Abstract
Background
Adiposity is consistently positively associated with postmenopausal breast cancer and inversely associated with premenopausal breast cancer risk, though the reasons for this difference remain unclear.
Methods
In this nested case–control study of 1649 breast cancer cases and 1649 matched controls from the Nurses’ Health Study (NHS) and the NHSII, we selected lipid and polar metabolites correlated with BMI, waist circumference, weight change since age 18, or derived fat mass, and developed a metabolomic score for each measure using LASSO regression. Logistic regression was used to investigate the association between this score and breast cancer risk, adjusted for risk factors and stratified by menopausal status at blood draw and diagnosis.
Results
Metabolite scores developed among only premenopausal or postmenopausal women were highly correlated with scores developed in all women (r = 0.93–0.96). Higher metabolomic adiposity scores were generally inversely related to breast cancer risk among premenopausal women. Among postmenopausal women, significant positive trends with risk were observed (e.g., metabolomic waist circumference score OR Q4 vs. Q1 = 1.47, 95% CI = 1.03–2.08, P-trend = 0.01).
Conclusions
Though the same metabolites represented adiposity in pre- and postmenopausal women, breast cancer risk associations differed suggesting that metabolic dysregulation may have a differential association with pre- vs. postmenopausal breast cancer.
Subject terms: Risk factors, Predictive markers, Metabolomics
Introduction
Body mass index (BMI) is a recognised risk factor for postmenopausal breast cancer, with higher adiposity associated with increased risk [1–4]. However, evidence consistently shows higher BMI is associated with a decreased risk of premenopausal breast cancer [3–5], though the reasons for these conflicting relationships remain unclear. There are several different ways to examine adiposity among women. Measures of waist circumference and weight change in adulthood have been used to capture adiposity [6, 7]; in addition, fat mass, while difficult to collect directly, may represent adiposity in adulthood better than BMI alone [8]. It is also possible that none of these measures adequately provides insight into one’s underlying metabolic health, which may be a more robust overall indicator of risk.
To better understand the relationship between adiposity and breast cancer we need to explore the biological mechanisms that potentially underlie the associations. Metabolomic analyses allow us to evaluate the contribution of metabolites, small molecules that are breakdown products within blood or urine, to various phenotypes. Several studies have identified metabolites that are associated with adiposity measures, such as BMI or waist circumference [9–13]. While one has explored the association between BMI-related metabolites and postmenopausal breast cancer [10], none to date have used metabolomic data to develop adiposity scores reflective of metabolic health. Here, we derived a metabolomic score for different adiposity measures and examined the association between this score and breast cancer risk, by menopausal status. We seek to add knowledge about the metabolic profiles of these adiposity measures, determine if metabolomic scores can be used to assess risk, and to explore how metabolic state is associated with the development of breast cancer and whether these associations differ by menopausal status.
Methods
Study population
Participants included women in the Nurses’ Health Study (NHS) and NHSII who were part of breast cancer nested case–control study. Both the NHS and the NHSII are long-running prospective cohorts, started in 1976 and 1989, respectively. Case–control participants included 1186 NHS women who provided a blood sample (collected 2000–2002), and 2117 NHSII women who provided a blood sample (collected 1996–1999) and had metabolites profiled. Cases were women with a breast cancer diagnosis after blood collection and before 2010 (NHS) or 2011 (NHSII); controls were matched to cases by menopausal status at diagnosis (pre/post/unknown), and age (±1 year), date (±1 month), time of day (±2 h), fasting status (≥8 h since a meal vs. <8 h or unknown), menopausal status and hormone use (premenopausal, postmenopausal, hormone user, postmenopausal, non-user, unknown) at blood draw. Risk-factor information is collected in both cohorts via biennial questionnaires. Breast cancer cases were identified by self-report and confirmed by medical record review. Deaths were captured by next of kin, postal service, or review of the National Death Index.
Metabolomic profiles
Metabolites were profiled by Dr. Clary Clish’s lab at the Broad Institute of MIT and Harvard (Cambridge, MA) using liquid chromatography-tandem mass spectrometry (LC/MS-MS) platforms designed to measure polar metabolites and lipids [14–17]. Matched case–control pairs were distributed randomly within batches. Pooled reference samples were included every 20 samples and blinded quality control samples were also randomly distributed. Measures were standardised using the ratio of the value of the sample to the value of the nearest pooled reference multiplied by the median of all reference values for the metabolite. Metabolites impacted by delayed sample processing were removed (ICC and Spearman rho <0.75 comparing immediately vs. within 24–48-h post collection, N = 37 in NHS, N = 65 in NHSII). After this step, there were 321 metabolites measured in NHS and 382 metabolites measured in NHSII [17]. For the primary analysis, metabolites were excluded if the blinded quality control samples CV was ≥25% (NHS N = 47, NHSII N = 46), though all metabolites were included in a sensitivity analysis. Metabolites with 0-<10% missingness (N = 21 in NHS, N = 321 in NHSII) were imputed with ½ the minimum value; metabolites with ≥10% missingness were excluded (N = 2 in NHS, N = 15 in NHSII). Finally, all metabolites that were included in both NHS and NHSII (N = 263) were assessed in analyses.
Exposure and covariate measurement
Adiposity measures assessed included BMI (kg/m2), waist circumference (cm), weight change since age 18 (kg), and derived fat mass. BMI and weight change were assessed at blood draw. Waist circumference was assessed in 2000 for NHS and in 1993 for NHSII. Fat mass was derived from measures of age, weight, height, waist circumference and race, through the National Health and Nutrition Examination Survey (NHANES)-developed equation for women which strongly predicts dual-energy X-ray absorptiometry (DXA)-measured fat mass (R2 = 0.90) [8].
Additional covariates included standard breast cancer risk factors, selected at the time of blood draw from corresponding questionnaires or prior reports for non-time-varying covariates: age at menarche, age at first birth and parity combined (nulliparous, 1–2 kids & <25 y, 1–2 kids & 25 + y, 3+ kids & <25 y, 3+kids & 25 + y), breastfeeding history (yes/no), history of benign breast disease (yes/no), family history of breast cancer (yes/no), BMI at age 18 (kg/m2), alcohol consumption (g/day) at blood draw, and physical activity level (MET-h/week) at blood draw. We additionally adjusted for type of hormone use at blood draw (oestrogen alone, oestrogen + progesterone, other) among postmenopausal women and oral contraceptive use at blood draw for premenopausal women. Less than 2% of the sample was missing values for BMI or for alcohol intake; where this occurred, values were imputed with median values.
Statistical methods
Derivation of metabolomics score for adiposity measure
A metabolomics-based score was derived for each adiposity measure separately. First, all individuals missing the adiposity measure of interest were removed from the analysis. BMI analyses further excluded extreme outliers: women with BMI > 60 or <15 kg/m2. Spearman correlations between probit-transformed metabolites and the adiposity measure, adjusted for case–control status and age at blood draw, were calculated. Metabolites with a correlation ≥ |0.15| with the adiposity measure, and a Benjamini Hochberg FDR P value <0.05 were selected to be carried forward in model development [18]. LASSO regression with tenfold cross-validation to select the optimal tuning parameter, lambda, was used to select metabolites for inclusion in each model, resulting in a metabolomic adiposity score with LASSO-penalised coefficients.
Sensitivity analyses were also conducted by limiting derivation of the metabolomic equation to controls-only and comparing selected metabolites with those derived using both cases and controls. In addition, the derivation of metabolomic scores was done among women premenopausal at blood draw and postmenopausal at blood draw separately and compared with the results using all women.
Association of adiposity measures (self-reported/derived and metabolomic scores) with breast cancer
Unconditional logistic regression with adjustment for breast cancer risk factors and matching factors were used to assess the association between quartiles of self-reported adiposity measures and metabolomic adiposity scores and breast cancer risk, with quartile cutpoints determined among the full population. For comparison, a sensitivity analysis tested the use of quartile cutpoints based on controls only and defined by menopausal status at diagnosis. For breast cancer subtypes (ER+/PR+, ER−/PR−), tertiles were used due to the smaller sample size. All analyses were stratified by menopausal status at blood draw, and secondarily by menopausal status at diagnosis. Trends for quartile analyses were calculated by modelling the median of each quartile as a continuous variable. P values <0.05 (two-sided) were considered statistically significant. The risk of oestrogen receptor (ER) positive and negative breast cancer were assessed separately, using relevant ER subtype cases and all controls. We formally tested whether the association between self-reported adiposity and breast cancer risk is independent of metabolomic score using the mediation package in R [19]. We evaluated if ORs for self-reported adiposity measures changed comparing the model of self-reported adiposity measures on breast cancer risk alone to the model of self-reported adiposity measures on breast cancer risk, including metabolomic scores. We also tested whether the association between metabolomic adiposity scores and breast cancer was statistically significantly different by BMI group (defined dichotomously as <25 kg/m2 vs. >=25 kg/m2) using the likelihood ratio test.
Results
Descriptive characteristics
The analysis cohort included 1649 cases and 1649 controls. Descriptive characteristics are included in Table 1. The average age at blood draw was 52.3 y (SD = 11.7) for cases and 52.6 y (SD = 11.7) for controls. Most participants were fasting at blood draw (75% of cases, 81% of controls). About half of the participants (51%) were premenopausal at blood draw, and 27% were premenopausal at diagnosis. Missingness varied by adiposity measures, with <2% for BMI, and <4% for weight change since age 18. However, approximately one-third of the participants were missing waist circumference and predicted fat mass measures. Women missing these measures were more likely to be in NHS2 and premenopausal at blood draw but otherwise were similar to women not missing these measurements.
Table 1.
Descriptive characteristics of participants in the Nurses’ Health Studya.
| Characteristic | Case | Control |
|---|---|---|
| N participants | 1649 | 1649 |
| NHS2 | 1057 (64%) | 1057 (64%) |
| NHS | 592 (56%) | 592 (56%) |
| Age at blood draw (mean (SD)) | 52.32 (11.73) | 52.57 (11.72) |
| Fasting at blood draw (N (%)) | 1241 (75%) | 1337 (81%) |
| Menopausal status and PMH use at blood draw (N (%)) | ||
| Premenopausal | 829 (50.3%) | 826 (50.1%) |
| Postmenopausal—no hormone use | 223 (13.5%) | 231 (14.0%) |
| Postmenopausal—yes hormone use | 547 (33.2%) | 542 (32.9%) |
| Unknown | 50 (3.0%) | 50 (3.0%) |
| OC use (N (%))b | ||
| No | 8 (0.5%) | 7 (0.4%) |
| Yes | 670 (40.6%) | 672 (40.8%) |
| Unknown | 151 (9.2%) | 147 (8.9%) |
| Type of PMH use (among users) (N (%)) | ||
| Oestrogen alone | 200 (24.4%) | 240 (29.2%) |
| Oestrogen + Progesterone | 223 (27.2%) | 174 (21.1%) |
| Progesterone alone/other | 124 (15.1%) | 128 (15.6%) |
| Age at diagnosis (mean (SD)) | 59.0 (10.6) | NA |
| Menopausal status at diagnosis (N (%)) | ||
| Premenopausal | 452 (27.4%) | 452 (27.4%) |
| Postmenopausal | 1069 (64.8%) | 1080 (65.5%) |
| Unknown | 128 (7.8%) | 117 (7.1%) |
| Age at menarche, years (mean (SD)) | 12.46 (1.35) | 12.52 (1.42) |
| Nulliparous (N (%)) | 274 (16.6%) | 230 (13.9%) |
| Parity, children (mean (SD))c | 2.56 (1.16) | 2.69 (1.34) |
| Age at first birth, years (mean (SD))c | 25.90 (4.09) | 25.52 (4.11) |
| Breastfeeding history (N (%))c | 1066 (64.6%) | 1068 (64.8%) |
| History of benign breast disease (N (%)) | 617 (37.4%) | 511 (31.0%) |
| Family history of breast cancer (N (%)) | 319 (19.3%) | 201 (12.2%) |
| BMI at age 18 in kg/m2 (mean (SD)) | 20.84 (2.79) | 21.20 (3.03) |
| Alcohol consumption at blood draw, g/day (mean (SD)) | 4.85 (7.90) | 4.19 (6.51) |
| Activity level at blood draw, MET-hours/week (mean (SD)) | 20.73 (28.23) | 20.04 (22.81) |
| BMI, kg/m2 (mean (SD)) | ||
| Premenopausal | 24.8 (5.2) | 25.5 (6.1) |
| Postmenopausal | 26.5 (5.0) | 26.3 (5.5) |
| Missing (N (%)) | 26 (1.6%) | 14 (0.8%) |
| Weight change since age 18, kg (mean (SD)) | 12.96 (12.61) | 13.02 (13.21) |
| Missing (N (%)) | 57 (3.5%) | 47 (2.9%) |
| Waist circumference, cm (mean (SD)) | 81.83 (13.21) | 82.10 (13.72) |
| Missing (N (%)) | 537 (32.6%) | 534 (32.4%) |
| Fat Mass (kg) (mean (SD)) | 26.79 (8.87) | 26.86 (8.95) |
| Missing (N (%)) | 578 (35.1%) | 568 (34.4%) |
aAll measures taken at blood draw unless otherwise noted. Blood draw 2000–2002 for NHS, 1996–1999 for NHS2.
bAmong premenopausal women.
cAmong parous women
Metabolomic scores for adiposity measures
Selection of metabolites
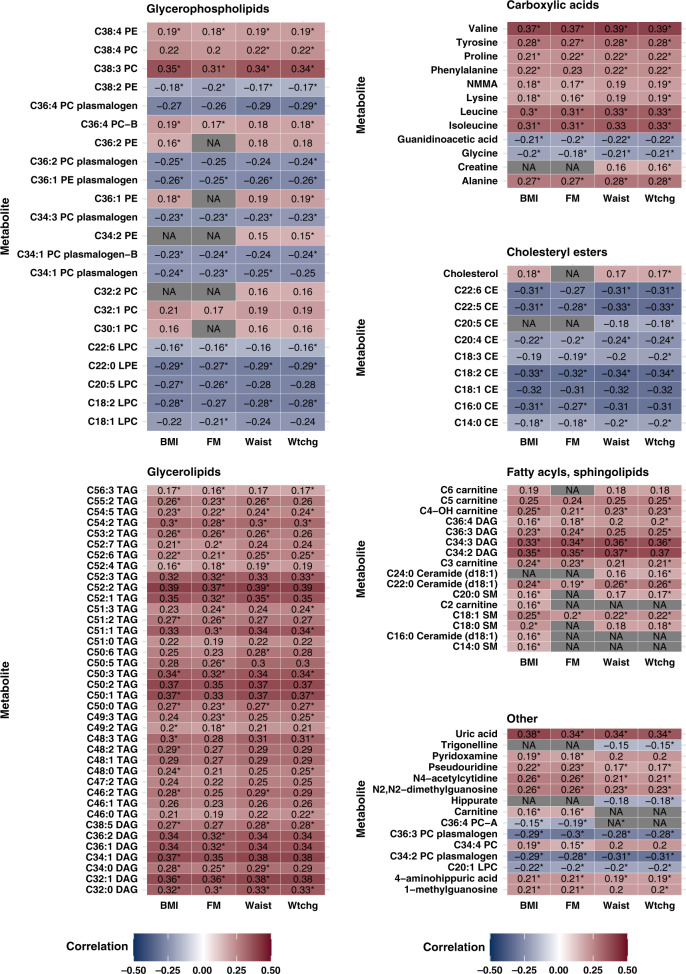
Out of 263 metabolites measured, a large proportion were selected as significantly correlated with each adiposity measure: 106 for BMI (N participants = 3245), 108 for weight change (N = 3194), 84 for waist circumference (N = 2209), and 96 for fat mass (N = 2137) (Fig. 1). Overlaps in selected metabolites for each adiposity measure were substantial. All metabolites with a significant Spearman correlation with waist circumference were also correlated with FM and BMI. All but one metabolite (C36:4 PC-A) correlated with waist circumference were also correlated with weight change. For fat mass-correlated metabolites, all were also significantly correlated with BMI, and all except for carnitine and C36:4 PC-A were significantly correlated with weight change. Only three metabolites selected for BMI did not overlap with other adiposity measures (C2 carnitine, C16:0 Ceramide, C14:0 SM), and seven metabolites were significantly correlated with weight change but no other adiposity measures. Metabolites with the highest correlations with adiposity measures tended to be consistent across all adiposity measures. Among the top ten correlated metabolites for each adiposity measure, overlapping metabolites included several triacylglycerols (TAGs) and diacylglycerols (DAGs) with 0–2 double bonds, such as C52:2 TAG, C50:1 TAG, C50:2 TAG, C32:1 DAG, C34:1 DAG, C34:2 DAG, as well as uric acid and valine. For example, C52:2 TAG was strongly correlated with BMI (r = 0.39), weight change (r = 0.39), waist circumference (r = 0.33), and fat mass (r = 0.37). Directions of correlations for metabolites were consistent across adiposity measures as well; for example, cholesteryl ester C18:2 CE was inversely correlated with all measures.
Fig. 1. Spearman correlations of metabolites with adiposity measures, by class of metabolite.
Correlations adjusted for age and case–control status. BMI body mass index, FM fat mass, Waist waist circumference, Wtchg weight change from age 18. Asterisks (*) represent the metabolites selected in LASSO regression for metabolite adiposity score creation.
LASSO selection of these significantly correlated metabolites resulted in 81 metabolites for BMI, 51 for waist circumference, 73 for fat mass, and 73 for weight change (Supplemental Table 1). Variability in adiposity measures explained by metabolite scores was generally high, as evidenced by R2 values from each equation: BMI = 0.53; weight change = 0.44; fat mass = 0.46; waist circumference = 0.33. The majority of selected metabolites were lipids.
Metabolites with large coefficients for a one-SD change in BMI included the glycerophosphocholines C38:3 PC and C34:3 PC plasmalogen, and sphingolipids C18:1 SM and C14:0 SM (Supplemental Table 1). The top metabolites associated with weight change also included glycerophosphocholine C38:3 PC, sphingolipid C18:1 SM, as well as cholesteryl esters C20:4 CE and C20:5 CE. Top metabolites selected for waist circumference were glycerophospholipids C38:3 PC and C34:3 PC plasmalogen, sphingolipid C18:1 SM, and cholesterol ester C20:4 CE. Fat mass metabolomic-score creation relied most heavily on glycerophosphocholines C38:3 PC and C34:3 plasmalogen, sphingolipid C18:1 SM, fatty acyl C34:3 DAG, and cholesterol ester C20:4 CE. Notably, glycerophosphocholines C38:3 PC and C34:3 PC plasmalogen, sphingolipid C18:1, and cholesteryl ester C20:4 CE were strongly predictive of all adiposity measures.
Sensitivity analyses—selection of metabolites in different populations
Metabolites identified as well-correlated with adiposity measures (r ≥ |0.15|) were very similar when examining only the controls compared to all participants. For BMI, 102 of 106 metabolites with correlations ≥|0.15| in the full participant group also had correlations ≥|0.15| among controls only. Six unique metabolites had correlations ≥|0.15| when examining controls only (r = |0.15–0.17|); the correlation coefficients were not materially different when examining contributions for the full participant dataset for these metabolites (r in the full group ranged from 0.12 to <0.15). In addition, metabolomic scores derived among controls only vs. cases and controls were strongly correlated, with Pearson correlations ≥0.94 for metabolic scores for BMI, weight change, waist circumference and fat mass.
Some minor differences were noted in metabolite correlations when examining women's premenopausal and postmenopausal at blood draw separately (Supplemental Table 2). Among participants premenopausal at blood draw, 116 metabolites were correlated with BMI (r > |0.15|); among participants postmenopausal at blood draw, 95 metabolites were correlated with BMI. However, 84 metabolites overlapped between both groups, and had similar correlation strengths with BMI, with the average difference in correlations between pre- and postmenopausal groups equal to 0.05 (range for difference = 0.003–0.15) for overlapping metabolites.
For those metabolites with strong correlations with BMI in women premenopausal at blood draw, but not in women postmenopausal at blood draw, the absolute value of the difference between correlations ranged from 0.03 to 0.27. Those with the largest differences in BMI correlations between groups (>0.15) included sphingolipid C20:0 SM, C14:0 SM, and several glycerophospholipids. For these metabolites, correlations were null among postmenopausal women (e.g.: for C20:0 SM r = 0.25 among premenopausal women vs. −0.02 among postmenopausal women). For metabolites strongly correlated with BMI in postmenopausal women but not in premenopausal women, the absolute value of the difference between correlations ranged from 0.01 to 0.24. Metabolites with differences above |0.15| in correlation coefficients were both glycerophospholipids: C38:6 PC, and C40:10 PC, with correlations of −0.16 and −0.18 among postmenopausal women, and null correlations among premenopausal women. Despite these noted differences, none of the non-overlapping metabolites was significantly associated with breast cancer.
Importantly, the impact of these uniquely selected metabolites to the prediction of BMI was minimal. For example, with respect to BMI, the correlations with derived scores separately by menopausal status at blood draw vs. all women combined were 0.96 for premenopausal and 0.93 for postmenopausal women.
Sensitivity analysis—including metabolites with high CVs
Several metabolites with high CVs (≥25%) were identified as being correlated with adiposity scores, though these metabolites were highly correlated with metabolites identified in the original set with low CVs. For example, C54:1 TAG and C52:0 TAG correlated with BMI (r = 0.27 and r = 0.25); these metabolites are highly correlated with TAGs with few double bonds, several of which were identified in the original correlation analysis. The metabolomic score derived using the larger set of metabolites, including those with CVs ≥25%, was strongly correlated with the score created when excluding these metabolites (e.g., for BMI, r = 0.97). Due to the similar nature of findings, all analyses reported exclude metabolites with high CVs.
Sensitivity analysis—comparing selection algorithms
As an alternative to LASSO regression, we performed stepwise selection with minimisation of Akaike’s information criterion (AIC) to obtain metabolomic scores for adiposity measures. Stepwise selection resulted in a model with fewer metabolites selected for each adiposity measure (e.g., for BMI, stepwise selection chose 50 metabolites as opposed to LASSO’s 81) (Supplemental Table 3). Only two metabolites (pseudouridine, C36:4 DAG) were selected in stepwise but not selected via LASSO. Further, the correlation between the metabolomic score for BMI using stepwise regression vs. LASSO was >0.99. Overall, results did not differ based on the selection algorithm used to produce the metabolomic score. Here we present results for the LASSO-derived models.
Association of metabolomic scores with breast cancer risk
Premenopausal women
Quartile cutpoints were defined in the overall population for consistency, as the metabolomic score was developed in all participants, and are given in Supplemental Table 4. A sensitivity analysis using quartile cutpoints defined by controls only, and separately by menopausal status, yielded similar results. Among women premenopausal at blood draw (N cases for BMI = 826, waist circumference = 508, weight change = 823, FM = 498), self-reported adiposity measures were generally inversely, though non-significantly, associated with breast cancer risk. For example, comparing quartile 4 with quartile 1, the BMI OR = 0.96 (95% CI = 0.68–1.35), and weight change OR = 0.89 (95% CI = 0.66–1.21). Metabolomic scores for all adiposity measures were also inversely, though non-significantly, associated with risk. Odds ratios were similar for women premenopausal at blood draw for lean and non-lean women (lean defined as BMI < 25 kg/m2). Inverse trends remained for ER + breast cancers for both self-reported and metabolomic-score measures, with self-reported fat mass being significantly inversely associated with breast cancer (P-trend = 0.004) though no significant trends were noted for ER- breast cancer (Supplemental Table 5a, b). Although the associations for self-reported measures were generally attenuated with the addition of metabolomic score, there was no evidence that the association between self-reported measure and breast cancer was dependent on the metabolomic score (P > 0.05).
When considering women who were premenopausal at both blood draw and diagnosis, associations with breast cancer were more strongly inverse than among women premenopausal at blood draw only. For example, comparing quartile 4 with quartile 1, the BMI OR = 0.69 (95% CI = 0.43–1.11). However, the inverse trends for this group were not significant, apart from self-reported derived fat mass (P-trend = 0.04) (Table 2). This pattern was also observed in ER + breast cancers (Supplemental Table 5a).
Table 2.
Association between self-reported and metabolomic adiposity scores & breast cancer incidence for women premenopausal at blood draw.a
| Premenopausal at blood draw | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Adiposity measures | Quartile 1b | Quartile 2b | Quartile 3b | Quartile 4b | |||||
| Self-reported | N cases/controls | OR (95% CI) | N cases/controls | OR (95% CI) | N cases/controls | OR (95% CI) | N cases/controls | OR (95% CI) | P-trendc |
| BMI (kg/m2) | 270/262 | 1.0 (ref) | 225/197 | 1.20 (0.92–1.57) | 179/180 | 1.11 (0.83–1.49) | 152/181 | 0.96 (0.68–1.35) | 0.69 |
| Waist circumference (cm) | 134/141 | 1.0 (ref) | 183/152 | 1.29 (0.92–1.79) | 111/124 | 0.91 (0.63–1.32) | 80/82 | 1.06 (0.68–1.66) | 0.83 |
| Weight change (kg) | 220/210 | 1.0 (ref) | 251/253 | 0.91 (0.69–1.19) | 194/191 | 0.95 (0.71–1.26) | 158/169 | 0.89 (0.66–1.21) | 0.54 |
| Fat mass | 176/171 | 1.0 (ref) | 141/108 | 1.33 (0.94–1.87) | 83/107 | 0.78 (0.53–1.14) | 98/99 | 0.97 (0.64–1.47) | 0.04 |
| Metabolomic score | |||||||||
| BMI (kg/m2) | 256/217 | 1.0 (ref) | 227/217 | 0.91 (0.70–1.19) | 176/203 | 0.79 (0.60–1.05) | 167/183 | 0.82 (0.61–1.12) | 0.14 |
| Waist circumference (cm) | 144/132 | 1.0 (ref) | 149/126 | 1.12 (0.79–1.59) | 111/130 | 0.78 (0.54–1.12) | 104/111 | 0.90 (0.62–1.32) | 0.27 |
| Weight change (kg) | 251/233 | 1.0 (ref) | 230/204 | 1.08 (0.83–1.41) | 177/190 | 0.91 (0.69–1.20) | 165/196 | 0.83 (0.62–1.11) | 0.14 |
| Fat mass | 150/123 | 1.0 (ref) | 141/128 | 0.92 (0.65–1.30) | 96/123 | 0.65 (0.45–0.94) | 111/111 | 0.83 (0.57–1.21) | 0.15 |
| Premenopausal at blood draw and at diagnosis | |||||||||
| BMI (kg/m2) | 158/148 | 1.0 (ref) | 119/111 | 1.01 (0.71–1.44) | 98/99 | 0.92 (0.62–1.36) | 73/91 | 0.69 (0.43–1.11) | 0.12 |
| Waist circumference (cm) | 79/79 | 1.0 (ref) | 100/75 | 1.31 (0.83–2.07) | 62/65 | 0.86 (0.52–1.42) | 39/45 | 0.77 (0.42–1.42) | 0.23 |
| Weight change (kg) | 126/122 | 1.0 (ref) | 141/141 | 0.92 (0.64–1.32) | 103/102 | 0.94 (0.64–1.38) | 75/83 | 0.80 (0.53–1.22) | 0.34 |
| Fat mass | 112/85 | 1.0 (ref) | 69/60 | 0.79 (0.49–1.26) | 45/55 | 0.52 (0.31–0.88) | 48/54 | 0.52 (0.29–0.92) | 0.04 |
| Metabolomic score | |||||||||
| BMI (kg/m2) | 146/131 | 1.0 (ref) | 124/121 | 0.94 (0.66–1.35) | 97/108 | 0.81 (0.56–1.18) | 81/89 | 0.78 (0.52–1.19) | 0.19 |
| Waist circumference (cm) | 83/77 | 1.0 (ref) | 85/67 | 1.18 (0.74–1.88) | 62/63 | 0.85 (0.52–1.38) | 50/57 | 0.79 (0.47–1.33) | 0.23 |
| Weight change (kg) | 143/140 | 1.0 (ref) | 124/109 | 1.17 (0.82–1.68) | 91/107 | 0.86 (0.59–1.25) | 87/92 | 0.90 (0.61–1.35) | 0.39 |
| Fat mass | 88/70 | 1.0 (ref) | 78/65 | 0.94 (0.58–1.51) | 54/61 | 0.67 (0.40–1.12) | 54/58 | 0.67 (0.39–1.12) | 0.07 |
aMultivariable logistic regression models adjusted for age at menarche, age at first birth and parity combined, breastfeeding history, history of benign breast disease, family history of breast cancer, BMI at age 18 (kg/m2), alcohol use (g/day) at blood draw, and activity level (MET-h/week) at blood draw, oral contraceptive use at blood draw, and matched factors (age, fasting status, and month of blood draw).
bQuartile values were determined from the full cohort (pre-and postmenopausal at blood draw) for each measure. See Supplemental Table 3 for values.
cP-trend calculated using the median of quartiles (defined by the overall cohort).
Among women with menopausal status at both blood draw and diagnosis known (N = 2560), approximately 23% (N = 582) were premenopausal at blood draw and postmenopausal at diagnosis. Among these women, self-reported and metabolomic-score weight change were associated with inverse risk of breast cancer; this was significant for metabolomic score (Q4 vs. Q1 OR = 0.53, 95% CI = 0.31–0.89, P-trend = 0.02). All ORs for the association between metabolomic score and breast cancer were <1, while self-reported BMI, weight change, and fat mass were positively associated with breast cancer risk (though this was non-significant) (Supplemental Table 6).
Postmenopausal women
For women postmenopausal at blood draw, higher self-reported adiposity measures were associated with higher breast cancer risk, though these relationships were non-significant (Table 3). For example, self-reported BMI was associated with a 17% increase in breast cancer risk (95% CI = 0.84–1.64, P-trend = 0.07), and self-reported weight change was associated with a 34% increase in breast cancer risk (95% CI = 0.97–1.85, P-trend = 0.08). All metabolomic scores for adiposity were positively associated with breast cancer risk, with significant trends observed for BMI (Q4 vs. Q1 OR = 1.33, 95% CI = 0.98–1.82, P-trend = 0.05), waist circumference (Q4 vs. Q1 OR = 1.47, 95% CI = 1.03–2.08, P-trend = 0.01), and fat mass (OR = 1.62, 95% CI = 1.13–2.33, P-trend = 0.01). The association between metabolomic score and breast cancer risk was more positive among women in the non-lean group compared to the lean group, though there was no significant interaction of adiposity metabolomic score and BMI group.
Table 3.
Association between self-reported and metabolomic adiposity scores and breast cancer incidence for women postmenopausal at blood draw and diagnosis.a
| Adiposity measures | Quartile 1b | Quartile 2b | Quartile 3b | Quartile 4b | |||||
|---|---|---|---|---|---|---|---|---|---|
| Self-reported | N cases/controls | OR (95% CI) | N cases/controls | OR (95% CI) | N cases/controls | OR (95% CI) | N cases/controls | OR (95% CI) | P-trendc |
| BMI (kg/m2) | 133/126 | 1.0 (ref) | 169/195 | 0.80 (0.57–1.11) | 212/219 | 0.96 (0.70–1.33) | 228/219 | 1.17 (0.84–1.64) | 0.07 |
| Waist circumference (cm) | 52/57 | 1.0 (ref) | 130/116 | 1.27 (0.80–2.04) | 148/173 | 0.96 (0.61–1.52) | 231/235 | 1.19 (0.76–1.88) | 0.60 |
| Weight change (kg) | 120/149 | 1.0 (ref) | 161/156 | 1.16 (0.82–1.62) | 203/214 | 1.10 (0.80–1.53) | 235/210 | 1.34 (0.97–1.85) | 0.08 |
| Fat mass | 87/84 | 1.0 (ref) | 121/151 | 0.74 (0.50–1.09) | 153/175 | 0.82 (0.56–1.21) | 170/155 | 1.19 (0.80–1.77) | 0.99 |
| Metabolomic score | |||||||||
| BMI (kg/m2) | 147/174 | 1.0 (ref) | 170/178 | 1.15 (0.84–1.57) | 216/198 | 1.33 (0.99–1.81) | 209/209 | 1.33 (0.98–1.82) | 0.05 |
| Waist circumference (cm) | 121/141 | 1.0 (ref) | 120/142 | 0.96 (0.67–1.37) | 149/152 | 1.14 (0.81–1.61) | 171/146 | 1.47 (1.03–2.08) | 0.01 |
| Weight change (kg) | 141/158 | 1.0 (ref) | 165/180 | 0.99 (0.72–1.36) | 211/198 | 1.21 (0.89–1.65) | 202/193 | 1.25 (0.91–1.71) | 0.09 |
| Fat mass | 109/138 | 1.0 (ref) | 119/135 | 1.12 (0.78–1.61) | 146/158 | 1.20 (0.85–1.71) | 157/134 | 1.62 (1.13–2.33) | 0.01 |
aMultivariable logistic regression models adjusted for age at menarche, age at first birth and parity combined, breastfeeding history, history of benign breast disease, family history of breast cancer, BMI at age 18 (kg/m2), alcohol use (g/day) at blood draw, and activity level (MET-h/week) at blood draw, type of hormone use at blood draw, and matched factors (age, fasting status, and month of blood draw)
bQuartile values were determined from the full cohort (pre-and postmenopausal at blood draw) for each measure. See Supplemental Table 3 for values.
cP-trend calculated using the median of quartiles (defined by the overall cohort).
For ER+ cases, self-reported and metabolomic-score measures were more strongly positively associated with breast cancer risk compared to overall cases, and positive associations were particularly strong for metabolomic-score measures (e.g.: BMI OR Q4 vs. Q1 among ER+ cases = 1.98, 95% CI = 1.37–2.88, P-trend < 0.001). In opposition, ORs comparing tertiles of metabolomic scores for adiposity and risk of ER- breast cancer were generally inverse. Metabolomic-score BMI and weight change were significantly inversely associated with ER− breast cancer among women postmenopausal at blood draw (Supplemental Table 5b). Among postmenopausal women, the association between self-reported adiposity and breast cancer was generally not statistically accounted for by metabolomic score. An exception occurred with waist circumference, although in this case, the contribution of metabolomic score to the association remained quite small. Generally, the ORs for the associations of self-reported adiposity measures with breast cancer risk were only slightly attenuated when adding metabolomic score to regressions among postmenopausal women (e.g., BMI OR Q4 vs. Q1 before adjustment for a metabolomic score vs. after adjustment = 1.17 (0.84–1.64) vs. 1.07 (0.69–1.65), P-value statistical mediation > 0.05). The attenuation was stronger for waist circumference (OR Q4 vs. Q1 before adjustment for a metabolomic score vs. after adjustment = 1.19 (0.76–1.88) vs. 0.91 (0.55–1.51), P-value statistical mediation = 0.02), though in this case the contribution of metabolomic score to the association remained quite small (statistically mediated effect size = 0.0002).
Discussion
We generated metabolomic scores for adiposity measures including BMI, waist circumference, weight change since age 18, and fat mass, to gain insight into the relationship between adiposity and pre- and postmenopausal breast cancer. With a panel of 263 metabolites, including lipids and amino acids and derivatives, we identified many metabolites associated with adiposity measures. Metabolomic scores for adiposity measures were representative of the most predictive metabolites. The scores were generally associated with a lower risk of breast cancer among premenopausal women, and a higher risk among postmenopausal women. Classes of metabolites positively associated with adiposity included branched-chain amino acids (BCAAs), TAGs and DAGs with a low number of double bonds, and glycerophosphocholines. The individual metabolites selected for adiposity scores are reflective of metabolic dysregulation, providing insights into the complex relations between adiposity and breast cancer risk among premenopausal and postmenopausal women.
A large proportion of the metabolites measured were significantly associated with adiposity measures. Notably, the composition of metabolites for each metabolomic score was largely overlapping. This provides evidence that self-reported measures we typically use, whether it be BMI, waist circumference, fat mass or weight change, are similar in their underlying biology with respect to metabolomics.
Branched-chain amino acids (BCAAs) had particularly strong positive associations with adiposity measures in our study, and have been associated with BMI in prior literature [12, 20]. A study within the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Cohort (PLCO), the Navy Adenoma Study, and the Shanghai Physical Activity study, that assessed pre-diagnostic serum metabolites, also found significant positive associations between valine and isoleucine and BMI [11]. The relationship between BCAAs and adiposity may be driven in part through insulin resistance, a key marker of metabolic dysregulation [21, 22].
Lipids, including triacylglycerols (TAGs) and diacylglycerols (DAGs) with low numbers of double bonds were strongly associated with adiposity measures. Our finding of TAGs and DAGs as key contributors to adiposity measures is consistent in the literature [11]. In diabetes literature, the structure of TAGs appears important for determining the influence on insulin action [12, 23]; TAGs with fewer double bonds are associated with insulin resistance. Thus, as with BCAAs, TAGs and DAGs with few double bonds may represent metabolic dysregulation.
Glycerophosphocholine metabolites, notably C38:3 PC, were strongly associated with adiposity measures in our study and may reflect metabolic dysregulation. A recent study compared metabolic profiles in individuals who were classified as metabolically healthy vs. unhealthy (defined as having one or more abnormal metabolic indexes: hyperglycaemia, hypertension, dyslipidemia). Metabolite profiles were distinct between groups, and featured glycerophosphocholines, along with BCAAs, and metabolites involved in phenylalanine metabolism and fatty acid biosynthesis [24]. Carnitines, essential for fatty acid β-oxidation and therefore energy production [25], were also positively and relatively strongly associated with adiposity measures, a finding consistent with other literature [11, 26]. Chen et al.’s study of metabolically healthy vs. unhealthy individuals also demonstrated a different acylcarnitine profile between the two groups [24]. Moreover, acylcarnitine metabolism has been linked to obesity and insulin resistance [27].
Because, after menopause, stronger associations with breast cancer risk were observed for some metabolomic adiposity scores compared to self-reported measures, metabolic dysregulation may be an important underlying contributor to adiposity as a breast cancer risk factor. Evidence suggests metabolic syndrome (MetS), defined as a combination of factors including obesity (waist circumference or BMI), triglyceride levels, high-density lipoprotein (HDL) levels, hypertension, and hyperglycaemia [28], contributes more than adiposity alone to risk and progression of the disease. For example, in the National Institute of Health-American Association of Retired Persons (NIH-AARP) Diet and Health Study, MetS increased breast cancer risk in postmenopausal women by 13%, and women with more components of MetS had a higher risk of breast cancer compared to those with only one component (HR = 1.45, 95% CI = 0.99–2.13) [29].
Notably, we observed similar metabolites associated with adiposity in premenopausal and postmenopausal women. Thus, the different association between adiposity and breast cancer risk in premenopausal and postmenopausal women is not due to different metabolite profiles themselves, but perhaps to the distinct roles these metabolites, and more broadly, metabolic dysregulation, play at different stages in a woman’s life. Inclusion of metabolomic scores in regressions generally resulted in a greater change in OR between self-reported adiposity measures and breast cancer risk among women premenopausal at blood draw compared to postmenopausal women, with the exception of waist circumference, though the change was minimal in both groups.
BCAAs, which, as noted, are closely linked to insulin resistance, have been associated with breast cancer risk. Within PLCO, an increased risk for postmenopausal breast cancer was observed with higher postmenopausal levels of isoleucine and valine [10]. This finding was replicated in the NHS, with the same participants as in the present study, with a 63% higher risk of breast cancer for top vs. bottom quartile of isoleucine (P-trend = 0.01) among women postmenopausal at blood draw [30]. On the other hand, among women who were premenopausal at blood draw, BCAA levels were associated with a lower risk of breast cancer, demonstrating that the same metabolites may have differential associations pre- vs. post-menopause. There is some discrepancy in the literature, as BCAAs in the Women’s Health Study (WHS) were not associated with risk, with the exception of leucine [30, 31]. C-peptide, a byproduct of insulin processing, and therefore closely linked to BCAAs, is also associated with breast cancer risk [32], though this relationship may differ by menopausal status. Within the European Investigation into Cancer and Nutrition (EPIC), investigators found that serum C-peptide levels were inversely associated with breast cancer before age 50 (OR = 0.70, 95% CI = 0.39–1.24, P-trend = 0.05), but positively associated with breast cancer for women above age 60 (OR = 2.03, 95% CI = 1.20–3.43, P-trend = 0.01) [33]. Similarly, in the NHSII we observed that C-peptide was inversely associated with risk for premenopausal women with fasting blood samples [34].
Glycerophosphocholines have also been associated with breast cancer risk, though it is unclear whether the risk differs between pre- and postmenopausal women. For example, C32:1 PC was associated with higher breast cancer risk in the Cancer Prevention Study II, a cohort of postmenopausal women [9]. However, within EPIC, a cohort including approximately 25% premenopausal women at blood draw, glycerophosphocholines C38:3 PC and C18:2 LPC were both associated with lower breast cancer risk (e.g.: OR for a 1 SD change in C18:2 LPC = 0.89, 95% CI = 0.81–0.96) [35].
Carnitines have been implicated in breast cancer risk as well. However, it does not appear that carnitines are driving the differential association between pre- and postmenopausal breast cancer risk, as acylcarnitine levels were associated with elevated breast cancer risk in both pre- and postmenopausal women in EPIC [35].
The differential associations observed between levels of adiposity-related metabolites and pre vs. postmenopausal breast cancer risk may be explained in part by the role of oxidative stress associated with MetS. Oxidative stress is related to metabolic disorders, such as cardiovascular disease development and obesity [36, 37]. Thus, it is posited that adiposity-related metabolite profiles contributing to metabolic syndrome (MetS) may also be informative of overall oxidative stress levels. In premenopausal women, some effects of oxidative stress have been shown to prevent cancer [38], and higher oxidative stress has been suggestively associated with lower breast cancer risk in premenopausal women in prospective studies [39–41]. In contrast, levels of oxidative stress have been associated with higher breast cancer risk in postmenopausal women [42]. Thus, different functions of oxidative stress in pre- vs. postmenopausal breast cancer development may contribute to the observed differences of these metabolites on breast cancer risk before and after menopause.
It is possible that the relationship between adiposity and breast cancer, and the differential associations among pre-and postmenopausal women, may be due, in part, to hormonal influence. Adiposity in postmenopausal breast cancer is associated with higher levels of circulating oestrogens [43]. Aromatase, active in adipose tissue, contributes to breast cancer development through the conversion of androgens to oestrogens in postmenopausal women [44, 45], and changes in aromatase expression in breast tissue are related to metabolic dysregulation markers such as insulin resistance [46]. Thus, higher adiposity may increase the risk of breast cancer for postmenopausal women through higher aromatase levels. In premenopausal women, the relationship between adiposity and oestrogen is less clear. At the extreme, adiposity may cause anovulatory cycles and lower hormone exposure [47]. There is further evidence that BMI is inversely related to oestrogen levels in premenopausal women, even in normally ovulating individuals, suggesting another potential mechanism of risk reduction [48, 49]. This may be related to the correlation between current BMI and BMI in young adulthood. Body fatness at younger ages, measured up to age 20, is associated with lower levels of urinary parent oestrogens [50]. It is possible that the impact of early life adiposity on circulating oestrogens may have the longevity that allows for continued lower oestrogen levels in premenopausal women with higher BMI overall. Given that associations we found in pre- and postmenopausal women are stronger in ER+ cases compared with ER− cases, the metabolomic scores may represent the correlation between adiposity and oestrogen levels. This makes it difficult to tell if the mechanisms driving adiposity-related associations are metabolic or oestrogenic. Because dietary and other factors may impact metabolomic profiles, including those metabolites we selected based on their correlation with adiposity [51], it is possible that the metabolites included in our adiposity scores are not specific to adiposity. As such, the observed associations of metabolomic scores with breast cancer may be due to factors other than adiposity.
Previously, we established metabolomic scores for fat mass, BMI and waist circumference among men in the Health Professionals Follow-Up Study (HPFS) [26]. While fewer adiposity-related metabolites were identified compared to our study, there was high overlap between metabolites identified for each adiposity measure, consistent with our findings among women. Classes of metabolites associated with adiposity measures did not differ between men and women.
This study has several strengths. The use of both the NHS and NHSII allowed us to thoroughly investigate adiposity and metabolomics in both premenopausal and postmenopausal women, particularly important given the complicated relationship between adiposity and breast cancer risk by menopausal status. We leveraged metabolomics to evaluate associations between measures of metabolic health and breast cancer risk and had large numbers of breast cancer cases to evaluate these associations. Despite the advantages of this study, there are also limitations. We did not have a large subset of women who were premenopausal at blood collection and postmenopausal at diagnosis, and ER− cases were limited, which limited the precision of these estimates. Importantly, because the metabolomic scores for adiposity measures were developed based on the most predictive metabolites, they may not represent the most biologically important metabolites, and individual metabolite contributions should be interpreted with caution.
In this study of 3298 premenopausal and postmenopausal breast cancer cases and controls, we identified many metabolites associated with adiposity measures of BMI, waist circumference, weight change since age 18, and derived fat mass, the majority of which overlapped significantly between adiposity measures. Some metabolomic scores for adiposity were more predictive of breast cancer than self-reported measures, suggesting metabolomics may better capture metabolic dysregulation and improve our understanding of breast cancer risk. Because the association between metabolomic score and breast cancer risk appeared stronger among women with a BMI > 25 kg/m2 compared to women with a BMI < 25 kg/m2, the metabolomic score may prove more useful for predicting risk among this subgroup of women, though further investigation is needed. Further, we found that the metabolic composition of adiposity did not differ between premenopausal and postmenopausal women. This suggests that the opposing associations for adiposity and breast cancer risk by menopausal status reflect the differential associations between metabolic dysregulation and breast cancer risk over the life course. Further exploration of how metabolic dysregulation intersects with menopausal status to differentially predict breast cancer risk is warranted.
Supplementary information
Acknowledgements
We would like to thank the participants and staff of the Nurses’ Health Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA and WY. The authors assume full responsibility for the analyses and interpretation of these data.
Author contributions
KB and AHE designed the study. CC and JA performed metabolomic profiling. KB and OZ performed the statistical analysis. BD, RB, RT, BR and AHE performed interpretation of results. AHE, BR and RT supervised the study. All authors reviewed the manuscript and approved its final version.
Funding
This study was funded by the National Institutes of Health (NIH)/National Cancer Institute (NCI) with the following grants: UM1 CA186107, P01 CA87969, R01 CA49449, R01 CA050385, U01 CA176726, R01 CA67262 and T32 CA009001.
Data availability
The data generated in this study are not publicly available due to participant confidentiality and privacy concerns but are available upon request. Further information including the procedures to obtain and access data from the Nurses’ Health Studies is described at https://www.nurseshealthstudy.org/researchers.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The return of the self-administered questionnaires and blood samples were considered to imply consent. The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required. The study was performed in accordance with the Declaration of Helsinki.
Consent to publish
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-022-01873-9.
References
- 1.Lahmann PH, Hoffmann K, Allen N, van Gils C, Khaw KT, Tehard B, et al. Body size and breast cancer risk: findings from the European Prospective Investigation into Cancer And Nutrition (EPIC) Int J Cancer. 2004;111:762–71. doi: 10.1002/ijc.20315. [DOI] [PubMed] [Google Scholar]
- 2.Morimoto LM, White E, Chen Z, Chlebowski RT, Hays J, Kuller L, et al. Obesity, body size, and risk of postmenopausal breast cancer: the Women’s Health Initiative (United States) Cancer Causes Control. 2002;13:741–51. doi: 10.1023/A:1020239211145. [DOI] [PubMed] [Google Scholar]
- 3.Cheraghi Z, Poorolajal J, Hashem T, Esmailnasab N, Doosti, Irani A. Effect of body mass index on breast cancer during premenopausal and postmenopausal periods: a meta-analysis. PLoS ONE. 2012;7:e51446. doi: 10.1371/journal.pone.0051446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 5.Premenopausal Breast Cancer Collaborative Group. Schoemaker MJ, Nichols HB, Wright LB, Jones ME, O’Brien KM, Adami HO, et al. Association of body mass index and age with subsequent breast cancer risk in premenopausal women. JAMA Oncol. 2018;4:e181771. doi: 10.1001/jamaoncol.2018.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo J, Chen X, Manson JE, Shadyab AH, Wactawski-Wende J, Vitolins M, et al. Birth weight, weight over the adult life course and risk of breast cancer. Int J Cancer. 2020;147:65–75. doi: 10.1002/ijc.32710. [DOI] [PubMed] [Google Scholar]
- 7.Hartz A, He T, Rimm A. Comparison of adiposity measures as risk factors in postmenopausal women. J Clin Endocrinol Metab. 2012;97:227–33. doi: 10.1210/jc.2011-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee DH, Keum N, Hu FB, Orav EJ, Rimm EB, Sun Q, et al. Development and validation of anthropometric prediction equations for lean body mass, fat mass and percent fat in adults using the National Health and Nutrition Examination Survey (NHANES) 1999-2006. Br J Nutr. 2017;118:858–66. doi: 10.1017/S0007114517002665. [DOI] [PubMed] [Google Scholar]
- 9.Moore SC, Mazzilli KM, Sampson JN, Matthews CE, Carter BD, Playdon MC, et al. A metabolomics analysis of postmenopausal breast cancer risk in the cancer prevention study II. Metabolites. 2021;11:95. [DOI] [PMC free article] [PubMed]
- 10.Moore SC, Playdon MC, Sampson JN, Hoover RN, Trabert B, Matthews C, et al. A metabolomics analysis of body mass index and postmenopausal breast cancer risk. J Natl Cancer Inst. 2018;110:588–97. doi: 10.1093/jnci/djx244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore SC, Matthews CE, Sampson JN, Stolzenberg-Solomon RZ, Zheng W, Cai Q, et al. Human metabolic correlates of body mass index. Metabolomics. 2014;10:259–69. doi: 10.1007/s11306-013-0574-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng S, Rhee EP, Larson MG, Lewis GD, McCabe EL, Shen D, et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation. 2012;125:2222–31. doi: 10.1161/CIRCULATIONAHA.111.067827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaudet MM, Falk RT, Stevens RD, Gunter MJ, Bain JR, Pfeiffer RM, et al. Analysis of serum metabolic profiles in women with endometrial cancer and controls in a population-based case-control study. J Clin Endocrinol Metab. 2012;97:3216–23. doi: 10.1210/jc.2012-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mascanfroni ID, Takenaka MC, Yeste A, Patel B, Wu Y, Kenison JE, et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nat Med. 2015;21:638–46. doi: 10.1038/nm.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Sullivan JF, Morningstar JE, Yang Q, Zheng B, Gao Y, Jeanfavre S, et al. Dimethylguanidino valeric acid is a marker of liver fat and predicts diabetes. J Clin Invest. 2017;127:4394–402. doi: 10.1172/JCI95995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paynter NP, Balasubramanian R, Giulianini F, Wang DD, Tinker LF, Gopal S, et al. Metabolic predictors of incident coronary heart disease in women. Circulation. 2018;137:841–53. doi: 10.1161/CIRCULATIONAHA.117.029468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Townsend MK, Clish CB, Kraft P, Wu C, Souza AL, Deik AA, et al. Reproducibility of metabolomic profiles among men and women in 2 large cohort studies. Clin Chem. 2013;59:1657–67. doi: 10.1373/clinchem.2012.199133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benjamini Y, Krieger AM, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika. 2006;93:491–507. doi: 10.1093/biomet/93.3.491. [DOI] [Google Scholar]
- 19.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. “mediation: R package for causal Mediation analysis.”. J Stat Softw. 2014;59:1–38. doi: 10.18637/jss.v059.i05. [DOI] [Google Scholar]
- 20.Ho JE, Larson MG, Ghorbani A, Cheng S, Chen MH, Keyes M, et al. Metabolomic profiles of body mass index in the Framingham Heart Study Reveal Distinct Cardiometabolic Phenotypes. PLoS ONE. 2016;11:e0148361. doi: 10.1371/journal.pone.0148361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–26. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Q, Holmes MV, Davey Smith G, Ala-Korpela M. Genetic support for a causal role of insulin resistance on circulating branched-chain amino acids and inflammation. Diabetes Care. 2017;40:1779–86. doi: 10.2337/dc17-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhee EP, Cheng S, Larson MG, Walford GA, Lewis GD, McCabe E, et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest. 2011;121:1402–11. doi: 10.1172/JCI44442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen H-H, Tseng YJ, Wang S-Y, Tsai Y-S, Chang C-S, Kuo T-C, et al. The metabolome profiling and pathway analysis in metabolic healthy and abnormal obesity. Int J Obes. 2015;39:1241–8. doi: 10.1038/ijo.2015.65. [DOI] [PubMed] [Google Scholar]
- 25.Rebouche CJ. Kinetics, pharmacokinetics, and regulation of L-carnitine and acetyl-L-carnitine metabolism. Ann N. Y Acad Sci. 2004;1033:30–41. doi: 10.1196/annals.1320.003. [DOI] [PubMed] [Google Scholar]
- 26.Dickerman BA, Ebot EM, Healy BC, Wilson KM, Eliassen AH, Ascherio A, et al. A Metabolomics analysis of adiposity and advanced prostate cancer risk in the health professionals follow-up study. Metabolites. 2020;10:99. [DOI] [PMC free article] [PubMed]
- 27.Schooneman MG, Vaz FM, Houten SM, Soeters MR. Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes. 2013;62:1–8. doi: 10.2337/db12-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srikanthan K, Feyh A, Visweshwar H, Shapiro JI, Sodhi K. Systematic review of metabolic syndrome biomarkers: a panel for early detection, management, and risk stratification in the west virginian population. Int J Med Sci. 2016;13:25–38. doi: 10.7150/ijms.13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dibaba DT, Braithwaite D, Akinyemiju T. Metabolic syndrome and the risk of breast cancer and subtypes by race, menopause and BMI. Cancers. 2018;10:299. [DOI] [PMC free article] [PubMed]
- 30.Zeleznik OA, Balasubramanian R, Ren Y, Tobias DK, Rosner BA, Peng C, et al. Branched chain amino acids and risk of breast cancer. JNCI Cancer Spectr. 2021;5:pkab059. [DOI] [PMC free article] [PubMed]
- 31.Tobias DK, Hazra A, Lawler PR, Chandler PD, Chasman DI, Buring JE, et al. Circulating branched-chain amino acids and long-term risk of obesity-related cancers in women. Sci Rep. 2020;10:16534. doi: 10.1038/s41598-020-73499-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li M, Song L, Yuan J, Zhang D, Zhang C, Liu Y, et al. Association between serum insulin and C-peptide levels and breast cancer: an updated systematic review and meta-analysis. Front Oncol. 2020;10:553332. doi: 10.3389/fonc.2020.553332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verheus M, Peeters PHM, Rinaldi S, Dossus L, Biessy C, Olsen A, et al. Serum C-peptide levels and breast cancer risk: results from the European Prospective Investigation into Cancer and Nutrition (EPIC) Int J Cancer. 2006;119:659–67. doi: 10.1002/ijc.21861. [DOI] [PubMed] [Google Scholar]
- 34.Eliassen AH, Tworoger SS, Mantzoros CS, Pollak MN, Hankinson SE. Circulating insulin and c-peptide levels and risk of breast cancer among predominately premenopausal women. Cancer Epidemiol Biomark Prev. 2007;16:161–4. doi: 10.1158/1055-9965.EPI-06-0693. [DOI] [PubMed] [Google Scholar]
- 35.His M, Viallon V, Dossus L, Gicquiau A, Achaintre D, Scalbert A, et al. Prospective analysis of circulating metabolites and breast cancer in EPIC. BMC Med. 2019;17:178. doi: 10.1186/s12916-019-1408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davies SS, Roberts LJ. F2-isoprostanes as an indicator and risk factor for coronary heart disease. Free Radic Biol Med. 2011;50:559–66. doi: 10.1016/j.freeradbiomed.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Il’yasova D, Wang F, Spasojevic I, Base K, D’Agostino RB, Wagenknecht LE. Urinary F2-isoprostanes, obesity, and weight gain in the IRAS cohort. Obesity. 2012;20:1915–21. doi: 10.1038/oby.2011.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nemoto S, Finkel T. Ageing and the mystery at Arles. Nature. 2004;429:149–52. doi: 10.1038/429149a. [DOI] [PubMed] [Google Scholar]
- 39.Dai Q, Gao Y-T, Shu X-O, Yang G, Milne G, Cai Q, et al. Oxidative stress, obesity, and breast cancer risk: results from the Shanghai Women’s Health Study. J Clin Oncol. 2009;27:2482–8. doi: 10.1200/JCO.2008.19.7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sisti JS, Lindström S, Kraft P, Tamimi RM, Rosner BA, Wu T, et al. Premenopausal plasma carotenoids, fluorescent oxidation products, and subsequent breast cancer risk in the nurses’ health studies. Breast Cancer Res Treat. 2015;151:415–25. doi: 10.1007/s10549-015-3391-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nichols HB, Anderson C, White AJ, Milne GL, Sandler DP. Oxidative stress and breast cancer risk in premenopausal women. Epidemiology. 2017;28:667–74. doi: 10.1097/EDE.0000000000000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fortner RT, Katzke V, Kühn T, Kaaks R. Obesity and breast cancer. Recent Results Cancer Res. 2016;208:43–65. doi: 10.1007/978-3-319-42542-9_3. [DOI] [PubMed] [Google Scholar]
- 43.Simpson ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biol. 2003;86:225–30. doi: 10.1016/S0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- 44.Zhou C, Zhou D, Esteban J, Murai J, Siiteri PK, Wilczynski S, et al. Aromatase gene expression and its exon I usage in human breast tumors. Detection of aromatase messenger RNA by reverse transcription-polymerase chain reaction. J Steroid Biochem Mol Biol. 1996;59:163–71. doi: 10.1016/S0960-0760(96)00100-8. [DOI] [PubMed] [Google Scholar]
- 45.Sasano H, Nagura H, Harada N, Goukon Y, Kimura M. Immunolocalization of aromatase and other steroidogenic enzymes in human breast disorders. Hum Pathol. 1994;25:530–5. doi: 10.1016/0046-8177(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 46.Brown KA, Iyengar NM, Zhou XK, Gucalp A, Subbaramaiah K, Wang H, et al. Menopause is a determinant of breast aromatase expression and its associations with BMI, inflammation, and systemic markers. J Clin Endocrinol Metab. 2017;102:1692–701. doi: 10.1210/jc.2016-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rich-Edwards JW, Spiegelman D, Garland M, Hertzmark E, Hunter DJ, Colditz GA, et al. Physical activity, body mass index, and ovulatory disorder infertility. Epidemiology. 2002;13:184–90. doi: 10.1097/00001648-200203000-00013. [DOI] [PubMed] [Google Scholar]
- 48.Tworoger SS, Eliassen AH, Missmer SA, Baer H, Rich-Edwards J, Michels KB, et al. Birthweight and body size throughout life in relation to sex hormones and prolactin concentrations in premenopausal women. Cancer Epidemiol Biomark Prev. 2006;15:2494–501. doi: 10.1158/1055-9965.EPI-06-0671. [DOI] [PubMed] [Google Scholar]
- 49.Potischman N, Swanson CA, Siiteri P, Hoover RN. Reversal of relation between body mass and endogenous estrogen concentrations with menopausal status. J Natl Cancer Inst. 1996;88:756–8. doi: 10.1093/jnci/88.11.756. [DOI] [PubMed] [Google Scholar]
- 50.Houghton LC, Sisti JS, Hankinson SE, Xie J, Xu X, Hoover RN, et al. Estrogen metabolism in premenopausal women is related to early life body fatness. Cancer Epidemiol Biomark Prev. 2018;27:585–93. doi: 10.1158/1055-9965.EPI-17-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu K, Shi L, Zhang B, Mi B, Yang J, Sun X, et al. Distinct metabolite profiles of adiposity indices and their relationships with habitual diet in young adults. Nutr Metab Cardiovasc Dis. 2021;31:2122–30. doi: 10.1016/j.numecd.2021.03.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are not publicly available due to participant confidentiality and privacy concerns but are available upon request. Further information including the procedures to obtain and access data from the Nurses’ Health Studies is described at https://www.nurseshealthstudy.org/researchers.