Abstract
Osteoarthritis occurs frequently after joint injury. Currently, osteoarthritis is diagnosed by radiographic changes that are typically found after the disease has progressed to multiple tissues. The primary objective was to compare potential metabolomic biomarkers of joint injury between synovial fluid and serum in a mouse model of post-traumatic osteoarthritis. The secondary objective was to gain insight into the pathophysiology of osteoarthritis by examining metabolomic profiles after joint injury. 12-week-old adult female C57BL/6 mice (n=12) were randomly assigned to control, day-1, or day-8 post-injury groups. Randomly selected stifle joints were subjected to a single rapid compression. At days 1 and 8 post-injury, serum was extracted before mice were euthanized for synovial fluid collection. Metabolomic profiling detected ~2500 metabolites across serum and synovial fluid. Of these 179 were positively correlated and 51 were negatively correlated between synovial fluid and serum, indicating potential for development of metabolomic biomarkers. Synovial fluid captured injury-induced differences in metabolomic profiles at both days 1 and 8 after injury whereas serum did not. However, synovial fluid and serum were distinct at both time points after injury. In synovial fluid, pathways of interest mapped to amino acid synthesis and degradation, bupropion degradation, and tRNA charging. In serum, pathways were amino acid synthesis and degradation, the phospholipase pathway, and nicotine degradation. These results provide a rich picture of the injury response at early time points after joint injury. Furthermore, the correlations between synovial fluid and serum metabolites suggest potential to gain insight into intra-articular pathophysiology through analysis of serum metabolites.
Keywords: osteoarthritis, post-traumatic osteoarthritis, joint injury, biomarkers, metabolomics
Introduction
Osteoarthritis (OA) is the most common joint disease in the United States, affecting over 50 million people with a total annual cost exceeding $128 billion1. OA is characterized by loss of cartilage, osteophyte formation, subchondral sclerosis, cysts, and joint space narrowing that manifest as pain and loss of function2. OA has long been considered a disease caused by degeneration of articular cartilage and bone. However, since the late 1990’s, low-grade inflammation and other systemic factors have been increasingly recognized as relevant to the initiation and progression of OA3; 4. Joint injury results in post-traumatic osteoarthritis (PTOA). 50% of people with a diagnosed anterior cruciate ligament (ACL) or meniscus tear develop PTOA within 10–20 years5. Furthermore, imaging techniques can lack sensitivity and do not correlate well with symptoms6. PTOA is commonly diagnosed after significant tissue degeneration leading to current treatments being focused on pain management rather than prevention. These treatments include anti-inflammatories, cortisone injections, arthroscopic surgery, joint arthroplasty, and sometimes joint fusions. However, an emerging paradigm includes metabolic and structural changes within the joint that develop shortly after the initial injury thus providing a potential window for intervention7.
Preclinical mouse models are important tools for studying PTOA. Common ways to induce PTOA in mice include ACL transection (ACLT), destabilization of the medial meniscus (DMM), and intra-articular injections of agents that induce joint degradation8. ACLT and DMM in mice can be challenging procedures and require incisions that prevent interpretation of early time points (e.g., < 10 days). Intra-articular injections, while less invasive, often induce only a single inflammatory pathway which while having major scientific value does not fully replicate in vivo pathophysiology. Non-invasive mouse models of PTOA can provide ACL rupture using tibial compression overload8; 9. This method offers the opportunity to evaluate early time points after injury and observe natural joint processes induced by injury while simulating clinical ACL tears.
Because OA is a heterogenous disease, both diagnosis and treatment are complex10. Current American College of Rheumatology guidelines for diagnosing OA indicate that it is a clinical diagnosis confirmed with radiographs11. However, at the point of radiographic detection, irreversible changes have often already begun12. Additionally, there are currently no FDA-approved drugs to stop or reverse the progression of OA motivating the need to study the window immediately after joint injury.
Metabolites are small molecules that are generated from cellular processes. Metabolomic profiling characterizes large numbers of metabolites (i.e., thousands) from biological samples including blood, urine, synovial fluid, and others via mass spectrometry and other advanced methods13; 14. Metabolomic profiles represent metabolic processes driven by cellular biochemistry and offer insight into phenotypical processes15. Thus, metabolomics has the potential to detect metabolic changes early in the course of PTOA before symptomatic changes which may be useful for clinical treatment including early diagnosis and phenotyping15–17.
PTOA induces injury responses within the joint and systemically18. Synovial fluid is in contact with most of the joint tissues, and metabolomic profiling of synovial fluid has potential to detect biomarkers of injury and characterize mechanisms of OA initiation and development19; 20. However, there are challenges to clinical implementation of synovial fluid biomarker analysis including technical difficulty and expense. Therefore, examination of serum metabolomic profiles may also yield biomarkers and phenotypic data for PTOA. Thus, the objective of this study is to examine metabolomic profiles of synovial fluid and serum in PTOA. To better understand the initiation of PTOA, we characterize changes in metabolomic profiles at early time points after injury. Toward developing serum biomarkers of PTOA, we examine correlations between metabolites co-detected in both the serum and synovial fluid. These results provide greater understanding of the local and systemic response to joint injury and support further development of metabolomic biomarkers for OA as well as potential therapeutic targets.
Materials and methods
Preclinical Mouse Model of PTOA
Adult female C57BL/6 mice (n=12, mass 20.5±0.3g) were purchased from Charles River Labs and housed in the Animal Resource Center at Montana State University. The rationale for using female mice is that arthritis is more prevalent in female patients with an age-adjusted rate of 24.2% in women versus 18.5% in men21. Husbandry conditions included 3–5 mice per cage in specific pathogen free housing in individually-ventilated cages with SaniChip bedding on a 12 hour light-dark cycle at 22°C. After a two-week quarantine, mice were subjected to an established injury model8; 9 at 12 weeks of age. Their care followed NIH guidelines and the protocol was approved by the Montana State University Institutional Animal Care and Use Committee.
Mice were randomly assigned to either control or injury groups and marked on their tails for identification. Of the 12 mice, 8 were injured while 4 served as uninjured controls. For injured mice, the injured stifle (i.e., knee) joint (right or left) was randomized. The experimental sequence was the same for each mouse. Before injury, each mouse was placed in a chamber with 4% isoflurane and 2L/min O2 until anesthetized. Mice were then transferred to a nose cone with 3% isoflurane and 1L/min O2. All mice (injured and control) were then placed into the compression apparatus in a prone position.
The non-invasive injury apparatus consisted of a mechanical testing machine (Test Resources 510LE2, Shakopee, MN) outfitted with custom fixtures designed to position the hindlimb for stifle joint compression. The randomly selected stifle was placed in the cup with the ankle positioned directly above the knee in a fixture that provided ~30° of ankle dorsiflexion. A small pre-load (~1 N) stabilized the limb. For mice in the injured group, a single dynamic axial compression at 130 mm/s with a target of 12N was applied. This caused anterior translation of the tibia in relation to the femur. After each compression, injury was confirmed by comparing anterior-posterior laxity to the contralateral joint. At high displacement rates as used here, this procedure results in mid-substance rupture of the ACL and leads to joint damage8; 9; 22–24.
After injury, while still under anesthesia, all mice were administered 0.04 mL buprenorphine SR subcutaneously and monitored in a single-animal recovery cage until conscious and ambulatory. After recovery, demonstrated by walking, each mouse was placed in a fresh cage with its previous three cage-mates. Control mice went through the exact same procedure as the injured mice including anesthesia, pre-load, and buprenorphine injection without the applied joint overload.
Serum and Synovial Fluid Sample Collection
To examine early time point dynamics of PTOA, n=4 samples were harvested at 1- and 8-days post-injury along with n=2 controls at each time point which were pooled for analysis of n=4 controls. The rationale for these timepoints is that it allows comparison between early and sustained biological processes after injury. Blood was obtained by facial vein stick and collected in a standard red top serum tube microtainer (BD Microtainer Red Tubes No Additive, part number 365963). Approximately 200 μL of blood was collected for each mouse, and tubes were then inverted gently six times to begin activating the clotting factors. Each mouse was then immediately euthanized via cervical dislocation to avoid altering the blood pH through CO2 induced acidification. After allowing 30–60 minutes for activation of clotting factors, serum samples were centrifuged at 1,100 × g for 20 minutes to remove cells and clotting factors. This procedure was repeated for each mouse at their predetermined time point.
Synovial fluid was then extracted using established methodology25. Skin was removed from the injured leg and the tibial plateau was palpated with a scalpel to identify the joint line. The synovial membrane was accessed with an anterior incision through the patellar tendon that was then retracted proximally. A second incision was made through the joint capsule at which point, a 2 mm circular piece of Melgisorb (Tendra, part number 250600; Goteborg, Sweden) was dabbed onto the articular surfaces to adsorb synovial fluid. Once saturated, the Melgisorb was placed into a 1.5 mL microcentrifuge tube containing 35 μL of alginate lyase (Flavobacterium, Sigma-Aldrich A1603-100MG) in High Performance Liquid Chromatography (HPLC) water (1 U/mL concentration). Melgisorb was then digested in a water bath at 34°C for 30 minutes. To chelate calcium ions, 15 μL of 1.0M sodium citrate in HPLC water were added. After ~45 minutes of room temperature digestion, synovial fluid samples were centrifuged at 1100 × g for 15 minutes at 4°C. The supernatant was collected and transferred into a 1.5 mL microcentrifuge tube. All reagents and plasticware were HPLC-grade.
Metabolite Extraction
To extract metabolites, serum and synovial fluid samples followed the same extraction protocol. Both were centrifuged at 500 × g for 5 minutes at 4°C to remove cells and debris. The supernatant was collected and mixed with 80% methanol (80:20 MeOH:H2O, vol/vol) at a ratio of 3:1 (weight/weight) methanol mixture:supernatant. This mixture was kept at −20°C for 30 minutes to precipitate macromolecules. Samples were then vortexed for 3 minutes and centrifuged at 16,100 × g for 5 minutes at 4°C. The supernatant was again removed and transferred to a new 1.5 mL microcentrifuge tube then dried in a vacuum concentrator. Lipids and waxes were eliminated by re-extracting with 250 μL of aqueous acetonitrile solution (1:1 acetonitrile:water, vol/vol) at 0°C for 30 minutes. They were centrifuged at 16,100 × g for 5 minutes at 4°C and the supernatant was collected and dried in a vacuum concentrator. Samples were resuspended with 100 μL of aqueous acetonitrile solution (50:50 acetonitrile:water, vol/vol). They were vortexed for 3 minutes and 50 μL of each sample was transferred to a mass spectrometry tube and stored at −20°C until analysis.
Metabolomics and Data Analysis
Metabolomic profiling was performed as previously described19; 20 using LC-MS (liquid chromatography coupled to mass spectrometry). Chromatography used an Agilent 1290 UPLC system (Agilent, Santa Clara, CA, USA) and mass spectrometry was on an Agilent 6538 Q-TOF mass spectrometer (Agilent Santa Clara, CA, USA) in positive mode. The chromatography used a Cogent Diamond Hydride HILIC 150 × 2.1 mm column (MicroSolv, Eatontown, NJ, USA) in normal phase with established elution methods19. Spectra were analyzed as previously described19. This study used methods previously demonstrated to be effective in identifying possible biomarkers of OA. Raw mass spectrometry data files initially processed using XCMS26 for normalization, alignment, and peak picking.
Data were analyzed in Metaboanalyst27 using four groups: day 1 serum, day 1 synovial fluid, day 8 serum, and day 8 synovial fluid. Processing included normalization, log-transformation, and scaling to remove noise associated with mass spectrometry. Analyses assessed potential metabolic differences between the day 1, day 8, and control profiles for the serum and synovial fluid independently.
We used multivariate statistical methods to analyze metabolomic profiles to determine similarities and differences between serum and synovial fluid, as well as patterns of co-regulated metabolites. The overall variation in the dataset was assessed using unsupervised statistical methods including hierarchical cluster analysis (HCA) based on Euclidean distances and principal component analysis (PCA). To visualize the PCA, data were plotted as scatterplot projections on the principal axes. To assess differentially regulated metabolites, we used two-tailed Student’s t-tests with FDR corrections and volcano plot analysis. For metabolites co-detected between the serum and synovial fluid, we assessed relationships using Pearson correlation coefficients.
Finally, we used our established metabolomic profiling methods20; 28; 29 to identify metabolite clusters using the complete linkage function based on the median metabolite intensities of each group. From each cluster, pathways were assessed based on the clustered metabolites using the MS Peaks to Pathways function within Metaboanalyst. This analysis used a 10-ppm tolerance in positive mode searching mummichog database with a significance threshold of 0.05. The mus musculus pathway set was used from the BioCyc database30. All analysis completed using a priori significance levels of pfdr = 0.05 or for pairwise comparisons, false discovery rate (FDR) corrected significance levels of α = 0.05.
Results
Overview
To examine both the local and systemic response to joint injury, we characterized metabolomic profiles of synovial fluid and serum for control and injured mice. We detected 2544 total metabolite features across the synovial fluid and serum samples (Figure 1). Of these, 2544 were present in synovial fluid, 2543 in serum, and 2533 in both synovial fluid and serum. 11 metabolites were found only in synovial fluid while 10 were found only in serum. While most metabolites were co-detected in both the synovial fluid and serum, only 230 metabolites (~9.0%) were of similar intensity (p > 0.05) between the two media. Both principal components analysis and hierarchical clustering find major differences in metabolomic profiles between serum and synovial fluid (Figure 1B–C). Synovial fluid appears to capture differences in metabolomic profiles between injured mice at both days 1 and 8 whereas serum does not (Figure 1C).
Figure 1.
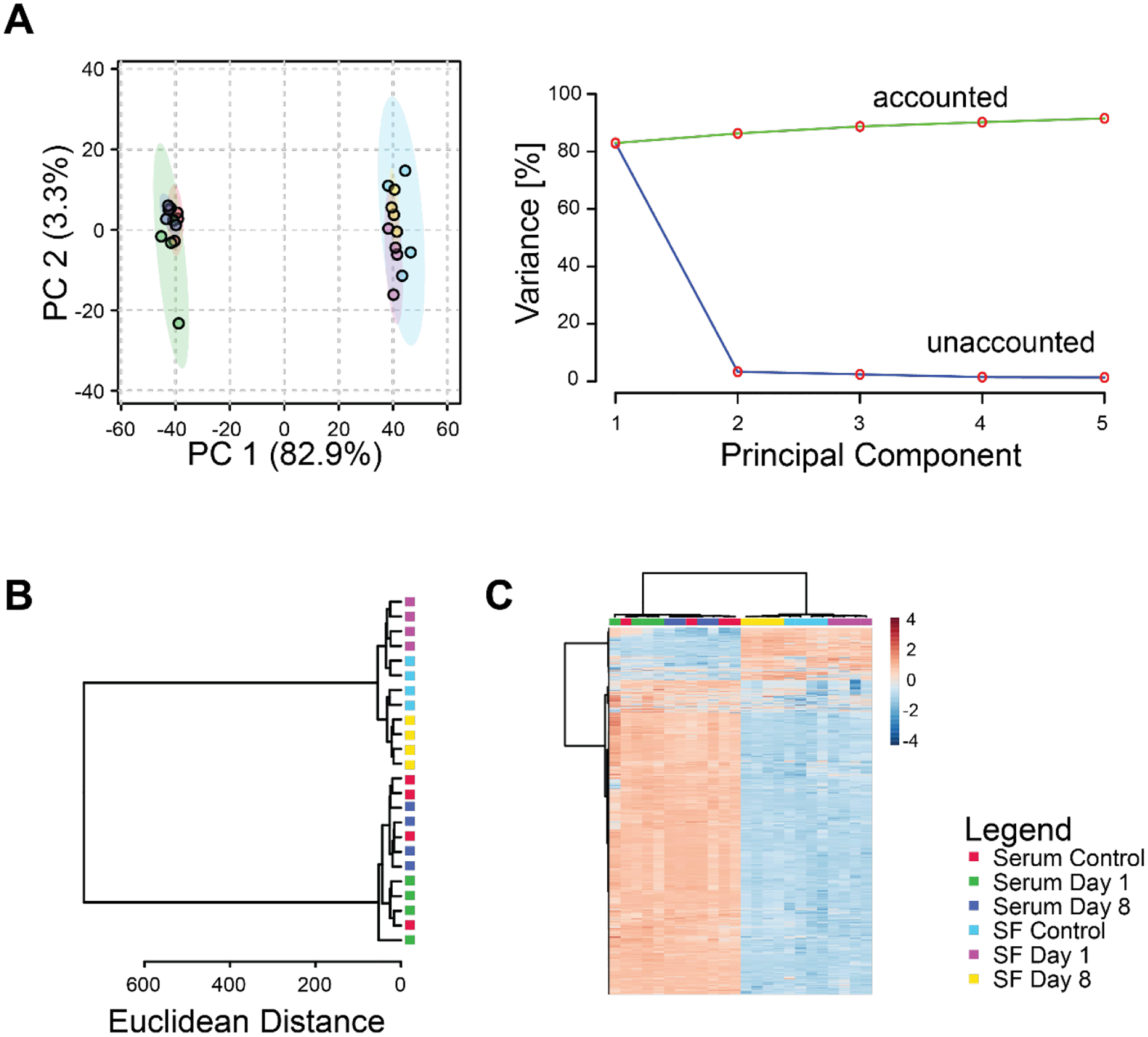
Differences in global metabolomic profiles between serum and synovial fluid. (A) Principal components analysis. Left projection of data onto the first 2 principal components shows distinct separation between metabolomic profiles of serum and synovial fluid. Right: scree plot shows that most of the variance in the overall dataset is associated with the first principal component. (B) Dendrogram of cluster analysis using the Euclidian distance metric and Ward linkage function shows complete separation between serum and synovial fluid samples. Furthermore, the synovial fluid metabolomic profiles were completely separated between experimental groups in contrast to the serum profiles that were not. (C) Heatmap of clustered metabolite intensities shows patterns of metabolite up- and down-regulation between experimental groups.
Injury response within the Synovial Fluid
Metabolomic profiles of synovial fluid showed substantial differences between control and injured mice. Principal components analysis found distinct clustering between controls and injured samples and more than 50% of the overall variance was associated with the first three principal components (Figure 2A). Unsupervised cluster analysis found that both the day 1 and day 8 metabolomic profiles were distinct from controls (Figure 2B–C).
Figure 2.
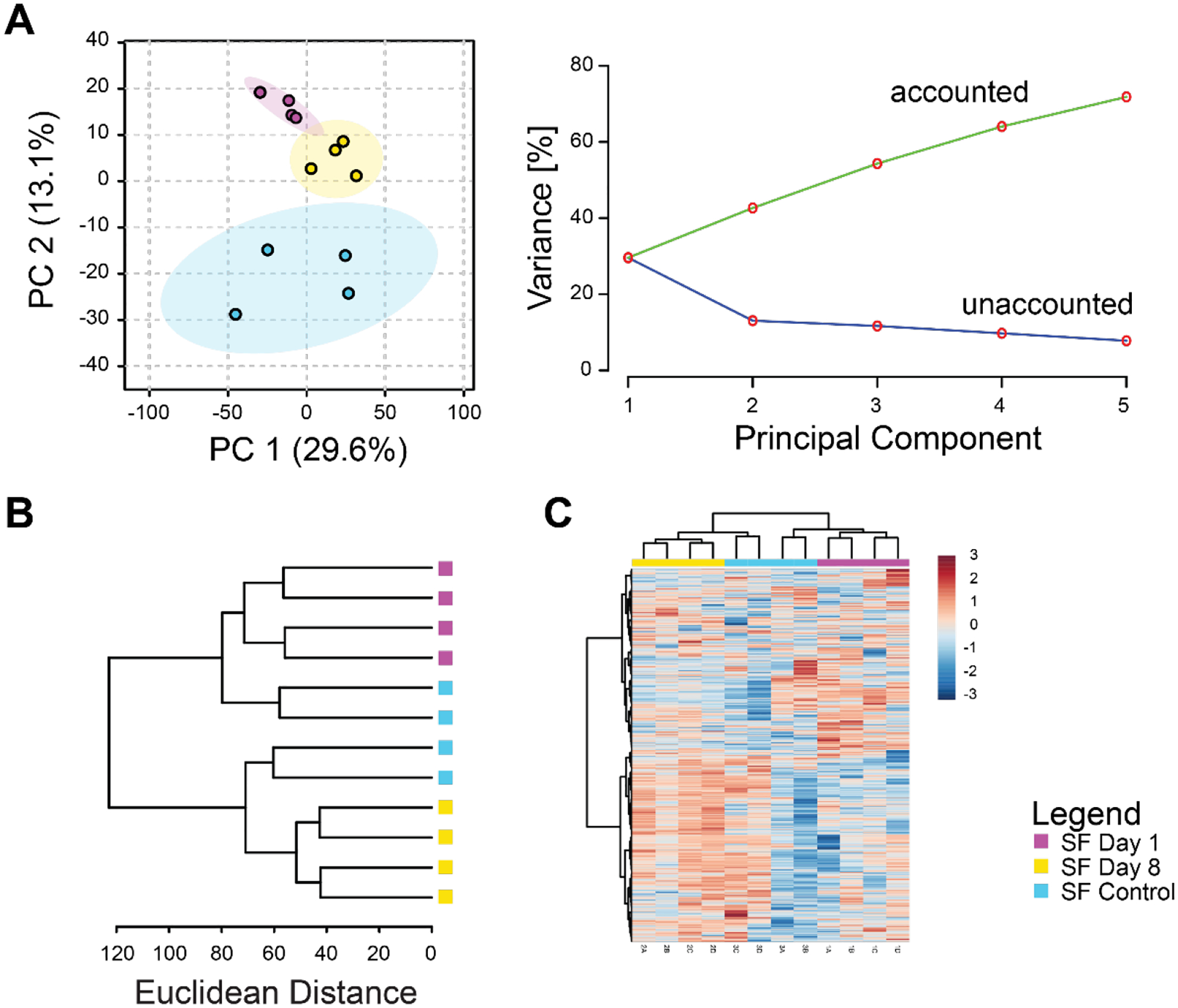
Synovial fluid metabolomic profiles capture changes induced by injury. (A) Principal components analysis. Left projection of data onto the first 2 principal components shows distinct separation between metabolomic profiles of control, 1-day post-injury, and 8-day post-injury synovial fluid. Right: scree plot showing that increasing the number of principal components results in increased modeled variance in the overall dataset. (B) Dendrogram of cluster analysis using the Euclidian distance metric and Ward linkage function shows complete separation between days 1 and 8 post-injury. (C) Heatmap of clustered metabolite intensities shows patterns of metabolite up- and down-regulation between experimental groups.
Injury response within the Serum
Serum metabolomic profiles were affected by joint injury, but less so than synovial fluid (Figures 2A and 3A). Principal components analysis found substantial overlap between controls and injured samples at day 8, but day 1 injured samples were more distinct (Figure 3A). The first three principal components were associated with 69% of the overall variance in the dataset. Unsupervised clustering failed to discriminate consistently between serum metabolomic profiles of control and injured mice with control samples occurring in each main branch of the dendrogram (Figure 3B–C). Nonetheless, clusters B, D, F, and G (Figure 6) all demonstrated changes in the serum at different time points.
Figure 3.
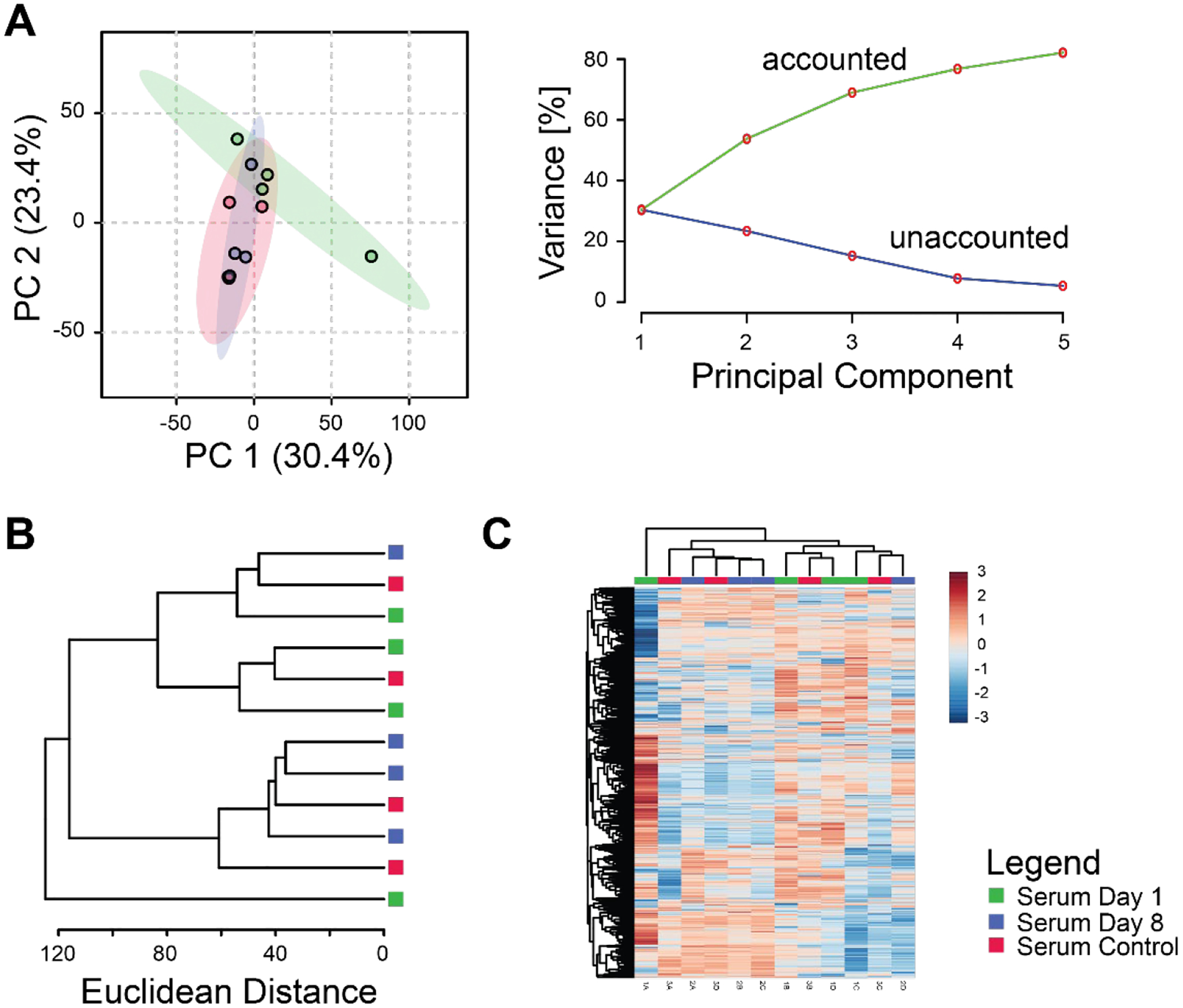
Injury response is partially captured using serum metabolomics. (A) Principal components analysis. Left: projection of data onto the first 2 principal components shows partial separation between serum metabolomic profiles of control, 1-day post-injury, and 8-day mice. Right: scree plot showing that increasing the number of principal components results in increased modeled variance in the overall dataset with a decreasing slope. (B) Dendrogram of cluster analysis using the Euclidian distance metric and Ward linkage function shows partial separation between experimental groups. (C) Heatmap of clustered metabolite intensities shows patterns of metabolite up- and down-regulation between experimental groups.
Figure 6.
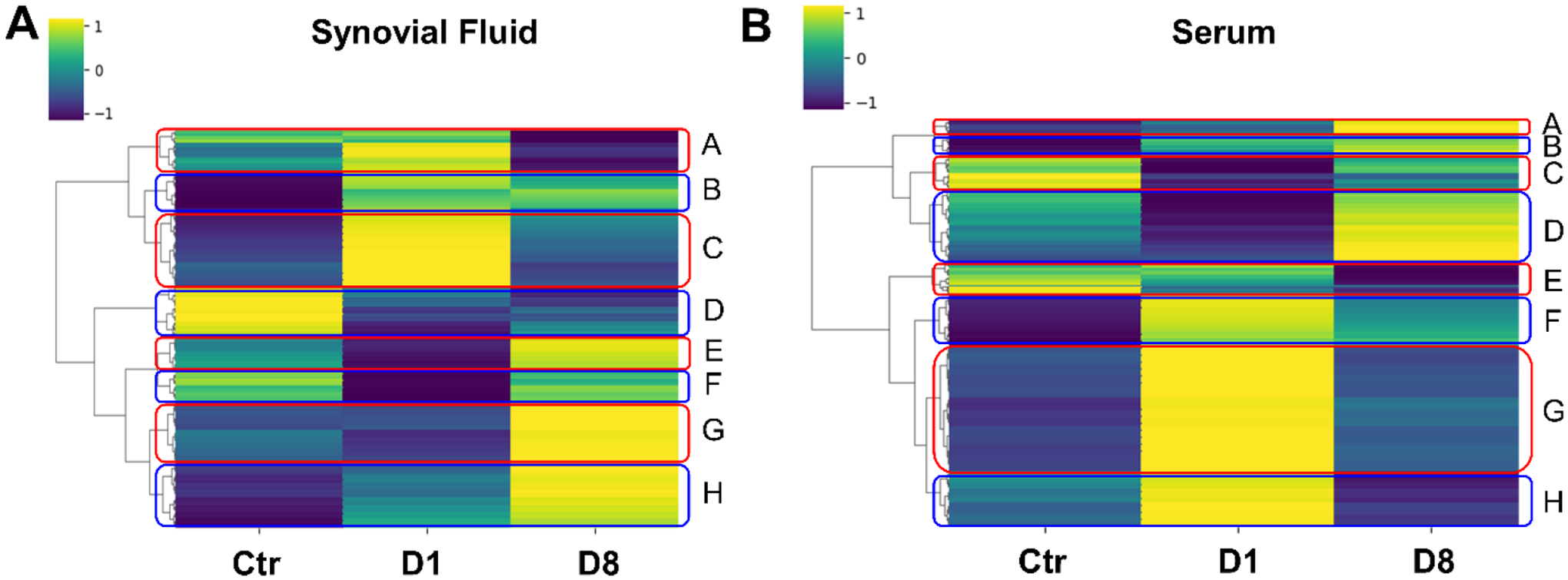
Clusters of coregulated metabolites within serum and synovial fluid indicate pathways relevant to joint injury. (A) Synovial fluid. (B) Serum. Median metabolite intensities for each group were subjected to clustering using the Euclidean distance metric. Clusters (A-H) of metabolites were then analyzed for pathways using Metaboanalyst.
Similarities and Differences between Metabolomic Profiles from Synovial Fluid and Serum
Metabolomic profiles capture responses to joint injury in both serum and the synovial fluid. However, there are substantial differences between metabolomic profiles of serum and synovial fluid at the same time point. At day 1 post-injury, PCA demonstrates clear separation between serum and synovial fluid (Figure 4A). Similar results are found using hierarchical clustering (Figure 4B–C), and volcano plot analysis shows many metabolites either up- or down-regulated in both groups, but the vast majority were upregulated in synovial fluid (Figure 4D). We found similar results when comparing synovial fluid and serum at day 8 post-injury (Figure 4E–H). These results indicate that distinct responses to injury occur between the synovial fluid and serum.
Figure 4.
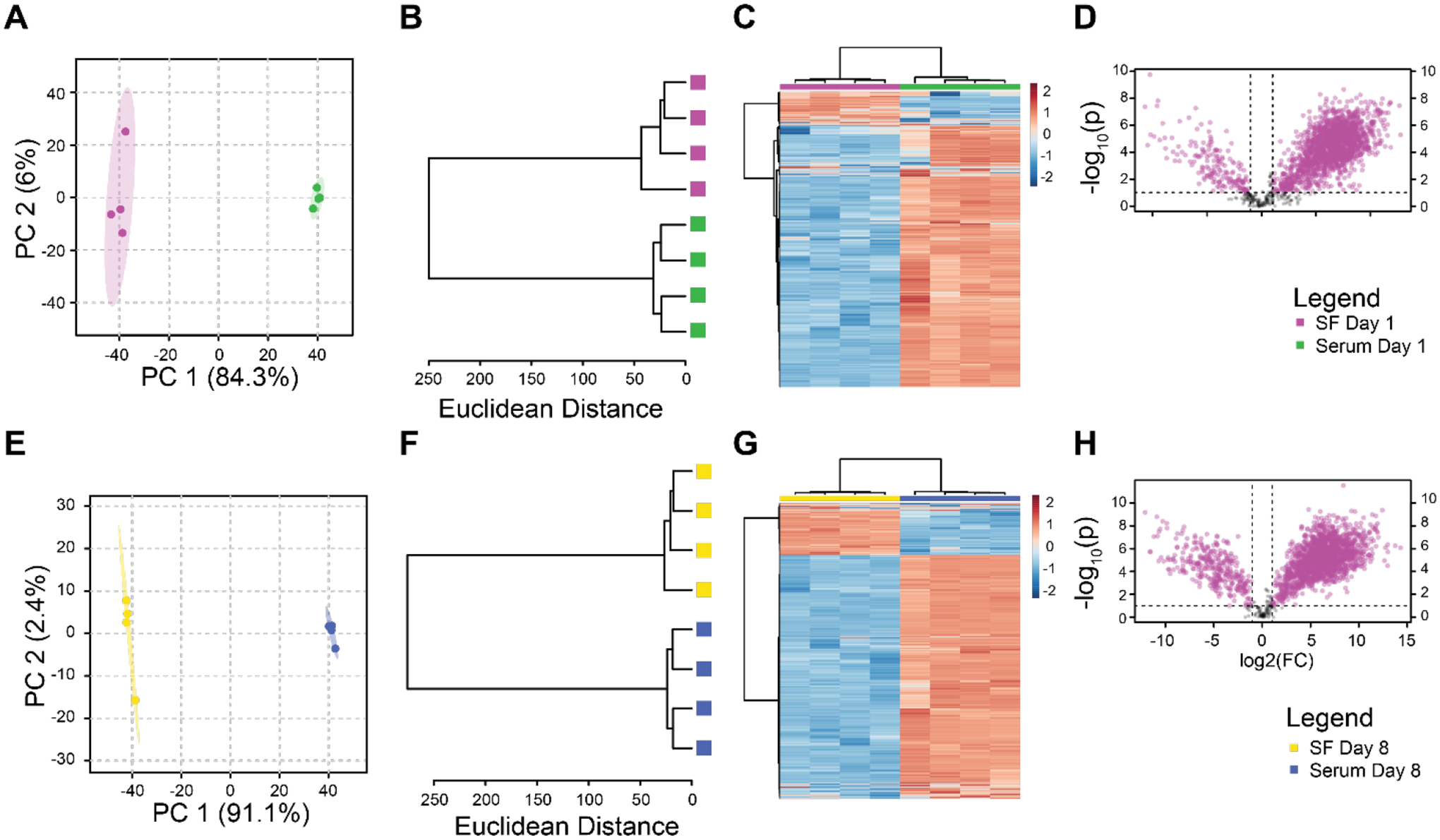
Paired analysis finds distinct metabolomic profiles between synovial fluid and serum at both day 1 and day 8 after injury. Day 1 panels A-D. Day 8 panels E-H. Principal components analysis (A, E). Unsupervised clustering (B, F). Clustered Heatmap (C, G). Volcano Plots (D, H).
While there are substantial differences in metabolite intensities, there were 2533 metabolites jointly detected in both serum and synovial fluid. To assess the potential for quantification of a synovial fluid metabolite level through measurement in the serum, we calculated correlation coefficients for the metabolite intensities between the serum and synovial fluid. There were 230 metabolite features with significant correlations between serum and synovial fluid (p < 0.05, Figure 5A). Of these correlated metabolites, 179 had positive correlations indicating that as the metabolite intensity increased in the serum it also increased in the synovial fluid. Of these positively correlated metabolite features, we identified 58 putative molecules including NADPH and steroid hormones (Figure 5B–C). 51 metabolites were negatively correlated indicating that increased intensity in the synovial fluid was associated with decreased intensity in the serum. Of these negatively correlated features, we identified 17 molecules including GAG monomers (Figure 5D). These significant correlations indicate that there is potential for using serum metabolite intensities to back-calculate synovial fluid metabolite intensities to provide insight into joint biology through assessment of serum metabolomic profiles.
Figure 5.
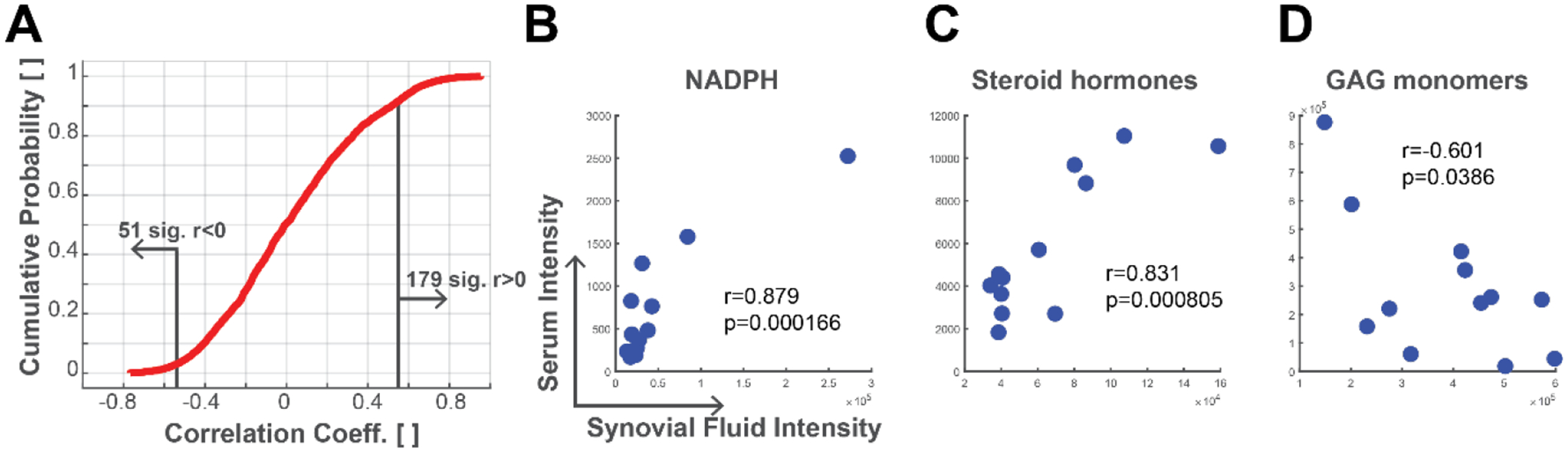
Correlations between synovial fluid and serum metabolites. Metabolite intensities were analyzed for correlations between serum and synovial fluid. (A) Cumulative probability plot of metabolite correlation coefficients. About 10% of co-detected metabolites have significant correlations (p<0.05). (B) Metabolite with putative identification of NADPH had a positive correlation between synovial fluid and serum. (C) Metabolite with putative database match for a steroid hormone including DHEA, testosterone, and androstenedione was positively correlated. (D) Metabolite with putative match representing the GAG monomers of N-acetyl-D-glucosamine and N-acetyl-D-galactosamine was negatively correlated.
Pathways related to Joint Injury in the Synovial Fluid and the Serum
To assess pathways related to joint injury in each of the synovial fluid and serum compartments we identified clusters of co-regulated metabolites (Figure 6). In synovial fluid (Figure 6A), cluster A was neutral in the control group, upregulated in day 1 post-injury, and downregulated in day 8 post-injury with no significant pathways. Cluster B was downregulated in the control group and upregulated in day 1 and day 8 post-injury, but without significant pathways. Cluster C was downregulated in the control group, upregulated in day 1 post-injury, and downregulated in day 8 post-injury, mapping to asparagine degradation. Cluster D was upregulated in the control group and downregulated in day 1 and day 8 post-injury, mapping to tryptophan degradation. Cluster E, which mapped to bupropion degradation, was neutral in the control group, downregulated in day 1 post-injury, and upregulated in day 8 post-injury. Cluster F mapped to tyrosine and phenylalanine degradation and was upregulated in the control group, downregulated in day 1 post-injury, and upregulated in day 8 post-injury. Cluster G was downregulated in the control group and day 1 post-injury but upregulated at day 8 post-injury. This cluster mapped to the tRNA charging pathway. Cluster H was also downregulated in the control group and day 1 post-injury and upregulated in day 8 post-injury but had no significant pathways.
In serum (Figure 6B), cluster A was downregulated in the control group and day 1 post-injury and upregulated at day 8 post-injury but did not map to any significant pathways. Cluster B was downregulated in the control group, neutral in day 1 post-injury, and upregulated at day 8 post-injury, mapping to the tyrosine degradation pathway. Cluster C which had no significant pathways was upregulated in the control group, downregulated in day 1 post-injury, and neutral in day 8 post-injury. Cluster D was neutral in the control group, downregulated in day 1 post-injury, and upregulated at day 8 post-injury and mapped to the phospholipase pathway. Cluster E was upregulated in the control group, neutral in day 1 post-injury, and downregulated in day 8 post-injury with no significant pathways. Cluster F was downregulated in the control group, upregulated in day 1 post-injury, and neutral in day 8 post-injury mapping to arginine synthesis and proline degradation. Cluster G was downregulated in the control group, upregulated in day 1 post-injury, and downregulated at day 8 post-injury. This cluster mapped to the nicotine degradation pathway. Cluster H did not have any significant pathways and was downregulated in the control group, upregulated at day 1 post-injury, and downregulated in day 8 post-injury.
Discussion
Overview
This project provides insight to multiple different aspects of traumatic joint injuries and their effects on homeostasis. First, we gathered data regarding basic joint biology through synovial fluid immediately following traumatic joint injury while simultaneously collecting systemic data using serum. We were then able to compare the two to develop a complex understanding of local and systemic changes following joint injury. Lastly, we worked toward developing a panel of potential biomarkers for osteoarthritis through analyzing correlations between co-detected metabolites between the synovial fluid and the serum compartments.
Injury Response within the Synovial Fluid
The synovial fluid samples demonstrated distinct changes in response to injury (Figure 2A). Five of the eight clusters (Figure 6A, clusters C, D, E, F, and G) related to pathways relevant to osteoarthritis initiation and progression. At day 1 post-injury, cluster C was upregulated and mapped to the asparagine degradation pathway. Amino acids and their metabolites are associated with end-stage osteoarthritis31; 32 but it appears that they may also play a role in acute injury33. However, this pathway became notably downregulated by day 8 post-injury indicating emphasis on an immediate response following injury.
At day 8 post-injury, clusters E, F, and G were upregulated. The pathway of interest in cluster E was bupropion degradation which likely indicates metabolism of the buprenorphine given for pain relief during the experiment. Interestingly, this pathway was downregulated in day 1 post-injury before becoming upregulated at day 8 post-injury. However, the extended-release form has a half-life of 43 to 60 days as described by the manufacturer which suggests why its metabolites were not found in the synovial fluid on day 1 post-injury.
In cluster F, the notable pathway mapped to tyrosine degradation. After a cell undergoes tyrosine hydroxylation it becomes catecholaminergic. One study showed that tyrosine hydroxylase positive cells were only present in the synovium of patients with OA, not the controls34. These anti-inflammatory releasing catecholaminergic cells may replace sympathetic nerve fibers which have grown into the cartilage over the progression of the disease and counteract the pro-inflammatory environment in the joint35; 36. While downregulated in our study at day 1 post-injury, by day 8 post-injury, upregulation had occurred which may mark the initiation of catecholaminergic cells infiltrating the cartilage. The tRNA charging pathway was the notable pathway in cluster G. Canonically tRNA carries specific amino acids and matches them with mRNA codons during translation. After joint injury, protein production is increased to perform numerous functions within the cells. Our data suggests that immediately following the injury this process is downregulated but becomes upregulated by day 8 post-injury.
Only cluster D was upregulated in the control group. Cluster D mapped to the tryptophan degradation pathway which has been cited as a promising biomarker for osteoarthritis37; 38. Indoleamine 2,3-dioxygenase is responsible for degrading tryptophan into kynurenine and is induced by pro-inflammatory cytokines to counteract inflammation33; 39. The effect of indoleamine 2,3-dioxygenase and its role in inflammation is further being explored.
Injury Response within the Serum
As a whole, the differences in serum between the control group, day 1 post-injury, and day 8 post-injury were less distinct than in the synovial fluid (Figure 3). That fewer changes are induced in the serum compartment compared to the synovial fluid is a novel finding of this study. This likely reflects the proximity of the synovial fluid to the injury location. Yet the limited hierarchical clustering of the serum (Figure 3B) may also indicate that joint-derived metabolites represent a small fraction of the overall serum metabolome, consistent with the fraction of correlations detected in this study. However in contrast, four of the eight serum clusters (Figure 6B, clusters B, D, F, and G) showed changes in the serum across different time points. The pathways of interest for each cluster are discussed in the following paragraphs.
The clusters upregulated at day 1 post-injury included clusters F and G. Cluster F’s metabolites mapped to synthesis of the amino acid arginine and degradation of the amino acid proline. Arginine is relevant to inflammatory disease given its role as an anti-inflammatory and has recently been shown to have decreased concentrations in patients with long-standing OA31; 40; 41. Arginine is also a precursor for proline which contributes to collagen and polyamine synthesis that results in fibrosis of tissue, further leading to OA42. Our data demonstrates upregulation of these pathways on day 1 post-injury which indicates an initial increase in arginine synthesis and proline breakdown, both of which are protective in the short-term but damaging in the long-term.
The metabolites of interest in cluster G mapped to the nicotine degradation pathway. Nicotine protects against joint inflammation and cartilage degradation in rodent models43; 44. Upregulation of nicotine degradation at day 1 post-injury in the serum indicates the protective role of such a pathway immediately following traumatic injury. However, at day 8 post-injury, nicotine degradation became downregulated which suggests that it provides only short-term anti-inflammatory properties.
At day 8 post-injury, clusters B and D had important upregulated pathways. The metabolites found in cluster B mapped to the tyrosine degradation pathway which consists of catecholamines and melanin. Not only are catecholaminergic cells upregulated within the joint as mentioned previously, but also throughout the body, most notably in lymphoid organs. During an in-vivo mouse study using collagen-induced arthritis, tyrosine hydroxylase positive cells were found to be denser in draining lymph nodes, the thymus, and joints even prior to the onset of arthritis (days 5–21) indicating measurable changes prior to objective evidence of osteoarthritis45. Our data supports this notion as this cluster was downregulated in the control group but became neutral in day 1 post-injury and upregulated by day 8 post-injury, indicating a gradual systemic catecholaminergic response to traumatic joint injury. Melanin, another byproduct of tyrosine, is the pigment that darkens skin. Additionally, it is regarded as a potent anti-oxidative agent. Zhong et al. found that intra-articular injection of dopamine melanin nanoparticles sequestered numerous reactive oxygen species including hydroxyl radicals and superoxides. This was chondroprotective and led to decreased inflammatory cytokine release, decreased proteoglycan loss, and slower cartilage degradation when tested in rodent OA models undergoing ACLT surgery46.
In cluster D, metabolites of interest mapped to the phospholipase pathway which showed an initial downregulation prior to upregulation at day 8 post-injury. Phospholipase and its derivatives such as phosphatidylinositol-3-kinase (PI3K) and phospholipase C (PLC) make up many of the cell signaling pathways including cell proliferation, intracellular trafficking, cell motility, cytoskeletal regulation, and ultimately cell death. Multiple studies have demonstrated that osteoblasts in joints with both PTOA and OA undergo modulated gene expression due to targeting of these pathways47; 48.
Differences between Metabolomic Profiles from Synovial Fluid and Serum
Overall, there were substantial differences between synovial fluid and serum metabolites across all time points (Figures 1, 4). PCA of all samples and all groups found complete separation between synovial fluid and serum with just a single component associated with 82.9% of the overall variation in the data. There was also distinct separation in synovial fluid between the control group and days 1 and 8 post-injury, with days 1 and 8 post-injury showing moderate differences (Figure 2). In contrast, the injury-induced differences in serum were not as strong, as all time points overlapped on PCA analysis (Figure 3). Paired analysis on day 1 post-injury showed that 84.3% variance between synovial fluid and serum was associated with the first principal component, and increased to 91.1% on day 8 post-injury. Previous studies have also found that a minority of metabolites are correlated between the two fluids49. These data show that in this mouse model of PTOA both synovial fluid and serum capture injury-induced differences in metabolomic profiles, but the synovial fluid profiles exhibit stronger and more sustained differences post-injury.
Limitations
While this study shows that metabolomic profiles of synovial fluid and serum are responsive to non-invasive mouse injury, there are important limitations that should be considered. We used n=4 mice per group including pooling two control mice from each time point as no prior data was available to perform a power analysis. While mice joints have similarities with human joints, there are inherent differences between mice and humans (e.g. mouse physes remain open in mature animals compared to adolescent closure in humans, quadruped versus bipedal gate, etc) that may limit eventual clinical application of these data. Furthermore, the timescale of 1- and 8-days post-injury likely represents a much longer period in humans. Additionally, these time points might have metabolic processes occurring between them50. While this study characterized thousands of metabolites, advances in metabolomics such as secondary fragmentation could provide more specific identifications of key metabolites that are affected by injury. Several key questions remain about joint injury including which cells and tissues are primarily characterized by synovial fluid analysis, potential differences between the male and female responses to injury, and the potential for translation of murine responses to humans.
Summary
In this study, mice were subjected to non-invasive traumatic joint injury before sampling of synovial fluid and serum. Metabolomic profiles differed between these compartments with synovial fluid providing a more detailed description of injury-induced changes than serum. Improved understanding of these metabolomic profiles and the underlying cellular pathways that are activated post-injury may yield novel treatment strategies for osteoarthritis. Additionally, key metabolites have the potential to serve as biomarkers of joint injury, and potentially other types of joint disease. That we observed several metabolites correlated between the synovial fluid and serum compartments indicates that future studies might develop serum metabolomic profiles that contain information from the synovial fluid for potential clinical use (e.g. biomarkers).
Supplementary Material
Acknowledgements
Funding provided by NSF (CMMI 1554708) and NIH (R01AR073964). Dr. June owns stock in Beartooth Biotech but this company was not involved in this study.
Footnotes
Supplementary Information
Supplemental File 1 contains the metabolomics peak data used for processing in this study. Column 1 contains the mass to charge ratios labeled as m/z for each metabolite, and the remaining columns are the peak intensities for each sample.
Supplemental File 2 contains the co-detected metabolites which are significantly correlated along with the correlation coefficients and p-values.
References
- 1.Ma VY, Chan L, Carruthers KJ. 2014. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil 95:986–995.e981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayashi D, Roemer FW, Guermazi A. 2016. Imaging for osteoarthritis. Ann Phys Rehabil Med 59:161–169. [DOI] [PubMed] [Google Scholar]
- 3.Bondeson J, Blom AB, Wainwright S, et al. 2010. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum 62:647–657. [DOI] [PubMed] [Google Scholar]
- 4.Pelletier JP, Martel-Pelletier J, Abramson SB. 2001. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum 44:1237–1247. [DOI] [PubMed] [Google Scholar]
- 5.Lohmander LS, Englund PM, Dahl LL, et al. 2007. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med 35:1756–1769. [DOI] [PubMed] [Google Scholar]
- 6.Hannan MT, Felson DT, Pincus T. 2000. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol 27:1513–1517. [PubMed] [Google Scholar]
- 7.Wu P, Holguin N, Silva MJ, et al. 2014. Early response of mouse joint tissue to noninvasive knee injury suggests treatment targets. Arthritis Rheumatol 66:1256–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christiansen BA, Guilak F, Lockwood KA, et al. 2015. Non-invasive mouse models of post-traumatic osteoarthritis. Osteoarthritis Cartilage 23:1627–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christiansen BA, Anderson MJ, Lee CA, et al. 2012. Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis. Osteoarthritis Cartilage 20:773–782. [DOI] [PubMed] [Google Scholar]
- 10.Driban JB, Sitler MR, Barbe MF, et al. 2010. Is osteoarthritis a heterogeneous disease that can be stratified into subsets? Clin Rheumatol 29:123–131. [DOI] [PubMed] [Google Scholar]
- 11.Altman R, Asch E, Bloch D, et al. 1986. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 29:1039–1049. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Oo WM, Linklater JM. 2018. What is the role of imaging in the clinical diagnosis of osteoarthritis and disease management? Rheumatology (Oxford) 57:iv51–iv60. [DOI] [PubMed] [Google Scholar]
- 13.Carlson AK, Rawle RA, Wallace CW, et al. 2019. Global metabolomic profiling of human synovial fluid for rheumatoid arthritis biomarkers. Clin Exp Rheumatol 37:393–399. [PubMed] [Google Scholar]
- 14.Guma M, Tiziani S, Firestein GS. 2016. Metabolomics in rheumatic diseases: desperately seeking biomarkers. Nat Rev Rheumatol 12:269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smolinska A, Blanchet L, Buydens LM, et al. 2012. NMR and pattern recognition methods in metabolomics: from data acquisition to biomarker discovery: a review. Anal Chim Acta 750:82–97. [DOI] [PubMed] [Google Scholar]
- 16.van der Greef J, Stroobant P, van der Heijden R. 2004. The role of analytical sciences in medical systems biology. Curr Opin Chem Biol 8:559–565. [DOI] [PubMed] [Google Scholar]
- 17.Lotz M, Martel-Pelletier J, Christiansen C, et al. 2014. Republished: Value of biomarkers in osteoarthritis: current status and perspectives. Postgrad Med J 90:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maerz T, Sherman E, Newton M, et al. 2018. Metabolomic serum profiling after ACL injury in rats: A pilot study implicating inflammation and immune dysregulation in post-traumatic osteoarthritis. J Orthop Res 36:1969–1979. [DOI] [PubMed] [Google Scholar]
- 19.Carlson AK, Rawle RA, Adams E, et al. 2018. Application of global metabolomic profiling of synovial fluid for osteoarthritis biomarkers. Biochem Biophys Res Commun 499:182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlson AK, Rawle RA, Wallace CW, et al. 2019. Characterization of synovial fluid metabolomic phenotypes of cartilage morphological changes associated with osteoarthritis. Osteoarthritis Cartilage 27:1174–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theis KA, Murphy LB, Guglielmo D, et al. 2021. Prevalence of Arthritis and Arthritis-Attributable Activity Limitation - United States, 2016–2018. MMWR Morb Mortal Wkly Rep 70:1401–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brodt MD, Silva MJ. 2010. Aged mice have enhanced endocortical response and normal periosteal response compared with young-adult mice following 1 week of axial tibial compression. J Bone Miner Res 25:2006–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lynch ME, Main RP, Xu Q, et al. 2010. Cancellous bone adaptation to tibial compression is not sex dependent in growing mice. Journal of Applied Physiology 109:685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Souza RL, Matsuura M, Eckstein F, et al. 2005. Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element. Bone 37:810–818. [DOI] [PubMed] [Google Scholar]
- 25.Seifer DR, Furman BD, Guilak F, et al. 2008. Novel synovial fluid recovery method allows for quantification of a marker of arthritis in mice. Osteoarthritis Cartilage 16:1532–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tautenhahn R, Patti GJ, Rinehart D, et al. 2012. XCMS Online: a web-based platform to process untargeted metabolomic data. Anal Chem 84:5035–5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia J, Wishart DS. 2016. Using MetaboAnalyst 3.0 for Comprehensive Metabolomics Data Analysis. Curr Protoc Bioinformatics 55:14 10 11–14 10 91. [DOI] [PubMed] [Google Scholar]
- 28.Jutila AA, Zignego DL, Hwang BK, et al. 2014. Candidate mediators of chondrocyte mechanotransduction via targeted and untargeted metabolomic measurements. Arch Biochem Biophys 545:116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zignego DL, Hilmer JK, June RK. 2015. Mechanotransduction in primary human osteoarthritic chondrocytes is mediated by metabolism of energy, lipids, and amino acids. J Biomech 48:4253–4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanehisa M 2017. Enzyme Annotation and Metabolic Reconstruction Using KEGG. Methods Mol Biol 1611:135–145. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Xiao W, Luo W, et al. 2016. Alterations of amino acid metabolism in osteoarthritis: its implications for nutrition and health. Amino Acids 48:907–914. [DOI] [PubMed] [Google Scholar]
- 32.McNearney T, Speegle D, Lawand N, et al. 2000. Excitatory amino acid profiles of synovial fluid from patients with arthritis. J Rheumatol 27:739–745. [PMC free article] [PubMed] [Google Scholar]
- 33.Leimer EM, Tanenbaum LM, Nettles DL, et al. 2018. Amino Acid Profile of Synovial Fluid Following Intra-articular Ankle Fracture. Foot Ankle Int 39:1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Capellino S, Cosentino M, Wolff C, et al. 2010. Catecholamine-producing cells in the synovial tissue during arthritis: modulation of sympathetic neurotransmitters as new therapeutic target. Ann Rheum Dis 69:1853–1860. [DOI] [PubMed] [Google Scholar]
- 35.Grassel S, Muschter D. 2017. Peripheral Nerve Fibers and Their Neurotransmitters in Osteoarthritis Pathology. Int J Mol Sci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suri S, Gill SE, Massena de Camin S, et al. 2007. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann Rheum Dis 66:1423–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang KY, Lee SH, Jung SM, et al. 2015. Downregulation of Tryptophan-related Metabolomic Profile in Rheumatoid Arthritis Synovial Fluid. J Rheumatol 42:2003–2011. [DOI] [PubMed] [Google Scholar]
- 38.Igari T, Tsuchizawa M, Shimamura T. 1987. Alteration of tryptophan metabolism in the synovial fluid of patients with rheumatoid arthritis and osteoarthritis. Tohoku J Exp Med 153:79–86. [DOI] [PubMed] [Google Scholar]
- 39.van der Goot AT, Nollen EA. 2013. Tryptophan metabolism: entering the field of aging and age-related pathologies. Trends Mol Med 19:336–344. [DOI] [PubMed] [Google Scholar]
- 40.Ohnishi A, Osaki T, Matahira Y, et al. 2013. Correlation of plasma amino acid concentrations and chondroprotective effects of glucosamine and fish collagen peptide on the development of osteoarthritis. J Vet Med Sci 75:497–502. [DOI] [PubMed] [Google Scholar]
- 41.Tootsi K, Vilba K, Martson A, et al. 2020. Metabolomic Signature of Amino Acids, Biogenic Amines and Lipids in Blood Serum of Patients with Severe Osteoarthritis. Metabolites 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rockel JS, Kapoor M. 2018. The Metabolome and Osteoarthritis: Possible Contributions to Symptoms and Pathology. Metabolites 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gu Q, Li D, Wei B, et al. 2015. Effects of nicotine on a rat model of early stage osteoarthritis. Int J Clin Exp Pathol 8:3602–3612. [PMC free article] [PubMed] [Google Scholar]
- 44.Teng P, Liu Y, Dai Y, et al. 2019. Nicotine Attenuates Osteoarthritis Pain and Matrix Metalloproteinase-9 Expression via the alpha7 Nicotinic Acetylcholine Receptor. J Immunol 203:485–492. [DOI] [PubMed] [Google Scholar]
- 45.Capellino S, Weber K, Gelder M, et al. 2012. First appearance and location of catecholaminergic cells during experimental arthritis and elimination by chemical sympathectomy. Arthritis Rheum 64:1110–1118. [DOI] [PubMed] [Google Scholar]
- 46.Zhong G, Yang X, Jiang X, et al. 2019. Dopamine-melanin nanoparticles scavenge reactive oxygen and nitrogen species and activate autophagy for osteoarthritis therapy. Nanoscale 11:11605–11616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zini N, Lisignoli G, Solimando L, et al. 2005. Quantitative immunodetection of key elements of polyphosphoinositide signal transduction in osteoblasts from arthritic patients shows a direct correlation with cell proliferation. Histochem Cell Biol 124:131–137. [DOI] [PubMed] [Google Scholar]
- 48.Zini N, Lisignoli G, Solimando L, et al. 2003. IL1-beta and TNF-alpha induce changes in the nuclear polyphosphoinositide signalling system in osteoblasts similar to that occurring in patients with rheumatoid arthritis: an immunochemical and immunocytochemical study. Histochem Cell Biol 120:243–250. [DOI] [PubMed] [Google Scholar]
- 49.Zhang W, Likhodii S, Aref-Eshghi E, et al. 2015. Relationship between blood plasma and synovial fluid metabolite concentrations in patients with osteoarthritis. J Rheumatol 42:859–865. [DOI] [PubMed] [Google Scholar]
- 50.Anderson DD, Chubinskaya S, Guilak F, et al. 2011. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res 29:802–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


