Abstract
Chemotherapy has been one of the most important treatments for advanced cancer in recent decades. Although the sensitivity rate of initial chemotherapy is high, patients with chemotherapy resistant tumors, experience tumor recurrence. In recent years, many studies have shown that homeobox transcript antisense intergenic RNA (HOTAIR) is involved in many pathological processes including carcinogenesis. The abnormal regulation of a variety of cell functions by HOTAIR, such as apoptosis, the cell cycle, epithelial-mesenchymal transition, autophagy, self-renewal, and metabolism, is associated with chemotherapy resistance. Therefore, there is an urgent need to understand the biology and mechanism underlying the role of HOTAIR in tumor behavior and its potential as a biomarker for predicting the effect of chemotherapy. In this manuscript, we review the mechanisms underlying HOTAIR-related drug resistance and discuss the limitations of current knowledge and propose potential future directions.
Subject terms: Long non-coding RNAs, Chemical genetics
Facts
Cancer has become a major threat to human health, but there is no effective way to stop its progress.
Chemotherapy plays an important role in controlling tumor growth, but chemotherapy resistance is still a difficult problem to be solved.
HOTAIR has been shown to be abnormally expressed in a variety of cancers and is associated with poor prognosis.
With the emergence of tumor resistance, HOTAIR has also been confirmed to be involved in chemotherapy resistance of tumors.
Open Questions
What is the specific mechanism of HOTAIR’s involvement in tumor chemotherapy resistance?
How to reduce drug resistance by inhibiting the expression of HOTAIR in clinic?
Introduction
Long non-coding RNAs (lncRNAs) are non-coding RNAs with a length of over 200 nucleotides [1]. LncRNAs are mainly transcribed by RNA polymerase II from different regions of the entire genome. Growing evidence shows that lncRNAs are involved in a variety of carcinogenic processes, such as tumor proliferation, invasion, and metastasis [2–4]. The role of lncRNAs in tumorigenesis and tumor progression has been widely investigated. However, studies of the role of lncRNAs in cancer resistance to chemotherapy are still at a nascent stage [5].
Homeobox transcript antisense intergenic RNA (HOTAIR) is an important polyadenylated and spliced lncRNA, containing 6 exons and 2158 nucleotides [6]. HOTAIR is transcribed from the antisense strand of the HOXC gene, which is located between HOXC11 and HOXC12 on chromosome 12q13.13 (Fig. 1) [7]. Abnormal overexpression of HOTAIR in cancer was first identified in breast cancer (BC) and has been associated with metastasis and poor survival [8]. Elevated expression of HOTAIR was found to induce genome-wide retargeting of polycomb repressive complex 2 (PRC2), leading to increase metastasis and invasion in BC cells [8]. Since then, HOTAIR has been attracting attention in the field of cancer.
Fig. 1. Schematic location of HOTAIR.
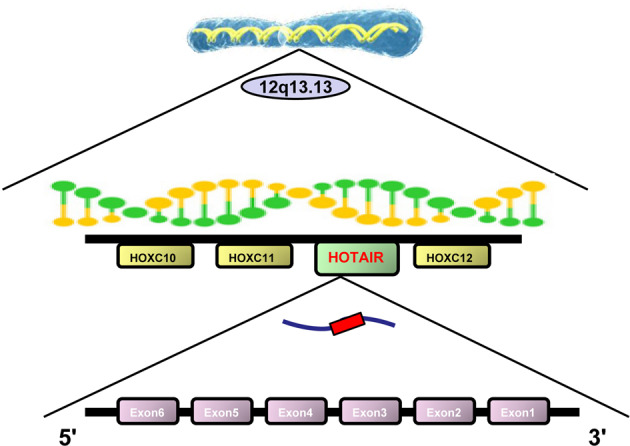
HOTAIR is transcribed from the antisense strand of the HOXC gene, which is located between HOXC11 and HOXC12 on chromosome 12. HOTAIR is a lncRNA, containing 6 exons and 2158 nucleotides.
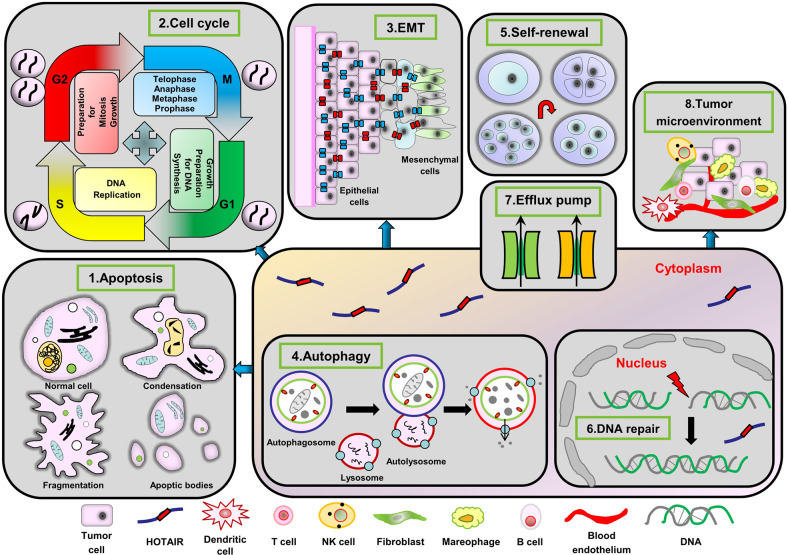
Chemotherapy plays an important role in controlling tumor growth and can increase the survival time of patients [9]. Several studies have shown that chemotherapy can significantly reduce the mortality rate of patients with advanced tumors and those who cannot undergo surgery [10, 11]. Despite the effectiveness of chemotherapy, the acquired chemotherapeutic resistance is a huge challenge to cancer treatment. Chemotherapy resistance can lead to tumor recurrence and increase patient mortality. The mechanisms underlying the development of resistance in cancer cells involve: (1) inhibition of cell apoptosis and protection of damaged cells, (2) avoidance of cell cycle checkpoints, (3) enhancement of epithelial-mesenchymal transition (EMT), (4) reconsitution of the cell autophagy system, (5) enhancement of self-renewal ability, (6) remodeling the repair ability of DNA, (7) alteration of drug metabolism and transport, which affects the pharmacokinetics in cancer cells, (8) and modulation of the tumor microenvironment (TME) (Fig. 2) [12–14]. In this review, we discuss the research progress on the relationship between HOTAIR and cancer chemotherapy resistance.
Fig. 2. Roles of HOTAIR in mechanisms mediating chemotherapy resistance of cancers.
This schematic shows the mechanism of HOTAIR-mediated drug resistance in cancer cells. (1) inhibition of cell apoptosis, (2) avoidance of cell cycle checkpoints, (3) enhancement of EMT, (4) reconstitution of cell autophagy, (5) enhancement of cell self-renewal, (6) remodeling DNA repair, (7) alteration of efflux pump, (8) and modulation of tumor microenvironment.
HOTAIR biogenesis and general functions
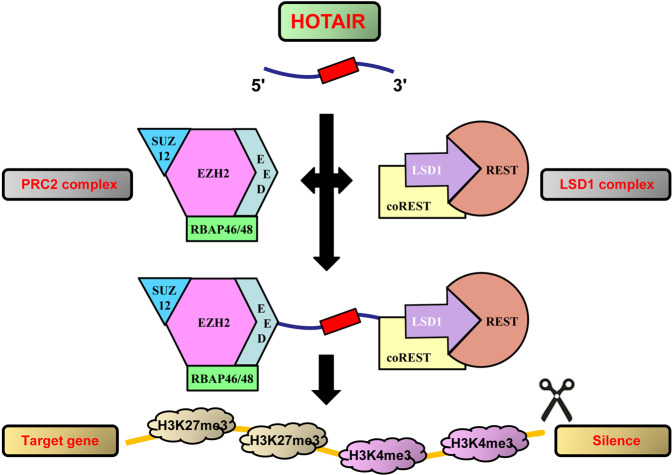
HOTAIR, first identified by Rinn et al. in 2007 [6], can inhibit the transcription of 40 kb at the HOXD site. HOTAIR is a key regulator of transcriptional silencing and chromatin remodeling. HOTAIR interacts with some vital epigenetic regulators, such as PRC2 and lysine-specific histone demethylase 1 A (LSD1) to regulate gene silencing [15, 16]. The PRC2 complex contains four main subunits, namely, SUZ12, EED, EZH2, and RBAP46/48, and interacts with a fragment at the 5ʹ end of HOTAIR [17]. Silencing of HOTAIR could activate the transcriptional repression of the HOXD site on chromosome 2 by reducing the tri-methylation of H3 lysine 27 trimethylation (H3K27me3), which is the feature of gene silencing modulated by EZH2, a member of the PRC2 family [18]. Another mechanism underlying the mode of interaction of HOTAIR with PRC2 has been proposed, according to which PRC2 interacts with short repeats of successive guanines in HOTAIR rather than with specific domains (Fig. 3) [19]. Recent studies revealed that RNA G-quadruplexes (G4s) at the 5ʹ end of HOTAIR mediated the reciprocal interaction with PRC2 [20]. RNA G4s are made by stacking two or more connected square planes of four guanines and are involved in many important cellular processes and the pathogenesis of various diseases, such as cancer [21]. Besides PRC2, the LSD1 complex is another important partner of HOTAIR, which interacts with a fragment at the 3ʹ end of HOTAIR (Fig. 3) [16]. The LSD1 complex consists of LSD1, REST, and CoREST, which inhibit gene expression by decreasing the tri-methylation of histone H3 Lys 4 (H3K4me3). In addition, recent studies have shown that HOTAIR influences miRNA-mediated inhibition of target gene expression through competitive binding with miRNA [22, 23]. Furthermore, HOTAIR alters gene expression by pairing with translation factors or ribosomal bases to control translation. In conclusion, HOTAIR functions as a scaffold to assemble the inhibitory complex composed of PRC2 and LSD1, thereby silencing its target genes through H3K27me3 (PRC2 activity) and H3K4me3 (LSD1 activity), respectively.
Fig. 3. Functions and molecular mechanisms of HOTAIR.
HOTAIR can recruit PRC2 and LSD1 complexes and act as a bridge. HOTAIR guides these complexes to the target genes, thereby regulating the trimethylation of H3K27 and demethylation of H3K4.
HOTAIR is found not only in humans, but also in mice and rats. However, it does not exist in non-mammalian vertebrates [24]. The sequence similarity between mouse HOTAIR and human HOTAIR is about 58%, while it is about 50% in rat HOTAIR [25, 26]. Therefore, HOTAIR in mammalian vertebrates is conservative. The human HOTAIR gene has 6 exons, whereas the rat and mouse HOTAIR gene has only 5 exons because exon 2 is not present [26]. There are two domains in exon 6 of rat and mouse HOTAIR, called domains A and B. Domain B is responsible for the interaction between HOTAIR and plenty of proteins [26]. However, the physiological function of domain A remains unclear.
HOTAIR is a vital modulator of chromatin dynamics. It acts on multiple genetic sites and modulates their expression [27]. HOTAIR interacts with RNA, DNA, and proteins to participate in a variety of cellular functions. During mammalian embryogenesis, HOTAIR is expressed in the forelimb and wrist, hindlimb bud and posterior trunk, and genital bud and tail [28]. In humans, HOTAIR is highly concentrated in the skin and reproductive system, mainly involving endometrium, prostate and testis [28]. HOTAIR can down-regulate osteogenic genes BMP2 and ALPL, indicating that HOTAIR has a negative regulatory role in the process of osteogenesis [29]. HOTAIR is involved in cell senescence by interacting with E3 ubiquitin ligases [30]. HOTAIR also participates in cell proliferation by regulating the expression of cell cycle-related proteins and kinases [28].
Mechanisms mediating HOTAIR-related chemotherapy resistance in cancer
HOTAIR regulates cell apoptosis and cell cycle
The ability of cancer cells to respond to chemotherapy drugs is thought to be due to their ability to undergo apoptosis. Drug-induced apoptosis is not only modulated by an upregulation of pro-apoptotic factors but also regulated by cell survival factors [31].
Recent studies revealed that HOTAIR is involved in the modulation of the apoptotic pathway in cancer cells, which may be related to chemotherapy resistance (Table 1). For example, a study showed that HOTAIR suppression promoted apoptosis and doxorubicin sensitivity in acute myeloid leukemia (AML). Furthermore, an investigation of the mechanism indicated that HOTAIR conferred multidrug resistance (MDR) to AML cells by modulating the expression of the AKT/Notch1 and P21 signaling pathways [32]. Another research group reported that HOTAIR inhibited PTEN expression in a manner dependent on DNMT3b upregulation, which resulted in doxorubicin resistance in AML [33]. In addition, downregulation of HOTAIR played a vital role in enhancing the acquired resistance to imatinib via PI3K/AKT signaling in chronic myelogenous leukemia (CML) [34]. Furthermore, the PI3K/AKT signaling pathway was found to be involved in HOTAIR-mediated cell apoptosis and chemotherapy resistance in BC and gastric cancer (GC) [35, 36]. Similar results have been widely reported in other digestive system tumors. The study by Zhang et al. [37] revealed that targeting HOTAIR sensitized esophageal cancer cells to 5-fluorouracil (5-FU) chemotherapy through the downregulation of methylenetetrahydrofolate reductase expression. In hepatocellular carcinoma (HCC), downregulation of HOTAIR weakened Taxol resistance through the Wnt/β-catenin and Akt phosphorylation pathways via antagonizing miR-34a [38]. In pancreatic cancer, high HOTAIR expression expedited cell resistance to TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through modulation of TRAIL receptor death receptor 5 expression [39]. Recently, it was reported that HOTAIR expression in non-small cell lung cancer (NSCLC) was dramatically higher than that in adjacent tissues and that high HOTAIR expression was associated with shortened overall patient survival. Small interfering RNA targeting HOTAIR (si-HOTAIR) reversed the sensitivity of NSCLC cells to cisplatin (DDP) [40]. Another study demonstrated that H3K27me3 affected cell apoptosis and HOXA1 methylation through HOTAIR modulation, indicating that targeting H3K27me3 could be an effective strategy against lung cancer chemotherapy resistance [41]. In osteosarcoma, HOTAIR increased DDP resistance by affecting cell apoptosis. Functional assays demonstrated that suppression of HOTAIR decreased DDP resistance and promoted cell apoptosis via miR-106a-5p/STAT3 signaling [42]. In endometrial cancer, HOTAIR repressed progesterone receptor B expression and mediated progesterone sensitivity. Hence, si-HOTAIR could be a potential therapeutic target for overcoming progesterone resistance [43]. In glioblastoma (GBM), the depletion of HOTAIR restrained HK2 expression by regulating miR-125, which facilitated cell apoptosis and elevated temozolomide (TMZ) sensitivity [44].
Table 1.
HOTAIR inhibits cell apoptosis.
| Cancer type | Expression | Related drugs | Target | Related genes or pathway | References |
|---|---|---|---|---|---|
| Leukemia | upregulation | doxorubicin | / | AKT/Notch1 and P21 | [32] |
| Leukemia | upregulation | doxorubicin | / | PTEN | [33] |
| Leukemia | upregulation | imatinib | / | PI3K/Akt | [34] |
| Breast cancer | upregulation | doxorubicin | / | PI3K/Akt/mTOR | [35] |
| Gastric cancer | upregulation | cisplatin | miR-34a | PI3K/Akt and Wnt/β-catenin | [36] |
| Esophageal cancer | upregulation | 5-fluorouracil | / | MTHFR | [37] |
| Hepatocellular cancer | upregulation | taxol | miR-34a | Akt and Wnt/β-catenin | [38] |
| Pancreatic cancer | upregulation | / | / | EZH2/DR5 | [39] |
| Lung cancer | upregulation | cisplatin | / | Wnt | [40] |
| Lung cancer | upregulation | / | / | HOXA1 | [41] |
| Osteosarcoma | upregulation | cisplatin | miR-106a-5p | STAT3 | [42] |
| Endometrial cancer | upregulation | progesterone | / | PRB | [43] |
| Glioblastoma | upregulation | temozolomide | miR-125 | HK2 | [44] |
In summary, many genes and proteins involved in the inhibition or induction of apoptosis, such as p53, inhibitor of apoptosis family (including X-linked inhibitor of apoptosis protein, neuronal apoptosis inhibitor protein, livin, survivin, and human inhibitor of apoptosis protein), Fas, TNF receptor, TRAIL receptor, PI3K/AKT, FAK, prohibitin, BAK, BID, BAD, BCL, and caspases, are abnormally regulated in various tumors [31, 45–49]. HOTAIR depletion could be an effective way to promote cell apoptosis and increase chemotherapeutic sensitivity in multiple cancers.
The cell cycle is regulated by various molecules, including cyclins, cyclin-dependent kinases, cyclin-dependent kinase inhibitors, and cell cycle checkpoints. Defects of cell cycle checkpoint kinases and promotion of cell cycle progression are associated with changes in the response of cancer cells to chemotherapy.
A large number of studies have shown the vital role of HOTAIR in modulating the cell cycle and driving chemotherapy resistance (Table 2). For example, the HOTAIR expression level was upregulated in DDP-resistant adenocarcinoma cells compared to that in A549 cells. si-HOTAIR-mediated recession of chemotherapy resistance was associated with the induction of G0/G1 arrest via modulation of p21 expression [50]. Similarly, the study by Fang et al. [51] revealed that suppression of HOTAIR expression prolonged the S phase and increased cell sensitivity to DDP, adriamycin (ADM), and etoposide (VP-16) by modulating HOXA1 methylation in small cell lung cancer (SCLC). The collection and analysis of bioinformatic data has attracted increasing attention. Liu et al. [52] proposed that HOTAIR was related to cell cycle pathways using Kyoto Encyclopedia of Genes and Genomes analysis of The Cancer Genome Atlas public database. The underlying molecular mechanisms indicated that HOTAIR heightened cell resistance to gefitinib by accelerating the progression of the cell cycle from the G1 to S phase by modulating the Rb-E2F pathway. In another study, a traditional Chinese medicine formula Xiaoji decoction (XJD) was demonstrated to suppress cell proliferation and induce cell arrest at the G2/M phase in NSCLC. Mechanism analysis showed that XJD reinforced the effect of the antineoplastic drug gefitinib through downregulation of EP4 induced by HOTAIR silencing [53]. In GC, upregulation of HOTAIR facilitated G1 to S phase transition and led to DDP resistance; HOTAIR performed its function by sponging miR-126 to promote PI3K/AKT/MRP1 signaling [54]. Another study showed that abnormally overexpressed HOTAIR stimulated cell proliferation and cell cycle transition to the S phase. Upregulation of HOTAIR increased doxorubicin and paclitaxel (PTX) resistance by suppressing the miR-217/GPC5/PTPN14 pathway [55]. In ovarian cancer (OC), HOTAIR enhanced PTX resistance through upregulation of CHEK1 expression. In contrast, si-HOTAIR, which could be used as a therapeutic target for OC, accelerated cell cycle arrest at the G2/M phase and boosted cell sensitivity to PTX [56]. The study by Xiang et al. [57] demonstrated that polyphyllin I (PPI), a main component extracted from Rhizoma Paridis saponins, played an anticancer role in prostate cancer. Further investigation into the mechanisms indicated that PPI suppressed cell growth at the S phase through downregulation of the expression of HOTAIR and its downstream targets DNMT1 and EZH2. In multiple myeloma (MM), downregulation of HOTAIR inhibited cell viability by inducing cell cycle arrest at the G0/G1 phase. Functional assays validated that HOTAIR enhanced cell activity and chemotherapy resistance to dexamethasone (DEX) via the JAK2/STAT3 pathway in MM [58].
Table 2.
HOTAIR participates in cell cycle regulation.
| Cancer type | Expression | Related drugs | Target | Related genes or pathway | References |
|---|---|---|---|---|---|
| Lung cancer | upregulation | cisplatin | / | P21 | [50] |
| Lung cancer | upregulation | cisplatin, doxorubicin, and etoposide | / | HOXA1 | [51] |
| Lung cancer | upregulation | gefitinib | / | Rb-E2F | [52] |
| Lung cancer | upregulation | gefitinib | / | EP4 | [53] |
| Gastric cancer | upregulation | cisplatin | miR-126 | PI3K/AKT/MRP1 | [54] |
| Gastric cancer | upregulation | doxorubicin and paclitaxel | miR-217 | GPC5/PTPN14 | [55] |
| Ovarian cancer | upregulation | paclitaxel | / | CHEK1 | [56] |
| Prostate cancer | upregulation | / | / | DNMT1 and EZH2 | [57] |
| Myeloma | upregulation | dexamethasone | / | JAK2/STAT3 | [58] |
Taken together, the above data show that si-HOTAIR induces cell cycle arrest and decreases chemotherapy resistance in many cancers. Two important apical kinases, ataxia telangiectasia and Rad3-related and ataxia telangiectasia-mutated, are involved in the regulation of the relevant signal pathway through downstream signal relay proteins and effector proteins, such as CHK1, CHK2, MDM2, P53, BRCA1, PIK3, and CDC25 [59, 60]. Thus, it could be considered as a diagnostic biomarker and therapeutic target in cancer.
HOTAIR enhances EMT
EMT refers to the transformation of epithelial cells into cells with mesenchymal phenotypes. EMT plays important roles in embryonic development, chronic inflammation, tissue remodeling, organ fibrosis, as well as cancer metastasis [61].
Several studies indicate that HOTAIR takes part in the regulation of EMT in cancer cells, affecting chemotherapy resistance (Table 3). It has been reported that the HOTAIR expression level was associated with chemotherapy resistance to epidermal growth factor receptor tyrosine kinase inhibitors through activating EMT in NSCLC. Upregulation of HOTAIR retarded gefitinib sensitivity in NSCLC cells, which served as a predictive biomarker for gefitinib resistance [62]. Another group reported that sorafenib resistance was accelerated in cells with abnormally overexpressed HOTAIR in HCC. An in-depth molecular mechanism analysis showed that si-HOTAIR enhanced sorafenib sensitivity by decreasing the Vimentin level and increasing the E-cadherin level by upregulating miR-217, which indicated that EMT may participate in HOTAIR-induced chemotherapy resistance [63]. The study by Jia et al. [64] showed that HOTAIR overexpression expedited the EMT process by regulating the miR-17-5p/PTEN pathway in GC. Therefore, anti-HOTAIR treatment boosted the sensitivity to chemotherapeutic drugs. In another study, suppression of HOTAIR expression influenced the expression of EMT-related genes in BC. HOTAIR downregulation using siRNA weakened trastuzumab resistance through activating the MEK/MAPK and PI3K/AKT/mTOR pathways [65]. In prostate cancer, propofol strengthened PTX sensitivity by regulating HOTAIR-mediated EMT [66]. In GBM, HOTAIR knockdown suppressed tumor growth, migration, and invasion and EMT, whereas exosomal HOTAIR accelerated TMZ resistance by the miR-519a-3p/RRM1 pathway [67].
Table 3.
HOTAIR enhances EMT.
| Cancer type | Expression | Related drugs | Target | Related genes or pathway | References |
|---|---|---|---|---|---|
| Lung cancer | upregulation | gefitinib | / | Rb-E2F | [62] |
| Hepatocellular cancer | upregulation | sorafenib | miR-217 | Vimentin and E-cadherin | [63] |
| Gastric cancer | upregulation | cisplatin, adriamycin, mitomycin, and 5-fluoroura | miR-17-5p | PTEN | [64] |
| Breast cancer | upregulation | trastuzumab | / | MEK/MAPK and PI3K/AKT/mTOR | [65] |
| Prostate cancer | upregulation | paclitaxel | / | Bcl-2 and Bax | [66] |
| Glioblastoma | upregulation | temozolomide | miR-519a-3p | RRM1 | [67] |
Overall, emerging evidence shows that this process is driven by some EMT-inducing transcription factors (EMT-TFs), such as SNAI1/2, ZEB1/2, and TWIST1/2 [68]. EMT-TFs have been found to change chemotherapy resistance through several molecular mechanisms [69]. These data support the conclusion that HOTAIR enhances EMT and decreases chemotherapeutic sensitivity in various cancers.
HOTAIR enhances autophagy
Autophagy is the process in which autophagosomes, consisting of cytoplasmic proteins or organelles enclosed into vesicles, fuse with lysosomes to form autophagy lysosomes, delivering their contents for degradation, to realize the metabolic needs of cells and the renewal of some organelles [70]. In cancer, autophagy is a double-edged sword, depending on the cellular environment and the characteristics of the tumors, including tumor stage, grade, type, and genetic relationships [71].
Recently, a series of studies revealed that HOTAIR plays a vital role in autophagy-mediated chemotherapeutic sensitivity in human cancers (Table 4). In NSCLC, HOTAIR silencing attenuated drug resistance to crizotinib through suppressing autophagy by inhibiting the ULK1 pathway [72]. Soon after, a study reported that HOTAIR knockdown inhibited autophagy by downregulating autophagy-related genes and increased drug sensitivity to DDP in oral squamous cell carcinoma [73]. The study by Zhang et al. [74] demonstrated that HOTAIR targeted miR-130a, the ATG2B inhibitor, to promote drug resistance to imatinib by elevating autophagy levels in gastrointestinal stromal tumors. Similar results indicated that HOTAIR modulated cisplatin resistance through the modulation of autophagy by affecting MDR, Beclin-1, and P-glycoprotein (P-gp) expression in endometrial cancer [75]. Another research group found that HOTAIR was overexpressed in OC and that ATG7 was the downstream gene of HOTAIR. Downregulation of HOTAIR enhanced DDP sensitivity by suppressing DDP-induced cell autophagy [76]. In renal cancer, HOTAIR targeted miR-17-5p to stimulate Beclin1-mediated autophagy, thereby increasing sunitinib resistance [77].
Table 4.
HOTAIR enhances autophagy.
| Cancer type | Expression | Related drugs | Target | Related genes or pathway | References |
|---|---|---|---|---|---|
| Lung cancer | upregulation | crizotinib | / | ULK1 | [72] |
| Oral cavity carcinoma | upregulation | cisplatin | / | Autophagy-related gene | [73] |
| Gastrointestinal stromal tumor | upregulation | imatinib | miR-130a | ATG2B | [74] |
| Endometrial cancer | upregulation | cisplatin | / | MDR, Beclin-1, and P-gp | [75] |
| Ovarian cancer | upregulation | cisplatin | / | ATG7 | [76] |
| Renal cancer | upregulation | sunitinib | miR-17-5p | Beclin1 | [77] |
Taken together, under normal or mild stress conditions, mild autophagy is conducive to cell survival, whereas excessive autophagy results in cell death [78]. The process of autophagy involves multiple steps, each of which is regulated by a set of core autophagy related proteins and transcription factors [79]. Therefore, the regulation of chemotherapeutic sensitivity by intervening with the regulatory factors involved in the autophagy process has become a promising new approach for cancer treatment. These data confirm that HOTAIR could enhance autophagy and decrease chemotherapeutic sensitivity in human tumors, providing the foundation for novel and promising antitumor therapy approaches.
HOTAIR enhances the self-renewal ability of cancer stem cells
Cancer stem cells (CSCs) are cells with self-renewal abilities that lead to tumorigenesis [80, 81]. The abnormal self-renewal mechanism of CSCs leads to tumor cell proliferation and tumor tissue enlargement.
Recent studies have shown that lncRNAs regulate the stem cell properties of cancer cells (Table 5). Liu et al. [82] found that HOTAIR upregulation enhanced NSCLC cell resistance to DDP. Overexpression of HOTAIR increased the expression of CSC-related biomarkers and was correlated with Klf4 expression, which could be used as a promising therapeutic target. A year later, another group found that gemcitabine may induce HOTAIR as a tumor promoter. HOTAIR promotes PACN-1 CSC self-renewal ability, growth, and migration by inhibiting the chemosensitivity of PACN-1 CSCs, supporting its potential as a new therapeutic target for pancreatic cancer [83]. In BC, HOTAIR was found to be a vital regulator of the self-renewal ability of CSCs, which occurred partly through modulation of the miR-34a/Sox2/p53 signal pathway [84]. In prostate cancer, HOTAIR elevated the quantity of CSCs by activating the STAT3 signal pathway. Investigation of the mechanisms showed that HOTAIR sponged miR-590-5p to prevent it from binding the upstream molecule of STAT3. Targeting HOTAIR eliminated Docetaxel resistance in prostate cancer [85]. In OC, HOTAIR retarded the miR-206-mediated suppression of TBX3 levels and enhanced DDP drug resistance, providing a novel biomarker for OC treatment [86]. In another study, the HOTAIR level was upregulated in OC CSCs, which stimulated their colony-forming and spheroid-forming abilities. Interrupting the HOTAIR-EZH2 interaction and DNA methylation is likely to boost chemosensitivity and restrain cancer recurrence [87].
Table 5.
HOTAIR enhances the self-renewal ability of cancer stem cells.
| Cancer type | Expression | Related drugs | Target | Related genes or pathway | References |
|---|---|---|---|---|---|
| Lung cancer | upregulation | cisplatin | / | Klf4 | [82] |
| Pancreatic cancer | upregulation | gemcitabine | / | / | [83] |
| Prostatic cancer | upregulation | docetaxel | miR-590-5p | STAT3 | [85] |
| Ovarian cancer | upregulation | cisplatin | miR-206 | TBX3 | [86] |
| Ovarian cancer | upregulation | platinum/paclitaxel | / | / | [87] |
Thus, several important signaling pathways have been shown to be involved in the self-renewal of CSCs, including the Bmi1, Notch, Wnt, and Hedgehog signaling pathways [88–91]. HOTAIR enhances the self-renewal ability of CSCs and plays a vital role in cancer chemosensitivity and recurrence.
HOTAIR interferes with the DNA repair pathway
The DNA in every cell of the human body is suffered tens of thousands damage each day. DNA repair is a cellular response to DNA damage aiming to restore the DNA structure and function. DNA repair protects the genome from damage and mutation and is therefore important for cell survival [92]. Fortunately, cells contain a variety of DNA repair mechanisms, including: (1) nucleotide excision repair (NER), which removes large amounts of DNA adducts; (2) base excision repair (BER), which removes damaged bases; (3) mismatch repair (MMR), which recognizes base damage and incorporation errors; (4) cross-linking repair (ICLR), which removes interstrand cross-links; (5) nonhomologous end joining (NHEJ) and homologous recombination (HR), which repairs DNA backbone damage; (6) DNA damage response (DDR), which deals with multi-step complex DNA damage repair.
Qian et al. [93] revealed that overexpression of HOTAIR promoted the expression of DDR factors, including DNA-protein kinases, Ku protein (Ku70 and Ku80), and ATM. Alternatively, these signaling molecules in the double-strand break repair pathway can be blocked by EZH2. Another research group demonstrated that DNA damage induced HOTAIR expression in a p53-dependent manner, indicating that HOTAIR involved in complex DDR. Therefore, HOTAIR might mediate the resistance of oral squamous cells to doxorubicin and irradiation [68]. Similar results indicated that HOTAIR silencing sensitized BCa cells to radiation, induced DNA damage, suggesting that HOTAIR may be a novel theraputic target for BCa [94]. In addition, Gao et al. [95] reported that HOTAIR was positively correlated with the degree of DNA damage, which indicated that HOTAIR may be involved in the modulation of DNA damage induced by PAHs exposure. Gupta et al. [8] demonstrated that enhanced expression of HOTAIR induced genome-wide retargeting of PRC2, resulting in altered H3K27me3 gene expression, and increased cancer invasion and metastasis in a PRC2-dependent manner during BC progression. Similar results indicated that the HOTAIR level was associated with genome-wide reprogramming of PRC2 function and that HOTAIR upregulation could be a key factor in the progression of colorectal cancer (CRC) metastasis [96]. Recently, HOTAIR was found to affect chemotherapy resistance by interfering with DNA repair (Table 6). Liu et al. [50] reported that HOTAIR recruited EZH2 to transcriptionally inhibit the expression of p21WAF1/CIP1 by modifying H3K27me3, thereby triggering DDP resistance by inhibiting p21-dependent DNA repair suppression in NSCLC. Another study showed that HOTAIR modulated the activation of NF-κB by reducing the NF-κB inhibitor Iκ-Bα in OC. Furthermore, they demonstrated that HOTAIR played a key role in platinum chemotherapy resistance and cellular senescence by inducing prolonged NF-κB activation and expression of NF-κB target genes during DNA damage [97].
Table 6.
HOTAIR participates in interfering with DNA repair pathway.
Together, these studies demonstrate that HOTAIR participates in chemotherapy resistance by interfering with the DNA repair pathway.
HOTAIR affects the drug efflux pump
Scientists had demonstrated that a major factor in the development of MDR in cancer cells was the reduction of drug accumulation. Approximately 40 years ago, Juliano et al. [98] discovered that P-gp mediates the active flow of chemotherapeutic drugs from cancer cells, resulting in pleiotropic drug cross-resistance.
A multitude of data has shown that HOTAIR causes chemotherapy resistance by modulating ABC transporters to affect the drug efflux pump (Table 7). In GC, the evidence demonstrated that the high expression of HOTAIR was associated with DDP resistance. HOTAIR could directly bind and inhibit the expression of miR-126, thereby activating the PI3K/AKT/MRP1 pathway [54]. A year later, similar results indicated that downregulation of HOTAIR might enhance the sensitivity of CML cells to imatinib through the PI3K/AKT/MRP1 pathway [34]. In HCC, Zhou et al. [99] reported that HOTAIR silencing impaired STAT3 activity and multi-drug resistant protein 1 (MDR1, ABCB1) expression and decreased chemotherapy resistance to DDP. Therefore, HOTAIR could become a new potential therapeutic target for the reversal of MDR in HCC. Subsequent studies showed that TGF-β1 was involved in a novel MDR mechanism by upregulating BCRP and P-gp through the SMAD4/HOTAIR/miR-145 pathway in HCC [100]. In NSCLC, an in-depth study revealed that si-HOTAIR enhanced the sensitivity of cells to DDP by inhibiting the expression levels of MRP1 and MDR1, as well as the Wnt signaling pathway [40].
Table 7.
HOTAIR participates in affecting the drug efflux pump.
| Cancer type | Expression | Related drugs | Target | Related genes or pathway | References |
|---|---|---|---|---|---|
| Gastric cancer | upregulation | cisplatin | miR-126 | PI3K/AKT/MRP1 | [54] |
| Leukemia | upregulation | platinum | / | PI3K/AKT/MRP1 | [34] |
| Hepatocellular cancer | upregulation | cisplatin | / | MDR1 | [99] |
| Hepatocellular cancer | upregulation | imatinib | miR-145 | P-gp and BCRP | [100] |
| Lung cancer | upregulation | cisplatin | / | MRP1, MDR1 and Wnt | [40] |
These results confirm that HOTAIR is involved in chemotherapy resistance by affecting the drug efflux pump in human tumors.
HOTAIR influnces the TME
The TME refers to the close relationship between the occurrence, growth, and metastasis of tumors and the internal and external environment of tumor cells. It is a hypoxic and acidic environment, with high osmotic pressure, containing microbiota, inflammatory factors, tumor-associated macrophages, extracellular matrix and molecules, and cancer-associated fibroblasts.
Several studies have revealed that HOTAIR influences drug resistance in an altered TME (Table 8). In 2017, a peptide nucleic acid (PNA)-mediated approach was discovered to block and inhibit the activity of the HOTAIR-EZH2 interaction, which resensitized resistant OC to platinum. Mechanically, PNAs were conjugated to pH-low insertion peptide (pHLIP) to facilitate the delivery of anti-HOTAIR effects to an acidic (pH about 6) TME. The pHLIP-PNA complex suppressed the activity of HOTAIR and decreased tumor formation, suggesting a new approach for the treatment of solid tumor drug resistance [101].
Table 8.
HOTAIR influnces tumor microenvironment.
| Cancer type | Expression | Related drugs | Target | Related genes or pathway | References |
|---|---|---|---|---|---|
| Ovarian cancer | upregulation | platinum | / | / | [101] |
Overall, these data indicate that HOTAIR influences chemoresistance in an altered TME.
Therapeutic potential of HOTAIR in cancer
A large number of studies have shown the effect of HOTAIR on the occurrence, progression, and metastasis of multiple tumors. Therefore, treatment strategies based on HOTAIR, including RNA interference (RNAi), small molecule inhibitors and antisense oligonucleotides (ASOs), have attracted wide attention. Gupta et al. [8] adopted RNAi technology to target HOTAIR and create HOTAIR knockout. Similarly, Lennox et al. [102] found that both RNAi and ASOs effectively inhibited HOTAIR. In addition, Bhan et al. [103] invented a synthetic small interfering nucleotide DNA complementary to the HOTAIR transcript to suppress the expression of HOTAIR.
Moreover, there are some inhibitors that suppress HOTAIR function without altering its expression level. For instance, Li et al. [104] discovered a small molecule compound, AC1Q3QWB (AQB), functioning as a HOTAIR-EZH2 inhibitor to block PRC2 recruitment. One year later, Shi et al. [105] demonstrated that the combination of AQB and palbociclib led to a higher inhibition of growth and metastasis than that obtained using the single drug in glioma cells with high HOTAIR expression. Ozes et al. [101] revealed that overexpression of HOTAIR blocked the binding of HOTAIR to EZH2, thereby decreasing tumor invasion and increasing chemotherapy sensitivity in breast and ovarian cancer cells. Therefore, screening a larger library of natural and synthetic compounds to identify compounds that target the interaction between HOTAIR and PRC2 complexes could be an alternative strategy for targeting HOTAIR/ EZH2-dependent cancers.
The exact molecular mechanisms by which HOTAIR is involved in cancer progression are more intricate than originally thought and have not yet been applied in the clinic. More research is needed to further understand the role of HOTAIR in the pathogenesis of human cancers.
Conclusions and prospects
Chemotherapy is a cancer treatment that involves the use of chemical drugs to prevent the proliferation, invasion, and metastasis of cancer cells and to finally kill them. It is a systemic treatment and together with surgery and radiation constitutes the three major cancer treatment types. However, tumor cells can develop resistance to chemotherapeutic drugs, resulting in a significant reduction in the chemotherapeutic effect of the drugs. Therefore, chemotherapy resistance is problem that needs to be urgently solved.
Recently, lncRNAs have been widely recognized as an important biological regulator of the tumor progression process. Furthermore, some lncRNAs, including HOTAIR, have been found to be involved in chemotherapy resistance [3, 32, 106, 107]. According to the literature, HOTAIR-induced MDR has been shown to increase drug resistance and decrease drug sensitivity in a variety of cancers. In this review, we explored the different mechanisms by which HOTAIR induces drug resistance in various tumors and showed that HOTAIR could confer resistance to multiple anticancer drugs, such as doxorubicin, ADM, imatinib, DDP, 5-FU, taxol, progesterone, TMZ, VP-16, gefitinib, PTX, DEX, sorafenib, mitomycin, trastuzumab, crizotinib, sunitinib, gemcitabine, docetaxel, platinum, PTX, and oxaliplatin. Furthermore, a series of miRNAs were involved in HOTAIR-mediated chemotherapy resistance, such as miR-34a, miR-106a-5p, miR-125, miR-126, miR-217, miR-17-5p, miR-519a-3p, miR-130a, miR-590-5p, miR-206, and miR-145. Moreover, the Wnt/β-catenin and PI3K/Akt signaling pathways were two important mediators of HOTAIR chemoresistance. In addition, we found that most studies used RNA-seq to screen lncRNAs with different expression patterns between resistant and non-resistant cell lines to explore the mechanisms of chemotherapy resistance, and targeting these differentially expressed lncRNAs might contribute to the elimination of chemotherapy resistance.
According to literature reports, we can see that the current research on the role of HOTAIR in tumor chemotherapy resistance is still in its infancy. There are still some challenges to be solved. First, studies of HOTAIR in cancer chemotherapy resistance are limited to a few classic drugs and a few cancer types. More drugs and cancer types need to be investigated. Second, HOTAIR polymorphisms and their relationship with chemotherapy response have not been evaluated in a large cohort of patients. Therefore, future studies should be performed to fill this research gap. Third, although many studies revealed that HOTAIR was associated with chemotherapy resistance, few studies investigated how drug resistance can be reduced by inhibiting HOTAIR expression in clinical practice. Further clinical studies may provide a new direction for exploring the mechanisms of HOTAIR-related drug resistance.
In conclusion, HOTAIR is closely involved in tumor development and associated with chemotherapy resistance. We summarized the various mechanisms by which HOTAIR leads to tumor resistance and conclude that HOTAIR may play an important role in cancer therapy in the future. Therefore, further exploration in this field could provide additional approaches to the treatment of tumor drug resistance.
Acknowledgements
We thank Yun Cui from the Department of Urology, National Urological Cancer Center, Peking University First Hospital and Institute of Urology for helping us prepare the manuscript.
Author contributions
KW and YW conceived the review; KW, CZ, and XW wrote the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China (Grant No. 82072835) to KW, Key Research and Development Joint Program of Liaoning Province (Grant No. 2020JH 2/10300139) to KW, Natural Science Foundation of Liaoning Province (Grant No. 2019-MS-360) to KW, Shenyang Science and Technology Bureau Plan Projects (Grant No. 20-205-4-076) to KW, 345 Talent Project of Shengjing Hospital of China Medical University (Grant No. M0366) to KW, Outstanding Scientific Fund of Shengjing Hospital to KW.
Competing interests
The authors declare no competing interests.
Consent for publication
Written consent for publication was obtained from all the participants.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yuan Wang, Email: wangy10@sj-hospital.org.
Kefeng Wang, Email: wang.kefeng@hotmail.com.
References
- 1.Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–66. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang K, Jin W, Song Y, Fei X. LncRNA RP11-436H11.5, functioning as a competitive endogenous RNA, upregulates BCL-W expression by sponging miR-335-5p and promotes proliferation and invasion in renal cell carcinoma. Mol Cancer. 2017;16:166. doi: 10.1186/s12943-017-0735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang M, Han D, Yuan Z, Hu H, Zhao Z, Yang R, et al. Long non-coding RNA H19 confers 5-Fu resistance in colorectal cancer by promoting SIRT1-mediated autophagy. Cell Death Dis. 2018;9:1149. doi: 10.1038/s41419-018-1187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao Y, Ma J, Xue Y, Wang P, Li Z, Liu Z, et al. Knockdown of long non-coding RNA XIST exerts tumor-suppressive functions in human glioblastoma stem cells by up-regulating miR-152. Cancer Lett. 2015;359:75–6. doi: 10.1016/j.canlet.2014.12.051. [DOI] [PubMed] [Google Scholar]
- 5.Deng H, Zhang J, Shi J, Guo Z, He C, Ding L, et al. Role of long non-coding RNA in tumor drug resistance. Tumour Biol. 2016;37:11623–31. doi: 10.1007/s13277-016-5125-8. [DOI] [PubMed] [Google Scholar]
- 6.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–23. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woo CJ, Kingston RE. HOTAIR lifts noncoding RNAs to new levels. Cell. 2007;129:1257–9. doi: 10.1016/j.cell.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–6. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69:363–85. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 10.Wakeam E, Acuna SA, Leighl NB, Giuliani ME, Finlayson SRG, Varghese TK, et al. Surgery versus chemotherapy and radiotherapy for early and locally advanced small cell lung cancer: a propensity-matched analysis of survival. Lung Cancer. 2017;109:78–88. doi: 10.1016/j.lungcan.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 11.Ashdown ML, Robinson AP, Yatomi-Clarke SL, Ashdown ML, Allison A, Abbott D, et al. Chemotherapy for late-stage cancer patients: meta-analysis of complete response rates. F1000Res. 2015;4:232. doi: 10.12688/f1000research.6760.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B. The different mechanisms of cancer drug resistance: a brief review. Adv Pharm Bull. 2017;7:339–48. doi: 10.15171/apb.2017.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luqmani YA. Mechanisms of drug resistance in cancer chemotherapy. Med Princ Pr. 2005;14:35–48. doi: 10.1159/000086183. [DOI] [PubMed] [Google Scholar]
- 14.Nunes T, Hamdan D, Leboeuf C, Bouchtaoui MEI, Gapihan G, Nguyen TT, et al. Targeting cancer stem cells to overcome chemoresistance. Int J Mol Sci. 2018;19:4036. doi: 10.3390/ijms19124036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhan A, Mandal SS. LncRNA HOTAIR: A master regulator of chromatin dynamics and cancer. Biochim Biophys Acta. 2015;1856:151–64. doi: 10.1016/j.bbcan.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majello B, Gorini F, Sacca CD, Amente S. Expanding the role of the histone lysine-specific demethylase LSD1 in cancer. Cancers (Basel) 2019;11:324. doi: 10.3390/cancers11030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Margueron R, Reinberg D. The polycomb complex PRC2 and its mark in life. Nature. 2011;469:343–9. doi: 10.1038/nature09784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhan A, Mandal SS. Long noncoding RNAs: emerging stars in gene regulation, epigenetics and human disease. ChemMedChem. 2014;9:1932–56. doi: 10.1002/cmdc.201300534. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Goodrich KJ, Gooding AR, Naeem H, Archer S, Paucek RD, et al. Targeting of polycomb repressive complex 2 to RNA by short repeats of consecutive guanines. Mol Cell. 2017;65:1056–67. doi: 10.1016/j.molcel.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Long Y, Bolanos B, Gong L, Liu W, Goodrich KJ, Yang X, et al. Conserved RNA-binding specificity of polycomb repressive complex 2 is achieved by dispersed amino acid patches in EZH2. Elife. 2017;6:e31558. doi: 10.7554/eLife.31558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fay MM, Lyons SM, Ivanov P. Quadruplexes in biology: principles and molecular mechanisms. J Mol Biol. 2017;429:2127–47. doi: 10.1016/j.jmb.2017.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y, Wang Y, Lin M, Wu D, Zhao M. LncRNA HOTAIR promotes proliferation and inhibits apoptosis by sponging miR-214-3p in HPV16 positive cervical cancer cells. Cancer Cell Int. 2021;21:400. doi: 10.1186/s12935-021-02103-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia S, Xie D, Jin H, Fan S, Wang X, Xu L. Long non-coding RNA HOTAIR as a competitive endogenous RNA to sponge miR-206 to promote colorectal cancer progression by activating CCL2. J Cancer. 2020;11:4431–41. doi: 10.7150/jca.42308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Y, Liu J, Zheng Y, You L, Kuang D, Liu T. Suppressed expression of long non-coding RNA HOTAIR inhibits proliferation and tumourigenicity of renal carcinoma cells. Tumour Biol. 2014;35:11887–94. doi: 10.1007/s13277-014-2453-4. [DOI] [PubMed] [Google Scholar]
- 25.Schorderet P, Doboule D. Structural and functional differences in the long non-coding RNA hotair in mouse and human. PLoS Genet. 2011;7:e1002071. doi: 10.1371/journal.pgen.1002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He S, Liu S, Zhu H. The sequence, structure and evolutionary features of HOTAIR in mammals. BMC Evol Biol. 2011;11:102. doi: 10.1186/1471-2148-11-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan SK, Pastori C, Penas C, Komotar RJ, Ivan ME, Wahlestedt C, et al. Serum long noncoding RNA HOTAIR as a novel diagnostic and prognostic biomarker in glioblastoma multiforme. Mol Cancer. 2018;17:74. doi: 10.1186/s12943-018-0822-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mozdarani H, Ezzatizadeh V, Parvaneh RR. The emerging role of the long non-coding RNA HOTAIR in breast cancer development and treatment. J Transl Med. 2020;18:152. doi: 10.1186/s12967-020-02320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carrion K, Dyo J, Patel V, Sasik R, Mohamed SA, Hardiman G, et al. The long non-coding HOTAIR is modulated by cyclic stretch and WNT/beta-CATENIN in human aortic valve cells and is a novel repressor of calcification genes. PLoS One. 2014;9:e96577. doi: 10.1371/journal.pone.0096577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoon JH, Abdelmohsen K, Kim J, Yang X, Martindale JL, Tominaga-Yamanaka K, et al. Scaffold function of long non-coding RNA HOTAIR in protein ubiquitination. Nat Commun. 2013;4:2939. doi: 10.1038/ncomms3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fraser M, Leung B, Jahani-Asl A, Yan X, Thompson WE, Tsang BK. Chemoresistance in human ovarian cancer: the role of apoptotic regulators. Reprod Biol Endocrinol. 2003;1:66. doi: 10.1186/1477-7827-1-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li M, Wang Y, Xu Y, Lu Q. Overexpression of LncRNA-HOTAIR promotes chemoresistance in acute leukemia cells. Int J Clin Exp Pathol. 2020;13:3044–51. [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou W, Xu S, Chen X, Wang C. HOTAIR suppresses PTEN via DNMT3b and confers drug resistance in acute myeloid leukemia. Hematology. 2021;26:170–8. doi: 10.1080/16078454.2021.1880733. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Li Q, Tang S, Li M, Feng A, Qin L, et al. The role of long noncoding RNA HOTAIR in the acquired multidrug resistance to imatinib in chronic myeloid leukemia cells. Hematology. 2017;22:208–16. doi: 10.1080/10245332.2016.1258152. [DOI] [PubMed] [Google Scholar]
- 35.Li Z, Qian J, Li J, Zhu C. Knockdown of lncRNA-HOTAIR downregulates the drug-resistance of breast cancer cells to doxorubicin via the PI3K/AKT/mTOR signaling pathway. Exp Ther Med. 2019;18:435–42. doi: 10.3892/etm.2019.7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng C, Qin Y, Zhi Q, Wang J, Qin C. Knockdown of long non-coding RNA HOTAIR inhibits cisplatin resistance of gastric cancer cells through inhibiting the PI3K/Akt and Wnt/β-catenin signaling pathways by up-regulating miR-34a. Int J Biol Macromol. 2018;107:2620–9. doi: 10.1016/j.ijbiomac.2017.10.154. [DOI] [PubMed] [Google Scholar]
- 37.Zhang S, Zheng F, Zhang L, Huang Z, Huang X, Pan Z, et al. LncRNA HOTAIR-mediated MTHFR methylation inhibits 5-fluorouracil sensitivity in esophageal cancer cells. J Exp Clin Cancer Res. 2020;39:131. doi: 10.1186/s13046-020-01610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duan Y, Chen J, Yang Y, Qu Z, Lu Y, Sun D. LncRNA HOTAIR contributes Taxol-resistance of hepatocellular carcinoma cells via activating AKT phosphorylation by down-regulating miR-34a. Biosci Rep. 2020;40:BSR20201627. doi: 10.1042/BSR20201627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang S, Xu F, Zhou T, Zhao X, McDonald JM, Chen Y. The long non-coding RNA HOTAIR enhances pancreatic cancer resistance to TNF-related apoptosis-inducing ligand. J Biol Chem. 2017;292:10390–7. doi: 10.1074/jbc.M117.786830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo F, Cao Z, Guo H, Li S. The action mechanism of lncRNA-HOTAIR on the drug resistance of non-small cell lung cancer by regulating Wnt signaling pathway. Exp Ther Med. 2018;15:4885–9. doi: 10.3892/etm.2018.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang S, Shen Y, Chen B, Wu Y, Jia L, Li Y, et al. H3K27me3 induces multidrug resistance in small cell lung cancer by affecting HOXA1 DNA methylation via regulation of the lncRNA HOTAIR. Ann Transl Med. 2018;6:440. doi: 10.21037/atm.2018.10.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo J, Dou D, Zhang T, Wang B. HOTAIR Promotes Cisplatin Resistance of Osteosarcoma Cells by Regulating Cell Proliferation, Invasion, and Apoptosis via miR-106a-5p/STAT3 Axis. Cell Transpl. 2020;29:963689720948447. doi: 10.1177/0963689720948447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chi S, Liu Y, Zhou X, Feng D, Xiao X, Li W, et al. Knockdown of long non-coding HOTAIR enhances the sensitivity to progesterone in endometrial cancer by epigenetic regulation of progesterone receptor isoform B. Cancer Chemother Pharm. 2019;83:277–87. doi: 10.1007/s00280-018-3727-0. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J, Chen G, Gao Y, Liang H. HOTAIR/miR-125 axis-mediated Hexokinase 2 expression promotes chemoresistance in human glioblastoma. J Cell Mol Med. 2020;24:5707–17. doi: 10.1111/jcmm.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verhagen AM, Coulson EJ, Vaux DL. Inhibitor of apoptosis proteins and their relatives: IAPs and other BIRPs. Genome Biol. 2001;2:REVIEWS3009. doi: 10.1186/gb-2001-2-7-reviews3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krammer PH. CD95(APO-1/Fas)-mediated apoptosis: live and let die. Adv Immunol. 1999;71:163–210. doi: 10.1016/S0065-2776(08)60402-2. [DOI] [PubMed] [Google Scholar]
- 47.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, et al. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–21. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 48.Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene. 2003;22:9030–40. doi: 10.1038/sj.onc.1207116. [DOI] [PubMed] [Google Scholar]
- 49.Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30:87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Z, Sun M, Lu K, Liu J, Zhang M, Wu W, et al. The long noncoding RNA HOTAIR contributes to cisplatin resistance of human lung adenocarcinoma cells via downregualtion of p21(WAF1/CIP1) expression. PLoS One. 2013;8:e77293. doi: 10.1371/journal.pone.0077293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fang S, Gao H, Tong Y, Yang J, Tang R, Niu Y, et al. Long noncoding RNA-HOTAIR affects chemoresistance by regulating HOXA1 methylation in small cell lung cancer cells. Lab Invest. 2016;96:60–8. doi: 10.1038/labinvest.2015.123. [DOI] [PubMed] [Google Scholar]
- 52.Liu M, Zhang H, Li Y, Wang R, Li Y, Zhang H, et al. HOTAIR, a long noncoding RNA, is a marker of abnormal cell cycle regulation in lung cancer. Cancer Sci. 2018;109:2717–33. doi: 10.1111/cas.13745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu J, Tang Q, Ren X, Zheng F, He C, Chai X, et al. Reciprocal interaction of HOTAIR and SP1 together enhance the ability of Xiaoji decoction and gefitinib to inhibit EP4 expression. J Ethnopharmacol. 2019;237:128–40. doi: 10.1016/j.jep.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 54.Yan J, Dang Y, Liu S, Zhang Y, Zhang G. LncRNA HOTAIR promotes cisplatin resistance in gastric cancer by targeting miR-126 to activate the PI3K/AKT/MRP1 genes. Tumour Biol. 2016;30:16345–55. [DOI] [PubMed]
- 55.Wang H, Qin R, Guan A, Yao Y, Huang Y, Jia H, et al. HOTAIR enhanced paclitaxel and doxorubicin resistance in gastric cancer cells partly through inhibiting miR-217 expression. J Cell Biochem. 2018;119:7226–34. doi: 10.1002/jcb.26901. [DOI] [PubMed] [Google Scholar]
- 56.Jiang J, Wang S, Wang Z, Cai J, Han L, Xie L, et al. HOTAIR promotes paclitaxel resistance by regulating CHEK1 in ovarian cancer. Cancer Chemother Pharm. 2020;86:295–305. doi: 10.1007/s00280-020-04120-1. [DOI] [PubMed] [Google Scholar]
- 57.Xiang S, Zou P, Tang Q, Zheng F, Wu J, Chen Z, et al. HOTAIR-mediated reciprocal regulation of EZH2 and DNMT1 contribute to polyphyllin I-inhibited growth of castration-resistant prostate cancer cells in vitro and in vivo. Biochim Biophys Acta Gen Subj. 2018;1862:589–99. doi: 10.1016/j.bbagen.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 58.Guan R, Wang W, Fu B, Pang Y, Lou Y, Li H. Increased lncRNA HOTAIR expression promotes the chemoresistance of multiple myeloma to dexamethasone by regulating cell viability and apoptosis by mediating the JAK2/STAT3 signaling pathway. Mol Med Rep. 2019;20:3917–23. doi: 10.3892/mmr.2019.10603. [DOI] [PubMed] [Google Scholar]
- 59.Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–96. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- 60.Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003;3:421–9. doi: 10.1016/S1535-6108(03)00110-7. [DOI] [PubMed] [Google Scholar]
- 61.Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 62.Wang Q, Li X, Ren S, Su C, Li C, Li W, et al. HOTAIR induces EGFR-TKIs resistance in non-small cell lung cancer through epithelial-mesenchymal transition. Lung Cancer. 2020;147:99–105. doi: 10.1016/j.lungcan.2020.06.037. [DOI] [PubMed] [Google Scholar]
- 63.Tang X, Zhang W, Ye Y, Li H, Cheng L, Zhang M, et al. LncRNA HOTAIR contributes to sorafenib resistance through suppressing miR-217 in hepatic carcinoma. Biomed Res Int. 2020;2020:9515071. doi: 10.1155/2020/9515071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jia J, Zhan D, Li J, Li Z, Li H, Qian J. The contrary functions of lncRNA HOTAIR/miR-17-5p/PTEN axis and Shenqifuzheng injection on chemosensitivity of gastric cancer cells. J Cell Mol Med. 2019;23:656–69. doi: 10.1111/jcmm.13970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen T, Liu Z, Zeng W, Huang T. Down-regulation of long non-coding RNA HOTAIR sensitizes breast cancer to trastuzumab. Sci Rep. 2019;9:19881. doi: 10.1038/s41598-019-53699-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang X, Qin J, Gong C, Yang J. Propofol enhanced the cell sensitivity to paclitaxel (PTX) in prostatic cancer (PC) through modulation of HOTAIR. Genes Genomics. 2021;43:807–14. doi: 10.1007/s13258-021-01093-0. [DOI] [PubMed] [Google Scholar]
- 67.Yuan Z, Yang Z, Li W, Wu A, Su Z, Jiang B. Exosome-mediated transfer of long noncoding RNA HOTAIR regulates temozolomide resistance by miR-519a-3p/RRM1 axis in glioblastoma. Cancer Biother Radiopharm. 2020;37:515–28. [DOI] [PubMed]
- 68.Liu H, Li Z, Wang C, Feng L, Huang H, Liu C, et al. Expression of long non-coding RNA-HOTAIR in oral squamous cell carcinoma Tca8113 cells and its associated biological behavior. Am J Transl Res. 2016;8:4726–34. [PMC free article] [PubMed] [Google Scholar]
- 69.Staalduinen JV, Baker D, Dijke PT, Dam H. Epithelial-mesenchymal-transition- inducing transcription factors: new targets for tackling chemoresistance in cancer? Oncogene. 2018;37:6195–211. doi: 10.1038/s41388-018-0378-x. [DOI] [PubMed] [Google Scholar]
- 70.Janku F, McConkey DJ, Hong DS, Kurzrock R. Autophagy as a target for anticancer therapy. Nat Rev Clin Oncol. 2011;8:528–39. doi: 10.1038/nrclinonc.2011.71. [DOI] [PubMed] [Google Scholar]
- 71.Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5:726–34. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- 72.Yang Y, Jiang C, Yang Y, Guo L, Huang J, Liu X, et al. Silencing of LncRNA-HOTAIR decreases drug resistance of Non-Small Cell Lung Cancer cells by inactivating autophagy via suppressing the phosphorylation of ULK1. Biochem Biophys Res Commun. 2018;497:1003–10. doi: 10.1016/j.bbrc.2018.02.141. [DOI] [PubMed] [Google Scholar]
- 73.Wang X, Liu W, Wang P, Li S. RNA interference of long noncoding RNA HOTAIR suppresses autophagy and promotes apoptosis and sensitivity to cisplatin in oral squamous cell carcinoma. J Oral Pathol Med. 2018;47:930–7. doi: 10.1111/jop.12769. [DOI] [PubMed] [Google Scholar]
- 74.Zhang J, Chen K, Tang Y, Luan X, Zheng X, Lu X, et al. LncRNA-HOTAIR activates autophagy and promotes the imatinib resistance of gastrointestinal stromal tumor cells through a mechanism involving the miR-130a/ATG2B pathway. Cell Death Dis. 2021;12:367. doi: 10.1038/s41419-021-03650-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun M, Zhu J, Zhang C, Zhang M, Song Y, Rahman K, et al. Autophagy regulated by lncRNA HOTAIR contributes to the cisplatin-induced resistance in endometrial cancer cells. Biotechnol Lett. 2017;39:1477–84. doi: 10.1007/s10529-017-2392-4. [DOI] [PubMed] [Google Scholar]
- 76.Yu Y, Zhang X, Tian H, Zhang Z, Tian Y, et al. Knockdown of long non-coding RNA HOTAIR increases cisplatin sensitivity in ovarian cancer by inhibiting cisplatin-induced autophagy. J BUON. 2018;23:1396–401. [PubMed] [Google Scholar]
- 77.Li D, Li C, Chen Y, Teng L, Cao Y, Wang W, et al. LncRNA HOTAIR induces sunitinib resistance in renal cancer by acting as a competing endogenous RNA to regulate autophagy of renal cells. Cancer Cell Int. 2020;20:338. doi: 10.1186/s12935-020-01419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moosavi MA, Haghi A, Rahmati M, Taniguchi H, Mocan A, Echeverria J, et al. Phytochemicals as potent modulators of autophagy for cancer therapy. Cancer Lett. 2018;424:46–69. doi: 10.1016/j.canlet.2018.02.030. [DOI] [PubMed] [Google Scholar]
- 79.Fullgrabe J, Klionsky DJ, Joseph B. The return of the nucleus: transcriptional and epigenetic control of autophagy. Nat Rev Mol Cell Biol. 2014;15:65–74. doi: 10.1038/nrm3716. [DOI] [PubMed] [Google Scholar]
- 80.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CHM, Jones DL, et al. Cancer stem cells-perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–44. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 81.Quintana E, Shackleton M, Foster HR, Fullen DR, Sabel MS, Johnson TM, et al. Phenotypic heteroge-neity among tumorigenic melanoma cells from patients that is reversible and not hierarchically organized. Cancer Cell. 2010;18:510–23. doi: 10.1016/j.ccr.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu M, Li X, Gao T, Cui Y, Ma N, Zhou Y, et al. Elevated HOTAIR expression associated with cisplatin resistance in non-small cell lung cancer patients. J Thorac Dis. 2016;8:3314–22. doi: 10.21037/jtd.2016.11.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang L, Dong P, Wang W, Huang M, Tian B. Gemcitabine treatment causes resistance and malignancy of pancreatic cancer stem-like cells via induction of lncRNA HOTAIR. Exp Ther Med. 2017;14:4773–80. doi: 10.3892/etm.2017.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deng J, Yang M, Jiang R, An N, Wang X, Liu B. Long non-coding RNA HOTAIR regulates the proliferation, self-renewal capacity, tumor formation and migration of the cancer stem-like cell (CSC) subpopulation enriched from breast cancer cells. PLoS One. 2017;12:e0170860. doi: 10.1371/journal.pone.0170860. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 85.Wang N, Jiang Y, Lv S, Wen H, Wu D, Wei Q, et al. HOTAIR expands the population of prostatic cancer stem-like cells and causes Docetaxel resistance via activating STAT3 signaling. Aging (Albany NY) 2020;12:12771–82. doi: 10.18632/aging.103188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Y, Guo J, Cai E, CCai J, Wen Y, Lu S, et al. HOTAIR maintains the stemness of ovarian cancer stem cells via the miR-206/TBX3 axis. Exp Cell Res. 2020;395:112218. doi: 10.1016/j.yexcr.2020.112218. [DOI] [PubMed] [Google Scholar]
- 87.Wang W, Fang F, Ozes A, Nephew KP. Targeting ovarian cancer stem cells by dual inhibition of HOTAIR and DNA methylation. Mol Cancer Ther. 2021;20:1092–101. doi: 10.1158/1535-7163.MCT-20-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kong Y, Ai C, Dong F, Xia X, Zhao X, Yang C, et al. Targeting of BMI-1 with PTC-209 inhibits glioblastoma development. Cell Cycle. 2018;17:1199–211. doi: 10.1080/15384101.2018.1469872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Harrison H, Farnie G, Howell SJ, Rock RE, Stylianou S, Brennan KR, et al. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res. 2010;70:709–18. doi: 10.1158/0008-5472.CAN-09-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Won HY, Lee JY, Shin DH, Park J, Nam J, Kim H, et al. Loss of Mel-18 enhances breast cancer stem cell activity and tumorigenicity through activating Notch signaling mediated by the Wnt/TCF pathway. FASEB J. 2012;26:5002–13. doi: 10.1096/fj.12-209247. [DOI] [PubMed] [Google Scholar]
- 91.Po A, Ferretti E, Miele E, Smaele ED, Paganelli A, Canettieri G, et al. Hedgehog controls neural stem cells through p53-independent regulation of Nanog. EMBO J. 2010;29:2646–58. doi: 10.1038/emboj.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang J, Lindahl T. Maintenance of Genome Stability. Genomics Proteom Bioinforma. 2016;14:119–21. doi: 10.1016/j.gpb.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qian L, Fei Q, Zhang H, Qiu M, Zhang B, Wang Q. et al. lncRNA HOTAIR promotes DNA repair and radioresistance of breast cancer via EZH2. DNA Cell Biol. 2020;39:2166–73. [DOI] [PubMed]
- 94.Hu X, Ding D, Zhang J, Cui J. Knockdown of lncRNA HOTAIR sensitizes breast cancer cells to ionizing radiation through activating miR-218. Biosci Rep. 2019;39:BSR20181038. doi: 10.1042/BSR20181038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gao C, He Z, Li J, Li X, Bai Q, Zhang Z, et al. Specific long non-coding RNAs response to occupational PAHs exposure in coke oven workers. Toxicol Rep. 2016;3:160–6. doi: 10.1016/j.toxrep.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, et al. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71:6320–6. doi: 10.1158/0008-5472.CAN-11-1021. [DOI] [PubMed] [Google Scholar]
- 97.Ozes AR, Miller DF, Ozes ON, Fang F, Liu Y, Matei D, et al. NF-κB-HOTAIR axis links DNA damage response, chemoresistance and cellular senescence in ovarian cancer. Oncogene. 2016;35:5350–61. doi: 10.1038/onc.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455:152–62. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- 99.Zhou J, Cheng D, He X, Meng Z, Ye H, Chen R. Knockdown of long non-coding RNA HOTAIR sensitizes hepatocellular carcinoma cell to cisplatin by suppressing the STAT3/ABCB1 signaling pathway. Oncol Lett. 2017;14:7986–92. doi: 10.3892/ol.2017.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kong J, Qiu Y, Li Y, Zhang H, Wang W. TGF-β1 elevates P-gp and BCRP in hepatocellular carcinoma through HOTAIR/miR-145 axis. Biopharm Drug Dispos. 2019;40:70–80. doi: 10.1002/bdd.2172. [DOI] [PubMed] [Google Scholar]
- 101.Ozes AR, Wang Y, Zong X, Fang F, Pilrose J, Nephew KP. Therapeutic targeting using tumor specific peptides inhibits long non-coding RNA HOTAIR activity in ovarian and breast cancer. Sci Rep. 2017;7:894. doi: 10.1038/s41598-017-00966-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lennox KA, Behlke MA. Cellular localization of long non-coding RNAs affects silencing by RNAi more than by antisense oligonucleotides. Nucleic Acids Res. 2016;44:863–77. doi: 10.1093/nar/gkv1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bhan A, Hussain I, Ansari KI, Kasiri S, Bashyal A, Mandal SS. Antisense transcript long noncoding RNA (lncRNA) HOTAIR is transcriptionally induced by estradiol. J Mol Biol. 2013;425:3707–22. doi: 10.1016/j.jmb.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li Y, Ren Y, Wang Y, Tan Y, Wang Q, Cai J, et al. A compound AC1Q3QWB selectively disrupts HOTAIR-mediated recruitment of PRC2 and enhances cancer therapy of DZNep. Theranostics. 2019;9:4608–23. doi: 10.7150/thno.35188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shi J, Lv S, Wu M, Wang X, Deng Y, Li Y, et al. HOTAIR-EZH2 inhibitor AC1Q3QWB upregulates CWF19L1 and enhances cell cycle inhibition of CDK4/6 inhibitor palbociclib in glioma. Clin Transl Med. 2020;10:182–8. doi: 10.1002/ctm2.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hu Y, Yu Y, You S, Li K, Tong X, Chen S, et al. Long noncoding RNA MALAT1 regulates autophagy associated chemoresistance via miR-23b-3p sequestration in gastric cancer. Mol Cancer. 2017;16:174. doi: 10.1186/s12943-017-0743-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Du P, Zhao H, Peng R, Liu Q, Yuan J, Peng G, et al. LncRNA-XIST interacts with miR-29c to modulate the chemoresistance of glioma cell to TMZ through DNA mismatch repair pathway. Biosci Rep. 2017;37:BSR20170696. doi: 10.1042/BSR20170696. [DOI] [PMC free article] [PubMed] [Google Scholar]