Abstract
Introduction
KRAS mutations (KRASmut), PIK3CAmut, BRAFmut, and mismatch repair deficiency (dMMR) have been associated with the Warburg-effect. We previously observed differential associations between energy balance-related factors (BMI, clothing-size, physical activity) and colorectal cancer (CRC) subtypes based on the Warburg-effect. We now investigated whether associations between energy balance-related factors and risk of CRC differ between subgroups based on mutation and MMR status.
Methods
Information on molecular features was available for 2349 incident CRC cases within the Netherlands Cohort Study (NLCS), with complete covariate data available for 1934 cases and 3911 subcohort members. Multivariable-adjusted Cox-regression was used to estimate associations of energy balance-related factors with risk of CRC based on individual molecular features (KRASmut; PIK3CAmut; BRAFmut; dMMR) and combinations thereof (all-wild-type + MMR-proficient (pMMR); any-mutation/dMMR).
Results
In men, BMI and clothing-size were positively associated with risk of colon, but not rectal cancer, regardless of molecular features subgroups; the strongest associations were observed for PIK3CAmut colon cancer. In women, however, BMI and clothing-size were only associated with risk of KRASmut colon cancer (p-heterogeneityKRASmut versus all-wild-type+pMMR = 0.008). Inverse associations of non-occupational physical activity with risk of colon cancer were strongest for any-mutation/dMMR tumors in men and women, and specifically for PIK3CAmut tumors in women. Occupational physical activity was inversely associated with both combination subgroups of colon cancer in men.
Conclusion
In men, associations did not vary according to molecular features. In women, a role of KRAS mutations in the etiological pathway between adiposity and colon cancer is suggested, and of PIK3CA mutations between physical activity and colon cancer.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-022-04019-9.
Keywords: Prospective cohort study, Energy balance, Colorectal cancer, Mutations, Mismatch repair/microsatellite instability, Etiological heterogeneity
Introduction
Colorectal cancer (CRC) risk was shown to be affected by energy balance-related factors (Moghaddam et al. 2007; Robsahm et al. 2013; Wolin et al. 2009; Samad et al. 2005). Adiposity measures, such as body mass index (BMI) and waist circumference, have been associated with an increased risk of CRC (Moghaddam et al. 2007; Robsahm et al. 2013), whereas physical activity has been associated with a decreased risk of CRC (Robsahm et al. 2013; Wolin et al. 2009; Samad et al. 2005). One of the proposed mechanisms underlying these associations is activation of the so-called Warburg-effect through upregulated PI3K/Akt-signaling (Huang and Chen 2009; Levine and Puzio-Kuter 2010; Feron 2009; Schwartz et al. 2017; Hanahan and Weinberg 2011). We have previously observed differential associations between energy balance-related factors (i.e. BMI; clothing-size, as a proxy for waist circumference; physical activity) and CRC subtypes expressing different levels of proteins involved in the Warburg-effect (Jenniskens et al. 2021a).
The Warburg-effect is a metabolic phenotype first discovered in the 1920s by Otto Warburg and colleagues (Warburg 1925). This phenotype is characterized by increased aerobic glycolysis (Levine and Puzio-Kuter 2010; Feron 2009) and is considered an important step in carcinogenesis (Schwartz et al. 2017; Hanahan and Weinberg 2011). Mutations in well-known oncogenes KRAS, PIK3CA, and BRAF have been reported to drive metabolic reprogramming towards the Warburg-effect (Levine and Puzio-Kuter 2010; Kimmelman 2015; Hutton et al. 2016; Jiang et al. 2018). Furthermore, we have previously shown in CRC that DNA mismatch repair deficiency (dMMR), a surrogate for microsatellite instability (MSI), was associated with the Warburg-effect (Offermans et al. 2021).
MSI and KRAS, PIK3CA, and BRAF mutations (KRASmut, PIK3CAmut, BRAFmut, respectively) are common molecular features in CRC (Li et al. 2020; Haluska et al. 2007; Boland and Goel 2010). Associations between energy balance-related factors (i.e. BMI, waist circumference, physical activity) and risk of CRC in relation to KRASmut, BRAFmut, and MSI/MMR status have been reported previously (Carr et al. 2018, 2020; Myte et al. 2019; Brändstedt et al. 2013, 2014; Slattery et al. 2000, 2001, 2007; Hughes et al. 2012; Campbell et al. 2010; Hoffmeister et al. 2013; Hanyuda et al. 2016). However, results thus far are inconsistent. To the best of our knowledge, there are no studies that have investigated associations between energy balance-related factors and risk of CRC in relation to PIK3CAmut status.
The aim of the current study was to investigate the associations of BMI, lower body clothing-size (as a proxy for waist circumference), and physical activity with risk of CRC subgroups based on KRASmut, PIK3CAmut, BRAFmut, and MMR status. First, we compared CRC subgroups based on a combination of these molecular features: I) all-wild-type + pMMR — cases wild-type for all genes (KRAS, PIK3CA, and BRAF) and MMR-proficient (pMMR); II) any-mutation/dMMR — cases with a mutation in any of the genes (KRAS, PIK3CA, and/or BRAF) and/or dMMR. Second, we investigated subgroups of these molecular features individually: KRASmut, PIK3CAmut, BRAFmut, and dMMR. The all-wild-type+pMMR subgroup served as the reference group for all other subgroups.
We hypothesized that associations between energy balance-related factors and risk of CRC differ between subgroups based on KRASmut, PIK3CAmut, BRAFmut, and MMR status, which could indicate involvement of the Warburg-effect in etiological associations. We reasoned that associations with subgroups of individual molecular features (KRASmut, PIK3CAmut, BRAFmut, or dMMR) and/or with the any-mutation/dMMR subgroup, but not the all-wild-type + pMMR subgroup, give an indication of involvement of the Warburg-effect in the etiological pathway between the exposure of interest and CRC.
Methods
Design and study population
Data from the Netherlands Cohort Study (NLCS), a large prospective cohort study, was used. At baseline (1986), 120,852 subjects aged 55–69 years completed a mailed, self-administered questionnaire on cancer risk factors (Brandt et al. 1990a). By completing and returning the questionnaire, participants agreed to participate in the study. The NLCS was approved by institutional review boards from Maastricht University and the Netherlands Organization for Applied Scientific Research. Ethical approval was obtained from the Medical Ethical Committee of Maastricht University Medical Center + . For data processing and analysis, a case-cohort approach was used (Prentice 1986). A subcohort (n = 5000) was randomly sampled from the total cohort immediately after baseline, and accumulated person-years were estimated from this subcohort. Vital status information of subcohort members was obtained biennially by active follow-up and by linkage with municipal population registries. Incident cancer cases from the total cohort were detected through annual record linkage with the Netherlands Cancer Registry and PALGA, the nationwide Dutch Pathology Registry (Brandt et al. 1990b), covering 20.3 years of follow-up (September 17, 1986 until January 1, 2007). Completeness of cancer follow-up by the Netherlands Cancer Registry and PALGA was estimated to be over 96% (Goldbohm et al. 1994). After excluding cases and subcohort members who reported a history of cancer (except skin cancer) at baseline, a total of 4,597 incident CRC cases and 4,774 subcohort members were available (Fig. 1). As described previously (Jenniskens et al. 2021a), formalin-fixed paraffin-embedded (FFPE) tissue blocks from primary tumor and matched normal colon tissue from 3,872 CRC cases were requested from participating laboratories as part of the Rainbow-TMA project during 2012–2017. Tissue blocks from 3,021 CRC cases were successfully collected from 43 pathology laboratories throughout the Netherlands (78% retrieval rate) (Fig. 1).
Fig. 1.
Flow diagram of the number of CRC cases and subcohort members; NLCS, 1986–2006. CRC colorectal cancer; NA not applicable; PALGA Dutch Pathology Registry; FFPE formalin-fixed paraffin-embedded; TMA tissue microarray; QC quality control; H&E Hematoxylin & Eosin; pan-CK pan-cytokeratin; MMR mismatch repair
Mismatch repair status
From the FFPE blocks, 78 tissue microarrays (TMAs) were constructed sampling three 0.6 mm tumor cores from 2,694 CRC cases (Fig. 1). Information on TMA construction has been published previously (Jenniskens et al. 2021a). Five μm thick sections were cut from all TMA blocks, stained with Hematoxylin & Eosin (H&E) according to a standard protocol, and subjected to immunohistochemistry (IHC) using an automated immunostainer (DAKO Autostainer Link 48, Glostrup, Denmark). MMR status, a surrogate for the presence or absence of MSI, was assessed using IHC staining of MLH1 and MSH2 as described previously (Offermans et al. 2021). All TMA sections were scanned using an Aperio scanner (Leica Microsystems, Milton Keynes, UK) at 40 × magnification at the University of Leeds (UK) Scanning Facility or at the Department of Pathology, Aachen University Hospital (Germany).
H&E-stained TMA sections combined with pan-cytokeratin stained sections (if necessary) were reviewed to confirm presence of adenocarcinoma for each core. Requiring at least one core per case with adenocarcinoma, 2497 cases passed quality control (Fig. 1). IHC scoring of MLH1 and MSH2 was performed according to the protocol published by Richman et al. (2016) by an experienced histopathologist (HG) as well as by three trained (Jenniskens et al. 2021b) non-pathologists (G.E. Fazzi: histology technician; K. Offermans: PhD student; J.C.A. Jenniskens: PhD student). Tumors with complete loss of either MLH1 or MSH2 expression were classified as MMR-deficient (dMMR), and those expressing both MLH1 and MSH2 were classified as MMR-proficient (pMMR). MMR status information was available for 2,455 CRC cases (Fig. 1).
DNA isolation and mutation detection
For DNA extraction, two 20 µm thick sections were cut from FFPE blocks containing primary tumor. Sections were deparaffinized manually using the Buffer ATL (Cat. No. 939011, Qiagen, Hilden, Germany), Proteinase K (Cat. No. 19131, Qiagen), and the Deparaffinization Solution (Cat. No. 19093, Qiagen), using an adapted version of the manufacturer’s protocol (Supplementary Methods). The QIAsymphony® DSP DNA Mini Kit (Cat. No. 937236, Qiagen) and the QIAsymphony® (Qiagen) instrument were used for DNA isolation following the manufacturer’s protocol (Tissue_HC_200 protocol). The Quantus™ Fluorometer (Promega, Madison, WI, USA) with a QuantiFluor® dsDNA system (Promega) was used to determine the double-stranded DNA concentrations. Mutations in tumor DNA were analyzed at Institut für Immunologie und Genetik (Kaiserslautern, Germany) with the ColoCarta panel (Agena Bioscience, Hamburg), which screens for 32 mutations in 6 genes (BRAF, HRAS, KRAS, MET, NRAS, PIK3CA; see Supplementary Table S1 for specific mutations) using Matrix Assisted Laser Desorption Ionization-Time of Flight (MALDI-TOF) mass spectrometry. To ensure valid mutation information, the following cut-offs were used: Z-score ≥ 4.00; spectrum quality ≥ 0.750; typer peak probability ≥ 0.850; primer extension rate cut-off ≥ 0.200. Detection of mutations at a frequency of ≥ 7.5% for any of the alleles was considered evidence of a mutation in the corresponding gene. A failed reaction at a single nucleotide position resulted in missing data for the corresponding gene status only if the reactions at all other positions were wild-type.
No mutations were observed in HRAS, and NRAS mutations were found in a total of 86 cases. NRAS mutations were not included in the current analyses as after stratification on sex and tumor location, subgroups would have less than 50 cases (range 10–42 cases). This would have led to empty cells or cells with less than five cases for models based on categories of exposures. Complete information on KRAS, PIK3CA, and BRAF mutation status as well as MMR status was available for 2,349 CRC cases (Fig. 1). Supplementary Table S2 shows baseline characteristics of CRC cases by availability of mutation and MMR status.
Subgroups of molecular features
The following subgroups were used for statistical analyses: (I) all-wild-type + pMMR — cases wild-type for all genes (KRAS, PIK3CA, and BRAF) and pMMR; (II) any-mutation/dMMR — cases with a mutation in any of the genes (KRAS, PIK3CA, and BRAF) and/or dMMR; (III) KRASmut — cases with a (non-exclusive) KRAS mutation; (IV) BRAFmut; (V) PIK3CAmut; and (VI) dMMR. Note: subgroups of individual mutation and MMR status might overlap since multiple mutations and/or dMMR can occur within the same tumor.
Energy balance-related factors
Baseline questionnaires provided information on anthropometry, physical activity, diet, and other risk factors (Brandt et al. 1990a). BMI at baseline (kg/m2) was calculated using baseline weight (kg) divided by height squared (m2). Lower body clothing-size (trouser/skirt) was used as a proxy for waist circumference (Hughes et al. 2009). Non-occupational physical activity included leisure activities like walking, cycling, or doing sports, as described in more detail previously (Simons et al. 2013). Occupational energy expenditure and sitting time were estimated for the longest held job, which was self-reported at baseline. Jobs were classified as low, moderate, or high activity, as described previously (Simons et al. 2013). Energy expenditure was classified as < 8, 8–12, and > 12 kJ/minute, and sitting time as sitting for > 6, 2–6, and < 2 working hours/day. Data on occupational physical activity were only available for the subcohort and for cases until 17.3 years of follow-up, since funding for later data-entry and classification of occupations was unavailable. Furthermore, we did not analyze occupational physical activity measures in women because many did not have paid jobs (Simons et al. 2013).
Statistical analyses
After exclusion of participants with incomplete or inconsistent data on exposure variables or confounders, 3911 subcohort members and 1934 CRC cases were available for analyses (Fig. 1). Descriptive statistics and frequency distributions were calculated for subgroups based on molecular features and cohort characteristics. Differences of molecular features between men and women and between colon and rectum were evaluated using Chi-square. Associations between energy balance-related factors and CRC subgroups based on molecular features were investigated stratified on sex and tumor location. Cox proportional hazard models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations between CRC and BMI (according to sex-specific quartiles, and per 5 kg/m2 increase), clothing-size (according to sex-specific quartiles, and per 2 sizes increase), non-occupational physical activity (in categories of < 30, 30–60, 60–90, > 90 min per day, and per 30 min/day increase), and, for men, occupational physical activity (energy expenditure in categories of < 8, 8–12, > 12 kJ/minute; sitting time in categories of > 6, 2–6, and < 2 working hours/day). Standard errors of the HRs were estimated using the Huber-White sandwich estimator to account for additional variance introduced by sampling from the cohort (Lin and Wei 1989). The proportional hazard assumption was tested using the scaled Schoenfeld residuals (Schoenfeld 1982) and by introducing time-covariate interactions into the models.
All multivariable models were adjusted for age, family history of CRC (yes/no), alcohol intake (0; 0.1–4; 5–14; > 15 g/day), energy intake at baseline (kcal/day), red meat consumption (g/day), and processed meat consumption (g/day), as used previously (Jenniskens et al. 2021a). In addition, BMI and clothing-size models were adjusted for non-occupational physical activity (minutes/day), and BMI models for height (cm). All physical activity models were adjusted for BMI. Moreover, an additional analysis was conducted with mutual adjustment for clothing-size and BMI, where clothing-size adjusted for BMI represents a proxy for abdominal fatness, and BMI adjusted for clothing-size a proxy for subcutaneous fatness (Hughes et al. 2009; Janssen et al. 2002). Sensitivity analyses were performed excluding the first two years of follow-up.
Heterogeneity in associations between energy balance-related factors and CRC subgroups based on molecular features was evaluated using an adapted version of the competing risks procedure in Stata developed specifically for the case-cohort design (Vogel et al. 2008). The original procedure assumes independence of both estimated HRs, which underestimates the standard error and thus overestimates the p-values for their difference. Therefore, the p-values and associated CIs were estimated based on a bootstrapping method developed specifically for the case-cohort design (Wacholder et al. 1989). Each bootstrap analysis was based on 1000 replications. The all-wild-type + pMMR subgroup was the reference group for heterogeneity tests of all subgroups. Since our analyses were hypothesis-driven and exposures reflect different aspects of energy balance, we did not correct for multiple testing. All analyses were conducted in Stata Statistical Software: Release 15 (StataCorp., 2017, College Station, TX).
Results
Frequencies of molecular features
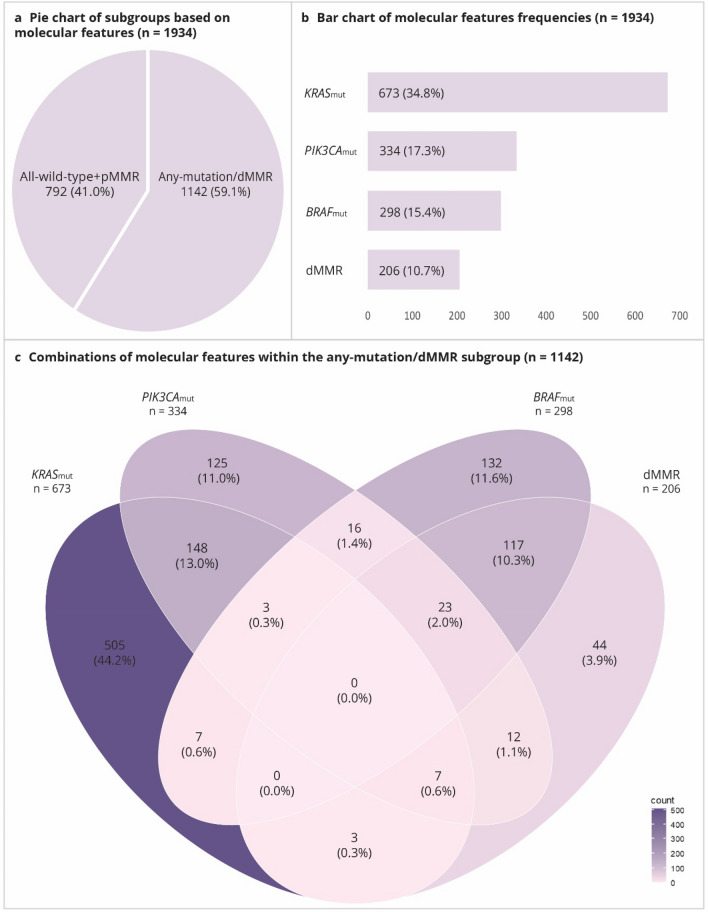
In total, 1142 (59.1%) tumors had a mutation in at least one of the genes (KRAS, PIK3CA, or BRAF) and/or were classified as dMMR (Table 1, Fig. 2a). The overall frequency of mutations and/or presence of dMMR was higher in women compared to men (66.4% vs 53.6%, respectively; p-value: < 0.001), and higher in tumors located in the colon compared to the rectum (64.7% vs 43.9%, respectively; p-value: < 0.001) (Table 1).
Table 1.
Frequenciesa [n (%)] of subgroups based on mutation and MMR status in CRC cases, by tumor location and sex; NLCS, 1986–2006
| CRC | pd | Colon | Rectum | pe | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total n = 1934 |
Men n = 1113 |
Women n = 821 |
Total n = 1384 |
Men n = 754 |
Women n = 630 |
Total n = 355 |
Men n = 224 |
Women n = 131 |
|||
| All-wild-type + pMMRb | 792 (41.0) | 516 (46.4) | 276 (33.6) | < 0.001 | 488 (35.3) | 309 (41.0) | 179 (28.4) | 199 (56.1) | 135 (60.3) | 64 (48.9) | < 0.001 |
| Any-mutation/dMMRc | 1142 (59.1) | 597 (53.6) | 545 (66.4) | 896 (64.7) | 445 (59.0) | 451 (71.6) | 156 (43.9) | 89 (39.7) | 67 (51.2) | ||
| KRASmut | 673 (34.8) | 376 (33.8) | 297 (36.2) | 0.275 | 478 (34.5) | 256 (34.0) | 222 (35.2) | 123 (34.7) | 68 (30.4) | 55 (42.0) | 0.969 |
| PIK3CAmut | 334 (17.3) | 196 (17.6) | 138 (16.8) | 0.645 | 266 (19.2) | 150 (19.9) | 116 (18.4) | 45 (12.7) | 30 (13.4) | 15 (11.5) | 0.004 |
| BRAFmut | 298 (15.4) | 117 (10.5) | 181 (22.1) | < 0.001 | 278 (20.1) | 105 (13.9) | 173 (27.5) | 14 (3.9) | 8 (3.6) | 6 (4.6) | < 0.001 |
| dMMR | 206 (10.7) | 72 (6.5) | 134 (16.3) | < 0.001 | 201 (14.5) | 70 (9.3) | 131 (20.8) | 3 (0.9) | 1 (0.5) | 2 (1.5) | < 0.001 |
(d/p)MMR mismatch repair (deficient/proficient); CRC colorectal cancer; NLCS Netherlands Cohort Study; mut mutated
aPercentages might not add up because multiple molecular characteristics (e.g. BRAF mutation and MMR deficiency) can occur per individual
bThis group excludes cases with mutations in any of the genes (KRAS, PIK3CA, or BRAF), as well as MMR deficient cases
cThis group includes cases with mutations in any of the genes (KRAS, PIK3CA, or BRAF) and/or cases that are MMR deficient
dDifference between men and women, based on total CRC, evaluated using Chi-square
eDifference between colon and rectum, based on men and women combined, evaluated using Chi-square
Fig. 2.
Graphical presentation of KRASmut, PIK3CAmut, BRAFmut, and MMR status in CRC cases from the NLCS. a Pie chart showing the distribution of the all-wild-type + pMMR and any-mutation/dMMR subgroups (based on all CRC cases; n = 1934). b Bar chart showing frequencies of KRASmut, PIK3CAmut, BRAFmut, and dMMR (based on all CRC cases; n = 1934). c Venn diagram showing combinations of KRASmut, PIK3CAmut, BRAFmut, and dMMR (based on any-mutation/dMMR subgroup; n = 1142). The color intensity indicates the frequency: a darker color indicates more cases; a lighter color indicates fewer cases. d/pMMR mismatch repair deficiency/proficiency; mut mutation; CRC colorectal cancer; NLCS Netherlands Cohort Study
KRASmut-tumors were observed in 673 (34.8%) cases, PIK3CAmut-tumors in 334 (17.3%) cases, BRAFmut-tumors in 298 (15.4%) cases, and dMMR-tumors in 206 (10.7%) cases (Table 1, Fig. 2b). The frequency of BRAFmut-tumors and dMMR-tumors was higher in women compared to men (BRAFmut: 22.1 vs 10.5%, p-value: < 0.001; dMMR: 16.3% vs 6.5%, p-value: < 0.001, respectively). PIK3CAmut-, BRAFmut-, and dMMR-tumors were more often observed in colon compared to rectum (PIK3CAmut: 19.2% vs 12.7%, p-value: 0.004; BRAFmut: 20.1% vs 3.9%, p-value: < 0.001; dMMR: 14.5% vs 0.9%, p-value: < 0.001, respectively) (Table 1).
Within the any-mutation/dMMR subgroup, exclusive KRASmut-tumors were observed in 505 (44.2%), exclusive PIK3CAmut-tumors in 125 (11.0%), exclusive BRAFmut-tumors in 132 (11.6%), and exclusive dMMR-tumors in 44 (3.9%) cases (Fig. 2c). Combinations of KRASmut and PIK3CAmut and of BRAFmut and dMMR were most common (13.0% and 10.3%, respectively). Other combinations of mutations and/or dMMR were relatively rare (i.e. < 5%) (Fig. 2c).
Cohort characteristics in subgroups based on molecular features
Information on cohort characteristics of CRC cases, overall and according to subgroups based on molecular features, is provided in Table 2. Cases in the any-mutation/dMMR subgroup were older than those in the all-wild-type + pMMR subgroup. Furthermore, cases in the any-mutation/dMMR subgroup were more often overweight compared to those in the all-wild-type + pMMR subgroup, with the exception of men with colon cancer. In general, overweight was most frequently observed amongst cases with KRASmut- and/or PIK3CAmut-tumors. Similarly, the any-mutation/dMMR subgroup showed a larger mean clothing-size compared to the all-wild-type + pMMR subgroup, with the exception of men with colon cancer. The mean clothing-size was largest for the KRASmut subgroup, again with the exception of men with colon cancer. Non-occupational physical activity was higher amongst the all-wild-type + pMMR subgroup than amongst the any-mutation/dMMR subgroup, with the exception of women with rectal cancer. In men, cases with a PIK3CAmut-tumor in the colon were least physically active, whereas in women cases with dMMR- or BRAFmut-tumors in the colon were least physically active. Colon cancer cases in the any-mutation/dMMR subgroup showed a higher occupational energy expenditure than those in the all-wild-type + pMMR subgroup. In particular, dMMR colon cancer cases showed the highest occupational energy expenditure and lowest occupational sitting time. In contrast, rectal cancer cases in the any-mutation/dMMR subgroup showed lower occupational energy expenditure compared to those in the all-wild-type + pMMR subgroup.
Table 2.
Characteristics [mean (SD) or %] of CRC cases in subgroups based on mutation and MMR status, by sex and tumor location; NLCS, 1986–2006
| Total | Wild-type + pMMRd |
Any-mutation/dMMRe | KRASmut | PIK3CAmutf | BRAFmutf | dMMRf | |
|---|---|---|---|---|---|---|---|
| Men—colon | |||||||
| N | 754 | 309 | 445 | 256 | 150 | 105 | 70 |
| Age (years) | 61.6 (4.2) | 61.2 (4.2) | 61.9 (4.2) | 62.0 (4.2) | 61.5 (4.3) | 62.2 (4.1) | 62.7 (4.1) |
| Overweight/obesitya (%) | 52.4 | 52.8 | 52.1 | 52.7 | 56.7 | 48.6 | 54.3 |
| Clothing sizeb | 52.2 (2.6) | 52.2 (2.5) | 52.2 (2.7) | 52.1 (2.7) | 52.1 (2.7) | 52.2 (2.9) | 52.3 (2.8) |
| Non-occupational PA > 60 min/day (%) | 50.4 | 53.7 | 48.1 | 51.6 | 42.0 | 49.5 | 55.7 |
| Occ. energy expenditure (> 12 kJ/min)c | 11.6 | 8.8 | 13.4 | 13.2 | 13.9 | 11.2 | 17.0 |
| Occ. sitting time (< 2 h/day)c | 23.2 | 23.5 | 22.9 | 23.5 | 27.1 | 20.2 | 28.8 |
| Men—rectum | |||||||
| N | 224 | 135 | 89 | 68 | 30 | 8 | 1 |
| Age (years) | 60.8 (3.9) | 60.4 (4.0) | 61.4 (3.9) | 61.7 (3.9) | |||
| Overweight/obesitya (%) | 48.2 | 46.7 | 50.6 | 52.9 | |||
| Clothing sizeb | 51.7 (2.5) | 51.6 (2.3) | 51.8 (2.8) | 52.3 (2.8) | |||
| Non-occupational PA > 60 min/day (%) | 59.4 | 60.0 | 58.4 | 54.4 | |||
| Occ. energy expenditure (> 12 kJ/min)c | 11.0 | 12.1 | 9.3 | 8.8 | |||
| Occ. sitting time (< 2 h/day)c | 30.4 | 31.0 | 29.3 | 29.8 | |||
| Women—colon | |||||||
| N | 630 | 179 | 451 | 222 | 116 | 173 | 131 |
| Age (years) | 62.0 (4.1) | 61.1 (3.9) | 62.3 (4.1) | 62.2 (4.1) | 62.2 (4.2) | 62.6 (4.1) | 62.3 (4.0) |
| Overweight/obesitya (%) | 44.8 | 39.7 | 46.8 | 52.7 | 49.1 | 43.4 | 38.2 |
| Clothing sizeb | 43.6 (3.4) | 43.4 (4.1) | 43.6 (3.0) | 43.9 (3.2) | 43.5 (2.9) | 43.6 (2.8) | 43.3 (3.0) |
| Non-occupational PA > 60 min/day (%) | 41.8 | 46.4 | 39.9 | 40.1 | 38.8 | 38.7 | 41.2 |
| Women—rectum | |||||||
| N | 131 | 64 | 67 | 55 | 15 | 6 | 2 |
| Age (years) | 61.5 (4.2) | 60.9 (4.3) | 62.0 (4.0) | 61.7 (4.0) | |||
| Overweight/obesitya (%) | 49.6 | 48.4 | 50.8 | 52.7 | |||
| Clothing sizeb | 43.5 (2.7) | 43.3 (2.7) | 43.8 (2.7) | 43.9 (2.8) | |||
| Non-occupational PA > 60 min/day (%) | 42.8 | 37.5 | 47.8 | 47.3 |
SD standard deviation; CRC colorectal cancer; (d/p)MMR mismatch repair (deficient/proficient); NLCS Netherlands Cohort Study; PA physical activity.; Occ occupational
aBody mass index ≥ 25
bLower body clothing size. Based on fewer participants due to extra missings
cBased on fewer participants due to shorter follow-up (17.3 years), only available for men
dThis group excludes cases with mutations in any of the genes (KRAS, PIK3CA, or BRAF), as well as MMR deficient cases
eThis group includes cases with mutations in any of the genes (KRAS, PIK3CA, or BRAF) and/or cases that are MMR deficient
fAnalyses for subgroups with < 50 cases were not performed
Associations of energy balance-related factors and CRC subgroups based on molecular features
Multivariable-adjusted Cox-regression models on energy balance-related factors and risk of CRC subgroups based on molecular features are shown in Tables 3, 4, 5, and 6. Age-adjusted Cox-regression models are shown in Supplementary Tables S3–S6. Results of associations between energy balance-related factors and risk of CRC wild-type and MMR-proficient subgroups separately are additionally presented in Supplementary Tables S7–S8. Age was included as a time-varying covariate in all models, because of violation of the proportional hazards assumption.
Table 3.
Multivariable-adjusted HRsa and 95%-CIs for associations between adiposity measures and CRC in subgroups based on mutation and MMR status, by sex and tumor location; NLCS, 1986–2006
| Person-years at risk | Total | Wild-type + pMMRb | Any-mutation/dMMRc | p-het | ||||
|---|---|---|---|---|---|---|---|---|
| ncases | HR (95% CI) | ncases | HR (95% CI) | ncases | HR (95% CI) | |||
| BMI quartiles (kg/m2): range (median) | ||||||||
| Men—colon | ||||||||
| < 23.4 (22.2) | 7993 | 167 | 1.00 (ref.) | 66 | 1.00 (ref.) | 101 | 1.00 (ref.) | |
| 23.4–24.9 (24.2) | 8343 | 188 | 1.06 (0.82–1.37) | 79 | 1.10 (0.76–1.57) | 109 | 1.04 (0.77–1.42) | |
| 25.0–26.6 (25.7) | 7683 | 200 | 1.21 (0.93–1.56) | 77 | 1.13 (0.78–1.64) | 123 | 1.26 (0.92–1.72) | |
| > 26.6 (27.8) | 7003 | 199 | 1.41 (1.09–1.83) | 87 | 1.47 (1.03–2.11) | 112 | 1.37 (0.99–1.88) | 0.710 |
| p-trend | 0.005 | 0.038 | 0.027 | |||||
| per 5 kg/m2 | 31,022 | 754 | 1.30 (1.12–1.51) | 309 | 1.34 (1.08–1.67) | 445 | 1.28 (1.07–1.53) | 0.454 |
| Men—rectum | ||||||||
| < 23.4 (22.2) | 7993 | 58 | 1.00 (ref.) | 34 | 1.00 (ref.) | 24 | 1.00 (ref.) | |
| 23.4–24.9 (24.2) | 8343 | 54 | 0.86 (0.57–1.28) | 35 | 0.95 (0.58–1.57) | 19 | 0.73 (0.39–1.37) | |
| 25.0–26.6 (25.7) | 7683 | 65 | 1.15 (0.78–1.69) | 42 | 1.29 (0.80–2.09) | 23 | 0.95 (0.51–1.74) | |
| > 26.6 (27.8) | 7003 | 47 | 0.93 (0.61–1.43) | 24 | 0.82 (0.47–1.44) | 23 | 1.09 (0.59–2.01) | 0.458 |
| p-trend | 0.851 | 0.870 | 0.636 | |||||
| per 5 kg/m2 | 31,022 | 224 | 1.02 (0.81–1.28) | 135 | 0.95 (0.71–1.26) | 89 | 1.14 (0.80–1.62) | 0.387 |
| Women—colon | ||||||||
| < 22.8 (21.5) | 9014 | 181 | 1.00 (ref.) | 56 | 1.00 (ref.) | 125 | 1.00 (ref.) | |
| 22.8–24.7 (23.8) | 8914 | 146 | 0.81 (0.63–1.05) | 43 | 0.78 (0.51–1.19) | 103 | 0.83 (0.62–1.11) | |
| 24.8–27.0 (25.7) | 8141 | 147 | 0.92 (0.71–1.20) | 36 | 0.73 (0.47–1.16) | 111 | 1.01 (0.75–1.36) | |
| > 27.0 (29.2) | 8158 | 156 | 1.01 (0.77–1.31) | 44 | 0.91 (0.59–1.41) | 112 | 1.05 (0.78–1.43) | 0.601 |
| p-trend | 0.805 | 0.595 | 0.516 | |||||
| per 5 kg/m2 | 34,228 | 630 | 1.04 (0.92–1.18) | 179 | 0.88 (0.69–1.11) | 451 | 1.11 (0.96–1.27) | 0.081 |
| Women—rectum | ||||||||
| < 22.8 (21.5) | 9014 | 37 | 1.00 (ref.) | 16 | 1.00 (ref.) | 21 | 1.00 (ref.) | |
| 22.8–24.7 (23.8) | 8914 | 26 | 0.69 (0.41–1.17) | 15 | 0.90 (0.43–1.89) | 11 | 0.53 (0.25–1.13) | |
| 24.8–27.0 (25.7) | 8141 | 32 | 0.91 (0.55–1.53) | 18 | 1.16 (0.58–2.34) | 14 | 0.75 (0.36–1.56) | |
| > 27.0 (29.2) | 8158 | 36 | 1.04 (0.63–1.72) | 15 | 0.93 (0.45–1.90) | 21 | 1.20 (0.61–2.38) | 0.491 |
| p-trend | 0.691 | 0.973 | 0.548 | |||||
| per 5 kg/m2 | 34,228 | 131 | 1.08 (0.86–1.34) | 64 | 1.05 (0.77–1.42) | 67 | 1.12 (0.82–1.53) | 0.872 |
| Clothing size: range (median) | ||||||||
| Men—colon | ||||||||
| ≤ 50 (50) | 10,903 | 211 | 1.00 (ref.) | 90 | 1.00 (ref.) | 121 | 1.00 (ref.) | |
| 52 (52) | 9750 | 247 | 1.30 (1.03–1.62) | 104 | 1.29 (0.94–1.77) | 143 | 1.30 (0.98–1.71) | |
| 54 (54) | 5156 | 136 | 1.36 (1.04–1.77) | 53 | 1.26 (0.86–1.84) | 83 | 1.43 (1.03–1.98) | |
| ≥ 56 (56) | 2619 | 90 | 1.80 (1.31–2.46) | 39 | 1.87 (1.22–2.86) | 51 | 1.75 (1.19–2.57) | 0.897 |
| p-trend | < 0.001 | 0.008 | 0.002 | |||||
| per 2 sizes | 28,428 | 684 | 1.33 (1.16–1.52) | 286 | 1.34 (1.12–1.61) | 398 | 1.32 (1.11–1.55) | 0.983 |
| Men—rectum | ||||||||
| ≤ 50 (50) | 10,903 | 78 | 1.00 (ref.) | 46 | 1.00 (ref.) | 32 | 1.00 (ref.) | |
| 52 (52) | 9750 | 69 | 1.00 (0.70–1.41) | 46 | 1.12 (0.73–1.72) | 23 | 0.80 (0.46–1.42) | |
| 54 (54) | 5156 | 43 | 1.20 (0.80–1.80) | 28 | 1.17 (0.70–1.96) | 18 | 1.23 (0.67–2.25) | |
| ≥ 56 (56) | 2619 | 16 | 0.90 (0.51–1.59) | 7 | 0.67 (0.29–1.51) | 9 | 1.23 (0.57–2.66) | 0.454 |
| p-trend | 0.801 | 0.760 | 0.470 | |||||
| per 2 sizes | 28,428 | 206 | 0.98 (0.81–1.20) | 124 | 0.95 (0.75–1.21) | 82 | 1.02 (0.74–1.41) | 0.711 |
| Women—colon | ||||||||
| ≤ 40 (40) | 6574 | 128 | 1.00 (ref.) | 46 | 1.00 (ref.) | 82 | 1.00 (ref.) | |
| 42 (42) | 8582 | 150 | 0.88 (0.67–1.17) | 34 | 0.58 (0.36–0.93) | 116 | 1.05 (0.76–1.46) | |
| 44 (44) | 9270 | 159 | 0.83 (0.63–1.10) | 48 | 0.74 (0.48–1.15) | 111 | 0.89 (0.64–1.23) | |
| ≥ 46 (46) | 9454 | 182 | 0.95 (0.72–1.26) | 50 | 0.78 (0.50–1.20) | 132 | 1.06 (0.77–1.46) | 0.104 |
| p-trend | 0.764 | 0.537 | 0.979 | |||||
| per 2 sizes | 33,880 | 619 | 1.08 (0.95–1.24) | 178 | 1.07 (0.82–1.40) | 441 | 1.09 (0.94–1.26) | 0.759 |
| Women—rectum | ||||||||
| ≤ 40 (40) | 6574 | 23 | 1.00 (ref.) | 11 | 1.00 (ref.) | 12 | 1.00 (ref.) | |
| 42 (42) | 8582 | 30 | 0.94 (0.53–1.67) | 17 | 1.13 (0.52–2.49) | 13 | 0.77 (0.34–1.73) | |
| 44 (44) | 9270 | 35 | 1.01 (0.58–1.75) | 20 | 1.28 (0.62–2.65) | 15 | 0.79 (0.35–1.77) | |
| ≥ 46 (46) | 9454 | 42 | 1.14 (0.66–1.97) | 16 | 0.96 (0.44–2.08) | 26 | 1.31 (0.62–2.78) | 0.319 |
| p-trend | 0.532 | 0.941 | 0.355 | |||||
| per 2 sizes | 33,880 | 130 | 0.99 (0.80–1.23) | 64 | 0.96 (0.71–1.28) | 66 | 1.02 (0.76–1.39) | 0.562 |
HR hazard ratio; CI confidence interval; CRC colorectal cancer; (d/p)MMR mismatch repair (deficient/proficient); NLCS Netherlands Cohort Study; BMI body mass index; p-het p-heterogeneity
aHazard ratios were adjusted for age (years; continuous), non-occupational physical activity (min/day; continuous), total energy intake (kcal/day; continuous), family history of CRC (yes/no), alcohol consumption (0; 0.1–4; 5–14; > 15 g/day), processed meat intake (g/day; continuous), red meat intake (g/day; continuous). Age was included as a time-varying covariate. BMI models were additionally adjusted for height (cm; continuous)
bThis group excludes cases with mutations in any of the genes (KRAS, PIK3CA, or BRAF), as well as MMR deficient cases
cThis group includes cases with mutations in any of the genes (KRAS, PIK3CA, or BRAF) and/or cases that are MMR deficient
Table 4.
Multivariable-adjusted HRsa and 95%-CIs for associations between adiposity measures and CRC for individual mutations and MMR status, by sex and tumor location; NLCS, 1986–2006
| Person-years at risk | KRASmut | PIK3CAmutb | BRAFmutb | dMMRb | |||||
|---|---|---|---|---|---|---|---|---|---|
| ncases | HR (95% CI) | ncases | HR (95% CI) | ncases | HR (95% CI) | ncases | HR (95% CI) | ||
| BMI quartiles (kg/m2): range (median) | |||||||||
| Men—colon | |||||||||
| < 23.4 (22.2) | 7993 | 61 | 1.00 (ref.) | 27 | 1.00 (ref.) | 25 | 1.00 (ref.) | 19 | 1.00 (ref.) |
| 23.4–24.9 (24.2) | 8343 | 58 | 0.89 (0.60–1.32) | 38 | 1.37 (0.81–2.30) | 28 | 1.11 (0.62–1.96) | 13 | 0.68 (0.33–1.42) |
| 25.0–26.6 (25.7) | 7683 | 69 | 1.12 (0.76–1.65) | 42 | 1.66 (0.98–2.81) | 29 | 1.22 (0.68–2.20) | 21 | 1.21 (0.63–2.34) |
| > 26.6 (27.8) | 7003 | 68 | 1.30 (0.88–1.92) | 43 | 1.97 (1.17–3.32) | 23 | 1.14 (0.61–2.14) | 17 | 1.17 (0.59–2.31) |
| p-trend | 0.112 | 0.007 | 0.603 | 0.378 | |||||
| per 5 kg/m2 | 31,022 | 256 | 1.25 (1.00–1.57) | 150 | 1.38 (1.05–1.82) | 105 | 1.23 (0.87–1.72) | 70 | 1.51 (1.01–2.26) |
| Men—rectum | |||||||||
| < 23.4 (22.2) | 7993 | 18 | 1.00 (ref.) | ||||||
| 23.4–24.9 (24.2) | 8343 | 13 | 0.67 (0.31–1.44) | ||||||
| 25.0–26.6 (25.7) | 7683 | 19 | 1.06 (0.53–2.15) | ||||||
| > 26.6 (27.8) | 7003 | 18 | 1.21 (0.59–2.47) | ||||||
| p-trend | 0.415 | ||||||||
| per 5 kg/m2 | 31,022 | 68 | 1.17 (0.79–1.73) | ||||||
| Women—colon | |||||||||
| < 22.8 (21.5) | 9014 | 52 | 1.00 (ref.) | 30 | 1.00 (ref.) | 53 | 1.00 (ref.) | 43 | 1.00 (ref.) |
| 22.8–24.7 (23.8) | 8914 | 46 | 0.88 (0.58–1.35) | 27 | 0.89 (0.52–1.53) | 40 | 0.76 (0.49–1.19) | 34 | 0.78 (0.49–1.26) |
| 24.8–27.0 (25.7) | 8141 | 65 | 1.48 (0.99–2.20) | 30 | 1.08 (0.64–1.84) | 39 | 0.82 (0.52–1.29) | 24 | 0.61 (0.36–1.03) |
| > 27.0 (29.2) | 8158 | 59 | 1.37 (0.90–2.08) | 29 | 1.01 (0.60–1.73) | 41 | 0.90 (0.57–1.41) | 30 | 0.78 (0.48–1.30) |
| p-trend | 0.031 | 0.798 | 0.678 | 0.221 | |||||
| per 5 kg/m2 | 34,228 | 222 | 1.31 (1.10–1.57)* | 116 | 1.09 (0.84–1.42) | 173 | 0.99 (0.81–1.22) | 131 | 0.90 (0.70–1.15) |
| Women—rectum | |||||||||
| < 22.8 (21.5) | 9014 | 18 | 1.00 (ref.) | ||||||
| 22.8–24.7 (23.8) | 8914 | 7 | 0.40 (0.17–0.98) | ||||||
| 24.8–27.0 (25.7) | 8141 | 9 | 0.57 (0.24–1.33) | ||||||
| > 27.0 (29.2) | 8158 | 21 | 1.46 (0.72–2.96) | ||||||
| p-trend | 0.312 | ||||||||
| per 5 kg/m2 | 34,228 | 55 | 1.21 (0.87–1.67) | ||||||
| Clothing size: range (median) | |||||||||
| Men—colon | |||||||||
| ≤ 50 (50) | 10,903 | 73 | 1.00 (ref.) | 40 | 1.00 (ref.) | 30 | 1.00 (ref.) | 18 | 1.00 (ref.) |
| 52 (52) | 9750 | 84 | 1.26 (0.89–1.78) | 52 | 1.45 (0.94–2.24) | 29 | 1.04 (0.61–1.78) | 23 | 1.37 (0.71–2.64) |
| 54 (54) | 5156 | 48 | 1.33 (0.88–2.02) | 22 | 1.20 (0.69–2.09) | 21 | 1.40 (0.77–2.55) | 12 | 1.37 (0.64–2.95) |
| ≥ 56 (56) | 2619 | 29 | 1.63 (1.00–2.65) | 17 | 1.80 (0.98–3.31) | 9 | 1.19 (0.54–2.61) | 7 | 1.55 (0.63–3.81) |
| p-trend | 0.040 | 0.094 | 0.360 | 0.280 | |||||
| per 2 sizes | 28,428 | 234 | 1.24 (1.01–1.54) | 131 | 1.31 (1.01–1.70) | 89 | 1.18 (0.85–1.64) | 60 | 1.33 (0.90–1.96) |
| Men—rectum | |||||||||
| ≤ 50 (50) | 10,903 | 21 | 1.00 (ref.) | ||||||
| 52 (52) | 9750 | 17 | 0.90 (0.45–1.77) | ||||||
| 54 (54) | 5156 | 17 | 1.71 (0.86–3.40) | ||||||
| ≥ 56 (56) | 2619 | 9 | 1.78 (0.78–4.06) | ||||||
| p-trend | 0.073 | ||||||||
| per 2 sizes | 28,428 | 64 | 1.17 (0.80–1.73) | ||||||
| Women—colon | |||||||||
| ≤ 40 (40) | 6574 | 35 | 1.00 (ref.) | 24 | 1.00 (ref.) | 29 | 1.00 (ref.) | 32 | 1.00 (ref.) |
| 42 (42) | 8582 | 54 | 1.13 (0.72–1.78) | 25 | 0.75 (0.42–1.34) | 47 | 1.23 (0.75–2.03) | 35 | 0.82 (0.49–1.37) |
| 44 (44) | 9270 | 58 | 1.10 (0.70–1.72) | 33 | 0.84 (0.48–1.45) | 45 | 1.02 (0.62–1.68) | 25 | 0.51 (0.29–0.88) |
| ≥ 46 (46) | 9454 | 70 | 1.33 (0.86–2.05) | 30 | 0.74 (0.42–1.30) | 49 | 1.11 (0.67–1.83) | 37 | 0.76 (0.45–1.28) |
| p-trend | 0.229 | 0.423 | 0.957 | 0.174 | |||||
| per 2 sizes | 33,880 | 217 | 1.26 (1.03–1.53) | 112 | 1.05 (0.82–1.35) | 170 | 1.04 (0.84–1.27) | 129 | 0.90 (0.71–1.14) |
| Women—rectum | |||||||||
| ≤ 40 (40) | 6574 | 10 | 1.00 (ref.) | ||||||
| 42 (42) | 8582 | 9 | 0.65 (0.26–1.62) | ||||||
| 44 (44) | 9270 | 12 | 0.76 (0.32–1.84) | ||||||
| ≥ 46 (46) | 9454 | 23 | 1.41 (0.63–3.13) | ||||||
| p-trend | 0.243 | ||||||||
| per 2 sizes | 33,880 | 54 | 1.07 (0.77–1.51) | ||||||
HR hazard ratio; CI confidence interval; CRC colorectal cancer; (d/p)MMR mismatch repair (deficient/proficient); NLCS Netherlands Cohort Study; BMI body mass index; p-het p-heterogeneity
*Statistically significant p-heterogeneity, p = 0.008 (reference group: wild-type for KRAS, PIK3CA, and BRAF, and pMMR). Note: other p-heterogeneity tests were not statistically significant
aHazard Ratios were adjusted for age (years; continuous), non-occupational physical activity (minutes/day; continuous), total energy intake (kcal/day; continuous), family history of CRC (yes/no), alcohol consumption (0; 0.1–4; 5–14; > 15 g/day), processed meat intake (g/day; continuous), red meat intake (g/day; continuous). Age was included as a time-varying covariate. BMI models were additionally adjusted for height (cm; continuous)
bAnalyses for subgroups with < 50 cases were not performed
Table 5.
Multivariable-adjusted HRsa and 95% CIs for associations between physical activity measures and CRC in subgroups based on mutation and MMR status, by sex and tumor location; NLCS, 1986–2006
| Person-years at risk | Total | Wild-type + pMMRb | Any-mutation/dMMRc | p-het | ||||
|---|---|---|---|---|---|---|---|---|
| ncases | HR (95% CI) | ncases | HR (95% CI) | ncases | HR (95% CI) | |||
| Non-occupational physical activity (min/day): range (median) | ||||||||
| Men—colon | ||||||||
| ≤ 30 | 4997 | 132 | 1.00 (ref.) | 49 | 1.00 (ref.) | 83 | 1.00 (ref.) | |
| 31–60 | 10,100 | 242 | 0.89 (0.69–1.16) | 94 | 0.95 (0.64–1.39) | 148 | 0.86 (0.63–1.17) | |
| 61–90 | 6001 | 156 | 0.99 (0.75–1.32) | 62 | 1.08 (0.71–1.63) | 94 | 0.95 (0.67–1.33) | |
| > 90 | 9925 | 224 | 0.85 (0.65–1.11) | 104 | 1.10 (0.76–1.60) | 120 | 0.71 (0.51–0.97) | 0.232 |
| p-trend | 0.356 | 0.402 | 0.050 | |||||
| per 30 min/day | 31,022 | 754 | 0.99 (0.95–1.03) | 309 | 1.02 (0.97–1.07) | 445 | 0.97 (0.92–1.02) | 0.204 |
| Men—rectum | ||||||||
| ≤ 30 | 4997 | 18 | 1.00 (ref.) | 13 | 1.00 (ref.) | 5 | 1.00 (ref.) | |
| 31–60 | 10,100 | 73 | 1.92 (1.12–3.30) | 41 | 1.49 (0.78–2.85) | 32 | 3.03 (1.16–7.89) | |
| 61–90 | 6001 | 57 | 2.57 (1.47–4.47) | 38 | 2.33 (1.21–4.47) | 19 | 3.13 (1.15–8.52) | |
| > 90 | 9925 | 76 | 2.09 (1.22–3.59) | 43 | 1.62 (0.85–3.08) | 33 | 3.32 (1.28–8.60) | 0.450 |
| p-trend | 0.012 | 0.104 | 0.033 | |||||
| per 30 min/day | 31,022 | 224 | 1.04 (0.98–1.09) | 135 | 1.04 (0.96–1.11) | 89 | 1.03 (0.96–1.11) | 0.850 |
| Women—colon | ||||||||
| ≤ 30 | 7756 | 169 | 1.00 (ref.) | 52 | 1.00 (ref.) | 117 | 1.00 (ref.) | |
| 31–60 | 10,923 | 198 | 0.83 (0.65–1.06) | 44 | 0.58 (0.38–0.89) | 154 | 0.94 (0.71–1.25) | |
| 61–90 | 8000 | 148 | 0.84 (0.64–1.09) | 47 | 0.85 (0.56–1.30) | 101 | 0.83 (0.61–1.13) | |
| > 90 | 7550 | 115 | 0.70 (0.53–0.93) | 36 | 0.69 (0.44–1.08) | 79 | 0.71 (0.51–0.98) | 0.145 |
| p-trend | 0.021 | 0.344 | 0.024 | |||||
| per 30 min/day | 34,228 | 630 | 0.97 (0.91–1.03) | 179 | 0.98 (0.88–1.10) | 451 | 0.96 (0.89–1.03) | 0.623 |
| Women—rectum | ||||||||
| ≤ 30 | 7756 | 31 | 1.00 (ref.) | 14 | 1.00 (ref.) | 17 | 1.00 (ref.) | |
| 31–60 | 10,923 | 44 | 1.03 (0.63–1.67) | 26 | 1.30 (0.66–2.56) | 18 | 0.78 (0.39–1.55) | |
| 61–90 | 8000 | 34 | 1.06 (0.64–1.75) | 12 | 0.79 (0.36–1.76) | 22 | 1.27 (0.67–2.39) | |
| > 90 | 7550 | 22 | 0.72 (0.41–1.26) | 12 | 0.83 (0.38–1.82) | 10 | 0.61 (0.28–1.37) | 0.214 |
| p-trend | 0.285 | 0.325 | 0.563 | |||||
| per 30 min/day | 34,228 | 131 | 1.00 (0.88–1.14) | 64 | 1.07 (0.89–1.28) | 67 | 0.93 (0.79–1.08) | 0.206 |
| Occupational energy expenditure (kJ/min) | ||||||||
| Men—colon | 25,073 | 564 | 226 | 338 | ||||
| < 8 | 15,144 | 365 | 1.00 (ref.) | 152 | 1.00 (ref.) | 213 | 1.00 (ref.) | |
| 8–12 | 6368 | 133 | 0.83 (0.65–1.05) | 54 | 0.80 (0.57–1.12) | 79 | 0.86 (0.64–1.15) | |
| > 12 | 3561 | 66 | 0.71 (0.52–0.97) | 20 | 0.51 (0.30–0.84) | 46 | 0.85 (0.59–1.23) | 0.201 |
| p-trend | 0.017 | 0.006 | 0.274 | |||||
| Men—rectum | 25,073 | 185 | 114 | 71 | ||||
| < 8 | 15,144 | 107 | 1.00 (ref.) | 65 | 1.00 (ref.) | 42 | 1.00 (ref.) | |
| 8–12 | 6368 | 57 | 1.35 (0.95–1.91) | 35 | 1.38 (0.89–2.13) | 22 | 1.30 (0.75–2.24) | |
| > 12 | 3561 | 21 | 0.84 (0.51–1.39) | 14 | 0.91 (0.49–1.70) | 7 | 0.73 (0.33–1.61) | 0.956 |
| p-trend | 0.905 | 0.746 | 0.801 | |||||
| Occupational sitting time (h/day) | ||||||||
| Men—colon | 25,073 | 564 | 226 | 338 | ||||
| > 6 | 6511 | 187 | 1.00 (ref.) | 85 | 1.00 (ref.) | 102 | 1.00 (ref.) | |
| 2–6 | 11,617 | 244 | 0.70 (0.55–0.88) | 87 | 0.55 (0.39–0.77) | 157 | 0.82 (0.62–1.09) | |
| < 2 | 6944 | 133 | 0.63 (0.48–0.83) | 54 | 0.56 (0.38–0.81) | 79 | 0.70 (0.50–0.97) | 0.102 |
| p-trend | 0.001 | 0.003 | 0.034 | |||||
| Men—rectum | 25,073 | 185 | 114 | 71 | ||||
| > 6 | 6511 | 60 | 1.00 (ref.) | 39 | 1.00 (ref.) | 21 | 1.00 (ref.) | |
| 2–6 | 11,617 | 69 | 0.62 (0.43–0.89) | 40 | 0.55 (0.35–0.87) | 29 | 0.75 (0.42–1.33) | |
| < 2 | 6944 | 56 | 0.88 (0.60–1.30) | 35 | 0.84 (0.52–1.37) | 21 | 0.96 (0.52–1.79) | 0.730 |
| p-trend | 0.541 | 0.500 | 0.912 | |||||
HR hazard ratio; CI confidence interval; CRC colorectal cancer; (d/p)MMR mismatch repair (deficient/proficient); NLCS Netherlands Cohort Study; p-het p-heterogeneity
aHazard Ratios were adjusted for age (years; continuous), BMI (kg/m2; continuous), total energy intake (kcal/day; continuous), family history of CRC (yes/no), alcohol consumption (0; 0.1–4; 5–14; > 15 g/day), processed meat intake (g/day; continuous), red meat intake (g/day; continuous). Age was included as a time-varying covariate
bThis group excludes cases with mutations in any of the genes (KRAS, PIK3CA, or BRAF), as well as MMR deficient cases
cThis group includes cases with mutations in any of the genes (KRAS, PIK3CA, or BRAF) and/or cases that are MMR deficient
Table 6.
Multivariable-adjusted HRsa and 95%-CIs for associations between physical activity measures and CRC for individual mutations and MMR status, by sex and tumor location; NLCS, 1986–2006
| Person-years at risk | KRASmut | PIK3CAmutb | BRAFmutb | dMMRb | |||||
|---|---|---|---|---|---|---|---|---|---|
| ncases | HR (95% CI) | ncases | HR (95% CI)s | ncases | HR (95%-CI) | ncases | HR (95% CI) | ||
| Non-occupational physical activity (min/day): range (median) | |||||||||
| Men—colon | |||||||||
| ≤ 30 (21.4) | 4997 | 41 | 1.00 (ref.) | 24 | 1.00 (ref.) | 19 | 1.00 (ref.) | 13 | 1.00 (ref.) |
| 31–60 (42.9) | 10,100 | 83 | 0.97 (0.64–1.46) | 63 | 1.30 (0.79–2.13) | 34 | 0.87 (0.49–1.55) | 18 | 0.66 (0.31–1.40) |
| 61–90 (73.6) | 6001 | 63 | 1.31 (0.85–2.02) | 28 | 0.97 (0.55–1.72) | 19 | 0.83 (0.43–1.61) | 16 | 1.00 (0.47–2.15) |
| > 90 (130.0) | 9925 | 69 | 0.84 (0.55–1.28) | 35 | 0.73 (0.42–1.26) | 33 | 0.82 (0.46–1.48) | 23 | 0.80 (0.39–1.64) |
| p-trend | 0.575 | 0.041 | 0.548 | 0.925 | |||||
| per 30 min/day | 31,022 | 256 | 0.95 (0.89–1.01) | 150 | 0.96 (0.87–1.06) | 105 | 1.02 (0.94–1.11) | 70 | 1.02 (0.92–1.13) |
| Men—rectum | |||||||||
| ≤ 30 (21.4) | 4997 | 5 | 1.00 (ref.) | ||||||
| 31–60 (42.9) | 10,100 | 26 | 2.38 (0.90–6.31) | ||||||
| 61–90 (73.6) | 6001 | 16 | 2.66 (0.96–7.40) | ||||||
| > 90 (130.0) | 9925 | 21 | 2.04 (0.76–5.45) | ||||||
| p-trend | 0.372 | ||||||||
| per 30 min/day | 31,022 | 68 | 0.98 (0.89–1.08) | ||||||
| Women—colon | |||||||||
| ≤ 30 (19.3) | 7756 | 55 | 1.00 (ref.) | 36 | 1.00 (ref.) | 47 | 1.00 (ref.) | 32 | 1.00 (ref.) |
| 31–60 (42.9) | 10,923 | 78 | 1.01 (0.69–1.47) | 35 | 0.73 (0.45–1.20) | 59 | 0.91 (0.60–1.37) | 45 | 1.00 (0.63–1.61) |
| 61–90 (75.0) | 8000 | 48 | 0.84 (0.56–1.27) | 28 | 0.79 (0.47–1.32) | 35 | 0.72 (0.45–1.16) | 34 | 1.00 (0.60–1.67) |
| > 90 (115.7) | 7550 | 41 | 0.80 (0.52–1.23) | 17 | 0.51 (0.28–0.93) | 32 | 0.70 (0.44–1.14) | 20 | 0.64 (0.36–1.14) |
| p-trend | 0.197 | 0.042 | 0.095 | 0.150 | |||||
| per 30 min/day | 34,228 | 222 | 0.98 (0.90–1.08) | 116 | 0.90 (0.77–1.04) | 173 | 0.94 (0.84–1.05) | 131 | 0.97 (0.85–1.09) |
| Women—rectum | |||||||||
| ≤ 30 (19.3) | 7756 | 14 | 1.00 (ref.) | ||||||
| 31–60 (42.9) | 10,923 | 15 | 0.79 (0.37–1.68) | ||||||
| 61–90 (75.0) | 8000 | 20 | 1.40 (0.71–2.78) | ||||||
| > 90 (115.7) | 7550 | 6 | 0.45 (0.17–1.18) | ||||||
| p-trend | 0.366 | ||||||||
| per 30 min/day | 34,228 | 55 | 0.86 (0.74–0.99) | ||||||
| Occupational energy expenditure (kJ/min) | |||||||||
| Men—colon | 25,073 | 190 | 114 | 87 | 58 | ||||
| < 8 | 15,144 | 115 | 1.00 (ref.) | 72 | 1.00 (ref.) | 59 | 1.00 (ref.) | 32 | 1.00 (ref.) |
| 8–12 | 6368 | 50 | 1.04 (0.72–1.49) | 26 | 0.81 (0.50–1.32) | 18 | 0.68 (0.38–1.20) | 16 | 1.08 (0.56–2.08) |
| > 12 | 3561 | 25 | 0.93 (0.58–1.49) | 16 | 0.84 (0.47–1.50) | 10 | 0.65 (0.32–1.33) | 10 | 1.02 (0.45–2.27) |
| p-trend | 0.855 | 0.425 | 0.139 | 0.919 | |||||
| Men—rectum | 25,073 | 53 | |||||||
| < 8 | 15,144 | 31 | 1.00 (ref.) | ||||||
| 8–12 | 6368 | 17 | 1.43 (0.77–2.64) | ||||||
| > 12 | 3561 | 5 | 0.70 (0.27–1.82) | ||||||
| p-trend | 0.892 | ||||||||
| Occupational sitting time (h/day) | |||||||||
| Men—colon | 25,073 | 190 | 114 | 87 | 58 | ||||
| > 6 | 6511 | 57 | 1.00 (ref.) | 34 | 1.00 (ref.) | 22 | 1.00 (ref.) | 14 | 1.00 (ref.) |
| 2–6 | 11,617 | 87 | 0.81 (0.56–1.17) | 48 | 0.76 (0.48–1.20) | 47 | 1.15 (0.67–1.96) | 27 | 0.98 (0.49–1.94) |
| < 2 | 6944 | 46 | 0.75 (0.49–1.14) | 32 | 0.84 (0.51–1.39) | 18 | 0.73 (0.37–1.41) | 17 | 0.99 (0.46–2.13) |
| p-trend | 0.181 | 0.508 | 0.325 | 0.992 | |||||
| Men—rectum | 25,073 | 53 | |||||||
| > 6 | 6511 | 17 | 1.00 (ref.) | ||||||
| 2–6 | 11,617 | 20 | 0.61 (0.32–1.19) | ||||||
| < 2 | 6944 | 16 | 0.92 (0.46–1.86) | ||||||
| p-trend | 0.826 | ||||||||
HR hazard ratio; CI confidence interval; CRC colorectal cancer; (d/p)MMR mismatch repair (deficient/proficient); NLCS Netherlands Cohort Study; p-het p-heterogeneity
p-heterogeneity tests (reference group for all tests: wild-type for KRAS, PIK3CA, and BRAF, and pMMR) were not statistically significant
aHazard ratios were adjusted for age (years; continuous), BMI (kg/m2), total energy intake (kcal/day; continuous), family history of CRC (yes/no), alcohol consumption (0; 0.1–4; 5–14; > 15 g/day), processed meat intake (g/day; continuous), red meat intake (g/day; continuous). Age was included as a time-varying covariate
bAnalyses for subgroups with < 50 cases were not performed
Adiposity
BMI and clothing-size were both associated with an increased risk of overall colon cancer in men (Table 3). Associations were similarly positive for the all-wild-type + pMMR subgroup [BMI: HR5kg/m2 (95%-CI): 1.34 (1.08–1.67), p-trendquartiles: 0.038; clothing-size: HRtwo sizes: 1.34 (1.12–1.61), p-trendquartiles: 0.008] and the any-mutation/dMMR subgroup [BMI: HR5kg/m2 (95%-CI): 1.28 (1.07–1.53), p-trendquartiles: 0.027; clothing-size: HRtwo sizes: 1.32 (1.11–1.55), p-trendquartiles: 0.002]. Although positive associations were found across all subgroups of individual molecular features (Table 4), associations were strongest for the PIK3CAmut subgroup [BMI: HR5kg/m2 (95%-CI): 1.38 (1.05–1.82), p-trendquartiles: 0.007; clothing-size: HRtwo sizes: 1.31 (1.01–1.70), p-trendquartiles: 0.094], and weakest for the BRAFmut subgroup [BMI: HR5kg/m2 (95%-CI): 1.23 (0.87–1.72), p-trendquartiles: 0.603; clothing-size: HRtwo sizes: 1.18 (0.85–1.64), p-trendcategories: 0.360]. In women, BMI and clothing-size were not associated with risk of overall colon cancer, nor with the all-wild-type + pMMR or any-mutation/dMMR subgroups (Table 3). For individual molecular features, both BMI and clothing-size were associated with an increased risk of KRASmut [BMI: HR5kg/m2 (95% CI): 1.31 (1.10–1.57), p-trendquartiles: 0.031; clothing-size: HRtwo sizes: 1.26 (1.03–1.53), p-trendquartiles: 0.229], but not with PIK3CAmut, BRAFmut, or dMMR colon cancer in women (Table 4). No associations between BMI or clothing-size and risk of overall rectal cancer were observed in men or in women, and stratification on subgroups did not lead to clear associations (Tables 3, 4). None of the models with mutual adjustment for BMI and clothing-size showed clear associations of BMI or clothing-size with CRC subgroups based on molecular features (Supplementary Tables S9–S10).
Non-occupational physical activity
Non-occupational physical activity was not associated with overall colon cancer risk in men (Table 5). However, a borderline significant inverse association was found between non-occupational physical activity and risk of the any-mutation/dMMR subgroup [HR30min/day (95% CI): 0.97 (0.92–1.02), p-trendcategories: 0.050], whereas no association was found for the all-wild-type + pMMR subgroup. Other subgroups of molecular features in colon cancer did not show clear associations (Table 6). In contrast, non-occupational physical activity was associated with an increased risk of overall rectal cancer in men, which was stronger for the any-mutation/dMMR subgroup [HR>90vs≤30 min/day (95% CI): 3.32 (1.28–8.60), p-trendcategories: 0.033], whereas no clear association was found for the all-wild-type + pMMR or KRASmut subgroups (Tables 5, 6). However, it should be noted that the reference group (≤ 30 min/day) in the any-mutation/dMMR and KRASmut subgroups had a limited number of cases (n = 5). In women, non-occupational physical activity was associated with a decreased risk of overall colon cancer (Table 5). Although inverse associations were found for all subgroups, most did not reach statistical significance (Tables 5, 6). Only the any-mutation/dMMR subgroup [HR>90vs≤30 min/day (95% CI): 0.71 (0.51–0.98), p-trendcategories: 0.024] and the subgroup with a PIK3CAmut-tumor [HR>90vs≤30 min/day (95% CI): 0.51 (0.28–0.93), p-trendcategories: 0.042] showed statistically significant inverse associations. Non-occupational physical activity was not associated with overall rectal cancer in women, and stratification on subgroups did not lead to clear associations (Tables 5, 6).
Occupational physical activity
Occupational energy expenditure was associated with a decreased risk of overall colon cancer in men (Table 5). Even though inverse associations were observed for both combination subgroups, only the association with the all-wild-type + pMMR subgroup reached statistical significance [HR>12 kJ/min (95% CI): 0.51 (0.30–0.84), p-trendcategories: 0.006]. Furthermore, lower occupational sitting time was associated with a decreased risk of overall colon cancer in men (Table 5), and associations were slightly stronger for the all-wild-type + pMMR subgroup [HR<2 h/day (95% CI): 0.56 (0.38–0.81), p-trendcategories: 0.003] compared to the any-mutation/dMMR subgroup [HR<2 h/day (95% CI): 0.70 (0.50–0.97), p-trendcategories: 0.034]. No associations were observed for occupational physical activity measures and subgroups of individual molecular features in colon cancer (Table 6). Occupational physical activity measures were not associated with risk of rectal cancer in men, and stratification on subgroups did not lead to clear associations (Tables 5, 6).
Heterogeneity testing
For heterogeneity analyses, the all-wild-type + pMMR subgroup served as the reference group for all other subgroups (i.e. any-mutation/dMMR, KRASmut, PIK3CAmut, BRAFmut, and dMMR). Statistically significant heterogeneity was observed only for BMI associations between KRASmut versus all-wild-type + pMMR colon cancer in women (p = 0.008), but not for any other subgroup.
Sensitivity analyses
Sensitivity analyses excluding the first two years of follow-up did not lead to essential changes (data not shown).
Discussion
In this large prospective cohort study, we investigated associations between energy balance-related factors and risk of CRC subgroups based on KRASmut, PIK3CAmut, BRAFmut, and MMR status. Associations between energy balance-related factors and risk of CRC varied by abovementioned molecular features, as well by sex and tumor location. A statistically significant difference in associations was only found between all-wild-type + pMMR and KRASmut subgroups of colon cancer in women regarding BMI associations. In women, we observed positive associations for BMI and clothing-size with risk of KRASmut colon cancer, but not with any other subgroup. In men, BMI and clothing-size were positively associated with risk of colon, but not rectal cancer, regardless of molecular features subgroups. While positive associations of BMI and clothing-size with risk of colon cancer were observed in men for all individual molecular features, associations were strongest for PIK3CAmut tumors and weakest for BRAFmut tumors. Non-occupational physical activity was inversely associated with any-mutation/dMMR colon cancer in men and women, but not with all-wild-type + pMMR colon cancer. In men, no clear associations were observed between non-occupational physical activity and individual molecular features in colon cancer. In women, inverse associations were observed for all individual molecular features, but associations were strongest for PIK3CAmut colon cancer. Occupational physical activity was associated with a decreased risk of colon cancer for both combination subgroups in men, but associations were strongest for all-wild-type + pMMR tumors.
Several studies have focused on investigating associations between energy balance-related factors (i.e. BMI, waist-circumference, physical activity) and risk of CRC in relation to specific (individual) mutations and/or MSI/MMR status, but results have been inconsistent (Carr et al. 2018, 2020; Myte et al. 2019; Brändstedt et al. 2014; Slattery et al. 2000, 2001; Hughes et al. 2012; Campbell et al. 2010; Hoffmeister et al. 2013). To our knowledge, the current study is the first to combine cases into subgroups based on KRASmut, PIK3CAmut, BRAFmut, and MMR status, and study potential etiological differences between these subgroups. Instead of comparing wild-type versus mutated tumors for individual genes and proficient versus deficient tumors for MMR, as done in previous studies, the all-wild-type + pMMR subgroup served as the reference group for all other subgroups in the current study. Combining mutation and MMR status into subgroups has some advantages. First, it has been suggested that mutations in KRAS, PIK3CA, and BRAF drive metabolic reprogramming toward the Warburg-effect (Levine and Puzio-Kuter 2010; Kimmelman 2015; Hutton et al. 2016; Jiang et al. 2018), and we have shown previously that MMR deficiency is associated with presence of the Warburg-effect (Offermans et al. 2021). Combining these molecular features, presumed to be involved in the same metabolic phenotype, thus results in a cleaner reference group compared to groups based on individual features (e.g. KRAS mutated versus wild-type). Our results show that co-occurrence of KRASmut and PIK3CAmut is relatively common, as is co-occurrence of BRAFmut and dMMR. Using the all-wild-type + pMMR subgroup as the reference for all subgroups of individual mutations and MMR status, this reference group is less heterogeneous compared to, e.g., the KRAS wild-type (KRASwt) group, which still contains a large number of cases with a PIK3CA mutation. Second, differentiating subgroups on the basis of the combination of presence or absence of mutations and/or dMMR leads to increased statistical power, since most individual molecular features occurred in < 20% of CRC cases (e.g., MMR deficiency: 10.7%).
Previous studies on adiposity and risk of CRC in relation to molecular features mainly focused on BMI (Carr et al. 2018, 2020; Myte et al. 2019; Brändstedt et al. 2013, 2014; Slattery et al. 2000, 2001, 2007; Hughes et al. 2012; Campbell et al. 2010; Hoffmeister et al. 2013; Hanyuda et al. 2016), though some used additional adiposity measures like waist circumference (Brändstedt et al. 2013, 2014; Hughes et al. 2012). Two cohort studies (Myte et al. 2019; Brändstedt et al. 2014) and two case–control studies (Carr et al. 2020; Slattery et al. 2001) investigated adiposity in relation to KRASmut status in CRC. Our results are in line with those of Slattery et al. (2001), which showed positive associations of adiposity with KRASmut but not KRASwt colon cancer in women, whereas similar associations were observed for KRASmut and KRASwt in men. A study by Brändstedt et al. (2014) also reported positive associations between adiposity and KRASmut but not KRASwt CRC, but in men, not women. These and our results are in contrast with those of Carr et al. (2020) and Myte et al. (2019), who reported positive associations of adiposity with KRASwt CRC (note: KRASwt + BRAFwt in the study by Myte et al.) but no or weak associations with KRASmut CRC. Three cohort studies (Myte et al. 2019; Brändstedt et al. 2014; Hughes et al. 2012), including one study that used data from the NLCS with 7.3 years of follow-up (Hughes et al. 2012), and two case–control studies (Carr et al. 2020; Slattery et al. 2007) studied adiposity in relation to BRAFmut status in CRC. Our results are in line with all but one of these studies (Myte et al. 2019; Brändstedt et al. 2014; Hughes et al. 2012; Slattery et al. 2007), as these reported either a weaker positive association of adiposity with BRAFmut compared to BRAFwt CRC (Brändstedt et al. 2014; Hughes et al. 2012), or no association with BRAFmut CRC (Myte et al. 2019; Slattery et al. 2007). Even though Carr et al. (2020) observed this same difference in associations for men, associations between adiposity and CRC were stronger for BRAFmut CRC than BRAFwt CRC in women. For MSI/MMR status, our results are in line with those of a recent meta-analysis by Carr et al. (2018), in which no difference in associations was observed between adiposity and MSI status in CRC. Our study is the first to investigate the association between adiposity and CRC risk in relation to PIK3CAmut status, and therefore cannot be compared to any previous data.
To our knowledge, associations between physical activity and colon cancer risk in relation to molecular features have only been investigated in a case–control study by Slattery et al. for KRASmut (Slattery et al. 2001), BRAFmut (Slattery et al. 2007), and MSI (Slattery et al. 2000) status. Our results are partly in line with these studies, which showed stronger positive associations between physical inactivity and risk of KRASmut colon cancer compared to KRASwt colon cancer in men, whereas associations did not differ according to KRASmut status in women (Slattery et al. 2001). For BRAF, they observed no association between physical activity and BRAFmut colon cancer (Slattery et al. 2007). Lastly, physical activity was associated with both MSS and MSI colon cancer in men, but only with MSS colon cancer in women (Slattery et al. 2000). Our results for PIK3CAmut CRC cannot be compared to any previous data, since studies investigating associations between physical activity and PIK3CAmut status in CRC are currently lacking.
The contradicting results across molecular pathological epidemiology (MPE) studies regarding associations of energy balance-related factors with risk of CRC according to KRASmut, BRAFmut, and/or MSI/MMR status might be attributed to several factors. For example: use of different methods for assessing molecular features (e.g. assessment of different mutations or MSI versus MMR status); different timing and method of exposure measurements (i.e. BMI, waist circumference, physical activity); different study designs (i.e. cohort versus case–control); different approaches for (outcome) stratification (for example stratification on sex and tumor location); and/or chance findings due to multiple testing, caused by repeatedly splitting CRC into different molecular pathological subgroups. We therefore believe it is important that large prospective cohort studies replicate the current analyses, preferably stratified on tumor location and sex.
The current results suggest a role of KRAS mutations in the etiological pathway between adiposity and colon cancer risk in women (adiposity was only associated with KRASmut colon cancers). In contrast, our results do not indicate a clear role of one of the molecular features in the etiological pathway between adiposity and colon cancer in men (adiposity was associated with all subgroups of molecular features in colon cancer). As mentioned above, the molecular features used in the current study have all been associated with the Warburg-effect (Levine and Puzio-Kuter 2010; Kimmelman 2015; Hutton et al. 2016; Jiang et al. 2018; Offermans et al. 2021). Associations with the all-wild-type + pMMR group indicate a low likelihood of Warburg-effect involvement, whereas associations with the any-mutation/dMMR subgroup or subgroups of individual molecular features indicate a higher likelihood of Warburg-effect involvement. Therefore, the current results indicate a potential role of the Warburg-effect in the etiological pathway between adiposity and colon cancer in women through KRAS mutations, but not other molecular features. In men, a role of the Warburg-effect in the etiological pathway between adiposity and colon cancer is not indicated by the current results. In a previous study, we investigated associations between energy balance-related factors and risk of Warburg-subtypes in CRC, based IHC expression of proteins involved in the Warburg-effect (Jenniskens et al. 2021a). The results of this previous study indicated involvement of the Warburg-effect in associations between adiposity and colon cancer risk in both men and women, though additional mechanisms could be at play in women as well.
For physical activity, the current results indicate a role of molecular features (KRASmut, PIK3CAmut, BRAFmut, and/or MMR deficiency) in the etiological pathway between physical inactivity and colon cancer risk in women (physical activity was associated with any-mutation/dMMR colon cancer), and it seems that in particular PIK3CA mutations are involved in this association (strongest association observed with PIK3CAmut colon cancer). In men, the current results do not give a clear indication of involvement of molecular features in the association between physical activity and colon cancer. While non-occupational physical activity was inversely associated with the any-mutation/dMMR subgroup, occupational physical activity was mainly associated with the all-wild-type + pMMR subgroup. It is assumed that occupational physical activity gives a better indication of physical activity for men than non-occupational physical activity. That is, while occupational physical activity represents long-term physical activity (median duration of longest held job: 29 years), non-occupational physical activity probably reflects the last few years before baseline. Therefore, the current results suggest that the molecular features studied here are not involved in the etiological pathway between physical inactivity and colon cancer risk in men. All in all, the current results indicate involvement of the Warburg-effect in associations between physical activity and colon cancer risk in women, but not men. Results of our previous study on Warburg-subtypes in CRC indicated that inverse associations between physical activity and colon cancer risk are explained by mechanisms other than the Warburg-effect (Jenniskens et al. 2021a).
Altogether, results from our previous study on Warburg-subtypes in CRC are only partly in line with the current results. Although the molecular features that were considered in the current study have been associated with the Warburg-effect (Levine and Puzio-Kuter 2010; Kimmelman 2015; Hutton et al. 2016; Jiang et al. 2018; Offermans et al. 2021), they are additionally known for their involvement in numerous diverse (oncogenic) cellular pathways for cell growth, differentiation, proliferation, and survival (Li et al. 2020; Haluska et al. 2007; Boland and Goel 2010). Therefore, the molecular features used in the current study might not always be a good reflection of the Warburg-effect. Furthermore, tumors of cases in the all-wild-type + pMMR subgroup might express other molecular features, possibly also associated with the Warburg-effect, that were not assessed in the current study. This may have potentially influenced our results. Still, combining these molecular features into all-wild-type + pMMR and any-mutation/dMMR subgroups seemed to be a straightforward way of subgrouping CRC cases, especially for physical activity associations.
A major strength of the current study is the prospective cohort design with long follow-up (20.3 years) and availability of DNA from FFPE tumor material from a large number of incident CRC cases. Another strength was the detection of mutations using MassARRAY technology, which has been shown to be a suitable technique for mutation typing in (older) FFPE material (Fleitas et al. 2016). The ColoCarta panel that was used includes assays for most of the KRAS (99%) and BRAF (98%) mutations, but it identifies only 78% of known PIK3CA mutations (Fumagalli et al. 2010). However, the most common PIK3CA mutations are included (Gray et al. 2017). This makes it unlikely that additional detection of less common mutations would alter the current results, since the number of additional cases with a PIK3CA mutation would be rather small. As an indicator of MSI status, we used IHC expression of MLH1 and MSH2, which might have led to misclassification of some of the cases. However, it has been shown that loss of MLH1 or MSH2 expression was observed in ~ 90% of MSI cases (Lanza et al. 2002).
In conclusion, results from this large prospective cohort study provide further insights in the associations between energy balance-related factors and CRC risk according to KRASmut, PIK3CAmut, BRAFmut, and MMR status. Associations between energy balance-related factors and risk of CRC varied by these molecular features, as well by sex and tumor location. Our results suggest a role of KRAS mutations in the etiological pathway between adiposity and colon cancer in women. For men, our results do not indicate a role of one of the molecular features in the etiological pathway of adiposity and colon cancer. Furthermore, the current results indicate a role of mutations in KRAS, PIK3CA, and/or BRAF, and/or MMR deficiency in the etiological pathway between physical inactivity and colon cancer risk in women, but not men, and it seems that in particular PIK3CA mutations are involved in this association. Our findings need to be replicated in additional large-scale MPE-studies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the participants of the Netherlands Cohort Study (NLCS), the Netherlands Cancer Registry, and the Dutch Pathology Registry. They are grateful to Ron Alofs and Harry van Montfort for data management and programming assistance; and to Jaleesa van der Meer, Edith van den Boezem, and Peter Moerkerk for TMA construction; and Jakob Kather (University Hospital Aachen, Germany) for scanning of slides. The Rainbow-TMA consortium was financially supported by BBMRI-NL, a Research Infrastructure financed by the Dutch government (NWO 184.021.007, to P.A. van den Brandt), and Maastricht University Medical Center, University Medical Center Utrecht, and Radboud University Medical Centre, the Netherlands. The authors would like to thank all investigators from the Rainbow-TMA consortium project group [P.A. van den Brandt, A. zur Hausen, H. Grabsch, M. van Engeland, L.J. Schouten, J. Beckervordersandforth (Maastricht University Medical Center, Maastricht, Netherlands); P.H.M. Peeters, P.J. van Diest, H.B. Bueno de Mesquita (University Medical Center Utrecht, Utrecht, Netherlands); J. van Krieken, I. Nagtegaal, B. Siebers, B. Kiemeney (Radboud University Medical Center, Nijmegen, Netherlands); F.J. van Kemenade, C. Steegers, D. Boomsma, G.A. Meijer (VU University Medical Center, Amsterdam, Netherlands); F.J. van Kemenade, B. Stricker (Erasmus University Medical Center, Rotterdam, Netherlands); L. Overbeek, A. Gijsbers (PALGA, the Nationwide Histopathology and Cytopathology Data Network and Archive, Houten, Netherlands)] and collaborating pathologists [Amongst others: A. de Bruïne (VieCuri Medical Center, Venlo); J.C. Beckervordersandforth (Maastricht University Medical Center, Maastricht); J. van Krieken, I. Nagtegaal (Radboud University Medical Center, Nijmegen); W. Timens (University Medical Center Groningen, Groningen); F.J. van Kemenade (Erasmus University Medical Center, Rotterdam); M.C.H. Hogenes (Laboratory for Pathology OostNederland, Hengelo); P.J. van Diest (University Medical Center Utrecht, Utrecht); R.E. Kibbelaar (Pathology Friesland, Leeuwarden); A.F. Hamel (Stichting Samenwerkende Ziekenhuizen Oost-Groningen, Winschoten); A.T.M.G. Tiebosch (Martini Hospital, Groningen); C. Meijers (Reinier de Graaf Gasthuis/ S.S.D.Z., Delft); R. Natté (Haga Hospital Leyenburg, The Hague); G.A. Meijer (VU University Medical Center, Amsterdam); J.J.T.H. Roelofs (Academic Medical Center, Amsterdam); R.F. Hoedemaeker (Pathology Laboratory Pathan, Rotterdam); S. Sastrowijoto (Orbis Medical Center, Sittard); M. Nap (Atrium Medical Center, Heerlen); H.T. Shirango (Deventer Hospital, Deventer); H. Doornewaard (Gelre Hospital, Apeldoorn); J.E. Boers (Isala Hospital, Zwolle); J.C. van der Linden (Jeroen Bosch Hospital, Den Bosch); G. Burger (Symbiant Pathology Center, Alkmaar); R.W. Rouse (Meander Medical Center, Amersfoort); P.C. de Bruin (St. Antonius Hospital, Nieuwegein); P. Drillenburg (Onze Lieve Vrouwe Gasthuis, Amsterdam); C. van Krimpen (Kennemer Gasthuis, Haarlem); J.F. Graadt van Roggen (Diaconessenhuis, Leiden); S.A.J. Loyson (Bronovo Hospital, The Hague); J.D. Rupa (Laurentius Hospital, Roermond); H. Kliffen (Maasstad Hospital, Rotterdam); H.M. Hazelbag (Medical Center Haaglanden, The Hague); K. Schelfout (Stichting Pathologisch en Cytologisch Laboratorium West-Brabant, Bergen op Zoom); J. Stavast (Laboratorium Klinische Pathologie Centraal Brabant, Tilburg); I. van Lijnschoten (PAMM laboratory for Pathology and Medical Microbiology, Eindhoven); K. Duthoi (Amphia Hospital, Breda)].
Author contributions
Conceptualization: JCAJ, KO, CCJMS, HIG, PAvdB; Methodology: PAvdB; Formal analysis and investigation: JCAJ; Writing—original draft preparation: JCAJ, KO, CCJMS, HIG, PAvdB; Writing—review and editing: IS, GEF, JRMvdM, KMS, LJS, MPW; Funding acquisition: PAvdB, HIG; Supervision: PAvdB.
Funding
This project was funded by The Dutch Cancer Society (KWF 11044 to P.A. van den Brandt).
Declarations
Conflict of interest
H. I. Grabsch: Honorarium from Astra Zeneca and BMS for scientific advisory board activities not related to the current study.
Ethics approval
Ethical approval was obtained from Medical Ethical Committee MUMC.
Consent to participate
Individuals consented to participate in the NLCS by completing and returning the questionnaire.
Consent to publish
Not applicable.
Footnotes
Heike I. Grabsch and Piet A. van den Brandt are co-last author.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Heike I. Grabsch, Email: h.grabsch@maastrichtuniversity.nl
Piet A. van den Brandt, Email: pa.vandenbrandt@maastrichtuniversity.nl
References
- Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138(6):2073–2087.e3. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brändstedt J, Wangefjord S, Borgquist S, Nodin B, Eberhard J, Manjer J, Jirström K. Influence of anthropometric factors on tumour biological characteristics of colorectal cancer in men and women: a cohort study. J Transl Med. 2013;11(1):1–13. doi: 10.1186/1479-5876-11-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brändstedt J, Wangefjord S, Nodin B, Eberhard J, Sundström M, Manjer J, Jirström K. Associations of anthropometric factors with KRAS and BRAF mutation status of primary colorectal cancer in men and women: a cohort study. PLoS ONE. 2014;9(6):e98964. doi: 10.1371/journal.pone.0098964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell PT, Jacobs ET, Ulrich CM, Figueiredo JC, Poynter JN, McLaughlin JR, Haile RW, Jacobs EJ, Newcomb PA, Potter JD. Case–control study of overweight, obesity, and colorectal cancer risk, overall and by tumor microsatellite instability status. J Natl Cancer Inst. 2010;102(6):391–400. doi: 10.1093/jnci/djq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr P, Alwers E, Bienert S, Weberpals J, Kloor M, Brenner H, Hoffmeister M. Lifestyle factors and risk of sporadic colorectal cancer by microsatellite instability status: a systematic review and meta-analyses. Ann Oncol. 2018;29(4):825–834. doi: 10.1093/annonc/mdy059. [DOI] [PubMed] [Google Scholar]
- Carr PR, Amitay EL, Jansen L, Alwers E, Roth W, Herpel E, Kloor M, Schneider M, Bläker H, Chang-Claude J. Association of BMI and major molecular pathological markers of colorectal cancer in men and women. Am J Clin Nutr. 2020;111:562–569. doi: 10.1093/ajcn/nqz315. [DOI] [PubMed] [Google Scholar]
- De Vogel S, Bongaerts BW, Wouters KA, Kester AD, Schouten LJ, de Goeij AF, de Bruïne AP, Goldbohm RA, van den Brandt PA, van Engeland M. Associations of dietary methyl donor intake with MLH1 promoter hypermethylation and related molecular phenotypes in sporadic colorectal cancer. Carcinogenesis. 2008;29(9):1765–1773. doi: 10.1093/carcin/bgn074. [DOI] [PubMed] [Google Scholar]
- Feron O. Pyruvate into lactate and back: from the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother Oncol. 2009;92(3):329–333. doi: 10.1016/j.radonc.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Fleitas T, Ibarrola-Villava M, Ribas G, Cervantes A. MassARRAY determination of somatic oncogenic mutations in solid tumors: Moving forward to personalized medicine. Cancer Treat Rev. 2016;49:57–64. doi: 10.1016/j.ctrv.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Fumagalli D, Gavin PG, Taniyama Y, Kim S-I, Choi H-J, Paik S, Pogue-Geile KL. A rapid, sensitive, reproducible and cost-effective method for mutation profiling of colon cancer and metastatic lymph nodes. BMC Cancer. 2010;10(1):101. doi: 10.1186/1471-2407-10-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbohm RA, van den Brandt PA, Dorant E. Estimation of the coverage of Dutch municipalities by cancer registries and PALGA based on hospital discharge data. Tijdschr Soc Gezondheidszorg. 1994;72(72):80–84. [Google Scholar]
- Gray RT, Cantwell MM, Coleman HG, Loughrey MB, Bankhead P, McQuaid S, O’neill RF, Arthur K, Bingham V, McGready C. Evaluation of PTGS2 expression, PIK3CA mutation, aspirin use and colon cancer survival in a population-based cohort study. Clin Transl Gastroenterol. 2017;8(4):e91. doi: 10.1038/ctg.2017.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haluska F, Pemberton T, Ibrahim N, Kalinsky K. The RTK/RAS/BRAF/PI3K pathways in melanoma: biology, small molecule inhibitors, and potential applications. Semin Oncol. 2007;34(6):546–554. doi: 10.1053/j.seminoncol.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hanyuda A, Ogino S, Qian ZR, Nishihara R, Song M, Mima K, Inamura K, Masugi Y, Wu K, Meyerhardt JA. Body mass index and risk of colorectal cancer according to tumor lymphocytic infiltrate. Int J Cancer. 2016;139(4):854–868. doi: 10.1002/ijc.30122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmeister M, Bläker H, Kloor M, Roth W, Toth C, Herpel E, Frank B, Schirmacher P, Chang-Claude J, Brenner H. Body mass index and microsatellite instability in colorectal cancer: a population-based study. Cancer Epidemiol Prevent Biomarkers. 2013;22(12):2303–2311. doi: 10.1158/1055-9965.EPI-13-0239. [DOI] [PubMed] [Google Scholar]
- Huang XF, Chen JZ. Obesity, the PI3K/Akt signal pathway and colon cancer. Obes Rev. 2009;10(6):610–616. doi: 10.1111/j.1467-789X.2009.00607.x. [DOI] [PubMed] [Google Scholar]
- Hughes LA, Schouten LJ, Goldbohm RA, van den Brandt PA, Weijenberg MP. Self-reported clothing size as a proxy measure for body size. Epidemiology. 2009;20(5):673–676. doi: 10.1097/EDE.0b013e3181a66eb5. [DOI] [PubMed] [Google Scholar]
- Hughes LA, Williamson EJ, van Engeland M, Jenkins MA, Giles GG, Hopper JL, Southey MC, Young JP, Buchanan DD, Walsh MD. Body size and risk for colorectal cancers showing BRAF mutations or microsatellite instability: a pooled analysis. Int J Epidemiol. 2012;41(4):1060–1072. doi: 10.1093/ije/dys055. [DOI] [PubMed] [Google Scholar]
- Hutton JE, Wang X, Zimmerman LJ, Slebos RJ, Trenary IA, Young JD, Li M, Liebler DC. Oncogenic KRAS and BRAF drive metabolic reprogramming in colorectal cancer. Mol Cell Proteomics. 2016;15(9):2924–2938. doi: 10.1074/mcp.M116.058925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Allison DB, Kotler DP, Ross R. Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. Am J Clin Nutr. 2002;75(4):683–688. doi: 10.1093/ajcn/75.4.683. [DOI] [PubMed] [Google Scholar]
- Jenniskens JCA, Offermans K, Simons CC, Samarska I, Fazzi GE, Smits KM, Schouten LJ, Weijenberg MP, Grabsch HI, van den Brandt PA. Energy balance-related factors and risk of colorectal cancer expressing different levels of proteins involved in the Warburg-effect. Cancer Epidemiol Biomarkers Prev. 2021 doi: 10.1158/1055-9965.EPI-21-0678. [DOI] [PubMed] [Google Scholar]
- Jenniskens JCA, Offermans K, Samarska I, Fazzi GE, Simons CCJM, Smits KM, Schouten LJ, Weijenberg MP, van den Brandt PA, Grabsch HI. Validity and reproducibility of immunohistochemical scoring by trained non-pathologists on Tissue MicroArrays. Cancer Epidemiol Prev Biomarkers. 2021;30(10):1867–1874. doi: 10.1158/1055-9965.EPI-21-0295. [DOI] [PubMed] [Google Scholar]
- Jiang W, He T, Liu S, Zheng Y, Xiang L, Pei X, Wang Z, Yang H. The PIK3CA E542K and E545K mutations promote glycolysis and proliferation via induction of the β-catenin/SIRT3 signaling pathway in cervical cancer. J Hematol Oncol. 2018;11(1):1–15. doi: 10.1186/s13045-018-0674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmelman AC. Metabolic dependencies in RAS-driven cancers. Clin Cancer Res. 2015;21(8):1828–1834. doi: 10.1158/1078-0432.CCR-14-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza G, Gafà R, Maestri I, Santini A, Matteuzzi M, Cavazzini L. Immunohistochemical pattern of MLH1/MSH2 expression is related to clinical and pathological features in colorectal adenocarcinomas with microsatellite instability. Mod Pathol. 2002;15(7):741. doi: 10.1097/01.MP.0000018979.68686.B2. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330(6009):1340–1344. doi: 10.1126/science.1193494. [DOI] [PubMed] [Google Scholar]
- Li W, Qiu T, Dong L, Zhang F, Guo L, Ying J. Prevalence and characteristics of PIK3CA mutation in mismatch repair-deficient colorectal cancer. J Cancer. 2020;11(13):3827. doi: 10.7150/jca.37437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DY, Wei L-J. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84(408):1074–1078. doi: 10.1080/01621459.1989.10478874. [DOI] [Google Scholar]
- Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Prev Biomarkers. 2007;16(12):2533–2547. doi: 10.1158/1055-9965.EPI-07-0708. [DOI] [PubMed] [Google Scholar]
- Myte R, Gylling B, Häggström J, Häggström C, Zingmark C, Löfgren Burström A, Palmqvist R, Van Guelpen B. Metabolic factors and the risk of colorectal cancer by KRAS and BRAF mutation status. Int J Cancer. 2019;145(2):327–337. doi: 10.1002/ijc.32104. [DOI] [PubMed] [Google Scholar]
- Offermans K, Jenniskens JC, Simons CC, Samarska I, Fazzi GE, Smits KM, Schouten LJ, Weijenberg MP, Grabsch HI, van den Brandt PA. Expression of proteins associated with the Warburg-effect and survival in colorectal cancer. J Pathol Clin Res. 2021;8(2):169–180. doi: 10.1002/cjp2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73(1):1–11. doi: 10.1093/biomet/73.1.1. [DOI] [Google Scholar]
- Richman SD, Adams R, Quirke P, Butler R, Hemmings G, Chambers P, Roberts H, James MD, Wozniak S, Bathia R. Pre-trial inter-laboratory analytical validation of the FOCUS4 personalised therapy trial. J Clin Pathol. 2016;69(1):35–41. doi: 10.1136/jclinpath-2015-203097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robsahm TE, Aagnes B, Hjartåker A, Langseth H, Bray FI, Larsen IK. Body mass index, physical activity, and colorectal cancer by anatomical subsites: a systematic review and meta-analysis of cohort studies. Eur J Cancer Prev. 2013;22(6):492–505. doi: 10.1097/CEJ.0b013e328360f434. [DOI] [PubMed] [Google Scholar]
- Samad A, Taylor R, Marshall T, Chapman MA. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal Dis. 2005;7(3):204–213. doi: 10.1111/j.1463-1318.2005.00747.x. [DOI] [PubMed] [Google Scholar]
- Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239–241. doi: 10.1093/biomet/69.1.239. [DOI] [Google Scholar]
- Schwartz L, Supuran C, Alfarouk K. The Warburg effect and the hallmarks of cancer. Anti-Cancer Agents Med Chem. 2017;17(2):164–170. doi: 10.2174/1871520616666161031143301. [DOI] [PubMed] [Google Scholar]
- Simons CC, Hughes LA, Van Engeland M, Goldbohm RA, Van Den Brandt PA, Weijenberg MP. Physical activity, occupational sitting time, and colorectal cancer risk in the Netherlands cohort study. Am J Epidemiol. 2013;177(6):514–530. doi: 10.1093/aje/kws280. [DOI] [PubMed] [Google Scholar]
- Slattery ML, Curtin K, Anderson K, Ma K-N, Ballard L, Edwards S, Schaffer D, Potter J, Leppert M, Samowitz WS. Associations between cigarette smoking, lifestyle factors, and microsatellite instability in colon tumors. J Natl Cancer Inst. 2000;92(22):1831–1836. doi: 10.1093/jnci/92.22.1831. [DOI] [PubMed] [Google Scholar]
- Slattery ML, Anderson K, Curtin K, Ma K-N, Schaffer D, Edwards S, Samowitz W. Lifestyle factors and Ki-ras mutations in colon cancer tumors. Mutat Res. 2001;483(1–2):73–81. doi: 10.1016/S0027-5107(01)00228-7. [DOI] [PubMed] [Google Scholar]
- Slattery ML, Curtin K, Sweeney C, Levin TR, Potter J, Wolff RK, Albertsen H, Samowitz WS. Diet and lifestyle factor associations with CpG island methylator phenotype and BRAF mutations in colon cancer. Int J Cancer. 2007;120(3):656–663. doi: 10.1002/ijc.22342. [DOI] [PubMed] [Google Scholar]
- van den Brandt PA, Goldbohm RA, Veer PVT, Volovics A, Hermus RJ, Sturmans F. A large-scale prospective cohort study on diet and cancer in The Netherlands. J Clin Epidemiol. 1990;43(3):285–295. doi: 10.1016/0895-4356(90)90009-E. [DOI] [PubMed] [Google Scholar]
- van den Brandt PA, Schouten LJ, Goldbohm RA, Dorant E, Hunen PM. Development of a record linkage protocol for use in the Dutch Cancer Registry for Epidemiological Research. Int J Epidemiol. 1990;19(3):553–558. doi: 10.1093/ije/19.3.553. [DOI] [PubMed] [Google Scholar]
- Wacholder S, Gail MH, Pee D, Brookmeyer R. Alternative variance and efficiency calculations for the case-cohort design. Biometrika. 1989;76(1):117–123. doi: 10.1093/biomet/76.1.117. [DOI] [Google Scholar]
- Warburg O. The metabolism of carcinoma cells. J Cancer Res. 1925;9(1):148–163. doi: 10.1158/jcr.1925.148. [DOI] [Google Scholar]
- Wolin KY, Yan Y, Colditz GA, Lee I. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer. 2009;100(4):611–616. doi: 10.1038/sj.bjc.6604917. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.