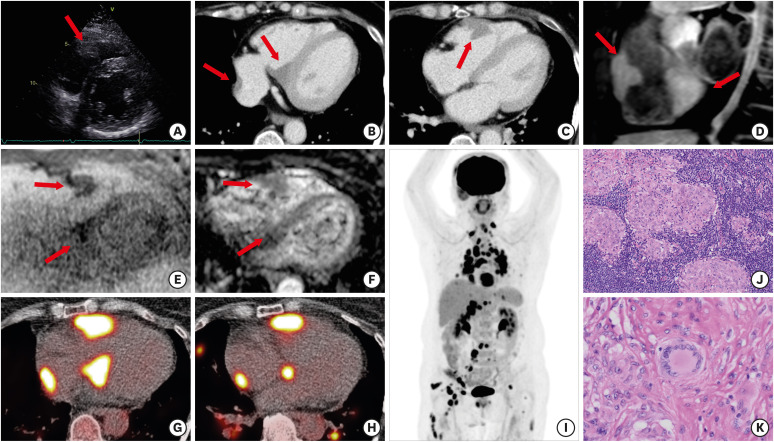
A 59-year-old woman with Sjogren’s syndrome (SS) and complete atrioventricular block who presented with dyspnea on exertion underwent chest computed tomography (CT). Laboratory examination revealed normal complete blood count and electrolytes and serum angiotensin-converting enzyme level was also normal (22.7 U/L; normal range 7.0–25.0 U/L). The CT images showed mediastinal and hilar lymphadenopathy and cardiac masses were suspected. Echocardiography, electrocardiography (ECG)-gated contrast enhanced CT (CECT) and magnetic resonance imaging (MRI) were performed for further evaluation. Echocardiography showed no wall motion abnormalities and a low echoic mass attached to the right ventricle free wall (Figure 1A). Axial ECG-gated CECT images identified masses attached to the myocardium (Figure 1B and C). Sagittal gadolinium enhanced MRI showed well-defined and lobulated masses with homogeneous enhancement (Figure 1D). Diffusion weighted imaging (DWI) and apparent diffusion coefficient map revealed slightly diffusion restriction within the masses compared with normal myocardium (Figure 1E and F). Based on the imaging findings, malignant lymphoma was initially suspected considering her medical history of SS. Subsequent transaxial images of 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/CT showed intense uptake in the cardiac masses (Figure 1G and H). Maximum intensity projection image of 18F-FDG PET showed avid uptake not only in the cardiac masses but also systemic enlarged lymph nodes (Figure 1I). Finally, inguinal lymph node biopsy revealed noncaseating granuloma (Figure 1J and K), and she was diagnosed with sarcoidosis.
Figure 1. Cardiac sarcoidosis mimicking lymphoma. (A) Parasternal short-axis echocardiogram shows a low-echoic mass attached to the right ventricle free wall (arrow). (B and C) Axial ECG-gated CECT images show multiple masses with homogenous enhancement attached to the right ventricle free wall, interventricular septum and right atrium (arrows). (D) Gd-FST1WI in sagittal view shows multiple masses with homogenous enhancement (arrows). (E and F) Axial DWI and corresponding ADC map image reveal slightly diffusion restriction within the masses compared with myocardium (arrows). (G and H) Axial fused 18F-FDG PET/CT images show intense uptake in the cardiac masses. (I) 18F-FDG PET MIP image shows multiple avid uptakes in not only cardiac masses but also systemic enlarged lymph nodes. (J and K) low-power and high-power view of the lymph node revealed non-caseating epithelioid-cell granulomas and multinucleated giant cells (hematoxylin and eosin staining; J, ×100 and K, ×200).
ADC = apparent diffusion coefficient; CECT = contrast enhanced computed tomography; DWI = diffusion weighted image; ECG = electrocardiogram; 18F-FDG = 18F-fluorodeoxyglucose; Gd-FST1WI = gadolinium-enhanced fat-suppressed T1-weighted image; MIP = maximum intensity projection; PET = positron emission tomography.
In the present case, there were two unique features including imaging findings and clinical manifestations. First, the cardiac lesions were imitating cardiac malignant tumor, especially on cardiac MRI. Cardiac MRI is a known useful imaging modality for diagnosing cardiac masses, whereas the feasibility of cardiac DWI remains controversial. Oda et al.1) reported the potential utility of cardiac DWI in the assessment of cardiac metastasis. However, cardiac DWI abnormalities may also be observed in benign diseases. Second, SS is associated with a well-established increased risk of lymphoma. On the other hand, coexistence of SS and sarcoidosis has been reported in some case reports,2),3) and the prevalence of SS and sarcoidosis to ranges from 1–2%.4) In addition, sarcoidosis occasionally mimics lymphoma,5) and therefore, cardiac sarcoidosis should be considered when diagnosing cardiac masses in patients with SS.
Informed consent was obtained from the participant for the publication.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest: The authors have no financial conflicts of interest.
Data Sharing Statement: The data generated in this study is available from the corresponding author upon reasonable request.
- Conceptualization: Fujimoto K, Norikane T, Takami Y.
- Writing - original draft: Fujimoto K, Norikane T.
- Writing - review & editing: Fujimoto K, Norikane T, Yamamoto Y, Takami Y, Murota M, Shimada H, Dobashi H, Nishiyama Y.
References
- 1.Oda S, Morita K, Okuaki T, Ogino T, Yamashita Y. Cardiac diffusion-weighted magnetic resonance imaging for assessment of cardiac metastasis. Eur Heart J Cardiovasc Imaging. 2018;19:683. doi: 10.1093/ehjci/jey039. [DOI] [PubMed] [Google Scholar]
- 2.Santiago T, Santiago M, Rovisco J, et al. Coexisting primary Sjögren’s syndrome and sarcoidosis: coincidence, mutually exclusive conditions or syndrome? Rheumatol Int. 2014;34:1619–1622. doi: 10.1007/s00296-014-3024-0. [DOI] [PubMed] [Google Scholar]
- 3.Mansour MJ, Al-Hashimi I, Wright JM. Coexistence of Sjögren’s syndrome and sarcoidosis: a report of five cases. J Oral Pathol Med. 2007;36:337–341. doi: 10.1111/j.1600-0714.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- 4.Patel R, Shahane A. The epidemiology of Sjögren’s syndrome. Clin Epidemiol. 2014;6:247–255. doi: 10.2147/CLEP.S47399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shetty A, Carter JD. Sarcoidosis mimicking lymphoma on FDG-PET imaging. Radiol Case Rep. 2015;6:409. doi: 10.2484/rcr.v6i2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]