Abstract
Background
In metastatic colorectal cancer (mCRC), regorafenib (RGF), a multi-kinase inhibitor with angiogenic inhibition has modest effects on survival. We reported that autophagy modulation using hydroxychloroquine (HCQ), enhances the anticancer activity of the histone deacetylase inhibitor, vorinostat (VOR), in mCRC, is well tolerated, and has comparable activity to RGF. Thus, we conducted a prospective study of VOR/HCQ versus RGF in mCRC.
Methods
This is a randomised, controlled trial of VOR 400 mg and HCQ 600 mg orally daily versus RGF 160 mg orally daily (3 weeks on/1 week off), every 4 weeks, in patients with mCRC. Primary endpoint: median progression-free survival (mPFS). Secondary endpoints: median overall survival (mOS); adverse events; pharmacodynamic analyses.
Results
From 2/2015-10/2017, 42 patients were randomised to VOR/HCQ and RGF. Median age was 58.4 years. mPFS on VOR/HCQ was 1.9 months versus 4.35 months with RGF (P = 0.032). There was no difference in mOS (P = 0.9). Treatment was tolerated in both arms. In both arms, there was improved anti-tumour immunity.
Conclusions
VOR/HCQ had an inferior PFS when compared to RGF, although there was an increase in anti-tumour immunity in mCRC. VOR/HCQ has a favourable safety profile, and immune or tumour biomarkers may be used to identify clinical benefit of autophagy modulation in mCRC.
Clinical trial registration
Subject terms: Colorectal cancer, Drug development
Background
In metastatic colorectal cancer (mCRC), systemic treatments have been limited to cytotoxic chemotherapy, agents targeting the vascular endothelial growth factor (VEGF), and agents targeting epidermal growth factor receptor (EGFR). Regorafenib (RGF), an oral multi-kinase inhibitor with predominant angiogenic or VEGF inhibition, has been approved for patients with mCRC who have progressed on standard therapies, including anti-VEGF agent bevacizumab [1]. Compared to placebo, the Phase III CORRECT study showed RGF improved median overall survival (mOS) from 5.0 to 6.4 months and median progression-free survival (mPFS) from 1.7 to 1.9 months, regardless of K-Ras mutational status [2]. Given the modest improvements in survival and clinical studies showing lack of efficacy with continued VEGF inhibition following the progression of anti-VEGF therapies, such as bevacizumab, novel therapies continue to be desperately needed in the third-line setting [3].
Therapeutic agents, such as the histone deacetylase (HDAC) inhibitor, vorinostat (VOR), have shown to induce an apoptotic response in mCRC, but also induce autophagy, which results in blunting of VOR’s anti-cancer activity [4]. Thus, therapeutics that derange the autophagy pathway may augment the efficacy of VOR as a novel therapeutic in mCRC [4]. Further, there is growing evidence that increased autophagy in tumours results in immune evasion by preventing effector T-cell-mediated cytotoxicity [5, 6]. Investigators at our institution have shown that autophagy inhibition using hydroxychloroquine (HCQ) enhanced the apoptotic activity of the histone deacetylase (HDAC) inhibitor, VOR, via ubiquitinated protein accumulation in pre-clinical CRC models [6]. An increase in lysosomal protease cathepsin D (CTSD) was found to be a key mediator of pro-apoptotic cell death [5, 6]. Based on this preclinical data, we completed a Phase I clinical study evaluating the safety, efficacy, and pharmacokinetics of the combination VOR and HCQ and found that patients with mCRC obtain prolonged clinical benefit with the combination therapy with VOR and HCQ [7]. Patient who received VOR in combination with HCQ had significantly increased intra-tumoral p21, cathepsin D, and LC3B, consistent with autophagy inhibition [7].
This study led to a Phase II single-arm study of VOR 400 milligrams (mg) orally daily plus HCQ 600 mg orally daily in patients with mCRC (NCT01023737) [8]. The primary endpoint was mPFS. Secondary endpoints include mOS, adverse events (AE), pharmacodynamics of inhibition of autophagy in primary tumours, immune cell analyses, and cytokine levels [8]. Twenty patients were enrolled (19 evaluable for survival) with a mPFS of 2.8 months and mOS of 6.7 months [8]. Grades 3–4 treatment-related adverse events (TR-AEs) occurred in eight patients (40%), with fatigue, nausea/vomiting, and anaemia being the most common [8]. Treatment significantly reduced CD4 + CD25hiFoxp3 + regulatory and PD-1 + (exhausted) CD4 + and CD8 + T cells and decreased CD45RO-CD62L + (naive) T cells, consistent with improved anti-tumour immunity [8]. On-study tumour biopsies showed increases in lysosomal protease cathepsin D and p62 accumulation, consistent with autophagy inhibition [8]. Taken together, VOR plus HCQ is active, safe, and well-tolerated in refractory CRC patients, resulting in potentially improved anti-tumour immunity and inhibition of autophagy.
These findings supported Phase II study VOR/HCQ versus standard therapy RGF in patients failing standard therapies. We hypothesised that VOR/HCQ will have improved efficacy when compared to RGF in treatment-refractory mCRC patients. The randomised nature of the study would also allow us to evaluate and compare anti-tumour immunity within both regimens. An interim analysis was pre-planned, and here, we present the findings for the pre-planned interim analysis.
Methods
Patient population
Patients at least 18 years of age, with histologically or cytologically confirmed colon adenocarcinoma who progressed despite standard therapy or for whom no standard therapy was available were eligible. Patients must have been treated in the past with irinotecan and/or oxaliplatin and/or anti-VEGF/EGFR therapy or intolerant to these agents. KRAS mutational status was documented. Other key inclusion criteria included measurable or evaluable disease defined by RECIST 1.0 [9]. All patients met the following inclusion criteria: Eastern Cooperative Oncology Group (ECOG) performance status ≤2; adequate bone marrow, liver, and kidney function (i.e., absolute neutrophil count ≥1000/mm3, platelets ≥75,000/mm3); creatinine ≤2 times the upper limits of normal; total bilirubin ≤1.5 mg/dL; alanine aminotransferase and aspartate aminotransferase ≤3 times above the upper limits of the institutional normal alanine aminotransferase (aspartate aminotransferase can be <5 times upper limits of normal if patients have hepatic involvement).
Patients were excluded if they had received prior RGF, VOR, and/or HCQ. Patients were excluded if they had one or more of the following conditions: previously documented macular degeneration or diabetic retinopathy, uncontrolled brain metastases, baseline QTc >500 milliseconds, or gastrointestinal dysfunction that may impair oral absorption. Patients with active, clinically significant and/or uncontrolled medical conditions were also excluded, including uncontrolled psoriasis. Patients should not have taken valproic acid or another histone deacetylase inhibitor for at least 2 weeks prior to enrollment.
Protection of human research subjects
All patients provided written informed consent before enrollment. This study followed the ethical principles of the Declaration of Helsinki, the International Conference on Harmonization Guidelines for Good Clinical Practice, and local regulations (European Directive 2001/20/EC and US Code of Federal Regulations Title 21). The Institutional Review Board at the University of Texas Health San Antonio approved the original protocol and all subsequent amendments (Clinical Trial Registration: NCT02316340).
Study design
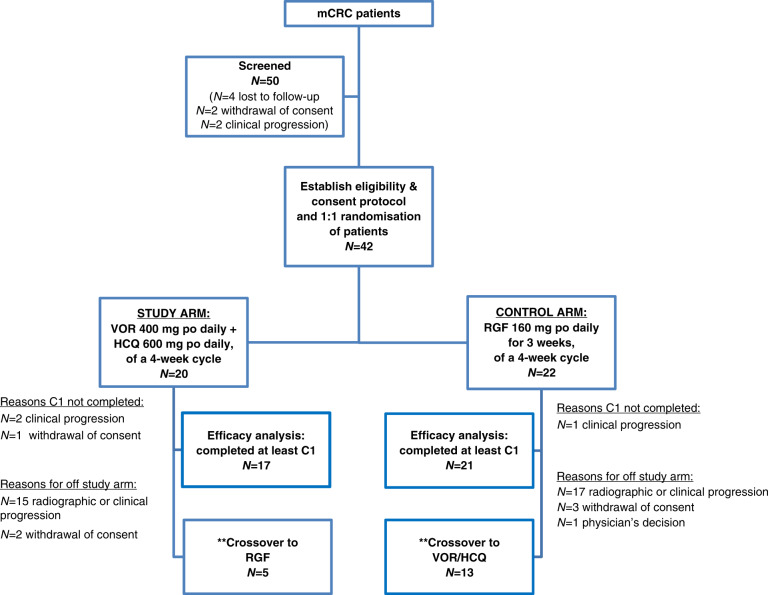
This is a Phase II randomised-controlled clinical trial of patients with mCRC, who have received local and currently approved standard therapies, excluding RGF. Patients were randomised 1:1 to RGF or VOR/HCQ (Fig. 1). We treated patients with mCRC with VOR 400 mg orally daily and HCQ 600 mg orally daily in 4-week cycles or regorafenib 160 mg by mouth daily, 3 weeks on, 1 week off on a 4-week cycle. Patients required imaging up to 6 weeks prior to enrollment and measurable evidence of mCRC. Crossover to the other arm was allowed, but optional, and was determined by the treating physician in the patient’s best interest. Patients who completed 100% of Cycle 1 of treatment were “evaluable” for efficacy, and all patients were evaluated for safety.
Fig. 1. Consort diagram for Phase II randomised controlled clinical trial for vorinostat (VOR)/hydroxychloroquine (HCQ) versus regorafenib (RGF) in refractory metastatic colorectal cancer (mCRC).
**Crossover is optional and at the discretion of the physician and in the best interest for the patient. po by mouth, C1 Cycle 1.
Randomisation
The investigators were blinded to the randomisation list. The study statistician sent the randomisation list to the pharmacy. Once the patient was registered onto the study, the pharmacist assigned the patient to one of the treatment arms. The patient started the treatment within 14 days of randomisation.
Follow-up
A repeat CT scan was performed after two cycles of the treatment regimen to evaluate response based on RECIST 1.1 criteria [9]. Clinical benefit was defined as a patient with stable disease (SD) for four or more cycles, partial response (PR), or complete response). Serum tumour marker carcinoembryonic antigen (CEA) and CT scans were repeated at least every 2 cycles, or 8 weeks, to ensure no progression of the disease. Patients would continue administering VOR/HCQ or RGF until disease progression, unacceptable toxicity, withdrawal of consent by the patient, or decision of the physician for patient’s best interest. Crossover to the other arm will be allowed, but optional, and was determined by the treating physician in the patient’s best interest. If patient was not given crossover treatment, then the patient would be off study and would be followed for survival and allowed to receive subsequent treatments. Dose reductions were allowed for both arms (Supplement Table 1S). Each patient was followed for 1 year.
Study endpoints
Our major objectives were to determine the clinical efficacy with progression-free survival of the combination of VOR/HCQ when compared to RGF in treatment-refractory mCRC. We also assessed the OS in refractory mCRC patients receiving VOR/HCQ when compared to RGF, PFS on the crossover arm, tumour response rate in refractory mCRC patients receiving VOR/HCQ when compared to RGF, the safety of the combination of VOR/HCQ when compared to RGF in treatment-refractory mCRC, and identify biomarkers associated with clinical efficacy of VOR/HCQ and RGF in mCRC. Safety was measured by NCI-CTCAEv3.0.
Biomarker analyses (optional for patients enrolled on the study)
Immune analysis
27-plex Human Cytokine Array (FGF basic, Eotaxin, G-CSF, GM-CSF, IFN-γ, IL1β, IL1ra, IL2, IL4, IL5, IL6, IL7, IL8, IL9, IL10, IL12 (p70), IL13, IL15, IL17A, IP10, MCP-1 (MCAF), MIP-1α, MIP1β, PDGF-BB, RANTES, TNFα, VEGF) (#M500KCAF0Y, Bio-Rad Inc, Carlsbad, CA, USA) was done on blood samples collected at Cycle 1 day 1 (C1D1, baseline) and on Cycle 2 days 1 (C2D1). Serum samples (30 µL) were aliquoted into microfuge tubes and diluted with 90 µL of assay buffer. Samples were run in duplicate (50 µL) in 96-well plates following the manufacturer’s instructions, with supplied cytokine standards. Multiplex standards and samples were analysed on a Bio-Plex 200 and data analysed with Bio-Plex Manager Software.
Whole blood samples from peripheral venipuncture or indwelling port were collected in sterile tubes containing lithium heparin, at C1D1 (baseline), C2D1, and at end of treatment. Total PBMCs were isolated by hypotonic lysis, washed in PBS then stained with Viability Dye BUV450 (Tonbo Biosciences, San Diego, CA). In total, 3 × 106 PBMCs were blocked with Human TruStainFcX (BioLegend) and subsequently labelled in PBS-1% FBS using anti-CD3, -CD4, -CD45RO (Tonbo Biosciences, San Diego, CA), -CD11b, -CD11c, -CD25 (BioLegend, San Diego, CA), -FoxP3, (eBioscience, San Diego, CA), -CD8, -CD16, -CD56, -CD62L (BD Biosciences, San Jose, CA) monoclonal antibodies and fixed with 4% paraformaldehyde. For cytokine detection, 3 × 106 PBMCs were stimulated for 5 h with Leukocyte Activation kit (BD bioscience), stained for surface markers, permeabilized using BD Fix/Perm protocol and stained intracellular cytokines using intracellular antibodies -IL- 17A and -IFN-γ (Biolegend, San Diego, CA). Cells were acquired using a BD FACS ARIA IIu flow cytometer (BD Biosciences), flow data analysed using FlowJo (Ashland, OR) and statistics done with GraphPad Prism software (La Jolla, CA).
Circulating tumour DNA (ctDNA)
Next-generation sequencing (NGS) analysis on cell-free, ctDNA were done at baseline and before Cycle 2 of treatment (Guardant Health). We used the maximum mutant allele fraction (MaxMAF) observed in each individual sample as a surrogate for plasma tumour fraction, as this typically represents the earliest initiating mutation shared by all tumour clones. Loss of function mutations in tumour suppressor genes such as TP53 and APC often have the highest MAF in mCRC. In cases where the highest MAF occurred in an amplified oncogene (e.g., KRAS), we used the next highest MAF, as amplification of the mutant allele can lead to an overestimation of tumour fraction.
Statistics
Historically, the mPFS for previously treated mCRC patients treated with RGF was 1.9 months [2]. We had based our hypothesis on preliminary data from the Phase II single-arm study of VOR plus HCQ in which 13 patients with mCRC had been enrolled, with 4 patients receiving 6+ cycles (or mPFS of over 3.5 months). Therefore, we predicted VOR/HCQ will improve mPFS to 3.8 months. We expected 5% lost to follow-up. Assuming a 36-month accrual period with 1 year for follow-up, 36 subjects per group were necessary to obtain 80% power. With 5% lost to follow-up the sample size required per group was 38, therefore, the total required sample size was 76 subjects.
Pre-planned interim analysis was built into the protocol with stopping rules. The O’Brien–Fleming procedure was used to conduct an interim analysis for efficacy when 50% of the patients completed the study. The interim results met the requirement for early stopping for efficacy because the findings were in a direction opposite to the expected efficacy. Therefore, the trial was stopped. This report summarises the interim analysis.
Continuously distributed outcomes were summarised with the median and range and categorical outcomes with frequencies and percentages. Treatment groups were contrasted on continuous outcomes with Wilcoxon tests and on categorical outcomes with Fisher’s Exact test. Time to progression and death were described with Kaplan–Meier curves and treatments were compared with proportional hazards models. Cytokine array results and Max MAF were descriptively summarised with bar charts and line plots. The significance of changes in cell surface markers with treatment was assessed with mixed-effects linear models. All statistical testing was two-sided with a significance level of 5%. R was used for demographic end points and survival analysis.
Results
At the interim analysis, 42 patients had enrolled in the study, from February 2015 to October 2017, of which 38 patients were evaluable (completed Cycle 1) for efficacy analysis. Therefore, enrollment was held until efficacy analysis completed.
Demographics
Twenty patients were randomised to VOR/HCQ and 22 patients were randomised to RGF (Fig. 1). Median age was 58.4 years (range 34.1–81.0), with patients 65 + (n = 11, 26.1%). Overall, 40.5% (n = 17) were Non-Hispanic, whereas 59.5% (n = 25) were Hispanic. The cohort had 35.7% (n = 15) female. Of the patients with mCRC, the primary location was rectum of 28.6% (n = 12). Molecular studies showed: 46.2% (n = 18) KRAS mutated, 2.8% (n = 1) microsatellite instability. Six patients received five or more cycles of treatment: 11.8% (n = 2) of those in the VOR/HCQ versus 19% (n = 4) in the RGF group. Most patient were ECOG performance status of 0 (61%); however, 34.1% were ECOG 1 and 4.9% were ECOG 2. Both groups were balanced in terms of demographics (Table 1).
Table 1.
Demographics for Phase II randomised controlled clinical trial for vorinostat (VOR)/hydroxychloroquine (HCQ) versus regorafenib (RGF) in refractory metastatic colorectal cancer (mCRC).
| Overall | RGF | VOR/HCQ | P | ||
|---|---|---|---|---|---|
| n | 42 | 22 | 20 | ||
| Median age (range) | 58.4 years (34.1–81.0) | 57.1 years (49.2–63.0) | 58.5 years (51.7–64.0) | 0.632 | |
| 65 years and older | Median 73.1 years (range 65–81) | 11 (26.1%) | 4 (18.2) | 5 (25.0) | 0.714 |
| Race (%) | Black | 3 (7.1) | 2 (9.1) | 1 (5.0) | 0.794 |
| Asian | 1 (2.4) | 0 (0.0) | 1 (5.0) | ||
| White | 38 (90.5) | 20 (90.9) | 18 (90.0) | ||
| Ethnicity (%) | Non-Hispanic/Latino | 17 (40.5) | 9 (40.9) | 8 (40.0) | 1 |
| Spanish/Hispanic/Latino | 25 (59.5) | 13 (59.1) | 12 (60.0) | ||
| Gender (%) | Female | 15 (35.7) | 5 (22.7) | 10 (50.0) | 0.107 |
| Male | 27 (64.3) | 17 (77.3) | 10 (50.0) | ||
| Primary location (%) | Colon | 30 (71.4) | 15 (68.2) | 15 (75.0) | 0.738 |
| Rectum | 12 (28.6) | 7 (31.8) | 5 (25.0) | ||
| KRAS (%) | Mutated | 18 (46.2) | 10 (47.6) | 8 (44.4) | 0.656 |
| N/A | 4 (10.3) | 3 (14.3) | 1 (5.6) | ||
| Wild-type | 17 (43.6) | 8 (38.1) | 9 (50.0) | ||
| NRAS (%) | N/A | 31 (86.1) | 19 (90.5) | 12 (80.0) | 0.63 |
| Wild-type | 5 (13.9) | 2 (9.5) | 3 (20.0) | ||
| MSI (%) | N/A* | 31 (86.1) | 18 (85.7) | 13 (86.7) | 1 |
| MSS | 4 (11.1) | 2 (9.5) | 2 (13.3) | ||
| MSI | 1 (2.8) | 1 (4.8) | 0 (0.0) | ||
| Cycles (%) | 2-Jan | 24 (63.2) | 10 (47.6) | 14 (82.4) | 0.061 |
| 4-Mar | 8 (21.1) | 7 (33.3) | 1 (5.9) | ||
| 5+ | 6 (15.8) | 4 (19.0) | 2 (11.8) | ||
| ECOG PS (%) | 0 | 25 (61.0) | 15 (71.4) | 10 (50.0) | 0.297 |
| 1 | 14 (34.1) | 5 (23.8) | 9 (45.0) | ||
| 2 | 2 (4.9) | 1 (4.8) | 1 (5.0) |
n number, N/A not available, MSS microsatellite stability, MSI microsatellite instability, ECOG PS Eastern Cooperative Oncology Group performance status.
*This study began enrolling patients with advanced refractory mCRC before MSI testing was universally done for all new patients diagnosed with colon cancer at our institution. Therefore, we were not able to go back retrospectively to capture data in patients who were deceased or had withdrawn consent from the study.
On the VOR/HCQ arm, the reasons for coming off study were as follows: 85% (n = 17) radiographic or clinical progression and 15% (n = 3) withdrawal of consent. On the RGF arm, the reasons for coming off study: 81.8% (n = 18) radiographic or clinical progression, 13.6% (n = 3) withdrawal of consent, and 4.5% (n = 1) physician’s decision. Of those on the VOR/HCQ arm, 5 patients (25.0%) crossed over to RGF, whereas 13 patients (59.1%) on the RGF arm crossed over to the VOR/HCQ arm.
Efficacy
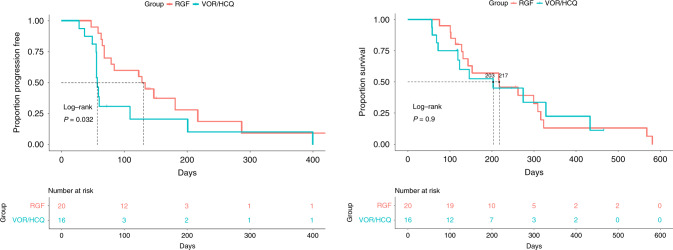
Of the 42 patients enrolled, four patients were not evaluable for efficacy as they did not complete one cycle of treatment (per protocol). The reasons for not completing Cycle 1 were the following: clinical progression (RGF n = 1, VOR/HCQ n = 2) and withdrawal of consent (VOR/HCQ n = 1). Of the 38 evaluable patients, the median PFS was 1.90 months (95% CI 1.87 to undefined) with VOR/HCQ versus 4.35 months (95% CI 2.63 to undefined) with RGF (P = 0.032, HR: 2.277, 95% CI: 1.058–4.898) (Fig. 2). The mOS was 6.77 months (95% CI 4.0 to undefined) with VOR/HCQ versus 7.23 months (95% CI 4.8– 10.8) with RGF (P = 0.90, HR: 1.05, 95% CI: 0.481–2.3) (Fig. 2).
Fig. 2. Left: progression-free survival (PFS) of VOR/HCQ versus RGF.
Median PFS was 1.90 months (95% CI 1.87 to undefined) with VOR/HCQ versus 4.35 months (95% CI 2.63 to undefined) with RGF (P = 0.032, HR: 2.277, 95% CI: 1.058–4.898). Right: overall survival (OS) with VOR/HCQ versus RGF in mCRC. The median OS was 6.77 months (95% CI 4.0 to undefined) with VOR/HCQ versus 7.23 months [95% CI 4.8–10.8] with RGF (P = 0.90, HR: 1.05, 95% CI: 0.481–2.3). VOR vorinostat, HCQ hydroxychloroquine, RGF regorafenib.
Most patients had stable disease as their best response, with 19% of RGF versus 11.8% of VOR/HCQ staying on treatment beyond four cycles. No partial or complete responses were observed. Clinical benefit was defined as a stable disease for four or more cycles (4 + cycles).
Regarding crossover arms (second treatment), median PFS was 2.1 months with RGF versus 1.9 months with VOR/HCQ (P = 0.81, HR 0.43, 95% CI for HR 0.09–1.99). Among those who crossed over from VOR/HCQ to RGF, mOS was 10 months (95% CI 9.1 to undefined) relative to those who crossed over from RGF to VOR/HCQ with mOS 8.7 months (95% CI 4.8 undefined) (P = 0.27).
Safety
Both treatments were tolerated, and the toxicity profile was similar to prior studies [2, 8]. Adverse events are in (Table 2). Grade 3–4 adverse events in the VOR/HCQ arm were n = 2 anaemia, n = 2 diarrhoea, n = 1 nausea, and n = 3 platelet count decreased, versus in the RGF arm were n = 1 blood bilirubin increased, n = 1 glucose intolerance, n = 1 hypertension, n = 5 transaminases increased, n = 1 palmar-plantar erythrodysesthesia syndrome, n = 2 platelet count decreased, n = 1 hypokalemia, and n = 1 fatigue. Grade 3 or 4 toxicities were observed in 8 (40%) patients in the VOR/HCQ group versus 9 (41%) patients in the RGF group. No grade 5 AEs were observed. Dose reductions or interruptions were necessary in 30% (n = 6) of patients receiving VOR/HCQ versus 41% (n = 9) of patients receiving RGF.
Table 2.
Adverse events in Phase II randomised controlled clinical trial for vorinostat (VOR)/hydroxychloroquine (HCQ) versus regorafenib (RGF) in refractory metastatic colorectal cancer (mCRC).
| RGF: adverse events by grade | ||||
|---|---|---|---|---|
| Grade | 1 | 2 | 3 | 4 |
| n (%) | 54 | 31 | 11 | 2 |
| AE | ||||
| Anaemia | 2 (3.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Anorexia | 1 (1.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Bloating | 1 (1.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Blood bilirubin increase | 0 (0.0) | 5 (16.1) | 1 (9.1) | 0 (0.0) |
| Chills | 1 (1.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Conjunctivitis | 1 (1.9) | 1 (3.2) | 0 (0.0) | 0 (0.0) |
| Constipation | 2 (3.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Diarrhoea | 3 (5.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dysgeusia | 1 (1.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Fatigue | 5 (9.3) | 3 (9.7) | 1 (9.1) | 0 (0.0) |
| Glucose intolerance | 0 (0.0) | 0 (0.0) | 1 (9.1) | 0 (0.0) |
| Hypertension | 1 (1.9) | 0 (0.0) | 1 (9.1) | 0 (0.0) |
| Hypoalbuminemia | 1 (1.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hypokalemia | 0 (0.0) | 0 (0.0) | 1 (9.1) | 0 (0.0) |
| Laryngeal inflammation | 1 (1.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Lung infection | 0 (0.0) | 1 (3.2) | 0 (0.0) | 0 (0.0) |
| Mucositis | 8 (14.8) | 3 (9.7) | 0 (0.0) | 0 (0.0) |
| Nail discoloration | 1 (1.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Nausea | 1 (1.9) | 1 (3.2) | 0 (0.0) | 0 (0.0) |
| Neutrophil count decreased | 0 (0.0) | 1 (3.2) | 0 (0.0) | 0 (0.0) |
| Palmar-plantar erythrodysesthesia syndrome | 9 (16.7) | 12 (38.7) | 1 (9.1) | 0 (0.0) |
| Platelet count decreased | 4 (7.4) | 1 (3.2) | 1 (9.1) | 1 (50.0) |
| Pruritus | 2 (3.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Rash maculopapular | 1 (1.9) | 1 (3.2) | 0 (0.0) | 0 (0.0) |
| Transferase increase | 3 (5.6) | 2 (6.5) | 4 (36.4) | 1 (50.0) |
| Voice alteration | 2 (3.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Vomiting | 1 (1.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| White blood cell decreased | 2 (3.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
n number.
Grade of adverse events by NCI CTCAE v3.0.
Immune markers
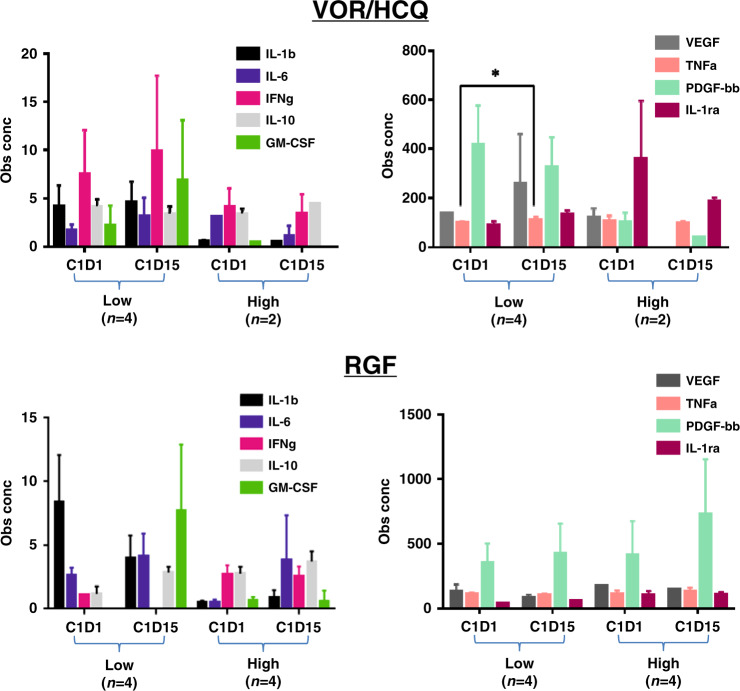
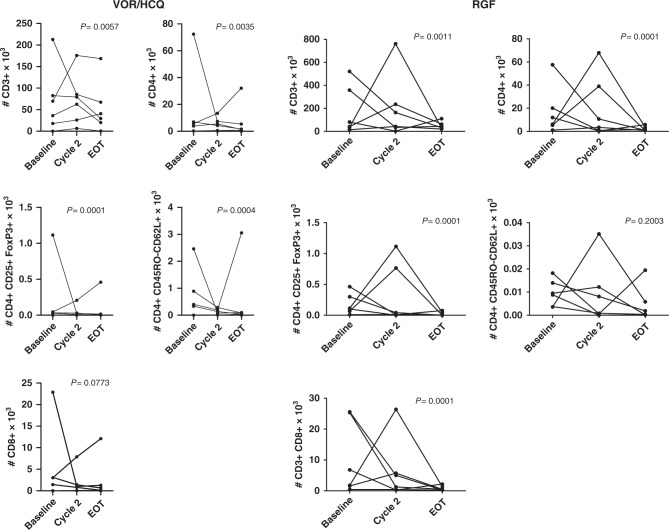
In RGF arm, there was a trend towards decreased IL-1b, IL-1ra, IL-6, IL-10, TNFa, IFNg but an increase in GM-CSF after treatment, whereas with VOR/HCQ arm, only a reduction in IL-6 (Fig. 3). There was a 10% increase TNFa after treatment with VOR/HCQ (P < 0.05). In the RGF arm, we did observe lower levels of VEGF in comparison to the VOR/HCQ, showing the effectiveness of RGF kinase inhibition. In the VOR/HCQ and RGF arms, treatment significantly reduced CD4 + CD25hiFoxp3 + regulatory and CD4 + and CD8 + T cells. In the VOR/HCQ arm, there was decreased CD45RO-CD62L + (naive) T cells, but this was not observed in the RGF arm (Fig. 4). This pattern was observed in patients who were on treatment, regardless of the duration of cycles received.
Fig. 3. 27-plex human cytokine array of patients who received RGF versus VOR/HCQ.
Assay done at Cycle 1 day 1 (C1D1, baseline) and Cycle 1 day 15 (C1D15). Low = 1–3 cycles, High = 4+ cycles completed. *P < 0.05, ~10% increase TNFa after treatment. VOR vorinostat, HCQ hydroxychloroquine, RGF regorafenib.
Fig. 4. VOR/HCQ versus RGF in mCRC results in a reduction in T cell and regulatory T cells.
In the VOR/HCQ arm, there was decreased CD45RO-CD62L + (naive) T cells, but this was not observed in the RGF arm. Flow cytometry analyses of absolute numbers of various T-cell populations (CD3 + , CD4 + , CD8 + , regulatory T cells) and surface markers (CD45RO) in total PBMCs for each individual patient at baseline and after Cycle 1. P values, paired t test. VOR vorinostat, HCQ hydroxychloroquine, RGF regorafenib.
ctDNA
As blood samples for biomarker analysis were optional, retrospective ctDNA analysis was done on 12 patients, six patients who received VOR/HCQ and six patients who received RGF. Six of six patients (100%) who received RGF had KRAS mutations versus five of six patients (83%) who received VOR/HCQ. Patients with clinical benefit (4 + cycles) appeared to exhibit lower baseline MaxMAF relative to patients with no clinical benefit for both treatment arms. In patients with clinical benefit, MaxMAF appeared to decrease at C2, and then increase at progression (Supplement Fig. 1S).
Discussion
This is the first Phase 2 randomised study evaluating the role of autophagy modulation in patients with mCRC in comparison to a standard of care treatment, regorafenib. At the pre-planned interim analysis, these data did not support improvement in mPFS with VOR/HCQ versus RGF in patients with mCRC, and therefore, continuing this study in an unselected population with mCRC was not warranted based on these results. As a result, the study was closed to further accrual. Despite our promising Phase 2 data [8], this study showed that VOR/HCQ was inferior to RGF. Our hypothesis to improve on the PFS of RGF was based on the history from the CORRECT study, which showed patients treated with RGF had a mPFS of 1.9 months [2]. However, in this study patients receiving RGF had a mPFS of 4.35 months, which was not expected as this cohort had wide inclusion criteria, including ECOG 2, comorbidities, and no age restrictions.
Our previous single-arm study in this same patient population, of the 19 evaluable patients mPFS of 2.8 months and mOS of 6.7 months [8]. Dosing of HCQ selected was able to inhibit autophagy induction related to HDAC inhibition, and survival analysis was comparable if not better than historic data with RGF with an acceptable safety profile. In this current study, VOR/HCQ had a similar toxicity profile to that observed in our earlier Phase I and II studies [7, 8]. Overall, VOR/HCQ had fewer adverse events than RGF. More patients required dosed reduction with RGF (41%) compared to VOR/HCQ (30%). The latter is comparable to the single-arm Phase II study of VOR/HCQ, which showed that 35% of patients required dose reduction [8].
In terms of efficacy, recent reported clinical studies that have reported higher mPFS of >3 months with RGF, include age, better patient selection, dose and schedule changes, and when evaluated in patients who were anti-angiogenic naive [10–12]. Despite the initial PFS of 1.9 months in the registration study [2], real-world data of patients with mCRC tend to have higher survivals when receiving RGF. For example, the Asian CONCUR study showed that RGF had PFS of 3.2 months [13]. In the prospective single-arm Phase 3b CONSIGN study, RGF had a mPFS of 2.7 months [14]. In addition, the interim analysis of the German, RECORA, study reported mPFS of 3.2 months [15]. In our US cohort of patients with CRC, all patients were treated with VEGF inhibitors prior to enrollment, therefore, given VOR/HCQ was inferior to RGF in our study, this would suggest the need to continue VEGF inhibition in heavily treated, refractory mCRC [16].
Of concern, however, is that within this same patient population, the mPFS of VOR/HCQ is certainly inferior to RGF, suggesting that inhibition of VOR-induced autophagy by HCQ in pre-clinical CRC models may not translate to the clinical efficacy of HDAC inhibitors in CRC. Regarding crossover arms, mPFS was similar in both groups. However, the number of patients in the crossover arms was small and lacked power to show a significant difference. The lack of efficacy of VOR/HCQ may be due to the heterogeneity of mCRC. For example, we found that patients with clinical benefit, as defined by receiving four or more cycles, appeared to exhibit lower baseline MaxMAF relative to those with no clinical benefit for both treatment arms. Therefore, baseline ctDNA showing lower MaxMAF may be a biomarker to identify patients with heavily-treated refractory mCRC who benefit from further systemic therapies. Of note, a limitation of our study was that our biomarker analyses was optional, and thus, we have a limited “n” for the biomarker analyses and correlative dataset. Thus, any correlatives are only hypothesis-generating.
Studies of VOR alone in colon cancers show induction of FOXP3 + regulatory cells [17, 18] and prevention of the activation of tumour-reactive NK and T cells, but this does not interfere with their cytolytic effector functions [19]. In our prior single arm study, VOR/HCQ significantly reduced CD4 + CD25hiFoxp3 + regulatory and PD-1 + (exhausted) CD4 + and CD8 + T cells and decreased CD45RO-CD62L + (naive) T cells, consistent with improved anti-tumour immunity [8]. In the VOR/HCQ arm of this study, treatment significantly reduced CD4 + CD25hiFoxp3 + regulatory and decreased CD45RO-CD62L + (naive) T cells, consistent with improved anti-tumour immunity with VOR/HCQ. We saw this effect sustained at progression (end of treatment) as well. This suggests the anti-tumour immunity effects of VOR/HCQ did not translate to clinical benefit. Other pathways may be driving cancer progression, such as VEGF and PDGFR, which were increased in expression in patients who received <4 cycles.
We observed a similar pattern in patients who received RGF, but we did not observe a significant change in CD45RO-CD62L + (naive) T cells. This pattern was observed in patients who were on treatment, regardless of the duration of cycles received. RGF inhibits CSF1R, a tyrosine kinase receptor involved in macrophage proliferation [20]. Tumour-associated macrophages (TAMs) dampen the immune system, and thus by reducing the recruitment of TAMs to the tumour, an immune response can be stimulated [21]. Based on this pre-clinical data, to enhance anti-tumour response of RGF, the Phase 1 study, REGONIVO, studied the combination of RGF with PDL-1 inhibitor, nivolumab, demonstrating mPFS of 7.9 months in patients with CRC [22]. However, the combination of RGF with pembrolizumab, PDL-1 inhibitor, in a Phase 1/2 study showed mPFS of 2 months in unselected microsatellite stable mCRC [23]. These studies highlight the heterogeneity of mCRC and the need of biomarkers to identify the subtype of patients who would benefit from these therapeutic combinations.
Other studies have investigated autophagy modulation with anti-angiogenic therapy, but toxicities have restricted further development [24]. Since the initiation of our Phase 2 study, pre-clinical studies have shown that RGF may also induce autophagy [25, 26]. In addition, pre-clinical models have shown resistance to sorafenib, an oral multi-kinase inhibitor similar to RGF, approved for hepatocellular carcinoma (HCC), as a result of autophagy induction [27, 28]. Autophagy inhibition with HCQ enhanced sorafenib-induced cell death and apoptosis in early pre-clinic and clinical studies [27, 28]. Therefore, we have also evaluated the efficacy of sorafenib and HCQ in advanced HCC patients, with preliminary data showing safety as well as better response rates with the combination than sorafenib alone (NCT03037437) [29].
Targeting autophagy and angiogenesis pathways together by combining the VOR with HCQ and RGF may lead to enhanced efficacy. A Phase 1 study of RGF, HCQ, and entinostat (an HDAC inhibitor) in mCRC was conducted on the premise that HDAC-induced autophagy through epigenetic mechanisms would be blunted by HCQ’s lysosomal acidification and synergise with anti-angiogenic therapies [30]. The mPFS was 1.8 months, the mOS was 5.2 months, the combination was poorly tolerated, and no patient remained on study longer than 4 months [30]. The poor tolerability may be due to the combination of RGF with an HDAC inhibitor, as well the lack in efficacy may be the starting RGF dose was reduced to 80 mg after three patients discontinued therapy early due to fatigue or rash due to RGF [30]. Therefore, it may be more prudent to investigate the efficacy of RGF with the better-tolerated HDAC inhibitor, VOR, and HCQ in mCRC.
Given the activity of RGF, perhaps its combination with HCQ and immunotherapy may result in increased anti-tumour responses and clinical activity. HCQ has been added to immunotherapy and doublet chemotherapy in pancreatic cancer in a Phase 2 clinic trial; however, the study was terminated due to increased serious AEs (43%) (NCT03344172). However, HCQ was found to be safe and effective as a steroid-sparing agent for immune checkpoint inhibitor-induced inflammatory arthritis [31]. Thus, future studies of immunotherapy in CRC should explore the combination of immunotherapy and HCQ to enhance the anti-tumour immune effects as well as possibly blunt toxicity from immune-checkpoint inhibitors. Further, HCQ-based combination therapies may be an option for patients who have autoimmune diseases, for which there are contraindications to immune-checkpoint inhibitors.
Patients with clinical benefit (stable disease for 4+ cycles) had lower baseline maximum mutant allele fraction (MaxMAF) versus those with no clinical benefit for both treatment arms. In patients with clinical benefit, MaxMAF at C2 was decreased and then increased at progression. Thus, ctDNA response may serve as a biomarker to predict which patients will have a long-term benefit versus which patients may be switched to another therapy or a clinical trial. Further, most patients had KRAS mutations followed by TP53 and PI3K, which did not correlate with treatment response in either arm. This is similar to the findings in a single-arm study of FOLFOX with HCQ, where responses were independent of genomic aberrations within tumour tissue, specifically KRAS, TP53, BRAF and PIK3CA [32]. However, our study has a limited sample size the lack of genomic data from all patients limits conclusions that can be made, because this overrepresentation of KRAS mutations may be due to sampling.
Conclusion
VOR/HCQ did not show efficacy when compared to RGF in patients with mCRC. The VOR/HCQ combination did lead to anti-tumour immunity changes that did not translate to clinical efficacy. The tolerability of VOR/HCQ was found to be similar to prior studies. Future studies in mCRC will need to focus on selective tumour and/or genetic alterations to identify subtypes of mCRC that would benefit from autophagy modulation and to perhaps explore novel combinations with HCQ.
Supplementary information
Acknowledgements
We would like to thank Merck for providing vorinostat for the study. We would like to thank the study coordinators, Leslie Wood RN and Lisa Longoria. Preliminary results were presented at ASCO 2019 conference, Chicago, IL (poster).
Author contributions
All authors conceived and/or designed the work that led to the submission, acquired data and/or played an important role in interpreting the results, drafted or revised the manuscript, approved the final version, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
All authors: Cancer Prevention Research Institute of Texas (CPRIT RP140685) funded this clinical trial. We thank Merck for providing the vorinostat for the study. SPA: NIH CA054174, NIA AG044271.
Data availability
The datasets generated and/or analysed during this study are available from the corresponding author on reasonable request
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All patients provided written informed consent before enrollment. This study followed the ethical principles of the Declaration of Helsinki, the International Conference on Harmonization Guidelines for Good Clinical Practice, and local regulations (European Directive 2001/20/EC and US Code of Federal Regulations Title 21). The Institutional Review Board at the University of Texas Health San Antonio approved the original protocol and all subsequent amendments.
Consent to publish
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-022-01892-6.
References
- 1.Package Insert: STIVARGA (regorafenib) tablets, oral [Internet]. Wayne, New Jersey: Bayer HealthCare Pharmaceuticals 2012. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203085lbl.pdf.
- 2.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–12. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 3.Patel SR, Karnad AB, Ketchum NS, Pollock BH, Sarantopoulos J, Weitman S, et al. Should we move beyond VEGF inhibition in metastatic colorectal cancer? Lessons from early phase clinical trials. J Gastrointest Oncol. 2014;5:99–103. doi: 10.3978/j.issn.2078-6891.2014.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carew JS, Nawrocki ST, Cleveland JL. Modulating autophagy for therapeutic benefit. Autophagy. 2007;3:464–7. doi: 10.4161/auto.4311. [DOI] [PubMed] [Google Scholar]
- 5.Carew JS, Nawrocki ST, Kahue CN, Zhang H, Yang C, Chung L, et al. Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl-mediated drug resistance. Blood. 2007;110:313–22. doi: 10.1182/blood-2006-10-050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carew JS, Medina EC, Esquivel JA, 2nd, Mahalingam D, Swords R, Kelly K, et al. Autophagy inhibition enhances vorinostat-induced apoptosis via ubiquitinated protein accumulation. J Cell Mol Med. 2010;14:2448–59. doi: 10.1111/j.1582-4934.2009.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahalingam D, Mita M, Sarantopoulos J, Wood L, Amaravadi R, Davis LE, et al. Combined autophagy and HDAC inhibition: A phase I safety, tolerability, pharmacokinetic, and pharmacodynamic analysis of hydroxychloroquine in combination with the HDAC inhibitor vorinostat in patients with advanced solid tumors. Autophagy. 2014;10:1403–14. [DOI] [PMC free article] [PubMed]
- 8.Patel S, Hurez V, Nawrocki ST, Goros M, Michalek J, Sarantopoulos J, et al. Vorinostat and hydroxychloroquine improve immunity and inhibit autophagy in metastatic colorectal cancer. Oncotarget. 2016;7:59087–97. doi: 10.18632/oncotarget.10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Riechelmann RP, Leite LS, Bariani GM, Glasberg J, Rivelli TG, da Fonseca LG, et al. Regorafenib in patients with antiangiogenic-naive and chemotherapy-refractory advanced colorectal cancer: results from a phase IIb trial. Oncologist. 2019;24:1180–7. doi: 10.1634/theoncologist.2019-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrioli R, Chirra M, Messuti L, Fiaschi AI, Savelli V, Martellucci I, et al. Efficacy and safety of regorafenib with 2/1 schedule for patients >/= 75 years with metastatic colorectal cancer (mCRC) after failure of 2 lines of chemotherapy. Clin Colorectal Cancer. 2018;17:307–12. doi: 10.1016/j.clcc.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Bekaii-Saab TS, Ou FS, Ahn DH, Boland PM, Ciombor KK, Heying EN, et al. Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (ReDOS): a randomised, multicentre, open-label, phase 2 study. Lancet Oncol. 2019;20:1070–82. doi: 10.1016/S1470-2045(19)30272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16:619–29. doi: 10.1016/S1470-2045(15)70156-7. [DOI] [PubMed] [Google Scholar]
- 14.Van Cutsem E, Martinelli E, Cascinu S, Sobrero A, Banzi M, Seitz JF, et al. Regorafenib for patients with metastatic colorectal cancer who progressed after standard therapy: results of the large, single-arm, open-label phase IIIb CONSIGN study. Oncologist. 2019;24:185–92. doi: 10.1634/theoncologist.2018-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulz H, Janssen J, Strauss UP, Langen M, Frey M, Fiala-Buskies S, et al. Clinical efficacy and safety of regorafenib (REG) in the treatment of metastatic colorectal cancer (mCRC) in daily practice in Germany: interim results of the prospective multicentre noninterventional RECORA study. J Clin Oncol. 2017;35(4_suppl):769. doi: 10.1200/JCO.2017.35.4_suppl.769. [DOI] [Google Scholar]
- 16.Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov. 2016;15:385–403. doi: 10.1038/nrd.2015.17. [DOI] [PubMed] [Google Scholar]
- 17.Lucas JL, Mirshahpanah P, Haas-Stapleton E, Asadullah K, Zollner TM, Numerof RP. Induction of Foxp3+ regulatory T cells with histone deacetylase inhibitors. Cell Immunol. 2009;257:97–104. doi: 10.1016/j.cellimm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Akimova T, Ge G, Golovina T, Mikheeva T, Wang L, Riley JL, et al. Histone/protein deacetylase inhibitors increase suppressive functions of human FOXP3+ Tregs. Clin Immunol. 2010;136:348–63. doi: 10.1016/j.clim.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmudde M, Friebe E, Sonnemann J, Beck JF, Broker BM. Histone deacetylase inhibitors prevent activation of tumour-reactive NK cells and T cells but do not interfere with their cytolytic effector functions. Cancer Lett. 2010;295:173–81. doi: 10.1016/j.canlet.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 20.Cannarile MA, Weisser M, Jacob W, Jegg AM, Ries CH, Ruttinger D. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer. 2017;5:53. doi: 10.1186/s40425-017-0257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abou-Elkacem L, Arns S, Brix G, Gremse F, Zopf D, Kiessling F, et al. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol Cancer Ther. 2013;12:1322–31. doi: 10.1158/1535-7163.MCT-12-1162. [DOI] [PubMed] [Google Scholar]
- 22.Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase Ib trial (REGONIVO, EPOC1603). J Clin Oncol. 2020;38:2053–61. [DOI] [PubMed]
- 23.Barzi A, Azad NS, Yang Y, Tsao-Wei D, Rehman R, Fakih M, et al. Phase I/II study of regorafenib (rego) and pembrolizumab (pembro) in refractory microsatellite stable colorectal cancer (MSSCRC) J Clin Oncol. 2022;40(4_suppl):15. doi: 10.1200/JCO.2022.40.4_suppl.015. [DOI] [Google Scholar]
- 24.Melnyk N, Xie X, Koh DJY, Rajpal M, Moss RA, Gibbon D, et al. CTEP #8342 autophagy modulation with antiangiogenic therapy: a phase I trial of sunitinib (Su) and hydroxychloroquine (HCQ) J Clin Oncol. 2013;31(15_suppl):2553. doi: 10.1200/jco.2013.31.15_suppl.2553. [DOI] [Google Scholar]
- 25.Weng Z, Luo Y, Yang X, Greenhaw JJ, Li H, Xie L, et al. Regorafenib impairs mitochondrial functions, activates AMP-activated protein kinase, induces autophagy, and causes rat hepatocyte necrosis. Toxicology. 2015;327:10–21. doi: 10.1016/j.tox.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Fondevila F, Méndez-Blanco C, Fernández-Palanca P, González-Gallego J, Mauriz JL. Anti-tumoral activity of single and combined regorafenib treatments in preclinical models of liver and gastrointestinal cancers. Exp Mol Med. 2019;51:1–15. doi: 10.1038/s12276-019-0308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimizu S, Takehara T, Hikita H, Kodama T, Tsunematsu H, Miyagi T, et al. Inhibition of autophagy potentiates the antitumor effect of the multikinase inhibitor sorafenib in hepatocellular carcinoma. Int J Cancer J Int du Cancer. 2012;131:548–57.. doi: 10.1002/ijc.26374. [DOI] [PubMed] [Google Scholar]
- 28.Shi YH, Ding ZB, Zhou J, Hui B, Shi GM, Ke AW, et al. Targeting autophagy enhances sorafenib lethality for hepatocellular carcinoma via ER stress-related apoptosis. Autophagy. 2011;7:1159–72. doi: 10.4161/auto.7.10.16818. [DOI] [PubMed] [Google Scholar]
- 29.Arora SP, Moseley JL, Tenner LL, Arellano L, Salazar M, Liu Q, et al. Phase II study of modulation of sorafenib (SOR)-induced autophagy using hydroxychloroquine (HCQ) in advanced hepatocellular cancer (HCC): planned interim efficacy and safety analysis. J Clin Oncol. 2021;39(3_suppl):305. doi: 10.1200/JCO.2021.39.3_suppl.305. [DOI] [Google Scholar]
- 30.Brown TJ, Karasic TB, Schneider CJ, Teitelbaum UR, Reiss KA, Mitchell TC, et al. Phase I trial of regorafenib, hydroxychloroquine, and entinostat in metastatic colorectal cancer. J Clin Oncol. 2021;39(15_suppl):e15580-e. doi: 10.1200/JCO.2021.39.15_suppl.e15580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts J, Smylie M, Walker J, Basappa NS, Chu Q, Kolinsky M, et al. Hydroxychloroquine is a safe and effective steroid-sparing agent for immune checkpoint inhibitor-induced inflammatory arthritis. Clin Rheumatol. 2019;38:1513–9. doi: 10.1007/s10067-019-04451-2. [DOI] [PubMed] [Google Scholar]
- 32.O’Hara MH, Karasic TB, Vasilevskaya I, Redlinger M, Loaiza-Bonilla A, Teitelbaum UR, et al. Phase II trial of the autophagy inhibitor hydroxychloroquine with FOLFOX and bevacizumab in front line treatment of metastatic colorectal cancer. J Clin Oncol. 2017;35(15_suppl):3545. doi: 10.1200/JCO.2017.35.15_suppl.3545. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during this study are available from the corresponding author on reasonable request