Abstract
Background
Macrophages are an important component of the tumour immune microenvironment (TME) and can promote tumour growth and metastasis. Macrophage-secreted chemokine-ligand-23 (CCL23) induces ovarian cancer cell migration via chemokine-receptor 1 (CCR1). However, the effect of CCL23 on other immune cells in the TME is unknown.
Methods
CCL23 levels were measured by ELISA. The expression of surface markers in exhaustion assays was quantified by flow cytometry. Signalling pathways were identified by phosphokinase array and validated by western blot.
Results
Ascites from patients with high-grade serous ovarian cancer (HGSC) contain high levels of CCL23. Similarly, significantly higher CCL23 levels were found in plasma from HGSC patients compared to healthy individuals. RNA-seq analysis of ovarian cancer tissues from TCGA showed that expression of CCL23 correlated with the presence of macrophages. In tissues with high levels of CCL23 and macrophage content, the fraction of CD8 + T cells expressing exhaustion markers CTLA-4 and PD-1 were significantly higher compared to low-level CCL23 tissues. In vitro, CCL23 induced upregulation of immune checkpoint proteins on CD8 + T cells, including CTLA-4, TIGIT, TIM-3 and LAG-3 via phosphorylation of GSK3β in CD8 + T cells.
Conclusions
Our data suggest that CCL23 produced by macrophages contributes to the immune-suppressive TME in ovarian cancer by inducing an exhausted T-cell phenotype.
Subject terms: Chemokines, Ovarian cancer
Introduction
The mainstay of ovarian cancer treatment is surgery and chemotherapy. Over the last decade, the development of novel therapies for ovarian cancer has focused mainly on the inhibition of DNA repair using poly-ADP-ribose polymerase (PARP) inhibitors and anti-angiogenic agents like bevacizumab [1]. In contrast, immunotherapy has so far shown only modest clinical benefits. The use of immune checkpoint inhibition for example has demonstrated clinical responses in only about 10–15% of patients with recurrent ovarian cancer [2].
The current lack of efficacy in the treatment of ovarian cancer with immunotherapy is due to a variety of factors including the presence of a highly immune-suppressive tumour microenvironment (TME) [3, 4]. The TME in ovarian cancer is complex and includes immune effector cells with tumour-promoting and immune-suppressive functions. Among these cell populations, tumour-associated macrophages (TAMs) can enhance tumour growth by various mechanisms, including the secretion of pro-angiogenic molecules like VEGF and suppression of anti-tumour T-cell immunity via PD-L1 expression [5]. TAMs can induce resistance to chemotherapy and immunotherapies [6, 7] and have the capacity to suppress anti-tumour immune responses [8–10] by expressing key immunomodulatory factors such as Interleukin (IL)-4, IL-10, IL-13, Arginase1 (Arg1) and chemokines.
In our prior work, we reported that murine omental macrophages secrete CCL6 as an important mediator of ovarian cancer cell migration and colonisation of the omentum [11]. In a mouse model of ovarian cancer, CRISPR-mediated deletion of CCR1, the chemokine receptor for CCL6, in ID8 ovarian cancer cells reduced metastasis to the omentum and was associated with longer survival due to significantly delayed tumour growth compared to wild-type ID8 cells. We also demonstrated that human omental macrophages secrete CCL23, the human homologue of CCL6. These studies suggested an important role of CCL23 in human ovarian cancer.
CCL23, also known as macrophage-inflammatory protein 3 (MIP-3), and myeloid progenitor inhibitory factor-1 (MPIF-1) is secreted by various immune cell types including eosinophils, neutrophils and monocytes [12–14]. CCL23 signals through its receptor CCR1, which is expressed on cancer cells, monocytes, macrophages, dendritic cells, lymphocytes (T cells), and endothelial cells [11, 15–19]. CCL23 can induce the proliferation of CCR1-expressing cancer cells, and acts as a chemoattractant for cancer and immune effector cells to tumour sites [20]. In addition, it induces angiogenesis by activating CCR1 on vascular endothelial cells [17, 21, 22]. However, little is currently known about the effects of CCL23 on the functional activity of immune-infiltrating cells in the TME.
In this study, we investigate the role of CCL23 in ovarian cancer and its effects on T cells. We demonstrate that ascites and plasma from patients with ovarian cancer contains high levels of CCL23 relative to plasma from healthy individuals. The expression of CCL23 in ovarian cancer tissue was found to correlate with poor prognosis. Furthermore, ovarian cancers with high levels of CCL23 showed increased fractions of CD8 + T cells with expression of exhaustion markers including CTLA-4 and PD-1. CCL23 induced an exhausted phenotype in CD8 + T cells in a GSK3β-dependent manner. In summary, our findings describe a novel function of CCL23 that might contribute significantly to the immunosuppression in the ovarian cancer TME in ovarian cancer.
Methods
Plasma and ascites samples
Ascites from patients with gynecologic malignancies were collected under an approved Institutional Review Board (IRB) protocol at Stanford University (Stanford, CA). Plasma from patients with HGSC (n = 5) was purchased from the tissue bank at Stanford University. Plasma from patients with HGSC (n = 20) and healthy donors (n = 20) was purchased from the biobank at The University of Pennsylvania Tumour Biotrust Collection (Philadelphia, PA). A separate set of plasma from healthy donors (n = 20) was purchased from Innovative Research (Novi, MI). Informed consent was obtained from all subjects.
ELISA
CCL23 was detected using an ELISA kit (Thermo Fisher Scientific (Waltham, MA), EHCCL23) according to the manufacturer’s protocol.
Isolation of primary human immune cells
LRS chambers were obtained from healthy human donors from Stanford Blood Bank. Blood was diluted with PBS containing 2% FBS and 1 mM EDTA (Wash Buffer), and carefully layered on Ficoll before spinning at 800×g for 30 min at room temperature. Mononuclear cells were collected from the interface and washed twice in wash buffer at 300×g for 8 min. Cells were resuspended in wash buffer and counted with trypan blue.
For isolation of monocytes and T cells, cell concentration was adjusted to 50 × 106 cells/ml and untouched cells were isolated with negative selection kits according to the manufacturer’s instructions. Three technical replicates were used for setting up the in vitro exhaustion assay for each donor.
In vitro exhaustion assays
CD8 + T cells were isolated using EasySep™ Human CD8 + T-cell Isolation Kit (STEMCELL™ Technologies (Cambridge, MA), 17953). Cells were resuspended in ImmunoCult-XF T-cell Expansion Medium (STEMCELL™ Technologies, 10981) with 20 IU/ml of human IL-2 (STEMCELL™ Technologies, 78036). Some wells were supplemented with human CCL23 at 200 ng/ml (PeproTech® (Rocky Hill, NJ), 300-29-100 µg). For each donor, CD8 + T cells were treated with 25 µl/well of ImmunoCult™ Human CD3/CD28 T-cell Activator (STEMCELL™ Technologies, 10971) as a positive control. Media was changed every 2 days and expression of exhaustion markers was carried out at transcriptomic level and proteomic level on day 8. For small-molecule inhibition of GSK3, SB-415286 (Tocris Bioscience (Minneapolis, MN), 16-171-0) was added to wells in addition to CCL23 treatment for 8 days. Small-molecule inhibition of GSK3 was conducted twice with different donors and three technical replicates for each donor.
Macrophage polarisation
CD14 + monocytes were isolated from peripheral blood using EasySep™ Human Monocyte Isolation Kit (STEMCELL™ Technologies, 19359) and resuspended in ImmunoCult™-SF Macrophage Medium (STEMCELL™ Technologies, 10961) supplemented with 50 ng/ml of Human Recombinant M-CSF (STEMCELL™ Technologies, 78057.1). Media was topped up on day 4. Polarisation was carried out by adding new media containing LPS + IFNg (STEMCELL™ Technologies, 78020) (for M1 macrophages) or IL-4 (STEMCELL™ Technologies, 78045.1) (for M2 macrophages) on day 6. Supernatants (macrophage conditioned media) were collected on day 8. Macrophage polarisation was performed with two donors, with three technical replicates.
Macrophage conditioned media neutralisation assay
Healthy CD8 + T cells were incubated in triplicate with M1 or M2 macrophage conditioned media, with or without CCL23 neutralising antibody (R&D systems (Minneapolis, MN), MAB371) at 5 µg/ml. Media was changed every 2 days and exhaustion markers were detected on day 8 by flow cytometry. Neutralisation assay was performed once, with three donors and three technical replicates for each donor.
Flow cytometry staining
CD8 + T cells were stained for expression of exhaustion markers at the end of 8-day in vitro experiments. Cells were incubated with live dead IR-dye (Life Technologies (Waltham,MA), L34975) at 1:1500 concentration. Cells were washed and surface markers were blocked by incubating cells in PBS supplemented with 10% FBS and Human TruStain FcX™(Biolegend (San Diego, CA), 422302) for 15 min. Cells were then stained with FITC anti-TIM-3 (Clone F38-2E2, Biolegend, 345021), APC anti-CD8a (Clone HIT8a, Biolegend, 300911), PE-Dazzle 594 anti-CTLA-4 (Clone BNI3, Biolegend, 369615), eFluor 450 anti-LAG-3 (Clone 3DS223H, eBioscience (Waltham, MA), 48-2239-42), BV711 anti-PD-1 (Clone EH12.2H7, Biolegend, 329927) and BUV395 anti-TIGIT (Clone 741182, BD Biosciences (Franklin Lakes, NJ), 747845). Cells were fixed with 4% paraformaldehyde for 15 min and then resuspended in PBS for analysis on BD FACSymphony. An experiment was performed once per donor, with three technical replicates for each donor.
Real-time RT–PCR
Total RNA was isolated with the RNeasy Mini Plus kit (Qiagen (Venlo, Netherlands), 74136) and reverse-transcribed into cDNA using the SuperScript™ III First-Strand Synthesis SuperMix (Invitrogen (Carlsbad, CA), 18080400). Real-time PCR was performed using SsoFast™ EvaGreen® Supermix (Biorad (Hercules, CA), 172-520) on an Applied Biosystems 7500 Fast Real-Time PCR System at the Stanford Functional Genomics Facility. Expression of the target genes was normalised to the housekeeping gene 18S. RNA from human PBMC-derived CD8 + T cells activated with CD3/CD28 was used as a positive control for all the gene expression studies.
The target gene-specific primers were obtained from ELIM Biopharm (Hayward, CA). The primer sequences used in this study are shown in Supplementary Table 1. All real-time RT-PCRs were performed in triplicates and the relative mRNA expression of each target gene was determined by using the formula 2−ΔCT (CT, cycle threshold) where ΔCT = CT (target gene) − CT (18S). The comparative expression level of each target gene between different samples was 2−ΔΔCT.
Protein isolation and western blotting
Protein was extracted at the 1h time point from CD8 + T cells treated with IL-2 or CCL23 + IL-2 with the Cell Lysis Buffer (Cell Signaling Technology® (Danvers, MA), 9803S) supplemented with Halt™ Protease and Phosphatase Inhibitor Cocktail (Thermo Scientific™, 78442), EDTA (0.5 M) and 1 mM PMSF. BCA (Thermo Scientific™, 23225) was performed to determine the total protein concentration. Samples were mixed with loading dye, boiled and equal amounts of protein were resolved on Invitrogen™ Bolt™ 4–12% Bis-Tris Plus Gels (Invitrogen™, NW04122BOX). Samples were transferred to PVDF membranes, blocked with 1:1 mix of TBS and Odyssey® Blocking Buffer (TBS) (Licor® (Lincoln, NE), 927-50000) for 1 h at room temperature. Membranes were incubated in primary antibody (Cell Signaling Technology, Phospho GSK3β (Ser9)—9323S, GSK3β—12456S, pAkt—4058S, Akt—9272, GAPDH—97166S) overnight followed by three washes in TBS with 0.2%Tween20® (TBS-T). Membranes were then incubated with secondary antibodies (Licor®, 926-32211, 92668070) for 1 h at room temperature. After three washes in (TBS-T), bands were detected on Licor Odyssey Fc imager in the 700 and 800 channel. The experiment was performed twice, once for GSK-3β and once for Akt.
Protein expression was determined based on the detection of a band. The intensity of the protein bands observed was semi-quantified using the Image studio software from Licor®, (Windows application VIO.02), with normalisation of each protein against GAPDH.
Phosphoprotein array
Proteome-profiler human phosphor array (R&D systems, ARY003B) was used to determine the effect of CCL23 on the phosphorylation of proteins in CD8 T cells. Protein was isolated from cells stimulated with IL-2 or IL-2 + CCL23 for 60 min and assayed for the array according to manufacturer’s protocol. Membranes were incubated with 500 µg of protein lysates for each condition. The membranes were imaged by chemiluminescence by using LICOR Odyssey Fc imaging system. Positivity of expression was determined based on the presence of a visible spot. The intensity of the chemiluminescent spots observed was semi-quantified using the Image studio software (Windows application VIO.02), using normalisation controls as per the manufacturer’s protocol. The values were used to plot graphs using GraphPad Prism Version 8.0.2 (GraphPad, San Diego, CA). The experiment was performed once.
Immune cell estimation from RNA-Seq data
Bulk RNA-Seq data from HGSC patients were downloaded from the Cancer Genome Atlas (TCGA) and normalised. Patients were stratified into three groups (High, Medium and Low) containing roughly equal number of samples based on the expression of CCL23 transcripts. Patients within the high and low groups were analysed using CIBERSORTx (https://cibersort.stanford.edu) and EPIC (http://epic.gfellerlab.org) with a Melanoma signature matrix with nine-cell subsets, including PDCD1 + CTLA-4 + CD8 T cells [23].
Quantification and statistical analysis
All grouped data are presented as mean ± SD. The sample size was limited by the maximum available patient samples and healthy donor cells for all experiments. No exclusion criteria were used. Significance between groups was analysed by one-way ANOVA or Student t test using GraphPad Prism. Testing for equal variance was performed by the Brown–Forsythe test. The KM plotter online tool (http://kmplot.com) was used to analyse the prognosis of CCL23 in ovarian cancer. The analysis was conducted for all serous samples with an auto-select best cutoff feature. Kaplan–Meier survival analysis was conducted via log-rank test.
Results
CCL23 is associated with poor prognosis in ovarian cancer
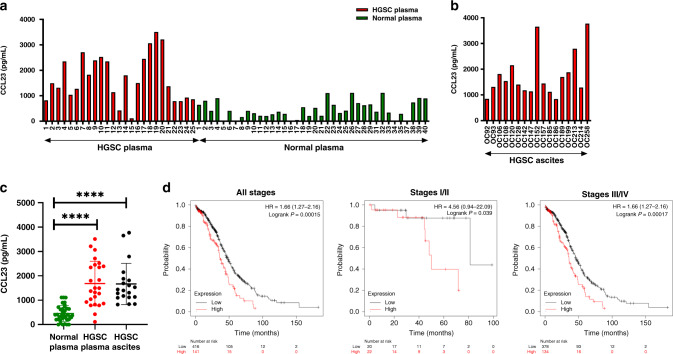
In our previous study, we found that human omental macrophages express CCL23 and demonstrated that CCL23 induces migration of human ovarian cancer cells. Our initial experiments therefore investigated whether CCL23 is present in the peripheral blood and ascites from patients with ovarian cancer. We determined serum levels of CCL23 in a cohort of patients with newly diagnosed high-grade serous ovarian cancer (HGSC) (n = 25). Blood samples were obtained prior to any treatment. (Fig. 1a, c). Plasma from healthy donors was used as control (n = 40). CCL23 plasma levels were found to be significantly higher in ovarian cancer patients (mean ± SD: 1684 ± 917.2 pg/ml) compared to healthy individuals (mean ± SD: 454.7 ± 328.3 pg/ml; P < 0.0001). In a separate cohort of HGSC patients, we found high levels of CCL23 (mean ± SD: 1756 ± 881.2 pg/ml) in ascites (n = 17) (Fig. 1b, c). Compared to patients with HGSC, ascites from patients with various other gynecologic malignancies, including cervical, endometrial and low-grade serous ovarian cancer demonstrated lower levels of CCL23 (mean ± SD: 1000 ± 765.8 pg/ml); P = 0.0137) (Supplementary Fig. 1a, b).
Fig. 1. CCL23 is elevated in plasma and ascites from patients with ovarian cancer.
a CCL23 levels in plasma from healthy donors (n = 40) and patients with HGSC (n = 25). b CCL23 levels in ascites samples from patients (n = 17) with HGSC. c Comparison of mean CCL23 levels in plasma from healthy donors, plasma from patients with HGSC and ascites samples from patients with HGSC. Each data point represents one patient sample. P values were calculated using Tukey’s multiple comparisons test (****P < 0.0001). d Kaplan–Meier survival analysis (log-rank test) of CCL23 expression in HGSC tumours and on survival of patients with ovarian cancer.
We next analysed mRNA-expression levels of CCL23 in HGSC using data from the Cancer Genome Atlas Program (TCGA). In all patients, the median OS for patients whose tumours showed high CCL23 expression levels was 36.73 months compared to 48.2 months in low CCL23 expressing tumours (hazard ratio (HR): 1.66 (1.27–2.16); P = 0.0015). In Stage I and II HGSC, high levels of CCL23 tumour expression were found to be associated with a significant shorter overall survival compared to patients with low levels of CCL23 (HR: 4.56 (0.94–22.09); median OS: 49.97 (high CCL23) vs. 81.23 (low CCL23) months, P = 0.039) (Fig. 1d). Similarly, in Stage III and IV ovarian cancer, high levels of CCL23 mRNA expression correlated with a poor clinical prognosis (HR: 1.66 (1.27–2.16); median OS: 35.77 (high CCL23) vs. 45.63 (low CCL23) months, P = 0.00017).
CCL23 expression is associated with the increased presence of macrophages and CD8 + PDCD1 + CTLA-4 + T cells in ovarian cancer
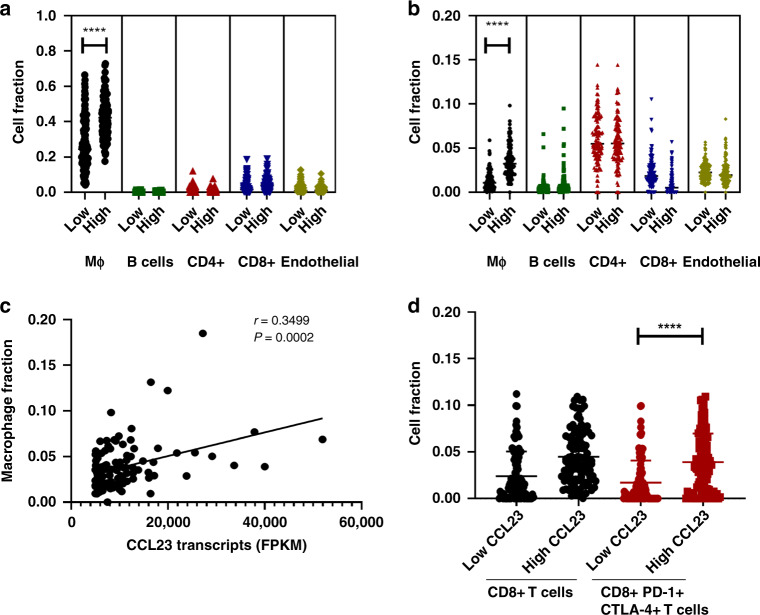
We next determined whether the expression of CCL23 is correlated with the presence of specific immune-infiltrating cells in ovarian cancer tissue. The TCGA HGSC mRNA-expression data was analyzed using CIBERSORT and EPIC for deconvolution (Fig. 2a). CIBERSORT and EPIC utilise gene expression data to estimate the total proportion of different immune cell populations in a sample. Independent CIBERSORT and EPIC analyses showed that ovarian cancers with high expression of CCL23 had a significantly higher cell fraction of macrophages (mean: 0.42 (0.17–0.72)) compared to ovarian cancers with low CCL23 expression (mean: 0.27 (0.04–0.66); P < 0.0001) (Fig. 2b). In contrast, the fraction of other immune cell subtypes such as B, CD4 + and CD8 + T cells, as well as endothelial cells did not differ between the high and low CCL23 expressing groups. The macrophage fraction and CCL23 transcript levels in the high CCL23 group showed a strong positive correlation (r = 0.35; P = 0.0002) (Fig. 2c), providing supportive evidence that macrophages are likely the major source of CCL23 in the tumour microenvironment of ovarian cancer.
Fig. 2. Higher CCL23 expression correlates with the increase in exhausted CD8 + T cells and macrophage infiltration in TCGA.
a Estimated immune cell fractions in HGSC stratified by CCL23 levels (CIBERSORT). b Estimated immune cell fractions in HGSC stratified by CCL23 levels (EPIC). c Correlation of macrophage content with CCL23 expression in HGSC with high CCL23. d Increased CD8 + PD-1+ CTLA-4 + T cells in HGSC with high CCL23. All statistical analyses were carried out by an unpaired t test (*P < 0.05; ****P < 0.0001).
We next used CIBERSORT analysis to study the expression of other markers expressed by subpopulations of immune effector cells in the TME. We focused our analysis on programmed death-1 (PD-1) and cytotoxic T-lymphocyte-associated antigen (CTLA-4) which are critical co-inhibitory immune checkpoints and important negative regulators of T-cell function. We found a significantly greater fraction of CD8 + T cells expressing both PDCD1 and CTLA-4 in ovarian cancer with high expression of CCL23 (mean: 0.03 (0.0–0.13)) compared to low CCL23 expressing samples (mean: 0.012 (0.0–0.05); P < 0.0001) (Fig. 2d).
CCL23 induces the expression of exhaustion markers on T cells
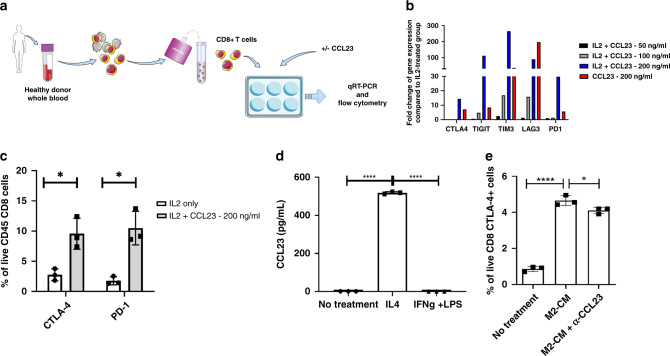
Based on the association between high levels of CCL23 in the tumour microenvironment and the presence of high proportions of CD8 + PDCD1 + CTLA-4 + T cells, we hypothesised that CCL23 increases the expression of exhaustion markers in CD8 + T cells. To test this hypothesis, we isolated CD8 + T cells from peripheral blood mononuclear cells (PBMCs) of healthy donors (Fig. 3a). CD8 + T cells were cultured in the presence of IL-2 and CCL23 at increasing concentrations for 8 days. qRT-PCR analysis was performed to assess the expression of T-cell exhaustion markers in CCL23-treated CD8 + T cells. Treatment of T cells with CCL23 resulted in a dose-dependent increase in the expression of genes encoding for CTLA-4, TIGIT, TIM-3, LAG-3 and PD-1. This increase was greater in IL-2-activated CD8 + T cells compared to cells treated with CCL23 alone for all exhaustion markers except for LAG-3 (Fig. 3b). The percentage of CD8 + CTLA-4 + -cells was 3.5-times higher in activated CD8 + T cells treated with CCL23 compared to IL-2-treated controls (P < 0.05) (Fig. 3c). Similarly, the percentage of PD-1 + T cells was three times higher in CCL23-treated CD8 + T cells compared to IL-2-treated cells as determined by FACS analysis (P < 0.05). The CCL23-induced upregulation of T-cell exhaustion markers suggests that CCL23 induces an immunosuppressed CD8 + T-cell phenotype. In contrast, CCL23 did not induce the expression of exhaustion markers on CD4 + T cells (Supplementary Fig. 2).
Fig. 3. CCL23 induces expression of exhaustion markers in CD8 + T cells.
a Schematic illustrating the process of isolating CD8 + T cells from PBMCs of healthy donors and treating the cells with exogenous CCL23 for qRT-PCR and flow cytometry analysis. b Expression of exhaustion markers on CD8 + T cells by qRT-PCR after treatment with CCL23. The experiment was performed once. c CTLA-4 and PD-1 expression is upregulated on CD8 + T cells after treatment with CCL23 by flow cytometry. The experiment was performed with n = 3 donors. d Expression of CCL23 in M1- and M2-polarised macrophage supernatant. The experiment was replicated once. e Neutralisation of CCL23 in M2-conditioned media partially reverses CTLA-4 expression on CD8 + T cells by flow cytometry. The experiment was performed with n = 3 donors. All error bars are mean + /− SD. Statistical analysis was carried out by an unpaired t test (*P < 0.05; ****P < 0.0001).
Our prior studies had demonstrated that macrophages extracted from human omentum express CCL23, but the effect of macrophage polarisation on CCL23 expression was not assessed [11]. Given our earlier observations that ovarian cancers with high levels of CCL23 have increased levels of macrophages and that CCL23 promotes immunosuppression by upregulating exhaustion markers in CD8 + T cells, we hypothesised that immunosuppressive M2 macrophages rather than M1 macrophages are the main source of CCL23. To test this hypothesis, we isolated CD14 + monocytes from healthy PBMCs and generated macrophages by incubating CD14 + cells with M-CSF over 6 days. We then polarised macrophages using IFNγ/LPS and IL-4 to obtain M1 and M2 macrophages, respectively. The culture supernatant of M2 macrophages contained significantly elevated levels of CCL23 (510 + /− 4 pg/ml; P < 0.0001) (Fig. 3d). In contrast, M1 macrophages did not produce any measurable levels of CCL23. T cells treated with conditioned media from M2-polarised macrophages showed a fivefold increase in levels of CTLA-4 + CD8 + T cells when compared to T cells cultured in control media (P < 0.001). The conditioned M2 medium is complex and likely contains factors other than CCL23 that might increase the expression of CTLA-4. However, the conditioned media-induced increase in the CTLA-4 + CD8 + T-cells population was at least partially reversed by antibody-mediated neutralisation of CCL23 (Fig. 3e) (P < 0.05). This data suggests that M2 macrophages are the main source of CCL23 which induces the expression of T-cell exhaustion markers in CD8 + T cells.
CCL23-mediated T-cell exhaustion is dependent on GSK3β
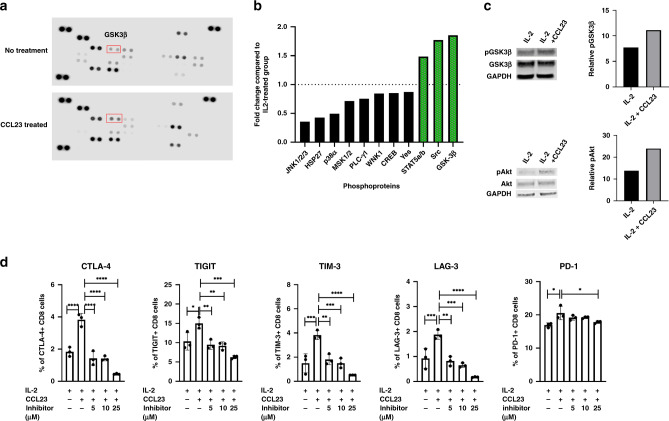
We next investigated the mechanisms underlying the CCL23-induced T-cell exhaustion in CD8 + T cells. Protein lysates isolated from CCL23-treated CD8 + T cells were analyzed by a phosphokinase array assay (Fig. 4a). CCL23 increased phosphorylation of several kinases such as Steroidogenic Acute Regulatory Protein 5A/B (STAT5A/B), Proto-oncogene tyrosine-protein kinase (SRC) and Glycogen synthase kinase 3β (GSK3β) (1.48-, 1.77- and 1.85-fold increase, respectively) compared to untreated controls (Fig. 4b, c). In contrast, c-Jun N-terminal kinases (JNK1/2/3), heat-shock protein (HSP27) and mitogen-activated protein kinase (MAPK) p38a showed decreased levels of phosphorylation (2.8, 2.33 and 2.02 times lower, respectively).
Fig. 4. CCL23-induced T-cell signalling.
a Phosphoprotein array of CCL23-treated T cells. The experiment was performed once. b Increased GSK3b phosphorylation induced by CCL23. The experiment was performed once. c Validation and quantification of phosphorylation of GSK3β and Akt by western blot. The experiment was performed once each. d GSK3 inhibition reduces the expression of exhaustion markers on CD8 + T cells. The experiment was replicated with n = 3 donors. Statistical analysis was conducted by one-way ANOVA (*P < 0.05; **P < 0.005; ***P < 0.001; ****P < 0.0001).
Interestingly, previous studies had demonstrated that GSK3β functions as a regulator of immune checkpoint expression [24]. Using western blot analysis, we confirmed the significant increase of GSK3β phosphorylation in IL-2-activated CD8 + T cells treated with CCL23. Inhibition of GSK3α/β using a small-molecule inhibitor (SB415286) during CCL23 treatment of CD8 + cells, showed a significant reduction of CCL23-induced T-cell exhaustion marker expression. The expression of CTLA-4, TIGIT, TIM-3, LAG-3 and PD-1 in CD8 + T cells decreased in a dose-dependent manner (Fig. 4d). This data demonstrates that CCL23-mediated T-cell exhaustion is dependent on the phosphorylation of GSK3β, and that inhibition of GSK3α/β prevents CCL23-induced T-cell exhaustion.
Discussion
Our study identifies a novel function of CCL23 which induces upregulation of exhaustion markers on T cells. This finding is of particular importance in the HGSC of the ovary since high levels of CCL23 were found in patients’ ascites and plasma. CCL23 secreted by TAMs might hence play an important role in the establishment of the immunosuppressive TME in ovarian cancer.
Tumour-associated macrophages (TAMs) found in the TME have important functions in the growth and metastasis of solid tumours including ovarian cancer [25]. TAMs secrete cytokines and chemokines that either directly affect T-cell effector function or stimulate the proliferation of immune-suppressive T-cell subtypes such as induced regulatory T cells (iTregs) and natural regulatory T cells (nTregs) [26]. CCL22 for example is secreted by TAMs and actively recruits CCR4 + nTregs to the TME [26]. Both IL-10 and TGF-β secreted by TAMs are able to interfere with the anti-tumour activity of T cells [27], and inhibit CD4 + T-cell proliferation [28]. IL-10 and TGF-β can recruit iTregs to suppress immune responses [29]. In addition, enzymes secreted by TAMs such as Arginase1 (ARG1) and matrix metalloproteinase (MMPs) inhibit T-cell effector cells [30]. Similarly, TGFβ production and TGFβ-dependent secretion of CCL22 by TAMs create an immunosuppressive TME [31]. In ovarian cancer, the majority of macrophages are of the M2 phenotype [32] that secrete hormones such as insulin-like growth factor-1 (IGF-1), immune-suppressive cytokines such as IL-8, IL-10, IL-13 and TGF-β and chemokines such as CCL2, CCL18, CCL20 and CCL22 [30, 33]. The high levels of CCL23 secreted by M2 macrophages as shown in our study add another mechanism underlying the immune-suppression function of this macrophage population.
Our current study finds that CCL23 is a modulator of the TME in ovarian cancer and induces T-cell exhaustion. Prior observations from our group have described that CCL23 functions as a chemoattractant for ovarian cancer cells [11]. Others have reported that CCL23 is expressed in osteophytic tissue and attracts osteoclast precursors to sites of bone resorption [34]. Apart from functioning as a chemoattractant, CCL23 regulates innate immune responses by suppressing the production of polymorphonuclear leucocytes (PMNs) and monocyte progenitors in the bone marrow [35]. High CCL23 levels are associated with numerous inflammatory conditions such as rheumatoid arthritis, atherosclerosis, eosinophilic chronic rhinosinusitis and systemic sclerosis [36–40]. Furthermore, elevated levels of circulating CCL23 following an ischaemic stroke serve as a biomarker for acquired cerebral lesions [41]. In endothelial cells, CCL23 upregulates the KDR/Flk-1 receptor which increases VEGF-mediated angiogenesis [22].
We identified GSK3β as an important mediator of CCL23-induced upregulation of T-cell exhaustion markers. The involvement of GSK3 in regulating T-cell immunity and immune checkpoints has been demonstrated in prior studies [24]. GSK3 isoforms α and β for example were shown to be a key upstream regulator of PDCD1, the gene that encodes for PD-1, on CD8 + T cells. siRNA knockdown of GSK3 in CD8 + T cells reduced PD-1 expression and increased cytotoxic T-lymphocyte (CTL) functions. In vivo, the GSK3 inhibitor SB415286 effectively enhanced CD8 + CTL function and reversed virus-induced T-cell exhaustion [24]. GSK3β, has a more profound impact on T-cell-mediated anti-tumour activity compared to GSK3α since T-cell-specific deletion of GSK3β in mice was sufficient to suppress tumour growth while GSK3α deletion did not prevent tumour growth [42]. The enhancement of CD8 + CTL function upon inhibiting GSK3 was also illustrated in mice using the B16 murine melanoma cells. Treatment of mice with established tumour using SB415286 showed effective tumour regression [43].
Exhausted T cells have been reported in numerous cancers. In CD8 + T cells isolated from metastases from patients with melanoma, expression of exhaustion markers was found to be high [44]. In NSCLC, intra-tumoral CD8 + T cells showed higher levels of exhaustion markers such as PD-1, TIM-3, CTLA-4 and LAG-3. The expression of PD-1 and CTLA-4 is associated with T-cell exhaustion which is characterised by T-cell dysfunction and reduced T-cell immunity. PD-1 and CTLA-4 participate in inhibiting T-cell activation and proliferation, therefore limiting T-cell-mediated anti-tumour responses. At a functional level, these exhausted T cells respond poorly to polyclonal activation [45]. In the context of ovarian cancers, CD4 + tumour-infiltrating lymphocytes (TILs) express high levels of PD-1 and the exhaustion transcription factor TOX and hence share transcriptomic and phenotypic features similar to exhausted CD8 + T cells [46, 47].
The impact of CCL23 on the response of patients to immunotherapy in ovarian cancer remains to be investigated. The immune-suppressive TME in ovarian cancer is however not the only obstacle to more clinical effective immunotherapy. Numerous studies now support the existence of intra-patient heterogeneity in the tumour immune microenvironment which has a significant impact on response to therapy and clinical outcomes [4, 48]. Jimenez-Sanchez et al. recently showed that exclusion of immune cells from metastatic sites was associated with progression of lesions whereas T-cell infiltration was associated with regression or stable disease [4]. In a separate study, ‘cold’ immune microenvironments present in HGSC patients pre-treatment can function as immune-privileged sites and harbour malignant clones contributing to future relapse [48]. Olalaken et al. performed a single-cell transcriptomic study and found that ovarian cancer can be divided into two distinct groups with high and low T-cell infiltration. Tumours with high T-cell infiltration included plasmablasts, granulysin-expressing CD4 + T cells and CD8 + TOX + T cells and showed better survival [49].
In summary, we describe a novel function of macrophage-derived CCL23. We demonstrate that CCL23 induces an exhaustion phenotype in CD8 + T cells by upregulation of several immune checkpoints, including CTLA-4, TIGIT, TIM-3 and LAG-3. The high levels of CCL23 in ovarian cancer ascites and plasma validate the importance of our findings in patients with ovarian cancer. Taken together, our data suggest that CCL23 secreted by macrophages contributes to the immunosuppressive TME in ovarian cancer.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Author contributions
KK, VK and OD designed experiments; KK carried out and analyzed experiments; KK, VK and OD prepared the figures and wrote the initial draft of the manuscript; all authors edited the manuscript; VK and OD provided supervision.
Funding
This work was supported by the Mary Lake Polan Gynecologic Oncology Endowment for Research (OD), the Vivian Scott Fellowship in Gynecologic Oncology (OD).
Data availability
RNA-seq data for patients with ovarian cancer were retrieved from TCGA, which is publicly available at https://portal.gdc.cancer.gov/.
Competing interests
KK and VK have no competing interests. OD has served on Advisory Boards for Merck, Eisai, PACT, GSK, IMV, Genentech. OD received funding for clinical research from AstraZeneca, IMV, Millenium, Pharmamar, Genentech, Bioeclipse.
Ethics approval and consent to participate
Patient samples were collected under an approved Institutional Review Board (IRB) protocol at Stanford University and the University of Pennsylvania. Informed consent for healthy plasma was obtained by Innovative Research (Novi, MI). This study was performed in accordance with the Declaration of Helsinki.
Consent to publish
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-022-01887-3.
References
- 1.Wang Y, Ren F, Song Z, Wang X, Zhang C, Ouyang L. PARP inhibitors in patients with newly diagnosed advanced ovarian cancer: a meta-analysis of randomized clinical trials. Front Oncol. 2020;10:1204. doi: 10.3389/fonc.2020.01204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borella F, Ghisoni E, Giannone G, Cosma S, Benedetto C, Valabrega G, et al. Immune checkpoint inhibitors in epithelial ovarian cancer: an overview on efficacy and future perspectives. Diagnostics. 2020;10:146. doi: 10.3390/diagnostics10030146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez GM, Galpin KJC, McCloskey CW, Vanderhyden BC. The tumor microenvironment of epithelial ovarian cancer and its influence on response to immunotherapy. Cancers. 2018;10:E242. doi: 10.3390/cancers10080242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jimenez-Sanchez A, Memon D, Pourpe S, Veeraraghavan H, Li Y, Vargas HA, et al. Heterogeneous tumor-immune microenvironments among differentially growing metastases in an ovarian cancer patient. Cell. 2017;170:927–38. doi: 10.1016/j.cell.2017.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krishnan V, Schaar B, Tallapragada S, Dorigo O. Tumor associated macrophages in gynecologic cancers. Gynecol Oncol. 2018;149:205–13. doi: 10.1016/j.ygyno.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Petty AJ, Yang Y. Tumor-associated macrophages: implications in cancer immunotherapy. Immunotherapy. 2017;9:289–302. doi: 10.2217/imt-2016-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiang X, Wang J, Lu D, Xu X. Targeting tumor-associated macrophages to synergize tumor immunotherapy. Signal Transduct Target Ther. 2021;6:75. doi: 10.1038/s41392-021-00484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang M, He Y, Sun X, Li Q, Wang W, Zhao A, et al. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res. 2014;7:19. doi: 10.1186/1757-2215-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dun EC, Hanley K, Wieser F, Bohman S, Yu J, Taylor RN. Infiltration of tumor-associated macrophages is increased in the epithelial and stromal compartments of endometrial carcinomas. Int J Gynecol Pathol. 2013;32:576–84. doi: 10.1097/PGP.0b013e318284e198. [DOI] [PubMed] [Google Scholar]
- 10.Petrillo M, Zannoni GF, Martinelli E, Pedone Anchora L, Ferrandina G, Tropeano G, et al. Polarisation of tumor-associated macrophages toward M2 phenotype correlates with poor response to chemoradiation and reduced survival in patients with locally advanced cervical cancer. PLoS ONE. 2015;10:e0136654. doi: 10.1371/journal.pone.0136654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishnan V, Tallapragada S, Schaar B, Kamat K, Chanana AM, Zhang Y, et al. Omental macrophages secrete chemokine ligands that promote ovarian cancer colonization of the omentum via CCR1. Commun Biol. 2020;3:524. doi: 10.1038/s42003-020-01246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poposki JA, Uzzaman A, Nagarkar DR, Chustz RT, Peters AT, Suh LA, et al. Elevated expression of CC Chemokine ligand 23 in eosinophilic chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128:73–81. doi: 10.1016/j.jaci.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arruda-Silva F, Bianchetto-Aguilera F, Gasperini S, Polletti S, Cosentino E, Tamassia N, et al. Human neutrophils produce CCL23 in response to various TLR-agonists and TNFα. Front Cell Infect Microbiol. 2017;7:176. [DOI] [PMC free article] [PubMed]
- 14.Novak H, Müller A, Harrer N, Günther C, Carballido JM, Woisetschläger M. CCL23 expression is induced by IL-4 in a STAT6-dependent fashion. J Immunol. 2007;178:4335–41. doi: 10.4049/jimmunol.178.7.4335. [DOI] [PubMed] [Google Scholar]
- 15.Cheng JF, Jack R. CCR1 antagonists. Mol Divers. 2008;12:17–23. doi: 10.1007/s11030-008-9076-x. [DOI] [PubMed] [Google Scholar]
- 16.Rabin RL, Park MK, Liao F, Swofford R, Stephany D, Farber JM. Chemokine receptor responses on T cells are achieved through regulation of both receptor expression and signaling. J Immunol. 1999;162:3840–50. [PubMed] [Google Scholar]
- 17.Hwang J, Son KN, Kim CW, Ko J, Na DS, Kwon BS, et al. Human CC chemokine CCL23, a ligand for CCR1, induces endothelial cell migration and promotes angiogenesis. Cytokine. 2005;30:254–63. doi: 10.1016/j.cyto.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 18.Foroughi F, Amirzargar A, Ahmadpoor P, Noorbakhsh F, Nafar M, Yekaninejad MS, et al. Increased levels of CD4(+) and CD8(+) T cells expressing CCR1 in patients developing allograft dysfunction; a cohort study. Transpl Immunol. 2016;38:67–74. doi: 10.1016/j.trim.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Conroy MJ, Galvin KC, Kavanagh ME, Mongan AM, Doyle SL, Gilmartin N, et al. CCR1 antagonism attenuates T cell trafficking to omentum and liver in obesity-associated cancer. Immunol Cell Biol. 2016;94:531–7. doi: 10.1038/icb.2016.26. [DOI] [PubMed] [Google Scholar]
- 20.Kim J, Kim YS, Ko J. CKβ8/CCL23 and its isoform CKβ8-1 induce up-regulation of cyclins via the Gi/Go protein/PLC/PKCδ/ERK leading to cell-cycle progression. Cytokine. 2010;50:42–9. doi: 10.1016/j.cyto.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Son KN, Hwang J, Kwon BS, Kim J. Human CC chemokine CCL23 enhances expression of matrix metalloproteinase-2 and invasion of vascular endothelial cells. Biochem Biophys Res Commun. 2006;340:498–504. doi: 10.1016/j.bbrc.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 22.Han KY, Kim CW, Lee TH, Son Y, Kim J. CCL23 up-regulates expression of KDR/Flk-1 and potentiates VEGF-induced proliferation and migration of human endothelial cells. Biochem Biophys Res Commun. 2009;382:124–8. doi: 10.1016/j.bbrc.2009.02.149. [DOI] [PubMed] [Google Scholar]
- 23.Newman AM, Steen CB, Liu CL, Gentles AJ, Chaudhuri AA, Scherer F, et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat Biotechnol. 2019;37:773–82. doi: 10.1038/s41587-019-0114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor A, Harker JA, Chanthong K, Stevenson PG, Zuniga EI, Rudd CE. Glycogen synthase kinase 3 inactivation drives T-bet-mediated downregulation of co-receptor PD-1 to enhance CD8(+) cytolytic T cell responses. Immunity. 2016;44:274–86. doi: 10.1016/j.immuni.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol. 2019;12:76. doi: 10.1186/s13045-019-0760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 27.Zhou J, Tang Z, Gao S, Li C, Feng Y, Zhou X. Tumor-associated macrophages: recent insights and therapies. Front Oncol. 2020;10:188. doi: 10.3389/fonc.2020.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao Q, Wang Y, Zheng D, Sun Y, Wang Y, Lee VW, et al. IL-10/TGF-beta-modified macrophages induce regulatory T cells and protect against adriamycin nephrosis. J Am Soc Nephrol. 2010;21:933–42. doi: 10.1681/ASN.2009060592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: the role of T regulatory cells. Immunology. 2006;117:433–42. doi: 10.1111/j.1365-2567.2006.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y, Song Y, Du W, Gong L, Chang H, Zou Z. Tumor-associated macrophages: an accomplice in solid tumor progression. J Biomed Sci. 2019;26:78. doi: 10.1186/s12929-019-0568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang D, Yang L, Yue D, Cao L, Li L, Wang D, et al. Macrophage-derived CCL22 promotes an immunosuppressive tumor microenvironment via IL-8 in malignant pleural effusion. Cancer Lett. 2019;452:244–53.. doi: 10.1016/j.canlet.2019.03.040. [DOI] [PubMed] [Google Scholar]
- 32.Reinartz S, Schumann T, Finkernagel F, Wortmann A, Jansen JM, Meissner W, et al. Mixed-polarization phenotype of ascites-associated macrophages in human ovarian carcinoma: correlation of CD163 expression, cytokine levels and early relapse. Int J Cancer. 2014;134:32–42. doi: 10.1002/ijc.28335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MP, Donners MM. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014;17:109–18. doi: 10.1007/s10456-013-9381-6. [DOI] [PubMed] [Google Scholar]
- 34.Votta BJ, White JR, Dodds RA, James IE, Connor JR, Lee-Rykaczewski E, et al. CKbeta-8 [CCL23], a novel CC chemokine, is chemotactic for human osteoclast precursors and is expressed in bone tissues. J Cell Physiol. 2000;183:196–207. doi: 10.1002/(SICI)1097-4652(200005)183:2<196::AID-JCP6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 35.Shih CH, van Eeden SF, Goto Y, Hogg JC. CCL23/myeloid progenitor inhibitory factor-1 inhibits production and release of polymorphonuclear leukocytes and monocytes from the bone marrow. Exp Hematol. 2005;33:1101–8. doi: 10.1016/j.exphem.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 36.Kim J, Kim YS, Ko J. CK beta 8/CCL23 induces cell migration via the Gi/Go protein/PLC/PKC delta/NF-kappa B and is involved in inflammatory responses. Life Sci. 2010;86:300–8. doi: 10.1016/j.lfs.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 37.Rioja I, Hughes FJ, Sharp CH, Warnock LC, Montgomery DS, Akil M, et al. Potential novel biomarkers of disease activity in rheumatoid arthritis patients: CXCL13, CCL23, transforming growth factor alpha, tumor necrosis factor receptor superfamily member 9, and macrophage colony-stimulating factor. Arthritis Rheum. 2008;58:2257–67. doi: 10.1002/art.23667. [DOI] [PubMed] [Google Scholar]
- 38.Castillo L, Rohatgi A, Ayers CR, Owens AW, Das SR, Khera A, et al. Associations of four circulating chemokines with multiple atherosclerosis phenotypes in a large population-based sample: results from the Dallas heart study. J Interferon Cytokine Res. 2010;30:339–47. doi: 10.1089/jir.2009.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poposki JA, Uzzaman A, Nagarkar DR, Chustz RT, Peters AT, Suh LA, et al. Increased expression of the chemokine CCL23 in eosinophilic chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128:73–81. doi: 10.1016/j.jaci.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yanaba K, Yoshizaki A, Muroi E, Ogawa F, Asano Y, Kadono T, et al. Serum CCL23 levels are increased in patients with systemic sclerosis. Arch Dermatol Res. 2011;303:29–34. doi: 10.1007/s00403-010-1078-8. [DOI] [PubMed] [Google Scholar]
- 41.Simats A, Garcia-Berrocoso T, Penalba A, Giralt D, Llovera G, Jiang Y, et al. CCL23: a new CC chemokine involved in human brain damage. J Intern Med. 2018;283:461–75.. doi: 10.1111/joim.12738. [DOI] [PubMed] [Google Scholar]
- 42.Steele L, Mannion AJ, Shaw G, Maclennan KA, Cook GP, Rudd CE, et al. Non-redundant activity of GSK-3α and GSK-3β in T cell-mediated tumor rejection. iScience. 2021;24:102555. doi: 10.1016/j.isci.2021.102555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rudd CE, Chanthong K, Taylor A. Small Molecule Inhibition of GSK-3 Specifically Inhibits the Transcription of Inhibitory Co-receptor LAG-3 for Enhanced Anti-tumor Immunity. Cell Rep. 2020;30:2075–82. doi: 10.1016/j.celrep.2020.01.076. [DOI] [PubMed] [Google Scholar]
- 44.Baitsch L, Baumgaertner P, Devêvre E, Raghav SK, Legat A, Barba L, et al. Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J Clin Invest. 2011;121:2350–60. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thommen DS, Schreiner J, Müller P, Herzig P, Roller A, Belousov A, et al. Progression of lung cancer is associated with increased dysfunction of T cells defined by coexpression of multiple inhibitory receptors. Cancer Immunol Res. 2015;3:1344–55. doi: 10.1158/2326-6066.CIR-15-0097. [DOI] [PubMed] [Google Scholar]
- 46.Balança C-C, Salvioni A, Scarlata C-M, Michelas M, Martinez-Gomez C, Gomez-Roca C, et al. PD-1 blockade restores helper activity of tumor-infiltrating, exhausted PD-1hiCD39+ CD4 T cells. JCI Insight. 2021;6:142513. doi: 10.1172/jci.insight.142513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foord E, Klynning C, Schoutrop E, Förster JM, Krieg J, Mörtberg A, et al. Profound functional suppression of tumor-infiltrating T-cells in ovarian cancer patients can be reversed using PD-1-blocking antibodies or DARPin® proteins. J Immunol Res. 2020;2020:e7375947. doi: 10.1155/2020/7375947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang AW, McPherson A, Milne K, Kroeger DR, Hamilton PT, Miranda A, et al. Interfaces of malignant and immunologic clonal dynamics in ovarian cancer. Cell. 2018;173:1755–69. doi: 10.1016/j.cell.2018.03.073. [DOI] [PubMed] [Google Scholar]
- 49.Olalekan S, Xie B, Back R, Eckart H, Basu A. Characterizing the tumor microenvironment of metastatic ovarian cancer by single-cell transcriptomics. Cell Rep. 2021;35:109165. doi: 10.1016/j.celrep.2021.109165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-seq data for patients with ovarian cancer were retrieved from TCGA, which is publicly available at https://portal.gdc.cancer.gov/.