Abstract
The presence of active viral infections has an impact on the prognosis of patients undergoing hematopoietic stem cell transplantation (HSCT). Nevertheless, the number of reports of cytomegalovirus infection in patients with inborn errors of immunity (IEI) who undergo HSCT is relatively low. To analyze the effect of cytomegalovirus infection acquired prior to curative treatment on patient survival in 123 children with IEI. An observational and retrospective study was performed with patients younger than 18 years diagnosed with IEI who were candidates for HSCT, gene therapy, or thymus transplantation at five hospitals in Spain between 2008 and 2019. We included 123 children, 25 infected by cytomegalovirus prior to undergoing curative treatment (20.3%). At IEI diagnosis, 24 of the patients were already infected, 21 of whom had symptomatic cytomegalovirus disease (87%), while the other three patients developed disease before undergoing curative treatment. The patients with cytomegalovirus infection had higher mortality than those without (p = 0.006). Fourteen patients developed refractory cytomegalovirus infection (56%), all of whom died, while no patients with non-refractory infection died (p = 0.001) All deaths that occurred before curative treatment and three of the five after the treatment were attributed to cytomegalovirus. Patients with refractory cytomegalovirus disease had the highest pre-HSCT mortality rate (64.3%), compared with the non-infected children and those with non-refractory cytomegalovirus disease (10.1%) (p < 0.0001).
Conclusion: Prevention and prompt control of cytomegalovirus infection, together with early HSCT/gene therapy, are crucial for improving the prognosis in children with IEI.
|
What is Known: • Cytomegalovirus is the most frequent viral infection in children with inborn errors of immunity who are candidates to hematopoietic stem cell transplantation (HSCT). • Active viral infections at the time of HSCT lead to worse prognosis. | |
|
What is New: • In children with inborn errors of immunity and indication of HSCT, refractory cytomegalovirus disease is associated with a very high mortality rate, compared with non-infected children and those with non-refractory cytomegalovirus disease. • In patients with novel transplantation indications, the presence and treatment response of CMV infection should be considered to decide the best possible moment for HSCT. |
Supplementary Information
The online version contains supplementary material available at 10.1007/s00431-022-04614-5.
Keywords: Inborn errors of immunity, Cytomegalovirus, Hematopoietic stem cell transplantation, Genetic therapy, Mortality
Introduction
Inborn errors of immunity (IEI) are genetic disorders that compromise innate and/or adaptive immune responses. Within these conditions, combined immunodeficiency (CID) and severe CID (SCID) are characterized by T, B, and NK cell defects that predispose patients to developing severe infections and immune dysregulation. Among patients with SCID, those undergoing hematopoietic stem cell transplantation (HSCT) or gene therapy (GT) in selected cases very early in life have the best prognosis [1, 2], even more so if promptly diagnosed by newborn screening [3, 4]. To completely restore T, B, and natural killer cell function and, consequently, improve survival, HSCT is currently recommended for patients with CID due to specific genetic defects such as CD40 ligand gene abnormalities and major histocompatibility complex class II deficiencies [3] and for many other patients such as those with severe clinical phenotypes irrespective of their genetic basis [5].
Early HSCT before the onset of a significant viral infection with organ dysfunction results in better outcomes for patients with IEI [1, 2]. The presence of active viral infections at the time of transplantation in patients with CID/SCID has a significant impact on prognosis and long-term survival [1, 6, 7], whereas there are hardly any data on patients with other IEI undergoing HSCT.
Although up to 70–90% of the world’s population has been exposed to cytomegalovirus [8], most of these infections are asymptomatic in immunocompetent individuals. In contrast, cytomegalovirus infections in immunocompromised patients can be life-threatening [9]. Cytomegalovirus is the most frequent viral pathogen in patients diagnosed with IEI prior to HSCT and is a risk factor for pre- and post-HSCT morbidity and mortality [9–11]. Cytomegalovirus disease prior to HSCT appears to be significantly more common in patients with IEI than in patients who undergo HSCT for other diseases, due to their profound pre-procedural lymphopenia and/or functional lymphocyte defects [1, 7–9]. Nevertheless, the number of reports on cytomegalovirus infection in IEI patients is relatively low [7–9, 12].
Our aim was to analyze the impact of cytomegalovirus infection on patient survival in a multicenter cohort of 123 children with IEI prior to potentially curative treatment (HSCT, GT, or thymus transplantation) by comparing those who acquired cytomegalovirus infection before treatment with those who did not.
Patients and methods
We conducted an observational, descriptive, and retrospective study with patients younger than 18 years diagnosed with IEI who were candidates for potentially curative treatment (HSCT, GT, or thymus transplantation) [13], at five tertiary university hospitals that are reference centers for IEI in Spain, between January 2008 and May 2019. At the time of the study, newborn screening for SCID in Spain had still not been implemented, except for Catalonia, which established it in January 2017.
The study included all children who were definitively diagnosed with an IEI (according to the International Union of Immunological Societies Expert Committee classification) in whom HSCT, GT, or thymus transplantation was indicated [14], except those with congenital phagocyte defects due to their low risk of pre-HSCT cytomegalovirus disease. Children with probable SCID/CID according to the European Society for Immunodeficiencies criteria were also included [15].
Cytomegalovirus infection was defined as virus isolation or detection of viral proteins (antigens) or nucleic acid in any body fluid or tissue specimen (plasma, serum, whole blood, peripheral blood leukocytes, cerebrospinal fluid, bronchoalveolar lavage fluid, urine, or tissue samples). Cytomegalovirus disease was defined as the presence of clinical symptoms and/or signs in the context of proven cytomegalovirus infection [16]. Resistant and refractory cytomegalovirus infections were established according to the Working Group of the Cytomegalovirus Drug Development Forum from the Infectious Disease Society of America. Resistant infection was defined as the presence of genetic alterations that decreased susceptibility to one or more antiviral drugs. Refractory and probable refractory CMV infection were established when CMV viremia increased more than 1 log10 or when viral load did not decrease at least 1 log10 after at least 2 weeks of appropriately dosed antiviral therapy, respectively [17].
The patients’ clinical and laboratory data were retrospectively reviewed by accessing their medical records. The following data were collected: sex, date of birth, age at clinical onset and at diagnosis of the IEI, confirmed genetic defect, breastfeeding, blood transfusions, cytomegalovirus infection or cytomegalovirus disease at diagnosis, and microbiological results. In addition, the study collected data regarding prescribed anti-cytomegalovirus therapies and their toxicity; cytomegalovirus resistance test results (if performed); indications for HSCT, GT, or thymus transplantation and procedure date (if performed); mortality; and cause of death. Patients were treated according to the physicians’ criteria, and the indication for curative treatment was based on current knowledge [13]. The patients were grouped according to the presence of cytomegalovirus infection. Those who were cytomegalovirus-positive were further classified depending on the development of refractory or probable refractory infection [17].
A statistical data analysis was performed using IBM Statistical Package for Social Sciences program (SPSS for Windows, version 25.0, IBM SPSS Corp.; Armonk, NY, USA). Qualitative data are expressed as absolute and relative frequencies, and quantitative data are expressed as median and interquartile range (IQR). The categorical variables were compared using chi-squared and Fisher’s exact test, and the continuous variables were compared with Student’s t-test or non-parametric tests as appropriate. The relative risks are expressed as odds ratio (OR) with 95% confidence intervals (CI). A two-tailed value of p < 0.05 was considered statistically significant.
Ethics approval for this study was first granted by the ethics committee of La Paz Hospital (decision number, PI-3807; decision date, 30th October 2019) and subsequently by the ethics committees of all participating hospitals.
Results
We included 123 children with IEI: 118 were initially considered candidates for HSCT, 3 were considered for GT (all of them diagnosed with adenosine deaminase deficiency), and 2 twins were considered for thymus transplantation (TBX1 deficiency) [18]. Eighty-five (69.1%) patients were male, and the median age at diagnosis was 6.97 months (IQR 3–15.52). Sixty-nine patients (56.1%) had SCID (five of whom had Omenn syndrome), 43 (35%) had CID, and 11 (8.9%) had other IEI. Genetic confirmation was achieved in 104 cases (84.6%), as summarized in Supplementary Table 1.
Of the candidates for HSCT, 86 (69.1%) ultimately underwent transplantation, and the median age at HSCT was 16.42 (IQR 9.15–45.09) months. Forty-three (36.4%) patients died, 18 before (15.2%) and 25 after the procedure (21.2%). Eleven children diagnosed with IEI other than SCID are currently stable without having undergone HSCT or other curative procedures. Only one of the patients (with lipopolysaccharide-responsive and beige-like anchor protein [LRBA] deficiency) had cytomegalovirus infection with a good clinical and microbiological response to ganciclovir. Three patients were lost during the follow-up period before HSCT. In addition, three patients were initially treated with GT (2.4%), all of whom survived. The twins with TBX1 deficiency died before undergoing a thymus transplantation due to severe congenital cytomegalovirus disease at the age of 3 and 4 months, respectively.
There were 25 patients infected by cytomegalovirus prior to undergoing potentially curative treatment (20.3%). Three of the cases involved congenitally acquired cytomegalovirus (12%), all of whom died. Genetic diagnoses were available for 21 of the 25 patients (84%) (Supplementary Table 1). At IEI diagnosis, 24 of the 25 patients were already infected, 21 of whom had symptomatic cytomegalovirus disease (87%), while the other three developed the disease before undergoing potentially curative treatment, with the remaining patient infected after the IEI diagnosis.
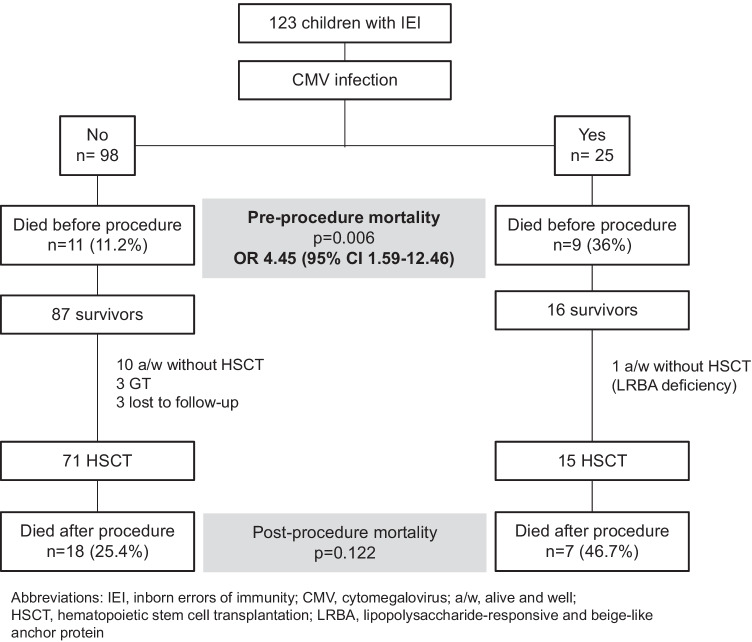
The median age at the diagnosis of cytomegalovirus infection was 6.39 months (IQR 3.37–19.16). Thirteen children had been breastfed (52%), but the cytomegalovirus PCR results in breast milk were available for only five cases, four of which were positive. Five patients (20%) had undergone non-irradiated non-leukodepleted blood transfusions before diagnosis. The main characteristics and treatments of the cytomegalovirus-infected children are summarized in Table 1. There were no significant differences in blood median peak viral loads between the survivors (138,000 copies/mL (IQR 4400–550,000)) and non-survivors (580,000 copies/mL (IQR 63,000–6,600,000)) (p = 0.322). The date of peak viral load was available in 21 cases and occurred before the initiation of antiviral treatment in 11 of them (52%). Three patients were tested for drug resistance due to unresponsiveness to antiviral therapy, and the UL97 mutation C603W was found in one of them, reported elsewhere [19]. The baseline characteristics and outcomes of the cytomegalovirus-infected and non-infected patients are summarized in Table 2. The cytomegalovirus-infected patients had significantly higher mortality than the non-infected ones (OR 4.23; 95% CI 1.68–10.66). The patient outcomes according to the presence of cytomegalovirus infection are summarized in Fig. 1. Fifteen cytomegalovirus-positive patients underwent HSCT, nine of whom had a cytomegalovirus-seropositive donor (mortality 44%; 4/9), and four had a cytomegalovirus-seronegative donor (mortality 50%; 2/4). For two patients, the donor’s cytomegalovirus serostatus was unknown (mortality 50%; 1/2). Reduced-intensity conditioning was employed in nine patients, four of whom died (44%). A myeloablative conditioning regimen was employed in four patients, three of whom died (75%). In two patients (both of whom survived), we could not obtain information regarding the conditioning regimen used. Eight of the fifteen transplanted children had been diagnosed with SCID (53%), although none of them received an unconditioned stem cell infusion. Up to 60% of the CMV-infected patients who underwent HSCT received serotherapy in their conditioning regimen (9/15) dying 44% of them (4/9), while three out of the six CMV-infected patients who did not received it died (50%).
Table 1.
Clinical and microbiological characteristics of the children with inborn errors of immunity and cytomegalovirus infection (n = 25)
| n (%) | |
|---|---|
| Clinical featuresa: | |
| • Respiratory symptoms/hypoxemia | 18 (72) |
| • Fever | 16 (64) |
| • Diarrhea | 11 (44) |
| • Hepatitis | 8 (32) |
| • Neurological symptoms | 8 (32) |
| • Retinitis | 7 (28) |
| • Asymptomatic (only viremia) | 2 (8) |
| Highest viral load prior to HSCT (copies/mL, median, and IQR) |
325 000 (5 400–2 100 000) |
| Coinfection by other virusesb | 10 (40) |
| Other coinfectionsc | 10 (40) |
| Oxygen therapy | 16 (64) |
| Invasive mechanical ventilation due to CMV infection | 10 (40) |
| PICU admission due to CMV infection | 11 (44) |
| Length of PICU stay, days; median (IQR) | 15 (8.5–29) |
| Antiviral therapy | |
| • Ganciclovir/valganciclovir | 25 (100) |
| • Foscarnet | 8 (32) |
| • Cidofovir | 3 (12) |
| • Leflunomide | 2 (8) |
| • Anti-CMV hyperimmune globulin | 4 (16) |
| • Adoptive therapy with CMV specific T-cells prior to HSCTd | 2 (8) |
| Number of drugs prescribed for CMV treatment (sequentially and/or in combination) | |
| • 1 | 15 (60) |
| • 2 | 7 (28) |
| • 3 or more | 3 (12) |
| Treatment duration, days; median (IQR) | |
| • Ganciclovir/valganciclovir | 36 (20.8–90) |
| • Foscarnet | 29.5 (19.8–50.8) |
| Treatment toxicitye: | 4 (16) |
| • Hematologic | 3 (12) |
| • Hepatic | 1 (4) |
| • Renal | 1 (4) |
BAL bronchoalveolar lavage, CMV cytomegalovirus, CSF cerebrospinal fluid, HSCT hematopoietic stem cell transplantation, PICU pediatric intensive care unit, IQR interquartile range
aCMV was isolated from blood and BAL; blood and CSF; blood, BAL, and CSF; and blood and urine in one patient each
bViruses: Epstein-Barr virus (3 cases), respiratory syncytial virus (3), adenovirus (2), norovirus (2), enterovirus (1), parainfluenza (1), parvovirus B19 (1). One patient was infected by enterovirus, norovirus, and respiratory syncytial virus and another by Epstein-Barr virus, norovirus, and adenovirus
cOther coinfections: Pneumocystis jirovecii (6), Pseudomonas aeruginosa (2), Candida spp. (2), Campylobacter jejuni (1), Cryptosporidium parvum (1), Aspergillus spp. (1), Serratia marcescens (1), Stenotrophomonas maltophilia. One patient was infected by Candida and C. jejuni, another by C. parvum, P. aeruginosa, and Aspergillus spp., and other by P. jirovecii, Candida spp., and S. marcescens
dThis therapy failed to control CMV load in these two patients, dying both of them before receiving HSCT
eOne patient experienced both hematological and renal toxicity. He received ganciclovir, foscarnet, cidofovir, CMV-specific hyperimmune globulin, and CMV-specific T cells
Table 2.
Comparison of baseline characteristics and outcomes according to cytomegalovirus infection status
| CMV-non-infected patients (n = 98) | CMV-infected patients (n = 25)a | p | |
|---|---|---|---|
| Male sex, n (%) | 70 (71.4) | 15 (60) | 0.333 |
| Median (IQR) age at diagnosis, months | 6.94 (3.02–15.08) | 7.26 (2.98–20.03) | 0.772 |
| Type of immune defect, n (%) | 0.008 | ||
| - SCID | 60 (61.2) | 11 (44) | |
| - CID | 34 (34.7) | 7 (28) | |
| - Other | 4 (4) | 7 (28) | |
| Median (IQR) age at diagnosis according to type of immune defect, months | |||
| - SCID | 6.1 (2.77–9.62) | 6.32 (2.97–9.94) | 0.812 |
| - CID | 7.97 (3.13–24.81) | 6.69 (1.84–28.66) | 0.522 |
| - Other | 73.55 (22.87–92.42) | 18.84 (5.21–68.4) | 0.201 |
| Gene therapy, n (%) | 3 (3.1) | 0 | 1 |
| HSCT, n (%) | 71 (72.4) | 15 (60) | 0.333 |
| Median age at HSCT, months | |||
| - SCID | 11.69 (6.89–22.32) | 11.83 (6.42–51) | 0.846 |
| - CID | 26.68 (16.58–61.11) | 36.27 (11.64–58.13) | 0.633 |
| - Other | 81.89 (16.26–147.52) | 53.32 (7.77–104.61) | 0.564 |
| Mortality, n (%) | 29 (29.6) | 16 (64) | 0.002 |
| Mortality before HSCT, n (%) | 11 (11.2) | 9 (36) | 0.006 |
Significant differences are shown in bold
CID combined immunodeficiency, CMV cytomegalovirus, HSCT hematopoietic stem cell transplantation, IQR interquartile range, SCID severe combined immunodeficiency
a24 of these 25 patients developed CMV disease
Fig. 1.
Outcomes of patients with inborn errors of immunity according to the presence of cytomegalovirus infection
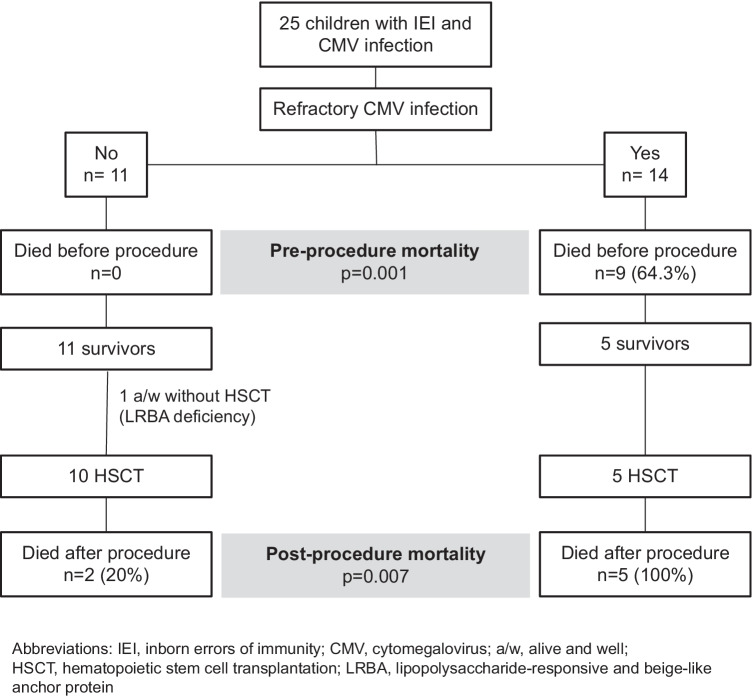
Fourteen patients (56%) developed refractory or probable refractory cytomegalovirus infection (56%), all of whom died, nine before HSCT and five after it (Fig. 2). All deaths that occurred before HSCT and three of the five that occurred after it were attributed to cytomegalovirus (86%). As for the other two cytomegalovirus-positive patients who died, one developed disseminated toxoplasmosis, and the other had non-filiated interstitial pneumonia with respiratory failure; however, the etiology remained unknown. Patients with refractory cytomegalovirus disease had the highest pre-HSCT mortality rate (9/14, 64.3%), compared with the non-infected children and those with non-refractory cytomegalovirus disease (11/109, 10.1%) [OR 16.04, 95% CI 4.56–56.45; p < 0.0001].
Fig. 2.
Outcomes of cytomegalovirus-positive patients according to the presence of refractory or probable refractory cytomegalovirus infection
Discussion
Our series reports on 123 children with IEI, 25 of whom were infected by cytomegalovirus prior to undergoing potentially curative treatment. All infected patients except one developed cytomegalovirus disease, which was refractory in 56% of cases. Mortality was significantly higher than for the cytomegalovirus-negative children, especially in pre-transplantation settings, with all the children with congenital cytomegalovirus or with refractory disease dying. Patients with refractory cytomegalovirus disease had the highest pre-HSCT mortality rate compared with the non-infected children and those with non-refractory cytomegalovirus disease.
Viral infections are one of the leading causes of death among patients with IEI [1, 6, 7]. Al-Herz et al. conducted a study to identify the risk factors that predict mortality in a national registry of 176 patients with IEI. Cytomegalovirus was the most frequently identified virus and an independent predictor of death, increasing this risk 2.8-fold. That series included all types of IEI, although the authors did not separately analyze patients with an indication for HSCT/GT. However, HSCT was associated with improved survival [7].
Since that study was performed, new indications for HSCT have been established in patients with IEI [20]. However, important questions are being raised, such as what type of IEI or which conditions could change the clinical outcome [3]. For many of these patients with novel transplantation indications, viral infections pose a significant risk when patients undergo HSCT, especially when they experience a pre-existing infectious burden that they bring to transplantation [20]. In our cohort, the children diagnosed with IEI other than SCID/CID, such as LRBA deficiency and immune dysregulation, polyendocrinopathy and enteropathy, and X-linked (IPEX) syndrome, were significantly more frequently infected by cytomegalovirus than those with SCID/CID, although they were also older.
Regarding children diagnosed with IEI other than SCID/CID, a large recently published cohort reported that patients with IEI associated with autoimmunity and/or inflammation present a poorer prognosis and lower survival rate after HSCT, compared with patients with other types of IEI [21]. Based on our results, cytomegalovirus infection could be a factor affecting these patients’ clinical outcomes and should be carefully considered when establishing a therapeutic strategy.
In the last decade, advances such as new antiviral drugs, adoptive immunotherapy with virus-specific T-cells, and GT have been developed [3, 19, 22]. However, managing cytomegalovirus infection in these patients is still a challenge, and, as we have observed in our cohort, mortality rates are still high among infected patients. Currently most antiviral drugs are virostatic and require functional T-cells to clear the virus [20]. In our cohort, the infection could not be controlled in up to 40% of the patients after starting an antiviral drug and ultimately required at least two drugs to treat the infection. Although early preemptive therapy could prevent organ damage, the risk of drug-related toxicity and developing antiviral resistance should also be considered [23]. Resistant strains seem to appear more easily and earlier in children with IEI compared with other immunosuppressed patients [24], probably due to the former’s more profoundly impaired T-cell immunity. Resistances can appear within 10 days to 3 weeks from the initiation of antiviral therapy in patients with combined immunodeficiencies [25]. Some authors suggest that ganciclovir therapy might be optimized using therapeutic drug monitoring (TDM) especially in pediatric populations and in immunocompromised patients, as ganciclovir pharmacokinetic is highly variable and the optimal exposure for the treatment of CMV disease is unknown [26, 27]. However, there is little information in this regard in children. Nguyen et al. recently characterized ganciclovir pharmacokinetics in patients younger than 18 years, suggesting increased doses to achieve a therapeutic exposure. However, these doses should be prospectively confirmed, and TDM could help to adjust them individually [27]. There is currently little evidence available regarding the use of combination therapy compared to the use of monotherapy for treating CMV infection in these patients. In our retrospective study, the use of several drugs in different national reference units reflects the clinicians’ difficulties to treat CMV disease in immunocompromised patients. Vora et al. have recently suggested that combined antiviral therapy could be a safe option for high-risk children with primary immunodeficiencies in pre-transplantation settings, especially for those with high viral loads [28]. Although these data have to be considered with caution and further studies are needed, dual therapy could be an option in infants with PID and severe CMV disease without viral load control. However, side effects are frequent and can be severe, causing cumulative organ dysfunction over time, especially when other toxic drugs are required before or during the HSCT [20].
All of our patients treated with GT survived, and its administration has been reported to have improved clinical outcomes [29]. Although until date the added value of GT over HSCT to control CMV has not been proven, GT could be a possible strategy in cases where a proper donor is not available, and a prompt improvement of T cell function is required for controlling CMV infection [30].
Adoptive immunotherapy with virus-specific T-cells appears to have limited efficacy for treating disseminated cytomegalovirus infections in pre-transplantation settings [19, 22]. Given that the presence of active cytomegalovirus infection adversely affects survival [1, 6, 28], adoptive immunotherapy could be administered as a rapid salvage therapy to reduce viremia until immunity is reestablished with an HSCT [19, 22]. Lastly, adjuvant therapy with intravenous immunoglobulin or with cytomegalovirus-specific hyperimmune globulin may be considered in cases of severe disease associated with hypogammaglobulinemia [6], although its effect remains controversial [31].
Another strategy that could reduce mortality is the selection of cytomegalovirus-seropositive donors, given that cytomegalovirus-specific memory T-cells transferred from the graft can help control the infection [32]. Nevertheless, the majority of data on cytomegalovirus infection in HSCT recipients are derived from patients with hematological malignancies [11]. In our cohort, although most infected patients received grafts from cytomegalovirus-seropositive donors, morbidity and mortality remained very high. Up to 96% of the patients developed cytomegalovirus disease, which was frequently refractory to antiviral therapy and led to multiple and severe organ damage at the time of HSCT. The effect of donor serostatus on patients with IEI and cytomegalovirus infection is not yet clearly elucidated. In addition, we have to take into account that the conditioning regimen could also modify the immune reconstitution and the transferred T cell immunity of the donor, the rate of CMV reactivations, and, therefore, the long-term survival [33]. Other approaches such as the use of ATG, ex vivo T-cell depletion using CD34 + positive selection, or post-transplant cyclophosphamide could affect the control of viral replication, leading to a higher incidence of CMV infection [33, 34].
Due to the poorer prognosis of patients with cytomegalovirus infection, preventing its acquisition is essential in children with IEI who require HSCT. Unfortunately, 96% of our patients were already infected at diagnosis, and almost all developed disease before undergoing potentially curative treatment. These findings highlight the importance of newborn screening to establish preventive measures for the infection, such as stopping breastfeeding [3, 6]. However, this screening is currently available only for detecting SCID. Other types of IEI associated with a risk of severe cytomegalovirus infection are not currently detected by newborn screening [22]. Clinicians should consider IEI in young patients with symptomatic cytomegalovirus disease, especially in cases of disseminated or severe infections or in the presence of other suggestive clinical findings.
Our study has several limitations. Data were collected retrospectively, and we have included a heterogeneity of diseases presenting different immunological profiles, although this is also a positive and valuable aspect of this study. We were unable to identify the source of the infection in most cases, and, due to our sample size, we were not able to analyze the relationship between mortality and clinical presentation or the presence of coinfections. Therefore, multicenter prospective studies are needed to obtain robust evidence. However, our results highlight both the importance of an early diagnosis of IEI, which is now possible in many cases through newborn screening programs, and the importance of prevention and prompt and aggressive treatment of cytomegalovirus infections in IEI patients who require HSCT/GT. Although further studies are needed to better define the therapeutic approach of cytomegalovirus infection in these patients, prompt control of the infection and early HSCT/GT are crucial to improve the prognosis.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- CI
Confidence interval
- CID
Combined immunodeficiency
- GT
Gene therapy
- HSCT
Hematopoietic stem cell transplantation
- IEI
Inborn errors of immunity
- IPEX
Immunodysregulation, polyendocrinopathy, and enteropathy X-linked
- IQR
Interquartile range
- LRBA
Lipopolysaccharide-responsive and beige-like anchor protein
- OR
Odds ratio
- SCID
Severe combined immunodeficiency
- TDM
Therapeutic drug monitoring
Authors’ contributions
All authors contributed to the study conception and design. The database for the study was designed by Cristian Quintana-Ortega and Ana Méndez-Echevarría. Data were collected by Cristian Quintana-Ortega, Angela Deyá-Martinez, Walter Alfredo Goycochea-Valdivia, Nerea Salmón, Luz Yadira Bravo-Gallego, and Laura Alonso. Data analysis was performed by Teresa del Rosal and Ana Méndez-Echevarría. All authors contributed to data interpretation. The first draft of the manuscript was written by Teresa del Rosal and Ana Méndez-Echevarría, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Availability of data and material
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Hospital Universitario La Paz (decision number, PI-3807; decision date, 30th October 2019) and subsequently by the ethics committees of all participating hospitals.
Consent to participate
This is a retrospective observational study. No informed consent was obtained from the parents.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Teresa del Rosal, Email: teredelrosal@yahoo.es.
Cristian Quintana-Ortega, Email: cristian.quintana.ortega@hotmail.com.
Angela Deyá-Martinez, Email: adeya@sjdhospitalbarcelona.org.
Pere Soler-Palacín, Email: psoler@vhebron.net.
Walter Alfredo Goycochea-Valdivia, Email: alfgova@gmail.com.
Nerea Salmón, Email: aeren28@hotmail.com.
Antonio Pérez-Martínez, Email: aperezmartinez@salud.madrid.org.
Laia Alsina, Email: lalsina@sjdhospitalbarcelona.org.
Andrea Martín-Nalda, Email: andmartin@vhebron.net.
Laura Alonso, Email: l.alonso@vhebron.net.
Olaf Neth, Email: olafneth@gmail.com.
Luz Yadira Bravo-Gallego, Email: yadira.bravo.gallego@transplantchild.eu.
Luis Ignacio Gonzalez-Granado, Email: luisigon@ucm.es.
Ana Mendez-Echevarria, Email: amendezes@yahoo.es.
References
- 1.Pai SY, Logan BR, Griffith LM, Buckley RH, Parrott RE, Dvorak CC, et al. Transplantation outcomes for severe combined immunodeficiency, 2000–2009. N Engl J Med. 2014;371:434–446. doi: 10.1056/NEJMoa1401177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lankester AC, Neven B, Mahlaoui N, von Asmuth EGJ, Courteille V, Alligon M, et al. Hematopoietic cell transplantation in severe combined immunodeficiency: the SCETIDE 2006–2014 European cohort. J Allergy Clin Immunol. 2022;149:1744–1754. doi: 10.1016/j.jaci.2021.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Cordero E, Goycoechea WA, Mendez-Echevarria A, Allende L.M, Alsina L, Bravo Garcia-Morato M et al (2020) Diagnosis and management of patients with primary immunodeficiencies. Consensus Document of SEIMC, SEI, SEIP-AEP and SEICAP-AEP. J Allergy Clin Immunol Pract [DOI] [PubMed]
- 4.Amatuni GS, Currier RJ, Church JA, Bishop T, Grimbacher E, Nguyen AA, et al. Newborn screening for severe combined immunodeficiency and T-cell lymphopenia in California, 2010–2017. Pediatrics. 2019;143(2):e20182300. doi: 10.1542/peds.2018-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Speckmann C, Doerken S, Aiuti A, Albert MH, Al-Herz W, Allende LM, et al. A prospective study on the natural history of patients with profound combined immunodeficiency: an interim analysis. J Allergy Clin Immunol. 2017;139:1302–10.e4. doi: 10.1016/j.jaci.2016.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Méndez-Echevarría A, Del Rosal T, Pérez-Costa E, Rodríguez-Pena R, Zarauza A, Ferreira-Cerdán A, et al. Clinical features before hematopoietic stem cell transplantation or enzyme replacement therapy of children with combined immunodeficiency. Pediatr Infect Dis J. 2016;35:794–798. doi: 10.1097/INF.0000000000001157. [DOI] [PubMed] [Google Scholar]
- 7.Al-Herz W, Moussa MA. Survival and predictors of death among primary immunodeficient patients: a registry-based study. J Clin Immunol. 2012;32:467–473. doi: 10.1007/s10875-011-9636-1. [DOI] [PubMed] [Google Scholar]
- 8.Zuhair M, Smit GSA, Wallis G, Jabbar F, Smith C, Devleesschauwer B, et al. Estimation of the worldwide seroprevalence of cytomegalovirus: a systematic review and meta-analysis. Rev Med Virol. 2019 doi: 10.1002/rmv.2034. [DOI] [PubMed] [Google Scholar]
- 9.Al-Herz W, Essa S. Spectrum of viral infections among primary immunodeficient children: report from a national registry. Front Immunol. 2019;10:1231. doi: 10.3389/fimmu.2019.01231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olaya M, Franco A, Chaparro M, Estupiñan M, Aristizabal D, Builes-Restrepo N, et al. Hematopoietic stem cell transplantation in children with inborn errors of immunity: a multi-center experience in Colombia. J Clin Immunol. 2020 doi: 10.1007/s10875-020-00856-w. [DOI] [PubMed] [Google Scholar]
- 11.Forlanini F, Dara J, Dvorak CC, Cowan MJ, Puck JM, Dorsey MJ. Unknown cytomegalovirus serostatus in primary immunodeficiency disorders: a new category of transplant recipients. Transpl Infect Dis. 2021;23(2):e13504. doi: 10.1111/tid.13504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7:1–16. doi: 10.1007/s40121-017-0180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castagnoli R, Delmonte OM, Calzoni E, Notarangelo LD. Hematopoietic stem cell transplantation in primary immunodeficiency diseases: current status and future perspectives. Front Pediatr. 2019;7:295. doi: 10.3389/fped.2019.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tangye SG, Al-Herz W, Bousfiha A, Chatila T, Cunningham-Rundles C, Etzioni A, et al. Human inborn errors of immunity: 2019 update on the classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol. 2020;40:24–64. doi: 10.1007/s10875-019-00737-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ESID Registry – Working Definitions for Clinical Diagnosis of PID (2019). Online available at: https://esid.org/Working-Parties/Registry-Working-Party/Diagnosis-criteria. Accessed 08 May 2021
- 16.Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. 2017;64:87–91. doi: 10.1093/cid/ciw668. [DOI] [PubMed] [Google Scholar]
- 17.Chemaly RF, Chou S, Einsele H, Griffiths P, Avery R, Razonable RR, et al. Definitions of resistant and refractory cytomegalovirus infection and disease in transplant recipients for use in clinical trials. Clin Infect Dis. 2019;68:1420–1426. doi: 10.1093/cid/ciy696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colmenero-Velázquez A, Esteso G, Del Rosal T, Calvo Apalategui A, Reyburn H, López-Granados E. Marked changes in innate immunity associated with a mild course of COVID-19 in identical twins with athymia and absent circulating T cells. J Allergy Clin Immunol. 2021;147:567–568. doi: 10.1016/j.jaci.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alonso L, Méndez-Echevarría A, Rudilla F, Mozo Y, Soler-Palacin P, Sisinni L, et al. Failure of viral-specific T cells administered in pre-transplant settings in children with inborn errors of immunity. J Clin Immunol. 2021;41:748–755. doi: 10.1007/s10875-020-00961-w. [DOI] [PubMed] [Google Scholar]
- 20.Slatter MA, Gennery AR. Hematopoietic cell transplantation in primary immunodeficiency - conventional and emerging indications. Expert Rev Clin Immunol. 2018;14:103–114. doi: 10.1080/1744666X.2018.1424627. [DOI] [PubMed] [Google Scholar]
- 21.Fischer A, Provot J, Jais JP, Alcais A, Mahlaoui N. Members of the CEREDIH French PID study group autoimmune and inflammatory manifestations occur frequently in patients with primary immunodeficiencies. J Allergy Clin Immunol. 2017;140:1388–1393.e8. doi: 10.1016/j.jaci.2016.12.978. [DOI] [PubMed] [Google Scholar]
- 22.Mendez-Echevarria A, Gonzalez-Granado LI, Allende LM, De Felipe B, Teresa DR, Calvo C, et al. Pneumocystis jirovecii and Cytomegalovirus infections in an infant with normal TRECs count: pitfalls of newborn screening for severe combined immunodeficiency. Pediatr Infect Dis J. 2019;38:157–160. doi: 10.1097/INF.0000000000002058. [DOI] [PubMed] [Google Scholar]
- 23.Camargo JF, Kimble E, Rosa R, Shimose LA, Bueno MX, Jeyakumar N, et al. Impact of cytomegalovirus viral load on probability of spontaneous clearance and response to preemptive therapy in allogeneic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2018;24:806–811. doi: 10.1016/j.bbmt.2017.11.038. [DOI] [PubMed] [Google Scholar]
- 24.Kim E, Asmar BI, Thomas R, Abdel-Haq N. Cytomegalovirus viremia and resistance patterns in immunocompromised children: an 11-year experience. Pediatr Hematol Oncol. 2020;37:119–128. doi: 10.1080/08880018.2019.1695031. [DOI] [PubMed] [Google Scholar]
- 25.Wolf DG, Yaniv I, Honigman A, Kassis I, Schonfeld T, Ashkenazi S, et al. Early emergence of ganciclovir-resistant human cytomegalovirus strains in children with primary combined immunodeficiency. J Infect Dis. 1998;178:535–538. doi: 10.1086/517468. [DOI] [PubMed] [Google Scholar]
- 26.Stockmann C, Sherwin CMT, Knackstedt ED, Hersh AL, Pavia AT, Spigarelli MG. Therapeutic drug monitoring of ganciclovir treatment for cytomegalovirus infections among immunocompromised children. J Pediatric Infect Dis Soc. 2016;5:231–232. doi: 10.1093/jpids/piw008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen T, Oualha M, Briand C, Bendavid M, Béranger A, Benaboud S, et al. Population pharmacokinetics of intravenous ganciclovir and oral valganciclovir in a pediatric population to optimize dosing regimens. Antimicrob Agents Chemother. 2021;65:e02254–e2320. doi: 10.1128/AAC.02254-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vora SB, Brothers AW, Waghmare A, Englund JA. Antiviral combination therapy for cytomegalovirus infection in high-risk infants. Antivir Ther. 2018;23:505–511. doi: 10.3851/IMP3238. [DOI] [PubMed] [Google Scholar]
- 29.Booth C, Romano R, Roncarolo MG, Thrasher AJ. Gene therapy for primary immunodeficiency. Hum Mol Genet. 2019;28(R1):R15–23. doi: 10.1093/hmg/ddz170. [DOI] [PubMed] [Google Scholar]
- 30.Chetty K, Houghton BC, Booth C. Gene therapy for inborn errors of immunity: severe combined immunodeficiencies. Hematol Oncol Clin North Am. 2022 doi: 10.1016/j.hoc.2022.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Ljungman P, de la Camara R, Robin C, Crocchiolo R, Einsele H, Hill JA et al (2019) 2017 European Conference on Infections in Leukaemia group. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis 19:e260-e272 [DOI] [PubMed]
- 32.Ljungman P, Brand R, Hoek J, de la Camara R, Cordonnier C, Einsele H, et al. Donor cytomegalovirus status influences the outcome of allogeneic stem cell transplant: a study by the European group for blood and marrow transplantation. Clin Infect Dis. 2014;59:473–481. doi: 10.1093/cid/ciu364. [DOI] [PubMed] [Google Scholar]
- 33.Luo XH, Zhu Y, Chen YT, Shui LP, Liu L. CMV infection and CMV-specific immune reconstitution following haploidentical stem cell transplantation: an update. Front Immunol. 2021;12:732826. doi: 10.3389/fimmu.2021.732826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaw P, Shizuru J, Hoenig M, Veys P. Conditioning perspectives for primary immunodeficiency stem cell transplants. Front Pediatr. 2019;7:434. doi: 10.3389/fped.2019.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Not applicable.