Abstract
Background
Vaccination against the coronavirus disease (SARS-CoV-2) is understood to be the key way out of the COVID-19 pandemic. Limited evidence exists on the determinants of vaccine rollouts and their health effects at the country level.
Objective
Examine the determinants of COVID-19 vaccine rollouts and their effects on health outcomes.
Methods
Ordinary least squares regressions with standard errors clustered at the country level for Cross-section and Panel daily data of vaccinations and various health outcomes (new COVID-19 cases, fatalities, intensive care unit (ICU) admissions) for an unbalanced sample of about 200 countries during the period 16 December 2020 to 20 June 2021.
Results
We find evidence that: (i) early vaccine procurement, domestic production of vaccines, the severity of the pandemic, a country’s health infrastructure, and vaccine acceptance are significant determinants of the speed of vaccination rollouts; (ii) vaccine deployment significantly reduces new COVID-19 infections, Intensive Care Unit (ICU) admissions, and fatalities, and is more effective when coupled with stringent containment measures, or when a country is experiencing a large outbreak; and (iii) COVID-19 cases in neighboring countries can lead to an increase in a country’s domestic caseload, and hamper efforts in taming its own local outbreak.
Conclusions
By providing an early broad overview of the quantitative empirical estimates of the determinants of vaccine rollouts and the effects of COVID-19 vaccines, our paper can help policymakers make informed decisions about local and global distributions of vaccines, as well as related policy tools, such as containment measure.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40258-022-00757-6.
Key Points for Decision Makers
| The success of a country’s vaccine deployment in the first half of 2021 was driven by five primary factors: the severity of its pandemic waves in 2020, its procurement strategies, the quantity of locally produced vaccines, the quality of health infrastructure, and vaccine acceptance. |
| Swift and broad administration of vaccines provides a significant boost to health outcomes, particularly in the midst of major outbreaks and accompanied by containment measures. |
| Cross-country health spillovers from vaccine rollouts mean the pandemic will not be over anywhere until it is over everywhere. |
Introduction
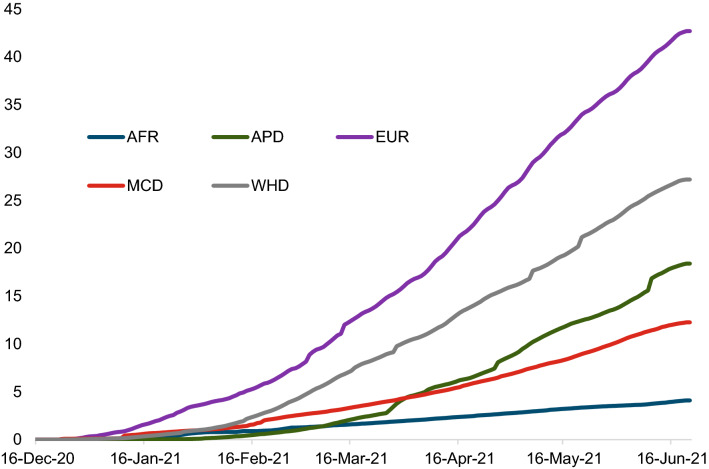
Vaccination against the coronavirus disease (SARS-CoV-2) is understood to be the key way out of the COVID-19 pandemic and the economic crisis it has brought about [1, 2]. However, access to and uptake of COVID-19 vaccines during the initial phase of the vaccination campaigns was heterogeneous and uneven, despite the improvement in vaccine availability. Countries in North America and Europe started vaccinations earlier on and were further along than other regions such as Africa and the Middle East by the middle of 2021. Vaccinations in Asia started later but then picked up (Fig. 1). Across income groups, during the initial phase, advanced economies vaccinated a much larger share of their populations than emerging and developing economies, on average (see Appendix Fig. A.1).
Fig. 1.
Vaccinations across regions (simple average, per 100 population). AFR Sub-Saharan Africa, APD Asia Pacific Department, EUR European Department, MCD Middle East and Central Asia Department, WHD Western Hemisphere Department.
Source: Our World in Data
The epidemiological literature has documented the effectiveness of vaccines on COVID-19 health outcomes thus far for individual countries or a small set of two to four countries (see literature review below), but there is little cross-country empirical evidence on the determinants of COVID-19 vaccine rollouts in the initial phase and their impacts on health outcomes during the initial phases of vaccinations.
Our paper contributes to two strands of the literature. The first is the one that looks at the role of supply and demand side factors that may impede or accelerate the rollout (and uptake) of vaccines, including COVID-19 vaccines. [3] conduct the largest country study to date of global vaccine confidence across 149 countries and find that confidence in the importance of vaccines (rather than their safety or effectiveness) has the strongest association with vaccine uptake compared to other determinants considered. [4] study the determinants of COVID-19 vaccine acceptance in the USA in May 2020, and find an average 67% acceptance rate, which is higher among males (compared to females), older adults (compared to younger adults), and college or graduate degree holders (compared to people with less than a college degree). [5] also look at the drivers of vaccine administration and delivery efficiency for 50 US states and find factors such as more COVID-19 deaths, demographics, and health infrastructure play an important role. [6] use survey data for 17 countries to examine the drivers of COVID-19 vaccine demand and find that vaccine hesitancy is a significant deterrent for vaccine uptake, and concerns over the severity of COVID-19 and trust in government as drivers of vaccine demand. This paper adds to this growing literature on the determinants of COVID-19 vaccine rollouts by examining the role of demand and supply side factors in explaining rollouts across a sample of nearly 200 countries.1
The paper also contributes to a second strand of the literature examining the health effects of COVID-19 vaccines. In a noncontrolled setting, [7] study the effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA-based COVID-19 vaccines in Israel across diverse populations. The outcomes 20 days after the first dose and 7 days after the second dose were 46% and 92% for preventing documented infection; 74% and 98% for hospitalization, and, for severe disease, 62%% and 92%%, respectively. Using data for healthcare workers in the UK, [8] estimate vaccine effectiveness against infection for the BNT162b2 vaccine to be 70% (21 days after the first dose), increasing to 85% (7 days after the second dose). [9] find similar results for BNT162b2 and also document that with ChAdOx1-S (Oxford-AstraZeneca) non-mRNA vaccine, effects were seen from 14 to 20 days after vaccination, reaching an effectiveness of 60% from 28 to 34 days, increasing to 73% from day 35 onwards. [10]find a 95% efficacy in preventing SARS-Cov-2 infections 7 days after the second dose of the BTN162b2 mRNA-based vaccine in randomized trials of a large sample size pooled from within the USA, Argentina, Brazil, and South Africa. [11] uses a cross-country regional database of 17 countries (326 regions) to analyze the effects of COVID-19 vaccines on health outcomes. They find that a 10% increase in the share of the population with one vaccine dose (which is comparable to moving from a region thatis relatively unvaccinated, as captured by the 25th percentile of the distribution of vaccinations as a share of population, to a region at the 75th percentile of the distribution) reduces infections after 21 days by 0.10 percentage point. This paper contributes to this strand of the literature by extending on [11] to examine the health outcomes of COVID-19 vaccines across a much larger sample of 126 countries, and explore the role of country-specific conditions in shaping the effect of COVID-19 vaccines and the effect of the COVID-19 pandemic in neighboring countries on the country’s caseload.
Our paper provides some evidence on multiple dimensions and adds to the existing literature by: (i) empirically assessing the determinants and drivers of vaccine rollouts across countries; (ii) analyzing the health impact of vaccinations at the country level for an extensive sample of 126 advanced and developing countries; (iii) studying the role of country-specific conditions—such as containment measures, the severity of the COVID-19 outbreak, the dominant COVID-19 variant, or the type (mRNA vs non-mRNA) of vaccine used—in amplifying/dampening the effect of vaccinations; and (iv) examining the health spillover effects of COVID-19 cases in neighboring countries. The goal of this paper is to provide an early broad overview of these issues and help inform policy making, and while a starting point for looking at the empirical evidence, more rigorous and in-depth analysis of many of these topics is left for future research.
Methods
Data
Our empirical analysis relies on the assembly of a comprehensive country-level database of daily data on vaccinations (first and second doses) per capita, confirmed COVID-19 infections, deaths, intensive care unit (ICU) admissions of COVID-19 patients, nonpharmaceutical interventions (henceforth known as containment measures), procurements of vaccines, vaccine acceptance proxies, vaccine production, and various metrics of health infrastructure and mobility indices for a broad range of countries, spanning from 16 December 2020 to 20 June 2021. Appendix Table A.2 provides further details on the data, including key descriptive statistics.
COVID-19-Related Variables
COVID-19 cases and fatalities. Daily data on COVID-19 cases and fatalities are collected from the COVID-19 Data Repository by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University.2 Coverage begins from 22 January 2020 for 208 countries.
COVID-19 vaccines and ICU admissions are sourced from the Our World in Data COVID-19 repository.3 Vaccination data are disaggregated by first and second shots, with data covering up to 202 countries starting in December 2020. Data starts from 1 January 2020 covering 23 countries for intensive care admissions.
COVID-19 variants. We collect data from CoVariants, which provides a weekly overview of 19 SARS-CoV-2 variants for 85 countries starting in the last week of April 2020.4 The dataset reports the share of a particular variant amongst all samples sequenced in a country for a given week.
Vaccine type. Data on administered vaccine are available for 14 brands (two of which are mRNA, Pfizer and Moderna) in 156 countries from Airfinity.5 We construct a variable that captures the share of mRNA vaccines as of 20 June 2021.
Vaccine production location. Airfinity provides data on vaccine production location for 15 countries starting on 19 November 2020.6 We use this to create a dummy variable if a country is a producer of a COVID-19 vaccine.
Government Responses
Vaccine procurement deals. We use data on vaccine procurement from the Duke Global Health Innovation Center.7 The daily data include confirmed doses (deals that have been signed and finalized) and potential doses (deals that are under negotiation or additional doses for existing deals), covering 102 countries starting on 1 May 2020. We also use procurement and supply data from Airfinity to check the robustness of our results.8
Stringency of containment measures. For containment measure indices, we use data from Oxford’s COVID-19 Government Response Tracker (OxCGRT).9 OxCGRT collects information on government policy responses across eight dimensions, namely: (i) school closures; (ii) workplace closures; (iii) public event cancellations; (iv) gathering restrictions; (v) public transportation closures; (vi) stay-at-home orders; (vii) restrictions on internal movement; and (viii) international travel bans. The database scores the stringency of each measure ordinally, for example, depending on whether the measure is a recommendation or a requirement and whether it is targeted or nationwide. We normalize each measure to range between 0 and 1 to make them comparable. In addition, we compute and aggregate a Stringency Index as the average of the sub-indices, again normalized to range between 0 and 1. The data start on 1 January 2020 and cover 151 countries/regions.
Mobility Indicators
Retail mobility. Data on retail and recreation mobility are collected from Google Mobility Reports.10 The reports provide daily data by country and highlight the percentage change in visits to places related to retail and recreation activity (e.g., restaurants, cafes, shopping centers, movie theaters, museums, and libraries). The data are reported as the change relative to a baseline value for that corresponding day of the week, the baseline is calculated as the median value for that corresponding day of the week, during the 5-week period between 3 January and 6 February 2020. Daily data are available for 135 countries in our dataset, with coverage beginning from 15 February 2020.
Public Opinion Proxies
Vaccine acceptance. Data on vaccine acceptance is collected from The University of Maryland Social Data Science Center Global COVID-19 Trends and Impact Survey based on a representative sample of Facebook users who are invited to report on topics including, for example, symptoms, social distancing behavior, and vaccine acceptance. Over 200,000 daily responses are collected. Weights are assigned to reduce nonresponse and coverage bias.11 Our sample covers 100 countries starting on 21 December 2020.
Attitude towards authorities. We capture the attitude of the population towards authorities, and by extension, towards the vaccination campaigns by using proxies for trust in government available form the World Economic Forum (WEF) and political stability from the World Bank.
Country-Specific Characteristics
Health infrastructure. We use two sources to measure a country’s health preparedness and competitiveness. Firstly, Health Index data from the 2019 Global Competitiveness Report of the WEF are obtained, which measures the overall health status of the population and the health infrastructure for 137 countries. Secondly, we collect data from the 2019 Global Health Security (GHS) Index, which is the first comprehensive assessment and benchmarking of health security and related capabilities across 195 countries.12 The GHS Index seeks to illuminate preparedness and capacity gaps to increase both political will and financing to fill them at the national and international levels. In addition, to measure countries’ health infrastructure, we use hospital beds and physicians per 1,000 people at the country level from the World Bank DataBank.13
Geographical distances between each country and the rest of the world’s capitals are obtained from the CEPII GeoDist Database, which incorporates country-specific geographical variables for 225 countries in the world.14
Methodology
This section lays out the methodology used to assess: (i) the determinants of vaccine rollouts; (ii) the impact of vaccines on health outcomes; (iii) the heterogeneity in the impact of vaccines depending on country conditions, COVID-19 variant, and type of vaccine; and (iv) the adverse health spillovers from increased infections in neighbors.
Determinants of Vaccine Rollouts
We exploit cross-sectional variation in vaccination rollout across countries to assess the role of demand and supply side factors. The cross-sectional setting allows us to explore the association between vaccine rollout and time invariant factors, as well as factors captured at a particular point of time—for example, the scale of the pandemic before the vaccination rollout that may have affected the attitude of authorities and the population towards vaccines; or procurement of vaccines early in the year, which affected supply later during the rollout phase. We begin by using univariate regressions to look at how total vaccinations to date are correlated with various factors such as vaccine procurement in January 2021, severity of the pandemic in the country, etc. Using the results from these univariate regressions, we select the most significant indicators from each category and try to explain how much of the overall heterogeneity in vaccine rollout is explained by these factors considered together. Specifically, we estimate the following equation:
| 1 |
where i is an index for country, is the level of vaccination as a share of population as of 20 June 2021 (or the average daily vaccinations since the start of the vaccination campaign), is the number of doses procured or being negotiated as a share of populations of January 2021, denotes a dummy variable that takes value 1 if the country is a producer of vaccines, indexes the country’s overall health status captured by the WEF index. measures the magnitude of the COVID-19 pandemic in the country in question at the end of 2020 as the share of cases per 100,000 population. Lastly, captures the attitude of the population towards vaccination at the start of the campaign in January 2021. Equation (1) is estimated with OLS with robust standard errors to account for heteroskedasticity.15 Kernel density plots suggest that the errors are normally distributed, which is confirmed by formal normality tests such as the Shapiro-Wilk W test.
Baseline Effect of Vaccinations on Health Outcomes
For the analysis of the health impact of vaccinations, we move to a country-time panel dataset at the daily frequency that allows for high-frequency identification of the impact of vaccinations on health outcomes. Establishing causality is difficult because vaccine rollout may depend on the current and expected evolution of the virus. We try to mitigate reverse causality by allowing for several lags in the response of new COVID-19 cases/deaths or the reproduction rate to vaccines, and by also controlling for lags in the change of the number of infected cases (deaths and ICU cases). We also control for country fixed effects, which at daily frequency effectively controls for slow moving factors such as vaccine procurement as well as structural factors (such as health capacity) affecting the speed of vaccine rollout, which remained invariant during the short window under consideration. To further account for expectations about the country-specific evolution of the pandemic, we also control for a set of variables that may affect future infections such as mobility, non-pharmaceutical interventions (NPIs)—including containment measures, enhanced testing, contact tracing, and public information campaigns aimed at increasing social awareness—and country-specific time trends.16 Finally, we also include time fixed effects to account for global factors affecting the evolution of the virus (such as new variants) and vaccination (supply disruptions). In particular, the following specification is estimated, with standard errors clustered at the country level:
| 2 |
where alternatively denotes: the cumulative number of COVID-19 cases or deaths as a share of the population, the number of COVID-19 ICU patients as a share of the population or a share of cases (lagged by 21 days), and the COVID-19 reproduction rate—the expected number of secondary cases generated by an index patient—of a country i at time t.17 The reproduction rate is estimated using the number of new infections per currently infected individual, multiplied by the duration of illness (see [12]).18 denotes the share of the individuals in the population who have received at least one vaccine shot. and are country and time fixed effects. X is a vector of control variables that includes the lagged level of cases as well as the stringency of containment measures index and mobility indices at lag t-l, as well as country-specific time trends. denotes the lags in the response of new COVID-19 cases/deaths or the reproduction rate to vaccines depending on specification. We follow the literature on vaccinations [7, 11], 13, 10] and opt for 21-day lags as a baseline to allow for delays in the development of immunity but examine various lags as a robustness check subsequently. For deaths, we use a longer lag structure of 42 days to account for the delay with which infections turn into fatalities.
Role of Country-Specific Conditions in Vaccine Effectiveness
Next, we test the role of country-specific conditions in shaping the effects of vaccinations. In particular, we explore whether the impact of vaccines on health outcomes depends on factors such as the stringency of containment measures, the severity of the outbreak itself, the variant of COVID-19 in circulation, or the type of vaccine used. We start off with linear interactions (or a dummy) to assess the role of different country specific factors. In particular, we estimate:
| 3 |
where denotes alternatively stringency of containment measures or the level of new COVID-19 cases in a country, share of Delta variant in the country, or the share of mRNA vaccines. Equation (3) imposes that the effect of vaccines on cases varies linearly with the interacting variable I. We relax this assumption using two alternative specifications. First, we use the smooth transition autoregressive model developed by [14] to directly test whether the effect of vaccinations varies across different country-specific “regimes.” This allows the effect of vaccines to vary smoothly across regimes by considering a continuum of states, thus making the functions more stable and precise. Specifically, we estimate:
| 4 |
where z is a country-specific characteristic normalized to have zero mean and a unit variance. The weights assigned to each regime vary between 0 and 1 according to the weighting function , so that can be interpreted as the probability of being in a given regime. The coefficients and capture the impact of vaccinations in cases of very low levels of z ( when z goes to minus infinity) and very high levels of z ( when z goes to plus infinity), respectively.
Second, we use a semi-parametric approach in which we interact vaccinations per capita with quartiles (“bins”) of country-specific conditions. This approach allows us to flexibly explore variation in vaccine effectiveness across the distribution of country conditions. We augment equation (2) with the following:
| 5 |
where , , and are dummy variables that denote alternatively quartiles of the stringency of containment measures, the level of new COVID-19 cases in a country, the share of Delta variant in all COVID-19 variants in circulation, or the share of mRNA vaccine. Quartiles are interacted with the percentage of the population that has received at least one dose of the vaccine. Interaction terms are also lagged 21 days, consistent with the vaccine variable. If the coefficients on the interaction terms of higher quartiles differ from those at lower quartiles, it signifies that the effectiveness of vaccines depends on country-specific conditions.
Effect of COVID-19 Cases in Neighboring Countries on Local Health Outcomes
We further test whether the pandemic outbreak in neighboring countries can affect (or worsen) a country’s own COVID-19 caseload. To investigate whether this may be the case, we examine empirically the effect of a country’s “neighboring” COVID-19 cases on its own pandemic evolution. Namely, we create the following:
| 6 |
where is a spillover term for COVID-19 cases in neighboring countries. are bilateral distance weights constructed between country i and country j based on the inverse of the distance between the ten closest foreign capital cities and country i's own capital city, and where =1. refer to country j’s own COVID-19 infections as a share of population at time t. Then, the spillover term captures COVID-19 cases in any given country’s closest ten foreign countries and capital cities. This term is introduced to equation (2) as the following:
| 7 |
All equations are estimated using OLS, with standard errors clustered at the country level.
We employ an Instrumental Variable (IV) approach to address endogeneity that may arise from uncontrolled factors that affect domestic and neighboring cases. For this purpose, we consider the share of the population that has been vaccinated in the ten closest neighboring countries, based on distance between capital cities as an instrument. The basic identifying assumption is that vaccination levels in foreign countries are strongly correlated with new COVID-19 cases in the corresponding foreign country but are not correlated with daily shocks affecting domestic COVID-19 cases or the evolution of the pandemic locally, after accounting for local vaccination. In particular, our IV strategy reads as follows:
| 8 |
with
| 9 |
where denotes the share of population vaccinated in neighboring countries 28 days before—the 21-day gap is thus kept consistent with our baseline results given that neighbor cases are lagged 7 days, so that the peak impact of vaccinations materializes after 21 days.
Results
Factors Affecting Vaccine Rollouts
We begin by exploring the factors that are associated with a faster pace of vaccine rollout in a given country. We use cross-country data for this analysis, focusing on both supply and demand aspects that may affect the speed of vaccination and rollouts. From the supply side, notwithstanding the recent increase in production, the overall supply of vaccines has remained scarce. Hence, we focus on the timing and size of vaccine procurement (determined by the procurement deals made by countries with producers in 2020 and 2021) as well as a dummy variable to capture whether the majority of vaccine is produced domestically or imported from abroad. We also look at various metrics that capture the health infrastructure of the country, which determines the country’s ability to roll out vaccines quickly and efficiently. From the demand side, we look at factors such as how badly the country was affected by the pandemic—capturing the urgency on the part of both governments and the general public on getting vaccinated; and the attitude of the population towards getting vaccinated.
Figure 2 shows that there was considerable variation in the pace of vaccine procurement. In general, the USA and the EU were faster in procuring vaccines, putting in orders even before the vaccines were approved and fully tested. This allowed them to capture the initial supply of vaccines as they became available at the end of 2020 and the early part of 2021. Lower-income countries in general were not able to procure vaccines as quickly while others were more conservative with regard to early negotiations with potential (not approved) vaccine producers (Appendix Fig. A.2). The latter was particularly true in countries that had the pandemic under control in the last quarter of 2020 (such as several Asian economies).
Fig. 2.
Vaccine procurements per region (orders including potential orders, per 100 population). The chart includes confirmed vaccine orders, potential procurement deals and donations. AFR Sub-Saharan Africa, APD Asia Pacific Department, EUR European Department, MCD Middle East and Central Asia Department, WHD Western Hemisphere Department.
Source: Duke University Heath Innovation Center and IMF Staff calculations
Table 1 reports results for univariate regressions of vaccine rollout on various factors (columns 1 through 5) as well as the multivariable regression as described in Eq. 1 in columns 6 and 7. Given endogeneity issues with this kind of an analysis, the focus here is on associations and not causation. We find that each factor has the expected sign and is statistically significant in the univariate regressions. Notably, the multivariable regressions based on the four factors are able to explain almost 60% of the observed cross-country variation in vaccination rollout.
Table 1.
Cross-sectional regression of vaccine rollout
| Variables | (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) |
|---|---|---|---|---|---|---|---|---|
| Share of vaccinated population (1 dose) | Share of vaccinated population (1 dose) | Share of vaccinated population (1 dose) | Share of vaccinated population (1 dose) | Share of vaccinated population (1 dose) | Share of vaccinated population (1 dose) | Share of vaccinated population (1 dose) | Share of vaccinated population (1 dose) | |
| Potential procurement (Jan 2021) | 0.0690*** | 0.0480*** | 0.0534*** | |||||
| (0.0159) | (0.0151) | (0.0161) | ||||||
| Domestic production | 20.61*** | 7.435* | 8.897** | |||||
| (3.075) | (3.770) | (3.702) | ||||||
| Cumulative cases (end-2020) | 5.717*** | 5.401*** | 4.293*** | 4.617*** | ||||
| (0.807) | (0.977) | (1.047) | (1.081) | |||||
| Health index (WEF) | 14.07*** | 8.760*** | 9.535*** | 7.360*** | ||||
| (1.433) | (2.347) | (2.378) | (2.164) | |||||
| Vaccine acceptance (Jan 2021) | 52.45*** | 29.80*** | 31.98*** | 25.99** | ||||
| (11.86) | (11.19) | (10.88) | (10.74) | |||||
| Constant | 22.55*** | 20.23*** | 14.15*** | − 62.97*** | − 11.35 | − 64.32*** | − 68.67*** | − 54.17*** |
| (1.703) | (1.795) | (1.777) | (8.453) | (8.003) | (14.29) | (13.92) | (13.05) | |
| Observations | 202 | 202 | 196 | 133 | 95 | 85 | 85 | 85 |
| R-squared | 0.042 | 0.122 | 0.251 | 0.355 | 0.135 | 0.570 | 0.549 | 0.602 |
Table reports results for Eq. (1). The dependent variable is the share of population that is vaccinated with at least one dose. Robust standard errors
***, **, and * represent statistical significance at 1%, 5%, and 10%, respectively
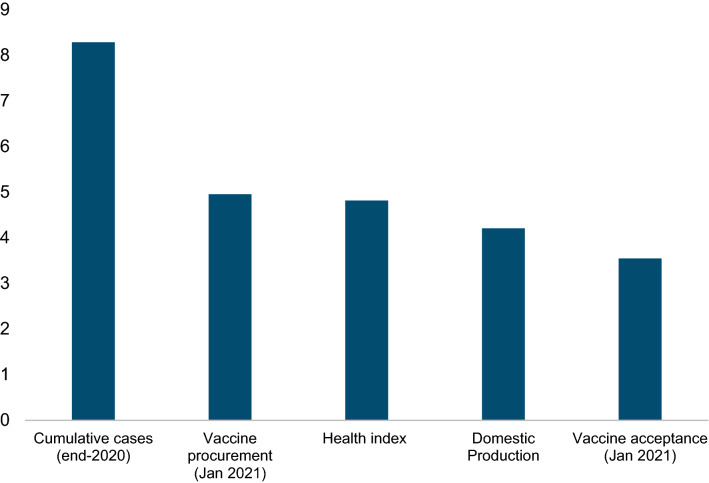
Figure 3 summarizes the results from column 8 of Table 1, showing the association between a one standard deviation change in different factors and vaccine rollout. From the supply side, it confirms that early procurement is significantly correlated with the pace of subsequent vaccination rollout. A one standard deviation increase in procurement (confirmed orders plus potential deals) in January 2021 (corresponding to the difference between procurement for Israel, which secured supply quickly, versus Germany, where negotiations were more protracted) is associated with around a 5 percentage-point higher vaccination rate at the end of June. Domestic production of vaccines is also associated with higher and faster vaccinations (see Table 1), probably reflecting the ability of producing countries to secure a larger share of vaccine and administer them faster (because of shorter delivery time).19 In particular, we find that on average, producer countries have vaccinated around 41% of their populations by 20 June 2021 relative to 20% for non-producers. Also of importance are countries’ health infrastructure (Appendix Fig. A.3, bottom right panel), with a one standard deviation higher health infrastructure score based on the health infrastructure index constructed by the World Economic Forum associated with a 6% increase in vaccinations. Note that one standard deviation higher health infrastructure roughly corresponds to the gap between an average Asian country and an average country in Africa.
Fig. 3.
Factors affecting vaccine rollouts (vaccinations per 100 population, impact of 1 standard deviation change in factor). The figure reports the impact of one standard deviation change in different factors on the share of population that is vaccinated with at least one dose based on estimates using Eq. (1)
Turning to demand side factors, the largest correlation with the pace of vaccinations was for the severity of the pandemic in a given country during the first COVID-19 wave. There was wide variation in how badly countries were affected during the first wave, with countries in Europe and America affected more than countries in Asia (Appendix Fig. A.3, top left panel), and this influenced how quickly countries vaccinated their population in the first half of 2021. On average, a one standard deviation increase in the number of confirmed COVID-19 cases per capita in 2020 is associated with an 8 percentage-point increase in vaccinations till June 2021. The willingness of the population to accept the vaccine also varied (Appendix Fig. A.3, top right panel) and was significantly related to the difference in vaccination rollout—a one standard deviation change in vaccine acceptance (difference between Denmark, the country in our sample with the highest vaccine acceptance in January 2021, and Australia) was associated with a 3.5% increase in vaccinations. Similar results are obtained for other factors such as trust in government or political stability (Appendix Fig. A.3, bottom left panel), which capture the attitude of the population towards authorities, and by extension, towards the vaccination campaigns (see Appendix Table A.5, columns 6 and 7).
The results presented above are robust to alternate specifications. In particular, the results hold for alternate measures of procurement (e.g., confirmed orders vs. potential orders), procurement at different times (in October 2020, latest available data), and different data sources (Airfinity instead of Duke University)—see Appendix Table A.4, columns 1–5. Results also hold for alternative measures of health infrastructure, such as the Global Health Security Index or alternative measures such has doctors per capita or hospital beds per capita (Appendix Table A.4, columns 6–8). On the demand side, results are robust to alternate measures of COVID-19 impact—latest COVID-19 caseload (as of 20 June 2021), average number of daily confirmed cases in 2020, size of peak daily cases in 2020, and measures based on COVID-19 deaths as opposed to cases (Appendix Table A.4, columns 1–4). Finally, the dependent variable used for this analysis is the number of vaccinations per capita in June 2021. All the results are similar with alternative measures, for example, the average number of daily vaccinations in 2021 (Appendix Table A.5, column 5).
Effects of Vaccinations on Health Outcomes
Baseline
We start by examining the effect of increased vaccine coverage on new COVID-19 cases and deaths, the reproduction rate, and COVID-19-related ICU hospitalizations using Eq. (2). Our results suggest that vaccinations have a large and statistically significant effect on confirmed COVID-19 cases. Under our baseline specification (Table 2, column 1), a 20 percentage-point increase in the number of daily vaccinations per 100 population results in about a 0.02 decline in the daily COVID-19 cases per 100 population after 21 days, which is equivalent to around one standard deviation of daily COVID-19 cases in our sample.20 This is statistically significant since we measure COVID-19 cases at the daily frequency, hence the measured decline in cases adds up over time. A similar result holds for the reproduction rate of the virus as well as COVID-19-related deaths and ICU hospitalizations as a share of population related to COVID-19.21 ICU hospitalization rates also decline, indicating that fewer confirmed cases translate into serious illness as vaccination rates increase. The second dose of the vaccine further reduces the number of daily COVID-19 cases, but the impact is statistically significant only in the case of reproduction rate.
Table 2.
Baseline results on the impact of vaccination on health outcomes
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | |
|---|---|---|---|---|---|---|---|---|---|
| Cases/pop | Cases/pop | R value | R value | Deaths/pop | Deaths/pop | ICU/pop | ICU/pop | ICU/cases | |
| First vaccine dose/population | − 0.000986*** | − 0.000898*** | − 0.013707*** | − 0.010507*** | − 0.000008* | − 0.000009* | − 0.000127*** | − 0.000122** | − 0.007801*** |
| (0.000) | (0.000) | (0.004) | (0.004) | (0.000) | (0.000) | (0.000) | (0.000) | (0.002) | |
| Second vaccine dose/population | − 0.000222 | − 0.007932** | 0.000003 | − 0.000015 | |||||
| (0.000) | (0.004) | (0.000) | (0.000) | ||||||
| Containment measures | − 0.009603 | − 0.010365 | − 0.505722*** | − 0.543493*** | − 0.000214* | − 0.000208* | 0.001605 | 0.001454 | |
| (0.008) | (0.008) | (0.165) | (0.164) | (0.000) | (0.000) | (0.002) | (0.002) | ||
| Mobility | 0.000100** | 0.000103*** | 0.002664** | 0.002756** | 0.000002** | 0.000002** | 0.000020 | 0.000020 | |
| (0.000) | (0.000) | (0.001) | (0.001) | (0.000) | (0.000) | (0.000) | (0.000) | ||
| Lagged cases/pop | 0.001610 | 0.001816 | 0.002710*** | 0.002742*** | |||||
| (0.003) | (0.003) | (0.001) | (0.001) | ||||||
| Lagged reproduction rate | − 1.059113*** | − 1.057343*** | |||||||
| (0.018) | (0.018) | ||||||||
| Lagged deaths/pop | 0.003925 | 0.003964 | |||||||
| (0.004) | (0.004) | ||||||||
| Lagged ICU/Cases | 0.000475 | ||||||||
| (0.008) | |||||||||
| Constant | − 1.973306 | − 1.318626 | 49.398938** | 67.91842*** | 0.002448 | 0.002879 | − 0.058064 | − 0.045988 | − 4.735857 |
| (1.544) | (1.768) | (23.416) | (22.226) | (0.014) | (0.014) | (0.211) | (0.233) | (4.652) | |
| Observations | 13,542 | 13,455 | 13,468 | 13,385 | 11,122 | 11,096 | 3,258 | 3,257 | 3100 |
| R-squared | 0.624 | 0.625 | 0.537 | 0.535 | 0.720 | 0.720 | 0.834 | 0.834 | 0.633 |
| Lags 1st dose/2nd dose | 21 | 21/7 | 21 | 21/7 | 42 | 42/28 | 21 | 21/7 | 21 |
| Health policy controls | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Country FE | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Time FE | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| No. of countries | 126 | 126 | 125 | 125 | 123 | 123 | 22 | 22 | 23 |
Table reports results for equation (2). The dependent variable is new COVID-19 cases, reproduction rate, COVID-19 deaths, and ICU admissions due to COVID-19 as a share of population. The regressions control for stringency of containment measures, other non-pharmaceutical interventions and health policy controls, mobility, lagged cases, deaths or reproduction rate, country specific time trends, as well as country and time fixed effects. First vaccine and control variables are lagged by 42 days for deaths (columns 5 and 6) and 21 days for all other columns. Standard errors are clustered at the country level
***, **, and * represent statistical significance at 1%, 5%, and 10%, respectively
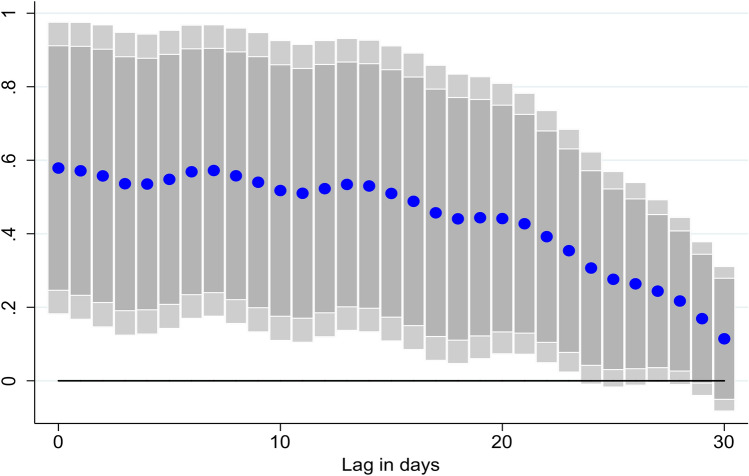
While our baseline specification measures the impact of vaccinations with a lag of 21 days, further exploration of the lag structure of the results suggests that the impact increases over time, peaking at around 2–3 weeks after vaccination (Fig. 4).
Fig. 4.
Effect of vaccinations on new COVID-19 cases, at different lags. Coefficient β is reported for each lag ℓ (1–30), and based on for a sample of 126 countries using daily data from December 20, 2020–June 16, 2021. where denotes: the number of cumulative COVID-19 cases. . denotes the share of the individuals in the population which have received at least one vaccine shot. and are country and time fixed effects. X is a vector of control variables that includes the lagged level of cumulative cases, the stringency of containment measures index, and mobility indices. ℓ denotes the lags in the response of new COVID-19 cases. Lightly shaded bars denote 95% confidence bands, and dark-shared bars denote 90% confidence bands
The results are robust to different subsamples. Appendix Table A.6 summarizes the robustness results: (1) the results hold when the data is winsorized to ensure that the results are not driven by outliers; (2) our results also hold if we drop countries that started vaccinating late—started their vaccination campaigns after 1 March—such as Colombia and Vietnam; (3) the results are also robust to dropping countries that started vaccinations very early such as the USA and Israel—already reached 5% of the population by 1 February; (4) the results are not driven by a particular region as our results go through if we drop one region at a time from the analysis, though the impact of vaccination is not statistically significant if we drop European countries as the sample size shrinks significantly. Finally, the results are not driven by a particular country and all our results hold if we drop countries with high levels of vaccinations (such as the USA, the UK, or Israel).22
Role of Containment Measures, the Severity of the Outbreak, Variants, and Type of Vaccine
We extend our baseline specification to assess the role of factors such as the stringency of containment measures and the severity of the outbreak in shaping the impact of vaccines on health outcomes using Eqs. (3–5).
Stringency of containment measures. Columns 1–3 of Table 3 extend our baseline regression for COVID-19 cases by adding an interaction term between the share of population that has been vaccinated with one dose with the stringency of containment measures. The interaction terms are negative, suggesting that an increase in vaccines reduces new COVID-19 cases more when they are complemented with more stringent containment measures. The results from the simple interaction are not statistically significant (column 1, Eq. 3), but the smooth transition (column 2, Eq. 4) and quartiles (column 3, Eq. 5) are significant, with the absolute value of the coefficient for the third and fourth quartiles being larger than the second quartile. This suggests that the effect of containment measure in shaping the effectiveness of vaccines is not linear and it becomes significant at higher levels of containment. In particular, we find that at higher levels of stringency, the efficacy of vaccines in reducing cases is about 50% higher than at lower levels of stringency. This indicates complementarity between vaccines and containment measures, with the two policy tools reinforcing each other in containing outbreaks.
Table 3.
Role of stringency measures and pandemic severity on vaccination outcomes
| (1) | (2) | (3) | (4) | (5) | (6) | |
|---|---|---|---|---|---|---|
| Cases | Cases | Cases | Cases | Cases | Cases | |
| Vaccine first dose | − 0.000216 | − 0.000623* | − 0.000443** | − 0.000024 | ||
| (0.001) | (0.000) | (0.000) | (0.000) | |||
| Interaction with containment measures | ||||||
| Containment measures * Vaccine first dose | − 0.000982 | |||||
| (0.001) | ||||||
| Low containment measures * Vaccine first dose | 0.000053 | |||||
| (0.000) | ||||||
| High containment measures * Vaccine first dose | − 0.001504*** | |||||
| (0.000) | ||||||
| 2nd Quartile of containment measures * Vaccine first dose | − 0.000292* | |||||
| (0.000) | ||||||
| 3rd Quartile of containment measures * Vaccine first dose | − 0.000350** | |||||
| (0.000) | ||||||
| 4th Quartile of containment measures * Vaccine first dose | − 0.000354* | |||||
| (0.000) | ||||||
| Interaction with new cases | ||||||
| New cases * Vaccine first dose | − 0.012338** | |||||
| (0.005) | ||||||
| Low new cases * Vaccine first dose | − 0.000892*** | |||||
| (0.000) | ||||||
| High new cases * Vaccine first dose | − 0.000885** | |||||
| (0.000) | ||||||
| 2nd Quartile of new cases * Vaccine first dose | − 0.000271** | |||||
| (0.000) | ||||||
| 3rd Quartile of new cases * Vaccine first dose | − 0.000586*** | |||||
| (0.000) | ||||||
| 4th Quartile of new cases * Vaccine first dose | − 0.000801*** | |||||
| Observations | 13,455 | 13,455 | 13,455 | 13,455 | 13,455 | 13,455 |
| R-squared | 0.625 | 0.627 | 0.628 | 0.637 | 0.624 | 0.637 |
| Country FE | Yes | Yes | Yes | Yes | Yes | Yes |
| Time FE | Yes | Yes | Yes | Yes | Yes | Yes |
| No. of countries | 126 | 126 | 126 | 126 | 126 | 126 |
| P-value F-test | 0.00574 | 0.987 |
The dependent variable is new COVID-19 cases as a share of population. The% of population that has received 1 vaccine dose is interacted with the stringency of containment measures (columns 1–3) and the level of new cases (moving average over 7 days, columns 4–6). Columns 1 and 4 allow for the simple interaction (Eq. 3). Columns 2 and 5 uses a smooth transition function (Eq. 4), while columns 3 and 6 categorize the interaction variables into 4 quartiles (Eq. 5). The vaccine variable as well as the interaction terms are lagged 21 days. All regressions control for stringency of containment measures and other non-pharmaceutical interventions (21 lags), the% of population that has received two doses (7 lags), mobility (21 lags), country specific time trends, as well as country and time fixed effects. Standard errors are clustered at the country level
***p < 0.01, **p < 0.05, *p < 0.1
Severity of the outbreak. The impact of vaccines on COVID cases is also likely to depend on the stage of the outbreak. If a country is in the middle of a significant outbreak, an increase in vaccine rollout is likely to lead to a bigger decline in new cases. To test this hypothesis, columns 4–6 of Table 3 add an interaction term between the share of population that has been vaccinated with one dose with the number of new cases (smoothed by a moving average over 7 days) in the country. Column 4 uses simple interaction, column 5 smooth transition, and in column 6 the number of new cases is categorized into quartiles. The interaction terms are negative and significant (the absolute value of the coefficient increasing for each higher quartile), once again indicating that an increase in vaccines reduce new cases by more when initial cases were high to begin with.23 Given the larger health gains in countries with severe outbreaks, and conversely the diminishing returns to vaccine rollout in countries with limited COVID cases, this highlights the scope for countries to share their vaccine supply with other countries once they reach a high level of vaccination.24
Variants and type of vaccine. The spread of new variants of COVID-19, in particular the Delta variant that has spread rapidly since Spring 2021, has raised concerns that existing vaccines may not be as effective against new variants. While data are still emerging, we find early evidence consistent with epidemiological studies that suggests that a larger share of the Delta variant makes vaccines less effective. Table 4 presents our regression results where we interact vaccine first dose with the share of Delta variant in the total number of samples sequenced. Column 1 allows for the simple interaction between share of population vaccinated with the first dose with the share of Delta variant detected (Eq. 3). The interaction term is positive and significant, indicating that a higher share of Delta decreases the impact of vaccines on COVID-19 cases. Column 2 uses a smooth transition function (Eq. 4) and shows that while vaccines remain effective in both cases (low and high share of Delta variant), the effectiveness is reduced by half when the Delta variant is dominant. The results for different quartiles of the share of the Delta variant (Eq. 5) are not statistically significant (column 3), but point in the same direction and are likely to improve as more data become available, allowing us to estimate the effects more precisely. We get similar results when using the vaccine second dose.
Table 4.
Role of variants on vaccination outcomes
| (1) | (2) | (3) | |
|---|---|---|---|
| Cases | Cases | Cases | |
| Variables | Simple interaction | Smooth transition | Quartiles |
| Vaccine first dose per capita | − 0.000790*** | − 0.000936*** | |
| (0.000) | (0.000) | ||
| Interaction with Delta variant | |||
| Delta share * Vaccine first dose | 0.000707*** | ||
| (0.000) | |||
| Low share of Delta * Vaccine first dose | − 0.001331*** | ||
| (0.000) | |||
| High share of Delta * Vaccine first dose | − 0.000584* | ||
| (0.000) | |||
| 2nd quartile of Delta * Vaccine first dose | − 0.000044 | ||
| (0.000) | |||
| 3rd quartile of Delta * Vaccine first dose | 0.000126 | ||
| (0.000) | |||
| 4th quartile of Delta * Vaccine first dose | 0.000333 | ||
| (0.000) | |||
| Observations | 8,485 | 8,485 | 8,485 |
| R-squared | 0.591 | 0.588 | 0.590 |
| Health policy controls | Yes | Yes | Yes |
| Country FE | Yes | Yes | Yes |
| Time FE | Yes | Yes | Yes |
| No. of countries | 75 | 75 | 75 |
The dependent variable is new COVID-19 cases as a share of population. The percent of population that has received one vaccine dose is interacted with the share of Delta variant in the total number of samples sequenced. Column 1 allows for the simple interaction (Eq. 3) between share of population vaccinated with the first dose with the share of Delta variant detected. Column 2 uses a smooth transition function (Eq. 4), while column 3 categorizes the share of Delta variant into 4 quartiles (Eq. 5). The vaccine variable is lagged 21 days. All regressions control for stringency of containment measures and other non-pharmaceutical interventions (21 lags), mobility (21 lags), country-specific time trends, as well as country and time fixed effects. Standard errors are clustered at the country level
***p < 0.01, **p < 0.05, *p < 0.1
A related question is about the efficacy of different types of COVID-19 vaccines. While the medical-scientific literature is best placed to answer this question, tentative results based on the share of mRNA vaccines relative to non-mRNA vaccine presented in Table 5 suggest that mRNA vaccines may be more effective. Although consistent with recent epidemiological studies (see [15]), this result needs to be interpreted with caution given data limitations—our data on mRNA vaccines is static and captures a snapshot on 20 June 2021, which may not capture the timing of when the different vaccines became available; and the majority of early vaccine adopters (advanced countries in North America and Europe) used mRNA vaccines, and this may bias the results against finding an effect for non-mRNA vaccines.25
Table 5.
Role of type of vaccine on vaccination outcomes
| Variables | (1) | (2) | (3) | (4) |
|---|---|---|---|---|
| Cases | Cases | Cases | Cases | |
| Dummy interaction | Simple interaction | Smooth transition | Quartiles | |
| Vaccine first dose per capita | − 0.000404 | − 0.000320 | 0.000129 | |
| (0.000) | (0.000) | (0.000) | ||
| Interaction with share of mRNA vaccine | ||||
| mRNA share * Vaccine first dose | − 0.000745* | |||
| (0.000) | ||||
| mRNA share * Vaccine first dose | − 0.000942* | |||
| (0.000) | ||||
| Low share of mRNA * Vaccine first dose | 0.000415 | |||
| (0.000) | ||||
| High share of mRNA * Vaccine first dose | − 0.001431*** | |||
| (0.000) | ||||
| 3rd quartile of mRNA * Vaccine first dose | − 0.000588 | |||
| (0.001) | ||||
| 4th quartile of mRNA * Vaccine first dose | − 0.001310** | |||
| (0.001) | ||||
| Observations | 13,542 | 13,239 | 13,239 | 13,239 |
| R-squared | 0.625 | 0.625 | 0.625 | 0.625 |
| Health policy controls | Yes | Yes | Yes | Yes |
| Country FE | Yes | Yes | Yes | Yes |
| Time FE | Yes | Yes | Yes | Yes |
| No. of countries | 126 | 122 | 122 | 122 |
The dependent variable is new COVID-19 cases as a share of population. The percent of population that has received one vaccine dose is interacted with the share of mRNA vaccines to total vaccines as of 20 June 2021. Column 1 uses a dummy variable (Eq. 3), which takes the value 1 if the share of mRNA vaccines is greater than 50%, 0 otherwise. Column 2 allows for the simple interaction (Eq. 3) between share of population vaccinated with the first dose with the share of mRNA vaccines. Column 3 uses a smooth transition function (Eq. 4), while column 4 categorizes the share of mRNA vaccines into quartiles (Eq. 5). Given the uneven distribution, only the 3rd and 4th quartiles are included, with all countries that do not use mRNA vaccines comprising of the residual omitted group. The vaccine variable is lagged 21 days. All regressions control for stringency of containment measures and other non-pharmaceutical interventions (21 lags), mobility (21 lags), country-specific time trends, as well as country and time fixed effects. Standard errors are clustered at the country level
***p < 0.01, **p < 0.05, *p < 0.1
Evidence of Pandemic Spillovers from Neighboring Countries
The analysis has thus far provided evidence on the importance of vaccines in controlling the COVID-19 pandemic, lowering infections and fatalities, and reducing the reproduction rate. However, while it may be that a country quickly and efficiently vaccinates its population while putting in place stringent containment measures, there may be countering effects that are not related to a country’s own policies, but that nonetheless may diminish the progress that a country makes in controlling its local outbreak. Indeed, progress in vaccinations may be hampered by “spillovers” of COVID-19 cases from other countries, namely those that are closer in proximity, share borders, or neighbor each other. This in turn can lengthen the duration of the pandemic and worsen its health outcomes. To investigate whether this may be the case, we use Eqs. (6) and (7) to empirically assess the effect of a country’s neighboring COVID-19 cases on its own pandemic evolution.
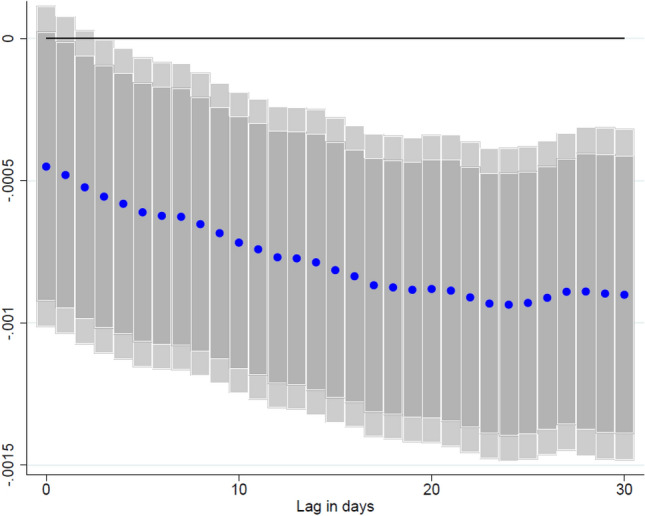
The results, reported in Table 6, provide evidence that pandemics in a country’s neighbors can derail efforts to reduce COVID-19 infections domestically. Namely, the results show that a one percentage-point increase in the neighboring COVID-19 caseload as a share of the population is likely to “spill over” to close-by countries, where domestic COVID-19 infections as a share of the population will increase by 0.5 percentage point after 7 days. This effect is persistent across our analysis’ time horizon and at different lags (Fig. 5), though it diminishes in magnitude over time. For additional robustness, we also create spillover terms using alternative sets of weights. In particular, we first broaden our specification to create bilateral distance weights to all capital cities worldwide, so that we capture a country’s linkages with all other countries.26 Second, we also create bilateral trade weights based on a country i’s imports and exports exposure to country j to factor in the economic relationship in addition to proximity. The results, reported in Appendix Table A.7, are robust to these alternative specifications, and show a higher magnitude of spillovers from a neighboring country’s COVID-19 caseload.
Table 6.
Effect of neighboring new COVID-19 cases on domestic new COVID-19 cases
| OLS | OLS | IV | IV | |
|---|---|---|---|---|
| (1) | (2) | (3) | (4) | |
| New COVID-19 Cases | New COVID-19 Cases | New COVID-19 Cases | New COVID-19 Cases | |
| Vaccinated persons, 1 dose | − 0.000973*** | − 0.000866*** | − 0.000325 | − 0.000183 |
| (0.000) | (0.000) | (0.000) | (0.000) | |
| Vaccinated persons, 2 doses | − 0.000285 | − 0.000199 | ||
| (0.000) | (0.000) | |||
| Neighbor cases (7 days lag) | 0.566195*** | 0.572086*** | 0.638675* | 0.680740** |
| (0.204) | (0.203) | (0.364) | (0.325) | |
| Containment measures index (lag) | − 0.010388 | − 0.011402 | − 0.001193 | − 0.001202 |
| (0.008) | (0.008) | (0.002) | (0.003) | |
| COVID-19 cases (lag) | − 0.000113 | 0.000155 | 0.000579 | − 0.000085 |
| (0.003) | (0.003) | (0.006) | (0.005) | |
| Mobility (lag) | 0.000098*** | 0.000103*** | 0.000018 | 0.000022 |
| (0.000) | (0.000) | (0.000) | (0.000) | |
| Observations | 13,241 | 13,154 | 13,241 | 13,154 |
| R-squared | 0.639 | 0.640 | 0.136 | 0.138 |
| Health Policy Controls | Yes | Yes | Yes | Yes |
| Country FE | Yes | Yes | Yes | Yes |
| Time FE | Yes | Yes | Yes | Yes |
| Kleibergen-Paap rk Wald F statistic | 45.025 | 11.175 | ||
| No. of countries | 123 | 123 | 123 | 123 |
| Vaccination 1 Lags | 21 days | 21 days | 21 days | 21 days |
| Vaccination 2 Lags | 7 days | 7 days | 7 days | 7 days |
Table reports results for Eq. (7). The dependent variable is new COVID-19 cases. A spillover term “Neighbor cases” (lag 7 days) is introduced to the equation to capture the effects of neighboring COVID-19 new cases on a country’s own caseload using bilateral distance weights (Eq. 6). The regressions control for stringency of containment measures, other non-pharmaceutical interventions and health policy controls (21 lags), lags of mobility (21 lags), lagged new cases, country-specific time trends, as well as country and time fixed-effects. Standard errors are clustered at the country level
***, **, and * represent statistical significance at 1%, 5%, and 10%, respectively
Fig. 5.
Effect of neighboring new COVID-19 cases on domestic COVID-19 cases at different lags. Coefficient is reported for each lag ℓ (1-30), and based on for a sample of 123 countries using daily data from December 20, 2020–June 16, 2021. where denotes: the number of cumulative COVID-19 cases. is a spillover term for COVID-19 cases in neighboring countries. denotes the share of the individuals in the population which have received at least one vaccine shot. and are country and time fixed effects. X is a vector of control variables that includes the lagged level of cumulative cases, the stringency of containment measures index, and mobility indices. ℓ denotes the lags in the response of new COVID-19 cases. Lightly shaded bars denote 95% confidence bands, and dark-shared bars denote 90% confidence bands
Large negative health spillovers from neighboring countries, in conjunction with the result from the previous sub-section that vaccines provide larger health gains in countries with severe outbreaks, thus provide a compelling rationale for vaccine sharing (especially with countries facing high COVID cases). Vaccinating early and broadly, not only a country’s own population but also all other countries’ populations, can then limit COVID-19 spillovers into an own nation and bring a swifter end to the pandemic abroad. These results are consistent with [1], who stress the importance of vaccinating a large share of the global population as quickly as possible, noting that “the pandemic is not over anywhere unless it is over everywhere.”
Our results are not driven by reverse causality. The first stage estimates of Eqs. (8) and (9) shown in Table 6 suggest that the instrument is statistically significant: The Kleibergen-Paap rk Wald F statistic, equivalent to the F-effective statistic for non-homoscedastic error in case of one endogenous variable and one instrument [16]—is 45 (column 3) and 11.75 (column 4), respectively, in the first case well above the associated Stock-Yogo critical values for non-spheric disturbances. The IV results are reported in Table 6 columns (3) and (4), and are consistent with the baseline OLS results shown in columns (1) and (2).The results also indicate that neighboring COVID-19 cases can significantly lead to an increase in the number of domestic COVID-19 cases 7 days later, though the magnitude of the IV coefficient is slightly larger than that of the OLS estimates.27
Discussion
This paper provides an early empirical overview of the determinants of vaccine rollouts in a cross-country setting, as well as the impact of COVID-19 vaccinations on health outcomes. We use a novel daily database on vaccine rollouts, new COVID-19 cases and deaths, the COVID-19 reproduction rate, COVID-19 ICU admissions, as well as data on non-pharmaceutical interventions and mobility. The daily database is combined with data on vaccine procurements, production, vaccine acceptance, and health infrastructure. To the best of our knowledge, this is the first attempt to empirically assess the effects of COVID-19 vaccines on such a large scale (with a country sample of 126 countries), while also examining the role of country-specific conditions, and the impact of the pandemic in neighboring countries. The goal of this paper is to provide a timely initial overview of a broad set of empirical relationship to guide policy, with more rigorous and detailed analysis of each of these points left for future in-depth research as more data becomes available.
The results on the factors affecting vaccine rollout suggest that from the supply side, early procurement, domestic production of vaccines, and countries’ health infrastructure are important factors associated with the speed of rollout in a given country. Meanwhile, looking at demand-side factors, the largest correlation between the pace of vaccinations was with the severity of the pandemic in a given country during the first COVID-19 wave, while the willingness of the population to accept the vaccine also contributed to a more rapid pace of vaccination in a country.
Turning to the effects of COVID-19 vaccines on health outcomes, we find that vaccinations have a large and statistically significant effect on new COVID-19 cases, deaths, and ICU admissions as a share of population, and the reproduction rate of the virus. Vaccinations also reduce the number of ICU patients per infected person, thereby enhancing the health system’s resilience to cope with the spread of the virus and potentially reducing the need for very strict and broad-based containment measures. Meanwhile, the second dose of the vaccine further reduces the number of daily COVID-19 cases, but the impact is statistically significant only in the case of the reproduction rate. These results are robust to alternative specifications and samples. Our results are also in line with findings of the epidemiological literature, where the protection from vaccine builds up over time. The more immediate effect of vaccinations (statistically significant impact after 2–3 days) may be explained by behavioral factors—people who were waiting to get vaccinated were taking greater precautions before, practicing more social distancing, and reducing their mobility in anticipation of developing COVID-19 immunity soon [17]. These results, consistent with [18], show that that the stringency of containment measures also has a significant and negative impact on the spread of COVID-19, while higher mobility is associated with worse health outcomes.
In addition, we find that the effect of COVID-19 vaccines varies depending on country-specific conditions, such as the level of stringency measures imposed in a country during the vaccine rollout, as well as the severity of the pandemic outbreak in a country. Specifically, the results provide evidence that COVID-19 vaccines are more effective in reducing new COVID-19 infections when complimented with stringent containment measures. Similarly, we find that the impact of vaccines on COVID cases varies depending on the stage of the outbreak, with an increase in vaccine rollouts being more likely to lead to a bigger decline in new cases if a country is in the middle of a significant outbreak. This suggests that vaccines should be channeled where possible to countries facing more acute outbreaks. Finally, while the data are still emerging, we find early evidence consistent with epidemiological studies that suggests that the presence of more infectious variations of COVID-19, such as the Delta variant, makes vaccines less effective, while vaccinations using mRNA vaccines have a greater marginal impact relative to their non-mRNA counterparts.
The results also provide evidence on the importance of controlling the pandemic not only locally, but also globally (see [1]). We find that spillovers from COVID-19 cases in neighboring countries are significant and lead to an increase in an own country’s caseload, therefore hampering efforts in taming its own local outbreak despite vaccinations and containment measures. In conjunction with the result that vaccines provide larger health gains in countries with severe outbreaks (or conversely there are diminishing returns to vaccine rollout in countries with limited COVID cases), this highlights the potential gains from vaccine sharing. Vaccinating early and broadly not only a country’s own population but also all other countries’ populations, especially those with large outbreaks, can thus limit COVID-19 spillovers into an own nation, minimize the loss of lives, and bring a swifter end to the pandemic abroad.28
The findings in this paper, combined with results from [19] on the beneficial effects of vaccines on economic outcomes, highlight the importance of vaccines to address the crisis instigated by the COVID-19 pandemic (see also [20]). In addition to the direct health and economic benefits of vaccines, this paper finds evidence for the role of containment measures in complementing COVID-19 vaccines, and the importance of vaccine-sharing to limit pandemic spillovers. By providing an early broad overview of the quantitative empirical estimates of the determinants of vaccine rollouts and the effects of COVID-19 vaccines, our paper can help policymakers make informed decisions about local and global distributions of vaccines, as well as related policy tools, such as containment measures.
While we have put together a novel and comprehensive dataset, it is important to note some limitations of the data. We use data from the early part of vaccination rollout, when vaccine penetration was low and supply constrained. Such limitation is relevant for assessing the impact of vaccine hesitancy and other non-linearities. The quality of the data also varies across countries, including due to countries showing uneven testing, and due to measurement errors in containment measures (especially the degree of enforcement of mask mandates and others), which could be a source of error, although we do control for country fixed effects in all our analysis.
Supplementary Information
Below is the link to the electronic supplementary material.
Authors’ Contributions
Each author contributed equally to this work.
Declarations
Funding
None.
Conflicts of interest/competing interests
None.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication (from patients/participants)
Not applicable.
Availability of data and material
Data are available upon request to the authors.
Code availability
Code is available upon request to the authors.
Footnotes
When examining demand and supply side factors of vaccine rollouts at the cross-sectional level we use a sample of 202 countries. For evaluating health outcomes, our panel data set is composed of 126 countries, due to limited data availability. In Appendix Table A.1 we detail the countries used in the two analyses.
https://www.airfinity.com/. All EU countries are considered to be producers as these countries have a vaccine-sharing arrangement.
As of June 2021, many countries were still in the early phases of their vaccination drive or had not begun vaccinations at all. Hence the cross-sectional data on vaccination as a share of population is not uniformly distributed, with a large mass around zero. To take this into account, we prefer to use robust standard errors for our baseline. However, the results remain unaffected by this choice.
It can be argued that controlling for NPIs may bias the results downwards if NPIs are affected by vaccinations. While we are primarily interested in the partial effect of vaccinations after controlling for NPIs, our results continue to hold if we exclude NPIs as controls.
We do not include other control variables when ICU patients as a share of lagged cases is the dependent variable, as containment measures, mobility, and other controls are only expected to impact the absolute level of health outcomes, not the share of cases requiring ICU admission.
The effective reproduction rate can be approximated based on the number of new infections per currently infected individual, multiplied by the duration of illness. Actual new infections on any day are not directly observable, but an unbiased estimator can be obtained by using lags of actual new infections, with the number of lags corresponding to the estimated incubation period of COVID-19, adjusted for delays between the onset of symptoms and testing and recording of a case. For the baseline, we use 7 days of lag, but the results are similar with 10, 14, and 21 days and are available upon request.
Cross-country analysis suggests that domestic production is significantly and positively associated with greater vaccine procurement. In addition, on average, procurement is higher for domestic producers relative to countries relying on the import of vaccines. Finally, controlling for the amount of vaccine, vaccine producer countries had higher rollouts.
Excess variability of COVID-19 cases could be a concern. To address this issue, we repeated the analysis by filtering the series alternatively using the Hodrick-Prescott filter [21] and the Hamilton filter [22]. The results reported in Appendix Table A.3 confirm our baseline findings.
The reproduction rate in the baseline is estimated using 7 days of lag. This represents the average duration of illness during which the index patient infects others. We get similar results with 10, 14, and 21 days.
Estimated coefficient when dropping one country at a time remains statistically significant and ranges from − 0.00107 to − 0.00071 (compared with the estimated coefficient of -0.000986 for the full sample). Results available upon request.
The results reported in columns 1 and 2 of Table 3 are of course related, in the sense that higher stringency in containment measures may be a response to higher COVID-19 cases. If we include the interaction terms of the quartiles for stringency as well as new cases together in the same regression, the coefficient signs remain the same though the stringency interactions become insignificant, indicating that the severity of the outbreak may be the more important factor determining the effect of the vaccine rollout.
We also explored whether vaccination rates impact health outcomes non-linearly by including square and cubic terms of the vaccination to population ratio in the baseline regression. These higher-order terms were insignificant, potentially reflecting the fact that not enough countries have reached high enough vaccination rates to approach herd immunity, in part because the new, more transmissible, variants of the virus may have raised herd immunity thresholds.
To account for the latter, we controlled for the share of vaccination (both linear and non-linear), and the results – not reported – remain robust, albeit weaker. The results – not reported – continue to hold also when controlling for early adopters.
Given that bilateral distance weights are created using the inverse of the distance between two cities, the closer the city, the higher its weight.
Domestic vaccinations are statistically insignificant in the IV regressions because of their high correlation with vaccinations abroad.
As the number of countries with high vaccination rates remain limited at the time of writing, the paper was not able to explore the potential non-linear effects of vaccines on health outcomes. Similarly, an exploration of whether health outcomes are worse in countries with higher levels of vaccine hesitancy require more countries to reach levels of vaccination where hesitancy becomes a binding factor in vaccine rollouts. Exploring such effects could be an interesting avenue for future research. If returns to vaccine were to diminish after a certain point, then this would add another rationale for sharing vaccine doses more equitably across countries.
The views expressed in this paper are those of the author(s) and do not necessarily represent the views of the IMF, its Executive Board, or IMF management. The authors would like to thank the editor Tim Wrightson and two anonymous referees for their helpful comments.
Contributor Information
Pragyan Deb, Email: pdeb@imf.org.
Davide Furceri, Email: dfurceri@imf.org.
Daniel Jimenez, Email: djimenez@imf.org.
Siddharth Kothari, Email: skothari@imf.org.
Jonathan D. Ostry, Email: jdo58@georgetown.edu
Nour Tawk, Email: ntawk@imf.org.
References
- 1.Agarwal R, Gopinath MG. A proposal to end the COVID-19 pandemic. Int Monet Fund. 2021.
- 2.Castillo JC, Ahuja A, Athey S, Baker A, Budish E, Chipty T, Glennerster R, Kominers SD, Kremer M, Larson G, Lee J.. Market design to accelerate COVID-19 vaccine supply. Science. 2021:1107–1109. [DOI] [PubMed]
- 3.De Figueiredo A, Simas C, Karafillakis E, Paterson P, Larson HJ. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study. Lancet. 2020: 898–908. [DOI] [PMC free article] [PubMed]
- 4.Malik AA, McFadden SM, Elharake J, Omer SB. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine. 2020: 100495. [DOI] [PMC free article] [PubMed]
- 5.Goel RK, Nelson MA. Drivers of COVID-19 vaccinations: vaccine delivery and delivery efficiency in the United States. NETNOMICS Econ Res Electron Netw. 2021: 53–69.
- 6.Khan H, Dabla-Norris ME, Lima F, Sollaci A. Who doesn’t want to be vaccinated? Determinants of vaccine hesitancy during COVID-19. Int Monet Fund. 2021.
- 7.Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, Hernán MA, Lipsitch M, Reis B, Balicer RD. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021. [DOI] [PMC free article] [PubMed]
- 8.Hall VJ, Foulkes S, Saei A, Andrews N, Oguti B, Charlett A, Wellington E, Stowe J, Gillson N, Atti A, Islam J. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021: 1725–1735. [DOI] [PMC free article] [PubMed]
- 9.Bernal JL, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, Simmons R, Cottrell S, Roberts R, O’Doherty M, Brown K. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. Br Med J. 2021. [DOI] [PMC free article] [PubMed]
- 10.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S. C4591001 Clinical trial group: safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2021. [DOI] [PMC free article] [PubMed]
- 11.Ganslmeier M, Deb P, Furceri D, Ostry J, Tawk N. Vaccinate early and vaccinate broadly: on the health and economic effects of COVID-19 vaccines. Res Sq. 2021.
- 12.Xu R, Rahmandad H, Gupta M, DiGennaro C, Ghaffarzadegan N, Amini H, Jalali MS. The modest impact of weather and air pollution on COVID-19 transmission. MedRXiv. 2020. [DOI] [PMC free article] [PubMed]
- 13.Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, Kovyrshina AV, Lubenets NL, Grousova DM, Erokhova AS, Botikov AG. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021: 671–681. [DOI] [PMC free article] [PubMed]
- 14.Granger CW, Terasvirta T. Modelling non-linear economic relationships. OUP Catalogue. 1993.
- 15.Olliaro P, Torreele E, Vaillant M. COVID-19 vaccine efficacy and effectiveness—the elephant (not) in the room. Lancet Microb. 2021: 279–280. [DOI] [PMC free article] [PubMed]
- 16.Andrews I, Stock JH, Sun L. Weak instruments in instrumental variables regression: theory and practice. Annu Rev Econ. 2019.
- 17.Engler, P., Pouokam, N., Rodriguez Guzman, D. and Yakadina, I.V., 2020. The Great Lockdown: International Risk Sharing Through Trade and Policy Coordination. Available at SSRN 3758075. https://scholar.google.com/scholar? hl=en&as_sdt=0%2C9&q=The+Great+Lockdown%3A+International+Risk+Sharing+Through+Trade+and++Policy+Coordination&btnG=
- 18.Deb P, Furceri D, Ostry JD, Tawk N. The effect of containment measures on the COVID-19 pandemic. CEPR Discussion Papers. 2020.
- 19.Deb P, Furceri D, Jimenez D, Kothari S, Ostry JD, Tawk N. The effects of COVID-19 vaccines on economic activity. Swiss J Econ Stat. 2022: 1–25. [DOI] [PMC free article] [PubMed]
- 20.International Monetary Fund. Leveraging opportunities from COVID-19 vaccines: early lessons from Asia. Asia and Pacific Regional Economic Outlook. 2021.
- 21.Hodrick RJ, Prescott EC. Postwar US business cycles: an empirical investigation. J Money Credit Bank. 1997: 1–16
- 22.Hamilton JD. Why you should never use the Hodrick-Prescott filter. Rev Econ Stat. 2018;100:831–843. doi: 10.1162/rest_a_00706. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.