Abstract
The increased population in megacities has recently exacerbated the need to combat air pollution. This study examined the concept that the sensitivity and tolerance of urban plant species to air pollution might be used to determine Tehran, Iran's air quality and obtain suitable urban greening. The air pollution tolerance index (APTI) was derived using the total chlorophyll, relative water content, pH, and ascorbic acid content of leaf extract from Morus alba, Ailanthus altissima, and Salix babylonica trees as an indicator of the sensitivity and tolerance of urban plant species. A. altissima and S. babylonica, with APTI values of 11.15 and 11.08, respectively, were sensitive to air pollution and can be employed as bioindicators, whereas M. alba, with an APTI value of 14.08, exhibited moderate resistance to air pollution and is therefore recommended for urban planting. Furthermore, the content of enzymatic and non-enzymatic parameters (carotenoid, phenol, and flavonoids) and proline concentration in the polluted seasons and sites (3 and 4) have been increased in M. alba. Collectively, we expect our findings to contribute to the rapidly growing body of research aiming to find a suitable urban greening for a wide range of polluted megacities.
Subject terms: Plant sciences, Environmental sciences, Environmental impact
Introduction
Air pollution is the main cause of various diseases, and it is one of the most concerning environmental issues, causing more than 40,000 deaths each year1,2. Among diverse air pollutants, particulate matter (PM), nitric oxide (NO), lead (Pb), and carbon monoxide (CO) are the most dangerous pollutants, which have negative impacts on humans, animals, and plant species3. By filtering air pollution and reducing human exposure to anthropogenic contaminants, tree planting in urban settings is a low-cost, effective strategy4. Tree parts such as leaves and bark can absorb air pollutants, and they are good proxies to assess the status of air quality in polluted urban areas and parks3.
One of the parameters related to the responses of plant species is the air pollution tolerance index (APTI). It consists of total chlorophyll, relative water content, pH of leaf extracts, and ascorbic acid, and it is used to indicate plants sensitivity or tolerance to air pollution5. When plants are exposed to air pollutants, reactive oxygen species (ROS) are enhanced, which first leads to oxidative stress and eventually causes the cell death procedure6,7. Different mechanisms are used for detoxification of ROS in plants, including a) activating enzymatic antioxidants such as catalase, ascorbate peroxidase (APX), and phenylalanine ammonia-lyase (PAL); b) activating hydrophilic (such as ascorbate and glutathione) and lipophilic (carotenoid) compounds8–10. According to Krishnaveni11, phenol and flavonoid compounds are utilized as antioxidants as their amounts change under oxidative stresses and the scavenging of ROS. When plants are subjected to abiotic stress, proline is accumulated in their tissues with osmoprotectant activities12. Proline has four functions: (a) alimental storage for growth, (b) proteins and membrane stabilization, (c) the scavenging of free radicals, and (d) acting as an antioxidant system13–15.
According to Alotaibi et al.16, the responses of five tree species to air pollution were assessed in Riyadh City, Saudi Arabia. The APTI value of the trees in four sites was measured and the highest tolerance of them, which is Ficus altissima Benth, was proposed to be applied in the green spaces of the city. Manjunath and Reddy17 reported that Bougainvillea spectabilis willd. and Vinca rosea L. plants had the highest tolerance to the pollution stressors and can be used in urban areas to develop greenbelts. In two urban regions of Tehran, the physiological responses, including proline, protein content, and activity of the nitrate reductase enzyme, of Laurus nobilis L. to air pollution were studied18. Additionally, in response to the urban air pollution by heavy metals (Cd, Pb, Ni, Zn, and Cr), the anatomical and certain physiological features (pigment contents and enzymatic antioxidants) of Platanus orientalis L. leaves in two urban areas of Tehran were measured19.
Tehran is one of the most air-polluted and most populated cities in western Asia. Urbanization, industrial development, consumption of low-quality fuels, rapid population growth, and the topography of the city are the main causes of air pollution in Tehran. Particulate matter (PM2.5) is the most significant air pollutant, with an annual concentration three times higher than the maximum range of national and WHO standards. PM poses high health risks by penetrating the lungs and entering the bloodstream20. Therefore, creating appropriate green areas and parks through choosing tolerant trees, which can control air pollution in Tehran, is vital.
This study aims to examine the reactions of trees to air pollution in several Tehran locations during the spring, summer, and autumn to obtain a suitable urban greening for controlling air pollution in this city. To determine tolerant and sensitive trees, the effects of air pollution on the leaves of Morus alba, Ailanthus altissima, and Salix babylonica, which are common deciduous trees in Tehran, were studied. These three tree species are utilized in the urban ecosystems of Iran due to their high absorption capacity for air pollutants and their ability to avoid various types of environmental pollution, as well as their economic and social benefits for the city's inhabitants4,21. The hypothesis is that trees with high APTI values are more resistant to air pollution and more suitable for urban greening than trees with low APTI values. The question is what the link is between the APTI value, the activity and content of antioxidants (enzymatic and non-enzymatic) and the proline concentration of various tree species in various regions and seasons. To identify tolerant and sensitive trees, the effects of air pollution on the leaves of Morus alba, Ailanthus altissima, and Salix babylonica, three common deciduous trees in cities, were investigated using antioxidant activity and content (enzymatic and non-enzymatic), as well as proline concentration. The APTI index was calculated using the overall chlorophyll content, relative water content, ascorbic acid concentration, and pH. Based on the APTI values, trees are classified as sensitive or tolerant. Based on the APTI values, trees are classified as sensitive or tolerant and recommended for future urban greening of megacities.
Material and methods
Study area
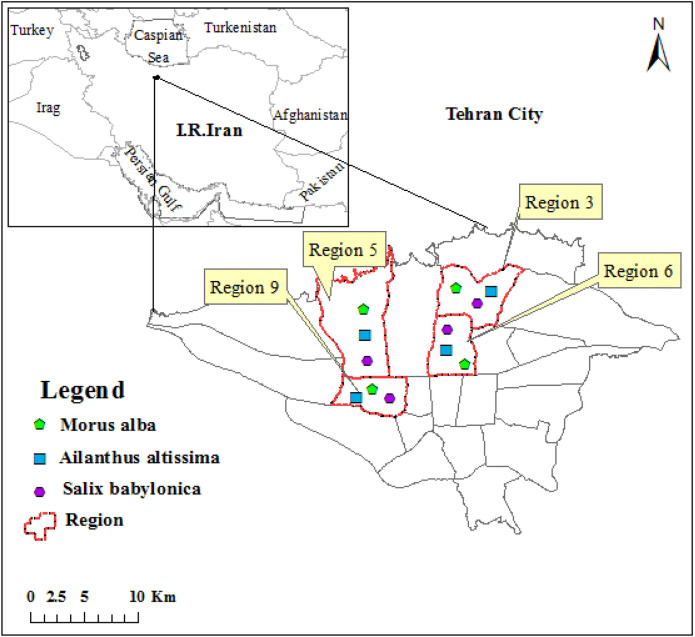
Tehran is one of the largest Middle Eastern megacity22,23, which has 22 regions with different air pollution monitoring stations in each region. The air quality index on the different days of plant species sampling has shown in the supplementary file. Given the information from air pollution monitoring stations data in the city, four sites were selected for sampling tree species in the different seasons of the year, including Mirdamad Street (region 3 or site 1), Kargar Shomali Street (region 6 or site 3), and Azadi Bus Terminal Street (region 9 or site 4) as polluted sites, and Mirza Babaei Street (region 5 or site 2) as a control site. The map of study area was drawn by software of ArcGIS version of 10.5, https://desktop.arcgis.com (Fig. 1). Mirza Babaei Street and Azadi Bus Terminal had the lowest and highest levels of air pollution, respectively, among the studied districts.
Figure 1.
Map of the study sites in Tehran, Iran (drawn by H. Dadkhah-Aghdash using software of ArcGIS Desktop. version of 10.5. ESRI, California, US. https://desktop.arcgis.com).
Plant samples collection
All procedures were followed in compliance with institutional, national, and international rules and legislation. The formal identification of Morus alba L. (white mulberry), Ailanthus altissima (Mill.) Swingle (tree-of-heaven), and Salix babylonica L. (willow) was performed based on the colorful flora of Iran24. Permissions or licenses were secured to collect these trees in the Tehran metropolis. These trees voucher specimens were gathered and deposited in a herbarium with the Department of Plant Biology, Tarbiat Modares University.
A total of 270 samples (three different tree species × three trees of each species × ten leaves from each tree × three seasons of the year) were sampled at each studied site. It is noteworthy that leaf samples were collected from different levels and heights of canopy cover trees with equal age and breast diameter in late spring (May 31), summer (September 22), and autumn (November 15) in 2019. Further details of the trees can be found in Supplementary Information (Figure S1). Details of the concentration of air pollutants on sampling days can be found in Supplementary Information (Figures S2, S3, and S4). Leaf samples were transported to the laboratory using a portable ice box for biochemical and physiological analysis. Leaf samples were washed and analyzed on the same day and were stored at −80 °C in the freezer5.
This study aims to examine the reactions of trees to air pollution in several Tehran locations during the spring, summer, and autumn. During these seasons, leaf samples from the examined trees were gathered. The total chlorophyll content, relative water content, ascorbic acid concentration, and pH were determined. The APTI value was computed using these four parameters. According to the obtained APTI value, the studied trees in the summer and autumn had a greater degree of tolerance than in the spring. On the other hand, according to the air quality index established by air pollution monitoring stations of the city, the spring season had relatively good and clean air quality compared to the summer and autumn seasons. Due to the aforementioned factors, the biochemical parameters associated with air pollution stress tolerance, including enzymatic and non-enzymatic antioxidants and proline, were examined in the summer and autumn seasons.
The air pollution tolerance index assays
Total chlorophyll content assay
Leaf samples with similar shapes and ages were selected for biochemical and physiological analyses. Crushed fresh leaves were extracted in 80% (v/v) acetone, and light absorbance at 646.8 and 663.2 nm was measured using a spectrophotometer (Perkin Elmer-USA). Acetone 80% was used as a control for the solution. Calculations were conducted using the following equation25:
| 1 |
where A663.2 and A646.8 refer to the absorbance values of the corresponding wavelengths. Chl T is the total chlorophyll concentration of the solution. Chlorophyll content was expressed according to the volume of the extract and the weight of the samples based on milligrams per gram of fresh weight (mg g−1 FW).
Relative water content (RWC) assay
| 2 |
where RWC is relative water content (%), was calculated according to the equation of 2. FW is the fresh weight (g), DW is the dry weight (g) of leaves under 3 h oven-drying treatment at 105 °C and TW is the leaf turgid weight (g) obtained by overnight immersion in deionized water26,27.
pH of leaf extracts assay
To measure the pH of the leaf extract, 0.5 g of fresh leaves were crushed and homogenized in 50 ml of deionized water and centrifuged at 3000 rpm for 15 min. The supernatant pH was determined using a digital pH meter (Fan Azma Gostar Company, Iran).
Ascorbic acid concentration assay
The ascorbic acid concentration was measured according to the method reported by Kampfenkel et al.28. Fresh leaves weighing as much as 0.5 g were grinded with 10 ml of 5% metaphosphoric acid solution, and the mixture was centrifuged at 10,000g for 15 min. Then, 0.3 ml of a centrifuged extract was thoroughly mixed with 0.75 ml of potassium phosphate buffer and 0.3 ml of distilled water by Vortex for 10 min at room temperature. The solution was incubated for 40 min at 40 °C with 10% Trichloroacetic acid (TCA), 0.6 ml of 44% Orthophosphoric acid, 0.6 ml of 4% Dipyridyl, and 10 L of FeCl3. Finally, the light absorbance was read at 525 nm by a spectrophotometer and the ascorbic acid content was calculated using the standard curve in mg g−1 fresh weight.
Air pollution tolerance index determination
APTI of plant species is determined by the formula introduced by29,30 as following:
| 3 |
A is the ascorbic acid content of the leaf in mg g−1 FW; T is the total chlorophyll content in mg g−1 FW, P is the pH of the leaf extract, and R is the percentage of relative water content. Plants with APTI values ranging between 17–30, 12–16, 1–11, and < 1 were considered tolerant, intermediate tolerant, sensitive, and very sensitive, respectively31.
The assay of enzymatic antioxidants
Fresh leaf samples (0.5 g) were homogenized in 0.8 mL of 100 mM potassium-phosphate buffer (pH 7) and then were centrifuged at 15,000g for 15 min. The supernatants were used for the determination of enzyme activities.
Assessment of catalase activity
According to the method of Dhindsa et al.32, the decrease of hydrogen peroxide absorbance at 240 nm was measured for 1 min. The reaction mixture is 3 mL (containing 2800 μL of 50 mM potassium phosphate buffer (pH 7.0), 100 μL of 15 mM hydrogen peroxide, and 100 μL of the enzyme extract). The reduction in the amount of hydrogen peroxide per minute at a wavelength of 240 nm using a spectrophotometer is considered the unit of enzymatic activity.
Assessment of ascorbate peroxidase activity
The reaction mixture contains 50 mM potassium phosphate buffer (pH 7), 0.5 mM ascorbate, 0.1 mM hydrogen peroxide, and 150 mM enzyme extract, which was measured with a spectrophotometer at 290 nm for 1 min based on ascorbic acid oxidation and decrease33.
Assessment of phenylalanine ammonia-lyase activity
According to the method of D'Cunha et al.34, about 600 μL of 50 mM Tris buffer (pH 8.8) was used with 900 μL of 2 mM phenylalanine and 800 μL of enzymatic extract for phenylalanine ammonia-lyase assay. The production of cinnamic acid from phenylalanine was stopped by adding 100 μL of hydrochloric acid. Then, 2 ml of toluene was added and centrifuged at 5000 rpm. The upper layer containing trans-cinnamic acid was read at 290 nm by a spectrophotometer.
The non-enzymatic antioxidant assays
Carotenoid, total phenol, and total flavonoid content in the leaves were assessed by different protocols.
Carotenoid concentration assessment
Based on Lichtenthaler's method25, crushed fresh leaves were extracted in 80% (v/v) acetone and the light absorbance was read by a spectrophotometer at 470 nm. Acetone 80% was used as a control for the solution. Calculations were conducted using the following formula:
| 4 |
where A470 refers to the absorbance value of the corresponding wavelength. Carotenoid content was expressed according to the volume of the extract and the weight of the samples based on milligrams per gram of fresh weight (mg g−1 FW).
Determination of total phenol
Leaf samples were crushed with 10 ml of 85% methanol. For the assay, 300 µl of the extract were combined with 1500 µl of diluted Folin reagent (1:10 ratio). After 5 min, 1200 µl of 7% sodium carbonate was added and after 90 min absorbance was read by spectrophotometer at 760 nm. Compared to the standard curve of Gallic acid, total phenolic content was expressed in micrograms of Gallic acid per dry weight35.
Determination of total flavonoid
Based on method Kaijv36, 75 µl (5% w/v NaNO2), 0.15 ml AlCl3 (10% w/v) and 0.5 ml NaOH (1 M) distilled water were added to the leaf extract sample (0.25 ml), and the final volume reached 2.5 ml. After 5 min, the absorbance of the solution was read by the spectrophotometer at 507 nm.
Proline concentration assay
Fresh leaves were dissolved in 10 ml of 3% sulfosalicylic acid solution and then centrifuged at 20,000g for 3 min. Also, 2 ml of supernatant was mixed with 2 ml of ninhydrin reagent and 2 ml of pure acetic acid for 1 h at 100 °C in a warm water bath. After the samples were cooled at room temperature, 4 ml of toluene was added to them. The colored upper layer containing toluene and proline was used to measure the l-proline concentration by a UV/vis spectrophotometer at 520 nm37.
Statistical analysis
Before analysis of variance, the normal distribution and homology of variances were checked by statistical analysis software (SAS) using Shapiro–Wilk and Kolmogorov–Smirnov tests. The physiological and biochemical traits of leaves have two factors, including the season in three levels (spring, summer, and autumn) and sites in four levels (1, 2, 3, and 4) with three replications were analyzed in a completely randomized design. Analysis of variance by two-way ANOVA and a comparison of the means of all measured parameters was performed using the Duncan test at P < 0.05 by SPSS software version 24.
Results
Air pollution tolerance index
Total chlorophyll content
The results of total chlorophyll contents confirmed that A. altissima in spring at site 3 has the highest chlorophyll content (1.72 mg g−1 FW) compared to all evaluated sites. The chlorophyll contents in summer were 6.3 and 2.52 mg g−1 FW for sites 2 and 1, respectively. Moreover, the highest and lowest contents were 1.04 and 0.57 mg g−1 FW at sites 4 and 2 during the autumn. The chlorophyll content of S. babylonica at site 4 was noticeably higher than at other sites for all three seasons (P < 0.05). At sites 3 and 4, M. alba showed a noticeable increment in chlorophyll content in comparison to sites 1 and 2 (P < 0.05, Table 1).
Table 1.
Comparing the effect of air pollution stress on the total chlorophyll concentration (fresh weight, FW) and relative water contents of A. altissima, S. babylonica, and M. alba trees.
| Trees | Sites | Total chlorophyll contents (mg g−1 FW) | Relative water contents (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Spring | Summer | Autumn | Spring | Summer | Autumn | |||
| A. altissima | 1 | 1.39op ± 0.005 | 2.52j ± 0.02 | 1.05t ± 0.01 | 50ghi ± 0.03 | 64.82d-h ± 0.13 | 66.4bcd ± 0.10 | |
| 2 | 1.26qr ± 0.01 | 6.3c ± 0.05 | 0.57w ± 0.01 | 50.66hij ± 0.14 | 70.77a-g ± 0.06 | 79.25abc ± 0.17 | ||
| 3 | 1.72n ± 0.01 | 5.37d ± 0.01 | 1.37op ± 0.05 | 56.3d-i ± 0.09 | 66.53a-g ± 0.10 | 72.69c-h ± 0.07 | ||
| 4 | 1.28q ± 0.01 | 4.69 g ± 0.05 | 1.04t ± 0.02 | 50.66hij ± 0.07 | 47.58hij ± 0.01 | 62.4c ± 0.02 | ||
| S. babylonica | 1 | 1.06t ± 0.02 | 3.31i ± 0.01 | 1.35qp ± 0.03 | 48.33hij ± 0.03 | 63.86c-h ± 0.08 | 65.69b ± 0.16 | |
| 2 | 1.27q ± 0.008 | 3.26i ± 0.005 | 1.9 lm ± 0.02 | 85.9c-h ± 0.19 | 48.55hij ± 1.8 | 84.89abc ± 0.85 | ||
| 3 | 1.7n ± 0.008 | 3.72 h ± 0.02 | 2.09 k ± 0.01 | 34.33 k ± 0.06 | 82.38a-d ± 0.08 | 89.5a ± 0.11 | ||
| 4 | 1.95 l ± 0.02 | 4.85f ± 0.08 | 1.87 lm ± 0.02 | 49ij ± 0.38 | 84.4cde ± 0.11 | 74.9a-e ± 0.20 | ||
| M. alba | 1 | 0.83u ± 0.02 | 4.94e ± 0.01 | 1.01t ± 0.04 | 54f-i ± 0.03 | 72.9a-g ± 0.14 | 86.3ab ± 0.45 | |
| 2 | 0.89u ± 0.006 | 5.35d ± 0.02 | 1.08° ± 0.01 | 67c-i ± 0.24 | 86.63c-h ± 0.02 | 89.22a ± 0.39 | ||
| 3 | 1.45° ± 0.02 | 6.4b ± 0.09 | 1.17rs ± 0.01 | 62.66e-i ± 0.24 | 60.9e-i ± 0.27 | 80.55abc ± 0.27 | ||
| 4 | 1.83 m ± 0.02 | 7.1a ± 0.005 | 1.17 s ± 0.08 | 63.3d-i ± 0.27 | 81.87a-d ± 0.05 | 76.17a-e ± 0.31 | ||
Three seasons of Spring, Summer, and Autumn at Sites 1 (Mirdamad Street), 2 (Mirza Babaei Street), 3 (Kargar Shomali Street), and 4 (Azadi Bus Station Street) Tehran. The means that have at least one common letter did not show a statistically significant difference (P < 0.05). Data are presented as mean ± SE.
Relative water content
As shown in Table 1, A. altissima showed no significant difference at all sites during the spring and autumn seasons. The lowest content was observed at site 4 (47.58%) in summer. Besides, we detected that S. babylonica had no significant changes in the spring and autumn seasons at sites 1, 2, and 4. In summer, the highest water content was observed at site 3 (4.85%). There was no significant difference (P > 0.05) in the relative water content of M. alba at all sites in the three seasons (Table 1).
pH
The leaf pH of A. altissima was 5.20 and 3.46, in spring at sites 3 and 4, respectively which are the highest and lowest pH values. Among all sites, trees at site 4 had high ascorbic acid concentration, whereas, site 2 had low value. Regardless of sites, the pH value concentrations measured for A. altissima increased with changing seasons from spring (4.76, site 1; 3.96, site 2; 3.46, site 4) and summer (5.4, site 1; 4.9, site 2; 5.13, site 4) to autumn (5.53, site 1; 5.46, site 2; 5.73, site 4). Besides, we detected pH values of 6.0 and 4.9 for S. babylonica leaves at sites 1 and 3, respectively. During the autumn and summer, when compared to all sites, site 1 had a high pH concentration. Based on the Table 2 data, the pH of M. alba leaves was in the range of 5.8 to 7.4, which was the highest measured value among all tree types.
Table 2.
Comparison of the air pollution stress effect on the pH and Ascorbic acid concentration (fresh weight, FW) of A. altissima, S. babylonica, and M. alba trees.
| Trees | Sites | pH | Ascorbic acid concentration (mg g−1 FW) | ||||
|---|---|---|---|---|---|---|---|
| Spring | Summer | Autumn | Spring | Summer | Autumn | ||
| A .altissima | 1 | 4.76° ± 0.12 | 5.4i-o ± 0.11 | 5.53 h-m ± 0.20 | 5.2ijk ± 0.20 | 4.55klm ± 0.14 | 7.01 g ± 0.06 |
| 2 | 3.96p ± 0.14 | 4.9no ± 0.10 | 5.46 h-n ± 0.08 | 3.66nop ± 0.14 | 3.91 m-p ± 0.14 | 5.72hi ± 0.17 | |
| 3 | 5.20 k-o ± 0.11 | 5.55 h-m ± 0.20 | 5.4i-o ± 0.05 | 6.71 g ± 0.33 | 5.4i ± 0.22 | 9.83 cd ± 0.12 | |
| 4 | 3.46q ± 0.26 | 5.13 l-o ± 0.08 | 5.73 g-k ± 0.23 | 7.8f ± 0.10 | 6.67 g ± 0.22 | 11.04b ± 0.07 | |
| S. babylonica | 1 | 5.36i-o ± 0.18 | 5.7 h-l ± 0.30 | 6d-h ± 0.5 | 3.89 m-p ± 0.20 | 4.32lmn ± 0.31 | 6.79 g ± 0.05 |
| 2 | 5.06mno ± 0.12 | 5.33i-o ± 0.03 | 5.86e-i ± 0.34 | 3.37p ± 0.26 | 3.66nop ± 0.18 | 5.58i ± 0.15 | |
| 3 | 4.90no ± 0.10 | 5.53 h-m ± 0.23 | 5.43 h-m ± 0.06 | 4.63j-m ± 0.22 | 5.31ij ± 0.12 | 8.56e ± 0.06 | |
| 4 | 5.53 h-m ± 0.13 | 5.23j-o ± 0.12 | 5.53 h-m ± 0.03 | 5.54i ± 0.42 | 6.34gh ± 0.16 | 9.34 cd ± 0.10 | |
| M. alba | 1 | 6.93ab ± 0.37 | 6.53bcd ± 0.14 | 6.26c-g ± 0.21 | 4.69jkl ± 0.61 | 4.12mno ± 0.09 | 9.25d ± 0.10 |
| 2 | 5.80f-j ± 0.6 | 6.53bcd ± 0.14 | 5.83e-i ± 0.16 | 4.52klm ± 0.44 | 3.49op ± 0.09 | 8.09ef ± 0.19 | |
| 3 | 6.33cde ± 0.08 | 7.4a ± 0.05 | 7.26a ± 0.12 | 7.75f ± 0.29 | 6.33gh ± 0.08 | 9.90c ± 0.10 | |
| 4 | 6.60bc ± 0.15 | 6.7bc ± 0.20 | 6.36cde ± 0.13 | 7.75e ± 0.20 | 7.8f ± 0.41 | 13.04a ± 0.13 | |
Three seasons of Spring, Summer, and Autumn at Sites 1 (Mirdamad Street), 2 (Mirza Babaei Street), 3 (Kargar Shomali Street), and 4 (Azadi Bus Station Street) Tehran. The means that have at least one common letter did not show a statistically significant difference (P < 0.05). Data are presented as mean ± SE.
Ascorbic acid
The next section of Table 2 illustrates the detected ascorbic acid concentration values. The ascorbic acid concentration varies between 3.37 to 13.04 mg g−1. Interestingly, the measured ascorbic acid content value in autumn was more than in summer and spring. In detail, for A. altissima, 7.8, 6.67, and 11.04 mg g−1 ascorbic acid concentrations were reported at site 4. Also, S. babylonica had 5.54, 6.34, and 9.34 mg g−1 ascorbic acid concentrations. M. alba had 7.75, 7.8, and 13.04 mg g−1 concentrations.
Air pollution tolerance index
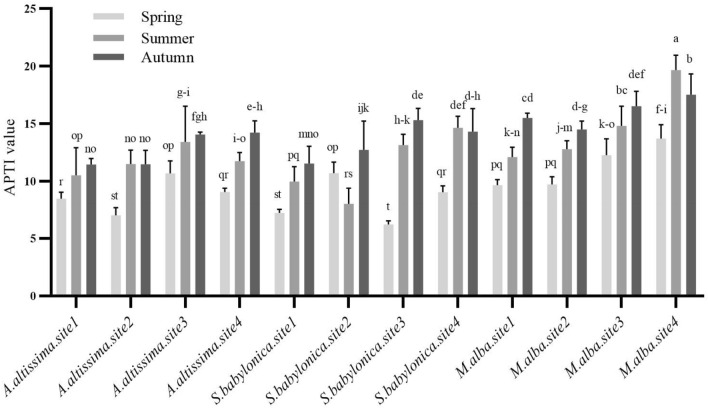
Compared to the spring and summer, A. altissima had high APTI values in autumn regardless of examined sites. A. altissima had 10.69 and 7.01 APTI values at sites 3 and 2, respectively. In summer, the highest APTI value of A. altissima was observed at site 3 (13.43). Further, the APTI values at sites 3 and 4 were significantly higher than at sites 1 and 2 in autumn (P < 0.05). On the other hand, the APTI value of S. babylonica peaked at 10.72 in spring at site 2, whereas the minimal APTI value, 6.21, was at site 3. In summer, the top and lowest APTI values at sites 4 and 2 were 14.65 and 8 for S. babylonica, respectively. In autumn sites 3 and 4 showed noticeable changes and had higher APTI values than sites 1 and 2 (P < 0.05). Finally, the APTI value of M. alba in the spring, summer and autumn seasons at sites 3 and 4 were significantly higher than sites 1 and 2 (P < 0.05). M. alba had the highest APTI value (14.08) among all tree types (Fig. 2).
Figure 2.
APTI values of A. altissima, S. babylonica, and M. alba trees in Spring, Summer, and Autumn seasons at sites 1 (Mirdamad Street), 2 (Mirza Babaei Street), 3 (Kargar Shomali Street), and 4 (Azadi Bus Station Street) of Tehran. The means that have at least one common letter did not show a statistically significant difference (P < 0.05). Data are presented as mean ± SE.
The enzymatic antioxidants
Among the investigated sites, the uppermost catalase activity was reported for A. altissima in summer at site 3 (0.95 ∆OD min−1; P < 0.05). While the highest catalase activity was observed in autumn, site 3, the lowest catalase activity was at sites 1 and 4. The catalase activity of S. babylonica in the summer and autumn seasons at sites 3 and 2 were significantly higher than at sites 1 and 4 (P < 0.05). Moreover, compared to sites 1 and 2, the catalase activity of M. alba was significantly high (P < 0.05) in the summer and autumn seasons at sites 3 and 4. The highest and lowest catalase activity of M. alba was observed at sites 3 and 2, respectively (Table 3). S. babylonica had significantly higher ascorbate peroxidase activity in summer, at sites 1 and 3 than at sites 2 and 4. In autumn, minimal ascorbate peroxidase activity was observed at site 2 (0.11 ∆OD min−1). Contrary to the summer, ascorbate peroxidase activity of M. alba in autumn at sites 3 and 4, was significantly higher than at sites 1 and 2 (P < 0.05). Notably, the phenylalanine ammonia-lyase activity of A. altissima, S. babylonica, and M. alba trees were significantly higher in the summer and autumn seasons at sites 3 and 4 than at sites 1 and 2 (P < 0.05; Table 3).
Table 3.
Comparing the effect of air pollution stress on the catalase, ascorbate peroxidase and PAL activities of A. altissima, S. babylonica, and M. alba trees.
| Trees | Sites | Catalase activity (∆OD min−1) | Ascorbate peroxidase activity (∆OD min−1) | PAL activity (μΜ cinnamic acid min−1) | |||
|---|---|---|---|---|---|---|---|
| Summer | Autumn | Summer | Autumn | Summer | Autumn | ||
| A. altissima | 1 | 0.45 fg ± 0.01 | 0.15j-l ± 0.01 | 0.20d-h ± 0.005 | 0.34bc ± 0.02 | 1.29p ± 0.38 | 2.96i ± 0.3 |
| 2 | 0.44 fg ± 0.03 | 0.49e-g ± 0.04 | 0.34bc ± 0.03 | 0.23c-g ± 0.21 | 0.92q ± 0.48 | 1.92 m ± 0.28 | |
| 3 | 0.95ab ± 0.01 | 0.62c-e ± 0.01 | 0.2d-h ± 0.02 | 0.5a ± 0.01 | 1.76n ± 0.23 | 3.8e ± 0.16 | |
| 4 | 0.45 fg ± 0.08 | 0.14j-l ± 0.01 | 0.35bc ± 0.01 | 0.04i ± 0.005 | 1.97 m ± 0.45 | 4.52c ± 1.1 | |
| S. babylonica | 1 | 0.12j-l ± 0.01 | 0.28 h-j ± 0.05 | 0.25c-f ± 0.01 | 0.3b-d ± 0.003 | 0.89q ± 0.21 | 3.22b ± 0.74 |
| 2 | 0.45 fg ± 0.01 | 0.33 g-i ± 0.01 | 0.03i ± 0.02 | 0.11f-i ± 0.02 | 0.64p ± 0.1 | 2.5 k ± 0.63 | |
| 3 | 0.53d-f ± 0.03 | 0.65 cd ± 0.01 | 0.31b-d ± 0.01 | 0.27c-e ± 0.02 | 1.24p ± 0.57 | 3.61f ± 1.01 | |
| 4 | 0.12j-l ± 0.01 | 0.21i-k ± 0.03 | 0.05i ± 0.003 | 0.35bc ± 0.01 | 1.55° ± 0.16 | 4.15d ± 0.19 | |
| M. alba | 1 | 0.71c ± 0.003 | 0.87ab ± 0.006 | 0.06hi ± 0.02 | 0.02i ± 0.02 | 2.57 k ± 0.55 | 4.12d ± 0.56 |
| 2 | 0.34 g-i ± 0.003 | 0.43f-h ± 0.01 | 0.06hi ± 0.10 | 0.07hi ± 0.03 | 2.42i ± 0.66 | 2.82j ± 0.86 | |
| 3 | 1.05a ± 0. 03 | 1.02ab ± 0.01 | 0.16e-i ± 0.01 | 0.25c-f ± 0.04 | 2.96i ± 0.82 | 6.41b ± 1.7 | |
| 4 | 0.89ab ± 0. 02 | 0.96b ± 0.02 | 0.1 g-i ± 0.02 | 0.43ab ± 0.02 | 3.5 g ± 1.1 | 8.17a ± 1.3 | |
Two seasons of Summer and Autumn at Sites 1 (Mirdamad Street), 2 (Mirza Babaei Street), 3 (Kargar Shomali Street), and 4 (Azadi Bus Station Street) Tehran. The means that have at least one common letter did not show a statistically significant difference (P < 0.05). Data are presented as mean ± SE.
PAL phenyalanine amonia lyase.
The non-enzymatic antioxidants
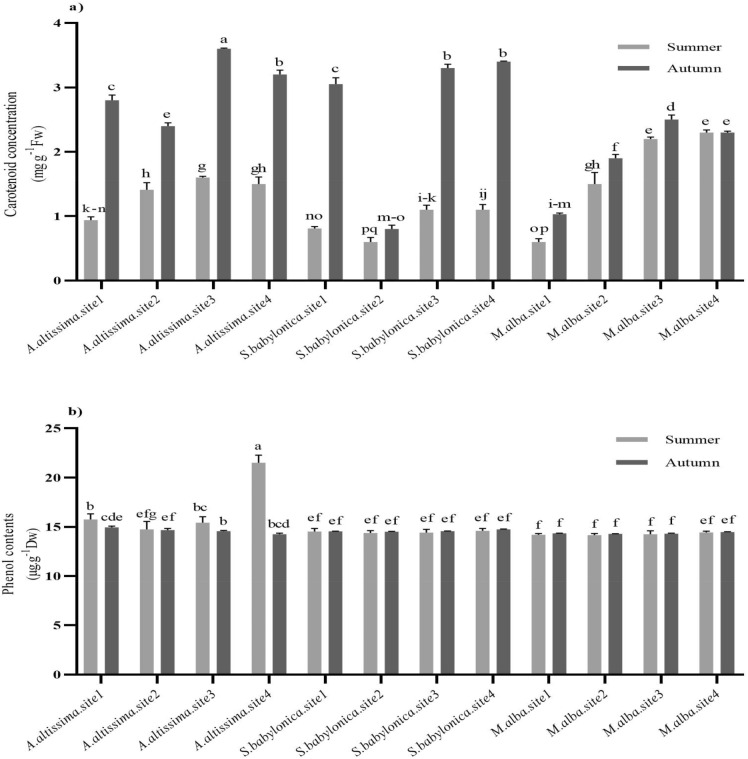
The carotenoid concentration of A. altissima, S. babylonica, and M. alba were significantly high in summer and autumn at sites 3 and 4 in comparison to sites 1 and 2 (P < 0.05; Fig. 3a). Among four evaluated sites, the total phenol content of M. alba and S. babylonica trees in summer and autumn did not change significantly (P > 0.05). A. altissima in summer at site 4 had maximum total phenol content between the other sites (21.51 µg g−1 Dw; Fig. 3b).
Figure 3.
Carotenoid concentrations (a) and phenol contents (b) of A. altissima, S. babylonica, and M. alba trees in spring, summer, and autumn at sites 1 (Mirdamad Street), 2 (Mirza Babaei Street), 3 (Kargar Shomali Street), and 4 (Azadi Bus Station Street) of Tehran. The means that have at least one common letter did not show a statistically significant difference (P < 0.05). Data are presented as mean ± SE.
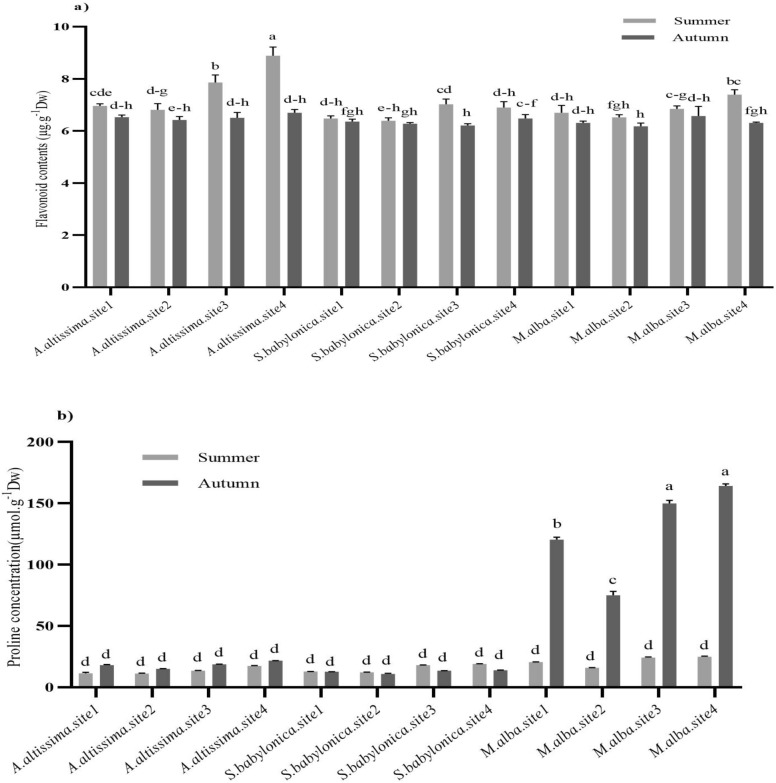
Flavonoid contents of M. alba and A. altissima were significantly higher in summer at sites 3 and 4 than at sites 1 and 2. In summer and autumn, the flavonoid content of S. babylonica did not significantly change at four sites. In autumn, there was no significant difference in flavonoid content of M. alba at four sites (Fig. 4a). Further, regarding the proline concentration of A. altissima and S. babylonica trees, there was no significant difference among the four sites in the summer and autumn seasons (P > 0.05). Proline concentration of M. alba in the summer season was not significantly changed among the four sites. In autumn, sites 3 and 4 had significantly (P < 0.05) higher proline than sites 1 and 2 (Fig. 4b).
Figure 4.
Flavonoid contents (a) and proline concentration (b) of A. altissima, S. babylonica, and M. alba trees in spring, summer, and autumn at sites 1 (Mirdamad Street), 2 (Mirza Babaei Street), 3 (Kargar Shomali Street), and 4 (Azadi Bus Station Street) of Tehran. The means that have at least one common letter did not show a statistically significant difference (P < 0.05). Data are presented as mean ± SE.
Discussions
Trees act as biomonitoring sensors and a remedy to urban air pollution by purifying PM2.5. Choosing suitable plant species is essential for designing urban greening to mitigate air pollution, and it is based on the plant sensitivity and tolerance to air pollution levels38. Although Morus alba, Ailanthus altissima, and Salix babylonica trees are broadleaf plants, they have an important role in the urban ecosystem of Tehran as these trees are distributed extensively on highways and urban green spaces in different areas of the city. Therefore, Morus alba, Ailanthus altissima, and Salix babylonica were selected because they influence directly and indirectly the life of 12 million people in Tehran. Importantly, their leaves are available until the beginning of the winter season, which allow us to measure the APTI values in different seasons. On the other hand, for the first time, this study aimed to evaluate the role of physiological and biochemical responses of Tehran's high distribution trees to air pollution.
Our result indicates that APTI values increased significantly during polluted seasons of the year and at sites in the city. Our findings reveal that M. alba with an APTI of 14.08 was intermediate tolerance to air pollution in Tehran. Likewise, Thespesia populnea and Pongamia pinnata trees exhibited the highest (APTI > 13) and were tolerant to air pollution when 25 tree species were evaluated for their air pollution tolerance in various urban locations of an Indian city. In addition, they performed admirably in controlling urban pollution levels39.
The A. altissima and S. babylonica trees, with APTI of 11.15 and 11.08, were sensitive, suggesting that these trees can be used for air pollution monitoring. Similarly, Anake et al., who evaluated the air pollution tolerance of six mature tree species in different parts of a Nigerian city, discovered that all trees with an APTI < 11 were sensitive to air pollution and might be used as bioindicators for the air pollution of this city31.
Proline regulates osmosis, protects photosynthetic components and proteins and cell membranes from osmotic stress, and increases antioxidants for free radical reduction40. Regarding proline concentration, whilst S. babylonica and A. altissima trees were not significantly different at polluted sites and seasons, M. alba had a higher concentration at the polluted sites. Hence, it appears that high proline concentration protected M. alba from oxidative stress damage and was accompanied by more tolerance to air pollution compared to other trees. These findings are consistent with the results of Rai et al.41 in India, who found that Magnifera indica L. compared to Thevetia neriifolia L. and Psidium guajava L. had the highest proline concentration in leaf organs.
Ascorbic acid is not only an antioxidant that protects plants from environmental threats like air pollution, but it is also a powerful reducing agent that activates many defenses and physiological mechanisms in the plant42,43. Besides, its reducing power is dependent on the pH of the cell and is active at a higher pH44. Our observation revealed that all trees had high ascorbic acid in pollution sites and seasons of the year for tolerance of abiotic stress. Some plants, such as Taraxacum officinale, increase their ascorbic acid levels in areas with high air pollution stress45.
To gain insights into the influence of pH, we analyzed the pH of leaf extracts of trees at all the mentioned sites and seasons. Our data revealed that almost all trees in spring had a low pH, whereas, in the autumn season, the pH was near neutral. Different pollutants could be inserted into tree cells and diminish the pH of their conditions (acidic pH), whereupon A. altissima and S. babylonica with lower pH may be sensitive to air pollution. Compared to other trees, M. alba approximately had neutral pH, showing that it was more tolerant to pollution. A similar result was reached by Joshi et al.46 that low leaf pH has a direct relationship with leaf susceptibility of different plant species to air pollution. Penetration of pollutants induces acidification of the intracellular pH, which is more common in sensitive species. rees in polluted seasons and locations with increased cell pH attempted to convert different hexoses carbohydrates to ascorbic acid. Thus, these mechanisms are different tolerance ways for trees to combat oxidative stress47.
We showed that trees had the highest chlorophyll concentration in summer. In summer and following autumn, air pollution levels were high and trees were tolerant as their chlorophyll levels increased. Besides, they had higher chlorophyll in pollution sites compared to other low pollution sites. In some plants, the dry and wet weight of the leaves and consequently their total chlorophyll increase in leaves that are exposed to air pollution stress. This increase can be a sign of their greater tolerance and resistance to this stress48,49. The chlorophyll content of the leaves of Taraxacum officinale, Plantago lanceolata, Betula pendula, and Robinia pseudoacacia L. is heavily influenced by pollution sources such as heavy traffic, polluted air, and industrial activities45.
The water content of trees changes in different seasons to maintain water potential for cells. M. alba trees maintain relatively higher water content than other trees in different sites and seasons. Other trees had irregular relative water content in pollution conditions, which means they were sensitive to osmotic stress. Therefore, maintaining the cell water content of studied trees was one of the indicators of tree tolerance to air pollution. When plants are confronted with non-biological stress such as air pollution, their water content increases, and physiological equilibrium is maintained, to resist the stress50,51. Our observation indicated that trees at pollution sites and seasons increased their intracellular water content, which led to the well-conducting of different physiological mechanisms. Thus, optimum water content is essential for cells when they are subjected to air pollution52.
It is known that enzymatic activity is high in polluted sites and can be termed a genetic adaptation to reduce the toxic level of hydrogen peroxide and avoid any damage to plant tissue53. Our results showed that examined trees had higher catalase, ascorbate peroxidase, and PAL activities in polluted sites and times of the year. As a sensitive indicator of plant defense changes against environmental stresses, the phenylalanine ammonium lyase enzyme is the initiator of the phenylpropanoid pathway, which is a major biosynthetic pathway of secondary metabolites in plant cells54,55. In our study, when trees were subjected to pollution, the phenylalanine ammonium enzyme was activated and reached its highest level within cells. By subjecting cells to oxidative stress, different metabolites that can respond to stress are activated through signaling pathways such as phenylpropanoid. So, this enzyme had an important function in the tolerance of cell components56.
In this study, all of the trees activate this pathway by phenylalanine ammonium enzymes, which is an important defense mechanism against abiotic stress. This is in line with the previous work by Rai57, which shows that roadside plants respond to air pollution in Aizawl City, India by increasing their catalase and peroxidase activities.
Phenolic metabolites are induced by stress and accumulate in tissues due to biological and non-biological stresses58. They may participate in the removal of reactive oxygen species through antioxidant enzymes or directly chelate metals. By reducing or inhibiting lipid auto-oxidation or decomposing peroxides, they are essential antioxidants to protect against the proliferation and progression of the oxidation chain and protect against reactive oxygen species59,60. Although phenols and flavonoids were present in plants as byproducts of related pathways to the phenylalanine ammonia-lyase enzyme, their concentrations could be increased under oxidative stress. In contrast to the M. alba and S. babylonica trees, in which the phenolic and flavonoid activities were unchanged, in A. altissima an increase was recorded. Under conditions of high air pollution, the genetic responses of these trees activate the phenylpropanoid pathway. Thus, different responses of trees and pollution conditions in the Tehran metropolis indicated that secondary metabolites such as total phenol and total flavonoid were different in our study. Our results were broadly in line with other results that oak species such as Quercus pubescens L. and Quercus ilex L. unchanged their total phenols because they have a superior ability to counteract oxidative stress56.
Carotenoid is one of the antioxidants that plays an important function in the tolerance of oxidative stresses61. Plants appear to be trying to protect chlorophyll from damage by increasing the concentration of carotenoids62. When cells are subjected to abiotic stress, the cell membrane is damaged and the level of thylakoid and chlorophyll in the chloroplast is diminished63.
Carotenoids are pigments whose biosynthesis pathway is similar to chlorophyll which protects them from photooxidation and oxidative stress. Therefore, the concentrations of chlorophyll and carotenoids were related to each other in the chloroplast structure64. Our findings demonstrated that carotenoid concentrations increased in different seasons in polluted sites to reduce photooxidative stress effects on chlorophyll components. This is consistent with a finding by Pellegrini et al.56, that showed where Quercus ilex L. trees are affected by different stresses, carotenoids concentrations increased with the diminishing of peroxidation free radicals from these stresses.
Tree species are the main components of urban greening, and different factors influence their survival in cities. This research measured the tolerance mechanisms of trees to environmental stress such as air pollution65. There were certain limitations, including the sampling of PM2.5 in the area surrounding the tree canopy and the evaluation of hydraulic pathways in the wood and phloem vessels of trees. These measurements are valuable for future research. In addition, it is suggested that managers and designers of urban ecosystems take into account the amount of evapotranspiration and the water requirements of trees in future urban greening in water-stressed cities such as Tehran.
Conclusions
To develop suitable green infrastructure to remediate and manage air pollution in Tehran, Iran, the tolerance of Morus alba, Ailanthus altissima, and Salix babylonica were evaluated in four regions of this megacity at different times of the year based on the APTI values. This research shows that Morus alba with a high APTI value had a medium-to-high tolerance to air pollution, while Ailanthus altissima and Salix babylonica with a low APTI value were sensitive to air pollution. All trees, especially M. alba in autumn and sites 3 and 4, had higher APTI values than other seasons and sites of the city. The PAL activity and carotenoid concentration of trees were significantly higher in summer and autumn at sites 3 and 4 than at sites 1 and 2. The total phenol content of trees did not change significantly at the four evaluated sites. The M. alba, unlike other trees that did not differ in proline concentration, had the highest proline in autumn and at sites 3 and 4. Considering these findings, Morus alba is suggested to be utilized as the main tree in designing resistant urban greening to mitigate air pollutant risks. Overall, our observations revealed that selecting suitable plants, which can be a remedy against air pollution and act as an indicator for measuring air quality, is an innovative and emerging strategy for future environmental management of megacities.
Supplementary Information
Author contributions
H.D.A.: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing—original draft. M.R.: Conceptualization, Investigation, Methodology, Software, Visualization, Writing—original draft. K.R.: Data curation, Resources, Supervision, Writing—review & editing. A.S.: Investigation, Supervision, Writing—review & editing. All authors reviewed the manuscript.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-19865-3.
References
- 1.Künzli N, et al. Public-health impact of outdoor and traffic-related air pollution: A European assessment. Lancet. 2000;356:795–801. doi: 10.1016/S0140-6736(00)02653-2. [DOI] [PubMed] [Google Scholar]
- 2.Dehghani M, Anushiravani A, Hashemi H, Shamsedini N. Survey on air pollution and cardiopulmonary mortality in shiraz from 2011 to 2012: An analytical-descriptive study. Int. J. Prevent. Med. 2014;5:734. [PMC free article] [PubMed] [Google Scholar]
- 3.Carvalho-Oliveira R, et al. Effectiveness of traffic-related elements in tree bark and pollen abortion rates for assessing air pollution exposure on respiratory mortality rates. Environ. Int. 2017;99:161–169. doi: 10.1016/j.envint.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Alahabadi A, et al. A comparative study on capability of different tree species in accumulating heavy metals from soil and ambient air. Chemosphere. 2017;172:459–467. doi: 10.1016/j.chemosphere.2017.01.045. [DOI] [PubMed] [Google Scholar]
- 5.Achakzai K, et al. Air pollution tolerance index of plants around brick kilns in Rawalpindi, Pakistan. J. Environ. Manag. 2017;190:252–258. doi: 10.1016/j.jenvman.2016.12.072. [DOI] [PubMed] [Google Scholar]
- 6.Jonak, C., Nakagami, H. & Hirt, H. Heavy metal stress. Activation of distinct mitogen-activated protein kinase pathways by copper and cadmium. Plant Physiol.136, 3276–3283 (2004). [DOI] [PMC free article] [PubMed]
- 7.Shahid M, et al. Heavy-metal-induced reactive oxygen species: Phytotoxicity and physicochemical changes in plants. Rev. Environ. Contam. Toxicol. 2014;232:1–44. doi: 10.1007/978-3-319-06746-9_1. [DOI] [PubMed] [Google Scholar]
- 8.Tausz M, Grulke NE, Wieser G. Defense and avoidance of ozone under global change. Environ. Pollut. 2007;147:525–531. doi: 10.1016/j.envpol.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 9.Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Foyer CH, Shigeoka S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011;155:93–100. doi: 10.1104/pp.110.166181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishnaveni M. Air pollution tolerance index and antioxidant activity of Parthenium hysterophorus. J. Pharm. Res. 2013;7:296–298. [Google Scholar]
- 12.Ashraf M, Foolad M. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007;59:206–216. doi: 10.1016/j.envexpbot.2005.12.006. [DOI] [Google Scholar]
- 13.Islam MM, et al. Exogenous proline and glycinebetaine increase antioxidant enzyme activities and confer tolerance to cadmium stress in cultured tobacco cells. J. Plant Physiol. 2009;166:1587–1597. doi: 10.1016/j.jplph.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Singh, M., Singh, V. P., Dubey, G. & Prasad, S. M. Exogenous proline application ameliorates toxic effects of arsenate in Solanum melongena L. seedlings. Ecotoxicol. Environ. Saf.117, 164–173 (2015). [DOI] [PubMed]
- 15.Aggarwal, M. et al. Exogenous proline application reduces phytotoxic effects of selenium by minimising oxidative stress and improves growth in bean (Phaseolus vulgaris L.) seedlings. Biol. Trace Elem. Res.140, 354–367 (2011). [DOI] [PubMed]
- 16.Alotaibi MD, et al. Assessing the response of five tree species to air pollution in Riyadh City, Saudi Arabia, for potential green belt application. Environ. Sci. Pollut. Res. 2020;27:29156–29170. doi: 10.1007/s11356-020-09226-w. [DOI] [PubMed] [Google Scholar]
- 17.Manjunath B, Reddy J. Comparative evaluation of air pollution tolerance of plants from polluted and non-polluted regions of Bengaluru. J. Appl. Biol. Biotechnol. 2019;7:63–68. [Google Scholar]
- 18.Sanaeirad, H., Majd, A., Abbaspour, H. & Peyvandi, M. The effect of air pollution on proline and protein content and activity of nitrate reductase enzyme in Laurus nobilis L. plants. J. Mol. Biol. Res.7, 99–105 (2017).
- 19.Khosropour E, et al. Response of Platanus orientalis leaves to urban pollution by heavy metals. J. For. Res. 2019;30:1437–1445. doi: 10.1007/s11676-018-0692-8. [DOI] [Google Scholar]
- 20.Motlagh SHB, Pons O, Hosseini SA. Sustainability model to assess the suitability of green roof alternatives for urban air pollution reduction applied in Tehran. Build. Environ. 2021;194:107683. doi: 10.1016/j.buildenv.2021.107683. [DOI] [Google Scholar]
- 21.Sadeghi, S. M. M., Van Stan II, J. T., Pypker, T. G. & Friesen, J. Canopy hydrometeorological dynamics across a chronosequence of a globally invasive species, Ailanthus altissima (Mill., tree of heaven). Agric. For. Meteorol.240, 10–17 (2017).
- 22.Faridi, S. et al. Long-term trends and health impact of PM2.5 and O3 in Tehran, Iran, 2006–2015. Environ. Int.114, 37–49 (2018). [DOI] [PubMed]
- 23.Heshmatol Vaezin, S. M. et al. The effectiveness of urban trees in reducing airborne particulate matter by dry deposition in Tehran, Iran. Environ. Monit. Assess.193, 1–14 (2021). [DOI] [PubMed]
- 24.Ghahraman, A. Colorful Flora of Iran. The Research Institute of Forest and Pastures, Tehran. Implication to Biodiversity Conservation. SINET Ethiop. J. Sci.30, 1–12 (1979).
- 25.Lichtenthaler, H.K, Chlorophylls and carotenoids, the pigments of photosynthetic biomembranes. in Methods Enzymology (Douce, R., Packer, L. eds.). Vol. 148. 350–382 (Academic Press Inc., 1987).
- 26.Liu Y-J, Ding H. Variation in air pollution tolerance index of plants near a steel factory: Implication for landscape-plant species selection for industrial areas. WSEAS Trans. Environ. Dev. 2008;4:24–32. [Google Scholar]
- 27.Zhang J, et al. Analysis of effects of a new environmental pollutant, bisphenol A, on antioxidant systems in soybean roots at different growth stages. Sci. Rep. 2016;6:1–10. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kampfenkel K, Vanmontagu M, Inzé D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995;225:165–167. doi: 10.1006/abio.1995.1127. [DOI] [PubMed] [Google Scholar]
- 29.Singh S, Rao D, Agrawal M, Pandey J, Naryan D. Air pollution tolerance index of plants. J. Environ. Manag. 1991;32:45–55. doi: 10.1016/S0301-4797(05)80080-5. [DOI] [Google Scholar]
- 30.Singh S, Rao D. Evaluation of plants for their tolerance to air pollution. Proc. Sympos. Air Pollut. Control. 1983;83:218–224. [Google Scholar]
- 31.Anake WU, Eimanehi JE, Omonhinmin CA. Evaluation of air pollution tolerance index and anticipated performance index of selected plant species. Indones. J. Chem. 2019;19:239–244. doi: 10.22146/ijc.35270. [DOI] [Google Scholar]
- 32.Dhindsa R, Plumb-Dhindsa P, Thorpe TA. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981;32:93–101. doi: 10.1093/jxb/32.1.93. [DOI] [Google Scholar]
- 33.Nakano Y, Asada K. Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 1987;28:131–140. [Google Scholar]
- 34.D'Cunha GB, Satyanarayan V, Nair PM. Stabilization of phenylalanine ammonia lyase containing Rhodotorula glutinis cells for the continuous synthesis of l-phenylalanine methyl ester/96/ Enzyme Microb. Technol. 1996;19:421–427. doi: 10.1016/S0141-0229(96)00013-0. [DOI] [Google Scholar]
- 35.Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]
- 36.Kaijv M, Sheng L, Chao C. Antioxidation of flavonoids of green rhizome. Food Sci. 2006;27:110–115. [Google Scholar]
- 37.Bates L, Waldren R, Teare I. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- 38.Zhang P-Q, et al. Pollution resistance assessment of existing landscape plants on Beijing streets based on air pollution tolerance index method. Ecotoxicol. Environ. Saf. 2016;132:212–223. doi: 10.1016/j.ecoenv.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Balasubramanian A, Prasath C, Gobalakrishnan K, Radhakrishnan S. Air pollution tolerance index (APTI) assessment in tree species of Coimbatore urban city, Tamil Nadu, India. Int. J. Environ. Clim. Change. 2018;8:27–38. doi: 10.9734/ijecc/2018/v8i127106. [DOI] [Google Scholar]
- 40.Bacelar EA, Moutinho-Pereira JM, Gonçalves BC, Lopes JI, Correia CM. Physiological responses of different olive genotypes to drought conditions. Acta Physiol. Plant. 2009;31:611–621. doi: 10.1007/s11738-009-0272-9. [DOI] [Google Scholar]
- 41.Rai PK. Biodiversity of roadside plants and their response to air pollution in an Indo-Burma hotspot region: Implications for urban ecosystem restoration. J. Asia-Pac. Biodivers. 2016;9:47–55. doi: 10.1016/j.japb.2015.10.011. [DOI] [Google Scholar]
- 42.Keller T, Schwager H. Air pollution and ascorbic acid. Eur. J. For. Pathol. 1977;7:338–350. doi: 10.1111/j.1439-0329.1977.tb00603.x. [DOI] [Google Scholar]
- 43.Lima JS, Fernandes E, Fawcett W. Mangifera indica and Phaseolus vulgaris in the bioindication of air pollution in Bahia, Brazil. Ecotoxicol. Environ. Saf. 2000;46:275–278. doi: 10.1006/eesa.1999.1894. [DOI] [PubMed] [Google Scholar]
- 44.Agbaire P, Esiefarienrhe E. Air pollution tolerance indices (APTI) of some plants around Otorogun Gas Plant in Delta State, Nigeria. J. Appl. Sci. Environ. Manag. 2009;13:11–14. [Google Scholar]
- 45.Nadgórska-Socha A, Kandziora-Ciupa M, Trzęsicki M, Barczyk G. Air pollution tolerance index and heavy metal bioaccumulation in selected plant species from urban biotopes. Chemosphere. 2017;183:471–482. doi: 10.1016/j.chemosphere.2017.05.128. [DOI] [PubMed] [Google Scholar]
- 46.Joshi N, Chauhan A, Joshi P. Impact of industrial air pollutants on some biochemical parameters and yield in wheat and mustard plants. Environmentalist. 2009;29:398–404. doi: 10.1007/s10669-009-9218-4. [DOI] [Google Scholar]
- 47.Dadkhah-Aghdash, H. et al. Variation in Brant’s oak (Quercus brantii Lindl.) leaf traits in response to pollution from a gas refinery in semiarid forests of western Iran. Environ. Sci. Pollut. Res.29, 10366–10379 (2022). [DOI] [PubMed]
- 48.Seyyednejad S, Niknejad M, Koochak H. A review of some different effects of air pollution on plants. Res. J. Environ. Sci. 2011;5:302–309. doi: 10.3923/rjes.2011.302.309. [DOI] [Google Scholar]
- 49.Singh, S. & Verma, A. Environmental Bioremediation Technologies. 293–314 (Springer, 2007).
- 50.Verma S, Dubey R. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2003;164:645–655. doi: 10.1016/S0168-9452(03)00022-0. [DOI] [Google Scholar]
- 51.Rai PK, Panda LL, Chutia BM, Singh MM. Comparative assessment of air pollution tolerance index (APTI) in the industrial (Rourkela) and non industrial area (Aizawl) of India: An ecomanagement approach. Afr. J. Environ. Sci. Technol. 2013;7:944–948. [Google Scholar]
- 52.Prajapati SK, Tripathi B. Seasonal variation of leaf dust accumulation and pigment content in plant species exposed to urban particulates pollution. J. Environ. Qual. 2008;37:865–870. doi: 10.2134/jeq2006.0511. [DOI] [PubMed] [Google Scholar]
- 53.Bansal P, Verma S, Srivastava A. Biomonitoring of air pollution using antioxidative enzyme system in two genera of family Pottiaceae (Bryophyta) Environ. Pollut. 2016;216:512–518. doi: 10.1016/j.envpol.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 54.Boudet A-M. Evolution and current status of research in phenolic compounds. Phytochemistry. 2007;68:2722–2735. doi: 10.1016/j.phytochem.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 55.Vogt T. Phenylpropanoid biosynthesis. Mol. Plant. 2010;3:2–20. doi: 10.1093/mp/ssp106. [DOI] [PubMed] [Google Scholar]
- 56.Pellegrini E, et al. Antioxidative responses of three oak species under ozone and water stress conditions. Sci. Total Environ. 2019;647:390–399. doi: 10.1016/j.scitotenv.2018.07.413. [DOI] [PubMed] [Google Scholar]
- 57.Rai PK. Impacts of particulate matter pollution on plants: Implications for environmental biomonitoring. Ecotoxicol. Environ. Saf. 2016;129:120–136. doi: 10.1016/j.ecoenv.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 58.Nayak R, Biswal D, Sett R. Biochemical changes in some deciduous tree species around Talcher thermal power station, Odisha, India. J. Environ. Biol. 2013;34:521. [PubMed] [Google Scholar]
- 59.Kovacik J, Backor M. Phenylalanine ammonia-lyase and phenolic compounds in chamomile tolerance to cadmium and copper excess. Water Air Soil Pollut. 2007;185:185–193. doi: 10.1007/s11270-007-9441-x. [DOI] [Google Scholar]
- 60.Egert M, Tevini M. Influence of drought on some physiological parameters symptomatic for oxidative stress in leaves of chives (Allium schoenoprasum) Environ. Exp. Bot. 2002;48:43–49. doi: 10.1016/S0098-8472(02)00008-4. [DOI] [Google Scholar]
- 61.Havaux M, Eymery F, Porfirova S, Rey P, Dörmann P. Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell. 2005;17:3451–3469. doi: 10.1105/tpc.105.037036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jaleel CA, et al. Antioxidant defense responses: Physiological plasticity in higher plants under abiotic constraints. Acta Physiol. Plant. 2009;31:427–436. doi: 10.1007/s11738-009-0275-6. [DOI] [Google Scholar]
- 63.Qadir SU, Raja V, Siddiqui WA. Morphological and biochemical changes in Azadirachta indica from coal combustion fly ash dumping site from a thermal power plant in Delhi, India. Ecotoxicol. Environ. Saf. 2016;129:320–328. doi: 10.1016/j.ecoenv.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 64.Assadi A, Pirbalouti AG, Malekpoor F, Teimori N, Assadi L. Impact of air pollution on physiological and morphological characteristics of Eucalyptus camaldulensis. J. Food Agric. Environ. 2011;9:676–679. [Google Scholar]
- 65.Yazdanpanahrostami, A. & Rasouli, K. Simulation of Tehran air pollution using artificial neural networks. in World Environmental and Water Resources Congress 2009: Great Rivers. 1–11 (2009).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files).