Abstract
The function of the N-terminal region of the Oenococcus oeni phage fOg44 lysin (Lys44) as an export signal was investigated. We observed that when induced in Escherichia coli, Lys44 was cleaved between residues 27 and 28 in a SecA-dependent manner. Lys44 processing could be blocked by a specific signal peptidase inhibitor and was severely reduced by modification of the cleavage site. The lethal effect of Lys44 expression observed in E. coli was ascribed to the presence of its N-terminal 27-residue sequence, as its deletion resulted in the production of a nontoxic, albeit active, product. We have further established that lytic activity in oenococcal cells was dependent on Lys44 processing. An active protein with the molecular mass expected for the cleaved enzyme was detected in extracts from O. oeni-infected cells. The temporal pattern of its appearance suggests that synthesis and export of Lys44 in the infected host progress along with phage maturation. Overall, these results provide, for the first time, experimental evidence for the presence of a signal peptide in a bacteriophage lysin. Database searches and alignment of protein sequences support the prediction that other known O. oeni and Lactococcus lactis phages also encode secretory lysins. The evolutionary significance of a putative phage lysis mechanism relying on secretory lytic enzymes is tentatively discussed, on the basis of host cell wall structure and autolytic capacity.
All tailed bacteriophages with double-stranded DNA genomes appear to accomplish lysis of the host cell by the concerted action of a peptidoglycan hydrolase (referred to as endolysin or lysin) and a small hydrophobic protein (holin) presumed to form nonspecific lesions upon oligomerization in the membrane (for a review, see reference 41). The latter function seems essential to allow access of the lytic enzyme to the cell wall compartment since in the phage lysins examined so far, the presence of a signal peptide (SP) that would target them to the translocase of the general secretion pathway (GSP) has never been demonstrated.
We have recently described the sequences of the lysin and holin genes from the Oenococcus oeni bacteriophage fOg44 and noted that the N-terminal region of its putative lysin (Lys44) was highly hydrophobic (23). A similar observation was made earlier concerning a related enzyme from the lactococcal phage Tuc2009 (2). In spite of its hydrophobic character, the function of the N-terminal sequence of the Tuc2009 lysin as a possible SP was not considered, presumably because the presence of a holin gene upstream of lys argued for a standard holin-dependent lysin export mechanism. This assumption was strengthened by the observation that the expression of an almost identical lysin in Escherichia coli (LysB from the Lactococcus lactis phage φLC3) did not result in a decrease in culture absorbance unless the corresponding holin was simultaneously induced (3).
Interestingly, however, during an attempt to overproduce Lys44 in an easily purifiable form (as a histidine-tagged fusion product, His-Lys44), we detected the production of two proteins, rather than a single polypeptide, in E. coli extracts. We then observed that only the larger product reacted with commercial anti-His6 antibodies (our unpublished results), suggesting that a processing event had removed part of the N-terminal region in a fraction of the synthesized proteins. From these preliminary observations came the idea that the hydrophobic N-terminal region of the fOg44 lysin could indeed be functioning as a cleavable SP. Also supporting this notion, an examination of the sequence by SP prediction algorithms (20, 21) indicated, with high probability, the presence of a peptidase cleavage site between residues 27 and 28 of Lys44 (see Fig. 1).
FIG. 1.
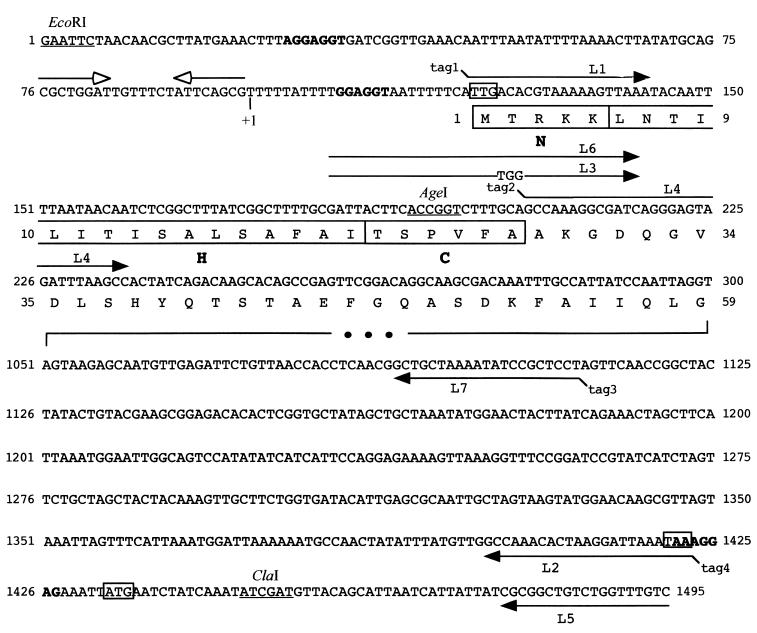
Relevant features of a DNA fragment including and surrounding the fOg44 lysin gene. Putative RBS are shown in bold. Translational start and stop codons are boxed. A “T” at position 99 in the sequence (indicated as +1) corresponds to the 5′ end of a late transcript formed in O. oeni during phage infection (reference 23 and our unpublished results). Convergent empty arrows above the nucleotide sequence represent a putative hairpin-like secondary structure. The region from nucleotide 301 to 1050 has been omitted. The entire sequence was previously deposited in GenBank (AF047001). The first 59 amino acid residues of Lys44 are indicated below the nucleotide sequence. The SP is boxed and divided into the N-terminal positively charged region (N), the hydrophobic domain (H), and the C-terminal region (C) preceding the cleavage site. Restriction sites relevant for this study are underlined and oligonucleotides used in PCRs (L1 to L7) are represented as full arrows above (forward primers) or below (reverse primers) the sequence. Noncomplementary 5′ overhangs in primers L1, L4, L7, and L2 are referred to as tag1 to tag4. These tags have the sequences AActgcag (tag1), CGgaattcAAGGAGGTAATTTTTCAATG (tag2), GTatcgatTCA (tag3), and CGgaattc (tag4), where lowercase, bold, and underlined sequences represent restriction sites, RBS, and translation start and stop codons, respectively.
We have therefore submitted the formulated hypothesis to experimental challenge. The results presented here unambiguously prove that when expressed in E. coli, the fOg44 lysin is synthesized as a precursor dependent on the GSP for translocation and is processed at the expected site by the LepB signal peptidase. Our results also imply that during fOg44 infection of O. oeni an analogous lysin maturation process must occur to produce the active enzyme.
MATERIALS AND METHODS
Bacterial strains, phages, plasmids and growth conditions.
E. coli strains (Table 1) were usually grown in Luria-Bertani medium (26) at the temperatures indicated below. M9 minimal medium (26) containing 0.4% glucose and 0.005% of each essential amino acid except glycine was used for culture growth prior to pulse-labeling (see below). When required, ampicillin (100 μg/ml) and/or kanamycin (40 μg/ml) was added to the culture medium for plasmid selection. O. oeni strain ML34-C10 (30) was used for phage fOg44 propagation. Preparation of fOg44 lysates was as previously described (29). Phage λCE6 (Sam7 cI857 int::T7 gene 1; Stratagene) was propagated in E. coli LE392 as described by the supplier. Plasmids pRSET-C (Invitrogen) and pBluescript II KS(+) (pKSII+) (Stratagene) were used as cloning vectors. pGP1-2 (35) was used as the delivery vehicle for T7 RNA polymerase by shifting cultures of the harboring strains (Table 1) from 26 to 28°C to 42°C. Plasmids of the pCSJ series encoding Lys44 or its derivatives were constructed in this work, as summarized in Table 2. The correctness of recombinant plasmids generated by PCR cloning was confirmed by DNA sequencing.
TABLE 1.
E. coli strains used in this work
| Strain | Relevant features | Use in this work | Reference |
|---|---|---|---|
| LE392 | e14−(McrA−) hsdR514 supE44 supF58 lacY1 | λCE6-propagating strain | Stratagene |
| XL1BlueMRF' | Δ(mcrA)183 Δ(mcrCB-hsdSMR-mrr)173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac[F′proAB lacIqΔM15 Tn10 (Tetr)] | Primary host (selection of plasmid constructs) | Stratagene |
| BL21 | E. coli B F−dcm ompT hsdS(rB− mB−) gal | Recipient for pGP1-2 | Stratagene |
| MC4100 | araD139 Δ(argF-lac) U169 rpsL150 relA1 flbB5301 deoCl ptsF25 rbsR | Recipient for pGP1-2 | 32 |
| KB1 | MC4100 secA51(Ts) | Recipient for pGP1-2 | 38 |
| ESS | Increased outer membrane permeability | Recipient for pGP1-2 | SmithKline Beecham |
| CG61 | BL21/pGP1-2 | Secondary host (allowing expression of T7 RNA polymerase) | This work |
| CG62 | MC4100/pGP1-2 | Secondary host (allowing expression of T7 RNA polymerase) | This work |
| CG63 | KB1/pGP1-2 | Secondary host (allowing expression of T7 RNA polymerase) | This work |
| CG64 | ESS/pGP1-2 | Secondary host (allowing expression of T7 RNA polymerase) | This work |
| CG601 | BL21/pCSJ1 | Expression of His-Lys44 | This work |
| CG612 | CG61/pCSJ2 | Expression of Lys44 | This work |
| CG622 | CG62/pCSJ2 | Expression of Lys44 | This work |
| CG632 | CG63/pCSJ2 | Expression of Lys44 | This work |
| CG642 | CG64/pCSJ2 | Expression of Lys44 | This work |
| CG613 | CG61/pCSJ3 | Expression of LysW27 | This work |
| CG614 | CG61/pCSJ4 | Expression of LysΔSP | This work |
| CG615 | CG61/pCSJ5 | Expression of LysΔC | This work |
| CG6100 | CG61/pKSII(+) | Control in expression studies | This work |
TABLE 2.
Plasmids expressing fOg44 lysin forms
| Plasmid | Construction procedurea | Product | Product description |
|---|---|---|---|
| pCSJ1 | pRSET-C cleaved with PstI and EcoRI ligated to similarly digested L1–L2 PCR productb | His-Lys44 | The 42-aa sequence MRGSH6GMASMTGGQQMGRDLYD4KDRWIRARDLQL replaces the N-terminal Met of the native fOg44 lysin |
| pCSJ2 | pKSII(+) cleaved with EcoRI and ClaI ligated to EcoRI/ClaI fOg44 DNA fragment | Lys44 | The fOg44 native lysin |
| pCSJ3 | pCSJ2 cleaved with AgeI and ClaI ligated to similarly digested L3–L5 PCR productc | LysW27 | A Trp residue replaces the original Ala27 in Lys44 |
| pCSJ4 | pKSII(+) cleaved with EcoRI and ClaI ligated to similarly digested L4–L5 PCR productc | LysΔSP | A single Met residue replaces the Lys44 SP |
| pCSJ5 | pCSJ2 cleaved with AgeI and ClaI ligated to similarly digested L6–L7 PCR productc | LysΔC | Truncated version of Lys44 (lacks the C-terminal 103 residues) |
Primer sequences (L1 to L7) are indicated in Fig. 1.
DNA template was fOg44 DNA.
DNA template was pCSJ2.
General recombinant-DNA techniques and sequence analysis.
fOg44 DNA extraction was performed as described in reference 29. Preparations of plasmid DNA, restriction endonuclease digestions, DNA ligations, and gel electrophoresis were performed by standard techniques (26). Plasmids were introduced into E. coli strains by electroporation using a Bio-Rad Gene Pulser II system and the conditions suggested by the supplier. Amplification of DNA by PCR was performed in a RoboCycler Gradient 96 thermocycler (Stratagene) using Pfu polymerase (Stratagene). PCR products were purified by agarose gel electrophoresis followed by DNA extraction using the QIAEX II kit (Qiagen). DNA sequencing reactions were performed by the chain termination method (27) using the Sequenase version 2 kit (United States Biochemicals) and appropriate primers. All oligonucleotides used in this work were obtained from GIBCO-BRL. DNA and protein sequences were analyzed with DNASIS-Mac v3.5 (Hitashi Software). Protein homology searches were carried out with PSI-BLAST (1). The public domain SignalP V2.0 (http://www.cbs.dtu.dk/services/SignalP) was used for predictions of SP structure and cleavage sites (20, 21).
Lysin expression in E. coli.
Unless stated otherwise, induction of lysin expression was carried out for 1 h in cells grown to an A600 of 0.5, either by infection with phage λCE6 (strain CG601) or by temperature upshift (pGP1-2-containing strains). Where indicated, induction of protein expression was carried out in the presence of 5 mM sodium azide (NaN3) or 0.1 mM Lep inhibitor (allyl (5S, 6S)-6-[(R)-acetoxyethyl]-penem-3-carboxilate; SmithKline Beecham) (4). Total-protein extracts were prepared by resuspending the pellet from 1-ml culture samples in 0.1 ml of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (19) followed by treatment at 100°C for 5 min. Proteins (5 μl per lane, unless otherwise indicated) were analyzed by electrophoresis on SDS–11% PAGE gels and visualized by Coomassie blue staining or blotted onto nitrocellulose membranes (Bio-Rad) as previously described (23). Lysin polypeptides were immunodetected using rabbit polyclonal antibodies raised against the 51-kDa His-Lys44 fusion protein (anti-Lys antibodies). Anti-Lys antibodies were prepared by Eurogentec (Belgium) according to their standard immunization protocol. The serum collected 10 days after the third immunization was used at a 1:10,000 dilution. Detection of protein–anti-Lys complexes was carried out with a chemiluminescence Western blotting kit (Roche Molecular Biochemicals) according to the manufacturer's instructions.
Lysin expression in O. oeni-infected cells.
To examine Lys44 production in O. oeni, an exponentially growing culture of strain ML34-C10 was infected with fOg44 at an approximate input multiplicity (IM) of 5. Two-milliliter samples were withdrawn at 10-min intervals and immediately frozen in liquid nitrogen. After thawing, cells were pelleted by centrifugation and concentrated 100-fold in Tris-EDTA buffer (26) supplemented with 20 mg of lysozyme per ml, 1 mM phenylmethylsulfonyl fluoride, and 100 μg of chloramphenicol per ml. After an incubation period at 37°C for 30 min, an equal volume of 2× SDS-PAGE sample buffer was added followed by incubation at 100°C for 5 min to complete cell lysis. O. oeni extracts were analyzed as described above for E. coli samples except that for immunodetection of Lys44, a serum collected 10 days after the fourth, rather than the third, immunization was used.
Pulse-chase analysis.
E. coli cells grown at 28°C in Luria-Bertani broth with appropriate antibiotics to an absorbance of 0.5 were washed, resuspended in supplemented M9 medium without glycine, and further incubated for 2 h at the same temperature. Following this period, cultures were shifted to the inducing temperature (42°C). Rifampin (200 μg/ml) was added 20 min later, and incubation proceeded at 42°C for another 10 min. Cultures were then transferred to 28°C and, if indicated, 0.1 mM Lep inhibitor was added. Incubation at 28°C was extended for 20 to 30 min before pulse-labeling with [U-14C]Gly (Amersham Pharmacia Biotech) for 1.5 min at 10 μCi/ml. Label incorporation was stopped by the addition of cold glycine to a final concentration of 0.5%, and samples (500 μl) were recovered in the same volume of 10% trichloroacetic acid at the indicated times after the chase. Cells were harvested by centrifugation, washed twice with acetone, and finally resuspended in 50 μl of SDS-PAGE sample buffer. Labeled polypeptides were detected by autoradiography following SDS-PAGE with 11% polyacrylamide gels.
Detection of cell wall hydrolase activity by in situ protein renaturation.
Detection of cell wall hydrolase activity in polyacrylamide gels was carried out essentially as described by Potvin et al. (25), with some modifications. Cells from stationary-phase cultures of O. oeni strain ML34-C10 were pelleted, washed with cold sterile water, frozen at −70°C for 30 min, dried at 60°C, resuspended in sterile water to a concentration of 3% (wt/vol), autoclaved, and finally stored at −20°C until use. SDS-PAGE was carried out in 11% polyacrylamide gels containing 0.4% autoclaved cell suspension. Due to the high sensitivity of the method, protein samples were usually applied in these gels at a 10-fold-lower concentration than usual (see above) to avoid false results arising from cross contamination between adjacent wells. After electrophoresis, gels were washed in water, incubated for 48 h in 50 mM phosphate buffer (pH 6.1) containing 1 mM CaCl2, 1 mM MgCl2, 0.5 M dithiothreitol, and 0.1% Triton X-100, washed again in water, and finally stained in a 0.1% methylene blue–0.01% KOH solution for 10 min at room temperature. The gels were then soaked in 0.5% SDS for 10 min followed by a final wash in water. Cell wall hydrolyzing activity appears as clear bands on a blue background.
N-terminal sequence analysis.
N-terminal sequence determination was performed by Edman degradation, after transfer of the proteins onto a polyvinylidene difluoride membrane (Bio-Rad), using an Applied Biosystems model 477A sequencer.
RESULTS
Precursor and mature forms of Lys44 In E. coli.
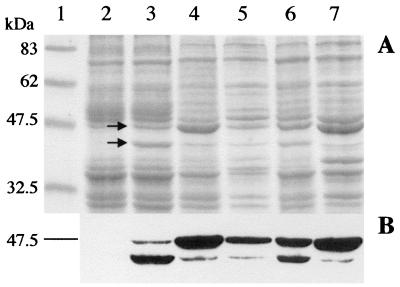
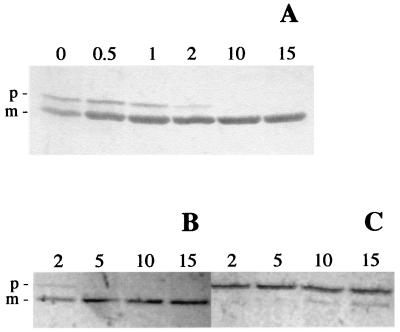
As reported above (see the introduction), the first experimental suggestion that Lys44 could be endowed with an SP came from the observation that induction of a recombinant His-tagged lysin in E. coli specifically produced two polypeptides. The occurrence of a cleavage event at the predicted location (Fig. 1) would be compatible with the difference in apparent molecular masses (51 and 43 kDa; Fig. 2) of the two proteins, considering that an additional 41-amino-acid-long peptide was N-terminally fused to Lys44 to produce His-Lys44 (Table 2). This was experimentally confirmed by sequencing the amino-terminal region of the 43-kDa protein. Indeed, the obtained sequence, AKGDQGVDLSHYQT, matched the deduced sequence of Lys44 from residues 28 to 41 (Fig. 1). These observations were then extended to the native lysin expressed from pCSJ2 (Table 2), which carries a 1.45-kb EcoRI/ClaI fragment from the phage DNA (Fig. 1), including the intact lysin gene and its own ribosomal binding site (RBS) under the control of the T7 φ10 promoter from pKSII+. As anticipated, induction of the native lysin also led to the production of two proteins (46 and 43 kDa) (Fig. 3, lane 3), both detected by immunoblotting with a polyclonal serum prepared against the 51-kDa recombinant protein (anti-Lys antibodies). Considering the lack of potential translational elements that could lead to direct synthesis of the smaller protein (Fig. 1), our observations clearly pointed to the production of a precursor and to an ensuing processing event. In agreement with this prediction, when induced cultures of strain CG612 (Table 1) were pulse-labeled with [14C]Gly and then chased with cold glycine, a time-dependent decrease of the 46-kDa form with a parallel increase of the labeled 43-kDa polypeptide was observed (Fig. 4A). This result strongly indicated that the larger and smaller proteins were not two separate translation products but rather a precursor and a processed form, respectively.
FIG. 2.
His-Lys44 expression in E. coli. Expression of the recombinant lysin in strain CG601 was induced by infection with λCE6 at an IM of ≈5. Samples were taken at 0 (lane 2) and 60 (lane 3) min after infection. NaN3 (5 mM) was then added to the culture and samples were withdrawn 30, 60, and 120 min afterwards (lanes 4, 5, and 6, respectively). (A) Total cell protein profiles after SDS-PAGE analysis (11% polyacrylamide gel) and Coomassie blue staining. The positions of induced polypeptides are indicated by arrows. Lane 1, prestained protein marker (New England Biolabs). (B) Western blot detection of lysin polypeptides with a polyclonal serum raised against the 51-kDa His-Lys44.
FIG. 3.
Lys44 and LysW27 expression in E. coli. Expression of the native fOg44 lysin (lanes 2 to 6) and its mutant derivative, LysW27 (lane 7) was induced by temperature upshift (see Materials and Methods). Samples were withdrawn immediately after the shift (lane 2) or after 60 min at 42°C (lanes 3 to 7). NaN3 (5 mM) or Lep inhibitor (0.1 mM) was added 5 or 30 min before induction (lanes 4 and 5, respectively). Lanes 2 to 4, CG622; lane 5, CG642; lane 6, CG632 (secA51[Ts]); lane 7, CG613. Panels A and B are as described in the legend for Fig. 2.
FIG. 4.
Pulse-chase analysis of Lys44 processing in E. coli strain CG612 (A) and strain CG642 pulse-labeled in the absence or presence of 0.1 mM Lep inhibitor (B and C, respectively). Radiolabeling with [14C]Gly for 1.5 min was followed by a chase with 0.5% cold glycine (see Materials and Methods). Time after the chase is indicated in minutes. Precursor (p) and mature (m) lysin forms are indicated.
SecA-dependent lysin maturation.
Signal sequence processing of preproteins occurs during or after their transit through the translocase of the general secretion system of the cell (11). One would therefore expect that inhibition of SecA function, one of the key elements in this pathway (11), would lead to precursor accumulation. We have tested this prediction, with both strain CG601 (expressing His-Lys44) and CG622 (expressing the native lysin). As shown in Fig. 2, infection of CG601 cultures with λCE6 in the presence of sodium azide (a widely used SecA inhibitor) (12) revealed the expected accumulation of the larger form of the recombinant lysin over the induction period. Similarly, when secretion was blocked by sodium azide or by incubation at a nonpermissive temperature in a secA51(Ts) strain (CG632), expression of Lys44 from pCSJ2 led to the expected increase in the precursor/mature lysin ratio (Fig. 3, lanes 4 and 6). Maturation of the fOg44 lysin in E. coli thus relies on the correct functioning of the GSP.
Processing of Lys44 is dependent on cleavage site structure and signal peptidase activity.
Preprotein processing by the LepB signal peptidase (7) is strongly affected by changes at particular sites in the cleavage region, namely the −1 and −3 positions, where small neutral amino acids are typically found (36). By PCR-based site-directed mutagenesis, the 27th codon of the lys44 reading frame (GCA) was changed to a TGG triplet, thus replacing an original Ala (−1 position) with a Trp residue (a bulky hydrophobic amino acid) in the protein product. Although a residual amount of processed enzyme was still produced upon induction of the mutant lysin (LysW27), most of the immunodetected protein exhibited the size of the precursor form (Fig. 3B, lane 7). A more direct demonstration of LepB involvement in Lys44 processing was achieved through the use of a specific inhibitory compound also used in recent studies to elucidate the membrane topology of the phage λ holin (14, 15). Pulse-chase experiments (as described above) were performed with inhibitor-treated and untreated cultures of strain CG642 (Table 1). As shown in Fig. 4B, whereas in untreated cells the preprotein was hardly detected 5 min after the addition of cold glycine, most of the label was still associated with the precursor after a 15-min chase in the presence of inhibitor, indicating a dramatic reduction in the cleavage rate. The effect of Lep inhibitor on lysin maturation was also observed by Western blotting (Fig. 3B, lane 5).
Growth and viability of E. coli strains expressing Lys44 or mutant derivatives.
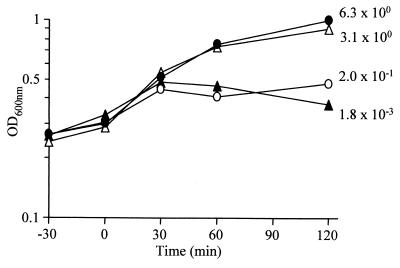
Initial attempts to introduce pCSJ1 (encoding His-Lys44) into E. coli BL21(DE3) (pLysS) resulted in very unstable clones even in the absence of IPTG (isopropyl-β-d-thiogalactopyranoside), an inducer of T7 RNA polymerase in that strain (34). This instability was in itself an indication of the lethal consequences of fOg44 lysin expression and therefore of its accessibility to the E. coli peptidoglycan. We have reasoned that replacement of the N-terminal 27 amino acids (aa) of Lys44 by a single methionine residue would convert the enzyme into a typical nonlethal endolysin when expressed alone (i.e., in the absence of a holin). Plasmid pCSJ4, encoding such an N-terminally modified lysin protein (LysΔSP) was therefore constructed (Table 2). A comparative examination of the growth curves and viability of CG6100 (control strain), CG612 (expressing Lys44), CG613 (expressing LysW27) and CG614 (expressing LysΔSP) was undertaken. Confirming our hypothesis, a similar increase in culture absorbances and viable counts was observed for strains CG6100 and CG614 within a period of 2 h after induction (Fig. 5). In contrast, after an identical induction period, a severe drop in viability was observed for the Lys44-producing strain (500- to 1,000-fold in three different experiments). Interestingly, although induction of LysW27 also led to a viability decrease in strain CG613, its survival rate was nevertheless 100-fold higher than that observed for strain CG612 (Fig. 5). Overall, these results indicate that lethality of lysin forms in E. coli is brought about by the presence of the SP and suggest a correlation between lysin activity and cleavage efficiency. Curiously, expression of the lethal enzymes did not result in a distinct lysis phenotype in E. coli: the absorbance of CG612- and CG613-induced cultures seemed to be halted rather than decreased over the induction period (Fig. 5).
FIG. 5.
Growth and viability of lysin-expressing strains. E. coli strains were grown at 28°C to mid-log phase and then shifted to 42°C (time, 0 min). Growth was monitored by absorbance measurements made at the indicated time points and by comparing the numbers of CFU at 0 and 120 min after induction. The ratio of CFU at 120 min to CFU at 0 min is given for each strain for this representative experiment. ●, CG6100 (control strain); ○, CG613 (expressing LysW27); ▴, CG612 (expressing Lys44); ▵, CG614 (expressing LysΔSP).
N-terminal processing of Lys44 is required for lytic activity in oenococcal cells.
Lytic activity in lysin-producing E. coli extracts was checked by in situ protein renaturation after SDS-PAGE, using gel-incorporated autoclaved oenococcal cells as the substrate. As shown in Fig. 6A, a lysis zone was only observed around the mature protein, even when most of the induced lysin present in the extract was in the precursor form (Fig. 6, lanes 2 and 3). This indicates that the Lys44 SP is a cis-inhibitory element which must be cleaved off for proper functioning of the enzyme. This inhibitory action of the SP could result from steric hindrance of the enzyme active site, presumed to be located in the N-terminal region of the mature lysin (13, 31) or, alternatively, to a negative effect of the SP on the lysin folding rate as previously documented for other preproteins (22). Activity bands corresponding to polypeptides with apparent molecular masses of 38 and 32.5 kDa were sometimes observed when more concentrated samples were applied in the gels. These have also been observed in overexposed Western blots (results not shown). The lower-molecular-mass species detected in activity gels migrates approximately at the same position as the mature form of a C-terminally truncated lysin derivative (LysΔC) which also retains activity (Fig. 6, lanes 8 and 9). LysΔC lacks the C-terminal 103 residues of Lys44 (Table 2), encompassing the pair of 48-aa repeats which were previously identified in its sequence (23).
FIG. 6.
Lytic activity of E. coli-produced lysin forms on oenococcal cells. (A) Lytic activity of lysin samples was assessed by in situ renaturation after SDS-PAGE using gel-incorporated autoclaved O. oeni cells as substrate. Lane 1, CG6100; lane 2, CG613; lanes 3 and 5, CG612 (with or without 5 mM NaN3); lane 4, prestained protein marker; lane 6, CG601; lane 7, CG614; lanes 8 and 9, CG615 (without or with 5 mM NaN3). One-tenth of the usual amount of protein samples (see Materials and Methods) was applied per lane. (B) A cell-free control gel was run in parallel with a 10-fold-higher amount of the same samples for immunoblotting detection of the lysin forms present. The positions of precursor (p) and mature (m) forms are indicated. p and m for Lys44 and LysW27 proteins, p′ and m′ for His-Lys44 proteins, and p" and m" for LysΔC proteins).
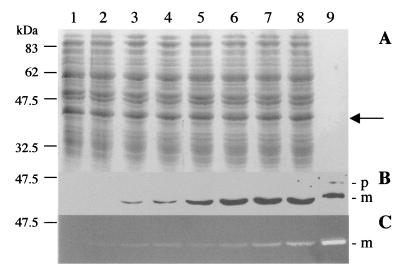
Lysin production in the course of O. oeni phage infection.
Infected O. oeni cells were examined for lysin production. Samples were collected immediately before and every 10 min following infection until near the end of the fOg44 latent period (150 min) (28). Protein extracts were prepared from such samples as described in Materials and Methods and checked for the presence of Lys44 by immunoblotting (Fig. 7B) and detection of lytic activity (Fig. 7C). Both methods revealed the presence of a single lysin band, first detected at 80 min postinfection, with a mobility indistinguishable from that exhibited by the mature form of the E. coli-expressed enzyme. Assuming that this lysin form results from a processing event analogous to that shown to occur in E. coli, our observations imply that in the natural fOg44-O. oeni system a mechanism for down-regulating lytic activity after SP cleavage must be operative to prevent lysis during the second half of the latent period (see Discussion).
FIG. 7.
Time course of lysin synthesis during fOg44 infection of O. oeni ML34-C10. Lysin production in O. oeni ML34-C10 was checked after infection with fOg44 at an IM of ≈5. Extracts were prepared from samples taken at 10-min intervals as described in Materials and Methods. Samples were processed for SDS-PAGE and Coomassie blue staining (A), Western blotting (B), and a lytic activity assay (C) as described in the legends for Fig. 2 and 6. Only the results for 0 (lane 1) and 80 to 140 (lanes 2 to 8) min postinfection are shown. An extract sample from induced, Lys44-expressing strain CG612 (1/20 of the standard amount) was run in parallel for comparison (lane 9). Anti-Lys antibodies used for Western blotting correspond to a 1:10,000 dilution of serum collected 10 days after the fourth rabbit immunization, whereas in previous figures an equivalent dilution of serum collected after the third immunization was used instead (see Materials and Methods). The arrow points to the expected position in the gel of the Lys44 mature protein. p, precursor protein; m, mature protein.
DISCUSSION
The evidence for an SP in Lys44.
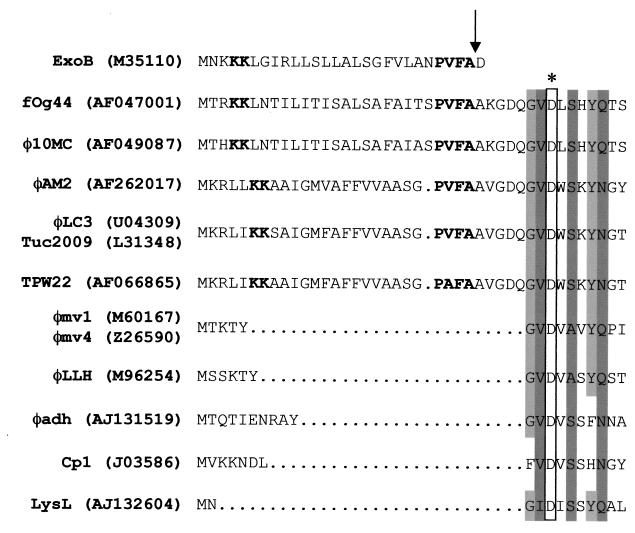
The term “endolysins” has been traditionally used to designate bacteriophage-encoded peptidoglycan hydrolases, owing to their cytoplasmic localization as long as membrane integrity is maintained (39, 40). However, the results presented in this work strongly suggest that the O. oeni bacteriophage fOg44 encodes a secretory lytic enzyme, or exolysin, which is structurally competent for export through the GSP. Primary structure analysis predicted that the first 27 residues of Lys44 should function as an SP in both gram-positive and gram-negative hosts. Database searches also revealed that several exported proteins contain putative or demonstrated SPs presenting variable degrees of similarity with the N-terminal 27-aa region of Lys44 (data not shown). From these, the best match was obtained with the SP of ExoB, an exported toxin produced by Streptococcus pyogenes (16) (Fig. 8). We have experimentally confirmed the SP prediction in E. coli, where expression of lys44 from its own cognate translational elements led to the synthesis of a 46-kDa preprotein which was then processed to a 43-kDa product. We have established that this cleavage event occurs at the predicted location and requires both the translocase-associated ATPase SecA and the signal peptidase LepB, two essential components of the GSP. Moreover, unlike most other phage lytic enzymes, which do not affect cell viability when overexpressed in E. coli, Lys44 proved to be lethal. This lethality could be directly ascribed to the presence of the SP, as its deletion converted the enzyme into a standard, nontoxic endolysin. The complete lack of information regarding protein secretion in O. oeni and the absence of genetic tools appropriate for homologous expression studies make it difficult to establish conclusively that lysin export through the GSP is also operative during normal phage development in its host. However, unlike the mature lysin, the precursor form of Lys44 does not exhibit lytic activity on oenococcal cells. In agreement with this necessity of SP cleavage for function, an active lysin form having the same mobility in SDS-PAGE as the E. coli-produced mature enzyme was observed in extracts from O. oeni-infected cells. Although the presence of a precursor was not evident in these experiments, this can be attributed to a high rate of lysin translocation and processing combined with the much lower level of enzyme production under native conditions compared to overexpression in E. coli.
FIG. 8.
Comparison of the N-terminal sequences of bacteriophage lysins (phage designation is given) with that of the SP of S. pyogenes exotoxin B (ExoB) and with a putative L. lactis autolysin (LysL). GenBank accession numbers are shown in parentheses. Two common tandem K residues, preceding the hydrophobic region in the ExoB sequence, and other putative SPs are shown in bold. An arrow indicates the peptidase cleavage site predicted using the public domain SignalP V2.0 web server. A conserved motif, P(V/A)FA, preceding the cleavage site is also represented in bold. The asterisk indicates an Asp residue presumed to be crucial for activity of muramidases (31). Sequence conservation around this residue among the indicated lytic enzymes is represented by light gray (conserved residues in at least half of the sequences) or dark gray (equivalent residues in all sequences) shading. For clarity, this conserved region is shown separated from the N-terminal residues by dotted lines, except in those cases where the existence of an SP is suggested.
Are there other phage lysins endowed with putative SPs?
The suggestion that bacteriophages may use a lysin export mechanism independent of holin-mediated membrane permeabilization, as indicated by our results, certainly deserves further investigation. However, for the reasons mentioned above, the fOg44-O. oeni system presents several shortcomings in this regard and alternative models should be sought. Using the N-terminal half of the Lys44 sequence to search for homologous proteins in databases, the sequences corresponding to several lytic enzymes have been retrieved. Two distinct patterns emerged, as depicted in Fig. 8. Apart from a short stretch of N-terminal amino acids, the protein sequences from one group could clearly be aligned with the first residues of the mature form of Lys44 (e.g., the φadh and the Cp1 phage lysins) (Fig. 8). Obviously, such lytic enzymes are synthesized without an SP. Five proteins, however, showed a notable similarity with the fOg44 lysin sequence even in the SP-corresponding region: the lysin of bacteriophage φ10MC (also infecting O. oeni) and the highly related lytic enzymes from the temperate L. lactis phages Tuc2009, φLC3, φAM2, and TPW22 (Fig. 8). We have recently tested the expression of a His-tagged version of the φAM2 lysin in E. coli, as reported here for His-Lys44, and obtained similar results with respect to the SecA-dependent production of two polypeptides and restricted activity to the shorter form (C. São-José, R. Parreira, G. Vieira, and M. A. Santos, Abstr. 100th Gen. Meet. Am. Soc. Microbiol., abstr. M-14, 2000). These preliminary findings suggest that the φAM2 lysin (and, by extension, the other related lysins) may be another example of a phage secretory lytic enzyme. It should be stressed that the previously observed phenotypes of E. coli strains bearing φLC3 lysis genes (see the introduction) are not incompatible with the presence of an SP in LysB, the φLC3 lysin. As described for LysB expression, we also failed to observe a clear lysis phenotype in E. coli upon Lys44 induction. Recent experiments in our group indicate that coexpression of the fOg44 lysin and holin genes does in fact result in a much more evident clearing of the culture than when either gene is expressed alone (São-José et al., Abstr. 100th Gen. Meet. Am. Soc. Microbiol.). Lysis phenotypes arising from heterologous gene expression should therefore be interpreted with caution. Presumably, the E. coli peptidoglycan is a poor substrate for these foreign lysins, their activity resulting in cells which are osmotically fragile (explaining the loss of colony-forming ability) but not structurally damaged enough to cause a significant reduction in the absorbance of the culture as a whole. On the other hand, whereas in the absence of peptidoglycan hydrolysis induction of a holin in E. coli is known to result in emptied but otherwise intact “ghost” cells (39), membrane disruption and extrusion of cellular contents may contribute effectively to envelope fragmentation in a situation where the murein sacculus has been previously weakened by limited degradation. There is, however, another possible interpretation for our results and those of Birkeland (3). In fact, the permeabilizing action of the phage EJ-1 holin on the membrane was previously suggested to activate the pneumococcal LytA amidase (10), claimed to be targeted to the periplasm in an inactive state, when expressed in E. coli (9). Although the putative mechanism by which LytA could be translocated across the membrane remains unknown, the concept of a holin as a triggering factor for lytic activity would certainly fit our data (also see below). According to this view, association with the energized membrane following export would negatively affect Lys44 (or LysB) activity. In any case, since in contrast to O. oeni, L. lactis is amenable to genetic manipulation, and since vectors appropriate for inducible-expression studies in this species have been described in recent years (8), the way seems open to address these questions in a natural phage-host context.
Significance of secretory lysins.
The active, presumably exported form of Lys44 was first detected in O. oeni-infected cells at 80 min postinfection, a time matching the appearance of a 1.8-kb transcript specifically hybridizing with probes internal to the fOg44 lysis genes (23). Considering that under the conditions used here, the fOg44 latent period extends for about 150 min (28), it would appear that the lysin is targeted to the cell wall compartment as it is being synthesized, rather than at the end of phage maturation, as observed in λ and related systems. An evolutionary advantage for the synthesis of secretory lysins may be rationalized in terms of the known differences between cell wall structure in gram-negative and gram-positive hosts. Since the latter have a much thicker peptidoglycan, composed of several layers, it seems reasonable to assume that a much more extensive lytic activity is required to promote lysis of gram-positive cells than is required to promote lysis of gram-negative cells. It has been recently argued (37, 41) that evolutionary pressure should favor an optimum balance between the duration of a lytic cycle (which, if delayed, would compromise the opportunity to infect new hosts) and effective progeny yield (which would be too low if lysis was premature). In keeping with this view, building up an increasing amount of lytic enzymes at their site of action as phage assembly progresses could be a sensible strategy for phages infecting hosts with thick murein walls in order to guarantee quick cell lysis once an adequate number of progeny virions is reached intracellularly. On the other hand, some extracytoplasmic regulatory mechanism must be operative to ensure that premature lysis does not take place. In fact, a similar problem is encountered during normal vegetative growth of gram-positive bacteria, which target to their cell walls a number of potentially lethal enzymes (autolysins) required for processes such as cell wall turnover and cell separation (for a recent review, see reference 33). We therefore suggest that an exported bacteriophage lysin may find its activity modulated in the cell wall environment by the same mechanism(s) the cell uses to keep appropriate levels of endogenous autolytic activity. Although the nature of these regulatory mechanisms remains largely unknown, several factors which induce autolysis in Bacillus subtilis have been identified and some information is available concerning the specific enzymes involved in each case. Whereas the LytC amidase and a putative endopeptidase (LytE) appear to mediate sodium azide-induced autolysis, inactivation of another B. subtilis major autolysin, the glucosaminidase LytD, does not affect this autolytic response. However, LytD plays a part, together with LytC, in autolysis induced by antibiotics, suggesting that a complex regulatory network is involved in autolysin regulation in this species (5, 33). Of particular relevance to the present discussion is the observation that energy poisons (such as sodium azide) or other conditions which destroy the proton motive force across the membrane lead to rapid autolysis in B. subtilis (5, 17). This implies that holin-mediated disruption of the membrane potential should contribute in itself to cell lysis, by recruiting host autolysin(s) to the task. It seems significant, in this regard, that the membrane-targeted XhlA and XhlB proteins, which presumably function as a holin complex during phage PBSX development in B. subtilis, have been shown to actually induce cell burst, even in the absence of the PBSX XlyA amidase (18). Although, as suggested by the authors of reference 18, a second PBSX-encoded lysin (XlyB) could be responsible for lysis in the absence of XlyA, the involvement of host autolysins (such as LytC) was not investigated and should be considered an additional possibility. We may thus envisage a general lysis strategy for many phages infecting gram-positive hosts which relies on the combined action of holin-released phage endolysins and holin-activated endogenous lytic enzymes already positioned in the cell wall. A crucial requirement for the success of such a strategy would be the presence in host cell walls of autolysins sensitive to the energized state of the membrane. This may not be the case for many strains, particularly those of simpler gram-positive bacteria encoding a limited number of autolytic enzymes. It is appropriate to note in this context that we failed to observe lysis bands in gel renaturation assays with samples from cultures of the fOg44 host strain, ML34-C10 (Fig. 7, lane 1). Also, whereas B. subtilis may encode many different and partially redundant autolysins (5), a survey of the completely sequenced genome of the L. lactis strain IL1403 revealed only two putative autolysin genes (6). We therefore speculate that the energetically costly process of phage lysin secretion through the GSP could be relevant to cell wall degradation of gram-positive strains with limited autolytic capacity. The presence of a holin gene in all phages predicted to encode secretory lysins (2, 3, 13, 23, 24; our unpublished results) would then be consistent with its role as a timing device for releasing the previously exported lysins from a putative inhibitory mechanism dependent on membrane energization. Experiments in our group are currently in progress to test some of the obvious predictions emerging from this conjectural model.
ACKNOWLEDGMENTS
We thank M. Regalla of the Protein Sequencing Laboratory of the Instituto de Tecnologia Química e Biológica for N-terminal sequence analysis and T. Silhavy and S. Tabor for the gift of plasmids and strains. Lep inhibitor and E. coli strain ESS were generous gifts from SmithKline Beecham (SB) Pharmaceuticals. We are also grateful to M.-C. Chopin, P. Tavares, and A. O. Henriques for helpful discussions, K. O'Dwyer (SB) for advice on the use of Lep inhibitor, and T. Silhavy for critically reading a first draft of the manuscript. We appreciate the useful suggestions of anonymous reviewers and, most particularly, the enthusiastic encouragement of Ry Young.
The financial support from the Fundação para a Ciência e Tecnologia through grants BIO/C/2041/95 to M.A.S. and BD/13390/97 to C.S.-J. is acknowledged.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arendt E K, Daly C, Fitzgerald G F, van de Guchte M. Molecular characterization of lactococcal bacteriophage Tuc2009 and identification and analysis of genes encoding lysin, a putative holin, and two structural proteins. Appl Environ Microbiol. 1994;60:1875–1883. doi: 10.1128/aem.60.6.1875-1883.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birkeland N-K. Cloning, molecular characterization, and expression of the genes encoding the lytic functions of the lactococcal bacteriophage φLC3: a dual lysis system of modular design. Can J Microbiol. 1994;40:658–665. doi: 10.1139/m94-104. [DOI] [PubMed] [Google Scholar]
- 4.Black M T, Bruton G. Inhibitors of bacterial signal peptidases. Curr Pharm Des. 1998;4:133–154. [PubMed] [Google Scholar]
- 5.Blackman S A, Smith T J, Foster S J. The role of autolysins during vegetative growth of Bacillus subtilis 168. Microbiology. 1998;144:73–82. doi: 10.1099/00221287-144-1-73. [DOI] [PubMed] [Google Scholar]
- 6.Bolotin A, Mauger S, Malarme K, Ehrlich S D, Sorokin A. Low-redundancy sequencing of the entire Lactococcus lactis IL1403 genome. Antonie Leeuwenhoek. 1999;76:27–76. [PubMed] [Google Scholar]
- 7.Dalbey R E. Leader peptidase. Mol Microbiol. 1991;5:2855–2860. doi: 10.1111/j.1365-2958.1991.tb01844.x. [DOI] [PubMed] [Google Scholar]
- 8.de Vos W M. Gene expression systems for lactic acid bacteria. Curr Opin Microbiol. 1999;2:289–295. doi: 10.1016/S1369-5274(99)80050-2. [DOI] [PubMed] [Google Scholar]
- 9.Díaz E, García E, Ascaso C, Méndez E, López R, García J L. Subcellular localization of the major pneumococcal autolysin: a peculiar mechanism of secretion in Escherichia coli. J Biol Chem. 1989;264:1238–1244. [PubMed] [Google Scholar]
- 10.Díaz E, Munthali M, Lunsdorf H, Holtje J V, Timmis K N. The two-step lysis system of pneumococcal bacteriophage EJ-l is functional in gram-negative bacteria: triggering of the major pneumococcal autolysin in Escherichia coli. Mol Microbiol. 1996;19:667–681. doi: 10.1046/j.1365-2958.1996.399929.x. [DOI] [PubMed] [Google Scholar]
- 11.Fekkes P, Driessen A J M. Protein targeting to the bacterial cytoplasmic membrane. Microbiol Mol Biol Rev. 1999;63:161–173. doi: 10.1128/mmbr.63.1.161-173.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fortin Y, Phoenix P, Drapeau G R. Mutations conferring resistance to azide in Escherichia coli occur primarily in the secA gene. J Bacteriol. 1990;172:6607–6610. doi: 10.1128/jb.172.11.6607-6610.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gindreau E, Lonvaud-Funel A. Molecular analysis of the region encoding the lytic system from Oenococcus oeni temperate bacteriophage φ10MC. FEMS Microbiol Lett. 1999;171:231–238. doi: 10.1111/j.1574-6968.1999.tb13437.x. [DOI] [PubMed] [Google Scholar]
- 14.Graschopf A, Bläsi U. Molecular function of the dual start motif in the lambda S holin. Mol Microbiol. 1999;33:569–582. doi: 10.1046/j.1365-2958.1999.01501.x. [DOI] [PubMed] [Google Scholar]
- 15.Grundling A, Bläsi U, Young R. Biochemical and genetic evidence for three transmembrane domains in the class I holin, lambda S. J Biol Chem. 2000;275:769–776. doi: 10.1074/jbc.275.2.769. [DOI] [PubMed] [Google Scholar]
- 16.Hauser A R, Schlievert P M. Nucleotide sequence of the streptococcal pyrogenic exotoxin type B gene and relationship between the toxin and the streptococcal proteinase precursor. J Bacteriol. 1990;172:4536–4542. doi: 10.1128/jb.172.8.4536-4542.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jolliffe L K, Doyle R J, Streips U N. The energised membrane and cellular autolysis in Bacillus subtilis. Cell. 1981;25:753–763. doi: 10.1016/0092-8674(81)90183-5. [DOI] [PubMed] [Google Scholar]
- 18.Krogh S, Jørgensen S T, Devine K M. Lysis genes of the Bacillus subtilis defective prophage PBSX. J Bacteriol. 1998;180:2110–2117. doi: 10.1128/jb.180.8.2110-2117.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen H, Krogh A. Prediction of signal peptides and signal anchors by a hidden Markov model. In: Glasgow J, et al., editors. Proceedings of the Sixth International Conference on Intelligent Systems for Molecular Biology (ISMB 6). Menlo Park, Calif: AAAI Press; 1998. pp. 122–130. [PubMed] [Google Scholar]
- 22.Park S, Liu G, Topping T B, Cover W H, Randall L L. Modulation of folding pathways of exported proteins by the leader sequence. Science. 1988;239:1033–1035. doi: 10.1126/science.3278378. [DOI] [PubMed] [Google Scholar]
- 23.Parreira R, São-José C, Isidro A, Domingues S, Vieira G, Santos M A. Gene organization in a central DNA fragment of Oenococcus oeni bacteriophage fOg44 encoding lytic, integrative and non-essential functions. Gene. 1999;226:83–93. doi: 10.1016/s0378-1119(98)00554-x. [DOI] [PubMed] [Google Scholar]
- 24.Petersen A, Josephsen J, Johnsen M G. TPW22, a lactococcal temperate phage with a site-specific integrase closely related to Streptococcus thermophilus phage integrases. J Bacteriol. 1999;181:7034–7042. doi: 10.1128/jb.181.22.7034-7042.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potvin C, Leclerc D, Tremblay G, Asselin A, Bellemare G. Cloning, sequencing and expression of a Bacillus bacteriolytic enzyme in Escherichia coli. Mol Gen Genet. 1988;214:241–248. doi: 10.1007/BF00337717. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor. N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 27.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. . Nucleotide sequence of bacteriophage λ DNA. J. Mol. Biol. 162:729–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santos R. Ph.D. thesis. Lisbon, Portugal: University of Lisbon; 1995. [Google Scholar]
- 29.Santos R, São-José C, Vieira G, Paveia H, Santos M A. Genome diversity in temperate bacteriophages of Oenococcus oeni. Arch Virol. 1998;143:523–536. doi: 10.1007/s007050050308. [DOI] [PubMed] [Google Scholar]
- 30.Santos R, Vieira G, Santos M A, Paveia H. Characterization of temperate bacteriophages of Leuconostoc oenus and evidence for two prophage attachment sites in the genome of starter strain PSU-1. J Appl Bacteriol. 1996;81:383–392. [Google Scholar]
- 31.Sanz J M, Garcia P, Garcia J L. Role of Asp-9 and Glu-36 in the active site of the pneumococcal Cpl1 lysozyme: an evolutionary perspective of lysozyme mechanism. Biochemistry. 1992;31:8495–8499. doi: 10.1021/bi00151a016. [DOI] [PubMed] [Google Scholar]
- 32.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions, p. xi–xii. Cold Spring Harbor. N.Y: Cold Spring Harbor Laboratory; 1984. [Google Scholar]
- 33.Smith T J, Blackman S A, Foster S J. Autolysins of Bacillus subtilis: multiple enzymes with multiple functions. Microbiology. 2000;146:249–262. doi: 10.1099/00221287-146-2-249. [DOI] [PubMed] [Google Scholar]
- 34.Studier F W. Use of bacteriophage T7 lysozyme to improve an inducible T7 expression system. J Mol Biol. 1991;219:37–44. doi: 10.1016/0022-2836(91)90855-z. [DOI] [PubMed] [Google Scholar]
- 35.Tabor S, Richardson C. A bacteriophage T7 RNA polymerase promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci USA. 1985;82:1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985;184:99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]
- 37.Wang I-N, Dykhuizen D E, Slobodkin L B. The evolution of phage lysis timing. Evol Ecol. 1996;10:545–558. [Google Scholar]
- 38.Wolfe P B, Rice M, Wickner W. Effects of two sec genes on protein assembly into the plasma membrane of Escherichia coli. J Biol Chem. 1985;260:1836–1841. [PubMed] [Google Scholar]
- 39.Young R. Bacteriophage lysis: mechanisms and regulation. Microbiol Rev. 1992;56:430–481. doi: 10.1128/mr.56.3.430-481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young R, Bläsi U. Holins: form and function in bacteriophage lysis. FEMS Microbiol Rev. 1995;17:191–205. doi: 10.1111/j.1574-6976.1995.tb00202.x. [DOI] [PubMed] [Google Scholar]
- 41.Young R, Wang I-N, Roof W D. Phages will out: strategies of host cell lysis. Trends Microbiol. 2000;8:120–128. doi: 10.1016/s0966-842x(00)01705-4. [DOI] [PubMed] [Google Scholar]