Abstract
Glioblastoma is the most common and aggressive primary malignant brain tumour. The prognosis of patients with glioblastoma is poor, and their overall survival averages at 1 year, despite advances made in cancer therapy. The emergence of immunotherapy, a strategy that targets the natural mechanisms of immune evasion by cancerous cells, has revolutionised the treatment of melanoma, lung cancer and other solid tumours; however, immunotherapy failed to improve the prognosis of patients with glioblastoma. This is attributed to the fact that glioblastoma is endowed with numerous mechanisms of resistance that include the intrinsic resistance, which refers to the location of the tumour within the brain and the nature of the blood–brain barrier, as well as the adaptive and acquired resistance that result from the tumour heterogeneity and its immunosuppressive microenvironment. Glioblastoma is notorious for its inter and intratumoral heterogeneity, which, coupled with its spatial and temporal evolution, limits its immunogenicity. In addition, the tumour microenvironment is enriched with immunosuppressive cells and molecules that hinder the reactivity of cytotoxic immune cells and the success of immunotherapies. In this article, we review the mechanisms of resistance of glioblastoma to immunotherapy and discuss treatment strategies to overcome them worthy of further exploration.
Subject terms: Immunosurveillance, Immunology
Introduction
Glioblastoma (GBM) is the most common primary malignant brain tumour, and it accounts for 14.5% of all central nervous system (CNS) masses [1]. Classified as a grade IV glioma according to the World Health Organization (WHO) classification, GBM is the most aggressive type of glioma [1, 2]. The current mainstay of treatment involves a maximal safe resection followed by radiotherapy and a six to twelve months course of chemotherapy with the alkylating agent Temozolomide (TMZ) [3, 4]. The median overall survival of patients with GBM is low (8–15 months) [1, 5, 6], and the current treatment strategy has only slightly improved the median survival by approximately two months in adults [5] and elderly patients [7]. The prognosis of patients with GBM is primarily dependent on the extent of resection, the molecular subclassification of the tumour, the Karnofsky Performance status at presentation (Table 1) [4], and is also influenced by age, gender and ethnicity [1].
Table 1.
Karnofsky performance Status Scale, adopted from Friedlander et al. [196].
| Functional evaluation | Score | Description |
|---|---|---|
|
Functionally independent Able to carry on normal activity and work No special care needed |
100 | Normal; no complaints, no evidence of disease |
| 90 | Minor signs or symptoms of the disease | |
| 80 | Normal activity with effort, some signs or symptoms of the disease | |
|
Unable to work Able to live at home and care for most personal needs Varying amount of assistance needed |
70 | Cares for self, unable to carry on normal activity or to do active work |
| 60 | Requires occasional assistance but is able to care for most of his personal needs | |
| 50 | Requires considerable assistance and frequent medical care | |
|
Functionally dependent Unable to care for self Requires the equivalent of institutional or hospice care Disease may be progressing rapidly |
40 | Disabled, requires special care and assistance |
| 30 | Severely disabled, hospital admission is indicated although death is not imminent | |
| 20 | Very sick, hospital admission necessary; active supportive treatment necessary | |
| Would not benefit from medical assistance or treatment | 10 | Moribund; fatal process progressing rapidly |
| 0 | Dead |
GBMs are endowed with numerous mechanisms of resistance that invariably result in the recurrence of the disease. GBM is an archetype of the tumour with high intrinsic, adaptive and acquired resistance [8], and failure of treatment is typically ascribed to the selection of resistant cells. The intrinsic resistance is typically related to the innate characteristics of the tumour such as location and heterogeneity. The adaptive resistance is attributed to the pressure-induced decrease in the expression of potential therapeutic targets, while the acquired resistance is due to the acquisition of novel genetic alterations [8]. Curative treatment has therefore been limited and attempts to intensify chemotherapy [9] and target dysregulated signalling pathways [10] or angiogenic factors [11, 12] have all failed to improve the prognosis. Despite decades of extensive research, the treatment modalities have not evolved, nor did the prognosis of patients with GBM improve [4, 13].
The immune system plays a major role in actively targeting and eliminating cancer cells [14]. The role of the immune system in cancer surveillance was first conceived by William Coley in 1893 [15] and further supported by Ehrlich in 1908 [14, 16]. As the understanding of immunobiology increased, Burnet [17–19] and Thomas [20] revisited the idea of cancer immunosurveillance. Burnet observed that cancer cells downregulate their expression of normal antigens (Ags), typically present on healthy cells, and favour the expression of Ags of viral origin or neoantigens that result from somatic mutations in normal genes, which subsequently become recognised as foreign by the immune system. Burnet therefore concluded that infections can partake in carcinogenesis [18, 19]. Thomas observed that patients with organ-transplant maintained on immunosuppressive treatment have an increased incidence of cancers, and therefore hypothesised that the mechanism of cancer immunosurveillance is similar to that of graft rejection [21]. The role of the immune system in cancer surveillance was finally confirmed with the identification of tumour-specific neoantigens and antibodies (Abs) [14, 22].
Considering that immunocompetent individuals develop cancer despite a functional immune system, Dunn et al. coined the three “Es” hypothesis of immunoediting that summarises the interplay between immune elimination and evasion of tumour cells [23]. According to this hypothesis, tumour growth results from a continuous process of Elimination, Equilibrium and Escape (three Es) [23]. The elimination phase refers to the original concept of immunosurveillance and, if successful, halts the proliferation of the tumour cells and the progression to the subsequent phases [23]. The equilibrium phase, which can last for many years in humans, constitutes a waging battle between the immune system and cancer cells. During this dynamic phase, cancer cells could acquire new mutations and form new resistant clones with reduced immunogenicity [23]. Finally, in the escape phase, the new resistant variants selected in the equilibrium phase prevail, and grow to clinically detectable pathologic levels [23].
Cancer immunotherapy targets the natural mechanisms of immunoresistance to favour the elimination of tumour cells. This approach has gained increasing interest in recent years, especially following the clinical success of inhibitors of signalling pathways (such as tyrosine kinase inhibitors and antiangiogenic factors) [24, 25], immune checkpoint inhibitors (ICIs) [8, 26], and chimeric Ag receptor T (CAR-T) cells [27, 28]. Cancer cells upregulate immune checkpoint molecules such as programmed death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) [29, 30], which are inhibitory proteins that normally tone down the function of Ag-specific T cells (Fig. 1). While the physiologic function of checkpoint molecules is to tone down the immune system and prevent the development of an exaggerated response against natural Ags which can lead to autoimmunity [31], cancer cells take advantage of this immunomodulatory role to tone down the immune response against the tumour [26]. ICIs that block PD-L1 and CTLA-4, as well as programmed death-1 (PD-1) expressed on activated lymphocytes [28], neutralise their ability to inhibit lymphocyte function, and have thus revolutionised cancer treatment and became the standard of care for advanced melanoma [32, 33], non-small cell lung carcinoma (NSCLC) [34], renal cell carcinoma (RCC) [35], hepatocellular carcinoma and urothelial carcinoma [36]. In addition to ICIs, chimeric Ag receptors (CAR) made of the intracellular domain of T-cell-specific activating molecules, such as the cluster of differentiation (CD) 3 and CD28, and the extracellular domain of the Fab portion of an Ab molecule specific to an Ag of choice, are largely being developed and expressed on autologous T cells (termed CAR-T cells) that can then be used to eliminate tumour cells [37]. CAR-T cells targeting CD19 expressed on B cells have recently been approved for the treatment of acute lymphoblastic leukaemia and B-cell lymphoma [38].
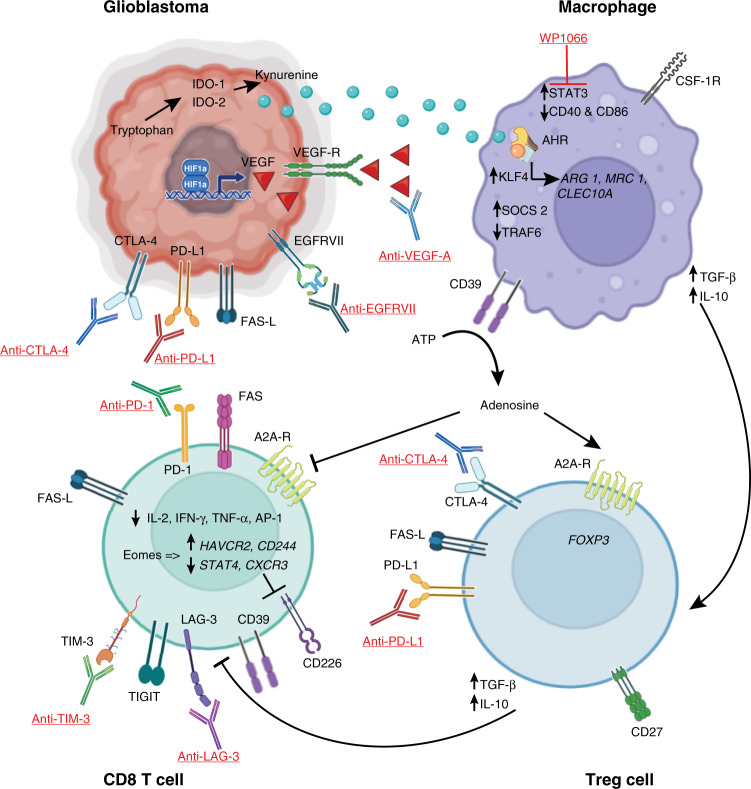
Fig. 1. Schematic representation of the molecules that favour the immunosuppressive tumour microenvironment in glioblastoma, and the immunotherapeutic drugs that have been investigated in clinical trials to enhance the anti-tumour response.
Under hypoxic conditions, glioblastoma cells activate the hypoxia-inducible factor-1 (HIF-1) pathway, which stimulates angiogenesis through the expression of the vascular endothelial growth factor (VEGF). To tone down the immune system, glioblastoma cells upregulate immune checkpoint molecules such as PD-L1 and CTLA-4, inhibiting cytotoxic T cells. Similarly, through the action of indoleamine 2,3-dioxygenase-1 (IDO-1), the tumour metabolises tryptophan into kynurenine, which is released into the tumour microenvironment. Kynurenine diffuses into macrophages where it acts as a ligand to the aryl-hydrocarbon receptor (AHR), upregulating the expression of the transcription factor Krüppel-like factor 4 (KFL4) and favouring an M2 phenotype. The activation of the AHR also stimulates the expression of the suppressor of cytokine signalling 2 (SOCS2) and increases the degradation of tumour necrosis factor-associated factor 6 (TRAF6), limiting the activity of NF-κB. Macrophages also upregulate the expression of the ectonucleoside triphosphate diphosphohydrolase-1 (ENTPD-1/CD39), which mediates the cleavage of ATP into adenosine. Adenosine acts through adenosine 2A receptors (A2AR) to activate regulatory T (Treg) cells and inhibit cytotoxic T cells. Both macrophages and Treg cells secrete inhibitory cytokines such as TGF-β and IL-10, further suppressing CD8+ T cells. In the inhibitory microenvironment, CD8+ T cells downregulate the expression of stimulatory cytokines such as IL-2, IFN-γ, TNF-α, and that of the activator protein 1 (AP-1) transcription factor. In contrast, Eomesodermins (Eomes) upregulate the transcription of genes associated with an exhausted state such as HAVCR2 and CD244, and downregulate that of effector genes such as STAT4 and CXCR3, which leads to the expression of inhibitory receptors such as PD-1, TIM-3, and inhibition of activating receptors such as CD226. Direct activation of cytotoxic T cells apoptosis is mediated by the activation of FAS, expressed on CD8+ T cells, by FAS ligand, expressed on glioblastoma and Treg cells. Underlined are the inhibitory antibodies and molecules that have been used to enhance the immune response against glioblastoma in clinical trials.
While immunotherapy showed promise in multiple types of cancer, this treatment failed to demonstrate superiority in primary malignant gliomas [38]. Bevacizumab, a monoclonal antibody (mAb) that targets the vascular endothelial growth factor-A (VEGF-A) expressed in GBM, is the only immunotherapy approved for the treatment of GBM [39]. While this drug failed to improve the overall survival of patients with GBM, it effectively enhanced progression-free survival and quality of life [12]. The CheckMate 143 Phase III clinical trial was the first large trial designed to evaluate the efficacy of nivolumab, an anti-PD-1 mAb, in comparison to bevacizumab, in the treatment of GBM. The results turned out disappointing and failed to demonstrate any superior benefit for nivolumab over bevacizumab in patients with recurrent GBM [40]. However, nivolumab effectively improved the mean overall survival in a subset of patients with methylated MGMT promoter GBM, and in patients who did not receive corticosteroids prior to treatment [40].
Because of the aggressive nature of GBM and the limited treatment available, it is essential to understand the reasons that hinder the success of immunotherapy in the treatment of this tumour. In this manuscript, we review the mechanisms of immunoresistance in GBM and discuss strategies to overcome them that are worthy of further exploration.
Mechanisms of GBM resistance
Intrinsic resistance: the brain as an immune-privileged organ and the blood–brain barrier
The brain was long considered to be an immunologically privileged organ based on early observations that foreign tissues transplanted into the CNS actively grow and escape rejection by the immune system [41]. In addition, the brain fails to stain with dyes that are injected into the peripheral system, in contrast to what is observed in peripheral organs [42]. The first evidence of an immune response in the CNS was provided in 1948 with the demonstration that homografts transplanted in the brain were successfully rejected after the peripheral immune system was primed [43]. The authors, however, concluded that the CNS succumbs to, but does not elicit an immune response, possibly due to the absence of a lymphatic system in the brain [43], a dogma that was debunked years later with the identification of a dural lymphatic system that transports fluid into the deep cervical lymph nodes [44, 45].
In addition, the brain was thought to be isolated by a mechanical barrier known as the blood–brain barrier (BBB) [46], a network of blood vessels and non-fenestrated endothelial cells closely held together by tight junctions and surrounded by astrocytic processes known as the glia limitans. The glia limitans limits the passive transit of cells and molecules, including peripheral immune cells and systemically administered drugs [47] from the systemic circulation into the brain area [48]. Hence, the success of immunotherapy against GBM will not only rely on its efficacy but also on its ability to penetrate the BBB and reach its target intracerebrally.
The BBB is however not rigid but rather acts as a dynamic barrier that becomes more permeable in pathological conditions such as brain tumours, allowing the entrance of peripherally derived immune cells and molecules [49–51]. In fact, a disrupted and more permeable BBB has been observed in the presence of gliomas [52, 53], and evidence to the permissibility of the BBB came when systemically administered CAR-T cells that recognise the epidermal growth factor receptor variant III (EGFRvIII) successfully crossed the BBB and reached the tumour in patients with EGFRvIII-positive GBM, resulting in reduced EGFRvIII expression [54]. These findings were paralleled by an increase in Foxp3+ regulatory T (Treg) cells and immunosuppressive molecules such as PD-L1, IL-10 and TGF-β in the tumour microenvironment (TME), further highlighting the permeability of the BBB, but possibly countering the efficacy of the CAR-T therapy [54].
Further evidence of blood–tumour barrier (BTB) permeability to ICIs can be ascribed to a Phase II clinical trial evaluating the effect of Pembrolizumab, an anti-PD-1 antibody, on untreated brain metastases [55]. Results from the trial showed that the drug effectively reduced the size of brain metastases in a subset of patients with metastatic melanoma or NSCLC [55]. Similar findings were also reported in a subgroup of patients suffering from lung adenocarcinoma with brain metastasis [56]. In the case of high-grade gliomas, Pembrolizumab was found to decrease the detection of PD-1 on CSF T cells from 39.3 to 3.8%, illustrating an efficient PD-1 blockade [57]. However, while enough evidence supports the permeability of the “broken” BTB to ICIs, the success of their use for GBM has been deceiving [40]. It has now become clearer that the BTB is highly heterogeneous and not uniformly permeable in GBM, as was demonstrated microscopically by areas of hypercellularity with intact BBB in non-contrast-enhancing regions [58, 59]. The tumour vessels also often upregulate the expression of efflux pumps, further contributing to the heterogeneous distribution of therapeutic drugs within the tumour and consequently to the failure of therapy [58, 60].
In recent years, with a better understanding of the BBB, several strategies have been devised to bypass this obstacle. For example, brain intraoperative perilesional administration has been attempted and is well documented with viral therapies [61–63]. In Phase III clinical trial, adenoviruses genetically engineered to express the herpes-simplex virus thymidine kinase (HSV-tk) gene under the control of the early growth response gene 1 (EGR-1) promoter have been used to transduce GBM cells by intraoperative administration locally to the peritumoral area after resection, following which the patient received intravenous ganciclovir against which the HSV-tk-expressing GBM cells became sensitive [61]. More recently, a Phase I clinical trial demonstrated the safety and feasibility of using neural stem cells, which are known to cross the BBB and distribute to the tumour bed [64], to deliver engineered oncolytic viruses to the peritumoral region in patients with newly diagnosed high-grade glioma [65]. Importantly, the intervention stimulated a lymphocytic anti-tumour response and was associated with an increase in CD8+ T cells at the tumour site [65]. Other strategies to improve drug delivery and penetration include hyperosmotic disruption of the BBB, intranasal drug delivery, and convection-enhanced delivery, which consists of delivering the therapy through an intracerebral catheter reaching the tumour [66, 67]. Strategies involving the use of nanoparticles coupled to the drug of choice have also gained attention, but no Phase III clinical trial has been reported to date [68]. More recently, the use of low-intensity pulsed ultrasound (LIPU) in conjunction with intravenous microbubble has gained in popularity after preclinical trials have demonstrated a substantial increase in the BBB’s permeability to chemotherapeutic (paclitaxel) and immunotherapeutic agents (ICI) after sonication, with an associated improvement in survival [69, 70]. Preliminary results from Phase I/II clinical trials have illustrated the safety and efficacy of this technology [69, 71], and the results of several ongoing clinical trials are highly anticipated (NCT03744026; NCT04528680; NCT04614493).
It is therefore evident that advances have been made to bypass the BBB and improve drug delivery, and substantial evidence demonstrates that immunotherapies reach their target intracerebrally. However, even when reaching the TME, the infiltration and distribution of the drugs within the TME remain heterogeneous, with areas of low permeability showing a limited response, which could be one of the reasons for the failure of the treatment and increased recurrences. To date, immunotherapies have largely failed to improve the prognosis of patients with GBM, and mechanisms of resistance other than the tumour location and the BBB further prevent a significant clinical response to immunotherapies.
Adaptive and acquired resistance
Tumour heterogeneity
The Cancer Genomic Atlas (TCGA), a joint programme between the National Cancer Institute (NCI) and the National Human Genome Research Institute (NHGRI), was established in 2006 to characterise various cancer cell types at the molecular level [72]. Based on the genomic variations identified, GBM was initially classified by the TCGA into four subtypes: Proneural, Neural, Classical, and Mesenchymal [73], however, the Neural subtype was later abandoned after it was found by single-cell RNA expression analysis to be normal Neural cell contamination of the tumour [74]. The Proneural subtype is associated with a high expression level of platelet-derived growth factor receptor alpha (PDGFRA), and expression of oligodendrocytes markers such as OLIG2 [73]. The Classical subtype is typified by a fourfold increase in the expression of the epidermal growth factor receptor (EGFR), alterations in the retinoblastoma (Rb) pathway and expression of neural precursor and stem cell markers, such as Nestin and Notch [73]. Finally, the Mesenchymal subtype expresses mesenchymal markers such as MET and CHI3L1 (also known as YKL40) and is characterised by low expression of neurofibromin (NF1) [73]. This variability among GBM subtypes is associated with a different activation of the immune system and distinct response to treatment [73, 74].
With the advances made in bulk and single-cell RNA sequencing (scRNAseq), it is now evident that the intertumoral heterogeneity is further complexed by intratumoral heterogeneity, where different subpopulations of cells exist within a single tumour [75, 76]. In that sense, sampling GBM cells from anatomically distinct regions within the same tumour mass reveals cells with different genomic profiles and subsequently different subclassification [77, 78]. This spatial heterogeneity is related to the accumulation of genetic alterations over time resulting in the clonal evolution of the tumour [77]. The spatial and temporal heterogeneity poses challenges for the treatment and could explain the variable responses between patients [77, 79] since genotypically and phenotypically distinct intratumor subclones have been shown to differ in their response to therapy by up to twofold [80, 81].
In addition to the intrinsic genetic evolution of the tumour, the treatment could also result in the selection of resistant clones, thus driving a further dynamic population shift and favouring tumour evolution [77, 78], which can be further accelerated by the acquisition of treatment-induced hypermutations [82]. While almost half of the driver mutations found in the initial tumour can be detected at recurrence, a high proportion of recurrences are driven by clone-specific divergent mutations not present in the primary tumour [81, 83]. To date, both linear and divergent models of subclones evolution have been reported, the latter coinciding more frequently with recurrences spatially distal from the primary tumour [84]. Consequently, intratumoral heterogeneity is unquestionably central to the failure of therapy, and personalised studies of subclones with genomic and RNA sequencing are crucial to predict the response of clones to the different treatment modalities, and subsequently guide the selection of adequate combination therapy.
Tumour microenvironment
Macrophages
The lack of clinically significant response to immunotherapies in GBM can also be attributed to the unique composition of brain-resident leukocytes and the immunosuppressive TME [85]. Compared to other organs, extremely low numbers of T cells infiltrate the brain [86], while the most abundant immune cells in the healthy brain are macrophages, which include brain-resident microglia and peripherally derived macrophages [85]. In addition, the most abundant immune cells infiltrating GBMs are myeloid cells, which can reach up to 70% of the total cell population in the tumour mass [38]. However, tumour-associated macrophages (TAMs) are frequently protumorigenic and lack the expression of activating molecules such as CD40 and CD86 that are required for their activation and stimulation of T cells, respectively [85].
Until recently, the proportion of bone marrow-derived macrophages (BMDM) and brain-resident microglia in the TME was not clear due to the lack of lineage-specific markers [85]. However, with the advances in flow cytometry and scRNAseq, lineage-specific markers have been identified and myeloid cell lineages can now be determined in detail [87]. CX3CR1 and the purinergic receptor P2RY12 were found to be specifically expressed by microglial TAM [87], while Itga4 (CD49D) was found specifically on BMDMs [88]. In addition, it became evident that BMDMs, rather than microglial TAMs, are enriched in tumours of higher grades and correlate with decreased survival [87].
Macrophages were typically classified as either immunostimulating (M1) or immunomodulatory (M2) macrophages; however, this binary model is now challenged [89]. Recent evidence suggests that GBM-infiltrating macrophages behave similarly to non-polarised M0 macrophages and co-express M1 and M2 markers [87, 90]. However, BMDMs isolated from the tumour show increased expression of immunosuppressive cytokine genes such as IL-10 and TGFβ, reminiscent of the M2 phenotype [87, 90]. This M2-like phenotype most likely correlates with the observed increased expression of the transcription factor STAT3 by BMDMs, which is involved in tumour-induced activation of M2 polarisation [91]. Constitutive STAT3 activation has been shown to increase the expression of immunosuppressive and pro-angiogenic cytokines, and limit anti-tumour mechanisms [92]. In turn, these immunosuppressive cytokines act in a positive feedback loop to stimulate the STAT3 pathway, resulting in an immunosuppressive TME that further enhances tumour growth (Fig. 1) [92]. Interestingly, inhibition of STAT3 in preclinical models of GBM reverses the suppression of immune cells and halts tumour progression; [93] however, this finding has yet to be translated to human patients [94, 95]. To date, there is one ongoing Phase 1 clinical trial evaluating the use of WP1066, a STAT3 inhibitor, in patients with recurrent malignant glioma (NCT01904123) but the results of that study are not reported yet.
The majority of infiltrating myeloid cells are recruited from the periphery to the brain where they contribute to the immunosuppressive GBM TME and failure of therapy. Therefore, a plausible approach to increase the success of therapy would involve targeting BMDMs in the circulation before they reach the tumour site [38]. Along this line, inhibition of the colony-stimulating factor-1 receptor (CSF-1R), which acts as a receptor for a potent macrophage chemoattractant, halted the invasion of the tumour with TAM and their polarisation [96, 97], and significantly improved survival when combined with radiotherapy in mouse models of GBM [98]. Therefore, combining therapeutic approaches seems inevitable for the successful treatment of GBM, and targeting the immunosuppressive myeloid cells could improve the effectiveness of conventional radiation [93] and chemotherapy, as well as T-cell-mediated therapies such as CAR-T cells therapy or immune checkpoint inhibitors [99].
Metabolites produced by the tumour cells, such as kynurenine and R-2-hydroxyglutarate, are also involved in the immunosuppressive phenotype (Fig. 1) [100, 101]. Kynurenine is typically produced in the context of inflammation by the enzymatic activity of indoleamine 2,3-dioxygenase-1 (IDO-1) and tryptophan 2,3-dioxygenase (TDO-2) [100]. It activates the aryl-hydrocarbon receptor (AHR), a transcription factor involved in the upregulation of M2-associated genes such as ARG1, MRC1 and CLEC10A through the activation of the transcription factor Kruppel-like factor 4 (KLF4) [100]. Moreover, AHR has been shown to upregulate the suppressor of cytokine signalling 2 (SOCS2) and increase the degradation of the tumour necrosis factor receptor-associated factor 6 (TRAF6), limiting the activity of NF-κB and consequently cancer immunosurveillance [100, 102]. In addition, AHR functions to increase the expression of the ectonucleoside triphosphate diphosphohydrolase-1 (ENTPD-1/CD39), which, along with the ecto-5’-nucleotidase (NT5E/CD73) that is upregulated on myeloid cells in the TME [103], mediates the extracellular enzymatic cleavage of ATP into adenosine (Fig. 1) [104, 105]. In turn, adenosine contributes to the immunosuppressive TME by inhibiting anti-tumour T lymphocytes and activating immunomodulatory Treg cells [104, 106]. Adenosine has been shown to primarily work via the adenosine 2A receptor (A2AR) on T cells to inhibit their activation and secretion of pro-inflammatory cytokines such as IL-2, interferon-γ and TNF-α, and upregulate the expression of FAS ligand (Fig. 1) [104]. While inhibition of the kynurenine-AHR-adenosine pathway has not made it into clinical trials yet, results from preclinical models have shown promising results [100, 107]. Inhibition of CD39 and CD73 to limit the generation of adenosine in rodent models of GBM resulted in a decrease in the population of Tregs and TAMs in the TME and was associated with a reduction in tumour growth and improved survival [100, 103, 107].
T lymphocytes
GBMs are notorious for being immunologically “cold” tumours, in that they are characterised by a weak lymphocytic infiltration and response [108]. In fact, tumour-infiltrating leukocytes (TILs) constitute only a small proportion of immune cells infiltrating the tumour [38]. Even when infiltration is successful, T cells are usually dysfunctional as a result of ignorance, anergy, tolerance, exhaustion and senescence [38, 109].
Ignorance refers to the failure of fully competent T lymphocytes to mount an effective immune response either due to anatomical barriers or inadequate Ag expression [110]. While ignorance in the case of GBM mainly results from systemic lymphopenia, which will be further discussed in the following section, ignorance in the context of the TME is caused by the limited availability of suitable tumour-specific Ags [38] that are exclusively expressed by cancer cells at detectable levels, and which should be differentiated from non-specific tumour-associated Ags that are overexpressed on tumour cells as compared to their normal expression levels on other cell types [108].
Compared with other tumour types, the GBM TME was found to be enriched with influenza-specific T cells [111], and extensive efforts were made to study the expression of cytomegalovirus (CMV) Ags in gliomas since it was first reported in 2002 [112, 113]. CMV proteins were demonstrated to be selectively expressed in more than 90% of GBM [114], and CMV-specific T cells have been detected in the TME and peripheral blood of patients with GBM [115]. However, within the immunosuppressive GBM TME, CMV-specific T cells were found to be largely immunologically dysfunctional [115] and the activation of these populations of T cells using dendritic cell vaccines is the subject of several clinical trials [116–118]. On the other hand, several tumour-specific Ags have been identified, however, their temporal expression and role in disease and therapy are not clear. GBM-specific Ags include the isocitrate dehydrogenase (IDH) R132H mutant and the EGFRvIII [108]. Trials with EGFRvIII-targeted CAR-T cells and vaccines were undertaken; however, they failed to improve survival and rather resulted in the adaptive downregulation of the Ag [54, 119]. The failure of single Ag therapies could be explained by the heterogeneity of Ags and the subsequent selection of subpopulations of cells lacking the targeted Ag [120], which stresses the importance of targeting multiple Ags and combining therapeutic approaches. Currently, there is a clinical trial investigating the use of personalised neoantigen vaccines in combination with ICIs for the treatment of GBM (NCT02287428).
Anergy refers to the mechanisms through which viable Ag-specific lymphocytes become inactive after exposure to an Ag [110]. It mostly results from the incomplete activation of lymphocytes such as in the absence of co-stimulatory signals or due to interaction with inhibitory signals such as CTLA-4 and PD-L1, and ultimately represents a state of growth arrest [121]. At the molecular level, anergy is maintained by a block in the Ras/Map kinase and defective activation of the activator protein (AP-1) transcription factor [122, 123]. Essentially, anergy and tolerance, further discussed below, result in decreased production of IL-2, which is observed in patients with GBM [110, 121]. Clinically, the expression of the co-inhibitory molecule CTLA-4 on GBM cells positively correlates with a higher tumour grade and more aggressive gliomas despite higher lymphocytic infiltration [124]. Similarly, GBMs release extracellular vesicles that contain PD-L1, which inhibits T cells activation and proliferation [125]. Interestingly, PD-L1 expression is not homogeneous in all GBMs but was specifically increased in the mesenchymal subtype of GBMs and is mostly localised in the perinecrotic and pseudopalisading regions of the tumour [125].
Tolerance is a physiologic mechanism of programmed induction of unresponsiveness that plays a major role in preventing autoimmunity [126]. There are two mechanisms of tolerance referred to as central and peripheral tolerance. Central tolerance is maintained through the negative selection of autoreactive T cells in the thymus, and autoreactive B cells in the bone marrow and liver [126]. However, central tolerance is not infallible, and peripheral tolerance serves as an additional regulator of self-reactivity [109], mediated by the peripheral elimination of self-reactive lymphocytes and their suppression by Treg cells [109]. Interestingly, both mechanisms of elimination and suppression are hijacked by tumour cells to escape the anti-tumour response. The earliest report of tumour-induced T cells apoptosis dates back to 1996 [110, 127]. Under normal conditions, Fas ligand (FasL/CD95L) is constitutively expressed on neurons but not astrocytes (Fig. 1) [128]. The ligand is however upregulated in astrocytomas and correlates with higher WHO grades [129]. Essentially, FasL enables the tumour cells to induce the death of invading lymphocytes, and evidence of Fas positive apoptotic lymphocytes has been detected in the vicinity of FasL-expressing GBM cells [130] where apoptotic cells can reach up to 22% of TILs in GBM samples [131]. In addition, apoptosis and suppression by Treg cells is another mechanism of tolerance and tumour evasion. While Treg cells are absent in healthy brains, they can reach up to 27% of GBM TILs [132]. This subclass of lymphocytes is notorious for promoting immunotolerance by expressing immunosuppressive molecules such as PD-L1 and CTLA-4, and secreting immunosuppressive cytokines such as TGF-β and IL-10 [110], which subsequently interferes with cancer immunosurveillance by cytotoxic T cells and the success of immunotherapies (Fig. 1). Treg cells are also more abundant in the tumour core under hypoxic conditions, where the increased expression of the hypoxia-inducible factor 1 (HIF-1) promotes the upregulation of attractant chemokines and VEGF [133]. Similarly, HIF-1 was found to correlate with PD-L1 expression, which was shown to further expand the pool of induced Treg cells [133–135]. In murine models of glioma, Treg cell depletion successfully restored immunosurveillance and prolonged survival [136]. It would therefore be important to investigate therapies that target Treg cell recruitment and induction and possibly combine them with other immunotherapeutic modalities. There is currently a Phase 2 clinical trial that evaluates the use of an anti-CD27 antibody to deplete Treg cells, in combination with dendritic cells (DC) vaccines, for the treatment of GBM (NCT03688178).
Lymphocyte exhaustion refers to a hyporesponsive state resulting from persistent antigenic exposure in the presence of inhibitory receptors [38, 110]. Under physiological conditions, it evolved to limit autoimmunity in the context of chronic inflammation [110] and is usually mediated by transcriptional and epigenetic changes [38]. T-bet, Eomesodermin (Eomes) and NFAT are transcription factors well-studied in the context of exhaustion and that regulate the expression of stimulatory and inhibitory receptors [110]. In the TME, exhausted T cells exhibit increased expression of Eomes, which upregulates the transcription of genes associated with an exhausted state such as HAVCR2 and CD244, downregulates that of effector genes such as Cxcr3 and Stat4, and antagonises T-bet function by binding to T-bet motifs [137]. Essentially, this transcription profile induces the expression of inhibitory receptors such as PD-1 and Tim-3 [137], and mediates the loss of activating receptors such as CD226 (DNAM-1) [138]. Clinically, high Eomes expression and loss of CD226 were shown to hamper the response to ICIs such as anti-PD-1 and anti-CTLA-4 [138, 139].
Cancers, including gliomas, induce T-cell dysfunction through mechanisms similar to the one described above, and the severity of the phenotype varies across tumour types [140]. GBM is known to elicit a severe exhausted state, and TILs upregulate the expression of several inhibitory molecules such as PD-1, TIM-3, TIGIT, LAG-3 and CD39 (Fig. 1) [140], and recent evidence demonstrates the adaptive upregulation of these alternative immune checkpoints following PD-1 blockade [141] and could, in fact, explain the failure of classical ICIs such as anti-PD-1 and anti-CTLA-4 in the context of GBM. This raises the importance of targeting several immune checkpoints simultaneously, and, to date, there are two Phase I clinical trials investigating the safety of anti-LAG-3 (NCT02658981) and anti-TIM-3 (NCT03961971) in combination with anti-PD-1 in the treatment of GBM. However, the immune profiling of T cells isolated from the blood and TME of patients with GBM has demonstrated that LAG-3 and TIM-3 are not widely expressed [142], and that T cells co-expressing PD-1, LAG-3 and TIM-3 are irreversibly exhausted [110, 143]. The blockade of these checkpoints is therefore not expected to be beneficial [142].
Senescence refers to a state of growth arrest that results from the shortening of telomeres with each round of cell division [110]. However, some cells express the Telomerase Reverse Transcriptase (TERT), an enzyme that mediates the addition of telomere repeats at the 3’ ends of chromosomes, conferring additional proliferative abilities [144]. This mechanism is prominent in cancer cells, and a transcription-enhancing mutation in the promoter region of TERT has been detected in 85% of GBMs [145, 146]. In contrast, immune cells lack telomerase activity and are therefore predisposed to telomere shortening and senescence, especially in the context of prolonged stimulation by chronic inflammation or tumour Ags [110, 147]. Recent evidence suggests that senescent cytotoxic CD8+ T cells downregulate the co-stimulatory molecule CD28 [148] and express CD57 [149]. Interestingly, cytotoxic T cells isolated from the blood and tumour microenvironment of patients with GBM show low CD28 expression, a phenotype that correlated with shorter overall survival [147, 150]. Functionally, CD8+CD28- T cells in the TME have lower levels of the inhibitory receptors PD-1 and TIM-3, further confirming that senescent and exhausted T cells constitute distinct populations [147]. To date, lymphocyte senescence and its effect on immunotherapy for patients with GBM remain poorly understood and further efforts are essential to elucidate the mechanisms behind this phenotype.
Other antigen-presenting cells-based strategies
To date, the standard of care and associated survival of patients with GBM remain unchanged despite tremendous efforts spent investigating potential immunotherapies [151]. Most strategies, however, rely on the function of cytotoxic T cells, which are known to be scarce and irreversibly exhausted in the TME [110]. More recently, there has been increased interest in DC and B-cell-based therapies in an attempt to bolster the activation and function of CD8+ T cells [117, 151]. DC have been designated as the most potent endogenous activator of T cells [152], and DC vaccines generated by the ex-vivo maturation and pulsation of autologous DC with tumour Ags have shown encouraging, yet modest results [153]. Clinical trials evaluating DC vaccines pulsed with CMV Ag pp65 showed improved survival, with ~30% survival at 5 years post diagnosis [116]. Confirmatory results from a larger clinical study (NCT02366728) using DC vaccines are highly anticipated.
B-cell-based vaccines also constitute a potential approach to boost anticancer immunity by enhancing cytotoxic T-cell activity and producing antitumor antibodies [151]. Yet, the strategy has so far been underinvestigated, possibly because B cells can quickly switch to a protumorigenic phenotype [151, 154]. To favour their antitumorigenic phenotype, B cells vaccines produced from autologous B cells specifically expressing the co-stimulatory marker 4-1BLL (CD137L) have been investigated in the preclinical setting [151]. 4-1BLL+ B cells have been demonstrated to be more potent activators of CD8+ T cells compared with 4-1BLL- B cells, and express higher levels of intracellular inflammatory cytokines such as TNFα and IFNγ. In rodents models of GBM, vaccination with 4-1BLL+ B cells activated with CD40 agonism and IFNγ and pulsed with tumour lysates conferred sustained T-cell activation in the TME and significantly improved survival [151]. The survival benefits were further potentiated by combining the treatment with PD-L1 blockade and radiation therapy, once again demonstrating the importance of combining therapies [151]. B-cell-based vaccines for the treatment of GBM are expected to move into clinical trials.
Systemic immunosuppressive state
Tumour-derived immunosuppression
Patients with GBM suffer from T-cell lymphopenia, and their CD4+ T helper cell numbers before treatment are low to levels comparable to those observed in patients with acquired immunodeficiency syndrome (AIDS) [109, 155]. In addition, several lines of evidence indicate that GBM plays an active role in suppressing the immune system, and impairment in cell-mediated immunity in patients with GBM has been reported since 1972 [156]. Despite being off corticosteroids, cytotoxic drugs, and radiation therapy, patients with intracranial tumours exhibited depressed delayed T-cell-mediated hypersensitivity reactions and sensitisation to primary Ags, although sensitisation to tumour Ags remains preserved [156, 157]. Lymphocytes isolated from the patients efficiently proliferated in vitro, however, their proliferation was inhibited by factors present in their plasma [156]. Furthermore, T cells isolated from patients with GBM show decreased secretion of the survival and proliferation cytokine IL-2, and a concomitant reduction in the expression of the IL-2Rα chain, compromising the cell’s successful proliferation and maturation [158–160].
The thymus is a primary lymphoid organ and the site of differentiation and maturation of CD4+ and CD8+ T cells [161]. Mice with intracranially implanted GL261 cells, frequently used as a model of GBM, exhibit thymic involution with decreased cellularity proportionally to the tumour burden [162]. This is specific to intracranial tumours as thymic involution is not observed when the neoplastic cells are implanted subcutaneously [162]. Concurrent with evidence from animal models, patients with GBM have an accumulation of thymocytes in the bone marrow [155, 162], which is related to the internalisation of sphingosine 1 phosphate receptor 1 (S1PR1) [155] that plays a central role in thymocyte trafficking [163]. This sequestration of thymocytes in the bone marrow was reversed when S1PR1 internalisation was halted, following which TILs were significantly increased and survival was improved, especially when S1PR1 stabilisation was combined with T-cell-activating therapies [155]. Therefore, therapies that stabilise S1PR1 or increase its expression on the surface of T cells, in combination with other immunotherapeutic modalities such as the use of CAR-T or ICIs, might be beneficial in the treatment of patients with GBM.
Exosomes are vesicles released from a variety of cell types into their environment or the circulation, transferring their biological molecules to distant targets [125]. Recently, there has been growing interest in the role of exosomes in cancer development, communication, and resistance to treatment. Characterisation of exosomes isolated from the sera of patients with GBM demonstrated the presence of the tumour-specific Ags EGFRvIII, IDH1-R132H and the immunomodulatory cytokine TGF-β [164]. GBM-derived exosomes were found to inhibit CD4+ T-cell proliferation by downregulating the expression of the IL-2Rα chain and the activation marker CD69 [165]. This inhibition did not result from a direct interaction between exosomes and the T cells but was rather mediated through the effect of exosomes on monocytes [165]. In fact, GBM-derived exosomes are preferentially incorporated by monocytes to which they transfer STAT3 and favour an immunosuppressive M2 polarisation with upregulation of PD-L1 [166]. In turn, immunosuppressive monocytes favour the differentiation of T helper cells to Th2 cells that secrete IL-4, IL-10 and IL-13, further potentiating the M2 polarisation [167]. This immune profile also favours the development and recruitment of monocytic myeloid-derived suppressor cells (Mo-MDSCs) and favours tumour growth. It is therefore essential to identify all the components of exosomes isolated from patients with GBM to elucidate the mechanisms through which tumour-derived exosomes drive immunosuppression, and to develop appropriate novel therapeutic strategies.
Iatrogenic immunosuppression
Patients with brain tumours frequently receive corticosteroids to attenuate oedema induced by the mass or the radiation [168]. Dexamethasone, the most commonly used steroid for patients with brain cancer, is a synthetic molecule characterised by a high potency, long half-life, and high BBB penetrance [169, 170]. Yet, its use has been associated with the occurrence of systemic adverse effects in up to 50% of patients with GBM [170]. Dexamethasone administration, in combination with temozolomide and radiation therapy, was associated with a decreased overall survival (12.7 vs 22.6 months, P = 0.003) and progression-free survival (3.6 vs 8.4 months, P < 0.0001) [171]. In addition, increasing evidence suggests that corticosteroids interfere with immunotherapies, including immune checkpoint inhibitors and viral therapies [172, 173]. A subgroup analysis in the Checkmate 143 Phase III trial indicated that corticosteroid use was associated with unfavourable outcomes in patients receiving nivolumab [40]. Furthermore, a retrospective study of patients with IDH-wild type GBM undergoing anti-PD-1 or anti-PD-L1 therapy revealed that dexamethasone decreased the overall survival in a dose-dependent manner, and constituted the strongest negative risk factor [174]. Likewise, patients receiving dexamethasone failed to generate neoantigen-specific lymphocytes after the administration of GBM neoantigen-targeting vaccines [175]. It is thus concluded that the associated negative outcomes in these studies are related to the immunosuppressive properties of corticosteroids [176], and supporting evidence from animal studies consistently indicated that the use of corticosteroids is associated with decreased tumour-infiltrating lymphocytes [174, 177]. Based on this, it is essential to cautiously use corticosteroids in patients with brain tumours, and further investigate immune-neutral agents with similar therapeutic properties such as anti-VEGF and anti-vascular endothelial protein tyrosine phosphatase (VE-PTP). Up to 46% of patients with recurrent GBM receiving corticosteroids were able to achieve a sustained corticosteroid dose reduction by at least 50% following initiation of anti-VEGF, with up to 20% of them completely stopping corticosteroids for at least a quarter of the study period [178]. However, while the use of anti-VEGF therapy has been shown to reduce the need for corticosteroids [178] and improve the progression-free survival of patients with GBM, it could potentially increase the rate of thromboembolic events, CNS haemorrhage, and delay wound healing [179], and its effect on overall survival has been limited [12]. Interestingly, progression-free survival has been measured by the delayed re-establishment of contrast enhancement on MRI [12, 180], which directly correlates with decreased BBB permeability [181]. The term “pseudoresponse” was subsequently designated to illustrate the discrepancy between the decreased contrast enhancement by MRI caused by antiangiogenic treatments, and the limited improvement in overall survival [181]. The use of antiangiogenic agents is not without drawbacks as VEGF inhibitors have been shown to promote an infiltrative GBM phenotype [182, 183] and favour a more hypoxic environment [180]. The GBM hypoxic environment has been previously demonstrated to induce the expression of STAT3, which further contributes to immunosuppression [180].
Chemotherapy can also have immunosuppressive effects, and the types and doses of chemotherapeutic agents have been shown to differentially affect the immunotherapeutic outcomes [184]. TMZ, the most commonly used chemotherapeutic agent for the treatment of GBM, is a potent inhibitor of lymphocyte proliferation [185]. However, its implication on the immune system and immunotherapies is still controversial. While in preclinical models of GBM, TMZ has been shown to abrogate the therapeutic effect of oncolytic viral therapies in a dose-dependent manner [186], the lymphodepletion was associated with a greater response to vaccine therapies in Phase II clinical trial [187]. Moreover, preclinical animal studies have shown that TMZ decreases the efficacy of anti-PD-1 when administered systemically [188], but facilitates the response to anti-PD-1 and improves survival when administered locally at the tumour site [188]. For this reason, it is important to consider the dosing, timing, and mode of delivery of TMZ when combining it with immunotherapeutic agents.
It is important to mention that, while radiation therapy contributes to brain oedema, it remains central to the current standard of care [5] and presents immunomodulatory roles that can be harnessed to enhance cancer immunosurveillance and response to immunotherapy [189]. By inducing inflammatory tumour cell death, radiation therapy increases the translocation of calreticulin to the cell surface [190] and the release of ATP and HMGB1 [189, 191]. These inflammatory changes subsequently stimulate the maturation of Ag presenting cells and their migration to regional lymph nodes where they activate T cells [189]. Likewise, radiation therapy enhances the function of cytotoxic T cells by permeabilizing the BBB, increasing the expression of immunostimulatory cytokines, and upregulating the expression of major histocompatibility complex (MHC) proteins on tumour cells [192].
Biomarkers of response
GBM is notorious for its microenvironmental and molecular heterogeneity, which account for the unpredictable response to therapy [193]. This variable response to treatment has been displayed in negative clinical trials where only a subset of patients respond to therapy [40, 193]. There has therefore been an increased interest in biomarkers of response, especially to ICI. A high tumour mutational burden (TMB-H) has so far been the principal biomarker considered to predict the response to ICI [194]. Accordingly, Pembrolizumab (anti-PD-1 antibody) has been approved by the United States Food and Drug Administration (FDA) for the treatment of unresectable or metastatic tumours with a hypermutated phenotype characterised by DNA mismatch repair deficiency (dMMR), microsatellite instability (MSI), or a high TMB-H (defined by >10 mutations/megabase) [194, 195]. However, most of the evidence supporting this decision is based on findings from hypermutator cancer types such as lung cancer and melanoma, and the predictive accuracy of TMB-H is, in contrast, limited in non-hypermutated tumour types such as glioma [194]. To address this limitation, McGrail and colleagues devised a transcriptional replication stress response defect (RSRD) score that evaluates the expression of genes indicative of RSR [195]. A high RSRD score was found to be predictive of improved overall survival in patients with GBM receiving ICI [195]. Similarly, phosphorylated ERK1/2 (p-ERK1/2) directly correlated with improved overall survival in patients with recurrent GBM receiving adjuvant PD-1 blockade [193]. GBM with high p-ERK1/2 also exhibited a distinct TME with an increased number of tumour-infiltrating myeloid cells with high MHC II expression [193]. While the predictive accuracy of p-ERK1/2 and the RSRD score has yet to be validated in a large prospective cohort, these biomarkers lay the foundations for further research and development in the field, and offer promising opportunities to personalise immunotherapeutic treatments.
Concluding remarks
GBM is the most common and lethal CNS tumour, and the prognosis of patients with GBM remains poor despite the advances made in cancer therapy. While immunotherapies have shown promising results in the treatment of tumours, their success in the treatment of GBM has been limited because GBM evades immune surveillance and has developed numerous mechanisms of immunoresistance. These mechanisms of evasion are related to a combination of tumour heterogeneity and location, as well as an immunosuppressive microenvironment that results in poor macrophage and T-cell immune response (Fig. 1). While several approaches have been used to treat GBM with modest outcomes, we believe that the current treatment modality (maximal resection, radiation therapy, and TMZ) should be supplemented by a multifaceted treatment approach that combines (1) bypassing the BBB to allow access for the treatment to reach the tumour, (2) recruiting the most cytotoxic macrophages and adaptive tumour-specific T cells or CAR-T cells to the tumour environment while neutralising the role of immunosuppressive macrophages and Treg cells, and (3) modulating the function of the recruited lymphocytes by the use of ICIs, stimulating cytokines, or signalling molecule inhibitors. It is also crucial to use immunosuppressive drugs such as corticosteroids carefully and to further investigate the use of drugs with similar therapeutic benefits but less immunosuppressive properties. Therefore, while in-depth studies and clinical trials are needed to determine the success of the combinatorial approach, it might be crucial to tackle the multiple aspects of resistance GBM is endowed with to provide superior benefits for the patients.
Author contributions
MJM and KJH contributed to the conceptualisation and outline. The first draft of the manuscript was written by KJH and corrected by MJM, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. RM contributed to the figure preparation.
Funding
The authors received no specific funding for this work.
Data availability
Not applicable.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
No ethics approval is required for this review of previously published literature.
Consent to publish
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro Oncol. 2020;22:iv1–96. doi: 10.1093/neuonc/noaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23:1231–51. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goenka A, Tiek D, Song X, Huang T, Hu B, Cheng SY. The many facets of therapy resistance and tumor recurrence in glioblastoma. Cells. 2021;10:484. [DOI] [PMC free article] [PubMed]
- 4.Oberheim Bush NA, Hervey-Jumper SL, Berger MS. Management of glioblastoma, present and future. World Neurosurg. 2019;131:328–38. doi: 10.1016/j.wneu.2019.07.044. [DOI] [PubMed] [Google Scholar]
- 5.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 6.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 7.Perry JR, Laperriere N, O'Callaghan CJ, Brandes AA, Menten J, Phillips C, et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376:1027–37. doi: 10.1056/NEJMoa1611977. [DOI] [PubMed] [Google Scholar]
- 8.Jackson CM, Choi J, Lim M. Mechanisms of immunotherapy resistance: lessons from glioblastoma. Nat Immunol. 2019;20:1100–9. doi: 10.1038/s41590-019-0433-y. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31:4085–91. doi: 10.1200/JCO.2013.49.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mao H, Lebrun DG, Yang J, Zhu VF, Li M. Deregulated signaling pathways in glioblastoma multiforme: molecular mechanisms and therapeutic targets. Cancer Invest. 2012;30:48–56. doi: 10.3109/07357907.2011.630050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–22. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 13.Kunigelis KE, Vogelbaum MA. Therapeutic delivery to central nervous system. Neurosurg Clin N. Am. 2021;32:291–303. doi: 10.1016/j.nec.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–48. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas: with a report of ten original cases.1: bibliography. Am J Med Sci. 1893;105:487. doi: 10.1097/00000441-189305000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Ehrlich P. Ueber den jetzigen Stand der Karzinomforschung. Beiträge zur experimentellen Pathologie und Chemotherapie. 1908. pp 117–64.
- 17.Burnet FM. Immunological surveillance in neoplasia. Transpl Rev. 1971;7:3–25. doi: 10.1111/j.1600-065x.1971.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 18.Burnet M. Cancer: a biological approach. III. Viruses associated with neoplastic conditions. IV. Practical applications. Br Med J. 1957;1:841–7. doi: 10.1136/bmj.1.5023.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burnet M. Immunological factors in the process of carcinogenesis. Br Med Bull. 1964;20:154–8. doi: 10.1093/oxfordjournals.bmb.a070310. [DOI] [PubMed] [Google Scholar]
- 20.Thomas L, Lawrence H. Cellular and humoral aspects of the hypersensitive states. New York: Hoeber-Harper; 1959. pp. 529–32.
- 21.Thomas L. On immunosurveillance in human cancer. Yale J Biol Med. 1982;55:329–33. [PMC free article] [PubMed] [Google Scholar]
- 22.Old LJ, Boyse EA. Immunology of experimental tumors. Annu Rev Med. 1964;15:167–86. doi: 10.1146/annurev.me.15.020164.001123. [DOI] [PubMed] [Google Scholar]
- 23.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–60. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 24.Amit L, Ben-Aharon I, Vidal L, Leibovici L, Stemmer S. The impact of Bevacizumab (Avastin) on survival in metastatic solid tumors–a meta-analysis and systematic review. PLoS ONE. 2013;8:e51780. doi: 10.1371/journal.pone.0051780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu W, Gong Y, Kuang M, Wu P, Cao C, Chen J, et al. Survival benefit and safety of bevacizumab in combination with erlotinib as maintenance therapy in patients with metastatic colorectal cancer: a meta-analysis. Clin Drug Investig. 2017;37:155–65. doi: 10.1007/s40261-016-0465-0. [DOI] [PubMed] [Google Scholar]
- 26.Medikonda R, Dunn G, Rahman M, Fecci P, Lim M. A review of glioblastoma immunotherapy. J Neurooncol. 2021;151:41–53. doi: 10.1007/s11060-020-03448-1. [DOI] [PubMed] [Google Scholar]
- 27.Chavez JC, Bachmeier C, Kharfan-Dabaja MA. CAR T-cell therapy for B-cell lymphomas: clinical trial results of available products. Ther Adv Hematol. 2019;10:2040620719841581. doi: 10.1177/2040620719841581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leary A, Tan D, Ledermann J. Immune checkpoint inhibitors in ovarian cancer: where do we stand? Ther Adv Med Oncol. 2021;13:17588359211039899. doi: 10.1177/17588359211039899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–7. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He X, Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30:660–9. doi: 10.1038/s41422-020-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013;14:1212–8. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- 32.Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–18. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–84. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 34.Paz-Ares L, Horn L, Borghaei H, Spigel DR, Steins M, Ready N, et al. Phase III, randomized trial (CheckMate 057) of nivolumab (NIVO) versus docetaxel (DOC) in advanced non-squamous cell (non-SQ) non-small cell lung cancer (NSCLC) J Clin Oncol. 2015;33:LBA109–LBA109. doi: 10.1200/jco.2015.33.18_suppl.lba109. [DOI] [Google Scholar]
- 35.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8. doi: 10.1186/s40425-018-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang C, Durer S, Thandra KC, Kasi A. Chimeric antigen receptor T-cell therapy. In: StatPearls. FL: Treasure Island; 2021. [PubMed]
- 38.Chuntova P, Chow F, Watchmaker PB, Galvez M, Heimberger AB, Newell EW, et al. Unique challenges for glioblastoma immunotherapy-discussions across neuro-oncology and non-neuro-oncology experts in cancer immunology. Meeting Report from the 2019 SNO Immuno-Oncology Think Tank. Neuro Oncol. 2021;23:356–75. doi: 10.1093/neuonc/noaa277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fisher JP, Adamson DC. Current FDA-approved therapies for high-grade malignant gliomas. Biomedicines. 2021;9:324. [DOI] [PMC free article] [PubMed]
- 40.Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, Wick A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6:1003–10. doi: 10.1001/jamaoncol.2020.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy JB, Sturm E. Conditions determining the transplantability of tissues in the brain. J Exp Med. 1923;38:183–97. doi: 10.1084/jem.38.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehrlich P. Das sauerstufbudurfnis des organismus. In: Eine Farbenanalytische Studie. Berlin: Hirschwald; 1885.
- 43.Medawar PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- 44.Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:991–9. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–41. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sampson JH, Gunn MD, Fecci PE, Ashley DM. Brain immunology and immunotherapy in brain tumours. Nat Rev Cancer. 2020;20:12–25. doi: 10.1038/s41568-019-0224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larsen JM, Martin DR, Byrne ME. Recent advances in delivery through the blood-brain barrier. Curr Top Med Chem. 2014;14:1148–60. doi: 10.2174/1568026614666140329230311. [DOI] [PubMed] [Google Scholar]
- 48.Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7:a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones KA, Maltby S, Plank MW, Kluge M, Nilsson M, Foster PS, et al. Peripheral immune cells infiltrate into sites of secondary neurodegeneration after ischemic stroke. Brain Behav Immun. 2018;67:299–307. doi: 10.1016/j.bbi.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 50.Rezai-Zadeh K, Gate D, Town T. CNS infiltration of peripheral immune cells: D-Day for neurodegenerative disease? J Neuroimmune Pharm. 2009;4:462–75. doi: 10.1007/s11481-009-9166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lossinsky AS, Shivers RR. Structural pathways for macromolecular and cellular transport across the blood-brain barrier during inflammatory conditions. Review. Histol Histopathol. 2004;19:535–64. doi: 10.14670/HH-19.535. [DOI] [PubMed] [Google Scholar]
- 52.Watkins S, Robel S, Kimbrough IF, Robert SM, Ellis-Davies G, Sontheimer H. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat Commun. 2014;5:4196. doi: 10.1038/ncomms5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vajkoczy P, Menger MD. Vascular microenvironment in gliomas. Cancer Treat Res. 2004;117:249–62. doi: 10.1007/978-1-4419-8871-3_15. [DOI] [PubMed] [Google Scholar]
- 54.O'Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9:eaaa0984. [DOI] [PMC free article] [PubMed]
- 55.Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17:976–83. doi: 10.1016/S1470-2045(16)30053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abid H, Watthanasuntorn K, Shah O, Gnanajothy R. Efficacy of pembrolizumab and nivolumab in crossing the blood brain barrier. Cureus. 2019;11:e4446. doi: 10.7759/cureus.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Portnow J, Wang D, Blanchard MS, Tran V, Alizadeh D, Starr R, et al. Systemic anti-PD-1 immunotherapy results in PD-1 blockade on T cells in the cerebrospinal fluid. JAMA Oncol. 2020; 10.1001/jamaoncol.2020.4508. [DOI] [PMC free article] [PubMed]
- 58.Sarkaria JN, Hu LS, Parney IF, Pafundi DH, Brinkmann DH, Laack NN, et al. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro Oncol. 2018;20:184–91. doi: 10.1093/neuonc/nox175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arvanitis CD, Ferraro GB, Jain RK. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2020;20:26–41. doi: 10.1038/s41568-019-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pitz MW, Desai A, Grossman SA, Blakeley JO. Tissue concentration of systemically administered antineoplastic agents in human brain tumors. J Neurooncol. 2011;104:629–38. doi: 10.1007/s11060-011-0564-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Westphal M, Yla-Herttuala S, Martin J, Warnke P, Menei P, Eckland D, et al. Adenovirus-mediated gene therapy with sitimagene ceradenovec followed by intravenous ganciclovir for patients with operable high-grade glioma (ASPECT): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:823–33. doi: 10.1016/S1470-2045(13)70274-2. [DOI] [PubMed] [Google Scholar]
- 62.Kicielinski KP, Chiocca EA, Yu JS, Gill GM, Coffey M, Markert JM. Phase 1 clinical trial of intratumoral reovirus infusion for the treatment of recurrent malignant gliomas in adults. Mol Ther. 2014;22:1056–62. doi: 10.1038/mt.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–74. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 64.Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000;97:12846–51. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fares J, Ahmed AU, Ulasov IV, Sonabend AM, Miska J, Lee-Chang C, et al. Neural stem cell delivery of an oncolytic adenovirus in newly diagnosed malignant glioma: a first-in-human, phase 1, dose-escalation trial. Lancet Oncol. 2021;22:1103–14. doi: 10.1016/S1470-2045(21)00245-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.D'Amico RS, Aghi MK, Vogelbaum MA, Bruce JN. Convection-enhanced drug delivery for glioblastoma: a review. J Neurooncol. 2021;151:415–27. doi: 10.1007/s11060-020-03408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peschillo S, Caporlingua A, Diana F, Caporlingua F, Delfini R. New therapeutic strategies regarding endovascular treatment of glioblastoma, the role of the blood-brain barrier and new ways to bypass it. J Neurointerv Surg. 2016;8:1078–82. doi: 10.1136/neurintsurg-2015-012048. [DOI] [PubMed] [Google Scholar]
- 68.Hsu JF, Chu SM, Liao CC, Wang CJ, Wang YS, Lai MY, et al. Nanotechnology and nanocarrier-based drug delivery as the potential therapeutic strategy for glioblastoma multiforme: an update. Cancers. 2021;13:195. [DOI] [PMC free article] [PubMed]
- 69.Idbaih A, Ducray F, Stupp R, Baize N, Chinot OL, Groot JFD, et al. A phase I/IIa study to evaluate the safety and efficacy of blood-brain barrier (BBB) opening with the SonoCloud-9 implantable ultrasound device in recurrent glioblastoma patients receiving IV carboplatin. J Clin Oncol. 2021;39:2049–2049. doi: 10.1200/JCO.2021.39.15_suppl.2049. [DOI] [Google Scholar]
- 70.Sabbagh A, Beccaria K, Ling X, Marisetty A, Ott M, Caruso H, et al. Opening of the blood-brain barrier using low-intensity pulsed ultrasound enhances responses to immunotherapy in preclinical glioma models. Clin Cancer Res. 2021; 10.1158/1078-0432.CCR-20-3760. [DOI] [PMC free article] [PubMed]
- 71.Carpentier A, Canney M, Vignot A, Reina V, Beccaria K, Horodyckid C, et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci Transl Med. 2016;8:343re342. doi: 10.1126/scitranslmed.aaf6086. [DOI] [PubMed] [Google Scholar]
- 72.Institute NC. The Cancer Genome Atlas Program. https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga Accessed 05/28.
- 73.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Q, Hu B, Hu X, Kim H, Squatrito M, Scarpace L, et al. Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell. 2017;32:42–56 e46. doi: 10.1016/j.ccell.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344:1396–401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khalafallah AM, Huq S, Jimenez AE, Serra R, Bettegowda C, Mukherjee D. "Zooming in" on glioblastoma: understanding tumor heterogeneity and its clinical implications in the era of single-cell ribonucleic acid sequencing. Neurosurgery. 2021;88:477–86. doi: 10.1093/neuros/nyaa305. [DOI] [PubMed] [Google Scholar]
- 77.Sottoriva A, Spiteri I, Piccirillo SG, Touloumis A, Collins VP, Marioni JC, et al. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci USA. 2013;110:4009–14. doi: 10.1073/pnas.1219747110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qazi MA, Vora P, Venugopal C, Sidhu SS, Moffat J, Swanton C, et al. Intratumoral heterogeneity: pathways to treatment resistance and relapse in human glioblastoma. Ann Oncol. 2017;28:1448–56. doi: 10.1093/annonc/mdx169. [DOI] [PubMed] [Google Scholar]
- 79.Bedard PL, Hansen AR, Ratain MJ, Siu LL. Tumour heterogeneity in the clinic. Nature. 2013;501:355–64. doi: 10.1038/nature12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee JK, Wang J, Sa JK, Ladewig E, Lee HO, Lee IH, et al. Spatiotemporal genomic architecture informs precision oncology in glioblastoma. Nat Genet. 2017;49:594–9. doi: 10.1038/ng.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reinartz R, Wang S, Kebir S, Silver DJ, Wieland A, Zheng T, et al. Functional subclone profiling for prediction of treatment-induced intratumor population shifts and discovery of rational drug combinations in human glioblastoma. Clin Cancer Res. 2017;23:562–74. doi: 10.1158/1078-0432.CCR-15-2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johnson BE, Mazor T, Hong C, Barnes M, Aihara K, McLean CY, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343:189–93. doi: 10.1126/science.1239947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim H, Zheng S, Amini SS, Virk SM, Mikkelsen T, Brat DJ, et al. Whole-genome and multisector exome sequencing of primary and post-treatment glioblastoma reveals patterns of tumor evolution. Genome Res. 2015;25:316–27. doi: 10.1101/gr.180612.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim J, Lee IH, Cho HJ, Park CK, Jung YS, Kim Y, et al. Spatiotemporal evolution of the primary glioblastoma genome. Cancer Cell. 2015;28:318–28. doi: 10.1016/j.ccell.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 85.Quail DF, Joyce JA. The microenvironmental landscape of brain tumors. Cancer Cell. 2017;31:326–41. doi: 10.1016/j.ccell.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Montoya ML, Kasahara N, Okada H. Introduction to immunotherapy for brain tumor patients: challenges and future perspectives. Neurooncol Pr. 2020;7:465–76. doi: 10.1093/nop/npaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Muller S, Kohanbash G, Liu SJ, Alvarado B, Carrera D, Bhaduri A, et al. Single-cell profiling of human gliomas reveals macrophage ontogeny as a basis for regional differences in macrophage activation in the tumor microenvironment. Genome Biol. 2017;18:234. doi: 10.1186/s13059-017-1362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bowman RL, Klemm F, Akkari L, Pyonteck SM, Sevenich L, Quail DF, et al. Macrophage ontogeny underlies differences in tumor-specific education in brain malignancies. Cell Rep. 2016;17:2445–59. doi: 10.1016/j.celrep.2016.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ginhoux F, Schultze JL, Murray PJ, Ochando J, Biswas SK. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat Immunol. 2016;17:34–40. doi: 10.1038/ni.3324. [DOI] [PubMed] [Google Scholar]
- 90.Gabrusiewicz K, Rodriguez B, Wei J, Hashimoto Y, Healy LM, Maiti SN, et al. Glioblastoma-infiltrated innate immune cells resemble M0 macrophage phenotype. JCI Insight. 2016;1:1–19. doi: 10.1172/jci.insight.85841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mu X, Shi W, Xu Y, Xu C, Zhao T, Geng B, et al. Tumor-derived lactate induces M2 macrophage polarization via the activation of the ERK/STAT3 signaling pathway in breast cancer. Cell Cycle. 2018;17:428–38. doi: 10.1080/15384101.2018.1444305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Piperi C, Papavassiliou KA, Papavassiliou AG. Pivotal role of STAT3 in shaping glioblastoma immune microenvironment. Cells. 2019;8:1398. [DOI] [PMC free article] [PubMed]
- 93.Ott M, Kassab C, Marisetty A, Hashimoto Y, Wei J, Zamler D, et al. Radiation with STAT3 blockade triggers dendritic cell-T cell interactions in the glioma microenvironment and therapeutic efficacy. Clin Cancer Res. 2020;26:4983–94. doi: 10.1158/1078-0432.CCR-19-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang L, Alizadeh D, Van Handel M, Kortylewski M, Yu H, Badie B. Stat3 inhibition activates tumor macrophages and abrogates glioma growth in mice. Glia. 2009;57:1458–67. doi: 10.1002/glia.20863. [DOI] [PubMed] [Google Scholar]
- 95.Hussain SF, Kong LY, Jordan J, Conrad C, Madden T, Fokt I, et al. A novel small molecule inhibitor of signal transducers and activators of transcription 3 reverses immune tolerance in malignant glioma patients. Cancer Res. 2007;67:9630–6. doi: 10.1158/0008-5472.CAN-07-1243. [DOI] [PubMed] [Google Scholar]
- 96.Coniglio SJ, Eugenin E, Dobrenis K, Stanley ER, West BL, Symons MH, et al. Microglial stimulation of glioblastoma invasion involves epidermal growth factor receptor (EGFR) and colony stimulating factor 1 receptor (CSF-1R) signaling. Mol Med. 2012;18:519–27. doi: 10.2119/molmed.2011.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–72. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Almahariq MF, Quinn TJ, Kesarwani P, Kant S, Miller CR, Chinnaiyan P. Inhibition of colony-stimulating factor-1 receptor enhances the efficacy of radiotherapy and reduces immune suppression in glioblastoma. Vivo. 2021;35:119–29. doi: 10.21873/invivo.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wei J, Chen P, Gupta P, Ott M, Zamler D, Kassab C, et al. Immune biology of glioma-associated macrophages and microglia: functional and therapeutic implications. Neuro Oncol. 2020;22:180–94. doi: 10.1093/neuonc/noaa215.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Takenaka MC, Gabriely G, Rothhammer V, Mascanfroni ID, Wheeler MA, Chao CC, et al. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat Neurosci. 2019;22:729–40. doi: 10.1038/s41593-019-0370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kohanbash G, Carrera DA, Shrivastav S, Ahn BJ, Jahan N, Mazor T, et al. Isocitrate dehydrogenase mutations suppress STAT1 and CD8+ T cell accumulation in gliomas. J Clin Invest. 2017;127:1425–37. doi: 10.1172/JCI90644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22:586–97. doi: 10.1038/nm.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Goswami S, Walle T, Cornish AE, Basu S, Anandhan S, Fernandez I, et al. Immune profiling of human tumors identifies CD73 as a combinatorial target in glioblastoma. Nat Med. 2020;26:39–46. doi: 10.1038/s41591-019-0694-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang B. CD73: a novel target for cancer immunotherapy. Cancer Res. 2010;70:6407–11. doi: 10.1158/0008-5472.CAN-10-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kazemi MH, Raoofi Mohseni S, Hojjat-Farsangi M, Anvari E, Ghalamfarsa G, Mohammadi H, et al. Adenosine and adenosine receptors in the immunopathogenesis and treatment of cancer. J Cell Physiol. 2018;233:2032–57. doi: 10.1002/jcp.25873. [DOI] [PubMed] [Google Scholar]
- 106.Ohta A, Sitkovsky M. Extracellular adenosine-mediated modulation of regulatory T cells. Front Immunol. 2014;5:304. doi: 10.3389/fimmu.2014.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Azambuja JH, Schuh RS, Michels LR, Iser IC, Beckenkamp LR, Roliano GG, et al. Blockade of CD73 delays glioblastoma growth by modulating the immune environment. Cancer Immunol Immunother. 2020;69:1801–12. doi: 10.1007/s00262-020-02569-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Frederico SC, Hancock JC, Brettschneider EES, Ratnam NM, Gilbert MR, Terabe M. Making a cold tumor hot: the role of vaccines in the treatment of glioblastoma. Front Oncol. 2021;11:672508. doi: 10.3389/fonc.2021.672508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Grabowski MM, Sankey EW, Ryan KJ, Chongsathidkiet P, Lorrey SJ, Wilkinson DS, et al. Immune suppression in gliomas. J Neurooncol. 2021;151:3–12. doi: 10.1007/s11060-020-03483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Woroniecka KI, Rhodin KE, Chongsathidkiet P, Keith KA, Fecci PE. T-cell dysfunction in glioblastoma: applying a new framework. Clin Cancer Res. 2018;24:3792–802. doi: 10.1158/1078-0432.CCR-18-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rosato PC, Wijeyesinghe S, Stolley JM, Nelson CE, Davis RL, Manlove LS, et al. Virus-specific memory T cells populate tumors and can be repurposed for tumor immunotherapy. Nat Commun. 2019;10:567. doi: 10.1038/s41467-019-08534-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cobbs CS, Harkins L, Samanta M, Gillespie GY, Bharara S, King PH, et al. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002;62:3347–50. [PubMed] [Google Scholar]
- 113.Dziurzynski K, Chang SM, Heimberger AB, Kalejta RF, McGregor Dallas SR, Smit M, et al. Consensus on the role of human cytomegalovirus in glioblastoma. Neuro Oncol. 2012;14:246–55. doi: 10.1093/neuonc/nor227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mitchell DA, Xie W, Schmittling R, Learn C, Friedman A, McLendon RE, et al. Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro Oncol. 2008;10:10–18. doi: 10.1215/15228517-2007-035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Weathers SP, Penas-Prado M, Pei BL, Ling X, Kassab C, Banerjee P, et al. Glioblastoma-mediated Immune dysfunction limits CMV-specific T cells and therapeutic responses: results from a phase I/II trial. Clin Cancer Res. 2020;26:3565–77. doi: 10.1158/1078-0432.CCR-20-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Batich KA, Mitchell DA, Healy P, Herndon JE, 2nd, Sampson JH. Once, twice, three times a finding: reproducibility of dendritic cell vaccine trials targeting cytomegalovirus in glioblastoma. Clin Cancer Res. 2020;26:5297–303. doi: 10.1158/1078-0432.CCR-20-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Reap EA, Suryadevara CM, Batich KA, Sanchez-Perez L, Archer GE, Schmittling RJ, et al. Dendritic cells enhance polyfunctionality of adoptively transferred T cells that target cytomegalovirus in glioblastoma. Cancer Res. 2018;78:256–64. doi: 10.1158/0008-5472.CAN-17-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mitchell DA, Batich KA, Gunn MD, Huang MN, Sanchez-Perez L, Nair SK, et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015;519:366–9. doi: 10.1038/nature14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28:4722–9. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rahman M, Sawyer WG, Lindhorst S, Deleyrolle LP, Harrison JK, Karachi A, et al. Adult immuno-oncology: using past failures to inform the future. Neuro Oncol. 2020;22:1249–61. doi: 10.1093/neuonc/noaa116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–34. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 122.Fields P, Fitch FW, Gajewski TF. Control of T lymphocyte signal transduction through clonal anergy. J Mol Med. 1996;74:673–83. doi: 10.1007/s001090050071. [DOI] [PubMed] [Google Scholar]
- 123.Kang SM, Beverly B, Tran AC, Brorson K, Schwartz RH, Lenardo MJ. Transactivation by AP-1 is a molecular target of T cell clonal anergy. Science. 1992;257:1134–8. doi: 10.1126/science.257.5073.1134. [DOI] [PubMed] [Google Scholar]
- 124.Liu F, Huang J, Liu X, Cheng Q, Luo C, Liu Z. CTLA-4 correlates with immune and clinical characteristics of glioma. Cancer Cell Int. 2020;20:7. doi: 10.1186/s12935-019-1085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ricklefs FL, Alayo Q, Krenzlin H, Mahmoud AB, Speranza MC, Nakashima H, et al. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci Adv. 2018;4:eaar2766. doi: 10.1126/sciadv.aar2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Theofilopoulos AN, Kono DH, Baccala R. The multiple pathways to autoimmunity. Nat Immunol. 2017;18:716–24. doi: 10.1038/ni.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hahne M, Rimoldi D, Schroter M, Romero P, Schreier M, French LE, et al. Melanoma cell expression of Fas(Apo-1/CD95) ligand: implications for tumor immune escape. Science. 1996;274:1363–6. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 128.Saas P, Walker PR, Hahne M, Quiquerez AL, Schnuriger V, Perrin G, et al. Fas ligand expression by astrocytoma in vivo: maintaining immune privilege in the brain? J Clin Invest. 1997;99:1173–8. doi: 10.1172/JCI119273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Frankel B, Longo SL, Ryken TC. Human astrocytomas co-expressing Fas and Fas ligand also produce TGFbeta2 and Bcl-2. J Neurooncol. 1999;44:205–12. doi: 10.1023/A:1006311231189. [DOI] [PubMed] [Google Scholar]
- 130.Didenko VV, Ngo HN, Minchew C, Baskin DS. Apoptosis of T lymphocytes invading glioblastomas multiforme: a possible tumor defense mechanism. J Neurosurg. 2002;96:580–4. doi: 10.3171/jns.2002.96.3.0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Walker DG, Chuah T, Rist MJ, Pender MP. T-cell apoptosis in human glioblastoma multiforme: implications for immunotherapy. J Neuroimmunol. 2006;175:59–68. doi: 10.1016/j.jneuroim.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 132.El Andaloussi A, Lesniak MS. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro Oncol. 2006;8:234–43. doi: 10.1215/15228517-2006-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tamura R, Ohara K, Sasaki H, Morimoto Y, Kosugi K, Yoshida K, et al. Difference in immunosuppressive cells between peritumoral area and tumor core in glioblastoma. World Neurosurg. 2018;120:e601–e610. doi: 10.1016/j.wneu.2018.08.133. [DOI] [PubMed] [Google Scholar]
- 134.DiDomenico J, Lamano JB, Oyon D, Li Y, Veliceasa D, Kaur G, et al. The immune checkpoint protein PD-L1 induces and maintains regulatory T cells in glioblastoma. Oncoimmunology. 2018;7:e1448329. doi: 10.1080/2162402X.2018.1448329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ding XC, Wang LL, Zhang XD, Xu JL, Li PF, Liang H, et al. The relationship between expression of PD-L1 and HIF-1alpha in glioma cells under hypoxia. J Hematol Oncol. 2021;14:92. doi: 10.1186/s13045-021-01102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66:3294–302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 137.Li J, He Y, Hao J, Ni L, Dong C. High levels of eomes promote exhaustion of anti-tumor CD8(+) T cells. Front Immunol. 2018;9:2981. doi: 10.3389/fimmu.2018.02981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Weulersse M, Asrir A, Pichler AC, Lemaitre L, Braun M, Carrie N, et al. Eomes-dependent loss of the co-activating receptor CD226 Restrains CD8(+) T cell anti-tumor functions and limits the efficacy of cancer immunotherapy. Immunity. 2020;53:824–39. doi: 10.1016/j.immuni.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 139.Sun R, Wu Y, Zhou H, Wu Y, Yang Z, Gu Y, et al. Eomes impedes durable response to tumor immunotherapy by inhibiting stemness, tissue residency, and promoting the dysfunctional state of intratumoral CD8(+) T cells. Front Cell Dev Biol. 2021;9:640224. doi: 10.3389/fcell.2021.640224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Woroniecka K, Chongsathidkiet P, Rhodin K, Kemeny H, Dechant C, Farber SH, et al. T-cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin Cancer Res. 2018;24:4175–86. doi: 10.1158/1078-0432.CCR-17-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ott M, Prins RM, Heimberger AB. The immune landscape of common CNS malignancies: implications for immunotherapy. Nat Rev Clin Oncol. 2021;18:729–44. doi: 10.1038/s41571-021-00518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ott M, Tomaszowski KH, Marisetty A, Kong LY, Wei J, Duna M, et al. Profiling of patients with glioma reveals the dominant immunosuppressive axis is refractory to immune function restoration. JCI Insight. 2020;5:1–17. doi: 10.1172/jci.insight.134386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Collins K, Mitchell JR. Telomerase in the human organism. Oncogene. 2002;21:564–79. doi: 10.1038/sj.onc.1205083. [DOI] [PubMed] [Google Scholar]
- 145.Pierini T, Nardelli C, Lema Fernandez AG, Pierini V, Pellanera F, Nofrini V, et al. New somatic TERT promoter variants enhance the telomerase activity in glioblastoma. Acta Neuropathol Commun. 2020;8:145. doi: 10.1186/s40478-020-01022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA, Jr., et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA. 2013;110:6021–6. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Huff WX, Bam M, Shireman JM, Kwon JH, Song L, Newman S, et al. Aging- and tumor-mediated increase in CD8(+)CD28(-) T cells might impose a strong barrier to success of immunotherapy in glioblastoma. Immunohorizons. 2021;5:395–409. doi: 10.4049/immunohorizons.2100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol Rev. 2005;205:158–69. doi: 10.1111/j.0105-2896.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 149.Focosi D, Bestagno M, Burrone O, Petrini M. CD57+ T lymphocytes and functional immune deficiency. J Leukoc Biol. 2010;87:107–16. doi: 10.1189/jlb.0809566. [DOI] [PubMed] [Google Scholar]
- 150.Fornara O, Odeberg J, Wolmer Solberg N, Tammik C, Skarman P, Peredo I, et al. Poor survival in glioblastoma patients is associated with early signs of immunosenescence in the CD4 T-cell compartment after surgery. Oncoimmunology. 2015;4:e1036211. doi: 10.1080/2162402X.2015.1036211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lee-Chang C, Miska J, Hou D, Rashidi A, Zhang P, Burga RA, et al. Activation of 4-1BBL+ B cells with CD40 agonism and IFNgamma elicits potent immunity against glioblastoma. J Exp Med. 2021;218:1–23. doi: 10.1084/jem.20200913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ashley DM, Faiola B, Nair S, Hale LP, Bigner DD, Gilboa E. Bone marrow-generated dendritic cells pulsed with tumor extracts or tumor RNA induce antitumor immunity against central nervous system tumors. J Exp Med. 1997;186:1177–82. doi: 10.1084/jem.186.7.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schaller TH, Sampson JH. Advances and challenges: dendritic cell vaccination strategies for glioblastoma. Expert Rev Vaccines. 2017;16:27–36. doi: 10.1080/14760584.2016.1218762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lee-Chang C, Rashidi A, Miska J, Zhang P, Pituch KC, Hou D, et al. Myeloid-derived suppressive cells promote B cell-mediated immunosuppression via transfer of PD-L1 in glioblastoma. Cancer Immunol Res. 2019;7:1928–43. doi: 10.1158/2326-6066.CIR-19-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chongsathidkiet P, Jackson C, Koyama S, Loebel F, Cui X, Farber SH, et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. 2018;24:1459–68. doi: 10.1038/s41591-018-0135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Brooks WH, Netsky MG, Normansell DE, Horwitz DA. Depressed cell-mediated immunity in patients with primary intracranial tumors. Characterization of a humoral immunosuppressive factor. J Exp Med. 1972;136:1631–47. doi: 10.1084/jem.136.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Roszman T, Elliott L, Brooks W. Modulation of T-cell function by gliomas. Immunol Today. 1991;12:370–4. doi: 10.1016/0167-5699(91)90068-5. [DOI] [PubMed] [Google Scholar]
- 158.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–79. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 159.Elliott LH, Brooks WH, Roszman TL. Cytokinetic basis for the impaired activation of lymphocytes from patients with primary intracranial tumors. J Immunol. 1984;132:1208–15. [PubMed] [Google Scholar]
- 160.Elliott LH, Brooks WH, Roszman TL. Inability of mitogen-activated lymphocytes obtained from patients with malignant primary intracranial tumors to express high affinity interleukin 2 receptors. J Clin Invest. 1990;86:80–86. doi: 10.1172/JCI114719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Singer A, Adoro S, Park JH. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat Rev Immunol. 2008;8:788–801. doi: 10.1038/nri2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ayasoufi K, Pfaller CK, Evgin L, Khadka RH, Tritz ZP, Goddery EN, et al. Brain cancer induces systemic immunosuppression through release of non-steroid soluble mediators. Brain. 2020;143:3629–52. doi: 10.1093/brain/awaa343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem. 2004;279:15396–401. doi: 10.1074/jbc.M314291200. [DOI] [PubMed] [Google Scholar]
- 164.Graner MW, Alzate O, Dechkovskaia AM, Keene JD, Sampson JH, Mitchell DA, et al. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J. 2009;23:1541–57. doi: 10.1096/fj.08-122184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Domenis R, Cesselli D, Toffoletto B, Bourkoula E, Caponnetto F, Manini I, et al. Systemic T cells immunosuppression of glioma stem cell-derived exosomes is mediated by monocytic myeloid-derived suppressor cells. PLoS ONE. 2017;12:e0169932. doi: 10.1371/journal.pone.0169932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gabrusiewicz K, Li X, Wei J, Hashimoto Y, Marisetty AL, Ott M, et al. Glioblastoma stem cell-derived exosomes induce M2 macrophages and PD-L1 expression on human monocytes. Oncoimmunology. 2018;7:e1412909. doi: 10.1080/2162402X.2017.1412909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Harshyne LA, Nasca BJ, Kenyon LC, Andrews DW, Hooper DC. Serum exosomes and cytokines promote a T-helper cell type 2 environment in the peripheral blood of glioblastoma patients. Neuro Oncol. 2016;18:206–15. doi: 10.1093/neuonc/nov107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kostaras X, Cusano F, Kline GA, Roa W, Easaw J. Use of dexamethasone in patients with high-grade glioma: a clinical practice guideline. Curr Oncol. 2014;21:e493–503. doi: 10.3747/co.21.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Roth P, Happold C, Weller M. Corticosteroid use in neuro-oncology: an update. Neurooncol Pr. 2015;2:6–12. doi: 10.1093/nop/npu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dubinski D, Hattingen E, Senft C, Seifert V, Peters KG, Reiss Y, et al. Controversial roles for dexamethasone in glioblastoma—opportunities for novel vascular targeting therapies. J Cereb Blood Flow Metab. 2019;39:1460–8. doi: 10.1177/0271678X19859847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shields LB, Shelton BJ, Shearer AJ, Chen L, Sun DA, Parsons S, et al. Dexamethasone administration during definitive radiation and temozolomide renders a poor prognosis in a retrospective analysis of newly diagnosed glioblastoma patients. Radiat Oncol. 2015;10:222. doi: 10.1186/s13014-015-0527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36:2872–8. doi: 10.1200/JCO.2018.79.0006. [DOI] [PubMed] [Google Scholar]
- 173.Upadhyayula PS, Higgins DM, Argenziano MG, Spinazzi EF, Wu CC, Canoll P, et al. The sledgehammer in precision medicine: dexamethasone and immunotherapeutic treatment of glioma. Cancer Investig. 2021;1–18; 10.1080/07357907.2021.1944178. [DOI] [PubMed]
- 174.Iorgulescu JB, Gokhale PC, Speranza MC, Eschle BK, Poitras MJ, Wilkens MK, et al. Concurrent dexamethasone limits the clinical benefit of immune checkpoint blockade in glioblastoma. Clin Cancer Res. 2021;27:276–87. doi: 10.1158/1078-0432.CCR-20-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Keskin DB, Anandappa AJ, Sun J, Tirosh I, Mathewson ND, Li S, et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565:234–9. doi: 10.1038/s41586-018-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tokunaga A, Sugiyama D, Maeda Y, Warner AB, Panageas KS, Ito S, et al. Selective inhibition of low-affinity memory CD8(+) T cells by corticosteroids. J Exp Med. 2019;216:2701–13. doi: 10.1084/jem.20190738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Badie B, Schartner JM, Paul J, Bartley BA, Vorpahl J, Preston JK. Dexamethasone-induced abolition of the inflammatory response in an experimental glioma model: a flow cytometry study. J Neurosurg. 2000;93:634–9. doi: 10.3171/jns.2000.93.4.0634. [DOI] [PubMed] [Google Scholar]
- 178.Vredenburgh JJ, Cloughesy T, Samant M, Prados M, Wen PY, Mikkelsen T, et al. Corticosteroid use in patients with glioblastoma at first or second relapse treated with bevacizumab in the BRAIN study. Oncologist. 2010;15:1329–34. doi: 10.1634/theoncologist.2010-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cohen MH, Shen YL, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14:1131–8. doi: 10.1634/theoncologist.2009-0121. [DOI] [PubMed] [Google Scholar]
- 180.de Groot J, Liang J, Kong LY, Wei J, Piao Y, Fuller G, et al. Modulating antiangiogenic resistance by inhibiting the signal transducer and activator of transcription 3 pathway in glioblastoma. Oncotarget. 2012;3:1036–48. doi: 10.18632/oncotarget.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Arevalo OD, Soto C, Rabiei P, Kamali A, Ballester LY, Esquenazi Y, et al. Assessment of glioblastoma response in the era of bevacizumab: longstanding and emergent challenges in the imaging evaluation of pseudoresponse. Front Neurol. 2019;10:460. doi: 10.3389/fneur.2019.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lucio-Eterovic AK, Piao Y, de Groot JF. Mediators of glioblastoma resistance and invasion during antivascular endothelial growth factor therapy. Clin Cancer Res. 2009;15:4589–99. doi: 10.1158/1078-0432.CCR-09-0575. [DOI] [PubMed] [Google Scholar]
- 183.Rubenstein JL, Kim J, Ozawa T, Zhang M, Westphal M, Deen DF, et al. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000;2:306–14. doi: 10.1038/sj.neo.7900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lake RA, Robinson BW. Immunotherapy and chemotherapy—a practical partnership. Nat Rev Cancer. 2005;5:397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 185.Pagani E, Pepponi R, Fuggetta MP, Prete SP, Turriziani M, Bonmassar L, et al. DNA repair enzymes and cytotoxic effects of temozolomide: comparative studies between tumor cells and normal cells of the immune system. J Chemother. 2003;15:173–83. doi: 10.1179/joc.2003.15.2.173. [DOI] [PubMed] [Google Scholar]
- 186.Saha D, Rabkin SD, Martuza RL. Temozolomide antagonizes oncolytic immunovirotherapy in glioblastoma. J Immunother Cancer. 2020;8:1–8. doi: 10.1136/jitc-2019-000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sampson JH, Aldape KD, Archer GE, Coan A, Desjardins A, Friedman AH, et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol. 2011;13:324–33. doi: 10.1093/neuonc/noq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mathios D, Kim JE, Mangraviti A, Phallen J, Park CK, Jackson CM, et al. Anti-PD-1 antitumor immunity is enhanced by local and abrogated by systemic chemotherapy in GBM. Sci Transl Med. 2016;8:370ra180. doi: 10.1126/scitranslmed.aag2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sahebjam S, Sharabi A, Lim M, Kesarwani P, Chinnaiyan P. Immunotherapy and radiation in glioblastoma. J Neurooncol. 2017;134:531–9. doi: 10.1007/s11060-017-2413-0. [DOI] [PubMed] [Google Scholar]
- 190.Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget. 2014;5:403–16. doi: 10.18632/oncotarget.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Liao Y, Liu S, Fu S, Wu J. HMGB1 in radiotherapy: a two headed signal regulating tumor radiosensitivity and immunity. Onco Targets Ther. 2020;13:6859–71. doi: 10.2147/OTT.S253772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–71. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Arrieta VA, Chen AX, Kane JR, Kang SJ, Kassab C, Dmello C, et al. ERK1/2 phosphorylation predicts survival following anti-PD-1 immunotherapy in recurrent glioblastoma. Nat Cancer. 2021;2:1372–86. doi: 10.1038/s43018-021-00260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.McGrail DJ, Pilie PG, Rashid NU, Voorwerk L, Slagter M, Kok M, et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol. 2021;32:661–72. doi: 10.1016/j.annonc.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.McGrail DJ, Pilie PG, Dai H, Lam TNA, Liang Y, Voorwerk L, et al. Replication stress response defects are associated with response to immune checkpoint blockade in nonhypermutated cancers. Sci Transl Med. 2021;13:eabe6201. doi: 10.1126/scitranslmed.abe6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Friendlander AH, Ettinger RL. Karnofsky performance status scale. Spec Care Dent. 2009;29:147–8. doi: 10.1111/j.1754-4505.2009.00088.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.