and carcinogenicity in addition to host-genome mutagenesis and cargo capacities.
On the other hand, nonviral gene delivery systems have emerged to overcome these hurdles of virus-mediated approaches. For the generation of human iPSCs, Yamanaka’s group has replaced retroviral- or lentiviral-mediated transduction system with nonviral repeated transfection methods using two genetically engineered plasmids.34) Thomson’s group also demonstrated that a single transfection with non-integrating episomal vectors can induce reprogramming of human foreskin fibroblasts.35) Indeed, they verified that the reprogrammed cells were completely free of transgene and vector sequences after the episomes are removed. Likewise, Kurian et al.22) established non-genome-integrative approach to introduce tissue-specific transcription factors to somatic cells in order to initiate the direct conversion of ECs through partial iPSC status. They transduced 4 transcription factors by retroviral infection or 6 factors by episomal nucleofection. It required 8 days to generate a plastic intermediate state in both procedures. Very recently, Cho et al.36) reported that they successfully generated reprogrammed cardiovascular tissue (rCVT), comprising reprogrammed cardiomyocytes (rCMs), rECs, and reprogrammed smooth muscle cells (rSMC), by transfecting mouse fibroblasts with synthetic miRNA mimics, miR-208b-3p. They used lipid-based transfection methods to generate reprogrammed cells and tissues in combination with ascorbic acid and BMP4 in the culture media. Thus, it is clear that the overexpression of lineage-specific transcription factors or small RNAs is achievable via various transfection techniques, such as episomal plasmid nucleofection, liposome or polymer carriers. However, low transfection efficiency of episomal electroporation or instability of desired genetic materials, such as mRNA and miRNAs, is another hurdle. Thus, more efficient and safer nonviral gene carrier, such as nanoparticles or purified recombinant proteins need to be further developed.
CHEMICALLY DRIVEN DIRECT REPROGRAMMING TOWARD ENDOTHELIAL CELL
Small molecules have been frequently used to enhance transdifferentiation efficiency in combination with growth factors in the cell culture media following transgene introduction in somatic cells.20),23),26),27),28),30),32) For example, Ginsberg et al.20) included SB431542 in EC growth media after lentiviral transduction of ETV2, FLI1 and ERG1. Li et al.23) also used SB431542 and 8-Br-cAMP together with VEGF and bFGF to induce iEnd cells after lentiviral transduction of OCT4 and KLF4. Lee et al.27) temporally treated valproic acid (VPA) at the second ETV2-induction period for the generation of late rEC. In other studies, forskolin or rosiglitazone was added in their culture media after viral transduction of lineage-specific transcription factors.30),32) At present, only one study demonstrated that ECs could be generated by pure use of small molecules without exogenous gene overexpression. Sayed et al.37) demonstrated a direct endothelial-fate conversion of human fibroblasts with sequential administration of small molecule cocktails. To activate innate immunity, they first treated poly I:C, as a TLR3 agonist, to human fibroblasts for 7 days. Next, they cultured these cells in transdifferentiation medium I, containing bFGF, VEGF, and BMP4, for another seven days. Then, they treated the cells with transdifferentiation medium II, an EGM-2 medium supplemented with bFGF, VEGF, BMP4 and 8-Br-cAMP, for additional two weeks. Finally, after PECAM1+ cell sorting, they maintained the iECs, in EGM-2 with SB431542. Moreover, they verified that the transcriptome profile of iECs was comparable to that of human microvascular endothelial cells (HMVECs). Their findings suggested that innate immunity stimulated global changes in transcriptome and epigenome and this signaling was crucial for the direct cell-fate conversion into ECs. However, the transdifferentiation efficiency with this strategy was low with a yield of about 2%.
SOMATIC CELL SOURCES FOR DIRECT ENDOTHELIAL REPROGRAMMING
While directly reprogrammed ECs can be served as the source cells for vascular regeneration, the various somatic source cell types to transdifferentiate into ECs must be considered before clinical development of manufacturing. For instance, human fibroblasts are commonly used in the studies described above (Tables 1 and 2). However, one should distinguish fetal, neonatal, or adult fibroblasts to use because the transdifferentiation efficiency may differ between them. Moreover, Ginsberg et al.20) used human ACs noting that they are an ideal source of genetically diverse nonvascular cells. However, cryopreserved ACs are not amenable for an autologous approach and their approach could not convert adult somatic cells. They discussed, for the inadequacy of their protocol for adult cells, that ACs express FOXC2 to partner with ETS element and that midgestational ACs hold a plastic chromatin state, permitting transcriptional reprogramming towards ECs. In addition, somatic stem cells may be used for direct reprogramming. Cheng et al.28) used human adipose-derived stem cells and human umbilical mesenchymal stem cells to induce direct endothelial reprogramming. Li et al.38) demonstrated that EC transdifferentiation via ETV2 transduction is feasible with human dental pulp stem cells. Both groups confirmed that these generated cells showed EC-specific markers at both mRNA and protein levels, and were functionally competent in vitro. Furthermore, other somatic cell types, such as blood and urine cells, can also be employed as possible sources for direct endothelial reprogramming, inferred from other transdifferentiation studies.39),40),41) For instance, urine cells are directly reprogrammed into hepatocytes, neural or skeletal muscle cells.42),43),44) In addition, mouse and human direct reprogramming studies have employed hepatocytes, astrocytes, umbilical cord blood cells, epithelial cells, and other cell types to transdifferentiate them into desired target cell types.45) Considering the fact that reprogramming efficiency and the yield are major concerns in clinical application, appropriate selection and sufficient acquisition of human source cells in non-invasive and cost-effective manner are critical.
THERAPEUTIC APPLICATIONS USING DIRECTLY REPROGRAMMED ENDOTHELIAL CELLS
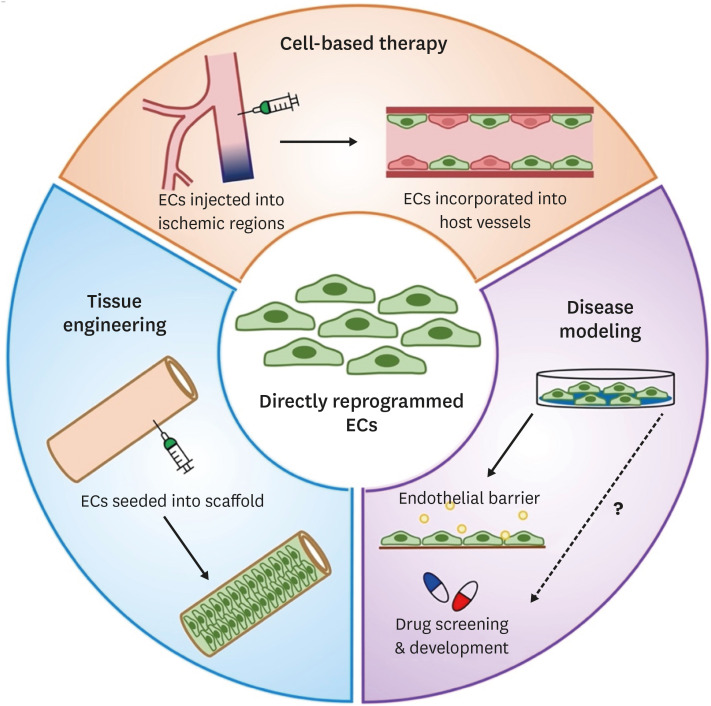
Thus far, at least three potential applications were suggested for directly reprogrammed ECs such as cell-based therapy, tissue engineering, and disease modeling (Figure 2). One of the most promising applications is to transplant or inject directly reprogrammed ECs into ischemic tissues to induce vascular regeneration. This cell-based therapy aims for formation of new blood vessels (neovascularization) in the ischemic regions, thereby re-establishing a functional vasculature and restoring blood perfusion and vascular function. Thus, the feasibility and efficacy of directly reprogrammed ECs for vascular regeneration have been investigated in various animal models.
Figure 2. Applications of directly reprogrammed EC. Directly rECs can be used for therapeutic applications, including cell-based therapy, tissue engineering, and disease modeling and potential drug screening and development.
EC = endothelial cell.
Ginsberg et al.20) demonstrated the vessel-forming properties of directly reprogrammed ECs in mice that have undergone partial hepatectomy. At 3 months after surgery, rAC-VECs were successfully engrafted and colocalized with the liver sinusoidal vessels that were perfused with isolectin B4, suggesting incorporation of these cells into the host vasculature. However, the animal model used by Ginsberg et al.20) only provided the in vivo behavior of rAC-VECs, but not their therapeutic effects. Henceforth, groups of investigators have utilized animal models of hindlimb ischemia to show the therapeutic effects of directly reprogrammed ECs. For one, Margariti et al.21) showed that intramuscular injection of PiPS-ECs improved blood flow in the ischemic hindlimbs as monitored by laser Doppler perfusion imaging (LDPI). In the PiPS-EC-injected ischemic tissues, blood capillary density was also significantly increased. This study showed the regenerative effects of PiPS-ECs. Similarly, Li et al.23) intramuscularly injected iEnd cells into the ischemic hindlimbs and conducted serial imaging with LDPI up to 14 days. Blood flow recovery and capillary density were enhanced in the iEnd cell-injected ischemic tissues. Of note, a second injection of iEnd cells into the ischemic hindlimbs were additionally carried out at seven days post-surgery, as their previous results showed enhanced blood perfusion in the repeatedly injected hindlimbs.9) Han et al.24) also demonstrated the therapeutic effects of iECs on a mouse model of hindlimb ischemia. When intramuscularly injected into the ischemic hindlimbs, iECs promoted the recovery of blood flow to ischemic hindlimbs and higher capillary density. Gross examination of the ischemic hindlimbs showed that mice that received iECs recovered well with reduced limb loss or necrosis. Histological analysis further showed that transplanted GFP-prelabelled iECs were colocalized with the Griffonia (Bandeiraea) Simplicifolia Lectin I (GSL I or BSL I)-perfused capillaries. However, only a fraction of transplanted iECs were incorporated into the microvasculature, even though these generated iECs were of murine origin. Sayed et al.37) determined the capacity of iECs for vascular regeneration in a mouse model of hindlimb ischemia. In accordance with their previous studies,9),23) Sayed et al.37) administered a second injection of iECs at 10 days post-surgery and enhanced blood flow and capillary density in the ischemic tissues. As a result, there was no considerable limb loss, but with the exception of discoloration of the toenails. Using the same animal model, Morita et al.25) also intramuscularly injected ETVECs, which not only showed better perfusion recovery but also protected ischemic hindlimbs from necrosis. At the histological level, Morita et al.25) demonstrated that ETVECs were able to survive and form functional perfused vasculature in vivo. While these studies20),21),23),24),25),37) revealed the capacity of directly reprogrammed ECs to rebuild blood vessels and restore vascular function in the ischemic regions, groups of investigators only looked at their short-term effects, which were up to 14 days. The study by Lee et al.27) was the only one to perform serial LDPI and to monitor the vessel-forming capacity of rECs in a mouse model of hindlimb ischemia up to 28 days. Injected rECs were incorporated into the host vessels and improved neovascularization of the ischemic hindlimbs. Kim et al.30) also showed that Fsk-iECs improved recovery of blood flow in ischemic hindlimbs. Transplanted Fsk-iECs were able to retain their angiogenic potential in vivo, enhancing vessel density. Recently, Cho et al.36) determined the therapeutic potential of rECs in a mouse model of experimental MI. Since Cho et al.36) simultaneously generated three types of cardiovascular cells (CMs, SMCs, and ECs) together with extracellular matrix by a direct reprogramming approach, the extent to which rECs alone exerted favorable therapeutic effects on acute MI remains to be elucidated. However, transplanted rECs from three-dimensional tissue-like structures not only were migrated in the host infarcted hearts but also newly formed blood vessels. These vessels formed by rECs were functional as determined by their colocalization with the BSL I-perfused vessels. Furthermore, Cho et al.36) showed that these rEC-incorporated vessels contained red blood cells, suggesting their connections to the systemic circulation. In summary, directly reprogrammed ECs have the capacity to restore vascular function to ischemic tissues in animal models. Thus, a readily available source of directly reprogrammed ECs has enormous therapeutic potential.
An alternative approach to induce vascular regeneration in the ischemic regions is to utilize directly reprogrammed ECs as a cell source for vascular tissue engineering. In particular, tissue-engineered vascular grafts using directly reprogrammed ECs have been developed to mimic the host vasculature. To date, there have been two groups of investigators. Margariti et al.21) were the first group to seed PiPS-ECs onto decellularized vessel scaffolds in a constructed bioreactors in which shear stress was applied. These PiPS-ECs in the tissue-engineered vessels showed the ability to mimic the architecture of a native blood vessel. Similarly, Chen et al. used decellularized mouse aortic grafts to allow iECs to attach and culture in an ex vivo circulation bioreactor system.29) The iEC-reconstructed vascular grafts were native vessel-comparable, forming the most inner layer with iECs and multiple outer layers of SMCs. Lastly, Kim et al.30) produced the Fsk-iEC-seeded scaffolds, which were derived from the acellular rat livers, in a customized bioreactor perfusion system. These Fsk-iECs successfully surrounded the vessel lumen of the scaffolds as determined by immunostaining with BSL I, CDH5, and CLDN5, maintaining their endothelial characteristics in tissue-engineered constructs. These tissue-engineered vascular grafts using directly reprogrammed ECs have provided blood vessel wall stability and integrity to a high level.
Another promising application using directly reprogrammed ECs in vitro is to model human diseases in a culture dish. To date, this “disease-in-a-dish” approach has been mostly practiced with human hiPSCs, as patient-specific iPSCs possess the inherited genetic disorders and genetic background.46),47) Specifically, ECs differentiated from patient-specific iPSCs have been proactively used to model a handful of CVDs, such as pulmonary arterial hypertension,48),49),50) calcified aortic valve disease,51) hemophilia A,52) and atrioventricular septal defects.53) These studies demonstrated that patient-specific iPSC-ECs could display a diseased EC phenotype. Although the hiPSC technology shed fascinating insights on the mechanisms of CVDs in a patient- and disease-specific fashion, it poses a major limitation for modeling age-related diseases. The reprogramming of somatic cells into iPSCs globally changes the epigenetic state of the cell, resetting the age of the initial somatic cell donor.54),55) Conversely, direct reprogramming using human fibroblasts circumvents such limitation, preserving the transcriptional aging signature of the initial somatic cell donor. Bersini et al.31) generated iVECs by isolating dermal fibroblasts from human young and elderly donors. While there were only a few differentially expressed genes identified between human young and elderly donors, the iVEC from elderly donors were functionally different from young donors as demonstrated by the compromised vascular permeability in vitro. Interestingly, Lee et al.27) were able to generate two types of rECs: early and late rECs. The study suggested that the late rECs showed features of more mature ECs, mimicking HUVEC and HMVEC while the early rECs could be more suitable for cell-based therapy. Therefore, these late rECs could be utilized to investigate the mechanisms of vascular diseases. Accordingly, the use of directly reprogrammed human ECs, while preliminary, may serve as an invaluable tool for the understanding of human CVDs.
REMAINING QUESTIONS AND CHALLENGES
Direct endothelial reprogramming is a viable strategy providing researchers not only to efficiently produce functional ECs for therapeutic purposes but also to accelerate the development of disease models for CVDs. However, many questions and technical challenges remain to be answered and overcome before directly reprogramed ECs can be extensively applied.
One major limitation in clinical application of directly reprogrammed ECs is the use of viral vectors for the delivery of reprogramming factors. These viral vectors, such as lentiviral20),21),23),24),25),27),56) and retroviral vectors,22) integrate into the genome of the host cells, causing insertional mutagenesis.57) This risk of mutagenesis has been highlighted by the induction of malignant phenotypes in the host cells by these viruses.58),59) Alternatively, non-integrating viral vectors, such as adenoviral and AAV vectors, can be employed. One study reported that transduction with adenoviral vector expressing DKK3 is sufficient to directly reprogram human embryonic lung fibroblasts into functional ECs.29) However, there are not enough studies on direct reprogramming into ECs by using these non-integrating viral vectors, questioning the feasibility of these viral gene delivery systems. The use of modified mRNA (modRNA) is currently an attractive alternative. Two recent studies by Suknuntha et al.60) and Wang et al.61) showed that hiPSCs could be differentiated into ECs by the delivery of modRNA expressing ETV2. Thus, modRNA encoding ETV2 may directly reprogram somatic cells into ECs, even though the short half-life and bioavailability of mRNAs should be taken into consideration.
Evidently, transplantation of directly reprogrammed ECs has been shown to enhance neovascularization and improve tissue perfusion in animal models of CVD. Nonetheless, concerns over cell engraftment, retention, and survival in the ischemic tissues remain unanswered. For one, most of adult stem cells injected into the ischemic hearts disappeared within a month.62),63) Likewise, groups of investigators showed low viability and survival of ECs differentiated from hiPSCs after 28 days in a mouse model of hindlimb ischemia.9),11),12) Pairing with biomaterial-based approaches should be considered to overcome low viability and survival of directly reprogrammed ECs in vivo, which have already been applied with generated cardiac-specific cell types.64) Such concerns await further investigation for directly reprogrammed ECs as well. In addition, the long-term fate of directly reprogrammed ECs in vivo was not addressed to determine whether transplanted ECs could manifest the expected characteristics of a bona fide EC (discussed in detail by our recent review65)). These transplanted directly reprogrammed ECs might display EC subtype heterogeneity, expressing arterial, venous, or even lymphatic lineage markers.66),67) Hence, further specification of EC subtypes need to be addressed in vivo. Of note, as most of these ECs injected into animal models were generated by virus-mediated gene delivery, it raises safety concerns, such as immune response and risk of tumorigenicity.20),21),23),24),25),27),37)
There are also challenges that have hampered the application of directly reprogrammed ECs for disease modeling. For one, this “disease-in-a-dish” approach is heavily dependent on the quality of ECs. Therefore, it is essential not only to provide a detailed characterization of directly reprogrammed ECs but also to demonstrate whether these cells faithfully mimic their in vivo counterparts. Further studies at the single-cell level are warranted to better understand the similarities and differences between directly reprogrammed ECs and human ECs. In addition, to model and understand CVD, EC heterogeneity should be taken into account, as ECs display remarkable heterogeneity depending on their localization on the vessels and tissues.66),67),68) Together, further investigation is required to reduce these variations to gain a better understanding of human CVDs.
CONCLUSION
Direct endothelial reprogramming of somatic cells has opened up new opportunities for both clinical application and disease modeling. Over the last decade, several groups developed novel methods to directly reprogram somatic cells into ECs, which have not only displayed enriched EC characteristics but also possessed the regenerative potential in animal models of CVDs. By avoiding pluripotency state, this direct reprogramming strategy could avoid potential side effects. Owing to the procedural simplicity and reasonable reprogramming efficiency in viral transduction methods, this technology has a potential to become a clinically viable strategy for treating ischemic CVDs via simple cell therapy or combination with tissue engineering technologies. As yet, multiple questions remain to be determined regarding the application of directly reprogrammed, including the development of appropriate vectors, their cell characteristics in vitro, and the short- and long-term fate of the cells in vivo including any potential side effects. Notwithstanding, this direct endothelial reprogramming strategy holds great promise for treating CVD and disease modeling.
Footnotes
Funding: This work was supported by National Research Foundation of Korea (NRF) funded by the Korean government (Ministry of Science and ICT; MSIT) (No. 2020R1A2C3003784, No. 2020M3A9I4038454), the Faculty Research Assistance Program of Yonsei University College of Medicine (6-2021-0178), the Parts/Materials Development Project in 2021 (20016564) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea), the Brain Korea 21 Project for Medical Science, Yonsei University, the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (HV22C0138), grants from NHLBI (R01HL150877), AHA Career Development Award (19CDA34760061) and AHA Transformational Project Award (20TPA35490282).
Conflict of Interest: Young-sup Yoon is a founder and CEO of Karis Bio Inc, a company that is commercializing technology related to the research described in this paper. Other authors have no financial conflicts of interest.
Data Sharing Statement: The data generated in this study is available from the corresponding authors upon reasonable request.
- Conceptualization: Jung C, Oh JE, Lee S, Yoon YS.
- Funding acquisition: Yoon YS.
- Supervision: Yoon YS.
- Visualization: Jung C, Oh JE.
- Writing - original draft: Jung C, Oh JE.
- Writing - review & editing: Lee S, Yoon YS.
References
- 1.Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 2.Bhagra SK, Pettit S, Parameshwar J. Cardiac transplantation: indications, eligibility and current outcomes. Heart. 2019;105:252–260. doi: 10.1136/heartjnl-2018-313103. [DOI] [PubMed] [Google Scholar]
- 3.Farber A. Chronic limb-threatening ischemia. N Engl J Med. 2018;379:171–180. doi: 10.1056/NEJMcp1709326. [DOI] [PubMed] [Google Scholar]
- 4.Kim W. Critical determinants of chronic limb threatening ischemia after endovascular treatment. Korean Circ J. 2022;52:441–443. doi: 10.4070/kcj.2022.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 7.Wang ZZ, Au P, Chen T, et al. Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat Biotechnol. 2007;25:317–318. doi: 10.1038/nbt1287. [DOI] [PubMed] [Google Scholar]
- 8.Taura D, Sone M, Homma K, et al. Induction and isolation of vascular cells from human induced pluripotent stem cells--brief report. Arterioscler Thromb Vasc Biol. 2009;29:1100–1103. doi: 10.1161/ATVBAHA.108.182162. [DOI] [PubMed] [Google Scholar]
- 9.Rufaihah AJ, Huang NF, Jamé S, et al. Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler Thromb Vasc Biol. 2011;31:e72–e79. doi: 10.1161/ATVBAHA.111.230938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SJ, Sohn YD, Andukuri A, et al. Enhanced therapeutic and long-term dynamic vascularization effects of human pluripotent stem cell-derived endothelial cells encapsulated in a nanomatrix gel. Circulation. 2017;136:1939–1954. doi: 10.1161/CIRCULATIONAHA.116.026329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clayton ZE, Yuen GS, Sadeghipour S, et al. A comparison of the pro-angiogenic potential of human induced pluripotent stem cell derived endothelial cells and induced endothelial cells in a murine model of peripheral arterial disease. Int J Cardiol. 2017;234:81–89. doi: 10.1016/j.ijcard.2017.01.125. [DOI] [PubMed] [Google Scholar]
- 12.Lai WH, Ho JC, Chan YC, et al. Attenuation of hind-limb ischemia in mice with endothelial-like cells derived from different sources of human stem cells. PLoS One. 2013;8:e57876. doi: 10.1371/journal.pone.0057876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ieda M, Fu JD, Delgado-Olguin P, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szabo E, Rampalli S, Risueño RM, et al. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 2010;468:521–526. doi: 10.1038/nature09591. [DOI] [PubMed] [Google Scholar]
- 17.Sekiya S, Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature. 2011;475:390–393. doi: 10.1038/nature10263. [DOI] [PubMed] [Google Scholar]
- 18.Pang ZP, Yang N, Vierbuchen T, et al. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee CS, Kim J, Cho HJ, Kim HS. Cardiovascular regeneration via stem cells and direct reprogramming: a review. Korean Circ J. 2022;52:341–353. doi: 10.4070/kcj.2022.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ginsberg M, James D, Ding BS, et al. Efficient direct reprogramming of mature amniotic cells into endothelial cells by ETS factors and TGFβ suppression. Cell. 2012;151:559–575. doi: 10.1016/j.cell.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Margariti A, Winkler B, Karamariti E, et al. Direct reprogramming of fibroblasts into endothelial cells capable of angiogenesis and reendothelialization in tissue-engineered vessels. Proc Natl Acad Sci U S A. 2012;109:13793–13798. doi: 10.1073/pnas.1205526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurian L, Sancho-Martinez I, Nivet E, et al. Conversion of human fibroblasts to angioblast-like progenitor cells. Nat Methods. 2013;10:77–83. doi: 10.1038/nmeth.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Huang NF, Zou J, et al. Conversion of human fibroblasts to functional endothelial cells by defined factors. Arterioscler Thromb Vasc Biol. 2013;33:1366–1375. doi: 10.1161/ATVBAHA.112.301167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han JK, Chang SH, Cho HJ, et al. Direct conversion of adult skin fibroblasts to endothelial cells by defined factors. Circulation. 2014;130:1168–1178. doi: 10.1161/CIRCULATIONAHA.113.007727. [DOI] [PubMed] [Google Scholar]
- 25.Morita R, Suzuki M, Kasahara H, et al. ETS transcription factor ETV2 directly converts human fibroblasts into functional endothelial cells. Proc Natl Acad Sci U S A. 2015;112:160–165. doi: 10.1073/pnas.1413234112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong WT, Cooke JP. Therapeutic transdifferentiation of human fibroblasts into endothelial cells using forced expression of lineage-specific transcription factors. J Tissue Eng. 2016;7:2041731416628329. doi: 10.1177/2041731416628329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S, Park C, Han JW, et al. Direct reprogramming of human dermal fibroblasts into endothelial cells using ER71/ETV2. Circ Res. 2017;120:848–861. doi: 10.1161/CIRCRESAHA.116.309833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng F, Zhang Y, Wang Y, et al. Conversion of human adipose-derived stem cells into functional and expandable endothelial-like cells for cell-based therapies. Stem Cell Res Ther. 2018;9:350. doi: 10.1186/s13287-018-1088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen T, Karamariti E, Hong X, et al. DKK3 (Dikkopf-3) transdifferentiates fibroblasts into functional endothelial cells-brief report. Arterioscler Thromb Vasc Biol. 2019;39:765–773. doi: 10.1161/ATVBAHA.118.311919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JJ, Kim DH, Lee JY, et al. cAMP/EPAC signaling enables ETV2 to induce endothelial cells with high angiogenesis potential. Mol Ther. 2020;28:466–478. doi: 10.1016/j.ymthe.2019.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bersini S, Schulte R, Huang L, Tsai H, Hetzer MW. Direct reprogramming of human smooth muscle and vascular endothelial cells reveals defects associated with aging and Hutchinson-Gilford progeria syndrome. eLife. 2020;9:e54383. doi: 10.7554/eLife.54383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han JK, Shin Y, Sohn MH, et al. Direct conversion of adult human fibroblasts into functional endothelial cells using defined factors. Biomaterials. 2021;272:120781. doi: 10.1016/j.biomaterials.2021.120781. [DOI] [PubMed] [Google Scholar]
- 33.Rezvani M, Español-Suñer R, Malato Y, et al. In vivo hepatic reprogramming of myofibroblasts with AAV vectors as a therapeutic strategy for liver fibrosis. Cell Stem Cell. 2016;18:809–816. doi: 10.1016/j.stem.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 35.Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho J, Kim S, Lee H, et al. Regeneration of infarcted mouse hearts by cardiovascular tissue formed via the direct reprogramming of mouse fibroblasts. Nat Biomed Eng. 2021;5:880–896. doi: 10.1038/s41551-021-00783-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sayed N, Wong WT, Ospino F, et al. Transdifferentiation of human fibroblasts to endothelial cells: role of innate immunity. Circulation. 2015;131:300–309. doi: 10.1161/CIRCULATIONAHA.113.007394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Zhu Y, Li N, et al. Upregulation of ETV2 expression promotes endothelial differentiation of human dental pulp stem cells. Cell Transplant. 2021;30:963689720978739. doi: 10.1177/0963689720978739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pavathuparambil Abdul Manaph N, Al-Hawwas M, Bobrovskaya L, Coates PT, Zhou XF. Urine-derived cells for human cell therapy. Stem Cell Res Ther. 2018;9:189. doi: 10.1186/s13287-018-0932-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou T, Benda C, Dunzinger S, et al. Generation of human induced pluripotent stem cells from urine samples. Nat Protoc. 2012;7:2080–2089. doi: 10.1038/nprot.2012.115. [DOI] [PubMed] [Google Scholar]
- 41.Zhang XB. Cellular reprogramming of human peripheral blood cells. Genomics Proteomics Bioinformatics. 2013;11:264–274. doi: 10.1016/j.gpb.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim EY, Page P, Dellefave-Castillo LM, McNally EM, Wyatt EJ. Direct reprogramming of urine-derived cells with inducible MyoD for modeling human muscle disease. Skelet Muscle. 2016;6:32. doi: 10.1186/s13395-016-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang W, Guo R, Shen SJ, et al. Chemical cocktails enable hepatic reprogramming of human urine-derived cells with a single transcription factor. Acta Pharmacol Sin. 2019;40:620–629. doi: 10.1038/s41401-018-0170-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Omrani MR, Yaqubi M, Mohammadnia A. Transcription factors in regulatory and protein subnetworks during generation of neural stem cells and neurons from direct reprogramming of non-fibroblastic cell sources. Neuroscience. 2018;380:63–77. doi: 10.1016/j.neuroscience.2018.03.033. [DOI] [PubMed] [Google Scholar]
- 45.Horisawa K, Suzuki A. Direct cell-fate conversion of somatic cells: toward regenerative medicine and industries. Proc Jpn Acad Ser B Phys Biol Sci. 2020;96:131–158. doi: 10.2183/pjab.96.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Engle SJ, Puppala D. Integrating human pluripotent stem cells into drug development. Cell Stem Cell. 2013;12:669–677. doi: 10.1016/j.stem.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 47.Merkle FT, Eggan K. Modeling human disease with pluripotent stem cells: from genome association to function. Cell Stem Cell. 2013;12:656–668. doi: 10.1016/j.stem.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 48.Gu M, Shao NY, Sa S, et al. Patient-specific iPSC-derived endothelial cells uncover pathways that protect against pulmonary hypertension in BMPR2 mutation carriers. Cell Stem Cell. 2017;20:490–504.e5. doi: 10.1016/j.stem.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sa S, Gu M, Chappell J, et al. Induced pluripotent stem cell model of pulmonary arterial hypertension reveals novel gene expression and patient specificity. Am J Respir Crit Care Med. 2017;195:930–941. doi: 10.1164/rccm.201606-1200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.West JD, Austin ED, Gaskill C, et al. Identification of a common Wnt-associated genetic signature across multiple cell types in pulmonary arterial hypertension. Am J Physiol Cell Physiol. 2014;307:C415–C430. doi: 10.1152/ajpcell.00057.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Theodoris CV, Li M, White MP, et al. Human disease modeling reveals integrated transcriptional and epigenetic mechanisms of NOTCH1 haploinsufficiency. Cell. 2015;160:1072–1086. doi: 10.1016/j.cell.2015.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu Y, Hu Z, Li Z, et al. In situ genetic correction of F8 intron 22 inversion in hemophilia A patient-specific iPSCs. Sci Rep. 2016;6:18865. doi: 10.1038/srep18865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ang YS, Rivas RN, Ribeiro AJ, et al. Disease model of GATA4 mutation reveals transcription factor cooperativity in human cardiogenesis. Cell. 2016;167:1734–1749.e22. doi: 10.1016/j.cell.2016.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maherali N, Sridharan R, Xie W, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 55.Studer L, Vera E, Cornacchia D. Programming and reprogramming cellular age in the era of induced pluripotency. Cell Stem Cell. 2015;16:591–600. doi: 10.1016/j.stem.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hong X, Margariti A, Le Bras A, et al. Transdifferentiated human vascular smooth muscle cells are a new potential cell source for endothelial regeneration. Sci Rep. 2017;7:5590. doi: 10.1038/s41598-017-05665-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baum C, Düllmann J, Li Z, et al. Side effects of retroviral gene transfer into hematopoietic stem cells. Blood. 2003;101:2099–2114. doi: 10.1182/blood-2002-07-2314. [DOI] [PubMed] [Google Scholar]
- 58.Ranzani M, Cesana D, Bartholomae CC, et al. Lentiviral vector-based insertional mutagenesis identifies genes associated with liver cancer. Nat Methods. 2013;10:155–161. doi: 10.1038/nmeth.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Z, Düllmann J, Schiedlmeier B, et al. Murine leukemia induced by retroviral gene marking. Science. 2002;296:497. doi: 10.1126/science.1068893. [DOI] [PubMed] [Google Scholar]
- 60.Suknuntha K, Tao L, Brok-Volchanskaya V, D’Souza SS, Kumar A, Slukvin I. Optimization of synthetic mRNA for highly efficient translation and its application in the generation of endothelial and hematopoietic cells from human and primate pluripotent stem cells. Stem Cell Rev Rep. 2018;14:525–534. doi: 10.1007/s12015-018-9805-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang K, Lin RZ, Hong X, et al. Robust differentiation of human pluripotent stem cells into endothelial cells via temporal modulation of ETV2 with modified mRNA. Sci Adv. 2020;6:eaba7606. doi: 10.1126/sciadv.aba7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cho HJ, Lee N, Lee JY, et al. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J Exp Med. 2007;204:3257–3269. doi: 10.1084/jem.20070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van der Bogt KE, Sheikh AY, Schrepfer S, et al. Comparison of different adult stem cell types for treatment of myocardial ischemia. Circulation. 2008;118:S121–S129. doi: 10.1161/CIRCULATIONAHA.107.759480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vasu S, Zhou J, Chen J, Johnston PV, Kim DH. Biomaterials-based approaches for cardiac regeneration. Korean Circ J. 2021;51:943–960. doi: 10.4070/kcj.2021.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oh JE, Jung C, Yoon YS. Human induced pluripotent stem cell-derived vascular cells: recent progress and future directions. J Cardiovasc Dev Dis. 2021;8:148. doi: 10.3390/jcdd8110148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garlanda C, Dejana E. Heterogeneity of endothelial cells. Specific markers. Arterioscler Thromb Vasc Biol. 1997;17:1193–1202. doi: 10.1161/01.atv.17.7.1193. [DOI] [PubMed] [Google Scholar]
- 67.Paik DT, Tian L, Williams IM, et al. Single-cell RNA sequencing unveils unique transcriptomic signatures of organ-specific endothelial cells. Circulation. 2020;142:1848–1862. doi: 10.1161/CIRCULATIONAHA.119.041433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nolan DJ, Ginsberg M, Israely E, et al. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell. 2013;26:204–219. doi: 10.1016/j.devcel.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]