Abstract
Background:
Many individuals with cystic fibrosis (CF) have chronic rhinosinusitis resulting in nasal obstruction, sinus infections, and repeated surgeries. Elexacaftor-tezacaftor-ivacaftor is a highly effective modulator therapy approved for individuals aged 6 years or older with CF who have at least one F508del allele or other responsive mutation. The current study tests the hypothesis that ELX/TEZ/IVA improves sinonasal disease in CF.
Methods:
The study was a pre/post, observational cohort study conducted at two sites. Participants underwent a study visit prior to starting ELX/TEZ/IVA and a second visit at a median of 9 months on therapy. Each visit included sinus CT scan, rigid nasal endoscopy, and sweat chloride measurement. Symptoms were measured with the 22 item Sinonasal Outcome Test at scheduled intervals during the study. Regression models were used to test for improvement in symptoms, endoscopy, and CT scales.
Results:
The study enrolled 34 individuals, with a median age of 27 years (range 12–60). Symptoms improved within 7 days of therapy and plateaued by day 28. Endoscopic crusting resolved and nasal polyposis improved, with a decrease in size or resolution of polyps. Sinus opacification and mucosal thickening improved on CT radiographs with treatment.
Conclusions:
Sinonasal symptoms improved rapidly and durably for at least 180 days on ELX/TEZ/IVA therapy. Objective measures of disease including endoscopic and CT findings improved with ELX/TEZ/IVA.
Keywords: highly effective modulator therapy, sinus disease, sinusitis, cystic fibrosis
1. Introduction:
Chronic rhinosinusitis (CRS) with or without nasal polyps causes symptomatic disease in 40–60% of pediatric and adult patients with Cystic Fibrosis (CF), and sinus mucosal thickening on CT scan is present in at least 90% of individuals with CF[1,2]. Anosmia, chronic congestion, nasal obstruction, chronic rhinorrhea, and facial pain are all frequent symptoms of CF-CRS[3]. Symptoms can start to manifest in young children under the age of 5 years [4] and may require aggressive medical and surgical management, including repeated sinus surgeries[5][6]. Sinonasal quality of life scores, as well as general health related quality of life scores, are lower in patients with moderate to severe CF-CRS[7], and CRS adds to the daily burden of medical care and maintenance therapy[8,9].
The pathogenesis of CF-CRS is caused by defects in the cystic fibrosis transmembrane conductance regulator protein (CFTR), leading to abnormal epithelial ion transport and a dehydrated mucus layer. The sinonasal cavity microbiome becomes dominated with a few pathogenic organisms and repeated cycles of infection and inflammation lead to anatomic remodeling[10]. The sinonasal cavity may also act as a reservoir for pathogenic bacteria in the lower airway with aspiration of sinonasal bacteria infecting the lung[11]. There is a close temporal association between pulmonary and sinus disease exacerbations[12], and pulmonary exacerbations are associated with worse sinonasal quality of life[13]. Thus, treating CF-CRS is medically important both because of the morbidity associated with the disease as well as the potential infectious interactions between the sinuses and lower airways in CF.
Recurrent nasal polyposis is present in approximately 40% of individuals with CF-CRS [2,14]. Nasal polyps in CF are neutrophil dominant, with abundant inflammatory mediators including IL-1β, IL-8, IL-17 and myeloperoxidase[15]. Polyps typically present in childhood and result in nasal obstruction[16]. Many patients will undergo sinus surgery and nasal polypectomy to relieve anatomic obstructions and create space for daily sinonasal irrigations. However, patients often have recurrence of occlusive polyps despite topical and systemic steroid use requiring repeated surgical procedures. Management of nasal polyposis is an unmet medical need in the care of people with CF.
Elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) is a highly effective CFTR corrector/potentiator drug that was approved by the Food and Drug Administration for the treatment of CF patients >6 years old who have at least one drug sensitive allele, most commonly F508del. Regarding sinus health, previous case reports demonstrated significant improvement in the CT scans of isolated adult patients with CF treated with ivacaftor alone who harbor the G551D gating mutation[17,18]. ELX/TEZ/IVA improves sinonasal symptoms at 3 months[19] and recent publication by Beswick et al also showed that ELX/TEZ/IVA improved sinus opacification from a mean volume of 64% to 41%, as well as improved health related quality of life measures out to six months[20]. However, treatment with ELX/TEZ/IVA does not improve quantitative measures of olfaction when measured out to 6 months[21,22]. As the era of ELX/TEZ/IVA begins, understanding the impact and outcomes of CFTR modulators on sinonasal disease is critical to managing CF-CRS in the pediatric and adult populations. By understanding how ELX/TEZ/IVA impacts the sinonasal cavity and status of nasal polyps we can more thoughtfully advise our patients with CF-CRS with or without polyps whether ELX/TEZ/IVA is likely to improve their CF-CRS in addition to improve other CF disease manifestations. Therefore, we conducted a prospective, observational study on the effects of ELX/TEZ/IVA on the sinonasal tract of patients ≥12 years old with CF who were homozygous or heterozygous for F508del mutations. The current cohort is unique in that it includes individuals as young as age 12 and includes endoscopic assessment of sinonasal disease. We hypothesized that ELX/TEZ/IVA would improve overall sinonasal outcomes, with sinonasal quality of life being our primary endpoint.
2. Methods:
2.1. Study Design:
An IRB- approved (Pitt STUDY1810016; UNC IRB 19-2298) pre-post study was conducted at two Cystic Fibrosis Foundation accredited centers: University of Pittsburgh and University of North Carolina. Participants were recruited from both CF pulmonary clinic and rhinology clinic. During the period between the publication of the Phase III study for ELX/TEZ/IVA and FDA approval of the drug, the clinical teams prepared eligibility lists based on genotypes, educational resources for patients, and flow process documents to streamline the prescription of the drug. We also informed eligible patients about the opportunities to participate in research surrounding this highly anticipated approval, including the current study. Potential participants were recruited from both pulmonary and rhinology clinics, depending on which appointment occurred first for any individual. Inclusion criteria included a diagnosis of cystic fibrosis, age 12 years and older, and the intent of the clinical care team to start ELX/TEZ/IVA following the educational process described above. Exclusion criteria included lung transplant, treatment for a pulmonary or sinus exacerbation with antibiotics within the previous 14 days, other recent CF therapy changes, and upper respiratory tract infection within the prior 14 days. Participants were enrolled between November 2019 and March 2020. The median time between the first visit and start of therapy was 3 days (IQR 1–11 days). A previous diagnosis of CF-CRS was not required for eligibility because we wanted to capture the full range of CRS presentations in the populations, including individuals with mild symptoms or without easy access to ENT care. There were no systematic changes in other therapies prior to initiation of ELX/TEZ/IVA, and previous medication adherence was not an inclusion criterion.
2.2. Study Endpoints:
The primary endpoint of the study was change in sinonasal quality of life as measured by the SNOT-22 scale [23]. The SNOT-22 scale is a patient reported outcome measure that grades 22 sinus-related symptoms on a scale of 0 to 5, with 5 indicating the most severe manifestation[23,24]. The scale covers common sinonasal symptoms such as: need to blow nose, nasal blockage, sneezing, cough, ear and facial symptoms, sleep related symptoms and mood related symptoms. The scale has been widely used in CF and has high internal consistency and test-retest reliability[23]. The minimal clinically important difference (MCID) for the scale is 8.9 points outside of CF; however, no MCID has been defined within the CF population. The median score for individuals without any sinonasal disease is 7 points[25]. Prespecified secondary endpoints were improvement in endoscopy findings and radiographic disease. The nasal endoscopies were recorded on video and scored using the Lund Kennedy nasal endoscopy scale[26]. The Lund-Kennedy scale rates each side of the nose on a 0–2 scale for polyps, discharge, edema, scarring, and crusting. Higher scores indicate worse disease, and the scale has moderate inter-rater and test/retest reliability. The pre and post ELX/TEZ/IVA maxillofacial CT scans were independently reviewed and scored by a head and neck radiologist using the modified Lund Mackay scale[27,28]. The observer was blinded to whether the scan was from before or after ELX/TEZ/IVA, and scans were presented in a random order. Each sinus was rated on a 0–2 scale based on the degree of opacification, with a score of 24 indicating the worst disease. The scale is weighted to account for sinus aplasia. Exploratory endpoints included the relationships between the clinical covariates and primary and secondary endpoints.
2.3. Study Procedures:
The study design is shown in Fig S1. Prior to starting ELX/TEZ/IVA, each study participant underwent height and weight measurements, spirometry, and a pilocarpine iontophoresis sweat test. Demographic information was obtained including genetic mutation, medication usage as reported by the participant, history of prior sinus surgeries, and other extra-pulmonary manifestations of CF. Each participant then underwent bilateral rigid nasal endoscopy by an experienced rhinologist, as well as thin-section low-dose non-contrast maxillofacial computerized tomography (CT) scan. After participants started ELX/TEZ/IVA, participants completed interval SNOT-22 scales at days 7 and 28 and months 2, 4, 6, and 9 or until visit 2. After 6 to 12 months of ELX/TEZ/IVA, participants returned for a second study visit with identical study procedures. All data were entered into the REDCap database [29].
2.4. Power and Sample Size Determination:
Statistical analysis was done using Stata Statistical Software: Release 16. (StataCorp LLC College Station, TX). We used our prior longitudinal study in adults with CF-CRS[12] to estimate sample size and power. A sample size of 30 individuals was estimated to have an 80% power of detecting the minimal clinically important difference of 8.9 points improvement in SNOT-22 score. Our initial recruitment goal was 40 participants to allow for attrition; however, due to study interruption by the COVID-19 pandemic we enrolled 34 participants. The pandemic also led to an extension to the time interval for the follow-up visit from 6 months to a range of 6–12 months after starting ELX/TEZ/IVA.
2.5. Analytic Approach: Primary and Secondary Outcomes
Data were displayed as median (interquartile range) or percentage of entire population. Primary covariates of interest were age, sex, baseline body mass index, spirometry, sweat chloride, SNOT-22 scale measurement, endoscopy score and CT scan score. For the primary outcome of change in sinonasal quality of life: a mixed effects linear regression was used to test the effect of time to determine if a treatment effect was present in the SNOT-22 scale measurements (margins plotted in Fig 1). The prespecified secondary outcome of change in Lund-Kennedy endoscopy scale was determined with a Wilcoxon signed rank test. Individual Lund-Kennedy subscale items were treated as binary variables (no disease vs any disease) and a McNemar test was used to determine significance. For the prespecified secondary outcome of change in Lund-Mackay CT scale, scores were binned around the pre-treatment median of 14.5 and treated as a binary variable because the distribution was highly skewed and the sample size was small, violating assumptions for linear regression. The McNemar test was used to test if scores changed with treatment.
Figure 1:
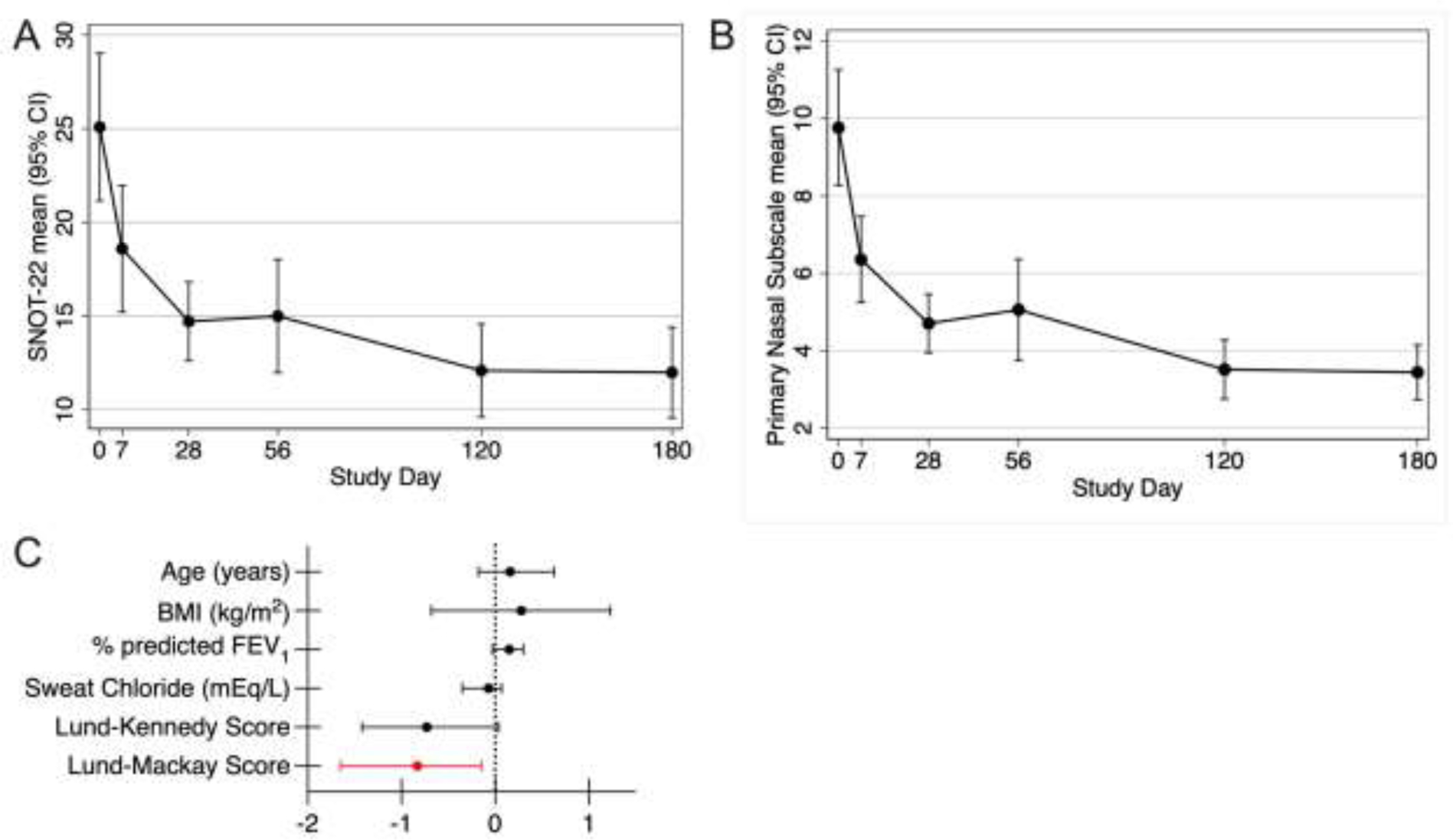
Change in sinonasal quality of life with treatment. Mixed effects linear regressions testing change in SNOT-22 scale measurements over time. Error bars show 95% confidence intervals at each time. A). SNOT-22 scores dropped within 7 days of starting ELX/TEZ/IVA, with a plateau by 4 weeks of treatment, p<0.0001 for change over time at all study days. B). Primary nasal symptom subscale dropped within 7 days of starting ELX/TEZ/IVA (p<0.0001 for all study days). C). Coefficient plot (with bootstrapped bias corrected 95% confidence interval) from ANCOVA models for final SNOT-22 score and indicated covariates.
2.6. Analytic Approach: Exploratory Outcomes:
Our overall goal was to determine if a predefined list of covariates (listed in Tables S3, S4, and S5) predicted response in the primary and secondary outcomes described above. For SNOT-22 scale data, separate bootstrapped ANCOVA model was used with SNOT-22 as the dependent variable and the covariates listed in Table S3 independent variables. Individual covariates were tested in these models, with data are shown as coefficient estimates with bootstrapped bias corrected 95% confidence intervals (Tables S3, Fig 1C). For exploratory analysis for predictors of final Lund-Kennedy endoscopy score, we did a similar analysis with covariates listed in Table S4. Lund-Mackay CT scan scores were binned around the pre-treatment median and treated as a binary variable. Then bootstrapped logistic regression models, which was adjustment for baseline Lund-Mackay CT score, were used to test the individual covariates listed in Table S5, with odds ratios (95% CI) being reported. For covariates with very small number of event (allergic rhinitis, prior modulator use, pancreatic insufficiency and ABPA), a Firth logistic regression was used. Analyses for Lund-Kennedy and Lund-Makay scores were done only with data on participants with both visits. Finally, simple linear regressions were done with the full (n=34) data set to determine predictors of baseline CRS disease severity (Fig S3)
3. Results:
3.1. Cohort description:
Thirty-four participants were enrolled in the study. Due to the COVID-19 pandemic, six participants withdrew from the study prior to the second visit; therefore, 28 participants completed both study visits, although 27 individuals had a complete set of SNOT-22 symptom measurements. Baseline characteristics of the study cohort are shown in Table 1 and self-reported medication use during the study are listed in Table S1. There were no significant differences between participants who enrolled in the study as compared to those who completed both study visits (Table 1). No patients underwent sinonasal surgery during the study period, and there were no systematic changes in general CF care during the study period, although participants generally reported less medication usage generally after 6 months of therapy (Table S1). After starting ELX/TEZ/IVA, participants aged 12 to 19 years saw an increase in median body mass index (BMI) percentile from 53 to 58 (p=0.047 by Wilcoxon match-pairs signed rank test), and participants age>20 years saw a median change in BMI from 23.2 to 24.0 m/kg2 (p=0.0048 by two-way paired t-test). On ELX/TEZ/IVA participants had a increase median % predicted FEV1 from 70.5 to 85.0 (p=0.0004 by two-way, paired t-test), and a drop in median sweat chloride from 92.5 to 43.8 mEq/L (<0.0001 by two-way, paired t-test) All comparisons only included participants who completed both visits Table S2).
Table 1:
Cohort Characteristics at Baseline: Data reported as median
| Characteristic | N=34 | Participants with both visits (N=28) |
|---|---|---|
| Male, n(%) | 21 (61.8%) | 18 (64%) |
|
| ||
| Age, years# | 27 (12–60) | 28.9 (12–60) |
| Age 12–21 years, n(%) | 12 (35.3%) | 10 (36%) |
|
| ||
| BMI (kg/m2)$ | 24.9 (20.0–27.5) | 23.2 (20.0–27.4) |
| BMI percentile@ | 53 (48–66) | 53 (47–69) |
|
| ||
| % predicted FEV1(median, IQR) | 80 (64–103) | 70.5 (58–103) |
|
| ||
| F508 homozygous genotype, n(%) | 23 (67.7%) | 19 (67.9%) |
|
| ||
| Prior modulator Use, n(%) | 20 (58.8%) | 16 (57.1%) |
|
| ||
| Pancreatic Insufficiency, n(%) | 29 (85.3%) | 25 (89.3) |
|
| ||
| CF Related Diabetes, n(%) | 9 (26.5%) | 9 (32%) |
|
| ||
| Prior sinus surgery | 24 (70.6%) | 21 (75.0%) |
|
| ||
| Nasal polyposis on endoscopy | 16 (47.1%) | 15 (53.6%) |
|
| ||
| Pseudomonas aeruginosa in prior year* | 19 (55.9%) | 14 (50.0%) |
|
| ||
| Staphylococcus aureus in prior year* | 27 (79.4%) | 23 (82.1%) |
|
| ||
| Allergic Bronchopulmonary Aspergillosis, n(%) | 5 (14.7%) | 4 (14.3%) |
|
| ||
| Allergic rhinitis, n(%) | 20 (58.8%) | 16 (57.1%) |
|
| ||
| Nasal Steroid, n(%) | 19 (55.9%) | 15 (53.4%) |
|
| ||
| Sweat chloride (mEq/L) | 92 (82–98) | 92 (82–98) |
|
| ||
| SNOT-22 (Symptom) Score | 21 (12–36) | 18 (12–38.5) |
|
| ||
| Lund-Makay (CT Scan) Score | 15.4 (12.0–18.9) | 16 (13.5–20.6) |
|
| ||
| Lund-Kennedy (Endoscopy) Score | 5.5 (3.0–8.0) | 6.0 (3–8.5) |
(IQR) except for as otherwise specified.
median (range).
Microbial cultures from lower respiratory source.
reported for participants age>19 years
reported for participants age 12–19 years. 8 particpants completed Visit 1, 8 participants completed both visits.
3.2. ELX/TEZ/IVA Improves Participant Reported Improved Sinonasal Quality of Life:
Participants completed serial Sinonasal Outcome Test-22 measurements over the course of the study. Sinonasal quality of life improved rapidly on ELX/TEZ/IVA, with a significant drop in SNOT-22 scale measurements seen by day 7, and persistent improvement seen out to 180 days (p<0.0001 for change over time, Fig 1A). Similar results were obtained when analyzing the data set with no missing time points. Using the primary nasal symptom subscale of the SNOT-22 scale, which contains items focused on nasal symptoms, a similar drop in scores was seen within 7 days and persistent improvement was seen out to day 180 (p<0.0001, Fig 1B). The magnitude of improvement with ELX/TEZ/IVA was greater than the MCID of the SNOT-22 scale of 8.9 points[23]. Baseline Lund-Mackay CT score was the only covariate that predicted the final SNOT-22 scale measurement, with each point increase in Lund-Mackay CT score at baseline predicting a 0.83 decrease (improvement) in final SNOT-22 scale measurement (Fig 1C, Table S3).
3.3. ELX/TEZ/IVA Improves Nasal Polyp Burden and Endoscopy Scores:
Participants underwent a rigid nasal endoscopy prior to starting ELX/TEZ/IVA and after a median of 9 months treatment. Endoscopies were scored using the Lund-Kennedy endoscopy scale[30]. The median score on enrollment was 6.0 (IQR 3–8.5) which dropped to 2 (IQR 1–3.5) on treatment (p=0.0002 by Wilcoxon signed-rank test, medians reported for participants who completed both visits). When subscale scores were examined, no participants demonstrated crusting post-treatment, and polyps were observed in significantly fewer patients (Fig 2E). Nasal endoscopy demonstrated less thick discharge and mucosal edema. Figs 2, 3 and S2 show representative images from nasal endoscopies. Of participants completing both visits, 15 (54%) had polyps present on endoscopy prior to treatment. On follow-up only 7 participants (25%) had visible polyps. On treatment, when polyps were present, they tended to be shrunken remnants of the prior polyp that were still visible (Fig 3D, Fig S1B, FigS1D). Radiographic severity was the only variable that predicted final Lund-Kennedy endoscopy score, with each increase in baseline Lund-Mackay CT score associated with a 0.19-point decrease in Lund-Kennedy endoscopy score (Fig3E, Table S4).
Figure 2:
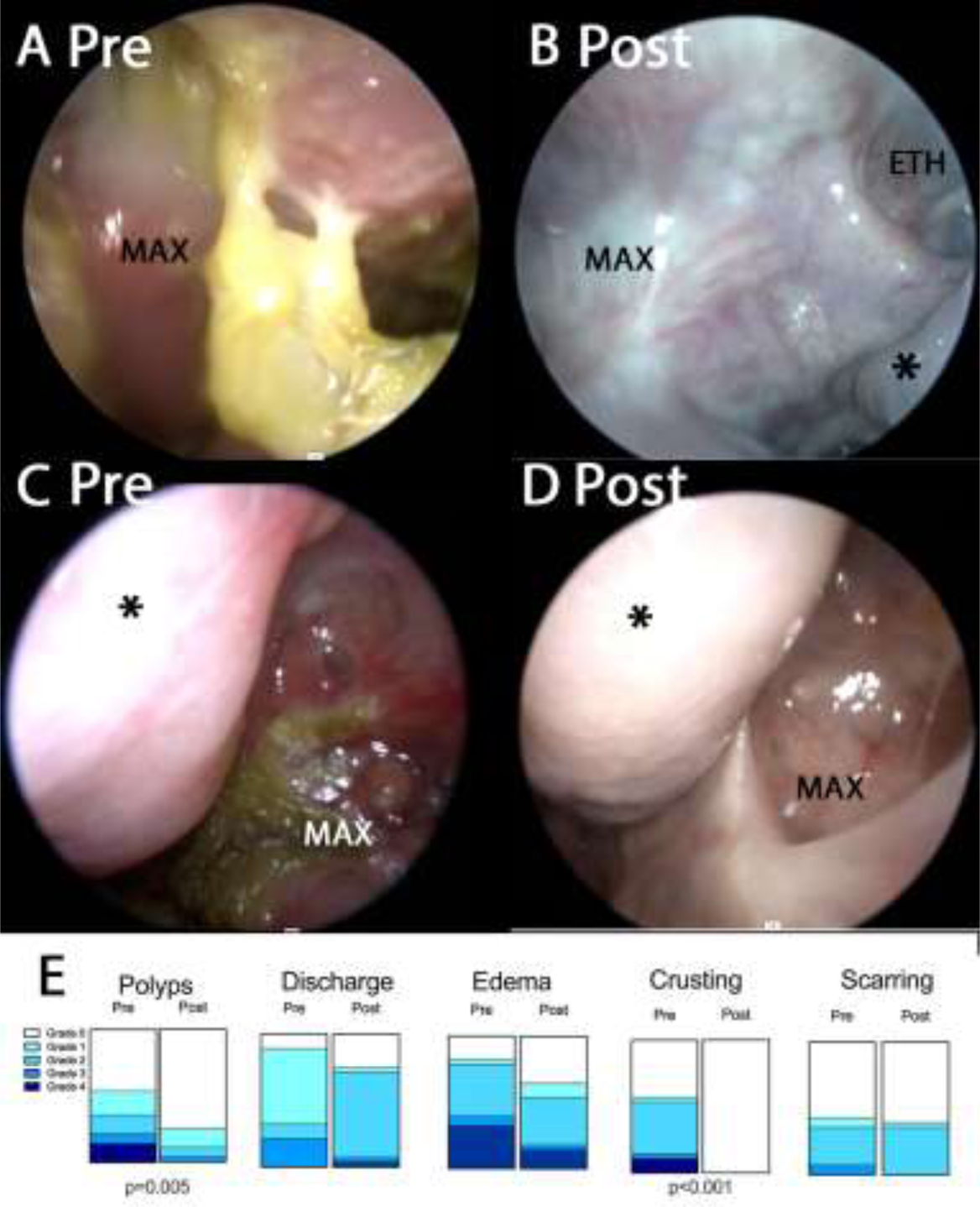
Rigid sinonasal endoscopic photographs pre (A, C) and post (B, D) ELX/TEZ/IVA treatment. Fig 2A–B: Right maxillary-ethmoid. Note adherent yellow mucus present in A prior to treatment. Fig 2C–D: Left maxillary. Note erythema, mucosal edema and discharge in C prior to treatment. * indicates middle turbinate. MAX indicates maxillary sinus. ETH indicates ethmoid. Fig 2E. Lund-Kennedy items scored before and after treatment with ELX/TEZ/IVA. p-values shown from McNemar test comparing grade 0 to grades >0.
Figure 3:
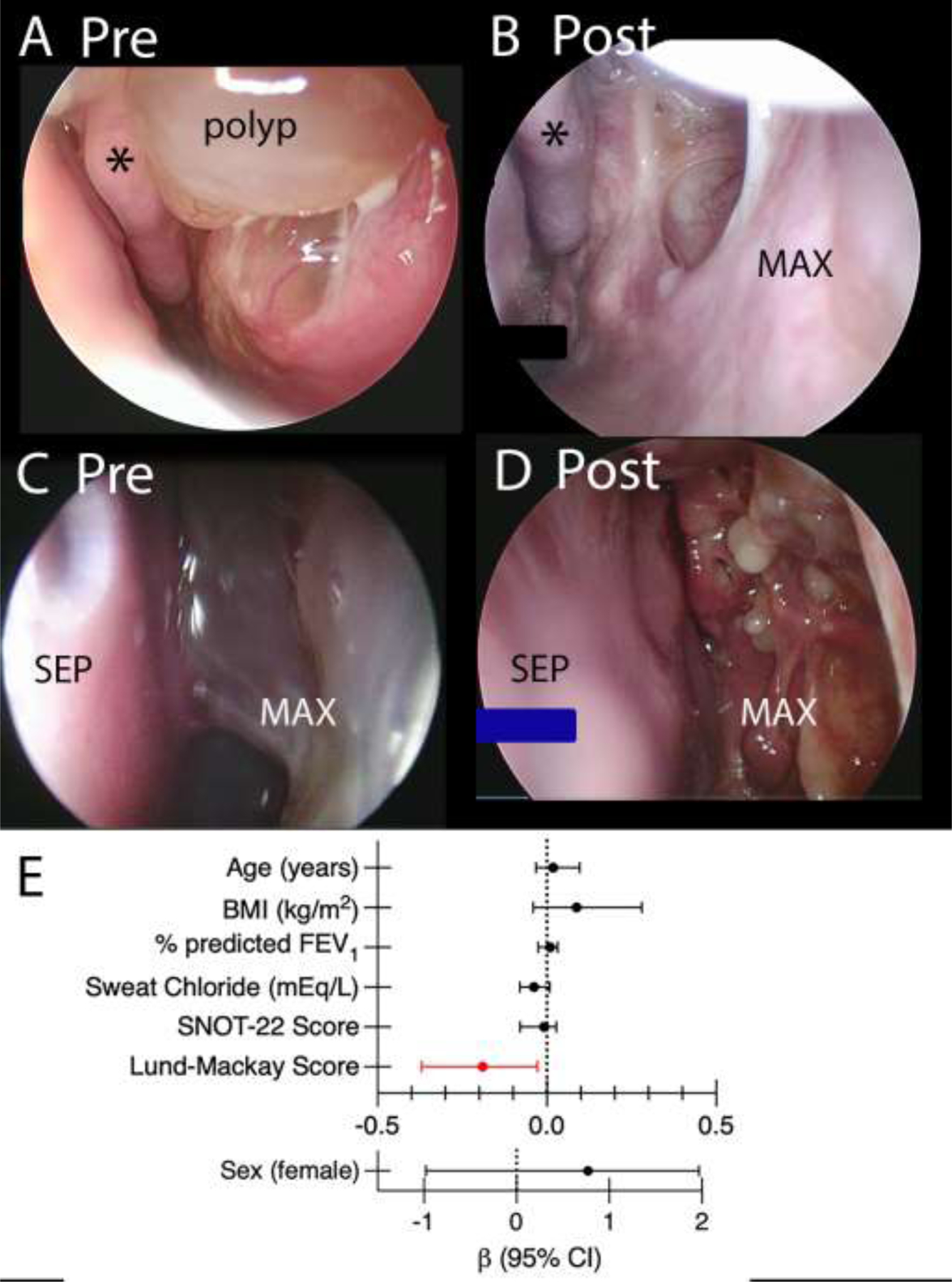
Rigid sinonasal endoscopic photographs (A, C) pre and (B, D) post ELX/TEZ/IVA treatment. A) Prior to treatment a large polyp is seen near the middle turbinate that has B) resolved with treatment. C): Maxillary cavity obscured by large polyp. D). Many residual, very small polyps are seen on the surface of the maxillary cavity after treatment. Maxillary cavity: MAX, septum: SEP, middle turbinate: * E). Coefficient plot (with bootstraped bias corrected 95% confidence interval) from ANCOVA models for final Lund-Kennedy Score.
3.4. ELX/TEZ/IVA improves radiographic evidence of CF sinus disease.
All participants received a low-dose dedicated sinus CT scan before and after starting ELX/TEZ/IVA. Using the Lund-Mackay CT scoring system, the median score decreased from 16 (IQR 13.5–20.6) to 12 (IQR 9.5–12.6), p=0.0001 (McNemar test, including only participants who completed both visits). After adjusting for baseline Lund-Mackay CT score, only having a history of prior sinus surgery predicted the final score, with a history of prior surgery leading to a odds ratio of 0.1 (0.025–0.57) of remaining above a score of 14.5 with treatment (the pre-treatment median). (Table S5). Overall, we saw a marked improvement in sinus opacification with a representative scan shown in (Fig 4A–B). However, there were a small number of individuals who continued to have significant sinus opacification (Fig 4C–D).
Figure 4.
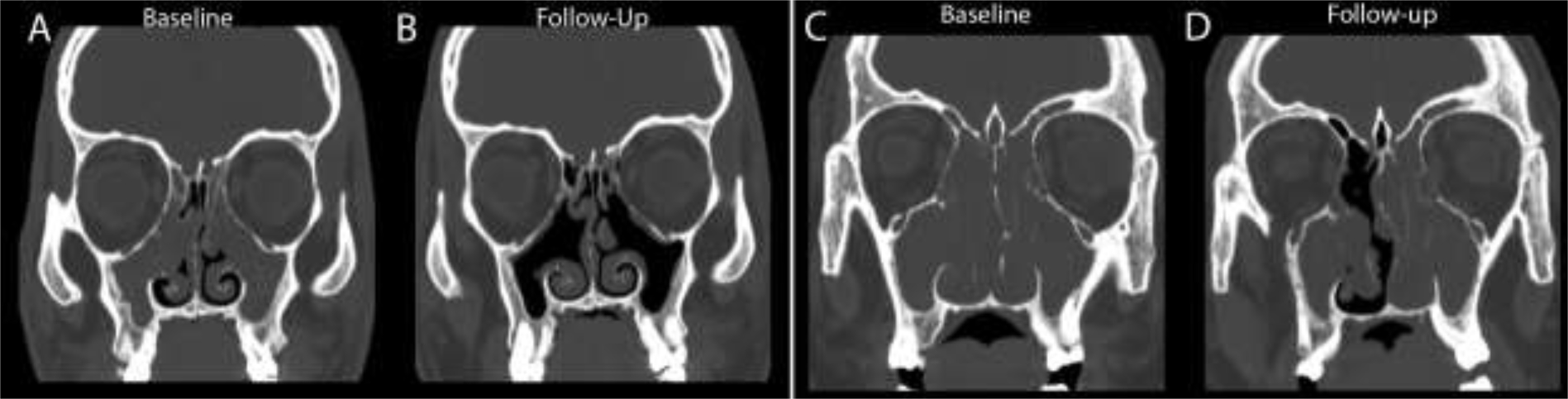
CT images of responsive and non-responsive patients. Pre (A) and post ELX/TEZ/IVA (B) coronal reformatted CT images through the maxillary and ethmoid sinuses of a 12-year-old male patient demonstrate complete opacification before treatment (A), but only minimal mucosal thickening after treatment (B). Pre (C) and post ELX/TEZ/IVA (D) coronal reformatted CT images through the maxillary sinuses of a 20-year-old male patient demonstrate complete opacification of the maxillary sinuses, ethmoid air cells, and nasal cavity prior to treatment (C). After treatment (D), there is improvement in the right anterior ethmoid air cells and nasal cavity, but no aeration of the maxillary sinuses.
3.5. Relationship between baseline sweat chloride concentration and disease severity.
The study also allowed us to determine baseline predictors of sinus disease severity (Fig S2). Older age and higher BMI predicted worse sinonasal quality of life, higher baseline sweat chloride predicted worse endoscopic and radiographic appearances.
4. Discussion:
CFTR modulator therapy has been a transformative treatment for many people with CF. Elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) was approved as highly effective modulator CFTR therapy in late 2019, and dramatically improves lung function and rate of CF pulmonary exacerbations [31]. As CFTR is expressed abundantly in the sinuses, the effects of improved chloride channel function are predicted to extend to the sinuses. In the current study we found improvements in sinonasal quality of life, nasal polyp burden, and radiographic disease severity.
4.1. ELX/TEZ/IVA Improves Sinonasal Quality of Life of CF Chronic Rhinosinusitis
We observed a large and sustained improvement in sinonasal quality of life. Improvement was noted within 7 days and persisted for at least 6 months. Our findings show a similar time course to those seen by Beswick et al, and is similar to that seen in people with G551D mutations and treated with ivacaftor [18,20]. It is important to recognize that the SNOT-22 scale contains multiple mood and sleep related items[23], and our study was conducted during the first phase of the COVID-19 pandemic, which may confound responses to those items. The scale also contains items including “fatigue” and “cough”, both of which might be improved on ELX/TEZ/IVA apart from any influence the drug might have on CF-CRS. When the primary nasal subscale alone was studied, we saw a similar improvement. Interestingly, unlike endoscopy and CT scan findings, sinonasal quality of life was not predicted by baseline sweat chloride levels, which may reflect additional factors in subjective disease severity including the effects of allergic rhinitis, chronic pain syndromes, or perhaps a CFTR-independent inflammatory component[7].
4.2. ELX/TEZ/IVA improves radiologic sinus disease in CF:
Radiologic evidence of CRS on CT or MRI studies is present in preschool children with CF [14] and includes findings such as sinus hypoplasia, sinus opacification and mucosal thickening. The extent of imaging findings correlates with CFTR mutation severity [32], and we indeed saw that higher baseline sweat chloride predicted higher (worse) Lund-Mackay CT scores. Multiple prior studies have shown a correlation between severity of radiographic and endoscopic disease, as did ours[2]. Our study is unique in that we directly measured sweat chloride in all participants, as opposed to numerous studies that link CFTR genotype and various sinonasal phenotypes. The association between sweat chloride and objective measures but lack of association with sinonasal quality of life may explain the variabilities in attempting to link genotype class and patient reported symptoms. A history of prior sinus surgery did not change the modified Lund-Mackay CT score at baseline but was associated with improvement in radiographic score on treatment as compared with those participants who had no prior surgeries. These results are intriguing and suggest perhaps the improved anatomic drainage afforded by prior surgery facilitates clearance of mucus when CFTR is restored. Notably, approximately 10% of participants did not show improvement on at least one measure of CRS disease severity despite biochemical improvement as measured by sweat chloride, so we infer additional patient level factors are at play.
Polyps, crusting and visual sinonasal inflammation improve with ELX/TEZ/IVA:
We also conducted rigid nasal endoscopies pre and post treatment on all participants. Nasal endoscopy scores significantly improved with ELX/TEZ/IVA treatment, however improvement varied by scale item. There was no change in scarring, which is unsurprising given the chronic nature of the process. The most striking finding was the resolution of polyps in most participants. In childhood, polyps increase in size over time without surgical intervention, so the finding is unlikely to be due to chance[4]. In many cases, we saw very minor residual polypoid tissue in the prior location of large polyps that appeared shrunken or deflated. The biologic mechanism of polyp formation in CF is not well understood, but polyp size seems to be related to CFTR dysfunction. Interestingly, final sweat chloride did not predict improvement in any variables we measured, which may reflect a threshold effect (i.e. all participants were beyond threshold), may be a limitation of our sample size, or perhaps reflects additional biological inputs to the disease.
4.3. Strengths and weakness:
Our study had several strengths: first, it was a prospectively designed study timed to start with the anticipated approval of ELX/TEZ/IVA in 2019. Second, the study design included sweat chloride as a measure of biochemical drug effect and included both patient reported and objective measures of anatomic disease. As there is geographic variability in non-CF CRS due to varying influences of allergic stimuli, inclusion of two sites strengthens the study. We also included individuals down to age 12, many of whom have near normal lung function. Inclusion of these young teens may help inform decision making regarding the initiation of ELX/TEZ/IVA in individuals with minimal pulmonary disease yet substantial sinonasal disease. Weaknesses of the study include a modest sample size, a follow-up interval that was wider than initially planned due to the COVID-19 pandemic, the potential for disease severity to influence any individual potential participant’s decision to enroll, and the lack of a control arm. Because the study occurred during the COVID-19 pandemic, there may have been unintended changes in participant access to general medical care. Recruiting a control arm of adults with CF who were eligible for, but not prescribed, ELX/TEZ/IVA was not feasible in the US due to expert opinion that rapidly made ELX/TEZ/IVA standard of care for CF lung disease. Longitudinal data on the natural history of CF-CRS does exist (37), and the magnitude of change we saw in our study was vastly greater than variation in disease over time due to chance.
4.4. Clinical Implications:
Some individuals have very symptomatic sinonasal disease or severe nasal polyposis in CF without concurrent severe lung disease. In these individuals, treatment with ELX/TEZ/IVA is likely to improve both sinonasal quality of life and polyp burden without the need for repeated surgical intervention. Improvement, at least in sinonasal quality of life, occurs within a month of starting treatment. Second, there are many CF lung transplant recipients with highly symptomatic sinus disease and ELX/TEZ/IVA might benefit these individuals in regards to their sinonasal symptoms. It remains to be answered if treating CF-CRS post lung transplant will lead to improve allograft function or decrease the incidence of lower airway infections. Finally, the residual polyp lesions are intriguing. It is unclear if there is an ongoing defect in epithelial maintenance in the presence of ELX/TEZ/IVA, and if these polyp remnants would enlarge if treatment were stopped. In conclusion, ELX/TEZ/IVA leads to regression of nasal polyps, improved nasal endoscopy scores, improved sinonasal CT scans, and improved sinonasal quality of life scores in patients 12 years old and older newly started on therapy.
Supplementary Material
Highlights.
HEMT improves symptoms of chronic rhinosinusitis within a week.
Improvement of symptoms lasts out to at least 6 months.
HEMT improves sinus opacification on CT scan
HEMT improves nasal polyposis and improving of crusting on endoscopic exam
Acknowledgements:
We thank our study participants and their families. The work would not have been possible without the clinical research teams at each site, whom we thank.
Funding Sources:
Cystic Fibrosis Foundation Grants ZEMKE19A0 and ZEMKE16Q0; National Institute of Health grants K23HL131930 to AZ and KL2TR002490 to AK, and Cystic Fibrosis Foundation Research Development Program (BOMBER19R0) to JMP.
Footnotes
CRediT authorship contribution statement
Amanda L. Stapleton: investigation, writing – original draft, writing – review & editing Adam J. Kimple: investigation, writing – review & editing Jennifer L Goralski: investigation, writing – review & editing S. M. Nouraie: formal analysis, data curation, writing – review & editing Amber D Shaffer: investigation, data curation, writing – review & editing Barton F Branstetter: formal analysis, visualization, writing – review & editing Joseph M Pilewski: conceptualization, supervision, funding acquisition, writing – review & editing Brent A Senior: investigation, writing – review & editing Stella E Lee: conceptualization, supervision, funding acquisition, writing – review & editing Anna C Zemke: conceptualization, formal analysis, resources writing – original draft, data curation, writing – review & editing, project administration, funding acquisition
Declaration of Competing Interest: No authors have competing interests with this work.
Supplementary Materials:
Supplementary material associated with this article can found online at doi:
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Gentile VG, Isaacson G. Patterns of sinusitis in cystic fibrosis. Laryngoscope 1996;106:1005–9. 10.1097/00005537-199608000-00018. [DOI] [PubMed] [Google Scholar]
- [2].Casserly P, Harrison M, O’Connell O, O’Donovan N, Plant BJ, O’Sullivan P. Nasal endoscopy and paranasal sinus computerised tomography (CT) findings in an Irish cystic fibrosis adult patient group. Eur Arch Otorhinolaryngol 2015;272:3353–9. 10.1007/s00405-014-3446-z. [DOI] [PubMed] [Google Scholar]
- [3].Wise SK, Lin SY, Toskala E, Orlandi RR, Akdis CA, Alt JA, et al. International Consensus Statement on Allergy and Rhinology: Allergic Rhinitis. International Forum of Allergy & Rhinology 2018;8:108–352. 10.1002/alr.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Suy P, Coudert A, Vrielynck S, Truy E, Hermann R, Ayari-Khalfallah S. Evolution of sinonasal clinical features in children with cystic fibrosis. International Journal of Pediatric Otorhinolaryngology 2019;124:47–53. 10.1016/J.IJPORL.2019.05.030. [DOI] [PubMed] [Google Scholar]
- [5].Okafor S, Kelly KM, Halderman AA. Management of Sinusitis in the Cystic Fibrosis Patient. Immunology and Allergy Clinics of North America 2020;40:371–83. 10.1016/j.iac.2019.12.008. [DOI] [PubMed] [Google Scholar]
- [6].Johnson BJ, Choby GW, O’Brien EK. Chronic rhinosinusitis in patients with cystic fibrosis—Current management and new treatments. Laryngoscope Investigative Otolaryngology 2020;5:368–74. 10.1002/lio2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Habib ARR, Buxton JA, Singer J, Wilcox PG, Javer AR, Quon BS. Association between chronic rhinosinusitis and health-related quality of life in adults with cystic fibrosis. Annals of the American Thoracic Society 2015;12:1163–9. 10.1513/AnnalsATS.201504-191OC. [DOI] [PubMed] [Google Scholar]
- [8].Kang SH, Meotti CD, Bombardelli K, Piltcher OB, de Tarso Roth Dalcin P Sinonasal characteristics and quality of life by SNOT-22 in adult patients with cystic fibrosis. European Archives of Oto-Rhino-Laryngology 2017;274:1873–82. 10.1007/s00405-016-4426-2. [DOI] [PubMed] [Google Scholar]
- [9].Chan DK, McNamara S, Park JS, Vajda J, Gibson RL, Parikh SR. Sinonasal Quality of Life in Children With Cystic Fibrosis. JAMA Otolaryngology–Head & Neck Surgery 2016;142:743. 10.1001/jamaoto.2016.0979. [DOI] [PubMed] [Google Scholar]
- [10].Whiteson KL, Bailey B, Bergkessel M, Conrad D, Delhaes L, Felts B, et al. The upper respiratory tract as a microbial source for pulmonary infections in cystic fibrosis. Parallels from island biogeography. American Journal of Respiratory and Critical Care Medicine 2014;189:1309–15. 10.1164/rccm.201312-2129PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nelson J, Karempelis P, Dunitz J, Hunter R, Boyer H. Pulmonary aspiration of sinus secretions in patients with cystic fibrosis. International Forum of Allergy & Rhinology 2018;8:385–8. 10.1002/ALR.22043. [DOI] [PubMed] [Google Scholar]
- [12].Zemke Anna C; Nouraie S. Mehdi; Moore John; Gaston Jordan; Rowan Nicholas; Pilewski Joseph; Bomerger Jennifer; Lee S Clinical Predictors of Cystic Fibrosis Chronic Rhinosinusitis Severity. Int Forum Allergy Rhinol 2019;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Safi C, DiMango E, Keating C, Zhou Z, Gudis DA. Sinonasal quality-of-life declines in cystic fibrosis patients with pulmonary exacerbations. International Forum of Allergy and Rhinology 2020;10:194–8. 10.1002/alr.22485. [DOI] [PubMed] [Google Scholar]
- [14].Rasmussen J, Aanæs K, Norling R, Nielsen KG, Johansen HK, von Buchwald C. CT of the paranasal sinuses is not a valid indicator for sinus surgery in CF patients. Journal of Cystic Fibrosis 2012;11:93–9. 10.1016/j.jcf.2011.09.009. [DOI] [PubMed] [Google Scholar]
- [15].Derycke L, Eyerich S, van Crombruggen K, Pé Rez-Novo C, Holtappels G, Deruyck N, et al. Mixed T Helper Cell Signatures In Chronic Rhinosinusitis with and without Polyps n.d. 10.1371/journal.pone.0097581. [DOI] [PMC free article] [PubMed]
- [16].Bock JMM, Schien M, Fischer C, Naehrlich L, Kaeding M, Guntinas-Lichius O, et al. Importance to question sinonasal symptoms and to perform rhinoscopy and rhinomanometry in cystic fibrosis patients. Pediatric Pulmonology 2017;52:167–74. 10.1002/ppul.23613. [DOI] [PubMed] [Google Scholar]
- [17].Chang EH, Tang XX, Shah VS, Launspach JL, Ernst SE, Hilkin B, et al. Medical reversal of chronic sinusitis in a cystic fibrosis patient with ivacaftor. International Forum of Allergy & Rhinology 2015;5:178–81. 10.1002/alr.21440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].McCormick J, Cho DY, Lampkin B, Richman J, Hathorne H, Rowe SM, et al. Ivacaftor improves rhinologic, psychologic, and sleep-related quality of life in G551D cystic fibrosis patients. International Forum of Allergy and Rhinology 2018. 10.1002/alr.22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].DiMango E, Overdevest J, Keating C, Francis SF, Dansky D, Gudis D. Effect of highly effective modulator treatment on sinonasal symptoms in cystic fibrosis. Journal of Cystic Fibrosis 2021;20. 10.1016/j.jcf.2020.07.002. [DOI] [PubMed] [Google Scholar]
- [20].Beswick D, Humphries S, Balkissoon C, Strand M, Vladar E, Lynch D, et al. Impact of CFTR Therapy on Chronic Rhinosinusitis and Health Status: Deep Learning CT Analysis and Patient Reported Outcomes. Annals of the American Thoracic Society 2021. 10.1513/ANNALSATS.202101-057OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bacon DR, Stapleton AL, Goralaski JL, Ebert CS, Thorp BD, Nouraie SM, et al. Olfaction Before and After Initiation of Elexacaftor-Tezacaftor-Ivacaftor in a Cystic Fibrosis Cohort. International Forum of Allergy and Rhinology 2021. 10.1002/alr.22891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].DM B, SM H, CD B, M S, EK V, VR R, et al. Olfactory dysfunction in cystic fibrosis: Impact of CFTR modulator therapy. Journal of Cystic Fibrosis : Official Journal of the European Cystic Fibrosis Society 2021. 10.1016/J.JCF.2021.09.014. [DOI] [PubMed] [Google Scholar]
- [23].Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clinical Otolaryngology 2009;34:447–54. 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- [24].Savastano V, Bertin S, Vittori T, Tripodi C, Magliulo G. Evaluation of chronic rhinosinusitis management using the SNOT-22 in adult cystic fibrosis patients. European Review for Medical and Pharmacological Sciences 2014;18:1985–9. [PubMed] [Google Scholar]
- [25].Gillett S, Hopkins C, Slack R, Browne JP. A pilot study of the SNOT 22 score in adults with no sinonasal disease. Clinical Otolaryngology 2009;34. 10.1111/j.1749-4486.2009.01975.x. [DOI] [PubMed] [Google Scholar]
- [26].Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngology--Head and Neck Surgery : Official Journal of American Academy of Otolaryngology-Head and Neck Surgery 1997;117:S35–40. 10.1016/S0194-59989770005-6. [DOI] [PubMed] [Google Scholar]
- [27].Woodworth BA, Ahn C, Flume PA, Schlosser RJ. The delta F508 mutation in cystic fibrosis and impact on sinus development. American Journal of Rhinology 2007;21:122–7. [DOI] [PubMed] [Google Scholar]
- [28].Do BA, Lands LC, Mascarella MA, Fanous A, Saint-Martin C, Manoukian JJ, et al. Lund-Mackay and modified Lund-Mackay score for sinus surgery in children with cystic fibrosis. Int J Pediatr Otorhinolaryngol 2015;79:1341–5. 10.1016/j.ijporl.2015.06.007. [DOI] [PubMed] [Google Scholar]
- [29].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics 2009;42:377–81. 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lund VJ, Kennedy DW. Quantification for staging sinusitis. The Staging and Therapy Group. The Annals of Otology, Rhinology & Laryngology Supplement 1995;167:17–21. [PubMed] [Google Scholar]
- [31].Uluer A, Ramsey BW, Taylor-Cousar JL, Prais D, Horsley A, Robertson S, et al. VX445–Tezacaftor–Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles. New England Journal of Medicine 2018. 10.1056/NEJMoa1807120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Halderman A, Lee S, London N, Day A, Jain R, Moore J, et al. Impact of high- versus low-risk genotype on sinonasal radiographic disease in cystic fibrosis. The Laryngoscope 2019;129:788–93. 10.1002/LARY.27595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


