Abstract
Background
Relatively little is known about various aspects of low-dose CT (LDCT) scan lung cancer screening in US clinical practice, including characteristics of cases diagnosed after screening. We assessed this using the Surveillance, Epidemiology, and End Results (SEER)-Medicare database.
Research Question
What were the characteristics of patients with lung cancer, including stage and survival, whose disease was diagnosed after LDCT scan screenings?
Study Design and Methods
We created an LDCT scan use cohort consisting of everyone in the 5% SEER-Medicare sample with ≥ 12 months of non-health maintenance organization (HMO) Part A and B coverage while 65 to 77 years of age from 2015 through 2019. LDCT scan use and lung cancer diagnosis rates were assessed in this cohort. Additionally, we created a lung cancer cohort consisting of patients who received a diagnosis between 2015 and 2017 at 65 to 78 years of age with complete (non-HMO Part A and B) coverage the year before diagnosis. The cases cohort comprised those screened or unscreened based on undergoing screening during that period; lung cancer characteristics and survival were compared between these groups.
Results
In the LDCT scan use cohort (n = 414,358), use rates increased from 0.10 (per 100 person-years) in 2015 to 1.3 in 2019. Among those with first screenings, 39.2% underwent a subsequent screen within 18 months. The 1-year cumulative lung cancer diagnosis rate after initial screenings was 2.4%. Claims for prescreen counseling were infrequent (about 10%). Of 48,891 patients in the lung cancer cohort, 1,150 (2.4%) underwent screening. Among screened patients, 52.3%, 11.0%, 20.7%, and 16.0% received diagnoses of stages I, II, III, and IV disease, respectively. Lung cancer-specific survival through 3 years was significantly greater in screened versus unscreened patients overall and for all stages except stage II; 3-year lung cancer-specific survival was 89.0% in screened patients with stage I disease.
Interpretation
LDCT scan use was low but increased over time. The lung cancer yield was substantial; cases among those who underwent screening primarily were in the early stage with high survival rates. Although screening rates were unacceptably low, screening outcomes in those Medicare recipients undergoing screening were favorable.
Key Words: low-dose CT scan, lung cancer, Medicare, screening, stage
Abbreviations: CMS, Centers for Medicare and Medicaid Services; LDCT, low-dose CT; HMO, health maintenance organization; NHIS, National Health Interview Survey; PY, person-year; SEER, Surveillance, Epidemiology, and End Results
Graphical Abstract
FOR EDITORIAL COMMENT, SEE PAGE 505
Take-home Points.
Study Question: What are the stage distribution and survival of patients with lung cancer among those with prior low-dose CT (LDCT) scan screening vs those without such screening in the Medicare population?
Results: For screened patients, 52.3% received a diagnosis of stage I disease compared with 27.1% for unscreened patients. Three-year lung cancer-specific survival rates for those with stage I disease and all stages combined were 89.0% and 78.5%, respectively, vs 81.75% and 53.0% for unscreened patients.
Interpretation: Among Medicare recipients with lung cancer, those with prior LDCT scan screening showed favorable stage and survival, suggesting that screening has been effective.
Evidence from randomized trials has shown that low-dose CT (LDCT) scan screening reduces mortality resulting from lung cancer in high-risk populations.1, 2, 3 Although organizations have recommended LDCT scan screening since 2013, uptake of screening among eligible people has been low, with estimates of screening rates in recent years in the range of 10% to 20%.4, 5, 6 Reasons for the low uptake involve factors at multiple levels and include patient financial barriers, lack of patient education about lung cancer risk and LDCT scan screening, physician skepticism about LDCT scan effectiveness, inadequate access to specialists for diagnostic follow-up, and lack of administrative commitment to LDCT scan screening.6, 7, 8
Although considerable research has been carried out on LDCT scan use, less is known about the downstream events after LDCT scan screening performed in standard clinical practice. Specifically, relatively little is known about rates of lung cancer after LDCT scan screenings and about the stage distribution, treatments, and survival of patients with lung cancer who receive a diagnosis after LDCT scan screening. Additionally, with respect to use, although prescreening counseling was mandated by the Centers for Medicare and Medicaid Services (CMS) for coverage of initial LDCT scan screening, evidence gaps exist regarding how often this actually occurs in practice and what its effects are.
In this analysis, we addressed some of these questions using real-world data on LDCT scan screening from Surveillance, Epidemiology, and End Results (SEER)-Medicare for patients enrolled in fee-for-service (ie, non-health maintenance organization [HMO]) Medicare from 2015 through 2019. We assessed use of LDCT scan screening, both initial and subsequent screenings, examined the use of prescreening counseling, and estimated cumulative rates of lung cancer diagnosis after LDCT scan screening. In addition, among patients with lung cancer, we compared lung cancer characteristics, including stage, treatment, and survival, for those who had vs had not undergone a recent LDCT scan screening.
Study Design and Methods
LDCT Scan Use Cohort
We obtained from SEER-Medicare the 5% cancer and noncancer sample of people enrolled in Medicare from 2015 through 2019.9 The Master Beneficiary Summary Files were used to ascertain Medicare enrollment status and demographics (age, race or ethnicity). Because the CMS eligibility criteria for LDCT scan screening covered only those 65 through 77 years of age, that age range was the focus of our analysis. The CMS criteria also included a 30-pack-year minimum smoking history and current smoking or quitting within the past 15 years; however, that information is not available in SEER-Medicare.10 Accordingly, we defined the LDCT scan use cohort as all those in the 5% sample with at least 12 months of non-HMO Part A and B and coverage while 65 to 77 years of age from 2015 through 2019.
The carrier claims and outpatient claims files were used to ascertain use of LDCT scan screening using the Health Care Common Procedure Coding System code G0297, as well as code S8032, which was valid through 2016.11 Additionally, these files were used to ascertain prescreening counseling visits (code G0296), which were required by CMS before an initial LDCT scan screening. Because those whose screening results were positive may receive diagnostic chest CT scans (code 71250), instead of subsequent LDCT scans, we also examined use of these procedures. The 5% sample SEER files within SEER-Medicare were used to ascertain lung cancer diagnosis after LDCT scan screening. Predefined chronic condition variables in SEER-Medicare were used to ascertain comorbid conditions, including COPD and tobacco use disorder, the latter of which covers claims for smoking cessation counseling, documented current smoking, or both. Chronic condition status in a given year was defined as positive if both coverage and claims were met in that year, negative if only coverage was met, and unknown otherwise. A comorbidity count was computed by summing up the number of diagnoses among the following conditions: COPD, myocardial infarction, diabetes, stroke, Alzheimer disease or dementia, chronic kidney disease, congestive heart failure, and ischemic heart disease. e-Table 1 summarizes the SEER-Medicare variables and codes used for this analysis.
Lung Cancer Cohort
The complete SEER files in SEER-Medicare (not limited to the 5% sample) were used to identify patients with a diagnosis of a first lung cancer from 2015 through 2017 (the latest year of available data). We defined the lung cancer cohort as all the above people who received a diagnosis at 65 to 78 years of age and who had complete Part A and B non-HMO coverage during the 12-month period before diagnosis. The upper age limit of 78 years allowed for 1 year after an LDCT scan screening at 77 years of age. In addition to the previously described files (Master Beneficiary Summary Files, etc.) available for this cohort, data from SEER, including stage of disease, histologic findings, treatment, and survival, also were available. For treatment, SEER captures whether surgery was performed at the primary site; additionally, it captures whether any radiation treatment or systemic therapy was administered, but not any details of those treatments. Within the cohort, those who underwent an LDCT scan screening in the year before diagnosis were classified as screened, and as unscreened otherwise.
Quantitative Methods
LDCT Scan Use Cohort
Primary outcome measures for this cohort were rates of LDCT scan screening (initial and subsequent), screening rates among eligible peoples, rates of prescreening counseling, and cumulative lung cancer incidence rates after LDCT scan screening. Rates of LDCT scan screening were defined as the number of screenings divided by person-years (PYs) of Part A and B non-HMO coverage while 65 to 77 years of age; rates were computed by year, age, and race or ethnicity. Because the detailed smoking history necessary to ascertain screening eligibility was not available in SEER-Medicare, we used data from the National Health Interview Survey (NHIS) on LDCT scan eligibility rates to estimate use rates among eligible people, denoted as adjusted rates. Specifically, adjusted rates were computed as the rate of LDCT scan screening (per 100 PYs) divided by the percent eligible according to CMS criteria as estimated from the 2015 NHIS in a recent study.10 The adjusted rates estimate the proportion of those eligible who underwent screening in a given year, assuming everyone screened was eligible according to CMS criteria. Because the NHIS study computed CMS eligibility based on a population 65 to 80 years of age, we adjusted the denominator to reflect a population 65 to 77 years of age by dividing by the proportion of the population 65 to 80 years of age who were 65 to 77 years of age.
Annual adjusted rates were compared by sex and race or ethnicity. For the latter, we concentrated on comparisons between non-Hispanic White people and non-Hispanic Black people because of the substantially elevated lung cancer risk of the latter group. Additionally, estimated eligibility rates for Hispanic and Asian people were imprecise because of low numbers in the NHIS sampling frame, making the adjusted rate estimates also imprecise. SEs of the adjusted rates were estimated using the SEs of the eligibility and annual use rates and the quotient method; P values for between group comparisons were computed using these SEs and assuming a normal distribution.
For patients’ first screening using Medicare, we assessed the proportion with a prescreen counseling visit before (within 6 months) the screening. Conversely, we assessed the rate of receiving a screen after (within 6 months) a counseling visit.
Kaplan-Meier survival analysis was used to estimate the cumulative proportions of those with an initial LDCT scan screening who received a first subsequent screening and who received either a first subsequent screening or a follow-up diagnostic chest CT scan; patients were censored at end of follow-up. Kaplan-Meier survival analysis also was used to estimate cumulative lung cancer incidence after initial LDCT scan screening, overall and by COPD status, with patients censored at the end of lung cancer follow-up (the end of 2017) or death, whichever came first.
Lung Cancer Cohort
Primary outcomes for the lung cancer cohort were disease stage, treatment, and survival, with a secondary outcome of histologic findings. Stage (TNM and SEER summary), histologic findings, and surgical treatment were compared between the screened and unscreened groups. Lung cancer-specific and overall survival by stage was assessed using Kaplan-Meier survival curves, with survival data complete through 2018. In addition, lung cancer-specific survival was examined with multivariate Cox proportional hazards models, which controlled for age, sex, race, and calendar year.
Results
LDCT Scan Use Cohort
Of 1,230,083 people in the 5% (cancer and noncancer) sample, 414,358 had the requisite Part A and B coverage while 65 to 77 years of age and constituted the LDCT scan use cohort. Table 1 shows demographics of the cohort. Women constituted 55%, and most (78.9%) were non-Hispanic White. The average time of coverage (for those 65-77 years of age with non-HMO coverage) was 3.6 years.
Table 1.
Demographics of Cohorts
| Variable | LDCT Scan Use Cohort | Lung Cancer Cohort |
|---|---|---|
| All | 414,358 (100) | 48,891 (100) |
| Sex | ||
| Male | 187,357 (45.2) | 24,653 (50.4) |
| Female | 227,027 (54.8) | 24,238 (49.6) |
| Race or ethnicity | ||
| Non-Hispanic White | 327,118 (78.9) | 42,129 (86.2) |
| Non-Hispanic Black | 33,247 (8.0) | 3,878 (7.9) |
| Hispanic | 11,341 (2.7) | 356 (0.7) |
| Asian | 16,317 (3.9) | 987 (2.0) |
| Other or unknown | 26,361 (6.4) | 1,541 (3.2) |
| COPDa | 83,727 (20.3) | 31,132 (64.1) |
| Tobacco use disordera | 48,062 (12.4) | 18,994 (28.9) |
| Average follow-up, yb | 3.6 | 1.4 |
Data are presented as No. (%). LDCT = low-dose CT.
Unknowns excluded from percentage calculations. Condition at any year for LDCT scan use cohort and in year of diagnosis for lung cancer cohort.
Years of coverage for LDCT scan use cohort and years of mortality follow-up for lung cancer cohort.
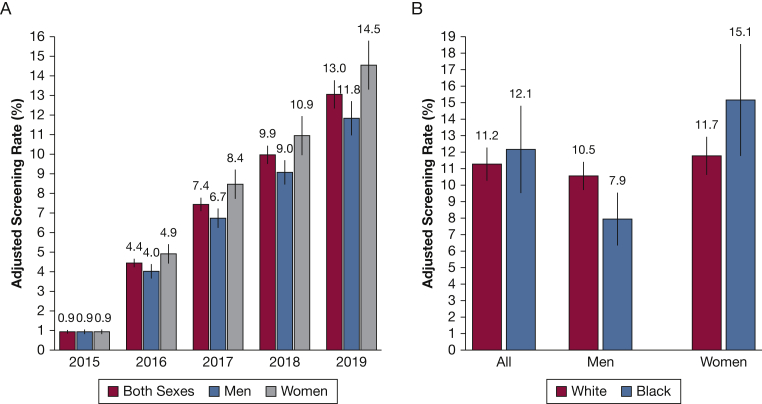
A total of 7,336 people (1.7%) had undergone at least one LDCT scan screening. Table 2 shows the LDCT scan screening rates per calendar year. Rates (per 100 PY) increased gradually from 2015 through 2019, from a very low of 0.10 in 2015 to 1.3 in 2019. For each year, rates were modestly and statistically significantly greater for men than women. By race or ethnicity for men, women, and both sexes combined, rates were significantly higher for non-Hispanic Whites than for the other racial or ethnic groups, both for the entire period (2015-2019) and for the latest 2 years (2018-2019). For example, in 2018 and 2019, rates among women were 1.14 for non-Hispanic Whites, compared with 0.79, 0.23, and 0.19 for non-Hispanic Blacks, Hispanics, and Asians, respectively.
Table 2.
LDCT Scan Use Rates (%) by Year, Sex, and Race or Ethnicity in the LDCT Scan Use Cohorta
| Period | Race or Ethnicity | Both Sexes | Men | Women | P Value (Men vs Women) |
|---|---|---|---|---|---|
| 2015 | All | 0.10 | 0.12 | 0.08 | .0014 |
| 2016 | All | 0.44 | 0.51 | 0.39 | < .001 |
| 2017 | All | 0.74 | 0.84 | 0.67 | < .001 |
| 2018 | All | 0.99 | 1.13 | 0.87 | < .001 |
| 2019 | All | 1.30 | 1.47 | 1.16 | < .001 |
| 2015-2019 | All | 0.72 | 0.82 | 0.64 | < .001 |
| Non-Hispanic White | 0.79 | 0.89 | 0.72 | < .001 | |
| Non-Hispanic Black | 0.51 | 0.58 | 0.47 | .011 | |
| Hispanic | 0.21 | 0.31 | 0.14 | .0013 | |
| Asian | 0.28 | 0.51 | 0.13 | < .001 | |
| 2018-2019 | All | 1.14 | 1.29 | 1.01 | < .001 |
| Non-Hispanic White | 1.26 | 1.41 | 1.14 | < .001 | |
| Non-Hispanic Black | 0.83 | 0.87 | 0.79 | .38 | |
| Hispanic | 0.36 | 0.51 | 0.23 | .007 | |
| Asian | 0.45 | 0.85 | 0.19 | < .001 |
LDCT = low-dose CT scan.
Use rates (%) defined as number of screenings per 100 person-years of those 65 to 77 years of age with non-HMO Part A and B coverage.
Among those screened in a given year with known COPD status, 46% had positive COPD findings, compared with 9% with positive COPD findings among those not screened in the year. Comparable figures for tobacco use disorder were 55% positive among those screened vs 7% among those not screened. Mean comorbidity counts were 1.43 and 0.89 for those screened and not screened, respectively, in a given year.
Figure 1A shows adjusted screening rates (observed rate divided by eligibility rate) for men, women, and both sexes by year. Overall adjusted rates reached 13.0% in 2019, with the rate borderline significantly higher in women (14.5%) than men (11.8%). Figure 1B shows comparisons for non-Hispanic Blacks vs non-Hispanic Whites. Among men, adjusted rates were higher in non-Hispanic Whites, whereas among women and overall, adjusted rates were higher among non-Hispanic Blacks; however, none of the differences approached statistical significance (P > .10 for all).
Figure 1.
A, B, Bar graphs showing adjusted screening rates (observed rate divided by eligibility rate): rates for men and women by year (A) and rates for non-Hispanic Blacks and non-Hispanic Whites for 2018 and 2019 (B). Vertical bars show SEs of estimates. A, P values for differences between men and women are .98, .12, .07, .12, and .08 for 2015, 2016, 2017, 2018, and 2019. B, P values for differences between non-Hispanic Blacks and non-Hispanic Whites are .75 for both sexes combined, .15 for men, and .35 for women.
The proportion of LDCT scan examinations that were subsequent examinations increased from near 0 in 2015 to 7.1%, 23.2%, 33.6%, and 45.0% in 2016, 2017, 2018, and 2019. Among patients with a first screening, the cumulative proportions receiving a second screening were 34.7%, 39.2%, 47.4%, and 53.5% at 15, 18, 24, and 30 months, respectively. The proportions were substantially higher for receiving either a second screening or a diagnostic chest CT scan after the initial screen: 54.5%, 60.1%, 68.7%, and 73.4% at 15, 18, 24, and 30 months, respectively. Cumulative proportions were not significantly different by sex, nor by race or ethnicity.
Among those screened, rates of receiving prescreening shared decision counseling before the initial screening were low: 5.4% in 2016 and 10.2% to 10.6% from 2017 through 2019. Conversely, among those receiving counseling, the rate of undergoing a screening within 6 months ranged from 66% to 73% for the years 2017 through 2019 (e-Table 2).
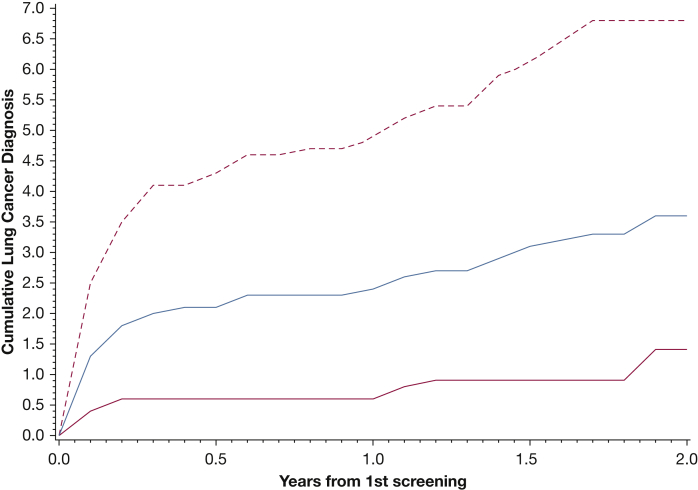
Lung cancer diagnoses after LDCT scan screening were assessed for the patients (n = 3,221) who underwent an LDCT scan screening from 2015 through 2017. Of those with known COPD status at the initial screening (92%), 44.7% had positive COPD findings. Figure 2 shows the cumulative lung cancer diagnosis rate after initial screenings, overall and by COPD status. The overall rate at 1 year was 2.4% (95% CI, 1.9%-3.0%), with the rate markedly higher for those with COPD, 4.9% (95% CI, 3.8%-6.3%), compared with those without COPD, 0.6% (95% CI, 0.3%-1.2%).
Figure 2.
Line graph showing cumulative rate (%) of lung cancer diagnosis after an initial screening. Blue curve is all participants, solid red curve is those without COPD, and dashed red curve is patients with COPD.
Lung Cancer Cohort
Of 161,803 patients with lung cancer who received a diagnosis from 2015 through 2017, 48,891 were included in the lung cancer cohort based on age at diagnosis and coverage status. Demographics of the cohort are given in Table 1. A total of 1,150 patients (2.4%) were screened in the year before diagnosis. Among those screened, 90% underwent only one screening before diagnosis. Median age was 71 and 72 years for the screened and unscreened patients, respectively; 80% of those screened vs 64% of the unscreened already had received a COPD diagnosis at the time of the lung cancer diagnosis.
Table 3 shows the stage distribution. Compared with the unscreened group, the screened group included substantially more TNM stage I cases (52.3% vs 27.1%) and substantially fewer stage IV cases (16.0% vs 45.1%). A similar pattern was seen for SEER summary stage, with the screened group having more localized and fewer distant cases. The stage distribution was similar for those patients who underwent only a single screening as for the overall screened group (Table 3). Histologic findings were similar between the two groups (e-Table 3). The proportions of patients with surgical treatment were 71.8% vs 58.9% for screened vs unscreened patients with stage I disease (P < .001) and 71.9% vs 51.6% for screened vs unscreened patients with stage II disease (P < .001). The mean comorbidity count (at diagnosis) was slightly higher among the unscreened (n = 2.2) than the screened (n = 2.0) patients.
Table 3.
Stage Distribution by LDCT Scan Screen Status in the Lung Cancer Cohort
| Variable | Unscreened | Screened |
|
|---|---|---|---|
| All | Single Screening | ||
| TNM stage | |||
| All | 47,741 (100) | 1,150 (100) | 1,036 (100) |
| I | 9,007 (27.1) | 391 (52.3) | 355 (51.8) |
| IA | 6,411 (19.3) | 300 (40.1) | 273 (39.8) |
| IB | 2,596 (7.8) | 91 (12.2) | 82 (11.9) |
| II | 2,685 (8.1) | 82 (11.0) | 79 (11.5) |
| IIA | 1,309 (3.9) | 48 (6.4) | 45 (6.6) |
| IIB | 1,376 (4.1) | 34 (4.5) | 34 (5.0) |
| III | 6,559 (19.7) | 155 (20.7) | 139 (20.3) |
| IIIA | 4,469 (13.5) | 122 (16.3) | 108 (15.7) |
| IIIB | 2,090 (6.2) | 33 (4.4) | 31 (4.5) |
| IV | 14,980 (45.1) | 120 (16.0) | 113 (16.5) |
| Unknown | 14,510 (30.4) | 402 (35.5) | 350 (34.1) |
| SEER summary stage | |||
| Local | 12,870 (28.2) | 576 (50.9) | 510 (50.0) |
| Regional | 10,933 (23.9) | 355 (31.4) | 325 (31.8) |
| Distant | 21,837 (47.9) | 200 (17.7) | 186 (18.2) |
| Unknown | 2,152 (4.4) | 20 (1.6) | 15 (1.4) |
Data are presented as No. (%). Percentages for nonunknown stages exclude unknowns. SEER = Surveillance, Epidemiology, and End Results
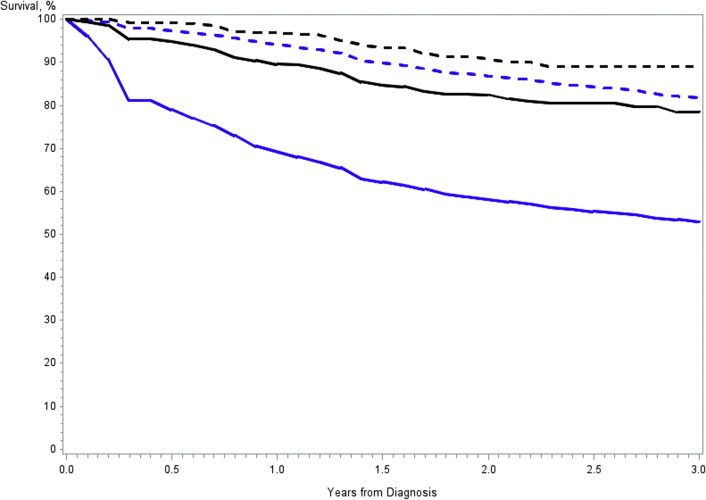
Median follow-up for survival was 1.2 years (interquartile range, 0.3-2.2 years) and 1.5 years (interquartile range, 1.1-2.1 years) in the unscreened and screened groups, respectively. Table 4 shows 3-year lung cancer-specific and overall survival rates by stage. For each stage and all stages combined, both lung cancer-specific and overall survival were significantly greater among the screened compared with unscreened patients, except for stage II disease, for which lung cancer-specific survival was borderline significant (e-Fig 1). Stage I 3-year lung cancer-specific survival was 89.0% among screened vs 81.7% unscreened patients. Hazard ratios by stage for the proportional hazards model for lung cancer-specific survival comparing screened with unscreened patients were 0.65 (95% CI, 0.45-0.93), 0.68 (95% CI, 0.41-1.12), 0.57 (95% CI, 0.43-0.76), and 0.72 (95% CI, 0.58-0.90) for stages I, II, III, and IV, respectively, and 0.36 (95% CI, 0.31-0.42) for all stages combined.
Table 4.
Three-Year Overall and Lung Cancer-Specific Survival by Screening Status in the Lung Cancer Cohort
| Variable | Lung Cancer-Specific Survival Rate (at 3 y) |
Overall Survival Rate (at 3 y) |
||||
|---|---|---|---|---|---|---|
| Screened | Unscreened | P Valuea | Screened | Unscreened | P Valuea | |
| All | 78.5 (74.1-82.2) | 53.0 (52.4-53.6) | < .001 | 60.9 (55.2-66.1) | 35.3 (34.8-35.8) | < .001 |
| Stage I | 89.0 (84.0-92.5) | 81.7 (80.7-82.7) | .01 | 76.8 (66.6-84.3) | 67.5 (66.4-68.7) | < .001 |
| Stage II | 72.4 (54.6-84.2) | 58.6 (56.2-60.9) | .05 | 58.8 (34.6-76.7) | 47.4 (45.1-49.4) | .01 |
| Stage III | 60.9 (50.3-69.8) | 36.8 (35.3-38.3) | < .001 | 46.1 (33.3-58.1) | 28.8 (27.4-30.0) | < .001 |
| Stage IV | 15.6 (4.7-32.2) | 13.1 (11.9-13.5) | .002 | 13.3 (4.1-27.9) | 8.8 (8.3-9.4) | < .001 |
Data are presented as rate (95% CI).
Log-rank test.
Discussion
In this analysis of SEER-Medicare data, we examined use rates of screening, lung cancer rates after screening, and lung cancer characteristics of patients who recently underwent screening. Although use was low, an appreciable yield of lung cancer was found after screenings. Furthermore, the stage distribution and survival of patients who underwent screening was very favorable compared with that of patients without prior screening.
In the age-eligible group by CMS LDCT scan screening criteria (65-77 years), screening rates (per 100 PY) were low but rising over time, from 0.44 in 2016 to 1.3 in 2019. Men showed modestly higher rates than women, and non-Hispanic White individuals showed higher rates than other racial or ethnic groups. The estimated adjusted rate (rate among eligible people) was 4.4% in 2016, increasing to 13% in 2019. Women showed borderline significantly higher adjusted rates than men, despite having significantly lower overall rates because of their lower eligibility rates. No significant differences were found in adjusted rates between non-Hispanic Black individuals and non-Hispanic White individuals. An analysis of 2016 data from all patients enrolled in non-HMO Medicare estimated a similar rate among eligible people of 4.1%.12 Estimates of use rates among eligible people for the years 2017 through 2019, based on survey responses, ranged from 12.5% to 17.5%.4, 5, 6
We found a low rate of receiving subsequent screening after a baseline screening, 39.2% at 18 months, but a much higher rate of receiving either a subsequent screening or a diagnostic chest CT scan, 60% at 18 months. Patients with positive screening results may continue with diagnostic CT scans in lieu of subsequent LDCT scans for some time, although some of the CT scans also could be for unrelated medical issues. A meta-analysis of adherence to subsequent screening after receiving a baseline screening, for time points varying from 12 to 24 months, found an average rate of 55%, but with a wide variation (9%-91%) across studies, about half of which were conducted at academic centers.13 Almost half of screenings (45%) in 2019 were subsequent screenings; this aligns closely with the American College of Radiology registry database, which shows that 41% of screenings in 2019 were subsequent screenings.14
Considering that counseling visits were required by CMS before the initial LDCT scan screening, the rate of receiving such counseling was quite low, around 10%, and steady for the last few years (2017-2019). It is possible that some patients received counseling, but for various reasons were not billed for it. About two-thirds of those receiving counseling went on to undergo screening (within 6 months). This could indicate that this counseling was working, with some deciding against receiving screening based on the counseling.
The lung cancer diagnosis rate after initial screenings was 2.4% within 1 year of an initial screening. For comparison, among those 65 to 74 years of age in the NLST, the rate was somewhat lower, at 1.8%.15 In the current study, 55% had a tobacco use disorder (suggesting current smoking), and 45% had COPD, both higher rates than in the NLST, so this may indicate that the general population undergoing screening is at higher risk than those in the NLST. A study of LDCT scan screening in an HMO population also found a substantially higher (about two-fold) lung cancer rate than did the NLST.16
The stage distribution among patients after initial screenings was similar to that of the NLST prevalent screening round, where 54% of patients received a diagnosis of stage I disease (including 46% with stage 1A disease), 22% of patients received a diagnosis of stage III disease, and 16% of patients received a diagnosis of stage IV disease.17 Comparable numbers in the present study are 51.8% of patients receiving a diagnosis of stage I disease (39.8% with 1A disease), 20.3% of patients receiving a diagnosis of stage III disease, and 16.5% of patients receiving a diagnosis of stage IV disease. This suggests that sensitivity by stage and the diagnostic workup process for Medicare patients was similar to that for the NLST.
Additionally, for each stage, screened patients showed better lung cancer-specific and overall survival than unscreened patients. Some of the survival benefit is likely the result of lead time, although lead time for patients with advanced stage disease presumably would be short. For early stage disease, improved survival also in part could be the result of the higher rate of surgical resection in the screened patients. A “healthy-screenee” effect could be contributing to the better all-cause survival seen in screened patients, where, among those with a diagnosis of lung cancer, those choosing to be screened were otherwise healthier than those not choosing to be screened. In addition, screened patients were slightly younger (median age, 71 years vs 72 years). Although the rate of COPD was high among those screened, and among patients with lung cancer, and greater among those screened compared with those unscreened, screened patients still showed better survival than unscreened ones. This, along with the much higher lung cancer yield after screenings in those with vs without COPD, may help to allay some concerns about those with COPD undergoing LDCT scan screening. Additionally, stage I survival for screened patients in this study was similar to that of the NLST, where 3-year lung cancer-specific survival for screening-detected patients was around 92%, only slightly higher than the 89% observed in the present study.18
This analysis had several important limitations. First, SEER-Medicare does not capture LDCT scan screening results, so our analysis was limited to outcomes after a screening, not after a screening with positive findings. However, in terms of lung cancer cases diagnosed within 1 year of the screen, it is likely that the vast majority occurred after screenings with positive results. Second, the database does not capture detailed smoking history, so we were unable to determine who was LDCT scan eligible; instead, we relied on eligibility rates derived from other sources. Additionally, the analysis was limited to those 65 years of age and older enrolled in non-HMO Medicare.
Interpretation
Using real-world data from a general population sample, we found that LDCT scan screening rates were low, but steadily increasing over time. Rates of subsequent screening and of those receiving prescreening counseling also were low. Diagnoses of lung cancer in those recently screened showed a favorable stage distribution and survival profile, consistent with the NLST.
Acknowledgments
Author contributions: P. F. P. completed the initial study design, participated in data collection and analysis, wrote the first draft of the manuscript, and edited future drafts. P. F. P. takes responsibility for the integrity of this work. E. M. participated in data analysis and edited future drafts of the manuscript.
Financial/nonfinancial disclosures: None declared.
Additional information: The e-Figure and e-Tables are available online under “Supplementary Data.”
Footnotes
FUNDING/SUPPORT: The authors have reported to CHEST that no funding was received for this study.
Supplementary Data
e-Figure 1.
References
- 1.Aberle D.R., Adams A.M., Berg C.D., et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Koning H.J., van der Aalst C.M., de Jong P.A., et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503–513. doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Preventive Services Task Force Screening for lung cancer. U.S. Preventive Services Task Force recommendation statement. JAMA. 2021;325:962–970. doi: 10.1001/jama.2021.1117. [DOI] [PubMed] [Google Scholar]
- 4.Richards T.B., Soman A., Thomas C., et al. Screening for lung cancer—10 states, 2017. MMWR Morb Mortal Wkly Rep. 2020;69:201–206. doi: 10.15585/mmwr.mm6908a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zgodic A., Zahnd W.E., Miller D.P., Studts J.L., Eberth J.M. Predictors of lung cancer screening utilization in a population-based survey. J Am Coll Radiol. 2020;17:1591–1601. doi: 10.1016/j.jacr.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 6.Zgodic A., Zahnd W.E., Advani S., Eberth J.M. Low-dose CT lung cancer screening uptake: a rural–urban comparison. J Rural Health. 2022;38:40–53. doi: 10.1111/jrh.12568. [DOI] [PubMed] [Google Scholar]
- 7.Rankin N.M., McWilliams A., Marshall H.M. Lung cancer screening implementation: complexities and priorities. Respirology. 2020;25(suppl 2):5–23. doi: 10.1111/resp.13963. [DOI] [PubMed] [Google Scholar]
- 8.Zeliadt S.B., Hoffman R.M., Birkby G., et al. Challenges implementing lung cancer screening in federally qualified health centers. Am J Prev Med. 2018;54:568–575. doi: 10.1016/j.amepre.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Cancer Institute, National Institutes of Health SEER-Medicare linked database. National Cancer Institute website. https://healthcaredelivery.cancer.gov/seermedicare/ Accessed June 5, 2021.
- 10.Pinsky P.F., Lau Y.K., Doubeni C. Potential disparities by sex and race or ethnicity in lung cancer screening eligibility rates. Chest. 2021;60(1):341–350. doi: 10.1016/j.chest.2021.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shieh Y., Bohnenkamp M. Low-dose CT for lung cancer screening: clinical and coding considerations. Chest. 2017;152(1):204–209. doi: 10.1016/j.chest.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Tailor T.D., Tong B.C., Gao J., et al. Utilization of lung cancer screening in the Medicare fee-for-service population. Chest. 2020;158(5):2200–2210. doi: 10.1016/j.chest.2020.05.592. [DOI] [PubMed] [Google Scholar]
- 13.Lopez M.A., Malki K.G., Choi N.J., et al. Patient adherence to screening for lung cancer in the U.S. A systematic review and meta-analysis. JAMA Network Open. 2020;3(11) doi: 10.1001/jamanetworkopen.2020.25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American College of Radiology National Radiology Data Registry Facility Report, Sep 2019. https://nrdrsupport.acr.org/support/solutions/articles/11000039219-lcsr-available-reports
- 15.Pinsky P.F., Gierada D.S., Hocking W., et al. National Lung Screening Trial findings by age: Medicare eligible versus under-65 population. Ann Int Med. 2014;161:627–633. doi: 10.7326/M14-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carroll N.M., Burnett-Hartman A.N., Joyce C.A., et al. Real-world clinical implementation of lung cancer screening—evaluating processes to improve screening guidelines-concordance. J Gen Int Med. 2020;35:1143–1152. doi: 10.1007/s11606-019-05539-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The National Lung Screening Trial Research Team Results of initial computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980–1991. doi: 10.1056/NEJMoa1209120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gierada D.S., Pinsky P.F. Survival following detection of stage I lung cancer by screening in the National Lung Screening Trial. Chest. 2021;159(2):862–869. doi: 10.1016/j.chest.2020.08.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.