Abstract
Obesity and the associated metabolic syndrome is considered a pandemic whose prevalence is steadily increasing in many countries worldwide. It is a complex, dynamic, and multifactorial disorder that presages the development of several metabolic, cardiovascular, and neurodegenerative diseases, and increases the risk of cancer. In patients with newly diagnosed cancer, obesity worsens prognosis, increasing the risk of recurrence and decreasing survival. The multiple negative effects of obesity on cancer outcomes are substantial, and of great clinical importance. Strategies for weight control have potential utility for both prevention efforts and enhancing cancer outcomes. Presently, time-restricted eating (TRE) is a popular dietary intervention that involves limiting the consumption of calories to a specific window of time without any proscribed caloric restriction or alteration in dietary composition. As such, TRE is a sustainable long-term behavioral modification, when compared to other dietary interventions, and has shown many health benefits in animals and humans. The preliminary data regarding the effects of time-restricted feeding on cancer development and growth in animal models are promising but studies in humans are lacking. Interestingly, several short-term randomized clinical trials of TRE have shown favorable effects to reduce cancer risk factors; however, long-term trials of TRE have yet to investigate reductions in cancer incidence or outcomes in the general population. Few studies have been conducted in cancer populations, but a number are underway to examine the effect of TRE on cancer biology and recurrence. Given the simplicity, feasibility, and favorable metabolic improvements elicited by TRE in obese men and women, TRE may be useful in obese cancer patients and cancer survivors; however, the clinical implementation of TRE in the cancer setting will require greater in-depth investigation.
Keywords: Obesity, Metabolism, Cancer, Dietary interventions time-restricted feeding
Introduction
Obesity has reached epidemic proportions globally, with nearly 39% of adults being classified as overweight and, of these, over 600 million being categorized as clinically obese in 2020 [1]. At the current pace, nearly half of the world’s population will be overweight or obese by 2030. Currently in the USA, 60% of the population is overweight and 30% is obese [2, 3]. The implications of this epidemic on the USA and global population health are enormous, as obesity has been linked to several metabolic, cardiovascular, and neurodegenerative diseases [4]. Furthermore, obesity is associated with an increased risk for developing cancer and predicts worse outcomes for a variety of malignancies [5–7]. Obesity may also worsen several aspects of cancer survivorship, including quality of life, cancer progression and recurrence, and disease-free survival [8]. Globally, 481,000 new cancer cases are attributed to overweight and obesity according to United Nations news report in 2014, establishing excessive body adiposity as a strong risk factor for cancer development [9]. The American Cancer Society reported in 2014 that 7.8% (122,536) of all cancers and 6.5% (38,188) of all cancer deaths in the USA were attributed to excess body weight [10]. After cigarette smoking, obesity represents the second greatest modifiable risk factor in the USA. The increased risk of cancer incidence and mortality is multi-factorial, but likely related to both the innate pro-inflammatory environment, dysregulation of growth factor and hormone expression, and altered circadian rhythms that occur in obesity. For instance, chronic low-level inflammation in viral hepatitis (a disease of the liver causing inflammation), obesity, or alcohol abuse is a risk factor for liver cancer [11]; increased levels of insulin and insulin-like growth factor-1 (IGF-1) may promote the development of colon, kidney, prostate, and endometrial cancers [12]; high levels of estrogen have been associated with increased risk of endometrial, breast, and ovarian cancer [13–15]; and circadian deregulation in night shift workers or in obesity has been connected with increased risk of breast cancer [10]. Given the common co-occurrence of obesity-related risk factors in many cancer patients that affect overall survival and increases risk of death, it is logical that strategies for weight control would be beneficial for both prevention and to improve cancer outcomes. Therefore, there is an urgent need to improve cancer care beyond novel therapeutics by elucidating the effects of different weight management strategies in cancer prevention and treatment. In this regard, many observational studies have provided consistent evidence that individuals with lower weight gain or weight loss have lower risk of colon cancer, kidney cancer, and breast, endometrial, and ovarian cancer [16–19]. Weight loss through dietary interventions such as caloric restriction (CR), intermittent fasting (IF), and fasting-mimicking diets (FMD) have beneficial metabolic effects and decrease cancer risk but are difficult to maintain. Surgical approaches such as gastric bypass are also beneficial in the short-term but long-term improvements are rare. Time-restricted eating (TRE) is a popular new intervention for improved metabolic health and weight control that does not involve calorie reduction. This method is a potentially easier way to maintain optimal body weight and health over a long period as it does not require reducing total food intake, calculating daily calorie intake, or changing diet. Small clinical studies have confirmed the effectiveness of this strategy to improve overall metabolic heath [20–22]. Preclinical studies have also reported the therapeutic benefits of TRE in mouse models of cancer [23–25]. Clinical trials are just starting to explore the role of TRE in cancer so it is too early to assess whether TRE has encouraging outcomes in cancer prevention and treatment. Although TRE is a promising dietary intervention for controlling weight and improving metabolic dysfunction in overweight or obese individuals, large-scale clinical trials are still needed to confirm the benefit of TRE for metabolic health and cancer prevention. In this review, we will give an overview of obesity as risk factor for cancer and the potentially useful role of time-restricted eating in cancer prevention and treatment.
Obesity and cancer: overview of a complex relationship
Obesity is defined by a body-mass index (BMI) of > 30 and over-weight as a BMI of 25–29.9. These cutoffs have been developed based on Caucasian data and it is important to recognize that they may not hold for other groups. For example, the Asia–Pacific classification uses a BMI 23–24.9 for over-weight and > 25 as obese. Obesity has been associated with an increased incidence of a variety of cancers such as colorectal, kidney, esophagus, endometrium, breast, pancreas, thyroid, liver, ovary, gallbladder, and prostate cancer, as well as non-Hodgkins lymphoma [26, 27]. In addition, obesity is increasingly recognized as an indicator of poor prognosis as data show that obesity is associated with higher rates of cancer progression and recurrence, reduced progression-free survival, and increased mortality, especially for breast, prostate, and colon cancer [28–33]. Cancer metastasis accounts for over 90% of cancer mortality and obesity increases distal metastasis, thereby increasing the severity of the disease and mortality [34, 35]. Unfortunately, weight gain after diagnosis is common in cancer patients, especially among breast cancer patients receiving systemic adjuvant therapy [36, 37]. In a study of 535 women with newly diagnosed breast cancer, 84.1% of the patients gained weight during the first year after diagnosis and the weight gain was significantly greater in patients on chemotherapy [37]. Obesity also increases the risk of complications from cancer treatment and the risk of several comorbidities. For example, obesity is associated with an increased risk of both treatment-related lymphedema in breast cancer survivors [38] and incontinence in prostate cancer survivors who have undergone radical prostatectomy [39]. Thus, obesity represents a significant modifiable risk factor affecting cancer health worldwide.
The mechanisms underlying the cancer-promoting effect of obesity are complex and likely multifactorial. There are several potential explanations for the link between increased adiposity and worse cancer prognosis, including hormonal, inflammatory, and immune system effects. Studies have documented links between obesity and elevated levels of free circulating hormones (e.g., insulin and estradiol) and their impact on hormone-dependent cancers [15, 40–42] such as breast and prostate cancer. These differences likely underlie the reported differential effects of obesity on cancer subtypes. A large meta-analysis of breast cancer studies reported that obesity in premenopausal women is a positive risk factor for triple-negative breast cancers (TNBC, odds ratio (OR) 1.4–3.7) but a negative risk factor in estrogen receptor (ER) positive breast cancers (OR 0.35–0.81) [43]. In contrast, obesity in postmenopausal women is a positive risk factor for ER-positive breast cancer (OR 1.2–2.7) when endogenous estrogen levels are low. The detrimental effect of obesity is not limited to cancer risk however the American Cancer Society Prevention Study II of 495,977 women reported an association of BMI and BrCa mortality. Women with BMI > 30 kg/m2 had > 65% increase in mortality [5]. In the UK Prospective Study of Outcomes in Sporadic and Hereditary Breast Cancer (POSH) study of 2,956 young (aged < 41 yrs) breast cancer survivors, obesity was associated with larger tumors, positive lymph node status, and higher percentage of TNBC. Overall (8-year) survival and disease-free interval were significantly shorter [44]. Lastly, a meta-analysis of 82 studies including 213,075 breast cancer survivors found a 40% increased risk of mortality due to obesity in both pre- and post-menopausal women [45]. These observations might indicate that dietary interventions to reduce obesity may only be beneficial in selected cancer subtypes, but obesity has a detrimental effect on mortality in all subtypes of breast cancer, so one cannot be guided by cancer risk analyses alone.
Obesity has been linked to increases in estradiol due to aromatase expression in adipose tissue [46]. In the HEAL study of 505 postmenopausal women with stage 0-IIIA breast cancer, adiposity was positively associated with circulating levels of estrone and estradiol [47]. A combined meta-analysis of nine cohort studies, which included data from 663 breast cancer cases and 1,765 women without breast cancer, found that postmenopausal women with serum hormone concentrations in the top quintile for androstenedione, testosterone, dehydroepiandrosterone (DHEA), and DHEA-sulfate were nearly twice as likely to develop breast cancer in comparison to women with serum hormones in the bottom quintile [48]. In the same analysis, a doubling of androgen concentration resulted in a 20% to 40% increase in risk for breast cancer. Other hormones have also been implicated. One of the best documented effects of obesity is to cause hepatic insulin resistance that triggers a compensatory increase in insulin secretion to maintain normoglycemia. This results in fasting hyperinsulinemia. Other tissues including tumors do not become insulin resistant so are exposed to elevated insulin levels. Hence, increased signaling via insulin and IGF-1 receptors, and the downstream phosphatidylinositol 3-kinase pathway, are observed in diverse cancers [49]. For example, in non-diabetic breast cancer patients, higher levels of fasting insulin have been associated with a 2–threefold increased risk of mortality [50–54]. Similarly, the Women’s Health Initiative Observational Study (WHI-OS) of 93,676 postmenopausal women, insulin levels were associated with a > 2.4-fold increase in breast cancer risk in women not on hormone-replacement therapy [55]. The increased risk may be restricted to postmenopausal women as the Nurse’s Health Study II of 29,611 women did not show an association of insulin with breast cancer incidence [56]. Elevated insulin levels may also be associated with cancer progression. Additionally, fasting insulin levels were significantly associated with both distant recurrence and death. In a study, women in the highest quartile of insulin levels had a 2.1 times increased risk of distant recurrence compared to those in the lowest quartile (95% CI = 1.2–3.6, P = 0.01) and a 3.3 times greater risk of death (95% CI = 1.5–7.0, P = 0.002) [52]. Similar findings are reported for colorectal cancer [57]. A meta-analysis of all cancer deaths in non-diabetics reported that fasting serum insulin was associated with increased mortality (HR 1.92) in men [58] and the French TELECOM study reported that elevated fasting insulin posed increased risk of cancer death (HR 2.30) in men over a 28-year follow-up [59].
Chronic tissue inflammation is a feature of obesity. Inflammation in itself makes individuals susceptible to many forms of cancer as it has been linked to different steps involved in tumorigenesis, including transformation of normal cells to cancerous cells, survival, proliferation, promotion, invasion, angiogenesis, and metastasis [60]. Immune cells such as tumor-associated macrophages, tumor-associated dendritic cells, and pro-inflammatory cytokines and chemokines are key players in initiating inflammation creating a pro-cancer microenvironment [61]. Obesity is associated with inflammatory markers including C-reactive protein, serum amyloid A, interleukin-6, interleukin-1, and tumor necrosis factor alpha, and importantly some of these are higher in patients with metastatic cancer compared with patients without cancer and with those with early cancer [2].
Circadian disruption in obesity and cancer
Circadian rhythms in physiology, metabolism, and behavior are vital part of homeostasis [62]. These rhythms occur from interactions between circadian clocks within brain and peripheral organs with cycles in light and dark, sleep and activity, and eating and fasting. Notably, obesity and its associated eating patterns have been shown to alter the circadian clocks in both the brain and peripheral tissues that generate 24 h rhythms in gene expression and diurnal behaviors [63–66]. Interestingly, daily rhythms in gene expression modulate several key aspects of cellular and tissue function with profound implications in disease prevention, and disease management including genes involved in glycolysis, gluconeogenesis, protein synthesis, lipid synthesis and oxidation, and mitochondrial function [67]. Acute circadian disruption can exacerbate chronic diseases, while chronic circadian disruption raises the risk for numerous diseases [62]. For example, forced circadian misalignment is associated with increased risk for obesity, diabetes, and cardiovascular disease. In a study involving ten adults (5 female) for 10-days, subjects were subjected to an artificial 28-h day, so they ate and slept at all phases of the circadian cycle during the 10-day stay. Subjects ate 4 isocaloric meals each 28-h day. When subjects ate and slept approximately 12 h out of phase from their normal 24-h circadian rhythms, increased both blood glucose and insulin (indicating insulin resistance), increased mean arterial pressure, reversed the daily cortisol rhythm, and reduced sleep efficiency. Notably, 3 of the 8 subjects developed a prediabetic state by this circadian misalignment [68].
Circadian clock disruption has been reported in some cancers and this is thought to promote tumor growth, owing to the dysregulation of key cell-cycle and tumor suppressor genes that are under clock control [69, 70]. In general, arrhythmic mice are susceptible to a variety of cancers [71–73]. In lung cancer, deletion of clock genes increases mutant Kras lung tumorigenesis [74]. Mechanistically, the loss of core clock gene components such as Per2 and Bmal1 leads to increased c-Myc expression, enhanced proliferation and metabolic dysregulation. A number of studies point to the role of MYC in both circadian disruption and cancer as it is a key player in cancer metabolism [75]. Deregulated expression of MYC or N-MYC disrupts the molecular clock by directly inducing REV-ERBα to dampen expression and oscillation of BMAL1, and both REV-ERBα and BMAL1 have key roles in N-MYC-driven human neuroblastomas. Importantly, these studies suggest a link between oncogenic transformation and circadian and metabolic dysrhythmia, which could be advantageous for cancer growth. In a similar study, overexpression of MYC in U2OS cells, severely attenuates circadian oscillations and promotes cell proliferation [76]. The authors showed that inhibition of the circadian clock was dependent on the formation of repressive complexes of MYC with MIZ1 leading to downregulation of the core clock genes CLOCK, BMAL1 and NPAS2. Interestingly, cancer stem cells display robust circadian rhythm with exquisite dependency on core clock transcription factors, BMAL1 and CLOCK, for optimal cell growth. It has been demonstrated that knockdown of either BMAL1 or CLOCK has been observed to induce cell cycle arrest and apoptosis in cancer stem cells in a patient-derived glioblastoma cell or murine leukemia stem cells in acute myeloid leukemia [77, 78]. Circadian disruption can also create a pro-tumor environment in the host. Chronic jet lag in mice induces persistent deregulation of liver gene expression and metabolism, promoting the development of spontaneous hepatocellular carcinoma [79]. Tumors may also influence normal circadian rhythms as Masri et al. demonstrated that lung cancer reprograms hepatic metabolism by rewiring hepatic circadian rhythms in gene expression and metabolites [80].
Epidemiological studies have also linked circadian disruption and clock genes to increased susceptibility to cancer development of diverse tissue types [for reviews see refs [81–86]. For example, there are several links between circadian clocks and breast cancer [71, 73, 87]. Women with SNPs in CRY2, NPAS2, and CLOCK are at a higher risk of breast cancer [88–90], and PER2 suppresses estrogen receptor-dependent transcription [73, 91, 92]. Low-grade and non-metastatic breast tumors have functional clocks, but aggressive carcinomas are arrhythmic [93]. Low CRY2 and PER1/2 expression is correlated with ER negativity, higher tumor grade and shorter overall survival in breast cancer patients [94, 95]. Breast cancer patients have higher methylation of the CRY2 promoter consistent with lower CRY2 expression [96] and loss of PER3 and CRY2 co-expression increases metastasis risk [93]. In hematological malignancies, BMAL1 expression levels correlate inversely with MYC levels [76], the PER genes are downregulated in CLL [97], NPAS2 is up-regulated in AML patients [98], and the CRY genes show both up- and down-regulation in CLL and AML [99, 100]. Similar associations have been reported in other cancers, including head and neck [101], colorectal cancer [83, 102], liver cancer [103], and lung cancer [104, 105] to name but a few. Overall, the accumulated data point to the importance of circadian rhythms in normal health and suggest that interventions to normalize disrupted rhythms in obesity and cancer could be beneficial.
Obesity management in cancer
Several methods for weight loss or control have been tested in the general population [106], including diets, exercise, and bariatric surgery [107–110]. Dietary interventions have received a lot of attention in both the scientific and lay community as a result of successful results in experimental animal models [111, 112]. The limited human data are consistent with the animal data. Sustained weight-loss after the age of 50 measured over 10 years reduces the risk of breast cancer (HR 0.68–0.82), whereas stable weight or short-term weight loss over one 5-year interval does not reduce risk [17]. This observation underscores the need for an intervention that is sustainable over a long period. Strong evidence for a causal link between obesity and cancer comes from bariatric surgery studies. Weight loss through bariatric surgery reduces the risk of colon, endometrial, pancreas, and pre-menopausal ER-negative and post-menopausal ER-positive breast cancer [113, 114]. Dieting or caloric restriction for weight loss can also prevent cancer. Experimentally, CR involves a 30% reduction in the daily caloric intake with the usual timing of meals [111] and CR without malnutrition remains the most robust intervention to date for cancer prevention in rodents, monkeys, and humans [111]. CR promotes anti-carcinogenic adaptations such as decreased production of inflammatory cytokines, growth factors, and anabolic hormones as well as decreased oxidative stress and DNA damage [115]. Despite of an abundance of the literature on the mechanisms and impact of CR, its clinical applicability remains limited because of challenges in long-term sustainability as most people regain weight lost during CR. Considering difficulties maintaining weight loss with CR, adopting a healthy diet to promote weight loss has been tested. A healthy diet, either with or without physical activity, however, does not alter disease-free survival or mortality in breast cancer [116]. Although physical activity does not alter cancer outcomes, there is evidence for a beneficial effect on quality-of-life, depression, anxiety, lymphedema, and fatigue [117].
Health benefits of time-restricted eating
There has been growing interest in intermittent fasting as an alternative to CR because of promising results in experimental animal models [112]. According to a survey by the International Food Information Council Foundation, IF has become the most popular dietary intervention and many cancer patients are seeking advice from oncologists about its beneficial effect for cancer prevention and treatment [118]. IF can take various forms, including alternate day fasting with 0–25% of normal daily calories on the fasting days, the 5:2 method with 2 days of 25% calorie intake every 5 days of normal eating, periodic fasting (calorie intake is restricted for multiple consecutive days, such as 5 days, once a month, and unrestricted on all other days), Sunnah fasting (fasting every Monday and Thursday), and many other variations. Preclinical studies have shown beneficial effect of IF on tumor growth. In p53-deficient cancer mouse model, a 1 day per week IF regimen delayed tumor onset, significantly reduced tumor metastasis, and improved overall survival [119]. A study in a human xenograft prostate cancer model, an IF regimen comprised of 2 separate 24-h fasting periods per week exhibited similar trends toward delayed tumor growth and improved survival compared to an iso-caloric control group [120]. When combined with a fasting-mimicking diet, IF blocks TNBC and cancer stem cell escape in mice [121]. Interestingly, several short-term randomized clinical trials have indicated promising effects of alternate day fasting or a 5:2 diet in improving some cancer risk factors, including decreased fasting glucose, insulin, and leptin levels and increased adiponectin [22]. A small nonrandomized study of 23 women at increased risk for breast cancer found that IF for 2 days per weeks resulted in 4.8% reduction in body weight, an 8.0% reduction in body fat, and an improvement in insulin resistance over 4 to 5 weeks [122]. Similarly, IF of a ketogenic diet in patients with grade 2–4 astrocytoma decreased body mass and insulin levels [123]. IF for 24 h before and after chemotherapy reduced hematologic toxicity and promote recovery of chemotherapy-induced DNA damage [124]. IF also improved quality of life in cancer patients undergoing chemotherapy [125, 126].
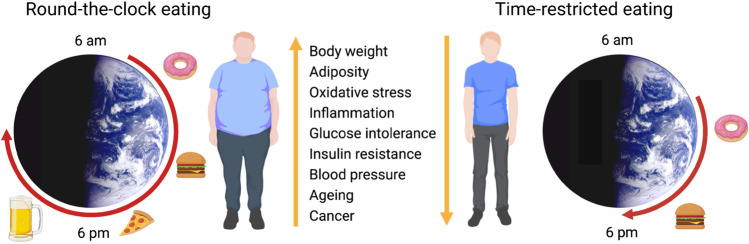
While IF emphasizes the ratio of fasting/feeding durations, time-restricted eating emphasizes the timing of eating within a limited window without involving CR. TRE is a type of intermittent fasting, which involves consuming all calories within a consistent 8–12 h daily window based on the normal circadian rhythm of eating (Fig. 1) [62, 67, 127]. TRE (also called time-restricted feeding or TRF in mice) improves metabolic health in animal models and potentially in humans and may facilitate adherence and long-term weight loss maintenance as it doesn’t involve calorie counting [22, 23, 128–130].
Fig. 1.
Health benefits of time-restricted eating
Mouse Studies on Time-Restricted Eating
The metabolic benefits of TRE were first demonstrated in mouse model of diet-induced obesity [131]. Mice were given 8-h access to a high-fat diet (HFD) during the night (TRE), which is when mice are active, and compared to mice with 24 h access to food. The mice were protected against obesity, fatty liver, hyperinsulinemia, and inflammation and had enhanced motor coordination. Interestingly the mice on the TRE regimen consumed equivalent calories as those with ad libitum access. Furthermore, the TRE regimen improved mTOR, and AMPK signaling and enhanced circadian oscillations of core clock genes. These studies have been expanded to a variety of obesogenic diets and TRE at night prevented obesity and metabolic diseases without reducing caloric intake. The response showed a time-dependence with better effects with a 9-h feeding window compared to 12 or 15 h of feeding [132]. Interestingly, the protective effects were still maintained when TRE was interrupted by ad libitum access to food during weekends, a modified 5/2 regimen that is especially attractive for human lifestyle. Many studies, including ours, have demonstrated a similar beneficial effect of TRE in various mouse models to improve metabolic profiles [23–25, 133]. The metabolic improvement observed with TRE without any weight loss has led to the presumption that eliciting a daily fasting response, or at certain times of the day, is in itself beneficial. This would explain why dietary dilution, a form of CR in which mice eat all day to compensate for the low density of energy in their diet, does not result in lifespan extension. By the same argument, CR may improve health, at least in part, through an extended period of fasting. When considering TRE, it is important to recognize that meal-timing and circadian synchronization influences the metabolic effects. In a recent study, TRE extended the lifespan of Drosophila and was able to delay the onset of aging when flies were fasted during the night rather than during the day [134]. In mice, providing food during the first half of the active phase (earlyTRE) was more beneficial than providing during the second half (lateTRE) [135], and providing food during the day, rather than at night, disrupts liver circadian rhythms [136]. Timing of feeding may also extend the lifespan of mice on CR as Acosta-Rodriguez et al. demonstrated that CR with food provided for 12 h during the dark phase extended life span by 35% in C57BL/6 J mice whereas CR alone only extended the life span by 10% [137] and furthermore ameliorated the aging-associated changes in gene expression. In mice, TRE can impart benefits irrespective of nutrition quantity and quality and seems to be both preventive and therapeutic for aging and metabolic diseases [138].
Human Studies on Time-Restricted Eating
Human data show a similar improvement in whole body metabolism (Table 1). For example, an isocaloric trial of TRE in pre-diabetic men for 5 weeks showed an improvement in glucose tolerance and a major decrease in systolic and diastolic blood pressure [20]. Another isocaloric study evaluating acute TRE for only 4 days showed a decrease in the average blood sugar level and reduced insulin resistance [139]. Likewise, a crossover-randomized trial [140] demonstrated that short-term TRE improved nocturnal glycemic control. Studies also support the impact of meal timing on metabolic health and indicate that eating at night is detrimental as it predisposes to obesity and metabolic dysregulation [141, 142]. For instance, women with metabolic syndrome on a daily three-meal schedule showed greater weight loss and metabolic improvement when the primary meal was at breakfast compared to women whose primary meal was at dinner [142]. In a small study with 19 men and women with metabolic syndrome, 10-h TRE reduced weight, blood pressure, and atherogenic lipids [130]. So, beneficial metabolic effects are seen in both sexes, which is consistent with studies in obese mice. Although, many metabolic studies support the beneficial effect of eating earlier in the day, not all studies support this idea. Evening protein ingestion leads to increased whole body and muscle protein synthesis [143], so TRE might not be advisable for sarcopenic patients. The effect of meal timing may even augment the impact of CR, as subjects in a weight-loss program who ate their main meal earlier in the day achieved greater weight loss than those subjects who ate later in the day [144], and in a separate study combined TRE and CR gave greater weight loss than CR alone although did not quite reach significance with the number of subjects studied [145]. Most human studies have focused on synchronizing the peripheral metabolic clocks to the central light-driven clock. It would be important to try TRE in individuals on night-shift workers with forced out-of-phase central and peripheral clocks or individuals with circadian rhythm sleep disorders [146] as mouse studies have shown desynchronization between central and peripheral clocks if food is provided during the daytime [147].
Table 1.
A list of recent Time-Restricted Eating trials in humans and their key outcomes
| Study Design | Duration | TRE Intervention | Participants | Age | Outcome | Study |
|---|---|---|---|---|---|---|
| Randomized control | 12 weeks | TRE: 10 h, 8 am-6 pm |
n = 60, diabetic |
18–70 yr |
↓ Body weight, HbA1c ↑ Insulin sensitivity |
[148] |
| Randomized control | 8 weeks | TRE: 10 h, |
n = 60, obese |
18–65 yr | ↓ Body weight, Fasting glucose, | [149] |
| Randomized control | 12 weeks | TRE: 8 h | n = 20 (17 females, 3 males), overweight | 33–58 yr | ↓ Body weight, lean mass, and visceral fat mass | [22] |
| Longitudinal | 12 weeks | TRE: 8 h (10 am–6 pm) | n = 14, overweight | 25–65 yr |
↓ Body weight, fat mass, systolic blood pressure ↔ Gut microbiome |
[150] |
| Cross-over | 5 days |
TRE: 8 h (10 am–6 pm) Extended eating: 15 h (7 am–10 pm) |
n = 11 males, overweight | 32–43 yr |
↓ Night-time glucose, glucose and insulin iAUC after lunch ↔ Daytime glucose ↑ TG after lunch |
[140] |
| Longitudinal | 12 weeks |
TRE: 10 h (self-selected, dinner before 8 pm): Baseline: ≥ 14 h |
n = 19 (6 females, 13 males), overweight | 48–70 yr |
↓ Body weight, fat mass, waist circumference, blood pressure, plasma cholesterol ↔ Fasting glucose, HbA1c, HOMA-IR, fasting insulin |
[130] |
| Longitudinal | 4 weeks | TRE: 8 h | n = 10 (6 females, 4 males), overweight, | ≥ 65 yr |
↓ Body weight ↑ Quality of life |
[151] |
| Longitudinal | 13 weeks | TRE: 8–9 h | n = 40 (31 females, 9 males), with abdominal obesity | 36–62 yr | ↓ Waist circumference, HbA1c | [152] |
| Randomized control | 8 weeks |
TRE: 8 h (12 pm–8 pm) TRE plus β-hydroxy β-methyl butyrate |
n = 40 females, resistance trained normal weight |
18–30 yr |
↓ Fat mass ↑ Muscle performance |
[153] |
| Cross-over | 4 days | TRE: 6 h (8 am–2 pm) | n = 11 (4 females and 7 males), overweight | 25–39 yr |
↓ Mean 24-h glucose, glycemic excursions, morning ghrelin, desire to eat ↑ metabolic flexibility, fullness, plasma ketones, fat oxidation |
[139, 154] |
| Cross-over | 1 week |
Early TRE: 9 h (8 am–5 pm) delayed TRE: 9 h (12 pm–9 pm) |
n = 15 males, overweight | 52–58 yr |
↓ Body weight, fasting TG, and hunger ↓ Mean fasting glucose by CGM in eTRE ↑ Glucose tolerance |
[21] |
| Cross-over | 5 weeks | TRE: 6 h (8 am–2 pm, dinner before 3 pm) | n = 8 males, overweight | 47–65 yr |
↓ Fasting TG, desire to eat in the evening ↑ Insulin sensitivity, β cell responsiveness ↔ Body weight |
[20] |
| Historical control | 12 weeks | TRE: 8 h (10 am–6 pm) | n = 23 (20 females, 3 males), obese | 25–65 yr |
↓ Body weight and blood pressure ↔ Fat mass, fasting glucose, LDL cholesterol, TG |
[155] |
| Randomized control | 8 weeks | TRE: 4 h (anytime 4 pm to midnight) for 4 days a week | n = 18 resistance trained males normal weight | 18–27 yr | ↔ Body weight, fat mass | [156] |
| Randomized control | 8 weeks | TRE: 8 h (1 pm–8 pm) | n = 34 males, normal weight | 25–33 yr | ↓ Fat mass, fasting glucose, fasting insulin, total testosterone, IGF-1, inflammation | [157, 158] |
| Longitudinal | 16 weeks | TRE: 10–11 h (self-selected) | n = 8 (3 females, 5 males), overweight | > 18 yr |
↓ Body weight Improved sleeping |
[159] |
| Cross-over | 7 days |
eTRE 70% calories before 5 pm vs TRE 8 h window vs ADF |
n = 32 (25 females, 8 males), obese | mean age 45.7 yr | No difference in weight loss between diets. TRE easiest to follow | [160] |
| Longitudinal | 12 weeks | eTRE + 35%CR 10 h (self-selected) vs 35% CR alone | n = 81 (69 females, 12 males | mean age 38 yr | No difference in weight loss | [161] |
| Longitudinal | 12-weeks |
TRE 8 h (self-selected) + CGM vs Control group 12 h |
n = 50 (14 males, 36 females), obese | 14–18 yr | No difference in weight loss | [162] |
| Longitudinal | 5-weeks |
eTRE 8 h (6am-3 pm) vs mTRE 8 h (11am-8 pm) vs Control |
n = 82 (64 females, 18 males), normal weight | mean age 31 | Weight loss and improved HOMA-IR in eTRE group | [163] |
| Longitudinal | 10-weeks | TRE 10 h (8am-6 pm) | n = 15 (males), overweight | 40–70 yr |
↓ Body weight Improved GTT, ↓ Fasting glucose, HbA1c |
[164] |
| Longitudinal | 8-weeks | TRE 8 h (10am-6 pm or 12 pm-8 pm) vs Control | n = 30 (females), normal weight | 40–65 yr |
↓ Body weight ↓ Diastolic BP |
[165] |
| Cross-over | 3-days | early dinner (6 pm) vs late dinner (9 pm) | n = 12 (2 males, 10 females) | > 20 yr |
↓ Mean 24 h glucose ↓ RQ after breakfast |
[166] |
| Cross-over | 4-weeks | TRE 8 h (1 pm-9 pm) | n = 12 (males), healthy | mean age 22 yr |
↑ Exercise performance ↑ Fat-free mass |
[167] |
| Longitudinal | 3-months | TRE 10 h (10am-7 pm) | n = 50 (41 females, 9 males), overweight | 30–75 yr |
↓ Body weight ↓ Systolic BP |
[168] |
| Longitudinal | 8-weeks |
TRE 8 h (12 pm-8 pm) + Exercise vs Exercise alone |
n = 21 (18 females, 3 males), overweight | 35–60 yr |
↓ Body weight ↓ Fat mass |
[169] |
| Longitudinal | 12-weeks | TRE 8 h | n = 20 (17 females, 3 males), overweight | mean 45 yr | ↑ Quality of life | [170] |
| Longitudinal | 6-weeks | TRE 8 h (8am-4 pm) | n = 18 women with PCOS | 18–31 yr |
↓ Body weight ↓ Fat mass ↓ Fasting insulin ↓ HOMA-IR |
[171] |
| Longitudinal | 12-weeks | TRE 8 h | n = 20 (17 females, 3 males), overweight and obese | 18–65 yr | ↑ Bone mineral content | [172] |
| Longitudinal | 6-months | TRE 12 h (self-selected) vs standard dietary advice | n = 213 (152 females, 61 males), normal to overweight | > 18 yr | ↓ Body weight in TRE group | [173] |
| Longitudinal | 10-weeks |
TRE 4 h (3-7 pm) vs TRE 6 h (1-7 pm) vs Control |
n = 58 (53 females, 5 males), obese | > 18 yr | ↓ Body weight and insulin resistance in TRE groups, no diff 4 h vs 6 h | [174] |
| Longitudinal | 12-weeks | TRE 8 h (self-selected) | n = 51 (37 females, 14 males), obese | > 18 yr | ↓ Body weight in TRE group | [175] |
↓ reduced; ↑increased; ↔ no change; iAUC, incremental area under the curve; BP, blood pressure; PCOS, poly-cystic ovary syndrome
Time-restricted eating and cancer
Given that TRE improves metabolic health in obese animals and humans, it might be expected to have anti-cancer effects in obesity-driven cancers. This has been borne out in a few rodent studies that evaluated the effect of TRE in modulating cancer risk or progression. In a recent study using mouse postmenopausal breast cancer models, our group reported that TRE, in the absence of caloric restriction or weight loss, could effectively inhibit the accelerated tumor initiation, progression, and metastasis due to obesity in comparison with mice with 24-h access to food. This beneficial effect of TRE was mediated, in part, by reduced insulin signaling as systemic insulin infusion through implanted pumps reversed the TRE-mediated protection and reducing insulin secretion mimicked the protection [23]. Sundaram and Yan have also shown that TRE of high-fat diet prevented cancer in the same transgenic MMTV-PyMT model of spontaneous breast cancer [24]. This group also demonstrated that TRE prevented high-fat diet enhanced metastasis in a subcutaneously injected Lewis lung cancer mouse model [25]. Aging increases the risk of cancer, and it has been proposed that the aged tissue microenvironment provides a pro-neoplastic niche. A recent study demonstrated that TRE could prevent the aging-associated changes in microenvironment and consequently decreases the growth of transplanted pre-neoplastic hepatocytes [176]. Colorectal cancer is also sensitive to the intestinal microenvironment and dysregulation of the gut microbiome has been connected to the pathogenesis of colorectal cancer. TRE was recently shown to improve the gut microbiota and prevent colon cancer [177]. Not all cancers respond to TRE however. Turbitt et. al. tested whether TRF alone or combined with anti-CTLA-4 immunotherapy would reduce tumor growth a murine model of kidney cancer. They found that TRF alone did not reduce tumor growth or metastasis in lean chow-fed or obese HFD-fed mice. Immune-checkpoint therapy had no effect in chow-fed mice but did reduce tumor growth in normal weight and obese mice on HFD irrespective of TRF [178]. Similarly, mice harboring LAPC-4 prostate cancer tumors did not show decreased tumor growth or increased survival [179].
As large prevention studies are lacking, most human studies to date have been epidemiological studies or small studies focused on assessing cancer biomarkers. In the Women’s Healthy Eating and Living study on a cohort of 2413 women with breast cancer, there was a significant increase in the risk of breast cancer recurrence with fasting < 13 h per night compared to fasting > 13 h per night (hazard ratio, 1.36; 95% CI, 1.05–1.76) [180]. An analysis of the NHANES data showed that each 3-h increase in night-time fasting was associated with improved glucose regulation and a decrease in hemoglobin A1c [181]. A case–control study in 922 Chinese women with incident BrCa and 913 controls [182] reported that eating after 10 pm was significantly associated with increased risk of breast cancer (OR 1.50). The association was strongest in women who had > 20 year history of eating after 10 pm (OR 2.28). A population case–control study of 1205 breast cancers and 621 prostate cancers in 1321 women and 872 men in Spain reported that a longer interval between the last meal and sleep was associated with lower cancer risk (prostate OR 0.74, breast OR 0.64)[183]. Similar protection was reported if meal eaten before 9 pm vs after 10 pm (OR 0.75 & 0.85) and in morning chronotypes (OR 0.65 & 0.67). As mentioned earlier, obesity causes hyperinsulinemia that can drive tumor growth and reducing insulin levels in mouse models inhibits tumor growth. Indeed, most of the obesity-associated increased risk for breast cancer can be accounted for by the increased risk due to the hyperinsulinemia [184–186]. Several small TRE studies have reported reductions in insulin resistance, and by inference insulin levels, that would be expected to reduce cancer risk [20, 187]. Breast cancer risk is also linked with hypertension, with several studies reporting a 7–38% higher risk of breast cancer among women with hypertension [188]. A meta-analysis of six TRE studies with 97 participants showed clinically significant decreases in systolic and diastolic blood pressure [128, 189]. All these epidemiological and observational studies support the potential beneficial role of TRE in cancer. Nonetheless, these findings strongly suggest that more TRE studies are needed to better understand the underlying mechanisms and differences in outcomes before clinicians may start to consider safely and confidently prescribing TRE for the treatment of cancer in humans.
What are the mechanisms underlying the beneficial effect of time-restricted eating?
As discussed earlier, obesity is tightly linked to the metabolic syndrome which is a collection of metabolic disturbances including hyperglycemia, hyperinsulinemia, dyslipidemia, and hypertension, many of which have been linked to cancer [190]. In a recent review, Mattson et al. discussed the metabolic and physiological responses to CR, IF, and TRE, and highlighted the importance of four mechanisms including the adaptive stress response to oxidative damage, the bioenergetics or normal and cancer cells, suppression of inflammation, and induction of autophagy to remove or repair damaged organelles [191]. Many of these pathways also have relevance to cancer development. Post-prandial hyperglycemia may provide excess glucose to cancer cells to support their rapid growth since many cancer cells are more glycolytic than normal cells [12, 192]. Hyperglycemia can cause overproduction of advanced glycation end-products and reactive oxygen species, which can cause DNA damage and may initiate cancer. Obesity can also cause oxidative stress through increased mitochondrial oxidation of lipids [193–196] and preliminary evidence suggests that TRE may reduce oxidative stress in men [20]. At the metabolic level, hyperinsulinemia increases the risk of both cancer incidence and death [197, 198]. This increase of cancer mortality is also observed in non-obese people with hyperinsulinemia [199]. Indeed, we recently demonstrated that TRF acts by correcting insulin resistance to prevent and inhibit breast tumor growth in mouse models of breast cancer [23]. Furthermore, obesity and diabetes alters the production of endotrophin, leptin, adiponectin, angiopoietins, bone morphogenic proteins, and other adipokines, which can also affect cancer cell growth and survival [200–204]. For example, endotrophin, which is a carboxy-terminal proteolytic cleavage product of collagen 6α3, is overexpressed in obesity, enhances progression of breast and liver cancer, enhances epithelial-mesenchymal transition, and causes chemoresistance [205–207]. As discussed earlier, obesity creates a state of sub-clinical, chronic tissue inflammation with immune cell infiltration due to elevated adipocyte inflammatory cytokine production [208, 209]. Such local inflammatory changes in the microenvironment have been shown to accelerate tumor initiation and growth [60, 61]. TRE reduces tissue macrophage infiltration and inflammation in mouse models [23, 131, 135, 210, 211]. Some human studies have shown that restricting food intake to 8 h, or a longer nighttime fast, significantly decreases proinflammatory markers [157] but other studies have not seen any changes in these markers [174, 212, 213].
In addition to the above-mentioned metabolic/inflammatory mechanisms, another mechanism to consider is circadian realignment. Most time-restricted eating protocols involve limiting food intake to a prescribed window, usually 6–10 h, but the timing of this window is also important. TRE during the normal active phase is more beneficial than TRE during the inactive phase in both animal and human studies. In-phase TRE reinforces the normal circadian rhythms of nutrient dependent clock genes, but out-of-phase TRE causes a phase shift in the normal oscillations. The circadian clock is essential for normal metabolic regulation and disruption of the clock causes obesity and insulin resistance [214–217]. Disruption of the clock also causes abnormal cellular division and promotes tumorigenesis [62, 69]. Indeed, clock genes have been implicated in cancer as many tumors are acyclic with deficient endogenous clocks [93, 218, 219], circadian gene variants are associated with cancer [89, 220], clock genes regulate oncogene expression and suppresses oncogenic signaling [221–223], and oncogenes regulate clock gene expression [224]. Our group has demonstrated that many of the disrupted tumor circadian rhythms were restored by TRE to patterns found in the normal tissues suggesting that TRE might suppress tumorigenesis by regulating tumor clock genes [23]. Despite the strong connection between circadian clock genes and cancer, no studies have shown a causative link between TRE-induced clock gene rhythms and tumor inhibition.
Time-restricted eating safety
Fasting has been safely practiced by individuals in various religious practices. For instance, over the 30 days of Ramadan, individuals fast from dawn-to-dusk which varies up to 21 h per day depending on latitude, and in Judaism individuals routinely undertake 25 h fasts [212, 225–227]. TRE is distinct from these religious fasts as the long fasting period is overnight rather than during the day, so is less associated with hunger. TRE also does not require total withdrawal from food and drink, as water and other zero-calorie beverages are allowed. Importantly, TRE has been reported not to cause major adverse events or negatively impact eating disorder symptoms among adults with obesity, metabolic syndrome, diabetes [128, 140], or pre-diabetes [20, 129], and TRE with a daytime feeding time window of 8 h does not cause occurrences of hypoglycemia, nor cause depression, anxiety or stress [228]. TRE has proven to be a more effective, safe, and convenient strategy than CR diet to lose weight [229, 230]. In obese individuals, TRE preserves healthy muscle in contrast to CR that causes 20–35% muscle loss [231–234]. This is an important finding, because weight loss interventions typically result in concomitant decreases in both fat and lean body mass [156, 157]. However, safety studies of longer duration are needed before recommending TRE as a healthy lifestyle intervention for body weight control. Furthermore, TRE may not be suitable for everyone, especially those with underlying metabolic conditions. Adhering to a TRE diet is likely not wise for type 1 diabetics, since metabolic switching, which can occur with TRE, may lead to diabetic ketoacidosis [235]. Similarly, the potential use of TRE in pediatric intensive care units may be complicated by the susceptibility of newborns and infants to fasting-induced ketogenesis [236]. People with impaired liver function may also be particularly sensitive to TRE [237, 238].
Time-restricted eating feasibility and adherence
TRE is a new treatment strategy for weight control, metabolic improvement, and diverse disease prevention without calorie reduction [190]. This method is an easier approach to maintain optimal body weight and health for a longer time because patients do not need to reduce total food intake, or calculate total daily calorie intake, or change the composition of their diet. Clinical studies have confirmed the effectiveness of this strategy. Dorothea et al. have reported that, 86% of participants achieved their weight target during the 3-month study period and TRE was well accepted by participants [152]. Studies in humans and animal models have reported the beneficial effects of TRE on obesity, diabetes, fatty liver, cardiometabolic dysfunctions, and lifespan [155, 239]. Several key features of TRE promote adherence relative to CR or other forms of IF. As TRE follows a cycle of fasting during the night with an 8–10 h eating window during the day with no calorie restriction, it may require less cognitive effort and facilitate dietary satisfaction. Additionally, TRE may reduce conflict with the homeostatic drive to eat and prevent dietary lapses resulting from prolonged negative energy balance [240]. In a large, randomized controlled trial of TRF in 116 overweight and/or obese men and women, high adherence to the TRF protocol (8-h feeding window) was reported [241]. Follow-up data from two small TRE trials reported promising data that subjects continued TRE even after the trial period had ended. In one study, long-term follow-up ~ 16 months after the end of the study reported that > 60% of the participants were still practicing some form of TRE [130]. In another study, it was reported that all participants were still doing TRE and maintained their weight loss one year after the end of the study [159]. While these observations are anecdotal, they do support the idea that TRE is easy to adopt and maintain. Long term adherence is very important if TRE is to have any preventative value for cancer, as Teras et al. found that sustained weight loss over two successive 5-year periods was needed to show a decreased risk of breast cancer, weight loss of a single 5-year period did not show a protective effect [17]. Although TRE may be easy to maintain once adopted, there are potential barriers to trying TRE in the general population. Work and family schedules may make adherence to a strict eating window difficult. Luckily, the animal data has shown that the benefits of TRE are maintained even if performed only during the week. Human data are lacking, but if this finding holds true, a five day "weekends-off" TRE regimen may prove attractive allowing participation in social events while maintaining adherence to TRE [242].
Conclusion and future directions
In conclusion, TRE is a promising therapeutic strategy for controlling weight and improving metabolic dysfunctions in those who are overweight or obese. As obesity represents a potential risk factor in cancer development and outcome, strategies that effectively modify obesity could potentially be harnessed as a means of cancer control. Preclinical studies support the potential beneficial effect of TRE in cancer prevention and growth. While definitive clinical trials showing the long-term effect of TRE on cancer prevention, treatment, and outcome are under investigation (Table 2), short-term TRE strategies for weight control may be helpful for some cancer patients and survivors. On a note of caution, TRE should still be regarded as a new dietary intervention with limited studies that have given mixed outcomes. For instance, small TRE studies have found significant decreases in weight and associated metabolic parameters, however, a large, randomized controlled trial of TRE in 116 overweight/ obese men and women for 12 weeks did not show a significant change in weight compared with the control group, although there were no measurements of energy intake or expenditure [241]. Therefore, large randomized clinical trials showing efficacy of TRE in obese individuals for 5-years or longer are needed before the adoption of TRE in the cancer clinical setting.
Table 2.
List of ongoing clinical trials on TRE and cancer
| Study | ClinicalTrials.gov Identifier: | Status | Disease condition, n | Time frame | Summary |
|---|---|---|---|---|---|
| Time-restricted Eating in Cancer Survivorship: A Single-arm Feasibility Pilot Study | NCT04243512 | Active, not recruiting | Cancer survivor, n = 40 |
10 h TRE, 14 days |
The investigators will assess the feasibility of delivering a time-restricted eating (TRE) intervention among cancer survivors with fatigue |
| Time-Restricted Eating (TRE) Among Endometrial Cancer Patients (TREND) | NCT04783467 | Recruiting |
Endometrial cancer patients, n = 15 |
8–10 h TRE, 16 weeks |
The long-term goal of this study is to determine the efficacy of Time-Restricted Eating (TRE) for improving metabolic health, preventing cardiometabolic comorbidities, and improving prognosis after endometrial cancer diagnosis. The study will also evaluate the feasibility, fidelity and preliminary acceptability of TRE among endometrial cancer patients |
| Time-Restricted Eating and Cancer: Clinical Outcomes, Mechanisms, and Moderators | NCT04722341 | Recruiting | Colorectal cancer, n = 300 |
8 h TRE starting 1–3 h after waking up, 6 months |
The purpose of this study is to test whether the timing of meals can improve treatment adverse events, influence tumor biology and alter a person’s mood and behaviors |
| Time-Restricted Eating During Chemotherapy for Breast Cancer | NCT05259410 | Recruiting |
Breast Cancer, n = 40 |
8 h TRE staring 10am-6 pm, 12 weeks |
The study will demonstrate that time-restricted eating, a form of intermittent fasting, will improve treatment related outcomes, patient related outcomes, and limit treatment related weight gain and fat mass accretion |
| Time-Restricted Eating (TRE) Among Native Hawaiian/Pacific Islander Women at Risk for Endometrial Cancer (TIMESPAN) | NCT04763902 | Recruiting |
Endometrial Neoplasms n = 30 |
8–10 h TRE, 14 weeks |
The primary objective of the study is to evaluate the feasibility, fidelity and preliminary acceptability of a TRE intervention among Native Hawaiian/Pacific Islander women at risk for developing endometrial cancer and to provide proof of principle that TRE can improve metabolic health in this population |
| Time-restricted Eating Versus Daily Continuous Calorie Restriction on Body Weight and Colorectal Cancer Risk Markers | NCT05114798 | Not yet recruiting |
Colorectal Cancer n = 255 |
8 h TRE starting from 11am – 7 pm, 1 month |
This research will demonstrate that time-restricted eating, a type of intermittent fasting, is an effective therapy to help obese individuals reduce and control their body weight and prevent the development of colorectal cancer |
| Time-Restricted Eating to Address Persistent Cancer-Related Fatigue | NCT05256888 | Not yet recruiting |
Cancer Survivor, n = 30 |
10 h TRE, 12 weeks |
This study will assess the feasibility of delivering a 12-week time-restricted eating intervention as well as the intervention’s preliminary efficacy on persistent cancer related fatigue among cancer survivors |
| Metformin and Nightly Fasting in Women With Early Breast Cancer | NCT05023967 | Not yet recruiting |
Breast Cancer, n = 120 |
8 h TRE, 4–6 weeks (until surgery) |
This study will explore the combined effect of prolonged nightly fasting and metformin hydrochloride extended release in decreasing breast tumor cell proliferation and other biomarkers of breast cancer |
| Effects of Time-Restricted Feeding on AGE-RAGE Signaling | NCT05038137 | Not yet recruiting |
Pre Diabetes Breast Cancer, n = 48 |
8 h TRE, 3½ months |
This study will explore the TRE on metabolic changes in women at high risk of breast cancer |
| Impact of Metabolic Health Patterns And Breast Cancer Over Time in Women | NCT05432856 | Recruiting |
Breast Cancer, n = 65 |
8 h TRE, 24-weeks |
This study will examine changes in fat volume, liver fat, metabolic syndrome score, Framingham risk score, peak VO2, insulin resistance, changes in hormonal markers and cytokines |
| Intermittent Fasting Accompanying Chemotherapy in Gynecological Cancers | NCT03162289 | Recruiting | Breast or Ovarian Cancer, n = 150 | 10 h TRE with 60–72 h modified fast during chemotherapy | Primary outcome will be FACT-G score, with complete remission or Millar Payne classification as secondary outcomes |
| Proof-of-Concept of Time-Restricted Eating as a Novel Lifestyle Intervention for Breast Cancer Prevention | NCT05454943 | Recruiting |
Women over 50 with metabolic dysfunction, n = 178 |
8 h TRE standard or personalized, with peer or external support | This study will assess adherence and HbA1c, with HOMA-IR, glucose control, body weight, metabolic syndrome score as secondary outcomes |
| Effect of Prolonged Nightly Fasting on Immunotherapy Outcomes in HNSCC—Role of Gut Microbiome | NCT05083416 | Recruiting |
Adults with newly diagnosed recurrent/metastatic HNSCC, n = 52 |
8–10 h TRE, 3-months | Primary outcome will be adherence, with changes in gut microbiome as secondary outcome |
| Intermittent Fasting and CLL/SLL | NCT04626843 | Active, not recruiting | Adults with CLL or SLL, n = 15 |
8 h TRE, 3-months |
This study will assess change in lymphocyte count, quality of, life, inflammation, metabolic profile, autophagy and immune cell gene expression |
| Metabolic Therapy Program In Conjunction With Standard Treatment For Glioblastoma Multiforme | NCT04730869 | Recruiting | Newly diagnosed GBM, n = 22 | 2 × 1 h eating intervals with ketogenic diet between 2 5-day fasts during chemotherapy | Primary outcome will be glucose-to-ketone ratio, with changes in weight, quality of life, activity, adverse events, progression-free and overall survival as secondary outcomes |
Declarations
Ethical Statement
The authors declare they have no conflicts of interest. The authors would like to acknowledge funding from the National Institutes of Health (R01 CA196853, P30 CA023100, and T32 DK007044) and the Department of Veterans Affairs (I01BX004848 and IBX005224). All authors contributed to the conception of the review. Dr. Das performed the literature search and prepared the original draft. Dr. Webster edited the draft, created the illustrations, and prepared the final version. All authors read and approved the final manuscript.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lobstein, T., Brinsden, H., & Neveux, M. (2022). World Obesity Atlas 2022. https://www.worldobesity.org/resources/resource-library/world-obesity-atlas-2022. Accessed 18 Aug 2022
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. Journal of the American Medical Association. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eheman C, Henley SJ, Ballard-Barbash R, Jacobs EJ, Schymura MJ, Noone AM, et al. Annual Report to the Nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer. 2012;118(9):2338–2366. doi: 10.1002/cncr.27514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haslam DW, James WP. Obesity. Lancet. 2005;366(9492):1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 5.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. The New England Journal of Medicine. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 6.Demark-Wahnefried W, Platz EA, Ligibel JA, Blair CK, Courneya KS, Meyerhardt JA, et al. The role of obesity in cancer survival and recurrence. Cancer Epidemiology, Biomarkers & Prevention. 2012;21(8):1244–1259. doi: 10.1158/1055-9965.EPI-12-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: Cohort study. BMJ. 2007;335(7630):1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmitz KH, Neuhouser ML, Agurs-Collins T, Zanetti KA, Cadmus-Bertram L, Dean LT, et al. Impact of obesity on cancer survivorship and the potential relevance of race and ethnicity. Journal of the National Cancer Institute. 2013;105(18):1344–1354. doi: 10.1093/jnci/djt223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyrgiou, M., Kalliala, I., Markozannes, G., Gunter, M. J., Paraskevaidis, E., Gabra, H., et al. (2017). Adiposity and cancer at major anatomical sites: umbrella review of the literature. Bmj-British Medical Journal, 356, ARTN j477 10.1136/bmj.j477 [DOI] [PMC free article] [PubMed]
- 10.Islami F, Goding Sauer A, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA: A Cancer Journal for Clinicians. 2018;68(1):31–54. doi: 10.3322/caac.21440. [DOI] [PubMed] [Google Scholar]
- 11.Bishayee A. The role of inflammation and liver cancer. Advances in Experimental Medicine and Biology. 2014;816:401–435. doi: 10.1007/978-3-0348-0837-8_16. [DOI] [PubMed] [Google Scholar]
- 12.Gallagher EJ, LeRoith D. Obesity and Diabetes: The Increased Risk of Cancer and Cancer-Related Mortality. Physiological Reviews. 2015;95(3):727–748. doi: 10.1152/physrev.00030.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho SM. Estrogen, progesterone and epithelial ovarian cancer. Reproductive Biology and Endocrinology. 2003;1:73. doi: 10.1186/1477-7827-1-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmandt RE, Iglesias DA, Co NN, Lu KH. Understanding obesity and endometrial cancer risk: Opportunities for prevention. American Journal of Obstetrics and Gynecology. 2011;205(6):518–525. doi: 10.1016/j.ajog.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cleary MP, Grossmann ME. Minireview: Obesity and breast cancer: The estrogen connection. Endocrinology. 2009;150(6):2537–2542. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keum, N., Greenwood, D. C., Lee, D. H., Kim, R., Aune, D., Ju, W., et al. (2015). Adult weight gain and adiposity-related cancers: a dose-response meta-analysis of prospective observational studies. Journal of the National Cancer Institute, 107(2),10.1093/jnci/djv088 [DOI] [PubMed]
- 17.Teras LR, Patel AV, Wang M, Yaun SS, Anderson K, Brathwaite R, et al. Sustained Weight Loss and Risk of Breast Cancer in Women 50 Years and Older: A Pooled Analysis of Prospective Data. Journal of the National Cancer Institute. 2020;112(9):929–937. doi: 10.1093/jnci/djz226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo J, Chlebowski RT, Hendryx M, Rohan T, Wactawski-Wende J, Thomson CA, et al. Intentional Weight Loss and Endometrial Cancer Risk. Journal of Clinical Oncology. 2017;35(11):1189–1193. doi: 10.1200/JCO.2016.70.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson RL, Newton RU, Taaffe DR, Hart NH, Lyons-Wall P, Galvao DA. Weight Loss for Obese Prostate Cancer Patients on Androgen Deprivation Therapy. Medicine and Science in Sports and Exercise. 2021;53(3):470–478. doi: 10.1249/MSS.0000000000002509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metabolism. 2018;27(6):1212–1221 e1213. doi: 10.1016/j.cmet.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutchison AT, Regmi P, Manoogian ENC, Fleischer JG, Wittert GA, Panda S, et al. Time-Restricted Feeding Improves Glucose Tolerance in Men at Risk for Type 2 Diabetes: A Randomized Crossover Trial. Obesity (Silver Spring) 2019;27(5):724–732. doi: 10.1002/oby.22449. [DOI] [PubMed] [Google Scholar]
- 22.Chow LS, Manoogian ENC, Alvear A, Fleischer JG, Thor H, Dietsche K, et al. Time-Restricted Eating Effects on Body Composition and Metabolic Measures in Humans who are Overweight: A Feasibility Study. Obesity (Silver Spring) 2020;28(5):860–869. doi: 10.1002/oby.22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das M, Ellies LG, Kumar D, Sauceda C, Oberg A, Gross E, et al. Time-restricted feeding normalizes hyperinsulinemia to inhibit breast cancer in obese postmenopausal mouse models. Nature Communications. 2021;12(1):565. doi: 10.1038/s41467-020-20743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sundaram S, Yan L. Time-restricted feeding mitigates high-fat diet-enhanced mammary tumorigenesis in MMTV-PyMT mice. Nutrition Research. 2018;59:72–79. doi: 10.1016/j.nutres.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 25.Yan L, Sundaram S, Mehus AA, Picklo MJ. Time-restricted Feeding Attenuates High-fat Diet-enhanced Spontaneous Metastasis of Lewis Lung Carcinoma in Mice. Anticancer Research. 2019;39(4):1739–1748. doi: 10.21873/anticanres.13280. [DOI] [PubMed] [Google Scholar]
- 26.Lozcano-Ponce E. Second Expert Report, Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective. Salud Publica De Mexico. 2009;51:S678–S680. doi: 10.1590/S0036-36342009001000024. [DOI] [Google Scholar]
- 27.Vainio H, Kaaks R, Bianchini F. Weight control and physical activity in cancer prevention: International evaluation of the evidence. European Journal of Cancer Prevention. 2002;11(Suppl 2):S94–100. [PubMed] [Google Scholar]
- 28.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Research and Treatment. 2010;123(3):627–635. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 29.Petrelli F, Cortellini A, Indini A, Tomasello G, Ghidini M, Nigro O, et al. Association of Obesity With Survival Outcomes in Patients With Cancer: A Systematic Review and Meta-analysis. JAMA Network Open. 2021;4(3):e213520. doi: 10.1001/jamanetworkopen.2021.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: A systematic review and meta-analysis. Cancer Prevention Research (Philadelphia, Pa.) 2011;4(4):486–501. doi: 10.1158/1940-6207.CAPR-10-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sinicrope FA, Foster NR, Sargent DJ, O'Connell MJ, Rankin C. Obesity is an independent prognostic variable in colon cancer survivors. Clinical Cancer Research. 2010;16(6):1884–1893. doi: 10.1158/1078-0432.CCR-09-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Troeschel AN, Hartman TJ, Jacobs EJ, Stevens VL, Gansler T, Flanders WD, et al. Postdiagnosis Body Mass Index, Weight Change, and Mortality From Prostate Cancer, Cardiovascular Disease, and All Causes Among Survivors of Nonmetastatic Prostate Cancer. Journal of Clinical Oncology. 2020;38(18):2018–2027. doi: 10.1200/JCO.19.02185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Bella CM, Howard LE, Oyekunle T, De Hoedt AM, Salama JK, Song H, et al. Abdominal and pelvic adipose tissue distribution and risk of prostate cancer recurrence after radiation therapy. Prostate. 2020;80(14):1244–1252. doi: 10.1002/pros.24054. [DOI] [PubMed] [Google Scholar]
- 34.McDowell SAC, Luo RBE, Arabzadeh A, Dore S, Bennett NC, Breton V, et al. Neutrophil oxidative stress mediates obesity-associated vascular dysfunction and metastatic transmigration. Nat Cancer. 2021;2(5):545–562. doi: 10.1038/s43018-021-00194-9. [DOI] [PubMed] [Google Scholar]
- 35.Annett, S., Moore, G., & Robson, T. (2020). Obesity and Cancer Metastasis: Molecular and Translational Perspectives. Cancers (Basel), 12(12), 10.3390/cancers12123798. [DOI] [PMC free article] [PubMed]
- 36.Greenlee H, Shi Z, Sardo Molmenti CL, Rundle A, Tsai WY. Trends in Obesity Prevalence in Adults With a History of Cancer: Results From the US National Health Interview Survey, 1997 to 2014. Journal of Clinical Oncology. 2016;34(26):3133–3140. doi: 10.1200/JCO.2016.66.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodwin PJ, Ennis M, Pritchard KI, McCready D, Koo J, Sidlofsky S, et al. Adjuvant treatment and onset of menopause predict weight gain after breast cancer diagnosis. Journal of Clinical Oncology. 1999;17(1):120–129. doi: 10.1200/JCO.1999.17.1.120. [DOI] [PubMed] [Google Scholar]
- 38.Paskett ED, Dean JA, Oliveri JM, Harrop JP. Cancer-related lymphedema risk factors, diagnosis, treatment, and impact: A review. Journal of Clinical Oncology. 2012;30(30):3726–3733. doi: 10.1200/JCO.2012.41.8574. [DOI] [PubMed] [Google Scholar]
- 39.Gacci M, Sebastianelli A, Salvi M, De Nunzio C, Schiavina R, Simonato A, et al. Role of abdominal obesity for functional outcomes and complications in men treated with radical prostatectomy for prostate cancer: Results of the Multicenter Italian Report on Radical Prostatectomy (MIRROR) study. Scandinavian Journal of Urology. 2014;48(2):138–145. doi: 10.3109/21681805.2013.803151. [DOI] [PubMed] [Google Scholar]
- 40.Brown KA, Simpson ER. Obesity and breast cancer: Progress to understanding the relationship. Cancer Research. 2010;70(1):4–7. doi: 10.1158/0008-5472.CAN-09-2257. [DOI] [PubMed] [Google Scholar]
- 41.McTiernan A. Obesity and cancer: the risks, science, and potential management strategies. Oncology (Williston Park) 2005;19(7):871–881. [PubMed] [Google Scholar]
- 42.Pike MC, Pearce CL, Wu AH. Prevention of cancers of the breast, endometrium and ovary. Oncogene. 2004;23(38):6379–6391. doi: 10.1038/sj.onc.1207899. [DOI] [PubMed] [Google Scholar]
- 43.Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA: A Cancer Journal for Clinicians. 2017;67(5):378–397. doi: 10.3322/caac.21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Copson ER, Cutress RI, Maishman T, Eccles BK, Gerty S, Stanton L, et al. Obesity and the outcome of young breast cancer patients in the UK: The POSH study. Annals of Oncology. 2015;26(1):101–112. doi: 10.1093/annonc/mdu509. [DOI] [PubMed] [Google Scholar]
- 45.Chan DSM, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Annals of Oncology. 2014;25(10):1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bulun SE, Chen D, Moy I, Brooks DC, Zhao H. Aromatase, breast cancer and obesity: A complex interaction. Trends in Endocrinology and Metabolism. 2012;23(2):83–89. doi: 10.1016/j.tem.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McTiernan A, Rajan KB, Tworoger SS, Irwin M, Bernstein L, Baumgartner R, et al. Adiposity and sex hormones in postmenopausal breast cancer survivors. Journal of Clinical Oncology. 2003;21(10):1961–1966. doi: 10.1200/Jco.2003.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Key T, Appleby P, Barnes I, Reeves G, Endogenous H, Breast Cancer Collaborative, G. Endogenous sex hormones and breast cancer in postmenopausal women: Reanalysis of nine prospective studies. Journal of the National Cancer Institute. 2002;94(8):606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 49.Bowers LW, Rossi EL, O'Flanagan CH, deGraffenried LA, Hursting SD. The Role of the Insulin/IGF System in Cancer: Lessons Learned from Clinical Trials and the Energy Balance-Cancer Link. Front Endocrinol (Lausanne) 2015;6:77. doi: 10.3389/fendo.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duggan C, Irwin ML, Xiao L, Henderson KD, Smith AW, Baumgartner RN, et al. Associations of insulin resistance and adiponectin with mortality in women with breast cancer. Journal of Clinical Oncology. 2011;29(1):32–39. doi: 10.1200/JCO.2009.26.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Emaus A, Veierod MB, Tretli S, Finstad SE, Selmer R, Furberg AS, et al. Metabolic profile, physical activity, and mortality in breast cancer patients. Breast Cancer Research and Treatment. 2010;121(3):651–660. doi: 10.1007/s10549-009-0603-y. [DOI] [PubMed] [Google Scholar]
- 52.Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Madarnas Y, et al. Fasting insulin and outcome in early-stage breast cancer: Results of a prospective cohort study. Journal of Clinical Oncology. 2002;20(1):42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- 53.Irwin ML, Duggan C, Wang CY, Smith AW, McTiernan A, Baumgartner RN, et al. Fasting C-peptide levels and death resulting from all causes and breast cancer: The health, eating, activity, and lifestyle study. Journal of Clinical Oncology. 2011;29(1):47–53. doi: 10.1200/JCO.2010.28.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pasanisi P, Berrino F, De Petris M, Venturelli E, Mastroianni A, Panico S. Metabolic syndrome as a prognostic factor for breast cancer recurrences. International Journal of Cancer. 2006;119(1):236–238. doi: 10.1002/ijc.21812. [DOI] [PubMed] [Google Scholar]
- 55.Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. Journal of the National Cancer Institute. 2009;101(1):48–60. doi: 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eliassen AH, Tworoger SS, Mantzoros CS, Pollak MN, Hankinson SE. Circulating insulin and c-peptide levels and risk of breast cancer among predominately premenopausal women. Cancer Epidemiology, Biomarkers & Prevention. 2007;16(1):161–164. doi: 10.1158/1055-9965.EPI-06-0693. [DOI] [PubMed] [Google Scholar]
- 57.Wolpin BM, Meyerhardt JA, Chan AT, Ng K, Chan JA, Wu K, et al. Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. Journal of Clinical Oncology. 2009;27(2):176–185. doi: 10.1200/JCO.2008.17.9945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghanavati M, Rahmani J, Rinaldi G, Zand H. Fasting Insulin and Risk of Cancer Related Mortality in Non-diabetic Adults: A Dose-response Meta-analysis of Cohort Studies. Current Diabetes Review. 2020;16(4):357–363. doi: 10.2174/1573399815666190906130544. [DOI] [PubMed] [Google Scholar]
- 59.Wargny M, Balkau B, Lange C, Charles MA, Giral P, Simon D. Association of fasting serum insulin and cancer mortality in a healthy population - 28-year follow-up of the French TELECOM Study. Diabetes & Metabolism. 2018;44(1):30–37. doi: 10.1016/j.diabet.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 60.Mantovani A. Cancer: Inflammation by remote control. Nature. 2005;435(7043):752–753. doi: 10.1038/435752a. [DOI] [PubMed] [Google Scholar]
- 61.Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Annals of African Medicine. 2019;18(3):121–126. doi: 10.4103/aam.aam_56_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sulli G, Manoogian ENC, Taub PR, Panda S. Training the Circadian Clock, Clocking the Drugs, and Drugging the Clock to Prevent, Manage, and Treat Chronic Diseases. Trends in Pharmacological Sciences. 2018;39(9):812–827. doi: 10.1016/j.tips.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eckel-Mahan KL, Patel VR, de Mateo S, Orozco-Solis R, Ceglia NJ, Sahar S, et al. Reprogramming of the circadian clock by nutritional challenge. Cell. 2013;155(7):1464–1478. doi: 10.1016/j.cell.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Engin A. Circadian Rhythms in Diet-Induced Obesity. Advances in Experimental Medicine and Biology. 2017;960:19–52. doi: 10.1007/978-3-319-48382-5_2. [DOI] [PubMed] [Google Scholar]
- 65.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes & Development. 2000;14(23):2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oosterman JE, Kalsbeek A, la Fleur SE, Belsham DD. Impact of nutrients on circadian rhythmicity. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2015;308(5):R337–350. doi: 10.1152/ajpregu.00322.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Manoogian ENC, Chow LS, Taub PR, Laferrere B, Panda S. Time-restricted Eating for the Prevention and Management of Metabolic Diseases. Endocrine Reviews. 2022;43(2):405–436. doi: 10.1210/endrev/bnab027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(11):4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sulli G, Lam MTY, Panda S. Interplay between Circadian Clock and Cancer: New Frontiers for Cancer Treatment. Trends Cancer. 2019;5(8):475–494. doi: 10.1016/j.trecan.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stenvers DJ, Scheer F, Schrauwen P, la Fleur SE, Kalsbeek A. Circadian clocks and insulin resistance. Nature Reviews. Endocrinology. 2019;15(2):75–89. doi: 10.1038/s41574-018-0122-1. [DOI] [PubMed] [Google Scholar]
- 71.Lin HH, Farkas ME. Altered Circadian Rhythms and Breast Cancer: From the Human to the Molecular Level. Frontiers in Endocrinology (Lausanne) 2018;9:219. doi: 10.3389/fendo.2018.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mteyrek A, Filipski E, Guettier C, Oklejewicz M, van der Horst GT, Okyar A, et al. Critical cholangiocarcinogenesis control by cryptochrome clock genes. International journal of cancer. Journal international du cancer. 2017;140(11):2473–2483. doi: 10.1002/ijc.30663. [DOI] [PubMed] [Google Scholar]
- 73.Blakeman V, Williams JL, Meng QJ, Streuli CH. Circadian clocks and breast cancer. Breast Cancer Research. 2016;18(1):89. doi: 10.1186/s13058-016-0743-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Papagiannakopoulos T, Bauer MR, Davidson SM, Heimann M, Subbaraj L, Bhutkar A, et al. Circadian Rhythm Disruption Promotes Lung Tumorigenesis. Cell Metabolism. 2016;24(2):324–331. doi: 10.1016/j.cmet.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Altman BJ, Hsieh AL, Sengupta A, Krishnanaiah SY, Stine ZE, Walton ZE, et al. MYC Disrupts the Circadian Clock and Metabolism in Cancer Cells. Cell Metabolism. 2015;22(6):1009–1019. doi: 10.1016/j.cmet.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shostak A, Ruppert B, Ha N, Bruns P, Toprak UH, Project MS, et al. MYC/MIZ1-dependent gene repression inversely coordinates the circadian clock with cell cycle and proliferation. Nature Communications. 2016;7:11807. doi: 10.1038/ncomms11807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dong Z, Zhang G, Qu M, Gimple RC, Wu Q, Qiu Z, et al. Targeting Glioblastoma Stem Cells through Disruption of the Circadian Clock. Cancer Discovery. 2019;9(11):1556–1573. doi: 10.1158/2159-8290.CD-19-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Puram RV, Kowalczyk MS, de Boer CG, Schneider RK, Miller PG, McConkey M, et al. Core Circadian Clock Genes Regulate Leukemia Stem Cells in AML. Cell. 2016;165(2):303–316. doi: 10.1016/j.cell.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kettner NM, Voicu H, Finegold MJ, Coarfa C, Sreekumar A, Putluri N, et al. Circadian Homeostasis of Liver Metabolism Suppresses Hepatocarcinogenesis. Cancer Cell. 2016;30(6):909–924. doi: 10.1016/j.ccell.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Masri S, Papagiannakopoulos T, Kinouchi K, Liu Y, Cervantes M, Baldi P, et al. Lung Adenocarcinoma Distally Rewires Hepatic Circadian Homeostasis. Cell. 2016;165(4):896–909. doi: 10.1016/j.cell.2016.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fu L, Kettner NM. The circadian clock in cancer development and therapy. Progress in Molecular Biology and Translational Science. 2013;119:221–282. doi: 10.1016/B978-0-12-396971-2.00009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yao J, He C, Zhao W, Hu N, Long D. Circadian clock and cell cycle: Cancer and chronotherapy. Acta Histochemica. 2021;123(8):151816. doi: 10.1016/j.acthis.2021.151816. [DOI] [PubMed] [Google Scholar]
- 83.Razi Soofiyani S, Ahangari H, Soleimanian A, Babaei G, Ghasemnejad T, Safavi SE, et al. The role of circadian genes in the pathogenesis of colorectal cancer. Gene. 2021;804:145894. doi: 10.1016/j.gene.2021.145894. [DOI] [PubMed] [Google Scholar]
- 84.Yang Y, Lindsey-Boltz LA, Vaughn CM, Selby CP, Cao X, Liu Z, et al. Circadian clock, carcinogenesis, chronochemotherapy connections. Journal of Biological Chemistry. 2021;297(3):101068. doi: 10.1016/j.jbc.2021.101068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Battaglin F, Chan P, Pan Y, Soni S, Qu M, Spiller ER, et al. Clocking cancer: The circadian clock as a target in cancer therapy. Oncogene. 2021;40(18):3187–3200. doi: 10.1038/s41388-021-01778-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Patel, S. A., & Kondratov, R. V. (2021). Clock at the Core of Cancer Development. Biology (Basel), 10(2), 10.3390/biology10020150. [DOI] [PMC free article] [PubMed]
- 87.Truong T, Liquet B, Menegaux F, Plancoulaine S, Laurent-Puig P, Mulot C, et al. Breast cancer risk, nightwork, and circadian clock gene polymorphisms. Endocrine-Related Cancer. 2014;21(4):629–638. doi: 10.1530/ERC-14-0121. [DOI] [PubMed] [Google Scholar]
- 88.Hoffman AE, Yi CH, Zheng T, Stevens RG, Leaderer D, Zhang Y, et al. CLOCK in breast tumorigenesis: Genetic, epigenetic, and transcriptional profiling analyses. Cancer Research. 2010;70(4):1459–1468. doi: 10.1158/0008-5472.CAN-09-3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reszka E, Przybek M, Muurlink O, Peplonska B. Circadian gene variants and breast cancer. Cancer Letters. 2017;390:137–145. doi: 10.1016/j.canlet.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 90.Dai H, Zhang L, Cao M, Song F, Zheng H, Zhu X, et al. The role of polymorphisms in circadian pathway genes in breast tumorigenesis. Breast Cancer Research and Treatment. 2011;127(2):531–540. doi: 10.1007/s10549-010-1231-2. [DOI] [PubMed] [Google Scholar]
- 91.Gery S, Virk RK, Chumakov K, Yu A, Koeffler HP. The clock gene Per2 links the circadian system to the estrogen receptor. Oncogene. 2007;26(57):7916–7920. doi: 10.1038/sj.onc.1210585. [DOI] [PubMed] [Google Scholar]
- 92.Rossetti S, Corlazzoli F, Gregorski A, Azmi NH, Sacchi N. Identification of an estrogen-regulated circadian mechanism necessary for breast acinar morphogenesis. Cell Cycle. 2012;11(19):3691–3700. doi: 10.4161/cc.21946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cadenas C, van de Sandt L, Edlund K, Lohr M, Hellwig B, Marchan R, et al. Loss of circadian clock gene expression is associated with tumor progression in breast cancer. Cell Cycle. 2014;13(20):3282–3291. doi: 10.4161/15384101.2014.954454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mao Y, Fu A, Hoffman AE, Jacobs DI, Jin M, Chen K, et al. The circadian gene CRY2 is associated with breast cancer aggressiveness possibly via epigenomic modifications. Tumour Biology. 2015;36(5):3533–3539. doi: 10.1007/s13277-014-2989-3. [DOI] [PubMed] [Google Scholar]
- 95.Lesicka M, Jablonska E, Wieczorek E, Seroczynska B, Siekierzycka A, Skokowski J, et al. Altered circadian genes expression in breast cancer tissue according to the clinical characteristics. PLoS ONE. 2018;13(6):e0199622. doi: 10.1371/journal.pone.0199622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu L, Shen H, Wang Y. CRY2 is suppressed by FOXM1 mediated promoter hypermethylation in breast cancer. Biochemical and Biophysical Research Communications. 2017;490(1):44–50. doi: 10.1016/j.bbrc.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 97.Rana S, Munawar M, Shahid A, Malik M, Ullah H, Fatima W, et al. Deregulated expression of circadian clock and clock-controlled cell cycle genes in chronic lymphocytic leukemia. Molecular Biology Reports. 2014;41(1):95–103. doi: 10.1007/s11033-013-2841-7. [DOI] [PubMed] [Google Scholar]
- 98.Song B, Chen Y, Liu Y, Wan C, Zhang L, Zhang W. NPAS2 regulates proliferation of acute myeloid leukemia cells via CDC25A-mediated cell cycle progression and apoptosis. Journal of Cellular Biochemistry. 2018 doi: 10.1002/jcb.28160. [DOI] [PubMed] [Google Scholar]
- 99.Rahman S, Al-Hallaj AS, Nedhi A, Gmati G, Ahmed K, Jama HA, et al. Differential Expression of Circadian Genes in Leukemia and a Possible Role for Sirt1 in Restoring the Circadian Clock in Chronic Myeloid Leukemia. J Circadian Rhythms. 2017;15:3. doi: 10.5334/jcr.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Habashy DM, Eissa DS, Aboelez MM. Cryptochrome-1 Gene Expression is a Reliable Prognostic Indicator in Egyptian Patients with Chronic Lymphocytic Leukemia: A Prospective Cohort Study. Turkish Journal of Haematology. 2018;35(3):168–174. doi: 10.4274/tjh.2017.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou J, Wang J, Zhang X, Tang Q. New Insights Into Cancer Chronotherapies. Frontiers in Pharmacology. 2021;12:741295. doi: 10.3389/fphar.2021.741295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.He A, Huang Z, Zhang R, Lu H, Wang J, Cao J, et al. Circadian Clock Genes Are Correlated with Prognosis and Immune Cell Infiltration in Colon Adenocarcinoma. Computational and Mathematical Methods in Medicine. 2022;2022:1709918. doi: 10.1155/2022/1709918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Crespo, M., Leiva, M., & Sabio, G. (2021). Circadian Clock and Liver Cancer. Cancers (Basel), 13(14), 10.3390/cancers13143631. [DOI] [PMC free article] [PubMed]
- 104.Liu B, Xu K, Jiang Y, Li X. Aberrant expression of Per1, Per2 and Per3 and their prognostic relevance in non-small cell lung cancer. International Journal of Clinical and Experimental Pathology. 2014;7(11):7863–7871. [PMC free article] [PubMed] [Google Scholar]
- 105.Xiang R, Cui Y, Wang Y, Xie T, Yang X, Wang Z, et al. Circadian clock gene Per2 downregulation in nonsmall cell lung cancer is associated with tumour progression and metastasis. Oncology Reports. 2018;40(5):3040–3048. doi: 10.3892/or.2018.6704. [DOI] [PubMed] [Google Scholar]
- 106.Pi-Sunyer FX, Becker DM, Bouchard C, Carleton RA, Colditz GA, Dietz WH, et al. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: Executive summary. American Journal of Clinical Nutrition. 1998;68(4):899–917. doi: 10.1093/ajcn/68.4.899. [DOI] [PubMed] [Google Scholar]
- 107.Courneya KS. Exercise in cancer survivors: An overview of research. Medicine and Science in Sports and Exercise. 2003;35(11):1846–1852. doi: 10.1249/01.Mss.0000093622.41587.B6. [DOI] [PubMed] [Google Scholar]
- 108.Brolin RE. Bariatric surgery and long-term control of morbid obesity. JAMA. 2002;288(22):2793–2796. doi: 10.1001/jama.288.22.2793. [DOI] [PubMed] [Google Scholar]
- 109.Chlebowski, R. T., Blackburn, G. L., Hoy, M. K., Thomson, C. A., Giuliano, A. E., McAndrew, P., et al. (2008). Survival analyses from the Women’s Intervention Nutrition Study (WINS) evaluating dietary fat reduction and breast cancer outcome. Journal of Clinical Oncology, 26(15), 10.1200/jco.2008.26.15_suppl.522.
- 110.Thomson CA, Rock CL, Giuliano AR, Newton TR, Cui HY, Reid PM, et al. Longitudinal changes in body weight and body composition among women previously treated for breast cancer consuming a high-vegetable, fruit and fiber, low-fat diet. European Journal of Nutrition. 2005;44(1):18–25. doi: 10.1007/s00394-004-0487-x. [DOI] [PubMed] [Google Scholar]
- 111.O'Flanagan CH, Smith LA, McDonell SB, Hursting SD. When less may be more: Calorie restriction and response to cancer therapy. BMC Medicine. 2017;15(1):106. doi: 10.1186/s12916-017-0873-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Clifton KK, Ma CX, Fontana L, Peterson LL. Intermittent fasting in the prevention and treatment of cancer. CA: A Cancer Journal for Clinicians. 2021;71(6):527–546. doi: 10.3322/caac.21694. [DOI] [PubMed] [Google Scholar]
- 113.Schauer DP, Feigelson HS, Koebnick C, Caan B, Weinmann S, Leonard AC, et al. Bariatric Surgery and the Risk of Cancer in a Large Multisite Cohort. Annals of Surgery. 2019;269(1):95–101. doi: 10.1097/SLA.0000000000002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Feigelson HS, Caan B, Weinmann S, Leonard AC, Powers JD, Yenumula PR, et al. Bariatric Surgery is Associated With Reduced Risk of Breast Cancer in Both Premenopausal and Postmenopausal Women. Annals of Surgery. 2020;272(6):1053–1059. doi: 10.1097/SLA.0000000000003331. [DOI] [PubMed] [Google Scholar]
- 115.Longo VD, Fontana L. Calorie restriction and cancer prevention: Metabolic and molecular mechanisms. Trends in Pharmacological Sciences. 2010;31(2):89–98. doi: 10.1016/j.tips.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rubinstein MM, Brown KA, Iyengar NM. Targeting obesity-related dysfunction in hormonally driven cancers. British Journal of Cancer. 2021;125(4):495–509. doi: 10.1038/s41416-021-01393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Medicine and Science in Sports and Exercise. 2019;51(11):2375–2390. doi: 10.1249/MSS.0000000000002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.International Food Information Council: Food & Health Survey (2020). https://foodinsight.org/wp-content/uploads/2020/06/IFIC-Food-and-Health-Survey-2020.pdf. Accessed 18 May 2022
- 119.Berrigan D, Perkins SN, Haines DC, Hursting SD. Adult-onset calorie restriction and fasting delay spontaneous tumorigenesis in p53-deficient mice. Carcinogenesis. 2002;23(5):817–822. doi: 10.1093/carcin/23.5.817. [DOI] [PubMed] [Google Scholar]
- 120.Buschemeyer WC, 3rd, Klink JC, Mavropoulos JC, Poulton SH, Demark-Wahnefried W, Hursting SD, et al. Effect of intermittent fasting with or without caloric restriction on prostate cancer growth and survival in SCID mice. Prostate. 2010;70(10):1037–1043. doi: 10.1002/pros.21136. [DOI] [PubMed] [Google Scholar]
- 121.Salvadori G, Zanardi F, Iannelli F, Lobefaro R, Vernieri C, Longo VD. Fasting-mimicking diet blocks triple-negative breast cancer and cancer stem cell escape. Cell Metab. 2021;33(11):2247–2259 e2246. doi: 10.1016/j.cmet.2021.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Harvie MN, Sims AH, Pegington M, Spence K, Mitchell A, Vaughan AA, et al. Intermittent energy restriction induces changes in breast gene expression and systemic metabolism. Breast Cancer Research. 2016;18(1):57. doi: 10.1186/s13058-016-0714-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schreck KC, Hsu FC, Berrington A, Henry-Barron B, Vizthum D, Blair L, et al. Feasibility and Biological Activity of a Ketogenic/Intermittent-Fasting Diet in Patients With Glioma. Neurology. 2021;97(9):e953–e963. doi: 10.1212/WNL.0000000000012386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.de Groot S, Vreeswijk MP, Welters MJ, Gravesteijn G, Boei JJ, Jochems A, et al. The effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients: A randomized pilot study. BMC Cancer. 2015;15:652. doi: 10.1186/s12885-015-1663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bauersfeld SP, Kessler CS, Wischnewsky M, Jaensch A, Steckhan N, Stange R, et al. The effects of short-term fasting on quality of life and tolerance to chemotherapy in patients with breast and ovarian cancer: A randomized cross-over pilot study. BMC Cancer. 2018;18(1):476. doi: 10.1186/s12885-018-4353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lugtenberg RT, de Groot S, Kaptein AA, Fischer MJ, Kranenbarg EM, Carpentier MD, et al. Quality of life and illness perceptions in patients with breast cancer using a fasting mimicking diet as an adjunct to neoadjuvant chemotherapy in the phase 2 DIRECT (BOOG 2013–14) trial. Breast Cancer Research and Treatment. 2021;185(3):741–758. doi: 10.1007/s10549-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Di Francesco A, Di Germanio C, Bernier M, de Cabo R. A time to fast. Science. 2018;362(6416):770–775. doi: 10.1126/science.aau2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Moon, S., Kang, J., Kim, S. H., Chung, H. S., Kim, Y. J., Yu, J. M., et al. (2020). Beneficial Effects of Time-Restricted Eating on Metabolic Diseases: A Systemic Review and Meta-Analysis. Nutrients, 12(5), 10.3390/nu12051267. [DOI] [PMC free article] [PubMed]
- 129.Parr, E. B., Devlin, B. L., Lim, K. H. C., Moresi, L. N. Z., Geils, C., Brennan, L., et al. (2020). Time-Restricted Eating as a Nutrition Strategy for Individuals with Type 2 Diabetes: A Feasibility Study. Nutrients, 12(11), 10.3390/nu12113228. [DOI] [PMC free article] [PubMed]
- 130.Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metabolism. 2020;31(1):92–104 e105. doi: 10.1016/j.cmet.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metabolism. 2012;15(6):848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metabolism. 2014;20(6):991–1005. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chung H, Chou W, Sears DD, Patterson RE, Webster NJ, Ellies LG. Time-restricted feeding improves insulin resistance and hepatic steatosis in a mouse model of postmenopausal obesity. Metabolism. 2016;65(12):1743–1754. doi: 10.1016/j.metabol.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ulgherait M, Midoun AM, Park SJ, Gatto JA, Tener SJ, Siewert J, et al. Circadian autophagy drives iTRF-mediated longevity. Nature. 2021;598(7880):353–358. doi: 10.1038/s41586-021-03934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Delahaye LB, Bloomer RJ, Butawan MB, Wyman JM, Hill JL, Lee HW, et al. Time-restricted feeding of a high-fat diet in male C57BL/6 mice reduces adiposity but does not protect against increased systemic inflammation. Applied Physiology, Nutrition and Metabolism. 2018;43(10):1033–1042. doi: 10.1139/apnm-2017-0706. [DOI] [PubMed] [Google Scholar]
- 136.Froy O, Chapnik N, Miskin R. Effect of intermittent fasting on circadian rhythms in mice depends on feeding time. Mechanisms of Ageing and Development. 2009;130(3):154–160. doi: 10.1016/j.mad.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 137.Acosta-Rodriguez V, Rijo-Ferreira F, Izumo M, Xu P, Wight-Carter M, Green CB, et al. Circadian alignment of early onset caloric restriction promotes longevity in male C57BL/6J mice. Science. 2022;376(6598):1192–1202. doi: 10.1126/science.abk0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Acosta-Rodriguez VA, Rijo-Ferreira F, Green CB, Takahashi JS. Importance of circadian timing for aging and longevity. Nature Communications. 2021;12(1):2862. doi: 10.1038/s41467-021-22922-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jamshed, H., Beyl, R. A., Della Manna, D. L., Yang, E. S., Ravussin, E., & Peterson, C. M. (2019). Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients, 11(6), 10.3390/nu11061234. [DOI] [PMC free article] [PubMed]
- 140.Parr, E. B., Devlin, B. L., Radford, B. E., & Hawley, J. A. (2020). A Delayed Morning and Earlier Evening Time-Restricted Feeding Protocol for Improving Glycemic Control and Dietary Adherence in Men with Overweight/Obesity: A Randomized Controlled Trial. Nutrients, 12(2), 10.3390/nu12020505. [DOI] [PMC free article] [PubMed]
- 141.Gallant AR, Lundgren J, Drapeau V. The night-eating syndrome and obesity. Obesity Reviews. 2012;13(6):528–536. doi: 10.1111/j.1467-789X.2011.00975.x. [DOI] [PubMed] [Google Scholar]
- 142.Jakubowicz, D., Barnea, M., Wainstein, J., & Froy, O. (2013). High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring), 21(12), 2504–2512, 10.1002/oby.20460. [DOI] [PubMed]
- 143.Snijders T, Trommelen J, Kouw IWK, Holwerda AM, Verdijk LB, van Loon LJC. The Impact of Pre-sleep Protein Ingestion on the Skeletal Muscle Adaptive Response to Exercise in Humans: An Update. Frontiers in Nutrition. 2019;6:17. doi: 10.3389/fnut.2019.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Garaulet M, Gomez-Abellan P, Alburquerque-Bejar JJ, Lee YC, Ordovas JM, Scheer FA. Timing of food intake predicts weight loss effectiveness. International Journal of Obesity . 2013;37(4):604–611. doi: 10.1038/ijo.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu D, Huang Y, Huang C, Yang S, Wei X, Zhang P, et al. Calorie Restriction with or without Time-Restricted Eating in Weight Loss. New England Journal of Medicine. 2022;386(16):1495–1504. doi: 10.1056/NEJMoa2114833. [DOI] [PubMed] [Google Scholar]
- 146.Liu C, Tang X, Gong Z, Zeng W, Hou Q, Lu R. Circadian Rhythm Sleep Disorders: Genetics, Mechanisms, and Adverse Effects on Health. Frontiers in Genetics. 2022;13:875342. doi: 10.3389/fgene.2022.875342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sunderram J, Sofou S, Kamisoglu K, Karantza V, Androulakis IP. Time-restricted feeding and the realignment of biological rhythms: Translational opportunities and challenges. Journal of Translational Medicine. 2014;12:79. doi: 10.1186/1479-5876-12-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Che T, Yan C, Tian D, Zhang X, Liu X, Wu Z. Time-restricted feeding improves blood glucose and insulin sensitivity in overweight patients with type 2 diabetes: A randomised controlled trial. Nutrition & Metabolism (London) 2021;18(1):88. doi: 10.1186/s12986-021-00613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Peeke PM, Greenway FL, Billes SK, Zhang D, Fujioka K. Effect of time restricted eating on body weight and fasting glucose in participants with obesity: Results of a randomized, controlled, virtual clinical trial. Nutrition & Diabetes. 2021;11(1):6. doi: 10.1038/s41387-021-00149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gabel K, Marcell J, Cares K, Kalam F, Cienfuegos S, Ezpeleta M, et al. Effect of time restricted feeding on the gut microbiome in adults with obesity: A pilot study. Nutrition and Health. 2020;26(2):79–85. doi: 10.1177/0260106020910907. [DOI] [PubMed] [Google Scholar]
- 151.Anton, S. D., Lee, S. A., Donahoo, W. T., McLaren, C., Manini, T., Leeuwenburgh, C., et al. (2019). The Effects of Time Restricted Feeding on Overweight, Older Adults: A Pilot Study. Nutrients, 11(7), 10.3390/nu11071500. [DOI] [PMC free article] [PubMed]
- 152.Kesztyus, D., Cermak, P., Gulich, M., & Kesztyus, T. (2019). Adherence to Time-Restricted Feeding and Impact on Abdominal Obesity in Primary Care Patients: Results of a Pilot Study in a Pre-Post Design. Nutrients, 11(12), 10.3390/nu11122854. [DOI] [PMC free article] [PubMed]
- 153.Tinsley GM, Moore ML, Graybeal AJ, Paoli A, Kim Y, Gonzales JU, et al. Time-restricted feeding plus resistance training in active females: A randomized trial. American Journal of Clinical Nutrition. 2019;110(3):628–640. doi: 10.1093/ajcn/nqz126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ravussin E, Beyl RA, Poggiogalle E, Hsia DS, Peterson CM. Early Time-Restricted Feeding Reduces Appetite and Increases Fat Oxidation But Does Not Affect Energy Expenditure in Humans. Obesity (Silver Spring) 2019;27(8):1244–1254. doi: 10.1002/oby.22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gabel K, Hoddy KK, Haggerty N, Song J, Kroeger CM, Trepanowski JF, et al. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutrition Healthy Aging. 2018;4(4):345–353. doi: 10.3233/NHA-170036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tinsley GM, Forsse JS, Butler NK, Paoli A, Bane AA, La Bounty PM, et al. Time-restricted feeding in young men performing resistance training: A randomized controlled trial. European Journal of Sport Science. 2017;17(2):200–207. doi: 10.1080/17461391.2016.1223173. [DOI] [PubMed] [Google Scholar]
- 157.Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, et al. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. Journal of Translational Medicine. 2016;14(1):290. doi: 10.1186/s12967-016-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Moro T, Tinsley G, Pacelli FQ, Marcolin G, Bianco A, Paoli A. Twelve Months of Time-restricted Eating and Resistance Training Improves Inflammatory Markers and Cardiometabolic Risk Factors. Medicine and Science in Sports and Exercise. 2021;53(12):2577–2585. doi: 10.1249/MSS.0000000000002738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gill S, Panda S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metabolism. 2015;22(5):789–798. doi: 10.1016/j.cmet.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Turner-McGrievy GM, Wirth MD, Bernhart JA, Aydin H. The Fasting and Shifted Timing (FAST) of Eating Study: A pilot feasibility randomized crossover intervention assessing the acceptability of three different fasting diet approaches. Appetite. 2022;176:106135. doi: 10.1016/j.appet.2022.106135. [DOI] [PubMed] [Google Scholar]
- 161.Thomas EA, Zaman A, Sloggett KJ, Steinke S, Grau L, Catenacci VA, et al. Early time-restricted eating compared with daily caloric restriction: A randomized trial in adults with obesity. Obesity (Silver Spring) 2022;30(5):1027–1038. doi: 10.1002/oby.23420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Vidmar, A. P., Naguib, M., Raymond, J. K., Salvy, S. J., Hegedus, E., Wee, C. P., et al. (2021). Time-Limited Eating and Continuous Glucose Monitoring in Adolescents with Obesity: A Pilot Study. Nutrients, 13(11), 10.3390/nu13113697 [DOI] [PMC free article] [PubMed]
- 163.Xie Z, Sun Y, Ye Y, Hu D, Zhang H, He Z, et al. Randomized controlled trial for time-restricted eating in healthy volunteers without obesity. Nature Communications. 2022;13(1):1003. doi: 10.1038/s41467-022-28662-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhao L, Hutchison AT, Liu B, Yates CL, Teong XT, Wittert GA, et al. Time-restricted eating improves glycemic control and dampens energy-consuming pathways in human adipose tissue. Nutrition. 2022;96:111583. doi: 10.1016/j.nut.2021.111583. [DOI] [PubMed] [Google Scholar]
- 165.Lin YJ, Wang YT, Chan LC, Chu NF. Effect of time-restricted feeding on body composition and cardio-metabolic risk in middle-aged women in Taiwan. Nutrition. 2022;93:111504. doi: 10.1016/j.nut.2021.111504. [DOI] [PubMed] [Google Scholar]
- 166.Nakamura, K., Tajiri, E., Hatamoto, Y., Ando, T., Shimoda, S., & Yoshimura, E. (2021). Eating Dinner Early Improves 24-h Blood Glucose Levels and Boosts Lipid Metabolism after Breakfast the Next Day: A Randomized Cross-Over Trial. Nutrients, 13(7), 10.3390/nu13072424. [DOI] [PMC free article] [PubMed]
- 167.Correia, J. M., Santos, I., Pezarat-Correia, P., Minderico, C., Schoenfeld, B. J., & Mendonca, G. V. (2021). Effects of Time-Restricted Feeding on Supramaximal Exercise Performance and Body Composition: A Randomized and Counterbalanced Crossover Study in Healthy Men. Int J Environ Res Public Health, 18(14), 10.3390/ijerph18147227. [DOI] [PMC free article] [PubMed]
- 168.Prasad, M., Fine, K., Gee, A., Nair, N., Popp, C. J., Cheng, B., et al. (2021). A Smartphone Intervention to Promote Time Restricted Eating Reduces Body Weight and Blood Pressure in Adults with Overweight and Obesity: A Pilot Study. Nutrients, 13(7), 10.3390/nu13072148. [DOI] [PMC free article] [PubMed]
- 169.Kotarsky CJ, Johnson NR, Mahoney SJ, Mitchell SL, Schimek RL, Stastny SN, et al. Time-restricted eating and concurrent exercise training reduces fat mass and increases lean mass in overweight and obese adults. Physiological Reports. 2021;9(10):e14868. doi: 10.14814/phy2.14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Crose, A., Alvear, A., Singroy, S., Wang, Q., Manoogian, E., Panda, S., et al. (2021). Time-Restricted Eating Improves Quality of Life Measures in Overweight Humans. Nutrients, 13(5), 10.3390/nu13051430. [DOI] [PMC free article] [PubMed]
- 171.Li C, Xing C, Zhang J, Zhao H, Shi W, He B. Eight-hour time-restricted feeding improves endocrine and metabolic profiles in women with anovulatory polycystic ovary syndrome. Journal of Translational Medicine. 2021;19(1):148. doi: 10.1186/s12967-021-02817-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lobene, A. J., Panda, S., Mashek, D. G., Manoogian, E. N. C., Hill Gallant, K. M., & Chow, L. S. (2021). Time-Restricted Eating for 12 Weeks Does Not Adversely Alter Bone Turnover in Overweight Adults. Nutrients, 13(4), 10.3390/nu13041155. [DOI] [PMC free article] [PubMed]
- 173.Phillips, N. E., Mareschal, J., Schwab, N., Manoogian, E. N. C., Borloz, S., Ostinelli, G., et al. (2021). The Effects of Time-Restricted Eating versus Standard Dietary Advice on Weight, Metabolic Health and the Consumption of Processed Food: A Pragmatic Randomised Controlled Trial in Community-Based Adults. Nutrients, 13(3), 10.3390/nu13031042. [DOI] [PMC free article] [PubMed]
- 174.Cienfuegos S, Gabel K, Kalam F, Ezpeleta M, Wiseman E, Pavlou V, et al. Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metabolism. 2020;32(3):366–378 e363. doi: 10.1016/j.cmet.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Przulj D, Ladmore D, Smith KM, Phillips-Waller A, Hajek P. Time restricted eating as a weight loss intervention in adults with obesity. PLoS ONE. 2021;16(1):e0246186. doi: 10.1371/journal.pone.0246186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Serra M, Marongiu F, Pisu MG, Serra M, Laconi E. Time-restricted feeding delays the emergence of the age-associated, neoplastic-prone tissue landscape. Aging (Albany NY) 2019;11(11):3851–3863. doi: 10.18632/aging.102021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hu DD, Mao YL, Xu G, Liao WJ, Yang HY, Zhang HB. Gut flora shift caused by time-restricted feeding might protect the host from metabolic syndrome, inflammatory bowel disease and colorectal cancer. Translational Cancer Research. 2018;7(5):1282-+. doi: 10.21037/tcr.2018.10.18. [DOI] [Google Scholar]
- 178.Turbitt WJ, Orlandella RM, Gibson JT, Peterson CM, Norian LA. Therapeutic Time-restricted Feeding Reduces Renal Tumor Bioluminescence in Mice but Fails to Improve Anti-CTLA-4 Efficacy. Anticancer Research. 2020;40(10):5445–5456. doi: 10.21873/anticanres.14555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Thomas JA, 2nd, Antonelli JA, Lloyd JC, Masko EM, Poulton SH, Phillips TE, et al. Effect of intermittent fasting on prostate cancer tumor growth in a mouse model. Prostate Cancer and Prostatic Diseases. 2010;13(4):350–355. doi: 10.1038/pcan.2010.24. [DOI] [PubMed] [Google Scholar]
- 180.Marinac CR, Nelson SH, Breen CI, Hartman SJ, Natarajan L, Pierce JP, et al. Prolonged Nightly Fasting and Breast Cancer Prognosis. JAMA Oncology. 2016;2(8):1049–1055. doi: 10.1001/jamaoncol.2016.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Marinac CR, Natarajan L, Sears DD, Gallo LC, Hartman SJ, Arredondo E, et al. Prolonged Nightly Fasting and Breast Cancer Risk: Findings from NHANES (2009–2010) Cancer Epidemiology, Biomarkers & Prevention. 2015;24(5):783–789. doi: 10.1158/1055-9965.EPI-14-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li M, Tse LA, Chan WC, Kwok CH, Leung SL, Wu C, et al. Nighttime eating and breast cancer among Chinese women in Hong Kong. Breast Cancer Research. 2017;19(1):31. doi: 10.1186/s13058-017-0821-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kogevinas M, Espinosa A, Castello A, Gomez-Acebo I, Guevara M, Martin V, et al. Effect of mistimed eating patterns on breast and prostate cancer risk (MCC-Spain Study) International Journal of Cancer. 2018;143(10):2380–2389. doi: 10.1002/ijc.31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Vona-Davis L, Rose DP. Type 2 diabetes and obesity metabolic interactions: Common factors for breast cancer risk and novel approaches to prevention and therapy. Current Diabetes Review. 2012;8(2):116–130. doi: 10.2174/157339912799424519. [DOI] [PubMed] [Google Scholar]
- 185.Shu X, Wu L, Khankari NK, Shu XO, Wang TJ, Michailidou K, et al. Associations of obesity and circulating insulin and glucose with breast cancer risk: A Mendelian randomization analysis. International Journal of Epidemiology. 2019;48(3):795–806. doi: 10.1093/ije/dyy201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Luque RM, Lopez-Sanchez LM, Villa-Osaba A, Luque IM, Santos-Romero AL, Yubero-Serrano EM, et al. Breast cancer is associated to impaired glucose/insulin homeostasis in premenopausal obese/overweight patients. Oncotarget. 2017;8(46):81462–81474. doi: 10.18632/oncotarget.20399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jones R, Pabla P, Mallinson J, Nixon A, Taylor T, Bennett A, et al. Two weeks of early time-restricted feeding (eTRF) improves skeletal muscle insulin and anabolic sensitivity in healthy men. American Journal of Clinical Nutrition. 2020;112(4):1015–1028. doi: 10.1093/ajcn/nqaa192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Han H, Guo W, Shi W, Yu Y, Zhang Y, Ye X, et al. Hypertension and breast cancer risk: A systematic review and meta-analysis. Science and Reports. 2017;7:44877. doi: 10.1038/srep44877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Williamson DA, Bray GA, Ryan DH. Is 5% weight loss a satisfactory criterion to define clinically significant weight loss? Obesity (Silver Spring) 2015;23(12):2319–2320. doi: 10.1002/oby.21358. [DOI] [PubMed] [Google Scholar]
- 190.Christensen, R. A. G., & Kirkham, A. A. (2021). Time-Restricted Eating: A Novel and Simple Dietary Intervention for Primary and Secondary Prevention of Breast Cancer and Cardiovascular Disease. Nutrients, 13(10), 10.3390/nu13103476. [DOI] [PMC free article] [PubMed]
- 191.Mattson MP, Allison DB, Fontana L, Harvie M, Longo VD, Malaisse WJ, et al. Meal frequency and timing in health and disease. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(47):16647–16653. doi: 10.1073/pnas.1413965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Saintot M, Mathieu-Daude H, Astre C, Grenier J, Simony-Lafontaine J, Gerber M. Oxidant-antioxidant status in relation to survival among breast cancer patients. International Journal of Cancer. 2002;97(5):574–579. doi: 10.1002/ijc.10099. [DOI] [PubMed] [Google Scholar]
- 194.Kang DH. Oxidative stress, DNA damage, and breast cancer. AACN Clinical Issues. 2002;13(4):540–549. doi: 10.1097/00044067-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 195.Senoner, T., & Dichtl, W. (2019). Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients, 11(9), 10.3390/nu11092090 [DOI] [PMC free article] [PubMed]
- 196.Sumida Y, Niki E, Naito Y, Yoshikawa T. Involvement of free radicals and oxidative stress in NAFLD/NASH. Free Radical Research. 2013;47(11):869–880. doi: 10.3109/10715762.2013.837577. [DOI] [PubMed] [Google Scholar]
- 197.Zhang AMY, Wellberg EA, Kopp JL, Johnson JD. Hyperinsulinemia in Obesity, Inflammation, and Cancer. Diabetes and Metabolism Journal. 2021;45(3):285–311. doi: 10.4093/dmj.2020.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Godsland IF. Insulin resistance and hyperinsulinaemia in the development and progression of cancer. Clinical Science (London, England) 2009;118(5):315–332. doi: 10.1042/CS20090399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tsujimoto T, Kajio H, Sugiyama T. Association between hyperinsulinemia and increased risk of cancer death in nonobese and obese people: A population-based observational study. International Journal of Cancer. 2017;141(1):102–111. doi: 10.1002/ijc.30729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Funcke JB, Scherer PE. Beyond adiponectin and leptin: Adipose tissue-derived mediators of inter-organ communication. Journal of Lipid Research. 2019;60(10):1648–1684. doi: 10.1194/jlr.R094060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Surmacz E. Leptin and adiponectin: Emerging therapeutic targets in breast cancer. Journal of Mammary Gland Biology and Neoplasia. 2013;18(3–4):321–332. doi: 10.1007/s10911-013-9302-8. [DOI] [PubMed] [Google Scholar]
- 202.Hebbard L, Ranscht B. Multifaceted roles of adiponectin in cancer. Best Practice & Research Clinical Endocrinology & Metabolism. 2014;28(1):59–69. doi: 10.1016/j.beem.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Miyoshi Y, Funahashi T, Tanaka S, Taguchi T, Tamaki Y, Shimomura I, et al. High expression of leptin receptor mRNA in breast cancer tissue predicts poor prognosis for patients with high, but not low, serum leptin levels. International Journal of Cancer. 2006;118(6):1414–1419. doi: 10.1002/ijc.21543. [DOI] [PubMed] [Google Scholar]
- 204.Otake S, Takeda H, Suzuki Y, Fukui T, Watanabe S, Ishihama K, et al. Association of visceral fat accumulation and plasma adiponectin with colorectal adenoma: Evidence for participation of insulin resistance. Clinical Cancer Research. 2005;11(10):3642–3646. doi: 10.1158/1078-0432.CCR-04-1868. [DOI] [PubMed] [Google Scholar]
- 205.Bu, D., Crewe, C., Kusminski, C. M., Gordillo, R., Ghaben, A. L., Kim, M., et al. (2019). Human endotrophin as a driver of malignant tumor growth. JCI Insight, 5, 10.1172/jci.insight.125094 [DOI] [PMC free article] [PubMed]
- 206.Wang J, Pan W. The Biological Role of the Collagen Alpha-3 (VI) Chain and Its Cleaved C5 Domain Fragment Endotrophin in Cancer. Oncotargets and Therapy. 2020;13:5779–5793. doi: 10.2147/OTT.S256654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kim M, Lee C, Seo DY, Lee H, Horton JD, Park J, et al. The impact of endotrophin on the progression of chronic liver disease. Experimental & Molecular Medicine. 2020;52(10):1766–1776. doi: 10.1038/s12276-020-00520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55(1):31–55. doi: 10.1016/j.immuni.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. The Journal of Clinical Investigation. 2017;127(1):1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sherman H, Frumin I, Gutman R, Chapnik N, Lorentz A, Meylan J, et al. Long-term restricted feeding alters circadian expression and reduces the level of inflammatory and disease markers. Journal of Cellular and Molecular Medicine. 2011;15(12):2745–2759. doi: 10.1111/j.1582-4934.2010.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wilson, R. B., Zhang, R., Chen, Y. J., Peters, K. M., Sawyez, C. G., Sutherland, B. G., et al. (2020). Two-Week Isocaloric Time-Restricted Feeding Decreases Liver Inflammation without Significant Weight Loss in Obese Mice with Non-Alcoholic Fatty Liver Disease. Int J Mol Sci, 21(23), 10.3390/ijms21239156. [DOI] [PMC free article] [PubMed]
- 212.Faris MA, Kacimi S, Al-Kurd RA, Fararjeh MA, Bustanji YK, Mohammad MK, et al. Intermittent fasting during Ramadan attenuates proinflammatory cytokines and immune cells in healthy subjects. Nutrition Research. 2012;32(12):947–955. doi: 10.1016/j.nutres.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 213.Halberg N, Henriksen M, Soderhamn N, Stallknecht B, Ploug T, Schjerling P, et al. Effect of intermittent fasting and refeeding on insulin action in healthy men. Journal of Applied Physiology (1985) 2005;99(6):2128–2136. doi: 10.1152/japplphysiol.00683.2005. [DOI] [PubMed] [Google Scholar]
- 214.Ferrell JM, Chiang JY. Circadian rhythms in liver metabolism and disease. Acta pharmaceutica Sinica B. 2015;5(2):113–122. doi: 10.1016/j.apsb.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Mayeuf-Louchart A, Zecchin M, Staels B, Duez H. Circadian control of metabolism and pathological consequences of clock perturbations. Biochimie. 2017;143:42–50. doi: 10.1016/j.biochi.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 216.Jacobi D, Liu S, Burkewitz K, Kory N, Knudsen NH, Alexander RK, et al. Hepatic Bmal1 Regulates Rhythmic Mitochondrial Dynamics and Promotes Metabolic Fitness. Cell Metabolism. 2015;22(4):709–720. doi: 10.1016/j.cmet.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD, Panda S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(50):21453–21458. doi: 10.1073/pnas.0909591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Yang C, Wu J, Liu X, Wang Y, Liu B, Chen X, et al. Circadian Rhythm Is Disrupted by ZNF704 in Breast Carcinogenesis. Cancer Research. 2020;80(19):4114–4128. doi: 10.1158/0008-5472.CAN-20-0493. [DOI] [PubMed] [Google Scholar]
- 219.Morgan MN, Dvuchbabny S, Martinez CA, Kerr B, Cistulli PA, Cook KM. The Cancer Clock Is (Not) Ticking: Links between Circadian Rhythms and Cancer. Clocks Sleep. 2019;1(4):435–458. doi: 10.3390/clockssleep1040034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Mocellin S, Tropea S, Benna C, Rossi CR. Circadian pathway genetic variation and cancer risk: Evidence from genome-wide association studies. BMC Medicine. 2018;16(1):20. doi: 10.1186/s12916-018-1010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Huber AL, Papp SJ, Chan AB, Henriksson E, Jordan SD, Kriebs A, et al. CRY2 and FBXL3 Cooperatively Degrade c-MYC. Molecular Cell. 2016;64(4):774–789. doi: 10.1016/j.molcel.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liu Z, Selby CP, Yang Y, Lindsey-Boltz LA, Cao X, Eynullazada K, et al. Circadian regulation of c-MYC in mice. Proceedings of the National Academy of Sciences of the United States of America. 2020;117(35):21609–21617. doi: 10.1073/pnas.2011225117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wang Z, Li F, Wei M, Zhang S, Wang T. Circadian Clock Protein PERIOD2 Suppresses the PI3K/Akt Pathway and Promotes Cisplatin Sensitivity in Ovarian Cancer. Cancer Management and Research. 2020;12:11897–11908. doi: 10.2147/CMAR.S278903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Repouskou A, Prombona A. c-MYC targets the central oscillator gene Per1 and is regulated by the circadian clock at the post-transcriptional level. Biochimica et Biophysica Acta. 2016;1859(4):541–552. doi: 10.1016/j.bbagrm.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 225.Boobes Y, Bernieh B, Al Hakim MR. Fasting Ramadan in kidney transplant patients is safe. Saudi Journal of Kidney Diseases and Transplantation. 2009;20(2):198–200. [PubMed] [Google Scholar]
- 226.Abazid RM, Khalaf HH, Sakr HI, Altorbak NA, Alenzi HS, Awad ZM, et al. Effects of Ramadan fasting on the symptoms of chronic heart failure. Saudi Medical Journal. 2018;39(4):395–400. doi: 10.15537/smj.2018.4.22011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Becker M, Karpati T, Valinsky L, Heymann A. The impact of the Yom Kippur fast on emergency room visits among people with diabetes. Diabetes Research and Clinical Practice. 2013;99(1):e12–13. doi: 10.1016/j.diabres.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 228.Currenti W, Godos J, Castellano S, Caruso G, Ferri R, Caraci F, et al. Time-restricted feeding is associated with mental health in elderly Italian adults. Chronobiology International. 2021;38(10):1507–1516. doi: 10.1080/07420528.2021.1932998. [DOI] [PubMed] [Google Scholar]
- 229.O'Connor SG, Boyd P, Bailey CP, Shams-White MM, Agurs-Collins T, Hall K, et al. Perspective: Time-Restricted Eating Compared with Caloric Restriction: Potential Facilitators and Barriers of Long-Term Weight Loss Maintenance. Advances in Nutrition. 2021;12(2):325–333. doi: 10.1093/advances/nmaa168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Rynders, C. A., Thomas, E. A., Zaman, A., Pan, Z., Catenacci, V. A., & Melanson, E. L. (2019). Effectiveness of Intermittent Fasting and Time-Restricted Feeding Compared to Continuous Energy Restriction for Weight Loss. Nutrients, 11(10), 10.3390/nu11102442. [DOI] [PMC free article] [PubMed]
- 231.Villanueva JE, Livelo C, Trujillo AS, Chandran S, Woodworth B, Andrade L, et al. Author Correction: Time-restricted feeding restores muscle function in Drosophila models of obesity and circadian-rhythm disruption. Nature Communications. 2020;11(1):2521. doi: 10.1038/s41467-020-16193-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cava E, Yeat NC, Mittendorfer B. Preserving Healthy Muscle during Weight Loss. Advances in Nutrition. 2017;8(3):511–519. doi: 10.3945/an.116.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Gasmi M, Sellami M, Denham J, Padulo J, Kuvacic G, Selmi W, et al. Time-restricted feeding influences immune responses without compromising muscle performance in older men. Nutrition. 2018;51–52:29–37. doi: 10.1016/j.nut.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 234.Tinsley GM, Paoli A. Time-restricted eating and age-related muscle loss. Aging (Albany NY) 2019;11(20):8741–8742. doi: 10.18632/aging.102384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Grajower MM, Horne BD. Clinical management of intermittent fasting in patients with diabetes mellitus. Nutrients. 2019;11(4):873. doi: 10.3390/nu11040873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Veldscholte K, Cramer ABG, Joosten KFM, Verbruggen S. Intermittent fasting in paediatric critical illness: The properties and potential beneficial effects of an overnight fast in the PICU. Clinical Nutrition. 2021;40(9):5122–5132. doi: 10.1016/j.clnu.2021.07.030. [DOI] [PubMed] [Google Scholar]
- 237.Yin C, Li Z, Xiang Y, Peng H, Yang P, Yuan S, et al. Effect of Intermittent Fasting on Non-Alcoholic Fatty Liver Disease: Systematic Review and Meta-Analysis. Frontiers in Nutrition. 2021;8:709683. doi: 10.3389/fnut.2021.709683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Memel ZN, Wang J, Corey KE. Intermittent Fasting as a Treatment for Nonalcoholic Fatty Liver Disease: What Is the Evidence? Clin Liver Dis (Hoboken) 2022;19(3):101–105. doi: 10.1002/cld.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, Rumpler WV, et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. American Journal of Clinical Nutrition. 2007;85(4):981–988. doi: 10.1093/ajcn/85.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annual Review of Nutrition. 2017;37:371–393. doi: 10.1146/annurev-nutr-071816-064634. [DOI] [PubMed] [Google Scholar]
- 241.Lowe DA, Wu N, Rohdin-Bibby L, Moore AH, Kelly N, Liu YE, et al. Effects of Time-Restricted Eating on Weight Loss and Other Metabolic Parameters in Women and Men With Overweight and Obesity: The TREAT Randomized Clinical Trial. JAMA Internal Medicine. 2020;180(11):1491–1499. doi: 10.1001/jamainternmed.2020.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Bjerre N, Holm L, Quist JS, Faerch K, Hempler NF. Watching, keeping and squeezing time to lose weight: Implications of time-restricted eating in daily life. Appetite. 2021;161:105138. doi: 10.1016/j.appet.2021.105138. [DOI] [PubMed] [Google Scholar]